Abstract
Ventilator-associated pneumonia (VAP) is a highly common hospital-acquired infection affecting people that require mechanical ventilation. The endotracheal tube (ETT) used during the ventilation process provides a surface that can allow bacterial colonization and biofilm formation, which can lead to VAP. Although various approaches, including ETT design and material selection, as well as antimicrobial coatings have been employed to minimize adverse events, VAP remains a significant unresolved clinical issue. In this study, we have utilized a novel styrylbenzene-based antimicrobial (BCP3) in a simple and robust coating that allows its long-term release at an effective level. BCP3 was applied onto PVC ETT segments blended together with poly(lactic-co-glycolic acid) via a facile dip-coating process with controlled loadings. In vitro studies demonstrated concentration-dependent release of BCP3 from the coatings for at least 31 days. Bacterial assays using major VAP culprits, Staphylococcus aureus and Pseudomonas aeruginosa, demonstrated significant growth inhibition, with a stronger effect on S. aureus. Despite its ability to inhibit bacterial growth, BCP3 showed no cytotoxicity toward mammalian (L929) fibroblasts, which makes it attractive from a clinical perspective. The coating procedure was successfully translated to coat the entire ETTs, making it highly amenable for large-scale manufacturing.
1. Introduction
Advances in medical device technologies have contributed significantly to the increase in life expectancy and, perhaps more importantly, the quality of life for aging population. However, some challenges associated with the application of these devices remain, including medical device-associated infections. Although the frequency of infection over the lifetime of the device varies significantly,1 the risk of an infection exists for all medical devices. In most cases, the infection is established by microbial colonization of the device, followed by the formation of a biofilm.2,3
To address this challenge, multiple approaches have been developed for the application of antimicrobial coatings to medical devices.4 However, many devices have been continuously used without any additional protection to reduce the risk of infection. In addition, many of the causative organisms have become increasingly resistant against commonly used antimicrobial agents,5−8 and new antimicrobial compounds with new drug targets are urgently required.
Ventilator-associated pneumonia (VAP) is one of the most common nosocomial infections which occurs in people who require intubation and mechanical ventilation.9−11 VAP typically affects people in intensive care units and represents a major unresolved clinical problem. For patients that contract VAP, the result can be an increased length of hospitalization or death (at a rate of 20–30%).12 Endotracheal tubes (ETTs) that are utilized during ventilation are considered to be a major risk factor for VAP.13,14 The ETT provides a surface15 that allows colonization and biofilm formation by bacteria including Staphylococcus aureus. In order to reduce the incidence of VAP associated with ETT use, various methods including prevention of aspiration of secretions, antimicrobial rinsing, photodynamic therapies, and antimicrobial releasing coatings have been explored.9,16,17 Particularly regarding antimicrobial coatings for ETTs, silver releasing coatings have been the most widely studied.17
The current study focuses on a new strategy to reduce the occurrence of VAP by utilizing a novel antimicrobial, BCP-3, as part of a release coating. BCP-3 is a first-generation antibiotic belonging to a new class of styrylbenzene-based derivative antibiotics (Figure 1).18,19 This generation of antibiotics has shown activity against the mechanosensitive ion channel of large conductance (MscL), a novel and highly sought protein after bacterial target.20 MscL is a highly conserved transmembrane protein found in all bacteria but not in the human genome, making it an ideal drug target. The channel is responsible for saving bacterial cells from lysis in a high osmotic environment. It responds to a high turgor pressure by opening up and allowing bacteria to release osmolytes, thereby reducing the pressure within. Styrylbenzene-based antibiotics lower the threshold at which these channels open and elongate their opening times,13 allowing the unnecessary loss of important osmolytes and other biomolecules, thereby weakening the bacteria. Although there are numerous advantages to the use of this new class including antioxidant properties,21 for the purpose of utilizing a compound in antimicrobial coating, properties such as high chemical and thermal stability and the ability of large-scale and cost-effective manufacturing are critical for the success of such an application. The compound BCP-3 offers these properties, making it an attractive antimicrobial agent for incorporation in polymer coatings.
Figure 1.
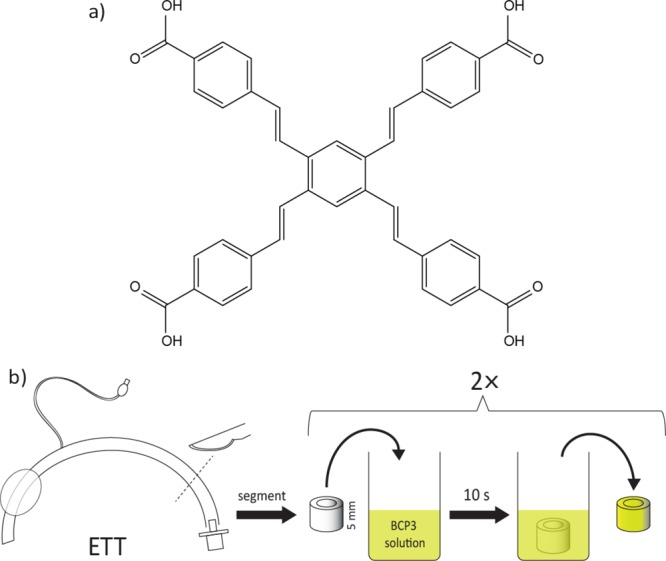
(a) BCP3 structure and (b) ETT segment coating procedure.
Although the current work focused on the use of BCP-3, we also put a lot of emphasis on establishing a simple and effective coating technology to enable the rapid translation of the technology into a commercial process. Moreover, our work focused on establishing a coating technology that allowed the self-limiting release of the antimicrobial compound over a time frame that is relevant for this specific medical device application.
2. Results and Discussion
2.1. Preparation of BCP3-PLGA Coatings on ETT Segments
For medical devices that are widely used, such as ETTs, an antimicrobial coating would need to be easily applicable using processes that can be adapted for large-scale manufacturing. Dip-coating is a robust and simple system that has been explored for the preparation of coatings for a variety of medical applications.22,23 In the case of ETTs, coating both the internal and outside surface of the ETT would maximize the defense against bacterial infection; hence, a dip-coating process was employed because of its simplicity and accessibility.
In this study, Medline 7.5 mm internal diameter poly(vinyl chloride) (PVC) ETTs were utilized as the coating substrate. The tubes were cut into 5 mm segments for ease of handling and for the purposes of in vitro studies.
Each segment was dipped into solutions of various formulations of poly(lactic-co-glycolic acid) (PLGA) and BCP3 in tetrahydrofuran (THF) (combinations of 5, 2.5, 1.25, 0.625, and 0.3125% w/v PLGA and 10, 5, 2.5, and 1.25 mg/mL of BCP3) for 10 s, coated twice (Figure 1), and allowed to dry at room temperature (the coated segments are labeled X/Y, where X is the concentration of PLGA in the coating solution as expressed % w/v and Y is the concentration of BCP3 in the coating solution expressed as mg/mL). It was observed that two coats resulted in a more consistent and uniform coating. PLGA is a copolymer of glycolic and lactic acids, with each of the monomeric units being linked by ester linkages providing biodegradable properties.24 PLGA has been approved by the FDA and widely studied for a variety of biomedical and therapeutic applications including release of bioactives, making it a highly attractive polymer.24 PLGA serves two purposes, not only does it act as a glue to hold the BCP3 but it also allows it to be released both by diffusion and in the longer term via the degradation of the polymer chains. Also, the hydrophobic nature of PLGA allows for a slower sustained release of BCP3 in aqueous environments.
Various BCP3 and PLGA combinations were utilized during the coating process to determine if the loading and the released amount of BCP3 could be controlled. The following sections determine the effect of PLGA and BCP3 concentration on the coating thickness, BCP3 loading, the release profile of BCP3, and ultimately the ability of the coatings to inhibit the growth of S. aureus and Pseudomonas aeruginosa.
2.2. Thickness Measurements
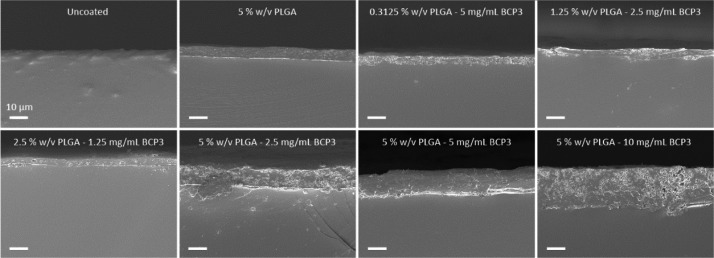
Coating thicknesses were determined via scanning electron microscopy (SEM) analysis of the tube segment cross sections. A clear contrast could be observed between the PVC tube material and the PLGA–BCP3 coating, which allowed for the facile determination of coating thickness. The coating appeared to be deposited uniformly on the ETT surface, with the addition of BCP3 increasing the presence of particulates throughout the coating of cross sections (Figure 2).
Figure 2.
SEM cross-sectional images of coated ETTs showing the presence of different coatings. Scale bar = 10 μm.
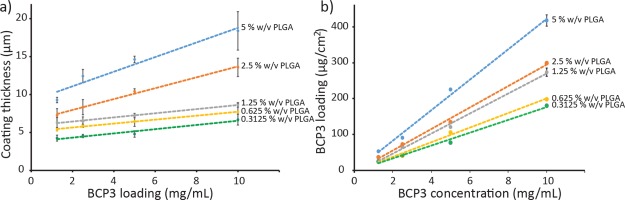
The thickness was determined via image analysis using ImageJ software, with thicknesses ranging from 4 to 20 μm for the various coating formulations used in this study. Figure 3a demonstrates the effect of PLGA concentration on the coating thickness with increasing BCP3 concentrations. The effect of BCP3 concentrations is more prominent at higher PLGA concentrations, whereby the thickness increases more significantly as the BCP3 concentration increases. PLGA effectively acts as a glue, allowing BCP3 to be bound in the coating, and with higher PLGA concentrations, more BCP3 can be incorporated and held together by the polymer following the coating process.
Figure 3.
Graph showing (a) coating thickness at different PLGA and BCP3 concentrations and (b) BCP3 loading (mg/cm2) on the ETT segments at each coating formulation (n = 3).
2.3. Determination of BCP3 Loading
BCP3 is minimally soluble in water; however, in the presence of a base such as NaOH, BCP3 becomes highly soluble as a result of the deprotonation of the carboxylic acid groups. Also, PLGA is a polyester polymer that is susceptible to base hydrolysis. Taking advantage of this, an extraction method with NaOH solution was utilized to determine the BCP3 loading on the ETT segments. Tube segments of 5 mm were initially coated with the 20 combinations of PLGA and BCP3. Subsequently, the segments were placed in a 2.5 M NaOH solution for 24 h to allow the BCP3 to dissolve and PLGA to degrade. After 24 h, it was observed that the coating was completely removed from the ETT surface, while BCP3 was completely dissolved. The concentration of each of the extraction solution was determined via spectroscopic measurement at 339 nm according to a previously determined BCP3 standard curve. Figure 3b summarizes the amount of BCP3 loading at the selected PLGA coating concentrations. It is possible to see a linear relationship between the concentration of PLGA and the concentration of BCP3 in the coating solution. As the PLGA concentration increases, the polymer provides better support and binding for the BCP3, thus leading to higher loading levels in the ETT coatings. The BCP3 loading also correlates very well with the thickness measurements, whereby the thickness of the coatings increases more significantly at higher PLGA concentrations, as the BCP3 concentration is increased during the coating process. It is also possible to see that by simply varying the BCP and PLGA ratios and concentrations, the BCP3 loading in the coating can be varied as desired.
2.4. In Vitro Release of BCP3
PLGA–BCP3 coatings are designed as release platforms to allow the release of BCP3 to inhibit bacterial growth around the ETT when in use. BCP3 is a hydrophobic small molecule and PLGA is a hydrophobic copolymer, so it is important to determine if BCP3 can be sufficiently released from the coatings. The release profile of BCP3 would be expected to be initially via diffusion, followed by a combination of diffusion and release via PLGA degradation owing to the degradable nature of PLGA as a result of the ester linkages present in the polymer backbone. To determine the release profile of BCP3 from the coatings, an in vitro release assay in phosphate-buffered saline (PBS) was conducted. PLGA–BCP3-coated ETT segments were initially cut into four equal pieces and were subsequently incubated in 500 μL of PBS for 31 days. At various time points, samples were taken from the incubated solution to determine the amount of BCP3 released using UV–vis spectroscopy. As can be seen in Figure 4a, the release profiles for all the 20 formulations of PLGA–BCP3 coatings for 31 days are presented. During the first 72 h (Figure 4b), a significant release of BCP3 takes place within the first 24 h, with a range of 9–105 μg/mL·cm–2 for the different coating formulations. The BCP3 and PLGA concentrations significantly affect the amount of BCP3 released in the first 24 h, with the higher BCP3 loadings leading to higher BCP3 release as expected. However, the amount of BCP3 released plateaus from 24 to 48 h for all the formulations. In the first 24 h, only 100 μL of incubated solution was removed for analysis to determine the release profile and maximum released amount; however, at all time points after 48 h, all of the incubated solution was removed and replaced with fresh PBS to determine if the release of BCP3 was concentration-dependent. As can be seen in Figure 4b, even though the release rate of BCP3 had significantly reduced by 48 h, following replacement with fresh PBS, the release rate of BCP3 increases significantly with a gradient similar to that between 4 and 24 h. This increase was more substantial for coatings with higher BCP3 loadings as expected. In Figure 4a, it is possible to see that especially for coatings with a higher BCP3 content, the total amount of released BCP3 continues to increase, although the release rate decreases at each time point as BCP3 in the coatings is depleted over time. While the release of BCP3 from the coatings with lower loadings plateaued at earlier time points, a significant measurable release could be observed for the higher loading coatings up to 31 days. Especially in the case of 5:10, 5:5, 2.5:10, and 1.25:10 coatings, the total amount of released BCP3 was still increasing after 31 days. By varying the BCP3 to PLGA ratios and concentrations in the coating solutions, we have demonstrated that a range of BCP3 release profiles can be obtained. This allows for the preparation of coatings that can be fine-tuned to specific release profiles for various target applications.
Figure 4.
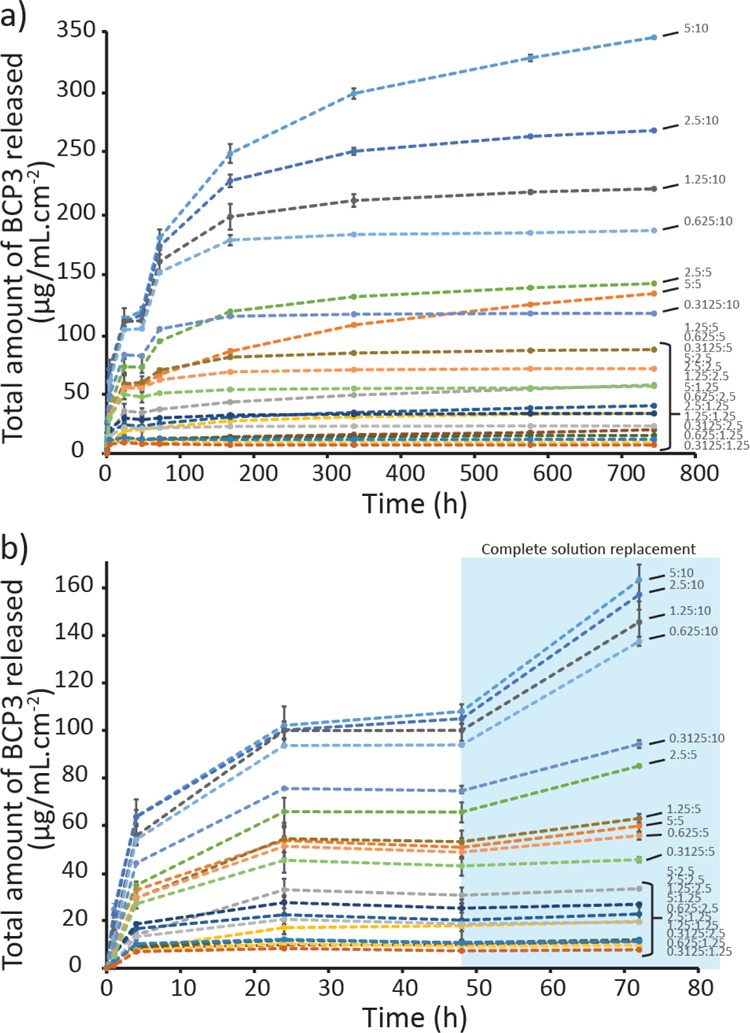
Release of BCP3 from the coatings over (a) 31 days and (b) 72 h (n = 3). A concentration-dependent release can be observed for at least 31 days. After 48 h, the ETT segments were incubated in fresh PBS.
2.5. Inhibition of S. Aureus and P. Aeruginosa Growth
S. aureus and P. aeruginosa are two major culprits associated with VAP.25 Various methods have been employed to reduce the incidence of VAP including the most widely studied use of silver-coated ETTs. Silver possesses broad spectrum efficacy against bacteria;26 however, it is also cytotoxic against mammalian cells.27,28 In this study, we utilized BCP3 as an active antimicrobial in PLGA–BCP3 coatings and determined its activity toward Gram-positive S. aureus: both methicillin-sensitive (MSSA) and methicillin-resistant (MRSA) strains and the Gram-negative P. aeruginosa. Initially, to determine the minimum inhibitory concentrations (MICs), all of the bacteria were exposed to various concentrations of BCP3 (1.6 × 10–4 to 1 mg/mL) for 24 h and the concentration which inhibited bacterial growth by greater than 90% was considered the minimal inhibitory concentration.29,30Table S1 summarizes the MIC values for MSSA, MRSA, and P. aeruginosa. For MSSA and MRSA, an MIC of 15.6 μg/mL was observed. On the other hand, even at 1 mg/mL, a maximum of 32% inhibition of P. aeruginosa growth was observed; hence, no MIC value was obtained for P. aeruginosa at these concentrations. This is not unexpected because Gram-negative bacteria including P. aeruginosa possess lipopolysaccharides in the outer membrane, which can act as a barrier to significantly reduce the effects of antimicrobials;31 hence, the interaction of BCP3 with the MscL channel could also be reduced as a result.
As demonstrated by the MIC study, BCP3 in its free form in solution is able to significantly inhibit the growth of MSSA and MRSA and reduce the growth of P. aeruginosa. From our in vitro studies, BCP3 could be released at a range of concentrations from the PLGA–BCP3 coatings, reaching concentrations above the MICs for MSSA and MRSA. It is important to determine if the released BCP3 from the coatings can also inhibit bacterial growth. In order to investigate this, an in vitro study was conducted, whereby ETT segments coated with various PLGA–BCP3 formulations were placed in MSSA, MRSA, and P. aeruginosa cultures for 24 h. Subsequently, the growth of these bacteria was determined relative to uncoated and only PLGA-coated ETT segments.
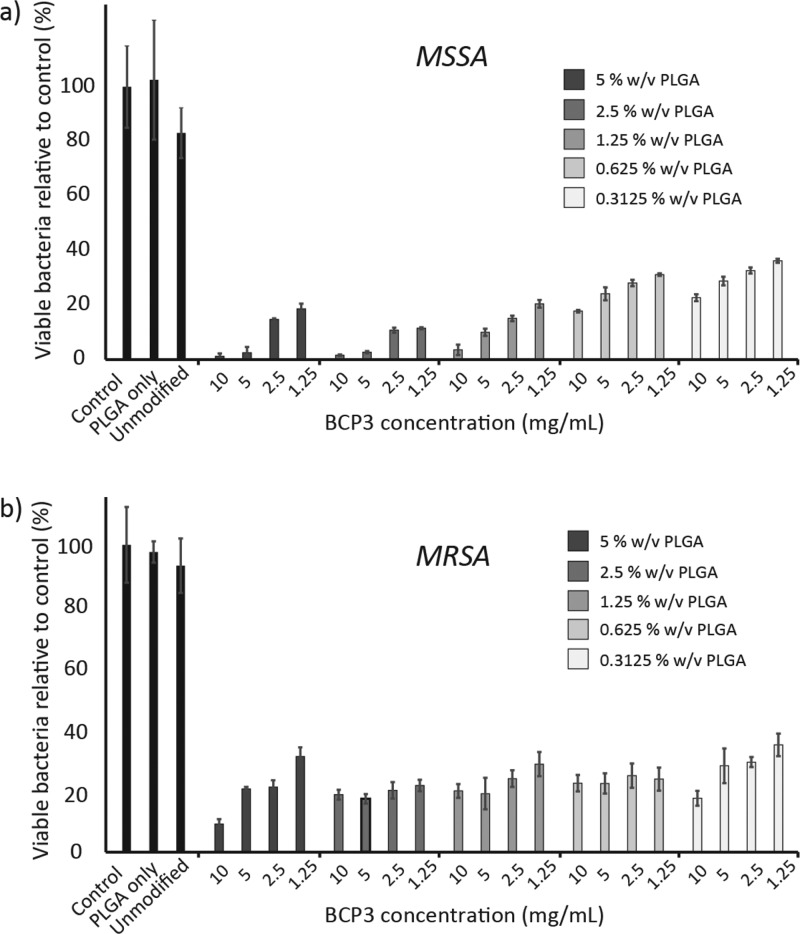
Figure 5 summarizes the bacterial growth inhibition for various PLGA–BCP3 formulations after 24 h. For both MSSA and MRSA, a significant growth inhibition can be observed for all of the coating formulations. For MSSA, a concentration-dependent inhibition can be observed for each PLGA concentration group, whereby a decrease in inhibition is observed as the BCP3 concentration is reduced (Figure 5a). Furthermore, the highest inhibition is observed for 5 and 2.5% w/v PLGA, and as the PLGA concentration is reduced, the inhibition to MSSA growth is also reduced for 1.25, 0.625, and 0.3125% w/v. Coatings 5:10, 5:5, 2.5:10, 2.5:5, and 1.25:10 were able to inhibit growth by >95%, and even at the lowest BCP3 loading (0.3125:10), a significant reduction in MSSA growth (>60% inhibition) can still be observed.
Figure 5.
Bacterial inhibition assays carried out using different coating formulations in vitro with (a) methicillin-sensitive and (b) methicillin-resistant S. aureus (n = 3).
For MRSA, a significant inhibition in growth is observed with an average of 80% reduction across all the coating formulations (Figure 5b). However, the maximum reduction observed was 91% at the highest PLGA and BCP3 loading (5:10); on the other hand, >95% reduction was observed for MSSA at various formulations. Furthermore, a strong concentration-dependent inhibition is not apparent for MRSA at different PLGA concentrations, with the inhibition being relatively constant across all coating formulations.
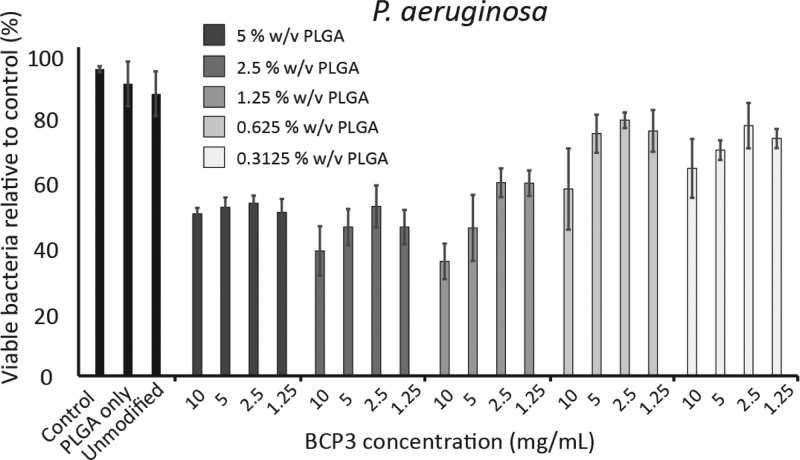
The MIC study demonstrated that P. aeruginosa was not as sensitive to BCP3 as were MSSA and MRSA; however, some growth inhibition was observed. P. aeruginosa was also exposed to coated ETT segments for 24 h. The inhibition of P. aeruginosa growth was to a lower extent compared to MSSA and MRSA (Figure 6). At 5, 2.5, and 1.25% w/v PLGA concentrations, an average of 50% reduction in P. aeruginosa growth was observed, with a maximum of 63% reduction at 1.25:10 coatings.
Figure 6.
Bacterial inhibition assays carried out using different coating formulations in vitro with P. aeruginosa (n = 3).
MIC assays demonstrated that BCP3 possessed excellent growth inhibition properties toward both MSSA and MRSA, while still being able to reduce the growth of P. aeruginosa. Furthermore, the PLGA–BCP3 coatings allowed the release of BCP3, such that in all formulations of the coating, the growth of MSSA and MRSA was significantly inhibited. However, to a lower extent, a significant inhibition of P. aeruginosa growth was also observed in the presence of the coated ETTs.
2.6. In Vitro Cytotoxicity Studies
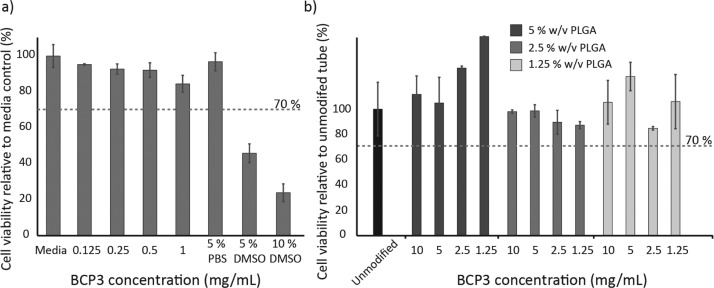
An ideal antimicrobial compound should be effective in inhibiting bacterial growth while remaining noncytotoxic toward the host. Although silver, which has been studied and utilized for ETTs,32 is effective against a range of bacteria, its cytotoxic nature toward mammalian cells is of concern. To determine whether BCP3 possesses cytotoxic properties, an in vitro cell viability assay was conducted at low and high concentrations of BCP3. All cell viability assays were conducted according to the International Standard ISO10993-5/12, and cell viability below 70% was considered cytotoxic. Preseeded L929 fibroblasts were exposed to 8 × 10–4 to 1 mg/mL of BCP3 in cell culture media for 24 h. Subsequently, an 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was utilized to determine cell viability. At all concentrations of BCP3, even at 1 mg/mL, no cytotoxicity was observed (Figure 7a). A minor reduction in cell viability compared to a TCPS control was observed with increasing concentrations; however, at all concentrations, the L929 cell viability was above 70% (85% at 1 mg/mL); hence, BCP3 was considered noncytotoxic.
Figure 7.
In vitro cell viability in the presence of (a) BCP3 up to 1 mg/mL concentration (concentrations below 0.125 mg/mL not shown) and (b) coated ETT segments (results for coatings below 1.25% w/v PLGA not shown). Cytotoxicity assessment of materials was performed according to the International Standard ISO10993-5/12 and cell viability below 70% was considered cytotoxic.
BCP3 in the free form showed noncytotoxic properties with L929 fibroblasts; however, for the preparation of PLGA–BCP3 coatings, the organic solvent THF is used. PLGA is a widely studied polymer which is approved by the FDA for a variety of applications and is considered nontoxic.33 However, it is important to determine if the coating process and the use of an organic solvent can lead to the leaching of potentially cytotoxic compounds from the coating. To determine if any cytotoxic leachables were present in the PLGA–BCP3 coatings, the coated ETT segments were incubated for 66 h in cell culture media. Subsequently, preseeded L929 cells were exposed to the incubated media for 24 h. Cell viability was determined via an MTS assay. No cytotoxicity was observed for any of the coating formulations (Figure 7b), even at the highest BCP3 loadings. BCP3 and the PLGA–BCP3 coatings show no cytotoxicity toward L929 fibroblasts, and combined with the strong inhibitory properties of BCP3 toward MSSA and MRSA, and even toward P. aeruginosa, the coating platform described in this study possesses highly desirable properties to reduce infections associated with ETT use.
2.7. Dip-Coating of Entire Devices
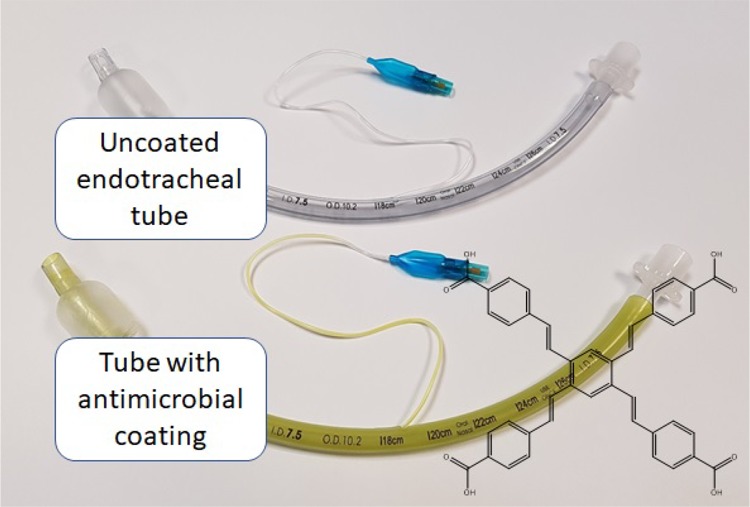
For the scale up of a coating on a medical device, it is necessary for the coating process to be simple, adaptable, and translatable for large-scale production. Dip-coating is a robust and simple coating method that is widely utilized for a variety of medical and nonmedical applications.34,35 In this study, we have demonstrated that small ETT segments can be easily coated by placing the segments into a PLGA–BCP3 solution; however, it is important to demonstrate if this coating procedure can be applied to the entire ETT as would be required for real-world applications. To demonstrate this, an entire Medline ETT was coated using a simple dip-coating procedure. Initially, a custom cylindrical container was filled with a precalculated volume (80 mL) of 1.25% w/v PLGA and 1.25 mg/mL of BCP3 solution. The ETT was also attached to a glass rod simply by inserting the tip of the rod into one end of the ETT. Subsequently, the ETT, via manipulation with the glass rod, was inserted into the PLGA–BCP3 solution in the glass container and removed. The dip-coating process was conducted over 10 s. Subsequently, the coated tube was allowed to dry hanging vertically in a fume cupboard for 5 min before repeating the process for a second coat. The coated tube was allowed to dry for 1 h, and then, the polytetrafluoroethylene (PTFE) tape was removed to expose the balloon section and a syringe was used to inflate the balloon to ensure function.
Observations on the ETT indicated a visually uniform coating along the tube both on the internal and on the external surface (Figure 8a). No streaks or patches were observed on any of the surfaces. Importantly, the visibility of the manufacturer’s printed features including branding and depth indicators were unaffected by the dip-coating process (Figure 8b). The balloon segment of the ETT was also unaffected by the dip-coating process and could be repeatedly inflated and deflated with a syringe.
Figure 8.
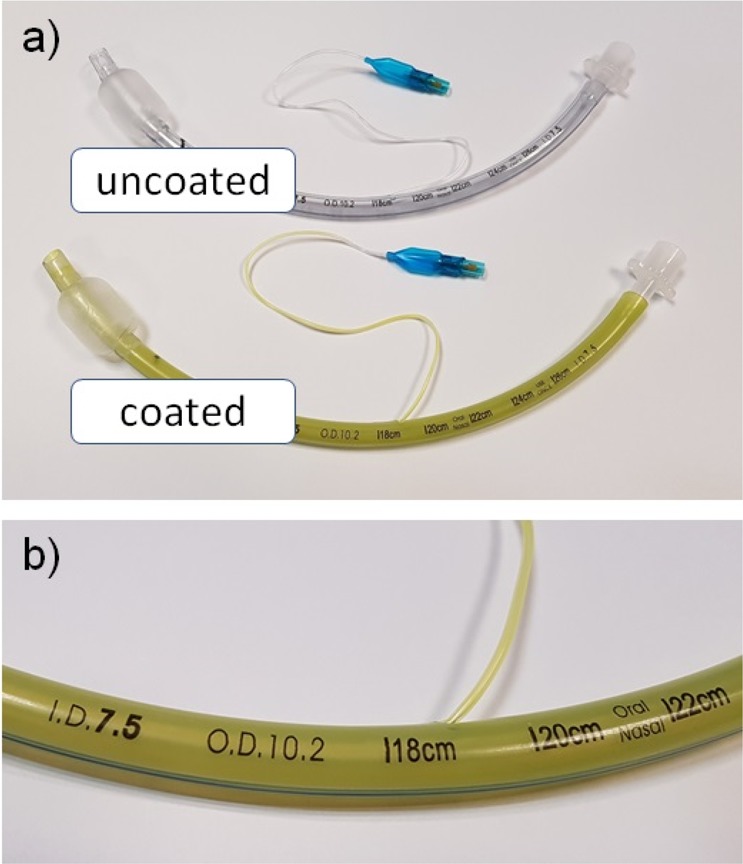
Images of (a) coated ETT compared to an uncoated ETT, and (b) magnified image of a coated ETT demonstrating that the device was not adversely affected by the coating process (photographs courtesy of B. Ozcelik. Copyright 2019).
Coating of the entire ETT was successfully achieved using a simple dip-coating process. We have demonstrated that uniform coating with PLGA–BCP3 in THF could easily be scaled up from coating 5 mm segments to the entire ETTs, allowing coating both inside and outside at the same time. The ease of coating application using the simple dip-coating process, combined with the commercial availability of PLGA, makes these coatings highly amenable for large-scale manufacturing and applications. Our approach solves a number of issues that are associated with antimicrobial ETT coatings, which are either approved or are seeking regulatory approval for clinical use.11
3. Conclusions
We have successfully developed a simple and robust coating method combining the highly biocompatible polymer PLGA and the novel antimicrobial compound BCP3. The PLGA–BCP3 coatings can be applied using a facile dip-coating process that allows the controlled loading of BCP3. Our in vitro studies have shown a concentration-dependent release of BCP3 for at least 31 days with a tunable release profile. The PLGA–BCP3 coatings were able to release BCP3 to significantly inhibit the growth of major VAP culprits, methicillin-sensitive and methicillin-resistant S. aureus and to a smaller extent the Gram-negative P. aeruginosa. While inhibiting bacterial growth, our in vitro studies demonstrated that even at concentrations significantly higher than bacterial MICs, no cytotoxicity was observed toward L929 fibroblasts. Furthermore, our experiments demonstrated that, using the facile coating method established in this study, the entire ETTs can be coated quickly and easily, making the PLGA–BCP3 coatings highly amenable for large-scale manufacturing. Our approach solves a number of issues that are associated with antimicrobial ETT coatings, which are either approved or are seeking regulatory approval for clinical use.
4. Experimental Section
4.1. BCP3-PLGA Coating of ETT Segments
Medline 7.5 mm internal diameter PVC ETTs (Medline Industries, IL, USA) were cut into 5 mm ring segments using a fresh scalpel to be used in the coating process. BCP3 (4,4′,4″,4‴-((1E,1′E,1″E,1‴E)-benzene-1,2,4,5-tetrayltetrakis(ethene-2,1-diyl))tetrabenzoic acid) (Boulos & Cooper Pharmaceuticals, WA, Australia) and poly(d,l-lactide-co-glycolide) (PLGA) (Sigma-Aldrich, lactide/glycolide 75:25, Mw 76,000–115,000 Da) were dissolved/suspended in THF. The solutions were then sonicated for 15 min using a bath sonicator. The final solutions obtained were combinations of 10, 5, 2.5, and 1.25 mg/mL of BCP3 and 5, 2.5, 1.25, 0.625, and 0.3125% w/v of PLGA. Previously prepared ETT segments were then dipped into 3 mL solutions of PLGA/BCP3 combinations for 10 s, removed using fine forceps, and allowed to dry in a laminar flow cabinet for 5 min prior to being dipped again in their respective solutions for another 10 s. All ETT segments were dip-coated twice. Uncoated ETT segments and segments only coated with a 5% w/v PLGA solution were used as controls.
4.2. Coating Thickness Measurement
Coated ETT segments were cut vertically using a fresh scalpel blade, mounted on aluminum stubs with double-sided carbon tabs, and coated with iridium (60 mA, 30 s) using a Cressington 208HRD sputter coater prior to imaging. Zeiss Merlin FESEM (field emission scanning electron microscope) operated in the secondary electron mode to highlight topographical features with an accelerating voltage of 3 kV was used for imaging. Images of the ETT segment cross sections were obtained from each of the different BCP3:PLGA formulations. The acquired images were subsequently analyzed using ImageJ (NIH, USA) software to determine the thickness of coating present on each ETT segment surface.
4.3. Determination of BCP3 Loading via NaOH Extraction
Each of the 20 different dip-coated ETT segments were placed in 12 mL of 2.5 M NaOH solution in glass vials for 24 h. From each vial, 100 μL of solution was transferred to a flat-bottom 96-well plate (Nunc) and was read using a BioTek plate reader at 339 nm. The concentrations in each extracted solution were determined via comparison to a standard curve of BCP3 in 2.5 M NaOH in water. These concentrations were then used to determine the loadings as milligrams of BCP3 per square centimeter of ETT surface.
4.4. In Vitro BCP3 Release Study
All formulations of coated ETT segments were cut vertically into equal quarters to produce curved rectangular pieces. Each quarter was placed into a well of tissue culture polystyrene (TCPS) 48-well plate. Fresh 1× PBS (500 μL) was pipetted into each well containing the ETT quarters, and the complete coverage of each piece with the PBS was ensured. The plate was sealed using parafilm and aluminum foil and placed in a shaker incubator at 37 °C (80 rpm). At 1, 4, 24, and 48 h time points, 100 μL of solution was removed and placed in a clear TCPS 96-well plate. The removed solution in each well was immediately replaced with 100 μL of fresh PBS at each time point. At 72 h and 8, 24, and 31 day time points, all 500 μL of solution was removed, 100 μL was transferred to a TCPS 96-well plate, and each well containing ETT segments was replaced with 500 μL of fresh PBS. The 96-well plates with the transferred solutions from each time point were read using a BioTek plate reader at 339 nm. The concentrations in each extracted solution were determined via comparison to a standard curve of BCP3 in 1× PBS. These concentrations were then used to determine the amount of BCP3 released at each time point as milligrams of BCP3 per square centimeter of ETT surface per milliliter of PBS.
4.5. Determination of the MIC
Three bacterial strains—Gram-positive S. aureus (ATCC 29213) (MSSA), methicillin-resistant S. aureus (MRSA) (ATCC 43300), and Gram-negative P. aeruginosa (ATCC 27853)—were used in this study. Bacterial stocks (stored at −80 °C in a nutrient broth with 15% glycerol) were streaked onto nutrient agar (Oxoid, Basingstoke, UK) plates for use as the working stock. From the stock, an overnight bacterial culture grown in the nutrient broth was diluted 1:100 into specific growth media, including a tryptic soya broth for S. aureus and the Luria–Bertani broth for P. aeruginosa. The final concentration of bacteria was made up to 1 × 105 cfu/mL for the inhibition assays. BCP3 was suspended in respective bacterial media to afford 5 mg/mL suspension. The stock suspension was then twice and 10 times diluted to finally obtain concentrations ranging from 8 × 10–4 to 5 mg/mL. Aliquots of 20 μL of each concentration were then added in quadruplicate to wells containing 80 μL of 1 × 105 cfu/mL bacterial solutions in a 96-well plate format for final well concentrations of 1.6 × 10–4 to 1 mg/mL of BCP3. The plates were then sealed with a parafilm and placed in a shaker incubator for 24 h at 37 °C. After 24 h, 100 μL from each well was transferred into a TCPS 96-well plate (Nunc), and the optical density was measured at 600 nm using a BioTek plate reader. The inhibition of bacterial growth was determined relative to the control (%).
4.5.1. Inhibition of S. aureus and P. aeruginosa Growth in the Presence of BCP3–PLGA Coatings
Three bacterial strains—Gram-positive S. aureus (ATCC 29213) (MSSA), methicillin-resistant S. aureus (MRSA) (ATCC 43300), and Gram-negative P. aeruginosa (ATCC 27853)—were used in this study. Bacterial stocks (stored at −80 °C in a nutrient broth with 15% glycerol) were streaked onto nutrient agar (Oxoid, Basingstoke, UK) plates for use as the working stock. From the stock, an overnight bacterial culture grown in the nutrient broth was diluted 1:100 into specific growth media, including a tryptic soya broth for S. aureus and the Luria–Bertani broth for P. aeruginosa. The final concentration of bacteria was made up to 1 × 105 cfu/mL for the inhibition assays. All formulations of coated ETT segments were cut vertically into equal quarters to produce curved rectangular pieces. Each quarter was placed into a well of TCPS 48-well plate in quadruplicates. From each bacterial solution, 500 μL was then added to wells containing the coated ETTs. PLGA-coated and uncoated ETT segments were used as controls. The plates were then sealed with parafilm and placed in a shaker incubator for 24 h at 37 °C. After 24 h, 100 μL from each well was transferred into a TCPS 96-well plate (Nunc), and the optical density was measured at 600 nm using a BioTek plate reader. The inhibition of bacterial growth was determined relative to the control (%).
4.5.2. Determination of Minimal BCP3 Cytotoxic Concentration
BCP3 was sterilized via incubation in 80% ethanol in a sterile glass vial for 1 h prior to being vacuum-dried at 120 °C for 5 h (0.2 mbar). Subsequently, BCP3 was suspended in a complete minimum essential medium (MEM, containing 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) nonessential amino acids) to afford a 5 mg/mL suspension. The stock suspension was then twice and 10 times diluted to finally obtain concentrations ranging from 8 × 10–4 to 5 mg/mL. Aliquots of 20 μL of each concentration were then added in quadruplicate to wells previously seeded with L929 cells containing 80 μL of complete MEM (18,000 cells per well, 16 h) in a 96-well plate format for final well concentrations of 1.6 × 10–4 to 1 mg/mL. Additional controls were added to wells containing preseeded cells; medium alone, 5%, and 10% (v/v) dimethylsulfoxide (DMSO, Sigma) in medium. The plate was subsequently incubated for 21 h (37 °C, 5% CO2). An MTS assay was then utilized to determine cell viability based on the metabolic activity of cells. Stock solutions of 4 mM MTS (Promega) in PBS and 3 mM of phenazine methosulfate (PMS, Sigma) in PBS were used to make up a mixture of MTS and PMS reagent. A working assay reagent solution was made up by the addition of 2 mL of MTS and 100 μL of PMS stock solutions per 10 mL of complete medium. The medium was removed from the sample and control wells and washed with 120 μL of fresh medium; subsequently, 100 μL of the assay reagent solution was added to the wells and then incubated for 3 h at 37 °C, 5% CO2. For colorimetric analysis, the plates were read in a BioTek plate reader at wavelengths 490 and 655 nm. The difference in readings at the two wavelengths was used to calculate cell attachment compared to controls.
4.6. Cytotoxicity Studies
Cytotoxicity assessment of coated ETTs was performed according to the International Standard ISO10993-5/12. All formulations of coated ETT segments were cut vertically into equal quarters to produce curved rectangular pieces. Each quarter was placed into the well of a TCPS 48-well plate. To each well containing ETT segments, 500 μL of complete MEM [containing 10% (v/v) FBS and 1% (v/v) nonessential amino acids] was added, and the plate was allowed to incubate for 66 h in a sealed humidified chamber placed on a rocker (Seoulin Mylab) at 20 rpm at 37 °C in a 5% CO2 incubator. PLGA-coated and uncoated ETT segments were used as controls.
Aliquots of 100 μL of incubated medium from each well were added in quadruplicate to wells previously seeded with L929 cells (18,000 cells per well, 16 h) in a 96-well plate format. Additional controls were added to wells containing preseeded cells: medium alone, 5, and 10% (v/v) DMSO (Sigma) in medium. The plate was subsequently incubated for 21 h (37 °C, 5% CO2). An MTS assay was performed to assess cell viability as previously described for the determination of BCP3 cytotoxic concentration.
4.7. Coating of an Entire ETT Using a Dip-Coating Method
For coating of an entire ETT, a Medline 7.5 mm internal diameter PVC ETT (Medline Industries, IL, USA) was utilized. The balloon section of the tube was wrapped and sealed with the PTFE tape to avoid swelling and damage to the balloon. A solution of 80 mL of 1.25 mg/mL BCP3 and 1.25% w/v PLGA was prepared and sonicated for 20 min before being transferred into a 2 cm ID, 40 cm length glass cylinder. For the dip-coating procedure, the ETT was held using a clean glass rod (8 mm diameter) by inserting the tip of the glass rod into the balloon end of the ETT. The ETT was then introduced into the cylinder with the coating solution and removed in a total of 10 s. Subsequently, the ETT was hung vertically and allowed to dry for 5 min before repeating the dip-coating procedure. The ETT was then allowed to dry in a laminar flow hood for 1 h before inspection and photographing.
4.8. Statistical Analysis
A minimum of three experimental repeats (n ≥ 3, unless otherwise specified) were used in each study, and the results are presented as mean ± standard error. Statistical significance was determined by an independent Student’s t-test, and a confidence level of 95% (p < 0.05) was considered to be statistically significant unless otherwise stated.
Acknowledgments
The authors acknowledge funding from Boulos & Cooper Pharmaceuticals for the project. The Commonwealth Scientific and Industrial Research Organisation (CSIRO) is acknowledged for providing an Office of the Chief Executive (OCE) Postdoctoral Fellowship to B.O. Mark Greaves (CSIRO) is acknowledged for his help with SEM image acquisition.
Glossary
Abbreviations
- VAP
ventilator-associated pneumonia
- ETT
endotracheal tube
- PLGA
poly(lactic-co-glycolic acid)
- BCP3
4,4′,4″,4‴-((1E,1′E,1″E,1‴E)-benzene-1,2,4,5-tetrayltetrakis(ethene-2,1-diyl))tetrabenzoic acid
- PVC
poly(vinyl chloride)
- MIC
minimum inhibitory concentration
- MSSA
methicillin-susceptible Staphylococcus aureus
- MRSA
methicillin-resistant Staphylococcus aureus
- TCPS
tissue culture polystyrene
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04178.
BCP3 MIC values for MSSA, MRSA, and P. aeruginosa (PDF)
Author Present Address
§ PolyNovo Ltd., 2/320 Lorimer Street, Port Melbourne, Victoria 3207, Australia.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The research described in the manuscript was supported by Boulos & Cooper Pharmaceuticals, which seeks to commercialize a range of novel antimicrobial compounds.
The authors declare the following competing financial interest(s): The research described in the manuscript was supported by Boulos & Cooper Pharmaceuticals, which seeks to commercialize a range of novel antimicrobial compounds.The authors declare that Ramiz A. Boulos is a founder of Boulos & Cooper Pharmaceuticals.
Supplementary Material
References
- Salwiczek M.; Qu Y.; Gardiner J.; Strugnell R. A.; Lithgow T.; McLean K. M.; Thissen H. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014, 32, 82–90. 10.1016/j.tibtech.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Ozcelik B.; Ho K. K. K.; Glattauer V.; Willcox M.; Kumar N.; Thissen H. Poly(ethylene glycol)-based coatings combining low-biofouling and quorum-sensing inhibiting properties to reduce bacterial colonization. ACS Biomater. Sci. Eng. 2017, 3, 78–87. 10.1021/acsbiomaterials.6b00579. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- Ozcelik B.; Chen R.; Glattauer V.; Kumar N.; Willcox M. P.; Thissen H. Crosslinked platform coatings incorporating bioactive signals for the control of biointerfacial interactions. Macromol. Biosci. 2016, 17, 1600315. 10.1002/mabi.201600315. [DOI] [PubMed] [Google Scholar]
- Butler M. S.; Blaskovich M. A.; Cooper M. A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. 2013, 66, 571–591. 10.1038/ja.2013.86. [DOI] [PubMed] [Google Scholar]
- Christoffersen R. E. Antibiotics-an investment worth making?. Nat. Biotechnol. 2006, 24, 1512–1514. 10.1038/nbt1206-1512. [DOI] [PubMed] [Google Scholar]
- Clardy J.; Fischbach M. A.; Walsh C. T. New antibiotics from bacterial natural products. Nat. Biotechnol. 2006, 24, 1541–1550. 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]
- Piddock L. J. V. Antibiotic action: helping deliver action plans and strategies. Lancet Infect. Dis. 2013, 13, 1009–1011. 10.1016/s1473-3099(13)70299-8. [DOI] [PubMed] [Google Scholar]
- Biel M. A.; Sievert C.; Usacheva M.; Teichert M.; Wedell E.; Loebel N.; Rose A.; Zimmermann R. Reduction of endotracheal tube biofilms using antimicrobial photodynamic therapy. Lasers Surg. Med. 2011, 43, 586–590. 10.1002/lsm.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes R. J.; Meduri G. U. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med. 1995, 21, 365–383. 10.1007/bf01705418. [DOI] [PubMed] [Google Scholar]
- Barnes M.; Feit C.; Grant T.-A.; Brisbois E. J. Antimicrobial polymer modifications to reduce microbial bioburden on endotracheal tubes and ventilator associated pneumonia. Acta Biomater. 2019, 91, 220–234. 10.1016/j.actbio.2019.04.042. [DOI] [PubMed] [Google Scholar]
- Raghavendran K.; Mylotte J. M.; Scannapieco F. A. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: the contribution of dental biofilms and periodontal inflammation. Periodontol 2007, 44, 164–177. 10.1111/j.1600-0757.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner R. J. Contribution of endotracheal tubes to the pathogenesis of ventilator-associated pneumonia. J. Hosp. Infect. 1997, 35, 83–89. 10.1016/s0195-6701(97)90096-7. [DOI] [PubMed] [Google Scholar]
- Berra L.; Curto F.; Li Bassi G.; Laquerriere P.; Pitts B.; Baccarelli A.; Kolobow T. Antimicrobial-coated endotracheal tubes: an experimental study. Intensive Care Med. 2008, 34, 1020–1029. 10.1007/s00134-008-1099-3. [DOI] [PubMed] [Google Scholar]
- Adair C. G.; Gorman S. P.; Byers L. M.; Jones D. S.; Goldsmith C. E.; Moore J. E.; Kerr J. R.; Curran M. D.; Hogg G.; Webb C. H.; McCarthy G. J.; Milligan K. R.; Feron B. M. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999, 25, 1072–1076. 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- Kollef M. H. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit. Care Med. 2004, 32, 1396–1405. 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- Deem S.; Treggiari M. M. New endotracheal tubes designed to prevent ventilator-associated pneumonia: do they make a difference?. Respir. Care 2012, 55, 1046–1055. [PubMed] [Google Scholar]
- Iscla I.; Wray R.; Blount P.; Larkins-Ford J.; Conery A. L.; Ausubel F. M.; Ramu S.; Kavanagh A.; Huang J. X.; Blaskovich M. A.; Cooper M. A.; Obregon-Henao A.; Orme I.; Tjandra E. S.; Stroeher U. H.; Brown M. H.; Macardle C.; van Holst N.; Ling Tong C.; Slattery A. D.; Gibson C. T.; Raston C. L.; Boulos R. A. A new antibiotic with potent activity targets MscL. J. Antibiot. 2015, 68, 453–462. 10.1038/ja.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.; Prestidge C. A.; Miesel L.; Sweeney D.; Shinabarger D. L.; Boulos R. A. Preclinical development of Ramizol, an antibiotic belonging to a new class, for the treatment of Clostridium difficile colitis. J. Antibiot. 2016, 69, 879–884. 10.1038/ja.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barh D.; Jain N.; Tiwari S.; Parida B. P.; D’Afonseca V.; Li L.; Ali A.; Santos A. R.; Guimarães L. C.; de Castro Soares S.; Miyoshi A.; Bhattacharjee A.; Misra A. N.; Silva A.; Kumar A.; Azevedo V. A novel comparative genomics analysis for common drug and vaccine targets in Corynebacterium pseudotuberculosis and other CMN group of human pathogens. Chem. Biol. Drug Des. 2011, 78, 73–84. 10.1111/j.1747-0285.2011.01118.x. [DOI] [PubMed] [Google Scholar]
- James E.; Viola H.; Hool L.; Eggers P. K.; Raston C. L.; Boulos R. A. A novel antimicrobial agent reduces oxidative stress in cells. RSC Adv. 2013, 3, 7277–7281. 10.1039/c3ra40658j. [DOI] [Google Scholar]
- Gollwitzer H.; Ibrahim K.; Meyer H.; Mittelmeier W.; Busch R.; Stemberger A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. 10.1093/jac/dkg105. [DOI] [PubMed] [Google Scholar]
- Zilberman M.; Elsner J. Antibiotic-eluting medical devices for various applications. J. Controlled Release 2008, 130, 202–215. 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Makadia H. K.; Siegel S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S. Y.; Kim T. O.; Park C. W.; Yu J. Y.; Lee B.; Lee H. S.; Kim Y. I.; Lim S. C.; Kwon Y. S. Bacterial pathogens of ventilator associated pneumonia in a tertiary referral hospital. Tuberc. Respir. Dis. 2012, 73, 32–37. 10.4046/trd.2012.73.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollef M. H. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA, J. Am. Med. Assoc. 2008, 300, 805–813. 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- Kim Y.-J.; Yang S. I.; Ryu J.-C. Cytotoxicity and genotoxicity of nano-silver in mammalian cell lines. Mol. Cell. Toxicol. 2010, 6, 119–125. 10.1007/s13273-010-0018-1. [DOI] [Google Scholar]
- AshaRani P. V.; Low Kah Mun G.; Hande M. P.; Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- Webb M. I.; Halcovitch N. R.; Bowes E. G.; Lee G. M.; Geier M. J.; Vogels C. M.; O’Neill T.; Li H.; Flewelling A.; Decken A.; Gray C. A.; Westcott S. A. Arylspiroborates derived from 4-tert-butylcatechol and 3,5-di-tert-butylcatechol and their antimicrobial activities. J. Heterocycl. Chem. 2014, 51, 157–161. 10.1002/jhet.1997. [DOI] [Google Scholar]
- Geier M. J.; Bowes E. G.; Lee G. M.; Li H.; O’Neill T.; Flewelling A.; Vogels C. M.; Decken A.; Gray C. A.; Westcott S. A. Synthesis and biological activities of arylspiroborates derived from 2,3-dihydroxynaphthalene. Heteroat. Chem. 2013, 24, 116–123. 10.1002/hc.21072. [DOI] [Google Scholar]
- Mann C. M.; Cox S. D.; Markham J. L. The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil). Lett. Appl. Microbiol. 2000, 30, 294–297. 10.1046/j.1472-765x.2000.00712.x. [DOI] [PubMed] [Google Scholar]
- Tokmaji G.; Vermeulen H.; Müller M. C.; Kwakman P. H.; Schultz M. J.; Zaat S. A. Silver-coated endotracheal tubes for prevention of ventilator-associated pneumonia in critically ill patients. Cochrane Database Syst. Rev. 2015, 8, CD009201. 10.1002/14651858.CD009201.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil K. A.; Fouad H.; Elsarnagawy T.; Almajhdi F. N. Preparation and characterization of electrospun PLGA/silver composite nanofibers for biomedical applications. Int. J. Electrochem. Sci. 2013, 8, 3483–3493. [Google Scholar]
- Simchi A.; Tamjid E.; Pishbin F.; Boccaccini A. R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine 2011, 7, 22–39. 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Hetrick E. M.; Schoenfisch M. H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.