Abstract
Background
Insulin resistance is an important defect associated with obesity and type 2 diabetes mellitus. Many studies have been reported that dietary fiber exerts beneficial metabolic effects. Resistant dextrin is a soluble fiber. The aim of this study was to investigate the effects of resistant dextrin on high-fat-high-fructose diet induced obese mice and to explore the underlying mechanisms.
Methods
Seventeen 4-week-old male C57BL/6 J mice were fed a normal diet (ND) or HFHFD for 22 weeks, and were gavaged with resistant dextrin (5 g/kg) for 10 weeks. Glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed, serum fasting insulin (FINS) and serum biochemical parameters were determined, the contents of triglyceride (TG) and total cholesterol (TC) in liver tissues were determined by enzymatic method. The pathological changes in liver were detected by HE staining. Real time PCR and Western blot were used to detect the expression of insulin signaling pathway and the fatty acid β oxidation pathway related genes and proteins respectively. The gut microbiota were analyzed via 16 s rRNA sequencing.
Results
Resistant dextrin significantly decreased serum FINS, improved serum lipid profiles, reduced the contents of liver TG and TC. The insulin signaling pathway and the fatty acid β oxidation pathway were promoted. The abundance of metabolically beneficial bacteria such as Prevotella and Akkermansia in the intestinal flora of the resistant dextrin group were increased.
Conclusions
Resistant dextrin can significantly ameliorate liver insulin resistance, improve serum lipid levels, as well as reduce hepatic lipid deposition. The modulation of gut microbiota might be responsible for the beneficial effects of resistant dextrin.
Keywords: Dietary fiber, Resistant dextrin, Insulin resistance, Fatty acid beta oxidation, Gut microbiota
Background
In recent decades, the incidence and prevalence of type 2 diabetes mellitus (T2DM) has been dramatically increased, contributing great burden to global health economies [1]. Insulin resistance (IR) is an important defect associated with obesity and T2DM, which is defined as ‘a relative impairment in the ability of insulin to exert its effects on glucose and lipid metabolism in target tissues’ [2]. Improving insulin sensitivity is an available strategy for the management of T2DM.
More and more studies have been reported that dietary fiber exerts many beneficial metabolic effects, including improvement of IR and lowing the risk of developing T2DM. A recent meta-analysis reported that higher intakes of fiber are associated with a reduced risk of diabetes [3]. The effects are associated with decreased gastric emptying, reduced blood cholesterol levels, enhanced production of short chain fatty acids (SCFAs) and modulated the composition of the gut microbiota.
Though substantial evidence has shown the beneficial effect of dietary fiber for health, there is still a gap between recommendations and intake worldwide, due the fact that it is difficult to get enough fiber from a traditional diet. Therefore, supplementing the extracts derived from fruits and vegetables or synthetic non-digestible carbohydrates may be an effective way to increase dietary fiber intake [4]. Various fiber subtypes such as oligosaccharides, inulin, β-glucan and pectin are well-studied. However, because of the production of gases, they often induce intestinal discomfort even at small dosage. Compare to other fibers, resistant dextrin showed better tolerance [5–8]. Resistant dextrin is a soluble fiber, derived from wheat or corn starch and is prepared by highly controlled partial hydrolysis and repolymerization of the dextrinization process. Animal studies showed that resistant dextrin could stimulate gut mucosal immunity and prevent colitis in piglets [9]. Clinical trials demonstrated that supplementation with resistant dextrin for 12 weeks alleviates insulin resistance and improves determinants of metabolic syndrome in overweight men [10]. In addition, a daily supplement of 10 g resistant dextrin improves insulin resistance and inflammation in women with type 2 diabetes [11].
Although a few clinical studies have reported that resistant dextrin exerts beneficial effects on insulin resistance and T2DM, the evidence is insufficient and the mechanisms have not yet been elucidated. The aim of this study was to investigate the effects of resistant dextrin on HFHFD induced obese mice, especially to observe its effects on insulin sensitivity and lipid metabolism, and to explore the underlying mechanisms.
Methods
Animals and treatment
Four-week-old male C57BL/6 mice were purchased from Shanghai slac corporation. The mice were housed under a constant temperature (22 ± 2 °C) and 12-h light/dark cycle and maintained on a normal diet with clean water ad libitum for 1 week. Mice were randomly divided into two groups and fed a normal diet (ND,10% fat, n = 5) or high-fat-high-fructose-diet (HFHFD, 35.5% fat, n = 12) for 12 weeks. Then the HFHFD group were randomly divided into the high-fat-high-fructose diet plus resistant dextrin (HFHFD+RD) group and the HFHFD group, with six mice in each group. The mice in the two groups were gavaged with resistant dextrin 5 g/kg body weight or distilled water respectively for 10 weeks. Both groups continued the HFHFD. The ND group were still on a normal diet, and they were gavaged with distilled water for 10 weeks. During the intervention, food intake was recorded, body weight and fasting blood glucose (FBG) were measured.
Glucose and insulin tolerance tests
Glucose tolerance test (GTT) was performed at the end of the intervention, mice were fasted 16 h. After measuring the baseline blood glucose level via a tail nick using a glucometer (Contour TS, Bayer, Germany), 2 g/kg glucose was administered via gavage, and glucose levels were measured at different time points of 15, 30, 60 and 120 min after glucose administration.1 week later, insulin tolerance test (ITT) was performed. Mice were fasted 6 h and they were injected intraperitoneally with recombinant human insulin (Novo Nordisk, Denmark) at 1 U/kg and their blood glucose concentrations were measured 0, 15, 30, 60 and 120 min after insulin injection.
Sampling
Blood was collected after mice were sacrificed, centrifuged at 3000 rpm for 15 min at 4 °C, and stored at − 80 °C before serum profile analysis. The liver tissues were removed, weighted, frozen in liquid nitrogen, and stored at − 80 °C.
Blood parameters
Serum FINS levels were detected using ELISA kit (EZRMI-13 K, Merckmilipore). HOMA-IR was calculated according to the formula FBG (mmol/L)*FINS (μU/L)/22.5. Serum TG, TC, Low-density lipoprotein cholesterol (LDL-Ch) and High-density lipoprotein cholesterol (HDL-Ch) levels were determined by automatic biochemical analyzer (ROCHE COBASc702).
Hepatic TG and total TC content
Hepatic TG and total TC content were determined using a commercially available kit (GPO-POD, Applygen Technologies Inc., Beijing, China) according to the manufacturer’s protocols.
Hematoxylin-eosin (H&E) staining
Take fresh liver tissue samples fixed, dehydrated and embedded in paraffin. After that, 4–6 μm serially sections were taken on a tissue microtome for routine HE staining, and pathological changes of liver tissue were observed under microscope.
Quantitative real-time PCR analysis
Total RNA was extracted using Trizol Reagent (Invitrogen). cDNA was synthesized from total RNA using the kit (Takara) according to the manufacturer’s instructions. Quantitative RT-PCR was carried out in triplicate using a real time PCR system (Applied Biosystems). The β-actin gene was used as an endogenous control, for normalization of gene expression levels. The relative gene expression levels were assessed by using the 2-ΔΔCt method. The primer sequences are listed in Table 1.
Table 1.
The primer sequences
| Primer | Sequences (5′ to 3′) |
|---|---|
| β-actin-F | GTGCTATGTTGCTCTAGACTTCG |
| β-actin-R | ATGCCACAGGATTCCATACC |
| PPARα-F | CACGCATGTGAAGGCTGTAA |
| PPARα-R | GCTCCGATCACACTTGTCG |
| CPT1A-F | AACCCAGTGCCTTAACGATG |
| CPT1A-R | GAACTGGTGGCCAATGAGAT |
| FXR-F | TTCCTCAAGTTCAGCCACAG |
| FXR-R | TCGCCTGAGTTCATAGATGC |
| ACOX-F | TAACTTCCTCACTCGAAGCCA |
| ACOX-R | AGTTCCATGACCCATCTCTGTC |
Western blot analysis
Liver tissue were lysed in RIPA buffer containing a protease inhibitor (Beyotime Institute of Biotechnology, China). 30 μg of total protein were separated using a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and followed by the transfer of proteins to a PVDF membrane. After blocking with 5% skim milk, membranes were incubated with the appropriate primary antibodies overnight at 4 °C. Primary antibodies including anti-phospho-IRS-1Ser612(CST), anti-IRS-1(CST), anti-phospho-AKTSer473(CST), anti-AKT (CST), anti-Glut-2 (Abclonal), anti-Sirt1(Abcam), anti-PPARα (Abclonal), anti-CPT1α(Abcam), anti-AMPK (Abcam). Blots were incubated with an appropriate horseradish conjugated secondary antibody (Beyotime Institute of Biotechnology, China) at room temperature for 1 h. Antibody bound protein was detected by ECL (Merkmillipore) and quantified by scanning densitometry. Relative protein expression was normalized to Tublin expression.
Gut microbiota composition
The 16 s rRNA analysis of the fecal samples was performed by OE Biotech Co., Ltd. (Shanghai, China). Fecal samples were used for DNA extraction with the QIAxtractor (QIAGEN). The V3–V4 region of the 16 s rRNA gene was amplified. The original double-ended sequence was de-interleaved using Trimmomatic software, and the de-duplexed double-ended sequence was spliced using FLASH software. Using of UCHIME to detect and remove chimeric sequences. After the sequencing data was preprocessed to generate high-quality sequences, the Vsearch software was used to classify the sequences into multiple OTUs based on the similarity of the sequences. A parameter with a sequence similarity greater than or equal to 97% is classified as an OTU unit. Use the QIIME package to pick out the representative sequences of the individual OTUs, and compare all the representative sequences to the database, using the Silva (version123) database alignment. The PCA principal component analysis was used to analyze the differences between the samples. Using linear discriminant analysis effect size (LEfSe), linear discriminant analysis (LDA) was used to determine the groups with significant differences in genus or higher taxonomic levels between groups of mouse populations.
Statistical analysis
For statistical analysis, we used the SPSS Statistical Package (version 22.0, SPSS Inc., Chicago, IL). Data followed normal distribution were presented as mean ± SD, and were analyzed by One-way ANOVA. Data which were not normally distributed were presented as median with interquartile range and were analyzed by Kruskal-Wallis test. P values< 0.05 were considered statistically significant.
Results
Effects of resistant dextrin on body weight and food intake
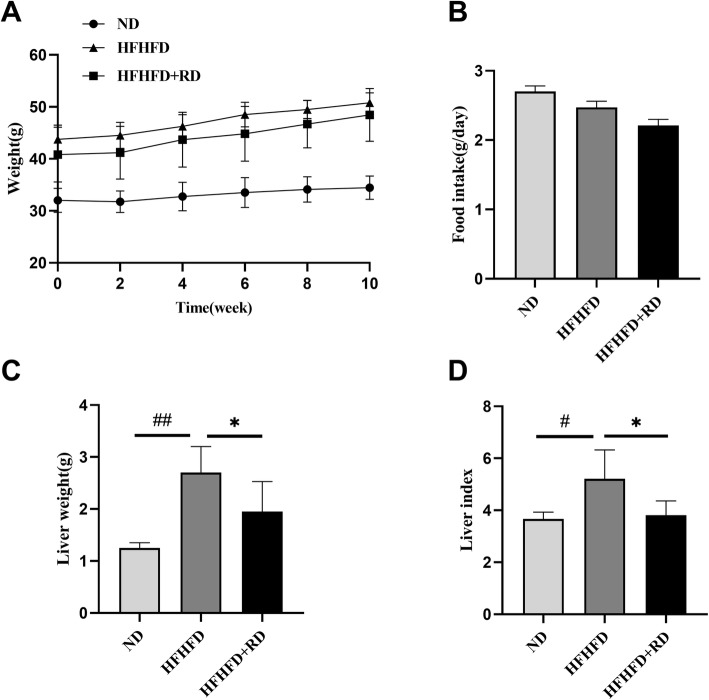
After 12 weeks, the HFHFD-fed mice weighted significantly (P<0.001, Fig.S1A), and the level of FBG were also significantly increased (P<0.001, Fig.S1B), indicating that the obesity mice model was successfully established. After the supplement of resistant dextrin for 10 weeks, HFHFD+RD mice gained progressively less body weight compared with HFHFD mice, under the circumstances of no food intake changing (Fig. 1a-b). As for the liver weight, data showed a decreased liver weight and a reduced liver to body weight ratio in HFHFD+RD mice when compared with HFHFD mice (P<0.05, Fig. 1c-d).
Fig. 1.
Effects of resistant dextrin (RD) on body weight and food intake. Body weight and food intake during the intervention of 10 weeks in the normal diet (ND) mice, HFHFD mice and HFHFD plus RD (HFHFD+RD) mice (a and b). Liver weight and liver index at the end of the intervention (c and d). Results were expressed as mean ± standard deviation. *P<0.05 vs. HFHFD group, #P<0.05, ##P<0.001 vs. ND group
Resistant dextrin ameliorated insulin resistance induced by HFHFD feeding in mice and enhanced insulin signaling pathway in the liver
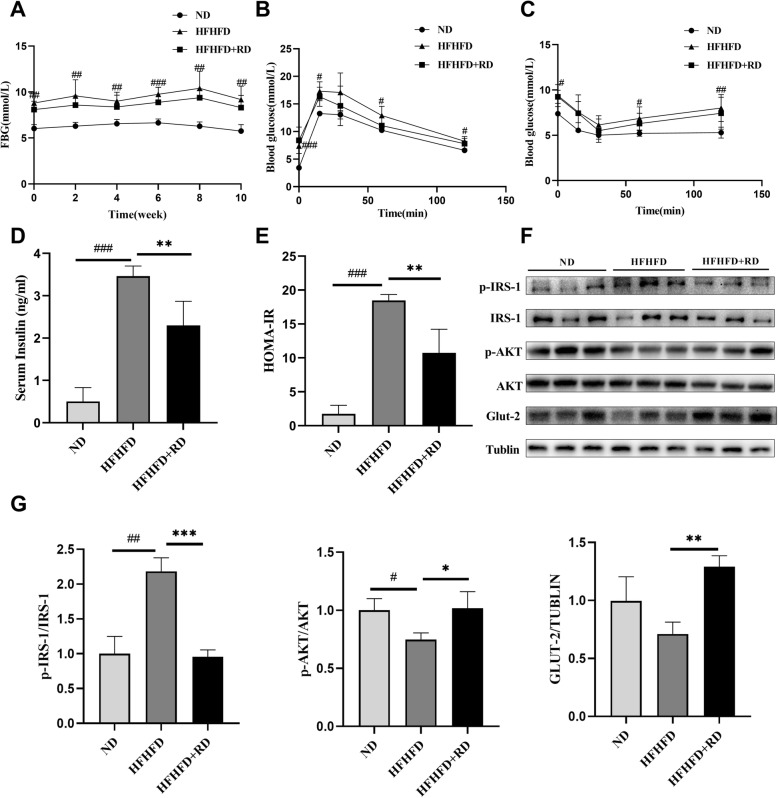
We measured FBG during the intervention and found that the levels of FBG in the HFHFD group was significantly higher than that in the ND group, however, the supplementation of resistant dextrin reduced the FBG levels induced by HFHFD (Fig. 2a). Similarly, the oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) showed a significant higher blood glucose in the HFHFD group compared to the ND group, while a decreasing trend in the HFHFD+RD group though there was no significant difference (Fig. 2b-c). In addition, serum insulin levels and HOMA-IR were significantly decreased after the supplement of resistant dextrin (Fig. 2d-e). As shown in Fig. 2f-g, the levels of insulin signaling pathway related proteins p-IRS-1ser612/IRS-1 were remarkably increased, p-AKTser473/AKT and GLUT-2 proteins were notably decreased in HFHFD group. Supplementation of resistant dextrin decreased the level of p-IRS-1ser612/IRS-1 and increased p-AKTser473/AKT and GLUT-2 protein levels.
Fig. 2.
Resistant dextrin ameliorated insulin resistance induced by HFHFD feeding in mice. a The level of FBG during the intervention. b Oral glucose tolerance test (OGTT) was performed at the end of the intervention. c Insulin tolerance test was performed at the end of the intervention. d Changes in fasting serum insulin of three groups. e Changes in HOMA-IR of three groups. f Western blot assay of insulin signaling pathway related proteins in the liver. g Relative levels of insulin signaling pathway related proteins of p-IRS-1, p-AKT and GLUT-2 were determined by normalizing protein expressions versus IRS-1, AKT and tublin expression. Results were expressed as mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs. HFHFD group. #P<0.05, ##P<0.01 and ###P<0.001 vs. ND group
Resistant dextrin improved serum lipid levels and reduced hepatic lipid deposition
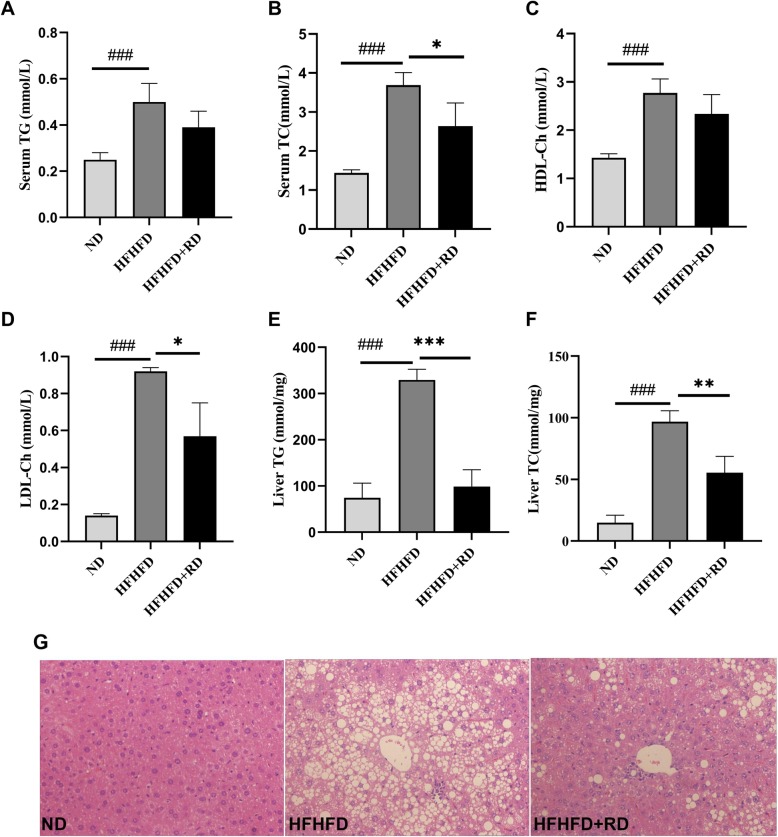
Serum TG, TC, LDL-Ch and HDL-Ch were significantly higher in the HFHFD group, and 10 weeks supplement of resistant dextrin reduced serum TC and LDL-Ch significantly, indicating that resistant dextrin improved blood lipid levels (Fig. 3a-d). The results of liver TG and TC contents demonstrated that resistant dextrin reduced hepatic lipid deposition, as the contents of TG and total TC were significantly reduced compared with the HFHFD group (Fig. 3e-f). The results of H&E staining (Fig. 3g) further revealed that the sizes and numbers of hepatic lipid droplets were decreased and fatty degeneration was alleviated in the HFHFD+RD group. This was consistent with the results of hepatic TG and TC contents.
Fig. 3.
Resistant dextrin improved serum lipid levels and reduced hepatic lipid deposition. a Serum TAG, b serum TC, c serum HDL-Ch, d serum LDL-Ch levels in three groups. e Hepatic TAG and f total TC contents. g H&E staining of liver morphology in three groups (200× magnification). Results were expressed as mean ± standard deviation. *P<0.05,**P<0.01 and ***P<0.001 vs. HFHFD group. ###P<0.001 vs. ND group
Resistant dextrin promoted fatty acid β oxidation in the liver
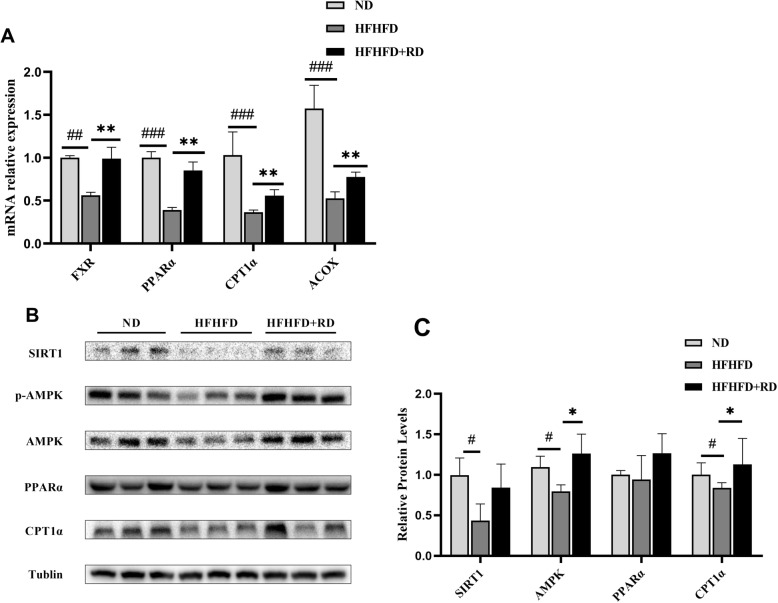
Relative to mice in the HFHFD group, mice in the HFHFD+RD group showed significantly changes in the expression of FXR, PPARα, CPT1α and ACOX genes, which are involved in fatty acid oxidation (Fig. 4a). We also analyzed the expression of proteins involved in β-oxidation by western blotting. As shown in Fig. 4b-c, expression level of AMPK was significantly increased in HFHFD+RD group compared with the HFHFD group. Moreover, expression levels of Sirt1, PPARα and its target protein CPT1α were higher than that in the HFHFD group.
Fig. 4.
Resistant dextrin promoted fatty acid β oxidation in the liver. a qRT-PCR analysis of genes involved in fatty acid metabolism. b Western blot assay of fatty acid β oxidation related proteins in the liver. c Relative levels of β oxidation related proteins of Sirt1, AMPK, PPARα and CPT1α were determined by normalizing protein expressions versus tublin expression. Results were expressed as mean ± standard deviation. *P<0.05 and **P<0.01 vs. HFHFD group. #P<0.05 vs. ND group
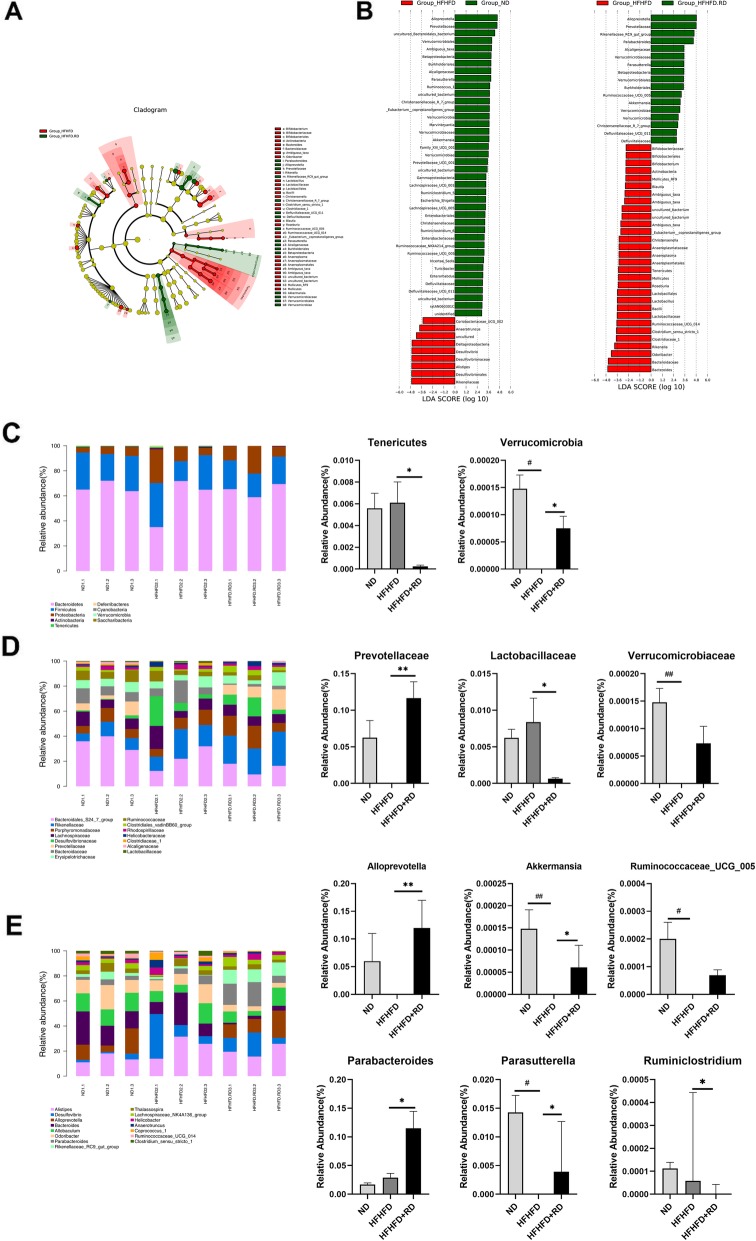
Gut microbiota composition analysis
Results from UniFrac-based principal coordinates analysis (PCoA) revealed a distinct clustering of microbiota composition for each group (Fig.S2). LEfSe analysis was carried out to identify discriminative features. The HFHFD+RD group was characterized by a higher amount of Alloprevotella and Prevotellaceae family, which is consistent with the results of ND group, while the HFHFD group was enriched by Bacteriodes genus.
To identify specific taxa related to resistant dextrin supplementation, relative abundance was assessed. At the phylum level, there was no significant differences in the ratio of Firmicutes/Bacteroidetes (Fig. S3). The relative abundance of Verrucomicrobia was notably higher compared to the HFHFD mice, and the relative abundance of Tenericutes was significantly decreased (Fig. 5c). At the family level, the results showed a lower level of Prevotellaceae and Verrucomicrobiaceae and a higher level of Lactobacillaceae in the HFHFD group. Conversely, these changes were restored in the mice of HFHFD+RD group (Fig. 5d). At the genus level, the HFHFD group presented a significantly lower level of Akkermansia, Alloprevotella, Parasutterella, Parabacteroides and Ruminococcaceae_UCG_005 but a higher level of Ruminiclostridium. While resistant dextrin treatment significantly restored the levels of the above bacteria to normal levels (Fig. 5e).
Fig. 5.
Gut microbiota composition analysis. a Cladogram generated from LEfSe analysis showing the relationship between taxon. b Linear discriminant analysis (LDA) scores derived from LEfSe analysis, showing the biomarker taxa LDA score of > 2 (the length of the bar represents the LDA score). c-e The composition of the gut microbiota in different classification levels. Data are expressed as the mean ± standard deviation or media with interquartile range. Differences were assessed by ANOVA or Kruskal-Wallis and denoted as follows, *P<0.05 vs. HFHFD group. #P<0.05 vs. ND group
Discussion
Resistant dextrin has been reported to improve insulin resistance and obesity. However, the molecular mechanisms underlying these effects remain unclear. In this study, we investigated the effects of resistant dextrin on HFHFD induced obesity mice. We found the beneficial effects of resistant dextrin on improving insulin resistance and reducing hepatic lipid deposition and these metabolic benefits of resistant dextrin may be associated with the modulation of gut microbiota.
As shown in our previous work, after 12 weeks of HFHFD feeding, mice exhibit obviously obesity and hyperglycemia [12]. In our current study, 10 weeks resistant dextrin intervention retarded the weight gain, which was under the circumstances of no change in food intake among three groups. Importantly, resistant dextrin remarkably improved hyperinsulinemia. This was consistent with the findings of previous studies showing that resistant dextrin can improve insulin resistance in overweight people or in those with type 2 diabetes [10, 11].
IRS-1-PI3K-AKT pathway is required for the maintenance of normal glucose metabolism. By activating this pathway, insulin promotes the utilization of hepatic glucose and reduces gluconeogenesis [13]. We studied the insulin signaling pathway in the liver, and found that the levels of p-AKTser473, GLUT-2 proteins were decreased in the HFHFD group, and p-IRS-1ser612 was increased. As expected, resistant dextrin up-regulated the expressions of p-AKTser473, GLUT-2 and reduced the phosphorylation of IRS-1 at ser612 site. Enhanced phosphorylation of the serine site of IRS-1 protein inhibits insulin signaling [14]. These results clearly showed that resistant dextrin successfully promoted insulin signaling pathway in the liver.
Hepatic lipid metabolism is closely linked with insulin resistance [15]. Our results showed that resistant dextrin improved serum lipid levels and reduced hepatic lipid deposition (Fig. 3). PPARɑ and FXR are crucial for fatty acid homeostasis [16, 17].Up-regulation of PPARα improves lipid metabolism [18]. Fxr−/− mice have elevated blood triglyceride and cholesterol levels [19]. In our study, we demonstrated that resistant dextrin could increase PPARα and FXR mRNA levels, as well as its target genes ACOX and CPT1α. We also demonstrated that resistant dextrin could increase the expression of PPARα and CPT1α at the protein levels. These results collectively suggest that resistant dextrin promoted fatty acid β-oxidation in the liver.
AMPK and Sirt1 play important roles in lipid homeostasis and insulin resistance. It has been reported that Sirt1 can stimulate the activation of AMPK, accelerating the decomposition of lipid to reducing liver fat accumulation [20]. Loss of liver Sirt1 leads to impaired PPARα signaling and decreased β-oxidation of fatty acids [21]. In addition, many studies have suggested that Sirt1 played a vital role in increasing insulin sensitivity. A study reported that Sirt1 agonists can improve glucose tolerance and insulin sensitivity in HFD mice [22]. It is well known that AMPK plays a central role in regulating insulin sensitivity, by reducing hepatic glucose production [23]. In our current study, we found that HFHFD obese mice treated with resistant dextrin expressed increased levels of Sirt1 and AMPK in liver. Thus, we suggest that resistant dextrin could effectively promote fatty acid β-oxidation and improve insulin sensitivity.
Dietary fiber can’t be hydrolyzed by digestive enzymes or be absorbed in the intestine, but can serve as a substrate for intestinal microbial fermentation [24], and many studies have indicated that the crucial roles of gut microbiota played in the host energy metabolism. Therefore, we employed 16 s rRNA sequencing to observe the effect of resistant dextrin on gut microbiota. PCoA analysis revealed a distinct separation in beta diversity of gut microbial communities among three groups. Besides, we analyzed the relative abundance of gut microbiota at different taxa levels. At the phylum level, Firmicutes and Bacteroidetes predominated in the majority. It is reported that the gut microbiota of obese rodent and human models display increased abundance of Firmicutes and decreased abundance of Bacteroidetes [25]. Our results showed that the Firmicutes/Bacteroidetes ratio was lower in mice treated with resistant dextrin group than that in the HFHFD group, which is positively correlated with obesity and diabetes. Interestingly, we also found that the relative abundance of Tenericutes was notably decreased by resistant dextrin supplementation, and the relative abundance of Verrucomicrobia, Verrucomicrobiaceae and Akkermansia were significantly higher compared to the HFFHD mice. Recent studies have reported that a reduction in the relative abundance of Tenericutes was detected in the inulin treated diabetic mice and rats [26, 27]. Everard et al. reported that Oligofructose increased the abundance of Akkermansia in the ob/ob mice and improved obesity-related phenotype [28], and Akkermansia is inversely correlated with metabolic disorders [29]. Supplementation of Akkermansia to HFD mice reduced obesity, decreased inflammatory markers, and improved insulin resistance [30].
Wu et al. suggested that dietary patterns affect gut microbial enterotypes. The long-term high-carbohydrate diet is characterized by Prevotella dominated enterotype, while the long-term high-protein and high-fat diet shows Bacteriodes enriched enterotype [31]. It has been reported that pectin-rich or whole grain diet enriches Prevotellaceae bacteria. Africa children with high fiber diet showed a significant enrichment in Prevotella, while European children who consume a typical low-fiber western diet lack this bacteria [32–34]. In our study, the HFHFD mice were dominated by Bacteriodes, whereas the ND mice were dominated by Allprevotella and Prevotellaceae. Importantly, mice with resistant dextrin supplementation also dominated by Allprevotella and Prevotellaceae, which is the same as the ND mice, indicating that resistant dextrin can switch HFHFD mice to Allprevotella and Prevotellaceae enterotype.
In addition, we found that resistant dextrin enhanced the relative abundance of Parasutterella and Parabacteroid, which were able to produce the SCFAs [35]. SCFAs were derived from the fermentation of non-digestible carbohydrates. Many studies have reported that they played a crucial role in the prevention and treatment of obesity-associated insulin resistance [36–38], and might via the upregulation of PPARα target genes, thereby increasing fatty acid oxidation [39]. This may help us to elucidate the mechanisms of the gut microbiota mediate the metabolic beneficial effects of resistant dextrin, though we didn’t detect the concentrations of SCFAs. Moreover, resistant dextrin increased Ruminococcaceae_UCG_005, which were beneficial to obesity and other associated metabolic disorders [40], while reduced Ruminiclostridium and Lactobacillaceae, which were increased in obesity [40]. Taken together, these results demonstrated that resistant dextrin can reduce HFHFD-induced gut microbiota dysbiosis.
Conclusions
Our results demonstrated that resistant dextrin can significantly ameliorate liver insulin resistance induced by HFHFD, improve serum lipid levels, as well as reduce hepatic lipid deposition. Although further studies are required to define the mechanisms underlying gut microbiota mediated protective effects in insulin resistance, we speculate that the modulation of gut microbiota might be responsible for these beneficial effects of resistant dextrin, and our work may provide a key strategy to the management of type 2 diabetes.
Supplementary information
Additional file 1: Figure S1. Body weight and FBG after 12 weeks of HFHFD feeding. Figure S2. The principal coordinates analysis (PCoA). Figure S3. The ratio of Firmicutes/Bacteroidetes in three groups.
Acknowledgments
Not applicable.
Abbreviations
- T2DM
Type 2 diabetes mellitus
- IR
Insulin resistance
- FINS
Fasting insulin
- TC
Total cholesterol
- TG
Triglyceride
- HDL-Ch
High-density lipoprotein cholesterol
- LDL-Ch
Low-density lipoprotein cholesterol
- SCFAs
Short chain fatty acids
- FBG
Fasting blood glucose
- GTT
Glucose tolerance test
- ITT
Insulin tolerance test
- FXR
Farnesoid X receptor
- CPT1α
Carnitine palmitoyl transferase 1α
- ACOX
Acyl-CoA oxidase
- PPARα
Peroxisome proliferators-activated receptorα
- AMPK
AMP activated protein kinase
- IRS-1
Insulin receptor substrate-1
- GLUT-2
Glucose transporter-2
- RD
Resistant dextrin
Authors’ contributions
Zhang HM and Su Q contributed to the conception or design of the work. Hu F and Niu YX conducted research and analyzed data, Hu F wrote and edited the manuscript for the work. Xu XY and Hu QY contributed to animal experiments, Zhang HM and Su Q critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This work was supported by Shanghai Pujiang Program (2019PJD033), National Natural Science Foundation of China (81300642,81970669) and Shanghai Sailing Program (18YF1415800).
Availability of data and materials
The data and materials used are available when requested.
Ethics approval and consent to participate
The animal study was conducted in accordance with the guidelines of the Laboratory Animal Ethical and Welfare Committee at Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fan Hu and Yixin Niu contributed equally to this work.
Contributor Information
Qing Su, Email: suqing@xinhuamed.com.cn.
Hongmei Zhang, Email: zhanghongmei02@xinhuamed.com.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12986-020-00450-2.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications [J] Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance?[J] Trends Endocrinol Metab. 2008;19(9):324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses [J] Lancet. 2019;393(10170):434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 4.Jones JM. CODEX-aligned dietary fiber definitions help to bridge the 'fiber gap’[J] Nutr J. 2014;13:34. doi: 10.1186/1475-2891-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timm DA, Stewart ML, Hospattankar A, et al. Wheat dextrin, psyllium, and inulin produce distinct fermentation patterns, gas volumes, and short-chain fatty acid profiles in vitro. [J] J Med Food. 2010;13(4):961–966. doi: 10.1089/jmf.2009.0135. [DOI] [PubMed] [Google Scholar]
- 6.Slavin JL, Savarino V, Paredes-Diaz A, et al. A review of the role of soluble fiber in health with specific reference to wheat dextrin [J] J Int Med Res. 2009;37(1):1–17. doi: 10.1177/147323000903700101. [DOI] [PubMed] [Google Scholar]
- 7.Pasman W, Wils D, Saniez MH, et al. Long-term gastrointestinal tolerance of NUTRIOSE FB in healthy men [J] Eur J Clin Nutr. 2006;60(8):1024–1034. doi: 10.1038/sj.ejcn.1602418. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Heuvel EG, Wils D, Pasman WJ, et al. Short-term digestive tolerance of different doses of NUTRIOSE FB, a food dextrin, in adult men [J] Eur J Clin Nutr. 2004;58(7):1046–1055. doi: 10.1038/sj.ejcn.1601930. [DOI] [PubMed] [Google Scholar]
- 9.Pouillart PR, Depeint F, Abdelnour A, et al. Nutriose, a prebiotic low-digestible carbohydrate, stimulates gut mucosal immunity and prevents TNBS-induced colitis in piglets [J] Inflamm Bowel Dis. 2010;16(5):783–794. doi: 10.1002/ibd.21130. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Guerin-Deremaux L, Pochat M, et al. NUTRIOSE dietary fiber supplementation improves insulin resistance and determinants of metabolic syndrome in overweight men: a double-blind, randomized, placebo-controlled study [J] Appl Physiol Nutr Metab. 2010;35(6):773–782. doi: 10.1139/H10-074. [DOI] [PubMed] [Google Scholar]
- 11.Aliasgharzadeh A, Dehghan P, Gargari BP, et al. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: a randomised controlled clinical trial [J] Br J Nutr. 2015;113(2):321–330. doi: 10.1017/S0007114514003675. [DOI] [PubMed] [Google Scholar]
- 12.Zhuhua Z, Zhiquan W, Zhen Y, et al. A novel mice model of metabolic syndrome: the high-fat-high-fructose diet-fed. ICR mice [J] 2015;64(4):435–442. doi: 10.1538/expanim.14-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czech MP. Insulin action and resistance in obesity and type 2 diabetes [J] Nat Med. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance [J] Physiol Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a Nexus of metabolic and hepatic diseases [J] Cell Metab. 2018;27(1):22–41. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montagner A, Polizzi A, Fouche E, et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD [J] Gut. 2016;65(7):1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka N, Aoyama T, Kimura S, et al. Targeting nuclear receptors for the treatment of fatty liver disease [J] Pharmacol Ther. 2017;179:142–157. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misawa E, Tanaka M, Nomaguchi K, et al. Oral ingestion of aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in zucker diabetic fatty rats [J] J Agric Food Chem. 2012;60(11):2799–2806. doi: 10.1021/jf204465j. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang X, Vales C, et al. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice [J] Arterioscler Thromb Vasc Biol. 2006;26(10):2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 20.Hou X, Xu S, Maitland-Toolan KA, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase [J] J Biol Chem. 2008;283(29):20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purushotham A, Schug TT, Xu Q, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation [J] Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes [J] Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action [J] J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics [J] Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 25.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity [J] Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 26.Li K, Zhang L, Xue J, et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice [J] Food Funct. 2019;10(4):1915–1927. doi: 10.1039/c8fo02265h. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Yu H, Xiao X, et al. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats [J] PeerJ. 2018;6:e4446. doi: 10.7717/peerj.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice [J] Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeberger M, Everard A, Gomez-Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice [J] Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity [J] Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes [J] Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivarsson E, Roos S, Liu HY, et al. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs [J] Animal. 2014;8(11):1777–1787. doi: 10.1017/S1751731114001827. [DOI] [PubMed] [Google Scholar]
- 33.Zhou AL, Hergert N, Rompato G, et al. Whole grain oats improve insulin sensitivity and plasma cholesterol profile and modify gut microbiota composition in C57BL/6J mice [J] J Nutr. 2015;145(2):222–230. doi: 10.3945/jn.114.199778. [DOI] [PubMed] [Google Scholar]
- 34.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa [J] Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Yang G, Wen Y, et al. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide [J]. Mol Nutr Food Res. 2017;61(11):1700466. [DOI] [PubMed]
- 36.De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits [J] Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites[J]. 2016;165(6):1332–45. [DOI] [PubMed]
- 38.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity [J] Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Chen H, Guan Y, et al. Acetic acid activates the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes [J] PLoS One. 2013;8(7):e67880. doi: 10.1371/journal.pone.0067880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Zhang Q, Ma W, et al. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota [J] Food Funct. 2017;8(12):4644–4656. doi: 10.1039/c7fo01383c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Body weight and FBG after 12 weeks of HFHFD feeding. Figure S2. The principal coordinates analysis (PCoA). Figure S3. The ratio of Firmicutes/Bacteroidetes in three groups.
Data Availability Statement
The data and materials used are available when requested.