Abstract
Background:
Stem cell therapy is an emerging treatment for tendon disorders.
Purpose:
To systematically review the efficacy of stem cell therapy for patients with tendon disorders.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
MEDLINE/PubMed, EMBASE, CINAHL, CENTRAL, PEDro, and SPORTDiscus; trial registers; and gray literature were searched to identify randomized controlled trials (RCTs) and non-RCTs, cohort studies, and case series with 5 or more cases. Studies investigating any type of stem cell therapy for patients with tendon disorders were eligible if they included patient-reported outcome measures or assessed tendon healing. Risk of bias was assessed through use of the Cochrane risk of bias tools.
Results:
This review included 8 trials (289 patients). All trials had moderate to high risk of bias (level 3 or 4 evidence). In Achilles tendon disorders, 1 trial found that allogenic-derived stem cells led to a faster recovery compared with platelet-rich plasma. Another study found no retears after bone marrow–derived stem cell therapy was used in addition to surgical treatment. There were 4 trials that studied the efficacy of bone marrow–derived stem cell therapy for rotator cuff tears. The controlled trials reported superior patient-reported outcomes and better tendon healing. A further 2 case series found that stem cell therapy improved patient-reported outcomes in patients with patellar tendinopathy and elbow tendinopathy.
Conclusion:
Level 3 evidence is available to support the efficacy of stem cell therapy for tendon disorders. The findings of available studies are at considerable risk of bias, and evidence-based recommendations for the use of stem cell therapy for tendon disorders in clinical practice cannot be made at this time. Stem cell injections should not be used in clinical practice given the lack of knowledge about potentially serious adverse effects.
Keywords: tendinopathy, tear, injection, Achilles, shoulder
Tendon disorders are common in athletes and include tendinopathy and partial or complete tendon tears.4 Tendinopathy is characterized by pain, swelling, and impaired performance.1,26 In some sport disciplines, such as volleyball, more than 50% of athletes develop tendinopathy during their athletic career.23 The Achilles tendon is the most commonly affected tendon in runners, and approximately 30% of runners will develop Achilles tendinopathy at some point.1,26 Tendon tears represent more severe injuries than tendinopathy, and outcomes of tendon tears are often poorer, particularly for rotator cuff tears of the shoulder.20
Various treatment modalities for tendon disorders have been described, such as exercise therapy,12,29 shockwave therapy, nonsteroidal anti-inflammatory drugs, and injection therapy with, for example, corticosteroids, sclerosing substances (aethoxysclerol),31 and autologous blood products.1 Surgery may be required in patients with persistent tendon symptoms or young patients with severe tendon tears.9 Despite the abundance of available treatment modalities, outcomes of tendon disorder treatments are often not satisfactory.5
Over the past decade, several studies have shown promising results of stem cell therapy in individuals with tendon disorders.3,37 Nevertheless, a systematic review performed by our research group in 2017 concluded that there was no evidence for use of stem cell therapy in tendon disorders.33 Given the increasing use of stem cell therapy in clinical practice, the absence of evidence for or against the use of stem cell therapy in 2017, and new studies having become available on the topic in the past 2 years, an update of our study was warranted.17,18,41,48 Therefore, the main purpose of the current systematic review was to reevaluate the efficacy of stem cell therapy based on patient-reported outcome measures (PROMs) and tendon healing in individuals with tendon disorders.
Methods
Protocol Registration and Reporting
This study was registered on PROSPERO (CRD42019116828) on January 10, 2019, before any synthesis was commenced. The PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines were followed for planning, conducting, and reporting this study.28
Research Question and Inclusion and Exclusion Criteria
The research question was to assess the efficacy of stem cell therapy (any type) on PROMs and tendon healing in patients with tendon disorders (any type). Our primary outcomes were PROMs, and our secondary outcome measures were tendon healing measured with magnetic resonance imaging (MRI) or ultrasonography. The inclusion and exclusion criteria are summarized in Table 1.
Table 1.
Inclusion and Exclusion Criteriaa
| Inclusion criteria |
|
| Exclusion criteria |
|
aThese criteria were similar to the criteria previously used by Pas et al.33 We reported recurrent tendon disorders, as measured with patient-reported outcomes, as an additional outcome measurement. MRI, magnetic resonance imaging; VISA-A, Victorian Institute of Sport Assessment–Achilles questionnaire.
Search Strategy
A sensitive search strategy was used as previously described.33 This strategy was based on indexed and free text terms and composed in collaboration with a research librarian (see Appendix 1, available as supplemental material). The electronic databases MEDLINE/PubMed, CENTRAL, EMBASE, CINAHL, PEDro, and SPORTDiscus were searched from inception to November 2018 by 2 independent authors (N.A.C.v.d.B. and M.H.M.). We did not apply any restrictions to our search strategy. Reference lists of included studies were scanned to identify any additional relevant reports. In addition, we searched for ongoing trials in several national (http://www.trialregister.nl) and international (http://www.controlled-trials.com) trial registries, the WHO trial register (apps.who.int/trialsearch), the EU clinical trial register (http://www.clinicaltrialsregister.eu), and ClinicalTrials.gov. We attempted to contact primary investigators to collect additional information about trials identified through these sources. Last, we searched for unpublished studies in OpenGrey (http://www.opengrey.eu), the British Library Inside (http://explore.bl.uk/primo_library/libweb/action/search.do?vid=BLVU1), Web of Science, and BIOSIS previews (http://www.ovid.com.). Authors of potentially eligible studies were contacted for additional information on their studies.
Study Selection
After removal of duplicates, 2 authors (N.A.C.v.d.B. and M.H.M.) independently screened titles and abstracts for potential eligible studies. Subsequently, both authors independently appraised full-text content and applied our inclusion and exclusion criteria. Discrepancies in inclusion of articles were resolved by a third reviewer (M.W.).
Data Extraction
A standardized data extraction sheet was adopted from the Cochrane Collaboration Centre and modified for the purpose of our study.43 Data extraction was performed by 2 authors (N.A.C.v.d.B. and M.H.M.) independently, including study design, year of publication, duration of follow-up, study population characteristics (eg, mean age, sex distribution), tendon injury type and location, stem cell type, injection frequency, primary and secondary outcome measurements, and main results of the studies. Disagreements were resolved by a third reviewer (M.W.).
Risk of Bias Assessments
Risk of bias was assessed independently by 2 authors (N.A.C.v.d.B., M.H.M.) for each outcome in each study. The Cochrane Risk of Bias (RoB 2) tool was used to assess the 5 major domains of biases.42 This tool provides an algorithm to reach an overall risk of bias judgment per study outcome. The 2 authors independently assessed bias (1) arising from the randomization procedure, (2) due to deviations from the intended interventions, (3) due to missing outcome data, (4) in measurement of the outcome, and (5) in selection of the reported result to make an overall risk of bias judgment for each study outcome. Each domain was completed after the signaling questions as proposed by the guidance of the RoB 2 tool.
Each separate domain was labeled as “low risk of bias,” “some concerns,” or “high risk of bias.” Outcomes of randomized controlled trials (RCTs) were considered to have low risk of bias for the overall judgment when all 5 domains of bias were scored “low risk of bias.” RCT outcomes were considered to entail some concerns for the overall risk of bias if at least 1 of the 5 domains was judged as having “some concerns” about bias. RCT outcomes were considered to have high risk of bias for the overall judgment if at least 1 of the 5 domains of bias was scored as “high risk of bias.” In case of disagreement between the 2 authors, a third reviewer (M.W.) made the final decision.
The Risk Of Bias In Non-randomised Studies–of Interventions (ROBINS-I) was used to assess risk of bias in non-RCTs, and the Newcastle Ottawa Scale was used to assess the quality of case series. Using the Newcastle Ottawa Scale, a maximum of 6 stars could be awarded. Six stars indicated perfect quality, whereas 0 stars indicated very low quality. Appendices 2 through 5 provide details.
Data Synthesis
Data synthesis was planned for RCTs if at least 2 studies investigated the same treatment comparison and studies were clinically homogeneous (ie, identical stem cell type and tendon disorder, cointerventions, mode of injection or transplant). The mean difference expressed the comparative efficacy if studies used the same outcome measure. Standardized mean differences expressed comparative efficacy for studies that used different measures to estimate treatment effects. P values less than .05 were considered significant for all analyses. Fixed-effects models were planned to pool data that were statistically homogeneous or when fewer than 3 studies were available for data synthesis. We used random effects models if statistical heterogeneity was present and when 3 or more studies were available. If heterogeneity was present and a sufficient number of studies were available (n ≥ 10), we planned a subgroup analysis or meta-regression analysis to explore potential sources for heterogeneity (age and sex). Descriptive synthesis was performed when data pooling was not possible.
Levels of Evidence
Levels of evidence were assigned to further specify the strength of the studies as proposed by the Oxford Centre for Evidence-Based Medicine (Table 2).44 Following this system, RCTs at low risk of bias were graded as level 2 evidence, non-RCTs at low risk of bias were considered level 3 evidence, and case series were considered level 4 evidence. The level of evidence of individual studies could be downgraded based on study imprecision, risk of bias, and study indirectness or because of small effect sizes. The level of evidence of individual studies could be upgraded based on large effect size.
Table 2.
Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence for Interventionsa
| Level 1 | Systematic reviews |
| Level 2 | Randomized controlled trials with low/moderate risk of bias or observational studies with dramatic effect |
| Level 3 | Non–randomized controlled trials with low/moderate risk of bias or randomized controlled trials at high risk of bias |
| Level 4 | Case series, case-control studies, historically controlled studies, or non–randomized controlled trials at high risk of bias |
| Level 5 | Mechanism-based reasoning/expert opinion |
aFrom Oxford Centre for Evidence-Based Medicine.44
Results
Study Selection
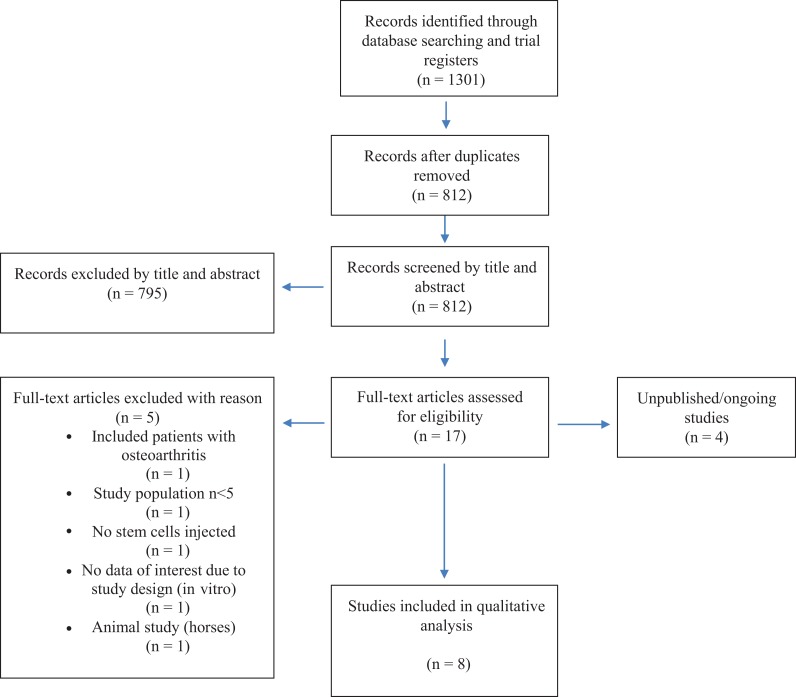
We included 8 trials, including 4 recent trials that were not included in our 2017 systematic review.16,23,41,48 Figure 1 shows the study flow diagram. We identified 4 unpublished trials in trial registries.15,39,40,47 We made attempts to contact the authors of these trials by email. The authors of 1 trial replied and reported that their trial was under review.9 The authors of the other 3 trials did not provide information about the status of their trials.
Figure 1.
Study flowchart based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Study Characteristics
Of the 8 studies included in this review, 1 was an RCT,48 3 were non-RCTs,14,17,18 and 4 were case series.12,22,34,41 These studies focused on stem cell therapy for tendon disorders of the lateral elbow22 and rotator cuff12,14,17,18 and the Achilles41,48 and patellar tendon,34 including a total of 289 patients (135 males, 154 females; age range, 14-74 years). The study populations consisted of athletes and nonathletes. Study characteristics are summarized in Table 3.
Table 3.
Study Characteristicsa
| Lead Author (Year) | Study Design | Sample Size | Injury Type | Demographicsb | Treatment | Outcome Measures | Follow-up | Level of Evidence |
|---|---|---|---|---|---|---|---|---|
| Usuelli48 (2018) | RCT | Group 1: n = 23 Group 2: n = 21 |
Achilles tendinopathy | Group 1 (C)
Group 2 (I)
|
Group 1: PRP Group 2: allo-ASC |
|
6 mo | 3 |
| Stein41 (2015) | Case series | N = 27 | Achilles tendon rupture |
|
BMAC |
|
24 mo | 4 |
| Kim18 (2017) | Non-RCT | Group 1: n = 35 Group 2: n = 35 |
Rotator cuff tear | Group 1 (C)
Group 2 (I)
|
Group 1: arthroscopic rotator cuff repair Group 2: allo-ASC |
|
24 mo | 3 |
| Hernigou14 (2014) | Non-RCT | Group 1: n = 45 Group 2: n = 45 |
Rotator cuff tear | Group 1 (C)
Group 2 (I)c |
Group 1: surgical repair with an arthroscopic protocol Group 2: MSC |
|
10 y | 3 |
| Kim17 (2018) | Non-RCT | Group 1: n = 12 Group 2: n = 12 |
Partial rotator cuff tear | Group 1 (C)
Group 2 (I)
|
Group 1: rotator cuff strengthening exercise Group 2: BMAC-PRP |
|
3 mo | 3 |
| Ellera Gomes12 (2012) | Case series | N = 14 | Rotator cuff tear |
|
BMMC |
|
12 mo | 4 |
| Pascual-Garrido34 (2012) | Case series | N = 8 | Patellar tendinopathy |
|
BMMC |
|
24 mo | 4 |
| Lee22 (2015) | Case series | N = 12 | Lateral epicondylar tendinopathy |
|
Allo-ASC |
|
52 wk | 4 |
aAOFAS, American Orthopaedic Foot and Ankle Society; ASC, adipose-derived stem cell; ASES, American Shoulder and Elbow Surgeons; ATRS, Achilles Tendon Rupture Score; BMAC, bone marrow aspirate concentrate; BMMC, bone marrow mononuclear cells; C, comparison group; I, intervention group; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MEPI, Mayo Elbow Performance Index; MMT, manual muscle test; MRI, magnetic resonance imaging; MSC, bone marrow-derived mesenchymal stem cells; PRP, platelet-rich plasma; RCT, randomized controlled trial; ROM, range of movement; SF-12, 12-Item Short Form Health Survey (mental and physical); SF-36, 36-Item Short Form Health Survey questionnaire; UCLA, University of California, Los Angeles; VAS, visual analog scale; VISA-A, Victorian Institute of Sport Assessment–Achilles questionnaire.
bAge is expressed in years as mean or mean ± SD, with range (if available) in parentheses. Data for female participants are expressed as n (%).
cAge and sex were not clearly described; the authors only mentioned there was no significance difference with comparison group.
Of the 8 studies, 3 studies evaluated the efficacy of allogenic adipose-derived stem cells (allo-ASCs).18,22,48 The efficacy of bone marrow mononuclear stem cells (BMMCs) was investigated in 2 studies,12,34 the efficacy of bone marrow aspirate concentration (BMACs) in 2 studies,17,41 of which 1study combined BMACs with platelet-rich plasma (PRP).17 Efficacy of stem cell therapy was measured using PROMs in 7 of the 8 studies.12,17,18,22,34,41,48 In 1 study,14 the investigators assessed efficacy of treatment using MRI and ultrasonography. In 7 of the 8 studies,12,14,17,18,22,34,48 investigators evaluated tendon healing using MRI or ultrasonography. Due to study heterogeneity, descriptive data synthesis was performed.
Risk of Bias in Individual Trial Outcomes
Domain-based risk of bias assessments for the non-RCTs and RCTs are presented in Table 4. The RCTs examined the efficacy of stromal vascular fraction (allo-ASC) versus PRP injection in patients with tendinopathy based on PROMs, MRI, and ultrasonography.48 High risk of bias was found for the overall judgment. The non-RCTs were found at high or moderate risk of bias for all PROMs. Ultrasound outcomes were found at moderate risk of bias for the overall judgment. Only MRI outcomes were found at low risk of bias in 1 non-RCT that investigated the efficacy of allo-ASC injection versus arthroscopic rotator cuff repair.18 Quality of case series varied; they received between 2 and 5 stars out of 6 stars in total. See Appendices 2 and 3 for all risk of bias judgements.
Table 4.
Risk of Bias Judgment for Randomized and Nonrandomized Controlled Trialsa
| Randomized Controlled Trial | |||
|---|---|---|---|
| Usuelli et al48 (2018) | |||
| Outcome |
Primary: VAS, VISA-A, AOFAS, SF-36 Secondary: MRI, US |
||
| Intervention vs control groups |
Intervention: allo-ASC injection Control: PRP injection |
||
| Risk of bias | |||
| Bias arising from the randomization process | Some concerns | ||
| Bias due to deviations from intended interventions | High risk | ||
| Bias due to missing outcome data | Low risk | ||
| Bias in measurement of the outcome |
Intervention: some concerns Control: low risk |
||
| Bias in selection of the reported result | Some concerns | ||
| Overall risk of bias judgment | High risk | ||
| Nonrandomized Controlled Trials | |||
| Kim et al17 (2018) | Kim et al18 (2017) | Hernigou et al14 (2014) | |
| Outcome |
Primary: VAS, MMT, ASES Secondary: US |
Primary: VAS, ROM, UCLA, Constant score Secondary: MRI |
Primary: PROMs (not measured) Secondary: MRI, US |
| Intervention vs control groups |
Intervention: BMAC-PRP injection Control: Exercise |
Intervention: allo-ASC with arthroscopy Control: Arthroscopy |
Intervention: MSC injection with arthroscopy Control: Arthroscopy |
| Risk of bias | |||
| Bias due to confounding | VAS, MMT, ASES: moderate risk US: low risk |
VAS, ROM, UCLA, Constant: moderate risk MRI: low risk |
Low risk |
| Bias in selection of participants into the study | Low risk | Moderate risk | Moderate risk |
| Bias in classification of interventions | Low risk | Low risk | Moderate risk |
| Bias due to deviations from intended interventions | Low risk | Low risk | Low risk |
| Bias due to missing data | Low risk | Low risk | Low risk |
| Bias in measurement outcomes | VAS, MMT, ASES: serious risk US: moderate risk |
VAS, ROM, UCLA, Constant: moderate risk MRI: low risk |
Moderate risk |
| Bias in selection of the reported result | Moderate risk | Low risk | Low risk |
| Overall risk of bias judgment | VAS, MMT, ASES: high risk US: moderate risk |
VAS, ROM, UCLA: moderate risk MRI: low risk |
Moderate risk |
aRisk of bias judgments apply to all study outcomes listed unless otherwise specified. AOFAS, American Orthopaedic Foot and Ankle Society; ASC, adipose-derived stem cell; ASES, American Shoulder and Elbow Surgeons; BMAC, bone marrow mononuclear stem cell; MMT, manual muscle test; MRI, magnetic resonance imaging; MSC, bone marrow-derived mesenchymal stem cells; PROM, patient-reported outcome measure; PRP, platelet-rich plasma; ROM, range of movement; SF-36, 36-Item Short Form Health Survey questionnaire; UCLA, University of California, Los Angeles; US, ultrasonography; VAS, visual analog scale; VISA-A, Victorian Institute of Sports Assessment–Achilles questionnaire.
Efficacy of Stem Cell Injections for Tendon Disorders
Achilles Tendon Disorders
Achilles Tendinopathy
Usuelli et al48 examined the efficacy of allo-ASC injection versus PRP injection in patients with noninsertional Achilles tendinopathy with a total follow-up of 6 months. At 15 days after treatment, the mean American Orthopaedic Foot and Ankle Society (AOFAS) score was significantly higher in the allo-ASC group than in the PRP group (80 vs 67, respectively; P < .05). The mean visual analog scale (VAS) score was significantly lower in the allo-ASC group than the PRP group at 15 days after treatment (2.5 vs 4.5, respectively; P < .05). There was no difference between the groups in the Victorian Institute of Sports Assessment–Achilles questionnaire (VISA-A) score at 15 days of follow-up (P > .05). At a follow-up of 30 days, the mean VAS pain score was 2.0 in the allo-ASC group and 4.0 in the PRP group (P < .05). The mean VISA-A score was 60 in the allo-ASC group and 47 in the PRP group (P < .05). The AOFAS score was 80 in the allo-ASC group and 70 in the PRP group (P > .05). After 60 days of follow-up, there were no differences in PROMs between patients treated with allo-ASC and PRP injection.
Achilles Tendon Tears
Stein et al41 examined the outcomes of primary Achilles tendon rupture repair with BMAC augmentation. In that study, 27 patients underwent open repair procedures augmented with BMAC injection. All patients could walk without a boot at 1.8 ± 0.7 months and participated in light activity at 3.4 ± 1.8 months, and 25 of 27 patients (92%) returned to sport at 5.9 ± 1.8 months. At a mean follow-up of 29.7 ± 6.1 months, no reruptures of Achilles tendons were reported. The mean Achilles Tendon Rupture Score at final follow-up was 91 points (range, 72-100 points).
Level of Evidence
There is level 3 evidence for a superior effect of allo-ASC injections for Achilles tendinopathy compared with PRP injections on PROMs at 15 and 30 days after injection, but no differences were observed at 60 days of follow-up. There is level 4 evidence for improved PROMs and absence of reruptures 2.5 years after open Achilles tendon repair with BMAC augmentation.
Rotator Cuff Tendon Tears
We identified 3 non-RCTs that examined the efficacy of stem cell injections in rotator cuff tendon tears. The first trial determined the efficacy of allo-ASC injection during arthroscopic rotator cuff repair, comparing the double-row suture bridge technique with injection versus the double-row suture bridge technique alone in patients with rotator cuff tears.18 At 21 months of follow-up, the mean Constant score was 78.3 ± 14.9 in the intervention group and 80.1 ± 13.2 in the control group (P = .634). The mean University of California, Los Angeles (UCLA) Shoulder Rating Scale score was 29.8 ± 5.1 in the intervention group and 30.5 ± 4.8 in the control group (P = .302). The mean VAS pain score at rest was 0.4 ± 0.6 for the allo-ASC group and 0.3 ± 0.5 for the control group (P = .776). The VAS pain score during motion was 2.4 ± 1.1 in the allo-ASC group and 2.1 ± 0.9 in the control group (P = .256). At minimum of 12 months after intervention, a retear rate of 28.5% was found with MRI in the control group versus 14.3% in the stem cell injection group (P < .001). Complete healing of the tendon, as measured with MRI, was observed in 85.7% of patients in the intervention group versus 71.4% of patients in the control group.
The second trial evaluated the efficacy of bone marrow–derived mesenchymal stem cells (MSCs) injection as an adjunct to single-row rotator cuff arthroscopy in comparison with single-row rotator cuff arthroscopy alone in patients diagnosed with rotator cuff tears.14 The total follow-up was 10 years. In the BMAC group, significantly fewer retears were reported after 10 years of follow-up as measured by MRI and ultrasonography (13% vs 56%, respectively; P < .05).
The third trial studied the efficacy of an injection with BMAC-PRP versus exercise therapy in patients diagnosed with a rotator cuff tear with a total follow-up of 3 months.17 At 3 weeks postoperatively, mean VAS pain scores were 2.3 ± 0.8 in the BMAC-PRP group and 3.6 ± 2.3 in the control group (P = .147). The mean American Shoulder and Elbow Surgeons (ASES) score was 74.1 ± 8.4 in the BMAC-PRP group versus 62.2 ± 12.2 in the control group at final follow-up (P = .011). The mean VAS pain score was 1.9 ± 0.7 in the intervention group and 3.7 ± 1.8 in the control group at final follow-up (P = .039).
In addition, 1 case series examined the efficacy of BMMC augmentation in patients undergoing surgery for complete rotator cuff tears. In that series, 14 consecutive patients were treated with transosseous stitches through mini-open incision and subsequent BMMC injection obtained from the iliac crest.12 The minimum follow-up was 12 months. The UCLA score was used as main outcome; this score increased from 12 ± 3.0 to 31 ± 3.2, and no statistical analysis was performed in this study.
Level of Evidence
There is level 3 evidence for a superior effect of allo-ASC injection augmentation for rotator cuff tears compared with arthroscopic double-row suture bridge technique alone on tendon healing and retears as measured by MRI. However, no significant differences have been observed between surgical rotator cuff repair with and without allo-ASC augmentation on PROMs (level 3). There is level 3 evidence for a superior effect of arthroscopic single-row rotator cuff repair with BMMC augmentation in comparison with arthroscopic single-row rotator cuff repair for retears as measured by MRI and ultrasonography. There is level 4 evidence for a superior effect of BMAC-PRP on rotator cuff tears compared with exercise therapy for the ASES score and VAS pain score at 3 months after injection. Table 2 provides more information about the level of evidence.
Patellar Tendinopathy
One case series studied the efficacy of BMMC injection for patients with chronic patellar tendinopathy (lasting at least 6 months).34 The follow-up time of the patients was at least 24 months. The following PROMs increased significantly over time: Tegner Activity Scale score (P = .006), International Knee Documentation Committee score (P = .047), and Knee injury and Osteoarthritis Outcome Score (KOOS) for the subdomains Activities of Daily Living (P = .008) and Function during Sports/Recreation (P = .0078). No statistical improvement was observed for the Lysholm score (P = .1043), KOOS for Pain (P = .2399), KOOS for Quality of Life (P = .0825), and 12-item Short Form Health Survey questionnaire for mental and physical domains (P = .5589 and P = .438, respectively). Tendon structures, as assessed with ultrasonography, did not change between baseline and follow-up.
Level of Evidence
There is level 4 evidence for improved PROMs after BMMC injections for patients with chronic patellar tendinopathy at least for 24 months of follow-up.
Lateral Elbow Tendinopathy
Lee et al22 studied the efficacy of an allo-ASC injection in a case series of patients with lateral elbow tendinopathy. The total follow-up period was 52 weeks. The Mayo Elbow Performance Index was 64.0 ± 13.5 at baseline and 87.1 ± 11.6, 89.2 ± 6.8, 92.1 ± 6.1, and 90.6 ± 5.8 after 6, 12, 23, and 52 weeks, respectively. Compared with baseline, these were significant improvements at all follow-up points (P = .002). The VAS pain score during motion was 66.8 ± 14.6 at baseline, 42.1 ± 23.2 at 6 weeks after injection (P = .004), 31.1 ± 20.6 at 12 weeks (P = .002), 15.3 ± 13.7 at 26 weeks (P = .002), and 14.8 ± 13.1 at final follow-up at 52 weeks (P = .002). The VAS scores after 26 and 52 weeks were also significantly lower than those at 6 weeks (P = .006 and P = .008, respectively) and at 12 weeks after injection (P = .013 and P = .034, respectively). For ultrasound evaluation of the tendon structure, the largest average defect area was 6.46 ± 3.37 mm2 at baseline, 2.34 ± 1.42 mm2 after 26 weeks, and 3.06 ± 1.32 mm2 (P = .003) after 52 weeks for the longitudinal axis. For the transverse axis, the average largest defect area was 8.14 ± 3.99 mm2 at baseline, 3.36 ± 1.94 mm2 after 26 weeks (P = .002), and 4.31 ± 2.10 mm2 after 52 weeks (P = .015).
Level of Evidence
There is level 4 evidence for improved PROMs and ultrasound evaluation of the tendon healing after allo-ASC injection at all follow-up periods.
Discussion
The purpose of this systematic review was to evaluate the evidence of stem cell therapy in the treatment of tendon disorders. To date, the efficacy of stem cell therapy has been examined in 8 studies of Achilles tendon, rotator cuff tendon, and patellar tendon disorders and lateral elbow tendinopathy.12,14,17,18,22,34,41,48 We found level 3 evidence for the superior effect of stem cell therapy compared with treatment without stem cells. This update shows that there is still a moderate to high risk of bias for studies evaluating stem cell therapy for tendon disorders.
How Does the Evidence for Stem Cell Therapy Compare With the Evidence for Other Treatments for Tendon Disorders?
Level 3 evidence for the use of stem cell therapy in tendon disorders implies that we cannot yet recommend its use in clinical practice. Except in the trial by Kim et al,18 stem cell therapy has not been compared with commonly used therapies such as exercise therapy or extracorporeal shockwave therapy (ESWT). Moreover, the duration of follow-up was relatively short in the trial by Kim et al.17 This makes it challenging to judge whether stem cell therapy should be preferred over conventional treatments in the management of tendon disorders.
Compared with stem cell therapy, other therapies have a larger body of evidence underpinning their efficacy, although their evidence is also moderate to low. A recent systematic review on the treatment of Achilles tendinopathy concluded that eccentric exercises may be better than wait-and-see and traditional physiotherapy.29 The evidence for this was, respectively, very low and low. Another commonly applied intervention for Achilles tendinopathy is ESWT. The recent systematic review by Korakakis et al19 reported that low-level evidence is available to show ESWT comparable with eccentric training and superior to wait-and-see. Those investigators also concluded that moderate evidence exists for ESWT being equal to sham ESWT for individuals with patellar tendinopathy. A systematic review published in 2016 described the results of eccentric training for tendinopathy of the rotator cuff and lateral elbow tendinopathy.32 It concluded that eccentric training may be more effective in treating an upper extremity condition than any other nonoperative form of therapy. However, this systematic review also lacked strong evidence.
All of the aforementioned systematic reviews suggested that the evidence for tendon disorder treatment is at best moderate to low. Yet, we do not recommend stem cell therapy as an alternative to these therapies. This is because of the invasive character of stem cells and their potential side effects. In the sections below, we explain the working mechanism and potential adverse effects of stem cells that merit further consideration before the limited evidence is put into practice.
Theoretical Evidence for the Efficacy of Stem Cell Therapy
Stem cells may be derived from many different tissues. In the studies included in this review, cells were derived from either bone marrow (BMACs, BMMCs, MSCs) or adipose tissue (allo-ASC). In most studies, plain unsorted cells were administered with an unknown number of stem cells. Only 1 study described a correlation between the actual number of stem cells (CD34+) and treatment outcome.14
There are 2 theories on the efficacy of stem cell treatments for tendon disorders. It is thought that tendon healing is supported by paracrine effects of injected stem cells, secreting various growth factors and cytokines that may mend injured tendons.2,6,8,24,26,27 Theoretically, tendon healing may also be improved by stem cell differentiation into tenocytes. In vitro studies have shown stem cell therapies to provide tendon regeneration rather than repair of the tendon tissue.49 This regeneration includes replacement of the damaged tendon fibers with identical “new” tissue.49 Regeneration occurs in all embryos, hardly ever occurs in neonates, and is never observed in adults.46 In contrast to regeneration, tendon repair is a more rapid process encompassing the inflammatory cell cascade, the deposition of matrix, and remodeling.30 Tendon tissue regeneration is an alternative for replacement of large damaged tissue based on a combination of embryonic or adult stem cells.36
Stem Cells and Their Potential to Harm
Stem cells may cause serious harm despite their potential to heal. Recently, the Food and Drug Administration warned of serious potential risks to patients from stem cell therapies administered for uses other than hematopoietic or immunologic reconstitution.35 Numerous infections were reported after receipt of bacterially contaminated umbilical cord blood–derived stem cell products. Autologous hematopoietic stem cells injected into the kidneys of a patient with renal failure were associated with the development of tumors that eventually led to nephrectomy.45 In another instance, autologous stem cells derived from adipose tissue and injected intravitreally into the eyes of people with macular degeneration were associated with worsening vision in 3 people, 2 of whom became blind.21 A patient who underwent intrathecal mesenchymal, embryonic, and fetal neural stem cell infusions for the treatment of an ischemic stroke developed a glioma that was derived from the injected stem cells.7 In cases of stem cell administration for tendon healing, adverse effects have not been reported. As shown in bone marrow–derived stem cells and ASCs, there is risk of ectopic bone and tumor formation under special circumstances.25
Given the paucity of studies following patients with tendon disorders after a stem cell injection for prolonged periods of time, the extent to which stem cell therapy is associated with serious harm is presently unknown.
Future Directions
Due to considerable bias and lack of high-level evidence supporting the use of stem cell therapy, we are currently not able to recommend stem cell therapy for patients with tendon disorders. Many gaps in our knowledge remain with regard to safety, indications, dosage, and concentration of the stem cells and harvesting methods.10,13,14,17 There is a need for large, methodologically sound RCTs that are reported according to the CONSORT (Consolidated Standards of Reporting Trials) statement.38 Considering the available evidence, RCTs should compare stem cell therapy to evidence-based treatments like ESWT and exercise therapy.11,29 Another issue that warrants attention is the standardized evaluation and reporting of potential harmful effects of stem cell therapies. Follow-up should be at least 5 years to monitor possible severe harmful effects.
Strengths and Limitations
We used a sensitive search strategy in 6 different databases and assessed risk of bias based on the latest standards. We also included 1 trial investigating the combination of stem cell therapy with PRP injection.17 We believe this combination of PRP with stem cells is relevant in the evaluation of the efficacy of stem cell therapy for tendon disorders and, therefore, is a strength of this review. A limitation is the absence of information about ongoing studies, despite our best efforts to obtain such information.
Conclusion
Despite promising results of various individual studies on the use of stem cells in the treatment of various tendon disorders, there is currently no high-level evidence that justifies an evidence-based recommendation of stem cell therapy. Stem cell injections should not be used in clinical practice given the lack of knowledge about potentially serious adverse effects.
Supplemental Material
Supplemental Material, DS_10.1177_2325967120915857 for Efficacy of Stem Cell Therapy for Tendon Disorders: A Systematic Review by Noortje Anna Clasina van den Boom, Marinus Winters, Hidde Jacobs Haisma and Maarten Hendrik Moen in Orthopaedic Journal of Sports Medicine
Acknowledgment
The authors thank Rik J. Molenaars, MD, Harvard Medical School, Sports Medicine Center (Massachusetts General Hospital), Boston, Massachusetts, USA, for proofreading this manuscript and optimizing the use of language.
Footnotes
Supplemental Material: Appendices 1-5 for this article are available at https://journals.sagepub.com/doi/suppl/10.1177/2325967120915857
Final revision submitted December 19, 2019; accepted January 10, 2020
One or more of the authors has declared the following potential conflict of interest or source of funding: This systematic review was supported by the Dutch National Olympic Committee. However, this funding had no influence on the collection, analysis, interpretation, and writing of the current manuscript. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad Z, Henson F, Wardale J, Noorani A, Tytherleigh-Strong G, Rushton N. Regenerative techniques for repair of rotator cuff tears. J Orthop Surg. 2013;21(2):226–231. [DOI] [PubMed] [Google Scholar]
- 3. Albano JJ, Alexander RW. Autologous fat grafting as a mesenchymal stem cell source and living bioscaffold in a patellar tendon tear. Clin J Sport Med. 2011;21(4):359–361. [DOI] [PubMed] [Google Scholar]
- 4. Albers IS, Zwerver J, Diercks RL, Dekker JH, Van den Akker-Scheek I. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC. 2016;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedi A, Maak T, Walsh C, et al. Cytokines in rotator cuff degeneration and repair. J Shoulder Elbow Surg. 2012;21(2):218–227. [DOI] [PubMed] [Google Scholar]
- 7. Berkowitz AL, Miller MB, Mir SA, et al. Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism.” N Engl J Med. 2016;375(2):196–198. [DOI] [PubMed] [Google Scholar]
- 8. Branford OA, Klass BR, Grobbelaar AO, Rolfe KJ. The growth factors involved in flexor tendon repair and adhesion formation. J Hand Surg Eur Vol. 2014;39(1):60–70. [DOI] [PubMed] [Google Scholar]
- 9. Braune C, von Eisenhart-Rothe R, Welsch F, Teufel M, Jaeger A. Mid-term results and quantitative comparison of postoperative shoulder function in traumatic and non-traumatic rotator cuff tears. Arch Orthop Trauma Surg. 2003;123(8):419–424. [DOI] [PubMed] [Google Scholar]
- 10. Chen JL, Zhang W, Liu ZY, et al. Physical regulation of stem cells differentiation into teno-lineage: current strategies and future direction. Cell Tissue Res. 2015;360(2):195–207. [DOI] [PubMed] [Google Scholar]
- 11. Dimitrios S, Pantelis M, Kalliopi S. Comparing the effects of eccentric training with eccentric training and static stretching exercises in the treatment of patellar tendinopathy: a controlled clinical trial. Clin Rehabil. 2012;26(5):423–430. [DOI] [PubMed] [Google Scholar]
- 12. Ellera Gomes JL, da Silva RC, Silla LM, et al. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaspar D, Spanoudes K, Holladay C, et al. Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev. 2015;84:240–256. [DOI] [PubMed] [Google Scholar]
- 14. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811–1818. [DOI] [PubMed] [Google Scholar]
- 15. Hospital San Carlos, Madrid. Mesenchymal stem cell (MSC) included in OrthADAPT membrane for rotator cuff tears repair (msctendonrep). ClinicalTrials.gov identifier: NCT01687777. https://clinicaltrials.gov/ct2/show/NCT01687777. Published September 19, 2012. Accessed December 15, 2019.
- 16. Jiang D, Gao P, Zhang Y, et al. Combined effects of engineered tendon matrix and GDF-6 on bone marrow mesenchymal stem cell-based tendon regeneration. Biotechnol Lett. 2016;38(5):885–892. [DOI] [PubMed] [Google Scholar]
- 17. Kim SJ, Kim EK, Kim SJ, et al. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res. 2018;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim YS, Sung CH, Chung SH, et al. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018. [DOI] [PubMed] [Google Scholar]
- 19. Korakakis V, Whiteley R, Tzavara A, et al. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med. 2018;52(6):387–407. [DOI] [PubMed] [Google Scholar]
- 20. Kuriyan AE, Albini TA, Townsend JH, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376(11):1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazarides AL, Alentorn-Geli E, Choi JH, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24(11):1834–1843. [DOI] [PubMed] [Google Scholar]
- 22. Lee SY, Kim W, Lim C, et al. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015;33(10):2995–3005. [DOI] [PubMed] [Google Scholar]
- 23. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561–567. [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Zhu S, Zhang C, et al. Crucial transcription factors in tendon development and differentiation: their potential for tendon regeneration. Cell Tissue Res. 2014;356(2):287–298. [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Hindieh J, Leong DJ, et al. Advances of stem cell based-therapeutic approaches for tendon repair. J Orthop Translat. 2017;9:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22(4):675–692. [DOI] [PubMed] [Google Scholar]
- 27. Meirelles Lda S, Fontes AM, Covas DT, et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5-6):419–427. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:B2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy MC, Travers MJ, Chivers P, et al. Efficacy of heavy eccentric calf training for treating mid-portion Achilles tendinopathy: a systematic review and meta-analysis. Br J Sports Med. 2019;53:1070–1077. [DOI] [PubMed] [Google Scholar]
- 30. Oakes BW. Orthopaedic tissue engineering: from laboratory to the clinic. Med J Aust. 2004;180(5)(suppl):S35–S38. [DOI] [PubMed] [Google Scholar]
- 31. Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36(3):173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ortega-Castillo M, Medina-Porqueres I. Effectiveness of the eccentric exercise therapy in physically active adults with symptomatic shoulder impingement or lateral epicondylar tendinopathy: a systematic review. J Sci Med Sport. 2016;19(6):438–453. [DOI] [PubMed] [Google Scholar]
- 33. Pas H, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51(13):996–1002. [DOI] [PubMed] [Google Scholar]
- 34. Pascual-Garrido C, Rolon A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perkins KM, Spoto S, Rankin DA, et al. Notes from the field: infections after receipt of bacterially contaminated umbilical cord blood-derived stem cell products for other than hematopoietic or immunologic reconstitution—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67(50):1397–1399. [DOI] [PubMed] [Google Scholar]
- 36. Protzman NM, Stopyra GA, Hoffman JK. Biologically enhanced healing of the human rotator cuff: 8-month postoperative histological evaluation. Orthopedics. 2013;36(1):38–41. [DOI] [PubMed] [Google Scholar]
- 37. Ruzzini L, Longo UG, Rizzello G, et al. Stem cells and tendinopathy: state of the art from the basic science to clinic application. Muscles Ligaments Tendons J. 2012;2(3):235–238. [PMC free article] [PubMed] [Google Scholar]
- 38. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:C332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seoul National University Hospital. Treatment of tendon injury using allogenic adipose-derived mesenchymal stem cells (rotator cuff tear). ClinicalTrials.gov identifier: NCT02298023. https://clinicaltrials.gov/ct2/show/NCT02298023. Posted November 21, 2014. Accessed December 15, 2019.
- 40. Seoul National University Hospital. Treatment of tendon injury using mesenchymal stem cells (ALLO-ASC). ClinicalTrials.gov identifier: NCT01856140. https://clinicaltrials.gov/ct2/show/NCT01856140. Posted May 17, 2013. Accessed December 15, 2019.
- 41. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901–905. [DOI] [PubMed] [Google Scholar]
- 42. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 43. The Cochrane Collaboration. Data extraction forms. https://dplp.cochrane.org/data-extraction-forms. Accessed August 22, 2019.
- 44. The Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence. https://www.cebm.net/index.aspx?o=5653. Accessed August 22, 2019.
- 45. Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol. 2010;21(7):1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toda A, Okabe M, Yoshida T, et al. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105(3):215–228. [DOI] [PubMed] [Google Scholar]
- 47. University College, London. Autologous stem cells in Achilles tendinopathy (ASCAT). ClinicalTrials.gov identifier: NTC0206406. https://clinicaltrials.gov/show/NCT02064062. Posted February 17, 2014. Accessed December 15, 2019.
- 48. Usuelli FG, Grassi M, Maccario C, et al. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2000–2010. [DOI] [PubMed] [Google Scholar]
- 49. Young M. Stem cell applications in tendon disorders: a clinical perspective. Stem Cells Int. 2012;2012:637836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, DS_10.1177_2325967120915857 for Efficacy of Stem Cell Therapy for Tendon Disorders: A Systematic Review by Noortje Anna Clasina van den Boom, Marinus Winters, Hidde Jacobs Haisma and Maarten Hendrik Moen in Orthopaedic Journal of Sports Medicine