Abstract
Central oxytocin potently reduces food intake and is being pursued as a clinical treatment for obesity. While sexually dimorphic effects have been described for the effects of oxytocin on several behavioral outcomes, the role of sex in central oxytocin modulation of feeding behavior is poorly understood. Here we investigated the effects of sex, estrous cycle stage, and female sex hormones (estrogen, progesterone) on central oxytocin-mediated reduction of food intake in rats. Results show that while intracerebroventricular (ICV) oxytocin potently reduces chow intake in both male and female rats, these effects were more pronounced in males than in females. We next examined whether estrous cycle stage affects oxytocin’s food intake-reducing effects in females. Results show that ICV oxytocin administration significantly reduces food intake during all estrous cycle stages except proestrous, suggesting that female sex hormones may modulate the feeding effects of oxytocin. Indeed, additional results reveal that estrogen, but not progesterone replacement, in ovariectomized rats abolishes oxytocin-mediated reductions in chow intake. Lastly, oxytocin receptor mRNA (Oxtr) quantification (via quantitative PCR) and anatomical localization (via fluorescent in situ hybridization) in previously established sites of action for oxytocin control of food intake revealed comparable Oxtr expression between male and female rats, suggesting that observed sex and estrous differences may be based on variations in ligand availability and/or binding. Overall, these data show that estrogen reduces the effectiveness of central oxytocin to inhibit food intake, suggesting that sex hormones and estrous cycle should be considered in clinical investigations of oxytocin for obesity treatment.
Keywords: feeding, obesity, estrogen, progesterone, oxytocin receptor, hypothalamus
Introduction
Obesity is a growing national health concern in the Unites States with rates exceeding one third of the adult population (Dietz WH, 2015;Flegal KM et al., 2016). While reduced physical activity may be contributing, excessive caloric intake is the primary determinant driving the obesity epidemic (Bray GA et al., 2004;Gonzalez-Muniesa P et al., 2017;Hill JO and Peters JC, 1998). Significant advances have been made toward developing effective obesity pharmacotherapies that reduce the excess consumption of calories; however, the vast majority of preclinical research has been conducted in male rodents (Zakiniaeiz Y et al., 2016;Zucker I and Beery AK, 2010). This is particularly problematic in light of emerging evidence that the neurobiology regulating feeding behavior is sexually dimorphic (Asarian L and Geary N, 2013;Sample CH and Davidson TL, 2018), and thus understanding the underlying mechanisms through which pharmacological treatments act in both males and females to affect feeding is important in developing effective obesity treatments. One neuropeptide currently under clinical investigation for the treatment of obesity is oxytocin. There is abundant evidence that central administration of oxytocin rapidly and potently reduces food intake (Altirriba J et al., 2014; Blevins JE and Baskin DG, 2015;Blevins JE et al., 2015;Ding C et al., 2019;Labyb M et al., 2018;Lawson EA et al., 2015;Maejima Y et al., 2011;Morton GJ et al., 2012;Olszewski PK et al., 2017;Thienel M et al., 2016). Consistent with these results, oxytocin null (−/−) mice demonstrate enhanced initial and sustained sucrose consumption in comparison to WT mice (Amico JA et al., 2005;Leng G and Sabatier N, 2017). Additional preclinical evidence from animal model studies suggests that oxytocin reduces feeding without adverse side effects such as nausea and malaise (Herisson FM et al., 2016;Noble EE et al., 2014). While it is known that oxytocin plays an important role in many sex-specific behaviors (e.g., lactation, parturition) (Kendrick KM, 2000;Nishimori K et al., 1996) and has sexually dimorphic effects on various social behaviors (Borland JM et al., 2019;Scott N et al., 2015), it is poorly understood as to whether there are sex differences in oxytocin’s regulation of feeding behavior.
The neuropeptide oxytocin is produced in the supraoptic nucleus and paraventricular nucleus of the hypothalamus (Lee HJ et al., 2009). Oxytocin has a multitude of behavioral effects, including modulating social behavior, stress responsivity, and energy balance (Campbell A, 2008;Dolen G et al., 2013;Ferguson JN et al., 2001;Ferguson JN et al., 2000;Hung LW et al., 2017;Leng G and Sabatier N, 2017;Reppucci C et al., 2018;Ross HE and Young LJ, 2009;Sabatier N et al., 2013) (see (Onaka T et al., 2012) for review). Early evidence for oxytocin’s function in food intake control revealed that either intraperitoneal (IP) or intracerebroventricular (ICV) administration of oxytocin significantly reduces food intake in male rats (Arletti R et al., 1989). Additionally, oxytocin neurons are activated by food consumption and contribute to meal termination in male rats (Hume C et al., 2017;Johnstone LE et al., 2006), whereas either suppressing exocytosis of oxytocin or genetic reduction of oxytocin expression increases food intake (Kublaoui BM et al., 2008;Zhang G et al., 2011). Furthermore, oxytocin receptor (Oxtr)-deficient male mice show late-onset obesity, reduced thermogenesis, and decreased energy expenditure (Leng G et al., 2008;Wu Q et al., 2012). While together these data implicate oxytocin as a viable candidate for obesity pharmacotherapy in males, a dearth of female preclinical oxytocin data render it unclear as to whether oxytocin is similarly effective in females. Therefore, the current study examined the hypothesis that oxytocin differentially regulates food intake in male and female rats, and that observed sex differences are driven, in part, by the female sex hormones estrogen and progesterone.
Experimental Procedures
Animals
Male and female Sprague-Dawley rats (Envigo, Indianapolis, IN; weighing 320–450 g) were individually housed in wire hanging cages in a temperature controlled (22–23°C) vivarium with ad libitum access to water and chow (LabDiet 5001, LabDiet, St. Louis, MO; except where noted). Rats were either on a 12hr:12hr light/dark cycle (lights on at 0600h) or reverse light/dark cycle (lights off at 1000h). All procedures were approved by the Institute of Animal Care and Use Committee at the University of Southern California.
Surgery
For all surgical procedures, rats were anesthetized and sedated via intramuscular injections of ketamine (90mg/kg), xylazine (2.8mg/kg), and acepromazine (0.72mg/kg) and were given analgesic (a subcutaneous injection of ketaprofen [5 mg/kg] after surgery and once daily for 3 subsequent days thereafter). All animals were allowed to recover for at least one-week post-surgery prior to experiments.
Intracerebroventricular (ICV) cannulation surgery
Rats were surgically implanted with indwelling guide cannula (26-gauge, Plastics One, Roanoke, VA) targeting the lateral ventricle using the following stereotaxic coordinates, which are relative to the location of bregma, with dorsal/ventral (DV) coordinates relative to the skull surface at the cannula implantation site (Paxinos and Watson, 2007): −0.9 mm anterior/posterior (AP), +1.8 mm medial/lateral (ML), and −2.6 mm DV. Cannula were affixed to the skull as previously described using jeweler’s screws and dental cement (Hsu TM et al., 2015). Following 7 days of recovery, cannula placement was evaluated via measurement of the cytoglucopenia-induced sympathoadrenal-mediated glycemic effect that occurs from 210 µg (in 2µl) of 5-thio-D-glucose (5TG) (Ritter RC et al., 1981). Food was removed 1 hour prior to the placement test. Animals were injected with 5TG via injectors that extended 2 mm beyond the end of the guide cannula, and blood glucose was measured at 30, 50, 90, and 120 min post-injection. Placement was deemed correct if blood glucose doubled within the measurement period. For animals who did not pass the placement test the injector length was adjusted until the correct placement was achieved (based on the 5TG test) and the adjusted injector length was used for that animal in all subsequent experiments.
Ovariectomies and implantation surgeries
Immediately prior to ICV cannulation surgery, female rats (experiment 3) were bilaterally ovariectomized (OVX) via dorsal flank incisions. Bilateral 2 mm incisions were made, ovaries were removed, and fallopian tubes were sutured, as previously described (Santollo J and Eckel LA, 2008;Wood RI and Rice R, 2013).
After a 1-week recovery period, females received either subcutaneous 5 mm empty sham Silastic implants or implants filled with crystalline 17ß-estradiol (Millipore, Burlington, MA) with the ends sealed with silicone adhesive (Schauwecker PE et al., 2009;Wood RI and Rice R, 2013). Estradiol is the major form of estrogen necessary for development and reproduction and has been shown to interact with oxytocin function in the brain to influence behavior (Jirikowski GF et al., 1988;Johnson AE et al., 1989). Females were allowed to recover from surgery for three weeks before testing.
Experiment 1: Effect of ICV oxytocin on food intake in male and randomly-cycling female rats
The effect of ICV oxytocin on standard chow intake was tested in males and randomly-cycling females (n=16/sex) using a within-subjects, counterbalanced design. Food was removed from the home cage 1 hr prior to injection, which occurred 10 min prior to the onset of the dark cycle. Rats were injected ICV with a volume of 1 µl containing a dose of either 0.3, 1, or 2 µg of oxytocin (Bachem, Torrance, CA) or artificial cerebrospinal fluid (aCSF; vehicle) via 33-gauge microsyringe injector. Injectors remained in place for 30 sec to allow for complete drug delivery. Food was weighed and returned to the home cage at dark onset and spill papers were placed underneath the suspended wire-bottom cages to collect crumbs for precise food intake measures. Food intake was measured at 30 min, 1 hr, 2 hr and 24 hr post injection, and cumulative food intake was calculated by subtracting the hopper weights at each time point from the food hopper weight at baseline (accounting for spillage).
Experiment 2: Estrous cycle effects on oxytocin-induced inhibition of food intake
Estrous cycles were monitored daily in a cohort of female rats (n=15) for one week prior to the start of the experiment. To identify estrous stage, vaginal cytology was investigated by vaginal lavage (Becker JB et al., 2005;Ekambaram G et al., 2017;McLean AC et al., 2012). Cytology samples were collected in 0.9% sterile saline (Teknova) 1.5 hr prior to the start of the dark cycle. A standard laboratory microscope (AmScope) was used to examine vaginal smears at 10X. Stages were assigned at the same time each day to minimize the incidence of transitional and ‘missed’ stages and were based on vaginal cell morphology (presence or absence of epithelial cells, cornified cells, and leucocytes) as described in (Becker JB,Arnold AP,Berkley KJ,Blaustein JD,Eckel LA,Hampson E,Herman JP,Marts S,Sadee W,Steiner M,Taylor J and Young E, 2005).
Rats received a 1 µl ICV injection of either 1 µg dose of oxytocin or aCSF using a within-subjects design such that each rat received each treatment once during each estrous stage (8 total treatments per animal). Treatments were separated by at least 2 days and counterbalanced such that no two treatments per stage occurred in a row. As in Experiment 1, food hoppers were removed from the home cage 1 hr prior to injections, and injections occurred 10 min prior to dark onset. Following injection, food hoppers were returned at dark onset and 30 min, 1 hr, 2 hr, and 24 hr cumulative chow intake (accounting for spillage) was measured as described in Experiment 1.
Experiment 3: Effect of estrogen and progesterone on oxytocin-induced inhibition of food intake
Female rats (n=16) were ovariectomized via bilateral dorsal flank incision, and indwelling cannula targeting the lateral ventricle were implanted as described in surgical methods above. Following a one-week surgical recovery period, rats received subcutaneous 5 mm Silastic implants filled with 17ß-estradiol (Millipore, Burlington, MA) or empty (sham) implants (n=8/group). These chronic Silastic implants slowly release physiological levels of estrogen (Wood RI and Rice R, 2013). After three weeks of surgical recovery, we utilized a counterbalanced mixed design, where ovariectomized female rats with either estrogen (n=8) or sham (n=6) implants were subdivided to receive 4 days of progesterone or vehicle injections (n=3 or 4/implant type) using a protocol modified from (Leibowitz SF et al., 2007). Using a within-subjects design, progesterone (Sigma-Aldrich, St. Louis, MO) in sesame oil (0.5 mg/0.2 mL) or sesame oil alone (vehicle) was subcutaneously injected 2 hrs prior to dark cycle onset. On days 2 and 4 of progesterone or vehicle injections, rats received 1 µl ICV injection of 1 µg dose of oxytocin or vehicle 10 min prior to dark cycle onset. Food intake was measured at 30 min, 1 hr, 2 hr, and 24 hrs (accounting for spillage) as described above.
Experiment 4a: Quantification of oxytocin receptor mRNA (Oxtr) expression in nucleus accumbens (ACB), ventromedial nucleus of the hypothalamus (VMH), and nucleus tractus solitarius (NTS) in male and female rats
To determine whether Oxtr expression varies as a function of sex or estrous stage, Oxtr gene expression was quantified in male (n=7) and female (n=8) rats. Vaginal cytology for female rats was examined daily for 1 week and on the day of tissue collection animals were categorized into either the proestrous/estrous stage (n=4) or metoestrous/diestrous stage (n=4). Prior to sacrifice, rats were 24 hr fasted and then given 5 g chow for re-feeding at the onset of the dark cycle. Thirty minutes after presentation of the meal, tissue was collected. Animals were first anesthetized and sedated via intramuscular injections of ketamine as described above, then decapitated. Brains were rapidly removed from the skull and flash frozen in −30°C isopentane on dry ice. Brains were then stored at −80°C until further analyses. Tissue punches of brain regions of interest (0.8 mm circumference) were collected using the Leica CM 1860 cryostat (Wetzlar, Germany) and anatomical landmarks were based on the Swanson rat brain atlas (Swanson LW, 2018). Tissue punches were collected from the nucleus accumbens (ACB; atlas levels 9–10), ventromedial nucleus of the hypothalamus (VMH; atlas levels 27-29), and nucleus tractus solitarius (NTS; atlas levels 69–70). Total RNA was extracted according to manufacturer’s instructions using RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). RNA was reverse transcribed to cDNA using the Quantitect Reverse Transcription Kit (Qiagen). qPCR was performed with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) using the BIO-RAD CFX96 Optics Model realtime PCR machine. Negative reverse-transcribed samples were generated and all reactions were carried out in triplicate. The following TaqMan probes were used: Otxr: Rn00563503_m1, Gapdh: Rn01775763_g1. To determine relative expression values, the 2−ΔΔCT method was used, where triplicate Ct values for each sample were averaged and subtracted from those derived from GAPDH as previously described (Davis EA et al., 2018).
Experiment 4b: Fluorescence in situ hybridization for Otxr expression in ACB, VMH, and NTS in male and female rats
Representative images for the relative anatomical locations within brain regions of interest (ACB, VMH, mNTS) were obtained using fluorescence in situ hybridization (FISH). Briefly, male and female rats were anesthetized and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5). Brains were post-fixed for 24 hours at 4°C in 12% sucrose in 4% paraformaldehyde, then flash-frozen in isopentane prior to sectioning on a freezing stage microtome at 30 μm. Serial sections were collected and stored in antifreeze solution at −20°C until further analyses. FISH protocols were conducted as previously described (Noble EE et al., 2018;Suarez AN et al., 2018) and utilized an Otxr (Advanced Cell Diagnostics; Newark, CA) probe. Briefly, tissue sections were mounted on subbed glass slides (Fisher brand Superfrost Plus) and placed in a vacuum desiccant chamber overnight (~16 hrs). Slides were postfixed in 4% PFA for 1hr 45 min, washed 5 x for 5 min in KPBS, placed in solution of 100 mM Tris (pH 8), 50 mM EDTA (pH 8), and 0.1% Proteinase K (10mg/mL, Sigma P2308) in water for 30 min, and then rinsed for 3 min in the same Tris and EDTA solution without Proteinase K all at 37°C. Slides were rinsed in 100mM triethanolamine (pH 8) in water for 3 min, incubated in 0.25% acetic anhydride in 100mM triethanolamine for 10 min at room temperature, and rinsed twice in 10% 20X saline-sodium citrate buffer for 2 min. Slides were then dehydrated in increasing concentrations of ethanol (50%, 70%, 95%, 100%, 100%) and air dried. Probes were applied to slides and incubated for 2 hrs for hybridization. Reagents from RNAscope® Fluorescent Multiplex Detection Reagent Kit V2 (ACD, 323119) were used to amplify the probe. Otxr signal was developed by incubating with the appropriate channel probe for 15 min, with the Cy3 fluorophore diluted 1:500 in TSA Buffer (ACD, 322809) for 30 min, and then with the horseradish peroxidase (HRP) blocker for 30 min. Between each incubation, slides were washed with RNAscope® Wash Buffer 1X (ACD, 320058) for 2 min. Lastly, slides were coverslipped with ProLong® Gold Antifade Reagent (Cell Signaling, 9071S). Representative images for Otxr expression in the ACB, VMH, and NTS were confined to Swanson Atlas levels 9–10, 27–29, and 69–70, respectively.
Statistical analyses
Except where noted, experiments were analyzed using repeated measures two-way analysis of variance (ANOVA) or mixed design two-way ANOVA. To adjust for multiple comparisons, post-hoc analyses were conducted with Tukey’s Honestly Significant Difference when significant main effects or interactions were obtained. For experiment 1, intake data were also analyzed with a repeated measures three-way ANOVA including time as a repeated-measures variable, and a linear regression for magnitude of 30-min food intake suppression of oxytocin (1 µg vs. vehicle) against body weight was conducted. For experiment 4, qPCR data were analyzed using an unpaired two-sample Student’s t-test. Statistical analyses were performed using Statistica (V7; StatSoft, Inc.) using an α level for significance of p<.05.
Results
ICV oxytocin’s anorexigenic effects are more pronounced in male vs. female rats
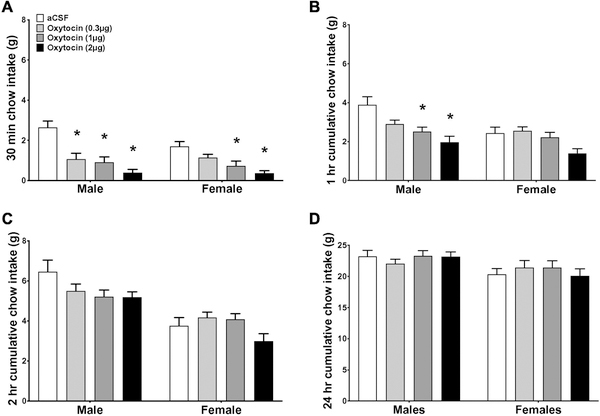
The efficacy of oxytocin to reduce food intake was compared in male and randomly-cycling female rats. When time was included as a variable, a repeated measures, 3-way ANOVA of non-cumulative intake revealed that there was no significant time x dose x sex interaction (F(9,261) = 0.44; p = 0.911). Since there was no time x oxytocin dose x sex interaction, cumulative intake at separate time points was examined for all other analyses. At the 30-min time point, a 2-way ANOVA with dose as a repeated measures variable revealed a significant dose x sex interaction (F (3,84) = 4.55, p < 0.01) and a significant main effect of dose (F(3,84) = 30.72, p < 0.0001). Post-hoc analyses reveal that ICV injection of oxytocin reduced 30 min food intake in male rats at all three doses given (0.3, 1, and 2 µg), but only the 1 and 2 µg dose reduced chow intake in randomly-cycling females (Fig. 1A; ps<0.05). Furthermore, the food intake suppressive effects of the 1 and 2 µg doses were sustained at 1 hr in male rats (ps<0.05), but not in randomly-cycling females (Fig. 1B). At 2 hrs post injection, ICV oxytocin was no longer effective at reducing intake in either males or females (Fig. 1C and 1D). A linear regression of the magnitude of suppression of oxytocin (1 µg; 1 hr vehicle intake minus 1 hr oxytocin intake) against body weight revealed no significant relationship between the magnitude of food intake reduction by oxytocin and body weight at the time of treatment (adjusted R2=0.07, p>0.05 for both sexes, adjusted R2=−0.04, p>0.05 males only, adjusted R2=−0.03, p>0.05 females only). Collectively, these results indicate that the efficacy of central oxytocin to reduce chow intake is not dependent on body weight and is greater in males than in female rats, as evident from results revealing that higher doses of oxytocin are required to reduce chow intake in females vs. males, as well as data revealing a longer duration of intake reduction in males vs. females following administration of doses that were effective in both sexes.
Figure 1:
Sex differences comparison for the effect of oxytocin on food intake for normal dark onset feeding. Effect of intracerebroventricular injection of 0 (aCSF), 0.3, 1, or 2 µg of oxytocin on cumulative chow intake in grams at 30 min (a), 1 hr (b), 2 hrs (c) and 24 hrs (d) post injection. Data are means ± SEM; *p<0.05.
Effects of ICV oxytocin on chow intake differ throughout the female estrous cycle
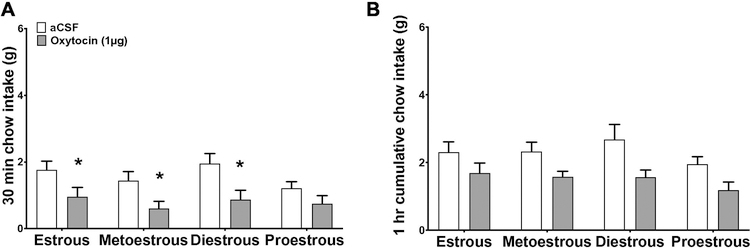
Since the efficacy of oxytocin was lower in female rats than in males, we reasoned that female sex hormones may affect oxytocin signaling. To investigate whether estrous stage alters the capacity of ICV oxytocin to reduce chow intake, female rats were injected with ICV oxytocin during each of the 4 stages of the rodent estrous cycle using a within-subjects design. A 2-way ANOVA of 30-min cumulative intake shows that there was no stage x oxytocin interaction (F(1,13) = 0.72, p > 0.05). There was a significant main effect of oxytocin (F(1,13) = 19.91, p < 0.01), but no main effect of stage (F(1,13) = 3.37, p > 0.05). However, post hoc analyses revealed that ICV oxytocin reduced 30-min chow intake in the estrous, metoestrous, and diestrous stages (p < 0.05), but not in the proestrous stage (Fig. 2A). There was no main effect of oxytocin for 1-hr cumulative intake.
Figure 2:
Comparison of the effects of oxytocin on dark onset food intake during different stages of the estrous cycle in naturally-cycling females. Effect of intracerebroventricular injection of 0 (aCSF), or 1 µg of oxytocin on cumulative chow intake in grams at 30 min (a), or 1 hr (b) post injection. Data are means ± SEM; *p<0.05.
Estrogen, but not progesterone replacement reduces the efficacy of central oxytocin to reduce chow intake
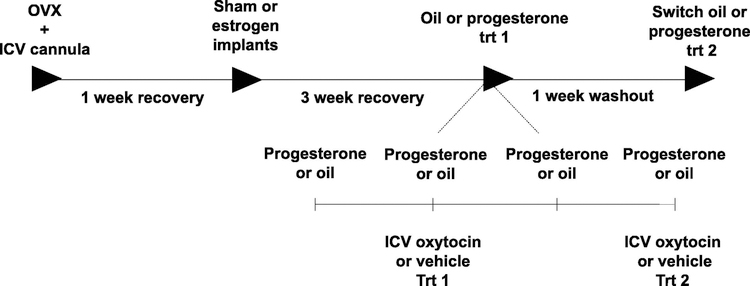
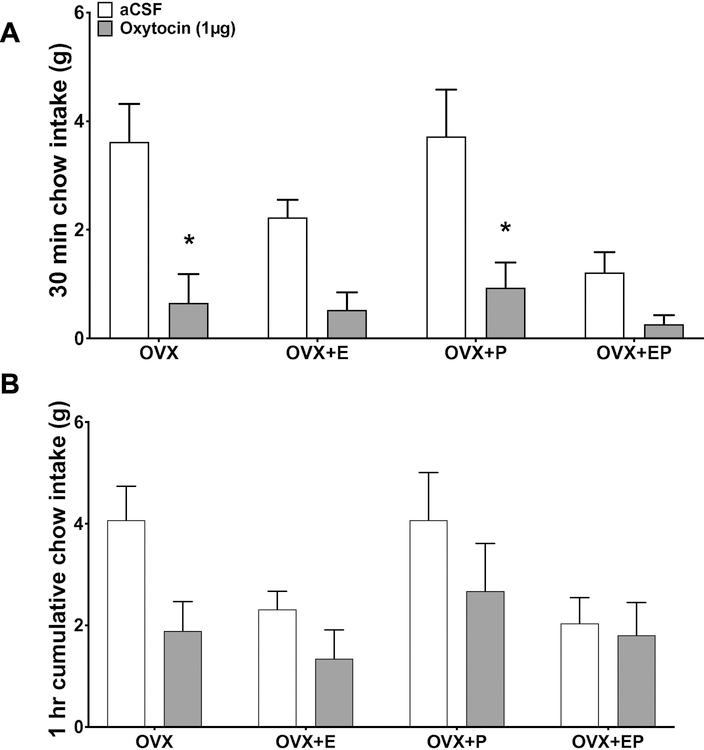
As estrogen and progesterone both surge together during the proestrous phase of the rat estrous cycle (Bauer-Dantoin AC et al., 1995), which is the stage in which the capacity for oxytocin to reduce chow intake in randomly cycling females was diminished in Exp. 2, we next tested whether estrogen in combination with progesterone alters the effectiveness of oxytocin to reduce chow intake in OVX female rats (timeline in Fig. 3). Results revealed that at 30 min post injection there was a significant oxytocin x estrogen interaction (F(1,12) = 7.56, p < 0.05), but neither the oxytocin x progesterone nor the oxytocin x estrogen x progesterone interactions were significant. These data suggest that estrogen modulates the effects of oxytocin on feeding, but progesterone does not, nor does progesterone influence estrogen’s effects on oxytocin-mediated food intake reduction (Fig. 4A). There was a significant main effect of both estrogen and oxytocin for food intake at 30 min (main effect of implant: F(1,12) = 9.25, p < 0.05; main effect of oxytocin (F(1,12) = 55.51; p < 0.0001) (Fig. 4A). Post hoc analyses revealed that ICV oxytocin reduced 30-min food intake for the OVX (sham implant + oil) and for the OVX+P (sham implant + progesterone treatment) treatments (ps < 0.05), but did not reduce intake for OVX+E (estrogen + oil) or OVX+EP (estrogen + progesterone) treatments (Fig. 4C). Oxytocin had no effect at 1 hr post injection for any treatment (Fig. 4B). Overall, estrogen, but not progesterone, interacts with oxytocin in the reduction of food intake.
Figure 3:
Timeline of oxytocin treatment in ovariectomized female rats with and without estrogen (E) and progesterone (P) replacement. All females received bilateral ovariectomies, and after one-week recovery, females received either sham or estrogen implants. After an additional three-week recovery period, females underwent 4 days of progesterone or oil treatment, and effect of ICV oxytocin on food intake was measured. After an additional 1-week washout, progesterone or oil treatment groups were switched and effect of ICV oxytocin on chow intake was examined.
Figure 4:
Comparison of the effects of oxytocin on dark onset food intake in ovariectomized female rats with and without estrogen (E) and progesterone (P) replacement. Effect of intracerebroventricular injection of 0 (aCSF), or 1 µg of oxytocin on cumulative chow intake in grams at 30 min (a), or 1 hr (b) post injection. Data are means ± SEM; *p<0.05.
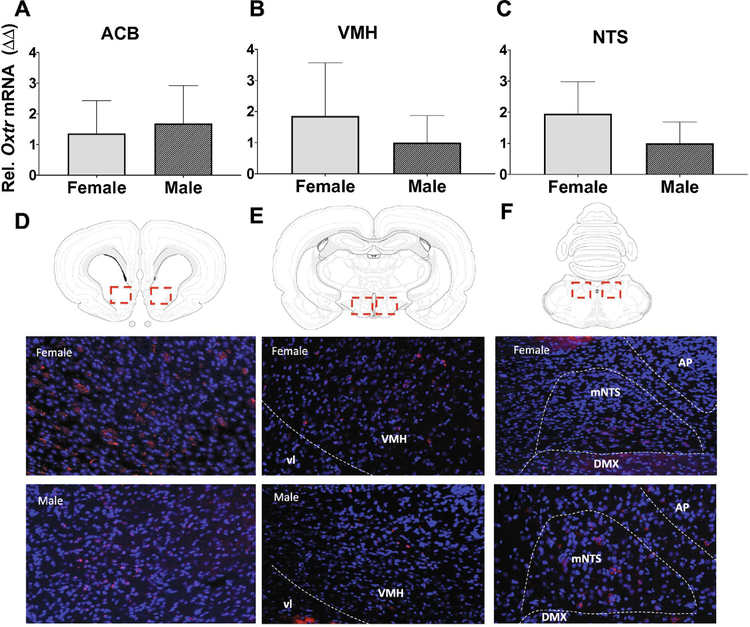
Oxtr expression does not differ in male and female rats in the ACB, VMH, or NTS
The nucleus accumbens (ACB) (Herisson FM,Waas JR,Fredriksson R,Schioth HB,Levine AS and Olszewski PK, 2016), ventromedial nucleus of the hypothalamus (VMH) (Noble EE,Billington CJ,Kotz CM and Wang C, 2014), and nucleus tractus solitarius (NTS) (Ong ZY et al., 2015;Ong ZY et al., 2017) have previously been shown to be sites of action for oxytocin-induced inhibition of food intake. Thus, we hypothesized that relative levels and anatomical distribution of Oxtr expression in these brain regions might differ between male and female rats, and by estrous cycle stage. To assess whether there are quantitative differences in Oxtr expression in males and females we performed quantitative polymerase chain reaction (qPCR) in tissue punches from these brain regions. qPCR relative Oxtr mRNA expression did not significantly differ across female estrous stages in the ACB, VMH, and NTS, and thus all further analyses combined all female estrous stages for comparison with males. Results revealed no quantitative differences in Oxtr mRNA expression in the ACB, VMH, or NTS between males and females (Fig. 5A–5C). To further examine possible anatomical differences in Oxtr expression between males and females within these brain regions, fluorescence in situ hybridization (FISH) analyses revealed that both males and females express Oxtr in the medial core of the ACB (figure 5D), as well as in central part of the VMH (VMHc) (Fig. 5E). Furthermore, Oxtr expression was confined to the ventral portion of the medial NTS (mNTS) (immediately dorsal to the dorsal motor nucleus of the vagus [DMX]) in both males and females (figure 5F). Overall, Oxtr expression was quantitatively and qualitatively similar across female estrous stages, as well as between sexes.
Figure 5:
Expression of Oxtr in feeding-relevant neural sites. Relative Oxtr mRNA expression (top panels) in tissue punches from the nucleus accumbens (ACB; a), ventromedial hypothalamus (VMH; b), and nucleus tractus solitarius (NTS; c) from male and female rat brains. Representative images of coronal brain sections (bottom panels) showing fluorescence in situ hybridization for Oxtr (red) in the ACB (a), VMH (b), and NTS (c) with dapi counterstain (blue). Data are means ± SEM.
Discussion
Oxytocin has potent inhibitory effects on food intake in both humans and experimental animal models (Arletti R et al., 1990;Blevins JE,Graham JL,Morton GJ,Bales KL,Schwartz MW,Baskin DG and Havel PJ, 2015;Herisson FM,Waas JR,Fredriksson R,Schioth HB,Levine AS and Olszewski PK, 2016;Iwasaki Y et al., 2015;Klockars A et al., 2017;Klockars OA et al., 2018;Klockars OA et al., 2017;Lawson EA,Marengi DA,DeSanti RL,Holmes TM,Schoenfeld DA and Tolley CJ, 2015;Maejima Y,Iwasaki Y,Yamahara Y,Kodaira M,Sedbazar U and Yada T, 2011;Maejima Y et al., 2015;Maejima Y et al., 2018;Mullis K et al., 2013;Noble EE,Billington CJ,Kotz CM and Wang C, 2014;Ott V et al., 2013;Sclafani A et al., 2007;Seelke AM et al., 2018;Striepens N et al., 2016). Similarly, data from oxytocin null rodent models reveal that the absence of oxytocin leads to the development of obesity, hyperleptinemia, and decreased peripheral insulin sensitivity (Camerino C, 2009). Thus, oxytocin is a promising target for pharmacotherapy development for the treatment of obesity. However, few preclinical studies have directly examined whether sex differences should be a consideration for the efficacy of oxytocin as an obesity pharmacotherapy. Specifically, there is a paucity of research directly examining the food intake suppressive effects of oxytocin in male compared to female animals. Furthermore, sex differences in oxytocin’s behavioral effects have been reported for the regulation of social behavior (Benelli A et al., 1995;Engelmann M et al., 1998), anxiety-related behavior (Lukas M and Neumann ID, 2014;Lukas M et al., 2011) and stress (Borland JM et al., 2018;Bredewold R and Veenema AH, 2018;Minhas S et al., 2016), and these effects stem in part from interactions with sex hormones (estrogen, progesterone, testosterone (Amico JA et al., 2002;Johnson AE, 1992;Sloan DK et al., 2018;Yamashita M et al., 2013)). While these studies provide evidence that oxytocin has sexually dimorphic effects on behavior and that sex hormones influence oxytocin signaling in the brain, the interaction between female sex hormones and oxytocin in the control of food intake is not well understood. To address this gap in the literature, the present study sought to investigate potential sex differences in chow intake in male vs. randomly cycling female rats following central (ICV) oxytocin administration. Consistent with previous reports, our results reveal robust, short-term reduction of food intake in both male and female rats. We also found that the capacity for oxytocin to reduce food intake was attenuated in females compared with males, where lower doses were effective at reducing food intake in males, and doses that were effective in both sexes reduced intake for a longer duration in males.
The observed sex differences in oxytocin’s anorexigenic effects in the present study were not based on body weight differences between males and females, as there was no significant correlation between the magnitude of central oxytocin-induced food intake reduction and body weight at the time of the treatment. We note, however, that previous findings have observed differences in oxytocin’s food intake reducing efficacy in female rats when comparing two different strains of rats with varying growth rates (Bjorkstrand E and Uvnas-Moberg K, 1996), suggesting that there may be interactions between oxytocin and growth factors, and/or rodent strain. In humans, oxytocin administration also has a greater effect on food intake reduction in obese compared to normal-weight men (Thienel M,Fritsche A,Heinrichs M,Peter A,Ewers M,Lehnert H,Born J and Hallschmid M, 2016). Our study examined food intake in healthy lean rats, and thus it is important for future work to examine sex differences in oxytocin’s hypophagic effects in obese vs. lean rats.
Sex differences in oxytocin’s anorexigenic effects may be in part due to the modulatory effects of the estrous cycle and interactions between sex hormones and oxytocin expression, signaling, and/or receptor binding. Indeed, the presence of an estrogen response element in the promotor region of the oxytocin gene (Adan RA et al., 1993;Peter J et al., 1990;Quinones-Jenab V et al., 1997) suggests that these two systems may interact. Furthermore, sequential exposure to long-term (2 week) estradiol and progesterone treatment followed by withdrawal of progesterone treatment increases oxytocin mRNA in the paraventricular and supraoptic nucleus of the hypothalamus in OVX female rats, as well in in castrated male rats (Amico JA et al., 1995;Amico JA et al., 1997;Thomas A and Amico JA, 1996). Based on these findings, as well as our results that central oxytocin was more effective in reducing food intake in males compared to randomly-cycling females and the fact that the female ovarian hormones estrogen and progesterone are highest during the proestrous phase (Eckel LA, 2011;Geary N, 1998), we hypothesized that oxytocin would differ in effectiveness in reducing food intake during proestrous. Indeed, the present study found that ICV oxytocin significantly reduced chow intake in all stages, except the proestrous stage. Consistent with these findings, oxytocin’s food intake suppressive effects in OVX female rats were reduced by estrogen replacement, whereas progesterone did not have affect estrogen’s modulation of oxytocin.
Whether there are sex-differences and interactions with sex hormones in oxytocin-mediated inhibition of food intake in humans is poorly understood. A recent meta-analysis concluded that peripheral oxytocin levels increase from the early follicular phase to ovulation, followed by a decrease in levels from ovulation to the mid-luteal phase (Engel S et al., 2019). These results suggest that oxytocin may also interact with sex hormones in humans, however, the extent to which these effects are relevant to feeding behavior is unknown. Moreover, another study revealed that the pattern of oxytocin concentrations in circulation was minimally influenced by menstrual cycle or by sex (Challinor SM et al., 1994). Thus, further research is clearly required to systematically understand the influence of menstrual cycle and sex on oxytocin’s pharmacological food intake reduction in humans.
Collectively our results raise several possible questions. Firstly, like oxytocin, estrogen rapidly reduces food intake (Eckel LA, 2011;Eckel LA and Geary N, 2001;Geary N et al., 1995), and thus it could be possible that estrogen and oxytocin act through disparate (vs. convergent or interacting) mechanisms to reduce intake. Along these lines, it may be the case that the reduced effectiveness of oxytocin during proestrous and in OVX rats with estrogen replacement is based on lower baseline intake when estrogen levels are high, thus yielding a floor effect in which CNS oxytocin cannot augment an already low level of intake. However, a previous study showed that vehicle- or estrogen-treated OVX rats refed after 48 hr of fasting demonstrated increased activation in oxytocin-producing neurons, but that expression was lower in estrogen-treated animals in comparison with vehicle-treated controls (Lucio-Oliveira F and Franci CR, 2012). These results are consistent with the present findings and further suggest that estrogen may act by directly modulating oxytocin peptide expression at the neuronal level. Further argument against a floor effect vs. a direct interaction between central oxytocin and estrogen is that the 2 µg dose of oxytocin yielded a near complete suppression of 30 min intake in randomly cycling females, suggesting that baseline intake during proestrous (and with estrogen replacement) was not at a floor, but rather, that oxytocin under these conditions was less effective at reducing intake.
Another consideration is that additional sex hormones, such as prolactin, may contribute to sex differences in the effectiveness of oxytocin to control food intake. While the present results do not support a role for progesterone in interacting with oxytocin’s feeding effects, Yamashita et al. found that prolactin-releasing peptide-deficient mice had reduced refeeding-induced oxytocin overexpression (Takayanagi Y et al., 2008;Yamashita M,Takayanagi Y,Yoshida M,Nishimori K,Kusama M and Onaka T, 2013), highlighting a possible role for oxytocin and prolactin interaction in food intake control. Future studies are needed to determine the extent to which prolactin interacts with estrogenic systems to modulate the efficacy of oxytocin to reduce feeding.
Another consideration for the sex-differences in the observed oxytocin-mediated feeding effects could be due to shifts in focus in behavior, such as the stimulatory effects of oxytocin on maternal behavior in female rodents. For example, oxytocin acts as a stimulator of maternal behavior in virgin female rats (Pedersen CA et al., 1982;Pedersen CA and Prange AJ, Jr., 1979), where central administration of oxytocin to an estrogen-primed adult reduces response latencies towards fostering pups in virgin female rats. Thus, it is possible that oxytocin administration during proestrous or in OVX female rats with estrogen replacement led to increased maternal or female-specific behaviors, and that this drive competed with food-motivated behavior. However, we find this interpretation unlikely given that the female rats used in this study had never been exposed to pups. Further, the anorexigenic effects of central oxytocin were reduced in females vs. males, which is inconsistent with the interpretation that elevated competing behavioral drives in females (increased maternal drive) is the basis for the observed sex differences in oxytocin-mediated feeding effects.
We investigated the possibility that there may be sex differences in Oxtr expression in sites of action previously established to be relevant for oxytocin’s control of food intake (Herisson FM,Waas JR,Fredriksson R,Schioth HB,Levine AS and Olszewski PK, 2016;Noble EE,Billington CJ,Kotz CM and Wang C, 2014;Ong ZY,Alhadeff AL and Grill HJ, 2015;Ong ZY,Bongiorno DM,Hernando MA and Grill HJ, 2017). Similar to previous reports, our data show that Oxtr is expressed in the ACB, VMH, and the NTS in adult male and female rats. However, we did not observe any sex or estrous stage differences in Oxtr expression ACB, VMH, and mNTS. In addition, anatomical FISH analyses revealed that Oxtr is expressed in similar subregions within these nuclei across estrous stage and in males and females. More specifically, Oxtr was expressed predominantly within the medial core of the ACB, the central part of the VMH (VMHc), and the ventromedial portion of the mNTS. One additional consideration is that from the current data set, we cannot say whether sex hormones differentially affect binding affinity of the oxytocin receptor. Estrogen and progesterone have been previously shown to regulate Oxtr binding in human cell cultures (Amico JA,Rauk PN and Cai HM, 2002), as well as in the rodent brain in a site-specific manner (Tribollet E et al., 1990;Worley NB et al., 2019). Thus, when considering the present findings with these previous studies revealing female sex hormone influences on oxytocin binding, it is likely that oxytocin binding affinity and/or oxytocin neuropeptide expression mediate the sex differences observed in the present study. Future work is needed to directly investigate this hypothesis.
With rising obesity trends, there is an increased need to develop effective pharmacotherapies that curb food intake and inhibit excessive caloric intake. While significant advances have been made in ingestive behavior research in the past 30 years, there is a paucity of data in the neuropeptidergic regulation of food intake control in females, specifically (Zakiniaeiz Y,Cosgrove KP,Potenza MN and Mazure CM, 2016;Zucker I and Beery AK, 2010). Here we show that oxytocin differentially affects food intake in male and female rodents, and that circulating estrogen interacts with oxytocin by attenuating the effectiveness of oxytocin at reducing food intake.
Acknowledgement
This study was supported by the National Institute of Health grants: DK104897 (SK), DK118402 (SK), DK116558 (AS), DK118000 (EN), and DK118944 (CL). The authors have nothing to disclose.
Abbreviations
- ICV
Intracerebroventricular
- OXTR
Oxytocin receptor
- E
Estrogen
- P
Progesterone
- OVX
Ovariectomized
- ACB
Nucleus accumbens
- VMH
Ventromedial nucleus of the hypothalamus
- NTS
Nucleus tractus solitarius
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Adan RA, Cox JJ, Beischlag TV, Burbach JP (1993), A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Molecular endocrinology (Baltimore, Md) 7:47–57. [DOI] [PubMed] [Google Scholar]
- Altirriba J, Poher AL, Caillon A, Arsenijevic D, Veyrat-Durebex C, Lyautey J, Dulloo A, Rohner-Jeanrenaud F (2014), Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology 155:4189–4201. [DOI] [PubMed] [Google Scholar]
- Amico JA, Crowley RS, Insel TR, Thomas A, O’Keefe JA (1995), Effect of gonadal steroids upon hypothalamic oxytocin expression. Advances in experimental medicine and biology 395:23–35. [PubMed] [Google Scholar]
- Amico JA, Rauk PN, Cai HM (2002), Estradiol and progesterone regulate oxytocin receptor binding and expression in human breast cancer cell lines. Endocrine 18:79–84. [DOI] [PubMed] [Google Scholar]
- Amico JA, Thomas A, Hollingshead DJ (1997), The duration of estradiol and progesterone exposure prior to progesterone withdrawal regulates oxytocin mRNA levels in the paraventricular nucleus of the rat. Endocrine research 23:141–156. [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L (2005), Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. American journal of physiology Regulatory, integrative and comparative physiology 289:R1798–1806. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A (1989), Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A (1990), Oxytocin inhibits food and fluid intake in rats. Physiology & behavior 48:825–830. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N (2013), Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305:R1215–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer-Dantoin AC, Weiss J, Jameson JL (1995), Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology 136:1014–1019. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, et al. (2005), Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673. [DOI] [PubMed] [Google Scholar]
- Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R (1995), Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides 28:251–255. [DOI] [PubMed] [Google Scholar]
- Bjorkstrand E, Uvnas-Moberg K (1996), Central oxytocin increases food intake and daily weight gain in rats. Physiology & behavior 59:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Baskin DG (2015), Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiology & behavior 152:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, Havel PJ (2015), Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. American journal of physiology Regulatory, integrative and comparative physiology 308:R431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Aiani LM, Norvelle A, Grantham KN, O’Laughlin K, Terranova JI, Frantz KJ, Albers HE (2018), Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed]
- Borland JM, Rilling JK, Frantz KJ, Albers HE (2019), Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM (2004), Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American journal of clinical nutrition 79:537–543. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Veenema AH (2018), Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Current opinion in neurobiology 49:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino C (2009), Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring, Md) 17:980–984. [DOI] [PubMed] [Google Scholar]
- Campbell A (2008), Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological psychology 77:1–10. [DOI] [PubMed] [Google Scholar]
- Challinor SM, Winters SJ, Amico JA (1994), Pattern of oxytocin concentrations in the peripheral blood of healthy women and men: effect of the menstrual cycle and short-term fasting. Endocrine research 20:117–125. [DOI] [PubMed] [Google Scholar]
- Davis EA, Zhou W, Dailey MJ (2018), Evidence for a direct effect of the autonomic nervous system on intestinal epithelial stem cell proliferation. Physiological reports 6:e13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH (2015), The response of the US Centers for Disease Control and Prevention to the obesity epidemic. Annual review of public health 36:575–596. [DOI] [PubMed] [Google Scholar]
- Ding C, Leow MK, Magkos F (2019), Oxytocin in metabolic homeostasis: implications for obesity and diabetes management. Obesity reviews : an official journal of the International Association for the Study of Obesity 20:22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC (2013), Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA (2011), The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiology & behavior 104:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA, Geary N (2001), Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. American journal of physiology Regulatory, integrative and comparative physiology 281:R738–746. [DOI] [PubMed] [Google Scholar]
- Ekambaram G, Sampath Kumar SK, Joseph LD (2017), Comparative Study on the Estimation of Estrous Cycle in Mice by Visual and Vaginal Lavage Method. Journal of clinical and diagnostic research : JCDR 11:Ac05–ac07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Klusmann H, Ditzen B, Knaevelsrud C, Schumacher S (2019), Menstrual cycle-related fluctuations in oxytocin concentrations: A systematic review and meta-analysis. Frontiers in neuroendocrinology 52:144–155. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Wotjak CT, Landgraf R (1998), Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behavioural brain research 90:89–94. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ (2001), Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT (2000), Social amnesia in mice lacking the oxytocin gene. Nature genetics 25:284. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL (2016), Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama 315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N (1998), The effect of estrogen on appetite. Medscape women’s health 3:3. [PubMed] [Google Scholar]
- Geary N, Trace D, Smith GP (1995), Estradiol interacts with gastric or postgastric food stimuli to decrease sucrose ingestion in ovariectomized rats. Physiology & behavior 57:155–158. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, et al. (2017), Obesity. Nature reviews Disease primers 3:17034. [DOI] [PubMed] [Google Scholar]
- Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK (2016), Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. Journal of neuroendocrinology 28. [DOI] [PubMed] [Google Scholar]
- Hill JO, Peters JC (1998), Environmental contributions to the obesity epidemic. Science (New York, NY) 280:1371–1374. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, Nakamoto EM, Kanoski SE (2015), Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume C, Sabatier N, Menzies J (2017), High-Sugar, but Not High-Fat, Food Activates Supraoptic Nucleus Neurons in the Male Rat. Endocrinology 158:2200–2211. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, et al. (2017), Gating of social reward by oxytocin in the ventral tegmental area. Science 357:1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, Kumari P, Nakabayashi H, et al. (2015), Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. American journal of physiology Regulatory, integrative and comparative physiology 308:R360–369. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Caldwell JD, Pedersen CA, Stumpf WE (1988), Estradiol influences oxytocin-immunoreactive brain systems. Neuroscience 25:237–248. [DOI] [PubMed] [Google Scholar]
- Johnson AE (1992), The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by gonadal steroids. Annals of the New York Academy of Sciences 652:357–373. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Coirini H, Ball GF, McEwen BS (1989), Anatomical localization of the effects of 17 beta-estradiol on oxytocin receptor binding in the ventromedial hypothalamic nucleus. Endocrinology 124:207–211. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G (2006), Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell metabolism 4:313–321. [DOI] [PubMed] [Google Scholar]
- Kendrick KM (2000), Oxytocin, motherhood and bonding. Experimental physiology 85:111s–124s. [DOI] [PubMed] [Google Scholar]
- Klockars A, Brunton C, Li L, Levine AS, Olszewski PK (2017), Intravenous administration of oxytocin in rats acutely decreases deprivation-induced chow intake, but it fails to affect consumption of palatable solutions. Peptides 93:13–19. [DOI] [PubMed] [Google Scholar]
- Klockars OA, Klockars A, Levine AS, Olszewski PK (2018), Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake. Neuroreport 29:504–510. [DOI] [PubMed] [Google Scholar]
- Klockars OA, Waas JR, Klockars A, Levine AS, Olszewski PK (2017), Neural Basis of Ventromedial Hypothalamic Oxytocin-Driven Decrease in Appetite. Neuroscience 366:54–61. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR (2008), Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Molecular endocrinology 22:1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyb M, Chretien C, Caillon A, Rohner-Jeanrenaud F, Altirriba J (2018), Oxytocin Administration Alleviates Acute but Not Chronic Leptin Resistance of Diet-Induced Obese Mice. International journal of molecular sciences 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ (2015), Oxytocin reduces caloric intake in men. Obesity (Silver Spring, Md) 23:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd (2009), Oxytocin: the great facilitator of life. Progress in neurobiology 88:127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Wang J, Alexander JT, Dourmashkin JT, Chang GQ (2007), Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol 19:753–766. [DOI] [PubMed] [Google Scholar]
- Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y (2008), Oxytocin and appetite. Progress in brain research 170:137–151. [DOI] [PubMed] [Google Scholar]
- Leng G, Sabatier N (2017), Oxytocin - The Sweet Hormone? Trends in endocrinology and metabolism: TEM 28:365–376. [DOI] [PubMed] [Google Scholar]
- Lucio-Oliveira F, Franci CR (2012), Effect of the interaction between food state and the action of estrogen on oxytocinergic system activity. The Journal of endocrinology 212:129–138. [DOI] [PubMed] [Google Scholar]
- Lukas M, Neumann ID (2014), Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. Journal of neuroscience methods 234:101–107. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011), The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36:2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T (2011), Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 3:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, Nishimori K, Gantulga D, Shimomura K, et al. (2015), Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology 101:35–44. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Yokota S, Nishimori K, Shimomura K (2018), The Anorexigenic Neural Pathways of Oxytocin and Their Clinical Implication. Neuroendocrinology 107:91–104. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA (2012), Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of visualized experiments : JoVE:e4389. [DOI] [PMC free article] [PubMed]
- Minhas S, Liu C, Galdamez J, So VM, Romeo RD (2016), Stress-induced oxytocin release and oxytocin cell number and size in prepubertal and adult male and female rats. General and comparative endocrinology 234:103–109. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE (2012), Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. American journal of physiology Endocrinology and metabolism 302:E134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K, Kay K, Williams DL (2013), Oxytocin action in the ventral tegmental area affects sucrose intake. Brain research 1513:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM (1996), Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proceedings of the National Academy of Sciences 93:11699–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Billington CJ, Kotz CM, Wang C (2014), Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. American journal of physiology Regulatory, integrative and comparative physiology 307:R737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Hahn JD, Konanur VR, Hsu TM, Page SJ, Cortella AM, Liu CM, Song MY, et al. (2018), Control of Feeding Behavior by Cerebral Ventricular Volume Transmission of Melanin-Concentrating Hormone. Cell metabolism 28:55–68.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Klockars A, Levine AS (2017), Oxytocin and potential benefits for obesity treatment. Current opinion in endocrinology, diabetes, and obesity 24:320–325. [DOI] [PubMed] [Google Scholar]
- Onaka T, Takayanagi Y, Yoshida M (2012), Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. J Neuroendocrinol 24:587–598. [DOI] [PubMed] [Google Scholar]
- Ong ZY, Alhadeff AL, Grill HJ (2015), Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. American journal of physiology Regulatory, integrative and comparative physiology 308:R800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ZY, Bongiorno DM, Hernando MA, Grill HJ (2017), Effects of Endogenous Oxytocin Receptor Signaling in Nucleus Tractus Solitarius on Satiation-Mediated Feeding and Thermogenic Control in Male Rats. Endocrinology 158:2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M (2013), Oxytocin reduces reward-driven food intake in humans. Diabetes 62:3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr. (1982), Oxytocin induces maternal behavior in virgin female rats. Science (New York, NY) 216:648–650. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ Jr. (1979), Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America 76:6661–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Burbach H, Adan RA, Tol HH, Verbeeck MA, Axelson JF, Leeuwen FW, Beekman JM, et al. (1990), Regulation of the rat oxytocin gene by estradiol. Journal of neuroendocrinology 2:633–639. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S, Ogawa S, Adan RA, Burbach JP, Pfaff DW (1997), Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology 65:9–17. [DOI] [PubMed] [Google Scholar]
- Reppucci C, Gergely C, Veenema A (2018), Activation patterns of vasopressinergic and oxytocinergic brain regions following social play exposure in juvenile male and female rats. Journal of neuroendocrinology:e12582. [DOI] [PMC free article] [PubMed]
- Ritter RC, Slusser PG, Stone S (1981), Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ (2009), Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in neuroendocrinology 30:534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Leng G, Menzies J (2013), Oxytocin, feeding, and satiety. Frontiers in endocrinology 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample CH, Davidson TL (2018), Considering sex differences in the cognitive controls of feeding. Physiology & behavior 187:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Eckel LA (2008), The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiology & behavior 93:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Wood RI, Lorenzana A (2009), Neuroprotection against excitotoxic brain injury in mice after ovarian steroid depletion. Brain research 1265:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA (2007), Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. American journal of physiology Regulatory, integrative and comparative physiology 292:R1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T (2015), A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525:519–522. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Rhine MA, Khun K, Shweyk AN, Scott AM, Bond JM, Graham JL, Havel PJ, et al. (2018), Intranasal oxytocin reduces weight gain in diet-induced obese prairie voles. Physiology & behavior 196:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DK, Spencer DS, Curtis KS (2018), Estrogen effects on oxytocinergic pathways that regulate food intake. Hormones and behavior 105:128–137. [DOI] [PubMed] [Google Scholar]
- Striepens N, Schroter F, Stoffel-Wagner B, Maier W, Hurlemann R, Scheele D (2016), Oxytocin enhances cognitive control of food craving in women. Human brain mapping 37:4276–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, Nakamoto EM, Hahn JD, de Lartigue G, et al. (2018), Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nature communications 9:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW (2018), Brain maps 4.0-Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. The Journal of comparative neurology 526:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Matsumoto H, Nakata M, Mera T, Fukusumi S, Hinuma S, Ueta Y, Yada T, et al. (2008), Endogenous prolactin-releasing peptide regulates food intake in rodents. The Journal of clinical investigation 118:4014–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienel M, Fritsche A, Heinrichs M, Peter A, Ewers M, Lehnert H, Born J, Hallschmid M (2016), Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. International journal of obesity (2005) 40:1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Amico JA (1996), Sequential estrogen and progesterone (P) followed by P withdrawal increases the level of oxytocin messenger ribonucleic acid in the hypothalamic paraventricular nucleus of the male rat. Life sciences 58:1615–1620. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ (1990), Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain research 511:129–140. [DOI] [PubMed] [Google Scholar]
- Wood RI, Rice R (2013), Ethanol-induced conditioned partner preference in female mice. Behavioural brain research 243:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley NB, Dumais KM, Yuan JC, Newman LE, Alonso AG, Gillespie TC, Hobbs NJ, Breedlove SM, et al. (2019), Estrogen and androgen receptor activation contribute to the masculinization of oxytocin receptors in the bed nucleus of the stria terminalis of rats. Journal of neuroendocrinology:e12760. [DOI] [PubMed]
- Wu Q, Clark MS, Palmiter RD (2012), Deciphering a neuronal circuit that mediates appetite. Nature 483:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Takayanagi Y, Yoshida M, Nishimori K, Kusama M, Onaka T (2013), Involvement of prolactin-releasing peptide in the activation of oxytocin neurones in response to food intake. Journal of neuroendocrinology 25:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Cosgrove KP, Potenza MN, Mazure CM (2016), Balance of the Sexes: Addressing Sex Differences in Preclinical Research. The Yale journal of biology and medicine 89:255–259. [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, et al. (2011), Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I, Beery AK (2010), Males still dominate animal studies. Nature 465:690. [DOI] [PubMed] [Google Scholar]