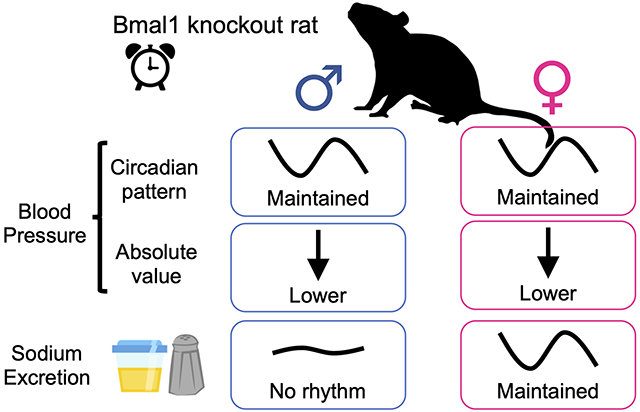
Abstract
The diurnal rhythms of sodium handling and blood pressure are thought to be regulated by clock genes, such as Bmal1. However, little is known about the regulation of these factors by Bmal1, especially in rats. Using a novel whole-body Bmal1 knockout rat model (Bmal1−/−), we hypothesized that time of day regulation of sodium excretion is dependent on Bmal1. Using telemetry to continuously record mean arterial pressure (MAP), we observed that male and female Bmal1−/− rats had significantly reduced MAP over the course of 24 hours compared to littermate controls. The circadian MAP pattern remained intact in both sexes of Bmal1−/− rats, which is in contrast to the Bmal1−/− mouse model. Male Bmal1−/− rats had no significant difference in baseline sodium excretion between 12 hour active and inactive periods, indicating a lack of diurnal control independent of maintained MAP rhythms. Female Bmal1−/− rats, however, had significantly greater sodium excretion during the active versus inactive period similar to controls. Thus, we observed a clear dissociation between circadian blood pressure and control of sodium excretion that is sex dependent. These findings are consistent with a more robust ability of females to maintain control of sodium excretion, and furthermore, demonstrate a novel role for Bmal1 in control of diurnal blood pressure independent of sodium excretion.
Keywords: Aryl hydrocarbon receptor nuclear translocator-like protein 1, circadian rhythms, blood pressure, sodium balance, sex differences, kidney
Summary
This study shows that the core clock gene, Bmal1, plays a role in maintaining blood pressure such that gene deletion of Bmal1 lowers blood pressure in both male and female rats. However, diurnal rhythms of sodium excretion were lost in knockout male, but not female rats consistent with 1) a dissociation between diurnal blood pressure and sodium excretion, and 2) females having redundant systems for maintaining diurnal patterns in excretory function compared to males.
Graphical Abstract
INTRODUCTION
Circadian rhythms are biological processes that occur in 24-hour periods or cycles and are present in essentially all life forms, including bacteria, fungi, plants, and animals.1 These biological rhythms are governed in part by transcription factors (clock genes) that oscillate in feedback loops to regulate the functions of different organs and tissues based on their level of expression at different times of the day. Following the development of gene editing techniques in mice that allowed for the knockout (KO) of specific clock genes, Bmal1 (brain and muscle Aryl hydrocarbon receptor nuclear translocator-like protein-1) has been recognized as a core regulator of the molecular clock.2 This is largely because whole-body loss of Bmal1 in mice abolishes all physiological circadian rhythms including activity, feeding, sleep, and blood pressure (BP) even when mice are maintained in a 12:12 hr. light:dark cycle that should synchronize rhythms driven by the suprachiasmatic nucleus (SCN) in the brain. The only other clock gene KO model that loses circadian BP rhythms is the Cryptochrome-1 (Cry1) and Cryptochrome-2 (Cry2) double knockout (Cry-null) mouse.3 However, the importance of Bmal1 in circadian control of physiological rhythms in larger species has yet to be directly tested.
In mammals, molecular clocks exist throughout the body with the central clock in the SCN generally acting as “the conductor” to reset and sync the peripheral clocks in other major organs in response to light. Peripheral molecular clocks have been implicated in control of circadian BP, as well as water and sodium (Na+) homeostasis by the kidneys.4 In one study, the kidney was reported to be the organ with the highest number of genes that oscillate in a circadian pattern second only to the liver.5 Further, we have known for many decades that renal functions, including the excretion of Na+, potassium (K+), and water, follow a strong circadian pattern independent of the time of day in which food and water are ingested as observed in both humans and non-human primates;6, 7 however, the mechanisms responsible for these rhythms have not been determined. While there is overwhelming evidence for renal control of Na+ excretion playing a critical role in BP control and hypertension, we do not know the relationship, if any, between circadian rhythms of Na+ excretion and BP.
Although genetically manipulated mice have revolutionized much of basic physiological research over the past few decades, this work has some limitations due to experimental approaches that are difficult or less accurate in mice, including renal balance studies. Focus on mouse models has resulted in reduced species diversity in preclinical studies that may limit the ability to predict human physiology and pathophysiology. Furthermore, in recent years, new genetic technology, has allowed for the development of gene deletion models in the rat. The current study describes what we believe is the first rat model of a clock gene knockout for Bmal1. Our studies were designed to determine the role of the core clock gene, Bmal1, on circadian control of BP and Na+ excretion in the rat.
MATERIALS AND METHODS
The data that support the findings of this study are archived within the University of Alabama at Birmingham Box.com account and are available from the corresponding author upon reasonable request.
Animals
Experiments were performed using male and female Bmal1KO (Bmal1−/−) rats created on a Sprague Dawley (SD) genetic background and their littermate controls (Bmal1+/+) obtained from our in-house breeding colony at the University of Alabama at Birmingham. These rats were created using Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas9) nuclease protein gene targeting technology with the help of the Gene Editing Rat Resource Center at the Medical College of Wisconsin. In our rat model, plasmids harboring three CRISPRs sgRNAs targeting the sequences (ggccccaccgacctgctctc, gactgcaaccgcaagaggaa, and gtccacaccactggtgctca) and expressing Cas9 were injected into the embryos of SD rats (Crl:SD, Charles River Laboratories). A founder animal harboring a 58 base pair deletion in exon 6 (first coding exon) of the Bmal1 gene was identified and confirmed by DNA sequencing (Supplemental Figure S1A). This founder was back crossed to the parental SD strain and colony of heterozygous breeders with the 58-bp mutation was established. To mitigate the effects of potential off-target mutational effects of CRISPR engineering8–10 and to maintain the outbred background of the model, a new SD breeder is introduced by back crossing into the colony at each generation. Otherwise, all breeding is conducted with male and female heterozygous rats. The deletion is predicted to cause a premature stop codon in exon 6 resulting in a severe truncation of the Bmal1 protein (Supplemental Figure S1A). To genotype, tail snip genomic DNA was extracted (Extracta DNA prep from QuantaBio, Beverly, MA), and polymerase chain reaction run using AccuSTart II PCR genotyping kit (QuantaBio) with the following primers(5’ to 3’): F1 GGAAACCTCATGCTTCCACAGGCA; R1 ACAATGCGGTTGTGGGCAGG. Control (+/+) rats have an amplicon of 333 base pairs (bp) while Bmal1 mutant (−/−) rats have an amplicon of 275 bp (Supplemental Figure S1B).
Animals were housed under conditions of constant temperature and humidity with a 12:12 hour light-dark cycle and maintained on standard rodent chow (Envigo 7917 Irradiated NIH-31 Open Formula Mouse/Rat Sterilizable Diet, 0.3% Na+ and 0.6% K+). Experimental protocols and animal care methods were approved by both the Medical College of Wisconsin and University of Alabama at Birmingham Institutional Animal Care and Use Committees in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Telemetry Blood Pressure Measurements
24-hour ambulatory BP measurements were made using PA-C40 or HD-S10 transmitters (Data Sciences International, Duluth, MN) following a protocol for implantation as previously described.11 In brief, 8–12 week old rats were anesthetized with 2–3% isoflurane, followed by exposure of the abdominal aorta by midline incision and brief occlusion of the aorta. The transmitter catheter was inserted into the distal portion of the abdominal aorta and held in place using tissue glue (Vetbond, 3M Corporation, St. Paul, MN). The transmitter body was sutured to the abdominal wall along the incision line as the incision was closed. Wound clips were used to close the skin. Rats were allowed to recover for at least one week after surgery before engaging in any experimental protocols at 14–18 weeks of age. Systolic arterial pressure, diastolic arterial pressure, mean arterial pressure (MAP), locomotor activity, and core body temperature was recorded for 10-second periods every 10 minutes.
Western Blot Analysis of Bmal1 Expression
Rats were anesthetized with 2–3% isoflurane, the kidneys were excised, decapsulated and cut longitudinally and dissected into three sections (cortex, outer and inner medulla), snap frozen in liquid nitrogen, and stored at −80°C. The cortex was used for determination of BMAL1 protein abundance. Cortex samples were homogenized using a nuclear isolation kit (Cayman Chemical Company, Ann Arbor, MI). Proteins from the nuclear compartment (20 μg) were used along with positive and negative controls taken from renal cortex of wildtype and Bmal1 knockout mice. Nuclear extracts were separated by a 15% SDS-PAGE and transferred to PVDF membranes. Non-specific protein binding was blocked by a 1 h incubation in blocking buffer (Odyssey® Blocking buffer in TBS, Li-Cor, Lincoln). Bmal1 was detected by a primary rabbit polyclonal antibody (Signalway Antibody LLC, Baltimore, MD, catalog # 21415) with a donkey anti-goat IgG-Alexa Fluor 680 (0.2 μg/ml, A32860, ThermoFisher) by the Odyssey CLx (Li-Cor). Specificity was confirmed in an identical blot with the exception of incubating the primary antibody with the antigenic peptide (catalog # 61415, Signalway Antibody LLC).
Immunohistochemical identification of Bmal1
Kidneys were formalin fixed and embedded in paraffin wax prior to being sectioned to 5 micron and placed on superfrost plus slides (Fisher Scientific). Slides were processed as previously described.12 Bmal1 primary antibody was incubated with the slide overnight at 4°C (rabbit monoclonal antibody at a dilution of 1:500; Cell Signaling Technology, catalog # 14020), and then visualized with horseradish peroxidase coupled secondary antibodies (Rabbit HRP-polymer, Cat # RMR622H, BioCare Medical, Pacheco, CA) and 3,3-diaminobenzidine (Vector Labs). Slides were counterstained for 1 min with hematoxylin (Hematoxylin, S3302, Dako North America, Inc. Carpinteria, CA).
Quantitative Droplet Digital PCR Analysis of Tissue Clock Gene Expression
Liver or renal tissue (50–100 mg) was homogenized in trizol reagent and RNA was extracted according to manufacturer’s instructions (ThermoFisher Corp., Waltham, MA). RNA from brain tissue containing primarily SCN (10–20 mg) was extracted using a MicroAqueous kit from the same manufacturer. Reverse transcription was carried out with 1 μg of DNAse-treated RNA using the iScript Reverse Transcription kit BioRad cDNA synthesis kit (Product # 1708891, BioRad Laboratories, Hercules, CA). Next, gene expression was carried out by digital droplet PCR. The digital droplet PCR reaction was set up using ddPCR probes Supermix, one μL of TaqMan primer/probes, and cDNA from 50 ng of total RNA. Primers were also designed within the deleted region (Forward- 5’-CTCTCCGGTTCCCTGAGCA-3’and reverse5’-ACTTTCTTGGTAGTCAGTGGCA-3’. For this reaction, ddPCR EvaGreen supermix was used in place of ddPCR probes supermix (BioRad, Hercules, CA). The reaction mix was separated into nanodroplets using the automated droplet generator (BioRad). PCR was carried out for 40 cycles per manufacturer’s instructions. Droplets were counted using the QX200 Droplet Reader and data was analyzed and copy count calculated using Quanta Soft software. The TaqMan probes used for digital droplet PCR (Per1: Rn01325256_m1, Per2: Rn01427704_m1, Cry1: Rn01503063_m1, Cry2: Rn01485701_m1, Bmal2: Rn01459351_m1, Dbp: Rn01498425_m1, Rev-ERB-α: Rn01460662_m1, and Clock: Rn00573120_m1) were purchased from ThermoFisher (Waltham, MA).
Baseline Urine Collections and Acute Salt Loading Protocol
We monitored food and water intake and collected urine in 12-hour intervals in metabolic cages in both male and female Bmal1+/+ and Bmal1−/− rats. On Day 3 of the experiment, rats were randomly divided into two groups. The first group was given an acute NaCl load (900 μEq Na+ in 1 mL H2O) by oral gavage at Zeitgeber Time (ZT) 12, while the second group was given the NaCl load at ZT0 as described previously.13 Measurements for urine volume, food and water were continued for two additional days in 12-hour increments. After the final collection period (5½ days: 11 consecutive 12-hour time points), rats were returned to regular cages for 3–4 days before repeating the protocol for a follow-up cross-over experiment. For the second week of experiments, the time of day for acute salt loading was reversed for each individual rat, meaning that all rats that received an acute salt load at ZT0 or ZT12 in the first week then alternatively received a salt load at ZT12 or ZT0. After completion of the experiment, the urine was analyzed for electrolyte content with an EasyLyte Na+/K+/Cl−/Li+ analyzer (Medica Corporation, Bedford, MA).
Statistical Analysis
Statistical analysis of each specific data set is indicated in the figure legends. For telemetry data, cosinor analysis was used to determine the Midline Estimating Statistic of Rhythm (MESOR), acrophase, and amplitude of the MAP data using the equation Y(x) = MESOR + Amplitude*cos(2πx/Period+Acrophase). The MESOR is a circadian rhythm-adjusted mean, the amplitude is a measure of half the extent of predictable variation within a cycle, the acrophase is a measure of the time of overall high values recurring in each cycle, and the period is the duration of one cycle.14 Period = 24 hours. Statistical analyses were performed using GraphPad Prism software version 7.0d for Mac OS X (GraphPad Software, San Diego, CA). Results with P < 0.05 were considered statistically significant.
RESULTS
Validation of Bmal1−/− Rat Model
Bmal1 mutant Sprague Dawley rats were generated using CRISPR/CAS technology.15 Genomic DNA sequencing of mutant (Bmal1−/−) rats determined that there was a 58 bp deletion in exon 6 of the Bmal1 gene (Arntl) just downstream of the start codon (Supplemental Figure S1A, S1B). This deletion results in a frameshift mutation and a premature stop codon leading to the prediction of a total loss of protein expression and function (Supplemental Figure S1A, S1B). To confirm the loss of Bmal1 expression, Western blots of renal cortex were performed with samples from male Bmal1+/+ and Bmal1−/− animals collected between ZT4–5, a time when Bmal1 protein expression is normally highest. In both renal cortical and liver nuclear extracts, there was no measurable Bmal1 expression from Bmal1−/− rats at the predicted molecular weight (Figure 1A). However, we observed what appears to be a truncated protein at a smaller molecular weight only evident in tissue from the Bmal1−/− rat. Immunohistochemical analysis of kidney cortex revealed a strong Bmal1 signal in nuclei of Bmal1+/+ rats (Figure 1B), but not Bmal1−/− rats (Figure 1C). Finally, we confirmed that the deletion was successful by performing PCR for Bmal1 using primers directed at the target region of our CRISPR. As expected, control animals had a much higher Bmal1 mRNA expression at ZT0 compared to ZT12 in the kidney, SCN and liver (Figure 1D–F), but no expression in Bmal1−/− rats was detected.
Figure 1:
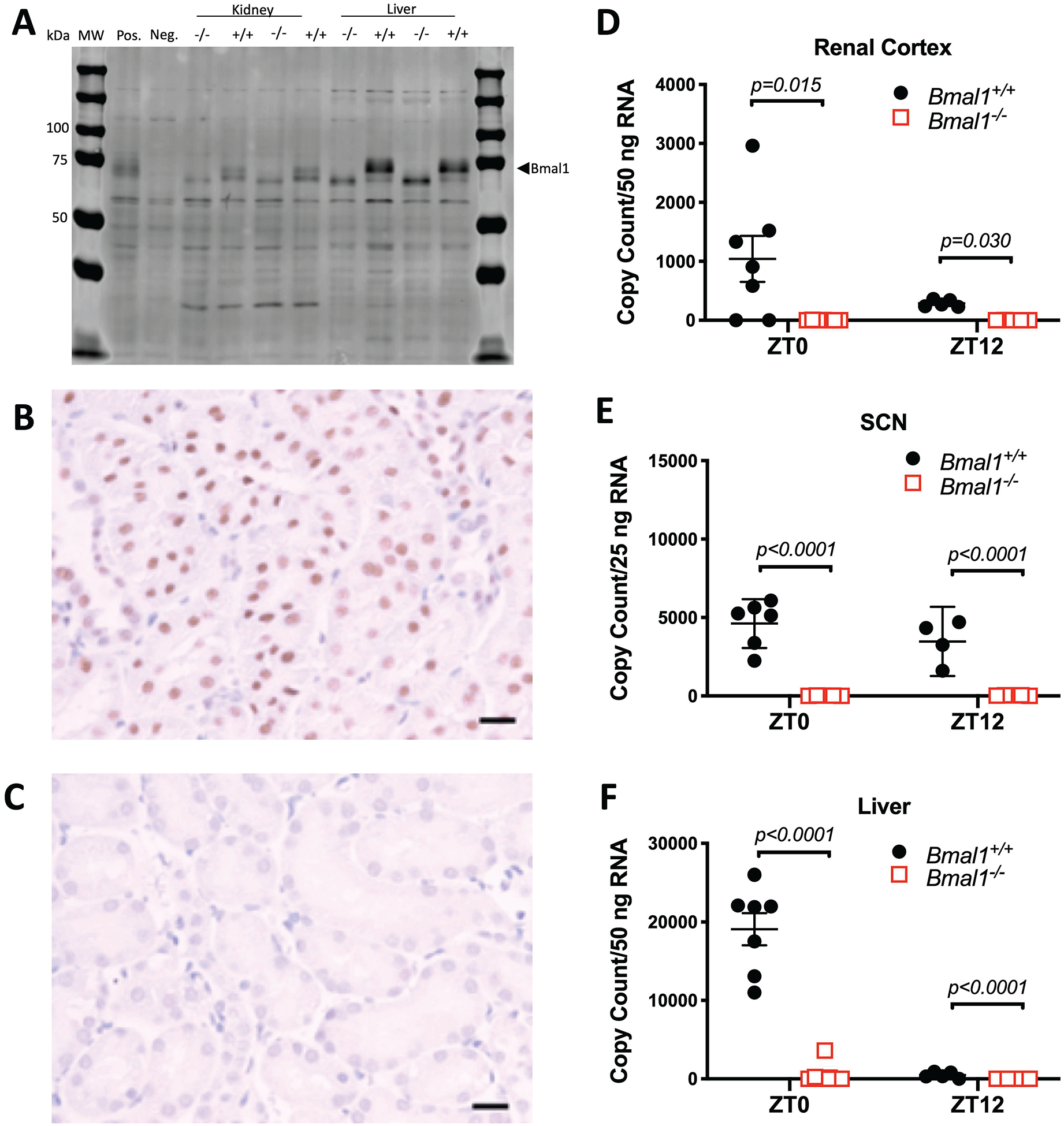
Western blot showing expression of Bmal1 in the nuclear compartment of the renal cortex and liver of control (+/+) and mutant (−/−) male rats (panel A). This band of 90 kDa was absent in the nuclear fraction of the −/− rats. Bmal1 is not expressed in the cytosol of either the +/+ or −/− rats. The positive (Pos.) and Negative (Neg.) controls are nuclear homogenates from kidneys of global Bmal1 knockout and wildtype littermate mice (MW, molecular weight). Additional validation of the Western blot approach is shown in Supplemental Figure S12. Immunohistochemical stain of renal cortex from control male rat (+/+) using hematoxylin as the counter stain (panel B). Bmal1 is indicated in brown and hematoxylin in blue. Horizontal bar indicates 20 μm. Immunohistochemical stain of renal cortex from male mutant rat (−/−) showing lack of Bmal1 staining in the nucleus (panel C). Digital droplet PCR for Bmal1 mRNA in tissues from renal cortex, SCN and liver (panels D, E, and F, respectively). As expected, Bmal1 mRNA expressed as copy number is elevated at zeitgeber time (ZT) 0 and lowest at ZT12 in control tissues while there is no detectable message in any tissue from mutant (−/−) rats. Data were analyzed by 2-way ANOVA with Tukey’s multiple comparison test.
To confirm the loss of Bmal1 function, we examined mRNA expression of two circadian clock-controlled genes known to be under the control of Bmal1. We observed a diurnal pattern for albumin promoter (albumin D-box) binding protein (Dbp) and nuclear receptor subfamily 1, group D, member 1 (NR1D1, also known as REV-ERBα) expression in the renal cortex of male Bmal1+/+ rats (Figure 2A and 2B, respectively). In contrast, the diurnal pattern of expression of both Dbp and of REV-ERBα was significantly reduced in the same tissues taken from male Bmal1−/− rats (Figure 2A and 2B, respectively). Importantly, Bmal1 mRNA expression in the central clock (SCN) was also significantly reduced and arrhythmic (Supplemental Figure S2). Similar findings were observed in the renal inner medulla (Supplemental Figure S4), outer medulla (Supplemental Figure S5), and liver (Supplemental Figure S6). These data indicate that we have a functional knockout of Bmal1 and that compensation has not occurred to promote the expression of these two established Bmal1 output genes.16, 17
Figure 2:
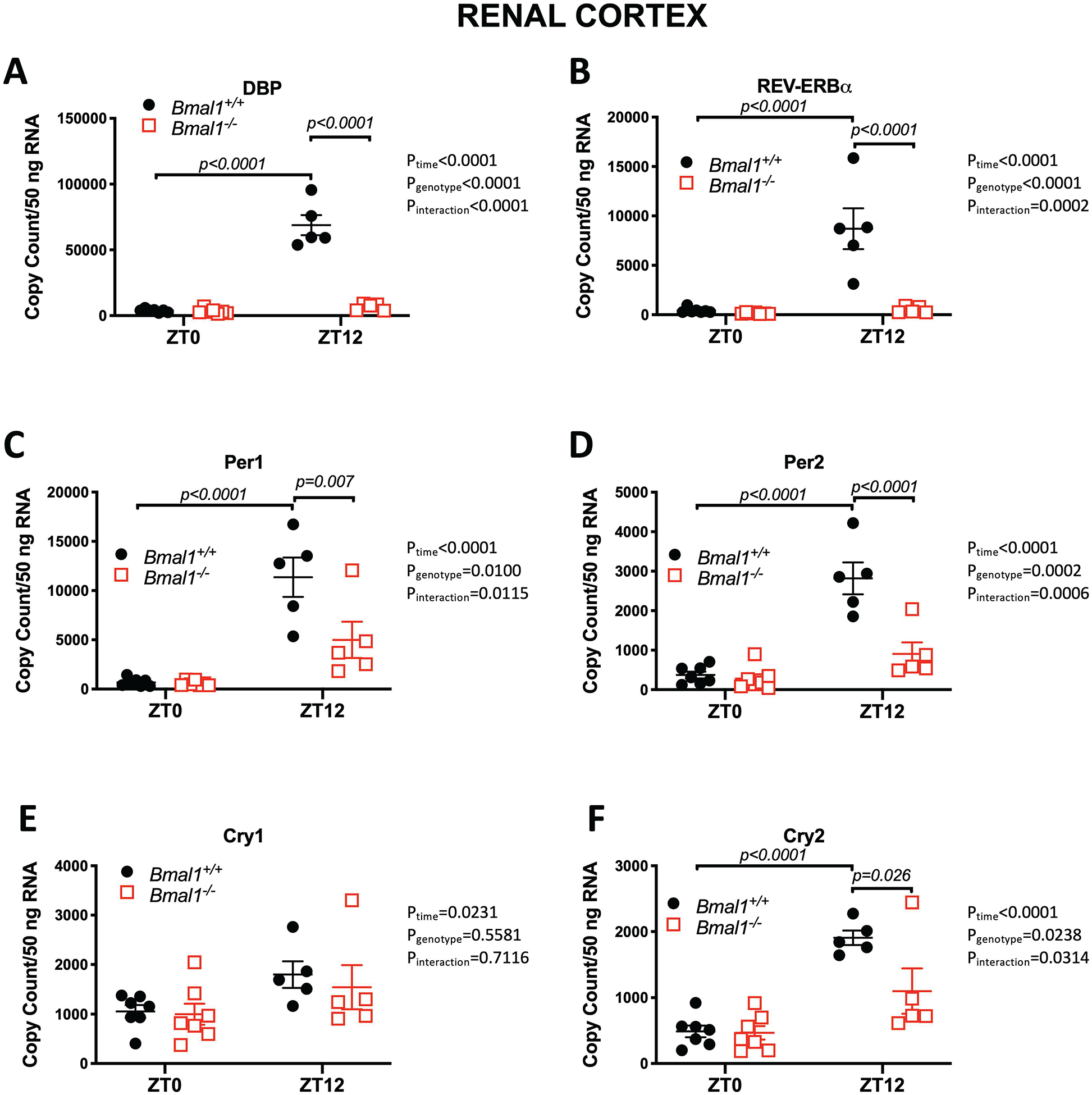
Effects of Bmal1 mutation on clock-controlled genes in the renal cortex of male rats. *P< 0.05 vs. Bmal1+/+ at ZT12. Results from two-way ANOVA indicated to the right of each individual graph. Tukey’s multiple comparison test was used for individual comparisons.
The Bmal1+/+ rats displayed expected time-of-day differences in Per1, Per2, Cry1 and Cry2 expression at ZT0 compared to ZT12 in both kidney and liver tissue (Figure 2 and Supplemental Figures S4–S6). In contrast, Bmal1−/− rats had a significant reduction in Per1, Per2, and Cry2 mRNA expression in the renal cortex at ZT12 compared to Bmal1+/+ rats (Figure 2C, 2D, and 2F). Cry1 expression was not different between genotypes (Figure 2E). Expression of Clock, the transcription factor that forms a heterodimer with Bmal1, was found to be similar between genotypes in the renal cortex (Supplemental Figure S3B). Inner medullary tissue showed significant differences in Cry2 and Clock mRNA expression, with Cry2 significantly lower and Clock significantly higher in Bmal1−/− rats compared to Bmal1+/+ at ZT12 (Supplemental Figure S4). There were no significant differences in Cry1, Cry2, and Clock expression between genotypes in outer medullary tissue (Supplemental Figure S5). In liver tissue, mRNA expression for Cry2 was significantly less in Bmal1−/− compared to Bmal1+/+ rats while Clock expression was significantly greater in the Bmal1−/− rats (Supplemental Figure S6).
To examine whether Bmal2 mRNA would be changed in compensation to the loss of Bmal1, we measured Bmal2 mRNA expression in SCN, kidney and liver tissue. First, it is important to note that Bmal2 expression was extremely low in all of these tissues with copy numbers well largely below 200 per 50 ng of RNA (Supplemental Figures S2H, S4H, S5H and S6H). We did not detect any significant differences in expression of Bmal2 between in SCN kidney or livers between the Bmal1+/+ or Bmal1−/− rats.
General Phenotype of Bmal1−/− Rats
Age-matched (11–16-week-old) male and female Bmal1−/− rats had significantly lower body and organ weights compared to their respective wild type controls (Supplemental Figure S7). We then examined if loss of Bmal1 had an effect on the diurnal pattern of urinary Na+ excretion (UNaV). As expected, male Bmal1+/+ rats display a regular diurnal pattern in UNaV with an average of 61 ± 2 % of their Na+ excreted during the active period (Figure 3A). However, Bmal1−/− rats did not exhibit this pattern in UNaV with only 45 ± 2 % being excreted during the active period. The difference in UNaV between the active and inactive periods (ΔUNaV) over this time period was significantly lower in Bmal1−/− rats (Figure 3B). In contrast, female Bmal1−/− rats show a diurnal UNaV similar to their littermate controls (Figure 3C). As a result, no significant difference in ΔUNaV was seen between genotypes in female rats (Figure 3D); female Bmal1+/+ and Bmal1−/− rats excreted 60 ± 2 % and 58 ± 2 % of their UNaV during the active period, respectively (Figure 3C).
Figure 3:
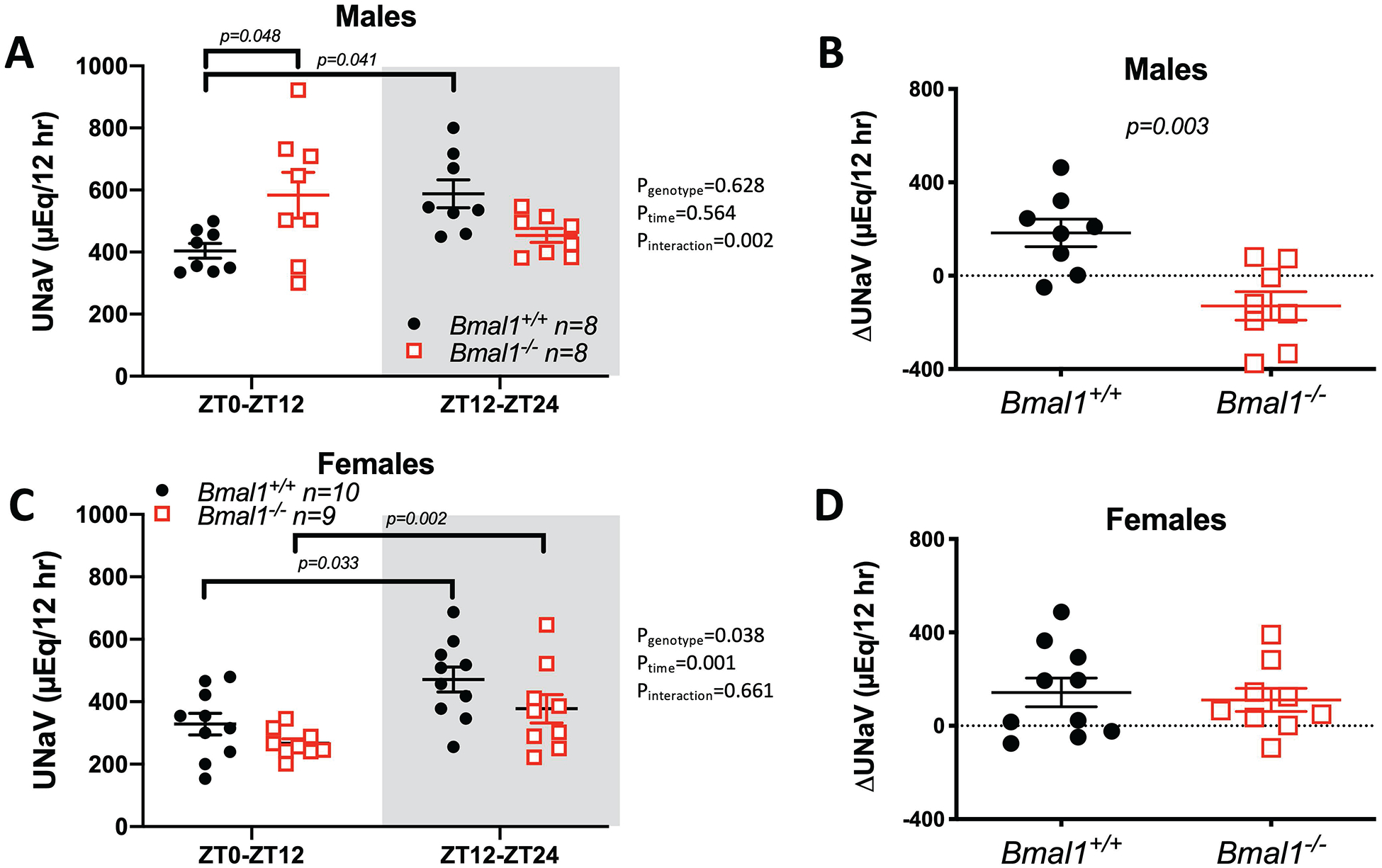
Diurnal pattern of urinary Na+ excretion (UNaV) in male and female Bmal1+/+ and Bmal1−/− rats (panels A, C, respectively). Shaded areas represents the 12 hour active period where lights are off. 2-way ANOVA was used for statistical analysis with post-hoc results indicated when statistically significant. Panels B and D show active minus inactive period differences in Na+ (ΔUNaV) in male and female rats, respectively (Student’s t-test results indicated).
Supplemental Figure S8 shows the urine volume data from the 12-hour urine collections. The time-of-day pattern of urine volume was not particularly robust in either male Bmal1+/+ or Bmal1−/− rats, although the male Bmal1−/− rats excreted significantly less urine compared to controls. There was no significant difference in the active versus inactive period urine volume. Urine volume in both female Bmal1+/+ and Bmal1−/− rats followed a diurnal pattern, with urine volume higher during the active period. The difference in urine volume between active and inactive periods was not significantly different between female genotypes.
Male Bmal1+/+ and Bmal1−/− rats showed a significant time-of-day difference in urinary potassium excretion (UKV), excreting significantly more K+ during the active period (Supplemental Figure S8E). The smaller night-day change in UKV (ΔUKV) in Bmal1−/− rats was not statistically significant (Supplemental Figure S8F). In female rats, Bmal1+/+ excreted significantly more K+ during the active period compared to the inactive period (Supplemental Figure S8G). However, UKV during the active period was also significantly greater in female Bmal1+/+ compared to Bmal1−/−. Female Bmal1−/− rats did not appear to have a diurnal pattern of UKV although the average active-inactive UKV (ΔUKV) was not significantly different between genotypes (Supplemental Figure S8H).
Food intake in both male and female rats of both genotypes followed a clear diurnal pattern (Supplemental Figure S9). Overall, the knockout rats consumed significantly less food, which was driven by lower consumption during the active period. This was evident when calculating the difference in food intake between active and inactive periods as Bmal1−/− rats had a significantly lower difference in food intake between time periods (Supplemental Figure S9B and S9D). Differences in water intake between genotypes were not as clear. In males, Bmal1−/− rats had slightly higher overall water intake compared to Bmal1+/+ (Supplemental Figure S9E), yet the active-inactive difference was not different between genotypes (Supplemental Figure S9F). In females, water intake tended to be lower in Bmal1−/− rats, but there were no significant differences between genotypes (Supplemental Figure S9G) or in the diurnal pattern between genotypes (Supplemental Figure S9H).
Continuous MAP recordings by telemetry revealed a diurnal rhythm in MAP in both male and female Bmal1+/+ and Bmal1−/− rats over a 72-hour period (Figure 4A and 4B). Using cosinor analysis, Bmal1−/− rats were found to have a significantly lower mesor than Bmal1+/+ rats, indicating a lower MAP that was largely driven by the lower pressure in female Bmal1−/− rats (Table 1). There were no significant differences in amplitude or acrophase between genotypes for either sex. Similar to MAP, both SBP and DBP were significantly lower in Bmal1−/− compared to Bmal1+/+ rats driven by lower pressures in females (Supplemental Figures S10A, S10B, S10C and S10D). Again, amplitude and acrophase of SBP and DBP were not significantly different between sex or genotypes (Table 1). For heart rate (HR), the mesor of female rats was consistently higher than males in both Bmal1+/+ and Bmal1−/− rats, but there was no significant genotype difference (Supplemental Figures S10E and S10F). However, females had an overall significantly lower amplitude in HR that was more pronounced in the Bmal1−/− female rats (Table 1). Although the acrophase was not significantly different between sexes, the acrophase was significantly delayed in Bmal1−/− rats. Spontaneous activity measured by telemetry displayed a somewhat regular diurnal rhythm, albeit quite variable, that was not different between sex or genotype (Figure 4C and 4D). Finally, body temperature was also rhythmic, and the mesor was slightly, but significantly higher in female versus males regardless of genotype or sex (Figure 4E and 4F). There was also a significantly lower amplitude in female rats that was driven by the lower amplitude in female Bmal1−/− rats (Table 1).
Figure 4:
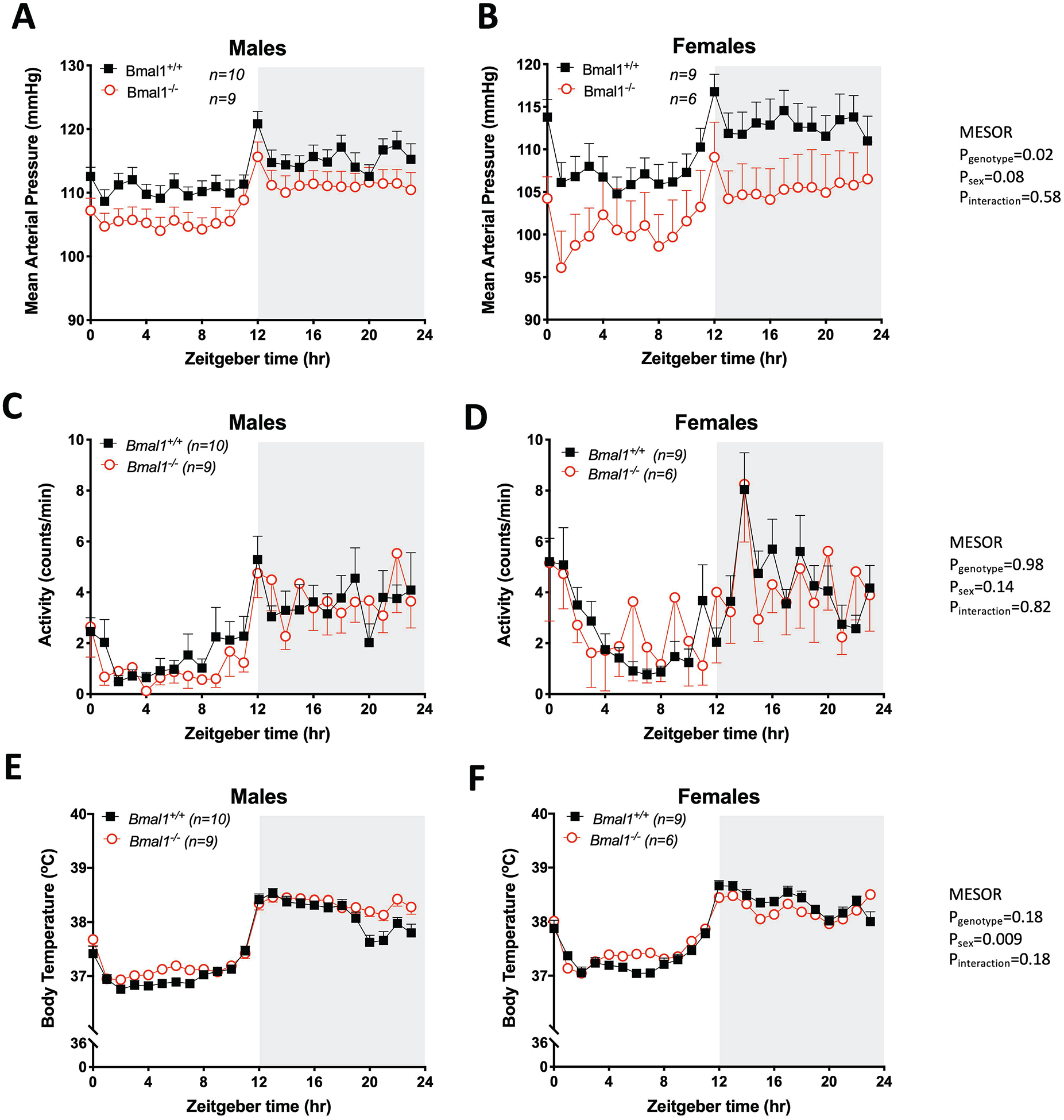
24-hour mean arterial pressure (MAP), locomotor activity and core body temperature as measured by telemetry in Bmal1+/+ and Bmal1−/− rats. Data were collected over 3 consecutive 24-hour periods in males (A,C, E) and females (B, D, F). 2-way ANOVA results for mesor are indicated. Remaining cosinor analysis comparing genotypes are indicated in Table 1.
Table 1 –
Cosinor Analysis of Telemetry Data
| Mean Arterial Blood Pressure | |||||||
|---|---|---|---|---|---|---|---|
| Bmal1+/+ | Bmal1−/− | ||||||
| Males (10) | Females (9) | Males (9) | Females (6) | Pgenotype | Psex | Pinteraction | |
| Mesor (mmHg) | 113.1 ± 1.6 | 110.2 ± 2.3 | 108.5 ± 2.1 | 103.0 ± 3.7a | 0.02 | 0.08 | 0.58 |
| Amplitude (mmHg) | 3.2 ± 0.4 | 4.0 ± 0.4 | 3.9 ± 0.4 | 3.2 ± 0.4 | 0.87 | 0.89 | 0.07 |
| Acrophase (ZT) | 17.0 ± 0.3 | 17.4 ± 0.2 | 17.2 ± 0.2 | 17.3 ± 0.3 | 0.99 | 0.33 | 0.65 |
| Systolic Blood Pressure | |||||||
| Bmal1+/+ | Bmal1−/− | ||||||
| Males (10) | Females (9) | Males (9) | Females (6) | Pgenotype | Psex | Pinteraction | |
| Mesor (mmHg) | 138 ± 1.7 | 136.5 ± 3.0 | 133.8 ± 3.0 | 124.6 ± 4.1a,b | 0.009 | 0.07 | 0.21 |
| Amplitude (mmHg) | 3.9 ± 0.4 | 5.0 ± 0.5 | 5.4 ± 0.4 | 3.7 ± 0.6 | 0.89 | 0.48 | 0.007 |
| Acrophase (ZT) | 17.5 ± 0.3 | 17.9 ± 0.2 | 17.9 ± 0.2 | 17.4 ± 0.6 | 0.79 | 0.79 | 0.20 |
| Diastolic Blood Pressure | |||||||
| Bmal1+/+ | Bmal1−/− | ||||||
| Males (10) | Females (9) | Males (9) | Females (6) | Pgenotype | Psex | Pinteraction | |
| Mesor (mmHg) | 93.6 ± 1.6 | 89.8 ± 2.2 | 89.2 ± 1.7 | 84.8 ± 3.9 | 0.04 | 0.08 | 0.88 |
| Amplitude (mmHg) | 3.2 ± 0.3 | 4.4 ± 0.7 | 3.5 ± 0.5 | 3.5 ± 0.3 | 0.52 | 0.23 | 0.22 |
| Acrophase (ZT) | 17.0 ± 0.4 | 16.8 ± 0.3 | 16.7 ± 0.2 | 16.7 ± 0.3 | 0.58 | 0.68 | 0.67 |
| Heart Rate | |||||||
| Bmal1+/+ | Bmal1−/− | ||||||
| Males (10) | Females (9) | Males (9) | Females (6) | Pgenotype | Psex | Pinteraction | |
| Mesor (mmHg) | 354.3 ± 4.9 | 360.5 ± 5.4a,c | 332.3 ± 4.8 | 367.9 ± 4.0a,c | 0.78 | <0.0001 | 0.98 |
| Amplitude (mmHg) | 46.4 ± 1.9 | 43.4 ± 2.5 | 42.7 ± 3.3 | 34.0 ± 3.3a | 0.028 | 0.047 | 0.32 |
| Acrophase (ZT) | 15.8 ± 0.1 | 15.9 ± 0.1c | 16.5 ± 0.1a | 16.2 ± 0.2 | 0.0002 | 0.42 | 0.17 |
| Activity | |||||||
| Bmal1+/+ | Bmal1−/− | ||||||
| Males (10) | Females (9) | Males (9) | Females (6) | Pgenotype | Psex | Pinteraction | |
| Mesor (mmHg) | 2.5 ± 0.3 | 3.3 ± 0.4 | 2.4 ± 0.5 | 3.6 ± 1.6 | 0.92 | 0.13 | 0.73 |
| Amplitude (mmHg) | 1.8 ± 0.3 | 1.9 ± 0.2 | 1.9 ± 0.8 | 1.4 ± 0.8 | 0.52 | 0.59 | 0.33 |
| Acrophase (ZT) | 17.8 ± 0.5 | 18.4 ± 0.3 | 17.6 ± 0.3 | 17.8 ± 0.8 | 0.37 | 0.36 | 0.66 |
| Body Temperature | |||||||
| Bmal1+/+ | Bmal1−/− | ||||||
| Males (12) | Females (10) | Males (9) | Females (7) | Pgenotype | Psex | Pinteraction | |
| Mesor (mmHg) | 37.2 ± 0.1 | 37.8 ± 0.1a | 37.7 ± 0.1 | 37.8 ± 0.1a | 0.18 | 0.009 | 0.18 |
| Amplitude (mmHg) | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.5 ± 0.1a,b,c | 0.002 | <0.001 | 0.006 |
| Acrophase (ZT) | 17.2 ± 1.7 | 15.7 ± 1.8 | 15.8 ± 0.9 | 18.9 ± 1.8 | 0.70 | 0.72 | 0.12 |
MESOR = mean estimated statistic of rhythm; () = n/group; 2-way ANOVA with Tukey’s post-hoc test for multiple comparisons;
P < 0.05 vs. Bmal+/+ male;
P < 0.05 vs. Bmal+/+ female;
P < 0.05 vs. Bmal−/− male
Response to Acute Salt Loading
To determine if the loss of Bmal1 impacts the ability of rats to excrete an acute salt load, male and female Bmal1+/+ and Bmal1−/− rats were given a 900 μEq NaCl load by oral gavage at either ZT0 or ZT12. Both male and female rats given a salt load at ZT0 excreted the majority of their salt load within the first 12 hours of the salt load regardless of genotype (Supplemental Figure S11A and S11C). The increase in 24-hour total UNaV following the ZT0 acute salt load was also similar between genotypes (Supplemental Figure S11B and S11D). Similar results were observed for a salt load given at ZT12, whereas both male and female rats excreted their salt load within the first 12 hours of the salt load without an effect of genotype (Supplemental Figure S11E and S11G). Once again, there was not a significant difference between genotypes in the amount of total excess salt excreted over the 24-hour period following the salt load (Supplemental Figure S11F and S11H).
DISCUSSION
The major new finding of the current study is the observation that genetic deletion of one of the core clock genes, Bmal1, in the rat results in an overall lowering of arterial pressure, yet BP rhythms remain intact. Given the previous reports and confirmation in our own lab that the Bmal1 knockout mouse has no BP rhythm,18 this calls into question the fundamental role presumed for this key clock gene in circadian control of BP based on studies in the mice. The second important new finding from the development of this model is that Bmal1 appears to be key for diurnal control of Na+ excretion in male rats, but not females. Countless studies have provided evidence for a link between BP and renal Na+ handling. Guyton’s well-known theory of renal-body fluid feedback system for controlling BP has become central dogma in much of the hypertension research community, but how this applies to circadian rhythms of BP have never been investigated to our knowledge. The current study provides compelling evidence that circadian rhythms in BP are dissociated from Na+ handling by the kidney.
Bmal1 is often considered the most indispensable clock gene because loss of expression in mice is associated with numerous pathophysiological phenotypes, such as skeletal muscle weakness and impaired glucose homeostasis.19, 20 Bmal1−/− mice generally lack circadian rhythms in a wide range of physiological variables including BP that is characterized by a loss of the normal increase in pressure during their active period.18 Therefore, much to our surprise, we observed that the Bmal1−/− rat has a normal BP rhythm albeit hypotensive relative to littermate controls. Given the complexity of BP control, the reasons for the species difference are far from clear and reconciling these differences will require considerable future work. Despite the difference in BP rhythms, characterization of this new rat model revealed that Bmal1−/− rats have lower body weight as well as overall food intake compared to controls, a phenotype that is similar to that seen in the Bmal1−/− mouse model.21–25 Given that both species have reduced growth, we can speculate that this could be related to differences in metabolism or growth hormones. Another distinction between the rat and mouse knockouts is that Bmal1−/− mice do not have a diurnal feeding pattern, whereas Bmal1−/− rats maintain a similar eating pattern as wild-type animals by consuming most of their food in the active period. The food consumption of Bmal1−/− rats was lower than controls during the active period, which could influence the hypotensive phenotype that was observed.26, 27 Future studies involving time- or calorie-restricted feeding and their effects on blood pressure would be necessary to elucidate a potential link in these rats. Although it is difficult to know at this point why Bmal1−/− rats are able to maintain a normal feeding pattern, it appears that the metabolic dysfunction that is well documented in Bmal1−/− mice is less severe in the Bmal1−/− rat. Whether the loss of diurnal feeding has an overall effect of loss of BP rhythms in mice will need to be investigated.
The mechanisms responsible for circadian BP rhythms are unknown. Traditional thinking about body fluid homeostatic control of BP might suggest that the rise in BP during the active period is in response to increases in extracellular fluid volume associated with increased intake of fluid and electrolytes, but this appears not to be the case because the rise in BP begins and often peaks well before intake significantly rises. More specifically, our data indicate that the circadian rise in BP is not associated with diurnal sodium intake. It is also worth noting that we previously observed a rather normal diurnal BP pattern in rats on an extremely low salt diet.11 Therefore, the BP rhythm would appear to be controlled by neurohumoral factors that have not yet been well characterized. Despite the clear species differences in Bmal1−/− models, the use of mouse models containing whole-body mutations or complete deletion of circadian clock genes have allowed us to gain more knowledge on how these transcription factors contribute to the overall BP phenotype independent of rhythmicity. The Bmal1−/− mouse lacks a circadian BP rhythm due to BP failing to rise during the active phase.18 Mice with a loss of Bmal1 in kidney tubules (Bmal1lox/lox/Pax8-rtTA/LC1-Cre) maintain a normal circadian BP rhythm, although with a slight but significantly lower systolic BP.28 A similar pattern of a maintained BP rhythm yet reduced overall BP is seen in smooth-muscle Bmal1KO mice.29 A mouse model with Bmal1 gene deletion driven by the renin promoter (Bmal1lox/lox/Ren1dCre) also displays a significantly lower BP compared to controls.30 However, in this model it is important to note that Bmal1 was deleted not only in the juxtaglomerular cells, but also in a fairly wide range of renin-producing cells including the thick ascending limb and collecting duct as well as the liver.30 In total, these findings suggest an important role for Bmal1 in maintaining overall BP, yet the mechanism for how Bmal1 impacts BP rhythms has yet to be resolved.
At this point, there is little published information on the role for Bmal1 in control of the diurnal pattern of Na+ excretion. Male Bmal1lox/lox/Ren1dCre mice have reduced Na+ excretion compared to controls during the active period, but it is unknown if a similar trend is apparent during the inactive period, or in female Bmal1lox/lox/Ren1dCre mice.30 We observed a blunted diurnal pattern in Na+ excretion in male Bmal1−/− rats. Because food intake of both Bmal1−/− rats and controls was higher during the active period, it is possible that there could be dysfunction not only in renal function of the Bmal1−/− male, but also in the ability of the gut to absorb nutrients or electrolytes, although our acute NaCl loading experiments would suggest otherwise. In Wistar rats, Soták et al reported that the amiloride-sensitive current follows a diurnal pattern in the colon.31 Future studies will need to explore the role of Bmal1 in NaCl absorption by the gut.
The finding that female Bmal1−/− rats do not have a blunted variation in Na+ excretion and display a pattern similar to controls suggests a sex difference in Na+ handling and a better ability to maintain Na+ balance in normal day-to-day functions compared to male Bmal1−/− rats. Of note, Veiras et al. recently observed a more rapid excretion of an acute NaCl load in female compared to male rats,32 which could account for the ability of female rats to maintain a pattern of diurnal Na+ excretion in coordination with Na+ intake. As far as other core clock gene KO animal models are concerned, it is unclear whether females have a disrupted rhythm in Na+ excretion due to studies involving those animals using only males.30, 33 Douma et al. found that female Per1-KO mice on a C57BL/6J background are protected from nondipping hypertension in response to high salt diet and treatment with the mineralocorticoid deoxycorticosterone pivalate (DOCP),34 while males are not.35 Female Per1-KO mice excreted similar levels of Na+ as controls over a 24-hour period, but it is unclear whether or not time-of-day differences in Na+ excretion are present between genotypes.34 Central and peripheral tissues from ovariectomized (OVX) female rats can display a tissue-specific advance or delay in the mean phase, along with an increase or decline in the phase synchrony of Per1-luciferase expression, suggesting that hormone levels could have an effect on the timing of circadian organization.36 It remains to be seen what effect OVX has on Bmal1 expression in peripheral tissues, in particular the kidney, and whether or not OVX would result in a blunted rhythm in Na+ excretion that mirrors the male Bmal1−/− rats. Premenopausal women have a lower prevalence of hypertension than men,37 and a greater protection against a rise in BP in female animal models with salt-sensitive hypertension suggests that the higher levels of estrogens in the female sex play a vital role in that protection. The hormonal protection could help compensate for the other deficiencies, such as a loss of Bmal1, to maintain the differences seen in BP. Future studies that examine the role of Bmal1 in regulating BP in premenopausal women garner consideration to explore this hypothesis.
Another sex difference we observed was in core body temperature rhythms. Regardless of genotype, we see that females have a small, but significantly higher overall mean body temperature compared to males, which is opposite to prior results reported in Wistar rats.38 This could be due to differences in strain of rat and that our rats were singly housed during the data collection period. Furthermore, we see that the amplitude in 24-hour body temperature was significantly lower in female Bmal1−/− rats compared to both male and female Bmal1+/+ rats. Whether this involves a sex difference in central clock control will need to be investigated.
Mice with a loss of Bmal1 in the kidney exhibit reduced expression of renal Na+ channels30 suggesting that a lack of this transcription factor provides a pro-natriuretic function. Because of this, we hypothesized that our Bmal1−/− rats would have a more efficient natriuretic response to an acute salt load compared to Bmal1+/+ rats. Although both male and female Bmal1−/− rats excreted the majority of their salt load within the first 12 hours of it being given at either ZT0 or ZT12, there was no difference in ΔUNaV between genotypes of either sex. Given how well Bmal1+/+ male and female rats excreted a salt load regardless of the time of day it was given, it would have been difficult to see a better natriuretic response given the 12-hour timeframe used in our experiment.
A fascinating result from the development of the Bmal1−/− rats is the observation that Na+ excretion and BP may not be directly linked to each other within the context of a 24-hour rhythm. Theoretically, the renal-body fluid mechanism regulates BP due to the phenomenon of pressure natriuresis and works to keep BP at a normal set point.39, 40 Defects in Na+ handling tend to lead to impaired Na+ reabsorption and, if issues in Na+ handling persist long-term, ultimately hypertension. When examining the female Bmal1−/− rats, their pattern of Na+ excretion and MAP is similar to controls, rising during the active period and falling during the inactive period. Male Bmal1−/− rats on the other hand, have no apparent rhythm in Na+ excretion despite a regular rhythm in MAP. The nondipping hypertension seen in male Per1-KO mice by Douma et al. was subsequently found to be caused by a defect in renal sodium handling.41 It is possible that circadian clock disruption in our Bmal1−/− rat model may have had the opposite effect in the males. Bmal1−/− male rats have a lower hypotensive phenotype that could be related to their sodium excretion remaining elevated during the inactive period leading to a lack of a diurnal pattern. However, female Bmal1−/− rats also have a hypotensive phenotype despite a diurnal pattern in sodium excretion. At this point it is unclear if there are tubular or hemodynamic factors that are causing this sex-specific loss of rhythm, or if there are extrarenal factors such as sex hormones that are contributing as well. Clearly, more work needs to be done to figure out how Na+ excretion and BP operate independently of each other in this animal model. The differences in the pattern of food intake between Bmal1−/− mice and rats may offer some insight for Na+ regulation. Bmal1−/− mice lack a diurnal pattern in food intake, whereas Bmal1−/− rats have higher levels of intake during the active period. It could be possible that the species difference in the pattern of food intake, and how each animal manages absorption of electrolytes in the colon, could have a greater influence on the pattern of Na+ excretion in mice more than rats.
Finally, it is worth noting that Per1 expression is reduced in the tissues of Bmal1−/− rats even though the diurnal pattern remained. While the canonical transcription-translation loop would predict that the other clock genes would become arrhythmic with the loss of one of the core clock components, we know that the system can remain operative.42 There was a tendency for Per1 to be reduced, albeit not significantly, as well as Cry2. In addition, the reduction of Per1 expression was not observed in the renal outer medulla of these Bmal1−/− rats despite a decrease in expression in the other renal sections. Clock also seems to show some region-specific differences in expression in the kidney. Given the documented importance of the Period and Clock genes in regulating renal tubular function,2, 33 further work is needed to discern what appears to be a complicated regulatory system.
PERSPECTIVES
In conclusion, our novel Bmal1−/− rat model has revealed the importance of Bmal1 in the diurnal regulation of Na+ excretion. However, this regulation is sex dependent. These differences are distinct from previous studies in a comparable mouse model. These findings highlight the need for more species diversity in the study of mechanisms that control BP and renal function, especially in relation to clock genes and their role in regulating physiological function. The issue with animal models not predicting human pathobiology (and even physiology) has most likely been exacerbated by over-reliance on a single species, the mouse. New technology that improves the efficiency of gene editing in rats will undoubtedly advance the field. With regards to the kidney, understanding the physiological significance of the interaction between the kidney and the circadian clock allows us to expand and redefine what we know about kidney function as well as provide valuable insight into future experimental designs. Potential sex differences in the circadian regulation of renal function, combined with the phenotype of metabolic dysfunction including advanced aging observed in mice deficient in Bmal121, 43, could reveal a mechanism linking increased protection against renal damage and decay in one sex that could provide benefits to the other. In addition, clock-controlled genes and proteins in renal tissues similar to both rodents and humans could potentially serve as biomarkers to predict future cardiovascular events, for which researchers and clinicians alike could offer prevention tactics rather than treatment.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This first reported rat model of a clock gene knockout.
Evidence for a role of the clock gene, Bmal1, in maintenance of blood pressure in a rat model.
Our study demonstrates a sex-dependent role for Bmal1 in diurnal control of sodium excretion.
What is relevant?
Demonstration that diurnal patterns in sodium excretion and blood pressure are not linked.
Additional evidence that females have more efficient control of sodium excretion even when the molecular clock is dysfunctional.
ACKNOWLEDGEMENTS
The authors would like to express their extreme thanks to Dr. Melinda Dwinell and the Medical College of Wisconsin Gene Editing Rat Resource Center for their key role in the creation of the Bmal1 knockout rat. The authors gratefully acknowledge the expert technical assistance of Liz Daugherty.
SOURCES OF FUNDING
This study was supported by grants from the NIH National Heart, Lung, and Blood Institute (P01 HL69999, P01 HL95499 to D.M.P. and J.S.P., K99 /R00 HL127178 to J.S.S., K99HL144817 to M.K.); the National Institute of Diabetes and Digestive and Kidney Diseases (DK105038 to K.A.H.); the American Heart Association (SFRN2390002 to J.S.P. and D.M.P.; 19POST34380109 to B.K.B.; 15PRE25560074 to J.G.J.). J.G.J. was also supported by the UAB Training Program in Cardiovascular Pathophysiology (T32 HL007918).
Footnotes
CONFLICT OF INTEREST/DISCLOSURES
None.
REFERENCES
- 1.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290 [DOI] [PubMed] [Google Scholar]
- 2.Richards J, Gumz ML. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal HSD3b6. Nat Med. 2010;16:67–74 [DOI] [PubMed] [Google Scholar]
- 4.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills JN, Stanbury SW. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol. 1952;117:22–37 [PMC free article] [PubMed] [Google Scholar]
- 7.Moore-Ede MC, Herd JA. Renal electrolyte circadian rhythms: Independence from feeding and activity patterns. Am J Physiol. 1977;232:F128–135 [DOI] [PubMed] [Google Scholar]
- 8.Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. Cas9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–150 [DOI] [PubMed] [Google Scholar]
- 12.De Miguel C, Obi IE, Ho DH, Loria AS, Pollock JS. Early life stress induces immune priming in kidneys of adult male rats. Am J Physiol Renal Physiol. 2018;314:F343–F355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston JG, Speed JS, Jin C, Pollock DM. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol. 2016;311:F991–F998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelissen G Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, Hatanaka F, Yamamoto M, Araoka T, Li Z, Kurita M, Hishida T, Li M, Aizawa E, Guo S, Chen S, Goebl A, Soligalla RD, Qu J, Jiang T, Fu X, Jafari M, Esteban CR, Berggren WT, Lajara J, Nunez-Delicado E, Guillen P, Campistol JM, Matsuzaki F, Liu GH, Magistretti P, Zhang K, Callaway EM, Zhang K, Belmonte JC. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–689 [PMC free article] [PubMed] [Google Scholar]
- 17.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260 [DOI] [PubMed] [Google Scholar]
- 18.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A. 2010;107:19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the mop3/bmal-1 locus. Genesis. 2005;41:122–132 [DOI] [PubMed] [Google Scholar]
- 23.Takarada T, Kodama A, Hotta S, Mieda M, Shimba S, Hinoi E, Yoneda Y. Clock genes influence gene expression in growth plate and endochondral ossification in mice. J Biol Chem. 2012;287:36081–36095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, Tezuka M, Kosuge Y, Ishige K, Ito Y, Komiyama K, Okamatsu-Ogura Y, Kimura K, Saito M. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6:e25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2016;30:1634–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–1221 e1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cote I, Toklu HZ, Green SM, Morgan D, Carter CS, Tumer N, Scarpace PJ. Limiting feeding to the active phase reduces blood pressure without the necessity of caloric reduction or fat mass loss. Am J Physiol Regul Integr Comp Physiol. 2018;315:R751–R758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, Henry H, Koesters R, Maillard M, Bonny O, Tokonami N, Firsov D. Nephron-specific deletion of circadian clock gene BMAL1 alters the plasma and renal metabolome and impairs drug disposition. J Am Soc Nephrol. 2016;27:2997–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML, Firsov D. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol. 2014;25:1430–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotak M, Polidarova L, Musilkova J, Hock M, Sumova A, Pacha J. Circadian regulation of electrolyte absorption in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1066–1074 [DOI] [PubMed] [Google Scholar]
- 32.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol. 2017;28:3504–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23:1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Physiol Regul Integr Comp Physiol. 2019;316:R50–R58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in PER1 knockout mice. Acta Physiol (Oxf). 2017;220:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy ZC, Pezuk P, Menaker M, Sellix MT. Effects of ovarian hormones on internal circadian organization in rats. Biol Reprod. 2013;89:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr., Whelton PK. Potential u.S. Population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol. 2018;71:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cambras T, Castejon L, Diez-Noguera A. Social interaction and sex differences influence rat temperature circadian rhythm under LD cycles and constant light. Physiol Behav. 2011;103:365–371 [DOI] [PubMed] [Google Scholar]
- 39.Guyton AC, Coleman TG, Cowley AV Jr., Scheel KW, Manning RD Jr., Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594 [DOI] [PubMed] [Google Scholar]
- 40.Guyton AC, Coleman TG, Young DB, Lohmeier TE, DeClue JW. Salt balance and long-term blood pressure control. Annu Rev Med. 1980;31:15–27 [DOI] [PubMed] [Google Scholar]
- 41.Douma LG, Holzworth MR, Solocinski K, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Renal Na-handling defect associated with PER1-dependent nondipping hypertension in male mice. Am J Physiol Renal Physiol. 2018;314:F1138–F1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10:466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G, FitzGerald GA. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


