Abstract
Objective:
To examine the association between income level and incident chronic kidney disease (CKD) in adults with normal baseline kidney function.
Patient and Methods:
We studied the association between income level categorized into deciles and incident CKD in a national cohort comprised of 7.4 million adults who underwent National Health Insurance Service health examinations over the period of January 1, 2009 and December 31, 2015 with baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73m2. Incident CKD was defined as de novo development of eGFR <60 mL/min/1.73m2 (model 1) or ≥25% decline in eGFR from the baseline values accompanied by eGFR <60 mL/min/1.73m2 (model 2).
Results:
During a median follow-up of 4.8 years, there were a total of 122,032 (1.65%) and 55,779 (0.75%) incident CKD events based on model 1 and 2 definitions, respectively. Compared with income levels in the sixth decile, there was an inverse association between lower income level and higher risk of CKD up to fourth decile, above which no additional reduction (model 1) or slightly higher risk of CKD (model 2) was observed at higher income levels. The multivariable-adjusted hazard ratios (95% confidence interval) from the lowest to fourth deciles were 1.30 (1.26–1.33), 1.16 (1.13–1.19), 1.07 (1.05–1.10), and 1.06 (1.03–1.09) in model 1 and 1.32 (1.27–1.37), 1.18 (1.14–1.22), 1.08 (1.04–1.13), and 1.05 (1.01–1.09) in model 2, respectively. These associations persisted across various subgroups of age, sex, and comorbidity status.
Conclusion:
In this large nationwide cohort, lower income levels were associated with higher risk of incident CKD.
Keywords: Socioeconomic status, income, chronic kidney disease
INTRODUCTION
Chronic kidney disease (CKD) is a major public health problem affecting approximately 10% of the adults worldwide.1 For example, patients with CKD have substantially higher risk of cardiovascular disease, as well as cardiovascular and all-cause death even in the early stages of CKD.2–4 While CKD patients are five to ten times more likely to die vs. progress to end stage renal disease (ESRD), those who survive may ultimately require dialysis treatment or kidney transplantation.5 These latter interventions pose exorbitant economic burden across many countries spending billions of dollars to treat ESRD patients, in addition to substantial financial costs incurred in the prevention of CKD and its complications.6, 7
Socioeconomic status (SES) has become increasingly recognized as an important factor for chronic diseases, vis-à-vis potential inadequate detection, treatment, and follow-up, and individuals of low SES are more likely to have hypertension, heart disease, and diabetes.8–11 Associations between low SES and kidney disease have also been reported, and an increasing body of literature suggests that socioeconomic deprivation can hasten progression of CKD and lead to ESRD and death.12, 13 Among measures of SES, income level is an important determinant of health status, given that inability to pay for medical costs precludes adequate access to medical care, timely screening and diagnosis, initiation of treatment, appropriate follow-up, particularly in patients of low income.14 Existing studies that have examined the association between SES and CKD have largely been cross-sectional, and to date there are a paucity of large longitudinal epidemiologic studies that examined this relationship. Moreover, little is known about the impact of gradients of income level upon the development of CKD. More specifically, there is a lack of data on income thresholds above which risk of CKD is mitigated or even reduced. To address these gaps in knowledge, using a large national longitudinal database of over seven million adults in South Korea (i.e., approximately one-third of the entire adult population 40 years and older in the nation), we conducted analyses examining the association between granular categorizations of income level with incident CKD.
PATIENTS AND METHODS
Source Population
We obtained data from the Korean National Health Insurance Service (NHIS) database, which is linked to national health screening examination information. The NHIS covers compulsory health insurance for all citizens in Korea with one of the following three types of insurance: employee insured (employee subscribers), self-employed insured (local subscribers), and medical aid beneficiary. All insured individuals, except those with very low income (medical aid beneficiary), pay monthly contributions to the NHIS which are calculated based on employees’ wages for those who are employee insured, versus household income level, property, vehicles owned, age, and sex for those who are self-employed insured. As a benefit, the NHIS provides cost-free annual or biennial health screening examinations to all insured individuals.15, 16
Participants who underwent NHIS health examination over the period of January 1, 2009 and December 31, 2015 were included in the study. We first identified 10,810,233 individuals who, at their first examination (baseline examination), were ≥40 years of age, had preserved kidney function (estimated glomerular filtration rate [eGFR] ≥60 ml/min/1.73 m2), and had at least three or more eGFR measurements over the follow-up period. To minimize errors in the estimation of income, we excluded individuals whose insurance status did not fall under the category of employee insured, given that those with self-employed insured and medical aid beneficiary status (1) tended to have unreliable information regarding income level, and also (2) required use of contribution formulas inconsistent with those of employee insured status. We then excluded those with missing data on income measures or core study variables (e.g., smoking status, alcohol consumption, physical activity, height, weight, lipid profiles, or urinalysis) at the time of baseline examination. We also excluded those with outlier eGFR values (defined as >99.75th percentile, i.e., >130.3 ml/min/1.73 m2). Therefore, the final study population was comprised of 7,405,715 participants (Figure S1). The Institutional Review Board of NHIS Ilsan Hospital approved this study and waived the requirement for informed consent as we used only deidentified data.
Data Collection and Measurements
Data on sociodemographic details, lifestyle behaviors, body anthropometry, and other laboratory results were collected on the date of baseline examinations. Information regarding smoking status, alcohol consumption, and physical activity were ascertained by self-administered questionnaires. Comorbidities (diabetes [E10~14], ischemic heart disease [I20~25], congestive heart failure [I10.1, I13.0, I13.2, I25.5, I42, I50], cerebrovascular disease [I60~64, G459], chronic obstructive pulmonary disease [J43, J44], and malignancy [C00~97]) were assessed using the International Classification of Disease, Tenth Revision. At least one diagnostic code identified during one year before the date of study entry (baseline examination) was used to determine these comorbidities. Use of antihypertensive drugs or HMG-CoA reductase inhibitors (i.e., statins) were defined as the prescription for these medications identified for ≥3 months within the year before the date of cohort entry. Blood pressure was measured using standardized methods while the participant was sitting on a chair after a five-minute rest. Body mass index was calculated as weight in kilograms divided by height in meters-squared. Lipid levels and serum creatinine concentrations were measured from specimens collected while fasting. eGFR was estimated by the CKD Epidemiology Collaboration equation for creatinine.17 Urinalysis was performed by urine dipstick based on random spot urine measurements, and albuminuria was categorized as trace or ≥1+.
Income data were derived from monthly insurance charge records between 2009 and 2015. The rates of insurance premiums for employee subscribers were 5.08%, 5.33%, 5.64%, 5.80%, 5.89%, 5.99%, and 6.07% of the monthly basic income in 2009, 2010, 2011, 2012, 2013, 2014, and 2015, respectively.18 Thus, the primary measure of monthly income was estimated as averaged monthly insurance charges (1 USD=1,100 KWN) divided by monthly insurance premium (%) in the year of the cohort entry.
Outcome Ascertainment
The primary outcome of interest was incident CKD. In this study, we used two-level hierarchical definitions of incident CKD. In primary analyses, incident CKD was defined as de novo development of an eGFR <60 mL/min/1.73m2 (designated as “model 1”), as suggested by current international guidelines.19, 20 To substantiate our findings, secondary analyses included kidney function decline in addition to an eGFR threshold, which was defined as an eGFR <60 mL/min/1.73m2 accompanied by a concomitant ≥25% decline in eGFR from the baseline values (designated as “model 2”), as used by previous epidemiologic studies.21, 22
These outcomes were considered to have occurred when the above criteria were observed for at least two consecutive measurements, and the first day of occurrence was designated as the study endpoint. Follow-up began on the date of baseline examination and ended on December 31, 2015.
Statistical Analysis
Data from descriptive analyses were summarized using means (standard deviation), medians (inter-quartile range, IQR), or proportions. The crude rates of developing CKD were calculated from the number of event occurrences and person-years during the follow-up period by using Poisson regression. Cox proportional hazard regression models were performed to study the associations between income levels and risk of incident CKD. Given a possible non-linear relationship with risk of CKD development, income level was treated as a categorical variable and was divided into deciles on the basis of each individual’s monthly income at baseline. The sixth decile was chosen as the reference because it included the mean and median values (1,329 and 1,083 USD, respectively) of income distribution and allowed for the most precise comparison with lower and higher income categories. In sensitivity analyses, we also treated monthly income as a continuous variable and modeled a non-linear effect by using restricted cubic spine functions. In addition, we performed analogous analyses after subjects were regrouped according to authentic distribution of resident income. All models were adjusted for age, sex, comorbidities, residential area, smoking status, alcohol intake, and physical activity, use of antihypertensive medications, use of statins, systolic blood pressure, body mass index, albuminuria, low-density lipoprotein cholesterol level, triglyceride level, high-density lipoprotein cholesterol level, and eGFR. The risk of incident CKD was expressed as hazard ratios (HRs) and 95% confidence intervals (CIs).
To test the robustness of our findings, we also performed subgroup analyses across the following clinically relevant subgroups: age (<60 and ≥60 years), sex (male and female), residential area (urban and rural), smoking status (never and former or current), diabetes, and use of antihypertensive medications. We utilized the same covariates for adjustment in multivariable models as described for the main analyses. All analyses were conducted using Stata version 15.1 (Stata Corporation, College Station, TX). P <.05 was used as the threshold for statistical significance for any tests.
RESULTS
Baseline Characteristics of Study Population
Baseline characteristics of the 7,405,715 participants who met eligibility criteria for the study are shown in Table 1. The median age of the study participants was 52 years, among whom 52% were male, 10% had diabetes, and 8% had receipt of antihypertensive medications at the time of study entry. In the study population, urban residents comprised 93% of the cohort. As income levels increased, subjects were more likely to be men, smokers, alcohol drinkers, and to have higher levels of physical activity. Overall, the mean age and systolic blood pressure levels were similar across the decile groups. In addition, comorbid conditions were similarly distributed among the groups, but malignancy was more prevalent in subjects with higher income levels.
Table 1.
Baseline characteristics of 7,405,515 participants stratified by income deciles
| Characteristics | Income deciles |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Decile 1 (Lowest) | Decile 2 | Decile 3 | Decile 4 | Decile 5 | Decile 6 | Decile 7 | Decile 8 | Decile 9 | Decile 10 (Highest) | |
| Number | 7.405,715 | 749,049 | 726,168 | 768,025 | 720,060 | 731,299 | 748,814 | 740,562 | 740,697 | 740,532 | 740,509 |
| Monthly income, USD | 1329±1048 | 322±62 | 441±30 | 572±46 | 743±56 | 958±69 | 1223±84 | 1519±89 | 1847±100 | 2245±139 | 3420±1594 |
| Age, years | 52.3±10.0 | 53.1±9.1 | 52.7±9.1 | 51.9±8.8 | 52.2±9.0 | 52.5±9.6 | 52.4±10.3 | 51.9±11.0 | 51.3±11.0 | 52.4±10.4 | 52.9±10.8 |
| 40–59 years, % | 74.9 | 74.0 | 73.9 | 79.1 | 77.4 | 74.1 | 71.9 | 71.5 | 74.9 | 77.5 | 74.9 |
| ≥60 years, % | 25.1 | 26.0 | 26.1 | 20.9 | 22.6 | 25.9 | 28.1 | 28.5 | 25.1 | 22.5 | 25.1 |
| Gender, % male | 52.4 | 37.7 | 39.6 | 43.1 | 50.6 | 55.3 | 57.7 | 57.9 | 58.4 | 60.1 | 64.0 |
| Residential area, % | |||||||||||
| Urban | 93.4 | 91.9 | 92.4 | 92.1 | 92.6 | 93.3 | 93.7 | 93.2 | 93.4 | 94.7 | 96.8 |
| Rural | 6.6 | 8.1 | 7.6 | 7.9 | 7.4 | 6.7 | 6.3 | 6.8 | 6.6 | 5.3 | 3.2 |
| Comorbidities, % | |||||||||||
| Diabetes | 9.8 | 10.1 | 9.8 | 9.3 | 9.6 | 10.1 | 10.2 | 9.9 | 9.2 | 9.5 | 10.1 |
| Ischemic heart disease | 4.8 | 4.4 | 4.4 | 4.2 | 4.5 | 4.8 | 5.0 | 5.0 | 4.8 | 5.2 | 5.6 |
| Congestive heart failure | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Cerebrovascular disease | 2.5 | 2.4 | 2.3 | 2.2 | 2.4 | 2.6 | 2.7 | 2.7 | 2.6 | 2.6 | 2.7 |
| COPD | 1.7 | 1.6 | 1.5 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 1.7 | 1.7 | 1.7 |
| Malignancy | 2.5 | 2.3 | 2.3 | 2.3 | 2.3 | 2.4 | 2.5 | 2.6 | 2.7 | 2.9 | 3.1 |
| Antihypertensive agents, % | 8.0 | 8.6 | 8.5 | 7.8 | 8.2 | 8.2 | 8.3 | 7.8 | 7.1 | 7.4 | 7.7 |
| Statins, % | 3.9 | 4.2 | 3.9 | 3.7 | 3.9 | 4.0 | 4.1 | 3.9 | 3.6 | 3.8 | 4.2 |
| Smoking status, % | |||||||||||
| Never | 62.3 | 71.7 | 71.2 | 67.9 | 62.4 | 59.0 | 57.3 | 58.2 | 59.0 | 58.6 | 57.0 |
| Former | 16.5 | 10.9 | 12.2 | 13.0 | 14.8 | 16.4 | 17.6 | 18.1 | 18.9 | 20.5 | 22.2 |
| Current | 21.3 | 17.4 | 16.6 | 19.1 | 22.9 | 24.6 | 25.1 | 23.7 | 22.1 | 20.9 | 20.8 |
| Alcohol intake, % | |||||||||||
| 0 g/day | 55.7 | 64.1 | 61.6 | 58.9 | 56.4 | 54.9 | 53.8 | 53.0 | 52.3 | 51.9 | 50.0 |
| 1–19 g/day | 31.5 | 26.5 | 28.8 | 30.0 | 30.8 | 31.3 | 31.9 | 32.8 | 33.6 | 34.1 | 35.4 |
| ≥20 g/day | 12.8 | 9.6 | 11.1 | 12.7 | 13.8 | 14.3 | 14.2 | 14.1 | 14.0 | 14.6 | 12.8 |
| Physical activity, % | |||||||||||
| <600 MET-min/week | 46.9 | 51.5 | 51.3 | 50.3 | 48.9 | 47.0 | 45.3 | 43.0 | 40.6 | 40.4 | 46.9 |
| 600–3000 MET-min/week | 44.8 | 40.7 | 41.1 | 41.1 | 41.8 | 43.0 | 44.5 | 46.1 | 48.5 | 50.2 | 50.6 |
| >3000 MET-min/week | 8.3 | 7.8 | 7.7 | 7.6 | 7.9 | 8.2 | 8.5 | 8.6 | 8.6 | 9.2 | 9.0 |
| Albuminuria % | 4.4 | 4.6 | 4.4 | 4.3 | 4.3 | 4.3 | 4.2 | 4.3 | 4.4 | 4.6 | 4.9 |
| SBP, mmHg | 123±15 | 123±16 | 123±15 | 123±15 | 124±15 | 124±15 | 124±15 | 124±15 | 123±15 | 123±15 | 122±14 |
| Body mass index, kg/m2 | 23.9±3.4 | 23.9±4.2 | 23.8±4.6 | 23.8±3.0 | 23.9±3.1 | 24.0±4.1 | 24.0±3.0 | 24.0±3.0 | 24.0±3.0 | 23.9±2.9 | 24.0±2.9 |
| GFR, mL/min/1.73m2 | 88.2±14.3 | 88.3±14.5 | 88.8±14.5 | 89.2±14.4 | 88.9±14.3 | 88.6±14.3 | 88.2±14.2 | 88.3±14.3 | 88.1±14.3 | 87.1±4.0 | 86.7±14.0 |
| LDL-C, mg/dL | 119±76 | 119±92 | 117±64 | 118±69 | 119±94 | 118±77 | 118±74 | 118±80 | 118±76 | 119±62 | 121±62 |
| HDL-C, mg/dL | 55±27 | 56±26 | 57±25 | 57±26 | 56±29 | 55±27 | 55±27 | 55±29 | 55±25 | 54±25 | 54±27 |
| Triglyceride, mg/dL | 138±102 | 134±98 | 129±96 | 131±99 | 137±103 | 142±106 | 144±108 | 143±107 | 141±104 | 140±101 | 139±98 |
Note: Data are presented as means ± standard deviation or percentages. COPD=chronic obstructive pulmonary disease; MET=metabolic equivalent of task; SBP=systolic blood pressure; GFR=glomerular filtration rate; LDL-C=low-density lipoprotein cholesterol; HDL-C=high-density lipoprotein cholesterol.
Income levels and Risk of Incident Chronic Kidney Disease
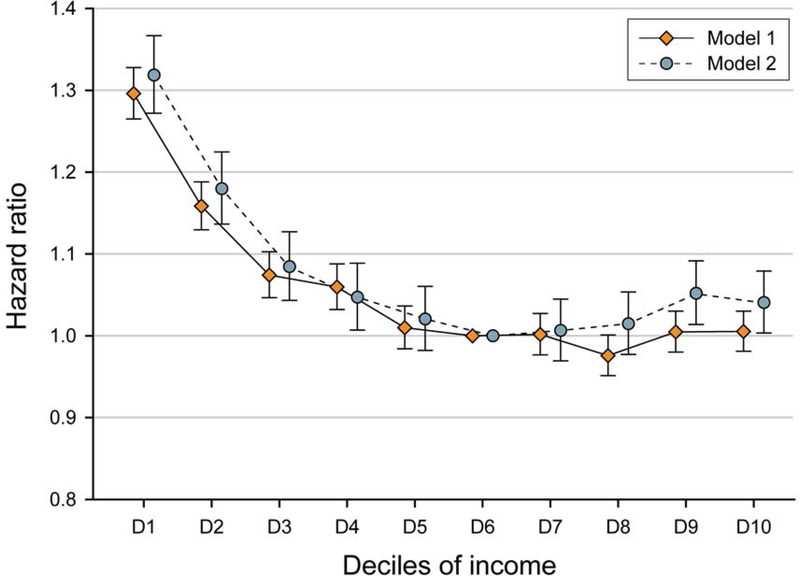
Using model 1 definitions of incident CKD, there were a total of 122,032 incident CKD events (i.e., 1.65% of the study population) over a follow-up of 34,777,829 person-years in the overall cohort. The crude incidence rate for incident CKD was 3.51 (95% CI, 3.49–3.53) per 1,000 person-years, and the median (IQR) follow-up among study participants was 4.8 (IQR 3.9–5.9) years. In Cox regression models that were adjusted for socio-demographic, comorbidity, laboratory, and medication data there was a graded association between lower income level and higher risk of incident CKD (Figure 1 and Table 2). Specifically, compared with individuals with sixth decile income category, we observed a 6% higher risk of incident CKD in those who belonged to the fourth decile: adjusted HR (aHR), 1.06 (95% CI, 1.03–1.09). This relationship between lower income and higher risk of incident CKD was increasingly stronger among individuals who belonged to the third decile (aHR, 1.07 [95% CI, 1.05–1.10]), second decile (aHR, 1.16 [95% CI, 1.13–1.19]), and the lowest decile (aHR, 1.30 [95% CI, 1.26–1.33]). However, we did not observe a reduction in the risk of incident CKD among individuals who belonged to the seventh income level decile and higher. The corresponding aHRs (95% CIs) from seventh to the tenth deciles were (reference group: sixth decile): 1.00 (0.98–1.03), 0.98 (0.95–1.00), 1.00 (0.98–1.03), and 1.01 (0.98–1.03), respectively.
FIGURE 1.
Associations between income deciles with incident chronic kidney disease. Incident chronic kidney disease was defined as an eGFR <60 mL/min/1.73m2 (model 1), or ≥25 % decline in eGFR from the baseline values accompanied by an eGFR <60 mL/min/1.73m2 (model 2). All models were adjusted for age, sex, residential area, comorbidities, smoking status, alcohol intake, physical activity, albuminuria, use of antihypertensive medications, use of statins, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, and estimated glomerular filtration levels. eGFR=estimated glomerular filtration.
Table 2.
Multivariate associations of income deciles with incident chronic kidney disease
| Income deciles | Crude event rates (per 1,000 PYs) |
Cox modes for CKD development |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||
| Incidence | 95% CI | Incidence | 95% CI | HR | 95% CI | p | HR | 95% CI | p | |
| Decile 1 (lowest) | 4.10 | (4.03–4.16) | 1.88 | (1.84–1.93) | 1.30 | (1.26–1.33) | <.001 | 1.32 | (1.27–1.37) | <.001 |
| Decile 2 | 3.59 | (3.52–3.65) | 1.67 | (1.63–1.72) | 1.16 | (1.13–1.19) | <.001 | 1.18 | (1.14–1.22) | <.001 |
| Decile 3 | 2.97 | (2.91–3.02) | 1.38 | (1.34–1.42) | 1.07 | (1.05–1.10) | <.001 | 1.08 | (1.04–1.13) | <.001 |
| Decile 4 | 3.08 | (3.02–3.14) | 1.41 | (1.37–1.45) | 1.06 | (1.03–1.09) | <.001 | 1.05 | (1.01–1.09) | .02 |
| Decile 5 | 3.20 | (3.14–3.26) | 1.48 | (1.44–1.52) | 1.01 | (0.98–1.04) | .45 | 1.02 | (0.98–1.06) | .30 |
| Decile 6 (reference) | 3.39 | (3.33–3.45) | 1.53 | (1.49–1.57) | 1.00 | 1.00 | ||||
| Decile 7 | 3.54 | (3.48–3.60) | 1.60 | (1.56–1.64) | 1.00 | (0.98–1.03) | .90 | 1.01 | (0.97–1.04) | .74 |
| Decile 8 | 3.40 | (3.34–3.46) | 1.58 | (1.53–1.62) | 0.98 | (0.95–1.00) | .06 | 1.01 | (0.98–1.05) | .45 |
| Decile 9 | 3.70 | (3.64–3.77) | 1.69 | (1.65–1.73) | 1.00 | (0.98–1.03) | .71 | 1.05 | (1.01–1.09) | .007 |
| Decile 10 (highest) | 4.12 | (4.05–4.19) | 1.83 | (1.79–1.88) | 1.01 | (0.98–1.03) | .67 | 1.04 | (1.00–1.08) | .03 |
Note: Incident CKD was defined as an eGFR <60 mL/min/1.73m2 (model 1), or ≥25 % decline in eGFR from the baseline values accompanied by an eGFR <60 mL/min/1.73m2 (model 2). All Cox models were adjusted for age, sex, residential area, comorbidities, smoking status, alcohol intake, physical activity, albuminuria, use of antihypertensive medications, use of statins, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, and estimated glomerular filtration levels. CKD=chronic kidney disease; PYs=person-years; eGFR=estimated glomerular filtration; HR=hazard ratio; CI=confidence interval.
Using model 2 definitions of incident CKD, we observed a similar pattern of findings (Figure 1 and Table 2). Using this secondary definition, there were a total of 55,779 incident CKD events (i.e., 0.75% of the study population) during 34,755,860 person-years of follow-up, and the crude rate for incident CKD was 1.60 (95% CI, 1.59–1.62) per 1,000 person-years. Compared with sixth decile, there was an inverse graded association between lower income levels and higher risk of incident CKD among individuals with lower income levels (i.e., lowest to fifth decile). We also observed an association between the two highest income levels and higher risk of incident CKD, although the magnitude of risk was small: aHR (95% CI) 1.05 (1.01–1.09) and 1.04 (1.00–1.08) for ninth and tenth deciles, respectively.
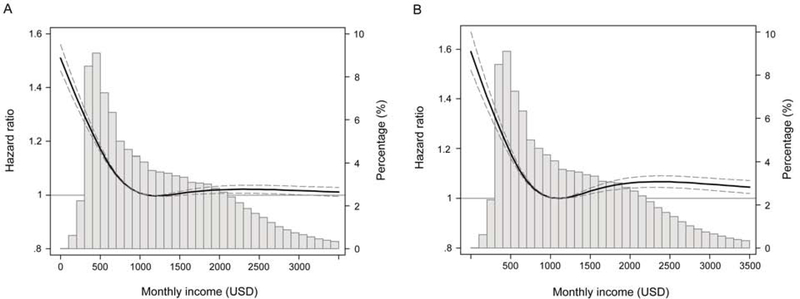
Spline analyses also showed a similar relationship between monthly income level and the risk of incident CKD, such that a progressively marked increase in risk was observed below the median value of monthly income level, above which risk plateaued for higher income levels (Figure 2). In analogous analyses using income levels based on authentic distribution of resident income, the results were consistent to those of the primary analyses above (Table S1).
FIGURE 2.
Association between monthly income level and incident chronic kidney disease using adjusted restricted cubic spine analyses. Incident chronic kidney disease was defined as an eGFR <60 mL/min/1.73m2 (A), or ≥25% decline in eGFR from the baseline values accompanied by an eGFR <60 mL/min/1.73m2 (B). All models were adjusted for age, sex, residential area, comorbidities, smoking status, alcohol intake, physical activity, albuminuria, use of antihypertensive medications, use of statins, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, and estimated glomerular filtration levels. A histogram of observed monthly income and a hazard reference ratio of 1 (solid line) is overlaid. eGFR=estimated glomerular filtration.
Subgroup Analyses
We also sought to examine the relationship between income level and risk of incident CKD across clinically relevant subgroups (Figure S2 and Table S2). We observed an increasingly higher risk of incident CKD with incrementally lower income levels, especially below 40 percentile of income levels, which was robust across all subgroups stratified by age, sex, residential area, smoking status, diabetes, and use of antihypertensive drugs. Notably, we observed that higher income levels in the top 20th percentile were also associated with higher risk of incident CKD among individuals who were of younger age (<60 years of age), male, smokers, and without diabetes or receipt of antihypertensive drugs. These associations were particularly prominent for model 2 definitions of incident CKD.
DISCUSSION
In this large national longitudinal cohort study, we found that incrementally lower income levels were associated with an increasingly higher risk of incident CKD. These relationships were robust in multiple secondary and sensitivity analyses that (1) utilized two established definitions of incident CKD, (2) accounted for potential confounders in multivariable models, (3) analyzed income level as a categorical vs. continuous variable, and (4) examined associations across subgroups of age, sex, residential area, smoking status, and comorbid conditions. Higher income levels above the median income threshold were also associated with higher risk of CKD, but the magnitude of risk was relatively small. Our findings suggest that income level is an important consideration in estimating an individual’s risk of incident CKD, and in devising strategies that can prevent its development at a population level.
It is well known that disparities in SES such as income level, education, and employment can adversely affect outcomes associated with chronic diseases and result in substantial morbidity and mortality.8–10, 23, 24 Income level is one of the key determinants of SES, and approaches to healthcare oftentimes vary depending upon income class. In a study by Chetty et al.23 examining the relationship between income level and mortality, investigators found that between the richest 1% and the poorest 1% of individuals, there was a gap in life expectancy of 15 years for men and 10 years for women. Given the numerous risk factors that are shared between CKD and other chronic diseases, it is also highly plausible that income level is an important determinant of the development of CKD and its complications, which has been supported by a growing number of studies.25–28 For example, two previous studies showed the significant association of lower income with early kidney function decline defined as a ≥0.4 mg/dL increase in serum creatinine level.29, 30 However, it should be noted that these previous studies have largely utilized a cross-sectional design, precluding ability to distinguish directionality of associations (i.e., low income level leading to incident CKD, or vice-versa), short-term follow-up, as well as examination of incident vs. prevalent CKD. To our knowledge, our study is the first effort to conduct a rigorous longitudinal examination of granularly-defined income levels and risk of incident CKD in a large national population of adults, and thus add important new knowledge to the field.
Notably, our study also observed that the relationship between income level and risk of incident CKD is non-linear, such that higher income levels showed mitigation but not reduction in risk. This stands in contrast to previous studies showing a reduction in risk with higher income levels (i.e., “the higher the income level, the better the outcome”).13, 31–33 While the reasons for these discrepant findings are not uncertain, they may be partly attributable to methodologic differences across studies including study design (i.e., cross-sectional vs. longitudinal), definitions and categorizations of income levels, designations of incident CKD, covariates selected for multivariable adjustment, and inherent differences in the study populations. Notably, our study also observed that nature of the relationship between income and CKD may vary depending on underlying characteristics of study population or definitions of incident CKD. For example, a markedly higher risk of incident CKD was observed with lower income categories across various subgroups and CKD definitions; however, we observed a slightly higher risk of incident CKD with higher income levels among individuals who were younger people, male, smokers, and absence of certain comorbidities (e.g., diabetes or receipt of antihypertensive drugs) when more stringent definitions of CKD were applied using model 2.
Another important potential explanation for these discrepant findings is differences in the social environment, educational levels, and government-driven healthcare policies across countries. For example, educational levels tend to be high in South Korea, and the South Korean government provides medical insurance coverage for the entire population.34 As previously mentioned, the NHIS also provides the cost-free annual or biennial health screening examination for all insured citizens, enabling earlier detection of CKD in a greater population of the population. While these government-sponsored public health initiatives improve access to health care screening, they alone may not be sufficient in ameliorating risk of CKD and its complications among individuals of lower income who demonstrated a high risk of incident CKD in our study. Presumably, lack of education, unhealthy work environments, as well as other social factors (e.g., limited social support, poor health literacy) particularly in those of low income may deter timely and effective treatment and follow-up after the initial detection of disease. Thus, from the perspective of prevention, our study’s findings may help inform healthcare policy with respect to individualizing interventions according to income level. While, income inequalities are pervasive and difficult to mitigate, further investment into interventions focused upon high-risk (i.e., low income) populations may have substantial impact upon improving outcomes and reducing societal health care costs.
There are several pathways that may potentially link low income with heightened risk of CKD. In addition to poor access to medical care, low income has been associated with unhealthy lifestyle and food consumption, particularly in the CKD population.35–38 This may eventually lead to the development of chronic diseases such as diabetes mellitus, obesity, and hypertension, which are potent risk factors for CKD.39, 40 Many epidemiologic studies have consistently supported these hypotheses showing that unfavorable comorbid conditions are more prevalent in low vs. high SES populations.41–45 Notably, in contrast to previous studies, our study did not find large differences in comorbidity burden across low and high income levels; in fact, certain unhealthy behaviors such as smoking and alcohol use were more prevalent among individuals of higher income level. Presumably, lower income may be a proxy for another unseen risk factor such as environmental exposures related to the work being done (with more poorly compensated work being associated with worse work conditions/environments). While the relationship between income level and the awareness of CKD among South Koreans has not been well-studied, our observations suggest that there may be limited understanding and awareness of the risk factors for and prevention of CKD in the broader population; indeed, previous research has shown that there is low awareness of CKD among South Koreans with an eGFR ≥60 ml/min/1.73 m2.46 Notably, Chin et al.46 reported that the World Kidney Day campaign had a positive impact on augmenting awareness and attention towards CKD and its risk factors among South Korean citizens. Hence, improving CKD awareness may be a critical and high-yield target for clinicians, policy makers, and regulatory bodies in improving the health and survival of the broader population.
The strengths of this study include its availability of detailed patient-level information on socio-demographics, comorbidities, and laboratory data from a large national cohort of 7.4 million Korean adults, which captures 30% of the nation’s adult population 40 years and older (i.e., largest study conducted to date); granular examination of income levels thresholds; utilization of two rigorous definitions of CKD; and comprehensive adjustment for potential confounders of the income level-incident CKD association.
However, several limitations of our study bear mention. First, potential selection bias cannot be excluded given our restriction of analyses to individuals age 40 years and older. However, separate examination of these associations in younger adults (i.e., 20–40 years of age) may be warranted given their differential patterns of kidney disease and low prevalence of CKD risk factors (e.g., hypertension and diabetes) as compared with middle-aged or older adults.47, 48 In addition, we included only individuals with employee’s insurance status, and individuals of self-employed insurer and medical aid beneficiary status were excluded given that in these latter groups the Korean NHIS applies different insurance premium rates and do not have accurate information on their wealth/assets. As the studies of wealth level with risk of CKD in these groups are important given their likely socio-economically deprived status, we are presently collecting the relevant information on these populations which will be the focus of corollary studies. Second, income levels were only assessed at study entry (i.e., baseline) and change in income level over the study period was not considered. However, the likelihood of economic status substantially changing during the five-years of follow-up in our study is unlikely. Third, due to data limitations, we lacked information regarding individuals’ education levels, another important marker of SES that has been associated with CKD outcomes,27, 33, 49 and the relationship of which may potentially differ among countries.28 Fourth, owing to the huge data size, we were able to ascertain only comorbidities and medication history during one year before the study entry. In this regards, it is possible that comorbid disease burden was under-estimated. Finally, our findings may not be generalizable to populations outside of South Korea, given the social factors, environmental exposures, national healthcare policies, and chronic disease burden that may be distinct from other countries.
CONCLUSION
In conclusion, we found a robust relationship between incrementally lower income levels and increasingly higher risk of incident CKD. While risk of CKD was mitigated among individuals with higher income level, we did not observe reduction in risk among those above the median income level. Our study’s findings highlight the importance of income level an individual’s risk of incident CKD, and the need for focused interventions upon high-risk (i.e., low income) populations which may have substantial impact upon improving outcomes and reducing health care costs at a population-level.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Korean National Health Insurance Service for access to their data (database no. NHIS-2018–1-206). Parts of this study were presented in abstract form at the 39th Annual Meeting of the Korean Society of Nephrology, Seoul, Korea, 23–26 May 2019.
Funding Source:
This work was supported by a grant (NHIMC 2017–12-013) funded by National Health Insurance Service Medical Center, Ilsan Hospital. The funding source had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript. KKZ is supported by NIH (NIDDK) grants K24-DK091419, U01-DK102163, and philanthropic grants from Mr. Harold Simmons, Mr. Louis Change, Joseph Lee, and AVEO, Inc. CMR is supported by NIH (NIDDK) grants K23-DK102903 and R03-DK114642.
Abbreviations and Acronyms:
- aHR
adjusted hazard ratio
- CKD
chronic kidney disease
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- ESRD
end stage renal disease
- HR
hazard ratio
- IQR
inter-quartile range
- NHIS
National Health Insurance Service
- SES
socioeconomic status
Footnotes
Conflict of interest disclosure: All authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease -A Systematic Review and Meta-Analysis. PLoS One. 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas B, Matsushita K, Abate KH, et al. Global Cardiovascular and Renal Outcomes of Reduced GFR. J Am Soc Nephrol. 2017;28(7):2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. [DOI] [PubMed] [Google Scholar]
- 5.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet.2017;389(10075):1238–1252. [DOI] [PubMed] [Google Scholar]
- 6.Jha V, Garcia-Garcia G, Iseki K, Li Z, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. [DOI] [PubMed] [Google Scholar]
- 7.World Kidney Day: Chronic Kidney Disease. 2015. http://www.worldkidneyday.org/faqs/chronic-kidney-disease. Assessed November 15, 2018.
- 8.Allen L, Williams J, Townsend N, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Health. 2017;5(3):e277–e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lago S, Cantarero D, Rivera B, et al. Socioeconomic status, health inequalities and non-communicable diseases: a systematic review. Z Gesundh Wiss. 2018;26(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas T, Islam MS, Linton N, Rawal LB. Socio-Economic Inequality of Chronic Non-Communicable Diseases in Bangladesh. PLoS One. 2016;11(11):e0167140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Report to the WHO Regional Office for the Western Pacific. http://www.wpro.who.int/publications/docs/WHOSESFINALforupload.pdf. Assessed November 15, 2018..
- 12.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51(4):563–572. [DOI] [PubMed] [Google Scholar]
- 14.Dodd R, Palagyi A, Guild L, Jha V, Jan S. The impact of out-of-pocket costs on treatment commencement and adherence in chronic kidney disease: a systematic review. Health Policy Plan. 2018;33(9):1047–1054. [DOI] [PubMed] [Google Scholar]
- 15.Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63–71. [DOI] [PubMed] [Google Scholar]
- 16.Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014;38(5):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Insurance statitical year book 2015. National Health Insurance Service, Health Insurance Review & Assessment Service, Seoul. http//www.nhis.or.kr/bbs7/boards/B0074/17042. Assessed November 15, 2018..
- 19.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. [DOI] [PubMed] [Google Scholar]
- 20.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. [DOI] [PubMed] [Google Scholar]
- 21.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Alrifai A, Gosmanova EO, et al. Age and Outcomes Associated with BP in Patients with Incident CKD. Clin J Am Soc Nephrol. 2016;11(5):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chetty R, Stepner M, Abraham S, et al. The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA. 2016;315(16):1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringhini S, Carmeli C, Jokela M, et al. Socioeconomic status, non-communicable disease risk factors, and walking speed in older adults: multi-cohort population based study. BMJ. 2018;360:k1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assari S, Lankarani MM. Income Gradient in Renal Disease Mortality in the United States. Front Med (Lausanne). 2017;4:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce MA, Beech BM, Crook ED, et al. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2010;55(6):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton RL, Schlackow I, Staplin N, et al. Impact of Educational Attainment on Health Outcomes in Moderate to Severe CKD. Am J Kidney Dis. 2016;67(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vart P, Gansevoort RT, Coresh J, Reijneveld SA, Bultmann U. Socioeconomic measures and CKD in the United States and The Netherlands. Clin J Am Soc Nephrol. 2013;8(10):1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krop JS, Coresh J, Chambless LE, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159(15):1777–1783. [DOI] [PubMed] [Google Scholar]
- 30.Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med. 2007;65(4):809–821. [DOI] [PubMed] [Google Scholar]
- 31.Crews DC, McClellan WM, Shoham DA, et al. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. Am J Kidney Dis. 2012;60(5):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peralta CA, Ziv E, Katz R, et al. African ancestry, socioeconomic status, and kidney function in elderly African Americans: a genetic admixture analysis. J Am Soc Nephrol. 2006;17(12):3491–3496. [DOI] [PubMed] [Google Scholar]
- 33.Vart P, Grams ME, Ballew SH, Woodward M, Coresh J, Matsushita K. Socioeconomic status and risk of kidney dysfunction: the Atherosclerosis Risk in Communities study. [published online Jun 11, 2018]. Nephrol Dial Transplant. doi: 10.1093/ndt/gfy142 [DOI] [PubMed] [Google Scholar]
- 34.Lee JC. Health care reform in South Korea: success or failure? Am J Public Health. 2003;93(1):48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutiérrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21(11):1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutiérrez OM, Isakova T, Enfield G, Wolf M. Impact of poverty on serum phosphate concentrations in the Third National Health and Nutrition Examination Survey. J Ren Nutr. 2011;21(2):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutiérrez OM, Katz R, Peralta CA, de Boer IH, Siscovick D, Wolf M, et al. Associations of socioeconomic status and processed food intake with serum phosphorus concentration in community-living adults: the Multi-Ethnic Study of Atherosclerosis (MESA). J Ren Nutr. 2012;22(5):480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutiérrez OM. Contextual poverty, nutrition, and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications. 2003;17(1):39–58. [DOI] [PubMed] [Google Scholar]
- 42.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21(4):518–524. [DOI] [PubMed] [Google Scholar]
- 43.Sampson UK, Edwards TL, Jahangir E, et al. Factors associated with the prevalence of hypertension in the southeastern United States: insights from 69,211 blacks and whites in the Southern Community Cohort Study. Circ Cardiovasc Qual Outcomes. 2014;7(1):33–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Signorello LB, Schlundt DG, Cohen SS, et al. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97(12):2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53(1):166–174. [DOI] [PubMed] [Google Scholar]
- 46.Chin HJ, Ahn JM, Na KY, et al. The effect of the World Kidney Day campaign on the awareness of chronic kidney disease and the status of risk factors for cardiovascular disease and renal progression. Nephrol Dial Transplant. 2010;25(2):413–419. [DOI] [PubMed] [Google Scholar]
- 47.Mahmood U, Healy HG, Kark A, et al. Spectrum (characteristics) of patients with chronic kidney disease (CKD) with increasing age in a major metropolitan renal service. BMC Nephrol. 2017;18(1):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neild GH. Primary renal disease in young adults with renal failure. Nephrol Dial Transplant. 2010;25(4):1025–1032. [DOI] [PubMed] [Google Scholar]
- 49.Choi AI, Weekley CC, Chen SC, et al. Association of educational attainment with chronic disease and mortality: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2011;58(2):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.