Abstract
Introduction
One out of seven women will develop a state of chronic postoperative pain following robot-assisted hysterectomy for endometrial cancer. Recently, metabolic studies have indicated that circulating lipids and lipoproteins could act as nociceptive modulators and thereby influence the induction and perpetuation of pain. The objectives of this explorative study were (1) to examine the preoperative serologic variations in concentrations of lipids, lipoproteins, and various low‐molecular metabolites in patients with and without chronic postoperative pain after robot-assisted hysterectomy and (2) to explore if any of these serological biomarkers were predictive for development of chronic postoperative pain.
Materials and Methods
The study was designed as a nested case–control study within a cohort of women treated for endometrial cancer with robot-assisted laparoscopic hysterectomy. Twenty-six women with chronic postoperative pain were matched on age and body mass index with fifty-two controls without chronic postoperative pain, and metabolic profiling of preoperatively drawn blood samples from a biobank was performed by means of nuclear magnetic resonance spectroscopy.
Results
Nineteen metabolites, including cholesterol, cholesteryl ester, linoleic acid, phospholipids, lipids, and triglycerides had statistically significant higher concentrations in a subgroup of patients who would develop chronic postoperative pain on a later stage compared to the group of patients who would not develop chronic postoperative pain (p < 0.05). A sparse Partial Least Squares-Discriminant Analysis model explained 38.1% of the variance and had a predictive accuracy of 73.1%.
Conclusions
This explorative study substantiates the hypothesis that certain lipids, lipoproteins, and fatty acids are associated with chronic postoperative pain.
Keywords: Chronic postoperative pain, endometrial cancer, robot-assisted laparoscopic hysterectomy
Introduction
The prevalence of chronic postoperative pain is highly procedure specific ranging from 12.3% after caesarean section,1 20.0% after total knee replacement,2 30.0% after hernia repair,3 47.0% after mastectomy4 and thoracotomy,5 and as high as 52.6% after limb amputation.6
A recent study demonstrated a prevalence of chronic postoperative pain of 14.9% in patients undergoing robot-assisted laparoscopic hysterectomy due to endometrial cancer and identified preoperative pelvic pain and high levels of acute postoperative pain intensity as independent risk factors for chronic postoperative pain.7
The surgical stress response following induced tissue injury triggers a physiologic cascade of cytokine-mediated immunologic and endocrinologic alterations as well as a sympathoadrenal response with increased cortisol secretion, thereby altering metabolism of glucose and circulating plasma lipids.8–11 The deafferentation of visceral nerves further elicits a release of sensitizing and proinflammatory mediators, lowering the excitatory threshold of nerve endings and thereby increasing the peripheral pain sensitivity.8,12
An emerging theory in pro- and antinociceptive factors suggests that lipoproteins like polyunsaturated fatty acids may have opposing effects on the inflammatory and nociceptive reactions via modulation of the cellular membrane microdomain composition.13 The theory has further been substantiated by studies showing an association between the occurrence of lower back pain and certain lipid profile compositions.14,15 Likewise, an association between chronic pain conditions and dyslipidemia has been demonstrated.16–18
The objectives of this explorative study were (1) to examine the preoperative serologic variations in concentrations of lipids, lipoproteins, and various low‐molecular metabolites by means of nuclear magnetic resonance (NMR) spectroscopy and (2) to explore if any of these serological biomarkers were predictive for development of chronic postoperative pain after robot-assisted hysterectomy.
Materials and Methods
Design
A nested case–control study within a cohort of women treated for endometrial cancer with robot-assisted laparoscopic hysterectomy.
The Study Cohort
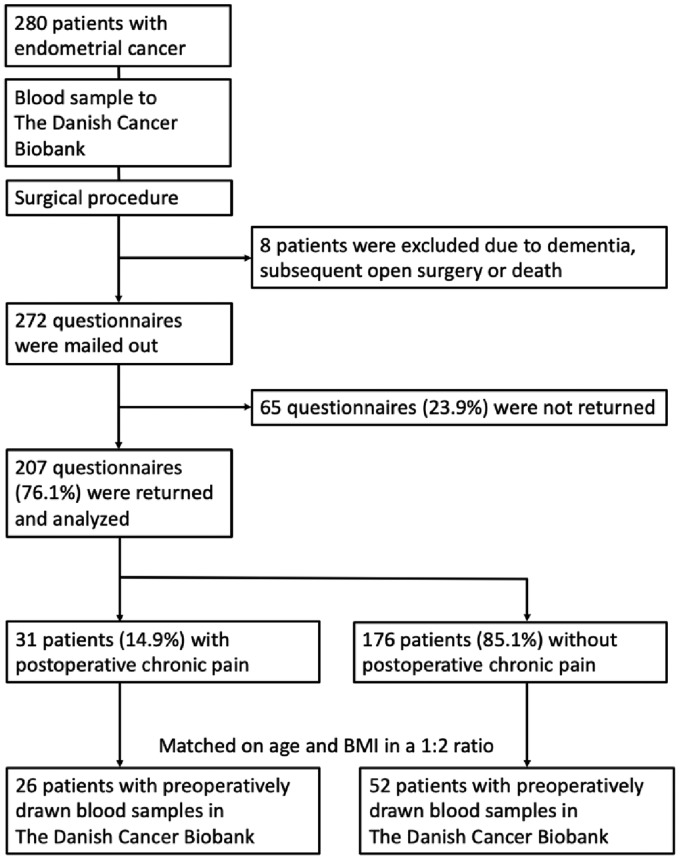
A recently published, questionnaire-based study defined a cohort of two hundred and seven women from a total of two hundred and eighty women, who all underwent robot-assisted laparoscopic hysterectomy at The Department of Obstetrics and Gynecology, Aalborg University Hospital, Aalborg, Denmark from 1 January 2010 till 31 July 2015 due to endometrial cancer.7 The study identified thirty-one women with chronic postoperative pain (defined as persistent, moderate to severe pain with a mean intensity of ≥3 on a Visual Analogue Scale 6 months after the surgical procedure), equal to a prevalence of 14.9% (Figure 1). The inclusion criteria were Danish speaking women, aged 18 to 85 years, diagnosed with endometrial cancer and scheduled for robot-assisted laparoscopic hysterectomy and bilateral salpingo-oophorectomy, while the exclusion criteria were conversion of the robot-assisted laparoscopic procedure to open surgery, subsequent open surgery, use of cannabis or opioids, and neurologic or mental disorders.
Figure 1.
Flow chart of the study.
The Treatment Algorithm and Surgical Procedure
The women were preoperatively stratified in risk categories based on the histologic type and grade according to the joint guideline from The European Society for Medical Oncology (ESMO), The European Society of Gynecological Oncology (ESGO), and The European Society for Radiotherapy and Oncology (ESTRO).19 The low-risk cases were treated with hysterectomy and bilateral salpingo-oophorectomy, intermediate-risk cases with additional pelvic lymphadenectomy, and high-risk cases with additional pelvic and paraaortic lymphadenectomy. All surgical procedures were performed on Da Vinci™ Si robotic systems (Intuitive Surgical Inc., Sunnyvale, CA, USA). All cases were postoperatively reviewed and staged at multidisciplinary tumor board meetings.
Blood Samples from The Danish Cancer Biobank
Since 2009, all cancer patients in Denmark have been offered to have blood and tissue samples stored in The Danish Cancer Biobank (DCB), part of a national collaboration between public hospitals entitled The Bio- and Genome Bank Denmark. Following an informed consent, the biologic materials are stored according to the Danish Data Protection Agency procedures. Twenty-six of the thirty-one women with chronic postoperative pain from the cohort had preoperatively drawn blood samples stored in the DCB.
Matching of Cases and Controls
The twenty-six cases with chronic postoperative pain were matched in a 1:2 ratio with fifty-two controls from the cohort without chronic postoperative pain, who also had preoperatively drawn blood samples stored in the DCB. The subjects were matched on age at the time of surgery and body mass index (BMI) in kg/m2 (Figure 1).
The NMR Spectroscopy
The metabolic profiles and biomarkers were quantified from serum samples using high‐throughput NMR metabolomics (Nightingale Health Ltd., Helsinki, Finland). This method provides simultaneous quantification of routine lipids, lipoprotein subclass profiling with lipid concentrations within fourteen subclasses, fatty acid composition, and various low‐molecular metabolites, including amino acids, ketone bodies, and gluconeogenesis-related metabolites, in molar concentration units. Details of the experimentation and applications of the NMR metabolomics platform have been described previously elsewhere.20
Approvals
The study was approved by The North Denmark Region Committee on Health Research Ethics (N-20150028), The Danish Data Protection Agency (2008–58-0028), and The Bio- and Genome Bank Denmark.
Statistical Analysis
In order to investigate any inherent clustering in the data and reduce possible overfitting and noise, we preprocessed the entire data set by using principal component analysis (PCA). Subsequent supervised modeling by sparse Partial Least Squares-Discriminant Analysis (sPLS-DA) was built in order to assign the respective metabolites for each group. The sPLS-DA algorithm can be used to effectively reduce the number of variables (metabolites) in high-dimensional metabolomics data to produce robust and easy-to-interpret models.21 For evaluation of the classification performance, fivefold cross-validation together with the receiver operating characteristic curve was chosen. With this model, the error rate was calculated to evaluate the performances.
Additionally, four machine learning algorithms (random forest, linear support vector machine, PLS-DA, and logistic regression) were applied to develop prediction models for chronic postoperative pain based on the identified metabolomic biomarkers. Finally, a permutation test was used to indicate whether the specific classification model was superior to random classifiers. All multivariate statistics, including unsupervised PCA and supervised sPLS-DA, were performed using MetaboAnalystR 2.0 packages.22
Results
Metabolic Profile Variance
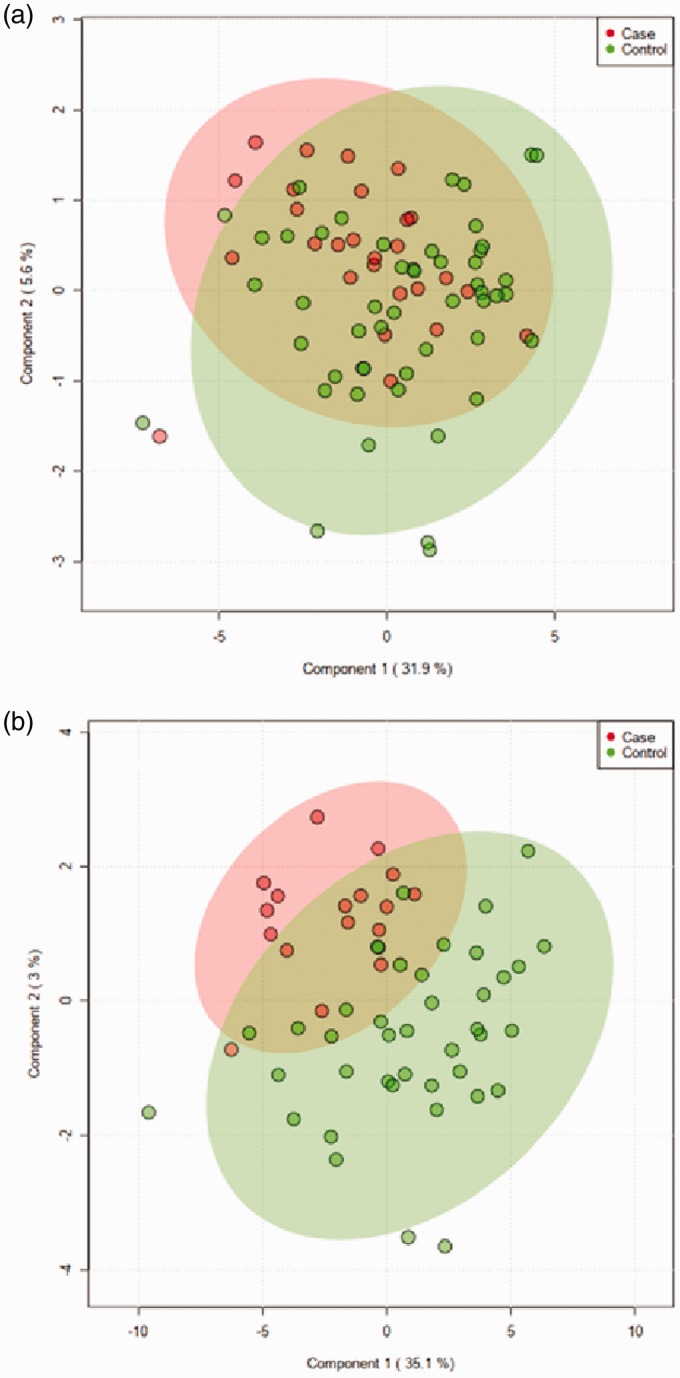
To compare the overall variation of metabolic profiles between the cases with chronic postoperative pain and controls without chronic postoperative pain, a classification model was built by the supervised sPLS-DA. The sPLS-DA model built on twenty-six cases and fifty-two controls showed no separation between the two groups (Figure 2(a)). The validation parameters calculated for this model had low values (data not shown).
Figure 2.
sPLS-DA score plot of the serum metabolome from the cases with chronic postoperative pain (red dots) and controls without chronic postoperative pain (green dots). (a) The sPLS model was used to discriminate between twenty-six cases and fifty-two controls. (b) The sPLS-DA model constructed for seventeen cases and forty-two controls after data set was synchronized.
Of the seventy-eight women in the cohort, fifty women were classified accordingly to the ESMO-ESGO-ESTRO guidelines as low risk, nine women as intermediate risk, and nineteen women as high risk based on histologic type and grade. In order to obtain a more synchronized data set, the high-risk cases were removed, and a second sPLS-DA model was built on the remaining seventeen cases and forty-two controls with low- and intermediate-risk assessment. A discrimination between the two groups could now be seen in the sPLS-DA score plots; even though the separation was not complete, and regions were overlapping, there was a visible tendency that allowed for clustering of case and control groups (Figure 2(b)). The sPLS-DA model explained 38.1% of the variance with the first two components and had a predictive accuracy of 73.1%.
Identification of Detected Metabolites
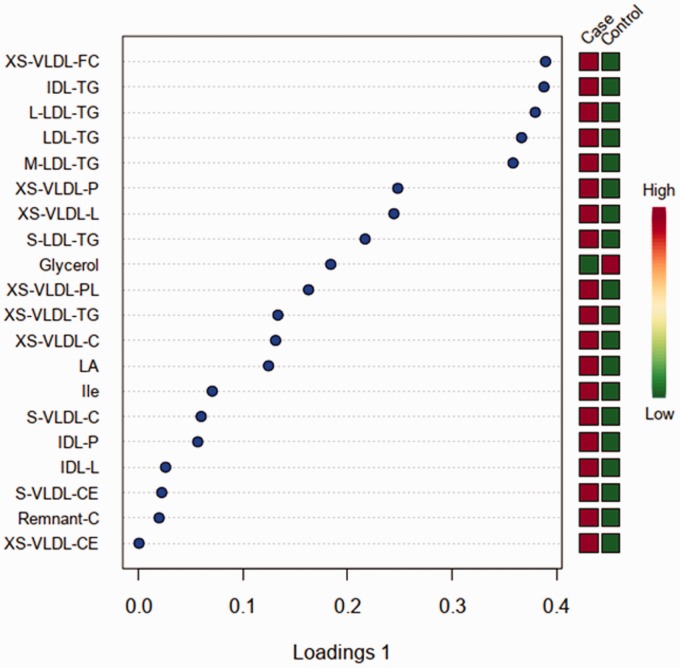
A total of one hundred and forty-seven metabolites were identified and classified in ten groups of cholesterol, glycerides and phospholipids, apolipoproteins, fatty acids, amino acids, glycolysis-related metabolites, ketone bodies, fluid balance, inflammation, and lipoproteins (see table in Supplementary material). Twenty metabolites which belong to fatty acids, amino acids, glycolysis-related metabolites, and lipoprotein groups were identified as the most influential factors for the differentiation between case and control groups. Of these, nineteen metabolites, including branched-chain amino acids, cholesterol, cholesteryl ester, free cholesterol, linoleic acid (LA), phospholipids, serum lipids, and triglycerides, demonstrated statistically significant higher concentrations in the case group than in the control group (p < 0.05), while the concentrations of glycerol was statistically significantly lower in the case group than in the control group (p < 0.05). A loading plot of these twenty metabolites was constructed, showing to which degree each metabolite contributed to the discrimination between the case and control groups (Figure 3).
Figure 3.
Loading plots from the cases with chronic postoperative pain and controls without chronic postoperative pain. High concentrations are depicted as red boxes, while low concentrations are depicted as green boxes.
LDL: low-density lipoproteins; IDL: intermediate-density lipoproteins; HDL: high-density lipoproteins; VLDL: very-low-density lipoproteins; XS-VLDL-FC: free cholesterol in very small VLDL; IDL-TG: triglycerides in IDL; L-LDL-TG: triglycerides in large LDL; LDL-TG: triglycerides in LDL; M-LDL-TG: triglycerides in medium LDL; XS-VLDL-P: concentration of very small VLDL particles; XS-VLDL-L: total lipids in very small VLDL; S-LDL-TG: triglycerides in small LDL; XS-VLDL-PL: phospholipids in very small VLDL; XS-VLDL-TG: triglycerides in very small VLDL; XS-VLDL-C: cholesterol in very small VLDL; LA: linoleic acid; Ile: isoleucine; S-VLDL-C: cholesterol in small VLDL; IDL-P: concentration of IDL particles; IDL-L: total lipids in IDL; S-VLDL-CE: cholesteryl esters in small VLDL; Remnant-C: remnant cholesterol, that is, non-HDL, non-LDL cholesterol; XS-VLDL-CE: cholesteryl esters in very small VLDL.
Fourteen of these metabolites were found to be the leading contributing metabolites correlated to chronic postoperative pain, based on a combination of high loadings scores and valid area under the curve (AUC) values (equal or higher than 0.7). These metabolites were cholesterol in very small, very-low-density lipoprotein (XS-VLDL-C), free cholesterol in very small, very-low-density lipoprotein (XS-VLDL-FC), glycerol, isoleucine, LA, particles in very small, very-low-density lipoprotein (XS-VLDL-P), phospholipids in very small, very-low-density lipoprotein (XS-VLDL-PL), total lipid in very small, very-low-density lipoprotein (XS-VLDL-L), triglycerides in intermediate-density lipoproteins (IDL-TG), triglycerides in large low-density lipoprotein (L-LDL-TG), triglycerides in low-density lipoprotein (LDL-TG), triglycerides in medium low-density lipoprotein (M-LDL-TG), triglycerides in small low-density lipoprotein (S-LDL-TG), and triglycerides in very small, low-density lipoprotein (XS-VLDL-TG). Their detailed information was summarized in Table 1.
Table 1.
Area under the curve, p value, and log2 fold change for a set of 14 metabolites.
| Metabolite | Area under the curve | p value | Log2 fold change |
|---|---|---|---|
| IDL-TG | 0.80 | 0.01 | 0.37 |
| LDL-TG | 0.80 | 0.01 | 0.35 |
| L-LDL-TG | 0.80 | 0.01 | 0.35 |
| M-LDL-TG | 0.79 | 0.01 | 0.34 |
| S-LDL-TG | 0.79 | 0.01 | 0.36 |
| XS-VLDL-FC | 0.78 | 0.01 | 0.30 |
| XS-VLDL-TG | 0.76 | 0.01 | 0.40 |
| XS-VLDL-L | 0.75 | 0.01 | 0.30 |
| XS-VLDL-P | 0.75 | 0.01 | 0.26 |
| Glycerol | 0.74 | 0.01 | –0.48 |
| XS-VLDL-PL | 0.73 | 0.01 | 0.28 |
| XS-VLDL-C | 0.73 | 0.01 | 0.28 |
| LA | 0.71 | 0.01 | 0.18 |
| Ile | 0.70 | 0.02 | 0.30 |
LDL: low-density lipoproteins; IDL: intermediate-density lipoproteins; VLDL: very-low-density lipoproteins; IDL-TG: triglycerides in IDL; LDL-TG: triglycerides in LDL; L-LDL-TG: triglycerides in large LDL; M-LDL-TG: triglycerides in medium LDL; S-LDL-TG: triglycerides in small LDL; XS-VLDL-FC: free cholesterol in very small VLDL; XS-VLDL-TG: triglycerides in very small VLDL; XS-VLDL-L: total lipids in very small VLDL; XS-VLDL-P: concentration of very small VLDL particles; XS-VLDL-PL: phospholipids in very small VLDL; XS-VLDL-C: cholesterol in very small VLDL; LA: linoleic acid; Ile: isoleucine.
Prediction Models for Chronic Postoperative Pain
The fourteen metabolites mentioned above were further applied to build prediction models for the detection of chronic postoperative pain through four machine learning algorithms (random forest, linear support vector machine, PLS-DA, and logistic regression). All four algorithms exhibited high AUC values (0.79–0.87) and coefficients of variation prediction (0.70–0.77) (Table 2).
Table 2.
Prediction models based on a set of fourteen metabolites distinguishing case from control groups.
| Algorithm | Area under the curve | Coefficient of variation prediction | p value |
|---|---|---|---|
| PLS-DA | 0.79 (0.53–0.93) | 0.70 | 0.01 |
| Linear support vector | 0.87 (0.69–0.97) | 0.77 | <0.001 |
| Logistic regression | 0.80 (0.54–0.97) | 0.74 | 0.005 |
| Random forest | 0.82 (0.70–0.93) | 0.71 | <0.001 |
Data in parentheses represent 95% confidence intervals. PLS-DA: Partial Least Squares-Discriminant Analysis.
Discussion
The present study demonstrated a significant difference in the preoperative metabolic profile in a subgroup of patients, who would later develop chronic postoperative pain compared to patients without, thereby supporting the hypothesis of circulating lipids and lipoproteins as nociceptive modulators and possible predictors for development of chronic postoperative pain. The predictive capabilities were shown in four predictive models using a set of fourteen metabolites of cholesterol, lipoproteins, and fatty acids closely correlated to chronic postoperative pain.
The study further showed that the preoperative levels of serum cholesterol in very small, very-low-density lipoprotein (XS-VLDL-C) and free cholesterol in very small, very-low-density lipoprotein (XS-VLDL-FC) were significantly associated to chronic postoperative pain after robot-assisted hysterectomy for endometrial cancer. Both metabolites were approximately 22% higher in the subgroup which developed chronic postoperative pain compared to the subgroup that did not develop chronic postoperative pain. This correlation between cholesterol metabolites and chronic postoperative pain is noteworthy, as cholesterol has been found to modulate the nociceptive capacity of opioid receptors in the cellular membrane.23,24 Moreover, studies have shown that animals with high cholesterol levels require less opioids to achieve the similar analgesic effect compared to animals with low cholesterol levels.25,26 A similar, negative correlation was shown in humans in a study of the required opioid doses for pain management in lung cancer patients.27
The delicate balance between pro- and antinociceptive lipoproteins and polyunsaturated fatty acids modulates the cellular membrane microdomain composition.13 Fatty acids can be divided into omega − 3 and omega − 6 fatty acids, where the first mentioned mainly have an antinociceptive capacity and the latter a pronociceptive capacity.28 The principal omega − 3 fatty acid derivatives are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), while the omega − 6 fatty acid derivatives are arachidonic acid and LA.29,30 A study by Ramsden et al.31 showed that a diet-induced reduction in the level of circulating LA in patients suffering from chronic headaches resulted in reduced frequency and severity of headaches. Furthermore, a high dietary intake of LA was shown to increase the abundance of LA derivatives and reduce the abundance of EPA and DHA, thus promoting a pronociceptive environment in the tissue.28 Likewise, a high dietary intake of LA was shown in rodents to induce hyperalgesia and allodynia.29,32,33
In the present study, significantly elevated serum concentrations of LA and other omega-6 fatty acids were shown in the preoperatively drawn blood samples among patients, who would develop chronic pain postoperatively. This indicates a preexisting, pronociceptive serologic environment in these patients, consequently increasing the susceptibility to induction and perpetuation of chronic pain.
Limitations
As mentioned above, this study was of an explorative nature. The study cohort was based on a retrospective questionnaire which has an inherent risk of recall bias. Moreover, there was risk of selection bias due to the questionnaire responder/nonresponder rate. Furthermore, it is important to note that the subgroup analysis only contained data from the patients with low- and intermediate-risk assessment which reduces the generalizability. Finally, the study was conducted at a single center which also could reduce the generalizability of the results.
Conclusion
This explorative study substantiates the hypothesis of certain lipids, lipoproteins, polyunsaturated fatty acids, and derivatives may be predictive for development of chronic pain in accordance with recent studies. The present study, however, is the first to demonstrate this association to chronic postoperative pain.
Supplemental Material
Supplemental material, sj-pdf-1-mpx-10.1177_1744806920923885 for Chronic Postoperative Pain After Hysterectomy for Endometrial Cancer: A Metabolic Profiling Study by Søren Lunde, Hien TT Nguyen, Kristian K Petersen, Lars Arendt-Nielsen, Henrik B Krarup and Erik Søgaard-Andersen in Molecular Pain
Acknowledgment
The Bio- and Genome Bank Denmark is acknowledged for biological material and for data regarding handling and storage.
Author Contributions
All authors contributed to the design of the study. SL conducted the study and collected the data. SL and HTTN performed the statistical analysis and wrote the draft of the manuscript. All authors contributed to the interpretation and discussion of results. Comments on the manuscript and approval of the final version were given by all authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an unrestricted research grant by The Danish Cancer Society (R134-RP13048).
ORCID iD
Søren Lunde https://orcid.org/0000-0001-7948-907X
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Nikolajsen L, Sørensen HC, Jensen TS, Kehlet H. Chronic pain following Caesarean section. Acta Anaesthesiol Scand 2004; 48: 111–116. [DOI] [PubMed] [Google Scholar]
- 2.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012; 2: e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, Smith W. Chronic pain and quality of life following open inguinal hernia repair. Br J Surg 2001; 88: 1122–1126. [DOI] [PubMed] [Google Scholar]
- 4.Gärtner R, Jensen M-B, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009; 302: 1985–1992. [DOI] [PubMed] [Google Scholar]
- 5.Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain 2014; 15: 887–897. [DOI] [PubMed] [Google Scholar]
- 6.Nikolajsen L, Finnerup NB, Kramp S, Vimtrup A-S, Keller J, Jensen TS. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology 2006; 105: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 7.Lunde S, Petersen KK, Kugathasan P, Arendt-Nielsen L, Søgaard-Andersen E. Chronic postoperative pain after robot-assisted laparoscopic hysterectomy for endometrial cancer. J Gynecol Surg 2019; 35: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet (London, England) 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 9.Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000; 85: 109–117. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Tian Y, Wang Z-F, Liu S-B, Mi W-L, Ma H-J, Wu G-C, Wang J, Yu J, Wang Y-Q. Involvement of the spinal NALP1 inflammasome in neuropathic pain and aspirin-triggered-15-epi-lipoxin A4 induced analgesia. Neuroscience 2013; 254: 230–240. [DOI] [PubMed] [Google Scholar]
- 11.Sheeran P, Hall GM. Cytokines in anaesthesia. Br J Anaesth 1997; 78: 201–219. [DOI] [PubMed] [Google Scholar]
- 12.Villarreal CF, Funez MI, Cunha F de Q, Parada CA, Ferreira SH. The long-lasting sensitization of primary afferent nociceptors induced by inflammation involves prostanoid and dopaminergic systems in mice. Pharmacol Biochem Behav 2013; 103: 678–683. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 2008; 47: 147–155. [DOI] [PubMed] [Google Scholar]
- 14.Heuch I, Heuch I, Hagen K, Zwart JA. Do abnormal serum lipid levels increase the risk of chronic low back pain? The Nord-Trøndelag Health Study. PLoS One 2014; 9: e108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starkweather A, Julian T, Ramesh D, Heineman A, Sturgill J, Dorsey SG, Lyon DE, Wijesinghe DS. Circulating lipids and acute pain sensitization: an exploratory analysis. Nurs Res 2017; 66: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodson NJ, Smith BH, Hocking LJ, McGilchrist MM, Dominiczak AF, Morris A, Porteous DJ, Goebel A. Cardiovascular risk factors associated with the metabolic syndrome are more prevalent in people reporting chronic pain: results from a cross-sectional general population study. Pain 2013; 154: 1595–1602. [DOI] [PubMed] [Google Scholar]
- 17.Fayaz A, Watt HC, Langford RM, Donaldson LJ. The association between chronic pain and cardiac disease: a cross-sectional population study. Clin J Pain 2016; 32: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 18.Üçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum 2006; 54: 2656–2664. [DOI] [PubMed] [Google Scholar]
- 19.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C, ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer 2016; 26: 2–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -Omic Technologies. Am J Epidemiol 2017; 186: 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lê Cao K-A, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics 2011; 12: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong J, Yamamoto M, Xia J. MetaboAnalystR 2.0: from raw spectra to biological insights. Metabolites 2019; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.André A, Gaibelet G, Le Guyader L, Welby M, Lopez A, Lebrun C. Membrane partitioning of various delta-opioid receptor forms before and after agonist activations: the effect of cholesterol. Biochim Biophys Acta 2008; 1778: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 24.Qiu Y, Wang Y, Law P-Y, Chen H-Z, Loh HH. Cholesterol regulates micro-opioid receptor-induced beta-arrestin 2 translocation to membrane lipid rafts. Mol Pharmacol 2011; 80: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfaro MJ, Ormazábal MJ, García-Arroba L, Martín MI. Cholesterol-fed rabbits: study of the response of the vas deferens to adrenergic and non-adrenergic stimulus and to a kappa-opioid agonist. J Pharm Pharmacol 1996; 48: 433–436. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Zou H, Liu X, Chu J, Zhou Y, Loh HH, Law P-Y. Cholesterol level influences opioid signaling in cell models and analgesia in mice and humans. J Lipid Res 2012; 53: 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, Liang L, Li L, Xu M, Li X, Sun H, He S, Lin L, Zhang Y, Song Y, Yang M, Luo Y, Loh HH, Law P-Y, Zheng D, Zheng H. Opioid doses required for pain management in lung cancer patients with different cholesterol levels: negative correlation between opioid doses and cholesterol levels. Lipids Health Dis 2016; 15: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsden CE, Ringel A, Majchrzak-Hong SF, Yang J, Blanchard H, Zamora D, Loewke JD, Rapoport SI, Hibbeln JR, Davis JM, Hammock BD, Taha AY. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: implications for idiopathic pain syndromes? Mol Pain 2016; 12: 174480691663614–174480691663638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci USA 2009; 106: 18820–18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morisseau C, Inceoglu B, Schmelzer K, Tsai H-J, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res 2010; 51: 3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, Hibbeln JR, Loewke J, Smith S, Coble R, Suchindran C, Gaylord SA. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials 2011; 12: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green DP, Ruparel S, Roman L, Henry MA, Hargreaves KM. Role of endogenous TRPV1 agonists in a postburn pain model of partial-thickness injury. Pain 2013; 154: 2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest 2010; 120: 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mpx-10.1177_1744806920923885 for Chronic Postoperative Pain After Hysterectomy for Endometrial Cancer: A Metabolic Profiling Study by Søren Lunde, Hien TT Nguyen, Kristian K Petersen, Lars Arendt-Nielsen, Henrik B Krarup and Erik Søgaard-Andersen in Molecular Pain