Abstract
Objective
Differences in body mass index (BMI) were used to analyze the survival and prognosis of SCCHN patients.
Patients and Methods
A retrospective cohort study was conducted to select 323 patients who underwent surgical treatment for SCCHN from June 2013 to June 2016. The patients were divided into a healthy BMI group (BMI<24kg/m2), an overweight group (24kg/m2≤BMI<28kg/m2) and an obese group (BMI≥28 kg/m2). Various statistical methods were used to summarize and analyze clinical data, complications, disease specific survival (DSS), the overall survival (OS), and recurrence-free survival (RFS) within the last 3 y.
Results
At 3 y, OS (54.40%) and DSS (51.94%) were slightly lower in the obese group compared with the overweight (64.62%, 61.92%) and healthy BMI groups (64.66%, 65.02%), but no statistical significance was found in DSS (P=0.178), OS (P=0.123) and RFS (P=0.362). The difference in operation duration (P=0.008) and bleeding volume (P=0.001) in obese patients was consistent with those in diabetes mellitus (P=0.002) and coronary heart disease (P=0.000). A high incidence of pharyngeal fistula was observed in obese (P=0.014) and overweight patients (P=0.025), but mouth floor fistula (P=0.038), lung infection (P=0.047), fat liquefaction (P=0.003) and lower extremities deep venous thrombosis (P=0.020) were only found in the obese group. Cox univariatable and multivariatable analysis showed that clinical stage, T stage, and N stage were independent prognostic factors for patients with SCCHN, which was not related to BMI.
Conclusion
BMI was associated with a higher probability of complications. However, BMI had no significant correlation with 3-year OS, RFS and DSS, and was not a prognostic indicator for patients with SCCHN.
Keywords: body mass index, SCCHN, DSS, OS, RFS, prognosis
Introduction
Overweight and obesity, a global disease, have become a research focus in many fields. According to a recent study published in 2019,1 more than one third (36.5%) of adults in the United States are obese (body mass index (BMI)≥30 kg/m2). NCD Risk Factor Collaboration (NCD-RisC)2 reported that 1698 studies, completed in 200 countries over the past 40 y, involving a total of 19.2 million participants, showed that the prevalence of overweight and obesity in adults has increased significantly from less than 25% to nearly 40% in both men and women, both in developed and developing areas. A study involving 6519 subjects from five provinces and cities in China showed that the total incidence of overweight and obesity in urban and rural residents in China was 35.2% in 2016.3 The rapid growth of overweight and obesity has become an important public health problem.4,5 To date, there have not been any successful strategies for preventing overweight and obesity, and more health challenges have appeared in children and the elderly in the past 33 years.4–6 An increasing number of studies have found that obesity is not only a risk factor for metabolic syndrome, diabetes, cardiovascular and cerebrovascular diseases, but also a variety of malignant tumors.7,8 The International Agency for Research on Cancer has indicated that obesity is a risk factor for gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, postmenopausal breast cancer, and other malignant tumors, and that BMI also has a significant dose-response relationship with cancer risk.9 Global population data for 2012 show that countries with a high incidence of overweight and obesity have a significantly increased number of patients with new malignant tumors comparison with BMI health groups.9,10
At present, most research on obesity in the field of head and neck surgery focuses on obstructive sleep apnea syndrome,11,12 few studies have reported on head and neck squamous cell carcinoma (SCCHN). Iyengar et al13,14 found that BMI is an independent variable affecting the prognosis of early tongue cancer, and that obese patients have a poor prognosis; these findings differ from the results reported by Hicks et al.15 In our clinical practice, we found that the incidence of complications such as pharyngeal fistula, mouth floor fistula, lung infection, fat liquefaction, and lower extremities deep venous thrombosis (LEDVT) in obese patients is greater than in patients at a healthy weight. These factors directly affect prognosis and quality of life.
Patients and Methods
Ethical Approval of the Study Protocol
The study protocol was approved by the Ethics Committee of the Affiliated Tumor Hospital of Zhengzhou University (Zhengzhou, China) compliance with the Declaration of Helsinki, all patient consent to review their medical records.
General Information
We selected 323 SCCHN patients who underwent head and neck surgery at the Affiliated Cancer Hospital of Zhengzhou University from January 2013 to January 2016. Clinical and pathological data, treatment information and the 3-year survival rate were analyzed for 323 patients, and all patients were followed up at the same time intervals. BMI was based on clinical statistics obtained for each patient. The BMI calculation formula is weight (kg)/height (m2). A total of 323 patients were divided into a healthy BMI group (BMI<24kg/m2), an overweight group (24kg/m2≤BMI <28kg/m2), and an obese group (BMI≥28kg/m2) in accordance with Chinese standards.16 The clinicopathological parameters of the patients enrolled included age, gender, smoking, alcohol consumption, Human Papillomavirus (HPV) status, weight loss, diabetes, coronary heart disease (CHD), tumor staging, lymph node staging, and clinical staging. Primary site, surgical methods, neck dissection, operation duration, intraoperative blood loss, and postoperative therapy were included in treatment-related indicators, as well as complications and mean hospitalization days.
Follow-Up
Each patient was regularly reviewed after surgery for at least 3 years, and all patients were followed up to November 1, 2019 or died. Patients were reviewed every 3 months in the first year after surgery, every 6 months in the second to third year, and once a year from the fourth year. If any discomfort was experienced during the follow-up period, the patients were free to attend an outpatient clinic at any time. The Database of medical record and outpatient appointment follow-ups were used to record data at each time point. All studies were independently checked twice by two experienced head and neck tumor surgeons to ensure data accuracy.
Study End Points and Statistical Analysis
Disease specific survival (DSS), the overall survival rate (OS), and recurrence-free survival (RFS) were the study end points. DSS was defined as the time from surgery to death because of SCCHN, and OS was the time from biopsy to all-cause mortality. RFS was defined as the time from surgery to any recurrence. All data were analyzed and plotted using SPSS l7.0 and GraphPad Prism 8.0 software. Differences between the healthy BMI group, the overweight group, and the obese group were evaluated through a Kruskal–Wallis test; then, a rank-sum test was performed for comparison of group pairs of continuous variables, and chi-squared tests were performed for categorical variables. Cox proportional hazard models were used to generate hazard ratios (HR) with 95% confidence intervals (95% CI) in univariable and multivariable analysis. Correlation between some clinical variables (such as age, gender, alcohol, smoking, T stage, N stage, and clinical stage) and BMI at diagnosis were calculated based on logistic regression treating the variables as outcomes and BMI as a predictor, only effective variables are analyzed in multivariate analysis with BMI. P<0.05 was considered to indicate statistical significance.
Results
A total of 323 patients were enrolled in this study, including 137 cases (42.41%) in the healthy BMI group (42.41%), 109 cases (33.75%) in the overweight group and 77 cases (23.84%) in the obese group (Table 1), and the median follow-up time is 47.6 weeks. Among the 323 SCCHN patients, older (≥60 y, 63.47%) men (63.78%) accounted for nearly two-thirds, as previously reported (11–13). However, there was no difference in these groups, and this was similar for T stage, N stage, and clinical stage. Frequent alcohol consumption (58.51%) and occasional alcohol consumption accounted for the majority of the 323 cases, and this was similar for smoking. Interestingly, a further investigation of 34 women who did not smoke showed that 15 (55.88%) of the women had a spouse who was a frequent smoker for several decades. In terms of 16 HPV viral infections, only eight patients were HPV-positive (2.48%), which is a lower HPV-positive rate than previously reported (13–14). We also found that the numbers of patients with diabetes mellitus (P=0.002) and CHD (P=0.001) were significantly different between the three groups, which may be related to the high incidence of diabetes and CHD in obese patients. Surprisingly, we did not find any difference in preoperative weight loss (P=0.264) between the three groups.
Table 1.
Baseline Characteristics of SCCHN Patients (N=323)
| Clinicopathological Variables | All (N = 323) | Healthy BMI (N = 137) | Overweight (N = 109) | Obese (N = 77) | P |
|---|---|---|---|---|---|
| Age (y) | |||||
| ≥60 y | 205 (63.47%) | 81 (59.12%) | 71 (65.14%) | 53 (68.83%) | |
| <60 y | 118 (36.53%) | 56 (40.88%) | 38 (34.86%) | 24 (31.17%) | 0.333 |
| Gender | |||||
| Female | 117 (36.22%) | 50 (36.50%) | 39 (35.78%) | 28 (36.37%) | |
| Male | 206 (63.78%) | 87 (63.50%) | 70 (64.22%) | 49 (63.63%) | 0.993 |
| Alcohol | |||||
| Frequent | 189 (58.51%) | 83 (60.58%) | 65 (59.63%) | 41 (53.25%) | |
| Occasional | 92 (28.48%) | 40 (29.20%) | 32 (29.36%) | 20 (25.97%) | |
| Never | 42 (13.01%) | 14 (10.22%) | 12 (11.01%) | 16 (20.78%) | 0.245 |
| Smoking | |||||
| Frequent | 177 (54.80%) | 80 (58.39%) | 58 (53.21%) | 39 (50.65%) | |
| Occasional | 89 (27.55%) | 38 (27.74%) | 29 (26.61%) | 22 (28.58%) | |
| Never | 57 (17.65%) | 19 (0.138%) | 22 (20.18%) | 16 (20.78%) | 0.624 |
| HPV-16 Ssub-Type | |||||
| Positive | 8 (2.48%) | 4 (2.92%) | 2 (1.83%) | 2 (2.58%) | |
| Negative | 315 (97.52%) | 133 (97.08%) | 107 (98.17%) | 75 (97.42%) | 0.860 |
| Pre-Diagnosis Weight Loss | |||||
| Yes | 206 (63.78%) | 87 (63.50%) | 75 (68.81%) | 44 (57.14%) | |
| No | 117 (36.22%) | 50 (36.50%) | 34 (31.19%) | 33 (42.86%) | 0.264 |
| Diabetes Mellitus | |||||
| Yes | 32 (9.91%) | 5 (3.65%) | 13 (7.41%) | 14 (20.51%) | |
| No | 291 (90.09%) | 132 (96.35%) | 96 (92.59%) | 63 (79.49%) | 0.002 |
| Coronary Heart Disease | |||||
| Yes | 35 (10.84%) | 6 (4.38%) | 12 (11.01%) | 17 (15.60%) | |
| No | 288 (89.16%) | 131 (95.62%) | 97 (88.99%) | 60 (84.40%) | 0.000 |
| T Stage | |||||
| T1/T2 | 204 (63.16%) | 93 (67.88%) | 60 (55.05%) | 51 (66.23%) | |
| T3/T4 | 119 (36.84%) | 44 (32.12%) | 49 (44.95%) | 26 (33.77%) | 0.095 |
| N Stage | |||||
| N0 | 202 (62.54%) | 85 (62.04%) | 68 (62.39%) | 49 (63.64%) | |
| N1–3 | 81 (25.08%) | 36 (26.28%) | 28 (25.69%) | 17 (22.08%) | |
| N x | 40 (12.38%) | 16 (11.68%) | 13 (11.93%) | 11 (14.29%) | 0.953 |
| Clinical Stage | |||||
| I/II | 231 (71.52%) | 98 (71.53%) | 77 (70.64%) | 56 (72.73%) | |
| III/IV | 92 (28.48%) | 39 (28.47%) | 32 (29.36%) | 21 (27.27%) | 0.893 |
Abbreviations: BMI, body mass index; HPV, human papilloma virus; T, tumor; N, node; SCCHN, squamous cell carcinoma of head and neck.
Laryngeal (20.12%) and tongue (14.86%) cancer were the two most common types of SCCHN, as previously reported (15), but there were no differences in the primary sites between the three groups (P=0.320) (Table 2). The operation duration for 233 patients (72.14%) was more than 4 h, and 191 patients had intraoperative blood loss greater than 400mL. Both these factors were significantly different (P=0.008, P=0.001) between the groups. Neck dissection patients accounted for 69.97%, which demonstrates the importance of radical surgery, especially in extended resection with flap repair (45.51%). In terms of postoperative adjuvant therapy, radiotherapy (72.45%) was much more acceptable than chemotherapy (22.91%) and targeted drug therapy (15.79%).
Table 2.
Treatment-Related Characteristics of SCCHN Patients (N=323)
| Treatment-Related Variables | All (N = 323) | Healthy BMI (N = 137) | Overweight (N = 109) | Obese (N = 77) | P |
|---|---|---|---|---|---|
| Primary Site | |||||
| Tongue | 48 (14.86%) | 20 (14.60%) | 14 (12.84%) | 14 (18.18%) | |
| Floor of mouth | 26 (8.05%) | 11 (8.03%) | 9 (8.26%) | 6 (7.79%) | |
| Gingiva | 32 (9.91%) | 14 (10.22%) | 8 (7.34%) | 10 (12.99%) | |
| Buccal mucosa | 39 (12.07%) | 11 (8.03%) | 14 (12.84%) | 14 (18.18%) | |
| Oropharyngeal | 25 (7.74%) | 11 (8.03%) | 9 (8.26%) | 5 (6.49%) | |
| Hypopharyngeal | 41 (12.69%) | 18 (13.14%) | 15 (13.76%) | 8 (10.39%) | |
| Laryngeal | 65 (20.12%) | 35 (25.55%) | 24 (22.02%) | 6 (7.79%) | |
| Maxillary sinus | 22 (6.81%) | 9 (6.57%) | 7 (6.42%) | 6 (7.79%) | |
| Other parts | 25 (7.74%) | 8 (5.84%) | 9 (8.26%) | 8 (10.39%) | 0.320 |
| Primary Tumor Resection | |||||
| Standard extended resection | 129 (39.94%) | 54 (39.42%) | 42 (38.53%) | 33 (42.86%) | |
| Extended resection + flap repair | 147 (45.51%) | 58 (42.34%) | 53 (48.62%) | 36 (46.75%) | |
| Others | 47 (14.55%) | 25 (18.25%) | 14 (12.84%) | 8 (10.39%) | 0.523 |
| Operation Duration | |||||
| ≥4 h | 233 (72.14%) | 87 (%) | 83 (76.15%) | 63 (81.82%) | |
| <4 h | 90 (27.95%) | 50 (%) | 26 (23.85%) | 14 (18.18%) | 0.008 |
| Intraoperative Blood Loss | |||||
| ≥400 mL | 191 (59.13%) | 66 (48.18%) | 69 (63.30%) | 56 (72.73%) | |
| <400 mL | 132 (40.87%) | 71 (51.82%) | 40 (36.70%) | 21 (27.27%) | 0.001 |
| Neck Dissection | |||||
| Yes | 226 (69.97%) | 101 (85.51%) | 68 (68.51%) | 57 (76.92%) | |
| No | 97 (30.03%) | 36 (14.49%) | 41 (31.49%) | 20 (23.08%) | 0.105 |
| Postoperative Chemotherapy | |||||
| Yes | 74 (22.91%) | 35 (7.24%) | 19 (7.40%) | 20 (5.13%) | |
| No | 249 (77.09%) | 102 (92.76%) | 90 (92.59%) | 57 (94.87%) | 0.246 |
| Postoperative Radiation | |||||
| Yes | 234 (72.45%) | 99 (82.61%) | 81 (75.92%) | 54 (79.49%) | |
| No | 89 (27.55%) | 38 (17.39%) | 28 (24.07%) | 23 (20.51%) | 0.819 |
| Postoperative Targeted Drug | |||||
| Yes | 51 (15.79%) | 22 (16.06%) | 15 (13.76%) | 14 (18.18%) | |
| No | 272 (84.21%) | 115 (83.94%) | 94 (86.24%) | 63 (81.82%) | 0.713 |
Abbreviations: BMI, body mass index; SCCHN, squamous cell carcinoma of head and neck.
We performed a paired analysis of the healthy BMI, overweight, and obese groups in terms of postoperative complications (Table 3). Although a high incidence of pharyngeal fistula was observed in obese (P=0.014) and overweight patients (P=0.025), mouth floor fistula (P=0.038), lung infection (P=0.047), fat liquefaction (P=0.003), and LEDVT (P=0.020) were found only in the obese group. An increased probability of postoperative complications with increased BMI (P=0.000) led indirectly to prolonged postoperative hospital stays. We found that postoperative hospital stays were greater in both the obese (P=0.000) and overweight groups (P=0.001) than in the healthy BMI group.
Table 3.
Complications in SCCHN Patients (N=323)
| Complications and Hospital Stay | All (N = 323) | Healthy BMI (N = 137) | Overweight (N = 109) | Obese (N = 77) | P |
|---|---|---|---|---|---|
| Complications | |||||
| Pharyngeal fistula | 12 (3.71%) | 1 (0.73%) | 5 (5.50%) | 5 (6.49%) | P a=0.014; P b=0.025 |
| Mouth floor fistula | 7 (2.17%) | 1 (0.73%) | 2 (1.85%) | 4 (5.20%) | P a=0.038; P b=0.432 |
| Hemorrhage | 10 (3.09%) | 5 (2.90%) | 3 (3.70%) | 2 (2.60%) | P a=0.691; P b=0.708 |
| Lung infection | 9 (2.80%) | 2 (1.45%) | 2 (3.70%) | 5 (6.49%) | P a=0.047; P b=0.818 |
| Fat liquefaction | 7 (2.17%) | 0 (0.00%) | 2 (1.85%) | 5 (6.49%) | P a=0.003; P b=0.111 |
| Flap necrosis | 3 (0.93%) | 1 (0.73%) | 1 (0.92%) | 1 (1.30%) | P a=0.678; P b=0.872 |
| LEDVT | 4 (1.24%) | 0 (0.00%) | 1 (0.92%) | 3 (3.90%) | P a=0.020; P b=0.261 |
| Lymphorrhagia | 4 (1.24%) | 1 (0.73%) | 1 (0.92%) | 2 (2.60%) | P a=0.265; P b=0.872 |
| Hospital Stay (d) | |||||
| ≥12 d | 139 (43.03%) | 41 (29.93%) | 56 (51.38%) | 42 (54.55%) | P a=0.000; P b=0.001 |
| <12 d | 184 (56.97%) | 96 (70.07%) | 53 (48.62%) | 35 (45.45%) | P =0.000 |
Notes: P a: Obese Group compared with Healthy BMI Group; P b: Overweight Group compared with Healthy BMI Group.
Abbreviations: LEDVT, lower extremities deep venous thrombosis; BMI, body mass index; SCCHN, squamous cell carcinoma of head and neck.
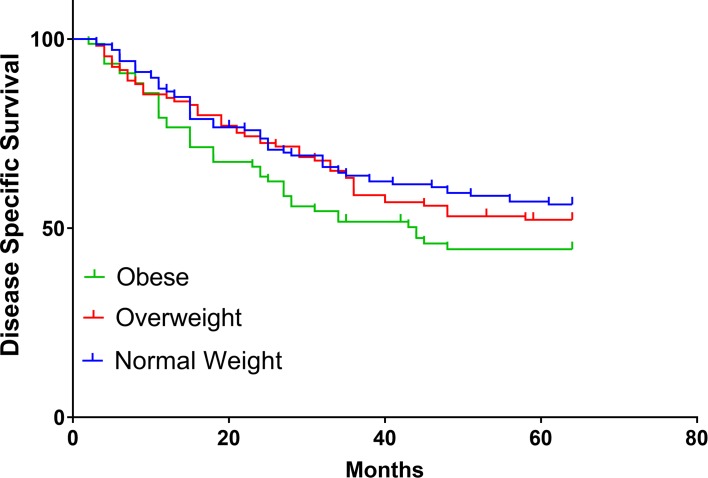
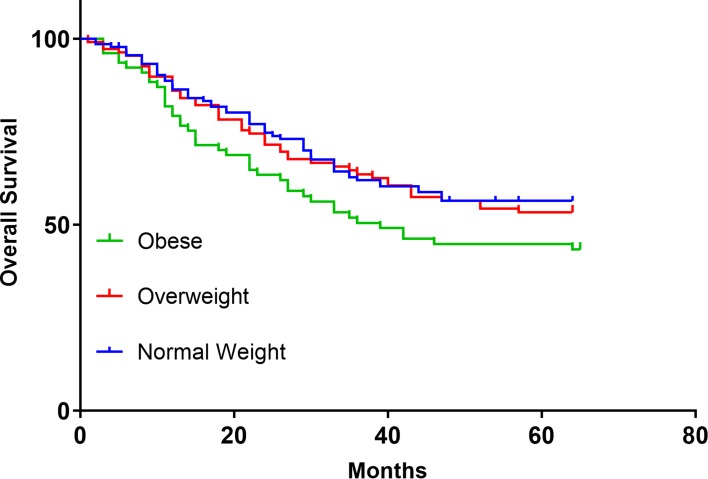
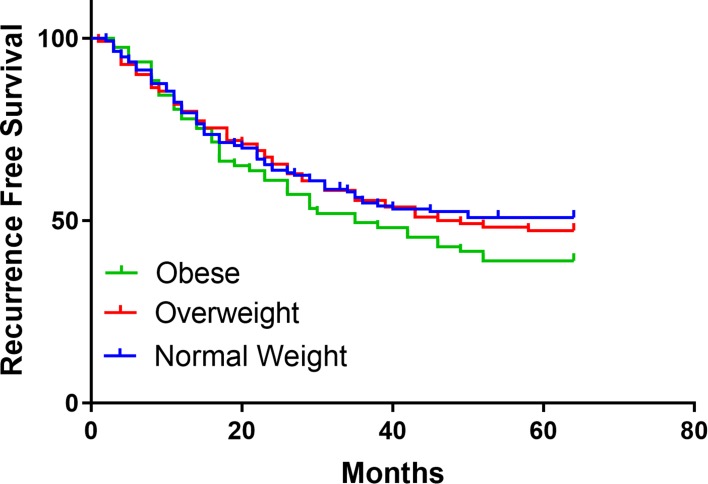
The differences in three-year (3-y) DSS (P=0.178; Figure 1 and OS (P=0.123; Figure 2) between the groups at 3 y were found no statistical significance, the same as RFS (P=0.362; Figure 3). OS (54.40%) and DSS (51.94%) in the obese group were slightly lower than in the overweight (64.62%, 61.92%) and healthy BMI groups (64.66%, 65.02%). Cox univariatable analysis found that overweight (HR=1.336; 95% CI, 0.572–2.892; P=0.681) and obese (HR=1.129; 95% CI, 0.408–2.404; P=0520) did not affect 3-y OS in patients with SCCHN, and the same findings were confirmed during Cox multivariate analysis. Using univariatable analysis or multivariatable analysis, it was found that smoking, drinking, age, and gender were not statistically significant in 3-y OS. In contrast, Cox univariatable analysis revealed that T-stage (HR=3.563; 95% CI, 2.071–9.053; P=0.023), N-stage (HR=4.561; 95% CI, 2.417–11.829; P=0.005) and clinical stage (HR=6.724; 95% CI, 3.809–13.296; P=0.002) can affect 3-y OS in the enrolled patients, and similar results were shown by the Cox multivariate data. These factors were not related to BMI (Table 4).
Figure 1.
Survival of DSS in BMI normal group, overweight group, and obese group.
Abbreviations: DSS, disease specific survival; BMI, body mass index.
Figure 2.
Survival of OS in BMI normal group, overweight group, and obese group.
Abbreviations: OS, overall survival; BMI, body mass index.
Figure 3.
Survival of RFS in BMI normal group, overweight group, and obese group.
Abbreviations: RFS, recurrence-free survival; BMI, body mass index.
Table 4.
Cox Univariatable and Multivariatable Analysis for SCCHN 3-y Overall Survival (N=323)
| Variables | HR | Cox-Univariatable | P | HR | Cox-Multivariatable | P |
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||
| Age (y) | ||||||
| <60 y | 1.000 | Ref. | – | – | – | – |
| ≥60 y | 1.098 | 0.523–2.709 | 0.801 | – | – | – |
| Gender | ||||||
| Female | 1.000 | Ref. | – | – | – | – |
| Male | 1.872 | 0.898–2.751 | 0.199 | – | – | – |
| BMI | ||||||
| Healthy | 1.000 | Ref. | - | 1.000 | Ref. | - |
| Overweight | 1.336 | 0.572–2.892 | 0.681 | 2.906 | 1.092–6.448 | 0.808 |
| Obese | 1.129 | 0.408–2.404 | 0.520 | 1.791 | 0.553–5.471 | |
| Alcohol | ||||||
| Never | 1.000 | Ref. | – | – | – | – |
| Occasional | 1.238 | 0.488–2.612 | 0.690 | – | – | – |
| Frequent | 0.909 | 0.400–1.774 | 0.594 | – | – | – |
| Smoking | ||||||
| Never | 1.000 | Ref. | – | – | – | – |
| Occasional | 1.218 | 0.602–2.791 | 0.677 | – | – | – |
| Frequent | 0.883 | 0.451–2.318 | 0.709 | – | – | – |
| T Stage | ||||||
| T1/T2 | 1.000 | Ref. | – | 1.000 | Ref. | – |
| T3/T4 | 3.563 | 2.071–9.053 | 0.023 | 4.981 | 1.453–13.609 | 0.019 |
| N Stage | ||||||
| N0 | 1.000 | Ref. | – | 1.000 | Ref. | – |
| N1–3 | 4.561 | 2.417–11.829 | 0.005 | 8.152 | 1.352–12.780 | 0.001 |
| Clinical Stage | ||||||
| I/II | 1.000 | Ref. | – | 1.000 | Ref. | – |
| III/IV | 6.554 | 3.809–13.296 | 0.002 | 3.772 | 1.393–8.668 | 0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; T, tumor; N, node; SCCHN, squamous cell carcinoma of head and neck.
Discussion
Is there an influence of the body mass index on the survival of patients with carcinoma in the head and neck region? SCCHN belongs to the most common pathological type of head and neck malignancy. In recent years, the incidence of SCCHN has increased with the rise in obesity rates. To the best of our knowledge, a large sample study of obesity and prognosis in SCCHN has not been conducted previously. In the current study, age, male gender, smoking, and drinking history were the main risk factors for SCCHN; however, the HPV infection rate was only 3.09%. This finding is different from previous research,15–17 and HPV-16 infection is excluded from the high-risk factors. These differences may be related to the primarily rural background of the enrolled patients, and the differences in their lifestyles from those of patients in developed countries. Surprisingly, we did not find any differences in preoperative weight loss between the three groups: some patients with early SCCHN, such as laryngeal cancer, have hoarseness as the first symptom and this does not affect eating, unlike in the case of cancers originating in the digestive tract, as previously reported.13,14
In the current study, we found that a lower BMI index was associated with a lower probability of postoperative complications. The incidence of pharyngeal fistula in the obesity group was higher than in the healthy BMI group because the thickness of the subcutaneous fat layer affected wound healing. All patients with pharyngeal fistula recovered after long-term dressing changes. Some patients had radiotherapy before surgery, and the dressing change time was more than 2 months or even longer. Floor of mouth fistulas are associated with significant increases in hospitalization time. Saliva exudation from the fistula, which occurs along with fat liquefaction, further aggravates the burden of obese patients, both economically and psychologically. The incidence of postoperative pulmonary infection in obese patients was higher than in the healthy BMI group. There are several potential reasons for this finding. One is that lung function in obese patients is generally poor. The other is that swelling in obese patients is more severe after surgery. Some patients required a tracheostomy because of swelling, which indirectly or directly leads to poor expectoration or aspiration after surgery, and an increased chance of pneumonia. In the obese group, one patient with a BMI over 33 kg/m2 died of serious aspiration difficulties because of swelling after horizontal partial laryngectomy. Therefore, it is very important to evaluate the preoperative pulmonary function of obese patients, especially before laryngeal surgery.
Some researchers have reported different or even opposing conclusions regarding the relationship between BMI and SCCHN prognosis. McRackan et al18 found that SCCHN patients undergoing chemoradiotherapy with a BMI greater than 25 kg/m2 had better RFS and total OS than patients with a BMI of 25 kg/m2 or less, which is similar to the results reported by Hicks et al15 and different from those of Iyengar et al.13–15 Iyengar et al13,14 found that obesity is an unfavorable independent prognostic variable for early tongue cancer and is associated with DSS but does not affect OS and RFS. This association may not have been previously recognized. In the current research, BMI had no significant correlation with 3-y OS, RFS and DSS, and was not a prognostic indicator for patients with SCCHN, which is different from the above studies. In obese patients 3-y DSS (P=0.054; Fig-1) and OS (P=0.057; Fig-2) were slightly lower than in the other groups, although no statistical significance was found. Cox univariatable and multivariatable analysis found that there was no significant difference in smoking, drinking, age, and gender between the different groups. The main factors affecting 3-y OS were T-stage, N-stage and clinical-stage, which is corroborated by some previous studies.15,17,18 However, in terms of BMI, no significant difference was found in the groups, through either Cox univariate or multivariate analysis, which is different from the results of Iyengar et al.13,14
In summary, with increasing BMI, the probability of complications such as pharyngeal fistula increases; BMI had no significant correlation with 3-year OS, RFS and DSS, and was not a prognostic indicator for patients with SCCHN. Although the present sample size was acceptable for the current study, it was still too small for the assessment of a single sub-group, such as gingival cancer or hypopharyngeal carcinoma patients, and the follow-up time was relatively short. Therefore, large-sample, multi-population, and longer-term prospective studies are needed to fully explore the relationship between BMI and SCCHN prognosis.
Conclusions
A greater BMI was linked to a higher probability of complications such as pharyngeal fistula, operation duration, hospitalization time and bleeding volume. However, BMI had no significant correlation with 3-year OS, RFS and DSS, and was not a prognostic indicator for patients with SCCHN.
Acknowledgments
The authors will thank Xu Zhang and Qigen Fang for great help in follow-up and case data collection.
Funding Statement
This paper is supported by National Natural Science Foundation of China (No. 81703298, 81402578); Medical Science and Technology Research Project of Henan Province (No.018020488); Special Research and Development and Promotion Project of Henan Province (No. 192102310354).
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Farag M, Ibraheem K, Garstka ME, et al. Thyroid surgery and obesity: cohort study of surgical outcomes and local specific complications. Am J Surg. 2019;217:142–145. doi: 10.1016/j.amjsurg.2018.07.038 [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang TT. Watching China’s weight. Obesity (Silver Spring). 2106;24:1407. doi: 10.1002/oby.21545 [DOI] [PubMed] [Google Scholar]
- 4.Wang JB, Gu MJ, Shen P, et al. Author information body mass index and mortality: a 10-year prospective study in China. Sci Rep. 2016;6:3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-201. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Angelantonio E, Bhupathiraju SN, Wormser D, Global BMI Mortality Collaboration, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 388;2016:776–786. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Luo R, Guo L, Li W, Qi J. Impact of the body mass index on hemorrhage after surgery for thyroid cancer. Cancer Manag Res. 2020;12:557–565. doi: 10.2147/CMAR.S239264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. International agency for research on cancer handbook working group: body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold M, Pandeya N, Byrnes G, et al. Soerjomataram: global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liguori C, Izzi F, Mercuri NB, et al. Vitamin D status of male OSAS patients improved after long-term CPAP treatment mainly in obese subjects. Sleep Med. 2017;29:81–85. doi: 10.1016/j.sleep.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 12.Papandreou C. Gluteal adipose tissue fatty acids and sleep quality parameters in obese adults with OSAS. Sleep Breath. 2013;17:1315–1317. doi: 10.1007/s11325-013-0818-3 [DOI] [PubMed] [Google Scholar]
- 13.Iyengar NM, Ghossein RA, Morris LG, et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer. 2016;122:3794–3802. doi: 10.1002/cncr.30251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyengar NM, Kochhar A, Morris PG, et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120:983–991. doi: 10.1002/cncr.28532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks DF, Bakst R, Doucette J, et al. Impact of obesity on outcomes for patients with head and neck cancer. Oral Oncol. 2018;83:11–17. doi: 10.1016/j.oraloncology.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 17.Gama RR, Song Y, Zhang Q, et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck. 2017;39:1226–1233. doi: 10.1002/hed.24760 [DOI] [PubMed] [Google Scholar]
- 18.McRackan TR, Watkins JM, Herrin AE, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope. 2008;118:1180–1185. doi: 10.1097/MLG.0b013e31816fca5c [DOI] [PubMed] [Google Scholar]