Abstract
Ketamine, a drug introduced in the 1960s as an anesthetic agent and still used for that purpose, has garnered marked interest over the past two decades as an emerging treatment for major depressive disorder. With increasing evidence of its efficacy in treatment-resistant depression and its potential anti-suicidal action, a great deal of investigation has been conducted on elucidating ketamine’s effects on the brain. Of particular interest and therapeutic potential is the ability of ketamine to exert rapid antidepressant properties as early as several hours after administration. This is in stark contrast to the delayed effects observed with traditional antidepressants, often requiring several weeks of therapy for a clinical response. Furthermore, ketamine appears to have a unique mechanism of action involving glutamate modulation via actions at the N-methyl-D-aspartate (NMDA) and -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, as well as downstream activation of brain-derived neurotrophic factor (BDNF) and mechanistic target of rapamycin (mTOR) signaling pathways to potentiate synaptic plasticity. This paper provides a brief overview of ketamine with regard to pharmacology/pharmacokinetics, toxicology, the current state of clinical trials on depression, postulated antidepressant mechanisms and potential biomarkers (biochemical, inflammatory, metabolic, neuroimaging sleep-related and cognitive) for predicting response to and/or monitoring of therapeutic outcome with ketamine.
Keywords: antidepressant, biomarkers, cognition, ketamine, mechanism of action, metabolism, neuroimaging, sleep
Introduction
Major depressive disorder (MDD) remains a significant contributor to the global burden of disease and has been reported to place second for causes of worldwide disability.1 In a 2012 epidemiological study of mental health in Canada, the lifetime prevalence of MDD was noted at 11.3%.2 Unfortunately, the pathophysiology of depression remains largely unknown. While the monoamine hypothesis, postulating that depression is the result of a functional deficiency of serotonin and/or noradrenaline neurotransmitters in the central nervous system,3,4 has been useful for explaining the pharmacology of many antidepressant drugs, it is overly simplistic and does not seem to fully encompass the pathways underlying depression. One significant limitation is the discrepancy in the time frame of antidepressant drugs’ effects on monoamine neurotransmitters (hours to days) and on clinical symptoms (several weeks).3–5 Following treatment with antidepressant drugs, only half of patients are noted to have a significant clinical response.6,7 Furthermore, up to one-third of patients are considered to have treatment-resistant depression (TRD), defined as a lack of response to two or more adequate trials of antidepressant medications.8 Given this large population of patients with TRD, there is a significant need for development of novel and more efficacious antidepressant treatments.
Ketamine is a non-competitive antagonist at glutamate N-methyl-D-aspartate (NMDA) receptors and has been traditionally used as a dissociative anesthetic. It was first reported to have antidepressant properties in 2000, when it was demonstrated that an intravenous (IV) administration of a sub-anesthetic ketamine dose resulted in a reduction of MDD symptoms rapidly and continuing to 72 h after treatment.9 Subsequent randomized controlled trials (RCTs) replicated this finding, demonstrating a 60–70% response rate of ketamine in TRD populations.10–14 Of specific interest is the finding that ketamine has a rapid clinical effect within 2–4 h after administration. However, ketamine’s antidepressant properties are also transient, lasting an average of 1 week following a single infusion10–14 and 18–19 days following repeated infusions.15,16 Retrospective, real-world clinical data demonstrated a response rate of 44% after six intravenous ketamine treatments in a population of complex patients with multiple comorbidities and ultra-resistant depression.17,18 Furthermore, ketamine has been reported to also have anti-suicidal and anti-anhedonic actions.14,19,20 Not only does ketamine exert a rapid clinical effect within several hours and demonstrate efficacy in patients unresponsive to other antidepressants, it appears to have a novel mechanism of action that is distinct from conventional antidepressant drugs. This paper is a review of the current state of knowledge on the pharmacology/pharmacokinetics, status of clinical trials, adverse effects and postulated mechanisms of action of ketamine as an antidepressant. In addition, biomarkers including sleep, cognition, inflammation and metabolism and neuroimaging will be discussed.
As preparation for this review, the authors performed a literature search on each specific topic of interest using PubMed/MEDLINE and the Web of Science Core Collection, including papers in English produced from 2000 to 2019. Search topics included ‘ketamine as an antidepressant’, ‘mechanisms of action of ketamine as an antidepressant’, ‘biomarkers for antidepressant response to ketamine’, ‘enantiomers and metabolites of ketamine as antidepressants’, ‘inflammation and antidepressant actions of ketamine’, ‘ketamine and metabolism’, ‘sleep and antidepressant actions of ketamine’, ‘adverse effects of ketamine as an antidepressant’, ‘effects of ketamine on cognition’ and ‘neuroimaging studies on antidepressant effects of ketamine’.
Basic chemistry, pharmacology and pharmacokinetics of ketamine
Ketamine, an arylcyclohexylamine derivative (see Figure 1), is a racemate, that is a mixture of R and S enantiomers. Chiral forms (enantiomers) of a drug have the same number and type of atom groupings, but have different arrangements in space, analogous to right and left hands. Usually the chiral center consists of four different groups attached to a carbon atom, and when the compounds are synthesized there are often nearly equal quantities of the two enantiomers; that mixture is called a racemate or racemic mixture. Pairs of enantiomers differ in their optical activity, and rotate plane polarized light to the left (–, or levorotatory enantiomer) or the right (+, or dextrorotatory). Thus the prefixes (+)- and (–)-, dextro- and levo-, or d- or l- are used. The terms R (rectus) and S (sinister) are also often used, and describe the enantiomers based on their absolute configuration. While optical activity can be influenced by temperature and light wavelength, the absolute configuration can be modified only by breaking and reforming chemical bonds, and there is no relationship between absolute configuration and optical activity; for example, some drugs are R(+), S(–) while others are R(–), S(+).21,22
Figure 1.
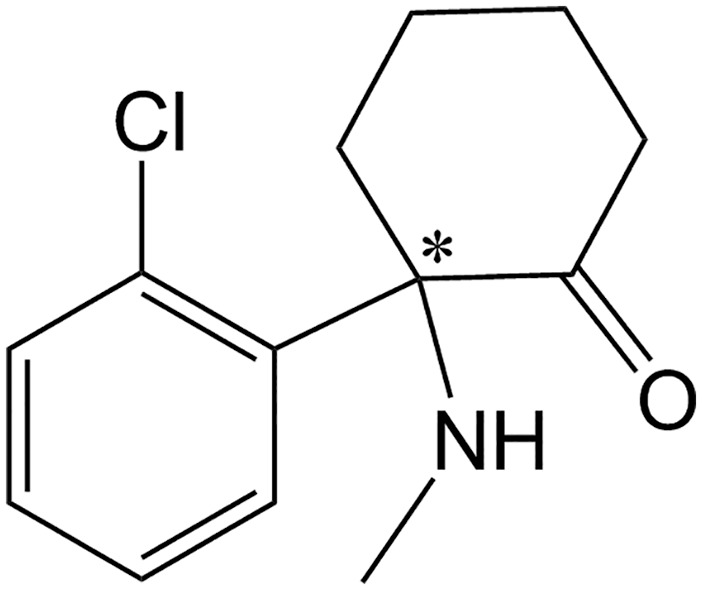
Chemical structure of ketamine, with the chiral center indicated by an asterisk.
There are many examples of antidepressants that contain a chiral center,21 and often these drugs have been used as racemates. However, often the two enantiomers may differ from one another significantly with regard to pharmacokinetics and pharmacodynamics. Racemates have often been used instead of individual enantiomers because of the difficulty and expense involved in separating the enantiomers from each other. Often the pharmacological activity may reside primarily in one enantiomer, and the two enantiomers may influence each other’s pharmacokinetics. Complicating factors that may arise when using racemates include: (1) association of therapeutic actions and/or adverse effects with one enantiomer; (2) differences in absorption, protein binding and/or renal clearance between the enantiomers; (3) preferential metabolism of one enantiomer over the other by enzymes; (4) one enantiomer having an effect on the pharmacokinetics of the other enantiomer; and (5) differing extents of the enantiomers interacting with co-administered drugs.21 However, there may also be cases in which the enantiomers produce complementary therapeutic effects or where one of the enantiomers counteracts adverse effects associated with the other enantiomer. Readers are referred to several review articles on this topic.21,23–25
Ketamine has been used for many years as an intravenous anesthetic, but has been the subject of intense interest in recent years in psychiatry after reports of its rapid-acting antidepressant and anti-suicidal effects.9,11,26 It contains a chiral center, and its R(–)- and S(+)-enantiomers have both been studied to varying extents. The S-enantiomer of ketamine (esketamine) is a more potent antagonist than R-ketamine at the phencyclidine site on the glutamate NMDA receptor,27–31 and has stronger analgesic potency than either R-ketamine or racemic ketamine.32 Esketamine is now being investigated as an antidepressant by several research groups.33 There is disagreement in the literature about the occurrence of adverse effects such as dissociation, psychoses and cognitive effects with the two enantiomers of ketamine.20,27,34 In animal studies, R-ketamine has been reported to have a rapid onset of antidepressant effects and a better side-effect profile than esketamine34–41; it has also been reported that R-ketamine improves phencyclidine-induced cognitive deficits in mice and that esketamine does not.42 Importantly, large head-to-head clinical comparisons of esketamine with R-ketamine and racemic ketamine have not yet been reported.41,43
Ketamine is metabolized extensively in the body via CYP2B6- and CYP3A4-mediated N-demethylation to norketamine. Norketamine then undergoes further catabolism to hydroxynorketamines (HNKs) and dehyronorketamine.20 Several researchers have investigated specific metabolites of ketamine for their antidepressant-like behavioral actions in animal models44–47; 2R,6R-HNK was reported to have antidepressant-like effects and no side effects in rodents.44 However, the literature on the antidepressant effects of 2R,6R-HNK is controversial, with several contradictory reports.41,48–52 S-norketamine, a metabolite of S-ketamine, has been shown in animal models to have useful antidepressant-like properties and fewer adverse effects than esketamine.35,53 In these animal models, S-norketamine has been reported to be equipotent to S-ketamine with regard to antidepressant-like activity, but less potent than R-ketamine.34,35
Bioavailability of ketamine differs with route of administration. Intravenous administration provides the most predictable dosing with 100% bioavailability, and availability via other routes of administration including intranasal (45%), sublingual (30%), oral (20%), intramuscular (93%) and rectal (30%) is discussed in the literature.31,34,35,46,54
Overview of the status of clinical trials with ketamine and its enantiomers
Since Berman et al.9 demonstrated a rapid antidepressant effect of intravenous ketamine, numerous studies have now replicated this finding. Multiple meta-analyses55–59 have now concluded that intravenous ketamine is effective as a rapid-acting antidepressant for major depressive episodes in both unipolar and bipolar depression, although one study suggested that there was a greater antidepressant effect size for unipolar depression than for bipolar.57 While several of these studies have supported claims that ketamine’s antidepressant effect lasts up to 7 days,55,56 other authors have suggested this to be true only for unipolar depression. One meta-analysis found that ketamine loses its effect in bipolar depression after day 3 or 4.58 Several RCTs have now looked at repeated infusions of six intravenous infusions over several weeks,60–62 but to date no long-term RCTs exist.
Studies on other modalities of ketamine administration are limited at this time. While one RCT on intranasal ketamine63 suggested that intranasal administration may be a viable alternative, another study64 was aborted early due to poor tolerance to the intranasal formulation. Other reports based on clinical experience65,66 have reported that although it remains experimental in nature, maintenance intranasal ketamine has been clinically useful in patients who have exhausted other treatment options. Studies on oral and sublingual ketamine have been the subject of a recent systematic review,67 but the studies reviewed include wide variations in dosing and do not appear to take into account limited bioavailability of oral formulations, so may underestimate efficacy.68 One previous pilot study has also suggested that intramuscular or subcutaneous routes may be viable options.69
Intranasal esketamine was approved by the FDA in the United States in March 2019 for major depression that has failed treatment with two or more antidepressants. This approval was based on three acute-phase studies and two maintenance-phase studies. A phase III trial of over 200 patients using esketamine adjunctive to an antidepressant demonstrated significant improvement in depression at 4 weeks compared with those using a placebo nasal spray.70 Two other phase III trials failed to meet primary endpoints.71,72 It has been suggested that the results of these studies were limited by a fixed dosing design in one71 and an elderly, more treatment-resistant population in the other.72 Of note, the esketamine acute studies have been conducted on populations more severely depressed than would be typical for FDA approval for antidepressant treatments of adjunctive medications.73 Two maintenance studies followed patients on maintenance esketamine up to 88 weeks, and reported decreased risk of depressive relapse when patients used esketamine weekly or every second week,74 and provided reassuring data on safety to over 1 year of esketamine use.75 Janssen has ongoing trials in progress, including one that will track safety outcomes to 5 years.33 It has been suggested that R-ketamine may confer antidepressant effects and greater tolerability than esketamine.34 Perception Pharmaceuticals has a phase I clinical study with R-ketamine underway from 2019, but to our knowledge no results are available to date.34
Potential adverse effects of ketamine
Ketamine administered at sub-anesthetic doses by infusion may result in several adverse effects, most of which occur during the infusion period and abate shortly thereafter. These acute and transient effects include an increase in blood pressure (usually asymptomatic), nausea and vomiting, perceptual disturbance, drowsiness, dizziness and dissociation.43,76–78 Blood pressure should be measured prior to ketamine administration and monitored after administration until it returns to normal values.79 As mentioned previously in this review, there appear to be differences in the degree of adverse effects between the enantiomers of ketamine, although the two enantiomers have not yet been compared in a comprehensive head-to-head clinical study.27,34 Incidence and severity of adverse effects may vary with route of administration and length of time administered, although more investigation must be done on these matters. Swainson et al.33 have provided a review of the adverse effects associated with intranasal administration of esketamine. It should also be remembered that ketamine is a potential drug of abuse, and high doses, particularly for long periods of time, can result in increased severity of the abovementioned side effects as well as severe urological side effects.80 In addition, as pointed out in a recent paper by Talbot et al.,81 further studies on the possible risks associated with cessation of ketamine antidepressant treatment are warranted.
Antidepressant mechanisms of ketamine and potential biochemical biomarkers
Glutamate is the main excitatory neurotransmitter in the central nervous system, acting on NMDA and -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) ionotropic receptors, as well as metabotropic glutamate receptors. Of interest, AMPA receptors are usually co-localized with NMDA receptors. Release of glutamate into the synaptic cleft initially activates AMPA receptors, which allow entry of sodium ions into the postsynaptic membrane. Subsequent depolarization of the postsynaptic membrane results in removal of the NMDA receptor channel voltage-dependent magnesium ion block and facilitates NMDA receptor activation, allowing entry of sodium and calcium ions. Ketamine has been long recognized as a non-competitive antagonist at NMDA receptors. To provide some background on other mechanisms of ketamine, brain-derived neurotrophic factor (BDNF) is a growth factor protein with roles in supporting survival of existing neurons as well as promoting neurogenesis and synaptogenesis in the central nervous system. Rapamycin, a compound isolated from bacteria in the 1960s and found to have antifungal, immunosuppressive and anti-tumor properties, was later discovered to interact with and inhibit a protein kinase named mechanistic target of rapamycin (mTOR). mTOR was noted to regulate metabolic cell growth by promoting lipid, nucleotide and protein synthesis while inhibiting cellular autophagy.82 Apart from its numerous metabolic functions, mTOR was also suggested to have roles in neural development and neuronal circuit formation.82 Interestingly, mTOR is a component of two separate protein complexes, namely mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2); ketamine appears to affect the mTORC1 pathway.
There has been suggestion that glutamatergic neurotransmission is dysregulated in MDD.83,84 This is supported by findings of elevated serum and plasma glutamate levels in patients85,86 and reduction of plasma glutamate levels following treatment with selective serotonin reuptake inhibitors (SSRIs).87 Of interest, the severity of depressive symptoms was found to be correlated with plasma glutamate levels.88 Elevation of extracellular glutamate in MDD may, in part, be caused by loss of glial cells that are responsible for glutamate/glutamine cycling.89 A consequence of increased extrasynaptic glutamate levels may actually be downstream suppression of glutamatergic neurotransmission via activation of metabotropic glutamate receptor 2 (mGluR2) autoreceptors.
However, ketamine’s mechanism of action is more complex than antagonism of NDMA receptors. This is supported by the finding that other NMDA receptor antagonists such as memantine, lanicemine and nitrous oxide do not exert a consistent antidepressant effect in RCTs.90 Furthermore, a meta-analysis of single-infusion non-ketamine NMDA receptor antagonists, including traxoprodil, lanicemine and rapastinel (GLYX-13), showed smaller effect sizes in depressive symptom change in comparison with ketamine and non-superiority in remission compared with placebo for unipolar and bipolar depression.55 It is likely that ketamine’s mechanism involves additional downstream targets, given that it is metabolized rapidly (within hours) but demonstrates longer-lasting antidepressant effects (days to weeks).
The mechanism of ketamine’s antidepressant action involves the following cascade of sequential events.41,91–93 Ketamine has a greater affinity for NDMA receptors on -aminobutyric acid (GABA) interneurons,94 which are inhibitory neurons and act to suppress excitation of downstream glutamatergic neurons. As a result of NMDA receptor antagonism, ketamine prevents activation of GABA interneurons and causes downstream disinhibition of glutamatergic neurons and a consequential glutamate surge. Increased extracellular glutamate initiates activation of postsynaptic AMPA receptors, leading to potentiation of BDNF and mTORC1 signaling pathways. The aforementioned pathways culminate in augmentation of synaptic plasticity and synaptic strength.
The majority of the literature on ketamine’s neurochemical effects has involved animal models. In the rat prefrontal cortex (PFC), ketamine was demonstrated to activate glutamate release and neurotransmission.95 AMPA receptor activation appears to be a critical step in ketamine’s mechanism of action as co-administration of an AMPA receptor inhibitor abolished its antidepressant effects.96–98 Ketamine administration was also shown to enhance AMPA-evoked electrophysiological responses in the rat hippocampus and medial PFC, suggesting that ketamine may augment AMPA receptor transmission.99,100 Furthermore, ketamine increased expression of AMPA receptor GluA1 and GluA2 subunits in the mouse hippocampus.44,101 Within 30 min of treatment, ketamine increased rat brain levels of BDNF96 and mTOR.102 This was further substantiated by observations that ketamine increased BDNF and mTOR expression in the rat hippocampus103 and that pre-treatment with the analgesic tramadol enhanced the antidepressant effects of ketamine in the forced-swim test and potentiated the upregulation of mTOR in the rat PFC and hippocampus.104 The increase in hippocampal and PFC BDNF and mTOR levels appears to be mediated by AMPA receptors as pre-treatment with an AMPA receptor antagonist increased forced-swim test immobility time and reduced levels of BDNF and mTOR, whereas pre-treatment with an AMPA receptor agonist reduced forced-swim test immobility times and increased levels of BDNF and mTOR.105
Several studies have reported that ketamine’s antidepressant effects are abolished when animals were pre-treated with rapamycin, an inhibitor of mTORC1.102,106 However, it should also be noted that the role of mTORC1 in ketamine’s antidepressant action may not be as clear-cut as originally surmised. In mice, ketamine administration was not noted to affect mTOR phosphorylation in hippocampal or cortical tissue44,96 and rapamycin did not block ketamine-induced antidepressant effects.96
There is significant support for the role of BDNF pathways in ketamine’s antidepressant mechanism. Use of genetic mutant mice lacking BDNF prevented the behavioral antidepressant responses of ketamine.96 The authors also proposed that ketamine-mediated antagonism of NMDA receptors deactivates eukaryotic elongation factor 2 (eEF2) kinase, resulting in de-suppressing BDNF translation. Mice with a Val66Met single-nucleotide polymorphism in the BDNF gene exhibit impairments in BDNF release and mRNA trafficking; ketamine administration was demonstrated to have reduced synaptogenesis in the PFC and impaired antidepressant behavioral effects in these animals.107
In rats vulnerable to chronic mild stress, ketamine was noted to result in reversal of anhedonic behavior, partial attenuation of hippocampal impairments in presynaptic release of glutamate and GABA, along with complete restoration of dendritic atrophy and dendritic BDNF mRNA trafficking.108 In a mouse model of social defeat stress, ketamine was noted to attenuate reductions in BDNF, dendritic spine density, GluA1 and PSD-95 (both markers of synaptogenesis) in the PFC, dentate gyrus and CA3 region of the hippocampus at 8 days following drug administration.109 In an investigation employing two-photon imaging in the PFC of living mice exposed to chronic stress, ketamine rescued elimination of postsynaptic dendritic spines and reversed the loss of coordinated activity of multicellular ensembles in projection neurons.110 Of note, the authors observed that ketamine’s rescue of dendritic spine formation occurred prior to its acute behavioral effects but was later correlated with behavioral effects 2–7 days after treatment. In addition, optogenetic ablation of newly formed dendritic spines disrupted the maintenance of ketamine’s behavioral effects. Taken together, this may suggest that ketamine’s effect on synaptogenesis may be related to longer-term maintenance of antidepressant activity. Furthermore, in a mouse social defeat model of depression, ketamine restored deficits in markers of neuronal and astroglial metabolic activity in the PFC to normal levels.111 This led the authors to suggest that ketamine may improve neurotransmitter cycling.
Ketamine’s neurochemical effects were also investigated, to a lesser extent, in depressed patients. Ketamine responders with TRD demonstrated rapid elevations in plasma BDNF levels112,113; however, another study did not support this finding.114 In the aforementioned investigations, higher levels of BDNF were correlated with lower severity of depressive symptoms on rating scales. Interestingly, patients with a Val66Met single-nucleotide polymorphism associated with impairments in BDNF release and mRNA trafficking were also found to have reduced responses to ketamine.115,116 In a study of three depressed patients responding to ketamine, the authors observed an increase in expression of plasma mTOR and eEF2 phosphorylation.117 While the increase in mTOR is supported by animal studies, it was surprising to note an increase in eEF2 phosphorylation that was previously shown to be reduced in animal models.96 A recent RCT of 20 patients demonstrated the surprising finding that pre-treatment with rapamycin, an mTORC1 inhibitor, actually tripled the response rate at 2 weeks after treatment.118 The authors suggested that rapamycin may have augmented ketamine’s effects by targeting neuroinflammation via its immunosuppressant actions or by promoting homeostasis of synaptic density. However, it has also been noted that it is unknown whether low-dose rapamycin would reach appropriate levels to inhibit mTOR in the brain and that it may exert its augmenting effects through dampening inflammation in the periphery.41
The possible interactions between D-serine, a potent co-agonist at the NMDA receptor that has been implicated as a possible therapeutic agent and/or biomarker in both depression and schizophrenia, are also of interest and warrant further investigation. Several animal studies and clinical investigations suggest that D-serine levels may be abnormal in depression and that D-serine has antidepressant properties.119–123 In this regard, it is interesting that ketamine inhibits transport of D-serine,124 ketamine metabolites decrease intracellular concentrations of D-serine in PC-12 cells,125 and that plasma D-serine levels predict a response to the antidepressant effects of ketamine.126,127
Other possible cellular targets of ketamine include binding to opioid (mu, delta and kappa) receptors, monoaminergic receptors and transporters, and muscarinic and nicotinic cholinergic receptors.46,47,128 It has been proposed that ketamine’s anti-suicidal and antidepressant effects may depend on activation of the opioid system, since pre-treatment with naltrexone (an opioid receptor antagonist) attenuated these effects in depressed patients.128,129 However, other studies have disputed the effect of naltrexone on ketamine’s mechanism of action.130,131 Agonists of the opioid receptors, such as buprenorphine and methadone, do not seem to affect ketamine’s antidepressant properties.131 Based on the results of studies on laboratory animals, Zhang and Hashimoto132 suggested that the opioid system may not play a role in ketamine’s antidepressant effects. Overall, it appears that the role of the opioid system in ketamine’s mechanism of action is still relatively unclear and controversial.
Other potential biomarkers for predicting response to ketamine
Sleep
It is natural to review the potential interactions of ketamine, sleep and MDD, given the well-known use of higher doses of ketamine as a general anesthetic as well as the myriad of clinical and neurophysiological interactions of sleep and MDD.
Ketamine has well-described effects on increasing total sleep and slow-wave sleep/slow-wave activity (SWS/SWA),133 and its antidepressant response has been linked to this effect.113 Improving SWS/SWA, especially early in the night, is thought to be a critical factor in ketamine’s mechanism of rapid antidepressant action in MDD, and similar results have been seen with repetitive transcranial magnetic stimulation (rTMS).134 This increase in SWA correlates strongly with increases in synaptic plasticity and plasma BDNF preclinically,135 and in ketamine-responsive MDD patients.113,133 BDNF is a well-known potential marker of antidepressant response,136 and the magnitude of increase has been seen to predict acute mood response to ketamine.112 Interestingly, this improvement in SWS/SWA may be unique to patients with unipolar depression. Ketamine responders with bipolar depression were found to experience the opposite effect – that is, a reduction of SWA. This may be due to known phenotypic differences of sleep (more hypersomnolence) associated with bipolar depression137 or the effect of mood-stabilizing medication.133 Low baseline delta sleep ratio, defined as decreased SWA earlier in the night compared with later in the night, has also been suggested to predict acute antidepressant effects of ketamine in MDD.138
Overall sleep improvement, especially reduction in objective electroencephalogram early-night awakening, may be a mechanism by which ketamine exerts its anti-suicidal effects.139 This should be an interesting area of future inquiry as non-antidepressant mechanisms need to be elucidated to completely explain the anti-suicidal effects of ketamine.140
Ketamine also appears to have significant effects on circadian rhythm systems, and its effects on glutamate likely underlie part of this. Synchronization of light/dark and the internal clock is partially mediated by glutamate in the retinothalamic tract. Small studies have shown ketamine responders exhibit more phase advance and a stronger amplitude increase in 24 h motor activity, indicating a more robust circadian rhythm.141,142 Baseline higher amplitude and a delayed 24 h motor activity pattern in the circadian rhythm were associated with nonresponse. Low and blunted amplitude 24 h activity patterns were also seen to associate with rapid relapse and brief response to ketamine, respectively.142
Some of these circadian rhythm changes are very similar to the effects of sleep deprivation and light therapy in MDD,143 and it may be these changes to the circadian clock that underlie some of ketamine’s rapid antidepressant effects. It is well known that circadian rhythm disruption is a key biological feature of MDD and it often appears to return to normal as symptoms remit.144 It has been postulated that people with disrupted circadian rhythms may be a subtype of mood disorders, but clinically they could be quite responsive to ketamine or other antidepressant treatments with rapid effects on the body clock.
Clock genes are known to control circadian rhythms, and ketamine has been noted to induce their rapid expression,145 suggesting that clock genes may play a role in ketamine’s rapid antidepressant effects.146,147 Newer preclinical data have shown an overlap between clock gene expression in both sleep deprivation and ketamine, both fast-acting treatments for depression.148 However, slower-acting treatments such as escitalopram149 and lithium150 have also demonstrated this effect. As such, clock gene expression could be more a long-term mechanism of ketamine, with the increase in sleep, SWS/SWA (which has been described as a proxy for sleep homeostasis) and more acute circadian rhythm changes being more linked to the rapid effects.141 The interaction is likely critical to sustain any antidepressant response overall.151
To date, data regarding ketamine’s effects on sleep are very limited, typically restricted to being short term with intravenous infusions. No reported data could be found on sleep effects of other less bioavailable forms of ketamine. The authors’ combined clinical experience is that drowsiness is seen in about 15–20%. Effects of ketamine on subjective sleep complaints in clinical trials is also not well reported. A major meta-analysis indicated no difference between ketamine and placebo groups on patient reports of ‘tired/fatigued’ and ‘vivid dreams’.55 However, when reviewing the major esketamine trials, significantly increased rates of somnolence versus placebo have been reported, especially in relapse-prevention studies,74,152 but this has not been seen consistently.153 While ketamine-specific data are limited, evidence from other MDD treatments indicates that both normalization of sleep homeostasis and circadian rhythm stabilization could be response predictors for ketamine. Associated rapid expression of clock genes could also be a marker for sustained improvement with ketamine in MDD. A prospective trial dosing ketamine at different points of the circadian rhythm is currently underway to shed more light on this question both clinically and biologically.154
Cognition
Cognition and cognitive symptoms (CCS) have a key role in recovery and functional outcome in MDD.155 Hence, looking at the effects of ketamine on CCS is critical to both understanding the mechanism and potentially predicting ketamine-responsive patient subtypes in MDD. There are emerging lines of evidence to support the theory that pro-cognitive effects of lower-dose ketamine are a foundational component in its putative efficacy.156
It can be confusing to examine the ketamine literature with respect to CCS, as it has traditionally been considered to have significant negative cognitive effects and has even been used as a model of schizophrenia, psychosis and cognitive dysfunction.157 Animal models show a differing, dose-dependent effect of ketamine on cognition, depression and anxiety, with a major postulated mechanism being altered BDNF levels. Sub-anesthetic doses of ketamine were seen to have positive effects on BDNF levels in the hippocampus, while the opposite is seen with anesthetic doses.158 A single infusion of low-dose ketamine (0.5 mg/kg) also increased hippocampal volume (often a proxy for increased BDNF) in a small group of unmedicated MDD patients as well.159 Infusion of much higher analgesic doses (8–20 mg/h) in healthy volunteers was also shown to produce significant deficits in cognition,160 indicating a potential dose-dependent effect with acute ketamine on CCS. Yet, there appears to be a distinct lack of long-term side effects of any sort with anesthetic doses,161 and sub-anesthetic doses appear to carry a very low risk in clinical trials.162 Animal models have also demonstrated impairment in episodic memory with a single ketamine infusion,163 but this may be an acute finding and unrelated to long-term cognitive dysfunction. Human results of acute ketamine use on memory are mixed,164 and a recent study on intranasal esketamine administration in healthy volunteers showed significant cognitive dysfunction at 40 min, but not at 2, 4, and 6 h post-dose.165
Ketamine treatment in actual MDD treatment protocols also shows promising results in CCS. Three groups of patients – with treatment-resistant unipolar and bipolar depression,166 TRD167 and anxious/non-anxious depression168 – were given six ketamine infusions over 12 days, with similar cognitive testing in a 2 week follow-up period. No deterioration in cognitive function was seen in any of the studies. Processing speed and verbal learning improved, but this was significantly correlated with improvement in depressive symptoms.166,167 Only the group of anxious depressed patients demonstrated a similar change in the third study.168 Improvement in many domains of memory in another study of repeated infusions over 12 days with a 4 week follow-up period was seen, but was not significant when controlling for depressive symptom improvement.169 A single infusion of 0.5 mg/kg in TRD patients was also seen to be slightly beneficial in attention and response control as well.170 In terms of predictive cognitive variables of response to ketamine, greater baseline visual learning predicted degree of MDD response to ketamine treatment in two of the above studies.166,168 Low attention169 and processing speed have also been seen to be predictive.171
There has long been concern that chronic ketamine use could lead to cognitive deficits. Heavy and chronic ketamine users have been seen to have a variety of cognitive function deficits across multiple domains. These include word reading and memory,172 verbal/visual memory, motor speed and executive function,173 as well as verbal fluency, processing speed and verbal learning specific to frontal and medial temporal cognition.174 A small group of chronic ketamine users have also demonstrated spatial memory disturbances and altered hippocampal activity.175
First, these cognitive deficits may be reversible. A large group recovered substantial cognitive function in domains of executive function, verbal and visual memory after stopping heavy ketamine use for 12 weeks.176 The ex-ketamine users who had been abstinent for a mean of 189 days in another study showed no cognitive deficits, even though their prior use had been just as significant as the chronic usage group.173
Second, the negative effects reported above could be related to the fact that abusers were likely using very high doses for long periods, creating an effect similar to ongoing anesthetic doses. Patients in a study demonstrating similar cognitive deficits of ketamine psychosis to schizophrenia had an average ketamine consumption of 3.8 g per day over 7 years.157 Even taking into account reduced bioavailability and purity, this would be an exponential order of magnitude greater than what is used in even regular TRD treatment. Cognitive deficits were also only seen in very frequent users (over four times weekly) in a study of chronic self-administered ketamine177 and the frequent user group in a small group of regional pain disorders178 versus people who used ketamine less often. Interestingly, the type of diagnosis may interact with ketamine in its potential to create cognitive deficits. Nonpsychotic ketamine abuse patients were seen to have significantly fewer cognitive deficits than either schizophrenic or ketamine-abusing psychotic patients.157 Clinically, this could indicate that more caution may be needed when treating patients who have a history of ketamine-induced psychotic disorders, psychotic illnesses and perhaps even psychotic depressions.
As mentioned previously, there are a number of potential mechanisms of the pro-cognitive effect of ketamine in depression. Some include de-emphasizing the link between cognition and emotion pathways in the brain, creating less interference of cognitive processing by negative emotional content.179 Glutamatergic modulation of numerous neural circuits (especially PFC networks) involved in cognition through mechanisms such as synaptogenesis, synapse stabilization, increased BDNF and mTORC1 production may also be involved.156 The specific anti-suicidal effect of ketamine may also be related to pro-cognitive effects. Better executive function and control could reduce the irrationality sometimes seen with the impulsivity of acute suicide attempts.180 This is partially supported by the finding of a single infusion of ketamine reducing explicit suicidal cognition and a performance-based index of implicit suicidal cognition compared with another anesthetic, although it was mediated by depressive symptom improvement.181 Another consideration supporting cognition as a key driver of ketamine response could be the potential decreased efficacy of ketamine clinically when benzodiazepines (which are known to impair cognitive processes) are co-administered.182–184
In summary, doses of ketamine used in TRD appear to have overall pro-cognitive effects that may mechanistically underlie their rapid effectiveness. The negative cognitive side effects of ketamine are present likely transiently in acute dosing of ketamine but only consistently in long-term heavy ketamine users, and appear to be reversible. These potential dose-dependent opposing actions of ketamine in CCS could be analogous to amphetamines, where low doses can greatly help cognitive measurements in disorders such as MDD179–185 and attention deficit hyperactivity disorder (ADHD),180–186 whereas much higher doses or different formulations of abuse can be detrimental cognitively or even cause psychosis in those predisposed.181–187 Indeed, there are significant overlaps in the cognitive dysfunction pattern between chronic ketamine and methamphetamine users.182–188 Cognitive impairment is not likely an issue in the doses given in MDD trials, but longer-term data are needed and, given the mechanistic underpinnings, consideration could be given to avoiding use in patients with psychosis.
Inflammation and metabolism
Metabolic syndrome, a constellation of symptoms including hypertension, hypercholesterolemia, hyperglycemia and increased waist circumference, is common in patients with mood disorders. A recent study reported a 38% prevalence of metabolic syndrome in patients with TRD, and it has been estimated that approximately one-third of depressed patients have elevated inflammatory markers.189 A significant yet complex relationship exists between mood disorders and metabolic syndrome, and this link appears to involve inflammation. Metabolic syndrome in patients with TRD is three times more common in patients with elevated C-reactive protein (CRP), an inflammatory marker.190 A systematic review looking at predictors of response in TRD suggested that the inflammatory markers interleukin-6 (IL-6), CRP and high-sensitivity CRP (hsCRP) may predict response to antidepressant medications with anti- inflammatory properties, including ketamine.191 While there is some suggestion from animal studies that racemic ketamine may have anti-inflammatory effects,192 human studies remain contradictory. Some authors have reported that decreases in inflammatory mediators IL-6 and interleukin-1 alpha (IL-1 alpha) following ketamine treatment have been only transient in the form of hours and have not correlated with antidepressant response,193,194 but changes in fibroblast growth factor (FG-2) were correlated with antidepressant response.193 Another study reported that a rapid decrease in the pro-inflammatory mediator tumor necrosis factor-alpha (TNF-α) was correlated with rapid antidepressant effects of ketamine, suggesting that changes in inflammatory cytokines may play a direct role.195 Of note, peripheral cytokine levels may not reflect central levels, and to date, studies have consisted of small sample sizes. Of particular interest, racemic ketamine has been suggested to protect against inflammation-induced vulnerability to stress behaviors in mouse models of depression.192
Mechanisms of the relationships between ketamine, depression, metabolism and inflammation remain unclear, but are likely mediated by multiple factors. Elevated body mass index (BMI) has been found to be a predictor of response to ketamine,196 but in another study197 elevated BMI was not correlated with ketamine response, and presence of metabolic syndrome was negatively correlated with ketamine response. It has been shown that fatty acid metabolism differs between depressed subjects and non-depressed controls,198–200 and it has been suggested that differences between patients who respond to ketamine and those who do not are due to alterations in the mitochondrial β-oxidation of fatty acids.201 Adipokines such as adiponectin, resistin and leptin regulate inflammatory and neuroplasticity pathways, as well as influence insulin sensitivity.202 It has been suggested that low levels of adiponectin, which typically acts as an anti-inflammatory and improves insulin sensitivity, may be predictive of ketamine response. Resistin is a pro-inflammatory molecule, and its levels have been noted to decrease with positive response to ketamine, suggesting that it may play a role in ketamine’s anti-inflammatory antidepressant action.202 Fat cells also release monocyte chemoattractant protein-1 (MCP-1), which leads to macrophage infiltration and more inflammation.203 It has been previously described that inflammation leads to glutamate excitotoxicity and synaptic destruction in depression.189 As noted previously in this review, ketamine’s antidepressant properties are thought to be at least in part due to its elevation of BDNF, which supports synaptic repair and regeneration.
Neuroimaging
There is a growing body of literature on neuroanatomical biomarkers of response to ketamine treatment. MDD has been shown to affect, among other areas, the PFC, the hippocampus and the anterior cingulate cortex (ACC), and there is evidence that ketamine affects these areas preferentially.204–206
Results from Lehmann et al.207 have implicated the ACC by utilizing task-related functional magnetic resonance imaging (fMRI) to investigate the effect of a single dose of intravenous ketamine versus placebo in a sample of healthy subjects. They found greater blood-oxygen-level-dependent (BOLD) reactivity in patients with high levels of rumination on negative experiences and a potentially larger effect at the pregenual ACC. A study using a magnetoencephalographic (MEG) task-related technique also found evidence for dysregulation in the ACC being implicated in a more favorable response to a single infusion of ketamine.208 This may correlate with findings of anhedonia being a possible clinical biomarker of response.17
Ketamine’s mechanism of action has also been associated with the glutamatergic system, especially in the PFC, as shown in neuroimaging studies. Using resting state fMRI, researchers have shown reduced PFC global connectivity to be implicated in MDD.209,210 Abdallah et al.211 demonstrated that 24 h after a single infusion of ketamine, PFC global connectivity could be normalized in responders and the extent of the PFC global connectivity increase was associated with response. Interestingly, lanicemine, another NMDA receptor antagonist, did not produce this.
Structural MRI has been performed on human subjects undergoing single-infusion ketamine, and evidence has been provided showing increased hippocampal volumes and decreased nucleus accumbens volumes 24 h post-infusion, which was correlated to treatment response.212
However, it should be mentioned that neuroimaging data overall are limited by small sample sizes and the low number of investigations to date.
Future directions
There has been increasing interest surrounding ketamine in recent years, largely owing to its rapid antidepressant and anti-suicidal properties in patients with TRD and its unique mechanism of action. The preclinical and clinical studies to date have led to further understanding of its use in psychiatry, and this will continue to be an active area of research as more investigations are conducted to determine optimum conditions for ketamine treatment in patients (see the work of Phillips et al.60, 213 for examples of very recent studies on comparisons of single, repeated and maintenance ketamine infusions on TRD and suicidal ideation in TRD). There will need to be further exploration of individual differences in response between patients (including sex differences214,215) and determination of appropriate regimens for maintenance therapy and discontinuation, given ketamine’s transient antidepressant effects. There is also a need to investigate further reliable biomarkers for prediction of ketamine response and adverse effects. As indicated in this review paper, studies on ketamine’s roles in sleep, cognition and inflammation have resulted in some interesting findings worthy of further research. Additional exploration of ketamine’s possible role in treatment of bipolar depression is also warranted.
It should be remembered that there are also many publications in the literature that urge caution in the use of ketamine as an antidepressant,76,216–222 and clinicians planning to use this drug would be well advised to be familiar with the extensive literature available on it. A 2017 consensus statement on ketamine use encourages consideration of the current data limitations and potential risks associated with the drug.222 Nonetheless, ketamine remains a promising option for those suffering from TRD, and it is exciting to surmise that understanding ketamine’s neurochemical mechanisms and related biomarkers will lead to the development of other, much needed, next-generation antidepressants.
Footnotes
Conflict of interest statement: JS has been paid speaking honoraria by Otsuka and Lundbeck and has served on advisory boards for Otsuka, Lundbeck, and Janssen. RKT and SMD have also served on advisory boards for Janssen. AK has been paid honoraria for speaking from, and has served on advisory boards for Pfizer, Takeda, Lundbeck, Janssen-Ortho, Purdue, Sunovion, Allergan, Otsuka and Merck, and has received speaking honoraria from Bausch Health; he has also received research funding from AstraZeneca, Sanofi-Aventis, Pfizer and Merck. None of the companies mentioned above has had a role in the preparation of this review paper.
Funding: The applicants are grateful to the Department of Psychiatry and the Faculty of Medicine & Dentistry, University of Alberta, for funding.
ORCID iD: Glen B. Baker  https://orcid.org/0000-0003-1581-6486
https://orcid.org/0000-0003-1581-6486
Contributor Information
Dmitriy Matveychuk, Department of Psychiatry, Neurochemical Research Unit, University of Alberta, Edmonton, Alberta, Canada.
Rejish K. Thomas, Grey Nuns Community Hospital and Department of Psychiatry, University of Alberta, Edmonton, Alberta, Canada
Jennifer Swainson, Misericordia Community Hospital and Department of Psychiatry, University of Alberta, Edmonton, Alberta, Canada.
Atul Khullar, Grey Nuns Community Hospital and Department of Psychiatry, University of Alberta, Edmonton, Alberta, Canada.
Mary-Anne MacKay, Department of Psychiatry, Neurochemical Research Unit, University of Alberta, Edmonton, Alberta, Canada.
Glen B. Baker, Department of Psychiatry, Neurochemical Research Unit, University of Alberta, 12-105B Clin Sci Bldg, Edmonton, Alberta T6G 2G3, Canada.
Serdar M. Dursun, Department of Psychiatry, Neurochemical Research Unit, University of Alberta, Edmonton, Alberta, Canada Grey Nuns Community Hospital, Edmonton, Alberta, Canada.
References
- 1. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013; 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patten SB, Williams JV, Lavorato DH, et al. Descriptive epidemiology of major depressive disorder in Canada in 2012. Can J Psychiatry 2015; 60: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker G, Mitchell N. Depression, chemical mechanisms of. In: Begley T. (ed.). Wiley encyclopedia of chemical biology. Hoboken, NJ: John Wiley and Sons, 2009. [Google Scholar]
- 4. Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 2000; 61(Suppl. 6): 4–6. [PubMed] [Google Scholar]
- 5. Liu B, Liu J, Wang M, et al. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci 2017; 11: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology 2012; 37: 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996; 19: 179–200. [DOI] [PubMed] [Google Scholar]
- 8. Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry 2007; 68: 17. [PubMed] [Google Scholar]
- 9. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 10. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zarate CAJ, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 12. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 2015; 30: 152–163. [DOI] [PubMed] [Google Scholar]
- 14. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2018; 175: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 2013; 74: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. aan hetRot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 2010; 67: 139–145. [DOI] [PubMed] [Google Scholar]
- 17. Thomas RK, Baker G, Lind J, et al. Rapid effectiveness of intravenous ketamine for ultraresistant depression in a clinical setting and evidence for baseline anhedonia and bipolarity as clinical predictors of effectiveness. J Psychopharmacol 2018; 32: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 18. Thomas R, Baker G, Dursun S. Rapid efficacy and antisuicidal actions of intravenous ketamine for ultraresistant depression in a clinical setting: a retrospective, database study. (ACNP 56th Annual Meeting: Poster Session I, December 4, 2017). Neuropsychopharmacology 2017; 42: S111. [Google Scholar]
- 19. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 2018; 175: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanos P, Thompson SM, Duman RS, et al. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 2018; 32: 197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker GB, Prior TI. Stereochemistry and drug efficacy and development: relevance of chirality to antidepressant and antipsychotic drugs. Ann Med 2002; 34: 537–543. [DOI] [PubMed] [Google Scholar]
- 22. Lane RM, Baker GB. Chirality and drugs used in psychiatry: nice to know or need to know? Cell Mol Neurobiol 1999; 19: 355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leonard BE. An introduction to enantiomers in psychopharmacology. Hum Psychopharmacol 2001; 16: S79–S84. [DOI] [PubMed] [Google Scholar]
- 24. McConathy J, Owens MJ. Stereochemistry in drug action. Prim Care Companion J Clin Psychiatry 2003; 5: 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen LA, He H, Pham-Huy C. Chiral drugs: an overview. Int J Biomed Sci 2006; 2: 85–100. [PMC free article] [PubMed] [Google Scholar]
- 26. DiazGranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 2010; 71: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol 2016; 6: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfenninger EG, Durieux ME, Himmelseher S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology 2002; 96: 357–366. [DOI] [PubMed] [Google Scholar]
- 29. Andrade C. Ketamine for depression, 3: does chirality matter? J Clin Psychiatry 2017; 78: e674–e677. [DOI] [PubMed] [Google Scholar]
- 30. Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol 2016; 6: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peltoniemi MA, Hagelberg NM, Olkkola KT, et al. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet 2016; 55: 1059–1077. [DOI] [PubMed] [Google Scholar]
- 32. Zielmann S, Kazmaier S, Schnull S, et al. S-(+)-Ketamine and circulation. Anaesthesist 1997; 46(Suppl. 1): S43–S46. [DOI] [PubMed] [Google Scholar]
- 33. Swainson J, Thomas RK, Archer S, et al. Esketamine for treatment resistant depression. Expert Rev Neurother 2019; 19: 899–911. [DOI] [PubMed] [Google Scholar]
- 34. Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci 2019; 73: 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hashimoto K, Yang C. Is (S)-norketamine an alternative antidepressant for esketamine? Eur Arch Psychiatry Clin Neurosci 2019; 269: 867–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang JC, Li SX, Hashimoto K. R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 2014; 116: 137–141. [DOI] [PubMed] [Google Scholar]
- 37. Yang C, Shirayama Y, Zhang JC, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 2015; 5: e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang C, Han M, Zhang JC, et al. Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res 2016; 239: 281–283. [DOI] [PubMed] [Google Scholar]
- 39. Fukumoto K, Toki H, Iijima M, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 2017; 361: 9–16. [DOI] [PubMed] [Google Scholar]
- 40. Hashimoto K, Kakiuchi T, Ohba H, et al. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 2017; 267: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang C, Yang J, Luo A, et al. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 2019; 9: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan Y, Fujita Y, Qu Y, et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine: role of BDNF-TrkB signaling. Pharmacol Biochem Behav 2019; 188: 172839. [DOI] [PubMed] [Google Scholar]
- 43. Andrade C. Ketamine for depression, 1: Clinical summary of issues related to efficacy, adverse effects, and mechanism of action. J Clin Psychiatry 2017; 78: e415–e419. [DOI] [PubMed] [Google Scholar]
- 44. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaki S. Is metabolism of (R)-ketamine essential for the antidepressant effects? Int J Neuropsychopharmacol 2018; 21: 154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Human Neurosci 2016; 10: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013; 19: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zanos P, Highland JN, Liu X, et al. (R)-ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol 2019; 176: 2573–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fukumoto K, Fogaca MV, Liu RJ, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci USA 2019; 116: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chou D, Peng HY, Lin TB, et al. (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 2018; 139: 1–12. [DOI] [PubMed] [Google Scholar]
- 51. Xiong Z, Fujita Y, Zhang K, et al. Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behav Brain Res 2019; 368: 111904. [DOI] [PubMed] [Google Scholar]
- 52. Yamaguchi JI, Toki H, Qu Y, et al. (2R,6R)-hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology 2018; 43: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang Y, Cui Y, Sang K, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018; 554: 317–322. [DOI] [PubMed] [Google Scholar]
- 54. Zhang K, Hashimoto K. An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev Neurother 2019; 19: 83–92. [DOI] [PubMed] [Google Scholar]
- 55. Kishimoto T, Chawla JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 2016; 46: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee EE, Della Selva MP, Liu A, et al. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry 2015; 37: 178–184. [DOI] [PubMed] [Google Scholar]
- 57. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 2015; 45: 693–704. [DOI] [PubMed] [Google Scholar]
- 58. Romeo B, Choucha W, Fossati P, et al. Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res 2015; 230: 682–688. [DOI] [PubMed] [Google Scholar]
- 59. Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 2014; 231: 3663–3676. [DOI] [PubMed] [Google Scholar]
- 60. Phillips JL, Norris S, Talbot J, et al. Single and repeated ketamine infusions for reduction of suicidal ideation in treatment-resistant depression. Neuropsychopharmacology 2020; 45: 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions for comorbid posttraumatic stress disorder and treatment-resistant depression. J Clin Psychiatry 2018; 79. [DOI] [PubMed] [Google Scholar]
- 62. Zheng W, Zhou YL, Liu WJ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res 2018; 106: 61–68. [DOI] [PubMed] [Google Scholar]
- 63. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 2014; 76: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Galvez V, Li A, Huggins C, et al. Repeated intranasal ketamine for treatment-resistant depression: the way to go? Results from a pilot randomised controlled trial. J Psychopharmacol 2018; 32: 397–407. [DOI] [PubMed] [Google Scholar]
- 65. Lee V, Archer S, Chrenek C, et al. A response to: Repeated intranasal ketamine for treatment resistant depression: the way to go? Results from a pilot randomised controlled trial. J Psychopharmacol 2019; 33: 258–259. [DOI] [PubMed] [Google Scholar]
- 66. Andrade C. Intranasal drug delivery in neuropsychiatry: focus on intranasal ketamine for refractory depression. J Clin Psychiatry 2015; 76: e628–e631. [DOI] [PubMed] [Google Scholar]
- 67. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry 2019; 80. [DOI] [PubMed] [Google Scholar]
- 68. Swainson J, Khullar A. Sublingual ketamine: an option for increasing accessibility of ketamine treatments for depression? J Clin Psychiatry 2020; 81(1): 19lr13146. [DOI] [PubMed] [Google Scholar]
- 69. Loo CK, Galvez V, O’Keefe E, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 2016; 134: 48–56. [DOI] [PubMed] [Google Scholar]
- 70. Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 2019; 176: 428–438. [DOI] [PubMed] [Google Scholar]
- 71. Fedgchin M, Trivedi M, Daly E, et al. Randomized, double-blind study of fixed-dosed intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression. J Affect Disord 2019; 254: 131–132. [Google Scholar]
- 72. Ochs-Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of intranasal esketamine plus an oral antidepressant in elderly patients with treatment-resistant depression. Biol Psychiatry 2018; 83: S391. [DOI] [PubMed] [Google Scholar]
- 73. FDA. FDA report on esketamine for treatment resistant depression, https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/UCM630970.pdf (2019).
- 74. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 2019; 76: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wajs E, Leah A, Morrison R, et al. Long-term safety of esketamine nasal spray plus an oral antidepressant in patients with treatment-resistant depression: SUSTAIN-2 phase 3 study. Eur Neuropsychopharm 2019; 29: S44–S45. [Google Scholar]
- 76. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs 2018; 32: 411–420. [DOI] [PubMed] [Google Scholar]
- 77. Meisner R. Ketamine for depression: new tool, new questions. Harvard Health Blog, 2019. www.health.harvard.edu/blog/ketamine-for-major-depression-new-tool-new-questions-2019052216673 [Google Scholar]
- 78. Wlodarczyk A, Cubala WJ, Szarmach J, et al. Short-term ketamine administration in treatment-resistant depression patients: focus on adverse effects on the central nervous system. Psychiatr Danub 2019; 31: 530–533. [PubMed] [Google Scholar]
- 79. Szarmach J, Cubala WJ, Wlodarczyk A, et al. Short-term ketamine administration in treatment-resistant depression: focus on cardiovascular safety. Psychiatr Danub 2019; 31: 585–590. [PubMed] [Google Scholar]
- 80. Jhang JF, Hsu YH, Kuo HC. Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int J Urol 2015; 22: 816–825. [DOI] [PubMed] [Google Scholar]
- 81. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci 2019; 44: 384–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 169: 361–371. [DOI] [PubMed] [Google Scholar]
- 83. Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012; 62: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mitchell ND, Baker GB. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr Scand 2010; 122: 192–210. [DOI] [PubMed] [Google Scholar]
- 85. Kim JS, Schmid-Burgk W, Claus D, et al. Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr 1982; 232: 299–304. [DOI] [PubMed] [Google Scholar]
- 86. Altamura C, Maes M, Dai J, et al. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol 1995; 5(Suppl. 1): 71–75. [DOI] [PubMed] [Google Scholar]
- 87. Küçükibrahimoğlu E, Saygın MZ, Çalışkan M, et al. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur J Clin Pharmacol 2009; 65: 571–577. [DOI] [PubMed] [Google Scholar]
- 88. Mitani H, Shirayama Y, Yamada T, et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1155–1158. [DOI] [PubMed] [Google Scholar]
- 89. Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 2013; 73: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 2015; 172: 950–966. [DOI] [PubMed] [Google Scholar]
- 91. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci 2017; 42: 160175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Strasburger SE, Bhimani PM, Kaabe JH, et al. What is the mechanism of ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther 2017; 42: 147–154. [DOI] [PubMed] [Google Scholar]
- 94. Zorumski CF, Izumi Y, Mennerick S. Ketamine: NMDA receptors and beyond. J Neurosci 2016; 36: 11158–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 2011; 224: 107–111. [DOI] [PubMed] [Google Scholar]
- 98. Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 2008; 63: 349–352. [DOI] [PubMed] [Google Scholar]
- 99. El Iskandrani KS, Oosterhof CA, El Mansari M, et al. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: an in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol 2015; 29: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bjorkholm C, Jardemark K, Schilstrom B, et al. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur Neuropsychopharmacol 2015; 25: 1842–1847. [DOI] [PubMed] [Google Scholar]
- 101. Nosyreva E, Szabla K, Autry AE, et al. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci 2013; 33: 6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li N, Lee B, Liu R-J, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang C, Hu YM, Zhou ZQ, et al. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci 2013; 118: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang C, Li WY, Yu HY, et al. Tramadol pretreatment enhances ketamine-induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex. J Biomed Biotechnol 2012; 2012: 175619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhou W, Wang N, Yang C, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 2014; 29: 419–423. [DOI] [PubMed] [Google Scholar]
- 106. Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu RJ, Lee FS, Li XY, et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tornese P, Sala N, Bonini D, et al. Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats: the rapid restorative action of ketamine. Neurobiol Stress 2019; 10: 100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dong C, Zhang JC, Yao W, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol 2017; 20: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Moda-Sava RN, Murdock MH, Parekh PK, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019; 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mishra PK, Kumar A, Behar KL, et al. Subanesthetic ketamine reverses neuronal and astroglial metabolic activity deficits in a social defeat model of depression. J Neurochem 2018; 146: 722–734. [DOI] [PubMed] [Google Scholar]
- 112. Haile CN, Murrough JW, Iosifescu DV, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 2014; 17: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Duncan WC, Sarasso S, Ferrarelli F, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 2013; 16: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Machado-Vieira R, Yuan P, Brutsche N, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry 2009; 70: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Laje G, Lally N, Mathews D, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 2012; 72: e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen MH, Lin WC, Wu HJ, et al. Antisuicidal effect, BDNF Val66Met polymorphism, and low-dose ketamine infusion: reanalysis of adjunctive ketamine study of Taiwanese patients with treatment-resistant depression (AKSTP-TRD). J Affect Disord 2019; 251: 162–169. [DOI] [PubMed] [Google Scholar]
- 117. Yang C, Zhou ZQ, Gao ZQ, et al. Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol Psychiatry 2013; 73: e35–e36. [DOI] [PubMed] [Google Scholar]
- 118. Abdallah CG, Averill LA, Gueorguieva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double-blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv 2018: 500959. [Google Scholar]
- 119. Ishiwata S, Hattori K, Sasayama D, et al. Cerebrospinal fluid D-serine concentrations in major depressive disorder negatively correlate with depression severity. J Affect Disord 2018; 226: 155–162. [DOI] [PubMed] [Google Scholar]
- 120. Hashimoto K, Yoshida T, Ishikawa M, et al. Increased serum levels of serine enantiomers in patients with depression. Acta Neuropsychiatr 2016; 28: 173–178. [DOI] [PubMed] [Google Scholar]
- 121. Malkesman O, Austin DR, Tragon T, et al. Acute D-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 2012; 15: 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wei IH, Chen KT, Tsai MH, et al. Acute amino acid D-serine administration, similar to ketamine, produces antidepressant-like effects through identical mechanisms. J Agric Food Chem 2017; 65: 10792–10803. [DOI] [PubMed] [Google Scholar]
- 123. Otte DM, Barcena de Arellano ML, Bilkei-Gorzo A, et al. Effects of chronic D-serine elevation on animal models of depression and anxiety-related behavior. PLoS One 2013; 8: e67131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Singh NS, Bernier M, Camandola S, et al. Enantioselective inhibition of D-serine transport by (S)-ketamine. Br J Pharmacol 2015; 172: 4546–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Singh NS, Rutkowska E, Plazinska A, et al. Ketamine metabolites enantioselectively decrease intracellular D-serine concentrations in PC-12 cells. PLoS One 2016; 11: e0149499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Moaddel R, Luckenbaugh DA, Xie Y, et al. D-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology (Berl) 2015; 232: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hashimoto K. Blood D-serine levels as a predictive biomarker for the rapid antidepressant effects of the NMDA receptor antagonist ketamine. Psychopharmacology (Berl) 2014; 231: 4081–4082. [DOI] [PubMed] [Google Scholar]
- 128. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry 2019; 24: 1779–1786. [DOI] [PubMed] [Google Scholar]
- 129. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 2018; 175: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 2019; 76: 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry 2019; 85: e75–e76. [DOI] [PubMed] [Google Scholar]
- 132. Zhang K, Hashimoto K. Lack of opioid system in the antidepressant actions of ketamine. Biol Psychiatry 2019; 85: e25–e27. [DOI] [PubMed] [Google Scholar]
- 133. Duncan WC, Jr, Zarate CA., Jr Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep 2013; 15: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Duncan WC, Jr, Ballard ED, Zarate CA. Ketamine-induced glutamatergic mechanisms of sleep and wakefulness: insights for developing novel treatments for disturbed sleep and mood. Handb Exp Pharmacol 2019; 253: 337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep 2007; 30: 129–139. [DOI] [PubMed] [Google Scholar]
- 136. Bjorkholm C, Monteggia LM. BDNF: a key transducer of antidepressant effects. Neuropharmacology 2016; 102: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Geoffroy PA, Hoertel N, Etain B, et al. Insomnia and hypersomnia in major depressive episode: prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J Affect Disord 2018; 226: 132–141. [DOI] [PubMed] [Google Scholar]
- 138. Duncan WC, Jr, Selter J, Brutsche N, et al. Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord 2013; 145: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vande Voort JL, Ballard ED, Luckenbaugh DA, et al. Antisuicidal response following ketamine infusion is associated with decreased nighttime wakefulness in major depressive disorder and bipolar disorder. J Clin Psychiatry 2017; 78: 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res 2014; 58: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Duncan WC, Jr, Slonena E, Hejazi NS, et al. Motor-activity markers of circadian timekeeping are related to ketamine’s rapid antidepressant properties. Biol Psychiatry 2017; 82: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Duncan WC, Jr, Slonena EE, Hejazi NS, et al. Are 24-hour motor activity patterns associated with continued rapid response to ketamine? Neuropsychiatr Dis Treat 2018; 14: 2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Benedetti F, Dallaspezia S, Fulgosi MC, et al. Phase advance is an actimetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiol Int 2007; 24: 921–937. [DOI] [PubMed] [Google Scholar]
- 144. Hasler BP, Buysse DJ, Kupfer DJ, et al. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res 2010; 178: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bellet MM, Vawter MP, Bunney BG, et al. Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One. 2011; 6: e23982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bunney BG, Bunney WE. Rapid-acting antidepressant strategies: mechanisms of action. Int J Neuropsychopharmacol 2012; 15: 695–713. [DOI] [PubMed] [Google Scholar]
- 147. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry 2013; 73: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 148. Orozco-Solis R, Montellier E, Aguilar-Arnal L, et al. A circadian genomic signature common to ketamine and sleep deprivation in the anterior cingulate cortex. Biol Psychiatry 2017; 82: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cuesta M, Clesse D, Pevet P, et al. New light on the serotonergic paradox in the rat circadian system. J Neurochem 2009; 110: 231–243. [DOI] [PubMed] [Google Scholar]
- 150. McCarthy MJ, Nievergelt CM, Kelsoe JR, et al. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One 2012; 7: e32091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Archer SN, Laing EE, Moller-Levet CS, et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci USA 2014; 111: E682–E691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 2019; 22: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 2018; 175: 620–630. [DOI] [PubMed] [Google Scholar]
- 154. Zhuo C, Tian H, Li G, et al. Effects of ketamine on circadian rhythm and synaptic homeostasis in patients with treatment-resistant depression: a protocol for mechanistic studies of its rapid and sustained antidepressant actions in humans. Brain Behav 2019; 9: e01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. McIntyre RS, Lee Y. Cognition in major depressive disorder: a ‘Systemically Important Functional Index’ (SIFI). Curr Opin Psychiatry 2016; 29: 48–55. [DOI] [PubMed] [Google Scholar]
- 156. Lee Y, Syeda K, Maruschak NA, et al. A new perspective on the anti-suicide effects with ketamine treatment: a procognitive effect. J Clin Psychopharmacol 2016; 36: 50–56. [DOI] [PubMed] [Google Scholar]
- 157. Cheng WJ, Chen CH, Chen CK, et al. Similar psychotic and cognitive profile between ketamine dependence with persistent psychosis and schizophrenia. Schizophr Res 2018; 199: 313–318. [DOI] [PubMed] [Google Scholar]
- 158. Wu C, Wang Y, He Y, et al. Sub-anesthetic and anesthetic ketamine produce different long-lasting behavioral phenotypes (24 h post-treatment) via inducing different brain-derived neurotrophic factor (BDNF) expression level in the hippocampus. Neurobiol Learn Mem 2019; 167: 107136. [DOI] [PubMed] [Google Scholar]
- 159. Abdallah CG, Salas R, Jackowski A, et al. Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol 2015; 29: 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hayley A, Green M, Downey L, et al. Neurocognitive and behavioural performance of healthy volunteers receiving an increasing analgesic-range infusion of ketamine. Psychopharmacology (Berl) 2018; 235: 1273–1282. [DOI] [PubMed] [Google Scholar]
- 161. Moretti RJ, Hassan SZ, Goodman LI, et al. Comparison of ketamine and thiopental in healthy volunteers: effects on mental status, mood, and personality. Anesth Analg 1984; 63: 1087–1096. [PubMed] [Google Scholar]
- 162. Perry EB, Jr, Cramer JA, Cho HS, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007; 192: 253–260. [DOI] [PubMed] [Google Scholar]
- 163. de Souza I, Meurer Y, Tavares PM, et al. Episodic-like memory impairment induced by sub-anaesthetic doses of ketamine. Behav Brain Res 2019; 359: 165–171. [DOI] [PubMed] [Google Scholar]
- 164. Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl) 2006; 188: 408–424. [DOI] [PubMed] [Google Scholar]
- 165. Morrison RL, Fedgchin M, Singh J, et al. Effect of intranasal esketamine on cognitive functioning in healthy participants: a randomized, double-blind, placebo-controlled study. Psychopharmacology (Berl) 2018; 235: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhou Y, Zheng W, Liu W, et al. Neurocognitive effects of six ketamine infusions and the association with antidepressant response in patients with unipolar and bipolar depression. J Psychopharmacol 2018; 32: 1118–1126. [DOI] [PubMed] [Google Scholar]
- 167. Zheng W, Zhou YL, Liu WJ, et al. Neurocognitive performance and repeated-dose intravenous ketamine in major depressive disorder. J Affect Disord 2019; 246: 241–247. [DOI] [PubMed] [Google Scholar]
- 168. Liu W, Zhou Y, Zheng W, et al. Repeated intravenous infusions of ketamine: neurocognition in patients with anxious and nonanxious treatment-resistant depression. J Affect Disord 2019; 259: 1–6. [DOI] [PubMed] [Google Scholar]
- 169. Shiroma PR, Albott CS, Johns B, et al. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 2014; 17: 1805–1813. [DOI] [PubMed] [Google Scholar]
- 170. Chen MH, Li CT, Lin WC, et al. Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. J Affect Disord 2018; 241: 1–7. [DOI] [PubMed] [Google Scholar]
- 171. Murrough JW, Burdick KE, Levitch CF, et al. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: a randomized controlled trial. Neuropsychopharmacology 2015; 40: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ke X, Ding Y, Xu K, et al. The profile of cognitive impairments in chronic ketamine users. Psychiatry Res 2018; 266: 124–131. [DOI] [PubMed] [Google Scholar]
- 173. Tang WK, Liang HJ, Lau CG, et al. Relationship between cognitive impairment and depressive symptoms in current ketamine users. J Stud Alcohol Drugs 2013; 74: 460–468. [DOI] [PubMed] [Google Scholar]
- 174. Chan KW, Lee TM, Siu AM, et al. Effects of chronic ketamine use on frontal and medial temporal cognition. Addict Behav 2013; 38: 2128–2132. [DOI] [PubMed] [Google Scholar]
- 175. Morgan CJ, Dodds CM, Furby H, et al. Long-term heavy ketamine use is associated with spatial memory impairment and altered hippocampal activation. Front Psychiatry 2014; 5: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tang WK, Lau CG, Ungvari GS, et al. Recovery of cognitive functioning following abstinence from ketamine. Addict Behav 2019; 99: 106081. [DOI] [PubMed] [Google Scholar]
- 177. Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction 2010; 105: 121–133. [DOI] [PubMed] [Google Scholar]
- 178. Kim M, Cho S, Lee JH. The effects of long-term ketamine treatment on cognitive function in complex regional pain syndrome: a preliminary study. Pain Med 2016; 17: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 179. Scheidegger M, Henning A, Walter M, et al. Effects of ketamine on cognition–emotion interaction in the brain. Neuroimage 2016; 124: 8–15. [DOI] [PubMed] [Google Scholar]
- 180. Zhang MW, Ho RC. Controversies of the effect of ketamine on cognition. Front Psychiatry 2016; 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Price RB, Iosifescu DV, Murrough JW, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety 2014; 31: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol 2015; 35: 334–336. [DOI] [PubMed] [Google Scholar]
- 183. Ford N, Ludbrook G, Galletly C. Benzodiazepines may reduce the effectiveness of ketamine in the treatment of depression. Aust N Z J Psychiatry 2015; 49: 1227. [DOI] [PubMed] [Google Scholar]
- 184. Albott CS, Shiroma PR, Cullen KR, et al. The antidepressant effect of repeat dose intravenous ketamine is delayed by concurrent benzodiazepine use. J Clin Psychiatry 2017; 78: e308–e309. [DOI] [PubMed] [Google Scholar]
- 185. Madhoo M, Keefe RS, Roth RM, et al. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology 2014; 39: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Weisler R, Ginsberg L, Dirks B, et al. Treatment with lisdexamfetamine dimesylate improves self- and informant-rated executive function behaviors and clinician- and informant-rated ADHD symptoms in adults: data from a randomized, double-blind, placebo-controlled study. J Atten Disord 2017; 21: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 187. Harro J. Neuropsychiatric adverse effects of amphetamine and methamphetamine. Int Rev Neurobiol 2015; 120: 179–204. [DOI] [PubMed] [Google Scholar]
- 188. Wang LJ, Chen CK, Lin SK, et al. Cognitive profile of ketamine-dependent patients compared with methamphetamine-dependent patients and healthy controls. Psychopharmacology (Berl) 2018; 235: 2113–2121. [DOI] [PubMed] [Google Scholar]
- 189. Haroon E, Miller AH. Inflammation effects on brain glutamate in depression: mechanistic considerations and treatment implications. Curr Top Behav Neurosci 2017; 31: 173–198. [DOI] [PubMed] [Google Scholar]
- 190. Godin O, Bennabi D, Yrondi A, et al. Prevalence of metabolic syndrome and associated factors in a cohort of individuals with treatment-resistant depression: results from the FACE-DR study. J Clin Psychiatry 2019; 80. [DOI] [PubMed] [Google Scholar]
- 191. Yang C, Wardenaar KJ, Bosker FJ, et al. Inflammatory markers and treatment outcome in treatment resistant depression: a systematic review. J Affect Disord 2019; 257: 640–649. [DOI] [PubMed] [Google Scholar]
- 192. Mastrodonato A, Cohensedgh O, LaGamma CT, et al. Prophylactic (R,S)-ketamine selectively protects against inflammatory stressors. Behav Brain Res 2020; 378: 112238. [DOI] [PubMed] [Google Scholar]
- 193. Rong C, Park C, Rosenblat JD, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health 2018; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Park M, Newman LE, Gold PW, et al. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J Psychiatr Res 2017; 84: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Chen MH, Li CT, Lin WC, et al. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatry Res 2018; 269: 207–211. [DOI] [PubMed] [Google Scholar]
- 196. Niciu MJ, Luckenbaugh DA, Ionescu DF, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 2014; 75: e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Dale RM, Bryant KA, Thompson NR. Metabolic syndrome rather than body mass index is associated with treatment response to ketamine infusions. J Clin Psychopharmacol 2020; 40: 75–79. [DOI] [PubMed] [Google Scholar]
- 198. Maes M, Smith R, Christophe A, et al. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 1996; 38: 35–46. [DOI] [PubMed] [Google Scholar]
- 199. Paige LA, Mitchell MW, Krishnan KR, et al. A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry 2007; 22: 418–423. [DOI] [PubMed] [Google Scholar]
- 200. Steffens DC, Wei J, Krishnan KR, et al. Metabolomic differences in heart failure patients with and without major depression. J Geriatr Psychiatry Neurol 2010; 23: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Villasenor A, Ramamoorthy A, Silva dos Santos M, et al. A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol 2014; 171: 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Machado-Vieira R, Gold PW, Luckenbaugh DA, et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry 2017; 22: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lotrich FE. Inflammatory cytokine-associated depression. Brain Res 2015; 1617: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther 2018; 190: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ionescu DF, Felicione JM, Gosai A, et al. Ketamine-associated brain changes: a review of the neuroimaging literature. Harv Rev Psychiatry 2018; 26: 320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhong M, Wang X, Xiao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol 2011; 88: 233–242. [DOI] [PubMed] [Google Scholar]
- 207. Lehmann M, Seifritz E, Henning A, et al. Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc Cogn Affect Neurosci 2016; 11: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry 2009; 65: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 2017; 42: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Abdallah CG, Averill CL, Salas R, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks, Calif) 2018; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Abdallah CG, Jackowski A, Salas R, et al. The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology 2017; 42: 1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Phillips JL, Norris S, Talbot J, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry 2019; 176: 401–409. [DOI] [PubMed] [Google Scholar]
- 214. Strong CE, Kabbaj M. On the safety of repeated ketamine infusions for the treatment of depression: effects of sex and developmental periods. Neurobiol Stress 2018; 9: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wright KN, Kabbaj M. Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Curr Opin Behav Sci 2018; 23: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Ho RCM, Zhang MW. Ketamine as a rapid antidepressant: the debate and implications. BJPsych Adv 2018; 22: 222–233. [Google Scholar]
- 217. Loo C. Can we confidently use ketamine as a clinical treatment for depression? Lancet Psychiatry 2018; 5: 11–12. [DOI] [PubMed] [Google Scholar]
- 218. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 2018; 5: 65–78. [DOI] [PubMed] [Google Scholar]
- 219. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry 2019; 176: 422–424. [DOI] [PubMed] [Google Scholar]
- 220. Turner EH. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 2019; 6: 977–979. [DOI] [PubMed] [Google Scholar]
- 221. Corriger A, Pickering G. Ketamine and depression: a narrative review. Drug Des Devel Ther 2019; 13: 3051–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 2017; 74: 399–405. [DOI] [PubMed] [Google Scholar]


