Abstract
Background:
High quality personal exposure data is fundamental to understanding the health implications of household energy interventions, interpreting analyses across assigned study arms, and characterizing exposure–response relationships for household air pollution. This paper describes the exposure data collection for the Household Air Pollution Intervention Network (HAPIN), a multicountry randomized controlled trial of liquefied petroleum gas stoves and fuel among 3,200 households in India, Rwanda, Guatemala, and Peru.
Objectives:
The primary objectives of the exposure assessment are to estimate the exposure contrast achieved following a clean fuel intervention and to provide data for analyses of exposure–response relationships across a range of personal exposures.
Methods:
Exposure measurements are being conducted over the 3-y time frame of the field study. We are measuring fine particulate matter [PM in aerodynamic diameter ()] with the Enhanced Children’s MicroPEM™ (RTI International), carbon monoxide (CO) with the USB-EL-CO (Lascar Electronics), and black carbon with the OT21 transmissometer (Magee Scientific) in pregnant women, adult women, and children of age, primarily via multiple 24-h personal assessments (three, six, and three measurements, respectively) over the course of the 18-month follow-up period using lightweight monitors. For children we are using an indirect measurement approach, combining data from area monitors and locator devices worn by the child. For a subsample (up to 10%) of the study population, we are doubling the frequency of measurements in order to estimate the accuracy of subject-specific typical exposure estimates. In addition, we are conducting ambient air monitoring to help characterize potential contributions of exposure from background concentration. Stove use monitors (Geocene) are being used to assess compliance with the intervention, given that stove stacking (use of traditional stoves in addition to the intervention gas stove) may occur.
Conclusions:
The tools and approaches being used for HAPIN to estimate personal exposures build on previous efforts and take advantage of new technologies. In addition to providing key personal exposure data for this study, we hope the application and learnings from our exposure assessment will help inform future efforts to characterize exposure to household air pollution and for other contexts. https://doi.org/10.1289/EHP6422
Introduction
Globally, nearly people burn solid fuels (e.g., wood, dung, charcoal) in inefficient and poorly vented combustion devices (i.e., open fires, traditional stoves) to meet daily cooking needs (Bonjour et al. 2013). The resulting household air pollution (HAP) is a leading risk factor for global morbidity and mortality (GBD 2017 Risk Factor Collaborators 2018). However, the burden of disease related to these exposures is highly uncertain, partly due to the relatively few studies with quantitative data on personal exposures. Furthermore, the implementation of household energy interventions intended to reduce the burden of disease has not been well-informed owing to the limited understanding of exposure–response relationships for HAP. Because of financial and technical constraints associated with conducting large-scale HAP measurements in low- and middle-income country settings, many studies have relied on imprecise proxy exposure measures (Dherani et al. 2008). Measures of fine particulate matter [PM in aerodynamic diameter ()] have been particularly challenging due to the limitations of affordable, feasible, and reliable instrumentation (Balakrishnan et al. 2014; Clark et al. 2013; Pillarisetti et al. 2017).
The Household Air Pollution Intervention Network (HAPIN) trial is a four-country (Rwanda, India, Guatemala, Peru) randomized controlled trial (RCT) evaluating the effects of a liquefied petroleum gas cookstove and fuel intervention vs. cooking on traditional biomass stoves among 800 households (split equally between control and intervention arms) in each of the four countries, for a total of 3,200 households. For the primary objective of the HAPIN trial, investigators will compare outcomes between the intervention and controls arms, including birthweight, severe pneumonia incidence, and stunting among infants, as well as blood pressure among older women. Although the primary analysis will not require data on exposure, describing the exposure contrast achieved between the intervention and control arms will inform the interpretation of health effect estimates. For the secondary objective of the HAPIN trial, exposure–response analyses will be conducted for these same health outcomes. The exposure–response analyses will produce results that may be transferable to other communities and stove types, given that for each proposed outcome, this information will help to refine existing exposure–response curves. Furthermore, this information, combined with our intensive evaluation of behaviors surrounding stove use, will be critical for benchmarking future stove dissemination efforts.
Here we summarize our methods used for estimating personal exposure for the HAPIN participants. A description of the overall trial methods can be found in the paper by Clasen et al. (2020) and a description of the biomarker methods, including repeated measures of biomarkers of exposure [e.g., urinary polycyclic aromatic hydrocarbons (PAHs), levoglucosan], can be found in the paper by Boyd Barr et al. (2020). Our methods build on previous efforts while making use of newer approaches and tools with the aim of maximizing the quality and accuracy of personal exposure estimates. In addition to providing key personal exposure data for this study, we hope that lessons from our exposure assessment will help inform future efforts to characterize exposure to HAP.
Study Setting and Exposure Sampling Design Overview
The HAPIN trial will be conducted across four sites in India, Rwanda, Guatemala, and Peru. Study settings are mainly rural, as described in more detail by Clasen et al. (2020). Briefly, each study site recruits 800 households (400 intervention and 400 control) with pregnant women who are between 18 and 35 years of age, demonstrate 9 to of gestation, primarily use biomass for cooking within the home, and are nonsmokers. The specific study areas at each site are in the rural areas of Tamil Nadu, India; Department of Puno, Peru; Eastern Province, Rwanda; and Jalapa Municipality, Guatemala. These areas were largely selected based on prevalence of biomass use, low background ambient concentrations, and accessibility for field staff. Following an 18-month period of planning and formative (pilot) research, the study began recruiting participants in May 2018 and completed enrollment in February 2020. During the formative research, 40 households were enrolled in a 3-month before-and-after gas stove and fuel intervention in three of the sites (Guatemala, India, Rwanda). In Peru, formative research was done within the context of the Cardiopulmonary Outcomes and Household Air Pollution (CHAP) trial in Peru (Fandiño-Del-Rio et al. 2017). Participant acceptance of instrumentation and wearing comfort were assessed through structured surveys and informal interviews at all sites during formative work.
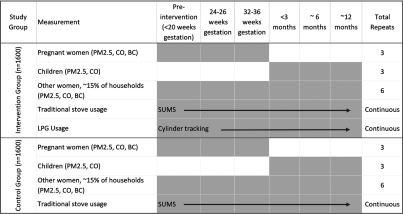
In the main trial, we are measuring personal exposure at multiple time points for three study populations of interest: pregnant women, infants, and older adult women (40–79 years of age). All three groups will come from the same households. We are collecting three measurements in pregnant women (one at baseline prior to randomization/intervention and two at follow-up during pregnancy), three measurements in infants in the first year of life, and six measurements in older adult women (one pre-intervention) during the approximately 18 months they will be under observation (Figure 1). The purpose of the multiple measurements will be to estimate subject-specific typical exposure levels during follow-up in order to characterize exposure–contrasts between the two study arms and to assess associations with health outcomes via exposure–response analyses. For example, the pregnancy period exposures may be associated with fetal growth, birthweight, and adverse birth outcomes; and exposures over the first year of life may be associated with pneumonia, growth, and development among infants. Exposure among older adult women may be associated with changes in mean blood pressure after baseline.
Figure 1.
Exposure assessment timeline including frequency of assessment for intervention and control households. The intervention arm will have gas stoves, whereas the control arm will use traditional biomass stoves. In each country, direct personal measurements will be collected for 800 pregnant women during gestation and an estimated 120 older women, 40–79 years of age, living in the same households. Indirect measurements of personal exposure using a microenvironmental approach will be conducted on 800 infants from birth to 1 year of age. Traditional biomass stove usage will be continuously measured by stove use monitors during the trial, whereas gas usage will be tracked by the number of cylinders used by each household throughout the trial. Note: BC, black carbon; CO, carbon monoxide; LPG, liquid petroleum gas; , particulate matter in aerodynamic diameter.
We are utilizing the Enhanced Children’s MicroPEM™ (ECM), a robust, lightweight, and validated gravimetric monitor and the Lascar CO logger, for repeated personal 24-h measurements of pregnant women and older adult women. For infants of age, we use a newly adapted and validated indirect assessment approach that pairs microenvironmental pollutant sampling and participant proximity sensing. The microenvironmental sampling occurs in the most commonly occupied rooms and on the mother (wearing a personal monitor as a mobile microenvironment). This approach will allow us to better reconstruct infant exposures to compared with the use of estimating location via participant recall, while also not having to rely on a proxy measure for such as CO (Carter et al. 2017).
An intensified exposure assessment that doubles the number of measurements over time is being conducted in a random subsample of 10% of participants per site. The random sample is selected monthly among newly recruited households. The purpose of collecting these additional measurements is to compare the average exposure level of subsample participants via the usual number of measurements with the average exposure level of subsample participants using more numerous measurements. Assuming these two averages differ only via random error, we can use the intensified assessment to correct for bias by calculating the intra-class correlation matrix in the 10% subsample [between variance/()], and use this to correct for classical measurement error (bias to the null) in the main study exposure–response analysis (Rosner et al. 1989). If there appears to be systematic error, for example due to seasonal effects, in our usual estimate compared with the intensified assessment, we can also use this comparison to correct our main study results (Rosner et al. 1989). We will judge that there is systematic error by whether the long-term average is significantly different (at the 0.05 level) from the short-term average from the same households. With approximately 320 short- and long-term samples across the four study sites, we should have good power to detect a systematic bias. For example, data from the formative phase indicate a mean personal exposure after intervention of about with a standard deviation of about across our four sites (https://ehp.niehs.nih.gov/doi/10.1289/isesisee.2018.O02.03.31). Let us assume that there are 320 women (80 in each of four sites, a 10% sample) with both short- and long-term measurements (each with three observations each for both short- and long-term samples). However, observations within a household are correlated; therefore, for our purposes here we consider that we have only 320 independent observations for each type of sample. We would then have 80% power (with ) to detect a significant difference between short- and long-term samples if their means differed by more than about .
Another important aspect of our sampling plan is employing stove use monitors to assess compliance with the intervention (Pillarisetti et al. 2017; Ruiz-Mercado et al. 2013). These monitors are small temperature sensors that can be installed inside a stove and give a continual readout of temperature that is stored for later downloading. Stove stacking (e.g., the use of baseline stoves in conjunction with the new intervention stove/fuel) has been common in studies of stove interventions (Masera et al. 2000; Rehfuess et al. 2014) and clean fuel stoves (Puzzolo et al. 2016; Quinn et al. 2018). As HAPIN is an efficacy trial, we are undertaking substantial efforts to ensure correct and consistent use of the intervention and to minimize stacking. Here, we note that it is important to monitor stove use, both to support behavioral reinforcement and to determine the extent to which stove use behaviors are associated with exposure.
Exposure Measurements
Measured Pollutants
Three primary pollutants were selected for measurement because of their health implications and associations with household fuel combustion: , CO, and black carbon. has the strongest evidence linking its exposure to a variety of key health outcomes (Adetona et al. 2016; Bruce et al. 2014), allowing for the estimation of integrated exposure–risk functions for several health outcomes (Burnett et al. 2014). CO is a major product of incomplete combustion in smoke, and elevated, short-term CO exposures are linked to acute symptoms and mortality due to CO binding with hemoglobin (Goldstein 2008; WHO 2010). Evidence also suggests chronic CO exposure may be linked with other health outcomes, including asthma, cardiovascular disease, and neurological development (Dix-Cooper et al. 2012; WHO 2010). and CO are also the pollutants included in the World Health Organization’s Air Quality Guidelines for Household Fuel Combustion (WHO 2014), highlighting their importance in this area of environmental exposures. Black carbon was included because evidence has shown that the black carbon fractions within may be more strongly linked with some specific health outcomes compared with as a whole (Cassee et al. 2013; Janssen et al. 2001), such as for blood pressure (Baumgartner et al. 2014), one of HAPIN’s primary health outcomes.
Instrumentation
Equipment selection, deployment protocols, and quality assurance procedures for the main trial were evaluated during the formative phases of HAPIN.
.
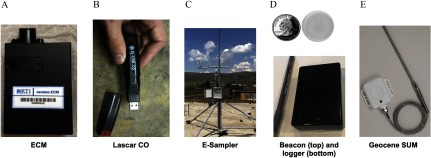
Our primary instrument for measuring is the ECM, which is well suited for our application due to its combination of small size and quiet operation compared with previous devices. The ECM (Figure 2), developed by RTI International, is a combined nephelometric and gravimetric sampler weighing approximately and capable of operating continuously at for up to 48 h. The ECM is virtually silent during operation; participants can wear the sampler on a shoulder band or in a pocket on a customized garment within their breathing zone. The ECM collects gravimetrically with a filter by drawing air through an impactor attached to a cassette containing Teflon® filters (PT15-AN-PF02; MTL Corporation). The ECM contains a calibrated mass–flow element, a six-axis accelerometer (to log activity rate and to verify the user complies with wearing the sampler), and measures real-time with a nephelometer (light scattering sensor). It also logs temperature, relative humidity, and filter-pressure drop. The ECM has been used for household energy studies (Fandiño-Del-Rio et al. 2017), as has the earlier version of the instrument (the MicroPEM™) (Bruce et al. 2018; Chartier et al. 2017; Dutta et al. 2017).
Figure 2.
(A) Enhanced Children’s MicroPEM™ (ECM) developed by RTI International; (B) CO data logger, model EL-USB-300 (Lascar Electronics); (C) E-Sampler (Met One Instruments) installed in the Peru site; Beacon (Model O Roximity Inc.); (D) Beacon Logger (Berkeley Air Monitoring Group); and (E) Geocene stove use monitors (Geocene). [Photo credits: Michael Johnson (A), Ricardo Piedrahita (B), Ajay Pillarisetti (C), and Ricardo Piedrahita (D), and Daniel Wilson (E).]
ECM preparation before deployment includes component cleaning using ethanol and lint-free wipes and device calibration. Three-point flow calibrations are performed before each deployment, as well as nephelometer, temperature, and humidity offsets. Flow calibration is done with National Institute of Standards and Technology–traceable flow calibrators. Post-deployment, ECMs are transported in coolers to the field offices, where the data is downloaded and viewed using a web-based analysis tool to assess data quality. Post-sample flows are checked and recorded, after which the filters are transferred to cold storage (quality controls for filter processing and analysis are described above). Maintenance is performed as needed for ECM components and is based on calibration performance and data analysis checks. The real-time data files are assessed biweekly using an automated system to check the volumetric flow rate, nephelometer, inlet pressure, compliance (accelerometry), temperature, and relative humidity. Flags are generated and reported to the sites based on predetermined thresholds for each variable. Data quality is also assessed through the use of duplicate ECM deployments and field blank filters. Duplicate ECM deployments, for which two ECMs are placed side-by-side, are being conducted on at least 30 personal and 30 area samples at each site, and field blank filters are being collected for 3% of all samples to correct for changes in mass associated with filter handling and processing. In addition, at least 20% of ECM microenvironmental area measures include a pre-weighed filter for gravimetric collection and analysis, while the remainder rely on those gravimetric values to adjust the nephelometer readings.
Carbon monoxide.
Real-time carbon monoxide (CO) concentrations are being measured with Lascar CO monitors (model EL-USB-300; Lascar Electronics). As with most personal CO monitors, the Lascar CO monitor uses an electrochemical cell to detect CO. The instrument is small (the size of a large pen), silent, can log continuously for days, has a range of , and has also been used to assess exposures and HAP in several other monitoring efforts (Das et al. 2018; Piedrahita et al. 2019a). Monthly two-point calibrations are performed with each Lascar CO logger. Data is also visually inspected after each deployment to ensure there are no signs of instrument malfunction. Side-by-side duplicate CO measures are being conducted for 10% of all data collected.
Black carbon.
Black carbon is being measured on the filters collected via the ECM and from the ambient monitors. Black carbon is being quantified on the filters using a SootScan™ Model OT21 transmissometer (Magee Scientific), which has been used often for characterizing black carbon for personal exposure and emissions studies (Baumgartner et al. 2014; Garland et al. 2017; Rajkumar et al. 2018). The instrument measures the light attenuation through the filter at the wavelength, which is then converted into a black carbon surface deposition.
Pregnant Women
Exposures of pregnant women (and prenatal exposures of their children) are measured with ECMs and CO loggers worn in a vest or apron for three 24-h periods during the pregnancy (, 24–36, and 32–36 weeks of gestation) (Figures 1–3). The women are asked to wear the vest or apron at all times during each measurement period except when sleeping, bathing, or when conducting other activities for which the equipment cannot be safely worn.
Figure 3.
Example photos of participants wearing customized vests and/or aprons with exposure monitoring equipment. (A) Guatemala; (B) India; (C) Peru; and (D) Rwanda. The picture of the sampling garment in Guatemala was taken when a pump and cyclone setup was also being compared with the Enhanced Children’s MicroPEM™ (ECM) during Household Air Pollution Intervention Network (HAPIN)’s formative research phase. [Photo credits: Eric Mollinedo (A), Thangavel Gurusamy (B), Vanessa Burrowes (C), and Ephrem Dusabimana (D)].
To estimate exposures to their children after birth, the mothers wear the sampling vest or apron during three 24-h periods (, , and ) after their child’s birth. During these time periods, mothers are asked to place the vest or apron holding the equipment near their child when they are not wearing it and to leave the sampling vest where the child is expected to spend most of their time if they leave the home without their child. The vests and aprons secure the ECMs and CO loggers near the breathing zone, a similar approach to that used in other HAP exposure studies (Balakrishnan et al. 2018; Bruce et al. 2018; Delapena et al. 2018; Hill et al. 2019; Nagel et al. 2016). Compliance is checked via the ECM’s accelerometer data to determine if motion is detected during normal daily activities and participants are also directly asked at the end of each sampling duration about wearing the monitors as part of the survey.
Older Adult Women
Exposures among older women living in the same home as a pregnant participant are also measured by ECMs and CO loggers worn in a vest or apron during three 24-h periods during the pregnancy (, 24–36, and 32–36 weeks of gestation) and three 24-h periods after the pregnancy (, , and after birth) (Figures 1–3). As with the pregnant women, the older women are asked to hang the vest or apron nearby when it is not being worn and compliance is checked via accelerometry and questionnaire.
Children
Children’s exposure is estimated using a microenvironmental approach. The primary environment comes from data collected by ECMs and CO loggers worn in a vest or apron by their mother, as described above (Figures 1–3). ECMs and CO loggers are also placed in the primary cooking area and the infant’s sleeping area. Two coin-sized location Beacons [EMBC-01, EM Microelectronics-Marin SA (Figure 2)] are worn by the children and linked to receivers where the ECMs are located, including in the mother’s sampling vest. Personal exposures for the child are estimated by integrating corresponding area concentrations over the time spent in that location.
The microenvironmental approach is used because even with the small size of the ECM, it is still impractical for use on young infants. The approach used here is similar to previous efforts (Balakrishnan et al. 2004; Ezzati et al. 2000; Saksena et al. 2003; Zuk et al. 2007) but adds an objective measure of location with the Beacons by tracking where the child is over 24 h (Piedrahita et al. 2019b; Liao et al. 2019). Objective measures of location are key for using this approach because participant recall of time–activity patterns can be unreliable and are often biased (Daum et al. 2018).
Results from the HAPIN formative work in Guatemala have shown that the Beacon system provides accurate time–location patterns, and this microenvironmental approach can predict personal exposure better compared with a single area measurement (Liao et al. 2019). The system was piloted by the four sites and found to be acceptable to participants. Specific results from our formative work indicated that indirect exposure measurements had high correlation with direct personal measurements for adult women cooking with either gas or solid fuel (, Spearman , concentration range: ), and indirect measurements had better agreement with direct measurements (bias: ) compared with kitchen area measurements (bias: ) (Liao et al. 2019). Performance of the Beacon localization approach is also checked via a walk-through procedure by the field enumerators after installation of the loggers and receivers in the home, but before sampling begins.
Real-time CO concentrations are also measured directly for the child when possible using the CO loggers situated in custom shirt pockets, depending on feasibility and consent of mother. Previous efforts for monitoring small children have also used CO as a proxy for or HAP exposure because small CO instrumentation is better suited for infants (Dionisio et al. 2012; Smith et al. 2010). The relationship between CO and varies with geography, fuel type, and combustion conditions. In a recent review of the literature, CO was deemed an inconsistent surrogate measure (Carter et al. 2017) for .
Ambient Air Pollution Monitoring
Ambient background is being measured every 6 d to capture weekly and daily variability. is measured at two or more sites in each study region, centered around locations where ongoing exposure assessment activities are underway. Monitor placement is meant to capture the background ambient concentrations in each region during other pollution monitoring exercises and follows U.S. Environmental Protection Agency (EPA) siting guidelines (U.S. EPA 2016). Ambient is being primarily collected using the E-Sampler (Met One Instruments), with comparable gravimetric systems also being deployed. The E-Sampler collects both real-time data, based on a forward-scattering nephelometer, and an integrated gravimetric sample on a filter. The E-Sampler can detect up to of PM and can be configured with different cut points. For the current work, a sharp cut cyclone is utilized at . The E-Sampler can be run off of an internal battery or from line current, has a user-configurable logging interval, conducts diagnostic tests at a user-set interval, and includes an inlet heater to address the impact of humidity on the nephelometer. The E-Sampler has been utilized in both low- (Rooney et al. 2018; Yip et al. 2017) and high-income settings, where it was validated against U.S. EPA Federal Reference Methods (Trent 2006). In India, the E-Sampler measurements are being complemented with integrated 24-h gravimetric measurements performed using traditional high-volume samplers equipped with a cyclone (APM 550EL, Envirotech Instruments).
Stove Use Monitoring
The Geocene Stove Usage Monitoring System (Geocene Inc.) is being employed to assess compliance of gas stove use in the intervention arm, as well as overall stove use patterns. The system is comprised of a Bluetooth®-enabled high-temperature data logger, an Android mobile app, a cloud-based data collection and analytics system, and an online dashboard. The hardware and software used in the Geocene platform are the culmination of prior work on advanced cookstove monitors and cookstove analytics techniques (Wilson et al. 2016, 2018), and a full description of the devices and platform as used for the HAPIN trial can be found in Wilson et al. (2020). The data logger is a stove usage monitor that records cookstove temperatures with a K-type thermocouple. The data logger uses thermocouples to allow for high, distinct temperature signals during cooking. The data from these sensors is processed with real-time cloud analytics. Stove use event summaries are automatically emailed to program managers and field staff who use these insights to improve data quality and participant adherence. Specifically, Geocene sends out weekly alert emails detailing households who have recently cooked on their traditional cookstove (and therefore are nonadherent) as well as technical issues with data loggers, such as overheating or broken probes.
In households receiving the gas stove intervention, stove use monitors are installed on the traditional cookstoves, where they remain for the duration of the intervention. Reinforcement of the gas stove intervention is provided in the case that traditional stove use is observed in intervention households. In a subset of up to 20% of these intervention households, the gas stoves are monitored in addition to the traditional stoves assess typical usage patterns. In a subset of up to 20% of control households, all stoves used more than once per week are monitored.
The deployment of Geocene stove use monitors is coordinated using the Geocene Android app. This app allows field staff to start and stop data recordings (missions, in Geocene parlance) as well as assign metadata tags (described below) to missions. When field staff provision a new Geocene stove use monitor for deployment, they begin by launching a new mission. The app prompts field staff to select a campaign (Guatemala, India, Rwanda, or Peru), then a short survey appears that allows field staff to assign metadata to the mission. The survey questions ask about household identification number (integer), stove type (multiple choice), whether the household is in the intervention group (true/false), and, if the household is in the intervention group, whether the intervention cookstove is installed (true/false). These metadata tags are used by the analytics pipeline to send alerts. For example, only households in the intervention group—with the intervention cookstove installed—are flagged for nonadherence when cooking on a traditional cookstove is detected.
The analytics system for the Geocene data is cloud-based and real-time. However, the stove use monitors do not stream data in real-time to the cloud. Instead, data it is collected from the dataloggers in biweekly batches on site by field staff using Bluetooth® and the Geocene Android app. After field staff return from the field, the Android app syncs cached data to the cloud once an internet connection is available. Once data enters the Geocene cloud system, automated analytics detect cooking events using the Geocene FireFinder cooking event detection algorithm. The FireFinder algorithm is part of an open-source package of cookstove analytics tools called SUMSarizer that is maintained by Geocene. The data is also checked for data quality issues, namely overheating thermocouples and broken or missing thermocouples. Using the analytics and mission metadata, weekly alert emails are sent to program managers and field staff regarding participant nonadherence and technical problems with stove use monitors. Field staff typically follow such alerts with visits to the households to investigate.
The Geocene mobile app includes a chart that displays a line graph of downloaded temperature vs. time data for each stove use monitor placement. This chart allows staff to visually detect historical cooking events. Field staff use cooking behavior data displayed on the chart to facilitate on-site discussions about behavior modifications with cooks. In addition, the app provides feedback about excessive probe temperature or probe errors, and field staff use these data to reposition or replace thermocouple probes. The recent cooking events and recent technical problems metrics are also emailed out to HAPIN data management core members on a weekly basis.
Filter Management and Analysis
Teflon® filters used for gravimetric measurement in HAPIN are weighed and stored following guidance from U.S. EPA protocols (U.S. EPA 2016). Filter weighing is performed at Sri Ramachandra University for all filters used in India and at the College of Public Health at the University of Georgia for filters used in other countries. The Universidad del Valle de Guatemala will weigh filters for the Guatemala field study once filter weighing facilities are finalized and validated by inter-laboratory comparisons. Pre- and post-weights are made on the same balance in the same facility.
Prior to pre-weighing, filters are visually inspected to discard filters with visible tears or other damage and conditioned for at least 24 h in a temperature- and humidity-controlled weighing room. Filters are then weighed on a resolution balance (Model MSA6.6S-000-DF; Sartorius) by a trained laboratory technician. Duplicate weighings are performed on all filters and, if different by more than , a third weight is taken, and the final two weights are used if within . Filters are weighed in sets of 10, with the calibration weight and the three laboratory blanks weighed at the beginning of each set. At the end of each set of 10 filters, the third filter from the set is re-weighed as a duplicate measurement. If the duplicate measurement differs by more than from its first measurement, the entire set of 10 filters is re-weighed. Laboratory blanks are weighed on a daily basis. All filter weights are recorded concurrently in standardized data collection forms, along with humidity, temperature, and barometric pressure in the weighing room, and uploaded to the centralized data management server.
After pre-weighing, filters are loaded into filter cassettes (Protolabs Inc.) or individual, bar-coded filter holders [Filter-Keepers (SKC; Eight Four) or petri dishes (Pall Corporation)]. A set of 10–12 filter holders is stacked in sealed plastic bags and transported in secure packaging that is insulated from shocks during transport and protected from possible condensation with additional aluminum foil and plastic bag wraps. Pre-weighed filters are shipped using ground transportation courier services in India and routine commercial shipping methods for the other sites, and they are shipped with the accompanying chain of custody forms.
Filters are loaded into ECMs at the field office, and upon completion of sampling, ECMs (containing the loaded filters) are transported back to the field office in cooler boxes. The filter cassettes are then unloaded from the ECMs, loaded back into the original labeled Filter-Keepers and stored in refrigerators or freezers. Filters are periodically transported back to the weighing laboratory following the same packaging procedures as described above for pre-weighed filters, and efforts are made to keep the temperature below 25°C (temperature loggers are included with the shipments). The filters are then stored in a freezer at the weighing institution. Post–weighings are performed following the same protocols as the pre-weighings. Following the post-weighing and black carbon measurements, filters are stored in a freezer in the original labeled Filter-Keepers.
Filters processed by the University of Georgia laboratory also undergo black carbon analysis. First, a reference or blank filter is scanned prior to every session to validate the long-term consistency of the SootScan™; this filter is unchanged. Second, a test filter is used at the start and end of every session that serves as a laboratory blank; this filter is changed every 2–3 months. Third, duplicate measures are taken every 20 filters to ensure stability among filters. Finally, neutral density validation filters of varying opaqueness are scanned periodically (per the manufacturer) and compared with known attenuation values to validate that the instrument performance is consistent. The Magee SootScan™ measures light attenuation for each filter before the pre-weight, and then again after the post-weight, which accounts for inter-filter differences and reduces variability associated with comparison to a blank reference filter. Filters are also being retained in cold storage for potential future source apportionment analysis using speciation by either X-ray fluorescence or ultraviolet absorbance of organics (UV-POC) (Mazumder et al. 2019). Source apportionment is of interest because of the varying health effects of different PM components (Janssen et al. 2011; Naeher et al. 2007).
Household Air Pollution Survey Data
Surveys—covering household characteristics, cooking behavior and preferences, exposure to other sources of smoke, and protocol compliance—are conducted with the pregnant woman at each of the visits depicted in Figure 1. These data provide contextual information that will be used in various modeling capacities and also allow for assessment of data quality, participant preferences, and potential exposure to other pollution sources. Sample metadata such as instrument start/stop times, instrument identification numbers, and others are also recorded by the enumerator. Data are input and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Emory University (Harris et al. 2009).
Based on previous studies (e.g., McCracken et al. 2009), survey data will provide primary data on household (e.g., ventilation, room size) and individual characteristics (e.g., height, weight), that can be used in mixed models, which consider multiple measurements of individual exposure, together with group level (e.g., same village) characteristics, to explain the large variability commonly reported in the HAP literature. An overall goal of such an exercise is to better estimate long-term exposures.
Data Processing and Quality
Multiple trainings were conducted at each site to standardize and implement the co-developed operating procedures. In collaboration with the data management core, file naming, data uploading, and data quality checking protocols and tools have been developed to ensure organization and timely resolution of issues. Exposure instrument data is downloaded on local computers and backed up on the cloud in secure folders. Data files are cleaned and processed using R (version 3.6) and stored as compressed text files on a server. Multiple quality control steps for each exposure data stream are taken.
Conclusions and Future Work
Household air pollution exposure is characterized by large variability and uncertainty due to differences in stove use and time–activity patterns, household room configuration and ventilation, fuel type and conditions, weather, instrument error, and others (Clark et al. 2013). The methods and tools described above build on previous efforts and take advantage of new technologies to address these challenges and characterize the exposure impacts of a household gas stove intervention.
The exposure assessment will inform the study arm comparisons by documenting the hypothesized large reduction in HAP due to the intervention that was observed in our formative work (https://ehp.niehs.nih.gov/doi/10.1289/isesisee.2018.O02.03.31). This is important because it seems likely that insufficient HAP reductions in prior improved biomass studies may have contributed to the lack of observed improvements in health (e.g., Mortimer et al. 2017; Hanna et al. 2016; Nightingale et al. 2019). Existing exposure–response curves suggest that modest reductions in HAP, such as seen with improved biomass stoves, may not have a strong health effect (Smith and Peel 2010; Steenland et al. 2018).
Furthermore, accurate exposure estimates may minimize classical measurement error (typical of personal measurements) that tend to bias exposure-response analyses to the null. Our relatively large numbers of repeated measurements should enable us to accurately characterize the longer-term exposure of our participants. In addition, our intensified exposure assessment in 10% of the population (doubling the number of measurements) will enable us to check whether our standard number of measurements accurately reflects long-term average exposure.
There are a number of novel aspects to our approach. With the extensive stove use monitoring data, accompanied by a large number of personal and microenvironmental HAP measurements, we will be able to examine whether stove use metrics could be used as reliable surrogates for exposures in large-scale implementation efforts. We also believe our approach to measuring infant exposure to via indirect measurements will be an advance on previous methods, based on the success of this method in our formative work (Liao et al. 2019). We will check the validity of the indirect method in our intensified exposure assessment by conducting and comparing the direct and indirect measurement methods for pregnant women.
Finally, although our analysis will focus on as our primary pollutant, black carbon and CO estimates will be incorporated into exposure–response models and may provide new insights into their health implications either as independent predictors or in combination. The exposure measurements will also be evaluated in conjunction with the planned biomarker assessments (e.g., urinary PAHs and levoglucosan) (Boyd Barr et al. 2020), allowing mediation analysis to assess whether the biomarkers may be intermediate variables between exposure and health effects.
Acknowledgments
The Household Air Pollution Intervention Network (HAPIN) trial is funded by the National Institutes of Health (NIH cooperative agreement 1UM1HL134590) in collaboration with the Bill & Melinda Gates Foundation [OPP1131279]. A.P. was partially supported by the HERCULES Center P30ES019776. We thank R. Chartier, C. Garland, A. Lovvorn, and S. Jabbarzadeh for their input and support in developing the exposure sampling materials and protocols. We are also thankful to the field teams at each of the research centers for their feedback and input. A multidisciplinary, independent Data and Safety Monitoring Board (DSMB) appointed by the National Heart, Lung, and Blood Institute (NHLBI) monitors the quality of the data and protects the safety of patients enrolled in the HAPIN trial. NHLBI DSMB: N.R. Cook, S. Hecht, C. Karr, K.H. Kavounis, D.-Y. Kim, J. Millum, L.A. Reineck, N. Sathiakumar, P.K. Whelton, and G.G. Weinmann. Program Coordination: G. Rodgers, Bill & Melinda Gates Foundation; C.L. Thompson, National Institute of Environmental Health Science; M.J. Parascandola, National Cancer Institute; D.M. Krotoski, Eunice Kennedy Shriver National Institute of Child Health and Human Development; J.P. Rosenthal, Fogarty International Center; C.R. Nierras, NIH Office of Strategic Coordination Common Fund; and A. Punturieri and B.S. Schmetter, NHLBI. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH, Department of Health and Human Services, or the U.S. Government.
References
- Adetona O, Reinhardt TE, Domitrovich J, Broyles G, Adetona AM, Kleinman MT, et al. 2016. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhal Toxicol 28(3):95–139, PMID: 26915822, 10.3109/08958378.2016.1145771. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Ghosh S, Thangavel G, Sambandam S, Mukhopadhyay K, Puttaswamy N, et al. 2018. Exposures to fine particulate matter (PM2.5) and birthweight in a rural-urban, mother-child cohort in Tamil Nadu, India. Environ Res 161:524–531, PMID: 29227900, 10.1016/j.envres.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Mehta S, Ghosh S, Johnson MA, Brauer M, Naeher L, et al. 2014. WHO indoor air quality guidelines: household fuel combustion. Review 5: population levels of household air pollution and exposures. http://www.who.int/airpollution/guidelines/household-fuel-combustion/Review_5.pdf?ua=1. [accessed 7 April 2020].
- Balakrishnan K, Sambandam S, Ramaswamy P, Mehta S, Smith KR. 2004. Exposure assessment for respirable particulates associated with household fuel use in rural districts of Andhra Pradesh, India. J Expo Sci Environ Epidemiol 14(suppl 1):S14–S25, PMID: 15118741, 10.1038/sj.jea.7500354. [DOI] [PubMed] [Google Scholar]
- Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. 2014. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc Natl Acad Sci USA 111(36):13229–13234, PMID: 25157159, 10.1073/pnas.1317176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, et al. 2013. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect 121(7):784–790, PMID: 23674502, 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Barr D, Puttaswamy N, Jaacks LM, Steenland K, Rajkumar S, Gupton S, et al. 2020. Design and rationale of the biomarker center of the Household Air Pollution Intervention Network (HAPIN) trial. Environ Health Perspect 128(4):047010, 10.1289/ehp5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce N, de Cuevas RA, Cooper J, Enonchong B, Ronzi S, Puzzolo E, et al. 2018. The government-led initiative for LPG scale-up in Cameroon: programme development and initial evaluation. Energy Sustain Dev 46:103–110, PMID: 31440016, 10.1016/j.esd.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce N, Smith KR, Balmes J, Pope D, Dherani M, Zhang J, et al. 2014. WHO indoor air quality guidelines: household fuel combustion. Review 4: health effects of household air pollution (HAP) exposure. https://www.who.int/airpollution/household/guidelines/Review_4.pdf?ua=1 [accessed 7 April 2020].
- Burnett RT, Pope CA III, Ezzati M, Olives C, Lim SS, Mehta S, et al. 2014. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect 122(4):397–403, PMID: 24518036, 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E, Norris C, Dionisio KL, Balakrishnan K, Checkley W, Clark ML, et al. 2017. Assessing exposure to household air pollution: a systematic review and pooled analysis of carbon monoxide as a surrogate measure of particulate matter. Environ Health Perspect 125(7):076002, PMID: 28886596, 10.1289/EHP767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassee FR, Héroux M-E, Gerlofs-Nijland ME, Kelly FJ. 2013. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal Toxicol 25(14):802–812, PMID: 24304307, 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier R, Phillips M, Mosquin P, Elledge M, Bronstein K, Nandasena S, et al. 2017. A comparative study of human exposures to household air pollution from commonly used cookstoves in Sri Lanka. Indoor Air 27(1):147–159, PMID: 26797964, 10.1111/ina.12281. [DOI] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. 2013. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect 121(10):1120–1128, PMID: 23872398, 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen T, Checkley W, Peel JL, Balakrishnan K, McCracken J, Rosa G, et al. 2020. Design and rationale of the HAPIN Study: a multicountry randomized controlled trial to assess the effect of liquefied petroleum gas stove and continuous fuel distribution. Environ Health Perspect 128(4):047008, 10.1289/ehp6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Pedit J, Handa S, Jagger P. 2018. Household air pollution (HAP), microenvironment and child health: strategies for mitigating HAP exposure in urban Rwanda. Environ Res Lett 13(4):045011, PMID: 29682002, 10.1088/1748-9326/aab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum T, Buchwald H, Gerlicher A, Birner R. 2018. Smartphone apps as a new method to collect data on smallholder farming systems in the digital age: a case study from Zambia. Comput Electron Agric 153:144–150, 10.1016/j.compag.2018.08.017. [DOI] [Google Scholar]
- Delapena S, Piedrahita R, Pillarisetti A, Garland C, Rossanese ME, Johnson M, et al. 2018. Using personal exposure measurements of particulate matter to estimate health impacts associated with cooking in peri-urban Accra, Ghana. Energy Sustain Dev 45:190–197, 10.1016/j.esd.2018.05.013. [DOI] [Google Scholar]
- Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. 2008. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ 86(5):390–398, PMID: 18545742, 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Howie SRC, Dominici F, Fornace KM, Spengler JD, Donkor S, et al. 2012. The exposure of infants and children to carbon monoxide from biomass fuels in the Gambia: a measurement and modeling study. J Expo Sci Environ Epidemiol 22(2):173–181, PMID: 22166810, 10.1038/jes.2011.47. [DOI] [PubMed] [Google Scholar]
- Dix-Cooper L, Eskenazi B, Romero C, Balmes J, Smith KR. 2012. Neurodevelopmental performance among school age children in rural Guatemala is associated with prenatal and postnatal exposure to carbon monoxide, a marker for exposure to woodsmoke. Neurotoxicology 33(2):246–254, PMID: 21963523, 10.1016/j.neuro.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Dutta A, Brito K, Khramstova G, Mueller A, Chinthala S, Alexander D, et al. 2017. Household air pollution and angiogenic factors in pregnant Nigerian women: a randomized controlled ethanol cookstove intervention. Sci Total Environ 599–600:2175–2181, PMID: 28575932, 10.1016/j.scitotenv.2017.05.130. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Saleh H, Kammen DM. 2000. The contributions of emissions and spatial microenvironments to exposure to indoor air pollution from biomass combustion in Kenya. Environ Health Perspect 108(9):833–839, PMID: 11017887, 10.1289/ehp.00108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandiño-Del-Rio M, Goodman D, Kephart JL, Miele CH, Williams KN, Moazzami M, et al. 2017. Effects of a liquefied petroleum gas stove intervention on pollutant exposure and adult cardiopulmonary outcomes (CHAP): study protocol for a randomized controlled trial. Trials 18(1):518, PMID: 29100550, 10.1186/s13063-017-2179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C, Delapena S, Prasad R, L’Orange C, Alexander D, Johnson M. 2017. Black carbon cookstove emissions: a field assessment of 19 stove/fuel combinations. Atmos Environ 169:140–149, 10.1016/j.atmosenv.2017.08.040. [DOI] [Google Scholar]
- GBD 2017 Risk Factor Collaborators. 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1923–1994, PMID: 30496105, 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. 2008. Carbon monoxide poisoning. J Emerg Nurs 34(6):538–542, PMID: 19022078, 10.1016/j.jen.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Hanna R, Duflo E, Greenstone M. 2016. Up in smoke: the influence of household behavior on the long-run impact of improved cooking stoves. Am Econ J Econ Policy 8(1):80–114, 10.1257/pol.20140008. [DOI] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381, PMID: 18929686, 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LD, Pillarisetti A, Delapena S, Garland C, Pennise D, Pelletreau A, et al. 2019. Machine-learned modeling of PM2.5 exposures in rural Lao PDR. Sci Total Environ 676:811–822, PMID: 31071563, 10.1016/j.scitotenv.2019.04.258. [DOI] [PubMed] [Google Scholar]
- Janssen NAH, Hoek G, Simic-Lawson M, Fischer P, van Bree L, ten Brink H, et al. 2011. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ Health Perspect 119(12):1691–1699, PMID: 21810552, 10.1289/ehp.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NAH, van Vliet PHN, Aarts F, Harssema H, Brunekreef B. 2001. Assessment of exposure to traffic related air pollution of children attending schools near motorways. Atmos Environ 35(22):3875–3884, 10.1016/S1352-2310(01)00144-3. [DOI] [Google Scholar]
- Liao J, McCracken JP, Piedrahita R, Thompson L, Mollinedo E, Canuz E, et al. 2019. The use of bluetooth low energy Beacon systems to estimate indirect personal exposure to household air pollution. J Expo Sci Environ Epidemiol 1–11, PMID: 31558836, 10.1038/s41370-019-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masera OR, Saatkamp BD, Kammen DM. 2000. From linear fuel switching to multiple cooking strategies: a critique and alternative to the energy ladder model. World Dev 28(12):2083–2103, 10.1016/S0305-750X(00)00076-0. [DOI] [Google Scholar]
- Mazumder S, Lee A, Dube B, Mehra D, Khaing P, Taneja S, et al. 2019. A clean fuel cookstove is associated with improved lung function: effect modification by age and secondhand tobacco smoke exposure. Sci Rep 9(1):2487, PMID: 30792415, 10.1038/s41598-018-37887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR, et al. 2009. Combining individual- and group-level exposure information: child carbon monoxide in the Guatemala woodstove randomized control trial. J Expo Sci Environ Epidemiol 20(1):127–36, PMID: 19057384, 10.1097/EDE.0b013e31818ef327. [DOI] [PubMed] [Google Scholar]
- Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, et al. 2017. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet 389(10065):167–175, PMID: 27939058, 10.1016/S0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, et al. 2007. Woodsmoke health effects: a review. Inhal Toxicol 19(1):67–106, PMID: 17127644, 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Nagel CL, Kirby MA, Zambrano LD, Rosa G, Barstow CK, Thomas EA, et al. 2016. Study design of a cluster-randomized controlled trial to evaluate a large-scale distribution of cook stoves and water filters in Western Province, Rwanda. Contemp Clin Trials Commun 4:124–135, PMID: 29736475, 10.1016/j.conctc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale R, Lesosky M, Flitz G, Rylance SJ, Meghji J, Burney P, et al. 2019. Noncommunicable respiratory disease and air pollution exposure in Malawi (CAPS). a cross-sectional study. Am J Respir Crit Care Med 199(5):613–621, PMID: 30141966, 10.1164/rccm.201805-0936OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita R, Coffey ER, Hagar Y, Kanyomse E, Verploeg K, Wiedinmyer C, et al. 2019a. Attributing air pollutant exposure to emission sources with proximity sensing. Atmosphere (Basel) 10(7):395, 10.3390/atmos10070395. [DOI] [Google Scholar]
- Piedrahita R, Coffey ER, Hagar Y, Kanyomse E, Wiedinmyer C, Dickinson KL, et al. 2019b. Exposures to carbon monoxide in a cookstove intervention in Northern Ghana. Atmosphere (Basel) 10(7):402, 10.3390/atmos10070402. [DOI] [Google Scholar]
- Pillarisetti A, Allen T, Ruiz-Mercado I, Edwards R, Chowdhury Z, Garland C, et al. 2017. Small, smart, fast, and cheap: microchip-based sensors to estimate air pollution exposures in rural households. Sensors (Basel) 17(8):1879, PMID: 28812989, 10.3390/s17081879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzolo E, Pope D, Stanistreet D, Rehfuess EA, Bruce NG. 2016. Clean fuels for resource-poor settings: a systematic review of barriers and enablers to adoption and sustained use. Environ Res 146:218–234, PMID: 26775003, 10.1016/j.envres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Quinn AK, Bruce N, Puzzolo E, Dickinson K, Sturke R, Jack DW, et al. 2018. An analysis of efforts to scale up clean household energy for cooking around the world. Energy Sustain Dev 46:1–10, PMID: 30886466, 10.1016/j.esd.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar S, Clark ML, Young BN, Benka-Coker ML, Bachand AM, Brook RD, et al. 2018. Exposure to household air pollution from biomass-burning cookstoves and HbA1c and diabetic status among Honduran women. Indoor Air 28(5):768–776, PMID: 29896912, 10.1111/ina.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfuess E, Pope D, Bruce N, Dherani M, Jagoe K, Naeher L, et al. 2014. WHO indoor air quality guidelines: household fuel combustion. Review 6: impacts of interventions on household air pollution concentrations and personal exposure. https://www.who.int/airpollution/guidelines/household-fuel-combustion/Evidence_Review_6.pdf?ua=1 [accessed 7 April 2020].
- Rooney B, Zhao R, Bates KH, Pillarisetti A, Sharma S, Kundu S, et al. 2018. Impacts of household sources on air pollution at village and regional scales in India. Atmos Chem Phys 19:7719–7742, 10.5194/acp-19-7719-2019. [DOI] [Google Scholar]
- Rosner B, Willett WC, Spiegelman D. 1989. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med 8(9):1051–1069, PMID: 2799131, 10.1002/sim.4780080905. [DOI] [PubMed] [Google Scholar]
- Ruiz-Mercado I, Canuz E, Walker JL, Smith KR. 2013. Quantitative metrics of stove adoption using stove use monitors (SUMs). Biomass Bioenergy 57:136–148, PMID: 25258474, 10.1016/j.biombioe.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksena S, Singh PB, Prasad RK, Prasad R, Malhotra P, Joshi V, et al. 2003. Exposure of infants to outdoor and indoor air pollution in low-income urban areas—a case study of Delhi. J Expo Anal Environ Epidemiol 13(3):219–230, PMID: 12743616, 10.1038/sj.jea.7500273. [DOI] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Thompson L, Edwards R, Shields KN, Canuz E, et al. 2010. Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE). J Expo Sci Environ Epidemiol 20(5):406–416, PMID: 19536077, 10.1038/jes.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Peel JL. 2010. Mind the gap. Environ Health Perspect 118(12):1643–1654, PMID: 20729177, 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Pillarisetti A, Kirby M, Peel J, Clark M, Checkley W, et al. 2018. Modeling the potential health benefits of lower household air pollution after a hypothetical liquified petroleum gas (LPG) cookstove intervention. Environ Int 111:71–79, PMID: 29182949, 10.1016/j.envint.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent A. 2006. Smoke Particulate Monitors: 2006 Update. 6E62F46–Collocation Study of New Smsoke Monitors. Missoula, MT: USDA Forest Service, Technology and Development Program; https://app.airsis.com/USFS/Content/pdf/Smoke_particulate_monitors_2006_update.pdf [accessed 7 April 2020]. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2016. Quality Assurance Guidance Document 2.12, Monitoring PM2.5 in Ambient Air Using Designated Reference or Class I Equivalent Methods. EPA-454/B-16-001. Research Triangle Park: U.S. EPA, Office of Air Quality Planning and Standards, Air Quality Division. [Google Scholar]
- WHO (World Health Organization). 2010. WHO Guidelines for Indoor Air Quality: Selected Pollutants. Copenhagen, Denmark: World Health Organization Regional Office for Europe. [PubMed] [Google Scholar]
- WHO. 2014. WHO Guidelines for Indoor Air Quality: Household Fuel Combustion. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- Wilson DL, Coyle J, Kirk A, Rosa J, Abbas O, Adam MI, et al. 2016. Measuring and increasing adoption rates of cookstoves in a humanitarian crisis. Environ Sci Technol 50(15):8393–8399, PMID: 27435285, 10.1021/acs.est.6b02899. [DOI] [PubMed] [Google Scholar]
- Wilson DL, Monga M, Saksena A, Kumar A, Gadgil AJ. 2018. Effects of USB port access on advanced cookstove adoption. Dev Eng 3:209–217, 10.1016/j.deveng.2018.08.001. [DOI] [Google Scholar]
- Wilson DL, Williams KN, Pillarisetti A. 2020. An integrated sensor data logging, survey, and analytics platform for field research and its application in HAPIN, a multi-center household energy intervention trial. Sustainability 12(5):1805, 10.3390/su12051805. [DOI] [Google Scholar]
- Yip F, Christensen B, Sircar K, Naeher L, Bruce N, Pennise D, et al. 2017. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ Int 99:185–191, PMID: 27923586, 10.1016/j.envint.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, Rojas L, Blanco S, Serrano P, Cruz J, Angeles F, et al. 2007. The impact of improved wood-burning stoves on fine particulate matter concentrations in rural Mexican homes. J Expo Sci Environ Epidemiol 17(3):224–232, PMID: 16721411, 10.1038/sj.jes.7500499. [DOI] [PubMed] [Google Scholar]