Abstract
Clinical and preclinical research have identified sex differences in substance use and addiction-related behaviors. Historically, substance use disorders are more prevalent in men than women, though this gap is closing. Despite this difference, women appear to be more susceptible to the effects of many drugs and progress to substance abuse treatment more quickly than men. While the glutamate system is a key regulator of addiction-related behaviors, much of the work implicating glutamate signaling and glutamatergic circuits has been conducted in men and male rodents. An increasing number of studies have identified sex differences in drug-induced glutamate alterations as well as sex and estrous cycle differences in drug seeking behaviors. This review will describe sex differences in the glutamate system with an emphasis on implications for substance use disorders, highlighting the gaps in our current understanding of how innate and drug-induced alterations in the glutamate system may contribute to sex differences in addiction-related behaviors.
Keywords: Sex differences, glutamate, alcohol, cocaine, nicotine, addiction, estrous cycle, substance use disorders
1. Sex Differences in Drug Use
Approximately 20 million people in the US were diagnosed with a substance use disorder in 2017 (Substance Abuse and Mental Health Services Administration, 2018). Alcohol use disorders are the most common type of substance use disorder, affecting approximately 14 million people (Substance Abuse and Mental Health Services Administration, 2018). For most drugs of abuse, men are more likely than women to be diagnosed with a substance use disorder. Approximately 7.5% of men and 4.0% of women have an alcohol use disorder (Substance Abuse and Mental Health Services Administration, 2018). Cocaine use disorders are also more prominent in men than women. However, for both cocaine and alcohol use disorders, the focus of this review, this gap is shrinking due to increases in use in women (Grant et al., 2017; Kerridge et al., 2019; White et al., 2015).
Men and women differ in blood concentrations of drugs of abuse when taking similar doses, which may contribute to propensity for ongoing drug misuse and neurobehavioral consequences of drug intake. Women exhibit higher blood alcohol concentrations than men when consuming similar amounts of alcohol (Mumenthaler et al. 1999). In contrast, peak plasma concentrations of cocaine achieved by men and women differ depending on the route of administration. Men achieve higher peak plasma concentrations when administering cocaine intranasally, but not intravenously (Lukas et al., 1996; Mendelson et al., 1999). Interestingly, when cocaine is administered intranasally, women achieve higher plasma concentrations during the follicular phase than the luteal phase (Lukas et al., 1996), while women administering cocaine intravenously achieve peak plasma levels more quickly during the follicular phase than men or women in the luteal phase (Mendelson et al., 1999). These hormone-dependent differences in plasma concentrations are also observed for other drugs of abuse, such as nicotine. Peak plasma concentrations of nicotine are achieved more quickly in women than in men and is further accelerated in women taking estrogen-, but not progesterone-based contraceptives (Benowitz et al., 2006).
Preclinical data has identified sex differences in blood and brain concentrations of drugs of abuse. Female rats administered ethanol intraperitoneally, intragastrically, or intravenously reach peak plasma levels more quickly than male rats (Crippens et al., 2006; Robinson et al., 2002). Further, ethanol concentrations in the nucleus accumbens peaked more quickly in female rats than male rats (Robinson et al., 2002). Cocaine concentrations in both plasma and brain of male and female rats following an intraperitoneal injection do not differ. However, males show higher levels of the cocaine metabolite benzoylecgonine, while females show higher levels of the metabolite ecgonine methyl ester (Bowman et al., 1999). In contrast, for other drugs, such as methamphetamine and nicotine, clearance is much slower in female rats than in male rats (Kyerematen et al., 1988; Milesi-Hallé et al., 2005). These sex and estrous contributions to the effects of delivery method on blood and brain drug concentrations should be considered in both understanding neurobehavioral consequences of drug use in preclinical settings, and health outcomes in patient populations.
In general, substance abusing women tend to experience worse health outcomes than men. Alcohol-dependent women are at an elevated risk of developing alcohol-related liver disease compared to men (Becker et al., 1996) and take significantly less time than men to develop a variety of health problems such as fatty liver, hypertension, obesity, and anemia (Ashley et al., 1977). Psychiatric disorders such as depression, anxiety, and PTSD are more common in women with substance use disorders than men (Torrens et al., 2011), but the direction of this relationship is unclear. This may result from differences in factors initiating drug and alcohol use, or from interactions with substance use as substance abuse may result in sex-specific differences in psychiatric symptom resolution. Following abstinence from cocaine, for example, women exhibited slower resolution of depressive symptoms than men (Griffin et al., 1989).
Men and women also exhibit differences in subjective response to drug use. Women also report less positive and negative effects of cocaine compared to men (Lukas et al., 1996), which is potentially due to increased peak plasma cocaine levels achieved by men. However, women in the follicular phase while taking cocaine and amphetamine reported a subjectively better experience than in the luteal phase (Evans et al., 2002; Sofuoglu et al., 1999; White et al., 2002), possibly due to women achieving higher plasma levels during the follicular phase (Lukas et al., 1996). In contrast, women report feeling more intoxicated when consuming ethanol than men (Wang et al., 2003), but this can potentially be attributed to higher blood alcohol concentrations achieved by women (Mumenthaler et al., 1999). The subjective effects of ethanol do not vary by menstrual cycle (Holdstock and De Wit, 2000). In contrast to cocaine and ethanol, women appear less sensitive to changes in nicotine dose (Perkins, 1999). These differences in subjective response to drugs of abuse may contribute to sex differences in initiation or maintenance of use. Further, differential brain concentrations of drugs of abuse may impact neural function in unique ways in males and females, including distinct effects on neurotransmitter systems.
2. The Glutamate System
Glutamate is the major excitatory neurotransmitter in the brain and has been implicated in normal brain functioning, such as learning and memory (Riedel et al., 2003), and in maladaptive functioning in a number of neurodegenerative disorders and addiction (Gass and Olive, 2008; Kalivas et al., 2009). In particular, glutamatergic projections from the prefrontal cortex, amygdala, hippocampus, and ventral tegmental area to the nucleus accumbens have been implicated in various aspects of drug-seeking behaviors. The nucleus accumbens, composed principally of GABAergic medium spiny neurons, can be further subdivided into the core and shell subregions. The core subregion receives glutamatergic projections from the prelimbic subregion of the medial prefrontal cortex and dorsal hippocampus (Brog et al., 1993; Groenewegen et al., 1987; Kelley and Domesick, 1982). The shell subregion receives glutamatergic input from the infralimbic subregion of the medial prefrontal cortex and ventral hippocampus (Britt et al., 2012; Brog et al., 1993; Groenewegen et al., 1987; Kelley and Domesick, 1982). The basolateral amygdala and ventral tegmental area project more broadly to the nucleus accumbens (Hnasko et al., 2012; Mcdonald, 1991; Stuber et al., 2010; Tecuapetla et al., 2010; Yamaguchi et al., 2011). While a majority of ventral tegmental projections to the nucleus accumbens are dopaminergic, a substantial subset either co-release glutamate or are exclusively glutamatergic (Hnasko et al., 2012; Yoo et al., 2016).
There are two major types of glutamate receptors, G protein coupled metabotropic glutamate receptors (mGluRs) and ligand-gated ionotropic receptors. Metabotropic receptors are composed of three subgroups: group I, group II, and group III. Group I, which includes mGluR1 and mGluR5, are located primarily postsynaptically and on astrocytes and generally signals via Gq (Niswender and Conn, 2010; Ribeiro et al., 2010). Group II, which is composed of mGluR2, mGluR3, GRM3A2, GRM3A4, and GRM3A2A3, are located both pre- and postsynaptically and on astrocytes and generally signals via Gi/o (Blümcke et al., 1996; Niswender and Conn, 2010). Group III generally signals via Gi/o and includes the predominantly presynaptic mGluR4, mGluR7, and mGluR8, and the postsynaptic mGluR6 (Niswender and Conn, 2010).
Ionotropic glutamate receptors are classified into three major groups: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartate (NMDA), kainate receptors (Stawski et al., 2010). Ionotropic glutamate receptors are composed of four large subunits that form a central pore that allows conductance of ions such as Ca2+, Na+, and Cl− (Traynelis et al., 2010). In contrast to NMDA and kainate receptors, AMPA receptors are generally Ca2+-impermeable (Dingledine et al., 1999). NMDA receptors require the cofactor glycine for activation and permit conductance of Ca2+, Na+, and Cl− (Qian and Johnson, 2002). Kainate receptors are somewhat unique in that they require coactivation by Na+ and Cl− (Wong et al., 2007).
Glutamate is cleared via sodium-dependent glutamate transporters expressed primarily on astrocytes, GLT1 and GLAST, with GLT1 being the predominant subtype in the forebrain (Anderson and Swanson, 2000). Upon uptake of glutamate from the synapse, astrocytes convert glutamate to glutamine via glutamine synthetase for use by neurons (Rose et al., 2013). Astrocytes can also release glutamate into the extracellular space by exchanging intracellular glutamate for extracellular cystine via the Na+-independent antiporter, system xc− (Bridges et al., 2012). Glutamate can also be cleared from the synapse via primarily neuronal glutamate transporters, EAAT3,4,5 (Arriza et al., 1997; Furuta et al., 1997) and vesicular glutamate transporters (vGLUTs) (Li et al., 2013).
2.1. Sex Differences in Glutamate Levels
A limited number of studies have explored sex differences in the glutamate system in humans. Those that exist have identified blood and brain region-specific sex differences in glutamate levels. In humans, prefrontal cortex glutamate levels are significantly higher in men than women (O’Gorman et al., 2011). In the striatum and cerebellum, women exhibit higher glutamate concentrations than men (Zahr et al., 2013). Blood glutamate levels are significantly higher in men than in women, but vary across the menstrual cycle with glutamate levels showing an inverse relationship with blood estrogen and progesterone levels (Zlotnik et al., 2011).
Glutamate concentrations also differ between male and female rodents in various brain regions. Male rats exhibit higher glutamate concentrations than female rats during diestrus in the lateral hypothalamic area, the ventromedial hypothalamic area, and the habenular nuclei, while diestrus females exhibit higher glutamate concentrations in the medial preoptic area than male rats (Frankfurt et al., 1984). Glutamate concentrations in proestrus females increase in the diagonal band of Broca and the medial septum and decrease in the lateral preoptic area and anterior hypothalamic area compared to diestrus females and increase in the lateral septum compared to estrus females (Frankfurt et al., 1984). These sex- and estrous phase-dependent alterations in glutamate levels appear to be regulated in part by gonadal hormones as estradiol administration has been shown to increase glutamate levels in the diagonal bands of Broca and the ventromedial nucleus of ovariectomized rats (Luine et al., 1997).
2.2. Sex Differences in Glutamate Receptor and Transporter Expression
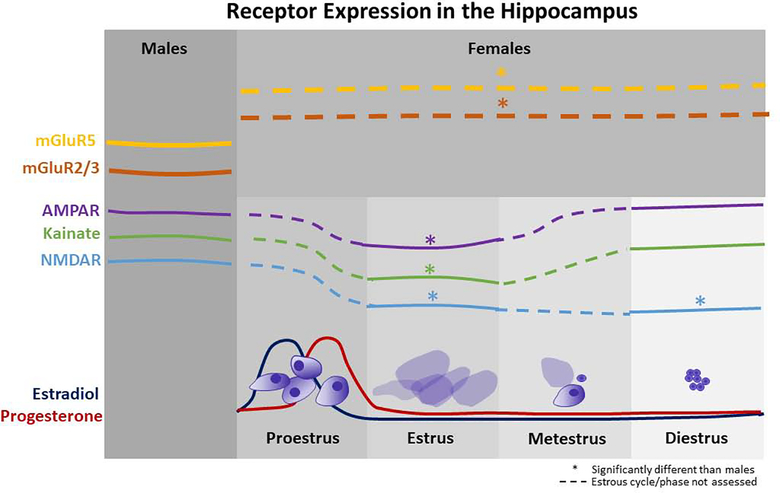
Rodents exhibit both sex and brain-region specific patterns of metabotropic and ionotropic glutamate receptor expression (Figure 1). Females show greater mGluR2/3 and mGluR5 expression in the hippocampus and greater mGluR5 expression in the PFC (Wang et al., 2015). Within the hippocampal subregions and layers, density of the ionotropic glutamate receptors -- AMPA, NMDA, and kainate – differs by sex and across the estrous cycle. Density of the AMPA receptor is significantly lower in all regions and layers of the hippocampus of estrus female rats compared to male rats and diestrus female rats. However, diestrus female rats do not differ from males (Palomero-Gallagher et al., 2003). Density of the NMDA receptor is also significantly lower in estrus and diestrus female rats in the oriens and radiatum layers of the CA1, CA2, and CA3 regions of the hippocampus compared to male rats (Palomero-Gallagher et al., 2003). Expression of the NR1 and NR2B subunits of the NMDA receptor in the hippocampus is higher in female rats than male rats (Wang et al., 2015). Density of the kainate receptor is significantly lower in all layers of the CA1 region of the hippocampus of estrus females compared to males (Palomero-Gallagher et al., 2003). Expression of the NR1 subunit is also higher in the prefrontal cortex and amygdala in females compared to males (Wang et al., 2015).
Figure 1. Sex- and estrous cycle-dependent alterations in hippocampal glutamate receptor expression.
Sex differences in expression of both metabotropic and ionotropic glutamate receptors have been identified within the hippocampus. The metabotropic receptors mGluR2/3 and mGluR5 are expressed at higher levels in females than males (Wang et al., 2015), but how this varies by estrous phase has not been investigated. Conversely, females express lower levels of ionotropic receptors within the hippocampus, but this appears mostly specific to the estrus phase of the estrous cycle (Palomero-Gallagher et al., 2003). Expression levels of ionotropic receptors have not been assessed for the either proestrus or metestrus, thus it remains unclear how the surge in estradiol and progesterone that occur during proestrus may alter expression patterns. These sex- and estrous cycle-dependent differences may underlie differential risk for addictive behavior and/or variance in response to treatment. Lines represent approximate increases or decreases in glutamate receptor expression in the hippocampus in males versus females and across the estrous cycle. Dashed lines represent receptor expression levels for which estrous cycle has not been assessed.
Spine density in various brain regions has been shown to vary across the estrous cycle due to alterations in estradiol levels (Peterson et al., 2015; Staffend et al., 2011; Woolley et al., 1997; Woolley and McEwen, 1993, 1992) and this appears to be mediated in part by estradiol regulation of glutamatergic receptors, NMDAR and mGluR5 (Peterson et al., 2015; Woolley and McEwen, 1994). Estradiol administration results in decreased spine density in the nucleus accumbens core and increased spine density in the CA1 region of the hippocampus (Peterson et al., 2015; Woolley and McEwen, 1992). This estradiol-induced effect on spine density is mediated by mGluR5 receptors in the nucleus accumbens core (Peterson et al., 2015) and mGluR1 and NMDA receptors in the CA1 (Peterson et al., 2015; Woolley and McEwen, 1994).
Glutamate transport is also modulated by sex and estrous cycle. In cortical synaptosomes, glutamate affinity for transport is highly dependent on sex and estrous cycle. The affinity of glutamate for high affinity transport is significantly greater in proestrus and estrus females compared to males and diestrus females, while the affinity of glutamate for low affinity transport is significantly greater in proestrus females than males or metestrus, diestrus, or estrus females (Mitrovic et al., 1999). In the striatum, mutation of the xCT subunit of system xc-decreases glutamate levels in male, but not female mice (Borra et al., 2014), suggesting that glutamate homeostasis in the striatum of female mice may be mediated by other factors.
Astrocytes, as mentioned previously, express many glutamate transporters and are critical to maintaining basal glutamate levels. In the hippocampus, the number of cells immunoreactive for the astrocytic cytoskeletal protein glial fibrillary acidic protein (GFAP) is significantly greater in females compared to males (Arias et al., 2009). This increase is even greater in proestrus females compared to both males and diestrus females (Arias et al., 2009). In the medial amygdala, there are more astrocytes in males than females (Pfau et al., 2016). Similar to what has been observed in the hippocampus, female rats exhibit increased GFAP expression in the medial amygdala during the proestrus phase (Martinez et al., 2006).
3. Regulation of Drug-seeking Behaviors by the Glutamate System: Known Sex Differences and Gaps in Understanding
Imaging studies have identified sex differences in the neural substrates that mediate drug seeking behaviors. In abstinent alcoholic men and women, alcohol-related cues elicit increased activation of the striatum and prefrontal cortex (PFC) compared to control subjects. Activation was greatest in those individuals that subsequently relapsed (Grüsser et al., 2004), indicating that cue reactivity may be predictive of risk of relapse. Alcohol-dependent men exhibit increased glutamate levels in the NAc (Bauer et al., 2013) and decreased mGluR2 in the anterior cingulate cortex (Meinhardt et al., 2013). The increased glutamate levels in the NAc correlated with craving (Bauer et al., 2013), but this relationship has not yet been evaluated in women. Among recreational drinkers, exposure to alcohol-related cues resulted in increased activity in the cortico-limbic-striatal circuit in both men and women, with women showing increased activity in the superior and middle frontal gyrus relative to men (Seo et al., 2011). Among cocaine dependent individuals, men exhibit corticostriatal-limbic hyperactivity in response to drug cues, while women are hyper-responsive to stress cues (Potenza et al., 2012).
3.1. Glutamatergic Regulation of Drug Seeking in Males: Implications for Females
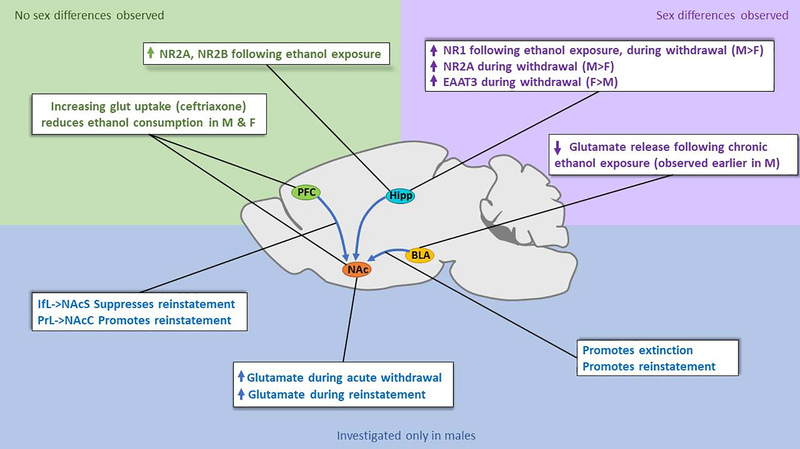
Despite clinical evidence suggesting that men and women may differentially engage particular circuits in response to drug cues or stress (Potenza et al., 2012; Seo et al., 2011), to our knowledge, no preclinical data has investigated the contribution of specific projections to drug seeking in females. Below we summarize the body of literature focusing on the role of glutamate in drug seeking behaviors collected in males to emphasize how much work is yet to be completed. Glutamate signaling within the corticolimbic and corticostriatal circuits -- including the nucleus accumbens and its glutamatergic projections from the prefrontal cortex, basolateral amygdala, and ventral tegmental area -- has been heavily implicated in drug seeking behaviors including self-administration, withdrawal, extinction, and reinstatement in male rodents (Figure 2).
Figure 2. State of knowledge about sex differences in ethanol-induced glutamatergic alterations.
Research investigating glutamatergic projections and alterations in glutamate levels, uptake, and receptor expression implicated in ethanol seeking and exposure for which no sex differences are present (green), for which sex differences have been identified (purple), and for which sex differences have not been investigated (blue). Identification of drug exposure-related sex differences in the glutamate system have been limited primarily to glutamate receptor subunit (Devaud and Morrow, 1999, Devaud and Alele, 2004) and transporter (Alele and Devaud, 2005) expression in the hippocampus and chronic ethanol exposure- induced effects on glutamate release in the amygdala. However, some receptor subunit alterations are observed in both sexes (Devaud and Alele, 2004). Pharmacological manipulation of glutamate uptake in the PFC and NAc also alters ethanol consumption similarly in both males and females Sari et al., 2011, 2013; Qrunfleh et al., 2013; Alhaddad et al., 2014; Rao and Sari, 2014). In general, the roles of specific projections in ethanol-seeking behaviors have not been investigated in females (Gass et al., 2011, Pati et al., 2016, Keistler et al., 2017, Millan et al., 2017, Millan and McNally, 2011).
3.1.1. Glutamatergic Regulation of Self-Administration
Consistent with the clinical literature, animal models have also identified sex differences in drug-seeking behaviors. Females acquire self-administration of drugs such as cocaine, heroin, and methamphetamine more quickly than males rats (Lynch and Carroll, 1999; Roth and Carroll, 2004a). Using low effort fixed ratio schedules, female rodents self-administer more ethanol (Almeida et al., 1998; Cailhol and Mormède, 2001; Dhaher et al., 2012; Juárez and De Tomasi, 1999; Lancaster et al., 1996; Lancaster and Spiegel, 1992; Lourdes De La Torre et al., 2015; Maldonado-Devincci et al., 2010; Priddy et al., 2017; Sluyter et al., 2000; Varlinskaya and Spear, 2014; Vetter-O’Hagen et al., 2009; Walker et al., 2008) and cocaine than males (Bechard et al., 2018a; Fuchs et al., 2005; Kerstetter et al., 2008; Kippin et al., 2005). When the effort required to gain access to drugs is increased using a progressive ratio schedule, however, females reach higher breakpoints for cocaine (Roberts et al., 1989) and other substances, including methamphetamine (Roth and Carroll, 2004b) and nicotine (Donny et al., 2000), but not ethanol (Middaugh and Kelley, 1999; Priddy et al., 2017).
Self-administration also appears to be modulated by the estrous cycle for some, but not all drugs of abuse. Estrus females achieve higher breakpoints when self-administering cocaine (Roberts et al., 1989) on a progressive ratio. Similarly, estrus females show an increased propensity to respond for increasing doses of cocaine compared to males and non-estrus females (Lynch et al., 2000). In contrast, females with synchronized estrous cycles self-administer less ethanol during estrus compared to other phases of the estrous cycle, though this effect was not present in female rats allowed to freely cycle (Roberts et al., 1998). The increased motivation to self-administer nicotine in females, however, does not appear to depend on the estrous cycle, as nicotine self-administration did not differ by estrous phase across four doses tested (Donny et al., 2000).
Glutamatergic signaling in the nucleus accumbens appears to be an important mediator of drug seeking. In male rats, glutamate is increased in the nucleus accumbens core and shell subregions during cocaine self-administration (Suto et al., 2010). Increased extracellular glutamate levels are observed in the nucleus accumbens following chronic ethanol exposure, which may drive increased ethanol drinking (Griffin et al., 2014). This is supported by work showing that increasing glutamate levels in the nucleus accumbens promotes ethanol self-administration in non-dependent mice (Griffin et al., 2014). In contrast, projections from the basolateral amygdala to the nucleus accumbens shell suppress both consumption of ethanol and cue-induced ethanol-seeking in males (Millan et al., 2017), While the role of glutamatergic projections in self-administration in females have not been examined, increased self-administration of ethanol in females could potentially be associated with less activity in this particular projection.
The role of glutamatergic receptors in the nucleus accumbens in self-administration has also been examined primarily in males. Antagonism or negative allosteric modulation of Gq-coupled mGluR5 receptors in the nucleus accumbens blocks cocaine seeking (Wang et al., 2013), while activation of mGluR2/3 within the nucleus accumbens appears to impair nicotine self-administration (Liechti et al., 2007). Cocaine seeking is also decreased by an AMPA/kainate receptor antagonist in the nucleus accumbens core (Di Ciano and Everitt, 2001). The GluA1 subunit of the AMPA receptor is increased in the nucleus accumbens of estrus females following cocaine self-administration (Bechard et al., 2018a) and thus may be driving the corresponding increased responding for cocaine during estrus (Lynch et al., 2000), though this remains to be determined experimentally (Table 1).
Table 1. Potential glutamatergic mechanisms of sex differences in drug seeking behaviors and the broader implications.
Sex differences have been reported in glutamate receptor expression, drug seeking behavior, and response to pharmacological manipulations of the glutamate system. Together, these findings suggest that a more thorough understanding of sex differences in drug seeking behavior and the underlying neurobiology may yield novel targeted treatment strategies. In rows a and b, examples of basal differences in glutamate receptors (Peterson et al., 2015) and receptor subunits (Wang et al., 2015) that may lead to sex and estrous cycle dependent outcomes following pharmacological manipulations previously studied only in males (Feltenstein and See, 2007; Knackstedt et al., 2014; Sinclair et al., 2012; Wang et al., 2013). In rows c-e, examples of sex differences in drug seeking behaviors (Bertholomey et al., 2016; Buffalari et al., 2012; Cox et al., 2013; Lynch et al., 2000; Roberts et al., 1989) and outcomes (Ignacio Pena-Bravo et al., 2019) that can potentially by explained by known sex and estrous cycle dependent glutamatergic sex differences at baseline (Wang et al., 2015), following stress (Wei et al., 2014), and following drug exposure (Bechard et al., 2018b). In row f, examples of the importance of considering sex when designing pharmacological interventions. Some therapies may be less effective in females (Goenaga et al., 2019) or work via different mechanisms (Alhaddad et al., 2014; Borra et al., 2014; Qrunfleh et al., 2013; Rao and Sari, 2014; Sari et al., 2011).
| Differences in Glutamate System | Drug Seeking Behavior | Specific Hypotheses | Broader Implications |
|---|---|---|---|
| a. mGluR5 mediates estradiol-induced spine density alterations in NAc | Antagonism of mGluR5 in NAc ↓cocaine seeking, cue-induced reinstatement of cocaine & ethanol seeking | Antagonism of mGluR5 in NAc may have sex and estrous cycle-dependent effects on drug seeking | Strategies effective at reducing drug seeking in males may be less effective or estrous cycle-dependent in females |
| b. ↑ NR1 subunit expression in amygdala of females | NMDAR antagonism ↓cue-induced reinstatement in amygdala | Antagonism of NMDAR in amygdala may have sex dependent effects on cue-induced reinstatement | |
| c. Females exhibit greater resilience to NMDA and AMPA receptor subunit alterations following chronic stress | Females are more vulnerable to stress-induced reinstatement of alcohol and methamphetamine seeking | Stress and drug exposure may differentially alter NMDAR and AMPAR subunit expression in females, thus enhancing susceptibility to stress-induced reinstatement | Sex differences in sensitivity to stress-induced reinstatement may be driven by sex differences in baseline, drug-induced, and stress-induced alterations in subunit compositions |
| d. ↑GluA1 in NAc of estrus females following cocaine self-administration | Estrus females exhibit increased responding for increasing doses of cocaine, achieve high break points when self-administering cocaine | ↑ GluA1 during estrus may be driving increased cocaine self-administration during estrus | Drugs of abuse may interact with estrous cycle to dysregulate drug seeking behavior |
| e. ↑ NR1 subunit expression in PFC of females | Females exhibit greater evoked glutamate release and increase in NMDA currents in PFC following meth self-administration | Baseline and/or drug-induced differences in NMDAR subunit composition may drive sex differences in response to psychostimulants | Innate differences in the glutamate system may interact with drug exposure to produce unique neurobiological consequences in females |
| f. System xc- appears to regulate glutamate levels in the NAc in males, not females | N-acetylcysteine reduces cue-induced reinstatement of nicotine seeking in male, but not female rats | Ability of N-acetylcysteine to reduce drug seeking may be selective to males for certain drugs of abuse | Lack of efficacy of certain pharmaceutical interventions in females may be driven by baseline differences in glutamatergic function |
| Ceftriaxone reduces ethanol consumption in both male and female alcohol-preferring rats | Ceftriaxone may work via different mechanisms in males and females | Pharmaceutical interventions may work via different mechanisms to produce the same outcome in males and females |
3.1.2. Glutamatergic Regulation of Conditioned Place Preference
Conditioned place preference models have been used to model drug seeking. In these models, a drug is paired with a particular environment and through learned association, animals develop a preference for that reward-paired context. Female rats require fewer reward-context associations in order to develop a cocaine-induced conditioned place preference (Russo et al., 2003). In contrast, in nicotine-induced conditioned place preference, using lower doses of nicotine (less than 0.6mg/kg), only males exhibit a conditioned place preference (Yararbas et al., 2010), but conditioned place preference was induced in females using higher doses (0.7–1.0 mg/kg) of nicotine (Kota et al., 2008). In a separate study, a conditioned place preference was observed in females using a low dose (0.32 mg/kg) (Isiegas et al., 2009). The reason for this difference is unclear but may be related to the strain of mice used in each study. In contrast to what is observed for nicotine, males and females acquire a conditioned place preference for ethanol at similar rates and to similar magnitudes (Cunningham and Shields, 2018). Under conditions where male and female mice acquire a cocaine conditioned place preference at the same rate, using a low dose of cocaine, male mice took longer to extinguish their conditioned place preference (Hilderbrand and Lasek, 2014).
Substance use disorders are also characterized by inflexible alcohol seeking that persists despite adverse consequences. Animals models of compulsive drug seeking utilize foot shock or adulteration of the drug to assess drug-seeking behavior despite adverse consequences. However, relatively little research has investigated sex differences in drug seeking despite adverse consequences. Female, but not male, rats exhibit increasing compulsive cocaine seeking in response to increasing cocaine dose, and this compulsive cocaine seeking was not impacted by the estrous cycle (Datta et al., 2018). Female, but not male, mice exhibit compulsive-like ethanol seeking in a modified conditioned place preference paradigm in which a previously ethanol-paired chamber is also associated with a foot shock (Xie et al., 2019). Further, this compulsive-like ethanol seeking that persists despite an aversive experience was attenuated in females with a history of chronic ethanol exposure (Xie et al., 2019), suggesting that chronic ethanol exposure enhanced sensitivity to aversive experiences in females. In this study, mice must recall both rewarding and aversive experiences in the same chamber. In contrast, following quinine adulteration of ethanol in a drinking paradigm in which the rewarding and aversive experiences are present simultaneously, levels of aversion-resistant intake did not differ between males and females (Sneddon et al., 2019).
3.1.3. Glutamatergic Regulation of Extinction
Extinction reduces drug seeking by removing the relationship between an action or a stimulus and a reward, such that the behavior or stimulus are no longer associated with reinforcement. Sex differences in the neurobiology of extinction of drug seeking have not been well characterized, and thus the majority of the work implicating the glutamate system has been conducted using males. In male rats, glutamate is increased in the nucleus accumbens core and shell subregions during extinction (Suto et al., 2010). Further, projections from the basolateral amygdala to the nucleus accumbens shell drive extinction of ethanol seeking in males (Keistler et al., 2017; Millan and McNally, 2011). The infralimbic cortex has been implicated in extinction of drug seeking as well. Activation of the infralimbic cortex prevented, while inhibition promoted, reinstatement of cocaine seeking in extinguished rats (Peters et al., 2008). This is perhaps mediated through projections to the nucleus accumbens shell as simultaneous unilateral inhibition of infralimbic cortex and nucleus accumbens shell promoted cocaine seeking in extinguished rats (Peters et al., 2008). Similarly, allosteric inhibition of small-conductance calcium-activated potassium channels within the infralimbic cortex promote extinction of alcohol seeking (Cannady et al., 2017). However, while reversible inactivation of the infralimbic cortex did not impact extinction expression or promote context-induced reinstatement of alcohol seeking in extinguished rats, it did increase response latencies in the extinction context (Willcocks and Mcnally, 2013).
Metabotropic and ionotropic glutamatergic receptors have also been implicated in extinction learning in male rodents. Extinction of ethanol seeking can be promoted by overexpression of Gi-coupled mGluR2 in infralimbic cortex projections (Meinhardt et al., 2013). Mice deficient in mGluR5 also display impaired extinction of cocaine conditioned place preference (Bird et al., 2014), suggesting that mGluR5 may be essential for extinction of cocaine seeking behaviors. While it remains unclear which brain regions are most important for this mGluR5 depletion-induced extinction impairment, baseline sex differences in mGluR5 expression in the prefrontal cortex and hippocampus may have interesting implications for extinction learning in females. AMPA receptors in the nucleus accumbens shell, have also been implicated in extinction of cocaine seeking, as overexpression of the GluR1/2 subunits of the AMPA receptor in the nucleus accumbens shell facilitates extinction learning (Sutton et al., 2003).
3.1.2. Glutamatergic Regulation of Reinstatement and Relapse Models
Substance use disorders are characterized by a high propensity to relapse, but the factors that drive relapse may be sex dependent. Drug-paired cues and stress can elicit intense feelings of craving (Cooney et al., 1987; Sinha et al., 2000) and drive drug-seeking behaviors, contributing to a risk of relapse (Grüsser et al., 2004; Sinha et al., 2006) even after extended periods of abstinence (Braus et al., 2001). Individuals with substance use disorders exhibit enhanced cue reactivity and have difficulty shifting their attention away from drug-paired stimuli (Baschnagel, 2013; Johnsen et al., 1994; Littel and Franken, 2011; Stormark et al., 1997; Wilcox et al., 2011). Clinical data has identified apparent sex differences in cue reactivity. Among cocaine-and nicotine-dependent individuals, women show enhanced reactivity to cues compared to men (Doran, 2014; Niaura et al., 2002; Robbins et al., 1999). Among recreational drinkers, alcohol-paired cues elicit craving in men, but not women (Willner et al., 1998), though this pattern may be absent in dependent individuals (Rubonis et al., 1994). Interestingly, women exhibit a reduction in drug cue-induced cocaine craving during the midluteal phase of the menstrual cycle when progesterone levels are elevated (Sofuoglu et al., 2007), but less cue-induced craving of nicotine in the follicular phase (Franklin et al., 2004). Women also exhibit greater sensitivity to stress-induced alcohol craving than men (Hartwell and Ray, 2013), with a reduction in stress-induced cocaine craving during the midluteal phase (Sofuoglu et al., 2007). Interestingly, depressive and anxiety symptoms correlate with methamphetamine craving in men, but not women (Hartwell et al., 2016). Thus, men and women are differentially vulnerable to cue- or stress-induced craving depending on the drug of abuse, and this can be further modulated by the menstrual cycle in women.
Cue-induced reinstatement of drug-seeking can be used to model cue-induced relapse-like behaviors in rodents. In these models, rodents are trained to self-administer drugs of abuse and the delivery of these drugs is paired with previously neutral stimuli. Responding is then extinguished and subsequently reinstated by exposure to the previously drug-paired cues. Ethanol-paired cues appear to drive reinstatement of ethanol-seeking in male rats, but not female rats (Randall et al., 2017). In contrast, cue-induced reinstatement of methamphetamine seeking is significantly attenuated in male rats compared to female rats (Cox et al., 2013), while cue-induced reinstatement of nicotine-seeking does not appear to differ between males and females (Feltenstein et al., 2012). When trained using either a low (0.25mg/kg) or a high (1.0mg/kg) doses of cocaine, females exhibit attenuated cue-induced reinstatement of cocaine-seeking compared to males (Fuchs et al., 2005). At the lowest dose of cocaine, this attenuated responding appears to be related to estrous phase as non-estrus females exhibited cue-induced reinstatement. However, attenuated responding at the highest dose was not related to estrous cycle (Fuchs et al., 2005). Using more intermediate doses, however, males and females display similar degrees of cue-induced reinstatement of cocaine seeking (Bechard et al., 2018b; Fuchs et al., 2005).
In studies using male rodents, glutamatergic projections to the nucleus accumbens have been implicated in cue-induced reinstatement of drug seeking. Glutamatergic projections from the prefrontal cortex to the nucleus accumbens mediate cue-induced reinstatement of ethanol (Keistler et al., 2017) and cocaine-seeking (Stefanik et al., 2016; Stefanik and Kalivas, 2013). Investigations into the specific role of prefrontal cortex and nucleus accumbens subregions in cue-induced reinstatement indicate that glutamatergic projections from the infralimbic cortex to the nucleus accumbens shell function to suppress cue-induced reinstatement of cocaine-seeking (Augur et al., 2016; Lalumiere et al., 2012; Peters et al., 2008), while the prelimbic cortex projections to the nucleus accumbens core promote cue-induced reinstatement of cocaine-seeking (Stefanik et al., 2016). Specific neuronal ensembles within the infralimbic cortex have also been implicated in inhibitory control over cue-induced reinstatement of alcohol seeking (Pfarr et al., 2015). Projections from the basolateral amygdala to the nucleus accumbens shell drive cue-induced reinstatement of ethanol (Keistler et al., 2017), and cocaine seeking (Stefanik and Kalivas, 2013). The contribution of these projections to cue-induced reinstatement in females remains to be determined. As cues have shown to drive reinstatement of ethanol-seeking in male rats, but not female rats (Randall et al., 2017), it is possible that glutamatergic projections from the prefrontal cortex to the nucleus accumbens to the nucleus accumbens are differentially engaged in females.
Extracellular glutamate levels are also elevated in the nucleus accumbens during cue-induced reinstatement of ethanol seeking (Gass et al., 2011). Viral overexpression of Gi-coupled mGluR2 in infralimbic cortex projections attenuates cue-induced reinstatement of ethanol seeking (Meinhardt et al., 2013). Activation of mGluR2/3 within the nucleus accumbens appears to impair cue-induced reinstatement of nicotine (Liechti et al., 2007). In contrast, antagonism or negative allosteric modulation of Gq-coupled mGluR5 receptors in the nucleus accumbens blocks cue-induced reinstatement of cocaine and ethanol seeking (Knackstedt et al., 2014; Sinclair et al., 2012). As mGluR5 has been shown to mediate estradiol-induced alterations in spine density in the nucleus accumbens (Peterson et al., 2015), it remains to be seen whether the efficacy of mGluR5 antagonism would vary across the estrous cycle in females (Table 1). Modulation of NMDA receptors results in contrasting effects on reinstatement depending on the brain region being targeted. Antagonism of NMDA receptors impairs cue-induced reinstatement of cocaine seeking when administered into the basolateral amygdala (Feltenstein and See, 2007) (Table 1), but increases reinstatement when administered into the nucleus accumbens shell (Famous et al., 2007). Though the effect of NMDA receptor antagonism on cue-induced reinstatement of cocaine seeking has not been investigated in females, females may be differentially affected due to higher levels of the NR1 subunit in the amygdala at baseline (Wang et al., 2015) (Table 1).
Reinstatement of drug seeking can also be achieved by exposure to the drug following extinction training. Males and females reinstate cocaine-seeking to a similar degree, estrus females exhibit potentiated cocaine-primed reinstatement of cocaine-seeking (Kippin et al., 2005), while females show enhanced sensitivity to methamphetamine-primed reinstatement (Cox et al., 2013). Although nicotine-primed reinstatement does not differ between males and females, when combined with cues, females exhibit attenuated reinstatement compared to males (Swalve et al., 2016). Together these data suggest that males and females may be differentially susceptible to risk factors for relapse-related behaviors.
Prelimbic cortex projections to the nucleus accumbens core appear to drive drug-primed reinstatement of cocaine seeking (McFarland et al., 2003) and methamphetamine (Rocha and Kalivas, 2010) seeking.. The prelimbic cortex and nucleus accumbens core both also appear to be involved in drug-primed reinstatement of methamphetamine seeking, though the contribution of t e specific prelimbic cortex to nucleus accumbens core projections has not been assessed in methamphetamine-primed reinstatement (Rocha and Kalivas, 2010). The nucleus accumbens core and prelimbic cortex may be preferentially engaged in a sex- and estrous phase-dependent manner driving the potentiation of drug-primed reinstatement in females for methamphetamine and in estrus females for cocaine. Further evidence for the critical role of the nucleus accumbens in drug-primed reinstatement comes from pharmacological data showing that activation of mGluR2/3 in the nucleus accumbens impairs cocaine-primed reinstatement (Peters and Kalivas, 2006).
Sex differences are also observed in stress induced relapse-like behaviors. Exposures to stressors such as restraint stress and forced swim stress and pharmacological manipulation of cortisol levels have all been used to model stress-induced reinstatement. Females are more sensitive to stress-induced and stress modulation of cue-induced reinstatement of ethanol seeking (Bertholomey et al., 2016) and corticotropin releasing factor-induced reinstatement of cocaine seeking in a subset of rats considered high responders (Buffalari et al., 2012). Female rats are also more sensitive to yohimbine-induced reinstatement of methamphetamine seeking compared to male rats (Cox et al., 2013). Male and female rats exhibit similar stress-induced reinstatement of nicotine (Feltenstein et al., 2012) and cocaine seeking, however, proestrus females exhibit enhanced cocaine reinstatement following stress prior to cue-induced reinstatement (Feltenstein et al., 2011).
Although sex differences in the glutamatergic mechanisms underlying stress-induced reinstatement of drug seeking behaviors have not been assessed, accumulating evidence indicates that stress alone may result in sex differences in glutamate transport, which may have important implications for stress-induced reinstatement. Repeated stress results in reduced glutamatergic neurotransmission in the PFC of male, but not female rats (Wei et al., 2014). Males also exhibited significant reductions in various subunits of AMPA and NMDA receptors, an effect which was not observed in females (Wei et al., 2014). This sex-specific response to repeated stress appears to be modulated by estradiol as blocking estrogen receptors in females resulted in similar reductions in PFC glutamate neurotransmission and glutamatergic receptor subunit expression, while estradiol administration in males blocked the effects of stress (Wei et al., 2014). While females may be more resilient to the effects of chronic stress on glutamate receptor alterations, it is unknown whether chronic drug exposure-induced glutamatergic alterations in females may contribute to enhanced susceptibility to stress-induced reinstatement of drug seeking. Further, it is also possible that as estradiol levels fluctuate across the estrous cycle, this resilience to stress-induced glutamatergic transmission and receptor composition may fluctuate as well. In contrast, females do exhibit stress-induced alterations in glutamate transporter expression. Sub-chronic variable stress results in increased VGLUT2 in the NAc in female mice to a much greater degree than in male mice (Brancato et al., 2017). Following chronic social defeat stress, both males and females exhibit a decrease in GLT1 in the striatum, while females exhibit a decrease in the PFC (Rappeneau et al., 2016). Females, but not males, also exhibit a reduction in GFAP immunoreactivity in the PFC and NAc following chronic social defeat stress. Despite differences in stress-induced alterations in GLT1 expression and GFAP immunoreactivity in the nucleus accumbens, both males and females exhibit similar reductions in extracellular glutamine (Rappeneau et al., 2016), demonstrating that similar outcomes may be mediated by different underlying mechanisms in males and females.
Importantly, while these behavioral assessments of the contribution of glutamatergic projections to reinstatement related behaviors were predominantly performed in males, the same circuits may contribute to relapse-related behaviors in females. Further, sex and estrous cycle-based differences in glutamate circuit function might contribute to observed sex differences in behavior. For example, NMDAR antagonism in the amygdala reduces cue-induced reinstatement in males but may have sex-dependent effects due to higher levels of NR1 in the amygdala of females (Table 1), but these differences remain to be assessed experimentally.
3.2. Sex Differences in Pharmacological Effects of Drug Seeking
Sex differences in the efficacy of pharmacologically altering glutamate uptake have been identified. The cystine prodrug, N-acetylcysteine, which increases glutamatergic tone on presynaptic glutamate receptors by enhancing cystine/glutamate exchange via system xc−, has been shown to reduce self-administration (Lebourgeois et al., 2018; Reichel et al., 2011; Zhou and Kalivas, 2008) and reinstatement of drug seeking behaviors (Reichel et al., 2011; Reissner et al., 2015). Recently, it was shown that N-acetylcysteine reduces cue-induced reinstatement of nicotine seeking in male, but not female rats, regardless of estrous phase (Goenaga et al., 2019), indicating either that reinstatement of nicotine seeking may be mediated by distinct mechanisms in male and females or that males and females are differentially impacted by N-acetylcysteine effects on glutamate levels (Table 1). Alternatively, system xc− may not be critical to glutamate homeostasis in females, as mutation of the xCT subunit in the striatum does not decrease glutamate levels as it does in males (Borra et al., 2014). Estrous phase also influences efficacy of pharmacologically manipulated glutamate uptake as upregulation of GLT1 and system xc− in the NAc of male and female rats using the β-lactam antibiotic, ceftriaxone, attenuates reinstatement of cocaine-seeking in male and non-estrus female rats (Bechard et al., 2018b). In contrast, increasing glutamate uptake in the NAc and PFC using ceftriaxone reduces ethanol consumption in both male and female alcohol-preferring rats (Alhaddad et al., 2014; Qrunfleh et al., 2013; Rao and Sari, 2014; Sari et al., 2013, 2011). Despite similar effects in males and females, it is possible that ceftriaxone may be mediating its effects primarily via GLT1 in females in light of the previously observed lack of effect of system xc− manipulation on glutamate uptake in females (Borra et al., 2014) (Table 1). Interestingly, while chronic alcohol exposure reduced GLT1 expression in male rats administering alcohol, other groups have reported that female P rats do not exhibit this change in protein expression (Ding et al., 2013). Together, these preclinical findings may suggest that GLT1 is a particularly promising target for pharmacotherapeutic strategy for reducing drug use in females.
3.3. Sex Differences in Drug-induced Glutamate Alterations
Drug exposure appears to alter glutamatergic signaling in a sex-specific manner (Figure 2). Recently, it was shown that in the prefrontal cortex, females exhibit reduced baseline spontaneous glutamate release, but greater evoked glutamate release following methamphetamine self-administration than males, despite similarities in the amount of methamphetamine self-administered by males and females (Ignacio Pena-Bravo et al., 2019). This increase in excitatory neurotransmission was accompanied by an increase in NMDA currents, leading the authors to propose that methamphetamine self-administration results in upregulation of NMDA receptors in the prefrontal cortex of females. It is also possible that basal (Wang et al., 2015) and drug exposure-induced sex differences in NMDA receptor subunit composition contribute to alterations in NMDA currents in females, though sex differences in the effects of methamphetamine on NMDA receptor subunit expression have not been investigated (Table 1).
Although minimal research has investigated sex differences in the role of glutamate receptors in drug seeking behaviors, sex differences in drug exposure-induced alterations in receptor and receptor subunit expression have been identified (Figure 2). Female, but not male, rats exhibit increased expression of the NR1 subunit of the NMDA receptor in the cerebral cortex as early as 3 days following exposure to a liquid diet containing ethanol (Devaud and Alele, 2004). In contrast, male, but not female, rats exhibit an increase in NR1 subunit expression in the hippocampus (Devaud and Alele, 2004). Exposure to ethanol also results in significant increases in expression of NR2A in the cortex and hippocampus at 9 days, but this is present in both males and females (Devaud and Alele, 2004). The impact of drugs of abuse on receptor expression may also be estrous stage dependent; for example, cocaine self-administration selectively increases GluA1 surface expression in estrus females relative to non-estrus female rats (Bechard et al., 2018b) (Table 1).
Withdrawal from drug exposure also results in glutamate receptor expression alterations in males and females (Figure 2). Ethanol withdrawn female rats exhibit a significant increase in NR1 in the cerebral cortex and hypothalamus compared to male rats and control females (Devaud and Morrow, 1999). The NR2A subunit is increased in the hippocampus of ethanol-withdrawn male rats, but increased in the hypothalamus of ethanol-withdrawn female rats (Devaud and Morrow, 1999). NR2B expression increases in the cortex of ethanol-withdrawn female, but not male rats (Devaud and Alele, 2004). NR2B expression also increases in the hippocampus of both males and females following ethanol withdrawal, but in the cortex, this is specific to females (Devaud and Alele, 2004). Cocaine withdrawal modulates receptor subunit expression in males, but this has not been investigated in females. Withdrawal from cocaine results in a transient increase in NR2A in the basolateral amygdala of males on day 1 of withdrawal from cocaine (Lu et al., 2005). In contrast, the NR2B subunit is altered by cocaine withdrawal in delayed manner, decreasing on day 30 in the basolateral amygdala (Lu et al., 2005). The GluR1 subunit of the AMPA receptor is increased in males in the basolateral amygdala for up to 30 days of withdrawal from cocaine (Lu et al., 2005) and in the nucleus accumbens shell following extinction of cocaine seeking (Sutton et al., 2003). Sex differences in drug-induced alterations in AMPA receptor subunits have not been investigated.
In addition to glutamate receptor subunit composition, withdrawal from an ethanol diet also appears to result in sex differences in glutamate uptake (Figure 2, Devaud and Alele, 2004). Female, but not male, rats also exhibit a significant increase in both glutamate uptake into cortical synaptoneurosomes and expression of the neuronal glutamate transporter, EAAT3, in the cortex and hippocampus following withdrawal (Alele and Devaud, 2005), suggesting that the resilience females exhibit during withdrawal may be in part mediated by an increased efficiency at clearing extracellular glutamate following withdrawal.
4. Implications for sex differences in glutamate signaling in drug related behaviors
Our understanding of sex differences in drug-seeking behaviors is far from complete, but both clinical and preclinical studies have identified sex differences in drug use patterns, motivation to take drugs, and factors that drive relapse. Further, the menstrual/estrous cycle plays a role in many drug seeking behaviors for some, but not all, drugs of abuse. Men are more likely to have a substance use disorder, but women with substance use disorders are more likely to experience worse health outcomes. The subjective experience of taking drugs is also different for men and women and depends on the drug of abuse.
Men and women are also differentially susceptible to relapse, depending on the drug of abuse. Women appear to be more vulnerable to cue-induced relapse of cocaine and nicotine seeking (Doran, 2014; Niaura et al., 2002; Robbins et al., 1999), with the menstrual cycle further modulating the susceptibility to relapse to cocaine use (Franklin et al., 2004; Sofuoglu et al., 2007). Although rodent models indicate that males and females are similarly susceptible to cue-induced reinstatement of cocaine and nicotine seeking (Feltenstein et al., 2012; Fuchs et al., 2005), cue-induced cocaine reinstatement is modulated by the estrous cycle (Fuchs et al., 2005). In contrast, men and male rodents are more susceptible to cue-induced relapse to alcohol use, while women and female rodents exhibit enhanced susceptibility to stress-induced alcohol seeking (Barker and Taylor, 2019). While clinical data has not investigated sex differences in susceptibility to relapse to methamphetamine use, preclinical data suggests that female rodents are more susceptible to both cue and stress-induced reinstatement (Cox et al., 2013). The menstrual cycle may further modulate the susceptibility to relapse to cocaine use. For example, women experience greater cue-induced craving of nicotine during the luteal phase relative to the follicular phase, suggesting a higher risk of relapse (Franklin et al., 2004). Women experience increased cue and stress-induced craving of cocaine when progesterone levels are lower (Sofuoglu et al., 2007). While both estrous cycle and menstrual cycle dependent changes in drug seeking have been identified, phases of the estrous cycle in rodents cannot be correlated exactly with phases of the menstrual cycle. Thus, in designing intervention strategies, it will be important to consider both the effects of menstrual phase and hormone level on craving as we can identify critical periods during which women may be at an elevated risk for relapse. Future studies should consider the factors that drive relapse in men and women as well as consider the role of hormones in relapse.
We also know that the glutamate system is differentially regulated in females compared to males at baseline, in response to drug use/exposure, and by stress (Table 1). These alterations appear to be modulated, in some cases, by the estrous cycle. For example, drug exposure induced alterations in GluA1 during estrus may represent a potential mechanism driving enhanced responding for cocaine during estrus. Further, spine density in the NAc is modulated by estradiol levels via mGluR5 and this may have implications for mGluR5 as a target for substance use disorders in females. Thus, estrous cycle regulation of glutamatergic alterations is an important factor to consider. Future research should also consider the role of hormones in glutamatergic alterations in males. Testosterone does not affect NR2A, NR2B, and NR1 mRNA in the hippocampus and cingulate cortex (Weiland et al., 1997), but it is unknown how testosterone levels may affect metabotropic glutamate receptor expression or glutamate transporters or in other brain regions. Similar to what has been observed with estradiol in females, testosterone and dihydrotestosterone have been shown to modulate spine density in the hippocampus in males (Hatanaka et al., 2015). Estradiol has also been shown to modulate glutamate synaptic potentiation via different receptors in males and females (Oberlander and Woolley, 2016). Thus, the same hormones may be acting via different mechanisms to modulate glutamate neurotransmission in males and females.
Observed sex and estrous cycle specific patterns of response to pharmacological manipulations may be mediated by either differences in response to pharmacological manipulations or by mechanistic differences underlying drug use, and a further understanding of these differences will enable development of personalized and targeted pharmacotherapeutic strategies (Table 1). For example, multiple small pilot studies have identified N-acetylcysteine as a promising therapeutic for the treatment of addiction-related behaviors. N-acetylcysteine treatment has been shown to reduce craving (Mousavi et al., 2015) in methamphetamine-dependent individuals and both craving (Amen et al., 2010) and relapse (Mardikian et al., 2007) in cocaine-dependent individuals, with mixed results in nicotine-dependent individuals (Froeliger et al., 2015; Schmaal et al., 2011). Most of these studies identify the major limitation that their studies contain insufficient numbers of female subjects to determine if N-acetylcysteine is effective in women. Preclinical research has indicated that modulation of glutamate signaling as an effective therapeutic strategy may be both sex- and estrous cycle-dependent as N-acetylcysteine is effective at reducing cue-induced reinstatement of nicotine seeking in males, but not females (Goenaga et al., 2019) and ceftriaxone was only effective at reducing reinstatement of cocaine seeking in male and non-estrus females (Bechard et al., 2018b). Consideration of these differences in the neurobiology of drug abuse may necessitate sex-specific treatment strategies for preventing relapse. This is supported by preclinical data suggesting that pharmacological therapeutics targeting the glutamate system are not equally effective in males and females. By continuing to investigate sex differences in the neurobiological underpinnings of drug seeking behaviors and considering important factors such as baseline and drug-induced differences in glutamate signaling and the role of the menstrual cycle, we can identify therapeutic targets that are more effective in both men and women.
Highlights.
Drug seeking behaviors are modulated by sex and menstrual/estrous cycle
Sex, estrous cycle, and drug exposure interact to regulate the glutamate system
Effects of modulation of the glutamate system on drug seeking are sex specific
Acknowledgements
This work was supported by funds from the Drexel University College of Medicine and NIH grant R00AA024499 (JMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alele PE, Devaud LL, 2005. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol. Clin. Exp. Res 29, 1027–1034. 10.1097/01.ALC.0000167743.96121.40 [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SHS, Wei Y, Sari Y, 2014. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front. Behav. Neurosci 8, 1–13. 10.3389/fnbeh.2014.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OFX, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK, 1998. Gender differences in ethanol preference and ingestion in rats: The role of the gonadal steroid environment. J. Clin. Invest 101, 2677–2685. 10.1172/JCI1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen SL, Risinger RC, Mantsch JR, Piacentine LB, Baker DA, Li S-J, Ahmad ME, 2010. Repeated N-Acetyl Cysteine Reduces Cocaine Seeking in Rodents and Craving in Cocaine-Dependent Humans. Neuropsychopharmacology 36, 871–878. 10.1038/npp.2010.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA, 2000. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia. [DOI] [PubMed] [Google Scholar]
- Arias C, Zepeda A, Hernández-Ortega K, Leal-Galicia P, Lojero C, Camacho-Arroyo I, 2009. Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus. Horm. Behav 55, 257–263. 10.1016/j.yhbeh.2008.10.016 [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG, 1997. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci 94, 4155–4160. 10.1073/pnas.94.8.4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MJ, Olin JS, Riche WH, Kornaczewski A, Schmidt W, Rankin JG, 1977. Morbidity in Alcoholics: Evidence for Accelerated Development of Physical Disease in Women. Arch. Intern. Med 137, 883–887. 10.1001/archinte.1977.03630190041012 [DOI] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J, 2016. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J. Neurosci 10.1523/JNEUROSCI.0773-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, 2019. Sex differences in incentive motivation and the relationship to the development and maintenance of alcohol use disorders. Physiol. Behav 203, 91–99. 10.1016/j.physbeh.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschnagel JS, 2013. Using mobile eye-tracking to assess attention to smoking cues in a naturalized environment. Addict. Behav 38, 2837–2840. 10.1016/j.addbeh.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P, 2013. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 38, 1401–1408. 10.1038/npp.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechard AR, Hamor PU, Schwendt M, Knackstedt LA, 2018a. The effects of ceftriaxone on cue-primed reinstatement of cocaine-seeking in male and female rats: estrous cycle effects on behavior and protein expression in the nucleus accumbens. Psychopharmacology (Berl). 235, 837–848. 10.1007/s00213-017-4802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechard AR, Hamor PU, Schwendt M, Knackstedt LA, 2018b. The effects of ceftriaxone on cue-primed reinstatement of cocaine-seeking in male and female rats: estrous cycle effects on behavior and protein expression in the nucleus accumbens. Psychopharmacology (Berl). 235, 837–848. 10.1007/s00213-017-4802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker U, Deis A, Sørensen TIA, Grønbæk M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G, 1996. Prediction of risk of liver disease by alcohol intake, sex, and age: A prospective population study. Hepatology 23, 1025–1029. 10.1053/jhep.1996.v23.pm0008621128 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P, 2006. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther 79, 480–488. 10.1016/j.clpt.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Bertholomey ML, Nagarajan V, Torregrossa MM, 2016. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl). 233, 2277–2287. 10.1007/s00213-016-4278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Lohmann P, West B, Brown RM, Kirchhoff J, Raymond CR, Lawrence AJ, 2014. The mGlu5 receptor regulates extinction of cocaine-driven behaviours. Drug Alcohol Depend. 137, 83–89. 10.1016/j.drugalcdep.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Blümcke I, Behle K, Malitschek B, Kuhn R, Knöpfel T, Wolf HK, Wiestler OD, 1996. Immunohistochemical distribution of metabotropic glutamate receptor subtypes mGluR1b, mGluR2/3, mGluR4a and mGluR5 in human hippocampus. Brain Res. 736, 217–226. 10.1016/0006-8993(96)00697-X [DOI] [PubMed] [Google Scholar]
- Borra S, McCullagh EA, Featherstone DE, Baker PM, Ragozzino ME, Shippy SA, 2014. Determining striatal extracellular glutamate levels in xCT mutant mice using LFPS CE-LIF. Anal. Methods 6, 2916–2922. 10.1039/c4ay00392f [DOI] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM, 1999. Effects of sex and gonadectomy on cocaine metabolism in the rat. J. Pharmacol. Exp. Ther [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE, 2017. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350, 180–189. 10.1016/j.neuroscience.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A, 2001. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J. Neural Transm 108, 887–894. 10.1007/s007020170038 [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Natale NR, Patel SA, 2012. System xc-cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol 165, 20–34. 10.1111/j.1476-5381.2011.01480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A, 2012. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 76, 790–803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS, 1993. The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol 338, 255–278. 10.1002/cne.903380209 [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE, 2012. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol. Behav 105, 209–214. 10.1016/j.physbeh.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, Mormède P, 2001. Sex and strain differences in ethanol drinking: Effects of gonadectomy. Alcohol. Clin. Exp. Res 25, 594–599. 10.1111/j.1530-0277.2001.tb02255.x [DOI] [PubMed] [Google Scholar]
- Cannady R, McGonigal JT, Newsom RJ, Woodward JJ, Mulholland PJ, Gass JT, 2017. Prefrontal Cortex K Ca 2 Channels Regulate mGlu 5 -Dependent Plasticity and Extinction of Alcohol-Seeking Behavior. J. Neurosci 10.1523/jneurosci.2873-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Gillespie RA, Baker LH, Kaplan RF, 1987. Cognitive Changes After Alcohol Cue Exposure. J. Consult. Clin. Psychol 55, 150–155. 10.1037/0022-006X.55.2.150 [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM, 2013. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology. 10.1016/j.psyneuen.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippens D, Lancaster FE, White ML, Brunner LJ, Gonzales RA, George MA, Jaworski JN, 2006. Gender Differences in Blood Levels, But Not Brain Levels, of Ethanol in Rats. Alcohol. Clin. Exp. Res 23, 414–420. 10.1111/j.1530-0277.1999.tb04131.x [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Shields CN, 2018. Effects of sex on ethanol conditioned place preference, activity and variability in C57BL/6J and DBA/2J mice. Pharmacol. Biochem. Behav 173, 84–89. 10.1016/j.pbb.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta U, Martini M, Fan M, Sun WL, 2018. Compulsive sucrose- and cocaine-seeking behaviors in male and female Wistar rats. Psychopharmacology (Berl). 235, 2395–2405. 10.1007/s00213-018-4937-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Alele P, 2004. Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol. Clin. Exp. Res 28, 957–965. 10.1097/01.ALC.0000128225.83916.40 [DOI] [PubMed] [Google Scholar]
- Devaud LL, Morrow AL, 1999. Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus. Eur. J. Pharmacol 369, 331–334. 10.1016/S0014-2999(99)00103-X [DOI] [PubMed] [Google Scholar]
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL, 2012. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol. Biochem. Behav 102, 540–548. 10.1016/j.pbb.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ, 2001. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology 25, 341–360. 10.1016/S0893-133X(01)00235-4 [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ, 2013. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol preferring (P) rats. Addict. Biol 157–168, 18 10.1111/ADB.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF, 1999. The glutamate receptor ion channels. Pharmacol. Rev [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S, 2000. Nicotine self-administration in rats: Estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl). 151, 392–405. 10.1007/s002130000497 [DOI] [PubMed] [Google Scholar]
- Doran N, 2014. Sex differences in smoking cue reactivity: Craving, negative affect, and preference for immediate smoking. Am. J. Addict 23, 211–217. 10.1111/j.1521-0391.2014.12094.x [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW, 2002. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl). 159, 397–406. 10.1007/s00213-001-0944-7 [DOI] [PubMed] [Google Scholar]
- Famous KR, Schmidt HD, Christopher Pierce R, 2007. When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci. Lett 420, 169–173. 10.1016/j.neulet.2007.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE, 2012. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 121, 240–246. 10.1016/j.drugalcdep.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE, 2011. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: Sex differences and the role of the estrous cycle. Psychopharmacology (Berl). 216, 53–62. 10.1007/s00213-011-2187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE, 2007. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol. Learn. Mem 88, 435–444. 10.1016/j.nlm.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Fuchs E, Wuttke W, 1984. Sex differences in γ-aminobutyric acid and glutamate concentrations in discrete rat brain nuclei. Neurosci. Lett 50, 245–250. 10.1016/0304-3940(84)90493-2 [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O’Brien CP, Childress AR, 2004. Retrospective study: Influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob. Res 6, 171–175. 10.1080/14622200310001656984 [DOI] [PubMed] [Google Scholar]
- Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM, 2015. The effects of N-Acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 156, 234–242. 10.1016/j.drugalcdep.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE, 2005. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl). 179, 662–672. 10.1007/s00213-004-2080-7 [DOI] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin CLG, Dykes-Hoberg M, Rothstein JD, 1997. Cellular and synaptic localization of the neuronal glutamate transporters excitatory amino acid transporter 3 and 4. Neuroscience. 10.1016/S0306-4522(97)00252-2 [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF, 2008. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol 75, 218–265. 10.1016/j.bcp.2007.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF, 2011. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict. Biol 16, 215–228. 10.1111/j.1369-1600.2010.00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenaga J, Powell GL, Leyrer-Jackson JM, Piña J, Phan S, Prakapenka AV, Koebele SV, Namba MD, McClure EA, Bimonte-Nelson HA, 2019. N-acetylcysteine yields sex-specific efficacy for cue-induced reinstatement of nicotine seeking. Addict. Biol. 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013. JAMA Psychiatry 74, 911 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U, 1989. A Comparison of Male and Female Cocaine Abusers. Arch. Gen. Psychiatry 46, 122–126. 10.1001/archpsyc.1989.01810020024005 [DOI] [PubMed] [Google Scholar]
- Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, 2014. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. 10.1038/npp.2013.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, der Zee E.V. Van, te Kortschot A, Witter MP, 1987. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23, 103–120. 10.1016/0306-4522(87)90275-2 [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Herta F, Mann K, Braus DF, Heinz A, 2004. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl). 175, 296–302. 10.1007/s00213-004-1828-4 [DOI] [PubMed] [Google Scholar]
- Hartwell EE, Moallem NR, Courtney KE, Glasner-Edwards S, Ray LA, 2016. Sex differences in the association between internalizing symptoms and craving in methamphetamine users. J. Addict. Med 10, 395–401. 10.1097/ADM.0000000000000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Ray LA, 2013. Sex moderates stress reactivity in heavy drinkers. Addict. Behav 10.1016/j.addbeh.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Hojo Y, Mukai H, Murakami G, Komatsuzaki Y, Kim J, Ikeda M, Hiragushi A, Kimoto T, Kawato S, 2015. Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: Dependence on synaptic androgen receptor and kinase networks. Brain Res. 1621, 121–132. 10.1016/j.brainres.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW, 2014. Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport 25, 105–109. 10.1097/WNR.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH, 2012. Ventral Tegmental Area Glutamate Neurons: Electrophysiological Properties and Projections. J. Neurosci 32, 15076–15085. 10.1523/jneurosci.3128-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, De Wit H, 2000. Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology (Berl). 150, 374–382. 10.1007/s002130000461 [DOI] [PubMed] [Google Scholar]
- Ignacio Pena-Bravo J, Penrod R, Reichel CM, Lavin A, 2019. Methamphetamine self-administration elicits sex related changes in postsynaptic glutamate transmission in the prefrontal cortex. eneuro ENEURO.0401–18.2018. 10.1523/ENEURO.0401-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA, 2009. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob. Res 10.1093/ntr/ntp076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen BH, Laberg JC, Cox WM, Vaksdal A, Hugdahl K, 1994. Alcoholic Subjects’ Attentional Bias in the Processing of Alcohol-Related Words. Psychol. Addict. Behav 8, 111–115. 10.1037/0893-164X.8.2.111 [DOI] [Google Scholar]
- Juárez J, De Tomasi EB, 1999. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol 19, 15–22. 10.1016/S0741-8329(99)00010-5 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, LaLumiere RT, Knackstedt L, Shen H, 2009. Glutamate transmission in addiction. Neuropharmacology. 10.1016/j.neuropharm.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keistler CR, Hammarlund E, Barker JM, Bond CW, DiLeone RJ, Pittenger C, Taylor JR, 2017. Regulation of Alcohol Extinction and Cue-Induced Reinstatement by Specific Projections among Medial Prefrontal Cortex, Nucleus Accumbens, and Basolateral Amygdala. J. Neurosci 37, 4462–4471. 10.1523/JNEUROSCI.3383-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, 1982. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde and retrograde-horseradish peroxidase study. Neuroscience 7, 2321–2335. 10.1016/0306-4522(82)90198-1 [DOI] [PubMed] [Google Scholar]
- Kerridge BT, Chou SP, Pickering RP, Ruan WJ, Huang B, Jung J, Zhang H, Fan AZ, Saha TD, Grant BF, Hasin DS, 2019. Changes in the prevalence and correlates of cocaine use and cocaine use disorder in the United States, 2001–2002 and 2012–2013. Addict. Behav 10.1016/j.addbeh.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE, 2008. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl). 10.1007/s00213-008-1089-8 [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE, 2005. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl). 182, 245–252. 10.1007/s00213-005-0071-y [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M, 2014. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict. Biol 10.1111/adb.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI, 2008. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl). 10.1007/s00213-008-1117-8 [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Owens GF, Chattopadhyay B, DeBethizy JD, Vesell ES, 1988. Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab. Dispos [PubMed] [Google Scholar]
- Lalumiere RT, Smith KC, Kalivas PW, 2012. Neural circuit competition in cocaine-seeking: Roles of the infralimbic cortex and nucleus accumbens shell. Eur. J. Neurosci 35, 614–622. 10.1111/j.1460-9568.2012.07991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB, 1996. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague-Dawley rats. Alcohol. Clin. Exp. Res 20, 1043–1049. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS, 1992. Sex differences in pattern of drinking. Alcohol 9, 415–420. 10.1016/0741-8329(92)90041-8 [DOI] [PubMed] [Google Scholar]
- Lebourgeois S, González-Marín MC, Jeanblanc J, Naassila M, Vilpoux C, 2018. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict. Biol 10.1111/adb.12521 [DOI] [PubMed] [Google Scholar]
- Li D, Herault K, Silm K, Evrard A, Wojcik S, Oheim M, Herzog E, Ropert N, 2013. Lack of Evidence for Vesicular Glutamate Transporter Expression in Mouse Astrocytes. J. Neurosci 33, 4434–4455. 10.1523/JNEUROSCI.3667-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A, 2007. Metabotropic Glutamate 2/3 Receptors in the Ventral Tegmental Area and the Nucleus Accumbens Shell Are Involved in Behaviors Relating to Nicotine Dependence. J. Neurosci 27, 9077–9085. 10.1523/jneurosci.1766-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littel M, Franken IH, 2011. Implicit and explicit selective attention to smoking cues in smokers indexed by brain potentials. J. Psychopharmacol 25, 503–513. 10.1177/0269881110379284 [DOI] [PubMed] [Google Scholar]
- Lourdes De La Torre M, Dolores Escarabajal M, Agüero Á, 2015. Sex differences in adult Wistar rats in the voluntary consumption of ethanol after pre-exposure to ethanol-induced flavor avoidance learning. Pharmacol. Biochem. Behav 137, 7–15. 10.1016/j.pbb.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shaham Y, Hope BT, 2005. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J. Neurochem 94, 161–168. 10.1111/j.1471-4159.2005.03178.x [DOI] [PubMed] [Google Scholar]
- Luine VN, Grattan DR, Selmanoff M, 1997. Gonadal hormones after hypothalamic GABA and glutamate levels. Brain Res. 747, 165–168. 10.1016/S0006-8993(96)01255-3 [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH, 1996. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl). 125, 346–54. 10.1007/BF02246017 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME, 2000. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl). 152, 132–139. 10.1007/s002130000488 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl). 144, 77–82. 10.1007/s002130050979 [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL, 2010. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol. Biochem. Behav 96, 476–487. 10.1016/j.pbb.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ, 2007. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Prog. Neuro-Psychopharmacology Biol. Psychiatry 31, 389–394. 10.1016/j.pnpbp.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Martinez FG, Hermel EES, Xavier LL, Viola GG, Riboldi J, Rasia-Filho AA, Achaval M, 2006. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity in the medial amygdala subnuclei across the estrous cycle and in castrated and treated female rats. Brain Res. 1108, 117–126. 10.1016/j.brainres.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Mcdonald AJ, 1991. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 44, 15–33. 10.1016/0306-4522(91)90248-M [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW, 2003. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci 23, 3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH, 2013. Rescue of Infralimbic mGluR2 Deficit Restores Control Over Drug-Seeking Behavior in Alcohol Dependence. J. Neurosci 10.1523/JNEUROSCI.4062-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM, 1999. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology 21, 294–303. 10.1016/S0893-133X(99)00020-2 [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, 1999. Operant ethanol reward in C57BL/6 mice: Influence of gender and procedural variables. Alcohol 17, 185–194. 10.1016/S0741-8329(98)00056-1 [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM, 2005. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol. Appl. Pharmacol 209, 203–213. 10.1016/j.taap.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, Janak PH, 2017. Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience. 10.1016/j.neuroscience.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, McNally GP, 2011. Accumbens shell AMPA receptors mediate expression of extinguished reward seeking through interactions with basolateral amygdala. Learn. Mem 18, 414–421. 10.1101/lm.2144411 [DOI] [PubMed] [Google Scholar]
- Mitrovic AD, Maddison JE, Johnston GAR, 1999. Influence of the oestrous cycle on L-glutamate and L-aspartate transport in rat brain synaptosomes. Neurochem. Int 34, 101–108. 10.1016/S0197-0186(98)00066-7 [DOI] [PubMed] [Google Scholar]
- Mousavi SG, Sharbafchi MR, Salehi M, Peykanpour M, Sichani NK, Maracy M, 2015. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: A double-blind controlled, crossover study. Arch. Iran. Med [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA, 1999. Gender differences in moderate drinking effects. Alcohol Res. Health 23, 55–64. [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Monti PM, Rohsenow DJ, Shadel WG, Sirota A, 2002. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict. Behav 23, 209–224. 10.1016/s0306-4603(97)00043-9 [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ, 2010. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu Rev Pharmacol Toxicol 50, 295–322. 10.1146/annurev.pharmtox.011008.145533.Metabotropic [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E, 2011. In vivo detection of GABA and glutamate with MEGA-PRESS: Reproducibility and gender effects. J. Magn. Reson. Imaging 33, 1262–1267. 10.1002/jmri.22520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS, 2016. 17β-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. J. Neurosci 36, 2677–2690. 10.1523/jneurosci.4437-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Bidmon HJ, Zilles K, 2003. AMPA, kainate, and NMDA receptor densities in the hippocampus of untreated male rats and females in estrus and diestrus. J. Comp. Neurol 459, 468–474. 10.1002/cne.10638 [DOI] [PubMed] [Google Scholar]
- Perkins KA, 1999. Nicotine discrimination in men and women, in: Pharmacology Biochemistry and Behavior. pp. 295–299. 10.1016/S0091-3057(99)00085-4 [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, 2006. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl). 186, 143–149. 10.1007/s00213-006-0372-9 [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW, 2008. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci 10.1523/JNEUROSCI.1045-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL, 2015. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct. Funct 220, 2415–2422. 10.1007/s00429-014-0794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schönig K, Bartsch D, Hope BT, Spanagel R, Sommer WH, 2015. Losing control: Excessive alcohol seeking after selective inactivation of cue-responsive neurons in the infralimbic cortex. J. Neurosci 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau DR, Hobbs NJ, Breedlove SM, Jordan CL, 2016. Sex and laterality differences in medial amygdala neurons and astrocytes of adult mice. J. Comp. Neurol 10.1002/cne.23964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KIA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R, 2012. Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. Am. J. Psychiatry 169, 406–414. 10.1176/appi.ajp.2011.11020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF, 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav 152, 61–67. 10.1016/j.pbb.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian A, Johnson JW, 2002. Channel gating of NMDA receptors. Physiol. Behav 77, 577–582. 10.1016/S0031-9384(02)00906-X [DOI] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y, 2013. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J. Psychopharmacol 27, 541–549. 10.1177/0269881113482529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Stewart RT, Besheer J, 2017. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol. Biochem. Behav 1–9. 10.1016/j.pbb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PSS, Sari Y, 2014. Effects of ceftriaxone on chronic ethanol consumption: A potential role for xCT and GLT1 modulation of glutamate levels in male P rats. J. Mol. Neurosci 10.1007/s12031-014-0251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappeneau V, Blaker A, Petro JR, Yamamoto BK, Shimamoto A, 2016. Disruption of the Glutamate–Glutamine Cycle Involving Astrocytes in an Animal Model of Depression for Males and Females. Front. Behav. Neurosci 10 10.3389/fnbeh.2016.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE, 2011. Chronic N-Acetylcysteine during Abstinence or Extinction after Cocaine Self-Administration Produces Enduring Reductions in Drug Seeking. J. Pharmacol. Exp. Ther 337, 487–493. 10.1124/jpet.111.179317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW, 2015. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict. Biol 20, 316–323. 10.1111/adb.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro F, Paquet M, Cregan SP, Ferguson SSG, 2010. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol. Disord. Drug Targets 9, 574–95. 10.2174/187152710793361612 [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J, 2003. Glutamate receptor function in learning and memory. Behav. Brain Res 10.1016/S0166-4328(02)00272-3 [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP, 1999. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 53, 223–230. 10.1016/S0376-8716(98)00135-5 [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF, 1998. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol. Clin. Exp. Res 22, 1564–1569. 10.1111/j.1530-0277.1998.tb03950.x [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ, 1989. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl). 98, 408–411. 10.1007/BF00451696 [DOI] [PubMed] [Google Scholar]
- Robinson DL, Brunner LJ, Gonzales RA, 2002. Effect of gender and estrous cycle on the pharmacokinetics of ethanol in the rat brain. Alcohol. Clin. Exp. Res 26, 165–172. 10.1111/j.1530-0277.2002.tb02521.x [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW, 2010. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur. J. Neurosci 31, 903–909. 10.1111/j.1460-9568.2010.07134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CF, Verkhratsky A, Parpura V, 2013. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem. Soc. Trans 41, 1518–1524. 10.1042/BST20130237 [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004a. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl). 172, 443–449. 10.1007/s00213-003-1670-0 [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004b. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl). 10.1007/s00213-003-1670-0 [DOI] [PubMed] [Google Scholar]
- Rubonis A, Colby S, Monti P, Rohsenow D, Gulliver S, Sirota A, 1994. Alcohol cue reactivity and mood induction in male and female alcoholics. J. Stud. Alcohol Drugs 55, 487–494. 10.15288/jsa.1994.55.487 [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V, 2003. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 10.1016/S0006-8993(03)02346-1 [DOI] [PubMed] [Google Scholar]
- Sari Y, Franklin KM, Alazizi A, Rao PSS, Bell RL, 2013. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience 241, 229–238. 10.1016/j.neuroscience.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL, 2011. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 10.1093/alcalc/agr023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, Van Den Brink W, 2011. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: A double-blind placebo-controlled pilot study. Eur. Addict. Res 17, 211–216. 10.1159/000327682 [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R, 2011. Sex differences in neural responses to stress and alcohol context cues. Hum. Brain Mapp 32, 1998–2013. 10.1002/hbm.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT, 2012. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol. Biochem. Behav 101, 329–335. 10.1016/j.pbb.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS, 2000. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl). 152, 140–148. 10.1007/s002130000499 [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ, 2006. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry 63, 324–331. 10.1001/archpsyc.63.3.324 [DOI] [PubMed] [Google Scholar]
- Sluyter F, Hof M, Ellenbroek BA, Degen SB, Cools AR, 2000. Genetic, sex, and early environmental effects on the voluntary alcohol intake in Wistar rats. Pharmacol. Biochem. Behav. 67, 801–808. 10.1016/S0091-3057(00)00425-1 [DOI] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice. Alcohol. Clin. Exp. Res 43, 243–249. 10.1111/acer.13923 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK, 1999. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol 7, 274–283. 10.1037/1064-1297.7.3.274 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Sinha R, Hong K-I, Morgan PT, Bergquist KT, Fox H, 2007. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: Implications for relapse susceptibility. Exp. Clin. Psychopharmacol 15, 445–452. 10.1037/1064-1297.15.5.445 [DOI] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL, 2011. Estradiol reduces dendritic spine density in the ventral striatum of female syrian hamsters. Brain Struct. Funct 215, 187–194. 10.1007/s00429-010-0284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski P, Janovjak H, Trauner D, 2010. Pharmacology of ionotropic glutamate receptors: A structural perspective. Bioorganic Med. Chem 10.1016/j.bmc.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW, 2013. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front. Behav. Neurosci 7 10.3389/fnbeh.2013.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW, 2016. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct. Funct 221, 1681–1689. 10.1007/s00429-015-0997-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormark KM, Field NP, Hugdahl K, Horowitz M, 1997. Selective processing of visual alcohol cues in abstinent alcoholics: An approach-avoidance conflict? Addict. Behav 22, 509–519. 10.1016/S0306-4603(96)00051-2 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A, 2010. Dopaminergic Terminals in the Nucleus Accumbens But Not the Dorsal Striatum Corelease Glutamate. J. Neurosci 30, 8229–8233. 10.1523/jneurosci.1754-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2018. Results from the 2017 National Survey on Drug Use and Health. Vol. I Summ. Natl. Find. [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA, 2010. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl). 211, 267–275. 10.1007/s00213-010-1890-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi K-H, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW, 2003. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421, 70–5. 10.1038/nature01249 [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME, 2016. Sex differences in attenuation of nicotine reinstatement after individual and combined treatments of progesterone and varenicline. Behav. Brain Res 308, 46–52. 10.1016/j.bbr.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T, 2010. Glutamatergic Signaling by Mesolimbic Dopamine Neurons in the Nucleus Accumbens. J. Neurosci 30, 7105–7110. 10.1523/jneurosci.0265-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens M, Gilchrist G, Domingo-Salvany A, 2011. Psychiatric comorbidity in illicit drug users: Substance-induced versus independent disorders. Drug Alcohol Depend. 113, 147–156. 10.1016/j.drugalcdep.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, 2010. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev 62, 405–496. 10.1124/pr.109.002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2014. Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol. Behav 148, 145–150. 10.1016/j.physbeh.2014.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L, 2009. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 44, 547–554. 10.1093/alcalc/agp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL, 2008. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol 42, 83–89. 10.1016/j.alcohol.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, Netusil N, Zhu W, Felder C, Ma Y, 2003. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcohol. Clin. Exp. Res 27, 909–917. 10.1097/01.ALC.0000071740.56375.BA [DOI] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW, 2013. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict. Biol 10.1111/j.1369-1600.2011.00432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma Y, Hu J, Cheng W, Jiang H, Zhang X, Li M, Ren J, Li X, 2015. Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience 301, 363–374. 10.1016/j.neuroscience.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z, 2014. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatry 19, 588–598. 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M, Tanapat P, 1997. Chronic corticosterone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neuroscience 78, 653–662. 10.1016/S0306-4522(96)00619-7 [DOI] [PubMed] [Google Scholar]
- White A, Castle IJP, Chen CM, Shirley M, Roach D, Hingson R, 2015. Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcohol. Clin. Exp. Res 39, 1712–1726. 10.1111/acer.12815 [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJH, De Wit H, 2002. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol. Biochem. Behav 73, 729–741. 10.1016/S0091-3057(02)00818-3 [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR, 2011. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 115, 137–144. 10.1016/j.drugalcdep.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, Mcnally GP, 2013. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur. J. Neurosci 10.1111/ejn.12031 [DOI] [PubMed] [Google Scholar]
- Willner P, Field M, Pitts K, Reeve G, 1998. Mood, cue and gender influences on motivation, craving and liking for alcohol in recreational drinkers. Behav. Pharmacol 9, 631–642. 10.1097/00008877-199811000-00018 [DOI] [PubMed] [Google Scholar]
- Wong AYC, MacLean DM, Bowie D, 2007. Na+/Cl- Dipole Couples Agonist Binding to Kainate Receptor Activation. J. Neurosci 27, 6800–6809. 10.1523/JNEUROSCI.0284-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1994. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J. Neurosci 14, 7680–7687. 10.1523/JNEUROSCI.14-12-07680.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336, 293–306. 10.1002/cne.903360210 [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci 12, 2549–2554. 10.1002/cne.903360210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA, 1997. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci 17, 1848–1859. 10.1523/jneurosci.1427-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Buck LA, Bryant KG, Barker JM, 2019. Sex differences in ethanol reward seeking under conflict in mice. Alcohol. Clin. Exp. Res 0, acer.14070. 10.1111/acer.14070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang H-L, Li X, Ng TH, Morales M, 2011. Mesocorticolimbic Glutamatergic Pathway. J. Neurosci 31, 8476–8490. 10.1523/JNEUROSCI.1598-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S, 2010. Nicotine-induced conditioned place preference in rats: Sex differences and the role of mGluR5 receptors. Neuropharmacology 58, 374–382. 10.1016/j.neuropharm.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, Hnasko TS, 2016. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun 10.1038/ncomms13697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Chanraud S, Gu M, Sullivan EV, Pfefferbaum A, 2013. In vivo glutamate measured with magnetic resonance spectroscopy: Behavioral correlates in aging. Neurobiol. Aging 34, 1265–1276. 10.1016/j.neurobiolaging.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW, 2008. N-Acetylcysteine Reduces Extinction Responding and Induces Enduring Reductions in Cue- and Heroin-Induced Drug-Seeking. Biol. Psychiatry 63, 338–340. 10.1016/j.biopsych.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Gruenbaum BF, Mohar B, Kuts R, Gruenbaum SE, Ohayon S, Boyko M, Klin Y, Sheiner E, Shaked G, Shapira Y, Teichberg VI, 2011. The Effects of Estrogen and Progesterone on Blood Glutamate Levels: Evidence from Changes of Blood Glutamate Levels During the Menstrual Cycle in Women. Biol. Reprod 84, 581–586. 10.1095/biolreprod.110.088120 [DOI] [PubMed] [Google Scholar]