Abstract
Pseudopalawania siamensisgen. et sp. nov., from northern Thailand, is introduced based on multi-gene analyses and morphological comparison. An isolate was fermented in yeast malt culture broth and explored for its secondary metabolite production. Chromatographic purification of the crude ethyl acetate (broth) extract yielded four tetrahydroxanthones comprised of a new heterodimeric bistetrahydroxanthone, pseudopalawanone (1), two known dimeric derivatives, 4,4′-secalonic acid D (2) and penicillixanthone A (3), the corresponding monomeric tetrahydroxanthone paecilin B (4), and the known benzophenone, cephalanone F (5). Compounds 1–3 showed potent inhibitory activity against Gram-positive bacteria. Compounds 2 and 3 were inhibitory against Bacillus subtilis with minimum inhibitory concentrations (MIC) of 1.0 and 4.2 μg/mL, respectively. Only compound 2 showed activity against Mycobacterium smegmatis. In addition, the dimeric compounds 1–3 also showed moderate cytotoxic effects on HeLa and mouse fibroblast cell lines, which makes them less attractive as candidates for development of selectively acting antibiotics.
Keywords: ascomycota, biological activity, multi-gene phylogenetic, new genus, new species, taxonomy, structure elucidation
1. Introduction
Fungi are potentially known as a promising source of bioactive compounds for drug discovery [1]. Mushrooms and other Basidiomycota, in particular, are widely used in traditional Chinese medicines and have been shown to provide beneficial activities against cancer and other ailments [2,3], but even the microfungi have various other potential benefits [4]. Dothideomycetes (Ascomycota) is a large and diverse class comprising of mostly microfungi. New species are constantly being discovered from this group and could be promising sources of novel bioactive compounds [5,6,7]. A few contemporary studies in Thailand have been focusing on saprobic fungi in Dothideomycetes as a source for finding novel bioactive compounds. For example, a novel Thai Dothideomycete, Pseudobambusicola thailandica, has yielded six new compounds with nematicidal and antimicrobial activity [8]. A new abscisic acid derivative with anti-biofilm activity against Staphylococcus aureus was isolated from cultures of a Roussoella sp. inhabiting Clematis subumbellata in northern Thailand [9], while Sparticola junci, another new Thai dothideomycete, yielded seven new spirodioxynaphthalenes with antimicrobial and cytotoxic activities [10]. Recently some phenalenones from another new Thai Pseudolophiostoma species were found to selectively inhibit α-glucosidase and lipase [11]. In spite of these recent discoveries, the study of bioactive compounds from Thai and other tropical Dothideomycetes is still in the initial stages of research.
In this study, we provide morphological descriptions and illustrations of a new Dothideomycetes fungus Pseudopalawania siamensis, collected from Caryota sp. (Arecaceae) in northern Thailand, based on multi-gene analyses and morphological comparison to confirm the current taxonomic placement of the fungus. In addition, we studied the new fungus for the production of bioactive compounds because its extracts showed significant antimicrobial activities in a preliminary screening. Thus, we here report the first secondary metabolites from this species, including their isolation, structure elucidation, and biological activity.
2. Materials and Methods
2.1. Sample Collection, Specimen Examination and Isolation of Fungi
Fresh material was collected from Nan Province, Thailand, in 2016. Fungal micromorphology was examined using a Motic, (Hongkong, China) SMZ 168 Series microscope. The appearance of ascomata on substrate was captured using a (stereo microscope fitted with an AxioCam ERC 5S camera (Carl Zeiss GmbH, Jena, Germany). Sections of ascomata were made by free hand. Fungal material was mounted in water and photographed with a Nikon (Bangkok, Thailand) ECLIPSE Ni compound microscope fitted with a Canon (Singapore) EOS 600D digital camera. Fungal photoplate was processed with Adobe Photoshop CS6 version 13.1.2 (Adobe Systems, CA, USA). All microscopic characters were measured using Tarosoft Image Frame Work program (IFW) version 0.97 (Nonthaburi, Thailand). Single spore isolations were obtained using the methods of Chomnunti et al. [12]. Germinating ascospores were transferred to a new malt extract agar (MEA) media and incubated at room temperature (25 °C) in the dark. Fungal cultures were used for molecular study and secondary metabolite production. The specimens and living cultures are deposited in the Herbarium of Mae Fah Luang University (Herb. MFLU) and Culture collection Mae Fah Luang University (MFLUCC), Chiang Rai, Thailand. Nomenclature and taxonomic information were deposited in MycoBank [13].
2.2. DNA Extraction, PCR Amplification and Sequencing
The genomic DNA from the fungal mycelium was extracted by using the ZR Soil Microbe DNA MiniPrep kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. DNA amplifications were performed by polymerase chain reaction (PCR). The partial large subunit nuclear rDNA (LSU) was amplified with primer pairs LROR and LR5 [14]. The internal transcribed spacer (ITS) was amplified by using primer pairs ITS5 and ITS4 [15]. The partial small subunit nuclear rDNA (SSU) was amplified with primer pairs NS1 and NS4 [15]. The translation elongation factor 1-alpha gene (TEF1) was amplified by using primers EF1-983F and EF1-2218R [16]. The partial gene encoding for the second largest RNA polymerase subunit (RPB2) was amplified by using primers fRPB2-5F and fRPB2- 7cR [17]. Methods for PCR amplification and sequencing were carried out according to previously described procedures [18,19].
2.3. Phylogenetic Analysis
The closest matched taxa were determined through nucleotide BLAST searches online in GenBank (http://www.ncbi.nlm.nih.gov/). Combined LSU: 28S large subunit of the nrRNA gene; ITS: internal transcribed spacer regions 1 and 2 including 5.8S nrRNA gene; SSU: 18S small subunit of the nrRNA gene; TEF1: partial translation elongation factor 1-α gene; and RPB2: partial RNA polymerase II second largest subunit gene sequence data from representative closest relatives to our strains were selected following Hongsanan et al. [20], Crous et al. [21], Hernández-Restrepo et al. [22], and Mapook et al. [23,24], to confirm the phylogenetic placement of our new strains. The phylogenetic analysis based on maximum likelihood (ML) and Bayesian inference (BI) were following the methodology as described in Mapook et al. [23,24]. The sequences used for analyses with accession numbers are given in Table 1. Phylogram generated from ML analysis was drawn using FigTree v. 1.4.2 [25] and edited by Microsoft Office PowerPoint 2013. The new nucleotide sequence data are deposited in GenBank.
Table 1.
Taxa used in this study and their GenBank accession numbers. New sequences generated in the present study are in bold.
| Taxa | Strain No. 1 | GenBank Accession Numbers 2 | References | ||||
|---|---|---|---|---|---|---|---|
| LSU | SSU | RPB2 | ITS | TEF | |||
| Acrospermum adeanum | M133 | EU940104 | EU940031 | EU940320 | EU940180 | - | Stenroos et al. [26] |
| Acrospermum compressum | M151 | EU940084 | EU940012 | EU940301 | EU940161 | - | Stenroos et al. [26] |
| Acrospermum gramineum | M152 | EU940085 | EU940013 | EU940302 | EU940162 | - | Stenroos et al. [26] |
| Alternaria alternata | KFRD-18 | KX609781 | KX609769 | - | KX346897 | KY094931 | Li et al. [27] |
| Alternariaster bidentis | CBS 134021 | KC609341 | - | KC609347 | KC609333 | - | Alves et al. [28] |
| Antennariella placitae | CBS:124785 | GQ303299 | - | - | MH863403 | - | Cheewangkoon et al. [29] |
| Arxiella dolichandrae | CBS 138853 | KP004477 | - | - | KP004449 | - | Crous et al. [30] |
| Arxiella terrestris | CBS 268.65 | MH870201 | - | - | MH858565 | - | Vu et al. [31] |
| Asterina fuchsiae | TH590 | GU586216 | GU586210 | - | - | - | Hofmann et al. [32] |
| Asterina phenacis | TH589 | GU586217 | GU586211 | - | - | - | Hofmann et al. [32] |
| Bambusicola massarinia | MFLUCC 11-0389 | JX442037 | JX442041 | KU940169 | JX442033 | - | Dai et al. [33] |
| Bambusicola splendida | MFLUCC 11-0439 | JX442038 | JX442042 | - | JX442034 | - | Dai et al. [33] |
| Botryosphaeria agaves | MFLUCC 11-0125 | JX646808 | JX646825 | - | JX646791 | JX646856 | Liu et al. [34] |
| Botryosphaeria tsugae | AFTOL-ID 1586 | DQ767655 | - | DQ767644 | - | DQ677914 | Schoch et al. [35] |
| Calicium salicinum | CBS 100898 | KF157982 | KF157970 | KF157998 | - | - | Beimforde et al. [36] |
| Calicium viride | 10-VII-1997 (DUKE) | AF356670 | AF356669 | AY641031 | - | - | Lutzoni et al. [37] |
| Camarosporium quaternatum | CBS 483.95 | GU301806 | GU296141 | GU357761 | KY929149 | GU349044 | Schoch et al. [38] |
| Capnodium salicinum | AFTOL-ID 937 | DQ678050 | DQ677997 | - | - | DQ677889 | Schoch et al. [37] |
| Caryospora minima | - | EU196550 | EU196551 | - | - | - | Cai and Hyde [39] |
| Chaetothyriothecium elegans | CPC 21375 | KF268420 | - | - | - | - | Hongsanan et al. [40] |
| Corynespora cassiicola | CBS 100822 | GU301808 | GU296144 | GU371742 | - | GU349052 | Schoch et al. [38] |
| Corynespora smithii | CABI 5649b | GU323201 | - | GU371783 | - | GU349018 | Schoch et al. [38] |
| Cucurbitaria berberidis | MFLUCC 11-0387 | KC506796 | KC506800 | - | - | - | Hyde et al. [41] |
| Cyphelium inquinans | Tibell 22283 (UPS) | AY453639 | U86695 | - | AY450584 | - | Tibell [42] |
| Cyphelium tigillare | Tibell 22343 (UPS) | AY453641 | AF241545 | - | AY452497 | - | Tibell [42] |
| Cystocoleus ebeneus | L161 | EU048578 | EU048571 | - | - | - | Muggia et al. [43] |
| Didymella exigua | CBS 183.55 | JX681089 | EU754056 | GU371764 | MH857436 | KR184187 | Verkley et al. [44] |
| Didymosphaeria rubi-ulmifolii | MFLUCC 14-0023 | KJ436586 | KJ436588 | - | - | - | Ariyawansa et al. [45] |
| Dothiora cannabinae | AFTOL ID 1359 | DQ470984 | DQ479933 | DQ470936 | - | DQ471107 | Spatafora et al. [46] |
| Dyfrolomyces phetchaburiensis | MFLUCC 15-0951 | MF615402 | MF615403 | - | - | - | Hyde et al. [47] |
| Dyfrolomyces rhizophorae | BCC15481 | - | KF160009 | - | - | - | Pang et al. [48] |
| Dyfrolomyces rhizophorae | JK 5456A | GU479799 | - | - | - | GU479860 | Suetrong et al. [49] |
| Dyfrolomyces thailandica | MFLU 16-1173 | KX611366 | KX611367 | - | - | - | Hyde et al. [50] |
| Dyfrolomyces thamplaensis | MFLUCC 15-0635 | KX925435 | KX925436 | - | - | KY814763 | Zhang et al. [51] |
| Dyfrolomyces tiomanensis | NTOU3636 | KC692156 | KC692155 | - | - | KC692157 | Pang et al. [48] |
| Elsinoe fawcettii | CPC 18535 | JN940382 | JN940559 | - | KX887207 | KX886853 | Schoch et al. [52] |
| Elsinoe verbenae | CPC 18561 | JN940391 | JN940562 | - | KX887298 | KX886942 | Schoch et al. [53] |
| Extremus antarcticus | CCFEE 5312 | KF310020 | - | KF310086 | KF309979 | - | Egidi et al. [54] |
| Gonatophragmium triuniae | CBS 138901 | KP004479 | - | - | KP004451 | - | Crous et al. [30] |
| Helicascus nypae | BCC 36751 | GU479788 | GU479754 | GU479826 | - | GU479854 | Suetrong et al. [49] |
| Julella avicenniae | BCC 20173 | GU371822 | GU371830 | GU371786 | - | GU371815 | Schoch et al. [38] |
| Karschia cezannei | Cezanne-Eichler B26 | KP456152 | - | - | - | - | Ertz and Diederich [55] |
| Katumotoa bambusicola | KT 1517a | AB524595 | AB524454 | AB539095 | NR_154103 | AB539108 | Tanaka et al. [56] |
| Labrocarpon canariense | Ertz 16907 (BR) | KP456157 | - | - | - | - | Ertz and Diederich [55] |
| Lentithecium fluviatile | CBS 123090 | FJ795450 | FJ795492 | FJ795467 | - | - | Zhang et al. [57] |
| Leptodiscella africana | CBS 400.65 | MH870275 | - | - | MH858635 | - | Vu et al. [31] |
| Leptodiscella brevicatenata | FMR 10885 | FR821311 | - | - | FR821312 | - | Madrid et al. [58] |
| Leptodiscella chlamydospora | MUCL 28859 | FN869567 | - | - | FR745398 | - | Madrid et al. [58] |
| Leptodiscella rintelii | CBS 144927 | LR025181 | - | - | LR025180 | - | Papendorf [52] |
| Leptosphaeria doliolum | MFLUCC 15-1875 | KT454719 | KT454734 | - | KT454727 | - | Ariyawansa et al. [59] |
| Leptosphaerulina australis | CBS 317.83 | EU754166 | GU296160 | GU371790 | MH861604 | GU349070 | de Gruyter et al. [60] |
| Leptoxyphium cacuminum | MFLUCC10-0049 | JN832602 | JN832587 | - | - | - | Chomnunti et al. [61] |
| Lophiotrema nucula | CBS 627 86 | GU301837 | GU296167 | GU371792 | LC194497 | GU349073 | Schoch et al. [38] |
| Lophium mytilinum | AFTOL-ID 1609 | DQ678081 | DQ678030 | DQ677979 | - | DQ677926 | Schoch et al. [35] |
| Massarina bambusina | H 4321 | AB807536 | AB797246 | - | LC014578 | AB808511 | Tanaka et al. [56] |
| Massarina eburnea | CBS 473.64 | GU301840 | GU296170 | GU371732 | - | GU349040 | Schoch et al. [38] |
| Melanomma pulvis-pyrius | CBS 371 75 | GU301845 | FJ201989 | GU371798 | - | GU349019 | Schoch et al. [38] |
| Melaspileopsis cf. diplasiospora | Ertz 16247 (BR) | KP456164 | - | - | - | - | Ertz and Diederich [55] |
| Melomastia maolanensis | GZCC 16-0102 | KY111905 | KY111906 | - | - | KY814762 | Zhang et al. [51] |
| Microsphaeropsis olivacea | CBS 233 77 | GU237988 | - | KT389643 | MH861055 | - | Aveskamp et al. [62] |
| Microthyrium buxicola | MFLUCC 15-0213 | KT306552 | KT306550 | - | - | - | Ariyawansa et al. [63] |
| Microthyrium microscopicum | CBS 115976 | GU301846 | GU296175 | GU371734 | - | GU349042 | Schoch et al. [44] |
| Multiseptospora thailandica | MFLUCC 11-0183 | KP744490 | KP753955 | - | KP744447 | KU705657 | Liu et al. [64] |
| Murispora rubicunda | IFRD 2017 | FJ795507 | GU456308 | - | - | GU456289 | Zhang et al. [57] |
| Muyocopron alcornii | BRIP 43897 | MK487708 | - | MK492712 | MK487735 | MK495956 | Hernández-Restrepo et al. [22] |
| Muyocopron atromaculans | MUCL 34983 | MK487709 | - | MK492713 | MK487736 | MK495957 | Hernández-Restrepo et al. [22] |
| Muyocopron castanopsis | MFLUCC 10-0042 | - | JQ036225 | - | - | - | Mapook et al. [23] |
| Muyocopron castanopsis | MFLUCC 14-1108 | KU726965 | KU726968 | KY225778 | MT137784 | MT136753 | Mapook et al. [23] |
| Muyocopron chromolaenae | MFLUCC 17-1513 | MT137876 | MT137881 | MT136761 | MT137777 | MT136756 | Mapook et al. [24] |
| Muyocopron chromolaenicola | MFLUCC 17-1470 | MT137877 | MT137882 | - | MT137778 | MT136757 | Mapook et al. [24] |
| Muyocopron coloratum | CBS 720.95 | MK487710 | - | MK492714 | NR_160197 | MK495958 | Hernández-Restrepo et al. [22] |
| Muyocopron dipterocarpi | MFLUCC 14-1103 | KU726966 | KU726969 | KY225779 | MT137785 | MT136754 | Mapook et al. [23] |
| Muyocopron dipterocarpi | MFLUCC 17-0075 | MH986833 | MH986829 | - | MH986837 | - | Senwanna et al. [65] |
| Muyocopron dipterocarpi | MFLUCC 17-0354 | MH986834 | MH986830 | - | MH986838 | - | Senwanna et al. [65] |
| Muyocopron dipterocarpi | MFLUCC 17-0356 | MH986835 | MH986831 | - | MH986839 | - | Senwanna et al. [66] |
| Muyocopron dipterocarpi | MFLUCC 18-0470 | MK348001 | MK347890 | - | MK347783 | - | Jayasiri et al. [67] |
| Muyocopron garethjonesii | MFLU 16-2664 | KY070274 | KY070275 | - | - | - | Tibpromma et al. [68] |
| Muyocopron geniculatum | CBS 721.95 | MK487711 | - | MK492715 | MK487737 | MK495959 | Hernández-Restrepo et al. [22] |
| Muyocopron heveae | MFLUCC 17-0066 | MH986832 | MH986828 | - | MH986836 | - | Senwanna et al. [66] |
| Muyocopron laterale | CBS 141029 | MK487712 | - | MK492716 | MK487738 | MK495960 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | IMI 324533 | MK487713 | - | MK492717 | MK487739 | MK495961 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 719.95 | MK487714 | - | MK492718 | MK487740 | MK495962 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 141033 | MK487715 | - | MK492719 | MK487741 | MK495963 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | URM 7802 | MK487716 | - | MK492720 | MK487742 | MK495964 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | URM 7801 | MK487717 | - | MK492721 | MK487743 | - | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 127677 | MK487718 | - | MK492722 | MK487744 | MK495965 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145310 | MK487719 | - | MK492723 | MK487745 | MK495966 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145315 | MK487720 | - | MK492724 | MK487746 | MK495967 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145313 | MK487721 | - | MK492725 | MK487747 | MK495968 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145309 | MK487722 | - | MK492726 | MK487748 | MK495969 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145314 | MK487723 | - | MK492727 | MK487749 | MK495970 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145311 | MK487724 | - | MK492728 | MK487750 | - | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145312 | MK487725 | - | MK492729 | MK487751 | MK495971 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | CBS 145316 | MK487726 | - | MK492730 | MK487752 | MK495972 | Hernández-Restrepo et al. [22] |
| Muyocopron laterale | FMR13797 | MK874616 | - | MK875802 | MK874615 | MK875803 | Hernández-Restrepo et al. [22] |
| Muyocopron lithocarpi | MFLUCC 10-0041 | JQ036230 | JQ036226 | - | - | - | Mapook et al. [23] |
| Muyocopron lithocarpi | MFLUCC 14-1106 | KU726967 | KU726970 | KY225780 | MT137786 | MT136755 | Mapook et al. [23] |
| Muyocopron lithocarpi | MFLU 18-2087 | MK347930 | MK347821 | - | MK347716 | - | Jayasiri et al. [66] |
| Muyocopron lithocarpi | MFLU 18-2088 | MK347931 | MK347822 | - | MK347717 | - | Jayasiri et al. [66] |
| Muyocopron lithocarpi | MFLUCC 16-0962 | MK348034 | MK347923 | - | - | - | Jayasiri et al. [66] |
| Muyocopron lithocarpi | MFLUCC 17-1465 | MT137878 | MT137883 | - | MT137779 | MT136758 | Mapook et al. [24] |
| Muyocopron lithocarpi | MFLUCC 17-1466 | MT137879 | MT137884 | - | MT137780 | MT136759 | Mapook et al. [24] |
| Muyocopron lithocarpi | MFLUCC 17-1500 | MT137880 | MT137885 | MT136762 | MT137781 | MT136760 | Mapook et al. [24] |
| Muyocopron zamiae | CBS 203.71 | MK487727 | - | MK492731 | - | MK495973 | Hernández-Restrepo et al. [22] |
| Mycoleptodiscus endophytica | MFLUCC 17-0545 | MG646946 | MG646978 | - | MG646961 | MG646985 | Tibpromma et al. [69] |
| Mycoleptodiscus suttonii | CBS 276.72 | MK487728 | - | MK492732 | MK487753 | MK495974 | Hernández-Restrepo et al. [22] |
| Mycoleptodiscus suttonii | CBS 141030 | MK487729 | - | MK492733 | - | MK495975 | Hernández-Restrepo et al. [22] |
| Mycoleptodiscus terrestris | CBS 231.53 | MK487730 | - | MK492734 | MK487754 | MK495976 | Hernández-Restrepo et al. [22] |
| Mycoleptodiscus terrestris | IMI 159038 | MK487731 | - | MK492735 | MK487755 | MK495977 | Hernández-Restrepo et al. [22] |
| Myriangium duriaei | CBS 260.36 | NG_027579 | AF242266 | KT216528 | MH855793 | - | Schoch et al. [35] |
| Myriangium hispanicum | CBS 247.33 | GU301854 | GU296180 | GU371744 | MH855426 | GU349055 | Schoch et al. [38] |
| Mytilinidion rhenanum | CBS 135.34 | FJ161175 | FJ161136 | FJ161115 | - | FJ161092 | Boehm et al. [70] |
| Natipusilla decorospora | AF236 1a | HM196369 | HM196376 | - | - | - | Ferrer et al. [71] |
| Natipusilla naponensis | AF217 1a | HM196371 | HM196378 | - | - | - | Ferrer et al. [71] |
| Neocochlearomyces chromolaenae | BCC 68250 | MK047514 | MK047552 | - | MK047464 | MK047573 | Crous et al. [21] |
| Neocochlearomyces chromolaenae | BCC 68251 | MK047515 | MK047553 | - | MK047465 | MK047574 | Crous et al. [21] |
| Neocochlearomyces chromolaenae | BCC 68252 | MK047516 | MK047554 | - | MK047466 | MK047575 | Crous et al. [21] |
| Neocylindroseptoria pistaciae | CBS 471.69 | KF251656 | - | KF252161 | KF251152 | KF253112 | Quaedvlieg et al. [65] |
| Neomycoleptodiscus venezuelense | CBS 100519 | MK487732 | - | MK492736 | MK487756 | MK495978 | Hernández-Restrepo et al. [22] |
| Palawania thailandensis | MFLUCC 14-1121 | KY086493 | KY086495 | KY086496 | MT137787 | - | Mapook et al. [24] |
| Palawania thailandensis | MFLU 16-1871 | KY086494 | - | - | MT137788 | - | Mapook et al. [24] |
| Paramycoleptodiscus albizziae | CPC 27552 | MH878220 | - | - | - | - | Vu et. al. [31] |
| Paramycoleptodiscus albizziae | CBS 141320 | KX228330 | - | MK492737 | KX228279 | MK495979 | Crous et. al. [72] |
| Phaeodimeriella cissampeli | MFLU 16-0558 | KU746806 | KU746808 | KU746810 | - | KU746812 | Mapook et. al. [73] |
| Phaeodimeriella dilleniae | MFLU 14-0013 | KU746805 | KU746807 | KU746809 | - | KU746811 | Mapook et. al. [73] |
| Phaeotrichum benjaminii | CBS 541.72 | AY004340 | AY016348 | GU357788 | MH860561 | DQ677892 | Lumbsch et. al. [74] |
| Physcia aipolia | AFTOL-ID 84 | DQ782904.1 | DQ782876 | DQ782862 | DQ782836 | DQ782892 | James et. al. [75] |
| Piedraia hortae | CBS 480.64 | GU214466 | - | KF902289 | GU214647 | - | Crous et. al. [76] |
| Platystomum crataegi | MFLUCC 14-0925 | KT026109 | KT026113 | - | NG_063580 | KT026121 | Thambugala et. al. [77] |
| Pleomassaria siparia | AFTOL-ID 1600 | DQ678078 | DQ678027 | DQ677976 | - | DQ677923 | Schoch et. al. [35] |
| Pleospora herbarum | IT 956 | KP334709 | KP334729 | KP334733 | KP334719 | KP334731 | Ariyawansa et. al. [78] |
| Preussia funiculata | CBS 659.74 | GU301864 | GU296187 | GU371799 | - | GU349032 | Schoch et. al. [38] |
| Pseudomassariosphaeria bromicola | IT-1333 | KT305994 | KT305996 | - | KT305998 | KT305999 | Ariyawansa et. al. [63] |
| Pseudopalawania siamensis | MFLUCC 17-1476a | - | MT137789 | - | MT137782 | MT136752 | This study |
| Pseudopalawania siamensis | MFLUCC 17-1476b | - | MT137790 | - | MT137783 | - | This study |
| Pseudostrickeria muriformis | MFLUCC 13-0764 | KT934254 | KT934258 | - | - | KT934262 | Tian et. al. [79] |
| Pseudovirgaria grisea | CPC 19134 | JF957614 | - | - | JF957609 | - | Braun et. al. [80] |
| Pseudovirgaria hyperparasitica | CPC 10753 | EU041824 | - | - | EU041767 | - | Arzanlou et. al. [81] |
| Ramularia endophylla | CBS 113265 | KF251833 | - | KP894673 | KF251220 | - | Verkley et. al. [82] |
| Rasutoria pseudotsugae | rapssd | EF114704 | EF114729 | - | EF114687 | - | Winton et al. [83] |
| Rasutoria tsugae | ratstk | EF114705 | EF114730 | GU371809 | EF114688 | - | Winton et al. [83] |
| Salsuginea ramicola | KT 2597.1 | GU479800 | GU479768 | GU479833 | - | GU479861 | Suetrong et al. [49] |
| Schizothyrium pomi | CBS 406.61 | EF134949 | - | KF902384 | - | - | Batzer et al. [84] |
| Setoapiospora thailandica | MFLUCC 17-1426 | MN638847 | MN638851 | - | MN638862 | MN648731 | Hyde et al. [85] |
| Stictographa lentiginosa | Ertz 17570 (BR) | KP456170 | - | - | - | - | Ertz and Diederich [55] |
| Sympoventuria capensis | CBS 120136 | KF156104 | KF156094 | - | KF156039 | - | Samerpitak et al. [86] |
| Teratosphaeria fibrillosa | CBS 121707 | GU323213 | GU296199 | GU357767 | MH863138 | KF903305 | Schoch et al. [38] |
| Trichodelitschia munkii | Kruys 201 (UPS) | DQ384096 | DQ384070 | - | - | - | Kruys et al. [87] |
| Tumidispora shoreae | MFLUCC 14-0574 | KT314074 | KT314076 | - | - | - | Ariyawansa et al. [63] |
| Uwebraunia commune | NC132C1d | - | - | KT216546 | - | - | Ismail et al. [88] |
| Venturia inaequalis | CBS 594.70 | GU301879 | GU296205 | GU357757 | KF156040 | GU349022 | Schoch et al. [38] |
| Xenolophium applanatum | CBS 123127 | GU456330 | GU456313 | GU456355 | - | GU456270 | Zhang et al. [89] |
| Zeloasperisporium hyphopodioides | CBS 218.95 | EU035442 | - | - | - | - | Crous et al. [90] |
| Zeloasperisporium siamense | IFRDCC 2194 | JQ036228 | JQ036223 | - | - | - | Mapook et. al. [73] |
| Zeloasperisporium wrightiae | MFLUCC 15-0225 | KT387737 | KT387738 | - | - | - | Hongsanan et al. [91] |
1 AFTOL-ID: Assembling the Fungal Tree of Life; BCC: BIOTEC Culture Collection; BRIP: Biosecurity Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CCFEE: Culture Collection of Fungi from Extreme Environments, The University of Tuscia; CPC: Culture collection of Pedro Crous, the Netherlands; FMR: Facultad de Medicina, Reus, Tarragona, Spain; GZCC: Guizhou Culture Collection; IFRDCC = International Fungal Research and Development Centre Culture Collection, China; IMI: The International Mycological Institute Culture Collections; JK: J. Kohlmeyer; MFLU: the Herbarium of Mae Fah Luang University; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; MUCL: Belgian Coordinated Collections of Microorganisms; URM: Universidade Federal de Pernambuco. 2 LSU: 28S large subunit of the nrRNA gene; SSU: 18S small subunit of the nrRNA gene; RPB2: partial RNA polymerase II second largest subunit gene; ITS: internal transcribed spacer regions 1 and 2 including 5.8S nrRNA gene; TEF1: partial translation elongation factor 1-α gene.
2.4. General Information of Chromatography and Spectral Methods
Specific optical rotations ([α]D) were measured using a Perkin-Elmer (Überlingen, Germany) 241 polarimeter in a 100 × 2 mm cell at 22 °C. ECD spectra were recorded on a J-815 spectropolarimeter (JASCO, Pfungstadt, Germany). UV spectra were obtained on a Shimadzu (Duisburg, Germany) UV-Vis spectrophotometer UV-2450 with 1 cm quartz cells. IR spectra were measured with a Nicolet Spectrum 100 FT-IR spectrometer (Perkin-Elmer, Waltham, MA, USA). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 700 MHz Avance III spectrometer with a 5 mm TXI cryoprobe (1H 700 MHz, 13C 175 MHz) and a Bruker 500 MHz Avance III spectrometer with a BBFO (plus) SmartProbe (1H 500 MHz, 13C 125 MHz). In all cases, spectra were acquired at 25 °C (unless otherwise specified) in solvents as specified in the text, with referencing to residual 1H or 13C signals in the deuterated solvents (CDCl3 or MeOH-d4). HPLC-DAD/MS analysis was conducted using an amaZon Speed ETD ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany). HR-ESI mass spectra was measured using an Agilent 1200 series HPLC-UV system (column 2.1 × 50 mm, 1.7 μm, C18 Waters Acquity UPLC BEH) combined with an maXis (Bruker) ESI-TOF-MS instrument The mobile phase was composed of H2O + 0.1% formic acid (solvent A) and acetonitrile + 0.1% formic acid (solvent B), with the following gradient: 5% solvent B for 0.5 min with a flow rate of 0.6 mL/min, increasing to 100% solvent B in 19.5 min and then maintaining 100% solvent B for 5 min. UV/Vis detection at 200–600 nm. Chemicals and solvents were obtained from AppliChem GmbH, Avantor Performance Materials, Carl Roth GmbH & Co. KG (Karlsruhe, Germany) and Merck KGaA (Darmstadt, Germany) in analytical and HPLC grade.
2.5. Fermentation and Extraction
Five mycelial plugs from actively growing colonies on malt extract agar (MEA) media (malt extract 20 g/L, D-glucose 20 g/L, peptone 6 g/L, pH 6.3) were cut using a sterile cork borer (0.7 × 0.7 cm2) and placed into a sterilized 500 mL Erlenmeyer flask containing 200 mL of liquid yeast malt (YM) medium (malt extract 10 g/L, D-glucose 4 g/L, yeast extract 4 g/L, pH 6.3). These seed cultures were incubated on a rotary shaker (140 rpm) at 23 °C in the dark for nine days. Ten milliliters of the seed culture were added into 25 × 500 mL sterile Erlenmeyer flasks with 200 mL of YM medium and incubated on a rotary shaker for 14 days. The extraction was conducted 3 days after glucose depletion as monitored by the glucose strip test using Bayer Harnzuckerstreifen, (Bayer, Leverkusen, Germany). Fungal mycelium and supernatant were separated by using vacuum filtration. The supernatant was mixed with 3% Amberlite XAD-16N adsorber resin (Sigma-Aldrich, Deisenhofen, Germany) and stirred for 1 h and filtrated to remove the culture broth. The XAD resin was eluted three times with an equal volume of ethyl acetate. The mycelia were extracted twice with an equal volume of acetone in an ultrasonic bath for 30 min and the combined extracts were passed through a filter, then dissolved in water/ethyl acetate. The aqueous phase (lower) was discarded while the organic phase (upper) was filtered through anhydrous sodium sulfate (Na2SO4) for water removal and then evaporated to dryness. This procedure yielded 1580 mg mycelial crude extract and 769 mg of supernatant crude extract. The mycelial extract contained mainly fatty acids and ergosterol derivatives and showed only weak bioactivity. It was therefore not further processed. The supernatant extract contained the majority of the active components and was therefore subjected to preparative isolation of its active ingredients.
2.6. Isolation of Compounds 1–5
The supernatant crude extract was dissolved in methanol and initially fractionated on preparative HPLC manufactured by Gilson (Middleton, Wi, USA), comprised of a GX-271 Liquid Handler, a 172 DAD, a 305 and 306 pump, with 50SC Piston Pump Head. A Phenomenex (Torrance, Ca., USA) Gemini 10u C18 110Å column (250 × 21.20 mm, 10 μm) was used as a stationary phase. The mobile phase was composed of deionised water (Milli-Q, Millipore, Schwalbach, Germany) with 0.05% of trifluoroacetic acid (TFA) as a solvent A and acetonitrile (ACN) HPLC grade with 0.05% TFA as a solvent B. The fractionation proceeded with the following gradient: linear gradient of 10% solvent B for 5 min with a flow rate of 35 mL/min, followed by 10% to 100 % solvent B for 30 min, and 100% solvent B for 10 min. The UV detection was carried out at 210, 254 and 350 nm. Final five compounds were purified from initially 16 fractions (Figure 1). Compound 1 (pseudopalawanone; 5.51 mg) eluted at tR = 7.8 min from fraction 12, compound 2 (4,4′-secalonic acid D; 5.48 mg) eluted at tR = 10.5 min from fraction 15, compound 4 (paecilin B; 1.08 mg) eluted at tR = 6.9 min from fraction 4, and compound 5 (cephalanone F; 1.52 mg) eluted at tR = 3.0 min from fraction 3, while compound 3 (penicillixanthone A; 0.86 mg) eluted at tR = 11.3 min was resulted from the purification of fraction 16 (4.12 mg) on a VarioPrep Nucleodur 100-10 C18 ec column (150 × 40 mm, 7 µm; Macherey-Nagel, Düren, Germany) using the following gradients: linear gradient of 30% solvent B for 5 min with a flow rate of 15 mL/min, followed by 30% to 100 % solvent B for 20 min, and 100% solvent B for 10 min.
Figure 1.
HPLC-(DAD)-UV chromatogram of the crude ethyl acetate extract of the culture filtrate of Pseudopalawania siamensis (MFLUCC 17-1476).
2.7. Spectral Data
Pseudopalawanone (1)
Pale yellowish gum. [a]25D = +30.0 (c 1.0, MeOH). 1H NMR (500 MHz, CDCl3): see Table 2; 13C NMR (125 MHz, CDCl3): see Table 2. HR-ESIMS m/z 641.1492 ([M + H]+, calcd for C31H29O15, 641.1501).
Table 2.
NMR spectroscopic data for pseudopalawanone (1).
| No. | δH, m, J (Hz) | δC, m | No. | δH, m, J (Hz) | δC, m |
|---|---|---|---|---|---|
| 1 | - | 160.1, C | 1′ | - | 161.8, C |
| 2 | - | 117.6, C | 2′ | 6.66, d (8.7) | 110.4, CH |
| 3 | 7.82, d (8.6) | 143.8, CH | 3′ | 7.54, d (8.7) | 141.2, CH |
| 4 | 6.77, d (8.6) | 108.3, CH | 4′ | - | 114.0, C |
| 4a | - | 158.3, C | 4a′ | - | 155.6, C |
| 5 | 4.44, d (4.0) | 74.1, CH | 5′ | 4.38, d (2.5) | 88.1, CH |
| 6 | 2.13, m | 30.4, CH | 6′ | 2.65, m | 29.9, CH |
| 7a | 2.36, dd (15.9, 13.6) | 33.8, CH2 | 7′a | 2.18, m | 35.8, CH2 |
| b | 2.12, m | b | 1.99, dd (18.3, 3.1) | ||
| 8 | - | 108.9, C | 8′ | - | 176.5, C |
| 8a | - | 73.6, C | 8a′a | 3.14, d (16.9) | 39.6, CH2 |
| b | 2.98, d (16.9) | ||||
| 9 | - | 194.9, C | 9′ | - | 193.6, C |
| 9a | - | 106.8, C | 9a′ | - | 107.6, C |
| 10a | - | 84.7, C | 10a′ | - | 84.8, C |
| 11 | 1.20, d (6.5) | 14.9, CH3 | 11′ | 1.16, d (7.2) | 20.9, CH3 |
| 12 | - | 176.6, C | 12′ | - | 168.5, C |
| 13 | - | - | 13′ | 3.80, s | 53.7, CH3 |
| 1-OH | 11.35, s | - | 1′-OH | 11.51, s | - |
2.8. Antimicrobial Activity and Cytotoxicity Assays
Minimum inhibitory concentrations (MIC) of compounds 1–5 were determined against various fungal and bacterial strains by using a 96-well serial dilution technique according to previously described procedures [92,93]. The tested organisms with results are given in Table 3 and Table 4. Gentamicin, kanamycin, nystatin, and oxytetracycline were used as positive controls against tested organisms. In vitro cytotoxicity (IC50) of compounds 1–5 were determined using the MTT assay according to previously described procedures [26,27] against the mouse fibroblast cell line (L929) and the human HeLa (KB-3-1) cell line. Epothilone B and methanol were used as positive and negative control, respectively.
Table 3.
Antimicrobial activity of compounds 1–5.
| Tested Organisms | Strain No. | Minimum Inhibitory Concentration (MIC) [μg/mL] | |||||
|---|---|---|---|---|---|---|---|
| Compounds | Positive Control * | ||||||
| 1 | 2 | 3 | 4 | 5 | |||
| Fungi | |||||||
| Candida albicans | DSM 1665 | - | 66.7 | - | - | - | 66.7 (20 µL N) |
| Cryptococcus neoformans | DSM 15466 | - | - | - | - | - | 66.7 (20 µL N) |
| Mucor hiemalis | DSM 6766 | - | - | 66.7 | - | - | 66.7 (20 µL N) |
| Pichia anomala | DSM 6766 | - | - | - | - | - | 66.7 (20 µL N) |
| Rhodoturula glutinis | DSM 10134 | - | - | - | - | - | 16.7 (20 µL N) |
| Schizosaccharomyces pombe | DSM 70572 | - | - | - | - | - | 33.3 (20 µL N) |
| Bacteria | |||||||
| Bacillus subtilis | DSM 10 | 66.7 | 1.0 | 4.2 | - | - | 8.3 (20 µL O) |
| Chromobacterium violaceum | DSM 30191 | - | - | - | - | - | 1.7 (2 µL O) |
| Escherichia coli | DSM 1116 | - | - | - | - | - | 3.3 (2 µL O) |
| Micrococcus luteus | DSM 1790 | 66.7 | 8.3 | 33.3 | - | - | 0.4 (2 µL O) |
| Mycobacterium smegmatis | ATCC 700084 | - | 66.7 | - | - | - | 3.3 (2 µL K) |
| Pseudomonas aeruginosa | PA14 | - | - | - | - | - | 0.8 (2 µL G) |
| Staphylococcus aureus | DSM 346 | 66.7 | 4.2 | 33.3 | - | - | 0.2 (2 µL O) |
* Positive drug controls: K = kanamycin, N = nystatin, O = oxytetracycline hydrochloride. (-): no inhibition. The starting concentration was 66.7 µg/mL.
Table 4.
Cytotoxic activity of compounds 1–5.
| Cell Lines | IC50 (µM) | |||||
|---|---|---|---|---|---|---|
| Compounds | Epothilone B | |||||
| 1 | 2 | 3 | 4 | 5 | ||
| HeLa cells KB3.1 | 29.7 | 3.9 | 17.2 | - | - | 8.9 × 10−5 |
| Mouse fibroblast L929 | 50.0 | 14.1 | - | - | - | 1.8 × 10−3 |
The in vitro cytotoxicity test of polyketides 1–5 was conducted against two mammalian cell lines, with epothilone B as positive control. Starting concentration for cytotoxicity assay was 66 μg/mL, substances were dissolved in MeOH (1 mg/mL). MeOH was used as negative control and showed no activity against the tested mammalian cell lines. Results were expressed as IC50: half maximal inhibitory concentration (µM). (-): no inhibition.
3. Results and Discussion
3.1. Phylogenetic Analysis
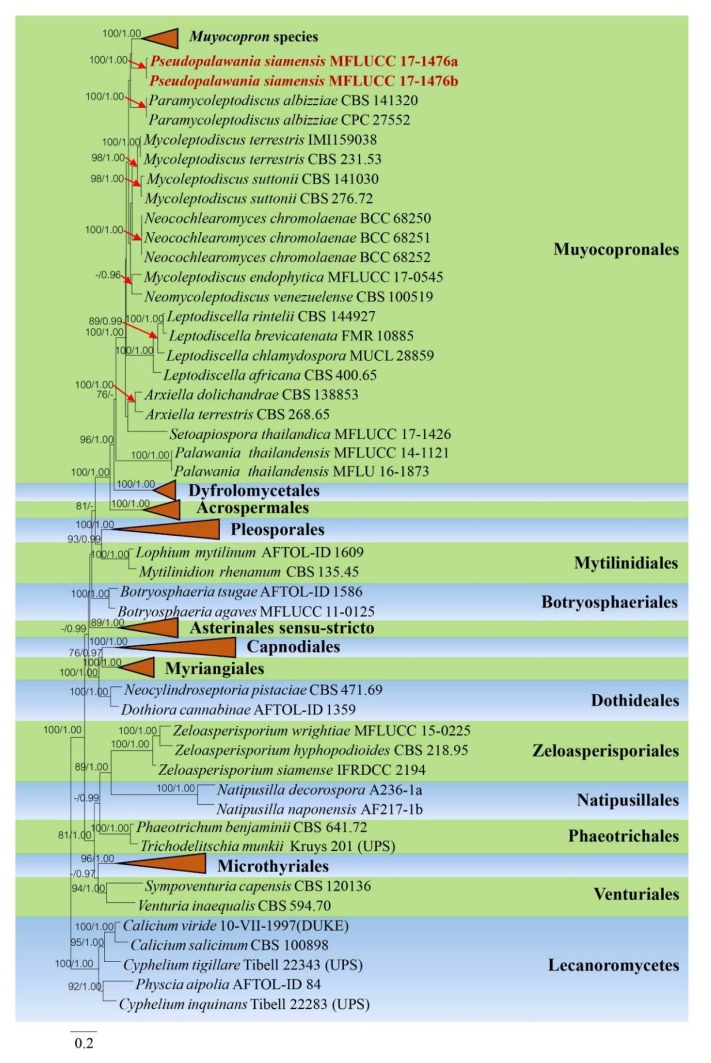
The combined dataset of LSU, SSU, RPB2, ITS and TEF sequence data including our new strains were analyzed by maximum likelihood (ML) and Bayesian analyses. The combined sequence alignment is comprised of 155 taxa (6131 characters with gaps), which include representative strains from Lecanoromycetes as outgroup taxa. A best scoring RAxML tree with a final likelihood value of -91,669.392085 is presented in Figure 2. The matrix had 3930 distinct alignment patterns, with 59.36% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.242975, C = 0.253394, G = 0.277569, T = 0.226061; substitution rates: AC = 1.292278, AG = 3.020191, AT = 1.589713, CG = 1.197479, CT = 6.661698, GT = 1.000000; gamma distribution shape parameter α = 0.357175. In a BLASTn search of NCBI GenBank, the closest matches of the ITS sequence of Pseudopalawania siamensis (MFLUCC 17-1476, ex-holotype) is Muyocopron geniculatum with 81.40% (MK487737) similarity, respectively, was strain CBS. 721.95, the closest matches of the SSU sequence with 98.90% similarity, was Neocochlearomyces chromolaenae (strain BCC 68250, NG_065766), the closest matches of the TEF sequence with 95.17% similarity, was Neomycoleptodiscus venezuelense (strain CBS 100519, MK495978). The phylogram generated from maximum likelihood analysis (Figure 2) shows that our new strains clustered within Dothideomycetes and form a distinct lineage in the Muyocopronales, even though the clade is lacking bootstrap support.
Figure 2.
Phylogram generated from maximum likelihood analysis based on combined dataset of LSU, SSU, RPB2, ITS and TEF sequence data. Bootstrap support values for maximum likelihood (ML) equal to or greater than 60% and Bayesian posterior probabilities (PP) equal to or greater than 0.90 are given above the nodes. Newly generated sequences are in dark red bold. The tree is rooted with Lecanoromycetes. Small red arrows point towards the bootstrap values of the clades representing genera of the order Muyocopronales, while some other monophyletic clades that represent monophyletic clades have been collapses (indicated by red triangles).
3.2. Taxonomy
3.2.1. Pseudopalawania Mapook and K.D. Hyde, gen. nov.
Mycobank number: MB834934.
Etymology: The generic epithet refers to the similarity to Palawania.
Saprobic on dead rachis of Arecaceae. Sexual morph: Ascomata superficial, solitary or scattered, sub-carbonaceous to carbonaceous, appearing as circular, flattened, dark brown to black spots, covering the host, without a subiculum, with a poorly developed basal layer and an irregular margin. Ostioles central. Peridium comprising dark brown or black to reddish-brown cells of textura epidermoidea to textura angularis. Hamathecium cylindrical to filiform, septate, hyaline, branching pseudoparaphyses. Asci eight-spored, bitunicate, fissitunicate, cylindric-clavate, straight or slightly curved, with an ocular chamber observed clearly when immature. Ascospores overlapping, 2–3-seriate, broadly fusiform to inequilateral, pointed ends, hyaline, 1-septate, constricted at the septum, guttulate when immature, surrounded by hyaline and thin layers of gelatinous sheath, observed clearly when mounted in Indian ink. Asexual morph: Undetermined.
Type species: Pseudopalawania siamensis Mapook and K.D. Hyde
3.2.2. Pseudopalawania siamensis Mapook and K.D. Hyde, sp. nov.
Mycobank number: MB834935; Figure 3
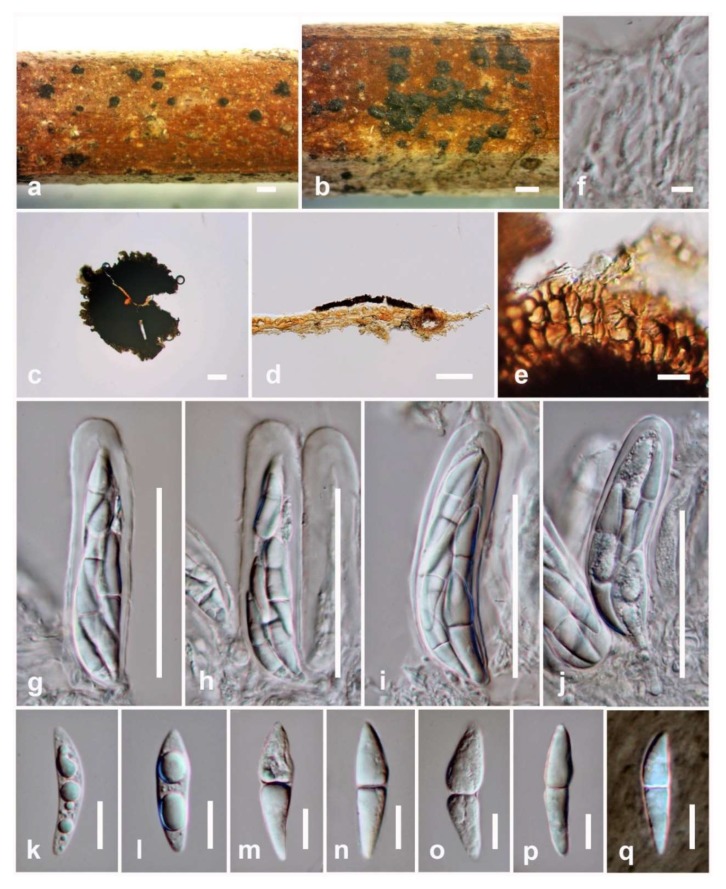
Figure 3.
Pseudopalawania siamensis (holotype) (a,b) Appearance of ascomata on substrate. (c) Squash mounts showing ascomata. (d) Section of ascoma. (e) Peridium. (f) Pseudoparaphyses. (g–j) asci. (k–p) Ascospores. (q) Ascospores in Indian ink. Scale bars: a, b = 500 µm, c, d = 100 µm, g–j = 50 µm, e, k–q = 10 µm, f = 5 µm.
Etymology: Named after the country from where the fungus was collected, using the former name of Siam.
Saprobic on dead rachis of Caryota sp. Sexual morph: Ascomata 29–40 µm high × 270–290(–315) µm diam. ( = 32.5 × 292 µm, n = 5), superficial, solitary or scattered, sub-carbonaceous to carbonaceous, appearing as circular, flattened, dark brown to black spots, covering the host, without a subiculum, with a poorly developed basal layer and an irregular margin. Ostioles central. Peridium 10–20 µm wide, comprising dark brown or black to reddish-brown cells of textura epidermoidea to textura angularis. Hamathecium comprising 1–2.5 µm wide, cylindrical to filiform, septate, hyaline, branching pseudoparaphyses. Asci 65–85 × 15–21 µm ( = 75 × 18 µm, n = 10), eight-spored, bitunicate, fissitunicate, cylindric-clavate, straight or slightly curved, with an ocular chamber observed clearly when immature. Ascospores 25–37 × 5–11 µm ( = 29 × 7 µm, n = 20), overlapping, 2–3-seriate, broadly fusiform to inequilateral, pointed ends, hyaline, 1-septate, constricted at the septum, guttulate when immature, surrounded by hyaline and thin layers of gelatinous sheath, observed clearly when mounted in Indian ink. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on MEA within 24 hrs. at room temperature and germ tubes produced from the apex. Colonies on MEA circular, slightly raised, filamentous, mycelium white at the surface and initially creamy-white to pale brown in reverse, becoming dark brown from the centre of the colony with creamy-white at the margin.
Pre-screening for antimicrobial activity: Pseudopalawania siamensis (MFLUCC 17-1476) showed antimicrobial activity against B. subtilis with a 16 mm inhibition zone and against M. plumbeus with a 17 mm inhibition zone, observable as full inhibition, when compared to the positive control (26 mm and 17 mm, respectively), but no inhibition of E. coli.
Material examined: THAILAND, Nan Province, on dead rachis of Caryota sp. (Arecaceae), 23 September 2016, A. Mapook (MFLU 20-0353, holotype); ex-type culture MFLUCC 17-1476.
Notes: Pseudopalawania is similar to Palawania in its superficial and flattened ascomata, with hyaline, 1-septate ascospores, but differs in its peridium wall patterns, shape of asci (cylindric-clavate vs. inequilateral to ovoid) with an ocular chamber and shape of ascospores (broadly fusiform to inequilateral vs. oblong to broadly fusiform) with a thin layer of gelatinous sheath. The gelatinous sheath in Palawania is thicker [24]. Pseudopalawania is also similar to Muyocopron in its superficial, flattened ascomata with similar peridium wall patterns, and asci with an ocular chamber; but differs in its sub-carbonaceous to carbonaceous ascomata, shape of asci and ascospores with surrounded by hyaline gelatinous sheath, 1-septate, while Muyocopron have coriaceous ascomata, aseptate ascospores with granular appearance and without gelatinous sheath [23]. In addition, the genus was compared with genera in Microthyriaceae of which no DNA sequence data are available, but the holotype specimens were re-examined in previous studies with morphological descriptions and illustrations [94,95,96,97,98,99], and neither of them matched our new fungus. Therefore, we introduce Pseudopalawania as a new genus with a new species P. siamensis from Thailand. The fungus is placed in Muyocopronaceae (Muyocopronales) with evidence from morphology and phylogeny.
3.3. Structure Elucidation of the New Compound
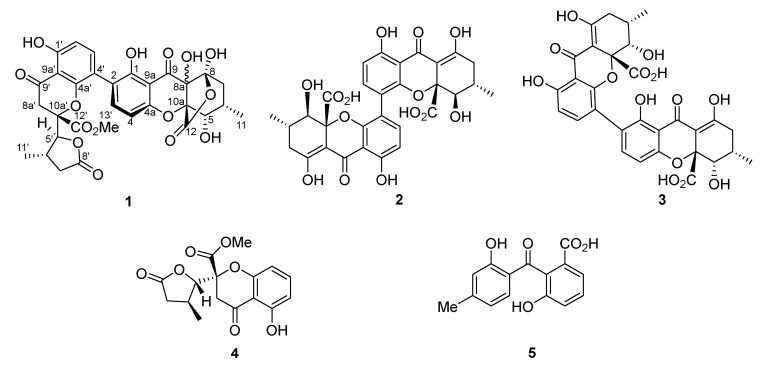
HPLC chromatographic fractionation of the crude ethyl acetate extract from the yeast malt (YM 6.3) broth of Pseudopalawania siamensis resulted in the isolation of a new heterodimeric bistetrahydroxanthone, pseudopalawanone (1) together with three known tetrahydroxanthones, 4,4′-secalonic acid D (2) [100], penicillixanthone A (3) [101], paecilin B (4) [102] and the benzophenone, cephalanone F (5) [103] (Figure 4).
Figure 4.
Secondary metabolites from Pseudopalawania siamensis.
Pseudopalawanone (1) was obtained as optically active, pale yellow gum. The IR spectrum showed the presence of hydroxyl groups (3387 cm−1), carbonyl functionalities (1787, 1741 cm−1) and aromatic residues (1648, 1622 cm−1) while the UV spectrum was indicative of absorptions due to chromanone units [102,104]. The molecular formula C31H28O15, indicating eighteen double bond equivalents, was established by HR-ESIMS based on its protonated pseudomolecular ion peak ([M + H]+) at m/z 641.1492. Observation of two sets of signals in the NMR spectra (Figures S1 and S2) and careful comparison of the 1H and 13C NMR spectroscopic data of 1 (Table 2) with those of 2–4 immediately revealed 1 to be an asymmetric dimer of an unfamiliar highly oxygenated tetrahydroxanthone subunit and 7-deoxyblennolide D [102]. Thus, the gross structure of the latter fragment along with its connection to 7-deoxyblennolide D was established through analysis of 1D and 2D NMR spectroscopic data and will be the subject of the following discussions. The 13C and HSQC-DEPT edited spectra (Figure S3) showed the presence of fifteen resonances comprised of a ketone (δC 194.9), a carboxyl group of an ester functionality (δC 176.6), a hemiacetal carbon (δC 108.9), four quaternary aromatic carbons (δC 106.8, 117.6, 158.3, 160.1), two aromatic methine carbons (δC 108.3, 143.8), two aliphatic quaternary carbons (δC 73.6, 84.7), two methine carbons (δC 30.4, 74.1), a methylene carbon (δC 33.8) and a methyl group (δC 14.9). The 1H and COSY NMR spectrum (Figure S4) revealed two ortho-coupled aromatic protons (3J = 8.6 Hz) for H–3 (δH 7.82) and H–4 (δH 6.77), and a seven-proton spin system comprised of H–5 (δH 4.44) – H–6 (δH 2.23) (H3–11) (δH 1.20) – H2–7 (δH 2.12, 2.36). A C–2 substituted 1-hydroxychromanone unit was elucidated on the basis of HMBC correlations of chelated 1-OH (δH 11.35) with C–1 (δC 160.1), C–2 (δC 117.6) and C–9a (δC 106.8) and of H–4 (δH 6.77) with C–2 and C–4a (δC 158.3). The remaining portion of the molecule was constructed through HMBC correlations of H–6 (δH 2.13) and H–11 (δH 1.20) with C–8 (δC 108.9), of H–5 (δH 4.44) with C–8a (δC 73.6), C–10a (δC 84.7) and C–12 (δC 176.6), and of H2–7 (δH 2.12, 2.36) with C–8 and C–8a. The chemical shifts assigned for C–8 and C–12 were ascribed to hemiacetal and γ-lactone moieties, respectively, by using a combination of 2D NMR experiments (Figure 5). The lactone ester was plausibly attached to C–8 forming a γ-hydroxylactone subunit of a [3.2.1] bicyclic structure. The remaining 17 mass units was attributed to a hydroxyl group attached to the 〈−carbon (C–8a) of the chromanone substructure. This unusual tetrahydroxanthone motif could putatively originate presumably from α hydroxylation of the keto form of blennolide A, followed by nucleophilic attack of the hydrolyzed C–12 methyl ester (Figure 6). The relative configurations of C–5 and C–6 were readily established to be similar with blennolide A by the coupling constant (3J5,6 = 4.0 Hz) and the chemical shifts as 5S*, 6S* while that of C–10a was assigned R* based on the observed positive n-π* CD transition at around 331 nm [104]. The chirality of C–8a cannot be established using available methods due to its remoteness to most protons in the molecule.
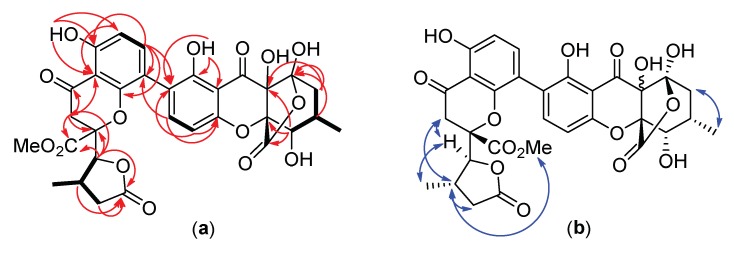
Figure 5.
COSY (bold bonds), HMBC (red arrows) (a), and ROESY (blue arrows) (b) correlations in pseudopalawanone (1).
Figure 6.
Plausible biogenetic pathway towards pseudopalawanone (1).
The linkage between the chromanone subunit and the ©−lactone in the 7-deoxyblennolide D monomer was indicated by the HMBC correlation of H–5′ (δH 4.38) with C–10a′ (δC 84.8) and C–12′ (δC 168.5). The C–5′S* and C–6′S* relative configurations in the lactone moiety were established by coupling constant analysis (3J5,6 = 2.5 Hz) depicting a pseudodiaxial orientation for H–5′/H–6′ and the NOE (Figures S6 and S7) noted between H–5′ and H–8a’a (δH 3.14), H–8a′b (δH 2.98) and H–6′ (δH 2.65), and that of H–6′ and H3–13 (δH 3.80) [102]. The spatial arrangements in ring C were similar to 7-deoxyblennolide D corroborated by NOE correlations between H–5′, H3–11′ (δH 1.16) and H–7′b (δH 1.99). Finally, the relative configuration of C-10a′ may be tentatively assigned as S* on the basis of negative π*-π* transitions below 330 nm and positive n-π* transitions at 346 nm in the ECD spectrum (Figure S9) of 1 [104]. The overall relative configuration of the blennolide-type tetrahydroxanthone substructure is 5S*, 6S*, and 10aS* thus, structurally similar to 7-deoxyblennolide D.
The planar structure of 1 was established by connecting the two monomers through the linkage of C–2 (δC = 117.6) of the oxidized secalonic acid subunit and C–4′ (δC 114.0) of 7-deoxyblennolide D evidenced by the diagnostic HMBC correlations of H–3 (δH 7.82) to C–4′ and H–3′ (δH 7.54) to C–2. The axial configuration of C-2/C-4′ was assigned as P based on the CD spectrum of 1 which showed a positive first Cotton effect (225 nm, De = −6.41) and a negative second cotton effect (250 nm, De = +3.15). Thus, compound 1 was given the trivial name pseudopalawanone. To establish unambiguously its relative and absolute configurations especially C–8a in the blennolide A substructure and C–10a’ in the 7-deoxyblennolide D substructure, we suggest additional experiments such as asymmetric total synthesis, derivatization with heavy atom/s followed by single crystal x-ray diffraction and/or further ECD-TDDFT measurements and calculations.
3.4. Biological Activity of Compounds 1–5
The polyketides 1–5 were evaluated for their antimicrobial activity against selected microorganisms (Table 3) and cytotoxicity against two mammalian cell lines, HeLa cells KB3.1 and mouse fibroblast cell line L929 (Table 4). The starting concentration for antimicrobial assay and cytotoxicity assay were 66.7 and 300 µg/mL, respectively and the substances were dissolved in MeOH (1 mg/mL). MeOH was used as the negative control and showed no activity against the tested organisms and mammalian cell lines. Results were expressed as MIC or minimum inhibitory concentration (μg/mL) and IC50 or half maximal inhibitory concentration (μM) (Table 3 and Table 4). The known compounds 4 and 5 showed neither antimicrobial nor cytotoxic activities. The dimeric tetrahydroxanthone 4,4′-secalonic acid D (2) showed inhibition against the pathogenic fungus Candida albicans while penicillixanthone A (3) inhibited Mucor hiemalis with activities comparable to the positive drug control nystatin. Prominent activities were observed for compounds 2 and 3 against Bacillus subtilis with MIC values of 1.0 and 4.2 μg/mL, respectively. Compound 2 also showed inhibitory activity against all Gram-positive bacteria (Bacillus subtilis, Micrococcus luteus, Mycobacterium smegmatis, and Staphylococcus aureus), while compounds 1 and 3 also showed inhibitory activity against the Gram-positive bacterium, Mycobacterium smegmatis. In general, only the dimeric tetrahydroxanthones 1–3 exhibited activity against fungi and bacteria with the secalonic acid-bearing derivatives 2 and 3 exhibiting better antimicrobial profile. However, the dimeric compounds 1–3 also showed moderate cytotoxic activities against two mammalian cell lines (Table 4). These inhibitory concentrations for cytotoxic activities are given traditionally in molar concentrations, but if they are calculated in µg/mL, the IC50 values would be equivalent to a range of 2–25 µg/mL (i.e., the same or only slightly higher activity range as compared to the MIC). This observation precludes the potential use of these metabolites as candidates for the development of antibiotics, because their selectivity indices are far too low. In addition, the fact that they are broadly active against both, prokaryotic and eukaryotic test organisms suggests that they may address multiple targets and are therefore less suitable for development of any drug.
Some information on these and chemically related compounds is even available from the literature. Compound 2 (4,4′-secalonic acid D; 4,4′-SAD) is a regioisomeric structure to SAD with 2,2′-biarylic connectivity, belonging to the secalonic acid family. This compound class has long been known to have non-selective antimicrobial and other biological activities [100,101,102,103,104,105,106]. The compound 4,4′-SAD (2) itself was recently reported to have low toxicity with “potent” antitumor activity against several cancer cell lines through cell proliferation inhibition and apoptosis induction [100]. However, when compared to the precursor for a marketed drug, epothilone, which we used as a positive control in our standard cytotoxicity assays (Table 4), the activities of all the metabolites from Pseudopalawania siamensis are much weaker. Promising candidate compounds for anticancer therapy should have at least activities in the 100 nM range such assays. Penicillixanthone A (3) was also already shown to possess moderate antibacterial activity against four tested bacterial strains (M. luteus, Pseudoalteromonas nigrifaciens, E. coli and B. subtilis [100], and its moderate cytotoxic effects on MDA-MB-435 human melanoma cells and SW620 human colorectal adenocarcinoma cell lines had been previously reported [101]. Furthermore, compound 3 was previously isolated from the marine-derived fungus Aspergillus fumigatus, and was reported to exhibit anti-HIV-1 activities by inhibiting CCR5-tropic HIV-1 and CXCR4-tropic HIV-1 infection [103]. These data also point toward non-selective effects of this metabolite in biological systems.
4. Conclusions
The current study showed that new genera and species of tropical fungi can still yield numerous new and interesting secondary metabolites. Even though the preliminary characterization of the metabolites 1–5 indicates that they act non-selectively in biological systems, their further evaluation could result in the discovery of additional, more specific biological effects. In any case, it is worthwhile to further explore tropical fungi whose cultures result from taxonomic and biodiversity studies for the production of secondary metabolites and other potentially beneficial properties [107].
Acknowledgments
The authors wish to thank Wera Collisi for conducting the biological assay; Christel Kakoschke for conducting the NMR spectroscopic measurements; Shaun Pennycook, Chayanard Phukhamsakda, Tian Cheng, Sae Kanaki, Boontiya Chuankid, Saowaluck Tibpromma, and Nimali Indeewari de Silva for their valuable suggestions and help.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/4/569/s1, Figure S1: 1H NMR spectrum (CDCl3, 700 MHz) of pseudopalawanone (1). Figure S2: 13C NMR spectrum (CDCl3, 175 MHz) of pseudopalawanone (1). Figure S3: HSQC-DEPT spectrum of pseudopalawanone (1). Figure S4: COSY spectrum of pseudopalawanone (1). Figure S5: HMBC spectrum of pseudopalawanone (1). Figure S6: ROESY spectrum of pseudopalawanone (1). Figure S7: NOESY spectrum of pseudopalawanone (1). Figure S8: LC-HRESIMS spectrum of pseudopalawanone (1). Figure S9: ECD spectrum of pseudopalawanone (1).
Author Contributions
All the authors listed made substantial contributions to the manuscript. A.M.: contributed in fungal specimen collection and isolation, fungal identification, fermentation, isolation of the compounds, and manuscript writing; A.P.G.M.: contributed in the experimental guidance, isolation of compounds, structure elucidation, and manuscript writing; B.T.: contributed in determination of biological activities, analyses of the spectral data; K.D.H. and M.S.: contributed to project organization, materials, facilities, experiment guidance and contributed in the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Research and Researchers for Industries (RRi) under Thailand Research Fund and the German Academic Exchange Service (DAAD) for a joint TRF-DAAD [PPP 2017–2018] academic exchange grant to K.D. Hyde and M. Stadler and the RRi for a personal grant to A. Mapook (PHD57I0012) and by the Alexander von Humboldt Foundation (Georg-Forster Research Fellowship to A.P.G.M.). K.D. Hyde thanks to Thailand Research grants entitled Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans (grant no: RSA5980068).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bills G.F., Gloer J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016;4:1–32. doi: 10.1128/microbiolspec.FUNK-0009-2016. [DOI] [PubMed] [Google Scholar]
- 2.De Silva D.D., Rapior S., Fons F., Bahkali A.H., Hyde K.D. Medicinal mushrooms in supportive cancer therapies: An approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 2012;55:1–35. doi: 10.1007/s13225-012-0151-3. [DOI] [Google Scholar]
- 3.Sandargo B., Chepkirui C., Cheng T., Chaverra-Munoz L., Thongbai B., Stadler M., Hüttel S. Biological and chemical diversity go hand in hand: Basidomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019;37:107344. doi: 10.1016/j.biotechadv.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Hyde K.D., Xu J., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S., et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- 5.Chomcheon P., Sriubolmas N., Wiyakrutta S., Ngamrojanavanich N., Chaichit N., Mahidol C., Ruchirawat S., Kittakoop P. Cyclopentenones, Scaffolds for organic syntheses produced by the endophytic fungus mitosporic Dothideomycete sp. LRUB20. J. Nat. Prod. 2006;69:1351–1353. doi: 10.1021/np060148h. [DOI] [PubMed] [Google Scholar]
- 6.Kim G.S., Ko W., Kim J.W., Jeong M.-H., Ko S.-K., Hur J.-S., Oh H., Jang J.-H., Ahn J.S. Bioactive α-pyrone derivatives from the endolichenic fungus Dothideomycetes sp. EL003334. J. Nat. Prod. 2018;81:1084–1088. doi: 10.1021/acs.jnatprod.7b01022. [DOI] [PubMed] [Google Scholar]
- 7.Wu B., Wiese J., Labes A., Kramer A., Schmaljohann R., Imhoff J.F. Lindgomycin, an unusual antibiotic polyketide from a marine fungus of the Lindgomycetaceae. Mar. Drugs. 2015;13:4617–4632. doi: 10.3390/md13084617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupcic Z., Chepkirui C., Hernández-Restrepo M., Crous P.W., Luangsa-ard J.J., Stadler M. New nematicidal and antimicrobial secondary metabolites from a new species in the new genus, Pseudobambusicola thailandica. MycoKeys. 2018;33:1–23. doi: 10.3897/mycokeys.33.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phukhamsakda C., Macabeo A.P.G., Yuyama K.T., Hyde K.D., Stadler M. Biofilm inhibitory abscisic acid derivatives from the plant-associated Dothideomycete fungus, Roussoella sp. Molecules. 2018;23:2190. doi: 10.3390/molecules23092190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phukhamsakda C., Macabeo A.P.G., Huch V., Cheng T., Hyde K.D., Stadler M. Sparticolins A–G, biologically active oxidized spirodioxynaphthalene derivatives from the ascomycete Sparticola junci. J. Nat. Prod. 2019;82:2878–2885. doi: 10.1021/acs.jnatprod.9b00604. [DOI] [PubMed] [Google Scholar]
- 11.Macabeo A.P.G., Pilapil L.A.E., Garcia K.Y.M., Quimque M.T.J., Phukhamsakda C., Cruz A.J.C., Hyde K.D., Stadler M. Alpha-Glucosidase- and lipase-inhibitory phenalenones from a new species of Pseudolophiostoma originating from Thailand. Molecules. 2020;25:965. doi: 10.3390/molecules25040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomnunti P., Hongsanan S., Aguirre-Hudson B., Tian Q., Peršoh D., Dhami M.K., Alias A.S., Xu J., Liu X., Stadler M., et al. The sooty moulds. Fungal Divers. 2014;66:1–36. doi: 10.1007/s13225-014-0278-5. [DOI] [Google Scholar]
- 13.Crous P.W., Gams W., Stalpers J.A., Robert V., Stegehuis G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004;50:19–22. [Google Scholar]
- 14.Vilgalys R., Hester M. rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990;172:4238–4246. doi: 10.1128/JB.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990;18:315–322. [Google Scholar]
- 16.Rehner S.A. Primers for Elongation Factor 1-Alpha (EF1-Alpha) [(accessed on 6 April 2020)];2001 Available online: http://ocid.nacse.org/research/deephyphae/EF1primer.pdf.
- 17.Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 18.Mapook A., Hyde K.D., McKenzie E.H.C., Jones E.B.G., Bhat D.J., Jeewon R., Stadler M., Samarakoon M.C., Malaithong M., Tanunchai B., et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed) Fungal Divers. 2020 doi: 10.1007/s13225-020-00444-8). in press. [DOI] [Google Scholar]
- 19.Mapook A., Boonmee S., Ariyawansa H.A., Tibpromma S., Campesori E., Jones E.B.G., Bahkali A.H., Hyde K.D. Taxonomic and phylogenetic placement of Nodulosphaeria. Mycol. Prog. 2016;15:34. doi: 10.1007/s11557-016-1176-x. [DOI] [Google Scholar]
- 20.Hongsanan S., Sánchez-Ramírez S., Crous P.W., Ariyawansa H.A., Zhao R.L., Hyde K.D. The evolution of fungal epiphytes. Mycosphere. 2016;7:1690–1712. doi: 10.5943/mycosphere/7/11/6. [DOI] [Google Scholar]
- 21.Crous P.W., Luangsa-ard J.J., Wingfield M.J., Carnegie A.J., Hernández-Restrepo M., Lombard L., Roux J., Barreto R.W., Baseia I.G., Cano-Lira J.F., et al. Fungal planet description sheets: 785–867. Persoonia. 2018;41:238–417. doi: 10.3767/persoonia.2018.41.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Restrepo M., Bezerra J.D.P., Tan Y.P., Wiederhold N., Crous P.W., Guarro J., Gené J. Re-evaluation of Mycoleptodiscus species and morphologically similar fungi. Persoonia. 2019;42:205–227. doi: 10.3767/persoonia.2019.42.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mapook A., Hyde K.D., Dai D.-Q., Li J., Jones E.B.G., Bahkali A.H., Boonmee S. Muyocopronales, ord. nov., (Dothideomycetes, Ascomycota) and a reappraisal of Muyocopron species from northern Thailand. Phytotaxa. 2016;265:225–237. doi: 10.11646/phytotaxa.265.3.3. [DOI] [Google Scholar]
- 24.Mapook A., Hyde K.D., Hongsanan S., Phukhamsakda C., Li J.F., Boonmee S. Palawaniaceae fam. nov., a new family (Dothideomycetes, Ascomycota) to accommodate Palawania species and their evolutionary time estimates. Mycosphere. 2016;7:1732–1745. doi: 10.5943/mycosphere/7/11/8. [DOI] [Google Scholar]
- 25.Rambaut A. FigTree v14: Tree Figure Drawing Tool. [(accessed on 6 April 2020)];2014 Available online: http://tree.bio.ed.ac.uk/software/figtree/
- 26.Stenroos S., Laukka T., Huhtinen S., Döbbeler P., Myllys L., Syrjänen K., Hyvönen J. Multiple origins of symbioses between ascomycetes and bryophytes suggested by a five-gene phylogeny. Cladistics. 2010;26:281–300. doi: 10.1111/j.1096-0031.2009.00284.x. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Pan H., Liu W., Chen M.Y., Zhong C.H. First report of Alternaria alternata causing postharvest rot of kiwifruit in China. Plant Dis. 2017;101:1046. doi: 10.1094/PDIS-11-16-1611-PDN. [DOI] [Google Scholar]
- 28.Alves J.L., Woudenberg J.H.C., Duarte L.L., Crous P.W., Barreto R.W. Reappraisal of the genus Alternariaster (Dothideomycetes) Persoonia. 2013;31:77–85. doi: 10.3767/003158513X669030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheewangkoon R., Groenewald J.Z., Summerell B.A., Hyde K.D., To-Anun C., Crous P.W. Myrtaceae, a cache of fungal biodiversity. Persoonia. 2009;23:55–85. doi: 10.3767/003158509X474752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crous P.W., Wingfield M.J., Schumacher R.K., Summerell B.A., Giraldo A., Gené J., Guarro J., Wanasinghe D.N., Hyde K.D., Camporesi E., et al. Fungal planet description sheets: 281-319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vu D., Groenewald M., de Vries M., Gehrmann T., Stielow B., Eberhardt U., Al-Hatmi A., Groenewald J.Z., Cardinali G., Houbraken J., et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann T.A., Kirschner R., Piepenbring M. Phylogenetic relationships and new records of Asterinaceae (Dothideomycetes) from Panama. Fungal Divers. 2010;43:39–53. doi: 10.1007/s13225-010-0042-4. [DOI] [Google Scholar]
- 33.Dai D., Bhat D.J., Liu J., Chukeatirote E., Zhao R., Hyde K.D. Bambusicola, a new genus from bamboo with asexual and sexual morphs. Cryptogam. Mycol. 2012;33:363–379. doi: 10.7872/crym.v33.iss3.2012.363. [DOI] [Google Scholar]
- 34.Liu J.-K., Phookamsak R., Doilom M., Wikee S., Li Y.-M., Ariyawansha H., Boonmee S., Chomnunti P., Dai D.-Q., Bhat J.D., et al. Towards a natural classification of Botryosphaeriales. Fungal Divers. 2012;57:149–210. doi: 10.1007/s13225-012-0207-4. [DOI] [Google Scholar]
- 35.Schoch C.L., Shoemaker R.A., Seifert K.A., Hambleton S., Spatafora J.W., Crous P.W. A Multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98:1041–1052. doi: 10.1080/15572536.2006.11832632. [DOI] [PubMed] [Google Scholar]
- 36.Beimforde C., Feldberg K., Nylinder S., Rikkinen J., Tuovila H., Dörfelt H., Gube M., Jackson D.J., Reitner J., Seyfullah L.J., et al. Estimating the phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogenetics Evol. 2014;78:386–398. doi: 10.1016/j.ympev.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Lutzoni F., Pagel M., Reeb V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature. 2001;411:937–940. doi: 10.1038/35082053. [DOI] [PubMed] [Google Scholar]
- 38.Schoch C.L., Crous P.W., Groenewald J.Z., Boehm E.W.A., Burgess T.I., de Gruyter J., de Hoog G.S., Dixon L.J., Grube M., Gueidan C., et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud. Mycol. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai L., Hyde K.D. Ascorhombispora aquatica gen. et sp. nov. from a freshwater habitat in China, and its phylogenetic placement based on molecular data. Cryptogam. Mycol. 2007;28:291–300. [Google Scholar]
- 40.Hongsanan S., Chomnunti P., Crous P.W., Chukeatirote E., Hyde K.D. Introducing Chaetothyriothecium, a new genus of Microthyriales. Phytotaxa. 2014;161:157–164. doi: 10.11646/phytotaxa.161.2.7. [DOI] [Google Scholar]
- 41.Hyde K.D., Jones E.B.G., Liu J.-K., Ariyawansa H., Boehm E., Boonmee S., Braun U., Chomnunti P., Crous P.W., Dai D.-Q., et al. Families of Dothideomycetes. Fungal Divers. 2013;63:1–313. doi: 10.1007/s13225-013-0263-4. [DOI] [Google Scholar]
- 42.Tibell L. Tholurna dissimilis and generic delimitations in Caliciaceae inferred from nuclear ITS and LSU rDNA phylogenies (Lecanorales, Lichenized Ascomycetes) Mycol. Res. 2003;107:1403–1418. doi: 10.1017/S0953756203008694. [DOI] [PubMed] [Google Scholar]
- 43.Muggia L., Hafellner J., Wirtz N., Hawksworth D.L., Grube M. The sterile microfilamentous lichenized fungi Cystocoleus ebeneus and Racodium rupestre are relatives of plant pathogens and clinically important Dothidealean fungi. Mycol. Res. 2008;112:50–56. doi: 10.1016/j.mycres.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Verkley G.J.M., Dukik K., Renfurm R., Göker M., Stielow J.B. Novel genera and species of Coniothyrium-like fungi in Montagnulaceae (Ascomycota) Persoonia. 2014;32:25–51. doi: 10.3767/003158514X679191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ariyawansa H.A., Camporesi E., Thambugala K.M., Mapook A., Kang J.-C., Alias S.A., Chukeatirote E., Thines M., McKenzie E.H.C., Hyde K.D. Confusion surrounding Didymosphaeria —Phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa. 2014;176:102–119. doi: 10.11646/phytotaxa.176.1.12. [DOI] [Google Scholar]
- 46.Spatafora J.W., Sung G.-H., Johnson D., Hesse C., O’Rourke B., Serdani M., Spotts R., Lutzoni F., Hofstetter V., Miadlikowska J., et al. A five-gene phylogeny of Pezizomycotina. Mycologia. 2006;98:1018–1028. doi: 10.1080/15572536.2006.11832630. [DOI] [PubMed] [Google Scholar]
- 47.Hyde K.D., Norphanphoun C., Abreu V.P., Bazzicalupo A., Thilini Chethana K.W., Clericuzio M., Dayarathne M.C., Dissanayake A.J., Ekanayaka A.H., He M.-Q., et al. Fungal diversity notes 603–708: Taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017;87:1–235. doi: 10.1007/s13225-017-0391-3. [DOI] [Google Scholar]
- 48.Pang K.-L., Hyde K.D., Alias S.A., Suetrong S., Guo S.-Y., Idid R., Gareth Jones E.B. Dyfrolomycetaceae, a new family in the Dothideomycetes, Ascomycota. Cryptogam. Mycol. 2013;34:223–232. doi: 10.7872/crym.v34.iss3.2013.223. [DOI] [Google Scholar]
- 49.Suetrong S., Schoch C.L., Spatafora J.W., Kohlmeyer J., Volkmann-Kohlmeyer B., Sakayaroj J., Phongpaichit S., Tanaka K., Hirayama K., Jones E.B.G. Molecular systematics of the marine Dothideomycetes. Stud. Mycol. 2009;64:155–173S6. doi: 10.3114/sim.2009.64.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyde K.D., Hongsanan S., Jeewon R., Bhat D.J., McKenzie E.H.C., Jones E.B.G., Phookamsak R., Ariyawansa H.A., Boonmee S., Zhao Q., et al. Fungal diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;80:1–270. doi: 10.1007/s13225-016-0373-x. [DOI] [Google Scholar]
- 51.Zhang J.-F., Liu J.-K., Hyde K.D., Chen Y.-Y., Liu Y.-X., Liu Z.-Y. Two new species of Dyfrolomyces (Dyfrolomycetaceae, Dothideomycetes) from Karst landforms. Phytotaxa. 2017;313:267–277. doi: 10.11646/phytotaxa.313.3.4. [DOI] [Google Scholar]
- 52.Papendorf M.G. Leptodiscus africanus sp. nov. Trans. Brit. Mycol. Soc. 1967;50:687–690. doi: 10.1016/S0007-1536(67)80102-5. [DOI] [Google Scholar]
- 53.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding Consortium Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egidi E., de Hoog G.S., Isola D., Onofri S., Quaedvlieg W., de Vries M., Verkley G.J.M., Stielow J.B., Zucconi L., Selbmann L. Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Divers. 2014;65:127–165. doi: 10.1007/s13225-013-0277-y. [DOI] [Google Scholar]
- 55.Ertz D., Diederich P. Dismantling melaspileaceae: A first phylogenetic study of Buelliella, Hemigrapha, Karschia, Labrocarpon and Melaspilea. Fungal Divers. 2015;71:141–164. doi: 10.1007/s13225-015-0321-1. [DOI] [Google Scholar]
- 56.Tanaka K., Hirayama K., Yonezawa H., Sato G., Toriyabe A., Kudo H., Hashimoto A., Matsumura M., Harada Y., Kurihara Y., et al. Revision of the Massarineae (Pleosporales, Dothideomycetes) Stud. Mycol. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y., Wang H.K., Fournier J., Crous P.W., Jeewon R., Pointing S.B., Hyde K.D. Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers. 2009;38:225–251. [Google Scholar]
- 58.Madrid H., Gené J., Cano J., Guarro J. A new species of Leptodiscella from Spanish soil. Mycol. Prog. 2012;11:535–541. doi: 10.1007/s11557-011-0768-8. [DOI] [Google Scholar]
- 59.Ariyawansa H.A., Phukhamsakda C., Thambugala K.M., Bulgakov T.S., Wanasinghe D.N., Perera R.H., Mapook A., Camporesi E., Kang J.-C., Gareth Jones E.B., et al. Revision and phylogeny of Leptosphaeriaceae. Fungal Divers. 2015;74:19–51. doi: 10.1007/s13225-015-0349-2. [DOI] [Google Scholar]
- 60.de Gruyter J., Woudenberg J.H.C., Aveskamp M.M., Verkley G.J.M., Groenewald J.Z., Crous P.W. Redisposition of Phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013;75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chomnunti P., Schoch C.L., Aguirre–Hudson B., Ko-Ko T.W., Hongsanan S., Jones E.B.G., Kodsueb R., Phookamsak R., Chukeatirote E., Bahkali A.H., et al. Capnodiaceae. Fungal Divers. 2011;51:103–134. doi: 10.1007/s13225-011-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aveskamp M.M., de Gruyter J., Woudenberg J.H.C., Verkley G.J.M., Crous P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related Pleosporalean genera. Stud. Mycol. 2010;65:1–60. doi: 10.3114/sim.2010.65.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ariyawansa H.A., Hyde K.D., Jayasiri S.C., Buyck B., Chethana K.W.T., Dai D.Q., Dai Y.C., Daranagama D.A., Jayawardena R.S., Lücking R., et al. Fungal diversity notes 111–252—Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015;75:27–274. doi: 10.1007/s13225-015-0346-5. [DOI] [Google Scholar]
- 64.Liu J.K., Hyde K.D., Jones E.B.G., Ariyawansa H.A., Bhat D.J., Boonmee S., Maharachchikumbura S.S.N., McKenzie E.H.C., Phookamsak R., Phukhamsakda C., et al. Fungal diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015;72:1–197. doi: 10.1007/s13225-015-0324-y. [DOI] [Google Scholar]
- 65.Quaedvlieg W., Verkley G.J.M., Shin H.-D., Barreto R.W., Alfenas A.C., Swart W.J., Groenewald J.Z., Crous P.W. Sizing up Septoria. Stud. Mycol. 2013;75:307–390. doi: 10.3114/sim0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senwanna C., Hongsanan S., Phookamsak R., Tibpromma S., Cheewangkoon R., Hyde K.D. Muyocopron heveae sp. nov. and M. dipterocarpi appears to have host-jumped to rubber. Mycol. Prog. 2019;18:741–752. doi: 10.1007/s11557-019-01484-4. [DOI] [Google Scholar]
- 67.Jayasiri S.C., Hyde K.D., Jones E.B.G., McKenzie E.H.C., Jeewon R., Phillips A.J.L., Bhat D.J., Wanasinghe D.N., Liu J.K., Lu Y.Z., et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere. 2019;10:1–186. doi: 10.5943/mycosphere/10/1/1. [DOI] [Google Scholar]
- 68.Tibpromma S., McKenzie E.H.C., Karunarathna S.C., Xu J., Hyde K.D., Hu D.M. Muyocopron garethjonesii sp. nov. (Muyocopronales, Dothideomycetes) on Pandanus sp. Mycosphere. 2016;7:1480–1489. doi: 10.5943/mycosphere/7/9/19. [DOI] [Google Scholar]
- 69.Tibpromma S., Hyde K.D., Bhat J.D., Mortimer P.E., Xu J., Promputtha I., Doilom M., Yang J.-B., Tang A.M.C., Karunarathna S.C. Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys. 2018;33:25–67. doi: 10.3897/mycokeys.33.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boehm E.W.A., Schoch C.L., Spatafora J.W. On the Evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycol. Res. 2009;113:461–479. doi: 10.1016/j.mycres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Ferrer A., Miller A.N., Shearer C.A. Minutisphaera and Natipusilla: Two new genera of freshwater Dothideomycetes. Mycologia. 2011;103:411–423. doi: 10.3852/10-177. [DOI] [PubMed] [Google Scholar]
- 72.Crous P.W., Wingfield M.J., Guarro J., Cheewangkoon R., van der Bank M., Swart W.J., Stchigel A.M., Cano-Lira J.F., Roux J., Madrid H., et al. Fungal planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mapook A., Boonmee S., Liu J.-K., Jones E.B.G., Bahkali A.H., Hyde K.D. Taxonomic and phylogenetic placement of Phaeodimeriella (Pseudoperisporiaceae, Pleosporales) Cryptogam. Mycol. 2016;37:157–176. doi: 10.7872/crym/v37.iss2.2016.157. [DOI] [Google Scholar]
- 74.Lumbsch H.T., Schmitt I., Lindemuth R., Miller A., Mangold A., Fernandez F., Huhndorf S. Performance of four ribosomal DNA regions to infer higher-level phylogenetic relationships of inoperculate Euascomycetes (Leotiomyceta) Mol. Phylogenet. Evol. 2005;34:512–524. doi: 10.1016/j.ympev.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 75.James T.Y., Kauff F., Schoch C.L., Matheny P.B., Hofstetter V., Cox C.J., Celio G., Gueidan C., Fraker E., Miadlikowska J., et al. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 76.Crous P.W., Schoch C.L., Hyde K.D., Wood A.R., Gueidan C., de Hoog G.S., Groenewald J.Z. Phylogenetic lineages in the Capnodiales. Stud. Mycol. 2009;64:17–47. doi: 10.3114/sim.2009.64.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thambugala K.M., Hyde K.D., Tanaka K., Tian Q., Wanasinghe D.N., Ariyawansa H.A., Jayasiri S.C., Boonmee S., Camporesi E., Hashimoto A., et al. Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Divers. 2015;74:199–266. doi: 10.1007/s13225-015-0348-3. [DOI] [Google Scholar]
- 78.Ariyawansa H.A., Thambugala K.M., Manamgoda D.S., Jayawardena R., Camporesi E., Boonmee S., Wanasinghe D.N., Phookamsak R., Hongsanan S., Singtripop C., et al. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers. 2015;71:85–139. doi: 10.1007/s13225-015-0323-z. [DOI] [Google Scholar]
- 79.Tian Q., Liu J.K., Hyde K.D., Wanasinghe D.N., Boonmee S., Jayasiri S.C., Luo Z.L., Taylor J.E., Phillips A.J.L., Bhat D.J., et al. Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales) Fungal Divers. 2015;74:267–324. doi: 10.1007/s13225-015-0350-9. [DOI] [Google Scholar]
- 80.Braun U., Crous P.W., Groenewald J.Z., Scheuer C. Pseudovirgaria, a fungicolous hyphomycete genus. IMA Fungus. 2011;2:65–69. doi: 10.5598/imafungus.2011.02.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arzanlou M., Groenewald J.Z., Gams W., Braun U., Shin H.-D., Crous P.W. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud. Mycol. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verkley G.J.M., Quaedvlieg W., Shin H.-D., Crous P.W. A new approach to species delimitation in Septoria. Stud. Mycol. 2013;75:213–305. doi: 10.3114/sim0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winton L.M., Stone J.K., Hansen E.M., Shoemaker R.A. The systematic position of Phaeocryptopus gaeumannii. Mycologia. 2007;99:240–252. doi: 10.1080/15572536.2007.11832584. [DOI] [PubMed] [Google Scholar]
- 84.Batzer J.C., Arias M.M.D., Harrington T.C., Gleason M.L., Groenewald J.Z., Crous P.W. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia. 2008;100:246–258. doi: 10.1080/15572536.2008.11832480. [DOI] [PubMed] [Google Scholar]
- 85.Hyde K.D., Dong Y., Phookamsak R., Jeewon R., Bhat D.J., Jones E.B.G., Liu N.G., Abeywickrama P.D., Mapook A., Wei D., et al. Fungal diversity notes 1151–1273: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020;100:5–277. doi: 10.1007/s13225-020-00439-5. [DOI] [Google Scholar]
- 86.Samerpitak K., Van der Linde E., Choi H.-J., Gerrits van den Ende A.H.G., Machouart M., Gueidan C., de Hoog G.S. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Divers. 2014;65:89–126. doi: 10.1007/s13225-013-0253-6. [DOI] [Google Scholar]
- 87.Kruys A., Eriksson O.E., Wedin M. Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol. Res. 2006;110:527–536. doi: 10.1016/j.mycres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Ismail S.I., Batzer J.C., Harrington T.C., Crous P.W., Lavrov D.V., Li H., Gleason M.L. Ancestral state reconstruction infers phytopathogenic origins of sooty blotch and flyspeck fungi on apple. Mycologia. 2016;108:292–302. doi: 10.3852/15-036. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y., Schoch C.L., Fournier J., Crous P.W., de Gruyter J., Woudenberg J.H.C., Hirayama K., Tanaka K., Pointing S.B., Spatafora J.W., et al. Multi-locus phylogeny of Pleosporales: A taxonomic, ecological and evolutionary re-evaluation. Stud. Mycol. 2009;64:85–102. doi: 10.3114/sim.2009.64.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crous P.W., Schubert K., Braun U., de Hoog G.S., Hocking A.D., Shin H.-D., Groenewald J.Z. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Stud. Mycol. 2007;58:185–217. doi: 10.3114/sim.2007.58.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hongsanan S., Tian Q., Bahkali A.H., Yang J.-B., Mckenzie E.H.C., Chomnunti P., Hyde K.D. Zeloasperisporiales ord. nov., and two new species of Zeloasperisporium. Cryptogam. Mycol. 2015;36:301–317. doi: 10.7872/crym/v36.iss3.2015.301. [DOI] [Google Scholar]
- 92.Kuephadungphan W., Macabeo A.P.G., Luangsa-ard J.J. Studies on the biologically active secondary metabolites of the new spider parasitic fungus Gibellula gamsii. Mycol. Prog. 2019;18:135–146. doi: 10.1007/s11557-018-1431-4. [DOI] [Google Scholar]
- 93.Macabeo A.P.G., Cruz A.J.C., Narmani A., Arzanlou M., Babai-Ahari A., Pilapil L.A.E., Garcia K.Y.M., Huch V., Stadler M. Tetrasubstituted α-pyrone derivatives from the endophytic fungus, Neurospora udagawae. Phytochem. Lett. 2020;35:147–151. doi: 10.1016/j.phytol.2019.11.010. [DOI] [Google Scholar]
- 94.Wu H., Hyde K.D. Re-appraisal of Scolecopeltidium. Mycotaxon. 2013;125:1–10. doi: 10.5248/125.1. [DOI] [Google Scholar]
- 95.Wu H.X., Schoch C.L., Boonmee S., Bahkali A.H., Chomnunti P., Hyde K.D. A Reappraisal of Microthyriaceae. Fungal Divers. 2011;51:189–248. doi: 10.1007/s13225-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu H., Jaklitsch W.M., Voglmayr H., Hyde K.D. Epitypification, morphology, and phylogeny of Tothia fuscella. Mycotaxon. 2011;118:203–211. doi: 10.5248/118.203. [DOI] [Google Scholar]
- 97.Wu H., Li Y., Chen H., Hyde K.D. Studies on Microthyriaceae: Some excluded genera. Mycotaxon. 2010;113:147–156. doi: 10.5248/113.147. [DOI] [Google Scholar]
- 98.Wu H., Hyde K.D., Chen H. Studies on Microthyriaceae: Placement of Actinomyxa, Asteritea, Cirsosina, Polystomellina and Stegothyrium. Cryptog. Mycol. 2011;32:3–12. doi: 10.7872/crym.v32.iss1.2012.003. [DOI] [Google Scholar]
- 99.Wu H., Tian Q., Li W., Hyde K.D. A reappraisal of Microthyriaceae. Phytotaxa. 2014;176:201–212. doi: 10.11646/phytotaxa.176.1.20. [DOI] [Google Scholar]
- 100.Chen L., Li Y.-P., Li X.-X., Lu Z.-H., Zheng Q.-H., Liu Q.-Y. Isolation of 4,4′-bond secalonic acid D from the marine-derived fungus Penicillium oxalicum with inhibitory property against hepatocellular carcinoma. J. Antibiot. 2019;72:34–44. doi: 10.1038/s41429-018-0104-5. [DOI] [PubMed] [Google Scholar]
- 101.Bao J., Sun Y.-L., Zhang X.-Y., Han Z., Gao H.-C., He F., Qian P.-Y., Qi S.-H. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J. Antibiot. 2013;66:219–223. doi: 10.1038/ja.2012.110. [DOI] [PubMed] [Google Scholar]
- 102.El-Elimat T., Figueroa M., Raja H.A., Graf T.N., Swanson S.M., Falkinham J.O., Wani M.C., Pearce C.J., Oberlies N.H. Biosynthetically distinct cytotoxic polyketides from Setophoma terrestris. Eur. J. Org. Chem. 2015;2015:109–121. doi: 10.1002/ejoc.201402984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan S., Yang B., Liu J., Xun T., Liu Y., Zhou X. Penicillixanthone A, a marine-derived dual-coreceptor antagonist as anti-HIV-1 agent. Nat. Prod. Res. 2019;33:1467–1471. doi: 10.1080/14786419.2017.1416376. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W., Krohn K., Zia-Ullah Flörke U., Pescitelli G., Di Bari L., Antus S., Kurtán T., Rheinheimer J., Draeger S. New mono- and dimeric members of the secalonic acid family: Blennolides A-G isolated from the fungus Blennoria sp. Chemistry. 2008;14:4913–4923. doi: 10.1002/chem.200800035. [DOI] [PubMed] [Google Scholar]
- 105.Wittine K., Saftić L., Peršurić Ž., Kraljević Pavelić S. Novel antiretroviral structures from marine organisms. Molecules. 2019;24:3486. doi: 10.3390/molecules24193486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asai T., Otsuki S., Sakurai H., Yamashita K., Ozeki T., Oshima Y. Benzophenones from an endophytic fungus, Graphiopsis chlorocephala, from Paeonia lactiflora cultivated in the presence of an NAD+-dependent HDAC inhibitor. Org. Lett. 2013;15:2058–2061. doi: 10.1021/ol400781b. [DOI] [PubMed] [Google Scholar]
- 107.Hyde K.D., Norphanphoun C., Chen J., Dissanayakem A.J., Doilom M., Hongsanan S., Jayawardena R.S., Jeewon R., Perera R.H., Thongbai B., et al. Thailand’s amazing diversity—An estimated 55–96% of fungi in northern Thailand are novel. Fungal Divers. 2018;93:215–239. doi: 10.1007/s13225-018-0415-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.