Abstract
Background
The frequency of childhood obesity has increased over the last 3 decades, and the trend constitutes a worrisome epidemic worldwide. With the raising obesity risk, key aspects to consider are accurate body mass index classification, as well as metabolic and cardiovascular, and hepatic consequences.
Data sources
The authors performed a systematic literature search in PubMed and EMBASE, using selected key words (obesity, childhood, cardiovascular, liver health). In particular, they focused their search on papers evaluating the impact of obesity on cardiovascular and liver health.
Results
We evaluated the current literature dealing with the impact of excessive body fat accumulation in childhood and across adulthood, as a predisposing factor to cardiovascular and hepatic alterations. We also evaluated the impact of physical and dietary behaviors starting from childhood on cardio-metabolic consequences.
Conclusions
The epidemic of obesity and obesity-related comorbidities worldwide raises concerns about the impact of early abnormalities during childhood and adolescence. Two key abnormalities in this context include cardiovascular diseases, and nonalcoholic fatty liver disease. Appropriate metabolic screenings and associated comorbidities should start as early as possible in obese children and adolescents. Nevertheless, improving dietary intake and increasing physical activity performance are to date the best therapeutic tools in children to weaken the onset of obesity, cardiovascular diseases, and diabetes risk during adulthood.
Keywords: Cardiovascular, Childhood, Liver health, Obesity
Introduction
Obesity in children and adolescents has emerged as one of the most serious health problems, condition which threatens future health and longevity. Over the past 30 years, childhood obesity rate has doubled and, in some cases, even tripled in developed countries [1].
According to the Global Health Observatory Data 2017 by World Health Organization (WHO), there are over 340 million obese children and adolescents aged 5–19.
The aim of this thematic review is to provide current data about the impact of excessive body fat accumulation in infancy across adulthood, as a sound predisposing factor of cardiovascular and hepatic consequences. We also discuss the impact of physical and dietary behaviors starting from childhood on obesity-related comorbidities.
Epidemiologic burden and consequences of obesity
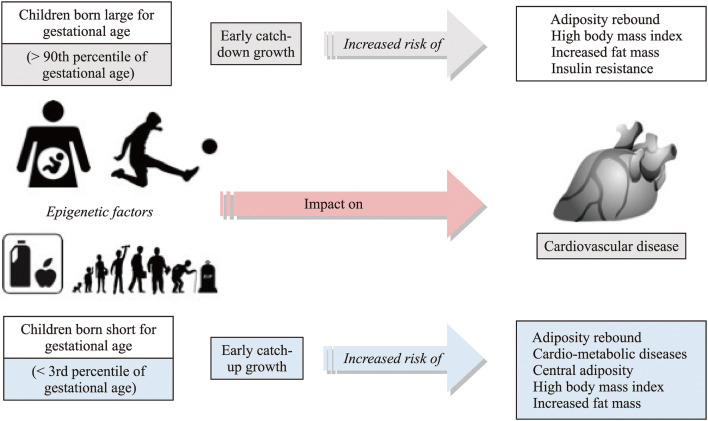
The rate of increase in obesity is faster in children than in adults [2]. Countries with a rapid development, as China, are displaying a remarkable increase in childhood obesity, with the number of overweight and obese Chinese children aged 7–18 years increasing by 28 times from 1985 to 2000 [3]. In the United States, the prevalence of obesity in the pediatric population reaches 18.5%, affecting almost 14 million of children and adolescents [4]. In Italy, about 21% of children are overweight and 10% are obese, with obesity trends expected to increase further [5]. A similar trend emerged in a previous ultrasonographic study in children and adolescents in southern Italy [6]. Obese children have a fivefold increased risk to stay obese during adulthood, as compared to normal-weight children [7, 8]. Infancy and childhood are critical moments in which key metabolic changes occur with health effects later in life. Both the children born large for gestational age or small for gestational age have the risk to develop obesity and metabolic consequences (Fig. 1). Genetic factors play only a minor role whilst social, economic, and environmental factors drive the increase of the obesity prevalence. The role of epigenetic factors at an early age is also important in determining adulthood metabolic abnormalities [9–13]. Table 1 depicts the potential weight-related comorbidities in obese children. Obese children are more susceptible to cardiovascular diseases (CVD) [14–17], metabolic alterations [18, 19], orthopedic complications, and psychosocial disorders, such as low self-esteem, anxiety, social isolation, and poor academic performance [20]. In addition, obese children may show abnormalities in the liver, reproductive system, brain, as well as increased blood pressure, impaired gluco-lipid metabolism, and sleep apnea. Thus, the priority is to design preventive measures to halt the number of obese children becoming unhealthy during adulthood [21].
Fig. 1.
Factors influencing obesity and its comorbidities
Table 1.
Potential weight-related comorbidities in obese children
| Hypertension |
| Nonalcoholic fatty liver disease |
| Dyslipidemia |
| Cardiovascular disease, early atherosclerosis |
| Insulin resistance, type 2 diabetes mellitus |
| Sleep apnea |
| Orthopedic disease |
| Precocious puberty |
| Polycystic ovary syndrome |
| Pseudotumor cerebri |
Assessment of childhood overweight and obesity
Obesity results from excessive fat accumulation in the body, usually assessed by body mass index (BMI), i.e., body weight (kg) divided by the square of height (m2). According to the International Classification by the WHO, adults are considered overweight when BMI ≥ 25 kg/m2 and obese if BMI ≥ 30 kg/m2. Incorrect evaluation of BMI as indicator of body adiposity can occur when muscle mass is increased or reduced in people engaged to frequent physical activity and to sedentary life, respectively.
In children up to 2 years, the diagnosis of overweight and obesity relies on the weight/length ratio using the WHO 2006 reference curves. For children over 2 years, the diagnosis is based on the use of BMI using the WHO 2006 reference curves up to 5 years, and the WHO 2007 reference curves thereafter. The diagnosis of overweight is made for BMI values ≥ 85th percentile and < 95th percentile, while obesity is defined by BMI values ≥ 95th percentile [22, 23]. The 99th percentile is the cut-off used to define severe obesity since this value corresponds to a higher prevalence of cardio-metabolic risk factors and persistence of obesity later in life, as compared with lower percentiles [14]. However, the WHO terminology used to define severe obesity differs from younger (up to 5 years of age) compared to older children and adolescents. Indeed, the 99th percentile identifies obesity in the former group, and severe obesity in the latter. The different cut-off used to define these conditions is justified by the differences in growth process at different ages [24, 25].
The recommendation to use the WHO reference curves relies on their higher sensibility in identifying overweight and obese children. Indeed, the Italian BMI thresholds [26] underestimated the prevalence, likely due to the curves based on dates collected in a time when the increase in obesity had already occurred [27].
Body composition can change among different ethnicities. For example, within the same BMI, non-Hispanic black children and adolescents show lower percentages of fat mass than non-Hispanic white or Mexican Americans. This condition explains why they are less prone to become obese [28]. Singapore Chinese adolescents, on the other hand, tend to have higher fat mass than Dutch Caucasian adolescents [29], and they are more predisposed to develop obesity-related consequences; hence, the cut-off to define overweight and obesity in Asian children and adolescents is lower [30]. Notably, in a recent meta-analysis, the number of genomic loci associated with height and BMI was disproportionately increased compared to previously published genome-wide-associated studies [31]. The authors suggest that the discovery of new loci will increase prediction accuracy and provide further data to explain complex trait biology.
The impact of childhood and adolescence obesity on CVD
CVDs are the most frequent cause of morbidity and mortality globally. Atherosclerosis is one of the main contributing factors for CVD. This process begins already during childhood mostly under the influence of environmental factors. Some studies suggest that maternal weight gain in early pregnancy may be a critical period for an adverse childhood cardiovascular risk profile [32].
Other studies found an association between BMI in childhood and increased risk of adulthood CVD and mortality. Twig et al. examined BMI in a cohort of 2,300,000 Israeli aged 16 and 19 years, in the search for a correlation between death and CVD later in life. In the 40-year follow-up, a correlation existed between increased risk of cardiovascular events and all-cause mortality in adults whose BMI increased during adolescence [33]. Baker et al. found similar results in a cohort of 276,853 Danish children. In particular, the increase of 1 unit in BMI Z score in 13-year-old children almost doubled the risk of adulthood CVD [34]. Bjorge et al. found a correlation of obesity with premature death, even if the critical age range associated with such increased risk remained uncertain [35]. Concerning the critical BMI threshold, Aune et al. conducted a meta-analysis of the available studies investigating the correlations of BMI with all-cause mortality. The nadir existed at BMI 23–24 kg/m2 among non-smokers, 22–23 kg/m2 among healthy non-smokers, and 20–22 kg/m2 when the analysis was limited to studies with longer durations of follow-up [36].
Due to the strong correlation between childhood obesity and the CVD risk during adulthood, several studies aimed to identify early markers of CVD. McGill et al. demonstrated the presence of early atherosclerotic lesions [37], which appear first in the distal aorta and then in the carotid arteries. Carotid intima–media thickness (cIMT) is considered a valid marker of pre-clinical atherosclerosis, and several studies support its role as independent predictor of CVD even in asymptomatic subjects [38]. In addition, cIMT may be a good marker of cardiovascular alterations in children, although studies show some discrepancies [39]. Freedman et al. analyzed a cohort of 513 subjects and detected a positive correlation between BMI measured throughout life and cIMT at age of 35 years. These associations, however, were restricted to adults who continued to be obese. In particular, they found the BMI-CVD correlation to be weak before the age of 11 years, but it progressively increased with age, reaching the strongest correlation among adolescents aged 15–18 years [40]. Juonala et al. found no significant association between BMI measured at 3, 6, 9 or 15 years and cIMT 21 years later, even though a positive correlation existed with BMI at ages 12 and 18 [41]. In a longer follow-up, Wright et al. did not find any significant correlations between childhood BMI and cIMT at the age of 50 [42].
The impact of childhood and adolescence obesity on liver health
The raising prevalence of obesity, metabolic syndrome together with insulin resistance [43], worldwide is associated with liver abnormalities encompassing the clinical spectrum of nonalcoholic fatty liver disease (NAFLD). NAFLD occurs in the absence of other triggering factors such as hepatitis C, alcohol consumption, parenteral nutrition, or steatogenic drugs. Whereas nonalcoholic fatty liver (NAFL)—a relatively benign condition [44]—implies more than 5% of fatty hepatocytes without hepatocellular injury, the term nonalcoholic steatohepatitis (NASH) is associated with fatty liver and hepatocellular injury revealed by the histological findings of hepatocyte ballooning, with or without fibrosis [45]. A third category is NASH cirrhosis, showing current or previous evidence of histologic NASH or NAFL. Ethnicity, age, metabolic syndrome, insulin resistance [46, 47], dyslipidemia [48], high intake of dietary fructose [49–51], all influence the development of NAFLD [52], with males showing higher risk than females [53]. In children, NAFLD is now the most common cause of liver disease [54–56], and this trend is somewhat worrisome because even in children NASH may evolve to fibrosis, cirrhosis (as early as 8 years) [57, 58], and even liver failure [59–61]. This correlation NAFLD/NASH, however, seems weaker in children than adults, suggesting a milder phenotype of NAFLD [62]. Since adults with NAFLD have high risk to die from cardiovascular disease, clinicians and the public should be aware that children with NAFLD must receive a full evaluation to detect or prevent important comorbidities listed in Table 1, and including type 2 diabetes mellitus, and cardiovascular disease. Based on elevated serum aminotransferases, imaging or liver biopsy, the prevalence of NAFLD in children and adolescents and in obese children ranges between 6 and 38%, depending on the context, the population studied, and the ethnicity (Table 2).
Table 2.
Studies relating diagnosis of nonalcoholic fatty liver disease with prevalence of nonalcoholic fatty liver disease in children/adolescents
| Diagnosis | Country | Populations | Prevalence of NAFLD | References |
|---|---|---|---|---|
| Aminotransferase elevationsa | USA | 2450 children (12–18 y) NHANES III |
6% in all 10% in obese |
[67] |
| USA | 12,714 children (12–19 y) | 11% in all | [68] | |
| USA | Meta-analysis on 23 studies (1–19 y) |
7% in all 13.7% in obese |
[59] | |
| Ultrasound | USA | Meta-analysis on 44 studies (1–19 y) |
7.6% in all 41.3% in obese |
[59] |
| Liver histology | USA |
Autopsy 742 children/adolescents (San Diego County) |
Whole 9.6% (NASH 3% of all, 23% of NAFL) 38% in obese Hispanics > White > Black |
[69] |
| USA |
Liver specimens within 48 h death 582 subjects (2–19 y) 50% Black, 33% Hispanic, 12% White, 3% Asian, and 2% other; 36% had a body mass index > 85% |
4.5% NAFLD (1.7% NASH) White 8.3%, Hispanics 7.9%b, Black 1% |
[70] |
As reported in adults, also children with NAFLD remain mostly asymptomatic [8] or describe mild symptoms such as pain in the right upper quadrant or nonspecific symptoms, including fatigue and abdominal discomfort [63, 64], or symptoms due to obesity-associated comorbidities (i.e., gallstones, gastroesophageal reflux disease, etc.) [65]. Thus, physical examination should look for comorbidities, splenomegaly, and end-stage liver disease (cirrhosis). Serum abnormalities include elevated liver transaminases, alkaline phosphatase, and gamma-glutamyl transpeptidase [8, 56, 58, 66, 67] which tend to improve upon adoption of healthy lifestyles (see below) [66, 68]. Notably, even in children with NASH the levels of aminotransferases may remain normal [69], and this possibility is intrinsic to the limited sensitivity and specificity of serum aminotransferase levels for clinically significant NAFLD. Due to the high and further raising prevalence of obesity and metabolic syndrome in children, recent guidelines recommend the screening of obese children as the primary screening for NAFLD. Steps vary according to the existence of concomitant comorbidities and levels of serum transaminases (i.e., normal, moderate, and > 2 upper normal limit persistent elevation) [15]. At least in children, imaging techniques for the screening diagnosis of NAFLD are not routinely recommended [15, 70], due to poor sensitivity and specificity (ultrasonography) [71, 72], poor correlation with steatohepatitis, fibrosis (magnetic resonance) [73–75], or detection of only advanced fibrosis, costs, lack of definitive cut-off values and need validation (magnetic resonance elastography) [76]. The role of liver biopsy (which ultimately confirms the diagnosis of NAFLD and determines the severity of the fatty liver disease with the presence and extent of inflammation and fibrosis [15, 56]) needs to be discussed on a case-by-case basis. Cases should include forms of more progressive NAFLD, possibility of other liver diseases, and morbidly obese scheduled for bariatric surgery.
In spite of the emerging epidemics of pediatric NAFLD worldwide, there is no established treatment so far, even when considering metformin (for improving insulin sensitivity), vitamin E (for reducing inflammatory changes in the liver), cysteamine bitartrate (as antioxidant agent), and ursodeoxycholic acid (for reducing the bile acid hepatotoxic effect) [15]. As a matter of fact, aggressive treatment of comorbidities and lifestyle intervention (diet, exercise, weight management, counseling) remain the mainstay of treatment even in pediatric NAFLD [77, 78].
Impact of lifestyles starting from childhood
Physical inactivity affects a vast majority of children and adolescents who become prone to high obesity rates and related diseases, including CVD and NAFLD. Promotion of programs involving physical activity has, therefore, become a relevant topic in health policy. Messing et al. conclude that “multi-component interventions in childcare facilities and schools stand out prominently” [79]. Molina–Molina et al. have recently discussed several mechanisms for which physical inactivity might affect CVD [80]. Between ages 5–6 and 8–9, there are similar increases in physical inactivity for both boys and girls, as reflected in a British cohort of 57 primary schools [81]. Already during adolescence, physical activity starts to decrease, contrarily to body weight [82]. A series of factors such as dietary intake and sedentary behaviors contribute to childhood obesity [83]. The phenotype of obesity could differ depending on the children’s age, gender, and family characteristics. Studies on television viewing and total recreational screen time in youth might be associated with adverse CVD risk factors, such as adiposity, increased triglycerides, and metabolic syndrome [84]. Other authors have linked sedentary time with diabetes and high blood pressure in obese and overweight adolescents [85, 86]. Several hours of television viewing by adolescents doubles the odds for metabolic syndrome later in adulthood, as observed in a study by Wennberg et al. [87]. Not only sedentary behaviors, but above 10 hours of night time sleep in primary school children from Germany was also associated with obesity [88].
Poor dietary habits in children also increase the risk of obesity. Among children, parents and caretakers have the greatest influence on their eating habits [89]. According to a study by Lipowska et al., children’s eating patterns are influenced by the parent–child interaction, shaping the nutritional status, which ultimately contributes to their health [90]. Nutritional status of children can also have direct effect on growth, development, and nutrition related-health problems [91].
The portion size of food consumed each day depends on age, sex, stage of growth, body weight and size, and level of physical activity [92]. Children who consume large portion sizes, hyper-caloric and high-energy-dense foods gain excess weight and body fat, while CVD risk increases [93]. Reduced consumption of fats, carbohydrates, and added sugars and more intakes of vegetables and fruits could decrease obesity in children and risk to CVD [94].
Both an optimal nutrition and regular physical activity increase the chances of healthy maturation during childhood [95]. According to Elmaogullari et al., age and BMI are the most important factors to be considered in childhood obesity [96]. Overall, changing dietary patterns seems the best treatment against obesity, CVD, diabetes, and NAFLD.
Conclusions and future perspectives
The epidemic of obesity and obesity-related comorbidities worldwide raises concerns about the impact of early abnormalities during childhood and adolescence. Exact evaluation of body composition parameters is required at an early age, to classify correctly the metabolic abnormalities, and to decrease the chances of further dysmetabolic changes at a later age.
Two key abnormalities in this context include cardiovascular diseases, and nonalcoholic fatty liver disease, a wide spectrum of conditions ranging from simple liver steatosis, steatohepatitis, and (metabolic) cirrhosis. Notably, nonalcoholic fatty liver is associated with increased risk of mortality in the adults. This means that appropriate metabolic screenings and associated comorbidities should start as early as possible in obese children and adolescents. Nevertheless, improving dietary intake and increasing physical activity performance are to date the best therapeutic tools in children to weaken the onset of obesity, CVD, and diabetes risk during adulthood.
Author contributions
MF and PP wrote the review. GD, MC, EMM, and HS revised the literature. FL and MK critically revised the manuscript. All the authors approved the final version of the manuscript.
Funding
The present par develops in the context of the project FOIE GRAS, which has received funding from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie Grant Agreement (No. 722619). EMM and HS are recipients of Foie Gras Early Research Training Grant.
Compliance with ethical standards
Ethical approval
This article does not contain any studies with human participants, but refers to previously published papers/studies that were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y. Overweight and obesity in China. BMJ. 2006;333:362–363. doi: 10.1136/bmj.333.7564.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;(288):1–8. [PubMed]
- 5.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92:251–265. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Palasciano G, Portincasa P, Vinciguerra V, Velardi A, Tardi S, Baldassarre G, et al. Gallstone prevalence and gallbladder volume in children and adolescents: an epidemiological ultrasonographic survey and relationship to body mass index. Am J Gastroenterol. 1989;84:1378–1382. [PubMed] [Google Scholar]
- 7.Simmonds M, Burch J, Llewellyn A, Griffiths C, Yang H, Owen C, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. 2015;19:1–336. doi: 10.3310/hta19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speiser PW, Rudolf MC, Anhalt H, Camacho-Hubner C, Chiarelli F, Eliakim A, et al. Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 9.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 10.Di Ciaula A, Portincasa P. Fat epigenome and pancreatic diseases. Interplay and common pathways from a toxic and obesogenic environment. Eur J Intern Med. 2014;25:865–873. doi: 10.1016/j.ejim.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Di Ciaula A, Portincasa P. Diet and contaminants: driving the rise to obesity epidemics? Curr Med Chem. 2019;26:3471–3482. doi: 10.2174/0929867324666170518095736. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Zou Z, Wang S, Yang Z, Ma J. Chinese famine exposure in infancy and metabolic syndrome in adulthood: results from the China health and retirement longitudinal study. Eur J Clin Nutr. 2019;73:724–732. doi: 10.1038/s41430-018-0211-1. [DOI] [PubMed] [Google Scholar]
- 13.Marzano F, Faienza MF, Caratozzolo MF, Brunetti G, Chiara M, Horner DS, et al. Pilot study on circulating miRNA signature in children with obesity born small for gestational age and appropriate for gestational age. Pediatr Obes. 2018;13:803–811. doi: 10.1111/ijpo.12439. [DOI] [PubMed] [Google Scholar]
- 14.Faienza MF, Santoro N, Lauciello R, Calabro R, Giordani L, Di Salvo G, et al. IGF2 gene variants and risk of hypertension in obese children and adolescents. Pediatr Res. 2010;67:340–344. doi: 10.1203/PDR.0b013e3181d22757. [DOI] [PubMed] [Google Scholar]
- 15.Faienza MF, Acquafredda A, Tesse R, Luce V, Ventura A, Maggialetti N, et al. Risk factors for subclinical atherosclerosis in diabetic and obese children. Int J Med Sci. 2013;10:338–343. doi: 10.7150/ijms.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nacci C, Leo V, De Benedictis L, Carratu MR, Bartolomeo N, Altomare M, et al. Elevated endothelin-1 (ET-1) levels may contribute to hypoadiponectinemia in childhood obesity. J Clin Endocrinol Metab. 2013;98:E683–E693. doi: 10.1210/jc.2012-4119. [DOI] [PubMed] [Google Scholar]
- 17.Ciccone MM, Faienza MF, Altomare M, Nacci C, Montagnani M, Valente F, et al. Endothelial and metabolic function interactions in overweight/obese children. J Atheroscler Thromb. 2016;23:950–959. doi: 10.5551/jat.31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano P, Del Vecchio GC, Cecinati V, Delvecchio M, Altomare M, De Palma F, et al. Metabolic, inflammatory, endothelial and haemostatic markers in a group of Italian obese children and adolescents. Eur J Pediatr. 2011;170:845–850. doi: 10.1007/s00431-010-1356-7. [DOI] [PubMed] [Google Scholar]
- 19.Faienza MF, Francavilla R, Goffredo R, Ventura A, Marzano F, Panzarino G, et al. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78:158–164. doi: 10.1159/000342642. [DOI] [PubMed] [Google Scholar]
- 20.Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 21.Faienza MF, Wang DQ, Fruhbeck G, Garruti G, Portincasa P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern Emerg Med. 2016;11:175–182. doi: 10.1007/s11739-015-1382-6. [DOI] [PubMed] [Google Scholar]
- 22.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 23.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes. 2010;5:458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- 25.de Onis M, Martinez-Costa C, Nunez F, Nguefack-Tsague G, Montal A, Brines J. Association between WHO cut-offs for childhood overweight and obesity and cardiometabolic risk. Public Health Nutr. 2013;16:625–630. doi: 10.1017/S1368980012004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) J Endocrinol Invest. 2006;29:581–593. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 27.Valerio G, Balsamo A, Baroni MG, Brufani C, Forziato C, Grugni G, et al. Childhood obesity classification systems and cardiometabolic risk factors: a comparison of the Italian, World Health Organization and International Obesity Task Force References. Ital J Pediatr. 2017;43:19. doi: 10.1186/s13052-017-0338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deurenberg P, Bhaskaran K, Lian PL. Singaporean Chinese adolescents have more subcutaneous adipose tissue than Dutch Caucasians of the same age and body mass index. Asia Pac J Clin Nutr. 2003;12:261–265. [PubMed] [Google Scholar]
- 30.Chung S. Body composition analysis and references in children: clinical usefulness and limitations. Eur J Clin Nutr. 2019;73:236–242. doi: 10.1038/s41430-018-0322-8. [DOI] [PubMed] [Google Scholar]
- 31.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374:2430–2440. doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 34.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–37. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 36.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGill HC, Jr, McMahan CA. Determinants of atherosclerosis in the young. Pathobiological determinants of atherosclerosis in youth (PDAY) research group. Am J Cardiol. 1998;82:30T–36T. doi: 10.1016/S0002-9149(98)00720-6. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 39.Faienza MF, Brunetti G, Delvecchio M, Zito A, De Palma F, Cortese F, et al. Vascular function and myocardial performance indices in children born small for gestational age. Circ J. 2016;80:958–963. doi: 10.1253/circj.CJ-15-1038. [DOI] [PubMed] [Google Scholar]
- 40.Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa heart study. Int J Obes Relat Metab Disord. 2004;28:159–166. doi: 10.1038/sj.ijo.0802515. [DOI] [PubMed] [Google Scholar]
- 41.Juonala M, Raitakari M, Viikari JSA, Raitakari OT. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The cardiovascular risk in young Finns study. Atherosclerosis. 2006;185:388–393. doi: 10.1016/j.atherosclerosis.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Wright CM, Parker L, Lamont D, Craft AW. Implications of childhood obesity for adult health: findings from thousand families cohort study. BMJ. 2001;323:1280–1284. doi: 10.1136/bmj.323.7324.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 44.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 45.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 46.Wang DQ, Portincasa P, Neuschwander-Tetri BA. Steatosis in the liver . Compr Physiol. 2013;3:1493–1532. doi: 10.1002/cphy.c130001. [DOI] [PubMed] [Google Scholar]
- 47.Martins MJ, Ascensao A, Magalhaes J, Collado MC, Portincasa P. Molecular mechanisms of NAFLD in metabolic syndrome. Biomed Res Int. 2015;2015:621080. doi: 10.1155/2015/621080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musso G, Gambino R, Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res. 2013;52:175–191. doi: 10.1016/j.plipres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring). 2011;19:868–874. doi: 10.1038/oby.2010.255. [DOI] [PubMed] [Google Scholar]
- 50.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from "two hit theory" to "multiple hit model". World J Gastroenterol. 2018;24:2974–2983. doi: 10.3748/wjg.v24.i27.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 54.Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8:549–558. doi: 10.1016/j.cld.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang JS, Barlow SE, Quiros-Tejeira RE, Scheimann A, Skelton J, Suskind D, et al. Childhood obesity for pediatric gastroenterologists. J Pediatr Gastroenterol Nutr. 2013;56:99–109. doi: 10.1097/MPG.0b013e31826d3c62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 58.Kinugasa A, Tsunamoto K, Furukawa N, Sawada T, Kusunoki T, Shimada N. Fatty liver and its fibrous changes found in simple obesity of children. J Pediatr Gastroenterol Nutr. 1984;3:408–414. doi: 10.1097/00005176-198406000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Schwimmer JB, Lavine JE, Wilson LA, Neuschwander-Tetri BA, Xanthakos SA, Kohli R, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology. 2016;151:1141–1154. doi: 10.1053/j.gastro.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conjeevaram Selvakumar PK, Kabbany MN, Alkhouri N. Nonalcoholic fatty liver disease in children: not a small matter. Paediatr Drugs. 2018;20:315–329. doi: 10.1007/s40272-018-0292-2. [DOI] [PubMed] [Google Scholar]
- 63.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 64.Baldridge AD, Perez-Atayde AR, Graeme-Cook F, Higgins L, Lavine JE. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr. 1995;127:700–704. doi: 10.1016/S0022-3476(95)70156-7. [DOI] [PubMed] [Google Scholar]
- 65.Phatak UP, Pashankar DS. Obesity and gastrointestinal disorders in children. J Pediatr Gastroenterol Nutr. 2015;60:441–445. doi: 10.1097/MPG.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 66.Franzese A, Vajro P, Argenziano A, Puzziello A, Iannucci MP, Saviano MC, et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. doi: 10.1023/A:1018850223495. [DOI] [PubMed] [Google Scholar]
- 67.Tazawa Y, Noguchi H, Nishinomiya F, Takada G. Serum alanine aminotransferase activity in obese children. Acta Paediatr. 1997;86:238–241. doi: 10.1111/j.1651-2227.1997.tb08881.x. [DOI] [PubMed] [Google Scholar]
- 68.Vajro P, Fontanella A, Perna C, Orso G, Tedesco M, De Vincenzo A. Persistent hyperaminotransferasemia resolving after weight reduction in obese children. J Pediatr. 1994;125:239–241. doi: 10.1016/S0022-3476(94)70202-0. [DOI] [PubMed] [Google Scholar]
- 69.Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD) Liver Int. 2013;33:1398–1405. doi: 10.1111/liv.12226. [DOI] [PubMed] [Google Scholar]
- 70.Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014;12:765–773. doi: 10.1016/j.cgh.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bohte AE, Koot BG, van der Baan-Slootweg OH, van Werven JR, Bipat S, Nederveen AJ, et al. US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology. 2012;262:327–334. doi: 10.1148/radiol.11111094. [DOI] [PubMed] [Google Scholar]
- 72.Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children with NAFLD. J Pediatr Gastroenterol Nutr. 2011;53:190–195. doi: 10.1097/MPG.0b013e31821b4b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. 2019;207:64–70. doi: 10.1016/j.jpeds.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kohli R, Sunduram S, Mouzaki M, Ali S, Sathya P, Abrams S, et al. Pediatric nonalcoholic fatty liver disease: a report from the expert committee on nonalcoholic fatty liver disease (ECON) J Pediatr. 2016;172:9–13. doi: 10.1016/j.jpeds.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61:1887–1895. doi: 10.1002/hep.27666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwimmer JB, Behling C, Angeles JE, Paiz M, Durelle J, Africa J, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017;66:1474–1485. doi: 10.1002/hep.29241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nobili V, Manco M, Ciampalini P, Alisi A, Devito R, Bugianesi E, et al. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther. 2008;30:1168–1176. doi: 10.1016/j.clinthera.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 79.Messing S, Rutten A, Abu-Omar K, Ungerer-Rohrich U, Goodwin L, Burlacu I, et al. How can physical activity be promoted among children and adolescents? A systematic review of reviews across settings. Front Public Health. 2019;7:55. doi: 10.3389/fpubh.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molina-Molina E, Lunardi Baccetto R, Wang DQ, de Bari O, Krawczyk M, Portincasa P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur J Clin Invest. 2018;48:e12958. doi: 10.1111/eci.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jago R, Solomon-Moore E, Macdonald-Wallis C, Sebire SJ, Thompson JL, Lawlor DA. Change in children's physical activity and sedentary time between year 1 and year 4 of primary school in the B-PROACT1V cohort. Int J Behav Nutr Phys Act. 2017;14:33. doi: 10.1186/s12966-017-0492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pietilainen KH, Kaprio J, Borg P, Plasqui G, Yki-Jarvinen H, Kujala UM, et al. Physical inactivity and obesity: a vicious circle. Obesity (Silver Spring). 2008;16:409–414. doi: 10.1038/oby.2007.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davison KK, Birch LL. Childhood overweight: a contextual model and recommendations for future research. Obes Rev. 2001;2:159–171. doi: 10.1046/j.1467-789x.2001.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barnett TA, Kelly AS, Young DR, Perry CK, Pratt CA, Edwards NM, et al. Sedentary behaviors in today’s youth: approaches to the prevention and management of childhood obesity: a scientific statement from the American Heart Association. Circulation. 2018;138:e142–e159. doi: 10.1161/CIR.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 85.Goldfield GS, Kenny GP, Hadjiyannakis S, Phillips P, Alberga AS, Saunders TJ, et al. Video game playing is independently associated with blood pressure and lipids in overweight and obese adolescents. PLoS One. 2011;6:e26643. doi: 10.1371/journal.pone.0026643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldfield GS, Saunders TJ, Kenny GP, Hadjiyannakis S, Phillips P, Alberga AS, et al. Screen viewing and diabetes risk factors in overweight and obese adolescents. Am J Prev Med. 2013;44(4 Suppl 4):S364–S370. doi: 10.1016/j.amepre.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 87.Wennberg P, Gustafsson PE, Dunstan DW, Wennberg M, Hammarstrom A. Television viewing and low leisure-time physical activity in adolescence independently predict the metabolic syndrome in mid-adulthood. Diabetes Care. 2013;36:2090–2097. doi: 10.2337/dc12-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobel S, Wartha O, Dreyhaupt J, Kettner S, Steinacker JM. Cross-sectional associations of objectively assessed sleep duration with physical activity, BMI and television viewing in German primary school children. BMC Pediatr. 2019;19:54. doi: 10.1186/s12887-019-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang A, Ji M, Zhang Y, Zou J, Li M, Yang L, et al. Dietary behaviors and caregiver perceptions of overweight and obesity among Chinese preschool children. Int J Environ Res Public Health. 2018;15:E716. doi: 10.3390/ijerph15040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lipowska M, Lipowski M, Jurek P, Jankowska AM, Pawlicka P. Gender and body-fat status as predictors of parental feeding styles and children's nutritional knowledge, eating habits and behaviours. Int J Environ Res Public Health. 2018;15:E852. doi: 10.3390/ijerph15050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 92.Britten P, Marcoe K, Yamini S, Davis C. Development of food intake patterns for the MyPyramid food guidance system. J Nutr Educ Behav. 2006;38(Suppl 6):S78–92. doi: 10.1016/j.jneb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 93.Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Circulation. 2011;124:1673–1686. doi: 10.1161/CIRCULATIONAHA.110.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hills AP, Andersen LB, Byrne NM. Physical activity and obesity in children. Br J Sports Med. 2011;45:866–870. doi: 10.1136/bjsports-2011-090199. [DOI] [PubMed] [Google Scholar]
- 96.Elmaogullari S, Demirel F, Hatipoglu N. Risk factors that affect metabolic health status in obese children. J Pediatr Endocrinol Metab. 2017;30:49–55. doi: 10.1515/jpem-2016-0128. [DOI] [PubMed] [Google Scholar]