Abstract
Most vaccines are so effective that they could lead to the control/elimination of the diseases they target and directly impact on intensive care admissions or complications. This is best illustrated by the use of vaccines against Haemophilus influenzae type b, Streptococcus pneumoniae, zoster, yellow fever, Ebola virus, influenza or measles—but also by third party strategies such as maternal, toddler and care-giver immunization. However, each of these vaccine-induced protection is threatened by insufficient vaccine uptake. Here, we briefly discuss how vaccine hesitancy has led to the resurgence of diseases that were considered as controlled and explore the effect of vaccine-hesitant healthcare workers on nosocomial infections. As intensive care physicians are in charge of polymorbid patients, we briefly summarize the current recommendations for vaccinations in high-risk patients. We finally give some perspective on ongoing research, and discuss how institutional policies and intensive care physicians could play a role in increasing the impact of vaccination, overall and in intensive care units.
Keywords: Vaccine efficacy, Vaccination coverage, Vaccine hesitancy, Maternal immunisation
Take-home message
| Vaccinations are life-saving preventive interventions victims of their own success; the rise of vaccine hesitancy has led to the resurgence of vaccine-preventable diseases. Strategies to increase vaccine impact include improved awareness of patients and healthcare workers, the implication of health policies, and the vaccination of third parties, such as pregnant women (to protect newborns and mothers), toddlers (to reduce microbial carriage in the community at large), and caregivers (to reduce direct transmission). |
Introduction
Vaccinology is a relatively young science (Table 1) [1], and intensive care medicine is even younger; both have more to share than may appear at first thought. Although the first approach to “intensive care” was delivered by Florence Nightingale during the Crimean War, and thereafter primarily reserved for postoperative patients or wounded soldiers, the birth of the Intensive Therapy Units began during the polio epidemic in Copenhagen in 1952 [2]. Although polio is now on the brink of eradication thanks to vaccines [3], intensive care medicine has since flourished. Deadly diseases such as smallpox have been completely eradicated or almost, but there are still too many unnecessary deaths due to tetanus, especially neonatal tetanus, in resource-limited countries [4]. Unfortunately, the global proportion of children with adequate vaccination coverage remains insufficient and has not improved in last decade [5].
Table 1.
Characteristics of selected diseases and their vaccines by date of discovery and estimates of vaccine efficacy
| Vaccine | Vaccine type (year available) | Mortality among unvaccinated | Vaccine efficacy | References |
|---|---|---|---|---|
| Smallpox | Live attenuated (1798) | 30% | 95% | [101, 102] |
| Rabies | Live attenuated (1882), killed (1980) | 100% | 100% (with post-exposure prophylaxis) | [102] |
| Cholera | Killed whole cell (1884), recombinant toxin B (1993), oral (2016) |
50–60% (historic) 3.3% (modern) |
53–86% (Cochrane injected vaccine: 48%) (Cochrane oral vaccine: 50–60%) |
[102–104] |
| Typhoid | Killed whole cell (1896), live oral (1989), polysaccharide (1994), conjugate (2008) |
10–20% (historic) < 1% (modern) |
51–88% (killed whole cell) 62–96% (live oral; Cochrane: 50%) 55–72% (polysaccharide; Cochrane: 55–69%) 100% (conjugate; Cochrane: 50–96%) |
[102, 105] |
| Plague | Killed whole cell (1897) |
100% (untreated pneumonic form) 20–40% (sepsis) 6.7% (recent estimate) |
60–100% (animal studies) | [102] |
| Diphtheria toxoid | Protein (1923) | 6% | 70–99% | [102] |
| Pertussis | Killed whole cell (1926), acellular (1996) | 1% (infants) |
64–90% (whole cell) 83–95% (infants pertussis) 90–95% (maternal immunization) |
[58, 106] |
| Tetanus toxoid | Protein (1926) |
25–100% (generalized tetanus) 10–20% (modern critical care unit) |
70–100% | [102] |
| Tuberculosis | Live attenuated (1927) | 23% |
20% (infection) 0–80% (pulmonary) 86% (meningitis and miliary disease) |
[102, 107] |
| Yellow fever | Live attenuated (1935) | 47% (severe cases) | 100%a | [34] |
| Influenza | Killed whole organism (1936), live attenuated (2003) | Up to 60% (pandemic) | 8–91% (Cochrane: 59%) | [81, 108] |
| Tick-borne encephalitis | Killed whole organism (1937, 1981) | Up to 35% (far eastern type) | 99% | [102] |
| Polio | Inactivated (1955), live attenuated oral (1963) | 0–57% |
80–96% (inactivated, paralytic polio) 90% (oral) |
[102] |
| Measles | Live attenuated (1963) | 2–15% (low-, middle-income countries) | 90–98% | [66, 80, 109] |
| Mumps | Live-attenuated (1967) | < 0.1% | 85% | [110] |
| Meningococcus | Polysaccharide (1974), conjugate (1999, group C; 2006, group ACWY), recombinant (2014, group B) |
70–85% (historic) 10–15% (antibiotic era) 40% (severe cases) |
61–97% (group C) 61–85% (group ACWY) 82.9% (group B) |
[102] |
| Pneumococcus | Polysaccharide (1977), conjugate (2000) | 11–30% (invasive diseases) | 77–100% (invasive diseases) | [19, 111, 112] |
| H. influenza type b | Polysaccharide (1985), conjugate (1990) | 40–90% (historic) |
55–92% (polysaccharide) 80–100% (conjugate) |
[15, 102] |
| Chickenpox | Live attenuated (1995) | < 0.1% | 77–100% | [102] |
| Shingles | Live attenuated (2006), recombinant (2017) | < 0.1% |
51–61% (live-attenuated) 89–97% (recombinant) |
[62, 63, 102] |
| Human papillomavirus | Recombinant (2006) | 3–66% (cervical cancer) | 43–100% (cancer or precursor lesions) | [102, 113, 114] |
| Dengue | Recombinant (2016) | 0.1–5% | 30–60% | [102] |
| Ebola | Recombinant (2017) | 36–90% | 100%a (rVSV-ZEBOV) | [52, 102] |
aLimited data available
Vaccine-preventable diseases and intensive care medicine have indeed always been intimately linked; during seasonal influenza epidemics, influenza alone is responsible for up to 3.4% of intensive care unit (ICU) admissions in the United States [6]. A single-centre study in a Chinese paediatric ICU showed that 9.3% of all admissions were due to vaccine-preventable infections [7]. Vaccines are not only highly effective in preventing disease, but are also highly cost-effective and may be cost saving [8]. Implementing vaccines has direct societal benefits, as illustrated by the expansion of a nationwide vaccination program in China which led to drastic decreases in vaccine-preventable diseases in the last 50 years [9]. By decreasing disease incidence, vaccination programmes indirectly lead to decreases in ICU admissions and/or complications, and it is safe to assume that any breach in vaccination programmes indirectly leads to increases in ICU admissions/complications.
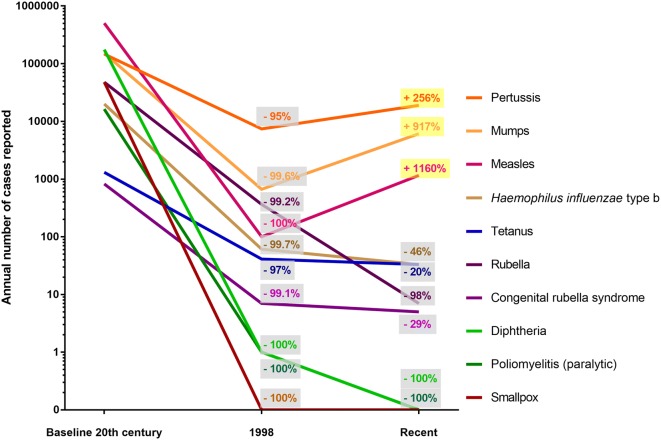
Despite their unmitigated success, the vaccine road has been rocky, with definite blunders, such as the Cutter Incident (where laboratory contaminations led to hundreds of vaccine-related polio cases) [1, 10], gross scientific misconduct around measles vaccine [11], and general disinformation (promotion of “fake news”) [12]. These, and a general trend towards more and more self-centred societies have led to increasing numbers of people who postpone or refuse vaccines in high-income countries (HICs) and worldwide [13] (Fig. 1). Consequently, the World Health Organization (WHO) recently included “vaccine hesitancy” (see below) among the main 10 threats to global health (https://www.who.int/emergencies/ten-threats-to-global-health-in-2019).
Fig. 1.
US annual morbidity from nine diseases with vaccines recommended before 1990 for universal use in children. Numbers were extracted from reports of the Centres for Disease Control and Prevention, available in the references [116–118]. The “recent” numbers are the one reported for 2017, except for the measles cases which are the provisional numbers for 2019 (weeks 1–31) [118]
In this review, we have selected vaccines that target diseases which may result in intensive care admissions or complications to briefly recall the main concepts behind their protective effects, the remaining challenges, and the potential role of intensive care physicians in promoting the health of their vulnerable patients.
Haemophilus influenzae: how vaccines may lead to the disappearance of intensive care admission
The Haemophilus influenzae type b (Hib) vaccine is a success story. In the pre-vaccination era, Hib was among the leading cause of serious bacterial infection such as meningitis in children and epiglottitis in adolescents, with a 40–90% case fatality rate, and a very high proportion (30–40%) of neurological sequelae among survivors [14, 15]. Since the Hib conjugated vaccine was introduced in routine immunization schedules in the 1990s, the incidence of Hib infections has dramatically decreased (Fig. 1) [16]. Most of today’s junior paediatricians practicing in high-income countries (HICs) have not seen a case of epiglottitis or Hib meningitis and its sequelae (deafness, death, etc.). Hib was also among the leading causes of invasive infections such as pneumonia, arthritis, sepsis and periorbital cellulitis, which are all rarer nowadays. Remarkably, the overall burden of invasive diseases resulting from non-type b or non-capsulated H. influenzae has remained low and active population-based surveillance has not reported any significant serotype shift following Hib vaccination [15]. Although the uptake of the Hib vaccine was slow in low- and middle-income countries (LMICs), all the Global Alliance for Vaccines and Immunization (GAVI) countries have now introduced Hib-containing vaccines in their program through to the Hib Initiative [17], and a significant impact on paediatric intensive care unit (PICU) admission is expected worldwide.
Streptococcus pneumoniae: the superiority of conjugate vaccines on community protection against invasive pneumococcal disease
Streptococcus pneumoniae is responsible for a large burden of disease and mortality worldwide, usually from community-acquired pneumonia, in which it is the most frequently isolated pathogen [18], and invasive pneumococcal disease. The latter is associated with an 11–30% fatality rate [19]. The disease burden is higher among both extremes of age, as well as patients with chronic medical conditions [20]: the risk of disease increases with the number of risk factors [21], a particularly relevant reality in patients admitted to intensive care.
Following the success of glycoconjugate vaccines against Hib, the same approach was applied to S. pneumoniae. A large observational study on recurrent community-acquired pneumonia has shown that the best predictor of pneumococcal pneumonia is the absence of vaccination, emphasizing its critical importance for prevention [22]. The impact of vaccinating children against S. pneumoniae has exceeded expectations, with a subsequent positive collateral effect on adults’ infection rate and mortality [23]. However, it has induced a shift in the incidence of non-vaccine circulating strains [24]. Thus, it is important to understand the difference between the two currently available types of vaccines: the 23-valent polysaccharide vaccine (PPSV23) versus the conjugate vaccines (PCVs) [25]. The PPSV23 (Pneumovax23®, Merck, Whitehouse Station, NJ, US) is composed of purified capsular polysaccharides. Therefore, it induces a mostly non-follicular B cell response with the short-term production of anti-capsular opsonizing immunoglobulins, but no B nor T-cell response; hence, immune memory is not elicited and hyporesponsiveness may be observed after repeat administrations [26]. The PCVs (Synflorix®, GlaxoSmithKline, London, UK; Prevenar®, Pfizer, New York, NY, US) were developed subsequently, essentially to protect infants who do not respond to polysaccharides. The pneumococcal polysaccharides are conjugated to a protein carrier, acquiring the capacity to induce both T follicular helper and B-cell responses, thus enabling the development of germinal centre responses, long-lived antibody-secreting cells and memory cells. Another advantage of PCVs is that they induce sufficiently high antibody titres to reduce pneumococcal carriage in vaccinees; unvaccinated contacts are thus indirectly protected from S. pneumoniae transmission and invasive pneumococcal disease through the reduction of circulating invasive serotypes, a brilliant example of community protection [23].
PPSV23 was traditionally recommended for all adults over 65 years, and for individuals older than 2 years with a high-risk medical condition, whereas PCVs are primarily recommended for children aged < 5 years. Despite the fact that PPSV23 induces protection against a larger number of serotypes, experts are less keen to recommend it nowadays, given the absence of memory response and the hyporesponsiveness observed after repeated administrations [26]. Given the superior immunogenicity of PCVs compared to PPSV23, it has been proposed to extend their indication to high-risk adult populations (65 years or older, immunosuppression, chronic diseases). Clinical trials have reported that PCVs elicit equivalent, or even superior, seroresponses than PPSV23 in adults [27, 28], including in immunocompromised hosts [29, 30]. A randomized clinical trial confirmed the protective efficacy of PCV against pneumococcal pneumonia in senior citizens [31]. Recommendations for PCV administration in high-risk adults vary across the world, although they are endorsed by several medical guidelines [29, 32, 33]. Their usefulness depends largely upon the vaccine coverage reached in infants, i.e. the remaining circulation of PCV-serotype in the community.
Yellow fever, Neisseria meningitidis, cholera, Ebola: how vaccines can control outbreaks
Yellow fever (YF) is a severe mosquito-borne haemorrhagic viral disease associated with a high case-fatality rate that occurs in tropical areas, mostly in Africa and Latin America [34]. The historical differences in yellow fever vaccination (YFV) strategies among African countries have proven at least twice that YFV can eliminate epidemics. A vaccine is available since the 1930s, enabling mass vaccination campaigns in the former French West African colonies, subsequently leading to adequate control of the disease. Routine recommendations for YFV was thus stopped in the 1980s. In the meantime, YF remained endemic in neighbouring former English-speaking African colonies that did not practice vaccination. In 2005, in response to increasing outbreaks in West Africa, the Yellow Fever Initiative was launched to reintroduce YFV into routine child immunization programmes in affected countries, in mass prevention and outbreak-control emergency campaigns. Consequently, epidemics were prevented in West Africa, whilst outbreaks still occurred in central and eastern African countries. An extended YFV strategy was launched in 2016 to improve control of YF all over Africa and worldwide [35]. This resulted into vaccine shortage, fortunately leading to the demonstration that one-fifth of the current vaccine dose could be used in outbreak control [36]. However, YF outbreaks are far from being controlled, vaccine shortages are a real threat and changes in travel patterns, habitations and global warming extend the territories at risk of YF—and thus of an ICU having to admit a patient with YF.
The implementation of N. meningitidis (Men) vaccination across the globe has repetitively demonstrated its ability to prevent severe meningococcal diseases, including meningitis, septicaemia and purpura fulminans, conditions that would result to intensive care admission [37]. The first meningococcal vaccine against serogroup C was introduced in the UK in 1999, rapidly demonstrating its highly beneficial impact among infants and toddlers [38]. In 2013, a Men W strain emerged in the UK and subsequently required the introduction of a quadrivalent vaccine (Men A–C–W–Y) in teenagers. In Africa, mass vaccination campaigns started in 2010 against Men A, leading to the control and near elimination of this deadly disease in 26 African countries of the so-called “meningitis belt” [39]. However, Men W and X persisted, and Men C emerged. Routine immunization with multivalent meningococcal conjugated vaccines could be the answer, would they be produced at an affordable price [40]. In parallel in the UK, a first innovative vaccine against Men B was introduced in infants. After having been shown to be safe [41] and effective in this age group [42], it is now licensed in several countries, but its recommendation varies greatly. Used in 2013 to manage Men B outbreaks in two US universities, it demonstrated the ability to quickly achieve high immunity rates, curbing the outbreak as no new cases occurred among vaccinees [43]. Thus, the development of meningococcal conjugate vaccines has had a significant impact on the incidence of these rare but severe diseases always requiring ICU admission, in which a more widespread use of Men B vaccines could reduce further.
Cholera is another example of outbreak control by mass vaccination. Cholera is an extremely virulent diarrhoeal infection caused by contaminated food or water. It remains a serious threat in many LMICs, with large epidemics and up to 50% fatality rate in the absence of effective therapy including aggressive fluid replacement [44]. Parenterally administered killed whole-cell vaccines may be used in HICs, but are not recommended by WHO for global use due to their limited efficacy and short duration of protection [45]. Yet cholera vaccines are game-changers in the fight against epidemics, explaining why the Global Task Force on Cholera Control included them in its eradication strategy [46]. Rapidly effective, oral cholera vaccines also induce a measurable community protection effect for non-vaccinated individuals [47]. Modelling studies suggest that vaccinating 50% of a population may result in a greater than 90% reduction in the incidence of cholera [48]; even a lower coverage of 30% may result in a 76% reduction in the disease incidence. Thus, the use of cholera vaccines in epidemic areas is predicted to be both life-saving and cost-effective [49].
More recently, the example of Ebola virus vaccines has shown how the integration of clinical research in emergency outbreak response could accelerate vaccine development. First identified in the 1970s, Ebola viruses are responsible for a frequently-fatal haemorrhagic fever disease. Their spread from Central to Western Africa led to a devastating outbreak between 2013 and 2016 [50]. Although initially delayed, the response strategy of the health community-integrated Phase I vaccination trials at an unprecedented pace. As a result, Phase I [51] and Phase II/III [52] trials of a new vectored Ebola virus vaccine candidate (rVSV-ZEBOV) were initiated only 5 months apart. The phase III African trial used an innovative design of ring vaccination strategy. Clusters (rings) of adults who were contacts (or contacts of contacts) of an Ebola disease index case were randomized 1:1 to vaccination, either immediately or 3 weeks later. After preliminary analysis by an independent safety monitoring board, randomization was stopped and immediate vaccination offered to all eligible: vaccine efficacy was 100% and the epidemic, which was already declining, was rapidly brought under control. This successful experience demonstrated how research can be accelerated in short delays when adequately supported/funded and appropriately championed. It also showed that clinical trials can be rapidly performed during outbreaks and could play a critical role in ending ongoing epidemics [50]. However, the use of rVSV-ZEBOV in response to a new epidemic that emerged in Democratic Republic of Congo at the end of 2017 and is not yet under control in September 2019 reminds us that even the most potent vaccines (> 97% estimated efficacy) must be administered timely to be effective [53].
Pertussis: the impact of maternal immunization
Pertussis remains an important cause of morbidity and mortality worldwide, especially among children less than 6 months old, in whom the case fatality rate is the highest. Indeed, younger infants are more likely to suffer from hypoxia during cough paroxysm or apnoea, potentially leading to encephalopathy or death. Moreover, the hyperviscosity syndrome secondary to pertussis toxin-induced hyperleucocytosis can lead to multiple organ failure and refractory pulmonary hypertension [54]. Thus, neonates or infants with pertussis are frequently admitted to PICU.
Despite vaccination, Bordetella pertussis infections have recently re-increased globally (Fig. 1) [55]. Although this could be partly explained by more reliable PCR-based diagnostic methods and epidemiological cycles, this resurgence is associated with the change from whole cell (wP) to acellular (aP) vaccines initiated in the late nineties. Whereas aPs are less reactogenic than wPs, they are also less potent. Neither pertussis infection nor immunization induce life-long immunity, but the immune response to aP differs from that following wP, resulting in an earlier waning of immunity [56].
Strategies to protect infants too young to be vaccinated include cocooning, and antenatal and early life immunization. The concept of cocooning is to protect infants by vaccinating all their contacts. Studies showed that close family members are nearly always the source of infection in infant pertussis cases [57]. However, as protection requires complete coverage of all contacts, cocooning is difficult to implement effectively. Antenatal maternal vaccination (first introduced in the UK in 2013) is the most efficient strategy against infant death from pertussis and has proven safe [58]. As maternofoetal antibody transfer requires high antibody concentration in the mothers and an active transfer across the placenta, maternal vaccination during the second trimester of pregnancy optimally protects infants during the highest risk period [59, 60]. Regrettably, aP vaccines are currently cost-prohibitive for many LMICs [61], although initiatives are ongoing to develop affordable aP vaccines for use during pregnancy.
Zoster vaccines: how boosting childhood immunity could prevent later complications
Shingles is a frequent complication of stress-, disease- or drug-induced immune suppression, all of which are common in ICU patients. The disease typically presents as a painful unilateral vesicular dermatomal rash and results from the reactivation of latent varicella-zoster virus located in sensory ganglia, usually decades after primary infection. To prevent post-herpetic neuralgia, which can significantly impact the quality of life for years, a first live-attenuated zoster vaccine was licensed for use in senior citizens in 2006 (Zostavax®, Merck, Whitehouse Station, NJ, US). The vaccine is significantly more potent that the one licensed to prevent chickenpox and, therefore, boosts T-cell-mediated immunity, which is essential to prevent viral reactivation. As this vaccine is contra-indicated in immunosuppressed patients, it is mostly not useful in ICU. However, recently, a novel glycoprotein-based adjuvanted vaccine (Shingrix®, GlaxoSmithKline, London, UK) has proven to be extremely effective and safe even in older adults [62, 63]. Several ongoing studies are currently assessing its immunogenicity in various groups of immunosuppressed patients. Should its efficacy be maintained, it could become a cornerstone of the patient preparation prior to any elective ICU admission (i.e. prior to surgery) to prevent shingles.
Tetanus: the importance of boosting immunity and physicians’ memory
Although tetanus is easily preventable with an inexpensive, safe, and widely available vaccine, the disease still causes approximately 50,000 deaths per year worldwide [4]. In LMIC, deaths are secondary to the lack of access to ICU, mainly in the neonatal period in the absence of maternal immunisation, or later in life, especially in countries where booster doses have not yet been introduced in the vaccination programme [4]. In HIC, individuals most at risk belong to the oldest or the poorest populations, and in particular the migrants. The recognition and management of tetanus cases are, however, likely to be delayed as the impact of immunization results into most physicians having never seen a case. Thus, “boosting” intensive care physicians’ memory on the importance of tetanus booster doses and on the various presentations of tetanus disease remains important.
Vaccine hesitancy, a threat for all vaccine-preventable diseases
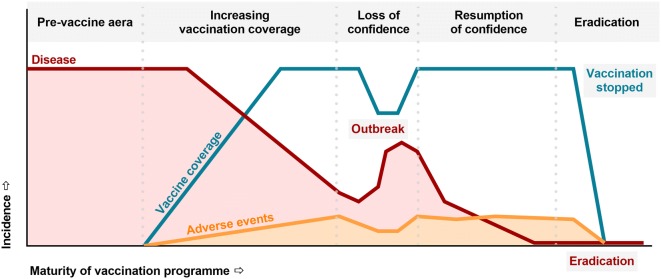
Although opposition to vaccination is as ancient as vaccine discovery [64], vaccines have been recognized as one of the most important public health achievements. As their large-scale use resulted into a > 99% decline in the incidence of vaccine-preventable diseases, a growing number of people are questioning their safety as well as the need for vaccination. Initially limited to small numbers of individuals with mostly philosophical or religious-based convictions, vaccine hesitancy (defined as “a delay in acceptance or refusal of vaccines despite availability of vaccination services”), is gaining ground and threatening community protection. Reasons underlying this tendency, and ways to respond to it, have been nicely reviewed recently [13]. Causes of increasing mistrust in vaccination are multiple. Given their high effectiveness, vaccine-preventable diseases progressively disappear from the community and collective history. The benefits of vaccination have become less obvious, whereas the smallest risks remain observable (Fig. 2). Other factors include fear or mistrust of Big Pharma and medical/government authorities, growing interest in “natural medicine”, fear of injections, and false perceptions such as the risk of overloading the infants’ immune system. The Internet and social networks have facilitated this movement, anti-vaccine materials being more easily accessible/diffused than scientific vulgarized information [13].
Fig. 2.
Evolution of a vaccination programme. Adapted with permission from [119]. The picture illustrates the dynamics of interactions between vaccination coverage, incidences of disease and increase in vaccine-related (or coincidentally related) adverse events
The vaccine hesitancy movement affects parents, individuals and even health professionals, with an increasing number of vaccine-hesitant physicians and nurses. Attitudes vary from dismissing families that refuse vaccination, proposing “personalized” vaccination schedules to some or all patients, or to being openly opposed to vaccination by personal conviction or interest. The legal and ethical issues underlying these conducts are challenging.
Measles: the impact of vaccine hesitancy on community protection
Measles infection is more than just a febrile rash: life-threatening complications can occur in almost every organ system [65]. These are more frequent in infants, adolescents, adults, and among immune-suppressed individuals in whom measles-attributable fatality rate is up to 70% [66]. Importantly, measles induces general immunosuppression, including lymphopenia, suppression of delayed-type hypersensitivity responses (e.g. tuberculin challenge test), impairment of both dendritic cells functions and lymphocytes proliferative responses to new antigens [67, 68], as well as a decreased accumulation of macrophages and neutrophils into the spleen, resulting in a lower ability to clear secondary bacterial infections [69]. It leads to a high frequency of secondary infections which all together are responsible for a high disease burden in LMICs [65, 70]. This immune suppression, which may persist for several weeks or months after wild-type measles infection, does not occur after administration of an attenuated measles vaccine [67].
Measles is an exclusively human pathogen which could be eradicated through sufficient vaccination coverage. With secondary attack rates of 75–90% among susceptible subjects [65], measles outbreaks occur whenever the population’s overall immunity is less than 95% [71, 72], be it nationwide or in small “pockets”. The re-emergence of major measles outbreaks in countries in which it had previously been well “controlled” or eliminated (Fig. 1) is the most dramatic example of the deleterious impact of vaccine hesitancy on community protection [73]: a substantial proportion of cases reported in the US were intentionally unvaccinated [74]. A mathematical simulation estimated that even a 5% decline in measles vaccine coverage in the US would have substantial public health and economic consequences [75]. Therefore, “every shot matters”. Unfortunately, a dramatic increase in measles cases has been reported in the US in 2019, the largest since 1994 and after measles was declared eliminated in 2000 [73]. Although global measles deaths have decreased by 80% from 2000 to 2017 in LMICs following accelerated immunization activities [39], vaccine hesitancy readily spreads from HIC to LMICs.
In HICs, the bad reputation of measles vaccine was secondary to a unique publication based on fraudulent data. In the 1990s, investigators from the UK suggested that measles-mumps-rubella vaccination could lead to inflammatory bowel disease and autism [11]. All subsequent studies were unable to replicate these findings [76], and well-conducted epidemiologic studies failed to demonstrate such associations [77]. In the 2010s, an investigative review found that the UK medical records had been purposefully incorrectly abstracted to falsely implicate the vaccine [78], leading the editors to subsequently retract the original article [79]. The principal investigator [11] lost his license to practise medicine but is still leading an anti-vaccine movement. Although all the evidence refutes the hypothesis that measles-containing vaccines contribute to inflammatory bowel disease and autism, this initial suspicion has a sustained dramatic effect on the population’s beliefs and subsequent vaccination coverage worldwide [80]. Consequently, intensive care physicians should remain alert to the eventuality of having to admit a patient with measles, a diagnosis which may be missed or delayed as disease incidence declines. Furthermore, lack of recognition or appropriate infection control measures -airborne precautions- might lead to subsequent nosocomial transmission among both patients and caregivers.
Influenza: how vaccine hesitancy among healthcare workers propagates nosocomial infections
Flu comes back every year, and it remains difficult to predict what the predominant strains will be. As influenza viruses undergo recurrent genetic and surface antigens antigenic changes, the influenza vaccine needs to be continually adapted to the circulating strains and vaccination repeated annually to ensure protection. With a vaccine efficacy ranging from 8 to 91%, influenza vaccines do not meet the expectations of vaccinologists, health authorities nor healthcare workers (HCWs). Nevertheless, annual vaccination campaigns of subjects at risks of complications or transmission are still considered as the most efficient preventive strategy against seasonal epidemics, and occasional pandemics, both associated with significant disease burden and costs [81].
The WHO strongly recommends influenza vaccination of all HCWs for their own protection, to maintain sufficient staffing during influenza epidemics, and to prevent nosocomial transmission [82]. HCWs are at increased risk of influenza exposure/infection and play an important role in spreading the virus during hospital outbreaks, as transmission can occur before symptoms. The number (of HCWs) needed to vaccinate to ensure a benefit to one patient ranges from 3 to 50 [83]. This benefit is most obvious in long-term care facilities hosting high-risk elderly patients. It is estimated that 60% of infections could be prevented if all HCWs in these settings were vaccinated [83]. However, acceptance of annual influenza vaccination is challenging. A systematic review showed that the strongest motivation for HCWs to get vaccinated was to protect themselves (or their families), and not their patients [83]; the strongest barriers against vaccination were lack of confidence about disease severity or vaccine effectiveness, concerns about vaccine safety, and lack of professional or ethical obligation to get vaccinated. A high level of misinformation on influenza disease and vaccination exists [84]. As a result, influenza vaccine coverage remains alarmingly low among HCWs in nearly all European countries but is generally high in Canada, US, Japan, and Australia—wherever institutional pressure is applied [85]. As HCWs should be role models in promoting vaccination, their hesitancy impacts the beliefs of the rest of the population. Correcting misinformation and strongly recommending the vaccines is essential, as is free and easy access to the vaccine. Mandatory vaccination remains the most successful intervention to increase vaccine uptake but is frequently not acceptable or feasible. “Soft-mandate” including opt-out programs, such as requiring mask use during the whole flu season for those who decline vaccination, may be an acceptable alternative [83].
Protecting the most vulnerable: how intensive care physicians can help
To improve vaccination uptake, all HCWs should feel concerned in updating their patients’ immunization status. Intensive care physicians are no exception as they are in contact with a variety of patients at high risk for vaccine-preventable diseases. This does not only imply knowing the most recent recommendation adapted to a given patient/situation (Table 2) but also taking the time to ascertain the patient’s vaccination status by checking their records or serologies, as relying on oral recall only can lead to undervaccination or overimmunization [86]. It can also imply updating the vaccination status of those in close contact with the patient (household members) to prevent transmission.
Table 2.
Summary of the Infectious Diseases Society of America guideline on vaccination of immunocompromised patients
| HIV | CTX | After CTX | Before HSCT | After HSCT | Before SOT | After SOT | Inflammatory diseases | Asplenia or SCD | Cochlear implants or CSF leak | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hib | C | U | U | U | H | U | U | U | R | U |
| HAV | U | U | U | U | R | R | R | U | U | U |
| HBV | H | U | R | U | R | H | R | U | U | U |
| DTP | U | U | U | U | H | U | U | U | U | U |
| HPV | H | U | U | U | H | U | H | U | U | U |
| IIV | R | R | U | R | R | R | R | R | U | U |
| LAIV | C | NO | U | NO | NO | NO | NO | NO | NO | U |
| MMR | C | NO | U | C | C | C | NO/C | C | U | U |
| MMRV | NO | NO | U | C | NO | C | NO | C | U | U |
| MEN | H | U | U | U | H | U | U | U | H | U |
| PCV | R | R | U | R | H | R | R | R | R | R |
| PPSV | R | R | U | R | R | R | R | R | R | R |
| IPV | U | U | U | U | H | U | U | U | U | U |
| ROT | U | NO | U | NO | NO | C | NO | C | U | U |
| VZV | C | NO | U | C | C | C | NO/C | C | U | U |
| ZV | NO | NO | U | C | NO | C | NO | C | U | U |
HIV: human immunodeficiency virus-infected patients. Vaccine recommendations differ according to lymphocyte count (CD4 counts of ≥ or < 200 cells/mm3 in adults and ≥ or < 15% CD4 T-lymphocyte percentage). CTX: during chemotherapy. After CTX: 3 months after chemotherapy or 6 months after anti-B-cell antibodies, patients should be vaccinated according to the routine vaccination schedule. Vaccines administered during cancer chemotherapy should not be considered valid doses unless there is documentation of a protective antibody level. Before HSCT: before hematopoietic stem cell transplantation, candidate should be updated with their vaccination according to routine schedule. Live-attenuated vaccine should be given at least 4 weeks and inactivated vaccines at least 2 weeks before starting the conditioning regimen. After HSCT: after hematopoietic stem cell transplantation patients should be fully re-immunized with more vaccine doses than for immunocompetent. Before SOT: before solid organ transplantation, candidate should be updated with their vaccination according to routine schedule. Live-attenuated vaccine should be given at least 4 weeks before transplantation. After SOT: after solid organ transplantation, recipient should be updated with inactivated vaccine according to routine schedule. Live-attenuated vaccines could be used with caution if patient is seronegative, clinically stable on low immunosuppression, after assessment of risk and benefits, with close follow-up and appropriate education of the patient and its primary care physician [115]. Inflammatory diseases: vaccine recommendations for patients with inflammatory diseases depend on the level of immunosuppression, whether it is planned, low-level or high-level. Low-level immunosuppression includes treatment with prednisone < 2 mg/kg with a maximum of ≤ 20 mg/day; methotrexate ≤ 0.4 mg/kg/week; azathioprine ≤ 3 mg/kg/day; or 6-mercaptopurine ≤ 1.5 mg/kg/day. High-level immunosuppression regimens include treatment with doses higher than those listed for low-dose immunosuppression and biologic agents such as tumor necrosis factor antagonists or rituximab. Asplenia or SCD: patients with asplenia or sickle cell disease should be continuously vaccinated against encapsulated bacteria. Influenza vaccination is essential given the high risk of pneumococcal infection following influenza. Cochlear implants or CSF leak: patients with profound hearing loss have or are scheduled to receive a cochlear implant, have an inner ear-cerebrospinal fluid communication or other sort of cerebrospinal fluid leak should be vaccinated against pneumococcus
C recommended in certain conditions (see Rubin et al. [89] for details, and Suresh et al. [115] for the latest recommendation on MMR and VZV after SOT), CSF cerebrospinal fluid, CTX chemotherapy, DTP diphtheria-tetanus-pertussis vaccine, H highly recommended, some patients will require more doses and/or higher dosage than immunocompetent person, HAV hepatitis A vaccine, HBV hepatitis B vaccine, Hib Haemophilus influenzae type b vaccine; HIV human immunodeficiency virus, HPV human papillomavirus vaccine, HSCT hematopoietic stem cell transplantation, IIV inactivated influenza vaccine, IPV inactivated poliovirus vaccine, LAIV live-attenuated influenza vaccine, MEN meningococcal conjugate vaccine, MMR measles-mumps-rubella vaccine, MMRV measles–mumps–rubella–varicella vaccine, NO not recommended, PCV pneumococcal conjugate vaccine, PPSV pneumococcal polysaccharide vaccine, R highly recommended (patient is at increased risk), ROT rotavirus vaccine, SCD sickle cell disease, SOT solid organ transplantation, U recommended as usually (in routine vaccination of immunocompetent person), VZV varicella vaccine, ZV zoster vaccine
This is of particular importance for neonatologists. The majority of patients admitted to neonatal intensive care are preterm. Preterm infants should be vaccinated according to their chronological (not corrected) age, using an accelerated schedule, to protect them as quickly as possible [87]. Indeed, not only do preterm newborns have immunologic immaturities, but they are deprived of maternal antibodies. The risk of delay in vaccine administration is high and increases with decreasing gestational age at birth [88]. Given their higher vulnerability, preterm infants also benefit from influenza vaccine (as of 6 months of age) and passive immunization against respiratory syncytial virus (RSV). The immunization status of their household contacts should be updated, especially with pertussis and influenza vaccines.
A substantial number of adults admitted to intensive care are at high-risk for vaccine-preventable infections due to multiple comorbidities, chronic underlying illnesses, malignancy, chronic organ failure or immune suppression. The Infectious Diseases Society of America published detailed clinical practice guidelines for vaccination of immunocompromised hosts, including individuals with HIV infection, cancer, haematopoietic stem cell transplant, solid organ transplant, chronic inflammatory disease (with planned, low- or high-level immune suppression), asplenia, sickle cell disease, cochlear implants, or cerebrospinal fluid leak (Table 2) [89]. Guidelines also include recommendations for vaccination of household members of immunocompromised patients. Improvement in patient care could result from simply informing a patient and anticipating a personalised administration schedule, which can be pointed out on the discharge letter, and updating vaccinations should be the matter of all. Discharge from critical care is probably an appropriate timing for immunization since near-death/life-threatening experience increases the awareness of disease-risk and acceptance of preventive interventions, such as vaccination. Although altered immune function, including lymphocyte depletion, is well-documented after septic injury [90–92] and reduces vaccine-responses in mice [93], the nature and magnitude of this “immunoparalysis” differ over time and between patients [94] and its impact on vaccine responses is unknown. We consider the risk of suboptimal vaccine responses as lower than that of a missed opportunity to vaccinate and propose immunization updates prior to ICU discharge.
Unfortunately, physicians’ compliance with immunization recommendations is still poor, as many studies report missed opportunities and low vaccination coverage among high-risk individuals. Reasons underlying these findings include lack of awareness, fear of vaccine-induced adverse events and misconception that immunosuppression could interfere with vaccine responses, in both patients’ and the physicians’ minds. Whereas the immune responses to nearly all vaccinations may be affected by the patient’s underlying condition, suboptimal response is better than no protection at all. Vaccination remains one of the best preventive strategies, and every effort should be made to increase the awareness of health practitioners on the importance of both vaccinating and verifying serological protection of high-risk patients against vaccine preventable diseases. Last, but not least, the immunization status of intensive care physicians and nurses should be controlled and maintained up-to-date.
Discussion and perspectives
WHO estimates that 2–3 million deaths are prevented every year by immunization. It is believed to be one of the most successful and cost-effective public health interventions. Should global vaccination coverage improve, a further 1.5 million deaths could be avoided [39]. On an individual perspective, a substantial benefit could result from better knowledge of vaccines and complete vaccination of both the patient and their caregiver(s) (Table 2).
There are vaccines in the pipeline that could have a significant impact on critical care admissions. Safe and effective vaccines against HIV, tuberculosis, and malaria would have a huge impact on the worlds’ burden of communicable diseases [95]. Children would also benefit from vaccination against deadly diarrhoeal diseases [96], and infants from efficient Streptococcus group B and RSV vaccines, given the high morbidity and mortality in this population. Vaccines against Staphylococcus aureus or Pseudomonas species could become important actors in preventing nosocomial infections [97]. In a nearer future, boosting varicella-immunity to prevent zoster could become a useful strategy prior to elective surgery in senior citizen or event at the time of admission to intensive care [62, 63].
Vaccination of third parties (patients’ caregivers, HCWs, and pregnant women) should be further encouraged. As an important proportion of neonatal deaths and stillbirths are due to infections, prenatal acquisition of disease-specific protection through maternal immunization increases survival in early life. Maternal immunization has gained its spurs in preventing tetanus, influenza and pertussis, and should be more widely used as a strategy for vaccines available or in development.
Vaccines can also help in the fight against antimicrobial resistance, a major public health concern and century challenge, due to overuse and misuse of antibiotics in humans, animals and the environment. Vaccination is an effective way to prevent infections by resistant bacteria and reduce subsequent antibiotic consumption [98].
Whichever the strategy, sufficient vaccination coverage is the mainstay for disease control or elimination. The main challenge in LMICs is to obtain vaccines at reasonable costs and enable their distribution to even the most isolated and deprived populations. The main challenge in HICs is vaccine hesitancy. Whether some vaccinations should be mandatory is subject of debate [99]: policies need to balance personal liberty with individual and public health, and mandatory vaccination may even increase opposing attitudes and reduce vaccine acceptance. In any case, intensified information strategies are necessary to improve trust, and rectify perceived risks and improve vaccines acceptability [100]. This information should be given both on a broad (public health policies) and individual scale, and intensive care physicians can have a critical role in the latter.
Author contributions
DP design the article structure. LFP and MA performed the literature search. LFP wrote the first draft of the manuscript. MA, CAS, and DP critically revised the work. All authors read and approved the final manuscript.
Funding
LFP is supported by the Swiss National Science Foundation (Early Postdoc.Mobility Grant No. P2GEP3_178155).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 1–15. [Google Scholar]
- 2.Reisner-Senelar L. The birth of intensive care medicine: Bjorn Ibsen’s records. Intensive Care Med. 2011;37:1084–1086. doi: 10.1007/s00134-011-2235-z. [DOI] [PubMed] [Google Scholar]
- 3.Martinez M, Shukla H, Nikulin J, Mbaeyi C, Jorba J, Ehrhardt D. Progress toward poliomyelitis eradication—Afghanistan, January 2018–May 2019. MMWR Morb Mortal Wkly Rep. 2019;68:729–733. doi: 10.15585/mmwr.mm6833a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyu HH, Mumford JE, Stanaway JD, Barber RM, Hancock JR, Vos T, Murray CJ, Naghavi M. Mortality from tetanus between 1990 and 2015: findings from the global burden of disease study 2015. BMC Public Health. 2017;17:179. doi: 10.1186/s12889-017-4111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanderEnde K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage—2017. MMWR Morb Mortal Wkly Rep. 2018;67:1261–1264. doi: 10.15585/mmwr.mm6745a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz JR, Neuzil KM, Shay DK, Rue TC, Neradilek MB, Zhou H, Seymour CW, Hooper LG, Cheng PY, Goss CH, Cooke CR. The burden of influenza-associated critical illness hospitalizations. Crit Care Med. 2014;42:2325–2332. doi: 10.1097/CCM.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang QL, Wan CM, MacDonald NE. Vaccine preventable infections and multiple organ dysfunction syndrome in critically ill children in China. Pediatr Infect Dis J. 2009;28:182–185. doi: 10.1097/INF.0b013e31818a65d2. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Shefer A, Wenger J, Messonnier M, Wang LY, Lopez A, Moore M, Murphy TV, Cortese M, Rodewald L. Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics. 2014;133:577–585. doi: 10.1542/peds.2013-0698. [DOI] [PubMed] [Google Scholar]
- 9.Yu W, Lee LA, Liu Y, Scherpbier RW, Wen N, Zhang G, Zhu X, Ning G, Wang F, Li Y, Hao L, Zhang X, Wang H. Vaccine-preventable disease control in the People’s Republic of China: 1949–2016. Vaccine. 2018;36:8131–8137. doi: 10.1016/j.vaccine.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offit PA. The Cutter incident: how America’s first polio vaccine led to the growing vaccine crisis. New Haven: Yale University Press; 2005. [Google Scholar]
- 11.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, Davies SE, Walker-Smith JA. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Tartari E, Lotfinejad N, Parneix P, Pittet D. Fighting the good fight: the fallout of fake news in infection prevention and why context matters. J Hosp Infect. 2018;100:365–370. doi: 10.1016/j.jhin.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 13.McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39:1550–1562. doi: 10.1016/j.clinthera.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, Cherian T. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 15.Nanduri SA, Sutherland AR, Gordon LK, Santosham M. Haemophilus influenzae type b vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 301–318. [Google Scholar]
- 16.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajjeh RA, Privor-Dumm L, Edmond K, O’Loughlin R, Shetty S, Griffiths UK, Bear AP, Cohen AL, Chandran A, Schuchat A, Mulholland EK, Santosham M. Supporting new vaccine introduction decisions: lessons learned from the Hib Initiative experience. Vaccine. 2010;28:7123–7129. doi: 10.1016/j.vaccine.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 19.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 20.Wotton CJ, Goldacre MJ. Risk of invasive pneumococcal disease in people admitted to hospital with selected immune-mediated diseases: record linkage cohort analyses. J Epidemiol Community Health. 2012;66:1177–1181. doi: 10.1136/jech-2011-200168. [DOI] [PubMed] [Google Scholar]
- 21.Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, Pelton SI. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1:ofu024. doi: 10.1093/ofid/ofu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Vidal C, Carratala J, Fernandez-Sabe N, Dorca J, Verdaguer R, Manresa F, Gudiol F. Aetiology of, and risk factors for, recurrent community-acquired pneumonia. Clin Microbiol Infect. 2009;15:1033–1038. doi: 10.1111/j.1469-0691.2009.02918.x. [DOI] [PubMed] [Google Scholar]
- 23.Grau I, Ardanuy C, Cubero M, Benitez MA, Linares J, Pallares R. Declining mortality from adult pneumococcal infections linked to children’s vaccination. J Infect. 2016;72:439–449. doi: 10.1016/j.jinf.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittet LF, Posfay-Barbe KM. Pneumococcal vaccines for children: a global public health priority. Clin Microbiol Infect. 2012;18(Suppl 5):25–36. doi: 10.1111/j.1469-0691.2012.03938.x. [DOI] [PubMed] [Google Scholar]
- 26.Borrow R, Heath PT, Siegrist CA. Use of pneumococcal polysaccharide vaccine in children: what is the evidence? Curr Opin Infect Dis. 2012;25:292–303. doi: 10.1097/QCO.0b013e3283531b0f. [DOI] [PubMed] [Google Scholar]
- 27.Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31:3577–3584. doi: 10.1016/j.vaccine.2013.04.085. [DOI] [PubMed] [Google Scholar]
- 28.Scott DA, Komjathy SF, Hu BT, Baker S, Supan LA, Monahan CA, Gruber W, Siber GR, Lockhart SP. Phase 1 trial of a 13-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2007;25:6164–6166. doi: 10.1016/j.vaccine.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Vaccine. 2015;33(Suppl 4):D60–D65. doi: 10.1016/j.vaccine.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 30.Pittet LF, Verolet CM, Michetti P, Girardin M, Juillerat P, Mottet C, Maillard MH, Siegrist CA, Posfay-Barbe KM, Swiss Inflammatory Bowel Disease Cohort Study Group High immunogenicity of the pneumococcal conjugated vaccine in immunocompromised adults with inflammatory bowel disease. Am J Gastroenterol. 2019;114:1130–1141. doi: 10.14309/ajg.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 31.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 32.Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther. 2014;31:1011–1044. doi: 10.1007/s12325-014-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DK, Bridges CB, Harriman KH. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older—United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:91–92. [PMC free article] [PubMed] [Google Scholar]
- 34.Staples JE, Monath TP, Gershman MD, Barrett ADT. Yellow fever vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 1181–1265. [Google Scholar]
- 35.World Health Organization . A global strategy to eliminate yellow fever epidemics 2017–2026. Geneva: World Health Organization; 2018. [Google Scholar]
- 36.Casey RM, Harris JB, Ahuka-Mundeke S, Dixon MG, Kizito GM, Nsele PM, Umutesi G, Laven J, Kosoy O, Paluku G, Gueye AS, Hyde TB, Ewetola R, Sheria GKM, Muyembe-Tamfum J-J, Staples JE. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak—final report. N Engl J Med. 2018;381:444–454. doi: 10.1056/NEJMoa1710430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Safadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–B36. doi: 10.1016/j.vaccine.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay ME, Andrews N, Kaczmarski EB, Miller E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet. 2001;357:195–196. doi: 10.1016/S0140-6736(00)03594-7. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization (2019) Immunization. https://www.who.int/news-room/facts-in-pictures/detail/immunization. Accessed 8 Aug 2019
- 40.Mustapha MM, Harrison LH. Vaccine prevention of meningococcal disease in Africa: major advances, remaining challenges. Hum Vaccines Immunother. 2018;14:1107–1115. doi: 10.1080/21645515.2017.1412020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;2:395–403. doi: 10.1016/S2352-4642(18)30103-2. [DOI] [PubMed] [Google Scholar]
- 42.Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388:2775–2782. doi: 10.1016/S0140-6736(16)31921-3. [DOI] [PubMed] [Google Scholar]
- 43.Kuhdari P, Stefanati A, Lupi S, Valente N, Gabutti G. Meningococcal B vaccination: real-world experience and future perspectives. Pathog Glob Health. 2016;110:148–156. doi: 10.1080/20477724.2016.1195072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. [PubMed] [Google Scholar]
- 46.Global Task Force on Cholera Control . Ending cholera—a global roadmap to 2030. Geneva: World Health Organization; 2017. [Google Scholar]
- 47.Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, Holmgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 48.Longini IM, Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Cost-effectiveness of new-generation oral cholera vaccines: a multisite analysis. Value Health. 2009;12:899–908. doi: 10.1111/j.1524-4733.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 50.Higgs ES, Dubey SA, Coller BAG, Simon JK, Bollinger L, Sorenson RA, Wilson B, Nason MC, Hensley LE. Accelerating vaccine development during the 2013–2016 West African Ebola virus disease outbreak. Curr Top Microbiol Immunol. 2017;411:229–261. doi: 10.1007/82_2017_53. [DOI] [PubMed] [Google Scholar]
- 51.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola ça suffit!) Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ilunga Kalenga O, Moeti M, Sparrow A, Nguyen VK, Lucey D, Ghebreyesus TA. The ongoing Ebola epidemic in the Democratic Republic of Congo, 2018–2019. N Engl J Med. 2019;381:373–383. doi: 10.1056/NEJMsr1904253. [DOI] [PubMed] [Google Scholar]
- 54.Straney L, Schibler A, Ganeshalingham A, Alexander J, Festa M, Slater A, MacLaren G, Schlapbach LJ. Burden and outcomes of severe pertussis infection in critically ill infants. Pediatr Crit Care Med. 2016;17:735–742. doi: 10.1097/PCC.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 55.Cherry JD. Epidemic pertussis in 2012—the resurgence of a vaccine-preventable disease. N Engl J Med. 2012;367:785–787. doi: 10.1056/NEJMp1209051. [DOI] [PubMed] [Google Scholar]
- 56.Esposito S, Stefanelli P, Fry NK, Fedele G, He Q, Paterson P, Tan T, Knuf M, Rodrigo C, Weil Olivier C, Flanagan KL, Hung I, Lutsar I, Edwards K, O’Ryan M, Principi N. Pertussis prevention: reasons for resurgence, and differences in the current acellular pertussis vaccines. Front Immunol. 2019;10:1344. doi: 10.3389/fimmu.2019.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, Munoz-Rivas F, Teyssou R, Guiso N, Van Rie A. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26:293–299. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 58.Lumbreras Areta M, Eberhardt CS, Siegrist CA, Martinez de Tejada B. Antenatal vaccination to decrease pertussis in infants: safety, effectiveness, timing, and implementation. J Matern Fetal Neonatal Med. 2019;32:1541–1546. doi: 10.1080/14767058.2017.1406475. [DOI] [PubMed] [Google Scholar]
- 59.Eberhardt CS, Blanchard-Rohner G, Lemaitre B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist CA. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62:829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eberhardt CS, Blanchard-Rohner G, Lemaitre B, Combescure C, Othenin-Girard V, Chilin A, Petre J, Martinez de Tejada B, Siegrist CA. Pertussis antibody transfer to preterm neonates after second- versus third-trimester maternal immunization. Clin Infect Dis. 2017;64:1129–1132. doi: 10.1093/cid/cix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sobanjo-Ter Meulen A, Duclos P, McIntyre P, Lewis KD, Van Damme P, O’Brien KL, Klugman KP. Assessing the evidence for maternal pertussis immunization: a report from the Bill & Melinda Gates foundation symposium on pertussis infant disease burden in low- and lower-middle-income countries. Clin Infect Dis. 2016;63:S123–S133. doi: 10.1093/cid/ciw530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC, Group ZOES Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 63.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 64.Jacobson RM, St Sauver JL, Finney Rutten LJ. Vaccine hesitancy. Mayo Clin Proc. 2015;90:1562–1568. doi: 10.1016/j.mayocp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189:S4–S16. doi: 10.1086/377712. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA. 1992;267:1237–1241. [PubMed] [Google Scholar]
- 67.Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153–164. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- 68.Avota E, Gassert E, Schneider-Schaulies S. Measles virus-induced immunosuppression: from effectors to mechanisms. Med Microbiol Immunol. 2010;199:227–237. doi: 10.1007/s00430-010-0152-3. [DOI] [PubMed] [Google Scholar]
- 69.Slifka MK, Homann D, Tishon A, Pagarigan R, Oldstone MB. Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection. J Clin Investig. 2003;111:805–810. doi: 10.1172/JCI13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halsey NA. Measles in developing countries. BMJ. 2006;333:1234. doi: 10.1136/bmj.39058.361620.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Serres G, Gay NJ, Farrington CP. Epidemiology of transmissible diseases after elimination. Am J Epidemiol. 2000;151:1039–1052. doi: 10.1093/oxfordjournals.aje.a010145. [DOI] [PubMed] [Google Scholar]
- 72.Hens N, Abrams S, Santermans E, Theeten H, Goeyvaerts N, Lernout T, Leuridan E, Van Kerckhove K, Goossens H, Van Damme P, Beutels P. Assessing the risk of measles resurgence in a highly vaccinated population: Belgium anno 2013. Euro Surveill. 2015;20:i20998. doi: 10.2807/1560-7917.es2015.20.1.20998. [DOI] [PubMed] [Google Scholar]
- 73.Patel M, Lee AD, Redd SB, Clemmons NS, McNall RJ, Cohn AC, Gastanaduy PA. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:402–404. doi: 10.15585/mmwr.mm6817e1. [DOI] [PubMed] [Google Scholar]
- 74.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315:1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo NC, Hotez PJ. Public health and economic consequences of vaccine hesitancy for measles in the United States. JAMA Pediatr. 2017;171:887–892. doi: 10.1001/jamapediatrics.2017.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iizuka M, Nakagomi O, Chiba M, Ueda S, Masamune O. Absence of measles virus in Crohn’s disease. Lancet. 1995;345:199. doi: 10.1016/s0140-6736(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 77.Taylor B, Miller E, Lingam R, Andrews N, Simmons A, Stowe J. Measles, mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: population study. BMJ. 2002;324:393–396. doi: 10.1136/bmj.324.7334.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:c5347. doi: 10.1136/bmj.c5347. [DOI] [PubMed] [Google Scholar]
- 79.Editors of The Lancet Retraction: ileal lymphoid nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 2010;375:445. doi: 10.1016/S0140-6736(10)60175-4. [DOI] [PubMed] [Google Scholar]
- 80.Strebel PM, Papania MJ, Gastañaduy PA, Goodson JL. Measles vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 579–618. [Google Scholar]
- 81.Bresee J, Fry AM, Sambhara S, Cox NJ. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 456–488. [Google Scholar]
- 82.World Health Organization . Global influenza strategy 2019–2030. Geneva: World Health Organization; 2019. [Google Scholar]
- 83.Jenkin DC, Mahgoub H, Morales KF, Lambach P, Nguyen-Van-Tam JS. A rapid evidence appraisal of influenza vaccination in health workers: an important policy in an area of imperfect evidence. Vaccine X. 2019;2:100036. doi: 10.1016/j.jvacx.2019.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dini G, Toletone A, Sticchi L, Orsi A, Bragazzi NL, Durando P. Influenza vaccination in healthcare workers: a comprehensive critical appraisal of the literature. Hum Vaccines Immunother. 2018;14:772–789. doi: 10.1080/21645515.2017.1348442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.To KW, Lai A, Lee KC, Koh D, Lee SS. Increasing the coverage of influenza vaccination in healthcare workers: review of challenges and solutions. J Hosp Infect. 2016;94:133–142. doi: 10.1016/j.jhin.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Miles M, Ryman TK, Dietz V, Zell E, Luman ET. Validity of vaccination cards and parental recall to estimate vaccination coverage: a systematic review of the literature. Vaccine. 2013;31:1560–1568. doi: 10.1016/j.vaccine.2012.10.089. [DOI] [PubMed] [Google Scholar]
- 87.Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccines Immunother. 2015;11:2556–2563. doi: 10.1080/21645515.2015.1074358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sisson H, Gardiner E, Watson R. Vaccination timeliness in preterm infants: an integrative review of the literature. J Clin Nurs. 2017;26:4094–4104. doi: 10.1111/jocn.13916. [DOI] [PubMed] [Google Scholar]
- 89.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, Kang I. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 90.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3:e98960. doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen IJ, Sjaastad FV, Griffith TS, Badovinac VP. Sepsis-induced T cell immunoparalysis: the ins and outs of impaired T cell immunity. J Immunol. 2018;200:1543–1553. doi: 10.4049/jimmunol.1701618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sjaastad FV, Condotta SA, Kotov JA, Pape KA, Dail C, Danahy DB, Kucaba TA, Tygrett LT, Murphy KA, Cabrera-Perez J, Waldschmidt TJ, Badovinac VP, Griffith TS. Polymicrobial sepsis chronic immunoparalysis is defined by diminished ag-specific T cell-dependent B cell responses. Front Immunol. 2018;9:2532. doi: 10.3389/fimmu.2018.02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bruse N, Leijte GP, Pickkers P, Kox M. New frontiers in precision medicine for sepsis-induced immunoparalysis. Expert Rev Clin Immunol. 2019;15:251–263. doi: 10.1080/1744666X.2019.1562336. [DOI] [PubMed] [Google Scholar]
- 95.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pletz MW, Uebele J, Gotz K, Hagel S, Bekeredjian-Ding I. Vaccines against major ICU pathogens: where do we stand? Curr Opin Crit Care. 2016;22:470–476. doi: 10.1097/MCC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 98.Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci USA. 2018;115:12896–12901. doi: 10.1073/pnas.1721095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bechini A, Boccalini S, Ninci A, Zanobini P, Sartor G, Bonaccorsi G, Grazzini M, Bonanni P. Childhood vaccination coverage in Europe: impact of different public health policies. Expert Rev Vaccines. 2019;18:693–701. doi: 10.1080/14760584.2019.1639502. [DOI] [PubMed] [Google Scholar]
- 100.Holzmann H, Wiedermann U. Mandatory vaccination: suited to enhance vaccination coverage in Europe? Euro Surveill. 2019;24:1900376. doi: 10.2807/1560-7917.ES.2019.24.26.1900376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radetsky M. Smallpox: a history of its rise and fall. Pediatr Infect Dis J. 1999;18:85–93. doi: 10.1097/00006454-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 102.Plotkin SA, Orenstein WA, Offit PA, Edwards KM. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. [Google Scholar]
- 103.Graves PM, Deeks JJ, Demicheli V, Jefferson T. Vaccines for preventing cholera: killed whole cell or other subunit vaccines (injected) Cochrane Database Syst Rev. 2010;8:Cd000974. doi: 10.1002/14651858.CD000974.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. Oral vaccines for preventing cholera. Cochrane Database Syst Rev. 2011;3:Cd008603. doi: 10.1002/14651858.CD008603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Milligan R, Paul M, Richardson M, Neuberger A. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2018;5:Cd001261. doi: 10.1002/14651858.CD001261.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edwards KM, Decker MD. Pertussis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 711–761. [Google Scholar]
- 107.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 108.Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018;2:Cd001269. doi: 10.1002/14651858.CD001269.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev. 2012;2:CD004407. doi: 10.1002/14651858.CD004407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rubin SA. Mumps vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 663–688. [Google Scholar]
- 111.Klugman KP, Dagan R, Malley R, Whitney CG. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 773–815. [Google Scholar]
- 112.Grabenstein JD, Musher DM. Pneumococcal polysaccharide vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. Philadelphia: Elsevier; 2018. pp. 816–840. [Google Scholar]
- 113.Harper DM, DeMars LR. HPV vaccines—a review of the first decade. Gynecol Oncol. 2017;146:196–204. doi: 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 114.Luckett R, Feldman S. Impact of 2-, 4- and 9-valent HPV vaccines on morbidity and mortality from cervical cancer. Hum Vaccines Immunother. 2016;12:1332–1342. doi: 10.1080/21645515.2015.1108500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suresh S, Upton J, Green M, Pham-Huy A, Posfay-Barbe KM, Michaels MG, Top KA, Avitzur Y, Burton C, Chong PP, Danziger-Isakov L, Dipchand AI, Hebert D, Kumar D, Morris SK, Nalli N, Ng VL, Nicholas SK, Robinson JL, Solomon M, Tapiero B, Verma A, Walter JE, Allen UD. Live vaccines after pediatric solid organ transplant: proceedings of a consensus meeting, 2018. Pediatr Transplant. 2019;23:e13571. doi: 10.1111/petr.13571. [DOI] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention Impact of vaccines universally recommended for children—United States, 1990–1998. MMWR Morb Mortal Wkly Rep. 1999;48:243–248. [PubMed] [Google Scholar]
- 117.Centers for Disease Control and Prevention Summary of notifiable diseases, United States, 1998. MMWR Morb Mortal Wkly Rep. 1999;47:ii-92. [PubMed] [Google Scholar]
- 118.Centers for Disease Control and Prevention (2019) National notifiable diseases surveillance system, weekly tables of infectious disease data. Atlanta, GA. CDC division of health informatics and surveillance. https://www.cdc.gov/nndss/infectious-tables.html. Accessed 14 Aug 2019
- 119.Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, Wassilak SG. The vaccine adverse event reporting system (VAERS) Vaccine. 1994;12:542–550. doi: 10.1016/0264-410x(94)90315-8. [DOI] [PubMed] [Google Scholar]