Abstract
Background
Native nephrectomy in pediatric kidney transplant recipients is performed for multiple indications. Posttransplant hypertension requiring medical management is common, and the effect of native nephrectomy on posttransplant hypertension is poorly studied. Our aim is to evaluate the impact of native nephrectomy on posttransplant hypertension.
Methods
One hundred thirty-six consecutive pediatric kidney transplant recipients from 2007 to 2012 were studied at a single institution and divided into 2 groups: no nephrectomy and native nephrectomy (unilateral and bilateral nephrectomy). Antihypertensive medication use was evaluated before nephrectomy/transplant, at discharge from transplant and at 1, 3, and 5 years posttransplant.
Results
In a bivariate analysis, nephrectomy was associated with a significant reduction in the percentage of patients requiring antihypertensive medication at the time of discharge (27.3%) and 1 year posttransplant (10.7%) as compared with patients without nephrectomy (71.7%, and 50%, respectively, P < 0.05). This trend toward reduction in antihypertensive medication in the nephrectomy group as compared with the no nephrectomy group persisted at 3 (18.6% versus 43.2%) and 5 years (19.7% versus 37.5%) posttransplant. Multivariable logistic regression demonstrated that patients without native nephrectomy had higher odds of requiring antihypertensive medication at the time of discharge (3.3) and 1 year (5.2) as compared with patients who underwent native nephrectomy (P = 0.036 and P = 0.013, respectively).
Conclusions
Native nephrectomy reduces the odds of needing antihypertensive medication after transplant. The impact of native nephrectomy is crucial to the comprehensive management of pediatric transplant recipients where medication compliance is challenging and lifelong hypertension is known to negatively impact cardiovascular health.
Native nephrectomy before or at the time of transplant is advocated in pediatric kidney transplant recipients with anatomical constraints, congenital anomalies, proteinuria, intractable hypertension, malignancy, and chronic infection.1–3 Specifically, pediatric patients often undergo nephrectomy when there is a perceived risk to the recipient or the graft. Cardiovascular disease is a significant cause of morbidity and mortality in pediatric patients with end-stage renal disease (ESRD) reducing lifespan by as much as 40 to 60 years for children with ESRD.4,5 Although renal transplantation improves renal-associated risk factors for cardiovascular disease, more than 50% of these patients still have hypertension after transplant.6–8 Even after transplant, the life expectancy of children with hypertension is 25 years shorter than age-matched controls with 25% of deaths due to cardiac morbidity.4
The impact of the native kidneys on the developing pathophysiology of pretransplant cardiovascular disease is multifactorial.7,9 However, the effects of the native kidney on persistent hypertension and cardiovascular disease after transplant are less clear.6,8 It has been postulated that native nephrectomy before or at the time of transplant in patients with ESRD may improve posttransplant hypertension. This largely stems from the idea that the native kidney still may exert inflammatory, hormonal, and biochemical effects on the cardiovascular system. The data on the actual impact of nephrectomy on hypertension is very limited. In a small study, Cavallini et al6 suggested that nephrectomy for the indication of hypertension before transplantation did not significantly influence long-term blood pressure control or the prevalence of left ventricular hypertrophy (LVH) after kidney transplantation. In another study, a small subset of patients with persistent arterial hypertension and large-volume proteinuria pretransplant had a significant reduction in hypertension after nephrectomy.2 These few studies limit our ability to draw a clear conclusion regarding the impact of nephrectomy on hypertension after transplant.
Given the limited findings in the literature, the primary aim of this study was to analyze the impact of native nephrectomy for all indications on posttransplant hypertension in pediatric renal transplant recipients.
MATERIALS AND METHODS
The institutional review board at Stanford University approved this study. We conducted a retrospective study of all pediatric patients younger than 18 years who underwent renal transplantation at Lucile Packard Children’s Hospital from 2007 to 2012. Data were gathered from the electronic medical record including demographic data, etiology of ESRD, history of prior transplants or multi organ transplants, history of nephrectomy, hypertensive medications pretransplant and posttransplant, immunosuppression, and biopsy-proven rejection. Indications for nephrectomy included congenital anomalies, proteinuria, polyuria, intractable hypertension, malignancy, and chronic infection. Patients were excluded if they were older than 18 years, had a history of a previous renal transplant (≥1) before the study period or underwent multiorgan transplant. If a patient proceeded to graft failure, defined as a creatinine clearance less than 15 mL/min per 1.73 m2, or required retransplantation during the study window, data from that time point forward were excluded. The remaining patients (n = 123) were categorized into 2 groups: no native nephrectomy (n = 46) and native nephrectomy (either unilateral or bilateral, n = 77) at discharge. Thirty-three patients were excluded from the study over the 5-year period. Nine patients in the no nephrectomy group and 4 patients in the nephrectomy group experienced graft failure over the 5-year study period (n = 13). Thirteen patients in the no nephrectomy group and 7 patients in the nephrectomy group transitioned care to outside hospitals due to relocation or progression to care at an unidentified adult hospital by 5 years posttransplant (n = 20). In review of the Scientific Registry of Transplant Recipients database, these patients had continued graft function but no additional data were available regarding their posttransplant management. The number of antihypertensive medications was evaluated immediately before nephrectomy/transplant, at the time of discharge from transplant and at 1, 3, and 5 years after transplant. Diuretics were not included as an antihypertensive medication. For the subset of patients that underwent nephrectomy before transplant, antihypertensive medications were evaluated before nephrectomy, postnephrectomy, and before transplant. Creatinine clearance as determined by the Schwartz formula10 was evaluated at 1, 3, and 5 years posttransplant.
STATISTICAL ANALYSIS
Univariate descriptive analysis was performed for demographic data and is expressed either as a percentage or a median and range for patients in each group. For bivariate analysis, the Pearson χ2 test was used for categorical variables, and the unpaired Student t test or the Mann-Whitney U test were used as appropriate for continuous variables. A 2-tailed analysis was performed in all tests. Multivariable logistic regression was performed to ascertain the effects of age, donor type, steroid-based immunosuppression, nephrectomy status, biopsy-proven rejection, and creatinine clearance on the odds that patients used antihypertensive medication at discharge from transplant and at 1, 3, and 5 years posttransplant. The first 4 variables were used at discharge from transplant, and all 6 variables were used at 1, 3, and 5 years posttransplant. Rejection and creatinine clearance were not included in the regression model at the time of discharge because they were not clinically relevant. For all statistical tests, an a priori critical P value less than 0.05 was used to determine significance. All statistical analyses were performed using IBM SPSS Statistics Version 24.0.
RESULTS
Demographics and Etiology of ESRD
Of the 136 pediatric patients who underwent pediatric renal transplant between 2007 and 2012, 7 patients were excluded given prior kidney transplant and 6 patients were excluded as they received a multiorgan transplant. Of the remaining 123 patients, 77 underwent native nephrectomy before or at the time of transplant and 46 did not undergo nephrectomy. Of the 77 patients who had a native nephrectomy, 39 underwent a unilateral nephrectomy and 38 underwent a bilateral nephrectomy. The majority of patients who underwent unilateral nephrectomy had the nephrectomy performed at the time of transplant (38 of 39 patients). In patients who had bilateral nephrectomies, 33 of 38 patients had both kidneys removed simultaneously with 26 patients having bilateral nephrectomies before kidney transplant and 7 patients at the time of transplant. Five patients underwent staged bilateral nephrectomies, 4 of which were completed before transplant.
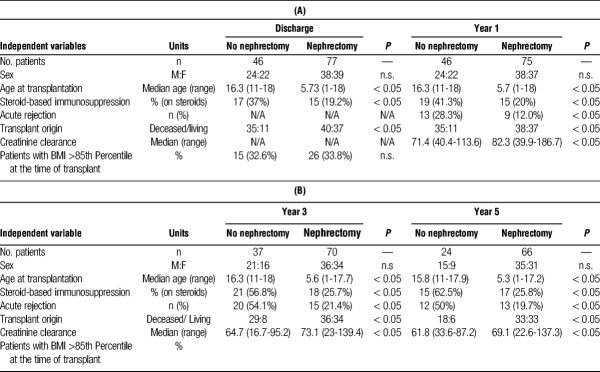
Between the 2 groups, there was no significant difference in sex or percentage of patients with a body mass index (BMI) of 85th percentile or greater at the time of transplantation (Table 1). HLA matches were compared between groups; the average HLA match was 1.8/6 for the no nephrectomy group and 2.1/6 for the nephrectomy group (data not shown, P = 0.262). Age at transplant, donor origin (deceased versus living), steroid-based immunosuppression, creatinine clearance and biopsy-proven rejection were significantly different between groups (Table 1, P < 0.05). Given these findings, the above variables were included in a multivariable logistic regression model to determine their relative impact on the use of antihypertensive medication at the time of discharge from transplant and at years 1, 3, and 5 posttransplant (Table 4A and B, Table 5A and B). These data will be discussed in greater detail below.
TABLE 1.
Demographic and clinical characteristics at each timepoint
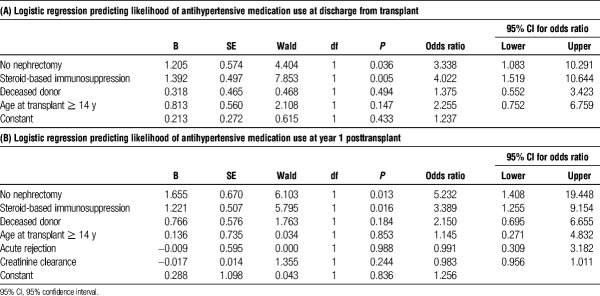
TABLE 4.
Multivariate regression at discharge (A) and year 1 (B) after transplant
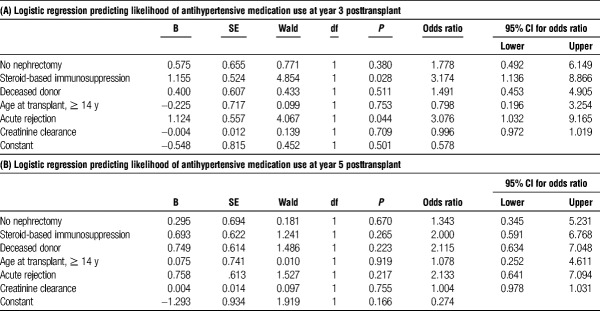
TABLE 5.
Multivariate regression at year 3 (A) and year 5 (B) after transplant
Among all patients, the most prevalent known etiology of ESRD was congenital anomalies of the kidney and urinary tract (CAKUT) followed by glomerulonephritis (Table 2). The 3 most common indications for nephrectomy were urologic/infectious reasons (27%), anatomic constraints (space for allograft, 20%) and proteinuria (14%). Refractory hypertension accounted for 5.2% of the patients in the study (Table 3).
TABLE 2.
Etiology of ESRD
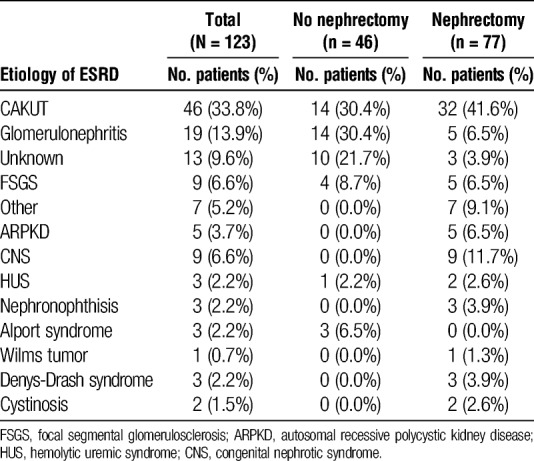
TABLE 3.
Nephrectomy indications of the 77 patients who underwent nephrectomy

Hypertension
There was no difference between the percentage of patients on antihypertensive medication in either the nephrectomy (63.6%) and no nephrectomy (73.9%) groups before transplant or nephrectomy (Figure 1). However, there was a significant reduction in the percentage of patients requiring antihypertensive medication at the time of discharge from transplant whom underwent nephrectomy (27.3%) as compared with patients without nephrectomy (71.7%, P < 0.001). At 1 year posttransplant, only 10.7% of patients that underwent nephrectomy remained on antihypertensive medication as compared with 50% of the patients in the no nephrectomy group (Figure 1, P < 0.001). Multivariable logistic regression analysis demonstrated that patients who did not undergo native nephrectomy had a 3.3 higher odds of requiring antihypertensive medication at the time of discharge from transplant compared with patients that underwent nephrectomy (Table 4A, P = .036). This difference persisted out to 1 year posttransplant, where patients in the no nephrectomy group had a 5.2 higher odds of requiring antihypertensive medication compared to patients in the nephrectomy group (Table 4B, P = 0.013).
FIGURE 1.
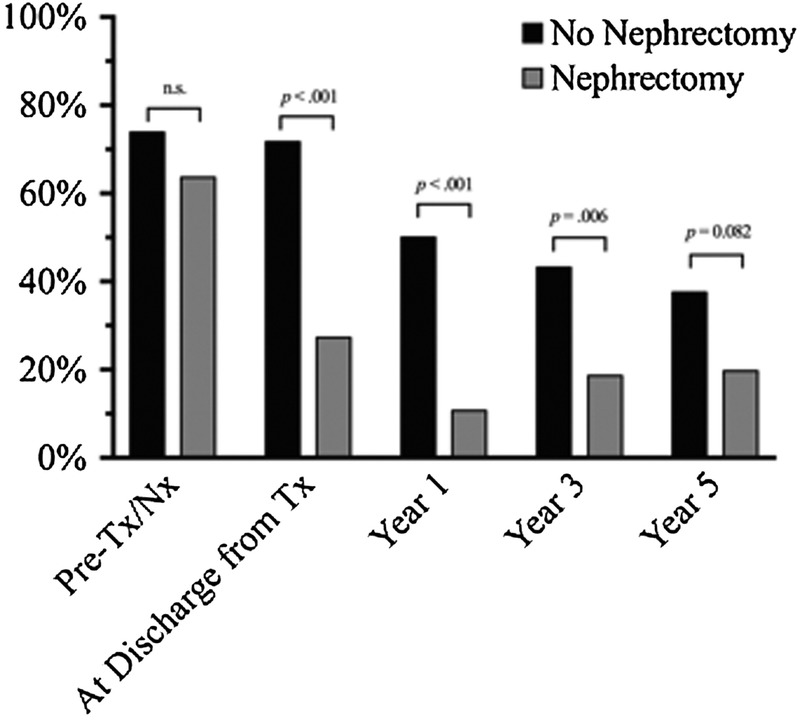
Percentage of patients on antihypertensive medication pretransplant, at discharge from transplant, 1, 3, and 5 years after transplant. Black indicates no nephrectomy and gray indicates nephrectomy. Specific P values between groups at a given time point are denoted with bars as determined by the Pearson χ2 test.
After nephrectomy, we saw a trend toward reduction in the use of antihypertensive medication in the nephrectomy group as compared to the no nephrectomy group at years 3 (18.6% vs 43.2%) and 5 (19.7% vs 37.5%) posttransplant (Figure 1). However, these data were not statistically significant in our multivariable regression at 3 and 5-years posttransplant (Table 5A and B). These findings may be influenced by a 27% loss to follow up at 5 years, as 33 patients were excluded from analysis by year 5 posttransplant (13 due graft failure and 20 secondary to transitions in care). Given this, we did not have significant power to assess the impact of nephrectomy on hypertension at these later time points.
As 31 of 77 patients underwent native nephrectomy before receiving their kidney transplant, we conducted a separate analysis to investigate if use of antihypertensive medication in these patients changed after receiving a pretransplant nephrectomy or after undergoing their subsequent kidney transplant. Twenty-three of 31 patients who received a pretransplant nephrectomy displayed a decreased use of antihypertensive medication by time of discharge from transplant. 55.3% of this decrease in antihypertensive medications occurred after nephrectomy but before transplantation.
Impact of Immunosuppression and Rejection on Hypertension
All patients received induction immunosuppression with antithymocyte immunoglobulin or daclizumab. Tacrolimus and mycophenolate mofetil were used for maintenance immunosuppression. Patients received steroid-based immunosuppression based on preoperative sensitization status and donor compatibility. Steroid-based immunosuppression was defined as the use of steroids as part of the daily immunosuppression regimen for each recipient after discharge from transplant. If patients were transitioned to steroid-based immunosuppression at a later time point, they were then included in the steroid-based group starting from that time point forward.
Thirty-seven percent of patients that did not undergo nephrectomy were started on maintenance steroid-based immunosuppression as compared to 19.5% in the native nephrectomy group (P < 0.05). This approximately twofold increase in steroid-based immunosuppression within the nephrectomy group as compared with the no nephrectomy group persisted at years 1, 3, and 5 posttransplant. In multivariable regression analysis, the use of steroid-based immunosuppression at was associated with a 4.0 and 3.4 higher odds of requiring antihypertensive medication at discharge and 1 year posttransplant, respectively (Table 4A, P < 0.01 and P < 0.02). At 3 years posttransplant, patients on steroid-based immunosuppression had a 3.2 higher odds of being on antihypertensive medication (Table 5A, P < 0.05) but this difference did not persist out to 5 years posttransplant (Table 5B).
Additionally, 13 patients in the no nephrectomy group experienced a rejection episode as compared with 9 patients who underwent nephrectomy (P < 0.05) at 1 year. This difference persisted out to years 3 and 5 posttransplant with a ~2.5-fold increase in the percentage of patients who experienced a rejection episode in the no nephrectomy group as compared with the nephrectomy group (Table 1, P < 0.05). Multivariable logistic regression analysis demonstrated that acute rejection was associated with a 3.0 higher odds of being on antihypertensive medication at year 3 posttransplant (P = 0.044), but was not a significant predictor at all other time points (Table 4&5).
Allograft Function
Using creatinine clearance as a proxy of allograft function, there was superior graft function in the nephrectomy group as compared to the no nephrectomy group at years 1, 3, and 5 after transplant (Table 1, P < 0.05). In our multivariable regression model, this difference did not contribute to the use of antihypertensive medication (Table 4B, Table 5).
DISCUSSION
The incidence of native nephrectomy before or at the time of transplant has decreased; however, there is a relative paucity of evidence regarding the impact of the native kidney on host physiology. Persistent cardiac morbidity and mortality after renal transplant remains a significant concern in the pediatric population and varying reports have implicated the role of nephrectomy in mitigating cardiac factors.11–14 To our knowledge, we are the largest study to demonstrate a benefit of nephrectomy on posttransplant hypertension in pediatric renal transplant recipients. We report a reduction in the percentage of patients requiring antihypertensive medication after nephrectomy at discharge from transplant and 1 year posttransplant as well an increased odds ratio for the use of antihypertensive medication in patients who did not undergo nephrectomy. Of added interest, in patients that underwent nephrectomy before transplant, there was a reduction in the use of antihypertensive medications after nephrectomy and preceding transplantation, suggesting a deleterious role of the native kidney independent of the benefits of transplantation. In support of our findings, a recent manuscript by Shumate et al described the benefit of native nephrectomy in autosomal dominant polycystic kidney disease patients.14 In this study, native nephrectomy was associated with a significant decrease in the defined daily dose of antihypertensive medication as compared to patients without nephrectomy at 24 and 36 months posttransplant.14
Although we saw a trend toward a decreased use of antihypertensive medication in the no nephrectomy group at 3 and 5 years posttransplant with bivariate analysis, nephrectomy was not an independent predictor of antihypertensive medication. Given the number of patients that were excluded at later time points either to graft rejection or transitions in care, we were not able to determine the independent impact of nephrectomy on hypertension at years 3 and 5. We hope that continued study of this patient cohort over the coming years will provide additional data to ascertain if nephrectomy acts to directly improves long-term hypertension posttransplant.
Cavallini et al6 found no difference in mean arterial pressure, number of antihypertensive medications and LVH in a small cohort of pediatric patients who had undergone transplant with or without nephrectomy. Different from our study design, the authors did not evaluate hypertension and use of antihypertensive medications longitudinally after transplant but rather at 1 time point at a variable interval after transplant. Also, the sole indication for all nephrectomies in that study was intractable hypertension, with a greater number of blood pressure medications used in patients before transplant who had undergone bilateral nephrectomies (n = 14) as compared with both unilateral (n = 18) and no nephrectomy groups (n = 35). After transplant, this difference in the number of blood pressure medications was the same across groups. Together, these data suggest that the benefits of nephrectomy were marginalized by the nature of the inclusion criteria and that, as demonstrated in our study, nephrectomy may actually impact the use of hypertensive medications after transplant.
Antihypertensive management in our cohort was initiated by pediatric transplant surgeons, nephrologists and intensivists. After discharge, blood pressures were monitored via home blood pressure measurement as well as resting clinic blood pressures. We recognize that lack of prescription antihypertensive medication may not indicate adequate blood pressure control; however, 24-hour ambulatory blood pressure monitoring data were not available for this cohort. Similarly, echocardiographic measurements of ejection fraction and presence of LVH posttransplant were not available for the majority of our patients. LVH has been shown to be reduced but not necessarily normalized after transplant.15 Multiple factors pretransplant (uremia, age, and diabetes) as well as posttransplant (inflammatory cascade, immunosuppression, hypertension, anemia, and malnutrition) have been identified as contributors.16 Hypertension is a modifier of cardiac hypertrophy and remodeling,17 and given that it is a treatable clinical parameter for cardiac morbidity, we chose this as our primary outcome. We recognize this limitation, specifically in light of the findings by Cavallini et al. Future work to delineate the impact of improved hypertension after nephrectomy as assessed by ambulatory blood pressure monitoring on cardiac remodeling with longitudinal echocardiographic assessment is warranted.
Long-term steroid use is known to predicate the development of hypertension. We did see that steroid-based immunosuppression increased the odds that a patient would be on antihypertensive medication as expected. However, in our multivariable regression, no nephrectomy was actually a stronger predictor of need for antihypertensive medication at 1 year after transplant. Although long-term steroid use is certainly a risk factor for hypertension,18,19 our data support that nephrectomy acts as an independent factor in reducing hypertension after transplant.
Arguments against nephrectomy cite the need for attentive pretransplant fluid management, prolonged anesthesia exposure, operative complications (ie, bleeding, infection, and injury to surrounding structures, such as the liver or spleen) and the benefits of having the native ureter available for reconstruction should a complication develop with the transplanted ureters.20 Interestingly, the patients who did undergo nephrectomy in the above study by Fraser et al did so with minimal morbidity and with favorable graft outcomes. These authors did not evaluate any long-term parameters of cardiac outcomes, and in light of our data, we would favor nephrectomy in the majority of these patients to limit hypertension. As with any surgical indication, careful assessment of each surgical candidate is necessary.
It is also noteworthy that in the pediatric transplant population, there is a high percentage of graft loss secondary to medication noncompliance. Numerous studies have demonstrated that compliance improves with medication simplification21,22 and reducing pill burden directly improves overall adherence rates.23 In the first year posttransplant, medication regimens are often the most elaborate. Fifty percent of patients without nephrectomies required antihypertensive medications compared with approximately 11% in nephrectomy group; reduction in antihypertensive regimens could positively impact adherence and compliance, in turn promoting graft longevity.
In addition to some of the limitations discussed above, this is a retrospective study, and future prospective trials to delineate the role of nephrectomy on hypertension and long-term cardiac morbidity are clearly warranted. Given the data presented here, we continue to support native nephrectomy for appropriate indications in pediatric renal transplant recipients as we demonstrate a positive effect of nephrectomy in reducing hypertension in the posttransplant period. The potential benefit of native nephrectomy on cardiac morbidity in a patient population plagued by decreased longevity with a disproportionate incidence of cardiac-related death should not be dismissed.
Footnotes
The authors declare no funding or conflicts of interest.
A.L.B. and D.J.S. share co-first authorship.
A.L.B. participated in data collection and analysis and principal writing of article. D.J.S. participated in data collection and analysis as well as writing of the article. A.C. participated in writing and editing of the article. L.M. participated in data collection. P.C.G. participated in writing and editing of the article. W.C. participated in writing and editing of the article. A.E.G. participated in the research inception and design, data analysis, and writing of the article.
Correspondence: Aleah L Brubaker, MD PhD, Division of General Surgery, Department of Surgery, Stanford University Medical Center 300 Pasteur Drive, Rm H3591 Stanford, CA 94305-5641. (abrubake@stanford.edu).
This single-center retrospective analysis including 136 pediatric kidney transplant recipients suggests that native nephrectomy is associated with lower number of antihypertensive medication following transplantation. This intervention may facilitate medication compliance and improve long-term outcomes.
REFERENCES
- 1.Cochat P, Ranchin B. Is there a need for a multicenter study to determine the optimal approach to recurrent nephrotic syndrome following renal transplantation? Pediatr Transplant 2001. 5394–397 [DOI] [PubMed] [Google Scholar]
- 2.Sharbaf FG, Bitzan M, Szymanski KM. Native nephrectomy prior to pediatric kidney transplantation: biological and clinical aspects Pediatr Nephrol 2012. 271179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JM, Ho PL, McDonald RA. Renal transplant outcomes in adolescents: a report of the North American Pediatric Renal Transplant Cooperative Study Pediatr Transplant 2002. 6493–499 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System: USRDS 2011 Annual Report. Bethesda; 2011. [Google Scholar]
- 5.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease J Am Soc Nephrol 2012. 23578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavallini M, Di Zazzo G, Giordano U. Long-term cardiovascular effects of pre-transplant native kidney nephrectomy in children Pediatr Nephrol 2010. 252523–2529 [DOI] [PubMed] [Google Scholar]
- 7.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease Adv Chronic Kidney Dis 2005. 12397–405 [DOI] [PubMed] [Google Scholar]
- 8.Becker-Cohen R, Nir A, Rinat C. Risk factors for cardiovascular disease in children and young adults after renal transplantation Clin J Am Soc Nephrol 2006. 11284–1292 [DOI] [PubMed] [Google Scholar]
- 9.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease N Engl J Med 2004. 3502654–2662 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Muñoz A, Schneider MF. New equations to estimate GFR in children with CKD J Am Soc Nephrol 2009. 20629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power RE, Calleary JG, Hickey DP. Pre-transplant bilateral native nephrectomy for medically refractory hypertension Ir Med J 2001. 94214–216 [PubMed] [Google Scholar]
- 12.Bales GT, Fellner SK, Chodak GW. Laparoscopic bilateral nephrectomy for renin-mediated hypertension Urology 1994. 43874–877 [DOI] [PubMed] [Google Scholar]
- 13.Vanrenterghem Y, Waer M, Christiaens MR. Bilateral nephrectomy of the native kidneys reduces the incidence of arterial hypertension and erythrocytosis in kidney graft recipients treated with cyclosporin. Leuven Collaborative Group for Transplantation Transpl Int 1992. 5S35–S37 [DOI] [PubMed] [Google Scholar]
- 14.Shumate AM, Bahler CD, Goggins WC. Transplantation/vascular surgery native nephrectomy with renal transplantation is associated with a decrease in hypertension medication requirements for autosomal dominant polycystic kidney disease J Urol 2016. 195141–146 [DOI] [PubMed] [Google Scholar]
- 15.De Lima JJ, Vieira ML, Viviani LF. Long-term impact of renal transplantation on carotid artery properties and on ventricular hypertrophy in end-stage renal failure patients Nephrol Dial Transplant 2002. 17645–651 [DOI] [PubMed] [Google Scholar]
- 16.Hernández D. Left ventricular hypertrophy after renal transplantation: new approach to a deadly disorder Nephrol Dial Transplant 2004. 191682–1686 [DOI] [PubMed] [Google Scholar]
- 17.Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden death Curr Cardiol Rep 2002. 4449–457 [DOI] [PubMed] [Google Scholar]
- 18.Knight SR, Morris PJ. Steroid sparing protocols following nonrenal transplants; the evidence is not there. A systematic review and meta-analysis Transpl Int 2011. 241198–1207 [DOI] [PubMed] [Google Scholar]
- 19.Bamgbola O. Metabolic consequences of modern immunosuppressive agents in solid organ transplantation Ther Adv Endocrinol Metab 2016. 7110–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser N, Lyon PC, Williams AR. Native nephrectomy in pediatric transplantation—less is more! J Pediatr Urol 2013. 984–89 [DOI] [PubMed] [Google Scholar]
- 21.Claes A, Decorte A, Levtchenko E. Facilitators and barriers of medication adherence in pediatric liver and kidney transplant recipients: a mixed-methods study Prog Transplant 2014. 24311–321 [DOI] [PubMed] [Google Scholar]
- 22.Lee JL, Eaton C, Gutierrez-Colina AM. Longitudinal stability of specific barriers to medication adherence J Pediatr Psychol 2014. 39667–676 [DOI] [PubMed] [Google Scholar]
- 23.Neri L, Martini A, Andreucci VE. Regimen complexity and prescription adherence in dialysis patients Am J Nephrol 2011. 3471–76 [DOI] [PubMed] [Google Scholar]