Abstract
Mitochondrial oxidative phosphorylation (oxphos) is the process by which the ATP synthase conserves the energy released during the oxidation of different nutrients as ATP. The yeast ATP synthase consists of three assembly modules, one of which is a ring consisting of 10 copies of the Atp9 subunit. We previously reported the existence in yeast mitochondria of high molecular weight complexes composed of mitochondrially encoded Atp9 and of Cox6, an imported structural subunit of cytochrome oxidase (COX). Pulse-chase experiments indicated a correlation between the loss of newly translated Atp9 complexed to Cox6 and an increase of newly formed Atp9 ring, but did not exclude the possibility of an alternate source of Atp9 for ring formation. Here we have extended studies on the functions and structure of this complex, referred to as Atco. We show that Atco is the exclusive source of Atp9 for the ATP synthase assembly. Pulse-chase experiments show that newly translated Atp9, present in Atco, is converted to a ring, which is incorporated into the ATP synthase with kinetics characteristic of a precursor-product relationship. Even though Atco does not contain the ring form of Atp9, cross-linking experiments indicate that it is oligomeric and that the inter-subunit interactions are similar to those of the bona fide ring. We propose that, by providing Atp9 for biogenesis of ATP synthase, Atco complexes free Cox6 for assembly of COX. This suggests that Atco complexes may play a role in coordinating assembly and maintaining proper stoichiometry of the two oxphos enzymes
Introduction
The OXPHOS pathway of mitochondria is composed of four respiratory complexes and the ATP synthase, all located in the inner membrane where they couple the oxidation of NADH and FADH2/FMH2 to the synthesis of most of the ATP made under aerobic conditions. In mammalian mitochondria the NADH-coenzyme Q reductase, the bc1 and cytochrome oxidase (COX) complexes are physically associated in a supercomplex or respirasome [1]. Mammalian NADH-coenzyme Q reductase is a hetero-oligomeric complex structured from some 45 different polypeptides, seven of which are encoded in mitochondrial DNA [2]. In contrast, reduction of coenzyme Q in the yeast Saccharomyces cerevisiae is catalyzed by an enzyme consisting of a single subunit NADH dehydrogenase [3]. The yeast supercomplexes are composed of the bc1 and COX complexes in a 2:2 or 2:1 stoichiometry, respectively [1]. The structures of the yeast [4, 5] and mammalian supercomplexes [6–8] have been solved by cryoelectron microscopy. The physical association of the respiratory complexes is thought to enhance their stability and allow for a more efficient inter-complex transfer of electrons by coenzyme Q and cytochrome c [9, 10]. An interesting feature of the supercomplexes is their relatively low mobility in the inner membrane when compared to proteins of the outer membrane [11].
Mitochondrial ATP synthase is a hetero-oligomeric protein that utilizes the energy of the proton gradient to synthesize ATP from ADP and inorganic phosphate. Both bacterial and mitochondrial ATP synthase have a similar structure characterized by a group of hydrophobic proteins forming the membrane F0 sector and a peripheral ATPase, termed F1, which is attached to F0 by a central and peripheral stalk. The yeast ATP synthase has been shown to be formed from at least three independent assembly modules: the F1 ATPase, the rotating ring consisting of 10 copies of Atp9 that anchors F1 to the membrane, and four subunits that form the peripheral stalk that is connected to Atp6 and Atp8 [12]. The yeast ATP synthase is composed of 14 different polypeptides of which only 3 are encoded in the mtDNA (mitochondrial DNA). The three mitochondrial gene products, Atp6, Atp8 and the Atp9 ring, jointly translocate protons across the inner membrane.
The ATP synthase, like the respiratory chain, is housed in the inner membrane but is physically separated from the supercomplexes. High resolution electron microscopic studies have revealed that the ATP synthase exists as ribbons with a dimer as the repeating unit. The two ATP synthases of the dimer have an angle of inclination of 86o [13]. The ribbons confer a strong local curvature on the membrane and are responsible for the inner folds of the cristae. The physical organization of the ATP synthase in shaping the cristae has been proposed to create a proton sink at the apex of the cristae curvature resulting in a 3.5 fold increase in surface charge [14].
We previously reported the existence in yeast mitochondria of high molecular weight complexes composed of Cox6, a nucleous encoded subunit of cytochrome oxidase and Atp9 [15], a mitochondrially encoded subunit of the ATP synthase ring. In this communication we refer to the Atp9-Cox6 complexes as Atco (Atp9 and Cox6). The presence in Atco of subunits from different oxphos complexes suggests a role in establishing the proper stoichiometry of the two complexes relative to each other. The present study was undertaken to further examine the role of Atco in the biogenesis of ATP synthase. We present evidence that most if not all of the newly translated Atp9 is associated with Atco, which based on pulse-chase labeling experiments, behaves as a precursor of the Atp9 ring in ATP synthase.
Results
Atco complexes are a source of Atp9 for ATP synthase assembly
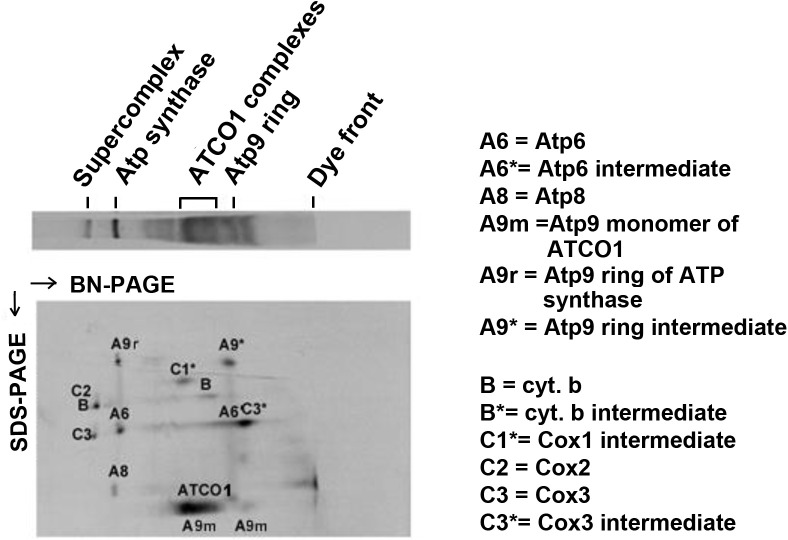
The extent to which Atco contributes to the biogenesis of ATP synthase was studied by measuring the size distribution of newly translated Atp9. More specifically, we were interested in determining if mitochondria pulsed with 35S-methionine/cysteine contained radiolabeled monomeric Atp9 or if most of this newly translated subunit of the ATP synthase is associated with Atco. Analysis of 35S-methionine/cysteine labeled mitochondria by BN-PAGE (blue native gel electrophoresis) in the 1st dimension combined with SDS-PAGE (sodium dodecyl sulfate gel electrophoresis) in the 2nd dimension, indicates that a large fraction of nascent Atp9 (A9 in Fig 1) is present in Atco complexes. A small fraction of newly translated Atp9 is present either as an independent ring (A9* in Fig 1) or as the ring of the fully assembled ATP synthase (A9r in Fig 1). None was detected in the region corresponding to monomeric Atp9. This indicated that Atco is the source of Atp9 for ATP synthase assembly.
Fig 1. Properties of newly translated Atp9.
Mitochondrial translation products, labeled in organello with 35S-methionine/cysteine, were extracted with 2% digitonin and separated in the 1st dimension on a 5–20% gel by BN-PAGE and in the 2nd dimension on a 12% gel by SDS-PAGE. The radiolabeled bands are identified in the X-ray. The identities of some minor bands are not known. The migrations of the supercomplex, ATP synthase, Atco complexes and Atp9 ring are indicated above the 1st dimension gel strip. The monomer is expected to migrate near the dye front in the blue native gel.
A role of Atco as a precursor of the Atp9 ring module is also supported by pulse-chase labeling of mitochondria from a strain expressing Cox6 with a tandem hemagglutinin plus protein C tag (HAC) and Atp6 with a tandem hemagglutinin plus poly histidine tag (HApH), the former to pull down Atco and the latter, the ATP synthase. Digitonin extracts of 35S-radiolabeled mitochondria were fractionated separately on protein C antibody beads, to purify Cox6 associated proteins and on Ni-NTA beads, to purify intermediates of ATP synthase. The decrease of radiolabeled Atp9 in Atco complexes (Fig 2B and 2C) was consistently found to be accompanied by an increase of labeled ring in the ATP synthase and as a stand-alone ring (Ni-NTA eluates in Fig 2A and 2B). As expected, the radiolabeled Atp9 monomer in the fraction eluted from the beads with EDTA (PC eluate) that corresponded to dissociated Atco complexes also decreases during the chase (Fig 2A and 2C).
Fig 2. Pulse-chase analysis of Atco complexes.
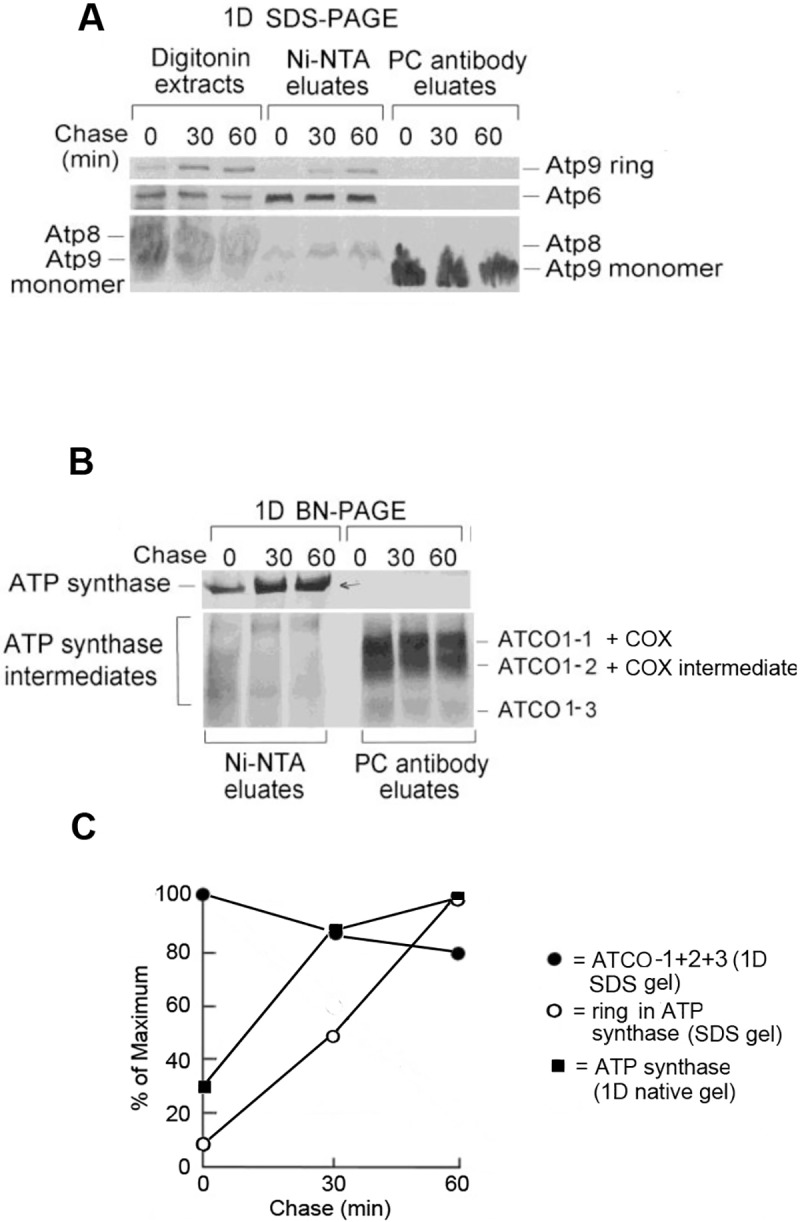
A. Mitochondria from W303/COX6-HAC,ATP6-HApH, a strain expressing Atp6 tagged with poly histidine and Cox6 with protein C epitope was pulse-labeled with 35S-methionine/cysteine for 10 minutes and chased for 0, 30 and 60 minutes. The mitochondria were extracted with 2% digitonin and purified on Ni-NTA beads to pull down partially and fully assembled ATP synthase. The same volume aliquots of the digitonin extracts were purified on protein C antibody (PC) beads to pull down Atco complexes. The affinity purified proteins were separated by SDS-PAGE on a 12% polyacrylamide gel and by BN-PAGE on a 4–13% polyacrylamide gel. B. The proteins purified on Ni-NTA and on the protein C antibody beads were separated by BN-PAGE. C. The radiolabeled Atco complexes (1D BN gel of PC eluates), ATP synthase intermediates and Atp9 ring (1D SDS gel of PC eluate) were quantified with a phosphorimager. The Atco complexes overlap with cytochrome oxidase and Cox1 intermediates, which contribute approximately 10% of the radiolabel in that region of the 1D blue-native gel.
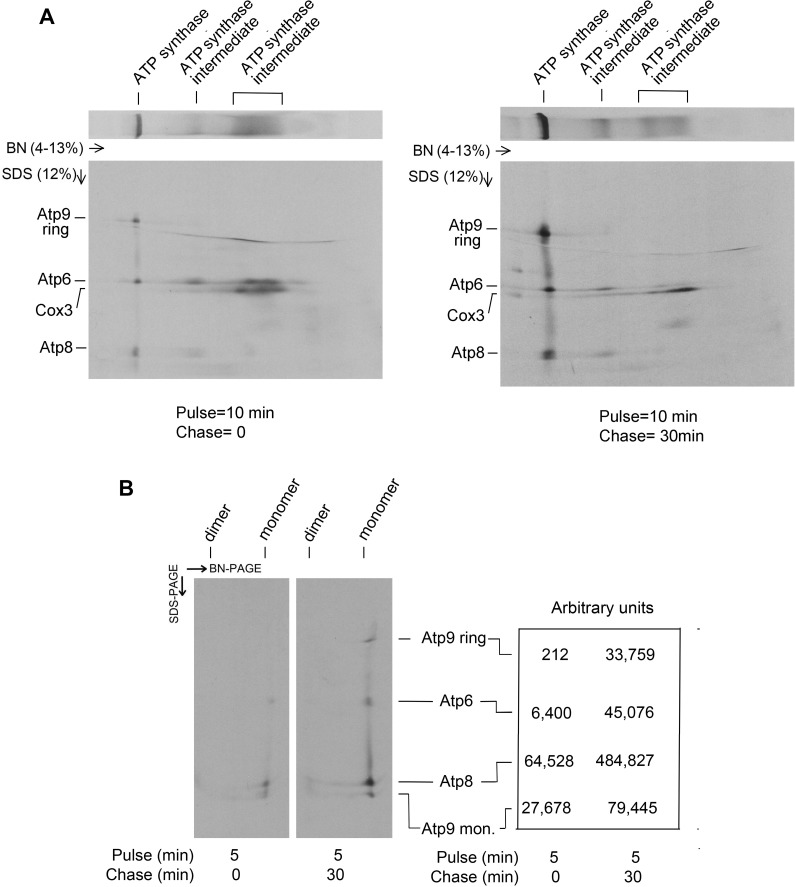
Although the pulse chase experiment shows a precursor-product relationship of newly translated Atp9 of Atco and the ring of ATP synthase, we could not exclude the possibility that the radiolabel measured in the ATP synthase was all attributable solely to Atp6 and Atp8. To exclude this possibility, the double tagged mitochondria were pulsed for 10 min and chased for 30 min. Assembly intermediates and mature ATP synthase containing Atp6 tagged with poly histidine were purified on Ni-NTA from digitonin extracts of mitochondria and were separated in two dimensions, first by BN-PAGE followed by SDS-PAGE to measure the radiolabel associated with the Atp9 ring in the ATP synthase. In agreement with the results of the pulse-chase experiment shown in Fig 2, the 1D BN-PAGE gel showed an increase of radiolabel in the Atp9 ring of the ATP synthase following a 30 min chase (Fig 3A and 3B). This was also true of Atp6 and Atp8. This experiment was repeated with a shorter 5 min pulse time to improve quantitation of the radiolabeled subunits after the chase. Taking dissociated Atp9 into account there was a 4-fold increase in radiolabeled Atp9 and a 7-fold increase in Atp6 and Atp8. A less efficient transfer of Atp9 from the gel to the PVDF membrane may account for the lower incorporation of Atp9 ring than Atp6 and Atp8 into the ATP during the chase. Since most of the Atp9 translated during the pulse is present in Atco complexes (Fig 1), they must serve as a source of Atp9 for oligomerization of the ring and its assembly into the ATP during the chase.
Fig 3. Pulse-chase analysis of ATP synthase.
A. Digitonin extracts of W303/COX6-HAC, ATP6-HApH mitochondria pulse-labeled with 35S-methionine/cysteine for 10 minutes and chased for 0 and 30 minutes were purified on Ni-NTA beads to pull down ATP synthase and its assembly intermediates. The purified proteins were then separated on the 1st dimension (1D) in a 4–13% polyacrylamide gel by BN-PAGE followed by separation in the 2nd (2D) dimension on a 12% gel by SDS-PAGE. B. Same as A. except that the pulse time was 5 min. Following transfer to a PVDF membrane the bands corresponding to each of the three mitochondrially encoded subunits of the fully assembled ATP synthase were quantified. In this experiment some of the ring associated with the synthase was depolymerized by SDS.
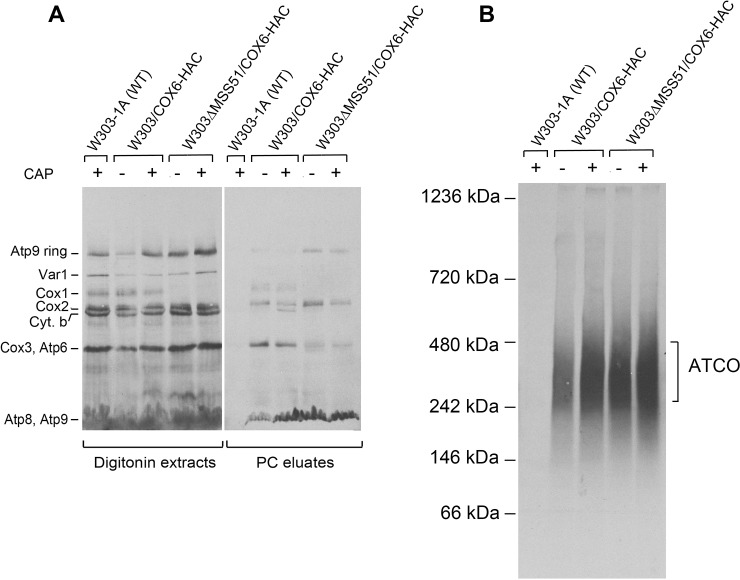
The migration of Atco complexes on blue native gels overlaps with Cox1 assembly intermediates D4 and D5 of COX [31]. To distinguish between radiolabeled Cox1 intermediates and Atco, mitochondria were isolated from an mss51 mutant that does not translate Cox1 and as a result lacks both Cox1 intermediates and COX. The blue native gel of the radiolabeled products from mitochondria of the mss51 mutant indicates that most of the radiolabeled material in the region of the Cox1 intermediate corresponds to Atp9 of Atco (Fig 4B).
Fig 4. Effect of growth in chloramphenicol on Atco.
Mitochondria were isolated from the parental strain W303-1B and from W303/COX6-HAC and W303ΔMSS51/COX6-HAC that had been grown to early stationary phase in rich galactose and grown for an additional 2 hours in fresh medium containing 2 mg/ml chloramphenicol. Mitochondria were also isolated from W303/COX6-HAC without and with the mss51 null mutation that had not been treated with chloramphenicol. Mitochondria were labeled with 35S-methionine and cysteine for 20 min as described in the Materials and Methods section; they were extracted with digitonin at a final concentration of 2% and purified on protein C antibody beads. The digitonin extracts and purified fractions were separated by SDS-PAGE on a 12% polyacrylamide gel (A) and by BN-PAGE on a 4–13% polyacrylamide gel (B). Proteins were transferred to a PVDF membrane and exposed to X-ray film. The radiolabeled mitochondrial gene products are identified in the margins.
Chloramphenicol inhibits mitochondrial but not cytoplasmic proteins synthesis [16]. Growth of yeast in chloramphenicol was previously shown to enhance the translation of Atp9 [17], presumably by increasing a limiting pool of one or more nuclear gene products for subsequent interaction with their mitochondrial partners during assembly of the ATP synthase. Digitonin extracts of mitochondria from cells grown for 2 hours in the presence of chloramphenicol displayed a significantly higher content of radiolabeled Atp9 in the ring form and in Atco than extracts of mitochondria of cells that had not been treated with chloramphenicol (Fig 4A and 4B). These data indicate that incorporation of Atp9 into the ring and ATP synthase correlates with an increase of Atp9 in Atco. Adsorption of Cox6-HAC on the protein C antibody beads also co-immunopurified cytochrome oxidase and the bc1 complexes of the supercomplex as evidenced by the presence of radiolabeled Cox1, Cox2, Cox3 and cytochrome b (Fig 4A, right panel). It is interesting to note that growth in the presence of chloramphenicol also elicits a substantial increase of radiolabeled cytochrome b in the fraction purified on the beads, indicative of a robust assembly of the bc1 complex in the supercomplex (Fig 4A).
Atco complexes in oxa1 and cox5 mutants
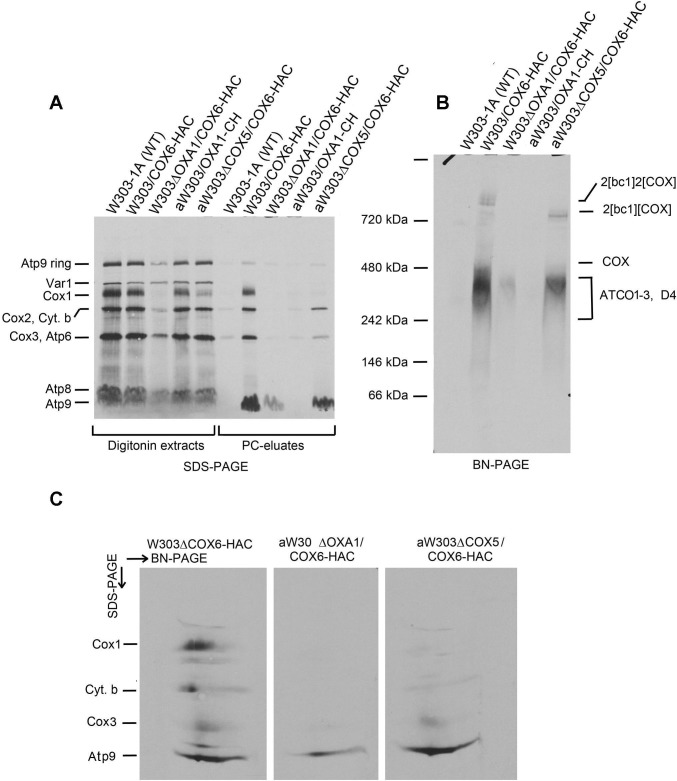
Mutations in OXA1, the yeast gene for the mitochondrial inner membrane insertase have been shown to elicit loss of COX and to severely reduce the levels of the bc1 complex and ATP synthase [18–20]. The decrease in ATP synthase has been attributed to a reduction in mitochondrially encoded Atp9 indicating that Oxa1 is needed for membrane insertion and assembly of Atp9 into the ATP synthase [20]. Although Atco complexes behave as membrane proteins, this does not necessarily indicate a proper insertion and orientation of Atp9 in the inner membrane. To confirm that Atp9 was correctly inserted into the inner membrane we compared the amount of Atco complexes in in wild type and in an oxa1 mutant expressing HAC tagged Cox6. Digitonin extracts of mitochondria that had been labeled with 35S-methionine/cysteine, when separated by SDS-PAGE, showed a decrease of the Atp9 ring and of Atp9 monomer derived from Atco (Fig 5A). A large decrease of Atco in the oxa1 mutant was observed when the digitonin extract was separated on a blue native gel alone or combined with SDS-PAGE in the second dimension to measure Atp9 of Atco as a monomer separated from Cox1 intermediates D3 and D4 (Fig 5B and 5C). The reduction of Atco in the mutant suggests that membrane insertion of Atp9 and its physical association with Cox6 is largely dependent on Oxa1. This in turn suggests a native conformation of Atp9 of Atco in the membrane.
Fig 5. Analysis of Atco complexes in oxa1 and cox5 mutants.
Mitochondria were prepared from the wild type W303-1A, a strain expressing Cox6-HAC without (W303/COX6-HAC) and with a null mutation in oxa1 (W303DOXA1/COX6-HAC) or cox5a (aW303DCOX5/COX6-HAC) and an oxa1 null mutant (aW303DOXA1). The mitochondria were labeled with 35S-methionine/cysteine for 20 min, extracted with 2% digitonin and the extracts purified on protein C antibody beads. A. The digitonin extracts and the purified protein fraction in the eluates from the beads (PC eluates) were separated by SDS-PAGE on a 12% polyacrylamide gel. B. The eluates from the protein C antibody beads were separated in a single dimension on a 4–13% polyacrylamide gel by BN-PAGE. C. The eluates from the indicated strains were separated by BN-PAGE in the first dimension and the region containing Atco, by SDS-PAGE in the second dimension. The radiolabeled band of each gel is identified in the margins.
Quantification of newly formed Atp9 ring in the digitonin extract separated by SDS-PAGE and of newly synthesized Atp9 monomer in the fraction purified on protein C antibody indicated an almost 10-fold reduction of both in the oxa1 mutant (Table 1). The residual 11% of Atco in the oxa1 mutant may account for the detection of only 12% Atp9 monomer in the PC eluate separated by SDS-PAGE (Table 1). Consistent with previous observations [18–20], the oxa1 mutations affected Cox1 of cytochrome oxidase and cytochrome b of the bc1 complex more severely than the ATP synthase (Table 1).
Table 1. Ratios of Atp9, Cox1 and Atco in strains with and without the oxa1 null allele.
| Component | Fraction | Ratio (Δoxa1/OXA1) | Gel system |
|---|---|---|---|
| Atp9 ring | Digitonin extract | 0.16 | SDS-PAGE |
| Cox1 | Digitonin extract | 0.04 | SDS-PAGE |
| Atp9 monomer | PC eluate | 0.124 | SDS-PAGE |
| Cox1 | PC eluate | 0.001 | BN-PAGE |
| Atp9 monomer | PC eluate | 0.123 | BN_>SDS-PAGE |
| Cox1 | PC eluate | 2D gel | |
| Atco1-3 | PC eluate | 0.06 | BN_>SDS-PAGE |
As Cox6 is a peripheral protein that is physically associated with Cox5 in cytochrome oxidase [21], Atco formation was studied in the background of a cox5a null mutant. Analysis of Atco in mitochondria labeled in organello indicated that the cox5a mutation affected biogenesis of COX but not of Atco (Fig 5A and 5C).
Physical property of Atp9 in Atco complexes
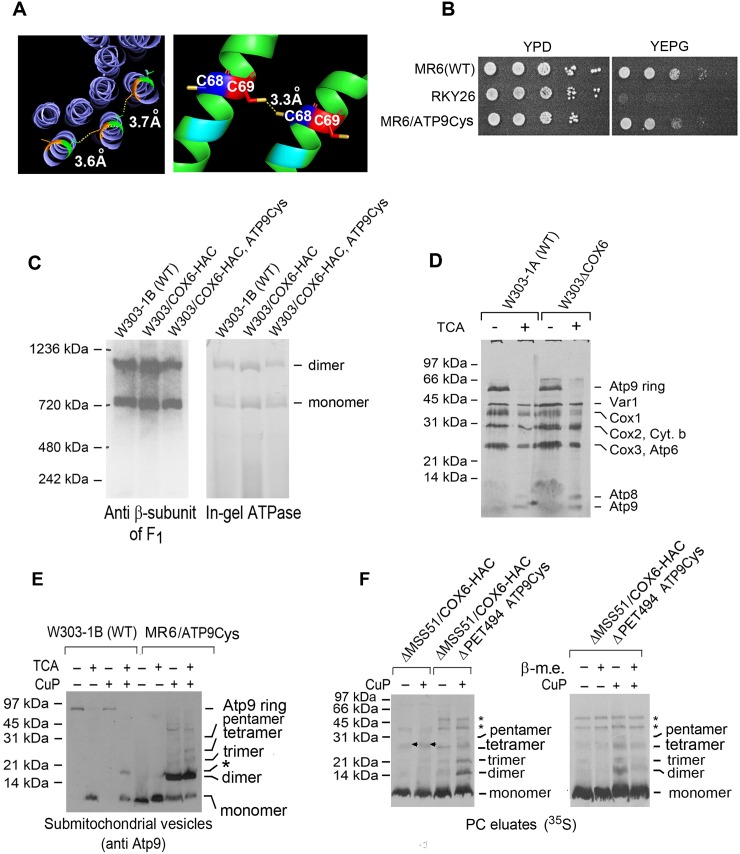
The requirement of Oxa1 for membrane insertion of Atp9 of Atco made it of interest to determine if the interactions of Atp9 monomers in Atco are similar to those of the native ring. The number of Atp9 subunits and their arrangement with respect to one another can be determined by Cu(II)-(phenanthroline)2 (CuP) oxidation of cysteine residues at sufficiently close positions in the neighboring intersubunit alpha helices to be cross-linked [22, 23]. Initial trials with cysteines residue introduced mutationally at positions matching those mutated in the E. coli subunit c were found to prevent growth on rich ethanol/glycerol medium indicating an inactivation of ATP synthase. However, substitutions of cysteines at residues 68 and 69, which based on the X-ray structure [24], are close enough (3.3 Å) to efficiently cross-link adjacent Atp9 subunits (Fig 6A), only partially inhibited growth on the two non-fermentable carbon sources (Fig 6B). The strain expressing the Atp9 with the cysteine substitutions displayed significant ATP synthase as measured by Westerns and in-gel ATPase activity (Fig 6C). This construct was first used to test cross-linking of cysteines 68 and 69 by CuP in neighboring Atp9 subunits of the ATP synthase ring.
Fig 6. Cross-linking of Atp9 in Atco.
A. Ribbon structure of the wild type dimeric Atp9 (left panel) and of the V68C and S69C mutant (right panel). The inter-subunit distance of 3.3 Å between cysteines at residues 68 and 69 from two adjacent monomers is based on the structure of the Atp9 reported by Srivastava et al [24]. B. Growth of aMR6/ATP9Cys containing Atp9 with the two cysteine mutations on non-fermentable carbon sources (YEPG). The wild type parental strain MR6, the atp9 deletion mutant (RKY26) and the mutant with the cysteine modified Atp9 were grown in liquid YPD and serial dilutions spotted on YPD and YEPG media and grown for 2 days at 30°C. C. Mitochondria of the wild type (W303-1B), a strain expressing Cox6-HAC without (W303/COX-HAC) and with Atp9 with the two cysteine mutation (W303/COX6-HAC,ATP9Cys) were extracted with 2% digitonin and separated on a 4–13% polyacrylamide gel by BN-PAGE (left panel). Proteins were transferred to a PVDF membrane and reacted with a primary rabbit antibody against the β-subunit of F1. The digitonin extract was also separated on a clear native 4–13% polyacrylamide gel by CN-PAGE (right panel). The gel was incubated in the presence of 4 mM ATP and 0.05% lead acetate to stain for ATPase activity [25]. D. Mitochondria from the wild type W303-1A and a cox6 null mutant (W303ΔCOX6) were labeled with 35S-methionine/cysteine for 20 min and extracted with 2% digitonin. The extracts were analyzed in a 12% polyacrylamide gel by SDS-PAGE. One half of each sample was precipitated with 5% TCA before addition of the SDS sample buffer to depolymerize the Atp9 ring. The migration of the ATP synthase monomer and dimer are indicated in the margin. E. Mitochondria from the wild type (W303-1B) and from the strain with the V68C and S69C mutations were converted to submitochondrial particles by sonication. The submitochondrial particles were sedimented at 70,000 x gave for 10 min, suspended in sample buffer [26] without β-mercaptoethanol (β-m.e.) and separated by SDS-PAGE on a 15% polyacrylamide gel without and with a prior treatment with 1.5 mM CuP for 1 h. One half of each sample was precipitated with 5% TCA before addition of the SDS sample buffer to depolymerize the Atp9 ring. F. (Left panel) Mitochondria of an mss51 null mutant strain expressing Cox6-HAC (ΔMSS51/COX6-HAC) and wild type Atp9, and from an mss51, pet494 double mutant expressing Cox6-HAC and Atp9 with the V68C and S69C mutations (ΔMSS51,PET494/COX6-HAC/ATP9Cys) were labeled with 35S-methionine/cysteine for 20 min, extracted with 2% digitonin and purified in protein C antibody beads. The band marked with an arrow that is missing in the pet494 null mutant is Cox3 that has a tendency to non-specifically adsorb to the protein C beads. (Right panel). Mitochondria of an mss51 and pet494 double mutant expressing Cox6-HAC and Atp9 with the two cysteine mutations (ΔMSS51ΔPET494/COX6-HAC/ATP9Cys) were labeled and Atco purified protein C antibody beads as above. The purified fraction was analyzed on a 15% polyacrylamide gel by SDS-PAGE. Equal size sample were dissolved in sample buffer with and without 1% β-mercaptoethanol to reduce the disulfide bonds. The identity of the two bands marked with asterisks that remain undiminished after treatment with β-mercaptoethanol have not been identified (left panel).
The ATP synthase ring is normally resistant to dissociation by SDS under standard conditions of electrophoresis but can be converted to the Atp9 monomer by treatment with chloroform base or trichloroacetic acid (TCA) prior to depolymerization with SDS [17, 27, see also Fig 6D]. Digitonin extracts of mitochondria from wild type strain, treated for 1 hour with CuP, were separated by SDS-PAGE without and with a prior treatment with 5% TCA to monomerize the ring in the SDS sample buffer. Western blots of extracts of wild type mitochondrial that had not been crosslinked indicated near complete conversion of the ring to the monomer following treatment with TCA (Fig 6E). In the presence of crosslinker and TCA the wild type digitonin extract showed a weak band that migrated like the dimer. This product is probably a dimer formed as a result of some crosslinking of the native cysteine at residue 65 that is 7 Å away from its counterpart on the adjacent Atp9 of the ring. Crosslinking followed by TCA treatment of the extract from the mutant with the V68C and S69C substitutions produced a major band corresponding to the dimer plus less abundant bands at positions expected for the trimer and tetramer (Fig 6E, last two lanes). The disproportionately large amount of dimer indicates that formation of the first crosslink probably affects the structure of the ring in a way that reduces the efficiency of cross-linking of the other Atp9 subunits in the ring. Also present were some other still less abundant products of larger molecular weight, the identity of which was not studied. It is noteworthy that the replacements at residues 68 and 69 decrease the stability of the ring as evidenced by its conversion by SDS to the monomer without a prior TCA treatment.
Crosslinking of Atp9 in Atco was studied in mitochondria of yeast expressing Cox6-HAC and Atp9 with the V68C and S69C mutations. To reduce the background from non-specific adsorption of radiolabeled Cox1, Cox2, Cox3 and cytochrome b of the supercomplexes that that co-immunoprecipitate with tagged Cox6, Atco was purified from COX mutants (mss51 and mss51, pet494 double mutant) lacking supercomplexes.
Atco was purified on protein C antibody beads from digitonin extracts of mitochondria that had been labeled with 35S-methionine/cysteine. The purified radiolabeled Atco was incubated with and without crosslinker and the products formed were analyzed by SDS-PAGE. The products formed were the same as those obtained with the ring in ATP synthase, except that dimer formation was only marginally greater than the larger oligomers (Fig 6F). As in the case of the native ring, crosslinking of the Atco Atp9 depended on the presence of CuP and the cysteines at residues 68 and 69 of Atp9 (Fig 6F). Longer exposures to X-ray film of purified Atco in the absence of the crosslinker, however, indicated the presence of very low concentrations of dimers and trimers, presumably as a result of CuP independent oxidation. The identity of the radiolabeled bands as products of sulfhydryl oxidation was confirmed by the decrease in their concentration in the presence of β-mercaptoethanol, which promotes the reduction of the disulfide bridges (Fig 6F, right panel). The dependence of Atp9 crosslinking in ATP synthase and Atco on the two cysteine substitution indicates that the interactions of Atp9 in both are similar.
Discussion
Previous pulse-chase experiments showed a time-dependent decrease of radiolabeled Atp9 of Atco complexes that correlated with an increase of Atp9 in the ring, suggesting a precursor product relationship of Atco complexes and the ring [15]. It was not excluded, however, that the ring was being formed from some other source of Atp9. Nor was the newly formed ring shown to be present in the fully assembled ATP synthase. The results of the pulse-chase experiments reported in this study addressed both points and constitute strong evidence that Atco is the sole precursor of the Atp9 ring module that interacts with the F1 and peripheral stalk modules during assembly of the ATP synthase [12]. The evidence may be summarized as follows: 1) Following pulse labeling of isolated mitochondria, all the newly synthesized Atp9 is found in either Atco, the ATP synthase or a stand-alone Atp9 ring. The absence of monomeric Atp9 implies a rapid incorporation of the newly translated subunit into both Atco and the ring for subsequent assembly of the ATP synthase. 2) The kinetics of Atp9 loss from Atco during the chase is similar to the kinetics of assembly of Atp9 ring into the ATP synthase. 3) Newly translated Atp9 is incorporated into the ring of ATP synthase during the chase. Atco must be a precursor of the ring in ATP synthase as all the radiolabeled Atp9 at the start of the chase is present in Atco with some in a stand-alone ring.
Oxa1 is a mitochondrial protein that functions in the insertion of most endogenously synthesized proteins, including Atp9, and some of the imported proteins into the inner membrane [20, 28]. Labeling of mitochondria from an oxa1 null mutant displayed significant less radiolabel in all the mitochondrial gene products. This is probably a consequence of high turnover rather than reduced translation of proteins that fail to be inserted into the membrane. The Oxa1-dependent membrane insertion of Atp9 component of Atco suggests that it is in a proper conformation for ring formation and assembly into the ATP synthase. The presence of some Atco and ATP synthase in the oxa1 mutant, however, implies that Oxa1 is not required for Atp9 oligomerization, which must occur subsequent to the co-translational insertion of Atp9 into the inner mitochondrial membrane.
Unlike Atp9 in the ATP synthase, which retains its oligomeric ring structure in the presence of SDS, the Atp9 of Atco dissociates into the monomer under the same conditions. Although this suggests that Atp9 in the Atco complexes is not a ring, it does not exclude the possibility that the interactions of Atp9 monomers in Atco are similar or identical to those in the native ring. This was tested by comparing crosslinking of Atp9 in Atco and in the bona fide ring of ATP synthase. The ring of ATP synthase in a strain expressing Atp9 with substitutions of cysteines separated by 3.3 Å in adjacent subunits of the ring was crosslinked in the presence of CuP. The predominant product was a dimer, although some trimer and tetramer were also detected. A similar pattern of cross-linked products was observed when Atco containing radiolabeled Atp9 was crosslinked, except that dimer formation was only marginally more efficient than trimer and tetramer. This argues against non-specific aggregation of Atp9 and instead suggests that the interaction of Atp9 oligomer in Atco is similar to that of the ring in the ATP synthase. A possible explanation for the disproportionate formation of the dimer in the case of the native Atp9 ring may be that crosslinking of a single Atp9 pair in the ring introduces a stress that distends the distance between remaining Atp9 subunits. In a linear arrangement of Atp9, as is likely to be the case in Atco, crosslinking of any Atp9 pair may not be as disruptive on the interaction of neighboring Atp9 in the oligomer.
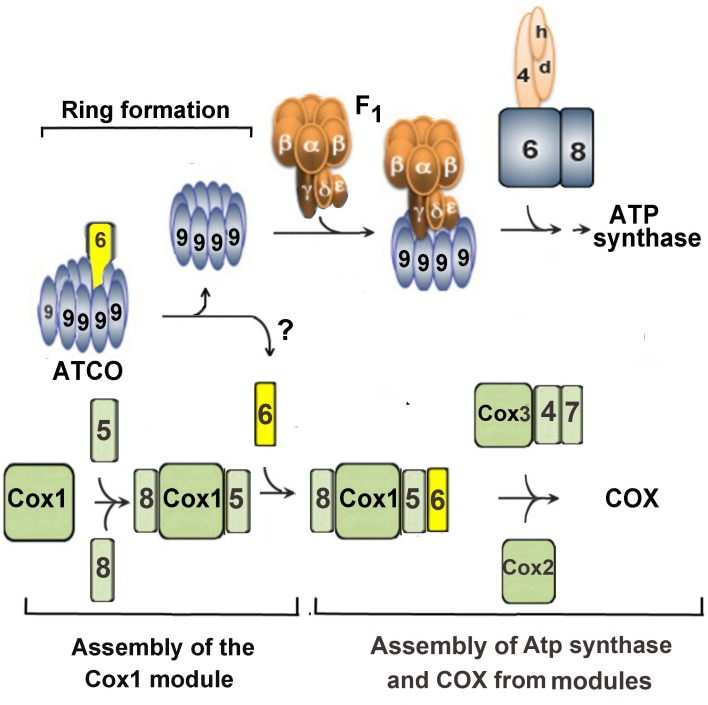
The presence of an ATP synthase and a COX subunit in Atco complexes suggests that the latter may function to regulate the stoichiometry of these two oxphos enzymes during their biogenesis as illustrated in the scheme of Fig 7. According to this model, Atco serves as the sole source of each subunit for assembly of the cognate enzyme. The pulse-chase labeling experiments presented here show that newly translated Atp9 of Atco is incorporated into the ATP synthase ring. Furthermore, as all of the Atp9 that is not in the ring form is present in Atco complexes, the latter must serve as the exclusive source of Atp9 for biogenesis of the ring module. At present, however, we do not know if Atco is a precursor of COX and the sole source of Cox6 for COX assembly.
Fig 7. Model of the role of Atco complexes in coordinating assembly of cytochrome oxidase and ATP synthase.
Atco is shown to co-regulate assembly of ATP synthase and cytochrome oxidase by supplying Cox6 and mitochondrially translated Atp9 to the respective enzymes.
Biogenesis of ATP synthase and COX in the proposed model depend on dissociation of the Atco complexes thereby releasing Atp9 and Cox6 for assembly with their partner subunits. It does not necessarily follow, however, that Atp9, in the absence of Cox6, is preempted from assembling into the ATP synthase and conversely that Cox6 on its own will not assemble into COX. In fact, the steady-state concentration of ATP synthase in a cox6 mutant, measured either immunologically or enzymatically, is not significantly different from that of wild type yeast. This indicates that Atp9 does not have to be associated with Cox6 for ring formation and ATP synthase assembly.
Pulse-labeling of mitochondria resulted in a very significant increase of Atco when they are isolated from cells incubated for two hours in the presence of chloramphenicol, which inhibits mitochondrial synthesis of Atp9 but does not affect synthesis of Cox6 in the cytoplasm and or its transport into mitochondria. A larger mitochondrial pool of Cox6 following incubation in chloramphenicol could explain the observed enhancement in the synthesis of Atp9 [17] and Atco. Cox6 may, therefore, be a positive regulator of ATP9 expression. This is consistent with previous evidence showing that cells undergoing derepression from glucose, assemble significantly less Atp9 ring in a cox6 but not in other oxidase mutants Su et al [15].
Similar to what has been reported for an atp6 mutant [29, 30], the atp9 null mutation elicits severe reduction in COX. The converse is not true as COX mutants contain normal steady-state levels of ATP synthase subunits [31]. This suggests a unidirectional regulatory mechanism that ensures assembly of ATP synthase even in cells that do not respire such as ρ0 and ρ- mutants. As Saccharomyces cerevisiae is a facultative anaerobe, it is capable of surviving and proliferating on the ATP produced from fermentation of sugars. The glycolytic ATP in the cytoplasm is exchanged for ADP by the electrogenic adenine nucleotide exchange carrier. Under these conditions generation and maintenance of a membrane potential depends on the hydrolysis of ATP by the synthase. Unlike the requirement of ATP synthase in non-respiring cells there is no obvious reason for the presence of COX in mitochondria deficient in ATP synthase. The effect of the atp9 mutation on COX is difficult to quantify because of its almost quantitative conversion to secondary ρ0 and ρ- mutants. Under certain conditions of growth it is possible to obtain cultures of the atp9 null mutant consisting of 50% cells with full length mtDNA. Despite the presence in the atp9 mutants of a normal mitochondrial genome, they were almost completely blocked in translation of the mitochondrially encoded Cox1, indicating that the ATP synthase regulates translation of the catalytic COX subunit (unpublished).
The proposed function of Atco in establishing the stoichiometry of ATP synthase relative to COX is predicated on the presence of all nascent Atp9 and Cox6 in Atco. This is supported by our failure to detect monomeric Atp9 in pulse-labeled mitochondria. Although we do not have similar direct evidence for Cox6, the observed increased synthesis of Atco by mitochondria isolated from cells that have been grown in the presence of chlroramphenicol, support the idea that synthesis of Atp9 and more to the point also of Atco depends on the supply of Cox6. Like other proteins that are transported by the TIM23 translocase of mitochondria into the matrix or inner membrane [32], uptake of Cox6 depends on a membrane potential. This could explain the increase of COX biogenesis in an atp6 mutant under conditions that favor substrate level ATP synthesis during the conversion of a-ketoglutarate to succinate [30]. The restoration of a membrane potential in the atp6 mutant would be expected to improve mitochondrial uptake of newly synthesized Cox6 needed for COX biogenesis.
A requirement of the ATP synthase in non-respiring cell is also consistent with the difference in the magnitude of repression of cytochrome oxidase and of ATP synthase by glucose. The mitochondrial concentrations of cytochrome c and large number proteins essential for assembly and activity of the respiratory chain complexes, including COX, are as much as 10-fold lower in yeast metabolizing glucose [33]. Under the same glucose repressed conditions the ATP synthase is at most only 2 times lower than in fully derepressed yeast [33]. Whether Cox6 plays a role in regulating COX biogenesis in respiratory deficient mutants and under conditions of glucose repression has not been determined.
Materials and methods
Strains and growth media
The genotypes and sources of the S. cerevisiae strains used in this study are described in Table 2. The compositions of solid and liquid YPD, YPGal, and YEPG have been described previously.
Table 2. Genotypes and sources of the S. cerevisiae strains used in this study.
| Strain | Relevant Genotype | mt DNA | Source |
|---|---|---|---|
| W303-1 A | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 | ρ+ | R. Rothstein Columbia University |
| W303-1 B | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 | ρ+ | R. Rothstein Columbia University |
| MR6 | MATa ade2-1 leu2-3,112 his3-11,15 trp1-1 ura3-1 his3::ARG8 | ρ+ | [29] |
| RKY26 | MATa ade2-1 leu2-3,112 his3-11,15 trp1-1 ura3-1 his3::ARG8 | Δatp9::ARG8m | [34] |
| DFKρ0 | MATα ρ0 ade2-101 leu2 Δura3-52 arg8::URA3 lys2 kar1-1 | ρ0 | [35] |
| aDFKρ0 | MATa ρ0 ade2-101 leu2 Δura3-52 arg8::URA3 lys2 kar1-1 | ρ0 | [35] |
| MR6/ATP9Cys | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 his3::ARG8 | atp9 (cys68,69) | This study |
| W303/COX6-HAC, ATP9Cys | MATα ade2-1 leu2-3,112 his3-11,15 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 | atp9 (cys68,69) | This study |
| W303/ΔMSS51ΔPET494 /COX6-HAC, ATP9Cys | MATα ade2-1 leu2-3,112 his3-11,15 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 mss51::HIS3 pet494::HIS3 | atp9 (cys68,69) | This study |
| W303ΔMSS51/COX6-HAC | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 mss51::HIS3 | ρ+ | This study |
| W303ΔCOX6 | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox6::URA3 | ρ+ | [35] |
| W303/COX6-HAC | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 | ρ+ | [35] |
| MRSIo /COX1-HAC | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8::HIS3 | ρ+ intronless COX1-HAC | [35] |
| MR6/ATP6-HapH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8∷HIS3 | ρ+ ATP6-HApH | [12] |
| W303/COX6-HAC, ATP6-HApH | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 | ρ+ ATP6-HApH | This study |
| aW303ΔCOX5a/COX6-HAC | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 cox5a::HIS3 | ρ+ | This study |
| aW303/OXA1-CH | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 oxa1::HIS3 leu2::pOXA1/ST8 | ρ+ | This study |
| aW303ΔOXA1/COX6-HAC | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 cox6::URA3 trp1::pG71/ST9 oxa1::HIS3 | ρ+ | This study |
Construction of MR6/ATP9Cys, W303ΔMSS51ΔPET494/COX6-HAC/ATP9Cys and W303/COX6-HAC/ATP9Cys
The V68C and S69C mutations were introduced by amplification of the ATP9 with primers 5’-GGCGAATTCGATATATAAATAAGTCCCTT and 5’- GGCGGTACCTTATATATATTAT-ACACCGAATAATAATAAGAAACAACACATTAAACAGAATAAACCTGTAGC. The 3’UTR of the gene was amplified with primers 5’-GGCGGTACCATAAATAAATAAAAAA-TAATG and 5’-GGCGGATCCAAAGTAATTATATATTATCC. The first PCR (polymerase chain reaction) product was digested with EcoR1 and KpnI and the second with KpnI and BamH1. The two digested products were cloned into pJM2 [36], previously cut with EcoRI and BamH1. Biolistic transformation of DFKρ0 and substitution of the modified ATP9 gene for the ARG8m allele in the atp9 null mutant to obtain MR6/ATP9Cys were performed as described previously [12].
A ρ0 derivative of W303/COX6-HAC was obtained by incubation for 30 minutes with ethidium bromide and purification of a respiratory deficient mutant that failed to be rescued when crossed to a panel of mit- testers with point mutations in mitochondrial genes. The resulting W303/COX6-HACρ0 was crossed to MR6/ATP9Cys on YPD and diploid cells selected on minimal glucose were sporulated on potassium acetate medium. Meiotic progeny were verified for the presence of COX6-HAC and ATP9Cys by their uracil and tryptophan prototrophy and growth on non-fermentable carbon sources. The same protocol was used to obtain W303ΔMSS51ΔPET494/COX6-HAC/ATP9Cys.
Construction of aW303/OXA1-CH
OXA1 was amplified with primers 5’-GGCGAGCTCCCACGTTCAGATGTTCC and 5’-GGCCTGCAGTTTTTTGTTATTAATGAAGTTTGATTTGTGAAC. The PCR product was digested with SacI and PstI and ligated to YIp352-CH, an integrative plasmid with a LEU2 selectable marker and the CH tag inserted between PstI and HindIII sites of the multiple cloning sequence. The resultant plasmid was linearized with BstXI and was integrated into the leu2 locus of an oxa1 null mutant strain. Transformants were selected for leucine prototrophy.
Growth, isolation of mitochondria, labeling of mitochondrial gene products, and purification of tagged proteins
Unless otherwise indicated, yeast grown in YPGal to early stationary phase were harvested and transferred to the same volume of fresh YPGal containing 2 mg/ml chloramphenicol and incubated at 30°C for another 2 hours. Mitochondria isolated by the method of Herrmann et al [27]. Small aliquots of mitochondria were frozen in liquid nitrogen and stored at -80°C. Unless otherwise indicated mitochondria were labeled for 20 min at 25°C with 35S-methionine/cysteine (3000 Ci/mmol) (MP Biochemicals, Solon, OH) as described previously [12]. The reaction was stopped with puromycin plus excess unlabeled methionine and further incubated for an additional 10 min. Digitonin extracts of the labeled mitochondria were purified on protein C antibody beads and analyzed by SDS-PAGE [26] and BN-PAGE [37].
Miscellaneous procedures
Purification, ligation, and transformation of Escherichia coli were done under standard conditions [38]. Yeast was transformed by the lithium acetate method [39]. Proteins were separated by SDS-PAGE on 12 or 15% polyacrylamide gels run in Laemmli buffer [26]. Proteins were separated by BN-PAGE on 4–13% polyacrylamide gels. Western blots were treated with monoclonal or polyclonal antibodies followed by a second reaction with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (Sigma) and proteins detected with SuperSignal chemiluminescent substrate kit (Pierce Biotechnology, Rockford, IL). The oxidation of cysteine was catalyzed by CuP [22]. The method of Lowry [40] was used to estimate protein concentration.
Supporting information
(PDF)
Acknowledgments
We thank Dr. Jean-Paul di Rago for providing us with the atp9 null mutant RKY26 and Dr. David Mueller for his suggestion of using residues 68 and 69 for the cysteine substitutions in Atp9.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by National Institutes of Health Grant 5RO1 GM111864 to (AT) and a FAPESP Post-Doctoral Fellowship 2019/02799-2 to (LVRF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19: 1777–1783. 10.1093/emboj/19.8.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma LK, Jianxin Lu J, Yidong Bai Y. Mitochondrial Respiratory Complex I, Structure, Function and Implication in Human Diseases. Curr Med Chem. 2009;16: 1266–1277. 10.2174/092986709787846578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luttik MA, Overkamp KM, Kotter P, De Vriess S, Van Dijken JP, Pronk JT. The Saccharomyces cerevisiae NDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J Biol Chem. 1998; 273: 24529–24534. 10.1074/jbc.273.38.24529 [DOI] [PubMed] [Google Scholar]
- 4.Heinemeyer J, Braun HP, Boekema EJ, Kouril R. A structural model of the cytochrome C reductase/oxidase supercomplex from yeast mitochondria. J. Biol. Chem. 2007; 282: 12240–12248 10.1074/jbc.M610545200 [DOI] [PubMed] [Google Scholar]
- 5.Rathore S, Berndtsson J, Marin-Buera L, Conrad J, Carroni M, Brzezinski P, et al. Cryo-EM structure of the yeast respiratory supercomplex. Nat. Struct. Mol Biol 2019;26: 50–57. 10.1038/s41594-018-0169-7 [DOI] [PubMed] [Google Scholar]
- 6.Wu M, Gu J, Guo R, Huang Y, Yang M. Structure of mammalian respiratory supercomplex I1III2IV1. Cell 2016;167: 1598–1609. 10.1016/j.cell.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 7.Letts JA, Fiedorczuk K, Sazanov LA. The architecture of respiratory supercomplexes. Nature 2016; 537: 644–648. 10.1038/nature19774 [DOI] [PubMed] [Google Scholar]
- 8.Sousa JS, Mills DJ, Vonck J, Kuhlbrandt W. Functional asymmetry and electron flow in the bovine respirasome. Elife 2016;5: e21290 10.7554/eLife.21290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schägger H. Respiratory chain supercomplexes. IUBMB Life 2011;52(3–5), 119–128. [DOI] [PubMed] [Google Scholar]
- 10.Acín-Pérez R, Bayona-Bafaluy MP, Fernández-Silva P, Moreno-Loshuertos R, Pérez-Martos A, Bruno C, et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell. 2004;13: 805–815. 10.1016/s1097-2765(04)00124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkens V, Kohl W, Busch K. Restricted diffusion of OXPHOS complexes in dynamic mitochondria delays their exchange between cristae and engenders a transitory mosaic distribution. J Cell Sci. 2013;126: 103–116. 10.1242/jcs.108852 [DOI] [PubMed] [Google Scholar]
- 12.Rak M, Gokova S, Tzagoloff A. Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 2011;30: 920–930. 10.1038/emboj.2010.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies KM, Strauss M, Daum B, Kief JH, Osiewacz HD, Rycovska A, et al. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc Natl Acad Sci USA. 2011;108: 14121–14126. 10.1073/pnas.1103621108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss M, Hofhaus G, Schröder RR, Kühlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008; 27: 1154–1160. 10.1038/emboj.2008.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su CH, McStay GP, Tzagoloff A. Assembly of the rotor component of yeast mitochondrial ATP synthase is enhanced when Atp9p is supplied by Atp9p-Cox6p complexes. J Biol Chem. 2014;289: 31605–1616. 10.1074/jbc.M114.602706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark-Walker GD, Linnane AW. In vivo differentiation of yeast cytoplasmic and mitochondrial protein synthesis with antibiotics. Biochem Biophys Res Commun. 1996;25: 8–13. [DOI] [PubMed] [Google Scholar]
- 17.Tzagoloff A, Barrientos A, Neupert W, Herrmann JM. Atp10p assists assembly of Atp6p into the F0 unit of the yeast mitochondrial ATPase. J Biol Chem. 2004;279: 19775–19780. 10.1074/jbc.M401506200 [DOI] [PubMed] [Google Scholar]
- 18.Altamura N, Capitanio N, Bonnefoy N, Papa S, Dujardin G. The Saccharomyces cerevisiae OXA1 gene is required for the correct assembly of cytochrome c oxidase and oligomycin-sensitive ATP synthase FEBS Lett. 1996;382: 111–115. 10.1016/0014-5793(96)00165-2 [DOI] [PubMed] [Google Scholar]
- 19.Szyrach G, Ott M, Bonnefoy N, Neupert W, Herrmann JM. Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 2003;22: 6448–6457. 10.1093/emboj/cdg623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia L, Dienhart MK, Stuart RA. Oxa1 directly interacts with Atp9 and mediates its assembly into the mitochondrial F1Fo-ATP synthase complex Mol Biol Cell 2007;18: 1897–1908. 10.1091/mbc.E06-10-0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272: 1136–1144. 10.1126/science.272.5265.1136 [DOI] [PubMed] [Google Scholar]
- 22.Jones PC, Fillingame RH. Genetic fusions of subunit c in the F0 sector of H+-transporting ATP synthase. Functional dimers and trimers and determination of stoichiometry by cross-linking analysis. J Biol Chem. 1998;273: 29701–29705. 10.1074/jbc.273.45.29701 [DOI] [PubMed] [Google Scholar]
- 23.Ballhausen B, Altendorf K, Deckers-Hebestreit G. Constant c10 ring stoichiometry in the Escherichia coli ATP synthase analyzed by cross-linking. J Bacteriol. 2009;191: 2400–2404. 10.1128/JB.01390-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava AP, Luo M, Zhou W, Symersky J, Bai D, Chambers MG, et al. High-resolution cryo-EM analysis of the yeast ATP synthase in a lipid membrane. Science. 2018;11: 360(6389). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittig I, Karas M, Schagger H. High Resolution Clear Native Electrophoresis for In-gel Functional Assays and Fluorescence Studies of Membrane Protein Complexes. Mol, Cell Proteomics. 2007;6: 1215–1225. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227: 680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 27.Herrmann JM, Foelsch H, Neupert W, Stuart RA. Isolation of yeast mitochondria and study of mitochondrial protein translation In: Cell Biology, Laboratory Handbook 1994. Vol. I, Celis J. R. (ed) pp. 538–544 San Diego, Academic Press. [Google Scholar]
- 28.Hell K, Neupert W, Stuart RA. Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 2001;20: 1281–128. 10.1093/emboj/20.6.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rak M, Tetaud E, Godard F, Sagot I, Salin B, Duvezin-Caubet S, et al. Yeast cells lacking the mitochondrial gene encoding the ATP synthase subunit 6 exhibit a selective loss of complex IV and unusual mitochondrial morphology. J Biol Chem. 2007;282: 10853–10864. 10.1074/jbc.M608692200 [DOI] [PubMed] [Google Scholar]
- 30.Su X, Rak M, Tetaud E, Godard F, Sardin E, Bouhier M, et al. Deregulating mitochondrial metabolite and ion transport has beneficial effects in yeast and human cellular models for NARP syndrome. Hum Mol Genet. 2019;28: 3792–3804. 10.1093/hmg/ddz160 [DOI] [PubMed] [Google Scholar]
- 31.Tzagoloff A, Akai A, Needleman RB, Zulch G. Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J Biol Chem. 1975;250: 8236–8242. [PubMed] [Google Scholar]
- 32.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76: 723–749. 10.1146/annurev.biochem.76.052705.163409 [DOI] [PubMed] [Google Scholar]
- 33.Tzagoloff A, Rubin MS, Sierra MF. Biosynthesis of mitochondrial enzymes. Biochim Biophys Acta. 1973;301(1): 71–104. 10.1016/0304-4173(73)90013-x [DOI] [PubMed] [Google Scholar]
- 34.Bietenhader M, Martos A, Tetaud E, Aiyar RS, Sellem CH, Kucharczyk R, et al. Experimental relocation of the mitochondrial ATP9 gene to the nucleus reveals forces underlying mitochondrial genome evolution. PLoS Genet. 2012;8: e1002876 10.1371/journal.pgen.1002876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McStay GP, Su CH, Tzagoloff A. Modular assembly of yeast cytochrome oxidase. Mol Biol Cell 2013;24: 440–452. 10.1091/mbc.E12-10-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulero JJ, Fox TD. Alteration of the Saccharomyces cerevisiae COX2 mRNA 5'-untranslated leader by mitochondrial gene replacement and functional interaction with the translational activator protein PET111. Mol Biol Cell. 1993;4: 1327–1335. 10.1091/mbc.4.12.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc. 206;1: 418–428. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. NY, Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Cur Genet. 1989;16: 339–346. [DOI] [PubMed] [Google Scholar]
- 40.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193: 265–275. [PubMed] [Google Scholar]