Abstract
Introduction
Acute exacerbation (AE) is a major cause of disease progression and death in patients with chronic obstructive pulmonary disease (COPD), accounting for majority of medical expenditures. Correct inhalation therapy is effective in preventing AE attacks. However, inappropriate usage of dry powder inhaler, partially due to the unrecovered peak inhalation flow rate (PIFR) after acute exacerbation of COPD (AECOPD), results in increased risk of early treatment failure. Therefore, we designed a multicentre, randomised clinical trial to determine whether PIFR-based optimised inhalation therapy and training on inhaler usage at discharge could effectively reduce early treatment failure events.
Methods and analysis
A total of 416 hospitalised patients just recovering from AECOPD will be recruited and equally randomised into the PIFR group and the control group at a 1:1 ratio. The PIFR group will receive additive support before discharge, including choice of PIFR-guided inhaler and education on its usage. PIFR is measured by InCheck DIAL. In comparison, the control group will receive inhalers based on judgement of the respiratory physician. The primary outcome of the study is 30-day treatment failure rate. Other endpoints include PIFR, error rate of inhalation device use, satisfaction with inhalation devices, 30-day mortality, 90-day mortality, symptoms and quality of life of patients, and COPD-related treatment costs.
Ethics and dissemination
The trial has been approved by the Ethics Committee of Zhongshan Hospital of Fudan University (B2019-142). Participants will be screened and enrolled from hospitalised patients with AECOPD by clinicians, with no public advertisement for recruitment. After the trial has completed, the results will be reported to the public through conference presentations and peer-reviewed journals.
Trial registration number
Keywords: respiratory medicine (see thoracic medicine), chronic airways disease, medical education & training
Strengths and limitations of this study.
To our knowledge, this is the first multicentre, randomised trial designed to study the efficacy of peak inhalation flow rate (PIFR)-based inhaler prescription in preventing short-term re-exacerbations in patients recovering from severe acute exacerbation of chronic obstructive pulmonary disease.
InCheck DIAL is used to measure PIFR and objectively evaluate the capacity of using dry powder inhalers.
Participants will be trained on inhaler techniques and on achieving optimal inhalation therapy.
Inhalers studied in this trial include Turbuhaler, HandiHaler, Respimat and pressure metered dose inhalers.
A limitation of the study is its single-blind design, which would yield bias, although blind evaluation is adopted to minimise bias.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease characterised by irreversible airflow limitation. It is the third leading cause of death and causes heavy socioeconomic burden worldwide.1 In China, COPD is also a serious challenge, with a prevalence of 8.6% among adults and with high mortality.2 Direct medical costs of COPD range from US$72 to US$3565 per capita per year, accounting for 33%–118% of the average annual income of Chinese people.3 As an important event throughout the course of COPD, acute exacerbation (AE) could accelerate decline in spirometry values and directly cause death, and could bring about huge health expenditure.4
Inhalation drugs, such as inhaled corticosteroid (ICS), long-acting β2 agonists (LABA) and long-acting muscarinic antagonists (LAMA), are the core pharmaceutical therapy in the management of stable COPD.5 However, inappropriate usage of inhalers is common in patients with chronic airway diseases, such as insufficient inspiratory force and no breath holding (or holding breath for less than 3 s).6 7 Previous studies showed that errors in usage of inhalers were significantly associated with poor outcomes (such as frequent exacerbations) and increased medical expenditure.8 Commonly used inhalers are classified into four types, with different characteristics: pressure metered dose inhaler (pMDI), dry powder inhalers (DPIs), soft mist inhalers and nebulisers.9 The use of pMDIs is relatively complex, requiring patients to slowly breathe in and ensuring coordination in many other processes to achieve a clinically effective dose. In comparison, the use of DPIs is simple, but requires increased inspiratory force to overcome the device’s internal resistance.10 Several in vitro studies have demonstrated that the efficacy of DPI is dependent on the inspiratory flow rate.
When the patient’s peak inhalation flow rate (PIFR) is less than a certain threshold required by the DPI device (60 L/min measured at no resistance),11 the DPI device releases a reduced dose of the drug and generates aerodynamically large drug particles that are inappropriate to meet therapeutic needs. Moreover, several studies demonstrated that insufficient PIFR in the stable COPD period was associated with poor prognosis when patients improperly used DPI.12 13 It should be noted that expiratory flow parameters are not linearly correlated with inspiratory flow rate, suggesting that postbronchodilator forced expiratory volume in 1 s (FEV1) is not a suitable predictor of PIFR. Other risk factors for early recurrence of acute exacerbation of COPD (AECOPD) include age, Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade, AE frequency in the previous year, pleural effusion, use of accessory respiratory muscles, non-invasive mechanical ventilation, controlled oxygen therapy and length of hospital stay, while inhaled LABA and ICS are protective factors.14
Short-term re-exacerbation is a prominent problem among patients hospitalised for AECOPD, with a 30-day readmission rate of 16%–20%.15 16 Many patients with COPD do not have enough PIFR to reach the threshold that DPI devices required both in the stable period (nearly 20%)17 and in the AE period (based on some small sample studies),13 18 influencing the effects of inhaled drugs on preventing AE recurrences. Moreover, the assessment of patients’ PIFR and their ability to use inhaler devices is not integrated into the clinical pathway of discharge for patients hospitalised for AECOPD. Clinicians are still inclined to choose the type of inhalers that patients used before admission.
We speculate that the PIFR of patients in the AE recovery period does not return to their baseline levels before AE, and untrained patients are more likely to use the inhalers incorrectly. Neglect of evaluation of inhalers might result in treatment failure and early re-exacerbation due to ineffective use of inhaled drugs. However, there is a lack of studies demonstrating whether the choice of inhalers based on the PIFR count could reduce the risk of short-term re-exacerbation in patients with AECOPD. Furthermore, although some research showed that inappropriate use of inhalers was associated with poor prognosis,7 8 it remains unclear whether training patients to correctly use inhalers could reverse poor outcomes.
Therefore, we plan to perform this clinical trial to prospectively determine whether an optimised inhaled drug administration based on PIFR and training with InCheck DIAL could reduce the rate of treatment failure and improve the prognosis of patients hospitalised for AECOPD. Our hypothesis is that AECOPD treatment failure rate is related to improper inhaler selection and lack of education on use of inhalers among patients.
Methods and analysis
Overview
This is a multicentre, single-blind, superiority, randomised clinical trial where patients hospitalised for AECOPD are randomly assigned into two groups at a 1:1 ratio: the PIFR group and the control group. Compared with the control group, the PIFR group will receive additive support before discharge, including PIFR-guided choice of inhaler and education on inhaler use. The primary outcome is 30-day treatment failure rate. Other endpoints include symptoms and quality of life of patients, error rate of inhalation device use, satisfaction with inhalation device, PIFR, 30-day mortality, 90-day mortality, and COPD-related treatment costs.
Patient enrolment, intervention and follow-up are performed at the Zhongshan Hospital of Fudan University, Shanghai Jing’an District Central Hospital, Shanghai Qingpu District Central Hospital and the North Branch of Shanghai Ninth People’s Hospital in China. The study is expected to last for 2 years. Recruitment of participants has started in November 2019.
Inclusion criteria
All patients with a diagnosis of COPD and hospitalisation for AE will be screened. COPD and AE are defined according to the criteria of the Expert Consensus on Acute Exacerbation of Chronic Obstructive Pulmonary Disease in the People’s Republic of China-2014 Edition.4 Briefly, AECOPD is defined as a sudden worsening of respiratory symptoms requiring additional treatment (typical manifestations include dyspnoea, aggravated cough, increased sputum volume and/or sputum purulence) and are not explained by normal day-to-day variations.4 19
Patients will be included if all of the following criteria are met: (1) aged 40–80 years old; (2) deteriorated respiratory symptoms being controlled and meeting the discharge criteria after standard treatment of 5–7 days for AECOPD; (3) a recorded spirometry measured during the stable period, with postbronchodilator FEV1/forced vital capacity <70% and FEV1% predicted value <80%; and (4) signed informed consent form.
Standard treatment during hospitalisation includes atomised or inhaled bronchodilators, broad-spectrum antibiotics and corticosteroids (oral or intravenous glucocorticoids daily equivalent to the 40–50 mg dose of prednisone, or Pulmicort 2 mg atomisation two times per day).
The discharge criteria are as follows: (1) the physician is confident that the patient can manage successfully at home; (2) either LABA and/or LAMA can be used for maintenance with or without ICS, and the frequency of short-acting inhaled β2 receptor agonists is less than every 4 hours; (3) the patient, if previously ambulatory, is able to walk across the room; (4) the patient is able to eat and sleep without frequent awakening due to dyspnoea; (5) the patient achieves clinically stable status lasting for 12–24 hours; and (6) values of arterial blood gases have been stable for 12–24 hours.4
Exclusion criteria
Exclusion criteria include the following: (1) already using home nebulisation therapy due to severe condition; (2) concomitant with asthma, interstitial lung disease, bronchiectasis, pulmonary embolism and other lung diseases; (3) with comorbidities including hypertension, heart diseases, chronic liver and kidney diseases, diabetes, chronic gastrointestinal diseases and malignant tumours, and is critically ill; (4) suffering from cognitive impairment or not cooperating with the study due to poor mental state; and (5) with PIFR less than 20 L/min.
Sample size
We plan to recruit 416 hospitalised patients with AECOPD whose deteriorated symptoms are relieved after 5–7 days of standard therapy. The sample size was calculated using PASS V.15.0 (Power Analysis and Sample Size Software, 2017; NCSS, Kaysville, Utah, USA) to ensure statistical power. Several studies have found that 30%–47% of patients hospitalised for AECOPD had a PIFR <60 L/min prior to discharge.5 10 For the control group, 30-day treatment failure rate after hospitalisation for AECOPD is approximately 20%, according to the literature and our retrospective cohort study.15 16 However, our preliminary research suggests 30-day treatment failure rate is 10% in the PIFR group. Thus, the expected effect size of superiority is around 10% between the two groups. The ratio of the number of people in the two groups is 1: 1. A significant two-sided p value is set at 0.05 and the power is set at 80%. Considering potential dropout risks (5%), 208 patients per group will be recruited, totalling 416 participants.
Study outline
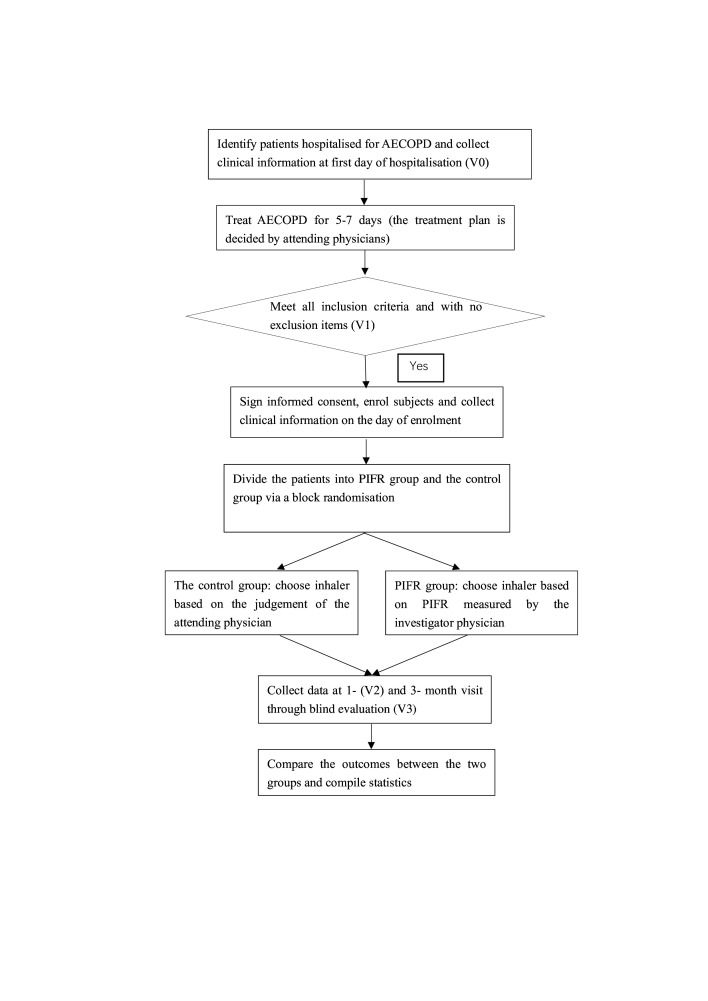
The flow chart of the study design is shown in figure 1.
Figure 1.
Flow chart of the study. Patients’ screening, recruitment, intervention, visits and data processing are described in the figure. Visit 0 (V0), visit 1 (V1), visit 2 (V2) and visit 3 (V3) are timepoints to collect data. AECOPD, acute exacerbation of chronic obstructive disease; PIFR, peak inspiratory flow rate.
The study will recruit 416 patients with AECOPD. After enrolment, the participants will be divided into the PIFR group and the control group at a 1:1 ratio. All participants in the two groups will receive standard treatment for AECOPD as described during the hospitalised period and will be given predesigned medications at discharge. At discharge, all patients will be prescribed with commercial ICS/LABA combination, including either Symbicort Turbuhaler (budesonide/formoterol, 160/4.5 µg two times per day, AstraZeneca) or Foster pressure pMDI (beclomethasone/formoterol, 100/6 µg two puffs two times per day, Chiesi Farmaceutici SpA). For patients with more respiratory symptoms during the stable period (modified Medical Research Council (mMRC) ≥2 and COPD Assessment Test (CAT) ≥10), Spiriva HandiHaler (18 µg once a day) or Spiriva Respimat (2.5 µg twice a day, Boehringer Ingelheim Pharma & Co KG) will be given in combination with ICS/LABA.
As for inhalers, participants in the control group will be given DPI or pMDI with a spacer based on judgement of the attending physician, while participants in the PIFR group will receive education on use of inhaler and additive support for evaluation of PIFR at the time of discharge. Attending physicians will show the proper use of inhalers and correct some common mistakes to patients in the PIFR group.
For the PIFR group, PIFR is measured by InCheck DIAL (Clement Clarke International, Harlow, UK and Alliance Tech Medical), which is designed to measure inspiratory flow and simulate the ‘internal resistance’ of common inhalers. The numerical values of PIFR provide reference for the attending physician to guide patients in improving their inspiratory techniques, such as increasing or decreasing inspiration forces, which is helpful to achieve a flow rate consistent with clinical efficacy. The coloured ‘flow’ icons show the clinically effective flow ranges for each inhaler device. The InCheck DIAL is accurate up to ±10% or 10 L/min and is a low-range inspiratory flow metre (15–120 L/min) with options for resistance ranging from high to low, shown by the coloured ‘flow’ icons calibrated to enable the measurement of airflow when the patient is using a different inhaler. When measuring PIFR, we will set the resistance of the InCheck DIAL to ‘Zero’ and to ‘Med High’ in line with pMDI and Turbuhaler, respectively. Before the measurement, patients will be trained on how to use the InCheck DIAL correctly. When patients’ PIFR steadily reached the maximum value, the PIFR will be measured three times, the average of which will be considered the final result. If PIFR is less than 60 L/min (measured without a resistance), the patients will be given the pMDI with spacer. Otherwise, they will be prescribed with the DPI. Patients using either pMDI or DPI will be taught on how to use the device on the spot and can access an educational video via a WeChat public account at any time.
Moreover, the InCheck DIAL is also a training device for inhalation muscles and helps to improve patients’ ability to use inhalers. When used for training of inspiratory muscles, the resistance threshold of InCheck DIAL is set according to the type of inhalers used by the patient.
Study steps
Researchers collect baseline information from participants on the day of enrolment and provide interventions to patients with COPD recovering from AE at the time of discharge. After discharge, all participants will be followed up for 3 months and will be asked for two separate visits at 1 and 3 months.
Table 1 shows the data that need to be collected at each visit. Baseline information in the stable period includes demographics, clinical characteristics, evaluation of respiratory symptoms and quality of life at stable phase, PIFR, chest imaging (X-ray or CT), and echocardiography or ECG at stable phase. Demographics include age, gender, age, height, weight, ethnicity, occupation (number of years of work), marital status, family address and so on. Clinical characteristics include disease history, history of drug sensitivity, history of vaccination, family disease history, current medical status, comorbidities, medications and so on. Respiratory symptoms are assessed by the mMRC Dyspnea Scale and CAT, while quality of life is evaluated by the St George’s Respiratory Questionnaire (SGRQ). PIFR and routine laboratory tests for AE patients (eg, blood routine, C reactive protein, liver and kidney function, blood electrolytes, B-type natriuretic peptide, D-dimer) were also recorded.
Table 1.
Data collected at each visit
| Visit 0 | Visit 1 | Visit 2 | Visit 3 | |
| Hospitalisation ±1 day | At the time of discharge (meet discharge standards) | 1 month after discharge | 3 months after discharge | |
| Basic information | √ | |||
| Information on COPD at stable phase | √ | |||
| Blood routine | √ | √ | ||
| Liver and kidney function | √ | √ | ||
| Electrolytes | √ | √ | ||
| C reactive protein | √ | √ | ||
| Procalcitonin | √ | √ | ||
| Brain natriuretic peptide | √ | √ | ||
| D-dimer, fibrinogen | √ | √ | ||
| Cardiac troponin T | √ | √ | ||
| CAT score | √ | √ | √ | √ |
| mMRC score | √ | √ | √ | √ |
| SGRQ score | √ | √ | √ | |
| Drug for COPD | Stable phase | AE phase | Stable phase | Stable phase |
| PIFR | √ | √ | √ | |
| Prognosis | √ | √ | ||
| Pulmonary function | √ | √ | √ | |
| Echocardiography at stable phase | ||||
| CT at stable phase | ||||
| CT at AE phase | ||||
| Error rate of inhaler use | √ | √ | ||
| Satisfaction with the inhaler | √ | √ | ||
| Daily cost of COPD-related treatment | √ | √ | ||
AE, acute exacerbation; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council Dyspnea Scale; PIFR, peak inhalation flow rate; SGRQ, St George’s Respiratory Questionnaire.
At 1 and 3 months, patients are required to have an outpatient department visit for assessment of the effects of intervention and to collect some data including CAT score, mMRC score, SGRQ score, PIFR, spirometry, error rate of inhalation device use, satisfaction with inhalation devices and COPD-related medications. To reinforce adherence, patients and their relatives will be contacted in advance by telephone to confirm the dates of evaluation. If it is inconvenient for the patient to come to the hospital, researchers will collect the abovementioned information as much as possible via phone.
Outcomes
The primary endpoint is 30-day treatment failure rate of AECOPD. Treatment failure means AECOPD recurrence resulting in an emergency visit, admission or need for intensified medication.
Secondary outcomes include PIFR, error rate of inhalation device use, satisfaction with inhalation devices, 30-day mortality, 90-day mortality, symptoms and quality of life of patients, and COPD-related treatment costs.
Patients’ satisfaction with inhalation devices will be assessed by the Feeling of Satisfaction with Inhaler (FSI-10) questionnaire. The FSI-10 questionnaire is supposed to be completed by the patients themselves, and has been widely applied to assess patients’ opinions about inhalers in terms of ease of use, portability and usability.20 The symptoms of patients are evaluated by CAT and mMRC, while quality of life is evaluated by SGRQ.
PIFR is measured by InCheck DIAL (Clement Clarke International and Alliance Tech Medical) under the guidance of respiratory physicians. Some common errors in the usage of different inhalation devices are described in table 2.6 7
Table 2.
Common errors in the usage of different inhalation devices
| Turbuhaler | HandiHaler/Accuhaler | pMDI | Respimat |
| Cover is not removed or not covered properly. | Cover is not removed or not covered properly. | Cover is not removed or not covered properly. | Cover is not removed or not covered properly. |
| Dose is reduced due to patients shaking or tilting the device during preparation. | – | The device is not installed correctly before use. | |
| Device is not held upright. | – | Device is not held upright. | – |
| Patient does not twist grip at the base or twist around and then back until click is heard. | – | – | Patient does not turn the device towards the arrow in the label until it clicks. |
| Inhalation force is insufficient. | Inhalation force is insufficient. | Patient does not inhale deeply and slowly. | Patient does not inhale deeply and slowly. |
| Patient does not tilt his/her head to make his/her chin slightly upturned. | Patient does not hold his/her head in a vertical position. | Patient does not tilt his/her head to make his/her chin slightly upturned. | Patient does not point the inhaler towards the back of throat. |
| Patient does not exhale to empty the lung before the next inhalation. | Patient does not exhale to empty the lung before the next inhalation. | Patient does not exhale to empty the lung before the next inhalation. | Patient does not exhale to empty the lung before the next inhalation. |
| – | Patient does not turn his/her head away from the device’s mouthpiece before exhalation. | Patient exhales into the device before the next inhalation. | Patient covers the air entries while inhaling. |
| Patient does not seal the mouthpiece with his/her lips. | Patient did not place the mouthpiece in his/her mouth nor closed his/her lips. | Patient does not seal the mouthpiece with his/her lips. | Patient does not seal the mouthpiece with his/her lips. |
| NA | NA | Patient does not inhale them in sync with the drug releasing. | Patient does not inhale them in sync with the drug releasing. |
| Patient does not hold breath (or hold breath less than 3 s). | Patient does not hold breath (or hold breath less than 3 s). | Patient does not hold breath (or hold breath less than 3 s). | Patient does not hold breath (or hold breath less than 10 s). |
| Patient does not cover the lid and wait for 30–60 s for the second dose. | Patient does not dispose of the capsule and cover the lid on the device. | Patient does not exhale and wait for 30–60 s before the second puff. | Patient does not inhale twice to achieve the total daily dosage. |
NA, not applicable; pMDI, pressure metered dose inhaler.
Randomisation and blinding
The participants will be assigned into two groups at a 1:1 ratio using a random number table generated by IBM SPSS Statistics V.23. Randomisation was performed by an independent researcher who will not participate in other research procedures. To maximise the objectivity and reliability of our study, single-blind and allocation concealment are adopted. Sealed envelopes containing an allocation number are distributed to attending physicians in advance to achieve allocation concealment, and they will not know the allocation group until giving intervention. Other researchers responsible for data collection and follow-up are not informed of which group the patient has been assigned to. Patients and their relatives are blinded to allocation during the whole process, and are only informed that they are participating in a study of COPD discharge plan. In addition, statistical analysis will be performed by an independent statistician, who will also be blinded to the group labels.
Statistical analysis
Statistical analysis data sets
Modified intent-to-treat set: subjects who have undergone randomisation and interventions, and carry out primary endpoint evaluation.
Safety set: subjects who are randomised, undergo the intervention and with safety evaluation data.
Statistical analysis methods
Statistical analyses will be performed using IBM SPSS Statistics V.22. Continuous variables are described as mean (SD) or median (IQR), while categorical variables are described as frequency and percentage. A two-tailed p value <0.05 is considered statistically significant. Student’s t-tests or Mann-Whitney test, depending on normality and homogeneity of variance, was used to compare continuous variables between the two groups, including PIFR, CAT score, mMRC score, SGRQ score and COPD-related treatment costs. For discrete variables, such as 30-day treatment failure rate, error rate of inhalation device use, satisfaction with inhalation devices, 30-day mortality and 90-day mortality, χ2 test, Fisher’s exact test or Cochran-Mantel-Haensel (CMH) χ2 test will be applied for comparison. To rule out the influence of confounding factors and identify optimal subpopulation, subgroup analysis will be performed based on exacerbation history and GOLD grade.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination of our research. The results will be available to the public if necessary.
Ethics and dissemination
This trial has been approved by the Ethics Committee of Zhongshan Hospital of Fudan University (B2019-142). In this study, diagnosis and treatment will be performed in accordance with the routine management of COPD. Neither additional drug intervention nor invasive examination and charges will be needed. Therefore, the study is relatively safe with minimal additional risks. Participants will be screened and recruited from hospitalised patients by physicians, with no public advertisement for recruitment. All participants are supposed to sign an informed consent. A blank copy of the original consent form is provided and shown as an online supplementary document. All information from the participants will be kept private and will not be provided to any company or institution. The results will be disseminated through peer-reviewed journals and conference presentations.
bmjopen-2019-034804supp001.pdf (132.3KB, pdf)
Discussion
DPI drug delivery depends on the inherent resistance of the inhaler and the PIFR of the patient. PIFR value is determined by an individual’s subjective effort as well as his/her respiratory muscle force, which may be decreased in patients with COPD due to airway stenosis, lung hyperinflation, hypoxaemia and muscle wasting. As a breath-actuated inhaler, DPI requires patients to create enough turbulent forces to disaggregate the powder into respirable particles which can reach the lower respiratory tract. Patients with a relatively high PIFR (>60 L/min) enable DPIs to release a sufficient amount of powder and disaggregate the drug to achieve sufficient drug deposition in the lung.
Sharma and colleagues10 reported that 31.7% of hospitalised patients for AECOPD had PIFR less than 60 L/min at the time of discharge. Patients with a PIFR less than 60 L/min have been considered not able to effectively inhale medications using a DPI into their lower respiratory tracts, while a PIFR less than 30 L/min was insufficient.21 22 For lack of availability of long-acting bronchodilators with pMDI, most Chinese clinicians routinely prescribe DPIs to patients recovering from AECOPD without measuring their PIFR values. Inappropriate inhaler selection may result in AECOPD treatment failure. To our knowledge, it remains unclear whether treatment failure rate is negatively related to improper inhaler description. A suitable inspiratory flow rate helps to improve treatment efficacy. In addition to appropriate inhaler selection, participants in the PIFR group also receive inhaler training to help them master the proper way of inhalation.
Our research proposed to measure the PIFR of patients recovering from AECOPD using InCheck DIAL to guide the choice of inhalers and training on inhalation techniques. The aim of this study is to determine whether treatment failure rate of patients just recovering from AECOPD could be reduced by the optimised inhalation therapy based on PIFR, which is measured against the simulated airway resistance. We anticipate that the positive results of this study will provide evidence for improving discharge protocols for AECOPD by including PIFR evaluation.
Supplementary Material
Footnotes
Contributors: JH and JZ planned the study. WZ planned the statistical analysis methods. All authors contributed to the design and development of the trial (JH, JZ, WZ, H-FC, C-LD, J-YM and Y-HZ). JH drafted the manuscript. JZ, H-FC, C-LD, J-YM and Y-HZ contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Not required.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 3.Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:1353–64. 10.2147/COPD.S161555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai B-qiang, Cai S-xi, Chen R-chang, et al. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the people's Republic of China. Int J Chron Obstruct Pulmon Dis 2014;9:381–95. 10.2147/COPD.S58454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2017;14:1103–7. 10.1513/AnnalsATS.201702-156PS [DOI] [PubMed] [Google Scholar]
- 6.Cho-Reyes S, Celli BR, Dembek C, et al. Inhalation technique errors with metered-dose inhalers among patients with obstructive lung diseases: a systematic review and meta-analysis of U.S. studies. Chronic Obstr Pulm Dis 2019;6:267–80. 10.15326/jcopdf.6.3.2018.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract 2017;5:1071–81. 10.1016/j.jaip.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res 2018;19:10. 10.1186/s12931-017-0710-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Campos JL, Soler-Cataluña JJ, Miravitlles M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: future challenges. Arch Bronconeumol 2020;56:65-67. 10.1016/j.arbres.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis 2017;4:217–24. 10.15326/jcopdf.4.3.2017.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross DL, Schultz RK. Effect of inhalation flow rate on the dosing characteristics of dry powder inhaler (DPI) and metered dose inhaler (MDI) products. J Aerosol Med 1996;9:215–26. 10.1089/jam.1996.9.215 [DOI] [PubMed] [Google Scholar]
- 12.Al-Showair RAM, Tarsin WY, Assi KH, et al. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med 2007;101:2395–401. 10.1016/j.rmed.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Mahler DA, Waterman LA, Ward J, et al. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J Aerosol Med Pulm Drug Deliv 2014;27:103–9. 10.1089/jamp.2013.1038 [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Peng S-H, Zhang J, et al. Prediction of short term re-exacerbation in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:1265–73. 10.2147/COPD.S83378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu W-P, Lhamo T, Liu D, et al. Development of a nomogram to predict the risk of 30-day Re-Exacerbation for patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease. COPD 2019;16:160–7. 10.1080/15412555.2019.1606187 [DOI] [PubMed] [Google Scholar]
- 16.Loh CH, Peters SP, Lovings TM, et al. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc 2017;14:1305–11. 10.1513/AnnalsATS.201611-903OC [DOI] [PubMed] [Google Scholar]
- 17.Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv 2013;26:174–9. 10.1089/jamp.2012.0987 [DOI] [PubMed] [Google Scholar]
- 18.Broeders MEAC, Molema J, Hop WCJ, et al. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med 2004;98:1173–9. 10.1016/j.rmed.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J 2018;52:1801261 10.1183/13993003.01261-2018 [DOI] [PubMed] [Google Scholar]
- 20.Perpiñá Tordera M, Viejo JL, Sanchis J, et al. [Assessment of patient satisfaction and preferences with inhalers in asthma with the FSI-10 Questionnaire]. Arch Bronconeumol 2008;44:346–52. 10.1016/S1579-2129(08)60060-9 [DOI] [PubMed] [Google Scholar]
- 21.Broeders MEAC, Molema J, Vermue NA, et al. In check dial: accuracy for Diskus and Turbuhaler. Int J Pharm 2003;252:275–80. 10.1016/S0378-5173(02)00650-6 [DOI] [PubMed] [Google Scholar]
- 22.Atkins PJ. Dry powder inhalers: an overview. Respir Care 2005;50:1304–12. discussion 12. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034804supp001.pdf (132.3KB, pdf)