Abstract
Background:
Emotion dysregulation is a key dimensional trait in psychopathology. It is of particular interest in ADHD because individual differences in emotion dysregulation predict impairment. Despite growing recognition of its importance, an understanding of emotional functioning in ADHD needs to be better integrated with the well-known non-emotional attentional impairments in the disorder. Here, we assess differences in early, reactive and later, regulatory attention to emotional stimuli, as well as how impairments in attentional control to non-emotional stimuli are affected under different emotional contexts.
Methods:
130 adolescents (nADHD =61) completed an emotional go/no-go task while 32-channel EEG data were recorded. Reaction time and accuracy were analyzed using the linear ballistic accumulator model.
Results:
The multimethod approach provided convergent evidence of increased early, reactive attention capture and over-arousal (faster drift rates, increased P1) by positively-valenced stimuli in ADHD, but no differences in later attention to emotional stimuli. Over-arousal in positive-valence contexts appeared to exacerbate existing ADHD-related impairments in attentional control to non-emotional stimuli as well (reduced N2 amplitude). In contrast, positive valence contexts facilitated attentional control to non-emotional stimuli for typically-developing adolescents.
Conclusions:
Results highlight the dynamic interaction of emotion with atttentional control in ADHD. Distinguishing reactive and regulatory contributions to emotion dysregulation has been informative for clarifying mechanisms and spurring development of novel interventions in other disorders. It can be informative in ADHD as well.
Keywords: ADHD, cognitive control, emotion dysregulation, event-related potentials, sequential sampling models, EEG
Introduction
Emotion dysregulation is at the core of many psychiatric illnesses and may, in fact, help explain high rates of overlap (1). Emotion dysregulation is of particular interest in ADHD. 24–50% of individuals are characterized by dysregulation of negative affect (2) that predicts individual differences in impairment (3–7). Dysregulation of positive affect also occurs in ADHD (8, 9) and neurobiological theories (10) and multiple pathway models (11) highlight how increased reactivity to both positive and negative stimuli combined with weak attentional control can be distinct routes by which ADHD may develop. Although many studies have described the prevalence rates of emotion dysregulation in ADHD using ratings scales, diagnostic interviews, and similar methods, the causes of emotion dysregulation in ADHD are not well-understood (2). It remains unclear how emotion dysregulation should be understood in relation to the well-documented impairments in attentional control in ADHD. Here, we characterize how cognitive and emotional processing interact in ADHD as a step towards integrating cognitive and emotional theories of the disorder.
Explanations of emotion dysregulation in ADHD
Although there are multiple relevant viewpoints on causes of emotion dysregulation, models converge (12–17) on the broad importance of attention, including 1) early, reactive (also called “bottom-up”) and 2) later, regulatory (also called “top-down”) attentional systems. Bottom-up and top-down processing interact continually (18–20) but these processes can also be distinguished in terms of behavioral, functional, and neural correlates (14, 21, 22). Here, we examine differences in early attention capture by emotional stimuli, later attention directed to emotional stimuli, and the ability to control attention to non-emotional stimuli when they occur in emotional contexts (i.e., during a task with other emotional stimuli).
In ADHD, weaknesses in top-down attentional control on non-emotional tasks are well-documented (23–27). Crucially, attentional control on non-emotional tasks relies on many of the same brain regions and networks that support control of attention in emotional contexts. For example, the dorsal lateral and ventral medial prefrontal cortex, the anterior cingulate cortex, and areas of the parietal cortex are all activated when individuals are asked to control attention on non-emotional tasks and when they are asked to control emotions (28). Because an overlapping set of neural networks support control of attention in both emotional and non-emotional contexts, one hypothesis has been that the emotion dysregulation observed in ADHD is the result of weak attentional control (29). In other words, children with ADHD have weak attentional control, and this weak attentional control also prevents them from controlling their emotions. However, relationships between attentional control deficits on non-emotional tasks and ratings of emotion dysregulation have not been consistently found in ADHD (29, 30), suggesting that this simple model is incomplete.
One limitation is that few studies have measured attentional control during emotional tasks. Because the same attentional resources are important in both non-emotional and emotional contexts, it may be that severity of attentional control impairments in ADHD vary based on the emotional context. More specifically, when attention is captured by, or directed to, emotional stimuli this may limit the attentional resources available for non-emotional tasks. In ADHD, this would lead to attentional impairments that are only evident under specific emotional contexts or are significantly worse in specific emotional contexts. This is in contrast to the simpler model that suggests a direct relationship between attentional control impairments on non-emotional tasks and dysregulated emotion in ADHD. It would explain the poor correspondence of non-emotional measures of attentional control with emotion dysregulation in ADHD.
Within ADHD, only a handful of studies directly address emotion-related changes in attentional control. Although other tasks have been used (31), studies have most often relied on variants of an emotional go/no-go task, which is the paradigm used here. Using standard cognitive performance measures (i.e., reaction time and accuracy), many studies have failed to find ADHD-related effects of emotion on attentional control (32–35), and at least one study unexpectedly found enhancements in attentional control using negatively-valenced stimuli (36). Factors such as small sample sizes may account for prior lack of findings; however, differences in peripheral (36) or central (33, 34) nervous system functioning have been identified even in the absence of reaction time or accuracy differences, suggesting that individuals with ADHD may also be achieving similar performance via recruitment of different cognitive or neurobiological resources. In addition, lack of effects in prior studies may be related to use of traditional measures (i.e., reaction time and accuracy) that cannot differentiate the multiple factors influencing performance. The current study addresses both limitations.
Sequential sampling models (SSMs)
From a developmental and computational cognitive science perspective, control of even relatively complex emotional states (37) and actions (38) are understood as the result of multiple more basic and quantifiable processes. Sequential sampling models are one approach to distinguishing these basic processes (39). Using the full distribution of reaction times and accuracy, separate parameters are estimated that quantify: 1) information processing speed/efficiency (i.e., drift rate), 2) speed-accuracy trade-offs (boundary height), 3) non-decision time (e.g., encoding and motor response), and 4) response biases. SSMs have already helped clarify the nature of non-emotional attentional impairments in ADHD by demonstrating that these are largely due to differences in speed/efficiency of information processing and to a lesser extent to differences in non-decisional processes (e.g., motor output), but are not related to speed-accuracy trade-offs (40–43). The granular computational parameters should be more sensitive to emotional effects than RT and accuracy measures (44), but this approach has not been applied to understand the effects of emotion on attentional control in ADHD.
Event-related potentials
Similar behavioral outputs can be generated by in different ways. Event-related potentials (ERPs), which reflect the neural response time-locked to events of interest, are ideal for differentiating these effects. ERP methods have been informative for understanding basic emotion in other populations (45). Several components are sensitive to interactions between emotion and attention. Early attention capture by emotional stimuli is reflected in changes in occipito-temporal P1 and N170 components (46–48). Our own prior work applying these approaches identified increased early attention capture to positive stimuli in children with ADHD who also experienced dysregulation of positive affect (49). However, recent systematic review identified no clear pattern of findings for early ERPs (50), suggesting additional studies are needed.
The centro-posterior late positive potential (LPP) is responsive to later, regulation of attention to emotional stimuli (51). In adults with ADHD, there is some evidence for increased LPP to emotional stimuli during passive viewing (52), while differences in children have not been found (53). In adolescents, one study failed to find differences in LPP to emotional stimuli, while another found increased LPP for some types of emotional stimuli (fear) but not others (sadness). Overall, ADHD-related differences in the LPP in response to emotional stimuli requires additional characterization.
ERPs can also be used to characterize differences in top-down attentional control to non-emotional stimuli. The no-go N2 is associated with conflict monitoring and inhibitory control (54, 55). It increases as inhibitory demands increase (54, 55) and is often smaller in adolescents with ADHD than typically-developing controls (56, 57). Lopez-Martin et al. (33) found larger amplitude no-go N2 to emotional distractors (both negative and positive) in ADHD than controls in the absence of other performance differences, which they interpreted as reflecting increased recruitment of resources to achieve similar performance. However, the sample was small (n=20) and additional studies looking at the response to task-relevant stimuli are also needed.
Current Study
The current study examined ADHD-related differences in attention to 1) emotional stimuli and 2) to non-emotional stimuli that occurred during an emotional task in adolescents with and without ADHD. The period from late childhood through adolescence reflects a period of high-risk for failures of emotion regulation due to the relatively earlier development of subcortical emotion processing regions as compared to prefrontal regions associated with cognitive control (58–61). This general risk may be even higher for adolescents with ADHD due to greater delays in maturation of frontal regulatory neural networks (62–65).
Participants completed three different emotional go/no-go tasks. Differences in 1) early attention capture by emotional stimuli, 2) later attention to the emotional stimuli, and 3) attentional control to the non-emotional stimuli that occurred during the emotional task were characterized using a linear ballistic accumulator model (LBA, one type of sequential sampling model) and ERPs. We compared these responses to a task condition where none of the stimuli were emotional. We predicted that all adolescents would have larger early attention capture to emotional than non-emotional stimuli (i.e., greater increases in drift rate, P1 and N170 in emotional versus neutral conditions), but that these effects would be particularly pronounced in ADHD. We also hypothesized that adolescents with ADHD would have reduced top-down control of attention to emotional stimuli compared to non-ADHD controls (i.e., greater increase in LPP in emotional versus neutral conditions), but the strength of this hypothesis was qualified by mixed prior findings. Finally, because emotional content captures attentional resources, we expected greater ADHD-related weaknesses in attentional control to the non-emotional stimuli that occurred in the emotional task conditions than when all stimuli were neutral (i.e., smaller no-go N2 in emotional than neutral conditions). The current study includes: 1) a larger sample than used in similar prior studies, 2) a novel SSM approach to decomposing the behavioral (reaction time and accuracy) effects, 3) integration of neurophysiological measures with the SSM framework, and 4) consideration of the response to both emotional and non-emotional stimuli to help clarify how emotion affects attentional impairments in ADHD.
Methods and Materials
Participants and Recruitment
130 individuals (nADHD = 61) were recruited from a larger, ongoing longitudinal study and were invited to participate in an additional EEG testing visit. Prior work has examined relationships between emotional traits and response to emotional stimuli in a subset of the current sample, as well as ADHD-related differences in resting-state EEG patterns associated with emotional functioning in an overlapping sample (49, 66). Recruitment for the larger study uses a community-based strategy based on public advertising and outreach. A parent/legal guardian provided written informed consent, and adolescents provided written assent for the study. All research was carried out in accordance with the World Medical Association Declaration of Helsinki. Ethics approval was obtained from the Institutional Review Board at Oregon Health & Science University.
Full description of screening and diagnostic procedures are found in the Supplemental material. Briefly, parents and teachers completed standardized ADHD rating forms and parents completed a semi-structured clinical interview. Parents also completed ratings of child emotional traits using the Temperament in Middle Childhood Questionnaire (67). IQ was estimated based on a reliable and valid two-subtest short form of the WISC-IV (Block Design and Vocabulary). Final DSM-IV diagnostic groups were determined following all criteria for symptoms count, cross-situational severity, and impairment. Adolescents with ADHD taking stimulant medications were included in the study but were required to be off medication for 24 (for short-acting preparations) to 48 hours (for long-acting preparations) prior to testing.
Experimental Procedure
Participants completed three separate conditions (positive [happy], negative [fear], neutral) of an emotional go/no-go task in counterbalanced order. For each condition, participants were shown a series of grey-scale faces from 12 individuals (6 female) from the NimStim set [54] and were asked to respond by button press to specified target faces (see Figure 1). Within each condition, 172 trials were presented in semi-random order in two equal blocks. The positive and negative conditions contained 70% go (emotional) and 30% no-go (neutral) stimuli. In the neutral condition, all faces had neutral expressions and participants responded to either male or female faces and withheld responding to the other sex. Each participant completed one block in which they responded to male faces and one block in which they responded to female faces (counterbalanced).
Figure 1.
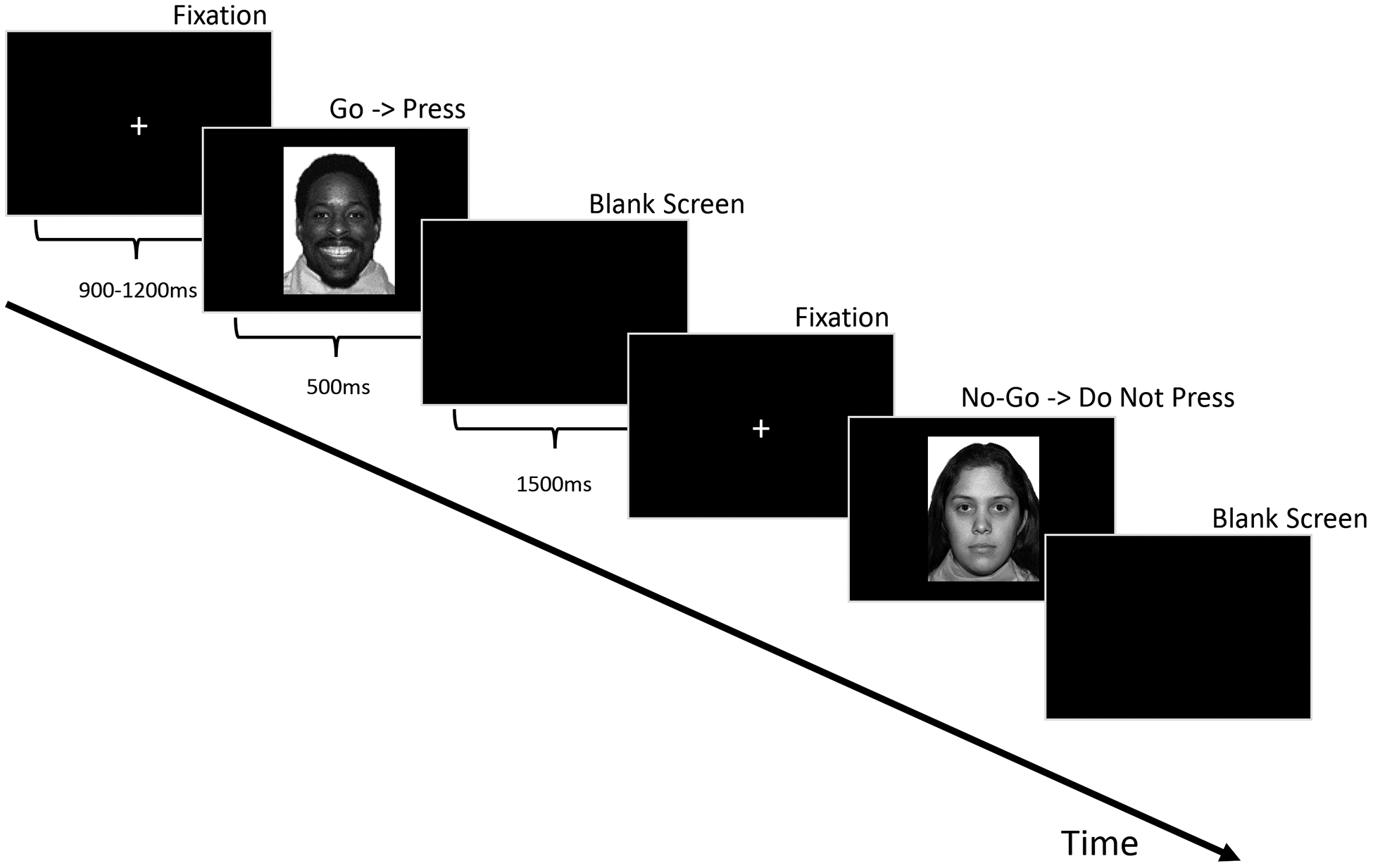
Experimental Task depicted for the Happy condition.
EEG Recordings
EEG was recorded at 500Hz with 32 Ag-AgCl active electrodes using PyCorder v1.0.9. The electrode array was based on the international 10–20 system centered at Cz. EEG signals were amplified by a BrainVision actiCHamp2 amplifier (Cary, NC). Recordings were referenced to Cz online then re-referenced offline.
ERP Measurement & Analyses
EEG data were analyzed using ERPLAB [55] and EEGLAB [56]. Full details of data processing are in the Supplement. Component amplitudes were measured as using ERPLAB’s numerical integration algorithms in a specified stimulus-locked time windows and at electrode sites selected based on those used most commonly in the literature: a) P1: between 60 and 160 ms and b) N170: between 150 and 250 ms at the right occipito-temporal electrode site P8 following our prior publication (49); c) LPP: between 600 and 1000 ms post stimulus at electrode site Pz; and d) no-go N2: between 200–400ms at Fz. Cases in which the algorithm returned a zero amplitude were excluded. Latency for the P1, N170, and N2 components was measured as the peak latency in the same time window but amplitude measures were considered the primary analyses due to concerns with reliability of latency measures. Components related to emotional stimulus processing (P1, N170, LPP) were analyzed for correct go trials (i.e. correct responses to emotional faces). Components related to cognitive control were analyzed for incorrect no-go trials (i.e., failures of inhibition for neutral stimuli). Standard multivariate repeated-measures ANOVA tests were used to examine effects of emotion condition between groups.
LBA Analyses
Prior to LBA analysis, all RTs ≤ 200 ms were excluded to prevent fast guesses from influencing parameter estimates (68). One person (control subject) was excluded because they were missing all trials from a single condition due to computer error. For the remaining subjects, a hierarchical Bayesian version of the LBA (69) was then fit to data in each group using Dynamic Models of Choice (DMC: 70, 71), a free set of R functions (72) for simulation and Bayesian analysis using the LBA and other choice RT models. Full details of these procedures are available in the Supplement. We report evidence for differences in group mean (μ) posterior distributions using odds ratios (ORs) (43, 73) and interpret these Jeffreys’ (74) recommendations. We report the diagnosis x emotion interactions from the LBA analyses that are directly relevant to our hypotheses. Results using standard RT and accuracy are in the Supplement along with group-level results for LBA parameters from the neutral condition. Briefly, we note that RT and accuracy measures identified main effects of emotion and group but no diagnosis x emotion interactions. Group differences in LBA parameters in the neutral condition were consistent with prior studies of non-emotional tasks (40, 41, 75, 76).
Relationships among measures.
Where applicable, correlation coefficients (Pearson’s r) for relationships between emotion-related changes in LBA parameters and corresponding changes in ERPs were estimated using a “plausible values” analysis (77). Additional details are in the Supplemental materials. A similar approach was used to test relationships between changes in LBA parameters and parent ratings of emotional traits. Relationships between ERP measures and emotional traits were examined using standard correlations.
Results
See sample demographics in Table 1. Consideration of covariates is described in the Supplement. Results reported below are without covariates.
Table 1.
Sample description
|
ADHD N=61 |
Control N=69 |
P | partial η2 | |
|---|---|---|---|---|
| Age (years) | 13.9 (1.5) | 13.8 (1.1) | .651 | 0.00 |
| Sex (% male) | 58.8 | 83.3 | .002 | |
| % on Stimulant Medication | 56.2 | --- | ||
| % White, non-Hispanic/Latino | 75.4 | 75.4 | .995 | |
| Median family income | $35–50K | $75–100K | .021 | |
| IQ | 108.9 (15.6) | 115.5 (12.6) | .009 | 0.05 |
| Parent Conners’ Inattention | 74.7 (10.8) | 47.6 (10.0) | < .001 | 0.41 |
| Parent Conners’ Hyp-Imp | 68.8 (15.5) | 48.1 (8.9) | < .001 | 0.63 |
| Parent Conners’ Aggression | 56.1 (12.9) | 47.3 (5.8) | < .001 | 0.17 |
| Teacher Conners’ Inattention | 65.9 (12.5) | 48.1 (7.4) | < .001 | 0.44 |
| Teacher Conners’ Hyp-Imp | 64.3 (15.5) | 48.2 (7.0) | < .001 | 0.32 |
| Teachers Conners’ Aggression | 57.1 (15.9) | 47.7 (5.8) | < .001 | 0.14 |
| Parent TMCQ Negative Affect | 2.7 (0.53) | 2.2 (0.39) | < .001 | 0.19 |
| Parent TMCQ Fear | 2.4 (0.67) | 2.1 (0.58) | .010 | 0.05 |
| Parent TMCQ Surgency | 3.6 (0.55) | 3.5 (0.64) | .233 | 0.01 |
| Parent TMCQ HIP | 3.3 (0.69) | 3.5 (0.78) | .121 | 0.02 |
LBA Parameters
Drift Rate.
There was substantial evidence for a diagnosis x emotion interaction for both go (OR=11.94) and no-go trials (OR=6.09) in the positive compared to neutral condition. On go trials, adolescents with (OR=93.96) and without ADHD (OR=3.53) both had faster drift rates to positive versus neutral stimuli; adolescents with ADHD had a larger increase in drift rate than non-ADHD controls. On no-go trials, typically-developing adolescents increased drift rates in the positive as compared to neutral condition (OR=31.20), but there was no change in no-go drift rate for the ADHD group (OR=1.38). See Figure 2a and 2c.
Figure 2.
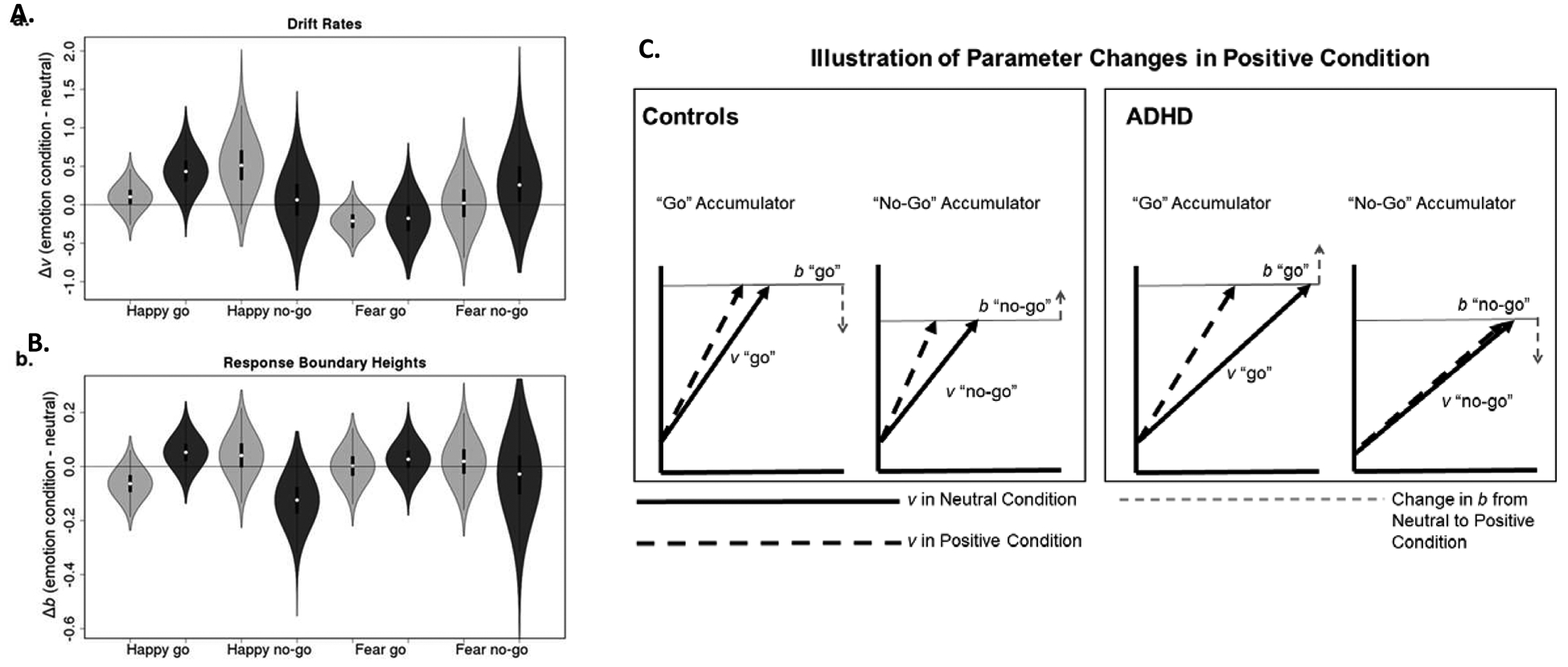
Panels A and B show violin plots depicting posterior difference distributions for the effects of emotion conditions (Happy - Neutral, Fear - Neutral) for the Control (light grey) and ADHD (dark grey) groups. These are presented as box plots of difference distribution samples surrounded by density plots of the same samples. Panel C illustrates the effects of the positive emotion condition on the LBA model parameters for each group.
Adolescents with (OR=3.52) and without (OR=20.64) ADHD both had slower drift rate to fearful than neutral stimuli. However, there was little evidence for diagnosis x emotion interactions for the fear condition (all OR<2.53).
Response Boundary Height.
There was strong evidence for diagnosis x emotion interactions for go trials in the positive compared to neutral condition (OR=24.88). On go trials, typically-developing adolescents reduced their boundary height in the positive compared to neutral condition (OR=10.58), requiring less evidence to make a go response. In contrast, adolescents with ADHD increased go boundary height (OR=6.35) in the positive compared to neutral condition, requiring more evidence to make a go response.
There was also strong evidence for diagnosis x emotion interactions for no-go trials (OR=24.93) in the positive compared to neutral condition. For no-go responses, there was little evidence that typically-developing adolescents changed their boundaries (OR=2.87) in positive as compared to neutral condition. In contrast there was strong evidence (OR=24.53) that adolescents with ADHD reduced their no-go boundary in positive versus neutral conditions, indicating they required less evidence to withhold responding. See Figure 2b and 2c.
In the fear condition, there was little evidence for group x emotion interactions or main effects of emotion (all OR < 2.57).
Event-related potentials
P1. A 3 (emotion) x 2 (diagnosis) multivariate repeated measures ANOVA identified a significant diagnosis x emotion interaction for P1 amplitude (F[2, 112] = 4.35, p = .015, partial η2 = .07). P1 amplitudes did not differ between conditions for typically-developing adolescents. However, adolescents with ADHD had larger P1 amplitudes to emotional (positive and negative) than to neutral stimuli. See Figure 3. There were no significant main or interaction effects for latency (all p > .33).
Figure 3.
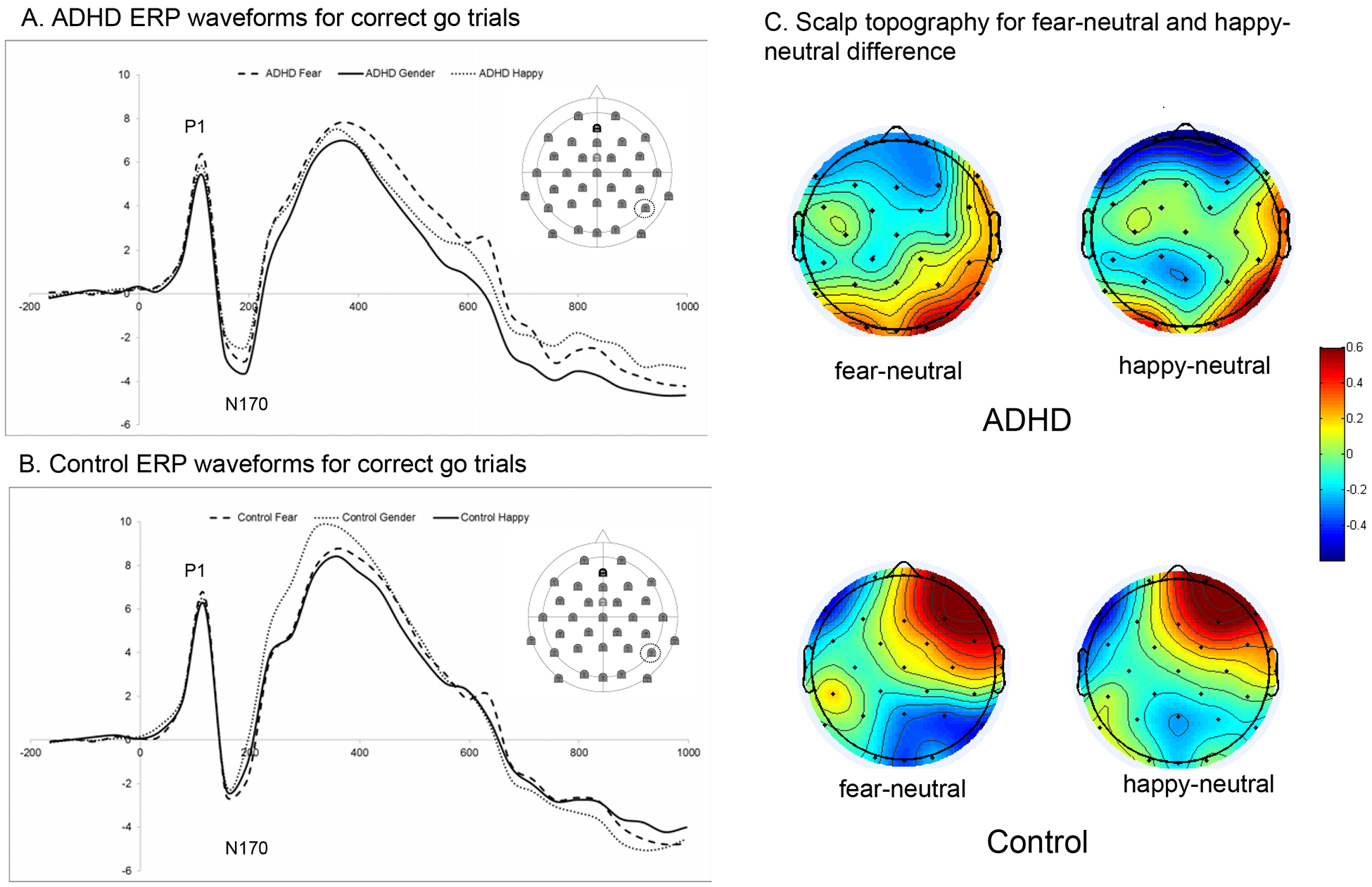
Effects of emotion condition on ERPs. Panels A and B show the ERP waveforms for each diagnostic group. Panel C shows the scalp topography for the emotion-neutral differences in each group. Scalp topography of the main effects for each emotion condition are in Table S1.
N170. A 3 (emotion) x 2 (diagnosis) multivariate repeated measures ANOVA identified a significant main effect of emotion for N170 amplitudes (F[2, 93] = 4.01, p = .021, partial η2 = .08). This was qualified by a significant diagnosis x emotion interaction (F[2, 93] = 5.00, p = .040, partial η2 = .07). Typically-developing adolescents had similar N170 in response to emotional and neutral stimuli, whereas adolescents with ADHD had reduced N170 amplitude in the positive as compared to neutral condition. See Figure 3. There were no significant main or interaction effects for latency (all p > .53).
LPP. A 3 (emotion) x 2 (diagnosis) multivariate repeated measures ANOVA identified a significant main effect of emotion on LPP amplitude (F[2, 110] = 18.19, p < .001, partial η2 = .25). All adolescents showed larger LPPs in the negative than in other conditions. No other main or interaction effects were significant (all p > .179).
No-go N2. For N2 amplitude, a 3 (emotion) x 2 (diagnosis) multivariate repeated-measures ANOVA identified a main effect of group (F[1, 91]=8.30, p = .005). This was qualified by a significant diagnosis x emotion interaction (F[2, 90]= 3.70, p = .029, partial η2 = .08). Adolescents with ADHD had smaller N2 amplitude in all conditions than non-ADHD controls. This difference between groups was largest in the positive condition. See Figure 4. There were no significant main or interaction effects for latency (all p > .25).
Figure 4.
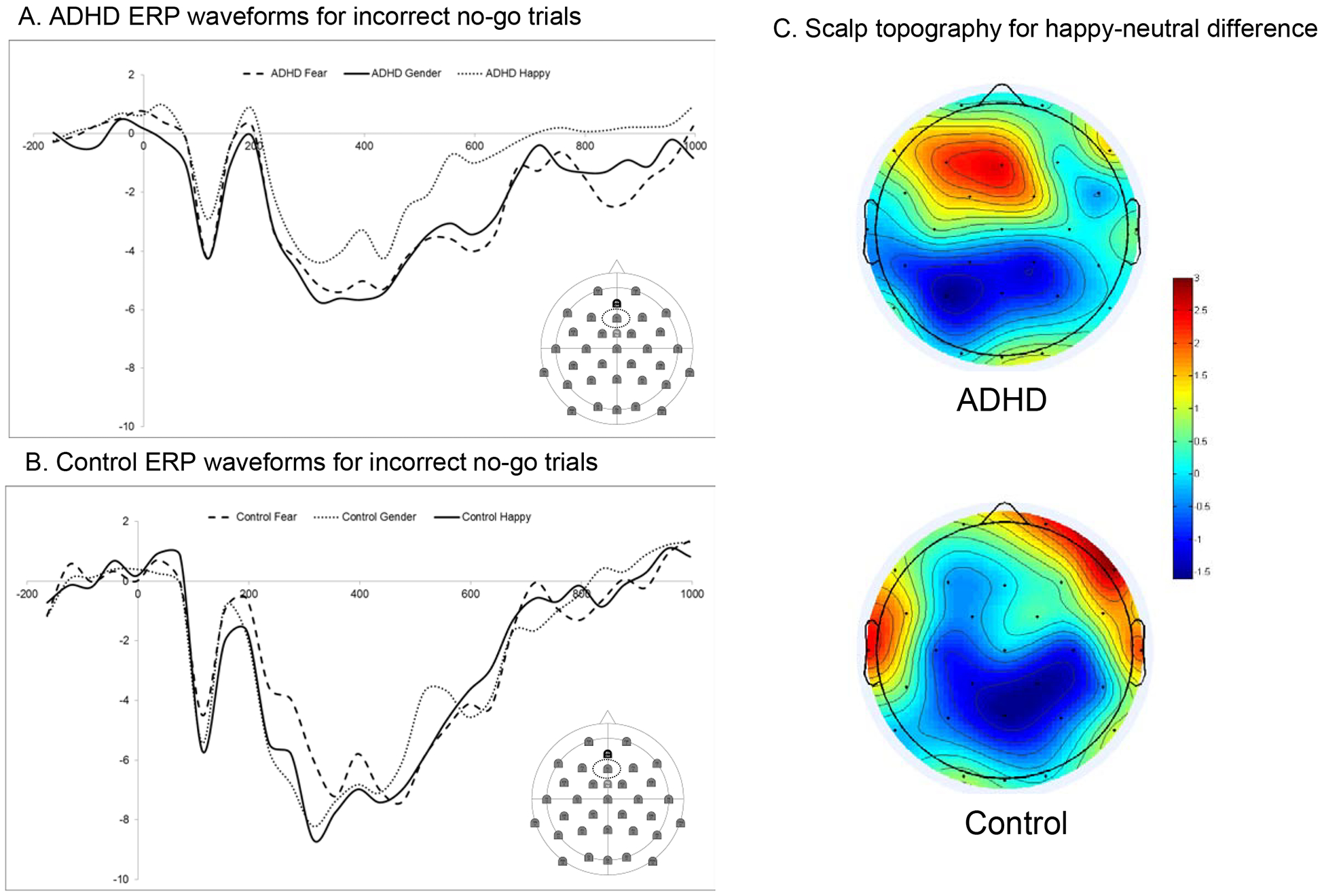
Effects of emotion condition on the no-go N2 ERP, which is related to cognitive control. Panels A and B show the ERP waveforms for each diagnostic group. Panel C shows the scalp topography for the happy-neutral condition. Scalp topography of the main effects for each emotion condition are in Table S1.
Relationships among measures
We examined relationships between changes in drift rate, changes in boundary separation, and changes in ERP amplitudes for the positive emotion condition. None of the relationships were credibly different than zero. See Table S1.
Changes in LBA parameters in the emotional conditions were not credibly related to parent ratings of emotional traits (see Table S2). However, larger P1 in the negative condition was associated with more parent-reported fear within the ADHD sample (r=.39, p=.002). No other relationships between ERPs and emotional traits were statistically significant (see Table S3).
Discussion
Emotion dysregulation is increasingly recognized as an important clinical feature for many children with ADHD (2). However, it remains unclear how emotion is related to well-studied, non-emotional attentional impairments in this population. In the current study, multimethod evidence partially converged to demonstrate that adolescents with ADHD have increased early, reactive attention capture by positively-valenced stimuli. These differences in emotional reactivity co-occur with disruptions in attentional control to non-emotional stimuli that are worse in positive-valence contexts than in non-emotional contexts. We speculate that dysregulation of positive affect in ADHD is related to increased reactive attention capture and that this increased reactivity may exacerbate impairments in attentional control in specific emotional contexts. Future studies that directly examine the pathways between early disruptions to attention and downstream impairments in attentional control will be important.
Traditional performance measures captured expected changes in cognitive performance with emotional stimuli: faster RTs in response to positive stimuli and slower RTs in response to negative stimuli. However, these measures were not sensitive to differences between groups. In contrast, use of a novel SSM approach identified ADHD-specific effects. All adolescents had faster drift rates to positively-valenced stimuli, but this effect was especially pronounced in the ADHD group. Changes in drift rate are associated with functioning of frontal-parietal networks (78) that are densely innervated by locus coeruleus-norepinephrine (LC-NE) inputs driving arousal-related attention processes. The large increase in drift rate in ADHD is consistent with increased arousal in response to positive stimuli.
Convergent evidence was also observed using ERP methods. The P1 component was larger in response to positive as compared to neutral stimuli for children with ADHD, an effect that was not present in the typically-developing group. The P1, which is generated by the extrastriate cortex, reflects basic early visual processing of stimuli. It is modulated by emotional face expression, reflecting prioritization of attention to emotional content (46). Thus, both ERP and SSM findings suggest increased reactive attention capture by positively-valenced stimuli in ADHD as compared to typical development.
Results also demonstrate the importance of considering emotional context in studies of attentional control in ADHD. Positive-valence conditions facilitated stimulus processing for typically-developing adolescents, evidenced by their faster drift rate for both go (emotional) and no-go (neutral) stimuli in the positive task condition as compared to the task condition with all neutral stimuli. Findings are consistent with evidence that positive emotion can facilitate aspects of attentional control (79). Given the overall enhancement of drift rate for both go and no-go stimuli, typically-developing adolescents adaptively decreased their go response height while maintaining their no-go response boundary, suggesting fronto-striatal driven changes in top-down response strategy (78, 80, 81) that would be expected to occur as task difficulty decreased (82, 83).
Unlike typically-developing controls, adolescents with ADHD did not have faster no-go drift rate in the positive condition (they only increased their go drift rate), suggesting they experienced a specific, strong bias towards the positively-valenced emotional stimuli rather than an overall facilitation of performance. In addition, adolescents with ADHD required more evidence to make a go response but also less to make a no-go response. Put another way, adolescents with ADHD experienced a strong go stimulus bias in positive conditions, but also shifted towards a no-go response bias (84). This differed from the adaptive reduction in both response thresholds demonstrated by their typically-developing peers and may indicate impaired top-down control over response threshold adjustments in the disorder.
Again, ERPs provide convergent evidence for impaired top-down attentional control in positive-valence conditions. Adolescents with ADHD had smaller no-go N2 amplitudes on failed inhibitory trials in all conditions, consistent with well-known difficulties on non-emotional tasks that require attentional control. However, the N2 was smallest in the positive condition, indicating less recruitment of neural resources to deal with response conflict in the positive context. Crucially, the no-go trials on which these effects were observed were always emotionally neutral stimuli. Thus, the overall emotional context (the fact that the no-go trials were occurring amidst many positively-valenced go trials) contributed to disruptions in attentional control that extended beyond processing of the emotional stimuli themselves.
Effects for positive stimuli were robust and apparent across multiple levels of measurement. However, the changes in LBA parameters were not correlated with changes in ERPs. This may be related to lack of reliability of change scores. Alternatively, because LBA parameters and ERPs are affected by only partially overlapping processes, it may be that the parallel effects we observed are due to different underlying influences. This possibility is also consistent with the lack of latency effects. The drift rate parameter is, as the name implies, related to speed of information processing and so changes in speed of the ERPs might also be predicted if the same processes were influencing both measures. However, the lack of latency findings may also be due to the generally lower reliability of those measures.
Relationships between cognitive parameters and neural response patterns may be clarified by use of other neuroimaging methodologies, such as functional MRI, to examine changes in frontoparietal brain networks in similar tasks. In addition, we focused on direct, linear relationships between ERPs and cognitive parameters. While this approach offers a reasonable starting point, Turner et al. (85) recently highlighted several new approaches that can account for the interaction among parameters within and across levels of analyses. Given the complexity of the processes of interest, these alternative models are likely to be informative in the future.
In addition, inconsistent effects were observed for negatively-valenced stimuli. Several explanations for the inconsistent effects require investigation. First, other emotions need to be considered. Studies using anger stimuli may be of particular interest given growing recognition that anger, specifically, is dysregulated in ADHD (86). In addition, variability in LBA parameters was larger for the negative than positive-valence stimuli. ADHD is an emotionally heterogeneous disorder (9, 87, 88). In our sample, correlations between LBA parameters and ratings of adolescents’ emotional traits were not credibly different than zero, however, larger P1 to positive stimuli predicted higher fear ratings. It is possible that a subgroup of children show differences in the negative condition that are obscured in the full ADHD sample. We are pursuing work to address each of these limitations now.
Summary
The current work demonstrates that adolescents with and without ADHD differ in the way emotion affects their attention. Findings provide partially convergent cognitive and neurophysiological evidence of greater early attention capture by positive stimuli and worsening attentional control impairments in positive emotional contexts. Future studies should focus on the potential relationships between these effects to determine if the differences in early attention capture may directly contribute to worsening attentional control later in the processing stream. Non-emotional attention impairments in ADHD remain important as potential mechanisms for symptom change (87). However, decision-making in the real-world is not emotionally neutral and fully integrating an understanding of emotion into cognitive accounts of ADHD will require simultaneously considering both cognitive and affective domains.
Supplementary Material
Acknowledgments
This project was supported by K23 MH108656 (PI: Karalunas) and R37 MH059105 (PI: Nigg). Ms. Alperin’s time was supported, in part, by National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR000129. Alexander Weigard’s time was supported by NIAAA T32 AA007477 (to Frederic Blow). Dr. Karalunas would also like to thank the lab’s research assistants and volunteers for their diligent work on data collection and cleaning and the children and families who make this work possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Karalunas, Dr. Weigard, and Dr. Alperin each reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Beauchaine TP (2015): Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology. 44:875–896. [DOI] [PubMed] [Google Scholar]
- 2.Shaw P, Stringaris A, Nigg J, Leibenluft E (2014): Emotion dysregulation in attention deficit hyperactivity disorder. American Journal of Psychiatry. 171:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, et al. (2004): Impact of Executive Function Deficits and Attention-Deficit/Hyperactivity Disorder (ADHD) on Academic Outcomes in Children. Journal of Consulting and Clinical Psychology. 72:757–766. [DOI] [PubMed] [Google Scholar]
- 4.Diamantopoulou S, Rydell AM, Thorell LB, Bohlin G (2007): Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Developmental Neuropsychology. 32:521–542. [DOI] [PubMed] [Google Scholar]
- 5.Leibenluft E, Cohen P, Gorrindo T, Brook JS, Pine DS (2006): Chronic Versus Episodic Irritability in Youth: ACommunity-Based, Longitudinal Study of Clinical and Diagnostic Associations. Journal of Child & Adolescent Psychopharmacology. 16:456–466. [DOI] [PubMed] [Google Scholar]
- 6.Stringaris A, Cohen P, Pine DS, Leibenluft E (2009): Adult outcomes of youth irritability: a 20-year prospective community-based study. The American journal of psychiatry. 166:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehmeier PM, Schacht A, Barkley RA (2010): Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. Journal of Adolescent health. 46:209–217. [DOI] [PubMed] [Google Scholar]
- 8.Bunford N, Evans SW, Wymbs F (2015): ADHD and emotion dysregulation among children and adolescents. Clin Child Fam Psychol Rev. 18:185–217. [DOI] [PubMed] [Google Scholar]
- 9.Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT (2014): Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA psychiatry. 71:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Keune PM, Schönenberg M, Wyckoff S, Mayer K, Riemann S, Hautzinger M, et al. (2011): Frontal alpha-asymmetry in adults with attention deficit hyperactivity disorder: Replication and specification. Biological psychology. 87:306–310. [DOI] [PubMed] [Google Scholar]
- 11.Nigg J, Goldsmith HH, Sachek J (2004): Temperament and Attention Deficit Hyperactivity Disorder: The Development of a Multiple Pathway Model. Journal of Clinical Child and Adolescent Psychology. 33:42–53. [DOI] [PubMed] [Google Scholar]
- 12.Derryberry D (2002): Attention and voluntary self-control. Self and Identity. 1:105–111. [Google Scholar]
- 13.Rothbart MK (2011): Becoming who we are: Temperament and personality in development. New York, NY: Guidford Press. [Google Scholar]
- 14.Posner MI, Rothbart MK (2000): Developing mechanisms of self-regulation. Development and psychopathology. 12:427–441. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan S, Berman MG (2010): Directed attention as a common resource for executive functioning and self-regulation. Perspectives on Psychological Science. 5:43–57. [DOI] [PubMed] [Google Scholar]
- 16.Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends in cognitive sciences. 9:242–249. [DOI] [PubMed] [Google Scholar]
- 17.Ochsner KN, Gross JJ (2008): Cognitive emotion regulation insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 17:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awh E, Belopolsky AV, Theeuwes J (2012): Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in cognitive sciences. 16:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor CE, Egeth HE, Yantis S (2004): Visual attention: bottom-up versus top-down. Current biology. 14:R850–R852. [DOI] [PubMed] [Google Scholar]
- 20.Sarter M, Givens B, Bruno JP (2001): The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain research reviews. 35:146–160. [DOI] [PubMed] [Google Scholar]
- 21.McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ (2011): Bottom-up and top-down emotion generation: implications for emotion regulation. Social cognitive and affective neuroscience. 7:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, et al. (2009): Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological science. 20:1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang-Pollock CL, Karalunas SL, Tam H, Moore AN (2012): Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J Abnorm Psychol. 121:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, et al. (2013): Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review. 33:795–811. [DOI] [PubMed] [Google Scholar]
- 25.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H (2005): A Meta-Analytic Review of Stopping Performance in Attention-Deficit/Hyperactivity Disorder: Deficient Inhibitory Motor Control? Journal of Abnormal Psychology. 114:216–222. [DOI] [PubMed] [Google Scholar]
- 26.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R (2005): A Meta-Analysis of Working Memory Impairments in Children With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 44:377–384. [DOI] [PubMed] [Google Scholar]
- 27.Willcutt EG, Doyle A, Nigg JT, Faraone SV, Pennington BF (2005): Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 57:1336–1346. [DOI] [PubMed] [Google Scholar]
- 28.Ochsner KN, Silvers JA, Buhle JT (2012): Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banaschewski T, Jennen-Steinmetz C, Brandeis D, Buitelaar JK, Kuntsi J, Poustka L, et al. (2012): Neuropsychological correlates of emotional lability in children with ADHD. Journal of child psychology and psychiatry. 53:1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjöwall D, Roth L, Lindqvist S, Thorell LB (2013): Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. Journal of Child Psychology and Psychiatry. 54:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang S, White SF, Nolan ZT, Williams WC, Sinclair S, Blair R (2015): Executive attention control and emotional responding in attention-deficit/hyperactivity disorder—A functional MRI study. NeuroImage: Clinical. 9:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balogh L, Kakuszi B, Papp S, Tombor L, Bitter I, Czobor P (2017): Neural Correlates of Error Monitoring in Adult Attention Deficit Hyperactivity Disorder After Failed Inhibition in an Emotional Go/No-Go Task. The Journal of neuropsychiatry and clinical neurosciences. 29:326–333. [DOI] [PubMed] [Google Scholar]
- 33.López-Martín S, Albert J, Fernández-Jaén A, Carretié L (2015): Emotional response inhibition in children with attention-deficit/hyperactivity disorder: neural and behavioural data. Psychological medicine. 45:2057–2071. [DOI] [PubMed] [Google Scholar]
- 34.Ibáñez A, Petroni A, Urquina H, Torrente F, Torralva T, Hurtado E, et al. (2011): Cortical deficits of emotional face processing in adults with ADHD: its relation to social cognition and executive function. Social neuroscience. 6:464–481. [DOI] [PubMed] [Google Scholar]
- 35.Schulz KP, Bédard A-CV, Fan J, Clerkin SM, Dima D, Newcorn JH, et al. (2014): Emotional bias of cognitive control in adults with childhood attention-deficit/hyperactivity disorder. NeuroImage: Clinical. 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenenbaum RB, Musser ED, Morris S, Ward AR, Raiker JS, Coles EK, et al. (2018): Response Inhibition, Response Execution, and Emotion Regulation among Children with Attention-Deficit/Hyperactivity Disorder. Journal of abnormal child psychology. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross JJ (2015): Emotion regulation: Current status and future prospects. Psychological Inquiry. 26:1–26. [Google Scholar]
- 38.Verbruggen F, McLaren IP, Chambers CD (2014): Banishing the control homunculi in studies of action control and behavior change. Perspectives on Psychological Science. 9:497–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratcliff R, Smith PL (2004): A comparison of sequential sampling models for two-choice reaction time. Psychological review. 111:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT (2014): Annual Research Review: Reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. Journal of Child Psychology and Psychiatry. 55:685–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karalunas SL, Huang-Pollock CL (2013): Integrating evidence of slow RTs and impaired executive functions using a diffusion model framework. Journal of Abnormal Child Psychology. 41:837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karalunas SL, Huang-Pollock CL, Nigg JT (2012): Decomposing attention-deficit/hyperactivity disorder (ADHD)-related effects in response speed and variability. Neuropsychology. 26:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weigard A, Huang-Pollock C, Brown S (2016): Evaluating the consequences of impaired monitoring of learned behavior in attention-deficit/hyperactivity disorder using a Bayesian hierarchical model of choice response time. Neuropsychology. 30:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White CN, Ratcliff R, Vasey MW, McKoon G (2010): Using diffusion models to understand clinical disorders. Journal of Mathematical Psychology. 54:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajcak G, MacNamara A, Olvet DM (2010): Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental neuropsychology. 35:129–155. [DOI] [PubMed] [Google Scholar]
- 46.Batty M, Taylor MJ (2003): Early processing of the six basic facial emotional expressions. Cognitive Brain Research. 17:613–620. [DOI] [PubMed] [Google Scholar]
- 47.Batty M, Taylor MJ (2006): The development of emotional face processing during childhood. Developmental science. 9:207–220. [DOI] [PubMed] [Google Scholar]
- 48.Blau VC, Maurer U, Tottenham N, McCandliss BD (2007): The face-specific N170 component is modulated by emotional facial expression. Behavioral and brain functions. 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alperin BR, Gustafsson H, Smith C, Karalunas SL (2017): The relationship between early and late event-related potentials and temperament in adolescents with and without ADHD. PloS one. 12:e0180627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feuerriegel D, Churches O, Hofmann J, Keage HA (2015): The N170 and face perception in psychiatric and neurological disorders: a systematic review. Clinical Neurophysiology. 126:1141–1158. [DOI] [PubMed] [Google Scholar]
- 51.Dennis TA, Hajcak G (2009): The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry. 50:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shushakova A, Ohrmann P, Pedersen A (2018): Exploring deficient emotion regulation in adult ADHD: electrophysiological evidence. European archives of psychiatry and clinical neuroscience. 268:359–371. [DOI] [PubMed] [Google Scholar]
- 53.Van Cauwenberge V, El Kaddouri R, Hoppenbrouwers K, Wiersema JR (2017): To make a molehill out of a mountain: An ERP-study on cognitive reappraisal of negative pictures in children with and without ADHD. Clinical Neurophysiology. 128:529–537. [DOI] [PubMed] [Google Scholar]
- 54.Bokura H, Yamaguchi S, Kobayashi S (2001): Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 112:2224–2232. [DOI] [PubMed] [Google Scholar]
- 55.Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD (2006): Neurophysiological correlates of emotion regulation in children and adolescents. Journal of cognitive neuroscience. 18:430–443. [DOI] [PubMed] [Google Scholar]
- 56.Doehnert M, Brandeis D, Imhof K, Drechsler R, Steinhausen H-C (2010): Mapping attention-deficit/hyperactivity disorder from childhood to adolescence—no neurophysiologic evidence for a developmental lag of attention but some for inhibition. Biological psychiatry. 67:608–616. [DOI] [PubMed] [Google Scholar]
- 57.Pliszka SR, Liotti M, Woldorff MG (2000): Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological psychiatry. 48:238–246. [DOI] [PubMed] [Google Scholar]
- 58.Casey B, Jones RM, Hare TA (2008): The adolescent brain. Annals of the New York Academy of Sciences. 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somerville LH, Casey B (2010): Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 20:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M (2009): Age differences in future orientation and delay discounting. Child Development. 80:28–44. [DOI] [PubMed] [Google Scholar]
- 61.Tottenham N, Hare TA, Casey B (2011): Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Frontiers in psychology. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilliam M, Stockman M, Malek M, Sharp W, Greenstein D, Lalonde F, et al. (2011): Developmental trajectories of the corpus callosum in attention-deficit/hyperactivity disorder. Biological Psychiatry. 69:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein D, Nugent T, et al. (2007): Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 164:647–655. [DOI] [PubMed] [Google Scholar]
- 64.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. (2007): Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. PNAS Proceedings of the National Academy of Sciences of the United States of America. 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. (2006): Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 63:540. [DOI] [PubMed] [Google Scholar]
- 66.Alperin BR, Smith CJ, Gustafsson HC, Figuracion MT, Karalunas SL (2019): The relationship between alpha asymmetry and ADHD depends on negative affect level and parenting practices. Journal of Psychiatric Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simonds J, Rothbart MK (2004): The Temperament in Middle Childhood Questionnaire (TMCQ): A computerized self-report measure of temperament for ages 7–10 Occasional Temperament Conference. Athens, GA. [Google Scholar]
- 68.Ratcliff R, Tuerlinckx F (2002): Estimating parameters of the diffusion model: Approaches to dealing with contaminant reaction times and parameter variability. Psychonomic bulletin & review. 9:438–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turner BM, Sederberg PB, Brown SD, Steyvers M (2013): A method for efficiently sampling from distributions with correlated dimensions. Psychological methods. 18:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heathcote A, Lin Y, Gretton M (2017): DMC: Dynamic Models of Choice. Retrieved from osf.io/pbwx8. [DOI] [PubMed]
- 71.Heathcote A, Lin Y-S, Reynolds A, Strickland L, Gretton M, Matzke D (2018): Dynamic models of choice. Behavior research methods. 1–25. [DOI] [PubMed] [Google Scholar]
- 72.Team RC (2013): R foundation for statistical computing. Vienna, Austria. [Google Scholar]
- 73.Winkel J, Hawkins GE, Ivry RB, Brown SD, Cools R, Forstmann BU (2016): Focal striatum lesions impair cautiousness in humans. cortex. 85:37–45. [DOI] [PubMed] [Google Scholar]
- 74.Jeffreys H (1961): Theory of probability (3rd edt) oxford university press; MR0187257. [Google Scholar]
- 75.Karalunas SL, Huang-Pollock CL (2010): Consistently inconsistent: effects of reward on patterns of intra-individual variability Poster presented at Eunethydis. Amsterdam, Netherlands. [Google Scholar]
- 76.Metin B, Roeyers H, Wiersema R, Van der Meere J, Thompson M, Barke E (2013): ADHD performance reflects inefficient but not impulsive information processing: a diffusion model analysis. Neuropsychology. [DOI] [PubMed] [Google Scholar]
- 77.Ly A, Boehm U, Heathcote A, Turner BM, Forstmann B, Marsman M, et al. (2017): A flexible and efficient hierarchical Bayesian approach to the exploration of individual differences in cognitive-model-based neuroscience. Computational models of brain and behavior. 467–480. [Google Scholar]
- 78.Mulder MJ, Wagenmakers E-J, Ratcliff R, Boekel W, Forstmann BU (2012): Bias in the brain: a diffusion model analysis of prior probability and potential payoff. Journal of Neuroscience. 32:2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gable PA, Harmon-Jones E (2008): Approach-motivated positive affect reduces breadth of attention. Psychological Science. 19:476–482. [DOI] [PubMed] [Google Scholar]
- 80.Benarroch EE (2009): The locus ceruleus norepinephrine system Functional organization and potential clinical significance. Neurology. 73:1699–1704. [DOI] [PubMed] [Google Scholar]
- 81.Mulder M, Van Maanen L, Forstmann B (2014): Perceptual decision neurosciences–a model-based review. Neuroscience. 277:872–884. [DOI] [PubMed] [Google Scholar]
- 82.Dutilh G, Krypotos A-M, Wagenmakers E-J (2011): Task-related versus stimulus-specific practice. Experimental Psychology. [DOI] [PubMed] [Google Scholar]
- 83.Dutilh G, Vandekerckhove J, Tuerlinckx F, Wagenmakers E-J (2009): A diffusion model decomposition of the practice effect. Psychonomic Bulletin & Review. 16:1026–1036. [DOI] [PubMed] [Google Scholar]
- 84.White CN, Poldrack RA (2014): Decomposing bias in different types of simple decisions. Journal of Experimental Psychology: Learning, Memory, and Cognition. 40:385. [DOI] [PubMed] [Google Scholar]
- 85.Turner BM, Palestro JJ, Miletić S, Forstmann BU (2019): Advances in techniques for imposing reciprocity in brain-behavior relations. Neuroscience & Biobehavioral Reviews. 102:327–336. [DOI] [PubMed] [Google Scholar]
- 86.Karalunas SL, Gustafsson HC, Fair D, Musser ED, Nigg JT (2019): Do we need an irritable subtype of ADHD? Replication and extension of a promising temperament profile approach to ADHD subtyping. Psychological Assessment. 31:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karalunas SL, Gustafsson HC, Dieckmann N, Tipsord J, Mitchell SH, Nigg JT (2017): Heterogeneity in development of aspects of working memory predicts longitudinal ADHD symptom change. Journal of Abnormal Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martel MM, Goth-Owens T, Martinez-Torteya C, Nigg JT (2010): A person-centered personality approach to heterogeneity in attention-deficit/hyperactivity disorder (ADHD). Journal of Abnormal Psychology. 119:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


