Abstract
With the ageing of the global population, interest is growing in the ‘geroscience hypothesis’, which posits that manipulation of fundamental ageing mechanisms will delay (in parallel) the appearance or severity of multiple chronic, non-communicable diseases, as these diseases share the same underlying risk factor—namely, ageing. In this context, cellular senescence has received considerable attention as a potential target in preventing or treating multiple age-related diseases and increasing healthspan. Here we review mechanisms of cellular senescence and approaches to target this pathway therapeutically using ‘senolytic’ drugs that kill senescent cells or inhibitors of the senescence-associated secretory phenotype (SASP). Furthermore, we highlight the evidence that cellular senescence has a causative role in multiple diseases associated with ageing. Finally, we focus on the role of cellular senescence in a number of endocrine diseases, including osteoporosis, metabolic syndrome and type 2 diabetes mellitus, as well as other endocrine conditions. Although much remains to be done, considerable preclinical evidence is now leading to the initiation of proof-of-concept clinical trials using senolytics for several endocrine and non-endocrine diseases.
Ageing is now generally accepted as the single largest risk factor for many of the major chronic diseases (for example, type 2 diabetes mellitus (T2DM), cardiovascular disease and cancer) that account for the bulk of morbidity, deaths and health costs in the USA and other developed countries1. For these conditions, the predictive ability of advanced chronological age exceeds the predictive ability of all other risk factors combined. Enormous progress has been made over the years in the development of specific drugs to treat individual diseases associated with ageing, including metabolic dysfunction and T2DM (for example, GLP1R agonists and DPP4 inhibitors), skeletal fragility and osteoporosis (for example, bisphosphonates and denosumab) and vascular dysfunction and disease (for example, statins), with potential new drugs on the horizon to treat skeletal muscle loss and frailty (for example, the activin type 2 receptor antibody bimagrumab); however, the combined effect of these drugs on reducing morbidity and mortality has been modest.
Age-associated chronic diseases rarely, if ever, exist in isolation in elderly individuals. Rather, they tend to occur in synchrony as multimorbidities, the prevalence of which increases exponentially after age 70 years2. This multimorbidity leads to substantial barriers to the appropriate treatment of each disease, including prioritization of each condition by the busy primary care physician and perhaps most importantly the growing problem of polypharmacy in older (aged >60 years) patients. Indeed, the most recent estimates indicate that 36–39% of adults aged 62 years or older take five or more prescription medicines3. When over-the-counter medication use is included, the prevalence of such adults taking five or more medications increases to 67% (REF.3). Fundamentally, polypharmacy occurs because most treatment strategies for chronic age-related morbidities are disease specific. This specificity inevitably leads to polypharmacy, which can result in problems related to adverse effects, unpredictable drug interactions and poor adherence.
Osteoporosis provides a good case study of the reasons why a disease-specific approach fails in older (aged >60 years) patients with multimorbidities. Currently, numerous options are available for the treatment of osteoporosis, including a selective oestrogen receptor modulator (raloxifene), four bisphosphonates (alendronate, risedronate, ibandronate and zoledronic acid), a human monoclonal antibody to receptor activator of nuclear factor-κB ligand (RANKL; denosumab), analogues for parathyroid hormone and parathyroid hormone-related protein (teriparatide and abaloparatide) and a monoclonal antibody to sclerostin (romosozumab)4. Despite these treatment options, most patients with osteoporosis, including following an event as devastating as hip fracture, remain untreated5. A number of reasons could explain the lack of appropriate treatment for age-associated co-morbidities, including osteoporosis, some of which are condition specific. For example, in osteoporosis, the fear of rare bisphosphonate-related adverse effects, such as osteonecrosis of the jaw or atypical femur fractures6, can reduce treatment uptake.
Within the context of multiple co-morbidities of ageing and the growing recognition by the gerontology community that ageing in itself is the largest risk factor for most age-related chronic diseases, the ‘geroscience hypothesis’ has gained accelerating momentum. This hypothesis posits that manipulation of fundamental mechanisms of ageing will delay (in parallel) the appearance or severity of multiple chronic diseases because these diseases share the same underlying risk factor — namely, ageing7 (FIG. 1). The potential efficiency of targeting fundamental ageing mechanisms, as opposed to treating each age-associated condition separately, is truly remarkable. Indeed, by one estimate, a 2% delay in the progression of ageing processes would lead to an increase often million healthy (as opposed to disabled) elderly people in the USA by 2060 compared with doing nothing, which would delay the onset of cancer or heart disease8. This increase would correspond to savings in US health costs of $7.1 trillion over 50 years8.
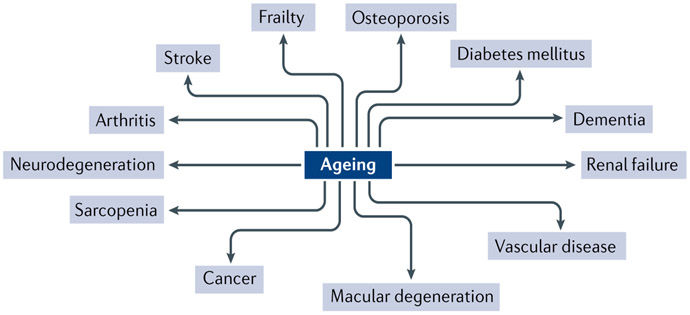
Fig. 1 ∣. The central role of ageing in chronic diseases.
This figure shows examples of chronic non-communicable diseases that have ageing as one of the main risk factors. Reprinted with permission from REF.11, JCI.
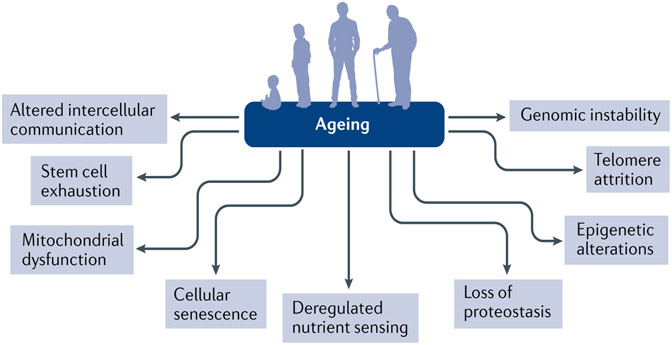
A number of common ageing mechanisms that influence lifespan and healthspan have been identified on the basis of studies across a range of species9. These ageing mechanisms can be categorized into nine hallmarks, which are genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication9 (FIG. 2). Although it is useful to consider these hallmarks individually in mechanistic studies related to ageing, they are highly interconnected, and, ultimately, systems biology approaches will be needed to fully understand interactions among these pathways as they contribute to ageing. Of these fundamental ageing mechanisms, cellular senescence has received considerable attention as a process that is potentially druggable to prevent or treat multiple ageing comorbidities.
Fig. 2 ∣. Nine fundamental hallmarks of ageing.
Nine fundamental physiological, cellular and molecular hallmarks of ageing that provide a useful framework for mechanistic studies. Reprinted with permission from REF.9, Elsevier.
In this Review, we discuss the biological mechanisms of cellular senescence and the evidence that this physiological process has a causative role in multiple diseases associated with ageing. Subsequently, we focus on the role of cellular senescence in a number of endocrine diseases, as well as approaches to target this mechanism to prevent or alleviate age-associated endocrine diseases.
Mechanisms of senescence
Cellular senescence, originally described by Hayflick10, is a cell fate that involves essentially irreversible replicative arrest, tumour suppressor activation, profound chromatin changes, apoptosis resistance and frequently increased protein synthesis11. The increase in protein synthesis includes excessive production of proinflammatory cytokines (the senescence-associated secretory phenotype (SASP)), which is responsible for tissue damage and contributes to ageing across tissues11.
Signals for senescence.
Multiple signals can induce a cell to enter senescence (FIG. 3). These include external signals, such as damage and/or danger signals (for example, damage-associated molecular patterns or pathogen-associated molecular patterns), metabolic signals (for example, high levels of glucose, ceramides, certain fatty acids, prostanoids, hypoxia and reactive oxygen species (ROS)), factors that induce repeated cell replication (for example, growth hormone or IGF1), inflammatory factors, radiation, shear or mechanical stress, certain extracellular vesicles and other factors (for example, signals originating from other senescent cells that lead to spread of senescence)11-23. Internal factors that can drive cells into senescence include telomeric dysfunction from repeated cell replication, DNA damage, aneuploidy, oncogene activation, mitochondrial dysfunction, intracellular metabolites (for example, ROS), nuclear envelope dysfunction and certain genetic variants or mutations (for example, those involving lamin A, DBC1 or p53)11,15,16,22,24-31. These senescence-inducing signals activate transcription factor cascades, including pathways involving the cell cycle inhibitors pl6INK4A/RB andp53/p21CIP1.
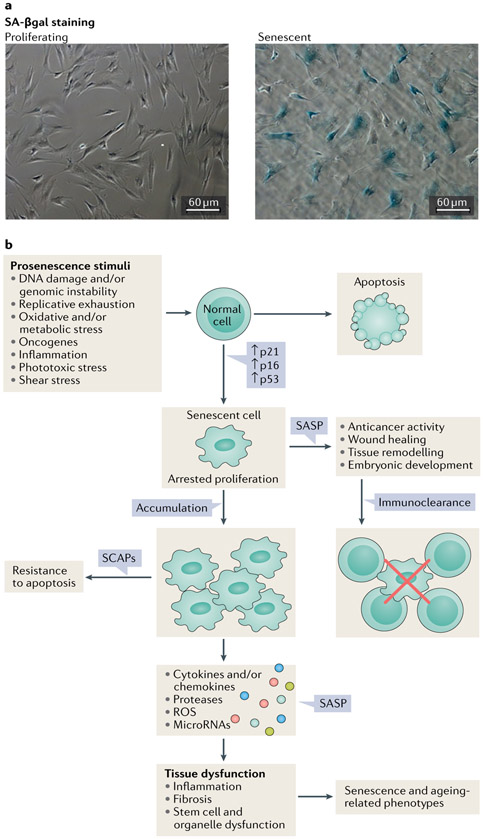
Fig. 3 ∣. Causes and consequences of cellular senescence.
a ∣ Images of senescence-associated β-galactosidase (SA-βgal) staining of proliferating and senescent human preadipocytic cells. Bars represent 60 μm. b ∣ Different stimuli can induce cellular senescence. The phenotypes of senescent cells are cell type and context dependent; however, cells can develop a senescence-associated secretory phenotype (SASP), arrested proliferation and resistance to proapoptotic pathways through senescence-associated antiapoptotic pathways (SCAPs). The formation of senescent cells in response to damage has an important role in tumour suppression and is also necessary for tissue repair (for instance during wound healing). By contrast, the persistent presence of fairly small numbers of senescent cells can promote a protumorigenic milieu and impair the function of multiple tissues. ROS, reactive oxygen species.
The senescent phenotype.
At least in cell culture, 2 weeks or more after the initiation of senescence, the senescent cell phenotype can become established and essentially irreversible. Cellular senescence is characterized by loss of proliferative potential, changes in expression of multiple genes, altered histone binding, changes in the levels of heterochromatin and/or euchromatin, resistance to apoptosis, increased metabolic activity, increased protein production and frequently a SASP11. The SASP involves secretion of inflammatory cytokines, chemokines that attract and anchor immune cells, tissue-destroying proteases, factors that affect stem and progenitor cell function (such as activin A), haemostatic factors (such as plasminogen-activated inhibitor 1), vasopressors, ceramides, prostanoids, bradykinins, nucleotides (such as microRNAs, mitochondrial DNA and nuclear DNA) and growth factors. These factors can act in a paracrine or endocrine manner to induce local and systemic inflammation, immune system activation, fibrosis, apoptosis, tissue damage, and stem and progenitor cell dysfunction and can induce the spread of senescence to other cells both locally and systemically11,15,16,26,32-34 (FIG. 3).
Senescent cells can undergo a partial Warburg shift with increased glycolytic metabolism, reduced fatty acid utilization, intracellular accumulation of lipids and increased ROS generation, which perturbs nearby cells and contributes to the spread of senescence to non-senescent cells in vitro and in vivo34,35. Several months after cells have entered senescence, transposons (such as LINE1 and other elements) can be produced within senescent cells along with reverse transcriptase. These elements lead to DNA rearrangements within the cell and further activation of DNA damage signals that can induce intracellular cGAS–STING damage-response pathways, thereby reinforcing and further exacerbating the proinflammatory proapoptotic SASP36.
The composition of the SASP differs depending on the cell type from which the senescent cells originated, the specific inducers that caused senescence and the hormonal milieu. For example, transcriptome analysis of senescent fibroblasts from different tissues showed considerable heterogeneity in the SASP37. Furthermore, the SASP varies over time and seems to be modifiable: hormones and drugs such as glucocorticoids, rapamycin, metformin or JAK1/JAK2 inhibitors can inhibit components of the SASP both in vitro and in vivo26,38-41. In addition, external signals can upregulate the SASP For example, at least in vitro, tumour necrosis factor (TNF) can exacerbate and reinforce the proinflammatory nature of the SASP14.
Markers of cellular senescence.
Much more research needs to be conducted on developing and optimizing sensitive and specific assays that track changes in the burden of senescent cells using biopsy samples, blood assays, assays of other body fluids or imaging42. Complicating such work, the definition of cellular senescence is somewhat vague: several potentially proinflammatory cell types, such as macrophages, osteoclasts or precancerous or cancer cells, share many features with senescent cells43. Furthermore, these proinflammatory ‘senescent-like’ cells could arguably even be the same as what are currently regarded as being senescent cells43. Few cellular or tissue markers are highly sensitive or specific for senescent cells, so combinations of these markers generally need to be analysed to draw firm conclusions about the effects of diseases or interventions on the burden of senescent cells. These in vitro and in vivo markers of senescent cells include increased cell size and intracellular protein content, accumulation of lipofuscin, increased expression of p16INK4A, p21CIP1 and SASP factors (for example IL-6, IL-1α, IL-8, monocyte chemoattractant protein 1, plasminogen-activated inhibitor 1 and plasminogen-activated inhibitor 2), increased cellular senescence-associated β-galactosidase (SA-βgal) activity, cytoplasmic HMGB1, the presence of senescence-associated distension of satellites (SADS) and telomere-associated DNA damage foci, among other markers44-48.
These markers of cellular senescence have their limitations. For example, both SA-βgal activity and p16INK4a expression can be high in activated macrophages, and p16INK4A expression can also be elevated in certain differentiated cell types in vitro and in vivo43,49. Furthermore, levels of p16INK4A are not elevated in all cells that could be considered to be senescent50. Thus, SA-βgal activity and p16INK4a expression are neither fully sensitive nor specific markers of senescent cells. A combination of assays is needed to determine senescent cell numbers in tissue samples.
Currently, it is unclear if circulating SASP factors or local senescent cell numbers in biopsy samples of skin, adipose tissue or other tissues reflect the overall burden of senescent cells. New or composite assays are needed for use in clinical trials, for example assays of circulating or urinary microvesicles originating from senescent cells, senescence-specific microRNAs or other nucleotides, or epigenetic profiles. Imaging methods also need to be developed to follow localized populations of senescent cells, for example in trials of senolytic drugs for idiopathic pulmonary fibrosis51 or osteoarthritis52.
Physiological relevance of cellular senescence.
Consistent with the notion of cellular senescence being a terminal cell fate that occurs in response to certain stimuli, senescent cells can develop at any point during life. For example, senescent cells can even occur in 32-cell-stage human blastocysts in response to hypoxia53. However, studies conducted using combinations of senescence markers suggest that the abundance of senescent cells increases with ageing in multiple tissues of mice, monkeys and humans20,54-56. In otherwise healthy humans, the abundance of senescent cells can increase from low or even nearly undetectable levels in skin or adipose tissue biopsy samples from young individuals (that is, aged 20–30 years) to as many as 5% of cells or even more in individuals aged 40–80 years; however, more research using a broader range of markers of cellular senescence needs to be undertaken57-60. Consistent with a relationship between cellular senescence and ageing, interventions that increase healthspan and lifespan, including caloric restriction or mutations in the growth hormone axis (for example, those in Ames dwarf mice (which lack growth hormone) and growth hormone receptor geneknockout mice) are associated with decreased burden of senescent cells in mice54,61.
Senescent cells that have a proinflammatory SASP can cause substantial pathogenic effects, resulting in phenotypes that are associated with ageing. For example, transplanting small numbers of senescent cells (mouse fibroblasts induced to become senescent by radiation) around the knee joints of young mice induces an age-related osteoarthritis-like condition52. Furthermore, transplanting autologous ear fibroblasts or syngeneic (genetically compatible) preadipocytes induced to become senescent by radiation or chemotherapeutic drug exposure intraperitoneally into lean middle-aged mice is sufficient to cause frailty, early onset of all age-related diseases and premature death compared with transplanting similar numbers of non-senescent cells. This effect occurs even though only 1 in 10,000 of the total number of cells in the recipient mice is a transplanted senescent cell34. Moreover, transplanting only half the number of senescent cells was sufficient to cause an early ageing phenotype and increased mortality in middle-aged diet-induced obese mice or lean old mice as opposed to lean middle-aged mice34. Thus, even small numbers of senescent cells can cause considerable dysfunction.
Targeting cellular senescence
Senescent cells can be beneficial in certain circumstances: for example in the defence against cancers, in the course of wound healing or in placental regulation of parturition62-64. Hence, interfering with the generation of new senescent cells is probably not a viable overall therapeutic strategy. For example, systemically reducing the expression of p16INK4A or p53 might delay the development of senescent cells but might also increase the risk of cancer63. However, once formed, senescent cells harbour oncogenic mutations and cause dysfunction owing to their SASP. Removing these already formed and persistent senescent cells might decrease cancer risk, decrease the spread of senescence and reduce tissue dysfunction.
SASP inhibitors.
SASP inhibitors (otherwise known as senomorphics) target processes or proteins in senescent cells that regulate or exacerbate the SASP, including mechanistic target of rapamycin complex 1 (mTORC1), JAK1/JAK2, STAT3, inflammatory mediators and mitochondrial dysfunction. SASP inhibitors include rapamycin and related mTORC1 inhibitors, ruxolitinib, glucocorticoids and metformin26,39,40,59,65,66. Most of these SASP inhibitors are segmental; that is, they reduce some sets of SASP components but not the entire range of SASP factors. Of note, these agents also have many other effects in addition to being SASP inhibitors.
Among other effects, rapamycin and related agents can reduce frailty in old mice, decrease heart failure, cancers, cognitive impairment and immune dysfunction in mouse models, and delay age-related adipose tissue loss and increase lifespan in mice67-72. Administering rapamycin at doses associated with lifespan extension in mice did not seemingly reduce the levels of at least some of the SASP factors in adipose tissue of old mice73. This finding indicates that much more remains to be learned about potential relationships among adipose tissue inflammation, SASP factors and the lifespan-extending effects of rapamycin, which is used in humans as an immunosuppressive agent following organ transplantation74.
Ruxolitinib is a JAK1/JAK2–STAT3 inhibitor in clinical use for various disorders (for example, polycythaemia vera, myelofibrosis and graft-versus-host disease) that also inhibits the SASP in vitro and in vivo in ageing mice59. Of note, in old mice this drug attenuates age-related adipose tissue dysfunction, decreases insulin resistance, reduces age-related osteoporosis and decreases stem cell dysfunction59,75. Furthermore, even in very old mice the drug reduces frailty, a condition once believed to be untreatable59. In older patients (median age, 65 years) with myeloproliferative syndrome, ruxolitinib partially attenuates the features of frailty, including reduced body weight, strength and appetite; however, no effect was seen on their underlying haematological condition, which was the original target of the drug76.
Of note, metformin, which among other mechanisms of action is a weak SASP inhibitor40, alleviates a number of age-related conditions in experimental animals and in humans; these disorders include metabolic dysfunction, insulin resistance, T2DM, cardiovascular disease, cognitive dysfunction and cancer development and spread42. In individuals with T2DM, one study has suggested that metformin could increase 5-year survival77.
On the basis of animal studies, although SASP inhibitors could help to alleviate conditions related to ageing and cellular senescence when administered intermittently41, generally these drugs would need to be administered continuously to maintain SASP suppression. Notably, SASP inhibitors act through a range of mechanisms, which makes disentangling effects on age-related phenotypes due to SASP modulation from other ‘off-target’ age-related processes difficult. This fact is especially true if continuous dosing of these inhibitors is used. Off-target effects that occur during continuous dosing could contribute to adverse effects that are unrelated to suppressing the SASP. For example, rapamycin can induce insulin resistance by inhibiting mTORC2 (REF78) and can cause cataract formation perhaps unrelated to mTORC2 inhibition70, among other effects in mice and humans. SASP inhibitors could see clinical application for age-related conditions, especially in situations where the short-term suppression of the effects of senescent cells is indicated. For example, SASP inhibitors could be used to promote recovery after myocardial infarction, to increase the effectiveness of immunizations or to decrease ventilator-induced myopathy; however, clinical studies specifically addressing these indications need to be done79,80.
Senolytics.
SASP inhibitors attenuate the SASP without killing senescent cells. To decrease the burden of senescent cells pharmacologically, small molecules, peptides and antibodies that selectively eliminate senescent cells, referred to as ‘senolytics’, are being developed15,32,81. Since the discovery of senolytic agents in 2015 (REF.82), considerable progress has been made in identifying additional small molecule senolytic drugs and their effects. In that first article82, a hypothesis-driven senolytic agent discovery paradigm was used. This system was based on the resistance of senescent cells to apoptosis83, despite their production of proapoptotic SASP factors, which should trigger their own death. Indeed, proapoptotic pathways were found to be upregulated in senescent cells82. Therefore, the hypothesis was tested that senescent cells depend on senescence-associated antiapoptotic pathways (SCAPs), which permit senescent cells to survive their own SASP.
SCAPs were identified by bioinformatics approaches based on senescent cell expression profiles of human preadipocytes induced to become senescent by radiation82. The reliance of senescent cells on SCAPs was verified in vitro by RNA interference studies, which identified SCAPs as the Achilles heel of senescent cells82,84. This finding led to the discovery of potential senolytic targets within these SCAP networks and the first senolytic drugs82, including the combination of dasatinib, which is an FDA-approved tyrosine kinase inhibitor85, and quercetin, which is a flavanol present in many fruits and vegetables86 (D+Q)82. Of note, a protein of the BCL-2 family that antagonizes apoptosis (BCL-XL) was identified as a SASP component82. Following this discovery, the third senolytic drug, navitoclax, which is a BCL-2 family inhibitor, was identified87,88. Researchers have now identified an increasing number of senolytics, including additional synthetic small molecules, compounds that are derived from natural products and peptide inhibitors that target known SCAPs. In addition, further SCAPs are being investigated as potential senolytic targets84,89,90.
SCAPs that are required for senescent cell survival differ among cell types. For example, the SCAPs needed for survival of senescent human primary adipose progenitors are different from those in senescent human embryonic venous endothelial cells (HUVECs)82. This difference means drugs that target a single SCAP will probably not be able to eliminate a wide range of senescent cell types. Indeed, most senolytics tested are effective against only a limited repertoire of senescent cell types. For example, navitoclax targets HUVECs; however, it is ineffective against senescent primary human adipocyte progenitors51,87. Evidence suggests that senolytics might have differing effectiveness even within one particular type of cell. For example, in human lung fibroblasts, navitoclax targets and kills senescent cells in the culture-acclimated IMR-90 lung fibroblast-like cell strain but is less effective against senescent primary human lung fibroblasts51,87. Thus, without extensive testing across a range of cell types, it is difficult to contend that any particular senolytic is universally effective.
Senolytics can act synergistically. For example, D+Q is effective in targeting senescent cultured mouse embryonic fibroblasts, whereas dasatinib or quercetin used alone are not82. Thus, different senolytics could prove to be effective for different indications. Moreover, combining different senolytics could extend the range of senescent cell types that can be eliminated.
The continuous presence of senolytics is not required for the drugs to be effective, as senescent cells take weeks or months to develop and acquire a SASP, at least in cell culture15,81,91. A brief disruption of prosurvival pathways is adequate to kill senescent cells in human adipose tissue explants, with senescent cells being eliminated within 18 hours of continuous exposure to the senolytic drug34. Thus, senolytics are seemingly effective in human tissue when administered intermittently75,82. Although D+Q has an elimination half-life of a few hours, a single dose of this combination decreases the effects of exposure of a leg to a 10-Gy dose of radiation for at least 7 months in mice82. Furthermore, as discussed later, intermittent administration of D+Q in doses that are weeks apart is effective in alleviating age-related osteoporosis in mice75.
The frequency of senolytic treatment for particular conditions will probably depend on the rates of senescent cell accumulation, which might differ among circumstances in which cellular senescence occurs. For example, repeated exposure to DNA-damaging cancer therapies or continued high-fat feeding could cause a more rapid reaccumulation of senescent cells after senolytic therapy than chronological ageing does. The intermittent administration of senolytics might reduce the risk of patients developing adverse effects and could permit the administration of senolytics during periods of good health. Moreover, intermittent dosing could decrease the risk of off-target effects and reduce the likelihood of patients developing drug resistance. Indeed, the development of proliferation-dependent drug resistance to senolytics is unlikely, as senescent cells do not divide (FIG. 3) and therefore cannot acquire advantageous mutations, which is in contrast to the situation with anticancer drugs or antibiotics.
Cellular senescence in ageing
Ageing is the leading risk factor for most of the chronic non-communicable diseases that account for the bulk of morbidity, death and health-care costs8,92. Most chronic diseases become more prevalent with increasing age, including T2DM, kidney disease, atherosclerosis, blindness, osteoporosis, osteoarthritis and dementias. The risk of various geriatric conditions, including frailty, immobility, mild cognitive impairment and incontinence, also increases with ageing. Furthermore, a loss of physiological resilience, with reduced capacity to recover following stresses such as surgery or pneumonia, occurs with advancing age93. These chronic diseases, geriatric syndromes and losses of resilience tend to cluster within older individuals (aged >60 years)94, which is consistent with strong links to upstream fundamental ageing processes, such as cellular senescence92.
The aforementioned observations led to the formulation of the geroscience hypothesis. If this hypothesis is true, then therapeutics that target fundamental ageing processes might be predicted to delay, prevent or alleviate age-related diseases and disorders as a group, as opposed to specific therapies that target one condition at a time. Moreover, if cellular senescence is indeed a key fundamental mechanism of ageing, then senolytics should be effective against a range of age-related conditions and chronic diseases.
Obesity, T2DM and their complications, including hepatic steatosis, diabetic kidney disease and obesity-related neuropsychiatric dysfunction, as well as age-related osteoporosis and growth hormone and/or IGF1 excess are among the endocrine conditions that have been associated with increases in senescent cells in pathogenically relevant tissues in mice and humans, as will be discussed later. In addition to these endocrine conditions, accelerated local or systemic senescent cell accumulation has been observed across a broad range of disorders and diseases in humans (BOX 1).
Box 1 ∣. Senescent cell accumulation in non-endocrine disorders.
Frailty and impaired ambulation in elderly women143
Pre-eclampsia137
Macular degeneration and other eye diseases145
Skin conditions, including psoriasis, scleroderma, melanocytic naevi, actinic keratosis, non-healing skin wounds and other skin disorders146
Chronic obstructive pulmonary disease147
Parkinson disease149
Sites damaged by radiation150
Cardiac dysfunction after myocardial infarction151
Atherosclerosis152
Sclerosis-related occlusion of arteriovenous fistulae used for dialysis17
Chronic kidney disease57
Cirrhosis153
Cancers154
Osteoarthritis155
Degenerated vertebral discs156
Evidencefrom mouse models.
In mouse models, a number of senescence-associated disorders analogous to those seen in humans (BOX 1) can be alleviated by treatment with senolytic agents, including D+Q, fisetin and navitoclax. For example, bleomycin inhalation results in a condition that models human idiopathic pulmonary fibrosis, and treatment with D+Q results in improved pulmonary function51. In mouse models of neurodegeneration and cognitive dysfunction generated by tau overexpression and amyloid-β overexpression, treatment with D+Q resulted in a reduction in markers of neuroinflammation and cognitive deficits95,96. Moreover, mouse models of myocardial damage repair and cardiac progenitor dysfunction24,97, as well as age-induced or high-fat diet-induced vascular calcification and hyporeactivity82,98, showed improvements in cardiac and vascular function following clearance of senescent cells with D+Q treatment. In addition, mouse models of arteriovenous fistula occlusion show upregulation of senescent cell markers in the fistula that are reduced following D+Q treatment17.
Other common age-related conditions that are characterized by senescent cell accumulation have been modelled in mice. For example, in an injury-induced osteoarthritis mouse model99, treatment with the senolytic drug UBX0101, which targets the BCL-2 family of antiapoptotic factors, attenuated the development of post-traumatic osteoarthritis. Furthermore, frailty and vertebral disc degeneration were reduced by D+Q in progeroid Ercc1−/Δ mice82 and cirrhosis was attenuated by another senolytic drug targeting the BCL-2 pathway (A-1331852) in Mdr2−/− mice100 (Mdr2 is also known as Abcb4). In old mice, physical dysfunction and frailty were decreased by treatment with D+Q34. Moreover, treatment with D+Q reduced frailty, early onset of all age-related diseases and premature death in mice into which senescent cells had been transplanted34. As described earlier, impaired gait in mice after radiation-induced damage of a leg was improved after treatment with D+Q82. Finally, mortality associated with a range of diseases was decreased in aged mice that were treated with D+Q or another flavonoid senolytic drug, fisetin34,101. Thus, as predicted for agents that target fundamental ageing processes, senolytics seemingly hold promise for multiple disorders if these findings in mice can be translated into clinical application.
Ex vivo human studies and causality studies.
In addition to showing effects in mouse models of human diseases, the efficacy of senolytic agents can be evaluated by treating tissue explants from patients with specific diseases using different candidate senolytic drugs. This approach has been used for T2DM by treating adipose tissue explants from patients with T2DM undergoing bariatric surgery with senolytics34. Similar ex vivo human studies are ongoing for other diseases.
Before proceeding to clinical trials, it is important to establish whether the effects of a candidate agent for a disease state are due to actual senolytic effects or are due to off-target effects. Such evidence can be gained from preclinical models by fulfilling a modified set of Koch’s postulates to establish causality (BOX 2). Such proof of causality is provided for D+Q by a study of physical dysfunction (that is, frailty) in mice34. Moreover, studies are under way to establish causality regarding treatment of osteoarthritis with fisetin in mice and attenuation of insulin resistance, age-related osteoporosis and cognitive dysfunction by D+Q in mouse models. We are not aware of work nearing completion that attempts to prove that navitoclax, nutlins or other potential senolytic agents alleviate disorders in preclinical animal models principally by clearing senescent cells as opposed to through off-target effects. Studies addressing this question would be a valuable direction for future research.
Box 2 ∣. Modified Koch’s postulates to prove the senolytic properties of a drug.
Senescent cells are present in association with the phenotype.
Individuals without senescent cells do not have the phenotype.
Inducing accumulation of senescent cells causes the phenotype.
Clearing these induced senescent cells alleviates the phenotype.
Clearing naturally occurring senescent cells alleviates the phenotype.
The drug has few or no effects related to the phenotype in young individuals without senescent cells.
Administering senolytics intermittently is effective.
Senolytics should alleviate multiple age-related conditions.
Clinical trials of senolytic drugs.
Clinical trials of senolytics are currently under way for several chronic non-communicable diseases. In the first clinical trial of senolytic agents, D+Q alleviated physical dysfunction defined as low gait speed, decreased gait distance, difficulty arising from a chair and impaired function assessed by the short physical performance battery in a small trial in patients with idiopathic pulmonary fibrosis102. A second trial indicated that the senolytic agent dasatinib might decrease the number of senescent skin cells in patients with scleroderma, as skin senescence signatures and SASP signatures decreased after a 6-week trial of dasatinib103. Preliminary data from an ongoing trial of senolytics to alleviate dysfunction in patients with diabetic kidney disease57 showed that a single 3-day course of D+Q is sufficient to reduce the burden of senescent cells in adipose tissue and SASP factors in the circulation; measurements were taken 11 days after the last dose. This finding suggests that, similarly to mice, in humans an intermittent ‘hit-and-run’ senolytic drug administration strategy might be effective, which reduces the risks of adverse effects compared with continuous treatment.
Other clinical studies currently under way include trials of senolytic drugs for frailty in older women (aged >60 years)104 and for the premature ageing-like state in bone marrow transplant recipients105. In addition, clinical trials of senolytics are about to start for several disorders, for example age-related osteoporosis. These trials will contribute to testing whether the geroscience hypothesis holds in humans. So far, it seems that targeting senescent cells might be a promising potential approach for delaying, preventing or alleviating multiple age-associated and cellular senescence-associated conditions.
Senescence in endocrine diseases
As in other diseases, it is becoming clear that fundamental ageing mechanisms, in particular cellular senescence, have an important role in a number of endocrine diseases. Our current state of understanding of the role of cellular senescence in endocrine diseases and endocrine physiology is discussed in this section.
Osteoporosis.
For many years in the bone field, the question as to whether senescent cells accumulate within bone in the context of natural, chronological ageing as they do in other tissues remained unanswered. The type (or types) of cells in the bone microenvironment that become senescent with ageing and the nature of their SASP was also unclear. In the first study to address these questions106, our group measured in vivo senescence and SASP markers in young versus old male and female mice in highly enriched populations of various cell lineages, all of which were rapidly isolated from the bone microenvironment without extensive manipulation. This survey revealed that expression of the senescent cell biomarker p16Ink4a (also known as Cdkn2a) increased consistently (approximately fivefold to tenfold) in both sexes with ageing in bone-derived enriched populations of B cells and T cells, myeloid cells, osteoprogenitors, osteoblasts and osteocytes. Despite the viewpoint held by some researchers at the time that postmitotic cells (such as osteocytes) cannot undergo cellular senescence, we found that osteocytes from old mice also displayed senescent cell features, including higher expression not only ofp16Ink4a but also of p21Cip1 (also known as Cdkn1a) as well as an accumulation of SADS106.
Although the SASP can be variable depending on the cellular senescence inducer or type of senescent cell37,38,107, our analysis revealed that with ageing, senescent myeloid cells and senescent osteocytes in particular upregulate their production of multiple key SASP factors in mice106. Several of these SASP factors, including Hmgb1, Il1a, Il6, Il8, Mmp9, Mmp12, Nfkb1 and Pai2 (also known as Serpinb2), seem to be conserved across different types of senescent cells with ageing37,108,109. Our observation of an accumulation of senescent osteocytes in the bones of old mice has since been independently confirmed110. These findings have added to mounting evidence demonstrating that differentiated, non-dividing postmitotic cells, including adipocytes111, neurons112,113, hepatocytes114 and cardiomyocytes24, can acquire multiple key features of cellular senescence with ageing, such as high expression of p16Ink4a and p21Cip1 as well as increased levels of SADS and SASP factors compared with normal cells of the same type.
Consistent with these observations in mice, our group additionally found that the expression levels of senescence biomarkers p16INK4A and p21CIP1 were statistically significantly increased in small needle bone biopsy samples isolated from the iliac crest of older postmenopausal women compared with younger premenopausal women106. Despite the heterogeneous nature of the biopsy samples, which contained various populations of bone marrow cells and bone cell lineages, multiple SASP factors were detected at statistically significantly higher levels in the bones of older women than in those of younger women106. Collectively, these data establish that senescent cells accumulate in old bone in mice and humans, thereby implicating a potential role for these cells in the pathogenesis of osteoporosis.
To determine whether cellular senescence has a causative role in mediating age-related bone loss, our group targeted senescent cells in old mice by genetic clearance using INK-ATTAC mice; this model allows inducible systemic elimination of p16Ink4a-expressing cells, many but not all of which are senescent, on administration of a drug (AP20187)115. This approach was compared with systemic elimination of senescent cells by intermittent administration of senolytics (D+Q) or with treatment with a SASP inhibitor (ruxolitinib)75. Each of these approaches in old mice (20 months or older) improved bone microarchitecture and increased strength75. Reducing the overall burden of senescent cells resulted in suppressed bone resorption on both trabecular and cortical surfaces, as well as bone formation that was increased on endocortical surfaces or maintained on trabecular surfaces75. Several lines of evidence indicate that these favourable effects on bone remodelling and metabolism were mediated partly by eliminating a subset of senescent osteocytes. For example, osteocyte-enriched bone samples from old INK-ATTAC mice treated with AP20187 (the drug used to activate the suicide transgene in INK-ATTAC mice) had reduced p16Ink4a expression115 as well as decreased SADS. Furthermore, intermittent senolytic therapy in old wild-type mice reduced p16Ink4a expression as well as SADS in osteocyte-enriched bone samples75.
Further evidence supporting the favourable effects of senescent cell clearance on bone formation included our findings of increased osteoblast numbers and bone formation rates in combination with a reduction in bone marrow adipose tissue in old INK-ATTAC mice treated with AP20187 compared with vehicle-treated mice75. This finding is consistent with an alteration in the lineage commitment of stem cells towards the osteoblast and away from the adipocyte lineage following senescent cell clearance. Thus, several hallmarks of ageing bone, including reduced bone formation and increased bone marrow adiposity, were rescued at least partially by reducing the senescent cell burden in old mice75.
Currently available treatments for elderly patients with osteoporosis and the associated fragility fractures are suboptimal. Furthermore, the fear of very rare adverse effects (for example, osteonecrosis of the jaw or atypical femur fractures) and complications has led to a crisis whereby many patients who are in need of pharmacological therapy are either not being prescribed any available medications or simply not adhering to their therapy6. Moreover, from a therapeutic perspective, targeting senescent cells using the intermittent administration of senolytics seems to offer substantial advantages over conventional antiresorptive therapy116-118. Indeed, with antiresorptive therapy (for example, bisphos-phonates, denosumab, oestrogen or raloxifene), bone resorption is decreased as a result of the elimination of osteoclasts, which in turn (owing to the coupling of bone resorption to bone formation119) leads to a reduction in bone formation (FIG. 4).
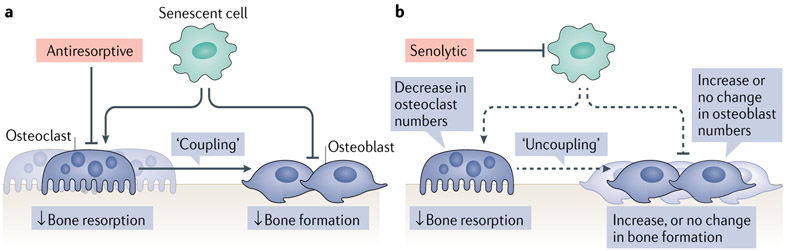
Fig. 4 ∣. The effects of antiresorptive versus senolytic therapies on bone metabolism.
a ∣ Senescent cells accumulate in the bone microenvironment with ageing, where they increase bone resorption by osteoclasts and reduce bone formation by osteoblasts. Antiresorptive drugs inhibit or eliminate osteoclasts and decrease bone resorption. Owing to coupling between osteoclasts and osteoblasts in the bone remodelling cycle, bone formation is also reduced. b ∣ Senolytic therapy reduces the burden of senescent cells, which leads to a reduction in bone resorption with either increased (cortical bone) or maintained (trabecular bone) bone formation, resulting in a beneficial ‘uncoupling’ between bone resorption and bone formation. Dashed lines indicate a reduction in the adverse effects of senescent cells on osteoclasts, osteoblasts, and the coupling between osteoclasts and osteoblasts. Reprinted from REF.75, Springer Nature Limited.
As described earlier, senescent cells accumulate in the bone microenvironment with ageing106, where they act to increase bone resorption and decrease bone formation75. Intermittent senolytic therapy reduces the burden of senescent cells, which in turn suppresses bone resorption with either an increase (cortical bone) or maintenance (trabecular bone) of bone formation, leading to favourable ‘uncoupling’ between osteoclasts and osteoblasts75 (FIG. 4). However, as with any novel therapeutic paradigm, a need exists for the discovery, development and optimization of interventions that target senescent cells to alleviate age-related bone loss. As such, further research is needed to better understand the underlying mechanisms that are altered in response to reducing the senescent cell burden in the setting of ageing. This knowledge is required to guide development and translation of senotherapeutics for treatment of osteoporosis in humans.
In addition to having a role in the pathophysiology of osteoporosis, cellular senescence might also be important in bone physiology in the growth plate and might be regulated by a local modulator of the growth plate, parathyroid hormone-related protein (BOX 3).
Box 3 ∣. Parathyroid hormone-related protein and regulation of the growth plate.
A series of studies157-159 have demonstrated that mice that express only a nuclear localization signal (NLS)-deficient form of parathyroid hormone-related protein (PTHrP) have growth retardation and an accelerated ageing phenotype in multiple tissues, which is characterized by increased cellular senescence in multiple tissues. Loss of the PTHrP NLS results in reduced expression of Bmi1, a member of the Polycomb–Trithorax gene family that regulates gene expression through chromatin remodelling157. Decreased Bmi1 expression leads, in turn, to increased expression of p16Ink4a and p21Cip1, triggering cellular senescence. Thus, PTHrP, through nuclear localization, serves to prevent senescence not only in bone but also in multiple other tissues.
These findings are perhaps related to a separate line of work showing that mesenchymal stem-progenitor cells (MSPCs) in the primary spongiosa of the long bones in mice at late puberty undergo programmed senescence, which is controlled by another member of the Polycomb family, the histone methyltransferase enhancer of zeste homologue 2 (EZH2)160. Temporally, this senescence of MSPCs is associated with cessation of longitudinal growth and fusion of the growth plates. The Indian hedgehog protein (IHH)–PTHrP signalling pathway is critical for maintaining the growth plate in an open phase161; therefore, nuclear PTHrP signalling could have a physiological role in preventing senescence of MSPCs in the primary spongiosa and thereby maintaining longitudinal growth. These actions would be complementary to the known effects of IHH-PTHrP signalling in delaying the differentiation of columnar chondrocytes into hypertrophic chondrocytes that also serve to keep the growth plate open161. However, further studies are needed to test this hypothesis.
Metabolic syndrome and T2DM.
The major risk factors for the development of T2DM are increasing age and obesity, each of which is associated with an increased burden of senescent cells. Senescent cells accumulate in adipose tissue of humans and mice in T2DM, obesity (primarily hypertrophic obesity) and age-related metabolic dysfunction18,20,23,26,111,120-123. In normoglycaemic individuals, adipocyte size is positively related to the number of markers of senescence present in subcutaneous fat20,124. Of note, in individuals with genetic predisposition for T2DM, increased senescent cell burden can occur even before T2DM develops124. Consistent with this finding, polymorphisms in genetic markers of cellular senescence (for example, p16INK4 (CDKN2A)) are associated with increased risk of developing both T2DM and cardiovascular disease125.
As described earlier, the cell cycle regulator p53 is also a senescence-associated regulator, particularly in adipose tissue. Furthermore, p53 expression is glucose responsive and a key mediator of adipogenesis126; p53 activity must be downregulated before adipose progenitors can undergo differentiation into insulin-responsive adipocytes. Activation of p53 and accumulation of ROS occur in adipose tissue early during the development of obesity, which prevents the normal differentiation of progenitors into adipocytes. This change can precede the appearance of insulin resistance, adipose tissue inflammation and glucose intolerance127. Additionally, activation of p53 reduces insulin-induced glucose transport and increases lipolysis in adipocytes, thereby contributing to both inflammation and insulin resistance127. In both ageing and T2DM, p53 activity and expression in adipose tissue is increased, and genetic activation of p53 in adipose tissue of mice leads to insulin resistance111.
Cellular senescence in adipose progenitors is a key negative regulator of adipogenesis, both through cell-autonomous mechanisms and by affecting neighbouring cells via the SASP23. For example, components of the SASP secreted by adipose-derived senescent cells can contribute to insulin resistance in metabolic tissues in vivo in mouse models by interfering with insulin signalling, impeding adipogenesis and attracting immune cells, each of which is a mechanism through which senescent cells can contribute to the risk of T2DM and metabolic syndrome26,34,121. Once formed, senescent adipose progenitors can affect the function of neighbouring cells by inhibiting adipogenesis, as demonstrated in co-culture experiments26. Additionally, senescent cells in general can directly promote insulin resistance through the secretion of SASP factors such as activin A, IL-6 and TNF59,128. In diet-induced obese (DIO) mice, senescent adipocytes can also chemoattract macrophages into visceral adipose tissue121. Chemokines released by these macrophages can further exacerbate T2DM.
Although cellular senescence can contribute to development of T2DM, the pathophysiological processes occurring in T2DM can themselves increase the burden of senescent cells. For example, elevated glucose and lipid levels, similarly to inflammation, can induce cellular senescence, which potentially amplifies the accumulation of senescent cells not only in adipose tissue but also systemically, particularly in uncontrolled T2DM18,129. Moreover, senescent-like pancreatic β-cells can accumulate in vivo in mice with T2DM and in ageing130. These cells might be capable of increased, rather than decreased, insulin secretion130. Perhaps paradoxically, type 1 diabetes mellitus in mouse models is partially alleviated and insulin production is increased in association with decreases in the abundance of these senescent-like β-cells following the administration of navitoclax (ABT263), a senolytic that targets senescent-like β-cells but not senescent adipocyte progenitors87,131. Thus, navitoclax acts by increasing insulin release and has little direct effect on peripheral tissue insulin sensitivity.
The senolytic combination therapy D+Q decreases the abundance of human senescent cultured adipocyte progenitors, a key cell type involved in the pathogenesis of T2DM and its complications82,121. Furthermore, D+Q is more effective in doing so than navitoclax131. In vivo, the abundance of senescent cells declines after intermittent administration of D+Q to DIO mice121. Mass cytometry analyses conducted after a single course of D+Q in these mice showed that adipose tissue senescent cells were statistically significantly reduced in number. Moreover, the main cell type targeted was adipose progenitor cells, rather than endothelial cells, macrophages or T cells. After D+Q administration, these DIO mice became more insulin sensitive, as indicated by insulin tolerance testing and an increased glucose infusion rate during hyperinsulinaemic clamping. In addition, AKT Ser473 phosphorylation (a marker of cellular insulin sensitivity) increased in response to insulin stimulation of adipose tissue freshly isolated from these mice121.
In addition to improved metabolic parameters, DIO mice treated intermittently with D+Q show decreased abundance of adipose tissue macrophages, as well as reductions in the crown-like structures that are associated with macrophage infiltration of adipose tissue in obesity and T2DM121. Notably, this decline in macrophage abundance was not due to the killing of macrophages by D+Q34. Rather, D+Q administration and associated reductions in the burden of senescent adipose progenitors caused decreased chemoattraction of monocytes into adipose tissue, despite the absence of changes in diet or weight. This finding is consistent with the hypothesis that senescent cells in adipose tissue attract, anchor and activate immune cells in T2DM associated with obesity23.
T2DM is associated with risk of complications involving the liver, heart, brain, nerves, eyes and kidneys. For example, T2DM is associated with an increased risk of non-alcoholic fatty liver disease (NAFLD), which is associated with metabolic syndrome. In humans, NAFLD is linked with increased abundance of senescent cells in the liver, and the extent of steatosis correlates with the senescent cell burden114. In mice, the induction of senescence in hepatocytes causes increased lipid deposition in the liver, suggesting a direct role of senescent hepatocytes in the pathogenesis of NAFLD. Furthermore, steatosis in these mice can be alleviated by administering D+Q114.
Cells in the media of the aorta and atherosclerotic plaques of both hypercholesterolaemic (Apoe−/−) and ageing mice have increased levels of senescence markers. Furthermore, the removal of senescent cells from these mice with D+Q leads to increased vascular smooth muscle reactivity in response to nitric oxide donors and decreased calcium deposition82,98, indicating that senescent cells have a role in vascular dysfunction associated with metabolic syndrome. Senolytic treatment of obese or ageing mice improves cardiac function, which has translational implications for patients with T2DM, in whom heart failure is common23,97. With ageing, cardiac progenitor cells develop a hypertrophic, profibrotic phenotype and undergo loss of replicative capacity. Senescent cell removal from mice alleviates the age-related dysfunction of cardiac progenitor cells and decreases the fibrotic area formed after myocardial infarction24,97,132.
Cellular senescence contributes to cognitive dysfunction in both DIO insulin-resistant mice and old mice113. Intermittent treatment with D+Q reduces the burden of senescent cells in the brain, restores neurogenesis and alleviates neuropsychiatric dysfunction in DIO mice113. Moreover, D+Q treatment also reduces neuroinflammation, restores neurogenesis, partly reverses brain atrophy and alleviates cognitive dysfunction in old mice with Alzheimer-like states due to tau or amyloid-β protein overexpression95,96.
Cellular senescence is increased in kidney cells from individuals with T2DM23,133. In addition, in DIO mice compared with lean mice, renal function is impaired, cortical oxygenation reduced and glomerulomegaly induced, which occur together with increases in markers of senescence (p16INK4A, p19 and p53) and the SASP in renal tubular cells. Quercetin improves renal function (decreases plasma levels of creatinine), improves oxygenation, decreases glomerulomegaly and reduces renal fibrosis in DIO mice133. Furthermore, D+Q treatment reduces proteinuria in DIO insulin-resistant mice121. In summary, the administration of senolytics alleviates a number of the complications of metabolic disease in mice.
Importantly, in patients with diabetic kidney disease, a 3-day course of oral D+Q treatment decreased the burden of senescent cells in adipose tissue and decreased adipose tissue macrophage infiltration and crown-like structures, together with decreased circulating levels of SASP factors57. Taken together, these findings in humans and mice support the importance of cellular senescence in the development of T2DM and its complications. They suggest that further clinical trials of reducing the senescent cell burden in both T2DM and its complications could be of value.
Cellular senescence in reproductive ageing.
A survey across tissues in rodent models of ageing showed increased p16Ink4a expression in multiple tissues, including the ovaries in old mice and rats and the testes in old rats but not old mice54. Caloric restriction markedly attenuated the age-related increase in p16Ink4a expression across many tissues, including the ovary and testes. To date, however, more data are available on the role of cellular senescence in ovarian versus testicular ageing. Accumulating evidence, principally from rodent models, suggests that cellular senescence has an important role in ovarian ageing. For example, shortened telomeres in ovarian cells can lead to replicative senescence and impair female reproduction. In addition, cellular senescence might limit the proliferation of primordial germ cells134.
Evidence suggests that cellular senescence could have a physiologically beneficial role during pregnancy by contributing to normal placental development. Furthermore, in the developing fetus, programmed cell senescence has been identified at multiple locations during embryonic development, where it facilitates tissue remodelling135,136. In addition, the SASP of senescent cells seems to contribute to the initiation of parturition. However, cellular senescence might also have a role in the impaired angiogenesis associated with pre-eclampsia137. Thus, for reproductive function, cellular senescence could represent an example of antagonistic pleiotropy, whereby it contributes to proper fetal development and timely parturition during the reproductive years but also to the risk of pre-eclampsia and a decline in ovarian function with ageing134. In addition, chemotherapy is a potent trigger of cellular senescence in multiple tissues64; therefore, cellular senescence might have an important role in the premature ovarian failure associated with chemotherapy, although further studies are needed to evaluate this.
Oncogene-induced cellular senescence in pituitary tumours.
Pituitary tumours, which are present at autopsy in ~20% of the population138, generally remain benign and rarely progress to carcinomas; indeed, malignant transformation occurs in less than 2% of all clinical pituitary tumours139. A number of mechanisms have been hypothesized to contribute to cessation of pituitary tumour cell proliferation, including G1 cell cycle arrest associated with DNA damage and p53-mediated induction of p21, leading to cellular senescence140. This process has been described as ‘oncogene-induced senescence’ and is believed to have a role in preventing malignant transformation by causing growth arrest of tumour cells. However, clinical evidence supporting this hypothesis is conflicting. One study found increased SA-βgal staining in pituitary adenomas compared with normal pituitary tissue in specimens from pituitary surgical operations141. By contrast, a second study found similar SA-βgal staining in both pituitary adenomas and carcinomas obtained from surgical operations, arguing against loss of cellular senescence as contributing to malignant transformation142. Thus, the role of cellular senescence in maintaining pituitary or other endocrine tumours in a cell cycle-arrested, benign state warrants further study.
Conclusions
It is now increasingly clear that cellular senescence has a key role in the pathogenesis of a number of age-associated disorders, including osteoporosis, metabolic syndrome, T2DM and probably several other endocrine diseases. Targeting senescent cells has emerged as an attractive therapeutic strategy to simultaneously treat several diseases, thereby reducing polypharmacy and the attendant risks of adverse events and drug interactions. Senolytic drugs are particularly attractive, as they seem to be effective even when given only intermittently, thus minimizing potential toxic effects. However, given the potential role of cellular senescence in reducing uncontrolled proliferation and cancer11, and potentially in wound healing64, carefully conducted clinical trials in humans are needed to better define the benefits and potential risks of these drugs.
Key points.
The ‘geroscience hypothesis’ posits that manipulation of fundamental ageing mechanisms will delay the appearance or severity of multiple chronic diseases because these diseases share the same underlying risk factor — namely, ageing.
Cellular senescence is a fundamental ageing mechanism that can contribute to or cause age-related phenotypes as well as multiple diseases, including endocrine disease, even in younger individuals (aged <40 years).
Some senescent cells, which accumulate with ageing, develop a proinflammatory, tissue-destructive and stem cell-disrupting or progenitor cell-disrupting senescence-associated secretory phenotype (SASP), which can spread senescence to nearby and distant non-senescent cells.
Senolytic agents have been discovered that selectively eliminate senescent cells by transiently disabling the survival pathways (senescent cell antiapoptotic pathways) that protect senescent cells against their own SASP.
Preclinical studies suggest that senolytics hold promise for delaying, preventing or treating many age-associated disorders.
Despite the growing experimental support for targeting cellular senescence to treat multiple age-associated diseases simultaneously, carefully conducted clinical trials in humans are needed to better define the benefits and risks of senolytic drugs.
Damage-associated molecular patterns
Extracellular nucleotides, Other cellular debris or proteins that are released by damaged cells (for example, HMCB1).
Pathogen-associated molecular patterns
Factors released by viral, fungal or bacterial pathogens.
Warburg shift
The preferential utilization of glycolysis rather than oxidative phosphorylation by a cell.
Lipofuscin
Lipid-containing pigment granules found in cells and associated with ageing.
Senescence-associated distension of satellites (SADS).
The distention of satellite DNA found in senescent cells.
Myeloproliferative syndrome.
Disorders of bone marrow and blood associated with the clonal proliferation of cells that may progress to leukaemia.
Koch’s postulates.
A set of criteria used to establish the cause of a disease.
Hypertrophic obesity.
Refers to enlarged adipocytes, typically found in abdominal obesity.
Acknowledgements
The authors acknowledge the support of the US National Institutes of Health through grants AG062413 (project 1, J.L.K.; project 2, S.K., J.N.F.), AG004875 (S.K.), AR027065 (S.K.), AR070241 (J.N.F.) and AG013925 (J.L.K.), the Translational Geroscience Network (AG061456; J.L.K.), Robert and Arlene Kogod, the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.) the Ted Nash Long Life Foundation (J.L.K.) and the Noaber Foundation (J.L.K.).
Footnotes
Competing interests
T.T. and J.L.K. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. Mayo Clinic has licensed patents on dasatinib and quercetin as senolytics to Unity Biotechnology. This research was reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic conflict of interest policies. All other authors declare no competing interests.
Peer review information
Nature Reviews Endocrinology thanks L. Hofbauer, D. Towler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirkland JL & Tchkonia T Clinical strategies and animal models for developing senolytic agents. Exp. Gerontol 68, 19–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocca WA et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin. Proc 89, 1336–1349 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy HB Polypharmacy reduction strategies: tips on incorporating American Geriatrics Society Beers and Screening Tool of Older People’s Prescriptions criteria. Clin. Geriatr. Med 33, 177–187 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Khosla S & Hofbauer LC Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 5, 898–907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SC et al. Impact of the U.S. Food and Drug Administration’s safety-related announcements on the use of bisphosphonates after hip fracture. J. Bone Miner. Res 31, 1536–1540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla S et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J. Bone Min. Res 32, 424–430 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Kennedy BK et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).This is a key review outlining the geroscience hypothesis, which posits that manipulation of fundamental mechanisms of ageing will delay (in parallel) the appearance or severity of multiple chronic diseases because these diseases share the same underlying risk factor — namely, ageing.
- 8.Goldman DP et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff. 32, 1698–1705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013).This is a landmark perspective summarizing nine fundamental hallmarks of ageing.
- 10.Hayflick L The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res 37, 614–636 (1965). [DOI] [PubMed] [Google Scholar]
- 11.Tchkonia T, Zhu Y, van Deursen J, Campisi J & Kirkland JL Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest 123, 966–972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stout MB, Tchkonia T & Kirkland JL Growth hormone in adipose dysfunction and senescence. Oncotarget 6, 10667–10668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchkonia T et al. Cellular senescence and inflammation in obesity. Obesity 17, S57 (2009). [Google Scholar]
- 14.Kandhaya-Pillai R et al. TNFalpha-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging 9, 2411–2435 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkland JL & Tchkonia T Cellular senescence: a translational perspective. EBioMedicine 21,21–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBrasseur NK, Tchkonia T & Kirkland JL Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr. Inst. Workshop Ser 83, 11–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath KA et al. The murine dialysis fistula model exhibits a senescence phenotype: pathobiologic mechanisms and therapeutic potential. Am. J. Physiol. Ren. Physiol 315, F1493–F1499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer AK et al. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64, 2289–2298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh P et al. Hyperoxia-induced cellular senescence in fetal airway smooth muscle cells. Am. J. Respir. Cell Mo!. Biol 61,51–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchkonia T et al. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran D et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell 13, 669–678 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Armstrong JL, Tchkonia T & Kirkland JL Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr. Opin. Clin. Nutr. Metab. Care 17, 324–328 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Palmer AK, Gustafson B, Kirkland JL & Smith U Cellular senescence: at the nexus between ageing and diabetes. Diabetologia 62, 1835–1841 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson R et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escande C et al. Deleted in Breast Cancer 1 regulates cellular senescence during obesity. Aging Cell 13, 951–953 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4, e12997 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andriani GA et al. Whole chromosome instability induces senescence and promotes SASP. Sci. Rep 6, 35218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baylis D et al. Inflammation, telomere length, and grip strength: a 10-year longitudinal study. Calcif. TissueInt 95, 54–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.d’Adda di Fagagna F et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003). [DOI] [PubMed] [Google Scholar]
- 30.von Zglinicki T, Petrie J & Kirkwood TB Telomere-driven replicative senescence is a stress response. Nat. Biotechnol 21, 229–230 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Freund A, Laberge RM, Demaria M & Campisi J Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tchkonia T & Kirkland JL Aging, cell senescence, and chronic disease: Emerging therapeutic strategies. JAMA 320, 1319–1320 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Coppé JP, Kauser K, Campisi J & Beauséjour CM Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem 281, 29568–29574 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Xu M et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med 24, 1246–1256 (2018).This study shows that treatment with senolytic agents has the potential to increase both healthspan and lifespan in aged mice.
- 35.Kim YM, Seo YH, Park CB, Yoon SH & Yoon G Roles of GSK3 in metabolic shift toward abnormal anabolism in cell senescence. Ann. NY Acad. Sci 1201, 65–71 (2010). [DOI] [PubMed] [Google Scholar]
- 36.De Cecco M et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez-Segura A et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol 27, 2652–2660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiley CD et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 23, 303–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laberge RM et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell 11,569–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moiseeva O et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 12, 489–498 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Laberge RM et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol 17, 1049–1061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huffman DM et al. Evaluating health span in preclinical models of aging and disease: guidelines, challenges, and opportunities for geroscience. J. Gerontol. A Biol. Sci. Med. Sci 71, 1395–1406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall BM et al. Aging of mice is associated with p16Ink4a- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 8, 1294–1315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debacq-Chainiaux F, Erusalimsky JD, Campisi J & Toussaint O Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc 4, 1798–1806 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Itahana K, Campisi J & Dimri GP Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol. Biol 371,21–31 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Hewitt G et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun 3, 708 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanson EC, Manning B, Zhang H & Lawrence JB Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J. Cell Biol. 203, 929–942 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davalos AR et al. p53-dependent release of alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol 201, 613–629 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall BM et al. p16Ink4a and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 9, 1867–1884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campisi J & d’Adda di Fagagna F Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol 8, 729–740 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Schafer MJ et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun 8, 14532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci 72, 780–785 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meuter A et al. Markers of cellular senescence are elevated in murine blastocysts cultured in vitro: molecular consequences of culture in atmospheric oxygen. J. Assist. Reprod. Genet 31, 1259–1267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnamurthy J et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest 114, 1299–1307 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeyapalan JC, Ferreira M, Sedivy JM & Herbig U Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev 128, 36–44 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waaijer ME et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 11, 722–725 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickson LJ et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).This is the first direct demonstration that administration of senolytic drugs reduces the number of senescent cells in humans.
- 58.Waldera Lupa DM et al. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J. Invest. Dermatol 135, 1954–1968 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Xu M et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, 301–310 (2015).Treatment with a JAK inhibitor reduces the SASP in vivo in mice and reduces indices of frailty.
- 60.Smith JR et al. Relationship between in vivo age and in vitro aging: assessment of 669 cell cultures derived from members of the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci 57, B239–B246 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Stout MB et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging 6, 575–586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menon R Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet. Gynecol. Sci 62, 199–211 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faget DV, Ren Q & Stewart SA Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Demaria M et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).This study demonstrates an important role for acute cellular senescence in the context of skin wound healing.
- 65.Singh M et al. Effect of low-dose rapamycin on senescence markers and physical functioning in older adults with coronary artery disease: results of a pilot study. J. Frailty Aging 5, 204–207 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Herranz N et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol 17, 1205–1217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison DE et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Kim SG & Blenis J Rapamycin: one drug, many effects. Cell Metab. 19, 373–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Majumder S et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell 11,326–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkinson JE et al. Rapamycin slows aging in mice. Aging Cell 11, 675–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y et al. Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci 69, 119–130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mannick JB et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Mau T, O’Brien M, Ghosh AK, Miller RA & Yung R Lifespan extension drug interventions affect adipose tissue inflammation in aging. J. Gerontol. A Biol. Sci. Med. Sci 75, 89–98 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arriola Apelo SI & Lamming DW Rapamycin: an inhibitor of aging emerges from the soil of Easter Island. J. Gerontol. A Biol. Sci. Med. Sci 71,841–849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farr JN et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med 23, 1072–1079 (2017).This study demonstrates that eliminating senescent cells or inhibiting their SASP prevents age-related bone loss in mice.
- 76.Verstovsek S et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med 363, 1117–1127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bannister CA et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab 16, 1165–1173 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Lamming DW et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mannick JB et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med 10, eaaq1564 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Larsson L et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev 99, 427–511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ & Robbins PD The clinical potential of senolytic drugs. J. Am. Geriatr. Soc 65, 2297–2301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu Y et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).This study provides the first identification of senolytic drugs, specifically the combination of dasatinib and quercetin.
- 83.Wang E Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55, 2284–2292 (1995). [PubMed] [Google Scholar]
- 84.Fuhrmann-Stroissnigg H et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun 8, 422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yi J-S et al. Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI Insight 1, e90220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Andrea G Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 106, 256–271 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Zhu Y et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang J et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med 22, 78–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baar MP et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Y et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 9, 955–963 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tchkonia T & Kirkland JL Therapeutic approaches to aging-reply. JAMA 321,901–902 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Kirkland JL Translating the science of aging into therapeutic interventions. Cold Spring Harb. Perspect. Med 6, a025908 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirkland JL, Stout MB & Sierra F Resilience in aging mice. J. Gerontol. A Biol. Sci. Med. Sci 71, 1407–1414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.St Sauver JL et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open. 5, e006413 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Musi N et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17, e12840 (2018).This study provides evidence that a mouse model of dementia is associated with cellular senescence in the brain.
- 96.Zhang P et al. Senolytic therapy alleviates A-beta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci 22, 719–728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis-McDougall FC et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roos CM et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeon OH et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med 23, 775–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moncsek A et al. Targeting senescent cholangiocytes and activated fibroblasts with Bcl-xL inhibitors ameliorates fibrosis in Mdr2−/− mice. Hepatology 67, 247–259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yousefzadeh MJ et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Justice JN et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martyanov V, Whitfield ML & Varga J Senescence signature in skin biopsies from systemic sclerosis patients treated with senolytic therapy: potential predictor of clinical response? Arthritis Rheumatol. 71, 1766–1767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03675724 (2020). [DOI] [PubMed]
- 105.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02652052 (2016). [DOI] [PubMed]
- 106.Farr JN et al. Identification of senescent cells in the bone microenvironment. J. Bone Min. Res 31, 1920–1929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiley CD et al. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16, 1043–1050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coppe JP et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coppe JP, Desprez PY, Krtolica A & Campisi J The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol 5, 99–118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piemontese M et al. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight 2, 93771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Minamino T et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med 15, 1082–1087 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Jurk D et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11,996–1004 (2012).This study provides in vivo evidence that postmitotic cells can develop a senescent-like phenotype.
- 113.Ogrodnik M et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061–1077 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogrodnik M et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun 8, 15691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baker DJ et al. Clearance of p16Ink4a-positive senescent cells delay aging-associated disorders. Nature 479, 232–236 (2011).This is a key article showing that clearance of senescent cells using a genetic approach delays ageing in a mouse model of premature ageing.
- 116.Khosla S, Farr JN & Kirkland JL Inhibiting cellular senescence: a new therapeutic paradigm for age-related osteoporosis. J. Clin. Endocrinol. Metab 103, 1282–1290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farr JN & Almeida M The spectrum of fundamental basic science discoveries contributing to organismal aging. J. Bone Min. Res 33, 1568–1584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farr JN & Khosla S Cellular senescence in bone. Bone 121, 121–133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khosla S Odanacatib: location and timing are everything. J. Bone Min. Res 27, 506–508 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Schafer MJ, Miller JD & LeBrasseur NK Cellular senescence: implications for metabolic disease. Mol. Cell Endocrinol 455, 93–102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palmer AK et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18, e12950 (2019).Clearance of senescent cells improves metabolic function in obese mice.
- 122.Palmer AK & Kirkland JL Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp. Gerontol 86, 97–105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kirkland JL, Hollenberg CH & Gillon WS Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am. J. Physiol 258, C206–C210 (1990). [DOI] [PubMed] [Google Scholar]
- 124.Gustafson B, Nerstedt A & Smith U Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat. Commun 10, 2757 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hannou SA, Wouters K, Paumelle R & Staels B Functional genomics of the CDKN2A/B locus in cardiovascular and metabolic disease: what have we learned from GWASs? Trends Endocrinol. Metab. 26, 176–184 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Krstic J, Reinisch I, Schupp M, Schulz TJ & Prokesch A p53 Functions in adipose tissue metabolism and homeostasis. Int. J. Mol. Sci 19, E2622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vergoni B et al. DNA damage and the activation of the p53 pathway mediate alterations in metabolic and secretory functions of adipocytes. Diabetes 65, 3062–3074 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Zaragosi LE et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 59, 2513–2521 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuki S et al. Hyperglycemia accelerated endothelial progenitor cell senescence via the activation of p38 mitogen-activated protein kinase. Circ. J 70, 1076–1081 (2006). [DOI] [PubMed] [Google Scholar]
- 130.Helman A et al. p16Ink4a-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med 22, 412–420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aguayo-Mazzucato C et al. Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 30, 129–142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walaszczyk A et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim SR et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl. Res 213, 112–123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Velarde MC & Menon R Positive and negative effects of cellular senescence during female reproductive aging and pregnancy. J. Endocrinol 230, R59–R76 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Munoz-Espin D et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Storer M et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).This study identifies cellular senescence as a physiological mechanism during embryonic growth.
- 137.Suvakov S et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol. Sex. Differ 10, 49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fernandez A, Karavitaki N & Wass JA Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol 72, 377–382 (2010). [DOI] [PubMed] [Google Scholar]
- 139.Scheithauer BW et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 59, 341–353 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Melmed S Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol 7, 257–266 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Manojlovic-Gacic E et al. Oncogene-induced senescence in pituitary adenomas-an immunohistochemical study. Endocr. Pathol 27, 1–11 (2016). [DOI] [PubMed] [Google Scholar]
- 142.Alexandraki KI et al. Oncogene-induced senescence in pituitary adenomas and carcinomas. Hormones 11, 297–307 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Justice JN et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci 73, 939–945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ashapkin VV, Kutueva II, Kurchashova SY & Kireev II Are there common mechanisms between the Hutchinson-Gilford progeria syndrome and natural aging? Front. Genet. 10, 455 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun S et al. HMGB1 and caveolin-1 related to RPE cell senescence in age-related macular degeneration. Aging 11,4323–4337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang AS & Dreesen O Biomarkers of cellular senescence and skin aging. Front. Genet 9, 247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Birch J, Barnes PJ & Passos JF Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol. Ther 183, 34–49 (2018). [DOI] [PubMed] [Google Scholar]
- 148.McShea A, Harris PL, Webster KR, Wahl AF & Smith MA Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am. J. Pathol 150, 1933–1939 (1997). [PMC free article] [PubMed] [Google Scholar]
- 149.Chinta SJ et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 22, 930–940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He Y et al. Cellular senescence and radiation-induced pulmonary fibrosis. Transl. Res 209, 14–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alam P et al. Inhibition of senescence-associated genes Rb1 and Meis2 in adult cardiomyocytes results in cell cycle reentry and cardiac repair post-myocardial infarction. J. Am. Heart Assoc 8, e012089 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Minamino T et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002). [DOI] [PubMed] [Google Scholar]
- 153.Papatheodoridi AM, Chrysavgis L, Koutsilieris M & Chatzigeorgiou A The role of senescence in the development of non-alcoholic fatty liver disease and progression to non-alcoholic steatohepatitis. Hepatology 71, 363–374 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Lee S & Schmitt CA The dynamic nature of senescence in cancer. Nat. Cell Biol 21,94–101 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Hou A et al. Cellular senescence in osteoarthritis and anti-aging strategies. Mech. Ageing Dev 175, 83–87 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Patil P et al. Systemic clearance of p16INK4a-positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell 18, e12927 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miao D et al. Severe growth retardation and early lethality in mice lacking the nuclear localization sequence and C-terminus of PTH-related protein. Proc. Natl Acad. Sci. USA 105, 20309–20314 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu M et al. The p27 pathway modulates the regulation of skeletal growth and osteoblastic bone formation by parathyroid hormone-related peptide. J. Bone Min. Res 30, 1969–1979 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y et al. DNA damage checkpoint pathway modulates the regulation of skeletal growth and osteoblastic bone formation by parathyroid hormone-related peptide. Int. J. Biol. Sci 14, 508–517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li C et al. Programmed cell senescence in skeleton during late puberty. Nat. Commun 8, 1312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karimian E, Chagin AS & Savendahl L Genetic regulation of the growth plate. Front. Endocrinol 2, 113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]