Abstract
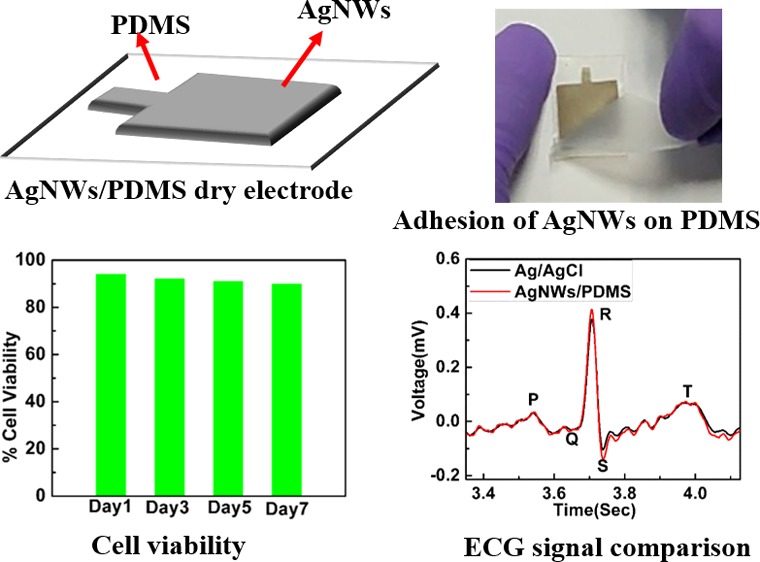
Flexible and dry electrodes have attracted huge attention due to their potential application in long-term electrophysiological signal monitoring. In this work, we present a novel method to pattern silver nanowires (AgNWs) on a polydimethylsiloxane (PDMS) substrate-based dry electrodes by a vacuum filtration method for electrophysiological signal monitoring. The Scotch tape peel-off test confirms the excellent adhesion of the patterned AgNWs on a PDMS substrate. The cytotoxicity of the proposed electrode is detected by an MTT assay method, and 90% cell viability is observed for the period of one week, indicating no cytotoxic effect on living cells. The signal to noise ratios of the conventional wet Ag/AgCl and dry AgNW/PDMS electrodes are 24.6 and 25.4 dB, indicating that AgNW/PDMS dry electrodes measure a high-quality electrophysiological signal when compared with that of the conventional Ag/AgCl wet electrodes.
1. Introduction
Wearable devices are revolutionizing the field of medical care by recording, transmitting, and monitoring real-time biopotential signals.1−3 In the case of biopotential signals like those of electroencephalograms (EEGs), electrocardiography (ECG), and electromyography (EMG), long-term recordings can provide useful medical information to patients in critical care, diagnosis, and home healthcare.4−6 However, the conventional Ag/AgCl electrodes that are currently used in hospitals are not suited for these long-term recordings. The use of a conductive gel increases their impedance with time as the gel dries out, which results in poor signal quality over long durations. The conductive gel also might cause irritation and discomfort to the patients during long-term recordings.7,8
In order to overcome the challenges associated with conventional electrodes for their use in continuous monitoring of biopotential signals, there has been a surge in research dedicated to developing flexible, conformable, and durable dry electrodes. The aim is to develop highly flexible, conductive, and biocompatible dry electrodes that do not require any skin preparation or application of a conductive gel for measuring electrophysiological signals. There are several methods that have been proposed to fabricate the flexible dry electrodes: (a) Metal-coated polymer substrate-based electrodes possess high conductivity, but most metal films or patterns prepared using vacuum deposition and photolithography exhibit poor adhesion with the flexible polymer substrate along with the issues of high costs and time consumption.9−11 (b) Polymer and conductive filler composite-based dry electrodes have been reported, but a uniform dispersion of such conductive fillers is difficult in order to obtain highly conductive polymer composites. Moreover, the obtained electrophysiological signals have low amplitude and poor signal to noise ratios because of the low conductivity of the polymer and conductive filler composite electrodes.12−14 (c) Microneedle-based dry electrodes provide good quality signals, but the fabrication of such electrodes requires clean-room facilities with sophisticated equipment and complicated procedures. Additionally, due to their invasive nature and the risk of microneedle breakage during the insertion process, they are not suitable for long-term signal monitoring.15−18 (d) Silver nanowires (AgNWs) and polydimethylsiloxane (PDMS)-based flexible dry electrodes have been previously fabricated by drop-casting methods. However, adhesion of AgNWs with PDMS and cytotoxicity of AgNWs and PDMS-based dry electrodes have not been evaluated in studies.19−21
To overcome the above issues, we propose a programmable mechanical cutter and vacuum filtration method to fabricate dry electrodes. A programmable mechanical cutter is a promising and cost-effective equipment to obtain direct patterns. The desired stencil for the pattern was designed using the programmable mechanical cutter. The vacuum filtration technique is used to obtain a thin film of silver nanowires on the patterned filter paper. The vacuum filtration technique is considered as a simple approach to get an ultrathin and homogenous film as compared to the drop-casting method. Moreover, the thickness of the films can be tuned by the amount and concentration of the solution to be filtered. When PDMS is poured onto the patterned AgNW network on filter paper, the PDMS impregnates into the patterned AgNW network. Simultaneously, the setup is attached to a vacuum pump, which further enhances the penetration of PDMS into the AgNW network, resulting in excellent adhesion between the PDMS and the patterned AgNW network making it highly reliable for long-term electrophysiological signal recording. The AgNW/PDMS dry electrodes fabricated using this method exhibited high conductivity (0.6 Ω/sq) and recorded good quality electrophysiological signals with a signal to noise ratio of 25.4 dB, which is comparable with the conventional Ag/AgCl electrodes (24.6 dB).
2. Experimental Section
2.1. Synthesis of Silver Nanowires (AgNWs)
In a modified polyol process,22,23 0.094 M silver nitrate (AgNO3) (Merck Specialities Co.), 4 M copper(II) chloride dihydrate (CuCl2·2H2O) (Merck Specialities Co.), and 0.147 M polyvinylpyrrolidone (PVP, Sigma-Aldrich) in ethylene glycol (Merck Specialities, Emparta) were prepared by ultrasonication for 1 h. Meanwhile, 25 mL of ethylene glycol in a glass flask was preheated at 152 °C in an oil bath with continuous magnetic stirring for 1 h. After 1 h, nitrogen gas was introduced in the glass flask through a three-way stopper, and 200 μL of 4 M CuCl2 solution was added to the preheated ethylene glycol. After 20 min, 7 mL of 0.147 M PVP solution and, subsequently, 7 mL of 0.094 M silver nitrate solution was added to the reaction mixture. The reaction was carried on for 1 h and 20 min; after which, it was stopped by quenching the flask in cold water. After quenching, the final AgNW solution was diluted with acetone and centrifuged at 3000 rpms for 5 min followed by washing with ethanol three times. As-obtained AgNWs were dispersed in IPA and stored in a glass vial for further characterization and use.
2.2. Fabrication of Silver Nanowires and PDMS-Based (AgNW/PDMS) Dry Electrodes
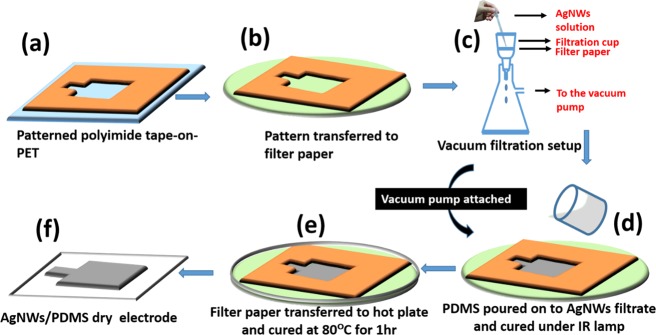
For the fabrication of AgNW/PDMS dry electrodes (see Figure S1 in the Supporting Information), first, an adhesive polyimide tape on the PET substrate was patterned using a programmable mechanical cutter (Silhouette Cutting Machine 3.0) as shown in Figure 1a. The pattern was a 15 × 15 mm square area with a 4 mm × 2 mm rectangular extension to make wire connections. The patterned polyimide tape was transferred onto a filter paper (Pall, 0.2 μm), which was then secured on the filtration assembly with a thermal resistant tape as shown in Figure 1b,c.
Figure 1.
(a–f) Schematic illustration of the fabrication process of AgNW/PDMS flexible dry electrodes.
In order to fabricate the patterned AgNW layer, the AgNW solution was added slowly using a 3 mL Pasteur pipette in the patterned area on the filter paper as shown in Figure 1c. The AgNW solution was vacuum-filtered for 10 min. Thereafter, the AgNWs deposited on the filter paper were left to dry for another 10 min. A vacuum-degassed 10:1 ratio of the PDMS base and curing agent mixture (Sylgard 184 Silicone, Dow Corning, U.S.A.) was then poured over the patterned AgNWs and cured for 2 h with an infrared lamp as shown in Figure 1d. The entire process was carried out with a vacuum pump attached to the filtration assembly to allow the penetration of PDMS into patterned AgNWs before it gets cured. The partially cured PDMS mixture on the AgNW network is transferred onto a hot plate along with the filter paper and cured for 60 min at 80 °C as shown in Figure 1e. When the PDMS film was peeled off of the filter paper after complete curing, the AgNW network was smoothly transferred from the filter paper to the PDMS film as shown in Figure 1f. The wire connections were made using silver conductive adhesive paste and epoxy. The metal snaps were soldered to the end of the wires to make connections to the Biopac MP36 system (Figure S2, Supporting Information).
2.3. Characterization
X-ray diffraction (XRD) was obtained by X’Pert Pro and EMPYREAN (PANalytical, The Netherlands) with Cu Kα (1.54184 Å). SEM micrographs were obtained by using ZIESS FIB-SEM (operating voltage of 5 kV). The sheet resistance of electrodes was measured using a Hall measurement system (HMS-3000). The electromechanical behavior of the AgNW/PDMS electrodes is carried out with Mark 10 Force torque measurement products (U.S.A.) and Precision Source Measure Units (Keysight B2900A). Skin-electrode impedance measurements were carried out by (Alpha and Beta Analyzer, Novocontrol Technology, Germany) a broadband dielectric spectrometer. Electrophysiological signals were monitored by using Biopac Systems Inc., U.S.A. MP36R. The active area of the fabricated AgNW/PDMS electrodes was maintained almost the same as that of the Ag/AgCl wet electrodes in order to compare the skin-electrode impedance values and the quality of electrophysiological signals (see Figure S3, Supporting Information). Velcro straps were used in the case of AgNW/PDMS dry electrodes for measuring electrophysiological signals. The ECG signals were obtained simultaneously from Ag/AgCl and AgNW/PDMS electrodes in the lead I configuration. For EMG signal recording, the electrodes were placed on the forearm of the subject.
2.4. Cytotoxicity of AgNW/PDMS Electrodes
The AgNW/PDMS electrodes were incubated with DMEM (Gibco, U.S.A.) and cell culture media for 7 days, and media aliquots were taken every alternate day (day 1, 3, 5, and 7). L929 (mouse fibroblast) cells were seeded in a 96-well plate (∼1 × 104 cells/well) and incubated overnight. The toxicity was assayed for media collected on days 1, 3, 5, and 7. The plate was then incubated for 24 h at 37 °C. After that, 10 μL of 5 mg/mL MTT dye (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma) was added to each well and incubated for 4 h. Subsequently, 100 μL of DMSO was added and incubated for 1 h. The absorbance at 560 nm and background scattering at 690 nm were measured in a SpectraMax M2 (Molecular Devices, California, U.S.A.) plate reader. The absorbance values were used to calculate the viability of cells in comparison to buffer control and plotted along with standard errors. The assay was repeated twice. The viability of cells was compared to the viability of control cells with fresh media, and the percentage viability was calculated. Triton X 100 was kept as a control, which showed maximum cell death. Cellular morphology was imaged using phase-contrast microscopy (Leica, Germany).
3. Results and Discussion
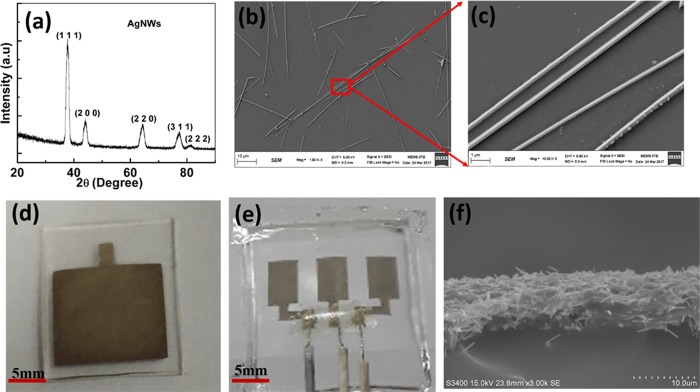
Figure 2a shows the XRD pattern of as-synthesized AgNWs. The five distinct peaks of (111), (200), (220), (311), and (222) confirmed the FCC structure of silver.24 The slight broadening of the peaks can be attributed to the nanoscale diameter of the silver nanowires. Figure 2b,c illustrates the SEM micrographs of as-synthesized silver nanowires at magnifications 1 k and 10 k, respectively. It was observed from the SEM micrographs that the average length and diameter of AgNWs were 20–30 μm and 200–250 nm, respectively, giving them a high aspect ratio. Figure 2d,e shows the optical images of the fabricated AgNW/PDMS electrode and electrode patch, respectively, while Figure 2f shows its cross-sectional SEM micrograph. The thickness of the patterned AgNWs on the PDMS substrate is approximately 10 μm as shown in Figure 2f.
Figure 2.
(a) XRD diffraction pattern, (b, c) SEM micrographs of silver nanowires, (d) optical images of the AgNW/PDMS dry electrode, (e) AgNW/PDMS electrode patch, and (f) cross-sectional SEM images of AgNW/PDMS dry electrodes.
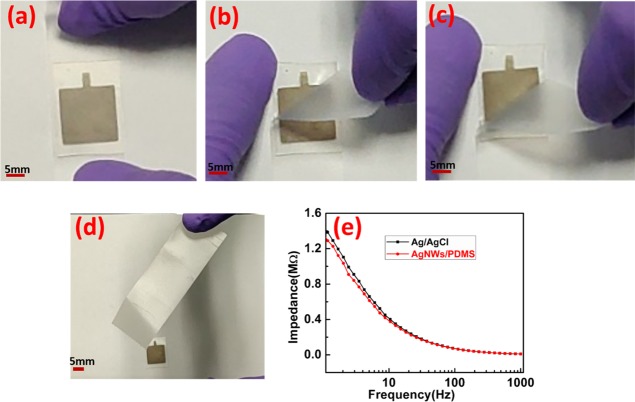
The purpose of dry electrodes is to ensure long-term continuous monitoring of electrophysiological signals. In order to fulfill that, it is desirable that the dry electrodes are durable and reliable over a long period of time. In this case, the active material is the AgNWs layer, which should remain intact. In order to confirm the adhesion of silver nanowires, a Scotch tape adhesion test was performed on the fabricated AgNW/PDMS dry electrodes as shown in Figure 3a–d. A strip of Scotch tape was removed and placed on the AgNW film on the PDMS substrate as shown in Figure 3a. It was smoothed using fingers and pressed to ensure good contact between the tape and the AgNW film. Within 30 s of applying the Scotch tape, it was removed in a single fast motion as shown in Figure 3b,c. Figure 3d shows an image of the Scotch tape after peeling it off from the electrode. The Scotch tape showed no traces of AgNWs (see Movie S1 of the Supporting Information). The adhesion strength of AgNWs on the PDMS film was measured using Scotch tape and Mark 10 Force torque measurement products (U.S.A.). The AgNW/PDMS electrode was firmly secured on the lower stage of Mark10. Scotch tape was placed on the AgNW/PDMS electrodes and pressed to ensure good contact between the Scotch tape and AgNW/PDMS electrode, and another end of the Scotch tape was attached to the upper grip of Mark 10 as shown in Figure S4a. After that, the Scotch tape was removed at the rate of 100 mm/min using Mark 10, as shown in Figure S4b,c. The adhesion strength versus distance curve is shown in Figure S4d. As shown in Figure S4d, the adhesion strength of patterned AgNWs on PDMS was calculated to be approximately 0.8 N. The adhesion test affirmed the conclusion drawn from the SEM images that the PDMS penetrated into the AgNW network resulting in very good adhesion. The film was completely intact even after multiple rounds of applying and peeling off the Scotch tape. It showed no deterioration even on applying on the skin for long hours. Thus, these electrodes could be used multiple times and for long durations for electrophysiological signal acquisition.
Figure 3.
(a–d) Scotch tape adhesion test of the pattern AgNWs on the PDMS substrate. (e) Skin-electrode impedance measurements of the Ag/AgCl and AgNW/PDMS dry electrodes at the forearm.
Next, we measured the electrical resistivity of the electrodes and skin-electrode impedance, which are major characteristics governing the quality of the electrophysiological signals. The sheet resistance of the fabricated AgNW/PDMS electrode before and after the adhesion test was estimated to be 0.6 and 0.8 Ω/sq, respectively. Further, we examined the electromechanical behavior of the AgNW/PDMS dry electrode (see Figure S5a–c, Supporting Information). As shown in Figure S5a, resistance and the relative change in resistance of the AgNW/PDMS electrode increases with the applied strain.23,25−27 The resistance values of the AgNW/PDMS electrode at 0% and 50% strain were calculated to be 1.54 and 2.44 Ω, respectively. Figure S5b shows the relative change in resistance of the AgNW/PDMS electrode versus time under cyclic stretching and releasing at a strain of 20%, indicating a stable and reliable response to cyclic strain. As shown in Figure S5c, the resistance of the AgNW/PDMS electrode is insensitive to the bending angle. For measuring the skin-electrode impedance, a pair of electrodes was placed 2 cm apart on the forearm without any surface preparation. It is known that the skin-electrode impedance changes with the location of the electrodes and from person-to-person. Hence, all the measurements were done on the same subject who participated in the recording of the electrophysiological signals. The impedance was measured in the frequency range of 1 Hz to 1 kHz. Figure 3e shows the impedance associated with the Ag/AgCl and AgNW/PDMS electrode. The skin-electrode impedance values at 10 Hz for Ag/AgCl wet electrodes and AgNW/PDMS dry electrodes were measured to be 400 and 374 kΩ, respectively. The order of the impedance values is similar to the values reported for AgNW-based dry electrodes.19
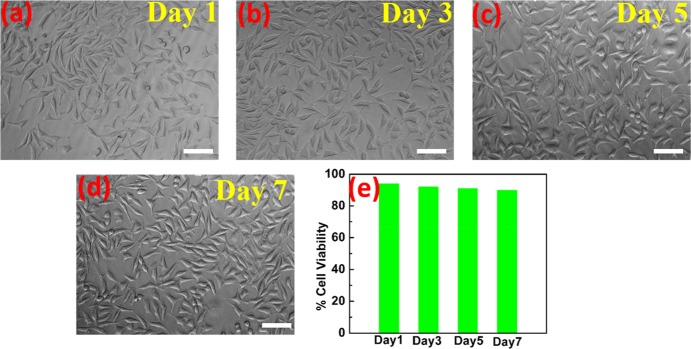
As the dry electrodes are fabricated for long-term signal recordings, it is important that the electrodes have no cytotoxicity effects during long-term contact with the skin. MTT assay tests were performed to check the cytotoxicity of the AgNW/PDMS electrodes.28,29 Since the electrodes are applied on the skin during the biopotential signal measurements, their cytotoxicity was assayed using L929, a mouse fibroblast cell line. Analysis of toxicity of the AgNW/PDMS dry electrodes was done by incubating the chips with cell culture media. The media in contact with the electrodes was collected and added on L929 cells to check the cytotoxicity. The cells remain viable in the presence of the media and did not show any change in morphology as shown in Figure 4a–d. As shown in Figure 4e, about 90% cell viability was observed over the course of one week, indicating that the AgNW/PDMS electrodes do not release any cytotoxic components as compared to control. Hence, the test results showed that the presence of silver nanowires had no cytotoxic effect on the living cells, and therefore we believe that these electrodes could be used for long-term continuous electrophysiological signal recordings.
Figure 4.
(a–d) Phase-contrast images of viable cells. (e) Percentage cell viability for days 1, 3, 5, and 7 by MTT assay (scale bar: 50 μm).
Figure 5a shows the ECG measurement results performed on the subject (male, 26 F) in a relaxed state using commercial Ag/AgCl electrodes as well as fabricated dry AgNW/PDMS. ECG measurements were recorded with a notch filter (50 Hz cutoff frequency) to reduce the power line noise. As we can see from Figure 5b, the recorded ECG signals from AgNW/PDMS electrodes were similar to the signal obtained from the wet commercial Ag/AgCl electrode. Also, typical ECG features like the P wave, QRS complex, and T wave could easily be identified for all the electrodes, as shown in Figure 5c. To measure the frequency response of the ECG signal, power spectral density (PSD) was estimated using the built-in MATLAB function for a non-parametric Welch periodogram (Hamming window) as shown in Figure S6, Supporting Information. As shown in Figure 5c and Figure S6, similar frequency response curves were observed for both Ag/AgCl and AgNW/PDMS electrodes where the typical ECG features like the P wave, QRS complex, and T wave were easily identifiable for all the electrodes, and the ECG signals were captured accurately in the frequency range of 0–30 Hz. Due to the excellent conformability of the PDMS polymer substrate and the high conductivity of the silver nanowire film, the intensity of the signal is slightly higher in the case of the AgNW/PDMS dry electrodes as shown in Figure 5c. The signal to noise ratios of the conventional wet Ag/AgCl and dry AgNW/PDMS electrodes were calculated to be 24.6 and 25.4 dB, respectively. Figure S7 (Supporting Information) shows the ECG signal from AgNW/PDMS dry electrodes for 120 se.
Figure 5.
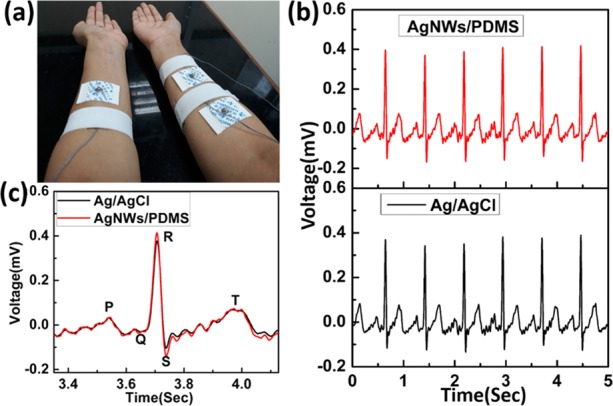
(a) Simultaneous ECG signal measurements from the Ag/AgCl and AgNW/PDMS electrodes. (b) Obtained ECG signal from the Ag/AgCl (black) and AgNW/PDMS (red) electrodes. (c) Enlarged view of ECG signal comparison from both electrodes.
Figure 6a,b shows the electrodes’ placements for simultaneous recording of EMG signals from both Ag/AgCl and AgNW/PDMS electrodes. As shown in Figure 6a,b, the subject was holding an air blower and squeezing it at an interval of 5 s to flex the muscles. Figure 6c shows the EMG signals measured from Ag/AgCl and AgNW/PDMS electrodes. The quality of EMG signals obtained from both electrodes was comparable, and the signal intensity of the AgNW/PDMS was slightly higher than that of the conventional Ag/AgCl electrodes, as shown in Figure 6c. In order to show the reusability of as-fabricated AgNW/PDMS dry electrodes, we measure the EMG signal after one month as shown in Figure S8 in the Supporting Information. As shown in Figure S8, the EMG signal is almost similar to the EMG signal in Figure 6c, indicating the reusability of our electrodes.
Figure 6.
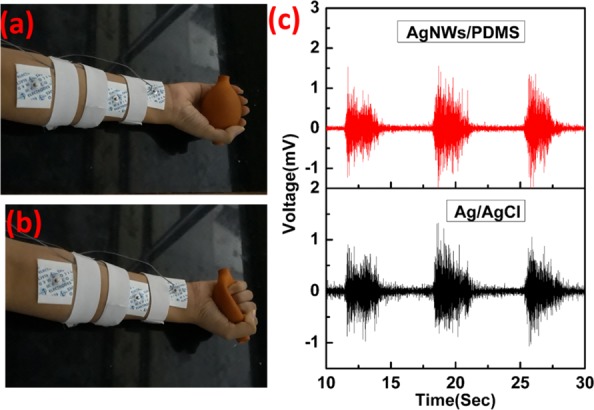
(a, b) Ag/AgCl and AgNW/PDMS electrode placement on the forearm for EMG measurements. (c) Simultaneously obtained EMG signals from Ag/AgCl and AgNW/PDMS electrodes.
4. Conclusions
In conclusion, flexible and highly conductive AgNW/PDMS dry electrodes are fabricated by a simple and cost-effective vacuum filtration method, which allows the excellent adhesion of patterned AgNWs on the PDMS substrate. The skin-electrode impedance of the AgNW/PDMS electrodes is slightly less than that of the conventional Ag/AgCl electrodes. The cytotoxicity test confirms 90% cell viability over the period of 7 days, and hence AgNW/PDMS electrodes are observed to have no cytotoxic effect on the living cells. The AgNW/PDMS dry electrodes are capable of recording better quality electrophysiological signals compared to those of the conventional Ag/AgCl wet electrodes.
Acknowledgments
This research work was supported by the Department of Science and Technology (DST), India (grant no. Do/2018-WRCB002-024). The authors acknowledge the Centre for Excellence in Nanoelectronics (CEN), Sophisticated Analytical Instrument Facility (SAIF), and Department of Metallurgical Engineering and Materials Science (MEMS) at the Indian Institute of Technology Bombay (IIT Bombay), Mumbai, for providing various facilities for fabrication and characterization of the device.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03678.
Fabrication process of the AgNW/PDMS dry electrode, optical images of conventional Ag/AgCl and AgNW/PDMS dry electrodes, adhesion strength of AgNWs on PDMS, electromechanical behavior of the AgNW/PDMS dry electrode, power spectral density of the ECG signal obtained from Ag/AgCl and AgNW/PDMS electrodes, continuous ECG signal from AgNW/PDMS dry electrodes for 120 s, and EMG signal from AgNWs/PDMS dry electrodes after one month (PDF)
Scotch tape showing no traces of AgNWs after peeling it off from the AgNW/PDMS electrode (MP4)
Author Contributions
§ R.R.K. and P.J. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Liu Y.; Pharr M.; Salvatore G. A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. 10.1021/acsnano.7b04898. [DOI] [PubMed] [Google Scholar]
- Khan Y.; Ostfeld A. E.; Lochner C. M.; Pierre A.; Arias A. C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. 10.1002/adma.201504366. [DOI] [PubMed] [Google Scholar]
- Jin H.; Abu-Raya Y. S.; Haick H. Advanced Materials for Health Monitoring with Skin-Based Wearable Devices. Adv. Healthcare Mater. 2017, 6, 1700024. 10.1002/adhm.201700024. [DOI] [PubMed] [Google Scholar]
- Chae H.; Kwon H.-J.; Kim Y.-K.; et al. Laser-Processed Nature-Inspired Deformable Structures for Breathable and Reusable Electrophysiological Sensors toward Controllable Home Electronic Appliances and Psychophysiological Stress Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 28387–28396. 10.1021/acsami.9b06363. [DOI] [PubMed] [Google Scholar]
- Jang K. I.; Jung H. N.; Lee J. W.; et al. Ferromagnetic, Folded Electrode Composite as a Soft Interface to the Skin for Long-Term Electrophysiological Recording. Adv. Funct. Mater. 2016, 26, 7281–7290. 10.1002/adfm.201603146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; Chen Y. C.; Nicolini L.; et al. “cut-and-Paste” Manufacture of Multiparametric Epidermal Sensor Systems. Adv. Mater. 2015, 27, 6423–6430. 10.1002/adma.201502386. [DOI] [PubMed] [Google Scholar]
- Yao S.; Zhu Y. Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. JOM 2016, 68, 1145–1155. 10.1007/s11837-016-1818-0. [DOI] [Google Scholar]
- Meziane N.; Webster J. G.; Attari M.; Nimunkar A. J. Dry electrodes for electrocardiography. Physiol Meas. 2013, 34, R47–R69. 10.1088/0967-3334/34/9/R47. [DOI] [PubMed] [Google Scholar]
- Baek J.-Y.; An J.-H.; Choi J.-M.; Park K.-S.; Lee S.-H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuators, A 2008, 143, 423–429. 10.1016/j.sna.2007.11.019. [DOI] [Google Scholar]
- Chen C.-Y.; Chang C.-L.; Chang C.-W.; et al. A low-power bio-potential acquisition system with flexible PDMS dry electrodes for portable ubiquitous healthcare applications. Sens. 2013, 13, 3077–3091. 10.3390/s130303077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y.; Li Z.; Chen J. A flexible dry electrode based on APTES-anchored PDMS substrate for portable ECG acquisition system. Microsyst. Technol. 2016, 22, 2027–2034. 10.1007/s00542-015-2490-y. [DOI] [Google Scholar]
- Kim T.; Park J.; Sohn J.; Cho D.; Jeon S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D-2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes. ACS Nano 2016, 10, 4770–4778. 10.1021/acsnano.6b01355. [DOI] [PubMed] [Google Scholar]
- Liu B.; Luo Z.; Zhang W.; Tu Q.; Jin X. Silver nanowire-composite electrodes for long-term electrocardiogram measurements. Sens. Actuators, A 2016, 247, 459–464. 10.1016/j.sna.2016.06.008. [DOI] [Google Scholar]
- Peng H.-L.; Liu J.-Q.; Tian H.-C.; et al. Flexible dry electrode based on carbon nanotube/polymer hybrid micropillars for biopotential recording. Sens. Actuators, A 2015, 235, 48–56. 10.1016/j.sna.2015.09.024. [DOI] [Google Scholar]
- Chen K.; Ren L.; Chen Z.; Pan C.; Zhou W.; Jiang L. Fabrication of micro-needle electrodes for bio-signal recording by a magnetization-induced self-assembly method. Sens. 2016, 16, 1–15. 10.3390/s16091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. K.; Bhartia B.; Mukhopadhyay K.; Sharma A. Long term biopotential recording by body conformable photolithography fabricated low cost polymeric microneedle arrays. Sens. Actuators, A 2015, 236, 164–172. 10.1016/j.sna.2015.10.041. [DOI] [Google Scholar]
- O’Mahony C.; Pini F.; Blake A.; Webster C.; O’Brien J.; McCarthy K. G. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens. Actuators, A 2012, 186, 130–136. 10.1016/j.sna.2012.04.037. [DOI] [Google Scholar]
- Ren L.; Jiang Q.; Chen K.; Chen Z.; Pan C.; Jiang L. Fabrication of a micro-needle array electrode by thermal drawing for bio-signals monitoring. Sens. 2016, 16, 908. 10.3390/s16060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. C.; Huang H.; Zhu Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. 10.1039/c4ra15101a. [DOI] [Google Scholar]
- Qin Q.; Li J.; Yao S.; Liu C.; Huang H.; Zhu Y. Electrocardiogram of a Silver Nanowire Based Dry Electrode: Quantitative Comparison with the Standard Ag/AgCl Gel Electrode. IEEE Access. 2019, 7, 20789–20800. 10.1109/ACCESS.2019.2897590. [DOI] [Google Scholar]
- Helgason R.; Banavali A.; Lai Y. Cohesive dry ECG sensor using silver nanowires and PDMS tuned for adhesion. Med. Devices Sens. 2019, 2, e10025 10.1002/mds3.10025. [DOI] [Google Scholar]
- Korte K. E.; Skrabalak S. E.; Xia Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2 -mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. 10.1039/b714072j. [DOI] [Google Scholar]
- Amjadi M.; Pichitpajongkit A.; Lee S.; Ryu S.; Park I. Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. 10.1021/nn501204t. [DOI] [PubMed] [Google Scholar]
- Liu S.; Sun B.; Li J.-G.; Chen J. Silver nanowires with rounded ends: Ammonium carbonate-mediated polyol synthesis, shape evolution and growth mechanism. CrystEngComm 2014, 16, 244–251. 10.1039/c3ce41738g. [DOI] [Google Scholar]
- Joo Y.; Byun J.; Seong N.; et al. Silver nanowire-embedded PDMS with a multiscale structure for a highly sensitive and robust flexible pressure sensor. Nanoscale 2015, 7, 6208–6215. 10.1039/c5nr00313j. [DOI] [PubMed] [Google Scholar]
- Huang G.-W.; Xiao H.-M.; Fu S.-Y. Wearable Electronics of Silver-Nanowire/Poly(dimethylsiloxane) Nanocomposite for Smart Clothing. Sci. Rep. 2015, 5, 1–9. 10.1038/srep13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Wu Z.; Lu X.; et al. Ultrastretchable and Stable Strain Sensors Based on Antifreezing and Self-Healing Ionic Organohydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 9405–9414. 10.1021/acsami.8b20267. [DOI] [PubMed] [Google Scholar]
- Wu X.; Ma Y.; Zhang G.; et al. Thermally stable, biocompatible, and flexible organic field effect transistors and their application in temperature sensing arrays for artificial skin. Adv. Funct. Mater. 2015, 25, 2138–2146. 10.1002/adfm.201404535. [DOI] [Google Scholar]
- Lee J. K.; Kim D. B.; Kim J. I.; Kim P. Y. In vitro cytotoxicity tests on cultured human skin fibroblasts to predict skin irritation potential of surfactants. Toxicol. In Vitro 2000, 14, 345–349. 10.1016/S0887-2333(00)00028-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.