Highlights
-
•
Evidence of a role for IL-6 in the pathology of SARS-CoV-2 and COVID-19.
-
•
IL-6 pathways: the classic (anti-inflammatory) and the trans-signaling (pro-inflammatory)
-
•
SGP130Fc could block the IL-6 trans-signaling pathway(pro-inflammatory) in COVID-19.
-
•
Inhibition of the JAK/STAT pathway activated by IL-6: Ruxolitinib and Baricitinib.
Keywords: SARS-CoV-2, COVID-19, Therapies, Drugs, Strategies, Targets, SARS-CoV-1, Coronavirus, Coronaviruses, Tocilizumab, Ruxolitinib, Baricitinib, IL-6, Interleukin-6, SGP130Fc, IL-6 trans-signaling
Abstract
Since the outbreak of COVID-19 many studies have been published showing possible therapies, here the author discusses the end of stage disease related drugs, like Tocilizumab which is currently being used in ARDS patients. In some patients, disease progression leads to an enormous secretion of cytokines, known as cytokine storm, among those cytokines IL-6 plays an important role. Here the author shows how IL-6 has both pro and anti-inflammatory properties, depending on the pathway of transduction: soluble (trans-signaling) or membrane-related (classic signaling), and suggests how targeting only the pro-inflammatory pathway, with SGP130Fc, could be a better option then targeting them both. Other possible IL-6 pathway inhibitors such as Ruxolitinib and Baricinitib are then analyzed, underlying how they lack the benefit of targeting only the pro-inflammatory pathway.
1. Introduction
Coronaviruses are enveloped, positive-sense, single stranded RNA viruses that are distributed broadly among humans which cause respiratory, enteric, hepatic, and neurologic diseases [1]. SARS-CoV-2 is the agent responsible for the COVID-19, it is a beta coronavirus of group 2B with over 70% similarity in genetic sequence to SARS-CoV-1 [2]. Acute respiratory distress syndrome (ARDS) is not an uncommon complication when disease can’t be controlled [3], the percentage of patients requiring ARDS treatment is about 10% for those who are actively infected. Total white blood count, lymphocytes, and platelets are lower than the average with extended activated thromboplastin time, increased C-reactive protein and muscle enzyme level. Lymphocytes decrease with diseases progression. Secretion of cytokine, such as IL1B, IL1RA, IL6, IL7, IL8, known as cytokine storm, is associated with disease severity [3], [4].
Therapeutic choices once disease has progressed will be discussed here.
2. Immunotherapy: fighting the cytokine storm
SARS-CoV-1, MERS-CoV and SARS-CoV-2 show a relatively higher mortality rates then other coronaviruses, all of which are associated with the presence of a cytokine storm, this might suggest that inflammatory responses play a role in the pathogenesis. If that is the case, targeting the coronavirus alone with antiviral therapy might not be sufficient to reverse highly pathogenic infections [5]. These observations led to explore the usage of therapies that included type I and II interferons. Interferon beta displayed the best efficacy in reducing MERS-CoV replication in tissue culture [6], [7]. A randomized control trial is ongoing in South Arabia (MIRACLE Trial) to determine whether the combination of antivirals used in HIV infection such as Lopinavir/Ritonavir and Interferon beta could improve clinical outcomes in MERS-CoV infections [8]. In a humanized transgenic mouse MERS-CoV infection model, Remdesivir (a drug already being used against SARS-CoV-2 in patients with severe and moderate disease, GS-US-540-5773/4 Studies) showed more activity and efficacy in prophylactic and therapeutic use then the combination of Lopinavir/Ritonavir and Interferon beta [9], this points towards the necessity to explore other options regarding immune system modulation and how control of viraemia is also essential.
The use of immunosuppressants like corticosteroids is quite controversial, for some it may be reasonable to counteract the effect of cytokine storm induced by the SARS-CoV-2. Although the recent open label study from Wu and colleagues showed a benefit for corticosteroids, for now clinical evidence does not support corticosteroid treatment for SARS-CoV-1 lung injury, as it could result in delayed viral clearance [10], [11].
3. Evidence of a role for IL-6 in the pathology of SARS-CoV-2 and COVID-19
IL-6 can be produced by almost all stromal cells and by immune system cells, such as B lymphocytes, T lymphocytes, macrophages, dendritic cells, monocytes, mast cells and many non-lymphocytes, such as fibroblast and endothelial cells [12]. Main activators of the Interleuking-6 (IL-6) expression are IL-1beta and tumor necrosis factor (TNF-alfa), but many other factors can contribute to its secretion such as Toll-like receptors (TLRs), prostaglandins, adipokines, stress response and other cytokines [13]. In the early stage of the infectious inflammation, IL-6 is produced by monocytes and macrophages stimulated by the TLRs [14].
IL-6 plays a crucial role in infectious diseases such as influenza where it has been shown how in a IL6-/- mice influenza specific CD4+ T cell response is impaired [15]. Loss of IL-6 results also in persistence of the influenza virus in the lung leading to extreme lung damage and death [16]. This shows how IL-6 limits influenza-induced inflammation and protects against lung damage [17] by promoting neutrophil survival in the lung [16]. IL-6 crucial role is not limited to influenza but also in other infections such as Herpes Simplex Virus-1 (HSV-1) infection where IL-6 deficient mice show an increase in infection susceptibility [18]. In other scenarios, such as Respiratory Syncytial Virus (RSV) infections in mice, early depletion of IL-6, but not late, resulted in significant increase of the disease with an associated influx of cytotoxic CD8+ T cells [19].
High levels of interleukin 6 (IL-6) and Interleukin 8 (IL 8) were found in the acute stage associated with lung lesions in SARS-CoV-1 patients. Especially IL-6 can induce the hyper-innate inflammatory response due to the SARS-CoV-1 invasion of the respiratory tract [20]. Interestingly, in human epithelial cells, SARS-CoV-1 was able to induce greater IL-6 when compared to influenza A virus [21]. Although in some murine viral infections IL-6 plays a protective and essential role in the resolution process, in others like in SARS-CoV-1 high levels of IL-6 were associated with severe inflammation and correlated with mortality in the mice [22], [23]. This happens also with SARS-CoV-2 in COVID-19 patients: some retrospective and meta-analysis studies show how elevated IL-6 and C-reactive protein (CRP) correlate with mortality and severe disease in comparison to moderate disease [24], [25], [26], [27]. More evidence suggests that critically ill patients with severe respiratory failure and SARS-CoV-2 have either immune dysregulation or macrophage-activation syndrome, both of which are characterized by pro-inflammatory cytokines. The immune dysregulation, in particular, is driven by the Interleukin-6 (IL-6) and not by Interleukin-1beta (IL-1beta) [27]. Two key features of this immune dysregulation are: over-production of pro-inflammatory cytokines by monocytes and lymphocyte dysregulation with CD4 lymphopenia [27].
A relevant study shows how IL-6 plays a major role in acute lung injury (ALI), proof of that was obtained in a murine model, where loss of IL-6 showed to alleviate the severity of ALI in response to acid respiration. Moreover, it was shown how SARS-CoV-1 has the ability of inducing production of compounds like oxidized phospholipid (OxPL) both in humans and animals. OxPLs in turn induce cytokine production and acute lung injury via Toll Like Receptor 4 (TLR4) [28]. This is evidence of SARS-CoV-1 ability to indirectly induce acute lung injury and cytokine production, like that of IL-6 [28], [29]. TLR4 is a transmembrane protein that belongs to the pattern recognition receptor family (PRR family), it recognizes molecules like lipopolysaccharide (LPS) thanks to an accessory protein known as MD-2 [30]. TLRs activate transcription factors like NFkB, AP-1 and IRF inducing proinflammatory cytokines expressions and Interferon 1 [31], [32], [33]. TLR4 is associated not only with infections but also with tissue damage, and this damage-dependent pathway may be amplified in the acute stages of the infection. Of note, TLR4-null mice are highly resistant to infection by the mouse adapted influenza A virus [34]. Protection against influenza infections in mouse models was shown to be achieved by targeting TLR4 with antagonists or with specific anti-receptor antibodies [35]. Another option in targeting host inflammatory response could then be targeting the cellular toll-like-receptor 4 (TLR4). Furthermore, TLR4 seems to regulate IL-6 secretion through the NFkB pathway [36]. This again points towards excessive activation of the innate immune response. Another proof of that is the damage shown to the pulmonary interstitial arteriolar walls that is more associated with the inflammatory response rather than the pathogenic effect of coronaviruses [37].
An interesting fact is that SARS-CoV-1 can directly promote IL-6 secretion, this of course is not the only way it happens since IL-6 can be induced by so many types of cells and cytokines as mentioned above. Among all SARS-CoV-1 structural proteins (nucleocapsid N, spike S, envelop E and membrane M) only the nucleocapsid protein (N) significantly induced the activation of IL-6 promoter in human airway epithelial cell cultures [38]. IL-6 gene expression is activated by the N protein which binds to the NF-kB regulatory element on IL-6 promoter and facilitates its translocation from cytosol to nucleus. The N protein is essential for IL-6 secretion to happen, since deletion of the C-terminus of the N protein resulted in the loss of function in the activation of IL-6 [38].
More evidence is emerging of many similarities between Macrophage Activation Syndrome (MAS) disease and COVID-19 pneumonia. Similarities include pathological findings and a “second wave” of cytokine secretion. The loss of “front line” anti-viral defence mechanism may be responsible for this “second wave” activation, thus prolonging IL-6 secretion [39]. Sustained IL-6 secretion has also been correlated to serum viral RNA load [40] in critically ill patients, and viral RNA load is in turn correlated to ARDS severity [41]: all evidence pointing to a possible detrimental role of IL-6 in SARS-CoV-2 infection.
Since high levels of IL-6 are associated with SARS-CoV-1 and SARS-CoV-2 infections as shown above and since high serum levels of IL-6 have been associated with lung lesions in SARS-CoV-2 patients in the acute and later stages [20], [24], [25], [26], [27], [42], a valid option could be targeting the expression of IL-6 with Tocilizumab a monoclonal antibody against IL-6 Receptor: this option is currently being used in some patients in Italy with important lung injuries (TOCIVID-19 study). Tocilizumab is currently approved for the treatment of rheumatoid arthritis and other autoimmune diseases [43]. This option should be approached only when there are radiological and clinical signs of progression of the lung lesions. Time decision as when to start clinical administration of anti-IL-6 drugs is maybe the most important decision in this scenario, since as mentioned before in murine models IL-6 is essential in the early stages of other infections to control disease progression [19].
The inclusion criteria for Tocilizumab administration for the TOCIVID trial in Italy, and for trials in other countries are summarized in Table 1.
Table 1.
This table summarizes inclusion criteria for Tocilizumab trials in the respective country.
| TOCIVID (Italy) | COVACTA (USA) | ChiCTR2000029765 (China) | 2020COVID-19TCZ (Belgium) | TOC-COVID(Germany) | |
|---|---|---|---|---|---|
| Diagnosis of COVID-19 | Virological diagnosis of SARS-CoV-2 infection (real-time PCR) AND Hospitalized due to clinical/instrumental diagnosis of pneumonia |
Hospitalized with COVID-19 pneumonia confirmed per WHO criteria (including a positive PCR of any specimen; e.g., respiratory, blood, urine, stool, other bodily fluid) AND evidenced by chest X-ray or CT scan | The patients who were diagnosed with the common type of Novel Coronavirus Pneumonia (NCP) (including severe risk factors) AND severe cases of new coronavirus pneumonia | PCR documented SARS-CoV-2 carriage in nasopharyngeal sample OR evocative thoracic scan images of COVID-19 associated with typical clinical presentation AND Hospitalized patients |
Proof of SARS-CoV2 |
| Severity of the disease | Oxygen saturation at rest in ambient air ≤ 93% (valid for not intubated patients and for both phase 2 and observational cohort) OR Intubated (<24 h before registration, eligible for phase 2 only, more than 24 h before registration eligible for observational cohort only) |
SPO2 </=93% OR PaO2/FiO2 < 300 mmHg |
Regular patients with COVID pneumonia (including severe risk factors): patients with dual pulmonary lesions based on common COVID pneumonia clinical symptoms accompanied by fever or no fever AND Severe cases, with any of the following: 1 Respiratory distress (≧30 breaths/min); 2 Oxygen saturation ≤ 93% at rest; 3 Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≦300 mmHg OR Critical COVID pneumonia patients: –Respiratory failure occurs and mechanical ventilation is required; –Shock; –Combined failure of other organs that requires ICU monitoring |
Signs of severe COVID-19 pneumonia (3 of the followings) –Patient wheezing or unable to speak in full sentences while at rest/with minimal effort –Respiratory rate > 22 –PaO2 < 65 mmHg or SpO2 < 90% –Repeated chest imaging is significantly worsening (despite being on standard of care, which may include anti-viral treatment, low dose steroids and antibiotics) |
Severe respiratory failure: a. ambient air SpO2 ≤ 92% or b. Need of ≥ 6 l O2/min or c. NIV (non-invasive ventilation) or d. IMV (invasive mechanical ventilation) |
| Laboratory findings | IL-6 elevated (using Elisa method, using the same company kit) | ||||
| Age | No age limit | No age limit | ≥ 18 and ≤ 85 years old | ≥ 18 and ≤ 75 years old | No age limit |
Here instead are the recommendation criteria used to decide when a patient should be given Tociluzumab, written by the Italian Society of Infectious and tropical diseases (SIMIT, Società Italiana di Malattie Infettive e Tropicali) last update to 13 March 2020:
-
–
End of the initial phase of high viral load of COVID-19 (patient with no fever for more than 72 h and/or more than 7 days from the symptoms onset)
-
–
Worsening of the respiratory condition requiring invasive or non invasive ventilation support
-
–
Elevated levels of IL-6 (more than 40 pg/ml); or high levels of D-Dimer and/or PCR and/or ferritin and/or progressive increasingly level of fibrinogen.
4. SGP130Fc and IL-6: the classic and the trans-signaling transduction pathway
The signal transduction of IL-6 is induced by binding of IL-6 to its alpha receptor, Interleukin-6 Receptor (IL-6R). The complex formed by IL-6 and its receptor in turn activates a homodimer of the signal transduction beta-receptor gp130 with high affinity [44]. The gp130 receptor thereupon dimerizes and initiates intracellular signal transduction by activating the JAK/STAT and ras/MAP kinase pathways [45]. GP130 is expressed on all cells of the human body. IL-6 Receptor instead is mainly found on hepatocytes, some leukocytes, and epithelial cells [46]. The IL-6 Receptor (IL-6R) protein is subjected to limited proteolysis by a metalloprotease activity which gives rises to a soluble form of IL-6R (sIL-6R) [47]. The majority of the soluble IL-6R found in the circulation is proteolytically cleaved from cells by the protease ADAM17 [48], [49]. A minor portion of the sIL-6R is generated from an alternatively spliced mRNA [50], but limited proteolysis remains the major mechanism by which the sIL-6R is generated [51].
For signal transduction the IL-6R can either be membrane bound (classic pathway) or soluble (trans-signaling pathway) [51]. Those two different pathways of signal transduction seem to have different and divergent functions.
In the trans signaling, the complex of sIL-6R and IL-6 binds to and activates gp130 even on cells that do not express IL-6R [52]. This is an important mechanism since only a few cells in the human body express IL-6R, whereas the majority of cells do not show IL-6R expression [53], [54]. Since all cells express gp130, all of them are in theory possible target cells of IL-6 trans signaling [52].
Trans-signaling is believed to be involved in chronic inflammation and cancer development [55]. Some animal models showed that when IL-6 was acting via the membrane bound IL-6R (classic signaling) it did so in a protective way, they also showed that inhibiting trans-signaling was superior to global blockade of IL-6 activity by neutralizing antibodies [56], this was done using the IL-6 trans-signaling inhibitor sgp130Fc, which is a fusion protein of the gp130 with the Fc portion of a human immunoglobulin antibody [57], [58]. In a murine polymicrobial sepsis model, selective blockade of IL-6 trans-signaling with sgp130Fc improved survival up to 100%. Interestingly, treating these mice with anti-IL-6R antibodies did not prevent death of the mice [56]. This is a first hint of how targeting both the IL-6R forms with drugs like Tocilizumab might not be enough in a sepsis infectious model. Furthermore, it all points to the trans-signaling transduction being the harmful one (pro-inflammatory response), and the classic signaling transduction being the protective one (anti-inflammatory response) [59]. More evidence of that in some studies showing that elevated serum levels of IL-6, which are a marker of disease severity in the inflammatory acute lung injury during acute pancreatitis, were mediated by the trans-signaling transduction way, as sgp130Fc blocked the pancreatitis induced acute lung injury [60]. Moreover in a mouse model of atherosclerosis sgp130Fc showed to reduce disease and showed significant regression of the atherosclerotic plaques, thus underlying the pro-inflammatory role of the trans-signaling pathway once more [61]. The sgp130Fc protein was shown not to interfere with IL-6 signaling via the membrane-bound IL-6R (mIL6-R). In contrast, IL-6 trans-signaling is completely blocked both in vitro and in vivo by the sgp130Fc protein [46]. Higher levels of the soluble IL-6 receptor(sIL-6R) are usually found in chronic inflammatory diseases and cancer [55], in multiple myeloma for example high sIL-6R levels were correlated with poor survival [62], sIL6R levels are also higher in chronic lymphocytic leukemia and lymphomas [63]. Locally increased sIL-6R concentrations were also found in the bronchoalveolar lavage fluid (BAL) of asthmatics [64], and in malignant ascites from ovarian cancer patients, where it was associated with poor prognosis [65].
Since sgp130 is expressed in all cells of the human body, under normal circumstances it is in molar excess over sIL-6R, therefore the concentration of the sIL-6R limits the capacity of the sIL-6R/sgp130 complex in the blood. During infections and inflammatory states, the concentration of sgp130 does not change, but the concentration of the sIL-6R increases up to 5 fold [66]. As a consequence, IL-6 and sIL-6R trans-signaling pathway stimulate all cells, which normally are not IL-6 target cells, like endothelial cells and smooth muscles cells [67].
Recent studies about polymorphism within the IL-6R genes, showed how some IL-6 Receptor variants could be a much better substrate for the shedding protease ADAM17, resulting in a reduced response to inflammation and infectious states, in terms of sIL-6R increase [68]. Those individuals are also protected from many chronic inflammatory diseases [69]. This is another evidence of the pro-inflammatory role of the trans-signaling pathway and it could also be the explanation as to why some patients show a higher inflammatory response mediated by IL-6, similarly to what is happening with SARS-CoV-2 infection.
The essential role of IL-6 in fighting and clearing influenza infection has already been discussed above [19]. This protective role could be mediated by IL-6 classic signaling. This also happens for other infections like Listeria and mycobacteria infections, where IL-6 classic signaling via the membrane bound IL-6R is responsible for the defense of the body against these pathogens. Furthermore this defense is not affected by the selective blockade of IL-6 trans-signaling [70], [71]. In the animal model of listeriosis global blockade of IL-6 led to a dramatic increase in bacterial titers in spleen and liver. In contrast, selective blockade of IL-6 trans-signaling with the sgp130Fc protein did not result in increased bacterial titers, and thus was concluded that the classic signaling was involved in the protection of the body from bacterial infection [71].
This shows the possibility to use the sgp130Fc protein as a potential inhibitor of the IL-6 pathway in COVID-19 patients, since it could preserve the regenerative and anti-inflammatory properties of the IL-6 classic pathway, and block the pro-inflammatory actions mediated by trans-signaling pathway [46]. It has been shown that the blockade of IL-6 trans-signaling using sgp130Fc was as efficient as the global blockade of IL-6 classic and trans-signaling using specific monoclonal antibodies [72]. SGP130Fc is mainly used in chronic inflammatory diseases (such as intestinal inflammation, rheumatoid arthritis, lupus erythematosus, neuroinflammation and cancer [51]), but it has shown its efficacy also in acute inflammation [57] and sepsis [73], where selective blockade of interleukin-6 trans-signaling improved survival in a murine polymicrobial sepsis model [56]. An infection where the inhibition of IL-6 trans-signaling has shown its efficacy is Malaria, another infection during which IL-6 levels correlate with the severity of the disease. In a murine model of IL-6R deficient mice the infection showed an increase in survival of otherwise lethal blood-stage malaria. Inducing IL-6 trans-signaling by injection of mouse recombinant soluble IL-6R in deficient mice restores the lethal outcome. In contrast, inhibition of IL-6 trans-signaling via injection of sgp130Fc protein in wild type mice results in 40% survival rate. This demonstrate that trans-signaling, rather than classic signaling, contributes to malaria lethality in mice [74].
IL-6 trans-signaling is also involved in the infiltration of granulocytes and macrophages during the late phase of an acute inflammation, and this happens when the trans-signaling triggers the secretion of chemokine MCP-1 in endothelial cells [57]. As most cells, endothelial cells lack the expression of membrane-bound IL-6R (mIL6-R) and are then unresponsive to IL-6 classic-signaling [75]. The infiltration of granulocytes and macrophages has been described as the possible cause of tissue damage in the acute lung injury, which is a severe complication of severe acute pancreatitis [60].
From these data can be concluded that IL-6 classic signaling via the membrane bound IL-6R is anti-inflammatory and protective, mainly via stimulation of intestinal regeneration, inhibition of epithelial cell apoptosis and defense against infections [46].
In contrast, trans-signaling via the sIL6-R is believed to act in a rather pro-inflammatory way via recruitment of mononuclear cells, inhibition of T-cell apoptosis, and inhibition of regulatory T-cells differentiation [46]. Interestingly, clinical pathological analysis of COVID-19 biopsy samples confirmed interstitial mononuclear inflammatory infiltrates in lung tissues, the same type of cells promoted by IL-6 trans-signaling [76], therefore suggesting a possible involvement of trans-signaling pathway of IL-6 in COVID-19 patients with ARDS. Moreover, IL-6 production sustained by circulating monocytes in COVID-19 is a different pathway from the one found in influenza infection, proof of that is Tocilizumab partially rescuing the immune dysregulation driven by SARS-CoV-2[27].
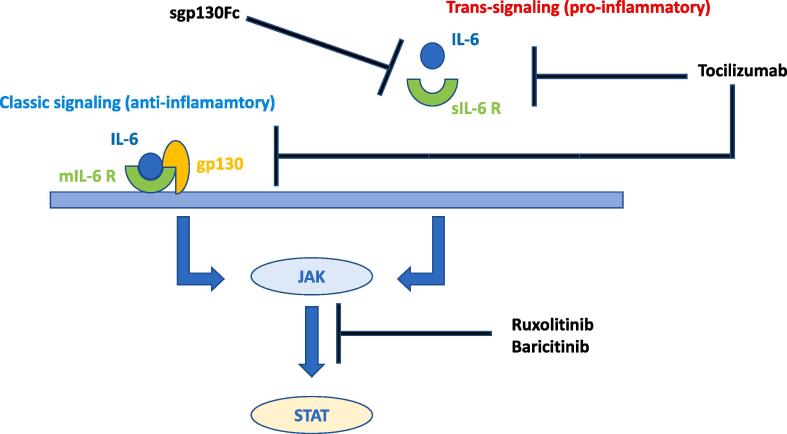
To the author’s knowledge sgp130Fc has not been tested in COVID-19 patients yet, thus the author suggests its possible use against SARS-CoV-2 in severe cases of COVID-19 patients, adopting the same criteria of Tocilizumab as to when to start the treatment. This option would provide the benefit of targeting only the pro-inflammatory trans-signaling pathway of IL-6, instead of targeting them both, which is something that happens with Tocilizumab [51]. IL-6 pathways and possible interventions are summerized in Fig. 1.
Fig. 1.
This diagram shows the classic signaling pathway (mediated by the membrane-bound form of IL-6 receptor, mIL6R) which is believed to be the anti-inflammatory one and the trans-signaling pathway (mediated by the soluble form of IL-6 receptor, sIL6-R) which is believed to be the pro-inflammatory one. Tocilizumab inhibits both of them, SGP130Fc inhibits only the trans-signaling pathway.
5. Inhibiting IL-6 signal transduction and the JAK/STAT pathway: Baricitinib and Ruxolitinib
Both the trans-signaling and the classic-signaling pathways of IL-6 converge in the activation of the JAK/STAT pathway and the MAPK cascade [77]. The next step after binding of IL-6 to IL-6R and sgp130 is then to initiate intracellular signal transduction. The signal transfer is performed by the Janus-kinase/Signal transducer and activator of transcription (Jak/STAT)-,mainly STAT1, STAT3 isoforms [78], [79], [80], mitogen-activated protein kinase (MAPK)- and phosphatidyl-inositol-3-kinase (PI3K)-pathway [44], [77]. Another possible way of counteracting IL-6 action is then to inhibit its intracellular transduction pathway, though in doing so the benefit of specifically targeting the pro-inflammatory trans-signaling pathway is lost.
This can be done by inhibiting the JAK 1/2 pathway with drugs like Ruxolitinib. Ruxolitinib is a small drug belonging to the class of Janus kinase (JAK) inhibitors and currently clinically used in the treatment of JAK2 mutated myeloproliferative neoplasms, including myelofibrosis and polycythemia vera [81], [82]. It shows activity against the JAK2 isoform and also the JAK1 isoform, which plays a major role in the signaling pathway of inflammatory cytokines [83]. JAK3 seems to be less sensitive to ruxolitinib [84], it also shows anti-inflammatory activity which may be beneficial in its clinical use [85]; it is also implicated in the suppression of the harmful consequences of macrophage activation hemophagocytic lymphohistiocytosis [86], which is an under-recognized hyperinflammatory syndrome characterized by fulminant and fatal hypercytokinemia with multi organ failure [87]. It has been proven that the expression of major inflammatory cytokines such as TNF alfa and IL-6 was highly reduced in inflammatory human macrophages exposed to ruxolitinib [85]. It has also been shown through an analysis of mRNA expression of cytokines by PCR array that the major inflammatory cytokines, IL-6 and TNF alfa, were highly reduced and down-regulated by ruxolitinib at both protein and mRNA level [88]. All these results point towards a possible use of Ruxolitinib as an IL-6 inhibitor like Tocilizumab in the advanced stages of COVID-19. This drug is currently being tested in some patients in Italy (CINC424, RUXOLITINIB).
Baricitinib has been suggested to be of therapeutic use against SARS-CoV-2 by Artificial Intelligence algorithms [89]. It is a selective and powerful JAK-STAT signaling inhibitor thus being effective against the consequences of the elevated levels of cytokines, not only being able of lowering IL-6 levels [90], but it also has the potential to inhibit clathrin-mediated endocytosis and thereby inhibit viral infection of cells. It targets members of the numb-associated kinase (NAK) family (AAK1 and GAK), the inhibition of which has been shown to reduce viral infection in vitro [91]. It can be administered orally and has acceptable side effect profile, besides having little interaction with CYP enzymes and drug transporters [89]. This makes Baricinitib a valid option in every phase of the viral infection, the early stages to reduce viral entry in the cells, and later stages for its anti-inflammatory properties.
6. Other anti-IL-6 drugs
Other monoclonal antibodies against IL-6 are sirukumab (CNTO136), olokizumab (CP6038), PF-423691, elsilimomab (BE-8), clazakizumab (BMS945429), which are in different phases of clinical trials to establish their efficacy and safety in varied disease states [92], [93], [94], [95], [96], [97]. Sarilumab, another monoclonal antibody against IL-6R, is being tested in a clinical trial against COVID-19 (Sarilimumab COVID-19). Another drug that showed potential inhibition of IL-6 related JAK/STAT pathway is glatiramer acetate which showed potential to downregulate both IL-17 and IL-6 in the central nervous system in an autoimmune encephalitis [98].
7. Conclusions
Here the author showed the viable options against the cytokine storm induced by SARS-CoV-2, showing that besides Tocilizumab, other options must be taken into account, and that inhibiting IL-6 is not a simple thing to do, since it has at least two different and divergent pathways; thus suggesting to inhibit only the pro-inflammatory response, the trans-signaling transduction with SGP130Fc, which seems a more reasonable choice then inhibiting both pathways. Here also other possible choices in counteracting IL-6 mediated activities are shown, like Ruxolitinib and Baricitnib, in the hope to find new possible strategies and discovering new properties of the drugs already available, since this would mean accelerating clinical trials as their side effects and tolerability in humans are already known.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HuiDS I.A., Madani T.A., Ntoumi F., Koch R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin. Transl. Med. 2020;9:19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 5.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J.F., Chan K.H., Kao R.Y. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart B.J., Dyall J., Postnikova E. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi Y.M., Alothman A., Balkhy H.H. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheahan T.P., Sims A.C., Leist S.R. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London, England) 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 13.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;105954 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longhi M.P., Wright K., Lauder S.N. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog. 2008;4:e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dienz O., Rud J.G., Eaton S.M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauder S.N., Jones E., Smart K. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 2013;43:2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy E.A., Davis J.M., Brown A.S., Carmichael M.D., Ghaffar A., Mayer E.P. Effect of IL-6 deficiency on susceptibility to HSV-1 respiratory infection and intrinsic macrophage antiviral resistance. J. Interferon Cytokine Res. 2008;28:589–595. doi: 10.1089/jir.2007.0103. [DOI] [PubMed] [Google Scholar]
- 19.Pyle C.J., Uwadiae F.I., Swieboda D.P., Harker J.A. Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog. 2017;13:e1006640. doi: 10.1371/journal.ppat.1006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W.K., Chen S.Y., Liu I.J. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2004;39:1071–1075. doi: 10.1086/423808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okabayashi T., Kariwa H., Yokota S. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J. Med. Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata N., Iwata N., Hasegawa H. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am. J. Pathol. 2008;172:1625–1637. doi: 10.2353/ajpath.2008.071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day C.W., Baric R., Cai S.X. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395:210–222. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J. Gong, H. Dong, S.Q. Xia, et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. medRxiv 2020:2020.2002.2025.20025643. [DOI] [PMC free article] [PubMed]
- 26.E.A. Coomes, H. Haghbayan, Interleukin-6 in COVID-19: A Systematic Review and Meta-Analysis. medRxiv 2020:2020.2003.2030.20048058. [DOI] [PMC free article] [PubMed]
- 27.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai Y., Kuba K., Neely G.G. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J., Duncan M.J., Li G. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park B.S., Song D.H., Kim H.M., Choi B.S., Lee H., Lee J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 31.Hiscott J., Nguyen T.L., Arguello M., Nakhaei P., Paz S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–6867. doi: 10.1038/sj.onc.1209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda K., Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 33.Puel A., Yang K., Ku C.L. Heritable defects of the human TLR signalling pathways. J. Endotoxin Res. 2005;11:220–224. doi: 10.1179/096805105X37367. [DOI] [PubMed] [Google Scholar]
- 34.Shirey K.A., Lai W., Scott A.J. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrin-Cocon L., Aublin-Gex A., Sestito S.E. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017;7:40791. doi: 10.1038/srep40791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyati K.K., Masuda K., Zaman M.M. TLR4-induced NF-kappaB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017;45:2687–2703. doi: 10.1093/nar/gkx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G., Fan Y., Lai Y. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Wu K., Wang D. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365:324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;102537 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.X. Chen, B. Zhao, Y. Qu, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv 2020:2020.2002.2029.20029520.
- 41.Chen W., Lan Y., Yuan X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes. Infect. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsueh P.R., Chen P.J., Hsiao C.H. Patient data, early SARS epidemic, Taiwan. Emerg. Infect. Dis. 2004;10:489–493. doi: 10.3201/eid1003.030571. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T., Narazaki M., Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 44.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Schaper F., Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 47.Mullberg J., Vollmer P., Althoff K., Marz P., Rose-John S. Generation and function of the soluble interleukin-6 receptor. Biochem. Soc. Trans. 1999;27:211–219. doi: 10.1042/bst0270211. [DOI] [PubMed] [Google Scholar]
- 48.Riethmueller S., Somasundaram P., Ehlers J.C. Proteolytic origin of the soluble human IL-6R in vivo and a decisive role of N-glycosylation. PLoS Biol. 2017;15:e2000080. doi: 10.1371/journal.pbio.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riethmueller S., Ehlers J.C., Lokau J. Cleavage site localization differentially controls interleukin-6 receptor proteolysis by ADAM10 and ADAM17. Sci. Rep. 2016;6:25550. doi: 10.1038/srep25550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lust J.A., Donovan K.A., Kline M.P., Greipp P.R., Kyle R.A., Maihle N.J. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 51.Rose-John S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin. Pharmacol. Ther. 2017;102:591–598. doi: 10.1002/cpt.782. [DOI] [PubMed] [Google Scholar]
- 52.Rose-John S., Heinrich P.C. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem. J. 1994;300(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose-John S., Schooltink H., Lenz D. Studies on the structure and regulation of the human hepatic interleukin-6 receptor. Eur. J. Biochem. 1990;190:79–83. doi: 10.1111/j.1432-1033.1990.tb15548.x. [DOI] [PubMed] [Google Scholar]
- 54.Oberg H.H., Wesch D., Grussel S., Rose-John S., Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int. Immunol. 2006;18:555–563. doi: 10.1093/intimm/dxh396. [DOI] [PubMed] [Google Scholar]
- 55.Scheller J., Garbers C., Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014;26:2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Barkhausen T., Tschernig T., Rosenstiel P. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 2011;39:1407–1413. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]
- 57.Rabe B., Chalaris A., May U. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 58.Rose-John S., Waetzig G.H., Scheller J., Grotzinger J., Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 59.Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020;28:115327. doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H., Neuhofer P., Song L. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Invest. 2013;123:1019–1031. doi: 10.1172/JCI64931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuett H., Oestreich R., Waetzig G.H. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 62.Kyrstsonis M.C., Dedoussis G., Baxevanis C., Stamatelou M., Maniatis A. Serum interleukin-6 (IL-6) and interleukin-4 (IL-4) in patients with multiple myeloma (MM) Br. J. Haematol. 1996;92:420–422. doi: 10.1046/j.1365-2141.1996.d01-1491.x. [DOI] [PubMed] [Google Scholar]
- 63.Lavabre-Bertrand T., Exbrayat C., Liautard J. Detection of membrane and soluble interleukin-6 receptor in lymphoid malignancies. Br. J. Haematol. 1995;91:871–877. doi: 10.1111/j.1365-2141.1995.tb05403.x. [DOI] [PubMed] [Google Scholar]
- 64.Doganci A., Eigenbrod T., Krug N. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo C.W., Chen M.W., Hsiao M. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71:424–434. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 66.Calabrese L.H., Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 2014;10:720–727. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- 67.Garbers C., Aparicio-Siegmund S., Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr. Opin. Immunol. 2015;34:75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Garbers C., Monhasery N., Aparicio-Siegmund S. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2014;1842:1485–1494. doi: 10.1016/j.bbadis.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Ferreira R.C., Freitag D.F., Cutler A.J. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sodenkamp J., Waetzig G.H., Scheller J. Therapeutic targeting of interleukin-6 trans-signaling does not affect the outcome of experimental tuberculosis. Immunobiology. 2012;217:996–1004. doi: 10.1016/j.imbio.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Hoge J., Yan I., Janner N. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J. Immunol. 2013;190:703–711. doi: 10.4049/jimmunol.1201044. [DOI] [PubMed] [Google Scholar]
- 72.Jones S.A., Scheller J., Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenhill C.J., Rose-John S., Lissilaa R. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J. Immunol. 2011;186:1199–1208. doi: 10.4049/jimmunol.1002971. [DOI] [PubMed] [Google Scholar]
- 74.Wunderlich C.M., Delic D., Behnke K. Cutting edge: Inhibition of IL-6 trans-signaling protects from malaria-induced lethality in mice. J. Immunol. 2012;188:4141–4144. doi: 10.4049/jimmunol.1102137. [DOI] [PubMed] [Google Scholar]
- 75.Romano M., Sironi M., Toniatti C. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 76.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Muller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villarino A.V., Kanno Y., O'Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PubMed] [Google Scholar]
- 80.Zegeye M.M., Lindkvist M., Falker K. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018;16:55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verstovsek S., Kantarjian H., Mesa R.A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vannucchi A.M., Harrison C.N. Emerging treatments for classical myeloproliferative neoplasms. Blood. 2017;129:693–703. doi: 10.1182/blood-2016-10-695965. [DOI] [PubMed] [Google Scholar]
- 83.Mesa R.A. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs. 2010;13:394–403. [PubMed] [Google Scholar]
- 84.Quintas-Cardama A., Vaddi K., Liu P. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bjorn M.E., Hasselbalch H.C. The impact of ruxolitinib treatment on inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Clin. Case Rep. 2015;3:499–503. doi: 10.1002/ccr3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maschalidi S., Sepulveda F.E., Garrigue A., Fischer A., de Saint Basile G. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood. 2016;128:60–71. doi: 10.1182/blood-2016-02-700013. [DOI] [PubMed] [Google Scholar]
- 87.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Febvre-James M., Lecureur V., Augagneur Y., Mayati A., Fardel O. Repression of interferon beta-regulated cytokines by the JAK1/2 inhibitor ruxolitinib in inflammatory human macrophages. Int. Immunopharmacol. 2018;54:354–365. doi: 10.1016/j.intimp.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 89.Stebbing J., Phelan A., Griffin I. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi J., Cooper M.L., Staser K. Baricitinib-induced blockade of interferon gamma receptor and interleukin-6 receptor for the prevention and treatment of graft-versus-host disease. Leukemia. 2018;32:2483–2494. doi: 10.1038/s41375-018-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bekerman E., Neveu G., Shulla A. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Invest. 2017;127:1338–1352. doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sieper J., Braun J., Kay J. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN) Ann. Rheum. Dis. 2015;74:1051–1057. doi: 10.1136/annrheumdis-2013-204963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smolen J.S., Weinblatt M.E., Sheng S., Zhuang Y., Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 2014;73:1616–1625. doi: 10.1136/annrheumdis-2013-205137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rovin B.H., van Vollenhoven R.F., Aranow C. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 2016;68:2174–2183. doi: 10.1002/art.39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weinblatt M.E., Mease P., Mysler E. The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol. 2015;67:2591–2600. doi: 10.1002/art.39249. [DOI] [PubMed] [Google Scholar]
- 96.Danese S., Vermeire S., Hellstern P. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn's disease (ANDANTE I and II) Gut. 2019;68:40–48. doi: 10.1136/gutjnl-2017-314562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li C., Shoji S., Beebe J. Pharmacokinetics and C-reactive protein modelling of anti-interleukin-6 antibody (PF-04236921) in healthy volunteers and patients with autoimmune disease. Br. J. Clin. Pharmacol. 2018;84:2059–2074. doi: 10.1111/bcp.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begum-Haque S., Sharma A., Kasper I.R. Downregulation of IL-17 and IL-6 in the central nervous system by glatiramer acetate in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2008;204:58–65. doi: 10.1016/j.jneuroim.2008.07.018. [DOI] [PubMed] [Google Scholar]