Abstract
The old-for-old allocation policy used for kidney transplantation (KT) has confirmed the survival benefit compared to remaining listed on dialysis. Shortage of standard donors has stimulated the development of strategies aimed to expand acceptance criteria, particularly of kidneys from elderly donors. We have systematically reviewed the literature on those different strategies. In addition to the review of outcomes of expanded criteria donor or advanced age kidneys, we assessed the value of the Kidney Donor Profile Index policy, preimplantation biopsy, dual KT, machine perfusion and special immunosuppressive protocols. Survival and functional outcomes achieved with expanded criteria donor, high Kidney Donor Profile Index or advanced age kidneys are poorer than those with standard ones. Outcomes using advanced age brain-dead or cardiac-dead donor kidneys are similar. Preimplantation biopsies and related scores have been useful to predict function, but their applicability to transplant or refuse a kidney graft has probably been overestimated. Machine perfusion techniques have decreased delayed graft function and could improve graft survival. Investing 2 kidneys in 1 recipient does not make sense when a single KT would be enough, particularly in elderly recipients. Tailored immunosuppression when transplanting an old kidney may be useful, but no formal trials are available.
Old donors constitute an enormous source of useful kidneys, but their retrieval in many countries is infrequent. The assumption of limited but precious functional expectancy for an old kidney and substantial reduction of discard rates should be generalized to mitigate these limitations.
The age of patients listed for kidney transplantation (KT) has raised due to the increased age of incident dialysis patients and their improved survival rates.1–3 In parallel, donor age has also increased in many countries,4,5 but not significantly in the United States (US).6,7 Historically, organs from old donors have been optimized in Spain.8,9 Particularly, age limits have been expanding, so that age itself is not usually a significant limiting parameter. In contrast, although candidates aged 65 years or older make up an increasing proportion of the waiting list in the United States,6 more than half of available kidneys from donors 65 years or older are discarded in this country,6 despite their argued benefits.10–12
The increase in donor age is associated with reduced graft function and decreased recipient and graft survival.11,13–15 To minimize this impact, age matching criteria between donor and recipient has been adopted, reasoning that elderly recipients have shorter life expectancy independently of the extended lifetime provided by the graft.16,17 The use of advanced age kidneys is beneficial for dialysis patients and provide extended survival over remaining listed.11,18,19 Consequently, given the increasing time in the waiting list and the mortality rates during this period, the use of kidneys from older donors should be encouraged.
We have reviewed the available literature on the use of kidneys from advanced age donors, their outcomes, and the potential strategies to expand their use. In particular, we tried to critically assess what is missing in the field by synthesizing and analyzing the material available.
MATERIALS AND METHODS
Literature Search
Relevant studies were obtained from a systematic literature search. Our start point was the systematic review performed in 2007.14 The literature search included MEDLINE and EMBASE (2007 to March 2016) within OVID system using the following terms:
Kidney Transplantation/.
(expand$ or extend$ or old$ or elderly or suboptimal or marginal or KDPI) adj25 (don$).tw.
1 and 2.
The reports’ selection was initially focused on retrieving all information about outcomes of kidneys from donors 60 years or older. The search strategy was used to obtain titles and abstracts of studies that may have been relevant to the review. Titles and abstracts were screened independently by 2 reviewers who discarded studies that were not applicable. The same reviewers assessed retrieved abstracts and, if necessary, the full text, to determine which studies satisfied the inclusion criteria. Data extraction was carried out by the 5 reviewers for each of the review sections. Special attention was given to the studies including a comparison between old and younger kidneys. Data on donor and recipient demographics, delayed graft function (DGF), graft function, acute rejection, and patient and kidney graft survival were of particular interest.
In the previously published review, a total of 177 reports were reviewed to extract information.14 They included observational reports of patients’ descriptions and outcomes using expanded criteria donors (ECD) (n = 95), or donors after cardiac death (DCD)-ECD (n = 6), value of donor kidney biopsy (n = 16), pulsatile perfusion (n = 3), dual KT (n = 22), and immunosuppression strategies (n = 18).
In the new search we found 1366 reports, and 1159 were discarded (not related to the topic [n = 957], narrative reviews or editorials [n = 58], observational descriptions of patients and outcomes using ECDs or advanced age donors reporting <100 recipients [n = 24], old living donors [n = 40], multiorgan or pediatric transplantation [n = 29], animal studies [n = 23], duplicates [n = 12], or already in the previous review [n = 16]). Reference lists of clinical practice guidelines, review articles, and relevant studies were also surveyed, and some of their references (n = 8) were used. Finally, the number of reports for full review was 215. They were grouped in outcomes of ECD kidneys (n = 49), Kidney Donor Profile Index (KDPI) policy (n = 4), outcomes of advanced age kidneys (n = 36), value of preimplantation biopsy (n = 32), dual KT (n = 27), impact of recipient age (n = 33), machine perfusion (n = 12) and immunosuppressive strategies (n = 22).
Measures of Effect
A global relative risk analysis summarizing the true effect of the different variables on the outcomes has been done when data could have been obtained from the reports. Statistical analyses were performed using Review Manager version 5.2.
For dichotomous outcomes (mortality, graft failure, and DGF), results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Mean difference was used where continuous scales of measurement were applied to assess the effects of the variables.
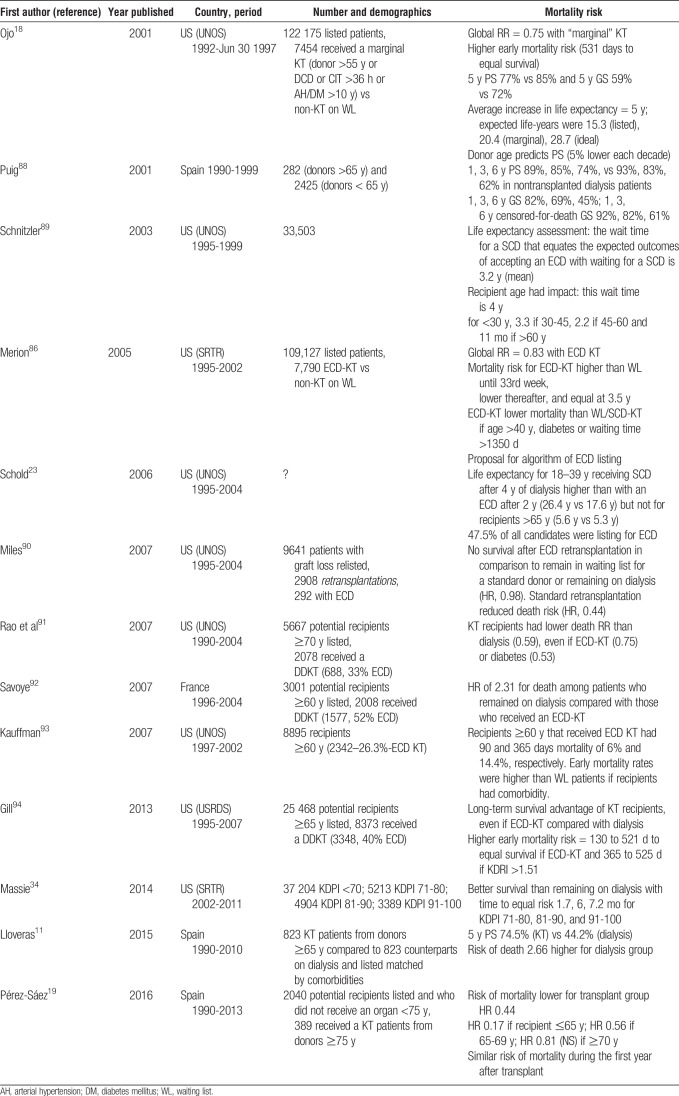
EXPANSION OF KIDNEY DONOR POOL, GENERAL CRITERIA TO USE OLD KIDNEYS, AND ALLOCATION STRATEGIES
ECD Allocation Policy
In 2002, the Organ Procurement Transplant Network (OPTN)/United Network for Organ Sharing (UNOS) adopted the ECD allocation policy, establishing an ECD definition based on age and 3 significant risk factors determined by a Scientific Registry for Transplant Research (SRTR) analysis: arterial hypertension history, serum creatinine (SCr) >1.5 mg/dL, or cause of death from cerebrovascular accident.20,21 ECDs were defined as any donor 60 years or older or 50 to 59 years with at least 2 of the cited risk factors. Each criteria was defined by a relative risk of graft failure that exceeded a relative risk of graft loss of 1.7 compared with a reference group of “ideal donors” aged 10 to 39 years, without hypertension, who did not die of cerebrovascular accident, and with a predonation SCr less than 1.5 mg/dL.20 During the following decade, this ECD program was evaluated in several studies, reporting an increase in the total number of kidneys procured and a marked variation in different US areas regarding the proportion of candidates listed for an ECD kidney and those who finally got an ECD kidney22–24 (Table 1). ECD-KT was increasing, however, the significant discard rates for ECD kidneys did not significantly change, with 40% of all ECD recovered kidneys discarded in 2005. This rate has probably been unnecessarily high. The long-term outcome of 170 kidneys refused by at least 2 US centers and subsequently transplanted were compared with 170 KT using kidneys initially accepted.22 Higher DGF rate, higher primary nonfunction rate, and lower creatinine clearance at 5 years in “marginal” kidneys were noted. However, 5-year patient survival and graft survival were not significantly different, justifying the use of this type of kidneys.30
TABLE 1.
Different kidney allocation policies during the last 20 years
KDPI to Guide Allocation
Recently, the Kidney Donor Risk Index (KDRI) and KDPI were introduced as a refined version of the ECD score.25 The KDRI is based on 10 donor factors associated with graft survival and estimates the relative risk of posttransplant kidney graft failure from a particular deceased donor compared with the median donor (values, 0.5-3.5). Based on the KDRI, the KDPI establishes the quality of the donor kidneys related to the other kidneys transplanted during the previous year (in percentage).25,31 The KDPI has also been made part of the “longevity matching” allocation in the United Sates, where the best kidneys are allocated to the recipients with the longest predicted posttransplant survival.32 This index highlights the fact that there is a large variability in the ECDs, with some standard criteria donors (SCD) having lower estimated quality (higher KDRI) than some ECDs. In fact, in each KDRI interval, survival is not significantly different between ECD and SCD, supporting the conclusion that ECD categorization does not alter graft survival above what is already predicted by the KDRI.33 Despite the KDRI has been related to poorer graft survival,26 patients transplanted from donors with the highest KDPI have better survival than their dialysis counterparts.34
Eurotransplant Senior Program
The Eurotransplant Senior Program (ESP) is a donor-to-recipient age matching policy developed in central Europe in 1999.27 The 5-year data showed no difference between patients who received grafts from elderly donors via ESP and those who received younger kidneys via the usual HLA-driven allocation. ESP data suggest that if care is taken to avoid the accumulation of additional risk factors such as long cold ischemic time and previous sensitization, old-for-old allocation can be operated successfully.27–29,35
All the reviewed allocation strategies with expanded kidney donor pools are summarized in Table 1.22–29,35 The outcomes of end-of-life care, critical care access (for donors), survival on dialysis, and transplant outcomes vary hugely from country to country. As a result, it is exceedingly difficult to compare what strategy to adopt for “marginal donor organs” by comparing the results of 1 country to another.
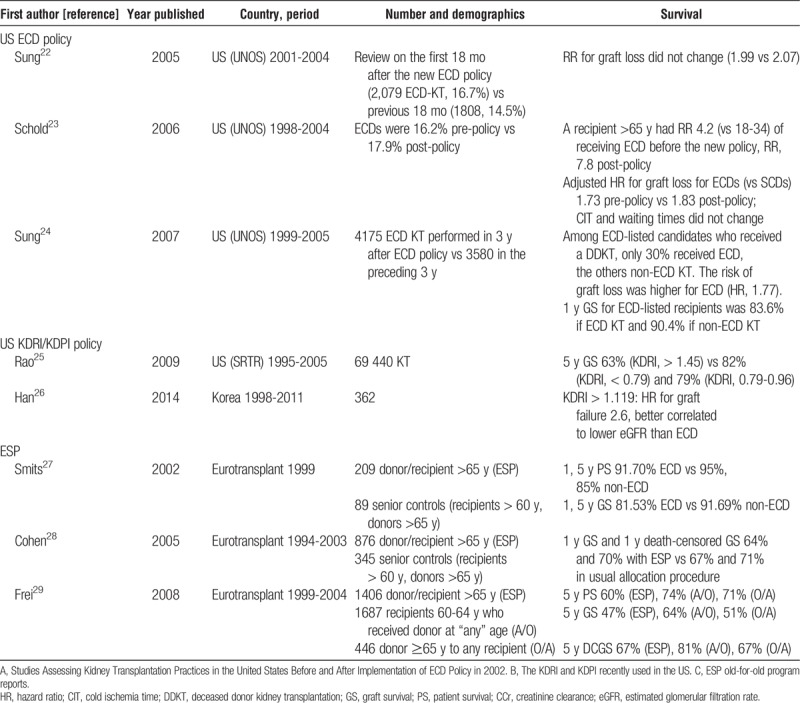
DONORS AFTER CARDIAC DEATH WITH EXPANDED CRITERIA
The particular group of ECD-DCD constitutes an increasing source of kidneys suitable for transplantation in many countries. They represented 14% of DCD in 2004 in the United Kingdom and increased to 43% in 2013.36 In Spain, controlled DCD constitute the most increasing donor modality.4 However, recent data show that around 50% of ECD-DCD kidneys in the United States are discarded compared with 30% to 40% of brain-dead ECD. Additionally, there is a significant overlap in KDRI scores among ECD-DCD kidneys that are discarded versus those used. This suggests that there may be a significant number of discarded ECD-DCD kidneys that could be acceptable for transplantation.37 Some reports have analyzed outcomes in Japan,38 the United States,39–41 and the United Kingdom42 (Table 2). In Japanese reports, as it occurs with brain-dead ECD, kidney grafts from ECD-DCD show inferior survival than those from standard DCD.38 However, the US Registry has pointed out that DGF, primary nonfunction and graft survival rates are not different between DCD-ECD and DCD-non-ECD when adjusted with multivariate analyses.40,41 The UK experience remarks a double risk of graft loss among ECD-DCD transplants compare to those younger than 40 years in the multivariate analyses, but similar graft survival than brain-dead ECD.42 An update of the UK Registry shows similar rates of primary nonfunction, 5-year estimated glomerular filtration rate and 5-year graft survival between ECD-DCD and brain-dead ECD KT.36 The report of graft losses and survival allowed to calculate RRs for 1- and 5-year graft loss38,41,42 (Figure 1). The events pooled are raw unadjusted ones. The RR for graft loss is higher with ECD-DCD than with non–ECD-DCD at 1 year (RR, 1.60 [1.28-1.99], P < 0.0001) and 5 years (RR, 1.62 [1.22-2.16], P = 0.0009).
TABLE 2.
Reports describing outcomes in kidney transplantation using organs from ECD after cardiac death (DCD)
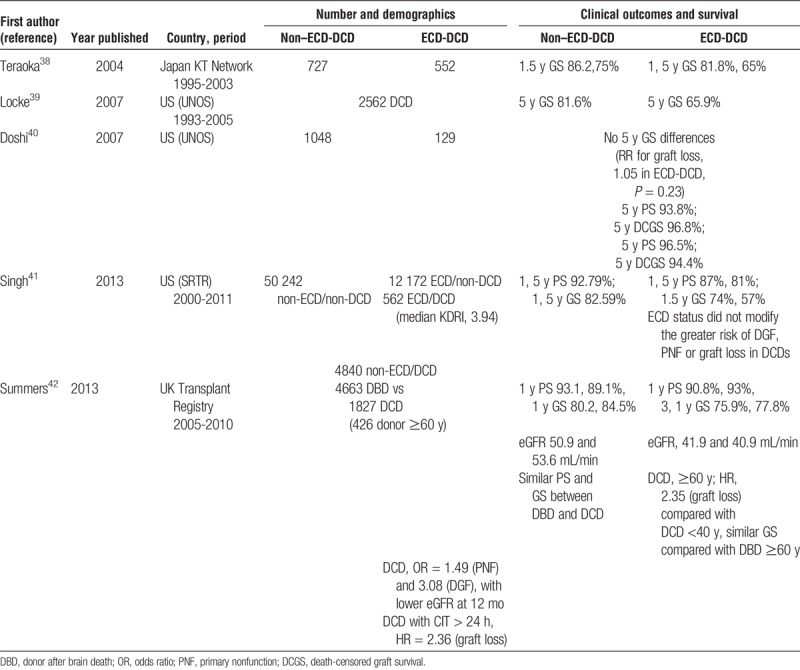
FIGURE 1.

Higher risk of graft loss using organs from ECD vs non-ECD after DCD.
Consequently, graft survival is lower using ECD-DCD than using non-ECD-DCD, but still reasonable to stimulate the use of this donor source. An effort should be made in selecting donors with enough kidney function potential, but based on the evidence available, selection criteria for DCD donor kidneys should not be different to those applied to brain-dead donor kidneys.
OUTCOMES: WORSE PATIENT AND GRAFT SURVIVAL WITH ECD KT OR ADVANCED AGE DONORS
ECD Versus SCD
Some observational studies have suggested that patient and graft survival achieved by using ECD kidneys are similar to those obtained with SCD (Table S1, SDC, http://links.lww.com/TP/B387)[43–49]. However, the majority of 1-center studies,43–56 and all available multicenter or registry reports20,57–74 show significantly worse graft survival for ECD kidneys, with an increased risk of graft failure (Tables S2 and S3, SDC, http://links.lww.com/TP/B387). The differences in outcomes regarding patient survival and death-censored graft survival after KT using ECD versus SCD are more variable. Great difficulties emerge when ECD KT outcomes are analyzed because all aspects in this area tend to be multifactorial and subject to great variability. The 1-center analyses are mainly European, and the multicenter reports mostly come from the consecutive publications from the UNOS registry. Graft survival is consistently decreased but patient survival and death-censored graft survival using ECD kidneys are not always worse, especially among older recipients.63,65,67,73 Other important outcomes using kidneys from ECD have been analyzed. In general, higher rate of DGF,64,73 primary nonfunction,60,64 and acute rejection have been described. Furthermore, worse kidney graft function has been the rule.66,71,73,75,76
Very Advanced Age
A few studies with the intention of stepping forward in the expansion of kidney donor pool have emerged from Europe in the last years reporting similar results with kidneys from very advanced aged donors (>70 or >75 years) than from traditional ECD kidneys (Table 3).19,63,77–84 Some of these reports contain numerical data that allowed us to calculate RRs for DGF,19,63,78,80,81,83 graft loss at 1 year19,63,77–82 and 5 years19,63,77,79,81,82 and mortality at 1 year19,63,77–81,83 and 5 years19,63,77,79–81 (Figure 2). DGF rates were similar in patients receiving a kidney from a very advanced age donor than in those receiving a kidney from a standard ECD (RR, 1.05 [0.92-1.21], P = 0.47). Graft loss was more frequent using a kidney from a very advanced age donor than a usual ECD one at 1 year (RR, 1.55 [1.12-2.15], P = 0.008) and 5 years (RR, 1.38 [1.04-1.84], P = 0.03). Mortality was also higher at 1 year (RR, 2.43 [2.07-2.86], P < 0.00001] but only marginally different at 5 years (RR, 1.41 [0.95-2.08], P = 0.08) (Figure 2). All these pooled analyses are performed including raw data, unadjusted by confounding factors, or multivariate analyses.
TABLE 3.
Studies showing outcomes in kidney transplantation using kidneys from very advanced age compared with traditional ECD or standard donors
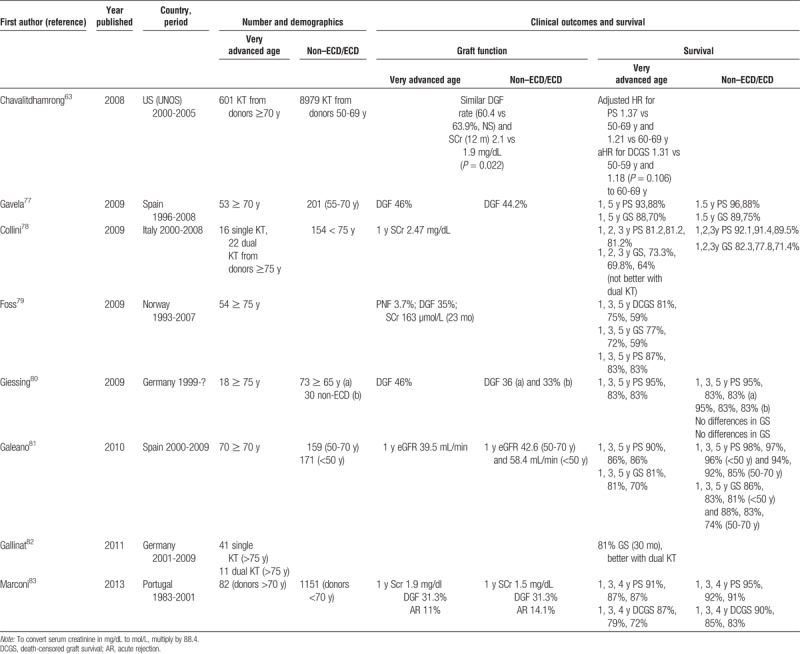
FIGURE 2.
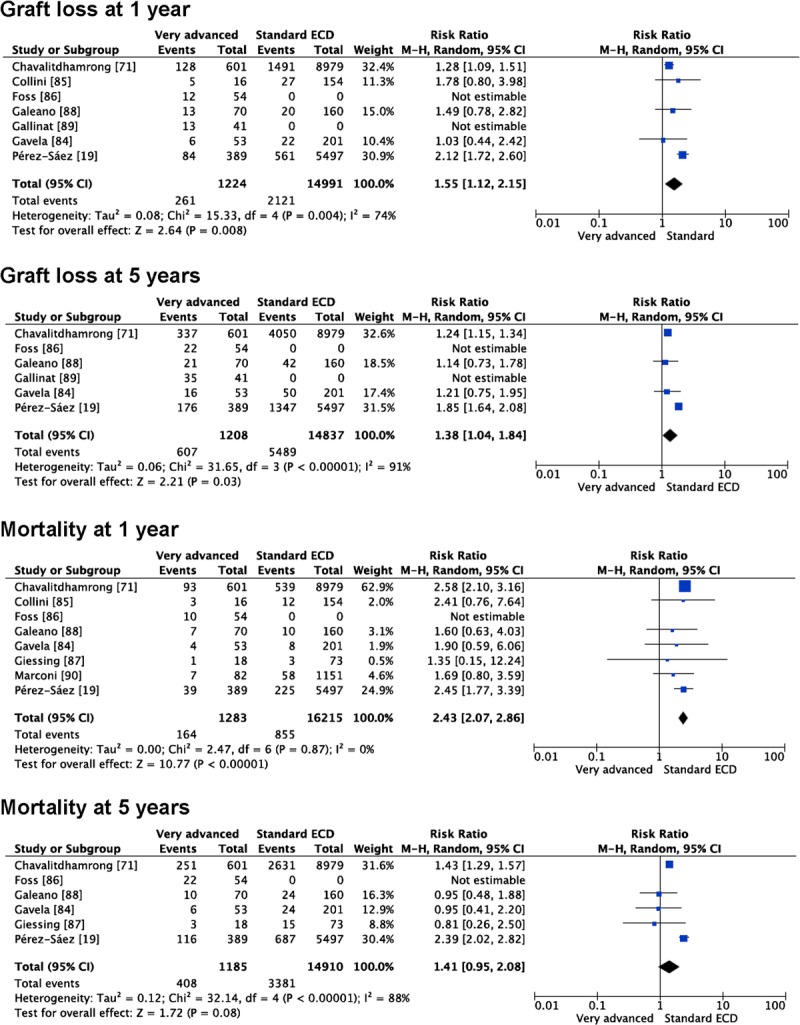
Outcomes using kidneys from very advanced age compared with classical ECDs.
ECD Versus KDPI
The OPTN/SRTR 2013 report76 is the last one published that depicted the deceased donor waiting list and waiting times under the previous US allocation system based on the classical deceased donor categories: SCD, ECD and DCD. Recently, Grams et al85 described that ECD listing by Merion’s recommendation86 is about 50% in the United States, despite the increasing evidence of improved survival in certain dialysis populations when an ECD donor is used compared with remaining on dialysis. Using the classical system, 3-year graft survival for ECD kidneys is 75%, and 5-year graft survival is 64%. Using the newest KDPI cuts, 3-year graft survival for KDPI greater than 85% kidneys is 72% and 5-year graft survival 58%.76
Increasing cold ischemia time is a risk factor for DGF among ECD KT, but DGF does not have a significant effect on graft survival: it is likely that many ECD kidneys not considered viable may be useful.87 In addition, donor/recipient size matching is important to optimize results using ECD kidneys.75
OUTCOMES: BETTER SURVIVAL AFTER KT WITH ECD OR ADVANCED AGE DONOR KIDNEYS THAN WAITLISTED AND ON DIALYSIS
Given the worse results with an ECD kidney than with an SCD, it is important to clarify if there is better patient survival using ECD kidneys compared with remaining on the waiting list on dialysis (Table 4).11,18,19,23,34,86,88–94 This is difficult to assess as the comparison between both populations implies unbridgeable biases. Ojo et al18 demonstrated that the average increase in life expectancy for recipients of “marginal” kidneys (defined then as those procured from old donors, with comorbidities, such as hypertension or diabetes or with prolonged cold ischemia time) compared with the waiting list nontransplanted dialysis cohort was 5 years, although there was an increase in the early mortality risk after transplant. Soon after this publication, the ECD definition was adopted trying to avoid the term “marginal” and to standardize this type of kidney. Years later, Merion et al86 studied survival benefit of KT using ECD compared with remaining on the waiting list or getting transplanted with an SCD. Due to excess mortality in the perioperative period, the ECD recipient survival did not equal the survival observed with SCD or remaining on the waiting list until 3.5 years after KT, in terms of cumulative mortality. In other words, according to data published more than a decade ago, it took 3.5 years to justify an ECD KT in terms of survival when this practice was compared with waiting until an SCD was available. The subgroups that showed significant ECD survival benefit included patients older than 40 years, non-Hispanics, unsensitized, recipients with hypertension, and diabetics, particularly in those programs with long (>4 years) waiting times.86 The long-time waiting for an SCD KT is a risk factor for patient mortality.89
TABLE 4.
Main studies assessing the benefit on survival of kidney transplantation over dialysis using ECDs or donors with advanced age
Albeit the benefits are clear for certain patient populations,23,91,92 patient survival is limited when an ECD KT is performed in high-risk recipients, such as retransplantation.90 Patients 60 years or older with associated comorbidities have particularly suboptimal survival results when receiving an ECD KT compared with SCD KT.93 Another study found similar results and high-risk recipients that receive an ECD KT, achieved equal survival at 521 days after transplant.94 The results about higher early mortality with an ECD transplant versus dialysis are consistent in the literature ranging the period to equal survival from 1.7 months to more than 1 year.18,34,88
In an attempt to minimize confounding factors in a comparison between patients listed who remained on dialysis and those who are transplanted, our group performed a paired-matched analysis between 823 recipients from donors over 65 years and counterparts listed with the same comorbidity. The risk for death was 2.66-fold higher in the dialysis group.11 Consequently, ECD-KT shows survival advantage over dialysis in the elderly, although undoubtedly SCD offers better survival. In a further analysis, a cohort of 389 KT recipients from donors 75 years or older was analyzed and compared with those who remained listed on dialysis. Even using these extreme aged kidneys, the benefit in survival over dialysis was clear, with 60% less mortality in the transplanted group. Notably, the youngest recipients, those younger than 65 years, obtained the highest benefit.19
Three of the referred studies were enough homogeneous and gave numerical data to calculate RRs for mortality at 1 and 5 years after KT with an ECD or an advanced age kidney in comparison with remaining in the waiting list on dialysis.11,86,92 Mortality at 1 year was quite similar in patients receiving an ECD/advanced age kidney or remaining on dialysis (RR, 0.49 [0.21-1.15], P = 0.10) but decreased at 5 years in those transplanted (RR, 0.47 [0.43-0.53], P < 0.00001) (Figure 3).
FIGURE 3.
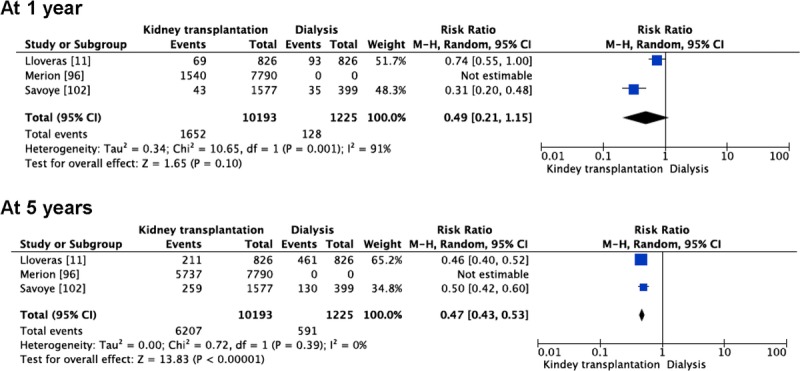
Comparison of mortality between patients undergoing kidney transplantation using ECDs and patients remaining on dialysis on the waiting list for kidney transplantation.
OUTCOMES: EFFECT OF RECIPIENT AGE
Patients older than 65 years represent the fastest growing group on the waitlist in the United States with the numbers increasing from 12.9% in 2003 to 21.2% in 2014.6 This trend, although encouraging, fails to highlight the low rate of elderly patients waitlisted or transplanted. For instance, less than 5% of dialysis patients older than 65 years are on the waiting list in the United Kingdom and only 10% are transplanted in the first 5 years.95 This patient population brings with them a unique set of problems, including frailty, cognitive impairment, and comorbidities less commonly seen in the other age groups.96 All these factors have been associated with morbidity and mortality after transplant,97–99 although the trend has improved.100 However, a number of studies have shown improvement in overall life expectancy (mortality risk, 40-60% lower) for those who have received a KT compared with those who remain listed on dialysis,11,18,19,23,34,86,91,92,94,101,102 even despite higher incidence of early mortality in some reports.18,86,93,94,101 A number of European and US studies (Table S4, SDC, http://links.lww.com/TP/B387)15,17,27–29,48–50,103–113 have confirmed that KT in advanced age patients is associated with prolonged graft survival, because patient survival is often the limiting survival factor for the kidney allograft.17,27,28,48,50,103,104,106–108,110,112,114 Contrarily, some studies have shown higher mortality and worse death-censored graft survival in older recipients using ECD kidneys.15,29,49,105,109,111 Although some studies showed similar survival using ECDs in younger recipients,15,106 suboptimal results are frequently reported.23,29,105,107,108,113–115
VALUE OF PREIMPLANTATION BIOPSY AND OTHER ASSESSMENT TOOLS
One possibility to expand more confidently the use of old donor kidneys may be the assessment of preimplantation biopsies. Wang et al116 performed recently a review on this topic, including a number of useful summarizing tables, concluding that routine use of biopsies to help determine whether or not to transplant a kidney should be reexamined. The reports published to date including a substantial number of biopsies, are of poor quality, heterogeneous and retrospective.107,116–144 In agreement with Wang et al, we have been unable to pool the results in a meta-analysis, as all studies have reported results and outcomes in very different ways. A substantial number of reports conclude that the time-zero or preimplantation biopsy is of very limited value to predict outcomes, particularly renal graft function or survival.117–128 It is likely that overestimation of glomerulosclerosis when the wedge biopsy is taken at a subcapsular level may mask the true importance of this parameter. SRTR reports including greater than 12 000 biopsies showed better 1-year graft function after transplanting kidneys with 0% to 5% glomerulosclerosis, compared with those showing higher percentages, but without any correlation with graft survival and loss of any discrimination power between 6% and 100% of sclerosed glomeruli.122,123 Of particular importance is the Spanish study performed by Azancot et al, confirming the limited value of the preimplantation biopsy findings when assessed by the local on-call pathologist.126 The histological parameters turned to be useful only when they were retrospectively re-assessed by an experienced renal pathologist, a resource unlikely available for most transplant programs. Some authors suggest that donor age correlates much better than histology with graft outcomes.121
Despite the negative results from the above mentioned studies, a good number of reports have underlined the value of time-zero or preimplantation biopsy in predicting outcomes.107,129–144 Severity of histological findings inversely correlates with graft outcome, particularly glomerulosclerosis,129,131,134 vascular disease and fibrous intimal thickening,133,136 or a combination of vascular, interstitial and glomerular damage joined in different scores.107,130,132,135–144 Remuzzi et al132 suggested that better graft survival using ECD kidneys might be achieved if histological evaluation is performed before kidney allocation. The limitation of this study is that dual KT was the modality chosen for the majority of patients, and it is not unexpected to have good results by performing KT with 2 ECD kidneys with minimal fibrosis and vasculopathy.
Wang et al116 have examined the value of 15 published semiquantitative scoring systems used to predict posttransplantation outcomes. Scores combining histological and clinical variables are of particular value.107,130,134,139 The first such mixed score used data from the UNOS during the nineties to include 5 donor variables related to creatinine clearance at 6 months.107 Six-year graft survival was 11% better in recipients scored greater than 20 versus those scored less than 20. In a further analysis, Nyberg score performed better to stratify survival than SCr at 2 to 4 years and ECD/non-ECD classification.130 A French group optimized prediction of a low estimated GFR combining donor SCr, the presence or absence of donor hypertension and glomerulosclerosis greater than 10% or less than 10%.134 The validation set in this study confirmed the weak prediction power of isolated clinical or histological parameters, which strongly improved in a combined composite score. De Vusser et al139 prospectively studied baseline biopsies in 548 patients showing that interstitial fibrosis, tubular atrophy and glomerulosclerosis associated significantly with death-censored graft survival, whereas hialynosis and vascular thickening did not. In parallel, donor age correlated significantly with the same 3 predictive histological parameters, and also with graft survival. They constructed a new scoring system for prediction of 5-year graft survival that improved prediction of allograft loss with respect with previously published histological scores,124,132,135 giving the strongest weight to donor age. Nonetheless, survival curves showed that those patients transplanted with a high scored kidney had around 80% graft survival al 5 years, and those getting a kidney with a low score had a great 90% 5-year graft survival. So in fact, the new score only confirmed that older kidneys had lower medium-term graft function and survival than younger kidneys, but not if they are worthy to be used or not.139
This literature overview confirms that preimplantation biopsy findings, in combination with other clinical and demographic donor characteristics may be useful to predict graft function in internal comparisons, but not to predict patient survival, graft survival or primary nonfunction. The extension of routine preimplantation biopsy has probably increased discard rate, which reaches 30% in biopsied kidneys versus 6.6% in not-biopsied ones, to the detriment of the large population in the waiting list for transplantation.6 Only a good randomized clinical trial may resolve the usefulness of pretransplant biopsy for assessing the kidney graft quality and outcomes. Of course, all the biopsied kidneys might be transplanted in this hypothetical trial, to make sure absence of selection biases in outcomes. In our standard practice, biopsy findings are not anymore a tool to discard kidneys, but a tool to assess kidney graft prospects and baseline pretransplant damage, serving as a good self-control for posttransplant assessment.
DUAL KT
Dual KT has been proposed as a strategy to increase KT with suboptimal, particularly old, donor kidneys.132 It is based in a prediction: the transplant physician considers that a single kidney from a given donor will not be sufficient to add sustained stable kidney function. Nonetheless, its practice is very limited, comprising only 2% to 4% of all KT performed in the US.145,146 Although a common practice in some Spanish units in the past,147–149 dual KT is very unusual nowadays in Spain, representing less than 1% of procedures. Most units prefer now transplanting a single kidney to optimize the kidney pool. Although some groups have tried to develop clinical algorithms to allocate single or dual KT according to donor renal function, histology and comorbidities, there is no uniform consensus.132,146,150–152 In Figure 4, we have summarized the different applied strategies by several groups.
FIGURE 4.
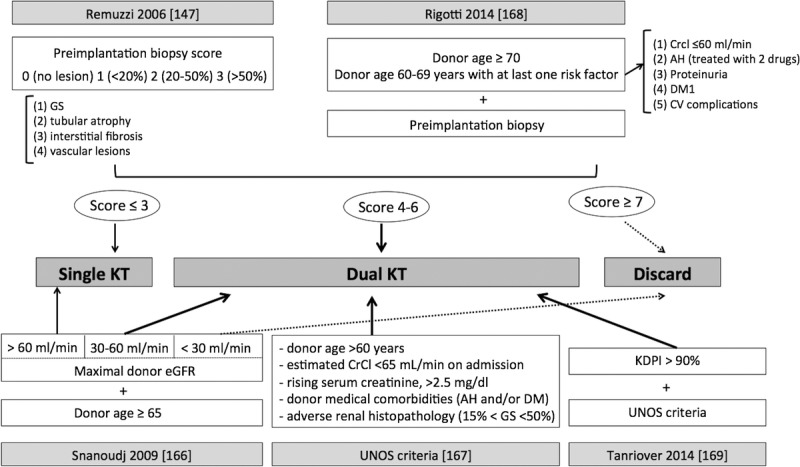
Different criteria for allocating kidneys to dual KT. According to Remuzzi et al,132 the allocation of a dual KT may be based in histopathological criteria in preimplantation donor biopsy with the assessment of 4 compartments (glomerulosclerosis, tubular atrophy, interstitial fibrosis and vascular lesions). The score ascribes 0 to 3 points to each compartment according to the degree of lesions. If the overall score is 3 points or less, a single KT is carried out, between 4 and 6 points, a dual KT, and 7 points or more lead to kidney discard. Rigotti et al, in addition to the histological score, takes into account donor age and donor comorbidities.152 If the donor is 70 years or older, or is 60 to 69 years old with at least 1 comorbid condition such as creatinine clearance below 61 mL/min, AH controlled with 2 drugs or more, proteinuria, diabetes or any cardiovascular complication, the recipient receives 2 kidneys in a dual KT. Snanoudj et al150 proposal is based in donor kidney function and donor age: a donor 65 years or older and eGFR between 30 and 60 mL/min is allocated to dual KT, if >60 ml/min to a single KT and if <30 ml/min discarded.150 UNOS criteria to allocate kidneys for dual KT are based in donor age (>60 years old), creatinine clearance (lower to 65 ml/min at admission), creeping creatinine after admission (to 2.5 mg/dl or higher) and comorbidities such as AH or DM, with glomerulosclerosis between 15-50%.151 Tanriover proposal for dual KT is based in UNOS criteria for kidneys with a KDPI higher than 90%.153 HTA, arterial hypertension; DM, diabetes mellitus; GS, glomerulosclerosis; eGFR, estimated glomerular filtration rate; CV, cardiovascular; CrCl, creatinine clearance.
During the last decade, some centers have reported their experience performing dual KT without a comparison with a control group. Eight reports (n = 290) showed 1-year graft survival of 87% to 96%.153–160 When outcomes are compared with those obtained after single KT with an ECD donor, many studies have reported similar patient and graft survival (Table S5, SDC, http://links.lww.com/TP/B387) [160–166,168,177-186]. We have been able to pool the results from 16 reports of dual KT in different outcomes.145,146,148–150,152,161–170 The incidence of DGF was lower performing dual KT (n = 2564) versus single KT (n = 23812; RR, 0.81 [0.68-0.98]; P = 0.03). SCr at 1-year posttransplantation was similar after dual or single KT (9 studies; mean difference, −0.24 [−0.55 to −0.07]; P = 0.13). Graft loss at 1 year was similar between dual and single KT (9 studies, RR, 0.92 [0.73-1.15]; P = 0.47). However, in the pooled analyses including the 6 relatively small reports with graft loss at 5 years available, dual KT (n = 507) was associated with lower graft loss than single KT (n = 695) (RR, 0.45 [0.30-0.67]; P < 0.0001) (Figure 5). Mortality at 1 year was similar after dual (n = 1135) or single KT (n = 8583) (7 studies; RR, 0.94 [0.52-1.69], P = 0.83]. The largest study included patients from the US Registry allocated according to UNOS criteria into dual KT (n = 625), single ECD (n = 7686), and single SCD (n = 6044).145 Mortality at 1 year was significantly higher after dual KT than after single KT (RR, 1.32 [1.02-1.71]), however, this difference disappeared when including the other 6 smaller studies. Mortality at 5 years was lower after dual KT (n = 443) versus single KT (n = 680) in the pooled analysis of 5 studies with this outcome available (RR, 0.61 [0.41-0.90]; P = 0.01) (Figure 5).
FIGURE 5.
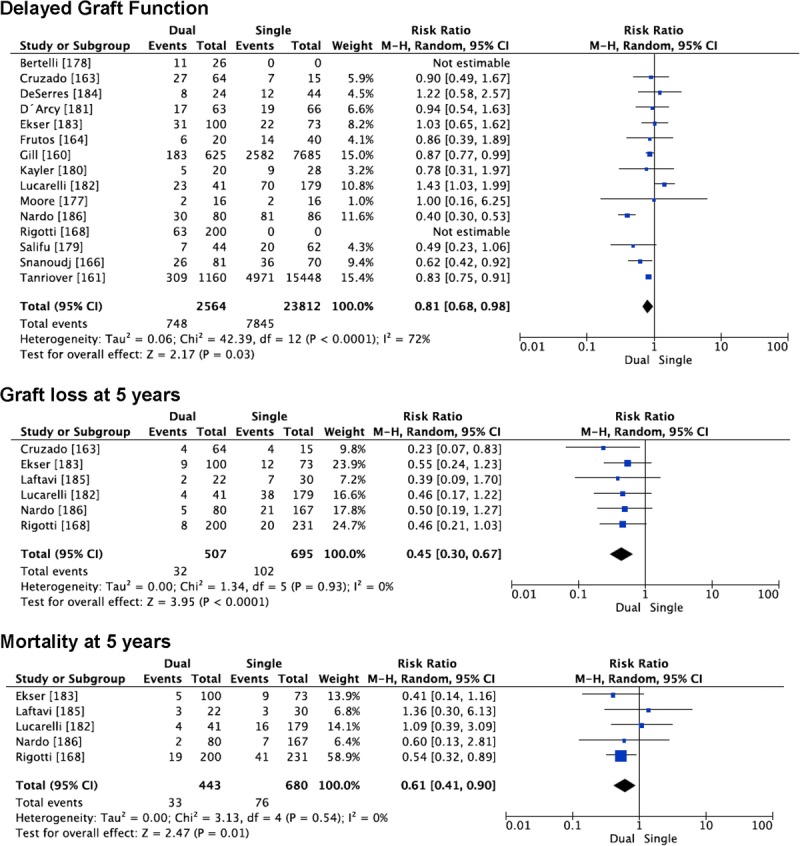
Outcomes after dual KT versus single transplantation using an ECD kidney.
More recently, Tanriover et al146 performed an analysis based in the KDPI allocation system. The innovative approach, quite different than those previously published, precluded the inclusion of this important report in our pooled analysis. In the group of patients receiving kidneys with KDPI greater than 90%, dual KT was associated with slightly better 3-year death-censored graft survival than single ECD (72.9% vs 67.6%). Those differences disappear when the analysis is performed with the kidneys with KDPI greater than 80%.The authors propose to reserve dual KT for kidneys with KDPI greater than 90%.
The results of our pooled literature analyses underline a better patient and graft survival at 5 years in those patients receiving a dual KT than a single ECD KT. However, in our opinion, these differences are based in few reports with a relatively low number of cases, and the actual reported differences in survival are not enough to justify the investment of 2 kidneys in 1 recipient as a routine practice, given the shortage of organs and mortality rates in the waiting list.6 But of course, given that 60% of kidneys from donors older than 65 years are currently discarded in the United States, their use in dual KT is better than full refusal. Better and larger studies would be needed to validate systematic selection of kidneys for dual KT, to optimize high KDPI/ECD organ use in those units with strict kidney selection criteria.
MACHINE PERFUSION WITH OLD KIDNEYS
Different studies have shown variable benefits of pulsatile machine perfusion to improve ECD kidney outcomes (Table 5).171–184 Pulsatile perfusion has increased the rates of ECD use.171,176 Recent meta-analysis showed reduced incidence of DGF and an increase in 1-year graft survival.185,186 The analysis of the effect of machine perfusion in ECD from a randomized controlled trial found that the better graft survival was more relevant when DGF occurred.176 Although this beneficial effect did not have significant impact in the 2- to 3-year patient survival rates,174–182,185 the use of machine perfusion decreased economic expenses (taking into account direct costs such as dialysis, readmission and preservation costs) in the short and long-term.186
TABLE 5.
Reports describing potential benefits of pulsatile perfusion machine use in kidneys from ECDs
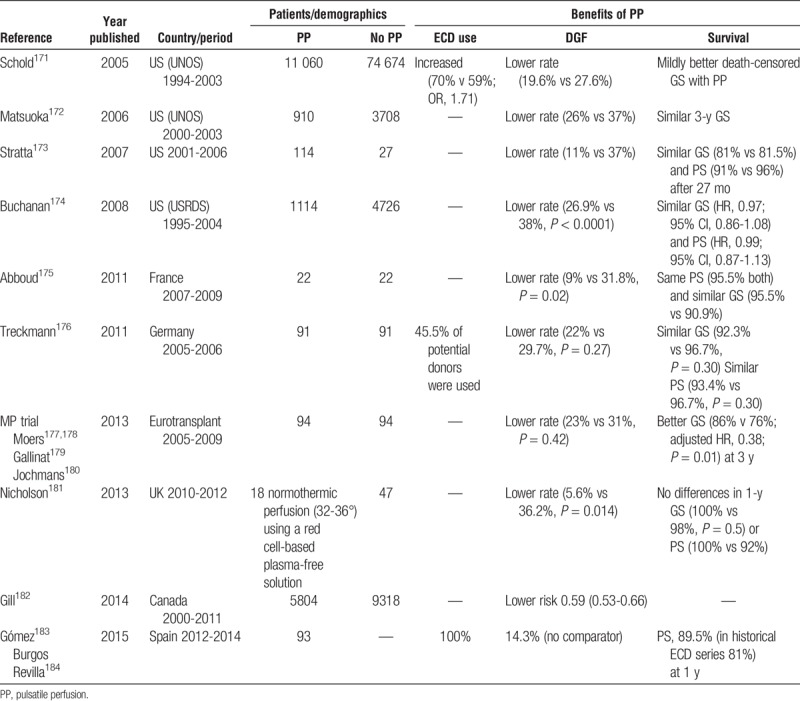
Some of the cited retrospective and prospective studies using hypothermic machine perfusion had available numerical data to perform a meta-analysis.172–179,183,184 DGF rate is lower with machine perfusion (n = 13498) than with cold storage (n = 83342) (11 reports; RR, 0.71 [0.67-0.74]; P < 0.00001). Mortality at 1 year (3 studies; RR, 1 [0.83-1.22]; P = 0.96] and 3 years (5 reports from 3 trials; RR, 0.94 [0.70-1.25], p = 0.66) and graft loss at 1 year (5 studies; RR, 0.87 [0.65-1.16]; P = 0.35) and 3 years (7 reports from 5 studies; RR, 0.98 [0.88-1.08]; P = 0.67) were not different using machine perfusion or cold storage. However, when we excluded retrospective registry articles and included only randomized clinical trials in our analyses,175–179,183,184 DGF rate remains lower with machine perfusion (n = 300) than with cold storage (n = 207) (7 reports, RR 0.71 [0.51-1]; P = 0.05); mortality at 1 year (2 studies; RR, 1 [0.07-15-1.22], P = 0.1] was not different but graft loss at 1 year (3 studies, RR 0.43 [0.25-0.75], p = 0.003) and 3 years (3 reports from 2 studies; RR, 0.44 [0.26-75], P = 0.002) were lower using machine perfusion.
Evaluation of graft viability is especially important in advanced age, and machine perfusion could be a useful tool. However, the renal resistance at the end of machine perfusion was not a useful predictor for outcomes.183,184
Machine perfusion is used in a minority of KT from deceased donors, and the inconsistency of the potential benefits reported, in addition to concerns regarding cost-effectiveness factors, does not permit a generalized advise for its use to optimize old donor kidney outcomes. This is an area in which new large prospective randomized studies are clearly needed, as preservation technique improvement should be a very relevant strategy to expand the use of advanced age kidneys and other damaged organs.
IMMUNOSUPPRESSIVE STRATEGIES FOR BETTER USE OF OLD KIDNEYS
Elderly recipients of an old renal graft are a special population with increased risk of poor graft function, calcineurin inhibitor (CNI)-induced nephrotoxicity, infections, cardiovascular events and malignancies. Amplification of senescence changes of the kidney allograft exaggerates the negative impact of acute rejection episodes.14,187 As a result, it is important to maintain adequate immunosuppression with a tailored drug regimen.
Our review confirms that the scarcity of immunosuppressive strategies especially designed for the elderly recipient receiving an old kidney. We have focused this review on the studies published along the last 10 years (Table S6, SDC, http://links.lww.com/TP/B387)[204–223], as the previous ones had already been reviewed.14 The great heterogeneity of the studies and the absence of many numerical outcomes in the different reports, precluded any meaningful pooled meta-analysis.
CNIs are nephrotoxic and 2 possible strategies have been proposed for CNI toxicity minimization: (1) to delay introduction until a certain level of renal graft function is achieved, and (2) more radical, complete CNI-free strategies. Delayed introduction has been analyzed in 3 European studies, all of them with induction with anti–interleukin-2-receptor antibodies (anti-IL2ra).188–190 Reduced CsA doses (3 mg/kg/d) initiated within the first 24 hours posttransplantation with mofetil mycophenolate (MMF), basiliximab and steroids, were not associated with an increased risk of acute rejection.188 A delayed initiation of cyclosporine after 7 days posttransplantation did not show any benefit in DGF prevention and increased acute rejection rates (25% vs 5.3%). Two controlled studies evaluating delayed-initiation of tacrolimus showed similar renal function and patient and graft survival at 6 months in delayed and immediate tacrolimus groups.189,190
Regarding CNI-free initial immunosuppression, a combined induction using antithymocyte globulin (ATG) and basiliximab using only MMF for low-risk allograft recipients brought high incidence of acute rejection and cytomegalovirus infections.191,192 When the elderly population was compared to the younger, there was a high risk of rejection because of a larger mismatch. Durrbach et al193 compared a strategy with early introduction of sirolimus vs CNI-based immunosuppression describing a higher incidence and longer duration of DGF, with lower graft survival in sirolimus patients. The comparison of CNI-MMF-steroids versus sirolimus-MMF-steroids using antibody-based induction therapy reported no differences between both groups.194 CNI-free treatment regimen using MMF plus a mammalian target of rapamycin inhibitor showed no difference in acute rejection with the CNI-treated patients, but a high incidence of switching to CNI in the initial CNI-free group.195
Old kidneys are generally transplanted in elderly recipients, so it seems reasonable to minimize induction therapy to prevent adverse effects in this vulnerable population. Old-for-old strategies, usually results in poor HLA matching, thus encouraging physicians to use induction therapy.29 Seven studies have compared different induction strategies in this population. A lower risk of DGF using ATG than anti-IL2ra and a higher risk of acute rejection with anti-IL2ra than using ATG or alemtuzumab is observed.196 Despite this apparent advantage of depletive induction agents, a greater 1-year mortality with alemtuzumab than ATG was described in KT using kidneys from ECD, DCD or with prolonged cold ischemia time. Two studies showed that ATG showed better acute rejection prevention than basiliximab, without differences in DGF or survival.197,198 However, higher acute rejection rates and lower survival were observed with a protocol of ATG in elderly recipients. Cumulative ATG dosage >6 mg/kg was associated with death with functioning graft, and the authors advise against high ATG dose in the elderly.199 These negative results were not confirmed in a similar study.200
A different strategy is the use of belatacept. Low-intense belatacept-based regimen was associated with better renal function compared to a cyclosporine-based regimen,201–206 with a better control of cardiovascular risk factors.204 A greater risk for posttransplant lymphoproliferative disease was observed in patients negative for Epstein-Barr virus at baseline and were treated with a belatacept-based regimen.201
The immunosuppressive drug protocol for KT using old kidneys should be based on potential nephron-protecting strategies.207 These include a tailored immunosuppression with early CNI minimization or delayed moderate dose CNI addition after induction, and adequate infection prophylaxis.
CONCLUSIONS: USE THESE KIDNEYS
Relying in donors with associated comorbidities and/or an advanced age is unavoidable to overcome the increasing waiting lists. Despite poorer results, the use of old kidneys targeted to a selected population may provide better survival than remaining on dialysis. The use of advanced age DCD kidneys is associated with outcomes not different to those seen with kidneys from ECD after brain dead. Preimplantation biopsy assessment has been overestimated for kidney graft discarding or use. Machine perfusion has decreased DGF and this beneficial effect has resulted in better graft survival in medium-size trials that should be confirmed in larger ones including advanced age kidneys. Investing 2 kidneys in 1 recipient does not make sense when a single KT would be enough, particularly in many elderly recipients. In these recipients, randomized trials with adapted immunosuppression strategies are urgently needed.
Old donors constitute an enormous potential source of useful kidneys, but their use in a vast majority of countries is limited. Strategies and policies should be fostered to solve it.
Footnotes
M.J.P.S. and N.M. contributed equally.
MJPS has support from a Rio Hortega contract, ISCIII. MC and JP are supported by grants FIS ISCIII-FEDER PI13/0598, Programa de Intensificación ISCIII 2015 and RedinRen RD12/0021/0024. NM did this work as part of her doctoral thesis at the Universitat Autònoma Barcelona.
The authors declare no conflicts of interest.
M.J.P.S. did data extraction and drafted the article. N.M. run the literature search, did data extraction, carried out analyses and drafted the article. D.R.-P. did data extraction and drafted the article. M.C. did data extraction and drafted the article. J.P. designed the review, did data extraction, and drafted the article.
All authors approved the final version.
The protocol of this systematic review is published in PROSPERO register (CR D42016036861).
Correspondence: Julio Pascual, Department of Nephrology, Hospital del Mar, Barcelona, Spain. (julpascual@gmail.com).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
The authors review strategies for organ utilization, including expanded criteria donors, advanced age kidneys, Kidney Donor Profile Index policy, preimplantation biopsy, dual kidney transplant, machine perfusion, and special immunosuppressive protocols. Many opportunities for increased utilization are identified. Supplemental digital content is available in the text.
REFERENCES
- 1.Catalan Renal Registry. Statistical report 2013. Barcelona, Spain: Catalan Transplant Organization, Health Department; 2015. http://trasplantaments.gencat.cat/ca/professionals/registres_d_activitat_i_seguiment/registre_de_malalts_renals/. [Google Scholar]
- 2.Himmelfarb J, Ikizler TA. Hemodialysis N Engl J Med 2010. 3631833–1845 [DOI] [PubMed] [Google Scholar]
- 3.2015 USRDS Annual Data Report. Volume 2 - ESRD in the United States. Chapter 1: Incidence, Prevalence, Patient Characteristics, and Treatment Modalities.: https://www.usrds.org/2015/download/vol2_01_IncidenceandPrevalence_15.pdf. [Google Scholar]
- 4.Ministry of Health, Social Services and Equality. Balance of Activity in Donation and Transplant 2015. http://www.ont.es/Documents/Balance_Actividad_2015.pdf. Published 2015. Accessed January 3rd, 2016. [Google Scholar]
- 5.Eurotransplant International Foundation. Annual Report 2014. https://www.eurotransplant.org/cms/mediaobject.php?file=ar_2014.pdf. Published 2014. Accessed January 3rd, 2016. [Google Scholar]
- 6.Hart A, Smith JM, Skeans MA. Kidney Am J Transplant 2016. 1611–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halldorson J, Roberts JP. Decadal analysis of deceased organ donation in Spain and the United States linking an increased donation rate and the utilization of older donors Liver Transpl 2013. 19981–986 [DOI] [PubMed] [Google Scholar]
- 8.Chang GJ, Mahanty HD, Ascher NL. Expanding the donor pool: can the Spanish model work in the United States? Am J Transplant 2003. 31259–1263 [DOI] [PubMed] [Google Scholar]
- 9.Lledó-García E, Riera L, Passas J. Spanish consensus document for acceptance and rejection of kidneys from expanded criteria donors Clin Transplant 2014. 281155–1166 [DOI] [PubMed] [Google Scholar]
- 10.Jay C, Washburn K, Dean P. Protecting older patients from dialysis: the survival benefit of preemptive transplant with high KDPI allografts. Am J Transplant. 2015;15:1. [Google Scholar]
- 11.Lloveras J, Arcos E, Comas J. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years Transplantation 2015. 99991–996 [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Missie A, Chow E. Survival benefit of kidney transplantation with expanded criteria kidney from donors after circulatory death. Am J Transplant. 2015;15:1. [Google Scholar]
- 13.Leichtman AB, Cohen D, Keith D. Kidney and pancreas transplantation in the United States, 1997-2006: the HRSA breakthrough collaboratives and the 58 DSA challenge Am J Transplant 2008. 8946–957 [DOI] [PubMed] [Google Scholar]
- 14.Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors Am J Kidney Dis 2008. 52553–586 [DOI] [PubMed] [Google Scholar]
- 15.Mezrich JD, Pirsch JD, Fernandez LA. Differential outcomes of expanded-criteria donor renal allografts according to recipient age Clin J Am Soc Nephrol 2012. 71163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waiser J, Schreiber M, Budde K. Age-matching in renal transplantation Nephrol Dial Transplant 2000. 15696–700 [DOI] [PubMed] [Google Scholar]
- 17.Al-Shraideh Y, Farooq U, Farney AC. Influence of recipient age on deceased donor kidney transplant outcomes in the expanded criteria donor era Clin Transplant 2014. 281372–1382 [DOI] [PubMed] [Google Scholar]
- 18.Ojo AO, Hanson JA, Meier-Kriesche H. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates J Am Soc Nephrol 2001. 12589–597 [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Sáez MJ, Arcos E, Comas J. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis Am J Transplant 2016. 162724–2733 [DOI] [PubMed] [Google Scholar]
- 20.Port FK, Bragg-Gresham JL, Metzger RA. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors Transplantation 2002. 741281–1286 [DOI] [PubMed] [Google Scholar]
- 21.Metzger RA, Delmonico FL, Feng S. Expanded criteria donors for kidney transplantation Am J Transplant 2003. 3114–125 [DOI] [PubMed] [Google Scholar]
- 22.Sung RS, Guidinger MK, Lake CD. Impact of the expanded criteria donor allocation system on the use of expanded criteria donor kidneys Transplantation 2005. 791257–1261 [DOI] [PubMed] [Google Scholar]
- 23.Schold JD, Howard RJ, Scicchitano MJ. The expanded criteria donor policy: an evaluation of program objectives and indirect ramifications Am J Transplant 2006. 61689–1695 [DOI] [PubMed] [Google Scholar]
- 24.Sung RS, Guidinger MK, Leichtman AB. Impact of the expanded criteria donor allocation system on candidates for and recipients of expanded criteria donor kidneys Transplantation 2007. 841138–1144 [DOI] [PubMed] [Google Scholar]
- 25.Rao PS, Schaubel DE, Guidinger MK. A comprehensive risk quantification score for deceased donor kidneys: the Kidney Donor Risk Index Transplantation 2009. 88231–236 [DOI] [PubMed] [Google Scholar]
- 26.Han M, Jeong JC, Koo TY. Kidney Donor Risk Index is a good prognostic tool for graft outcomes in deceased donor kidney transplantation with short, cold ischemic time Clin Transplant 2014. 28337–344 [DOI] [PubMed] [Google Scholar]
- 27.Smits JM, Persijn GG, van Houwelingen HC. Evaluation of the Eurotransplant Senior Program. The results of the first year Am J Transplant 2002. 2664–670 [DOI] [PubMed] [Google Scholar]
- 28.Cohen B, Smits JM, Haase B. Expanding the donor pool to increase renal transplantation Nephrol Dial Transplant 2005. 2034–41 [DOI] [PubMed] [Google Scholar]
- 29.Frei U, Noeldeke J, Machold-Fabrizii V. Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program Am J Transplant 2008. 850–57 [DOI] [PubMed] [Google Scholar]
- 30.Dahmane D, Audard V, Hiesse C. Retrospective follow-up of transplantation of kidneys from ’marginal’ donors Kidney Int 2006. 69546–552 [DOI] [PubMed] [Google Scholar]
- 31.Lee AP, Abramowicz D. Is the Kidney Donor Risk Index a step forward in the assessment of deceased donor kidney quality? Nephrol Dial Transplant 2015. 301285–1290 [DOI] [PubMed] [Google Scholar]
- 32.Israni AK, Salkowski N, Gustafson S. New national allocation policy for deceased donor kidneys in the United States and Possible Effect on Patient Outcomes J Am Soc Nephrol 2014. 251842–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodside KJ, Merion RM, Leichtman AB. Utilization of kidneys with similar Kidney Donor Risk Index values from standard versus expanded criteria donors Am J Transplant 2012. 122106–2114 [DOI] [PubMed] [Google Scholar]
- 34.Massie AB, Luo X, Chow EK. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys Am J Transplant 2014. 142310–2316 [DOI] [PubMed] [Google Scholar]
- 35.Fritsche L, Hörstrup J, Budde K. Old-for-old kidney allocation allows successful expansion of the donor and recipient pool Am J Transplant 2003. 31434–1439 [DOI] [PubMed] [Google Scholar]
- 36.Summers DM, Watson CJ, Pettigrew GJ. Kidney donation after circulatory death (DCD): state of the art Kidney Int 2015. 88241–249 [DOI] [PubMed] [Google Scholar]
- 37.Singh SK, Kim SJ. Epidemiology of kidney discard from expanded criteria donors undergoing donation after circulatory death Clin J Am Soc Nephrol 2016. 11317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teraoka S, Nomoto K, Kikuchi K, et al. Outcomes of kidney transplants from non-heart-beating deceased donors as reported to the Japan Organ Transplant Network from April 1995-December 2003: a multi-center report. Clin Transpl. 2004:91–102. [PubMed] [Google Scholar]
- 39.Locke JE, Segev DL, Warren DS. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation Am J Transplant 2007. 71797–1807 [DOI] [PubMed] [Google Scholar]
- 40.Doshi MD, Hunsicker LG. Short- and long-term outcomes with the use of kidneys and livers donated after cardiac death Am J Transplant 2007. 7122–129 [DOI] [PubMed] [Google Scholar]
- 41.Singh SK, Kim SJ. Does expanded criteria donor status modify the outcomes of kidney transplantation from donors after cardiac death? Am J Transplant 2013. 13329–336 [DOI] [PubMed] [Google Scholar]
- 42.Summers DM, Johnson RJ, Hudson A. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study Lancet 2013. 381727–734 [DOI] [PubMed] [Google Scholar]
- 43.Foley DP, Patton PR, Meier-Kriesche HU, et al. Long-term outcomes of kidney transplantation in recipients 60 years of age and older at the University of Florida. Clin Transplants. 2005:101–109. [PubMed] [Google Scholar]
- 44.Verran DJ, deLeon C, Chui AK. Factors in older cadaveric organ donors impacting on renal allograft outcome Clin Transplant 2001. 151–5 [DOI] [PubMed] [Google Scholar]
- 45.Persson NH, Omnell Persson M, Ekberg H. Renal transplantation from marginal donors: results and allocation strategies Transplant Proc 2001. 333759–3761 [DOI] [PubMed] [Google Scholar]
- 46.Berardinelli L, Beretta C, Raiteri M, et al. Early and long-term results using older kidneys from cadaver or living donors. Clin Transpl. 2001:157–166. [PubMed] [Google Scholar]
- 47.Lovén C, Nordén G, Nyberg G. Impact of cadaveric renal donor morbidity on long-term graft function Transpl Int 2003. 16857–860 [DOI] [PubMed] [Google Scholar]
- 48.Fabrizii V, Winkelmayer WC, Klauser R. Patient and graft survival in older kidney transplant recipients: does age matter? J Am Soc Nephrol 2004. 151052–1060 [DOI] [PubMed] [Google Scholar]
- 49.Cacho DT, Cusí LI, Piqué AA. Elderly donor kidney transplant: factors involved in graft survival Transplant Proc 2005. 373690–3692 [DOI] [PubMed] [Google Scholar]
- 50.Foss A, Tuvin D, Leivestad T. Should kidneys from older cadaveric donors be age-matched to the recipient? Transplant Proc 2005. 373280–3282 [DOI] [PubMed] [Google Scholar]
- 51.Végsö G, Máthé Z, Péter A. Improving results of renal transplantation with the use of elderly donors: the Budapest experience Transplant Proc 2005. 374225–4227 [DOI] [PubMed] [Google Scholar]
- 52.Messa P, Brezzi B, Cresseri D. Immediate graft function positively affects long-term outcome of renal allografts from older but not from younger donors Transplant Proc 2006. 383377–3381 [DOI] [PubMed] [Google Scholar]
- 53.Collini A, De Bartolomeis C, Ruggieri G. Long-term outcome of renal transplantation from marginal donors Transplant Proc 2006. 383398–3399 [DOI] [PubMed] [Google Scholar]
- 54.Diet C, Audard V, Roudot-Thoraval F. Immunological risk in recipients of kidney transplants from extended criteria donors Nephrol Dial Transplant 2010. 252745–2753 [DOI] [PubMed] [Google Scholar]
- 55.Praehauser C, Hirt-minkowski P, Saydam Bakar K. Risk factors and outcome of expanded-criteria donor kidney transplants in patients with low immunological risk. Swiss Med Wkly. 2013;143:w13883. doi: 10.4414/smw.2013.13883. [DOI] [PubMed] [Google Scholar]
- 56.Barba J, Zudaire JJ, Robles JE. Complications of kidney transplantation with grafts from expanded criteria donors World J Urol 2013. 31893–900 [DOI] [PubMed] [Google Scholar]
- 57.Cicciarelli J, Iwaki Y, Mendez R. The influence of donor age on kidney graft survival in the 1990s. Clin Transpl. 1999:335–340. [PubMed] [Google Scholar]
- 58.Gjertson DW. A multi-factor analysis of kidney regraft outcomes. Clin Transpl. 2002:335–349. [PubMed] [Google Scholar]
- 59.Mandal AK, Snyder JJ, Gilbertson DT. Does cadaveric donor renal transplantation ever provide better outcomes than live-donor renal transplantation? Transplantation 2003. 75494–500 [DOI] [PubMed] [Google Scholar]
- 60.Johnston TD, Thacker LR, Jeon H. Sensitivity of expanded-criteria donor kidneys to cold ischaemia time Clin Transplant 2004. 1828–32 [DOI] [PubMed] [Google Scholar]
- 61.Gjertson DW. Explainable variation in renal transplant outcomes: a comparison of standard and expanded criteria donors. Clin Transpl. 2004:303–314. [PubMed] [Google Scholar]
- 62.Schold JD, Kaplan B, Baliga RS. The broad spectrum of quality in deceased donor kidneys Am J Transplant 2005. 5757–765 [DOI] [PubMed] [Google Scholar]
- 63.Chavalitdhamrong D, Gill J, Takemoto S. Patient and graft outcomes from deceased kidney donors age 70 years and older: an analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing database Transplantation 2008. 851573–1579 [DOI] [PubMed] [Google Scholar]
- 64.Moers C, Kornmann NS, Leuvenink HG. The influence of deceased donor age and old-for-old allocation on kidney transplant outcome Transplantation 2009. 88542–552 [DOI] [PubMed] [Google Scholar]
- 65.Carrier M, Lizé JF. Impact of expanded criteria donors on outcomes of recipients after kidney transplantation Transplant Proc 2012. 442227–2230 [DOI] [PubMed] [Google Scholar]
- 66.Schnitzler MA, Lentine KL, Gheorghian A. Renal function following living, standard criteria deceased and expanded criteria deceased donor kidney transplantation: impact on graft failure and death Transpl Int 2012. 25179–191 [DOI] [PubMed] [Google Scholar]
- 67.Hernandez RA, Malek SK, Milford EL. The combined risk of donor quality and recipient age: higher-quality kidneys may not always improve patient and graft survival Transplantation 2014. 981069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris PJ, Johnson RJ, Fuggle SV. Analysis of factors that affect outcome of primary cadaveric renal transplantation in the UK. HLA Task Force of the Kidney Advisory Group of the United Kingdom Transplant Support Service Authority (UKTSSA) Lancet 1999. 3541147–1152 [DOI] [PubMed] [Google Scholar]
- 69.Pessione F, Cohen S, Durand D. Multivariate analysis of donor risk factors for graft survival in kidney transplantation Transplantation 2003. 75361–367 [DOI] [PubMed] [Google Scholar]
- 70.Miranda B, Vilardell J, Grinyó JM. Optimizing cadaveric organ procurement: the Catalan and Spanish experience Am J Transplant 2003. 31189–1196 [DOI] [PubMed] [Google Scholar]
- 71.Oppenheimer F, Aljama P, Asensio Peinado C. The impact of donor age on the results of renal transplantation Nephrol Dial Transplant 2004. 19iii11–iii15 [DOI] [PubMed] [Google Scholar]
- 72.Aubert O, Kamar N, Vernerey D. Long term outcomes of transplantation using kidneys from expanded criteria donors: prospective, population based cohort study. BMJ. 2015;351:h3557. doi: 10.1136/bmj.h3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins MG, Chang SH, Russ GR. Outcomes of transplantation using kidneys from donors meeting expanded criteria in Australia and New Zealand, 1991 to 2005 Transplantation 2009. 871201–1209 [DOI] [PubMed] [Google Scholar]
- 74.Lim WH, Dogra G, Chadban SJ. Lack of impact of donor age on patient survival for renal transplant recipients ≥60 years Transpl Int 2012. 25401–408 [DOI] [PubMed] [Google Scholar]
- 75.Goldberg RJ, Smits G, Wiseman AC. Long-term impact of donor-recipient size mismatching in deceased donor kidney transplantation and in expanded criteria donor recipients Transplantation 2010. 90867–874 [DOI] [PubMed] [Google Scholar]
- 76.Matas AJ, Smith JM, Skeans MA. OPTN/SRTR 2013 Annual Data Report: kidney Am J Transplant 2015. 151–34 [DOI] [PubMed] [Google Scholar]
- 77.Gavela E, Pallardó LM, Avila A. Renal allografts from donors older than 70 years are useful for single transplantation Transplant Proc 2009. 412047–2049 [DOI] [PubMed] [Google Scholar]
- 78.Collini A, Kalmar P, Dhamo A. Renal transplant from very old donors: how far can we go? Transplantation 2009. 871830–1836 [DOI] [PubMed] [Google Scholar]
- 79.Foss A, Heldal K, Scott H. Kidneys from deceased donors more than 75 years perform acceptably after transplantation Transplantation 2009. 871437–1441 [DOI] [PubMed] [Google Scholar]
- 80.Giessing M, Fuller TF, Friedersdorff F. Outcomes of transplanting deceased-donor kidneys between elderly donors and recipients J Am Soc Nephrol 2009. 2037–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galeano C, Marcén R, Jimenez S. Utilization of elderly kidney donors (>70 years) does not affect graft survival in the medium term Transplant Proc 2010. 423935–3937 [DOI] [PubMed] [Google Scholar]
- 82.Gallinat A, Feldkamp T, Schaffer R. Single-center experience with kidney transplantation using deceased donors older than 75 years Transplantation 2011. 9276–81 [DOI] [PubMed] [Google Scholar]
- 83.Marconi L, Figueiredo A, Campos L. Renal transplantation with donors older than 70 years: does age matter? Transplant Proc 2013. 451251–1254 [DOI] [PubMed] [Google Scholar]
- 84.Andrés A, Herrero JC, Gonzalez E. Long-term results of renal transplantation in elderly cadaver donor recipients 65 years old or older Transplant Proc 2002. 34356–357 [DOI] [PubMed] [Google Scholar]
- 85.Grams ME, Womer KL, Ugarte RM. Listing for expanded criteria donor kidneys in older adults and those with predicted benefit Am J Transplant 2010. 10802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merion RM, Ashby VB, Wolfe RA. Deceased-donor characteristics and the survival benefit of kidney transplantation JAMA 2005. 2942726–2733 [DOI] [PubMed] [Google Scholar]
- 87.Kayler LK, Magliocca J, Zendejas I. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis Am J Transplant 2011. 112647–2656 [DOI] [PubMed] [Google Scholar]
- 88.Puig JM, Solà R, Vela E. Renal transplantation using kidneys from elderly donors Transplant Proc 2001. 331141–1143 [DOI] [PubMed] [Google Scholar]
- 89.Schnitzler MA, Whiting JF, Brennan DC. The expanded criteria donor dilemma in cadaveric renal transplantation Transplantation 2003. 751940–1945 [DOI] [PubMed] [Google Scholar]
- 90.Miles CD, Schaubel DE, Jia X. Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys Am J Transplant 2007. 71140–1147 [DOI] [PubMed] [Google Scholar]
- 91.Rao PS, Merion RM, Ashby VB. Renal transplantation in elderly patients older than 70 years of age: results from the scientific registry of transplant recipients Transplantation 2007. 831069–1074 [DOI] [PubMed] [Google Scholar]
- 92.Savoye E, Tamarelle D, Chalem Y. Survival benefits of kidney transplantation with expanded criteria deceased donors in patients aged 60 years and over Transplantation 2007. 841618–1624 [DOI] [PubMed] [Google Scholar]
- 93.Kauffman HM, McBride MA, Cors CS. Early mortality rates in older kidney recipients with comorbid risk factors Transplantation 2007. 83404–410 [DOI] [PubMed] [Google Scholar]
- 94.Gill JS, Schaeffner E, Chadban S. Quantification of the early risk of death in elderly kidney transplant recipients Am J Transplant 2013. 13427–432 [DOI] [PubMed] [Google Scholar]
- 95.Stevens KK, Woo YM, Clancy M. Deceased donor transplantation in the elderly—are we creating false hope? Nephrol Dial Transplant 2011. 262382–2386 [DOI] [PubMed] [Google Scholar]
- 96.Ponticelli C, Podestà MA, Graziani G. Renal transplantation in elderly patients. How to select the candidates to the waiting list? Transplant Rev (Orlando 2014. 28188–192 [DOI] [PubMed] [Google Scholar]
- 97.Garonzik-Wang JM, Govindan P, Grinnan JW. Frailty and delayed graft function in kidney transplant recipients Arch Surg 2012. 147190–193 [DOI] [PubMed] [Google Scholar]
- 98.McAdams-Demarco MA, Grams ME, Hall EC. Early hospital readmission after kidney transplantation: patient and center-level associations Am J Transplant 2012. 123283–3288 [DOI] [PubMed] [Google Scholar]
- 99.Karim A, Farrugia D, Cheshire J. Recipient age and risk for mortality after kidney transplantation in England Transplantation 2014. 97832–838 [DOI] [PubMed] [Google Scholar]
- 100.McAdams-Demarco MA, James N, Salter ML. Trends in kidney transplant outcomes in older adults J Am Geriatr Soc 2014. 622235–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolfe RA, Ashby VB, Milford EL. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant N Engl J Med 1999. 3411725–1730 [DOI] [PubMed] [Google Scholar]
- 102.Macrae J, Friedman AL, Friedman EA. Live and deceased donor kidney transplantation in patients aged 75 years and older in the United States Int Urol Nephrol 2005. 37641–648 [DOI] [PubMed] [Google Scholar]
- 103.Humar A, Denny R, Matas AJ. Graft and quality of life outcomes in older recipients of a kidney transplant Exp Clin Transplant 2003. 169–72 [PubMed] [Google Scholar]
- 104.Foley DP, Patton PR, Meier-Kriesche HU, et al. Long-term outcomes of kidney transplantation in recipients 60 years of age and older at the University of Florida. Clin Transpl. 2005:101–109. [PubMed] [Google Scholar]
- 105.Heldal K, Hartmann A, Leivestad T. Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity Transplantation 2009. 871045–1051 [DOI] [PubMed] [Google Scholar]
- 106.Solá R, Guirado L, López-Navidad A. Is it appropriate to implant kidneys from elderly donors in young recipients? Transplantation 2010. 90286–291 [DOI] [PubMed] [Google Scholar]
- 107.Nyberg SL, Matas AJ, Kremers WK. Improved scoring system to assess adult donors for cadaver renal transplantation Am J Transplant 2003. 3715–721 [DOI] [PubMed] [Google Scholar]
- 108.Meier-Kriesche HU, Schold JD, Gaston RS. Kidneys from deceased donors: maximizing the value of a scarce resource Am J Transplant 2005. 51725–1730 [DOI] [PubMed] [Google Scholar]
- 109.Shah T, Bunnapradist S, Hutchinson I. The evolving notion of “senior” kidney transplant recipients Clin Transplant 2008. 22794–802 [DOI] [PubMed] [Google Scholar]
- 110.Huang E, Poommipanit N, Sampaio MS. Intermediate-term outcomes associated with kidney transplantation in recipients 80 years and older: an analysis of the OPTN/UNOS database Transplantation 2010. 90974–979 [DOI] [PubMed] [Google Scholar]
- 111.Molnar MZ, Streja E, Kovesdy CP. Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes Am J Kidney Dis 2012. 59841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rose C, Schaeffner E, Frei U. A lifetime of allograft function with kidneys from older donors J Am Soc Nephrol 2015. 262483–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma MK, Lim WH, Craig JC. Mortality among younger and older recipients of kidney transplants from expanded criteria donors compared with standard criteria donors Clin J Am Soc Nephrol 2016. 11128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tullius SG, Tran H, Guleria I. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome Ann Surg 2010. 252662–674 [DOI] [PubMed] [Google Scholar]
- 115.Swanson SJ, Hypolite IO, Agodoa LY. Effect of donor factors on early graft survival in adult cadaveric renal transplantation Am J Transplant 2002. 268–75 [DOI] [PubMed] [Google Scholar]
- 116.Wang CJ, Wetmore JB, Crary GS. The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review Am J Transplant 2015. 151903–1914 [DOI] [PubMed] [Google Scholar]
- 117.Pokorná E, Vítko S, Chadimová M. Proportion of glomerulosclerosis in procurement wedge renal biopsy cannot alone discriminate for acceptance of marginal donors Transplantation 2000. 6936–43 [DOI] [PubMed] [Google Scholar]
- 118.Pokorná E, Vítko S, Chadimová M. Adverse effect of donor arteriolosclerosis on graft outcome after renal transplantation Nephrol Dial Transplant 2000. 15705–710 [DOI] [PubMed] [Google Scholar]
- 119.Lehtonen SR, Taskinen EI, Isoniemi HM. Histological alterations in implant and one-year protocol biopsy specimens of renal allografts Transplantation 2001. 721138–1144 [DOI] [PubMed] [Google Scholar]
- 120.Edwards EB, Posner MP, Maluf DG. Reasons for non-use of recovered kidneys: the effect of donor glomerulosclerosis and creatinine clearance on graft survival Transplantation 2004. 771411–1415 [DOI] [PubMed] [Google Scholar]
- 121.Howie AJ, Ferreira MA, Lipkin GW. Measurement of chronic damage in the donor kidney and graft survival Transplantation 2004. 771058–1065 [DOI] [PubMed] [Google Scholar]
- 122.Sung RS, Christensen LL, Leichtman AB. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion Am J Transplant 2008. 8783–792 [DOI] [PubMed] [Google Scholar]
- 123.Bajwa M, Cho YW, Pham PT. Donor biopsy and kidney transplant outcomes: an analysis using the Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) database Transplantation 2007. 841399–1405 [DOI] [PubMed] [Google Scholar]
- 124.Snoeijs MG, Buurman WA, Christiaans MH. Histological assessment of preimplantation biopsies may improve selection of kidneys from old donors after cardiac death Am J Transplant 2008. 81844–1851 [DOI] [PubMed] [Google Scholar]
- 125.Carta P, Zanazzi M, Caroti L. Impact of the pre-transplant histological score on 3-year graft outcomes of kidneys from marginal donors: a single-centre study Nephrol Dial Transplant 2013. 282637–2644 [DOI] [PubMed] [Google Scholar]
- 126.Azancot MA, Moreso F, Salcedo M. The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors Kidney Int 2014. 851161–1168 [DOI] [PubMed] [Google Scholar]
- 127.Bodzin AS, Leiby BE, Ramirez CB. Expanded criteria donor kidneys where the paired kidney is discarded owing to biopsy results: a concept that needs revision Exp Clin Transplant 2014. 12499–505 [PubMed] [Google Scholar]
- 128.Tavares da Silva E, Oliveira R, Castelo D. Pretransplant biopsy in expanded criteria donors: do we really need it? Transplant Proc 2014. 463330–3334 [DOI] [PubMed] [Google Scholar]
- 129.Escofet X, Osman H, Griffiths DF. The presence of glomerular sclerosis at time zero has a significant impact on function after cadaveric renal transplantation Transplantation 2003. 75344–346 [DOI] [PubMed] [Google Scholar]
- 130.Nyberg SL, Baskin-Bey ES, Kremers W. Improving the prediction of donor kidney quality: deceased donor score and resistive indices Transplantation 2005. 80925–929 [DOI] [PubMed] [Google Scholar]
- 131.Cicciarelli J, Cho Y, Mateo R. Renal biopsy donor group: the influence of glomerulosclerosis on transplant outcomes Transplant Proc 2005. 37712–713 [DOI] [PubMed] [Google Scholar]
- 132.Remuzzi G, Cravedi P, Perna A. Long-term outcome of renal transplantation from older donors N Engl J Med 2006. 354343–352 [DOI] [PubMed] [Google Scholar]
- 133.Kayler LK, Mohanka R, Basu A. Correlation of histologic findings on preimplant biopsy with kidney graft survival Transpl Int 2008. 21892–898 [DOI] [PubMed] [Google Scholar]
- 134.Anglicheau D, Loupy A, Lefaucheur C. A simple clinico-histopathological composite scoring system is highly predictive of graft outcomes in marginal donors Am J Transplant 2008. 82325–2334 [DOI] [PubMed] [Google Scholar]
- 135.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC. The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure Am J Transplant 2008. 82316–2324 [DOI] [PubMed] [Google Scholar]
- 136.Cockfield SM, Moore RB, Todd G. The prognostic utility of deceased donor implantation biopsy in determining function and graft survival after kidney transplantation Transplantation 2010. 89559–566 [DOI] [PubMed] [Google Scholar]
- 137.Navarro MD, López-Andréu M, Rodríguez-Benot A. Significance of preimplantation analysis of kidney biopsies from expanded criteria donors in long-term outcome Transplantation 2011. 91432–439 [DOI] [PubMed] [Google Scholar]
- 138.Fernández-Lorente L, Riera L, Bestard O. Long-term results of biopsy-guided selection and allocation of kidneys from older donors in older recipients Am J Transplant 2012. 122781–2788 [DOI] [PubMed] [Google Scholar]
- 139.De Vusser K, Lerut E, Kuypers D. The predictive value of kidney allograft baseline biopsies for long-term graft survival J Am Soc Nephrol 2013. 241913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hofer J, Regele H, Böhmig GA. Pre-implant biopsy predicts outcome of single-kidney transplantation independent of clinical donor variables Transplantation 2014. 97426–432 [DOI] [PubMed] [Google Scholar]
- 141.Philosophe B, Malat GE, Soundararajan S. Validation of the Maryland Aggregate Pathology Index (MAPI), a pre-implantation scoring system that predicts graft outcome Clin Transplant 2014. 28897–905 [DOI] [PubMed] [Google Scholar]
- 142.Losappio V, Stallone G, Infante B. A single-center cohort study to define the role of pretransplant biopsy score in the long-term outcome of kidney transplantation Transplantation 2014. 97934–939 [DOI] [PubMed] [Google Scholar]
- 143.Gandolfini I, Buzio C, Zanelli P. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes Am J Transplant 2014. 142515–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kosmoliaptsis V, Salji M, Bardsley V. Baseline donor chronic renal injury confers the same transplant survival disadvantage for DCD and DBD kidneys Am J Transplant 2015. 15754–763 [DOI] [PubMed] [Google Scholar]
- 145.Gill J, Cho YW, Danovitch GM. Outcomes of dual adult kidney transplants in the United States: an analysis of the OPTN/UNOS database Transplantation 2008. 8562–68 [DOI] [PubMed] [Google Scholar]
- 146.Tanriover B, Mohan S, Cohen DJ. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation Am J Transplant 2014. 14404–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Andrés A, Morales JM, Herrero JC. Double versus single renal allografts from aged donors Transplantation 2000. 692060–2066 [DOI] [PubMed] [Google Scholar]
- 148.Cruzado JM, Fernandez L, Riera L. Revisiting double kidney transplantation: two kidneys provide better graft survival than one Transplant Proc 2011. 432165–2167 [DOI] [PubMed] [Google Scholar]
- 149.Frutos MA, Mansilla JJ, Cabello M. Optimization of expanded donors using dual kidney transplantation: case-control study Transplant Proc 2012. 442060–2062 [DOI] [PubMed] [Google Scholar]
- 150.Snanoudj R, Rabant M, Timsit MO. Donor-estimated GFR as an appropriate criterion for allocation of ECD kidneys into single or dual kidney transplantation Am J Transplant 2009. 92542–2551 [DOI] [PubMed] [Google Scholar]
- 151.OPTN/UNOS. Double kidney allocation. Jul 25. 2013 Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf. [Google Scholar]
- 152.Rigotti P, Capovilla G, Di Bella C. A single-center experience with 200 dual kidney transplantations Clin Transplant 2014. 281433–1440 [DOI] [PubMed] [Google Scholar]
- 153.Veroux M, Corona D, Gagliano M. Monolateral dual kidney transplantation from marginal donors Transplant Proc 2007. 391800–1802 [DOI] [PubMed] [Google Scholar]
- 154.Gaber AO, Shokouh-Amiri H, Nezakatgoo N. Ipsilateral placement in double-kidney transplantation Transplantation 2007. 84929–931 [DOI] [PubMed] [Google Scholar]
- 155.Navarro AP, Sohrabi S, Reddy M. Dual transplantation of marginal kidneys from nonheart beating donors selected using machine perfusion viability criteria J Urol 2008. 1792305–2309 [DOI] [PubMed] [Google Scholar]
- 156.Fontana I, Magoni Rossi A, Gasloli G. Single-center experience in double kidney transplantation Transplant Proc 2010. 421108–1110 [DOI] [PubMed] [Google Scholar]
- 157.Hugen CM, Polcari AJ, Skolek R. Illinois statewide dual kidney transplantation experience—are we appropriately selecting kidneys? J Urol 2011. 186996–1000 [DOI] [PubMed] [Google Scholar]
- 158.Kim YH, Jung JH, Song KB. Adult dual kidney transplantations obtained from marginal donors: two case reports Transplant Proc 2012. 4457–59 [DOI] [PubMed] [Google Scholar]
- 159.Impedovo SV, De Lorenzis E, Volpe A. Middle and long-term outcomes of dual kidney transplant: a multicenter experience Transplant Proc 2013. 451237–1241 [DOI] [PubMed] [Google Scholar]
- 160.Balaz P, Rokosny S, Wohlfahrt P. Dual kidney transplant: a single-center experience and review of the literature Exp Clin Transplant 2013. 11388–395 [DOI] [PubMed] [Google Scholar]
- 161.Medina-Polo J, Pamplona-Casamayor M, Miranda-Utrera N. Dual kidney transplantation involving organs from expanded criteria donors: a review of our series and an update on current indications Transplant Proc 2014. 463412–3415 [DOI] [PubMed] [Google Scholar]
- 162.Moore PS, Farney AC, Sundberg AK. Dual kidney transplantation: a case-control comparison with single kidney transplantation from standard and expanded criteria donors Transplantation 2007. 831551–1156 [DOI] [PubMed] [Google Scholar]
- 163.Bertelli R, Varotti G, Puviani L. Bologna transplant center results in double kidney transplantation: update Transplant Proc 2007. 391833–1834 [DOI] [PubMed] [Google Scholar]
- 164.Salifu MO, Norin AJ, O’Mahony C. Long-term outcomes of dual kidney transplantation-a single center experience Clin Transplant 2009. 23400–406 [DOI] [PubMed] [Google Scholar]
- 165.Kayler LK, Mohanka R, Basu A. Single versus dual renal transplantation from donors with significant arteriosclerosis on pre-implant biopsy Clin Transplant 2009. 23525–531 [DOI] [PubMed] [Google Scholar]
- 166.D’Arcy FT, O’Connor KM, Shields W. Dual kidney transplantation with organs from extended criteria cadaveric donors J Urol 2009. 1821477–1481 [DOI] [PubMed] [Google Scholar]
- 167.Lucarelli G, Bettocchi C, Battaglia M. Extended criteria donor kidney transplantation: comparative outcome analysis between single versus double kidney transplantation at 5 years Transplant Proc 2010. 421104–1107 [DOI] [PubMed] [Google Scholar]
- 168.Ekser B, Furian L, Broggiato A. Technical aspects of unilateral dual kidney transplantation from expanded criteria donors: experience of 100 patients Am J Transplant 2010. 102000–2007 [DOI] [PubMed] [Google Scholar]
- 169.De Serres SA, Caumartin Y, Noël R. Dual-kidney transplants as an alternative for very marginal donors: long-term follow-up in 63 patients Transplantation 2010. 901125–1130 [DOI] [PubMed] [Google Scholar]
- 170.Nardo B, Bertelli R, Cavallari G. Analysis of 80 dual-kidney transplantations: a multicenter experience Transplant Proc 2011. 431559–1565 [DOI] [PubMed] [Google Scholar]
- 171.Schold JD, Kaplan B, Howard RJ. Are we frozen in time? Analysis of the utilization and efficacy of pulsatile perfusion in renal transplantation Am J Transplant 2005. 51681–1688 [DOI] [PubMed] [Google Scholar]
- 172.Matsuoka L, Shah T, Aswad S. Pulsatile perfusion reduces the incidence of delayed graft function in expanded criteria donor kidney transplantation Am J Transplant 2006. 61473–1478 [DOI] [PubMed] [Google Scholar]
- 173.Stratta RJ, Moore PS, Farney AC. Influence of pulsatile perfusion preservation on outcomes in kidney transplantation from expanded criteria donors J Am Coll Surg 2007. 204873–882 [DOI] [PubMed] [Google Scholar]
- 174.Buchanan PM, Lentine KL, Burroughs TE. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors Am J Transplant 2008. 82391–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abboud I, Antoine C, Gaudez F. Pulsatile perfusion preservation for expanded-criteria donors kidneys: Impact on delayed graft function rate Int J Artif Organs 2011. 34513–518 [DOI] [PubMed] [Google Scholar]
- 176.Treckmann J, Moers C, Smits JM. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death Transpl Int 2011. 24548–554 [DOI] [PubMed] [Google Scholar]
- 177.Moers C, Smits JM, Maathuis MJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7e19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 178.Moers C, Pirenne J, Paul A. Machine perfusion or cold storage in deceased-donor kidney transplantation N Engl J Med 2012. 366770–771 [DOI] [PubMed] [Google Scholar]
- 179.Gallinat A, Moers C, Smits JM. Machine perfusion versus static cold storage in expanded criteria donor kidney transplantation: 3-year follow-up data Transpl Int 2013. 26E52–E53 [DOI] [PubMed] [Google Scholar]
- 180.Jochmans I, Moers C, Smits JM. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial Ann Surg 2010. 252756–764 [DOI] [PubMed] [Google Scholar]
- 181.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study Am J Transplant 2013. 131246–1252 [DOI] [PubMed] [Google Scholar]
- 182.Gill J, Dong J, Eng M. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time Transplantation 2014. 97668–674 [DOI] [PubMed] [Google Scholar]
- 183.Gómez V, Orosa A, Rivera M. Resistance index determination in the pre and post kidney transplantation time points in graft dysfunction diagnosis Transplant Proc 2015. 4734–37 [DOI] [PubMed] [Google Scholar]
- 184.Burgos Revilla FJ, Hevia V, Diez V. Machine perfusion: initial results in an expanded criteria donor kidney transplant program Transplant Proc 2015. 4719–22 [DOI] [PubMed] [Google Scholar]
- 185.Jiao B, Liu S, Liu H. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS One. 2013;8:e81826. doi: 10.1371/journal.pone.0081826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Groen H, Moers C, Smits JM. Cost-effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation Am J Transplant 2012. 121824–1830 [DOI] [PubMed] [Google Scholar]
- 187.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient Transplantation 2007. 84285–291 [DOI] [PubMed] [Google Scholar]
- 188.Andrés A, Marcén R, Valdés F. A randomized trial of basiliximab with three different patterns of cyclosporin A initiation in renal transplant from expanded criteria donors and at high risk of delayed graft function Clin Transplant 2009. 2323–32 [DOI] [PubMed] [Google Scholar]
- 189.Andrés A, Budde K, Clavien PA. A randomized trial comparing renal function in older kidney transplant patients following delayed versus immediate tacrolimus administration Transplantation 2009. 881101–1108 [DOI] [PubMed] [Google Scholar]
- 190.González-Roncero FM, Gentil-Govantes MÁ, González-Molina M. Late evolution of kidney transplants in elderly donors and recipients receiving initial immunosuppressant treatment with daclizumab, mycophenolate mofetil, and delayed introduction of tacrolimus Nefrologia 2012. 32446–454 [DOI] [PubMed] [Google Scholar]
- 191.Guba M, Rentsch M, Wimmer CD. Calcineurin-inhibitor avoidance in elderly renal allograft recipients using ATG and basiliximab combined with mycophenolate mofetil Transpl Int 2008. 21637–645 [DOI] [PubMed] [Google Scholar]
- 192.Arbogast HP, Hoffmann JN, Illner WD. Calcineurin inhibitor-free immunosuppressive strategy in elderly recipients of renal allografts from deceased donors: 1-year results from a prospective single center trial Transplant Proc 2009. 412529–2532 [DOI] [PubMed] [Google Scholar]
- 193.Durrbach A, Rostaing L, Tricot L. Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor Transplantation 2008. 85486–490 [DOI] [PubMed] [Google Scholar]
- 194.Luke PP, Nguan CY, Horovitz D. Immunosuppression without calcineurin inhibition: optimization of renal function in expanded criteria donor renal transplantation Clin Transplant 2009. 239–15 [DOI] [PubMed] [Google Scholar]
- 195.Sánchez-Escuredo A, Alsina A, Diekmann F. Polyclonal versus monoclonal induction therapy in a calcineurin inhibitor-free immunosuppressive therapy in renal transplantation: a comparison of efficacy and costs Transplant Proc 2015. 4745–49 [DOI] [PubMed] [Google Scholar]
- 196.Gill J, Sampaio M, Gill JS. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States Clin J Am Soc Nephrol 2011. 61168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sancho Calabuig A, Gavela Martínez E, Kanter Berga J. Safety and efficacy of induction treatment with low thymoglobulin doses in kidney transplantation from expanded-criteria donors Transplant Proc 2015. 4750–53 [DOI] [PubMed] [Google Scholar]
- 198.Hardinger KL, Brennan DC, Schnitzler MA. Rabbit antithymocyte globulin is more beneficial in standard kidney than in extended donor recipients Transplantation 2009. 871372–1376 [DOI] [PubMed] [Google Scholar]
- 199.Patel SJ, Knight RJ, Suki WN. Rabbit antithymocyte induction and dosing in deceased donor renal transplant recipients over 60 yr of age Clin Transplant 2011. 25E250–E256 [DOI] [PubMed] [Google Scholar]
- 200.Khanmoradi K, Knorr JP, Feyssa EL. Evaluating safety and efficacy of rabbit antithymocyte globulin induction in elderly kidney transplant recipients Exp Clin Transplant 2013. 11222–228 [DOI] [PubMed] [Google Scholar]
- 201.Durrbach A, Pestana JM, Pearson T. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant 2010. 10547–557 [DOI] [PubMed] [Google Scholar]
- 202.Larsen CP, Grinyó J, Medina-Pestana J. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-Year results from the BENEFIT and BENEFIT-EXT studies Transplantation 2010. 901528–1535 [DOI] [PubMed] [Google Scholar]
- 203.Pestana JO, Grinyo JM, Vanrenterghem Y. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys Am J Transplant 2012. 12630–639 [DOI] [PubMed] [Google Scholar]
- 204.Vanrenterghem Y, Bresnahan B, Campistol J. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation 2011. 91976–983 [DOI] [PubMed] [Google Scholar]
- 205.Charpentier B, Medina Pestana JO, Del C. Long-term exposure to belatacept in recipients of extended criteria donor kidneys Am J Transplant 2013. 132884–2891 [DOI] [PubMed] [Google Scholar]
- 206.Vincenti F, Rostaing L, Grinyo J. Belatacept and long-term outcomes in kidney transplantation N Engl J Med 2016. 374333–343 [DOI] [PubMed] [Google Scholar]
- 207.Montero N, Pérez-Sáez MJ, Pascual J. Immunosuppression in the elderly renal allograft recipient: a systematic review Transplant Rev (Orlando 2016. 30144–153 [DOI] [PubMed] [Google Scholar]