Abstract
CD4+ effector T cells effectuate T cell immune responses, producing cytokines to orchestrate the nature and type of immune responses. The non-receptor tyrosine kinase IL-2 inducible T cell kinase (ITK), a mediator of T cell Receptor signaling, plays a critical role in tuning the development of these effector cells. In this review we discussed the role that signals downstream of ITK, including the Ras/MAPK pathway, play in differentially controlling the differentiation of TH17, Foxp3+ T regulatory (Treg) cells, and Type 1 regulatory T (Tr1) cells, supporting a model of ITK signals controlling a decision point in the effector T cell differentiation process.
Summary sentence:.
TCR signals traveling via ITK differentially regulates distinct T helper cell differentiation.
Introduction
Naïve CD4+ T cells are activated by interaction with antigen presenting cells carrying their cognate antigen, and in the presence of specific cytokine environments, can differentiate into a number of different effector T helper (TH) cell lineages. These T helper effector cells produce critical cytokines that drive the immune response, including influencing the nature of the response that can lead to specific disease, or alternatively, immune suppression. Among these effector TH cells, this includes cells that produce inflammatory cytokines including TH1 cells (that express the transcription factor (TF) Tbet and secrete IFNγ), TH2 cells (that express TF GATA3 and secrete cytokines such as IL-4, IL-5 and IL-13), TH9 cells (that express TF PU.1 and secrete IL-9), and TH17 cells (that express TF RAR–related Orphan Receptor gamma T (RORγt) and secrete IL-17) [1–3]. In addition, there are effector TH cells that produce suppressive cytokines, including Foxp3+ T regulatory (Treg) cells (that can produce TGF-β and IL-10), and Foxp3− Type 1 regulatory T (Tr1) cells (that also produce high levels of IL-10 (see Fig. 1A)) [4, 5]. The balance of generation of these cells, and their production of cytokines, can control whether the immune response will be inflammatory or suppressive, and can alter the course of an immune response.
Figure 1. TCR tuning of TH differentiation.
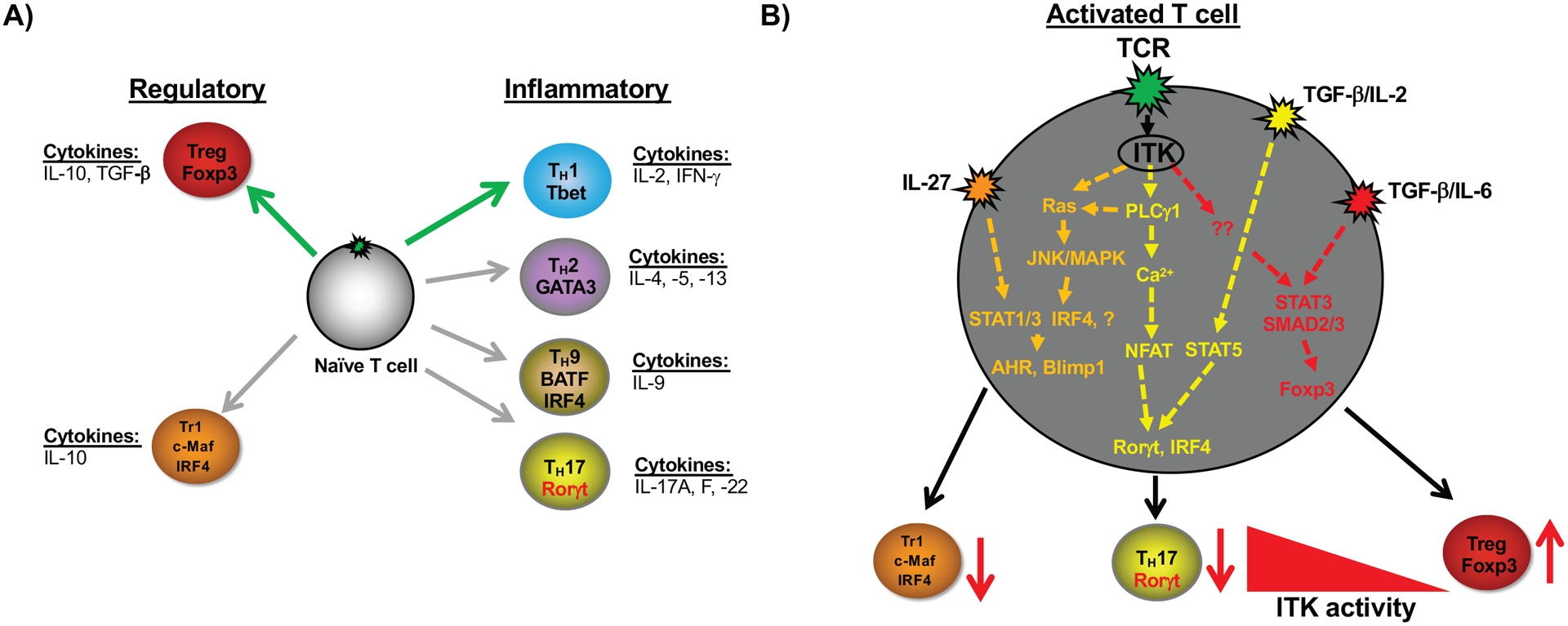
A) Different TH effector cell fates that can emerge from early T cell activation and differentiation, and the cytokines that they produce. Green arrows indicate enhanced differentiation in the absence of ITK. Gray arrows indicate reduced differentiation in the absence of ITK. B) Depiction of specific TCR signaling networks downstream of ITK that influence Tr1 cells, TH17 cells and Treg cells as reported [31, 37, 43, 58, 63, 76–78]. Inhibition or absence of Itk (depicted by the red arrows) results in reduced Tr1 cells, TH17 cells and enhanced Treg cells. Furthermore, inhibition or absence of Itk during TH17 differentiation results in enhanced Treg cells. Cytokines depicted are those involved in the specific TH cell fate differentiation.
Regardless of their subsequent fate, activation of CD4+ T cells via their antigen specific TCR by peptide/MHC complexes induce a series of downstream signaling networks that drive the production of cytokines, expression of cytokine receptors and critical TF that drive their differentiation, depending on the surrounding cytokine milieu [6, 7]. TCR triggering activates the Src family kinase Lck, which phosphorylates ITAMs located on the cytoplasmic tails of the TCR/CD3 complex proteins. The phosphorylated ITAMs recruit the tyrosine kinase ZAP70, which leads to the phosphorylation of adaptor proteins LAT and SLP-76 [8–11]. The lipid kinase PI3K, also activated by TCR triggered Lck activation, results in the production of Phosphatidylinositol (3,4,5)-trisphosphate (PIP3) lipids in the plasma membrane and recruits the Tec family tyrosine ITK to the plasma membrane [12], where it interacts with LAT and SLP-76, the latter via Tyrosine 145 (Y145) [7, 13–19]. An important substrate for the kinase activity of ITK is PLC-γ [20], which when activated, generates IP3 leading to increased intracellular calcium [21–24], and DAG. Increases in intracellular calcium activates the TF NFAT [25], and DAG activates PKCθ pathways leading to the activation of Akt and NF-κB [26–29], as well as the RAS/MAPK pathways [25]. The network of these activated pathways eventually leads to T cell proliferation and differentiation. However, the role of these pathways and networks in T cell differentiation is not well understood. Furthermore, although TCR signaling is necessary, and a positive regulator in the differentiation of CD4+ naïve progenitors to effector TH cells [30][31], its regulation of the differentiation of Foxp3+ Treg cells is more complex [31–34]. It should also be noted that these intracellular signaling networks triggered by the TCR intersect with the specific cytokine signals that regulate specific differentiation of TH subsets.
As a critical regulator of intracellular signaling downstream of the TCR, ITK plays an important role in the differentiation of effector TH cells, among other functions (for review of ITK functions, see [17, 19, 35]). While Itk has also been shown to be important in other T cell populations, including CD8+ T cells and intestinal ILC2 populations, [36–42], in this review, we focus on recent work indicating that the absence of ITK impairs differentiation into TH17 and Tr1 cell subsets [31, 43], but enhances the development of Foxp3+ T regulatory cells (Tregs, see Fig. 1B) [31, 43]. Thus ITK plays an important role in regulating signaling networks downstream of the TCR that govern the differentiation of effector TH cells.
ITK signaling negatively regulates differentiation of Foxp3+ Treg cells
Foxp3+ Treg cells are derived from the thymus (tTreg) and can also be induced in the periphery (iTreg). These Tregs are critical in maintaining tolerance and immunological homeostasis [44, 45]. Tregs suppress immune responses through the production of TGF-β and IL-10, depletion of IL-2, and/or the expression of coinhibitory receptors such as CTLA-4 and PD-1 [46–48]. In the periphery, naïve CD4+ T cells can differentiate into iTreg cells upon TCR activation in the presence of IL-2 and TGF-β [49]. Downstream of these cytokines, the STAT5 (IL-2) and Foxo3a (TGF-β) pathways induce Foxp3 expression, which drives Treg differentiation [50–52]. However, not all TCR signals have the same effect, as low antigen dose or low concentrations of anti-CD3 to stimulate cells leads to increased Treg differentiation both in vitro and in vivo, suggesting that strong TCR signals can inhibit Treg differentiation [32, 33]. Strong TCR signals activate the PI3K/mTOR pathway which suppresses Foxp3 expression by promoting nuclear export of Foxo3a and Foxo1 [34, 50]. Indeed, T cells carrying mutated TCR CD3/ζ chains that disrupt all six ITAMs, or a SLP-76 Y145F mutation which disrupts the interaction with ITK [53] and thus have attenuated TCR activity, show increased frequency of Treg cells. In support of this model, attenuating TCR signaling strength such as in the absence of ITK signaling, results in increased frequency of Treg cells in Itk−/− mice compared to WT mice (see Fig. 1B) [31, 43]. In addition, naïve Itk−/− CD4+ T cells and Treg cells are hyperresponsive to IL-2 in vitro and in vivo [31, 43]. IL-2 signals through both STAT5 and PI3K/mTOR pathways, however only STAT5 displays increased phosphorylation in the absence of ITK, due to enhanced degradation of PTEN [31]. PTEN is a negative regulator of PI3K, which is downregulated upon sufficient TCR signaling through ITK [31]. In addition, Foxp3 negatively regulates Itk expression adding another layer to how the Treg phenotype is maintained [49, 54]. This intersection between TCR signals via ITK, and the IL-2 and TGF-β signaling pathways results in a tuning function for Itk−/− Treg differentiation (Fig. 1). We note that experiments probing the function of ITK using kinase dead mutants reveal subtle differences in interpreting ITK function. T cells from mice expressing a kinase dead ITK exhibit a phenotype that is largely similar to those lacking Itk [55, 56], with the exception that no difference was observed in proportion of splenic Tregs [57]. Given the similarities in the Itk deficiency and ITK specific inhibition with regards to enhancing Treg development [43], it is possible that precursor development in the presence of a kinase deficient ITK leads to differences in Treg development in vivo. Indeed, in human T cells, knockdown of ITK leads to a similar increase in Treg development [58]. While the related kinase Rlk/Txk also plays a role in the activation of T cells, its role is less clear given the lack of clear phenotype observed in Rlk/Txk deficient T cells [23]. While a kinase inhibitor that affects both ITK and Rlk/Txk inhibits the development of Tregs, it should be noted that such inhibitors may affect other kinases and pathways as well, complication clear interpretation of such results [58].
ITK positively regulates TH17 differentiation
Initially considered a subset of either TH1 or TH2 cells, TH17 cells are generally characterized as proinflammatory [59]. These cells express the master TF RORγT and produce TH17 cytokines, IL-17A, IL-17F, IL-21 and IL-23 [59]. These cells have been shown to be involved in the immune response at mucosal surfaces, as well as in the development of autoimmune conditions such as rheumatoid arthritis [60, 61]. Differentiation of naïve CD4+ T cells by the TCR in the presence of IL-6 and TGF-β leads to TH17 cells [59], although other cytokine combinations can also generate TH17 cells e.g. IL-21 and TGF-β [62]. Although IL-6 and TGF-β ostensibly have opposing roles, both are critical for TH17 cell differentiation characterized by expression of RORγt. IL-6 signaling activates JAK1/2 and STAT3, and the absence of IL-6 in mice results in impaired TH17 responses, and enhanced generation of Treg cells [62]. TGF-β thus acts to regulate the balance of TH17 cells and Treg cells.
Although the precise molecular pathway driving TH17 differentiation following TCR activation is not fully understood, Itk deficient naive CD4+ T cells have defects in the differentiation of Th17 cells (see Fig. 1B) [63]. Similar findings have been reported for human Th17 cells [58]. In addition, humans with Itk deficiency have reduced proportions of TH17 cells, and biochemical inhibition of ITK blocks Th17 differentiation in vitro [37]. This is likely due to the ability of ITK to trigger increases in intracellular calcium and calmodulin activation via its substrate PLCγ1 leading the NFAT activation [63, 64]. Other studies have reported that STIM1, and PKCθ signals are important for TH17 differentiation as well [65].
Interestingly, differentiation of Itk deficient naïve CD4+ T cells under TH17 conditions results in reduced TH17 differentiation as described above, but also, instead, the development of Foxp3+ T cells (see Fig. 1B) [31]. This relationship between ITK, TH17 and Treg cells is interesting given the shared use of TGF-β signals along with IL-6 (for TH17) or IL-2 (for Treg cells). There are a number of shared pathways downstream of ITK that may regulate this relationship, including PKCθ (which can act downstream of ITK [29]) and inhibits the differentiation of iTreg cells and their function in vitro [66, 67], while acting as a positive mediator of TH17 cells [65].
ITK positively regulates differentiation of Type 1 Regulatory (Tr1) T cells
Type 1 regulatory (Tr1) cells are Foxp3− CD4+ cells that regulate immune responses via multiple mechanisms including, IL-10 production and coinhibitory receptors [5, 68, 69]. Their ability to promote immune tolerance has made Tr1 cells an attractive target for development of immunotherapies [70, 71]. Tr1 cells can differentiate from naïve CD4+ T cells upon TCR engagement in the presence of IL-27 [72], and although Tr1 cells have been reported to be able to express IFN-γ, production of IFN-γ or T-bet are not required for Tr1 cell development [72]. Additionally, TH17 are able to transdifferentiate into Tr1 cells during the resolution of inflammation [73]. Tr1 differentiation is controlled by a network of transcription factors which include IRF4, Blimp-1, Ahr, c-Maf and others [74, 75]. We have recently shown that Tr1 differentiation requires ITK, and that expression of these transcription factors is impaired in the absence of ITK signaling [76]. Furthermore, downstream of ITK, activation of the HRas/MAPK pathway upregulates IRF4 expression, which is upstream of Blimp-1 and Ahr [75, 76]. Importantly, Ahr agonists and reintroduction of Blimp-1 into Itk deficient naïve T cells were unable to rescue Tr1 differentiation, highlighting the importance of the HRas/IRF4 pathway in the Tr1 differentiation program [76].
Perspective
(i). Importance of the field.
The differentiation of naive CD4+ T cells into effector cells is critical for subsequent production of cytokines that can orchestrate the nature and type of immune response. This has implications for the response to infection, as well as autoimmunity and immunotherapy, including cellular immunotherapy.
(ii). Summary of the current thinking.
ITK, as a critical mediator of the strength of signal delivered by the TCR, plays a key function in tuning the development of these effector cells. These TCR signals regulated by ITK seem to be able to tune the cytokine response of the differentiating naïve CD4+ T cells, such that effector T cells that have so far been shown to depend on ITK signals, TH1, TH2, TH9, TH17 and Tr1 cells, as well Foxp3+ Treg cells, may utilize different pathways and TFs downstream of ITK to achieve those goals. This differential usage of the signaling network downstream of ITK may intersect with the relevant differentiating cytokines to tune TH differentiation. While a role for Rlk/Txk has not been evaluated in Treg, TH17 and Tr1 cell differentiation, and it is possible that the absence of Rlk could affect the differentiation of these cells, our work suggests that ITK plays a dominant role on the differentiation of these cells.
(iii). Comment on future directions.
The exploration of the signaling networks that dictate the differentiation of TH cells continues to be of significant interest given the potential ability to manipulate these pathways to control the nature of the immune response, but also to enhance immunotherapy. In addition, exploring the network of TFs that respond to these signals, as well as the changes in chromatin landscape that set up the transcriptional networks that are mobilized to drive this differentiation is of considerable interest. Temporal analysis of these systems promise to reveal the impact early TCR signals have on driving changes leading the specific TH cell types.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (AI120701 and AI138570 to A.A., AI129422 to A.A. and W.H., and GM130555 Sub-6610 to W.H.), and a Pilot Grant (to W.H.) from LSU-Tulane COBRE Center for Experimental Infectious Disease Research (funded by NIH P30GM110760). J.P.E. was supported by Grant Number T32EB023860 from the National Institute of Biomedical and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. M.C.M. is a recipient of the Pathobiological Sciences Graduate Program Fellowship in the School of Veterinary Medicine at the Louisiana State University.
Abbreviations used:
- Ahr
Aryl Hydrocarbon Receptor
- Blimp-1
B lymphocyte-induced Maturation Protein
- c-Maf
cellular musculoaponeurotic fibrosarcoma oncogene homolog
- DAG
Di-Acyl Glycerol
- Treg
Foxp3+ T regulatory cells
- IP3
Inositol tri-Phosphate
- IRF4
Interferon Regulatory Factor 4
- iTreg
Inducible Treg
- ITAMs
Immunoreceptor Tyrosine-based Activation Motif
- ITK
IL-2 inducible T cell kinase
- IL
Interleukin
- LAT
Linker of Activated T cells
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate
- PI3K
Phosphatidyl Inositol 3-Phosphate
- PLC-γ
Phospholipase C-gamma
- RORγt
RAR–related Orphan Receptor gamma T
- SLP-76
SH2 domain-containing leukocyte protein
- STIM1
Stromal interaction molecule 1
- TCR
T cell Receptor
- TF
Transcription factor
- TH
T helper
- Treg
- Tr1
Type 1 regulatory T cell
- tTreg
thymus derived Treg
- ZAP70
Zeta chain Associated protein 70
Footnotes
Conflict of Interest disclosure
A.A. receives research support from 3M Corporation. The other authors have no commercial or financial conflicts of interest.
References
- 1.Kaplan MH, Hufford MM, and Olson MR, The development and in vivo function of T helper 9 cells. Nat Rev Immunol, 2015. 15(5): p. 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coquet JM, Rausch L, and Borst J, The importance of co-stimulation in the orchestration of T helper cell differentiation. Immunol Cell Biol, 2015. 93(9): p. 780–8. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Yamane H, and Paul W, Differentiation of effector CD4 T cell populations. Annu Rev Immunol., 2010. 28: p. 445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagliani N, et al. , Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med, 2013. 19(6): p. 739–46. [DOI] [PubMed] [Google Scholar]
- 5.Huber S, et al. , Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity, 2011. 34(4): p. 554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson G, et al. , Cellular interactions in thymocyte development. Annu. Rev. Immunol, 1996. 14: p. 73–99. [DOI] [PubMed] [Google Scholar]
- 7.Kannan A, et al. , Signal transduction via the T cell antigen receptor in naive and effector/memory T cells. Int J Biochem Cell Biol, 2012. 44(12): p. 2129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irving B and A.W. A., The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell, 1991. 64: p. 891–901. [DOI] [PubMed] [Google Scholar]
- 9.Chan A, et al. , ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell, 1992. 71: p. 649–662. [DOI] [PubMed] [Google Scholar]
- 10.Finco T, et al. , LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity, 1998. 9: p. 617–626. [DOI] [PubMed] [Google Scholar]
- 11.Wardenburg JB, et al. , Phosphorylation of SLP-76 by the ZAP-70 Protein-tyrosine Kinase Is Required for T-cell Receptor Function. J. Biol. Chem, 1996. 271(33): p. 19641–19644. [DOI] [PubMed] [Google Scholar]
- 12.August A, et al. , Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA, 1997. 94: p. 11227–11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ching KA, et al. , TCR/CD3-Induced Activation and Binding of Emt/Itk to Linker of Activated T Cell Complexes: Requirement for the Src Homology 2 Domain. J Immunol, 2000. 165(1): p. 256–262. [DOI] [PubMed] [Google Scholar]
- 14.Bunnell S, et al. , Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem, 2000. 275: p. 2219–2230. [DOI] [PubMed] [Google Scholar]
- 15.Jordan M, et al. , Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity., 2008. 28: p. 359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S, et al. , Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J Immunol, 2011. 186: p. 4573–8. Epub 2011 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.August A and Ragin MJ, Regulation of T-cell responses and disease by tec kinase Itk. Int Rev Immunol, 2012. 31(2): p. 155–65. [DOI] [PubMed] [Google Scholar]
- 18.Berg L, et al. , Tec family kinases in T lymphocyte development and function. Annu Rev Immunol, 2005. 23: p. 549–600. [DOI] [PubMed] [Google Scholar]
- 19.Andreotti AH, et al. , T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol, 2010. 2(7): p. a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Villar J and Kanner S., Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-C gamma 1 in human T lymphocytes. J Immunol, 1999. 163: p. 6435–6441. [PubMed] [Google Scholar]
- 21.Hogan PG, Lewis RS, and Rao A, Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annual Review of Immunology, 2010. 28(Journal Article): p. 491–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowell D, et al. , Impaired NFATc Translocation and Failure of Th2 Development in Itk Deficient CD4 T Cells. Immunity, 1999. 11: p. 399–409. [DOI] [PubMed] [Google Scholar]
- 23.Schaeffer EM, et al. , Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science, 1999. 284(5414): p. 638–41. [DOI] [PubMed] [Google Scholar]
- 24.Liu K, et al. , T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med, 1998. 187: p. 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Garvin JE, Koretzky GA, and Jordan MS, T cell activation. Annu Rev Immunol, 2009. 27: p. 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wegener E, et al. , Essential role for IkappaB kinase beta in remodeling Carma1-Bcl10-Malt1 complexes upon T cell activation. Mol Cell, 2006. 23(1): p. 13–23. [DOI] [PubMed] [Google Scholar]
- 27.Narayan P, et al. , CARMA1 is required for Akt-mediated NF-kappaB activation in T cells. Mol Cell Biol, 2006. 26(6): p. 2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blonska M and Lin X, NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res, 2011. 21(1): p. 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman A, et al. , Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol, 2004. 34(7): p. 2001–11. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita M, et al. , T cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the interleukin 4 receptor signaling complex. J Exp Med, 2000. 191: p. 1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Rodriguez J, et al. , Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med, 2014. 211(3): p. 529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottschalk RA, Corse E, and Allison JP, TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. The Journal of experimental medicine, 2010. 207(8): p. 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner MS, Kane LP, and Morel PA, Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol, 2009. 183(8): p. 4895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauer S, et al. , T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A, 2008. 105(22): p. 7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh S, et al. , Interleukin-2-Inducible T-Cell Kinase Deficiency-New Patients, New Insight? Front Immunol, 2018. 9: p. 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho HS, et al. , The Tec kinase ITK is essential for ILC2 survival and epithelial integrity in the intestine. Nat Commun, 2019. 10(1): p. 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eken A, et al. , Genetic Deficiency and Biochemical Inhibition of ITK Affect Human Th17, Treg, and Innate Lymphoid Cells. J Clin Immunol, 2019. 39(4): p. 391–400. [DOI] [PubMed] [Google Scholar]
- 38.Huang F, et al. , The tyrosine kinase Itk suppresses CD8+ memory T cell development in response to bacterial infection. Sci Rep, 2015. 5: p. 7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linka RM, et al. , Loss-of-function mutations within the IL-2 inducible kinase ITK in patients with EBV-associated lymphoproliferative diseases. Leukemia, 2012. 26(5): p. 963–71. [DOI] [PubMed] [Google Scholar]
- 40.Kapnick SM, et al. , Inducible T Cell Kinase Regulates the Acquisition of Cytolytic Capacity and Degranulation in CD8(+) CTLs. J Immunol, 2017. 198(7): p. 2699–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prince AL, et al. , Development of innate CD4+ and CD8+ T cells in Itk-deficient mice is regulated by distinct pathways. J Immunol, 2014. 193(2): p. 688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W, et al. , ITK tunes IL-4-induced development of innate memory CD8+ T cells in a gammadelta T and invariant NKT cell-independent manner. J Leukoc Biol, 2014. 96(1): p. 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W, et al. , IL-2-inducible T cell kinase tunes T regulatory cell development and is required for suppressive function. J Immunol, 2014. 193(5): p. 2267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baecher-Allan C and Hafler DA, Human regulatory T cells and their role in autoimmune disease. Immunol Rev, 2006. 212: p. 203–16. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhry A, et al. , Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity, 2011. 34(4): p. 566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton AM, et al. , Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol, 2004. 172(11): p. 6519–23. [DOI] [PubMed] [Google Scholar]
- 47.Palomares O, et al. , Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun, 2014. 15(8): p. 511–520. [DOI] [PubMed] [Google Scholar]
- 48.Gianchecchi E and Fierabracci A, Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front Immunol, 2018. 9: p. 2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marson A, et al. , Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature, 2007. 445(7130): p. 931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada Y, et al. , Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med, 2010. 207(7): p. 1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tone Y, et al. , Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol, 2008. 9(2): p. 194–202. [DOI] [PubMed] [Google Scholar]
- 52.Laurence A, et al. , Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity, 2007. 26(3): p. 371–81. [DOI] [PubMed] [Google Scholar]
- 53.Caton AJ, et al. , Strength of TCR signal from self-peptide modulates autoreactive thymocyte deletion and Foxp3(+) Treg-cell formation. Eur J Immunol, 2014. 44(3): p. 785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan F, et al. , Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science, 2009. 325(5944): p. 1142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi Q, et al. , Interleukin-2-inducible T cell kinase (Itk) network edge dependence for the maturation of iNKT cell. J Biol Chem, 2011. 286: p. 138–46. Epub 2010 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sahu N, et al. , Differential sensitivity to Itk kinase signals for T helper 2 cytokine production and chemokine-mediated migration. J Immunol., 2008. 180: p. 3833–8. Erratum in: J Immunol. 2008 May 15;180(10):7047–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deakin A, et al. , Characterisation of a K390R ITK kinase dead transgenic mouse--implications for ITK as a therapeutic target. PLoS One, 2014. 9(9): p. e107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mamontov P, et al. , A negative role for the interleukin-2-inducible T-cell kinase (ITK) in human Foxp3+ TREG differentiation. PLoS One, 2019. 14(4): p. e0215963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korn T, et al. , IL-17 and Th17 Cells. Annu Rev Immunol, 2009. 27: p. 485–517. [DOI] [PubMed] [Google Scholar]
- 60.Cypowyj S, et al. , Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol, 2012. 42(9): p. 2246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinman L, A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med, 2007. 13(2): p. 139–45. [DOI] [PubMed] [Google Scholar]
- 62.Korn T, et al. , IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature, 2007. 448(7152): p. 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Rodriguez J, et al. , Differential expression of interleukin-17A and −17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity, 2009. 31(4): p. 587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, et al. , Calmodulin and PI(3,4,5)P(3) cooperatively bind to the Itk pleckstrin homology domain to promote efficient calcium signaling and IL-17A production. Sci Signal, 2014. 7(337): p. ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma J, et al. , T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol, 2010. 40(11): p. 3028–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma J, et al. , Protein kinase C-theta inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway. J Immunol, 2012. 188(11): p. 5337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brezar V, Tu WJ, and Seddiki N, PKC-Theta in Regulatory and Effector T-cell Functions. Front Immunol, 2015. 6: p. 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akdis M, T-cell tolerance to inhaled allergens: mechanisms and therapeutic approaches. Expert Opin Biol Ther, 2008. 8(6): p. 769–77. [DOI] [PubMed] [Google Scholar]
- 69.Haringer B, et al. , Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med, 2009. 206(5): p. 1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roncarolo MG, et al. , Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol, 2014. 380: p. 39–68. [DOI] [PubMed] [Google Scholar]
- 71.Zeng H, et al. , Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol, 2015. 12(5): p. 566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fitzgerald DC, et al. , Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol, 2007. 8(12): p. 1372–9. [DOI] [PubMed] [Google Scholar]
- 73.Gagliani N, et al. , Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature, 2015. 523(7559): p. 221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Apetoh L, et al. , The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol., 2010. 11: p. 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cretney E, et al. , The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol, 2011. 12(4): p. 304–11. [DOI] [PubMed] [Google Scholar]
- 76.Huang W, et al. , ITK signalling via the Ras/IRF4 pathway regulates the development and function of Tr1 cells. Nat Commun, 2017. 8: p. 15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kannan AK, et al. , Itk signals promote neuroinflammation by regulating CD4+ T-cell activation and trafficking. J Neurosci, 2015. 35(1): p. 221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kannan A, et al. , Allele-sensitive mutant, Itkas, reveals that Itk kinase activity is required for Th1, Th2, Th17, and iNKT-cell cytokine production. Eur J Immunol, 2015. 45(8): p. 2276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


