Abstract
Background:
In an effort to decrease the rates of smoking conventional tobacco cigarettes, electronic cigarettes (e-cigarettes) have been proposed as an effective smoking cessation tool. However, little is known about their toxicological impacts. This is concerning given that e-cigarette use is perceived as less harmful than conventional tobacco cigarettes during pregnancy for both the mother and fetus.
Objective:
The goal of this study was to test the neurodevelopmental consequences of maternal e-cigarette use on adult offspring behavior and neuroimmune outcomes.
Methods:
Pregnant female CD-1 mice were randomly assigned to one of three treatment groups () and exposed daily to either filtered air, propylene glycol and vegetable glycerol (50:50 PG/VG vehicle), or to PG/VG with nicotine (). Whole-body exposures were carried out for 3 h/d, 7 d/week, from gestational day (GD)0.5 until GD17.5. Adult male and female offspring (8 weeks old) were assessed across a battery of behavioral assessments followed by region-specific quantification of brain cytokines using multiplex immunoassays.
Results:
Adult offspring of both sexes exposed to exhibited elevated locomotor activity in the elevated plus maze and altered stress-coping strategies in the forced swim task. Moreover, male and female offspring exposed to PG/VG with and without nicotine had a 5.2% lower object discrimination score in the novel object recognition task. In addition to differences in offspring behavior, maternal e-cigarette exposure with nicotine led to a reduction in interleukin (IL)-4 and interferon-gamma () in the diencephalon, as well as lower levels of hippocampal (females only). E-cigarette exposure without nicotine resulted in a 2-fold increase of IL-6 in the cerebellum.
Discussion:
These findings support previous adverse findings of e-cigarette exposure on neurodevelopment in a mouse model and provide substantial evidence of persistent adverse behavioral and neuroimmunological consequences to adult offspring following maternal e-cigarette exposure during pregnancy. https://doi.org/10.1289/EHP6067
Introduction
Electronic cigarettes (e-cigarettes) are battery-powered nicotine delivery systems purported by some to be a safer alternative to tobacco cigarettes for pregnant women (Mark et al. 2015; Wagner et al. 2017) despite the lack of information on their toxicological effects on the developing fetus. Alarmingly, up to 15% of pregnant women use e-cigarettes based on survey data collected between 2007 and 2017 (Wagner et al. 2017; Whittington et al. 2018), with the highest prevalence of overall e-cigarette use among people of childbearing age (18–34 years of age) (Kasza et al. 2014; Parker and Villanti 2019; Stallings-Smith and Ballantyne 2019). In addition to the known detrimental effects of nicotine on the developing brain, potential health risks are being raised for the added flavors and vehicle [propylene glycol and vegetable glycerin (PG/VG)] used in most e-cigarettes due to their under-investigated toxicology (Burstyn 2014). This is of particular concern for those using low-dose nicotine e-cigarettes because their use often results in the consumption of higher amounts of the PG/VG vehicle while attempting to obtain nicotine doses equivalent to a conventional cigarette. Given the use of e-cigarettes among pregnant women and individuals of childbearing age [a survey published in 2018 suggested 6.52% of pregnant women use e-cigarettes and up to 8.54% use both electronic and tobacco cigarettes (Wagner et al. 2017)] and the perception of e-cigarettes as a safer alternative to tobacco cigarettes during pregnancy (Baeza-Loya et al. 2014; McCubbin et al. 2017; Whittington et al. 2018), there is an urgent need to better understand their toxicology, particularly in regard to the developing fetus.
Decades of research on conventional cigarette smoke have reported the toxicological effects of smoking and nicotine on brain development. For example, studies utilizing various exposure paradigms to assess the developmental effects of prenatal and/or postnatal nicotine exposure in rodents reported increased hyperactivity (Lacy et al. 2016; Newman et al. 1999), impaired cognitive ability in terms of memory and attention, altered pre-pulse inhibition (Alkam et al. 2013; Zhang et al. 2018), and increased prevalence of anxiety- or depressive-like behaviors in adulthood (Lee et al. 2016; Pinheiro et al. 2015; Zhang et al. 2019). Importantly, some of these behavioral alterations (e.g., increased activity) were reported in offspring of pregnant mice exposed to 2.4% nicotine through e-cigarette aerosol exposure (Smith et al. 2015). This is not surprising given that nicotine levels between tobacco and electronic cigarettes are often comparable, and sometimes higher, in e-cigarette devices. Despite the well-known developmental effects of maternal nicotine exposure, 43% of pregnant women surveyed in 2014 viewed e-cigarettes as a safer alternative to tobacco cigarettes during pregnancy (Mark et al. 2015). Such assertions are also concerning given that the toxicology of e-cigarette constituents (e.g., the PG/VG vehicle) and released by-products on offspring health, even in the absence of nicotine, produced adverse effects on neurodevelopment in mice (Lauterstein et al. 2016).
Evidence suggests that the PG/VG vehicle (commonly used to aerosolize nicotine) is potentially hazardous (for review, see Burstyn 2014). Animal studies have reported both maternal and direct PG/VG exposure impacts on offspring body weight, disease susceptibility, and brain development (Chen et al. 2018a, 2018b; Lau et al. 2012; Lauterstein et al. 2016; Nguyen et al. 2018; Smith et al. 2015). Uncertainty in these claims is likely due to the fact that previous preclinical literature often considered only male offspring in regard to behavioral effects and brain gene changes (Chen et al. 2018b; Smith et al. 2015), did not control for litter effects in primary data analysis and inconsistently reported any such differences in data analyses (Smith et al. 2015), or differed in timing of maternal e-cigarette aerosol exposure and nicotine dosing (Chen et al. 2018a, 2018b; Lauterstein et al. 2016; Nguyen et al. 2018; Smith et al. 2015). These differences hinder our ability to discern how the constituents and by-products of e-cigarettes could impart lifelong impairments in offspring development. Although the abovementioned studies begin to reveal the detrimental consequences of gestational e-cigarette exposure, understanding the long-term biological underpinnings, particularly in the brain, of these negative outcomes is essential for understanding the true risk of their use during pregnancy.
Information concerning the long-term impact of perinatal e-cigarette exposure in the offspring brain is limited (Lauterstein et al. 2016; Zelikoff et al. 2018), and the relationship between long-term neuroinflammation and altered behavioral development is even less well researched. Rodent studies have demonstrated that nicotine exposure negatively affects cortical neuronal morphology and connectivity when given prenatally (Muhammad et al. 2012), genetic expression of metabolism and feeding genes in the brain when dams were exposed prior to breeding through offspring weaning (Chen et al. 2018b), and expression of neurotransmitter receptors (reviewed by Dwyer et al. 2009 and Smith et al. 2010) in the developing nervous system. Interestingly, nicotine exposure in vitro was shown to directly activate immune cells (Hosseinzadeh et al. 2016), including placental macrophages (Belhareth et al. 2018), indicating a potential role for neuroinflammation in the detrimental effects that nicotine-containing e-cigarettes have on brain development and function. In particular, changes in brain cytokine signaling profoundly influence behavior and are often associated with clinical pathologies, including developmental disorders (Mittleman et al. 1997; Siniscalco et al. 2018). Importantly, perinatal exposure to e-cigarette aerosol without nicotine, beginning 6 weeks before pregnancy and continuing through offspring weaning, has been shown to induce long-term peripheral inflammation in the lungs of adult mouse offspring (Chen et al. 2018a). This suggests that the PG/VG vehicle and other component by-products of e-cigarette aerosols, in the absence of nicotine, may have unique and lasting effects on offspring nervous system health. Therefore, further investigation is needed to determine the behavioral and neuroinflammatory effects of maternal e-cigarette use in adult offspring, with particular emphasis on disentangling the specific developmental impact of nicotine compared with the PG/VG vehicle.
Given that e-cigarettes are perceived as a safer alternative to tobacco cigarettes for pregnant women (Baeza-Loya et al. 2014; Mark et al. 2015), despite our limited understanding of the neurotoxicological consequences to the developing fetus, we sought in this study to determine the long-term effects of prenatal e-cigarette aerosol exposure on adult offspring neurodevelopment and behavior. It was hypothesized that daily maternal exposure to e-cigarette aerosol, both PG/VG alone and PG/VG in combination with nicotine, impart unique and lasting behavioral and neuroimmunological alterations in both sexes of offspring. To test this, adult offspring of dams exposed throughout pregnancy to e-cigarette aerosol with or without nicotine were assessed for stress-coping behaviors, hyperactivity, and learning and memory deficits compared with age-matched filtered air (FA) control offspring. Following behavioral analyses, brains were assessed for differences in cytokine levels in the hippocampus, cortex, diencephalon, and cerebellum.
Methods
Animals
Twenty-seven female CD-1 mice, 8–10 weeks of age (Charles River Laboratories), were individually paired at proestrus with adult males and checked the following morning for the presence of a seminal plug (Blum et al. 2012; Church et al. 2018). Mice were housed in cages with high-efficiency particulate air (HEPA)-filtered lids and were provided free access to food and water at the New York University (NYU) Department of Environmental Medicine animal facility and, subsequently, in the same manner at Mount Holyoke College (Massachusetts). Female mice were group housed at NYU until gestational day (GD)15, at which time females were single housed until parturition. After weaning, offspring were group housed (2–5 mice per cage) with same-sex littermates and all mice were maintained at ambient room temperature on a 12-h light/dark cycle (lights on at 0600 hours). All breeding and e-cigarette exposure procedures were carried out at the NYU facility. Following weaning (at ), 135 offspring were transported from NYU (Sterling Forest, NY, Campus) in standard plastic cages in a temperature-controlled van (World Courier, AmerisourceBergen Corporation) to Mount Holyoke College. Upon arrival, mice were group housed with previous cage mates in individually ventilated cages and allowed 4 weeks to acclimate to the new facilities prior to behavioral testing. All behavioral assessments were carried out at Mount Holyoke College during the first 4 h of the light cycle. All mouse procedures were approved by both NYU Langone Medical Center’s and Mount Holyoke College’s Institutional Animal Care and Use Committees in accordance with the Guide for the Care and Use of Laboratory Animals (NRC 2011).
E-Cigarette Exposure
Upon successful mating, as demonstrated by the presence of a seminal plug, female mice were randomly assigned to one of three treatment groups () (see Table S1) and exposed daily to either 50% PG and 50% VG (50:50 PG/VG), 50:50 PG/VG with nicotine (), or ambient air filtered to remove particles (FA). E-cigarette aerosols were produced from an automated three-port e-cigarette aerosol generator (), as previously described (Lauterstein et al. 2016). Puff aerosols were generated with charcoal and HEPA-filtered air (i.e., FA) using a rotor-less diaphragm pump; the puff regimen consisted of puff volumes of 4-s duration at 30-s intervals. Each puff was mixed with filtered dilution air before entering the exposure chamber. Whole-body exposures were carried out for 3 h/d for 7 d/week from GD0.5 to GD17.5, and aerosol samples were collected daily using preweighed Teflon® filters (, pore size; PALL Life Sciences Teflo) for assessment of particulate matter (PM). The PM concentration from FA was determined on a weekly basis. Particle-laden filters were equilibrated overnight in a temperature- and humidity-controlled weigh room ( and relative humidity) and were weighed gravimetrically on an MT5 microbalance (Mettler Toledo).
Urine Collection
To confirm e-cigarette nicotine exposure in the appropriate treatment group, urinary cotinine levels in selected dams were measured at GD5.5, GD10.5, and GD15.5 using a commercially available enzyme-linked immunosorbent assay kit (Abnova). On the day of urine sample collection, pregnant dams were weighed and each was placed in a novel plastic container where they were allowed to freely explore until micturition. Urine was collected from the bottom of the container using a pipette and transferred to a microcentrifuge tube. Each container was thoroughly cleaned with 70% ethanol and water between animals, and separate containers were used between groups.
Maternal Growth, Litter Size, and Offspring Weight
Pregnant dams were weighed daily beginning on the first day of pregnancy (GD0) and continued through parturition. Litters were assessed for size (i.e., number of pups) and ratio of male mice, which was calculated as the number of male offspring divided by the total number of mice in the litter. Offspring were weighed at postnatal day (PND)21 and again before sacrifice at 12 weeks of age to assess differences in body mass between treatment groups.
Behavioral Testing
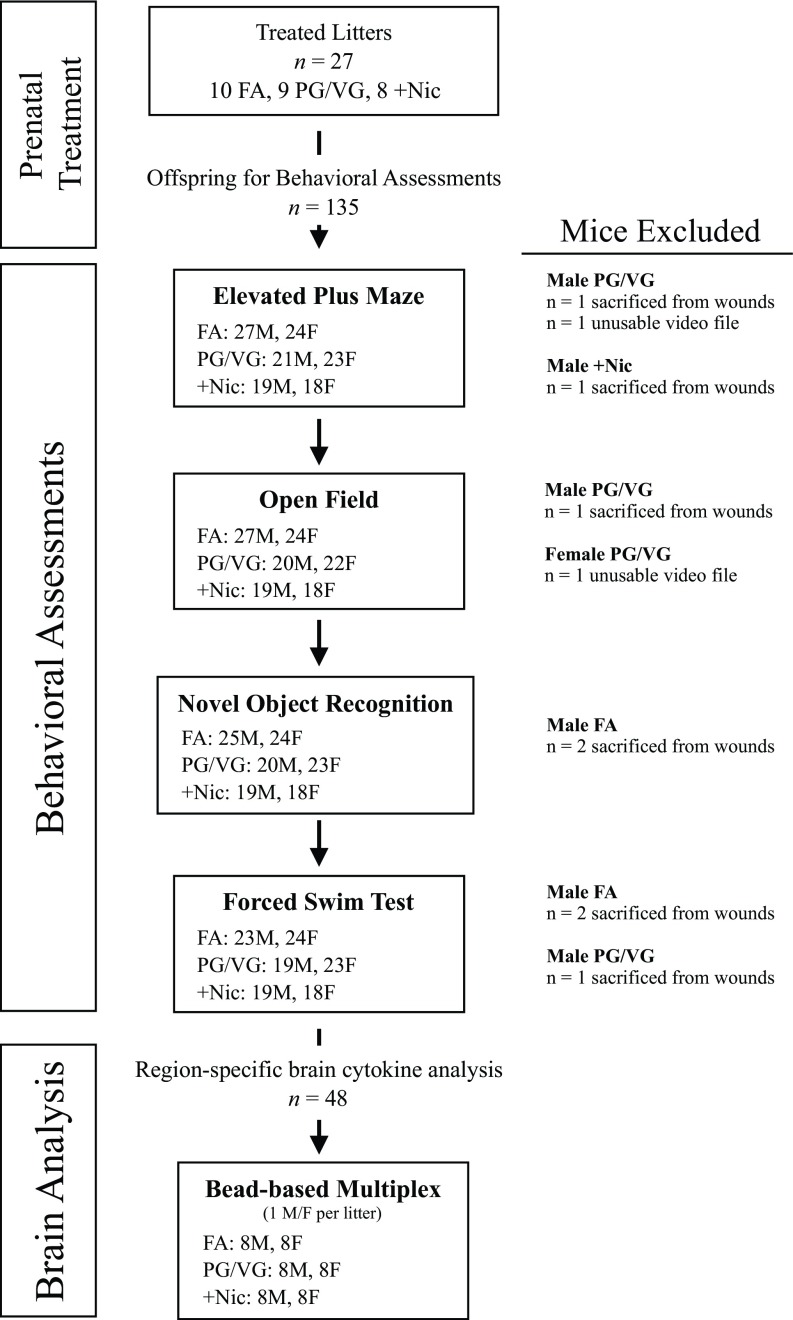
At 8 weeks of age, 67 male (27 FA, 21 PG/VG, ) and 65 female (24 FA, 23 PG/VG, ) offspring were assessed through a series of behavioral tasks beginning with the elevated plus maze followed by the open field task, the novel object recognition task, and then the forced swim task. Behaviors were carried out sequentially with at least 1 week between each behavioral task, and experimenters were blinded to treatment conditions. Mice were pseudorandomized to ensure offspring of all treatment groups and between sex were counterbalanced across each task. All mice completed one behavioral task before beginning the subsequent task. Eight male mice were removed throughout the study because of intra-cage aggression (Figure 1).
Figure 1.
Diagram of experimental procedures and sample size. The number of male and female mice in each treatment group is included for each behavioral task. Brains from a subset of mice were used for region-specific brain cytokine measures. Note: F, female; FA, filtered air; M, male; PG/VG, propylene glycol/vegetable glycerin; , propylene glycol/vegetable glycerin plus nicotine.
Elevated plus maze.
To evaluate differences in anxiety and risk aversion, adult offspring were assessed using an elevated plus maze constructed of black Plexiglas® in full light plus a portable halogen work light to serve as an aversive stimulus. The apparatus consisted of two open arms () and two perpendicular closed arms () extending from a central platform. The entire maze was elevated approximately 1 m from the floor. Mice were video recorded and placed in the central platform and allowed to freely explore the maze for 5 min, as previously described (Walf and Frye 2007). The videos were later scored using EthoVision XT (version 14; Noldus) for the number of entries into each arm and the total time spent in each arm. Reductions in open arm exploration (calculated as the exploration time in the open arm divided by the total exploration time in both the open and closed arms) were interpreted as increased anxiety.
Open field exploration.
Mice were placed in an open arena () and were video recorded under dim lighting during 20 min of free exploration. Videos were analyzed using EthoVision XT14 for total distance traveled and the total time spent in the center of the arena.
Novel object recognition.
Mice were returned to the open field arena 1 week later and assessed for memory performance in the novel object recognition task using the short habituation protocol outlined by Leger et al. (2013). During the initial familiarization (training) phase, mice were allowed to freely explore two identical objects for 10 min. Twenty-four hours later, one of the familiar objects was replaced with a novel object and experimental mice were returned to the arena and video recorded for a 10-min testing session. Object investigation, as defined by the time spent sniffing either the familiar or novel object, was measured using EthoVision XT14. Novel-object recognition, as described by Lueptow (2017), was calculated by measuring the time spent sniffing the novel object divided by the total time sniffing either object during the 10-min trial. Only mice who actively sniffed objects for a minimum of 20 s in the familiarization phase were included in the novel object recognition test phase. Novel-object sniff times greater than 50% indicated object recognition. All objects and arenas were cleaned with 70% ethanol between each testing session to remove any olfactory cues.
Forced swim test.
Mice were placed in a transparent Plexiglas® cylinder () filled with warm water () to a height of . Animals were then video recorded for 6 min and measured for time spent actively swimming or immobile (i.e., the period of time not spent actively exploring, swimming, or trying to escape) (Can et al. 2012; Porsolt et al. 1977); measurements were determined using EthoVision XT14. Following the 6-min task, mice were removed from the cylinder, toweled dry, and placed in a warmed, dry cage for 20 min before being returned to their home cage.
Tissue Processing
Between 2 and 3 weeks following the completion of the forced swim test, one male and one female from each litter was anesthetized by 3% isoflurane inhalation and sacrificed by decapitation followed by brain dissection. Freshly collected brains were rinsed in ice-cold phosphate-buffered saline and separated into four distinct regions: cortex, diencephalon, cerebellum, and hippocampus. Individual regions were separated into microcentrifuge tubes (Eppendorf), flash frozen in liquid nitrogen, and stored at . Each sample was disrupted using the Bio-Plex® cell lysis kit containing Factor 1 and Factor 2 (BioRad) and protease inhibitor phenyl-methylsulfonyl fluoride (Sigma-Aldrich) in dimethyl sulfate using disposable pestles. Brain regions were homogenized by triturating using and then pipette tips. Homogenates were then mixed on an orbital shaker at 4°C for 40 min followed by centrifugation at 4°C (at ) for 20 min. Supernatants were aliquoted into aliquots for multiplex assays (described below) and stored at until used. The protein concentration of each sample was determined using a bicinchoninic acid protein assay kit (Thermo Scientific) as directed by the manufacturer, with bovine serum albumin as the standard. Sample absorbance was read on a Synergy H1 Hybrid plate reader (BioTek) and protein content was estimated using a four-parameter logistic regression curve.
Brain Cytokine Measurements
The quantification of cytokines in sample brain section supernatants was carried out using multiplex bead-based immunoassays (Procarta Plex Mouse High Sensitivity Kit; Invitrogen). Cytokine analyses were performed as directed by the manufacturer. Samples were diluted to protein using Bio-Plex® Cell Lysis Buffer (BioRad) and then combined with of a bead solution containing four high-sensitivity antibody-coupled magnetic polystyrene beads [i.e., interleukin (IL)-2, IL-4, IL-6, interferon-gamma ()]. The samples were washed three times with wash buffer using a handheld magnet. Samples, of each, along with eight 4-fold serial dilutions of standards and blanks were added in duplicate and incubated overnight at 4°C in the dark. The following day, the plate was washed three times with wash buffer followed by the addition of of detection antibodies, and then agitated on a plate shaker at room temperature for 45 min. After another series of washes with wash buffer, of streptavidin, R-Phycoerythrin conjugate (SAPE) was added to each well and incubated in the dark at room temperature for 30 min. To increase detection and sensitivity, an additional two-step amplification process was carried out consisting of incubation with of Amplification Reagent 1 (Invitrogen) for 30 min followed by incubation of of Amplification Reagent 2 (Invitrogen) at room temperature for 30 min. After a final series of washes with wash buffer, the beads were resuspended in read buffer and the plate was read on a MAGPix system (Luminex) using xPONENT® software (version 4.1; Luminex). Unknown sample cytokine concentrations were estimated using a five-parameter logistic regression curve derived from the known reference cytokine concentrations supplied by the manufacturer. Supernatant aliquots did not undergo multiple freeze–thaw cycles. The sensitivity of this assay allowed for the detection of cytokine concentration with the following limits of detection: (), IL-2 (), IL-4 (), and IL-6 (). Wells with bead-read errors provided by the xPONENT® 4.1 software were excluded from analysis.
Statistical Analyses
All behavioral measures were assessed using multilevel linear mixed-effects models with maximum likelihood estimates and Type III sums of squares using R (version 3.5.3; R Development Core Team) and the nlme package. Models were constructed using a stepwise forward selection strategy beginning with an intercept-only model (Model 1) and then adding fixed main effects (Model 2) and interactions (Model 3). First, a basic two-level random-effects model was constructed with representing each behavioral observation for the ith animal of Level 1 nested in the jth litter at Level 2. is equal to the Level 1 intercept plus the unexplained variance, or residual, noted as . Then each animal was nested in litter at Level 2 represented by a fixed intercept for litter as , plus a litter-specific random intercept that varied by j litter, . The random intercept was assumed to be normally distributed with a mean of 0 and variance equal to . The random-effects model (Model 1) is represented by the following equation:
| (1) |
| (2) |
| (3) |
Next, fixed effects were added to the model and tested for model fit using the log-likelihood ratio test. In this mixed-effects model (Model 2), which included both fixed and random components, was estimated from the Level 1 intercept and the regression parameter of each predictor variable and for the main effect of treatment, PG/VG or , and for offspring sex (Female). A third model (Model 3) was then constructed that included the addition of predictor variables and for the two-way interactions of treatment by sex (PG/VG:Female and :Female) and tested for model fit compared with the random-effects and fixed main-effects models. The full model included fixed main effects and interaction as well as variance components for both Level 1 intercept, represented by , and random Level 2 error (litter), , plus the remaining residual, .
| (4) |
For repeated measures analysis of object sniffing in the novel object recognition task, models included an additional Level 1 random-effects component for each animal, nested within litter, to account for the repeated measure of objects (familiar or novel) within each mouse. Daily weights of pregnant dams were fitted with an autoregressive polynomial model that included a random-effects component for each dam repeated across days.
Model variations were assessed using the likelihood ratio test and the best model was selected based on the lowest Akaike information criterion (AIC). All models were first fit with a homogenous residual variance structure and then tested against the heterogeneous residual variance for each treatment group using the likelihood ratio test. The final model was assessed graphically to verify that the data met the assumptions of linearity, normality, and homogeneity of variance. Models that included a significant interaction component in the fixed effect were confirmed with Wald’s test using F-values estimated using Kenward-Rogers degrees of freedom and Type III sums of squares followed by simple main effects and post hoc analysis using Tukey corrections. Model summaries can be found in Tables S2–S15, with the fixed-effect parameters shown only for the final model selected with the lowest AIC.
Maternal cotinine, PM concentration, litter size, and ratio of male offspring were assessed by one-way analysis of variance (ANOVA) followed by Tukey post hoc comparisons. Offspring body mass and cytokine analytes were assessed using two-way factorial ANOVA (treatment by sex) followed by simple main effects analysis and Tukey comparisons when applicable. Cytokine values were correlated with behavioral measures using Spearman’s rho ().
Results
Maternal and Litter Measures
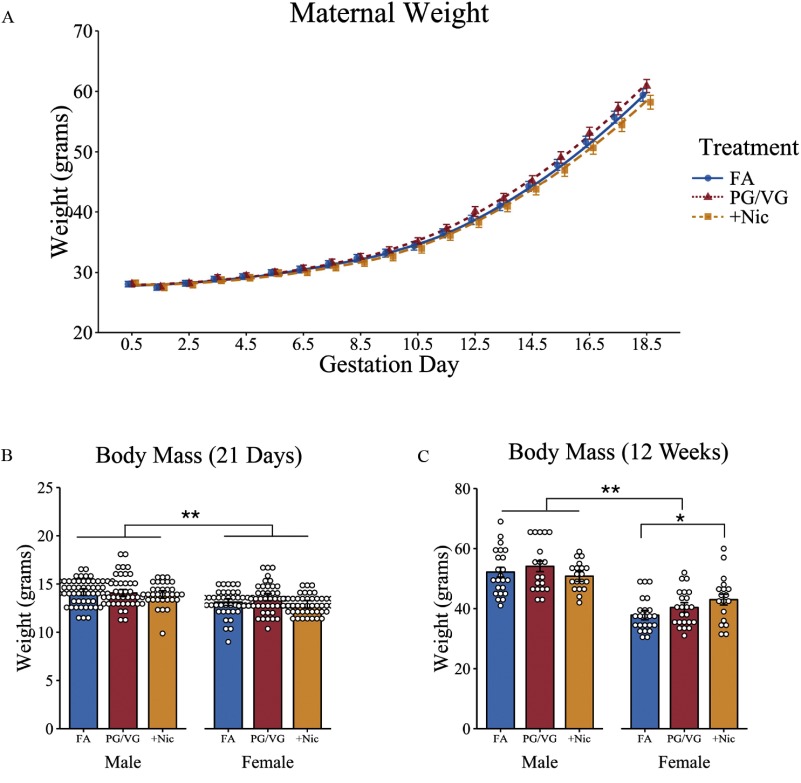
Pregnant dams in all three treatment groups showed similar weight gain throughout pregnancy (Figure 2A; Table S2). Litter sizes averaged 9–10 offspring per litter (see Table S1), with no differences in the number of pups across treatment groups, , . To verify exposure constituents, PM concentrations in aerosol and maternal cotinine (i.e., nicotine metabolite) levels were assessed for all three treatment groups. PM levels from e-cigarette aerosols were averaged across multiple Teflon® filters from all gestational exposure days. Compared with FA (), PG/VG aerosol had an average PM concentration of on each day of exposure, and these levels were similar to those measured in the aerosol, . Mean cotinine levels averaged from GD0.5, GD10.5, and GD15.5 were higher in maternal urine from the dams () compared with PG/VG alone (), and FA-exposed mice () (Table 1).
Figure 2.
Weights of pregnant dams and offspring exposed to filtered air (FA), e-cigarette aerosol (PG/VG), or e-cigarette aerosol with nicotine. (A) Pregnant dams were weighed daily throughout gestation (). Offspring were weighed on (B) PND21 and again in (C) adulthood at the completion of behavioral testing, and body mass (in grams) were compared across treatment groups. For maternal weight, plots are mean and trend lines represent estimated growth curves using linear mixed-effects modeling with treatment and day as fixed effects and dam as random effects. For offspring weights, *, ** as determined by linear mixed-effects modeling with treatment and sex as fixed effects and litters as random effects. Plots represent individual mice; bars represent marginal means . FA (10 litters), males [ (PND21) and (adulthood)], females [ (PND21) and (adulthood)]; PG/VG (9 litters) [males (PND21) and (adulthood)], females [ (PND21) and (adulthood)]; (8 litters) males [ (PND21) and (adulthood)], females [ (PND21) and (adulthood)]. Note: PG/VG, propylene glycol/vegetable glycerin; PND, postnatal day; SE, standard error; , propylene glycol/vegetable glycerin plus nicotine.
Table 1.
Maternal measures ().
Note: Ratio of male to female offspring was calculated as the number of male mice in a litter divided by the total number of pups in the litter. FA, filtered air; PG/VG, propylene glycol/vegetable glycerin; PM, particulate matter; , propylene glycol/vegetable glycerin plus nicotine.
compared with FA mice as determined by one-way analysis of variance.
Body Mass Analysis in Response to Prenatal E-Cigarette Exposure
On PND21 and again at 12 weeks of age, offspring were weighed and assessed for differences in body mass using mixed-effects models. Female offspring had a lower mean weight on PND21 compared with males, [95% confidence interval (CI): , ], , , with no differences observed across treatment groups (Figure 2B; Table S3). Similarly, in adulthood, male mice were on average heavier, (95% CI: 49.06, 55.28) compared with female offspring, (95% CI: 34.6, 41.1), (95% CI: , ), , . There was also a treatment by sex interaction, with female mice of -exposed dams weighing an estimated more than female offspring of FA dams, (95% CI: 1.05, 9.54), , . However, there were no differences in body mass between and PG/VG-alone offspring, , , and no effects on weight were observed between male offspring across treatment groups (Figure 2C; Table S4).
Stress-Coping Behavioral Measures in Offspring
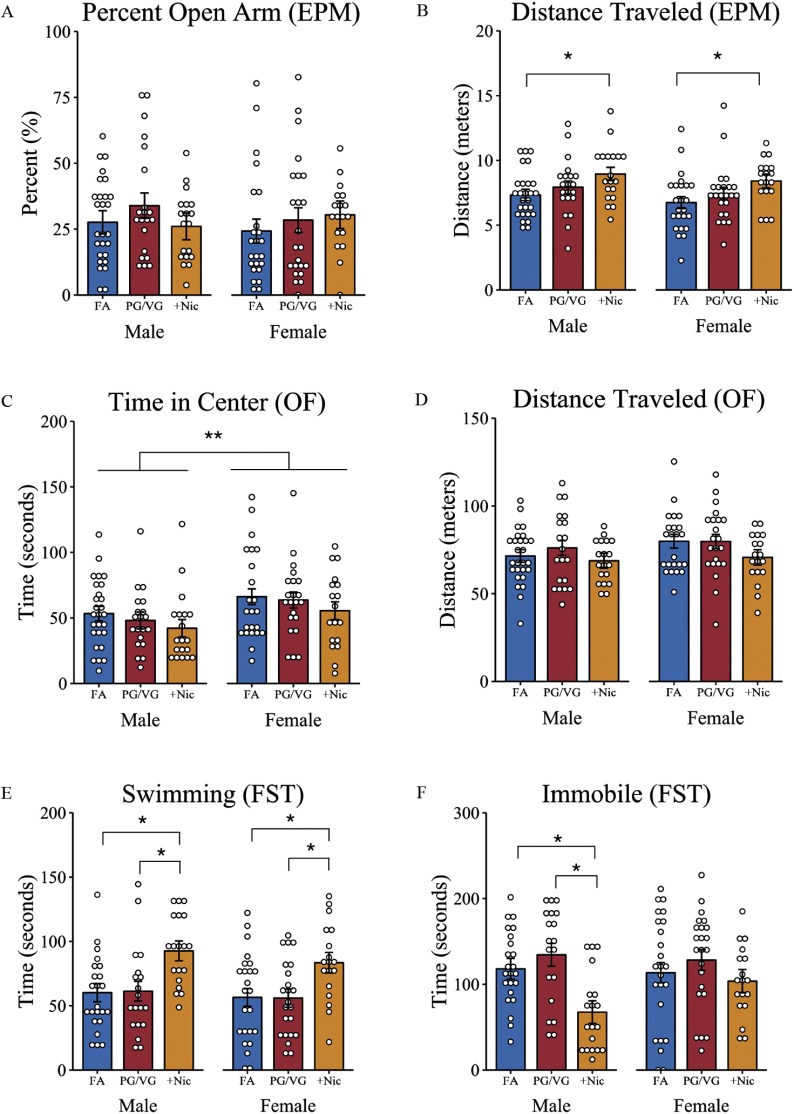
To determine whether CD-1 male and female offspring exposed to e-cigarette aerosols, with or without nicotine, during gestation leads to anxiety-like behaviors in adulthood, mice were tested for differences in open arm exploration in the elevated plus maze. For percentage open arm exploration, a random effects–only model (see Table S5) estimated that male and female CD-1 mice on average spent 28% (95% CI: 24%, 33%) of the time exploring the open arm of the maze, with no differences observed in total exploration time across sex and treatment groups (Figure 3A). Similarly, for total time in the open arm, a random effects–only model (see Table S6) confirmed that across all treatment groups male and female offspring spent a similar total time in the open arms [ (95% CI: 38.93, 56.88)] and an average of 10 entries (95% CI: 9.01, 11.18) into the open arms during the 5-min test (see Table S7; Figure S1). Despite these similarities in open arm exploration, there was a treatment-induced effect on locomotor activity, , , that was observed in both male and female offspring of dams exposed during pregnancy to . These offspring of both sexes displayed greater distance traveled compared with sex- and age-matched FA-exposed offspring, (95% CI: 54.57, 273.96), , (Figure 3B; Table S8).
Figure 3.
Stress-coping behaviors as determined by the elevated plus maze, open field, and forced swim tests, respectively, in male and female adult offspring of mice treated with filtered air (FA), E-cigarette aerosol (PG/VG), or E-cigarette aerosol with nicotine (). Pregnant female mice were exposed to either FA or e-cigarette aerosol (PG/VG) with or without nicotine, and offspring were tested using the (A,B) elevated plus maze, (C,D) open field, and (E,F) forced swim tasks. Reported is (A) the percentage of time offspring spent in the open arm of the elevated plus maze as a percentage of the total time spent in both open and closed arms; (B) total distance traveled during the 5-min elevated plus maze task; (C) time spent in the center of an open field arena during the 20-min open field task; (D) the total distance traveled in the open field task; and the total time spent (E) swimming and (F) immobile in the forced swim task. *, ** as determined by linear mixed-effects modeling with treatment and sex as fixed effects and litters as random effects. Plots represent individual mice; bars represent marginal means . FA (10 litters) [males (), females ()]; PG/VG (9 litters) [males (), females ()]; (8 litters) [males (), females ()]. Note: EPM, elevated plus maze; FST, forced swim task; OF, open field; PG/VG, propylene glycol/vegetable glycerin; SE, standard error; , propylene glycol/vegetable glycerin plus nicotine.
In the open field task, a model including fixed main effects (see Table S9) indicated that female mice from all treatment groups spent more time exploring the center of the arena compared with exposure- and age-matched male offspring, (95% CI: 4.23, 23.47), , , (Figure 3C). However, there were no differences in center time exploration across treatment groups of either male or female mice. In addition, male and female offspring showed similar locomotor activity across all treatment groups, random effects–only model (see Table S10), with a mean distance traveled of (95% CI: 6,711.98, 7,915.52) during the 20-min free exploration task. In addition, no differences were observed in total distance traveled between sex (Figure 3D).
Adult offspring across treatment groups were assessed for differences in immobility and swimming time in the forced swim test. For time swimming, a mixed-effects model with sex and treatment as fixed effects (see Table S11) revealed an effect of treatment on swimming behavior, , . Male and female offspring of exposed dams spent more time swimming, (95% CI: 13.00, 46.42) compared with FA , and PG/VG-alone treatment groups, , (Figure 3E). No difference in swimming time was observed between sexes, , . The mixed-effects model for immobility included both fixed main effects and a sex by treatment interaction (see Table S12) with male, but not female, offspring of e-cigarette aerosol with nicotine-exposed () dams showing less immobility, (95% CI: , ), , compared with age- and sex-matched FA control offspring. Simple main effects analysis confirmed a treatment effect in male mice, , , with male offspring of dams showing less immobility compared with both FA, , , and PG/VG-alone offspring, , . No differences in immobility were observed across treatment groups of female offspring, , (Figure 3F).
Memory Performance in Offspring of E-Cigarette–Exposed Dams
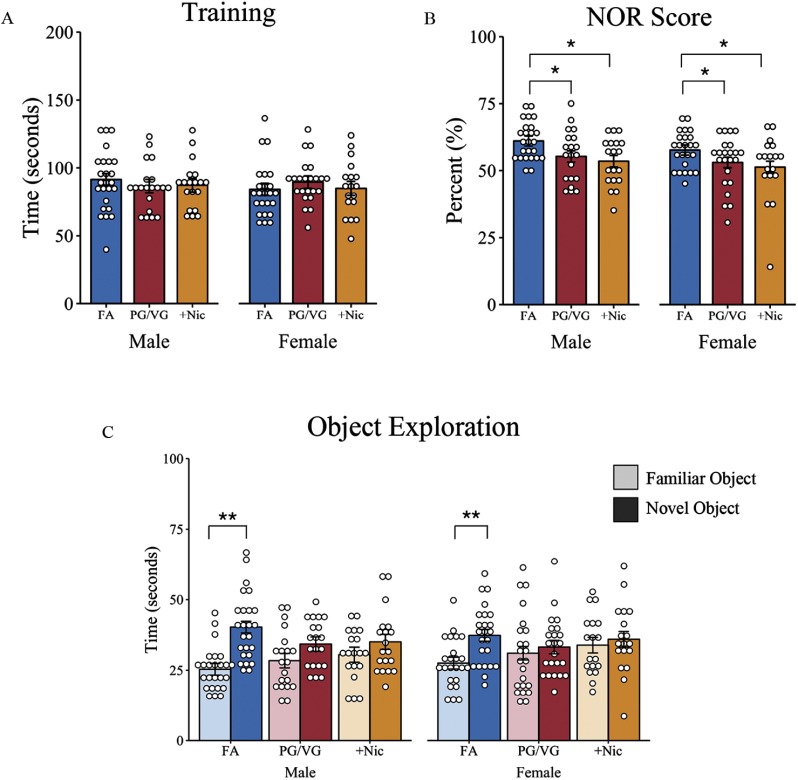
In the familiarization phase, all mice met the training criteria of a minimum of 20 s of object sniffing time to proceed to the testing phase. A random effects–only model confirmed no differences in total time spent sniffing objects across treatment groups and sex (Figure 4A; Table S13). Mice spent an average of 87.36 s (95% CI: 82.97, 91.75) exploring the two objects during the 10-min familiarization phase.
Figure 4.
Measure of short-term memory in offspring of dams exposed to filtered air (FA), e-cigarette aerosol (PG/VG), or e-cigarette aerosol with nicotine (). Offspring were given 10 min to explore two identical objects and then assessed 24 h later for memory performance by replacing one familiar object with a novel object. (A) Total time spent sniffing two objects during the initial learning phase; (B) percentage of novel object recognition determined as the time spent with the novel object over total object exploration time; and (C) time spent sniffing the familiar and novel object 24 h later during the testing phase. *, ** as determined by liner mixed-effects model with treatment and sex as fixed effects (A,B) or as separate models for each treatment group (C) and individual animals nested in litter as random effects. Plots represent individual mice; bars represent marginal means . FA (10 litters) [males (), females ()]; PG/VG (9 litters) [males (), females ()]; (8 litters) [males (), females ()]. Note: NOR, novel object recognition; PG/VG, propylene glycol/vegetable glycerin; SE, standard error; , propylene glycol/vegetable glycerin plus nicotine.
Memory performance was tested 24 h later when one familiar object was replaced with a novel object. Object exploration times were standardized across animals by calculating a novel object score equal to the time spent sniffing the novel object divided by total object sniff time. The final model, which included fixed main effects without interactions, revealed that adult male and female offspring of FA-exposed dams spent an average of 60.71% (95% CI: 57.61, 63.81) of their object exploration time with the novel object, with no differences observed between sex, , (see Table S14). Conversely, male and female offspring exposed to PG/VG without nicotine had an estimated 5.19% lower novel object recognition score, (95% CI: , ), , ; and offspring born to dams exposed to PG/VG had an estimated 6.97% lower object recognition score, (95% CI: , ), , (Figure 4B) compared with offspring of dams exposed to FA.
Differences in novel and familiar object sniffing was also compared within treatment groups to assess object preference (see Table S15). Male and female offspring from FA-exposed control dams spent an average of 12.45 s longer with the novel object compared with the familiar object, (95% CI: 9.46, 15.43), , . In contrast, male and female offspring of PG/VG-exposed dams only spent approximately 3.91 s longer with the novel object compared with the familiar object, (95% CI: 0.14, 7.68), , . Similar differences in novel object exploration time were observed in male and female offspring of dams exposed to PG/VG with nicotine throughout pregnancy (): (95% CI: , 7.93), , (Figure 4C).
Region-Specific Cytokine Levels in Adult Offspring
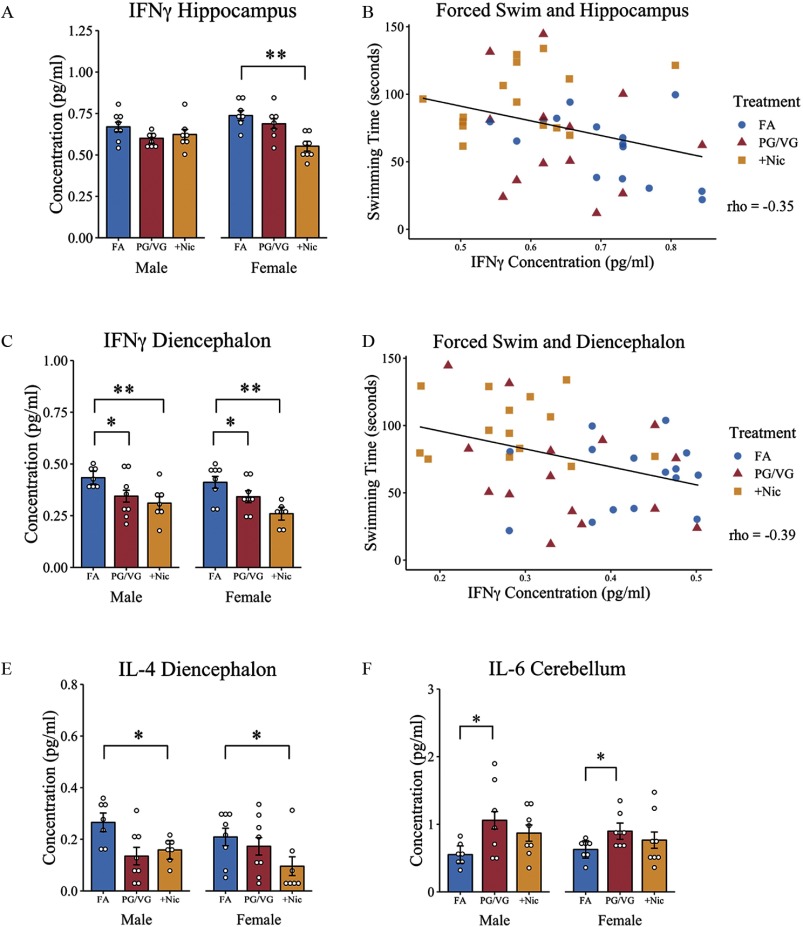
To determine whether maternal e-cigarette aerosol exposure during pregnancy, with or without nicotine, altered neuroimmune signaling in offspring, brains were collected from adult male and female offspring and microdissected to assess region-specific differences in several cytokine levels using multiplex arrays. There was a significant treatment by sex interaction for in the hippocampus, , . Simple main effects analysis confirmed an effect of treatment in female offspring, , , with lower levels of observed in the group compared with age-matched FA-exposed female mice, , , but not in the PG/VG-exposed group, , . These same differences in were not seen in the hippocampus of the age-matched male offspring (Figure 5A). The concentration of in the hippocampus was negatively correlated with time swimming in the forced swim test, , , with higher levels of associated with less time spent swimming (Figure 5B). There were no sex or treatment differences in IL-4, IL-6, or IL-2 levels in the hippocampus (Table 2).
Figure 5.
Brain cytokine measurement in offspring of dams exposed to filtered air (FA), e-cigarette aerosol (PG/VG), or e-cigarette aerosol with nicotine (). Following behavioral assessments, offspring from e-cigarette-treated and control dams were sacrificed and brains processed for cytokine quantification using bead-based multiplex assays. Reported are (A) levels in the hippocampus and (B) correlation with swimming behavior in the forced swim test, (C) in the diencephalon and (D) correlation with swimming behavior, (E) IL-4 concentrations in the diencephalon, and (F) IL-6 concentrations in the cerebellum. *, ** as determined by two-way factorial ANOVA followed by simple main effects analysis and Tukey post hoc. Correlations were tested using Spearman’s rho. Plots represent individual mice; bars represent marginal means ; scatterplots include both male and female offspring. FA (8 litters) [males (, females )]; PG/VG (8 litters) [males (), females ()]; (8 litters) [males (, females )]. Note: ANOVA, analysis of variance; IFN, interferon; IL, interleukin; PG/VG, propylene glycol/vegetable glycerin; SE, standard error; , propylene glycol/vegetable glycerin plus nicotine.
Table 2.
Cytokine concentrations [pg/mL ( error)] in brain homogenates of adult offspring.
| Region | Sex | Exposure | IL-4 | IL-6 | IL-2 | |
|---|---|---|---|---|---|---|
| Neocortex | Male | FA | ||||
| PG/VG | ||||||
| Female | FA | |||||
| PG/VG | ||||||
| Diencephalon | Male | FA | ||||
| PG/VG | * | |||||
| ** | ** | |||||
| Female | FA | |||||
| PG/VG | * | |||||
| ** | ** | |||||
| Hippocampus | Male | FA | ||||
| PG/VG | ||||||
| Female | FA | |||||
| PG/VG | ||||||
| ** | ||||||
| Cerebellum | Male | FA | ||||
| PG/VG | * | |||||
| Female | FA | |||||
| PG/VG | * | |||||
Note: FA, filtered air; IFN, interferon; IL, interleukin; PG/VG, propylene glycol/vegetable glycerin; , propylene glycol/vegetable glycerin plus nicotine.
compared with FA-exposed offspring.
compared with FA-exposed offspring.
In the diencephalon, there was an effect of treatment on , , , with male and female offspring of dams exposed to PG/VG without nicotine showing lower levels of compared with offspring of age and sex-matched FA control mice, , . Similar differences in were present in male and female offspring exposed to PG/VG with nicotine compared with FA controls, , (Figure 5C). Levels of in the diencephalon were negatively correlated with swimming time in the forced swim test, , (Figure 5D). In addition, IL-4 levels in the diencephalon differed across treatment groups, , , with male and female offspring of dams exhibiting lower levels of IL-4 compared with age- and sex-matched FA-exposed offspring, , , (Figure 5E). However, levels of IL-4 were not correlated with any behavioral measures. No differences were observed in IL-6 or IL-2 levels in the diencephalon across sex or treatment groups (Table 2).
Although there were no differences in , IL-4, or IL-2 in the cerebellum, there was a significant effect of treatment on IL-6 levels in the cerebellum, , . Post hoc analysis confirmed a significantly lower level of IL-6 in PG/VG-exposed offspring compared with FA controls, , . (Figure 5F). Finally, no differences were observed in any of the cytokine levels measured across treatment conditions or between sexes in offspring neocortex (Table 2).
Discussion
Given the prevalence of e-cigarette vaping during pregnancy and the documented effects of nicotine on offspring behavior and health, it is essential to determine the long-term behavioral and biological consequences of e-cigarette exposure in utero. We hypothesized that maternal exposure to e-cigarette aerosols during gestation, with and without nicotine, alters offspring behavioral and neuroimmune development and that these effects persist into adulthood. In this study, prenatal exposure to e-cigarette aerosol containing nicotine increased locomotor activity in male and female adult offspring as seen in the elevated plus maze and by increased swimming in the forced swim test. In the novel object recognition task, offspring of PG/VG-exposed dams spent similar lengths of time exploring the familiar and novel objects, and mice in both e-cigarette–exposed groups (with and without nicotine) spent a lower percentage of time with the novel object compared with controls. Brain cytokine analysis via bead-based multiplex immunoassay revealed lower and IL-4 levels in the diencephalon of offspring, and females in this group also showed lower concentrations of hippocampal . Furthermore, levels were inversely correlated with swimming time in the forced swim test in both the hippocampus and diencephalon. Interestingly, offspring of dams exposed to PG/VG alone expressed elevated IL-6 in the cerebellum. This work highlights the persistent behavioral and neuroinflammatory consequences of gestational e-cigarette aerosol exposure and furthers our understanding of the impact of e-cigarette aerosol on fetal neurodevelopment.
Although toxicological research on the developmental effects of e-cigarette aerosols and their component by-products are newly emerging, the adverse effects of nicotine exposure during pregnancy have been widely documented in the literature. These studies include clinical and epidemiological reports consistently demonstrating a link between maternal nicotine exposure, whether through tobacco smoking or nicotine replacement therapies, and negative health effects in their children (for review, see Holbrook 2016). These consequences include reproductive, endocrine, metabolic, immune, and neurobiological changes, as well as an increased risk of the offspring developing obesity (Ino 2010) or having an attention deficit hyperactivity disorder diagnosis (Tiesler and Heinrich 2014) later in life. In rats, maternal exposure to tobacco cigarette smoke 5 d/week between GD11.5 and GD21.5 transiently decreased offspring body weight at PND21, a difference no longer evident at 60 d of age (Zinkhan et al. 2014). Subcutaneous nicotine given to pregnant rat dams beginning 14 d prior to breeding and continuing until offspring weaning (PND21) resulted in increased body weight in male offspring (females not examined) beginning at 10 weeks of age (Gao et al. 2005). In our study, maternal e-cigarette exposure did not affect offspring weight at weaning (PND21), which is in contrast with the findings of Zinkhan et al. (2014); however, by 12 weeks of age female offspring of dams weighed significantly more than females of FA control dams, similar to the male offspring as reported by Gao et al. (2005) following perinatal maternal nicotine exposure. These discrepancies highlight the importance of also examining non-nicotine constituents when investigating the neurodevelopmental effects of gestational nicotine delivery systems and the importance of including female offspring in all analyses.
Maternal E-Cigarette Exposure during Pregnancy and Adult Offspring Behavior
In a study on nicotine exposure during pregnancy in mice, maternal consumption of nicotine in drinking water led to a behavioral phenotype in offspring characterized by increased motor activity and risk-taking behaviors that continued across subsequent generations (Buck et al. 2019). Similarly, nicotine exposure via e-cigarette exposure in our mice increased adult male and female offspring locomotor activity compared with controls as determined by total distance traveled in the elevated plus maze and total swim time in the forced swim test. Interestingly, spontaneous locomotor activity measured as total distance traveled in the open field test revealed no differences in motor activity among treatment groups. This discrepancy may be a response to differences in motor activity during high stress (i.e., elevated plus maze and forced swim test) compared with the lower stress environment of the open field task under dim lighting (Bouwknecht et al. 2007). Although behaviors in the elevated plus maze and forced swim test were more pronounced in males, evidence of hyperactivity was also observed in adult female offspring, highlighting potential sex-dependent effects of e-cigarette exposure. Locomotor hyperactivity in females is important to emphasize given that previous studies assessing the effects of e-cigarette exposure focused only on male BALB/c (Nguyen et al. 2018) or C57BL/6J (Smith et al. 2015) offspring behavior. Our behavior data both support previous prenatal e-cigarette studies noting hyperactivity in males and extend the findings to female offspring in the CD-1 strain. The findings here also substantiate a transcriptomic analysis study with young adult C57BL/6 mouse frontal cortex revealing up-regulation of gene expression associated with hyperactivity in response to perinatal e-cigarette exposure with and without nicotine (Lauterstein et al. 2016). These data underscore lasting developmental differences in locomotor activity in response to early life e-cigarette aerosol across mouse strains.
Although our data on prenatal nicotine-containing e-cigarette exposure are aligned with externalizing behaviors such as hyperactivity in both humans (Ashford et al. 2008; Brion et al. 2010; Indredavik et al. 2007; Menezes et al. 2013; Tiesler and Heinrich 2014) and animal model research (Buck et al. 2019; Zhu et al. 2012), the impact of both maternal nicotine exposure alone and e-cigarette aerosols containing nicotine on affective states are less clear. In our study, no differences were observed in open arm exploration across treatment groups or sex despite increases in overall maze exploration from male and female offspring from the group. Similarly, no differences in anxiety-like measures from the elevated zero maze were reported in a previous study of male C57BL/6 offspring exposed to e-cigarette aerosol containing 2.4% nicotine (Smith et al. 2015). Conversely, Nguyen et al. (2018) noted increases in open arm exploration in the elevated plus maze in adult male offspring of BALB/c dams exposed to e-cigarette aerosols (both with and without nicotine) from prior to pregnancy through lactation compared with control offspring of dams exposed to ambient air (Nguyen et al. 2018). However, the elevated plus maze measures from Nguyen et al. (2018) were carried out only in adult male offspring and were limited to a 2-min exploration time, in contrast to the typical 5-min exploration time that is validated and common practice in the field (Walf and Frye 2007). As a result, support for a sex-specific developmental impact of maternal e-cigarette use during pregnancy, both with and without nicotine, on offspring anxiety-like states remains weak and suggests that other brain systems, particularly those associated with stress coping, attention, and hyperactivity, may be more susceptible to the effects of developmental e-cigarette exposure.
In our study, both male and female offspring of -exposed dams showed increased swimming behavior and male offspring showed less immobility in the forced swim test. The sex-specific differences in immobility and concomitant increases in swimming, juxtaposed with locomotor differences in the elevated plus maze task, suggest differences in stress-coping behaviors among offspring. Specifically, passive immobility in the forced swim task is thought to represent an adaptive pro-survival (de Kloet and Molendijk 2016; Molendijk and de Kloet 2015) behavior governed by cognitive evaluation of inescapable stress. Therefore, reductions in immobility observed in our male offspring would suggest impaired stress coping in response to this acute stressor. In humans, a study of over 180,000 late adolescent/young adult males found no association between maternal tobacco use during pregnancy and later stress-coping skills (Kuja-Halkola et al. 2010). However, whether this finding translates to maternal e-cigarette use during gestation or female offspring is unclear. Although speculative, our behavioral findings suggest that gestational e-cigarette aerosol exposure containing nicotine has the potential to hinder stress-coping strategies in offspring of both sexes, with a greater vulnerability in males.
E-cigarette aerosols in general use PG/VG as the vehicle for the nicotine and flavorings (Wagner et al. 2018). Although some of these substances are deemed safe at low exposure levels, reports have noted adverse effects at higher exposures following commercial use in the entertainment and aviation industry (Varughese et al. 2005; Wieslander et al. 2001) and in preclinical toxicological studies (Suber et al. 1989; Werley et al. 2011). Assessments of PG/VG inhalation in e-cigarette use, particularly in low or no-nicotine liquids, are estimated to result in higher levels of PG near the acceptable threshold limit (Burstyn 2014). In fact, high exposures to PG and/or VG in animal studies increased apoptotic neurodegeneration and microglial activation in the hippocampus (Lau et al. 2012; Zelikoff et al. 2018), and increased levels of reactive oxygen species (Lerner et al. 2015), implicating elevated doses of PG/VG as a central nervous system toxicant. In the present study, adult male and female offspring of dams exposed to e-cigarette aerosol with and without nicotine showed declines in hippocampal-dependent novel object recognition scores. That is, daily exposure to PG/VG both with and without nicotine during pregnancy resulted in suppression of episodic memory performance in the adult offspring months after their initial exposure in utero. These behavioral differences, regardless of the presence of nicotine, suggest that exposure to PG/VG alone during critical periods of fetal development could have lasting consequences on the brain, a hypothesis supported by a recent transcriptomic analysis that revealed more severe gene expression changes in C57BL/6 offspring brain following PG/VG exposure without nicotine (Lauterstein et al. 2016). In fact, Golli et al. (2016) reported similar reductions in novel object memory performance in adult rats following a 4-week exposure to PG/VG and the deficits were mitigated by the presence of nicotine (Golli et al. 2016). Despite previous animal studies showing that nicotine alone positively impacted memory and had neuroprotective effects in the central nervous system (for review, see Toledano et al. 2010), our data do not support a beneficial role of nicotine in the presence of PG/VG. Our findings and that of others underscore a unique role for PG/VG aerosol and raise concerns about the safety and use of nicotine-free e-cigarettes among individuals of childbearing age (Golli et al. 2016; Lauterstein et al. 2016; Zelikoff et al. 2018).
Brain Cytokines and Neuroinflammation in Offspring Exposed Prenatally to E-Cigarette Aerosols
Much of the toxicological research on e-cigarettes has focused on the user (Kaisar et al. 2016), with far fewer studies specifically addressing the unique risks that e-cigarette use during pregnancy may have on the offspring. That is, the developmental and long-term neurotoxicological impact following gestational e-cigarette exposure has not been well defined. Initial animal studies on the impact of e-cigarette aerosol on fetal development have noted reductions in lung and brain health as well as altered gene expression. For example, maternal e-cigarette aerosol exposure of mice without nicotine beginning 6 weeks prior to mating and continuing through offspring weaning resulted in altered inflammatory profiles in adult offspring lungs as well as global epigenetic modification in immune signaling genes (Chen et al. 2018a). Similarly, perinatal exposure (pregnancy through lactation) to e-cigarettes with and without nicotine increased microglial activation and altered gene expression patterns in the hippocampus with concomitant decreases in peripheral serum cytokine levels (Zelikoff et al. 2018). These differences in immune signaling in adult offspring, both with and without nicotine, suggest that early life e-cigarette exposure may have lasting impacts across numerous fetal organ systems, including the brain. In the present study, both male and female offspring prenatally exposed to e-cigarette aerosols containing nicotine showed lower and IL-4 levels in the diencephalon as well as lower levels in the female hippocampus. These reductions in immune signals are hypothesized to be a result of nicotine’s well-documented immunosuppressive actions on the central nervous system (Piao et al. 2009) and could reflect nicotine’s ability to inhibit T-lymphocyte maturation and function through acetylcholine receptor activation (Middlebrook et al. 2002). In fact, maternal cigarette smoking was associated with significantly decreased levels of IL-4 and in cord blood of newborn infants (Macaubas et al. 2003; Noakes et al. 2006). This finding suggests that the immune modulating effects of prenatal nicotine exposure may begin during gestation and persist through adulthood. Our data also revealed that levels in the hippocampus and diencephalon negatively correlated with swimming time in the forced swim test. The e-cigarette with nicotine group had the longest swimming time, interpreted as impaired stress-coping behavior, and the highest average levels. In humans, adult males with positive stress-coping styles were reported to have altered T-cell–released in relation to perceived stress (positive association), whereas those with negative stress-coping styles had reduced numbers of cells (Sakami et al. 2004). Together these findings propose an interaction between (neuro)immune function and stress-coping strategies, with brain playing an important role in behavioral responses to stress.
In the present study, e-cigarette aerosol exposure in the absence of nicotine (i.e., PG/VG alone) resulted in elevations in IL-6 in the cerebellum, and these cytokine increases were not observed in offspring prenatally exposed to aerosol with nicotine. This finding further underscores the immunosuppressive potential of nicotine. Elevations in IL-6 have previously been reported in lung fluid of humans exposed to PG/VG in the absence of nicotine (Scott et al. 2018), and IL-6 mRNA was reportedly elevated in whole brain from offspring of dams exposed to cigarette smoke during pregnancy (Chan et al. 2017). In contrast, Zelikoff et al. (2018) noted decreases in serum cytokines of offspring exposed from gestation through lactation to e-cigarette aerosols with and without nicotine. These disparate findings, particularly in studies examining maternal–fetal exposures, are likely a reflection of variations in exposure timing, nicotine dose, vehicle delivery (i.e., PG/VG aerosol or cigarette smoke) and experimental design, including specific mouse strain. These variations notwithstanding, the impact of e-cigarette aerosol, with and without nicotine, on fetal development is evident and raises concerns for their use during pregnancy.
All cytokines measured in the current study were present in low concentrations and at levels typically found in the brain. Although more extreme variations in brain cytokine levels were found in neuroimmunological conditions ranging from multiple sclerosis to traumatic brain injury (DiSabato et al. 2016), less is known about the impact of smaller deviations from baseline. Studies examining differences in brain cytokine levels in mice following maternal environmental insults reported outcomes as relative fold-changes (Garay et al. 2013), making it difficult to compare differences in cytokine levels in this study to other animal models. That is, the approximately 2-fold differences we observed in IL-4, IL-6, and across brain regions may be statistically significant, but it is not clear whether these differences are biologically relevant. A further limitation with our findings is the potential contamination of our designated brain-specific regions with other portions of the brain. For example, differences in cytokine levels in the cortex were not observed between groups in this study, despite previous studies noting numerous differences in gene expression levels in mouse offspring frontal cortex following e-cigarette exposure during pregnancy and lactation (Lauterstein et al. 2016). Our analysis of the cortex included the entire neocortex and did not differentiate sections of frontal compared with occipital or temporo-parietal regions. As a result, some of the null finings we report may be a false negative outcome given that differences in cytokine expression in a subregion of the cortex or diencephalon may be masked or diluted by the inclusion of multiple subareas.
Comparison with Human E-Cigarette Use and Study Limitations
The chemical composition and exposure levels of e-cigarettes can vary greatly among users given that individuals are able to prepare their own formulations of PG/VG and desired concentration of nicotine. Estimates of human consumption for toxicology assessments of inhaled PG assume a range of 50–95% PG (Burstyn 2014), and many preclinical toxicology studies, including the one here, use a 50:50 formulation of PG/VG to model typical ratios found among human users (compare Garcia-Arcos et al. 2016; HW Lee et al. 2018; Olfert et al. 2018; Tang et al. 2019). JUUL®, a major manufacturer of e-cigarettes, sells nicotine-containing e-cigarette formulations that range from (JUUL 2019). In comparison, we used a nicotine concentration of to represent a low-to-moderate dose comparable to that of human consumption. Other e-cigarette toxicology studies in mice have used similar low-to-moderate doses ranging from (HW Lee et al. 2018) to (Tang et al. 2019). These ranges also approximate concentrations used by manufacturers and other research groups (Eaton et al. 2018). With respect to the inhalation exposure paradigm, user puff topography—including puff volume, duration, and daily puff rate—voltage and heating temperature vary widely across users, complicating our ability to accurately translate our paradigm to human consumption levels. Studies on user topography estimate an average experienced e-cigarette user to have a 4-s puff duration (Farsalinos et al. 2013; Hua et al. 2013), a duration comparable to that used in our study. Further, although other estimates report lower puff durations ranging from 2.25 (ECigStats 2019) to 3.3 s (YO Lee et al. 2018), differences in daily puff rates and puff volume place our aerosol regimen, puff at 30-s intervals for 3 h/d, at a moderate exposure level. Olfert et al. (2018) argue that mouse exposure paradigms may in fact underestimate human exposures given that mouse studies such as ours administer e-cigarette aerosols through nasal inhalation, compared with oral inhalation in humans, thereby filtering more airborne particles through the nasal passage compared with direct oral inhalation.
Our assessment of the e-cigarette aerosol both with and without nicotine had similarly elevated levels of PM compared with FA. Several studies have reported the presence of fine and ultra-fine PM in e-cigarette aerosols (Ingebrethsen et al. 2012; Melstrom et al. 2017; Zhao et al. 2016), as well as carbonyls, volatile organic compounds, and metals (Cheng 2014; Wagner et al. 2018). Although we were not able to specifically define the PM composition generated from the e-cigarette aerosols in this study, a previous report from our laboratories demonstrated behavioral alterations in response to maternal exposure (Church et al. 2018). Therefore, we cannot rule out a potential contribution of these particles in shaping the behavioral and neuroimmunological differences observed in the offspring of e-cigarette–exposed dams.
Several limitations of this study should be considered, including the unexamined potential effect of daily e-cigarette exposure on maternal behavior. The effect of e-cigarette exposure during pregnancy on maternal behavior has yet to be investigated. However, early postpartum nicotine consumption ( in drinking water) by mouse dams increased time spent passively nursing (Heath et al. 2010), a posture thought to be less advantageous to pups; no other adverse effects on maternal behavior have been noted. Thus, although there is limited evidence to suggest that nicotine-containing products alter maternal behavior, this factor cannot be ruled out as a potential contributor to the behavioral phenotype observed in our study. Importantly, any potential litter effects have been controlled for by incorporating mixed-effects modeling, assuring that the behavioral and neuroimmunological differences observed in this study are related to treatment rather than to litter-to-litter variations.
Dams were exposed to e-cigarette aerosols throughout gestation to specifically address the question of whether vaping during pregnancy affects neurodevelopment long term. However, brain development in rodents continues postpartum with the human equivalent of the third trimester occurring several days into the postpartum period (Clancy et al. 2001; Ohmura and Kuniyoshi 2017; Workman et al. 2013). Thus, although our findings support the argument that maternal e-cigarette aerosol impacts fetal brain development, there may be additional and unique consequences that emerge if exposure were to continue into the postpartum period. Similarly, dams were exposed only to e-cigarette aerosol after the presence of a seminal plug, with no exposures occurring prior to the start of pregnancy. A previous study in rats noted a developmental impact on offspring following daily paternal nicotine injections () to the males prior to the start of breeding (Hawkey et al. 2019). Therefore, e-cigarette aerosols with and without nicotine may have additive or synergistic effects when exposure occurs prior to pregnancy and during the gestational period and when both biological parents are exposed.
Despite the aforementioned limitations, our study provides compelling evidence in support of the notion that maternal exposure to e-cigarette aerosols during pregnancy, both with and without nicotine, negatively impacts brain and behavior development of the offspring in a sex-dependent manner and well into their adult life. Findings from this translational study should be considered by pregnant women who may be considering e-cigarette use during this vulnerable time period.
Conclusion
The adverse behavioral and neuroimmunological outcomes observed in our adult male and female offspring demonstrate the persistent health consequences of e-cigarette use during pregnancy. These findings should raise caution to those who perceive its use as less harmful than traditional tobacco cigarettes for pregnant smokers (Baeza-Loya et al. 2014; Mark et al. 2015). Even without nicotine, daily exposure to PG/VG throughout gestation has the potential to disrupt learning and memory performance in offspring and increase neuroinflammation. This is particularly troublesome given the targeted marketing of nicotine-free vaping products to younger populations of childbearing age (Padon et al. 2017). The relatively unknown neurodevelopmental and toxicological effects of these products coupled with unsubstantiated claims of their safety warrant careful scrutiny and further investigation to properly inform regulatory agencies and medical communities on the health risks associated with the use of e-cigarettes during pregnancy.
Supplementary Material
Acknowledgments
The authors acknowledge K. Byrne, R. Goodier, A. Kazakova, K. Ouimette, and E. Halzack for their technical expertise. Funding was provided by the National Institutes of Health (R15HD082638 and R15MH119500) and by the New York University–National Institute of Environmental Health Sciences Core Center Pilot (ES000260).
References
- Alkam T, Kim HC, Mamiya T, Yamada K, Hiramatsu M, Nabeshima T. 2013. Evaluation of cognitive behaviors in young offspring of C57BL/6J mice after gestational nicotine exposure during different time-windows. Psychopharmacology (Berl) 230(3):451–463, PMID: 23793357, 10.1007/s00213-013-3175-9. [DOI] [PubMed] [Google Scholar]
- Ashford J, van Lier PA, Timmermans M, Cuijpers P, Koot HM. 2008. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J Am Acad Child Adolesc Psychiatry 47(7):779–787, PMID: 18520960, 10.1097/CHI.0b013e318172eefb. [DOI] [PubMed] [Google Scholar]
- Baeza-Loya S, Viswanath H, Carter A, Molfese DL, Velasquez KM, Baldwin PR, et al. 2014. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bull Menninger Clin 78(3):243–252, PMID: 25247743, 10.1521/bumc.2014.78.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhareth R, Mezouar S, Ben Amara A, Chartier C, Azzouz EB, Chabrière E, et al. 2018. Cigarette smoke extract interferes with placenta macrophage functions: a new mechanism to compromise placenta functions? Reprod Toxicol 78:120–129, PMID: 29673796, 10.1016/j.reprotox.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Blum JL, Xiong JQ, Hoffman C, Zelikoff JT. 2012. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol Sci 126(2):478–486, PMID: 22240978, 10.1093/toxsci/kfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. 2007. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull 72(1):32–43, PMID: 17303505, 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion MJ, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C, et al. 2010. Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics 126(1):e57–e65, PMID: 20587678, 10.1542/peds.2009-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O’Neill HC. 2019. Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioral, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice. Neuropharmacology 149:66–82, PMID: 30742847, 10.1016/j.neuropharm.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn I. 2014. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health 14(1):18, PMID: 24406205, 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. 2012. The mouse forced swim test. J Vis Exp 59:e3638, PMID: 22314943, 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YL, Saad S, Machaalani R, Oliver BG, Vissel B, Pollock C, et al. 2017. Maternal cigarette smoke exposure worsens neurological outcomes in adolescent offspring with hypoxic-ischemic injury. Front Mol Neurosci 10:306, PMID: 29018327, 10.3389/fnmol.2017.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, et al. 2018a. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol 58(3):366–377, PMID: 28960086, 10.1165/rcmb.2017-0206RC. [DOI] [PubMed] [Google Scholar]
- Chen H, Li G, Chan YL, Nguyen T, van Reyk D, Saad S, et al. 2018b. Modulation of neural regulators of energy homeostasis, and of inflammation, in the pups of mice exposed to e-cigarettes. Neurosci Lett 684:61–66, PMID: 29981356, 10.1016/j.neulet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Cheng T. 2014. Chemical evaluation of electronic cigarettes. Tob Control 23(suppl 2):ii11–ii17, PMID: 24732157, 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Tijerina PB, Emerson FJ, Coburn MA, Blum JL, Zelikoff JT, et al. 2018. Perinatal exposure to concentrated ambient particulates results in autism-like behavioral deficits in adult mice. Neurotoxicology 65:231–240, PMID: 29104007, 10.1016/j.neuro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. 2001. Translating developmental time across mammalian species. Neuroscience 105(1):7–17, PMID: 11483296, 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML. 2016. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast 2016:6503162, PMID: 27034848, 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSabato DJ, Quan N, Godbout JP. 2016. Neuroinflammation: the devil is in the details. J Neurochem 139(suppl 2):136–153, PMID: 26990767, 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. 2009. The dynamic effects of nicotine on the developing brain. Pharmacol Ther 122(2):125–139, PMID: 19268688, 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Kwan LY, Stratton K. 2018. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- ECigStats. 2019. https://www.ecigstats.org/global-stats/ [accessed 22 December 2019].
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. 2013. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health 10(6):2500–2514, PMID: 23778060, 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG, et al. 2005. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res 13(4):687–692, PMID: 15897477, 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. 2013. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68, PMID: 22841693, 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, et al. 2016. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71(12):1119–1129, PMID: 27558745, 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golli NE, Dallagi Y, Rahali D, Rejeb I, Fazaa SE. 2016. Neurobehavioral assessment following e-cigarette refill liquid exposure in adult rats. Toxicol Mech Methods 26(6):435–442, PMID: 27401341, 10.1080/15376516.2016.1193585. [DOI] [PubMed] [Google Scholar]
- Hawkey AB, White H, Pippen E, Greengrove E, Rezvani AH, Murphy SK, et al. 2019. Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol Teratol 74:106808, PMID: 31103693, 10.1016/j.ntt.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Horst NK, Picciotto MR. 2010. Oral nicotine consumption does not affect maternal care or early development in mice but results in modest hyperactivity in adolescence. Physiol Behav 101(5):764–769, PMID: 20826170, 10.1016/j.physbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook BD. 2016. The effects of nicotine on human fetal development. Birth Defects Res C Embryo Today 108(2):181–192, PMID: 27297020, 10.1002/bdrc.21128. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. 2016. Nicotine induces neutrophil extracellular traps. J Leukoc Biol 100(5):1105–1112, PMID: 27312847, 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Yip H, Talbot P. 2013. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control 22(2):103–106, PMID: 22116832, 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- Indredavik MS, Brubakk AM, Romundstad P, Vik T. 2007. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr 96(3):377–382, PMID: 17407460, 10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. 2012. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol 24(14):976–984, PMID: 23216158, 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Ino T. 2010. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int 52(1):94–99, PMID: 19400912, 10.1111/j.1442-200X.2009.02883.x. [DOI] [PubMed] [Google Scholar]
- JUUL. 2019. https://www.juul.com/resources/What-is-JUUL-Vape-Juice-All-JUUL-Pod-Flavors [accessed 22 December 2019].
- Kaisar MA, Prasad S, Liles T, Cucullo L. 2016. A decade of e-cigarettes: limited research & unresolved safety concerns. Toxicology 365:67–75, PMID: 27477296, 10.1016/j.tox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Bansal-Travers M, O’Connor RJ, Compton WM, Kettermann A, Borek N, et al. 2014. Cigarette smokers’ use of unconventional tobacco products and associations with quitting activity: findings from the ITC-4 U.S. cohort. Nicotine Tob Res 16(6):672–681, PMID: 24376276, 10.1093/ntr/ntt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Halkola R, D’Onofrio BM, Iliadou AN, Långström N, Lichtenstein P. 2010. Prenatal smoking exposure and offspring stress coping in late adolescence: no causal link. Int J Epidemiol 39(6):1531–1540, PMID: 20719746, 10.1093/ije/dyq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Brown RW, Morgan AJ, Mactutus CF, Harrod SB. 2016. Intravenous prenatal nicotine exposure alters METH-induced hyperactivity, conditioned hyperactivity, and BDNF in adult rat offspring. Dev Neurosci 38(3):171–185, PMID: 27287203, 10.1159/000446563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K, Swiney BS, Reeves N, Noguchi KK, Farber NB. 2012. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatr Res 71(1):54–62, PMID: 22289851, 10.1038/pr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterstein DE, Tijerina PB, Corbett K, Akgol Oksuz B, Shen SS, Gordon T, et al. 2016. Frontal cortex transcriptome analysis of mice exposed to electronic cigarettes during early life stages. Int J Environ Res Public Health 13(4):417, PMID: 27077873, 10.3390/ijerph13040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chung S, Noh J. 2016. Maternal nicotine exposure during late gestation and lactation increases anxiety-like and impulsive decision-making behavior in adolescent offspring of rat. Toxicol Res 32(4):275–280, PMID: 27818729, 10.5487/TR.2016.32.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Park SH, Weng MW, Wang HT, Huang WC, Lepor H, et al. 2018. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA 115(7):E1560–E1569, PMID: 29378943, 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YO, Nonnemaker JM, Bradfield B, Hensel EC, Robinson RJ. 2018. Examining daily electronic cigarette puff topography among established and nonestablished cigarette smokers in their natural environment. Nicotine Tob Res 20(10):1283–1288, PMID: 29059416, 10.1093/ntr/ntx222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. 2013. Object recognition test in mice. Nat Protoc 8(12):2531–2537, PMID: 24263092, 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. 2015. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10(2):e0116732, PMID: 25658421, 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow LM. 2017. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 126:e55718, PMID: 28892027, 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, et al. 2003. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 362(9391):1192–1197, PMID: 14568741, 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- Mark KS, Farquhar B, Chisolm MS, Coleman-Cowger VH, Terplan M. 2015. Knowledge, attitudes, and practice of electronic cigarette use among pregnant women. J Addict Med 9(4):266–272, PMID: 25974378, 10.1097/ADM.0000000000000128. [DOI] [PubMed] [Google Scholar]
- McCubbin A, Fallin-Bennett A, Barnett J, Ashford K. 2017. Perceptions and use of electronic cigarettes in pregnancy. Health Educ Res 32(1):22–32, PMID: 28158490, 10.1093/her/cyw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melstrom P, Koszowski B, Thanner MH, Hoh E, King B, Bunnell R, et al. 2017. Measuring PM2.5, ultrafine particles, nicotine air and wipe samples following the use of electronic cigarettes. Nicotine Tob Res 19(9):1055–1061, PMID: 28340080, 10.1093/ntr/ntx058. [DOI] [PubMed] [Google Scholar]
- Menezes AM, Murray J, László M, Wehrmeister FC, Hallal PC, Gonçalves H, et al. 2013. Happiness and depression in adolescence after maternal smoking during pregnancy: birth cohort study. PLoS One 8(11):e80370, PMID: 24265817, 10.1371/journal.pone.0080370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook AJ, Martina C, Chang Y, Lukas RJ, DeLuca D. 2002. Effects of nicotine exposure on T cell development in fetal thymus organ culture: arrest of T cell maturation. J Immunol 169(6):2915–2924, PMID: 12218105, 10.4049/jimmunol.169.6.2915. [DOI] [PubMed] [Google Scholar]
- Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. 1997. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol 159(6):2994–2999, PMID: 9300724. [PubMed] [Google Scholar]
- Molendijk ML, de Kloet ER. 2015. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62:389–391, PMID: 26386543, 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Mychasiuk R, Nakahashi A, Hossain SR, Gibb R, Kolb B. 2012. Prenatal nicotine exposure alters neuroanatomical organization of the developing brain. Synapse 66(11):950–954, PMID: 22837140, 10.1002/syn.21589. [DOI] [PubMed] [Google Scholar]
- Newman MB, Shytle RD, Sanberg PR. 1999. Locomotor behavioral effects of prenatal and postnatal nicotine exposure in rat offspring. Behav Pharmacol 10(6–7):699–706, PMID: 10780512, 10.1097/00008877-199911000-00017. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA. 2018. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem Res Toxicol 31(7):601–611, PMID: 29863869, 10.1021/acs.chemrestox.8b00084. [DOI] [PubMed] [Google Scholar]
- Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. 2006. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J 28(4):721–729, PMID: 16870663, 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2011. Guide for the Care and Use of Laboratory Animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- Ohmura Y, Kuniyoshi Y. 2017. A translational model to determine rodent’s age from human foetal age. Sci Rep 7(1):17248, PMID: 29222462, 10.1038/s41598-017-17571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, et al. 2018. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985) 124(3):573–582, PMID: 29097631, 10.1152/japplphysiol.00713.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padon AA, Maloney EK, Cappella JN. 2017. Youth-targeted e-cigarette marketing in the US. Tob Regul Sci 3(1):95–101, PMID: 28083545, 10.18001/TRS.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Villanti AC. 2019. Patterns and frequency of current e-cigarette use in United States adults. Subst Use Misuse 54(12):2075–2081, PMID: 31226905, 10.1080/10826084.2019.1626433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD. 2009. Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin 30(6):715–722, PMID: 19448649, 10.1038/aps.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro CR, Moura EG, Manhães AC, Fraga MC, Claudio-Neto S, Younes-Rapozo V, et al. 2015. Maternal nicotine exposure during lactation alters food preference, anxiety-like behavior and the brain dopaminergic reward system in the adult rat offspring. Physiol Behav 149:131–141, PMID: 26048299, 10.1016/j.physbeh.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. 1977. Depression: a new animal model sensitive to antidepressant treatments. Nature 266(5604):730–732, PMID: 559941, 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Sakami S, Maeda M, Maruoka T, Nakata A, Komaki G, Kawamura N. 2004. Positive coping up- and down-regulates in vitro cytokine productions from T cells dependent on stress levels. Psychother Psychosom 73(4):243–251, PMID: 15184719, 10.1159/000077743. [DOI] [PubMed] [Google Scholar]
- Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, et al. 2018. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73(12):1161–1169, PMID: 30104262, 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalco D, Schultz S, Brigida AL, Antonucci N. 2018. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals (Basel) 11(2):56, PMID: 29867038, 10.3390/ph11020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dwoskin LP, Pauly JR. 2010. Early exposure to nicotine during critical periods of brain development: mechanisms and consequences. J Pediatr Biochem 1(2):125–141, PMID: 24904708, 10.3233/JPB-2010-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, et al. 2015. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One 10(9):e0137953, PMID: 26372012, 10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings-Smith S, Ballantyne T. 2019. Ever use of e-cigarettes among adults in the United States: a cross-sectional study of sociodemographic factors. Inquiry 56:46958019864479, PMID: 31328601, 10.1177/0046958019864479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suber RL, Deskin R, Nikiforov I, Fouillet X, Coggins CR. 1989. Subchronic nose-only inhalation study of propylene glycol in Sprague-Dawley rats. Food Chem Toxicol 27(9):573–583, PMID: 2807102, 10.1016/0278-6915(89)90016-1. [DOI] [PubMed] [Google Scholar]
- Tang MS, Wu XR, Lee HW, Xia Y, Deng FM, Moreira AL, et al. 2019. Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci USA 116(43):21727–21731, PMID: 31591243, 10.1073/pnas.1911321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesler CM, Heinrich J. 2014. Prenatal nicotine exposure and child behavioural problems. Eur Child Adolesc Psychiatry 23(10):913–929, PMID: 25241028, 10.1007/s00787-014-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano A, Alvarez MI, Toledano-Díaz A. 2010. Diversity and variability of the effects of nicotine on different cortical regions of the brain. Therapeutic and toxicological implications. Cent Nerv Syst Agents Med Chem 10(3):180–206, PMID: 20528766, 10.2174/1871524911006030180. [DOI] [PubMed] [Google Scholar]
- Varughese S, Teschke K, Brauer M, Chow Y, van Netten C, Kennedy SM. 2005. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med 47(5):411–418, PMID: 15828073, 10.1002/ajim.20151. [DOI] [PubMed] [Google Scholar]
- Wagner KA, Flora JW, Melvin MS, Avery KC, Ballentine RM, Brown AP, et al. 2018. An evaluation of electronic cigarette formulations and aerosols for harmful and potentially harmful constituents (HPHCs) typically derived from combustion. Regul Toxicol Pharmacol 95:153–160, PMID: 29567331, 10.1016/j.yrtph.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Camerota M, Propper C. 2017. Prevalence and perceptions of electronic cigarette use during pregnancy. Matern Child Health J 21(8):1655–1661, PMID: 28084577, 10.1007/s10995-016-2257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328, PMID: 17406592, 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werley MS, McDonald P, Lilly P, Kirkpatrick D, Wallery J, Byron P, et al. 2011. Non-clinical safety and pharmacokinetic evaluations of propylene glycol aerosol in Sprague-Dawley rats and beagle dogs. Toxicology 287(1–3):76–90, PMID: 21683116, 10.1016/j.tox.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Whittington JR, Simmons PM, Phillips AM, Gammill SK, Cen R, Magann EF, et al. 2018. The use of electronic cigarettes in pregnancy: a review of the literature. Obstet Gynecol Surv 73(9):544–549, PMID: 30265741, 10.1097/OGX.0000000000000595. [DOI] [PubMed] [Google Scholar]
- Wieslander G, Norbäck D, Lindgren T. 2001. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58(10):649–655, PMID: 11555686, 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. 2013. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33(17):7368–7383, PMID: 23616543, 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, Aschner M. 2018. Microglia activation and gene expression alteration of neurotrophins in the hippocampus following early-life exposure to e-cigarette aerosols in a murine model. Toxicol Sci 162(1):276–286, PMID: 29161446, 10.1093/toxsci/kfx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Fan SJ, Sun AB, Liu ZZ, Liu L. 2019. Prenatal nicotine exposure induces depression-like behavior in adolescent female rats via modulating neurosteroid in the hippocampus. Mol Med Rep 19(5):4185–4194, PMID: 30942466, 10.3892/mmr.2019.10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Spencer TJ, Biederman J, Bhide PG. 2018. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS One 13(5):e0198064, PMID: 29795664, 10.1371/journal.pone.0198064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pyrgiotakis G, Demokritou P. 2016. Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal Toxicol 28(14):658–669, PMID: 27829296, 10.1080/08958378.2016.1246628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG. 2012. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J Neurosci 32(27):9410–9418, PMID: 22764249, 10.1523/JNEUROSCI.1041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkhan EK, Lang BY, Yu B, Wang Y, Jiang C, Fitzhugh M, et al. 2014. Maternal tobacco smoke increased visceral adiposity and serum corticosterone levels in adult male rat offspring. Pediatr Res 76(1):17–23, PMID: 24727947, 10.1038/pr.2014.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.