Abstract
Glucose homeostasis depends on the coordinated secretion of glucagon, insulin and GLP-1 by pancreas and intestine. Obesity which is associated with an increased risk of developing insulin resistance and type 2 diabetes affects the function of these organs. Here we investigate the functional and molecular adaptations of proglucagon-producing cells in obese mice to better define their involvement in type 2 diabetes development.
We used GLU-Venus transgenic male mice specifically expressing Venus fluorochrome in proglucagon-producing cells. Mice were subjected to 16 weeks of low-fat (LFD) or high-fat (HFD) diets, and then subdivided by measuring glycated haemoglobin (HbA1c) in three groups: LFD mice and I-HFD (glucose-intolerant) mice with similar HbA1c and H-HFD (hyperglycemic) mice which exhibited higher HbA1c.
At 16 weeks, both HFD groups exhibited similar weight gain, hyperinsulinemia and insulin resistance. However, I-HFD mice exhibited better glucose tolerance compared to H-HFD mice. I-HFD mice displayed functional and molecular adaptations of enteroendocrine L-cells resulting in increased intestinal GLP-1 biosynthesis and release as well as maintained pancreatic alpha- and beta-cells functions. By contrast, H-HFD mice exhibited dysfunctional L, alpha- and beta-cells with increased beta- and L-cell numbers. Administration of the GLP-1R antagonist Exendin9-39 in I-HFD mice led to hyperglycemia and alterations of glucagon secretion without changes in insulin secretion.
Our results highlight the cross-talk between islet and intestine endocrine cells and indicate that a compensatory adaptation of L-cell function in obesity plays an important role in preserving glucose homeostasis through the control of pancreatic alpha-cell functions.
Keywords: Diet-induced obesity, GLP-1, glucagon, FACS-purified alpha-cells, FACS-purified L-cells, Exendin9-39
Introduction
The control of glycemia depends largely on the coordinated secretion of glucagon and insulin by pancreatic alpha and beta-cells respectively and GLP-1 by enteroendocrine L-cells. Obesity is associated with insulin resistance and an increased type 2 diabetes risk (1). When insulin resistance is accompanied by dysfunction of pancreatic islet cells, hyperglycemia results (2). The disrupted coordination of glucagon and insulin secretion observed in type 2 diabetes is characterized by impaired and delayed insulin secretion as well as basal hyperglucagonemia and non-suppressed glucagon secretion in response to glucose (3,4).
GLP-1, which is secreted from L-cells along the intestinal tract and from specific cells of the central nervous system, exerts pleiotropic biological actions including stimulation of glucose-dependent insulin secretion and biosynthesis as well as glucagon secretion inhibition, gastric emptying and food intake inhibition (5). Several studies have shown that the incretin effects on insulin secretion is diminished in type 2 diabetic patients due to defects of intestinal GLP-1 secretion and beta-cells responsiveness (6). GLP-1R agonists have been developed as type 2 diabetes treatment (7) where the acute glucose-lowering actions of GLP-1 are secondary to inhibition of gastric emptying and glucagon secretion as well as stimulation of insulin secretion (8). Indeed alpha-cells retain near normal responsiveness to GLP-1 infusion, with inhibition of glucagon secretion seen to the same extent in diabetic compared to non-diabetic subjects (9).
A large number of studies have examined the consequences of diabetes on islet and intestinal endocrine cell functions using different animal models among them diet-induced obesity (DIO) (10). HFD-fed mice exhibit impaired glucose tolerance and insulin resistance leading to hyperglycemia, hyperinsulinemia and dysregulated glucagon secretion (11). However, these mice exhibit a high variability in their metabolic response to diet with phenotypic variations involving sex, genetic background, food intake, stress, physical activity and microbiome composition (12,13).
Importantly, glucagon receptor knock-out HFD mice have been reported to exhibit better glycemic control and reduced hyperinsulinemia (11). Furthermore GLP-1R agonist treatment of HFD mice led to reversion of obesity and insulin resistance (14). These data underline the critical roles exerted by glucagon and GLP-1 in the development of hyperglycemia in obese rodents.
Functional and molecular alterations of pancreatic alpha- and intestinal L-cell in obesity and diabetes remain still largely unknown. Thus a better understanding of alpha- and L-cell function and dysfunction should allow a better design of therapeutic approaches for diabetes care.
We thus aimed to explore the functional and molecular alterations of proglucagon-producing cells from pancreas and intestine in obesity to bring new insights in the factors involved in the maintenance of normoglycemia or leading to diabetes.
Using transgenic GLU-Venus male mice subjected to 16 weeks of HFD or LFD, we demonstrated that adaptation of intestinal L-cell function, mainly by increases of GLP-1 biosynthesis and secretion along with adapted pancreatic alpha- and beta-cell function, maintains glycemic control in I-HFD compared to H-HFD obese mice. Our results indicate that diet-induced obesity associated with hyperglycemia involves specific functional and molecular alterations in pancreatic alpha- and L-cells where GLP-1 compensation represents a critical factor in the preservation of pancreatic alpha-cells function and glucose homeostasis in obese male mice.
Materials and Methods
Animals
The transgenic mice C57Bl/6J-Tg(GLU-Venus) were obtained from collaboration with Dr Fiona Gribble (Cambridge, UK). The GLU-Venus mice express specifically the Venus fluorochrome in proglucagon-producing cells as previously described (15,16). Mice, which were bred in conventional housing, were subjected to experimental procedures according to ethical approbation by Swiss federal committee in the University of Geneva Medical School. 10 weeks-old male GLU-Venus mice were fed with a low-fat diet (LFD-control, 10% of fat) or a high-fat diet (HFD, 60% of fat) during 16 weeks.
HbA1c measurements were performed after 15 weeks of diet. Oral glucose tolerance tests (OGTT), pancreatic perfusion and ex vivo experiments as well as sample collections were performed after 16 weeks of protocol (Supplemental Fig.1). Glycemia as well as plasma insulin, glucagon and GLP-1 levels were measured before and after D-glucose gavages on 8h-fasted mice. Areas under the curve (AUC) were calculated with the trapezoidal rule for each group. Insulin sensitivity index (ISI) was calculated with the formula: 10000/(√[8h-fasting-glycemia x 8h-fasting-insulinemia x mean OGTT-glycemia x mean OGTT-insulinemia]) (17).
Ex9-39 (Sigma, Basel, Switzerland), a GLP-1 antagonist, was administrated acutely in I-HFD mice by intraperitoneal injection after 16 weeks of diet or chronically delivered (0,5μ1/h) by subcutaneous implantation for 14 additional days using Alzet pumps (Alzet 2002; Charles River, L’Arbresle, France).
Glucagon, insulin, GLP-1, GIP, leptin, NEFA and HbA1c measurements
Plasma, tissues, cell extracts and supernatants from control and obese mice were evaluated with specific Elisa kits for mature glucagon, insulin (Mercodia AB, Uppsala, Sweden), total GLP-1 and leptin (Meso Scale Discovery, Rockville, MD, USA) and total GIP (Millipore Corporation, Billerica, MA, USA) peptide detections as well as NEFA (Wako Diagnostics, Richmond, VA, USA) and HbA1c by the Siemens DCA systems Hemoglobin A1c (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA).
Morphometric analyses
The pancreas and small intestine (distal part of jejunum and ileum organized in Swiss roll (18)) were fixed overnight in paraformaldehyde 4% and embedded in paraffin as described (16,19). 4’,6-diamidino-2-phenylindole (DAPI) (10μg/ml) and immunofluorescence stainings were performed with rabbit anti-glucagon (1:500; Millipore Corporation, Billerica, MA, USA) and guinea pig anti-insulin antibodies (1:500; Thermo Fischer Scientific, Waltham, MA, USA) for pancreatic islets as well as mouse anti-GLP-1 antibody (1:10,000; provided by Pr David D’Alessio (20)) for small intestine. Alexa Fluor 488 anti-rabbit (1:1,000), Alexa Fluor 568 anti-guinea pig and Alexa Fluor 488 anti-mouse (1:500) were used as secondary antibodies (Thermo Fischer Scientific). Whole pancreas and intestine sections were scanned using Zeiss Mirax (pancreas) and Axioscan.Z1 (intestine) after staining.
The pancreas section images obtained were displayed on a large screen to localize all areas containing alpha- and/or beta-cell labelling and analyzed with Metamorph software to obtain alpha- and beta-cell total areas, as well as alpha- and beta-cell number, in each islet and in whole sections. Islets areas (μm2) were obtained by adding the total alpha- and beta-cell area in each islet. Islet mean diameter was calculated with the formula 2X(√(islet area / π)) for each islet. Alpha- and beta-cell mean areas (μm2) were determined by dividing the total alpha- and beta-cell area by the total number of alpha- and beta-cells in each section as described (21).
The small intestine section images obtained were analysed with the Definiens Tissue Studio IF software to obtain whole section area (μm2) with the tissue background separation function. With the use of the nucleus detection function the software also detects and quantifies the total number of nuclei labeled by DAPI, then with the nucleus classification algorithm, it also determines the number of GLP-1 positive cells corresponding to double positive labelling (DAPI in the nucleus and GLP-1 in the cytoplasm).
Pancreas and intestine analyses were performed on the entire organs including the dorsal and ventral pancreas or jejunum and ileum parts of small intestine (Swiss roll). The analyses were repeated on 3 independent 5 μm thick sections per animals (spaced at least 250μm) of 6 mice per group (18 slides per group).
Pancreatic perfusion
After an initial 30min-stabilization step, pancreases of LFD, I-HFD and H-HFD mice were perfused during 3 successive periods using low (2.8mmol/1), high (16.7mmol/1) and again low glucose solutions as described (16). Pancreatic perfusion samples were next analyzed for glucagon and insulin.
Primary cell preparations and ex vivo secretion assays
After 16 weeks of protocol, primary Venus+ alpha and L-cells from LFD, I-HFD and H-HFD mice were separated from non-Venus cells by fluorescence-activated cell sorting (FACS) using Biorad S3 and Beckman Coulter Astrios, after standard isolation procedures on pancreas, small intestine and colon as described (15,16). FACS-purified alpha- and L-cells were collected in Acid/Ethanol mixture (1.5%HCl / 75%Ethanol) for glucagon and GLP-1 contents measurements or in RNAse-free lysis buffer (Qiagen RLT+ buffer) for gene expression analyses.
For glucagon secretion, collected alpha-cells were seeded in Krebs 5.6mmol/l Glucose medium during 1 hour recovery period and assessed for glucagon release during 30min in Krebs buffer containing 1 or 5.6 or 16.7mmol/l glucose or 5.6mmol/l glucose with insulin (100nmol/l, Sigma-Aldrich, Saint-Louis, MI, USA).
For GLP-1 release and glucose-stimulated secretion, mixed intestinal cells from small intestine (jejunum/ileum) or colon explants were isolated and seeded in Matrigel (Corning, Bedford, MA, USA) as described (22). After overnight recovery in 5.6mmol/l glucose DMEM medium supplemented with 10% FBS, 10mmol/l Na-pyruvate and antibiotics, intestinal or colonic cells were incubated 2 hours with fresh medium or in Krebs buffer ± 10mmol/l glucose for jejunum as described (23). Supernatants and cell lysates (Acid/Ethanol mixture) were collected for GLP-1 measurements.
Glucagon and GLP-1 releases were expressed relative to glucagon and GLP-1 cellular contents (% of content).
Target gene analysis
Total mRNA was isolated from mouse sorted-Venus+ pancreatic alpha-cells as well as intestinal (jejunum/ileum) and colonic L-cells with RNeasy plus micro kit (Qiagen, Hilden, Germany). After reverse transcription (Prime-script RT Reagent, Takara Bio Inc., Otsu, Japan) and pre-amplification (cDNA Pre-Amp Master, Roche Diagnostics, Rotkreuz, Switzerland) following manufacturer’s recommendations, specific cDNA levels were analyzed by real-time quantitative PCR (qPCR). qPCR are performed using Light-Cycler 480 SYBR Green technology (Roche Diagnostics). Each target gene amplification was previously validated by evaluation of the melting temperature of the products and of the slope obtained with the standard curve as well as by sequencing the PCR product. The analyses were performed using the Light-Cycler software and target gene levels are relative to three reference genes. Each quantification was corrected by real efficiency through the E-Method (Roche Diagnostics) to compensate for differences in target and references gene amplification efficiencies. Data obtained from the LFD, I-HFD and H-HFD groups are presented as fold of the littermates LFD values ± SEM.
Data analysis
Data are presented as means ± SEM and analyzed using GraphPad Prism software (v.5.0, San Diego, CA, USA). Statistical analyses were performed using ANOVA (One-Way for 3 groups in one condition and Two-Way for 3 groups in multiple conditions) with post hoc Boneferroni test, or by unpaired two-tailed Student t test for comparison between two groups in one condition when appropriate. Data are statistically significant at p<0.05.
Results
Phenotypic characterization of obese HFD mice
To better understand the functional and molecular changes of proglucagon-producing cells in obesity and diabetes, we generated control and obese mice using the GLU-Venus mouse strain; mice were fed either a 10% (LFD) or 60% (HFD) fat diet during 16 weeks.
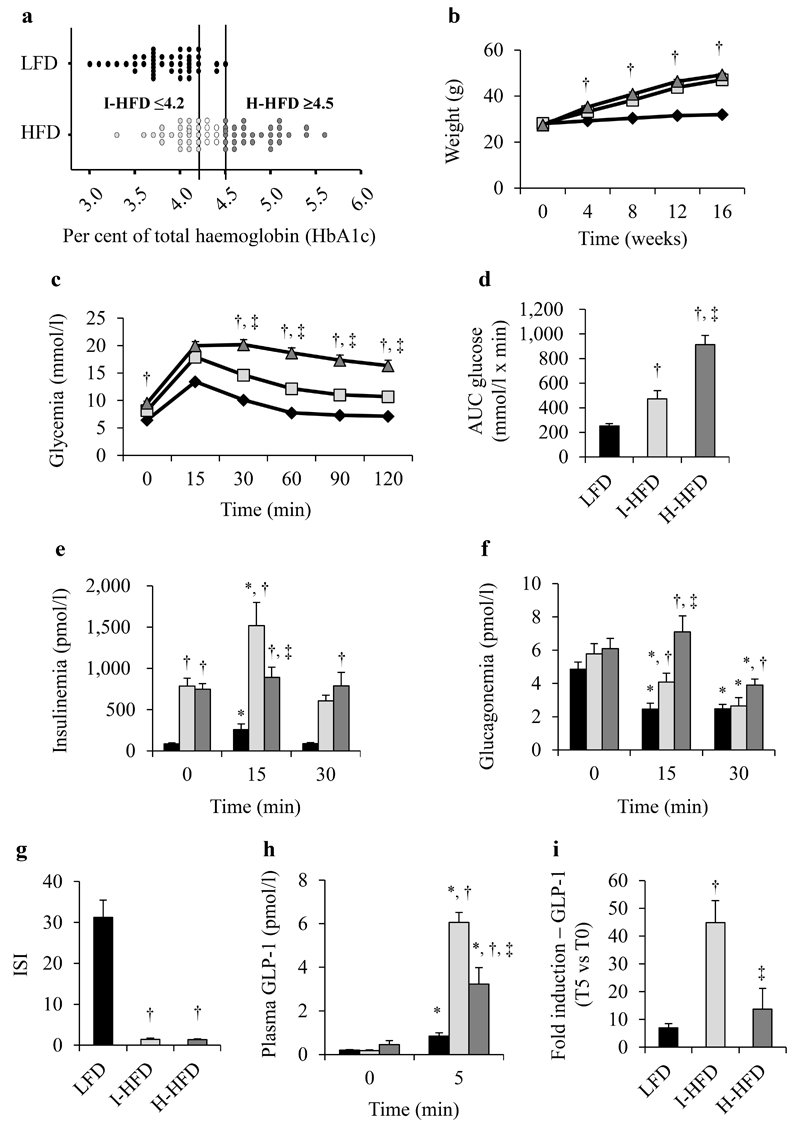
HFD mice exhibited high variability of HbA1c values (Fig.1a) from 3.3 to 5.6% reflecting differential impact of HFD feeding on glucose homeostasis, ranging from normoglycemia (LFD group) to hyperglycemia. To characterize which mechanisms are involved in this metabolic variation, HFD mice were then subdivided in 2 distinct groups depending on their HbA1c values. Mice with HbA1c≤4.2% (IFCC:22mmol/mol) were classified as normal range of HbA1c (I-HFD); while those with HbA1c≥4.5% (IFCC:26mmol/mol) as increased HbA1c (H-HFD) compared to LFD mice. The HbA1c values≤4.2% was chosen as 90% of LFD mice were at this value or below (Fig.1a), whereas HbA1c values≥4.5% was selected as no LFD mice exhibited higher values. HFD mice with intermediate values (4.2%<HbA1c<4.5%) were not included in the study.
Figure 1. Diet-fed mice characteristics.
HbA1c was measured after 15 weeks of LFD (black) or HFD (grey) feeding (a). HFD groups were subdivided in 2 distinct groups following HbA1c values. LFD (black losanges/bars), I-HFD (light-grey squares/bars) and H-HFD (dark-grey triangles/bars) mice weight evaluated retrospectively every 4 weeks during 16 weeks (b). Glycemia (c), AUC-Glucose (d), plasma insulin (e) and glucagon (f) levels during an OGTT (1g/kg) on LFD, I-HFD and H-HFD mice at 16 weeks. ISI calculated from insulinemia and glycemia values during OGTT (g). Plasma GLP-1 levels (h) and the relative glucose effects on GLP-1 secretion (i, T5/T0 values) were evaluated in separate OGTT (2g/kg) (n=25 mice per group except for plasma GLP-1 n=10 mice). *,† and ‡ mean significant compared to T0, LFD and I-HFD respectively.
We first observed that I-HFD and H-HFD mice exhibited comparable weight-gain all along the protocol (Fig.1b). We next assessed glycemia, insulinemia and glucagonemia during an OGTT and observed, before gavage, that 8h-fasted HFD mice exhibited hyperglycemia associated with hyperinsulinemia compared to LFD mice but similar glucagonemia (Fig.1c-f). H-HFD mice had higher glycemia compared to I-HFD mice from 30 min as confirmed by AUC measurements (Fig.1c,d). Insulin response to glucose was conserved in I-HFD mice as in LFD mice but lost in H-HFD mice (Fig.1e). Glucagonemia after glucose load was significantly higher in H-HFD mice (7.10±0.97 pmol/l at T15 and 3.91±0.36 pmol/l at T30) compared to LFD (2.45±0.37 pmol/l at T15 and 2.47±0.27 pmol/l at T30) and I-HFD mice (4.09±0.52 pmol/l at T15 and 2.64±0.61 pmol/l at T30) (Fig.1f).
These results indicate that while the I-HFD and H-HFD groups presented a similar insulin resistance state (Fig.1g), H-HFD mice exhibited alterations of insulin and glucagon secretions associated with hyperglycemia.
We measured plasma GLP-1 levels during OGTT (Fig.1h). Fasting GLP-1 levels were similar between groups whereas after glucose administration it was markedly increased in both HFD groups compared to LFD mice. However, the glucose response of GLP-1 secretion in I-HFD mice was much higher (44.86±7.93-fold increase) compared to LFD and H-HFD groups (6.97±1.54-fold and 13.68±7.49-fold, respectively) (Fig.1i). Our results indicate that L-cells from HFD mice exhibit considerable alterations during obesity corresponding to an adaptation with a marked increase in the GLP-1 response to glucose especially for I-HFD mice. We also measured the levels of another incretin, GIP (Supplemental Fig.2). Fasting GIP levels were increased in both HFD groups compared to LFD mice. GIP response to glucose was increased in both HFD groups compared to LFD mice with a higher level in H-HFD.
Of note, we also measured plasma leptin and NEFA levels at the end of protocol and observed that leptinemia was 11.8- and 10.2-fold higher for I-HFD and H-HFD mice respectively compared to LFD mice (Supplemental Fig.3a). By contrast, NEFA levels were significantly higher in obese mice compared to LFD mice only in the H-HFD group (Supplemental Fig.3b).
Functional alterations of pancreatic alpha and beta-cells in obese mice
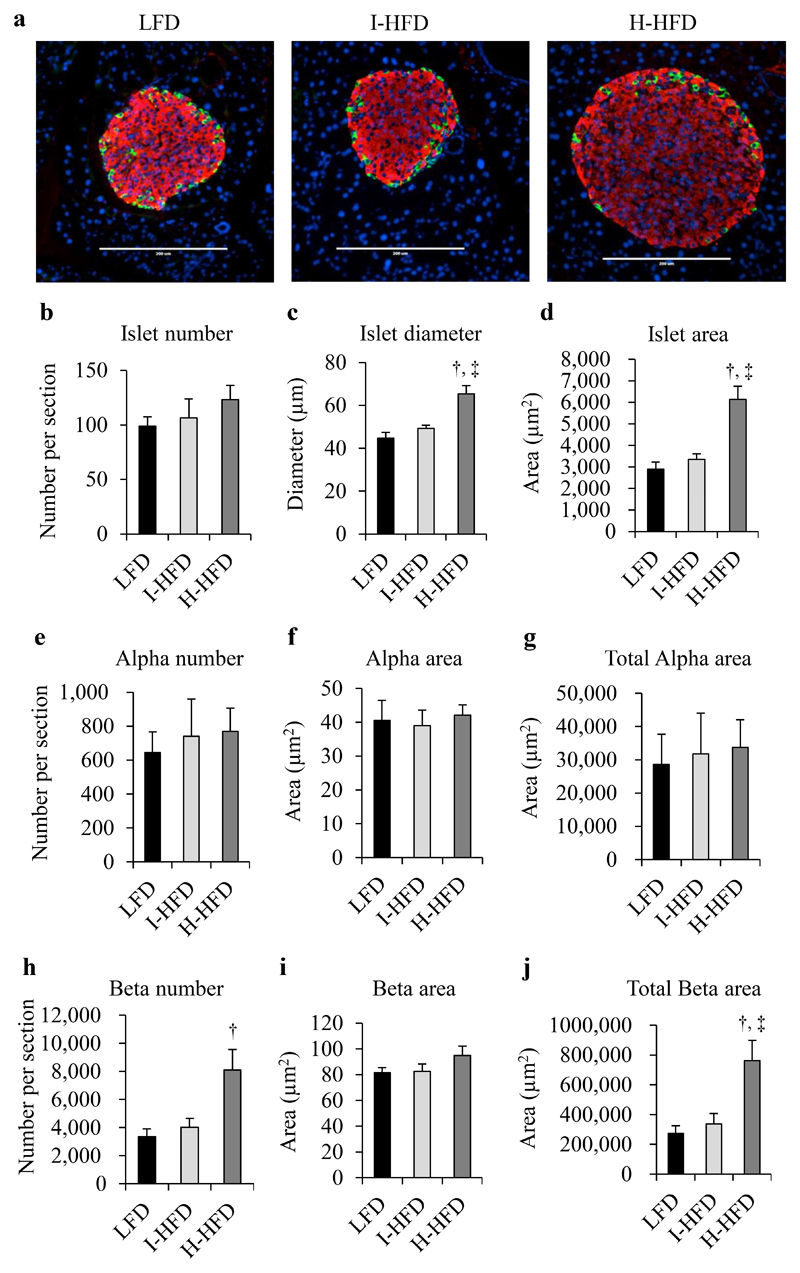
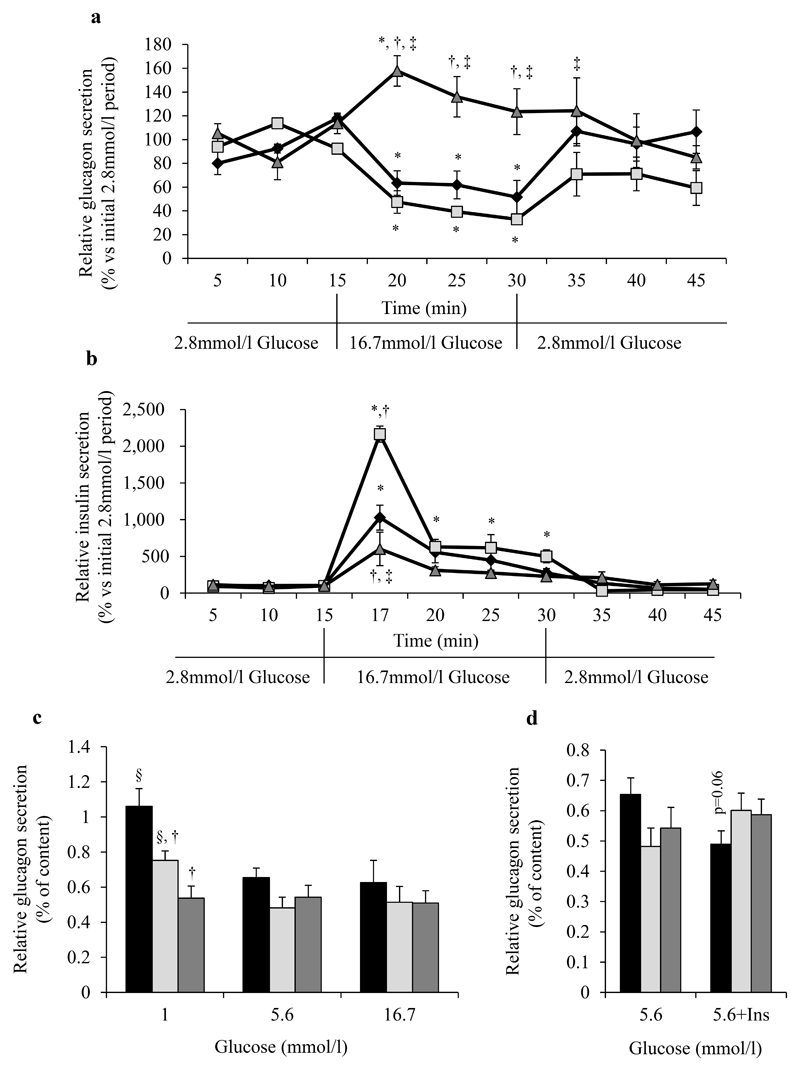
Both HFD groups presented similar values for islet number, alpha-cell number and size compared to LFD group, whereas islet diameter and area as well as beta-cells number and total beta-cell area were clearly increased only in H-HFD mice compared to LFD (Fig.2a-j). Pancreatic insulin contents of H-HFD mice were 2.70±0.41 fold-higher compared to LFD mice (Supplemental Fig.4a); glucagon contents were not different between groups (Supplemental Fig.4b). To further characterize insulin and glucagon secretions in obese mice, we performed pancreatic perfusion experiments using low (2.8mmol/l) and high glucose (16.7mmol/l) concentrations. In H-HFD mice, glucagon secretion was not inhibited by high glucose as observed in LFD and I-HFD mice and even increased after 5 minutes of 16.7mmol/l glucose perfusion (Fig.3a). Glucose-stimulated insulin secretion in H-HFD mice was lower compared to I-HFD and LFD mice (Fig.3b).
Figure 2. Morphometric analysis of pancreatic alpha and beta-cells in control and obese mice.
LFD (black bars), I-HFD (light-grey bars) and H-HFD (dark-grey bars). Staining (a) and quantification (b-j) of pancreatic slices against insulin (red), glucagon (green) and nuclei (blue) (n=6 animals per group, scale bar = 200μm). † and ‡ mean significant compared to LFD and I-HFD respectively.
Figure 3. Regulation of glucagon and insulin secretions in HFD mice.
Glucagon and insulin secretions from LFD (black losanges/bars), I-HFD (light-grey squares/bars) and H-HFD (dark grey triangles/bars) mice. Glucagon (a) and insulin (b) levels in samples collected from perfused pancreas (n=3 mice per group). Data were expressed relative to the average of the initial 2.8mmol/l glucose phase values. FACS-purified alpha-cells from LFD, I-HFD and H-HFD mice incubated for 30min in Krebs-medium supplemented with 1 or 5.6 or 16.7 mmol/l glucose (c) and 5.6mmol/l glucose + insulin (100ng/ml) (d) (n=4). Histograms represent the percentage of glucagon measured from the supernatant relative to glucagon cellular contents. *, §, † and ‡ mean significant compared to 2.8mmol/l glucose, 5.6mmol/l glucose, LFD and I-HFD respectively.
To further analyze the characteristics of dysfunctional alpha-cells in H-HFD mice we specifically investigated glucagon secretion from FACS purified alpha-cells after 16 weeks of diet. Glucagon release was evaluated during 30min in the presence of 1, 5.6 and 16.7mmol/l glucose after an initial 1h-period in 5.6mmol/l glucose solution. Glucagon secretion was similar in all 3 groups in the presence of 5.6 and 16.7mmol/l glucose; however, glucagon secretion in response to 1mmol/l glucose was significantly higher compared to 5.6mmol/l glucose conditions only in sorted alpha-cells from LFD and I-HFD and unchanged in H-HFD cells (Fig.3c). In addition, there was a trend to glucagon secretion inhibition in response to insulin only in LFD mice, suggesting insulin resistance of alpha-cells from obese mice whether glucose intolerant or hyperglycemic (Fig.3d). We also assessed the molecular footprint of pancreatic alpha-cells from control and obese mice and showed that expression of critical genes coding for proteins involved in function and differentiation are specifically altered in both HFD groups (Supplemental Table 1). Among 48 tested genes we observed specific increases of proglucagon (Gcg), Foxa1 and cMaf as well as maturation enzyme Pcsk1/3 mRNA levels only in H-HFD mice whereas genes coding for the properties and functions of alpha cells such as Pcsk2, Arx, Foxa2, InsR, Gck, Slc2a1 and Ffar1 were not. These observations were coupled to increases of FOXA1, cMAF proteins and of GLP-1 production from alpha-cells of H-HFD compared to LFD mice (Supplemental Fig.5).
Taken together these data clearly suggest that pancreatic alpha- and beta-cells of H-HFD mice fail to compensate and adapt to obesity, presenting marked alterations of glucagon and insulin secretions in response to glucose, resulting in hyperglycemia.
Functional and molecular alterations of intestinal L-cells in obese mice
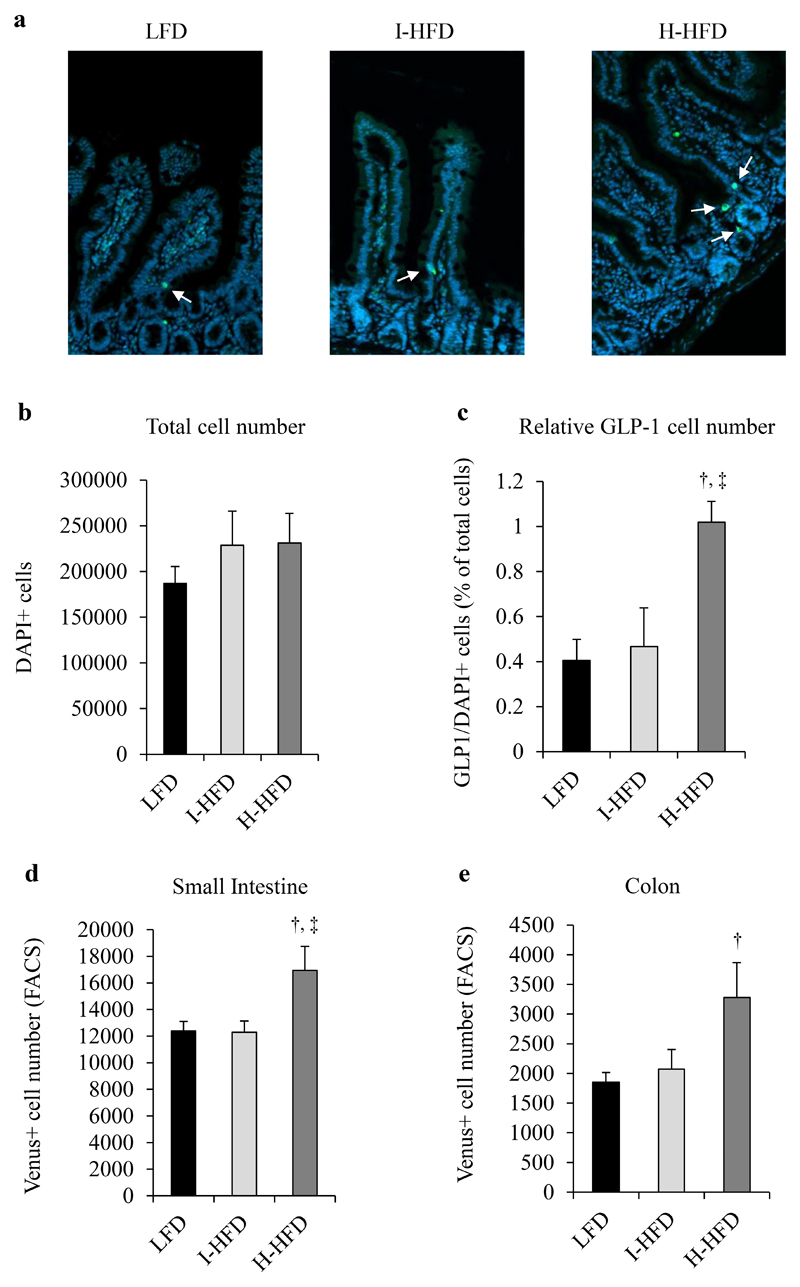
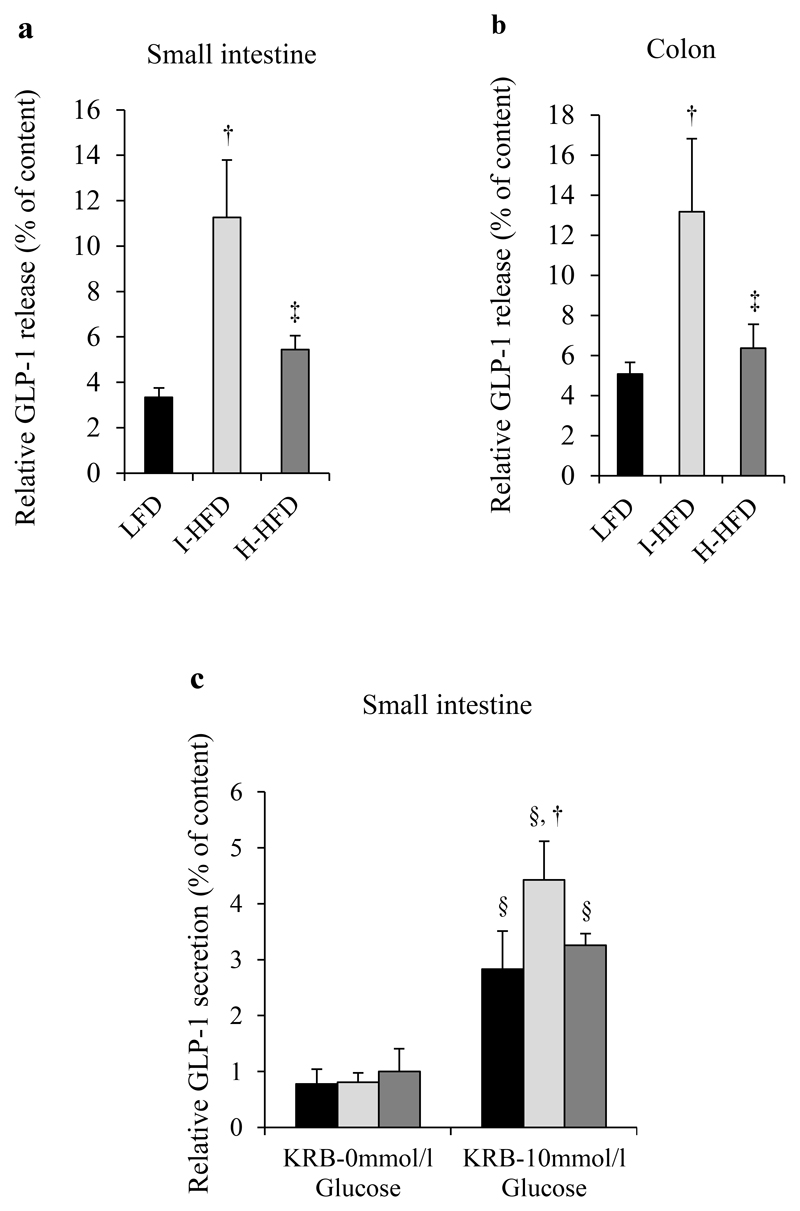
We first analyzed L-cell number from the small intestine of LFD, I-HFD and H-HFD mice after GLP-1 immunostaining. Whereas L-cell number of I-HFD mice was unchanged compared to LFD mice, H-HFD mice displayed increased GLP-1 positive cell number (Fig.4a-c). Furthermore, we observed higher numbers of sorted Venus+ L cells from small intestine and colon of H-HFD mice compared to I-HFD and LFD mice (Fig.4d,e). We next evaluated GLP-1 release from L-cells of small intestine and colon explants for each group. Explants of I-HFD mice exhibited much higher GLP-1 release rates compared to LFD and H-HFD mice in small intestine (3.35±0.41% for LFD, 11.26±2.52% for I-HFD and 5.45±0.61% for H-HFD) and colon (5.08±0.59% for LFD, 13.17±3.64% for I-HFD and 6.36±1.2% for H-HFD) (Fig.5a,b). GLP-1 secretion from intestinal L-cells was increased in response to glucose in each group and again higher in I-HFD mice (Fig.5c).
Figure 4. Morphometric analysis of intestinal L-cells in control and obese mice.
Sections of small intestine (jejunum and ileum) from LFD, I-HFD and H-HFD were evaluated for GLP-1 (green) and nuclei staining (DAPI, blue) (a). Intestine sections were analyzed for total cell (b) and GLP-1 positive cell numbers (c). GLP-1 expressing cell number (GLP-1+/DAPI+) were expressed relative to total cells (% of DAPI+ cells) (n=6 animals per group) (c). L-cell numbers (Venus+ cells) were also evaluated by FACS from small intestine (d) and colon (e). LFD (black bars, controls), I-HFD (light-grey bars) and H-HFD (dark-grey bars).
Figure 5. Functional analysis of intestinal L-cells in control and obese mice.
GLP-1 evaluated in supernatants and contents of dissociated mixed cells from small intestine (jejunum/ileum) (a) and colon (b) after 2h incubation in fresh complete medium and for small intestine in Krebs buffer ± 10mmol/l glucose (c). Histograms represent the percentage of GLP-1 measured from the supernatant of primary mixed cells relative to GLP-1 cell contents (n=6 animals per group). †, ‡ and § mean significant compared to LFD, I-HFD and KRB-0mmol/l glucose respectively. LFD (black bars, controls), I-HFD (light-grey bars) and H-HFD (dark-grey bars).
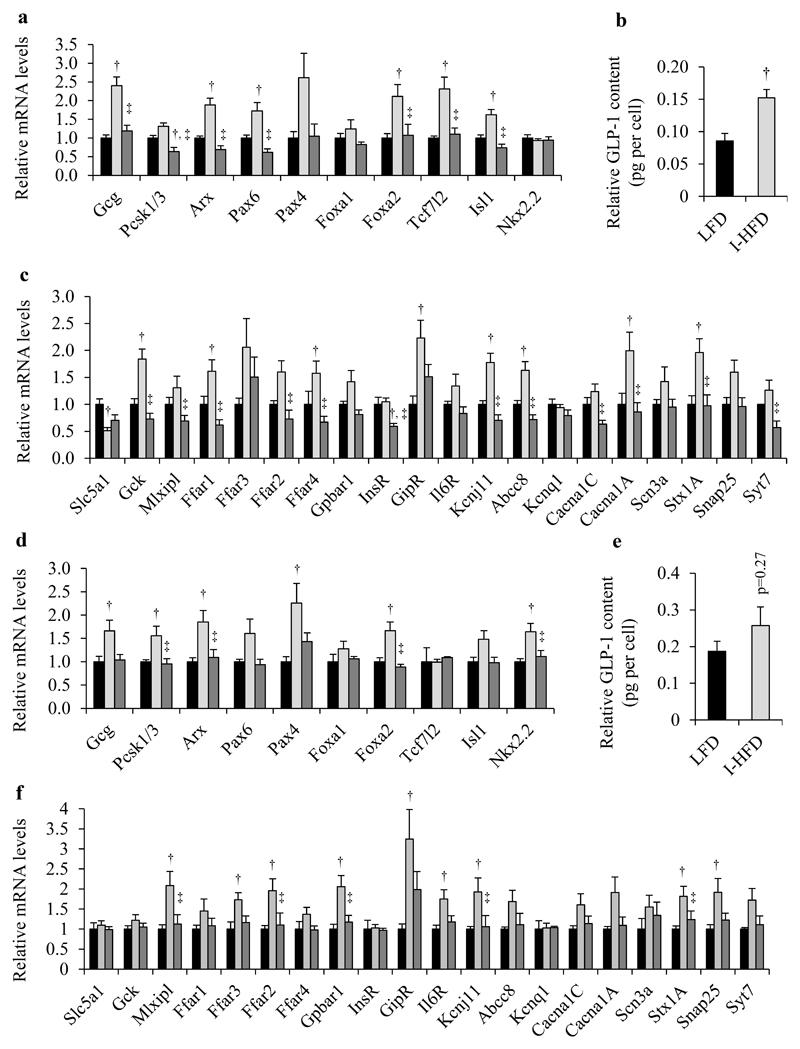
To analyze the molecular characteristics of enteroendocrine L-cells of obese mice we investigated mRNA levels coding for proteins involved in GLP-1 biosynthesis and secretion using FACS-purified Venus+ L cells from small intestine and colon of LFD, I-HFD and H-HFD mice (all data are listed in Supplemental Tables 2-3). We first observed that Gcg mRNA levels of L-cells from I-HFD were significantly increased compared to LFD and H-HFD mice in small intestine and to LFD mice in colon (Fig.6a,d) with significant increases in GLP-1 contents per cell only in the small intestine (Fig.6b,e), reflecting large increases of intestinal GLP-1 biosynthesis in I-HFD mice. Genes coding for proteins involved in GLP-1 biosynthesis such as Arx, Pax6, Foxa2 and Isl1 in small intestine as well as Pcsk1/3, Arx and Foxa2 in colon were also significantly up-regulated in I-HFD compared to LFD and H-HFD mice (Fig.6a,d).
Figure 6. Molecular analyses of Venus+ intestinal and colonic L-cells from control and obese mice.
LFD (black bars), I-HFD (light-grey bars) and H-HFD (dark-grey bars). Relative mRNA quantification of specific genes coding for proteins involved in L-cell identity, GLP-1 biosynthesis and in the secretion process in FACS-purified L-cells from small intestine (a, c) and colon (d, f). Data are analysed and presented as the means (Fold of littermate controls) ± SEM (n=8 mice for small intestine, n=6 for colon per group). GLP-1 cellular contents in sorted-L-cells of LFD and I-HFD mice from small intestine (b) and colon (e) (n=6 animals per group). † and ‡ mean significant compared to LFD and I-HFD respectively.
We also investigated the mRNA levels coding for proteins involved in GLP-1 secretion and observed that expression of GipR, Kcnj11 and Stx1A are up-regulated in both small intestine and colon of I-HFD mice (Fig.6c,f); most genes analyzed are in fact up-regulated in small intestine or in colon of I-HFD mice. By contrast, we noted decreased expression of Pcsk1/3 and InsR in small intestine and unchanged expression of genes such as the Arx, Pax6, Isl1, Ffar1, Ffar4, Cacna1C and Syt7 genes in small intestine and colon of H-HFD mice. Of note, the sodium/glucose cotransporter Slc5a1 was the only gene to be significantly down-regulated in small intestine of I-HFD mice.
Obese I-HFD mice with impaired glucose tolerance exhibit major functional and molecular adaptations of their intestinal and colonic L-cells towards an increased production and secretion of GLP-1 without changes of L-cell mass whereas hyperglycemic obese H-HFD mice adapt by an increase of L-cell number but with much less changes in gene expression and GLP-1 release capabilities.
Ex9-39 impairs glucagon secretion and increases glycemia in I-HFD mice
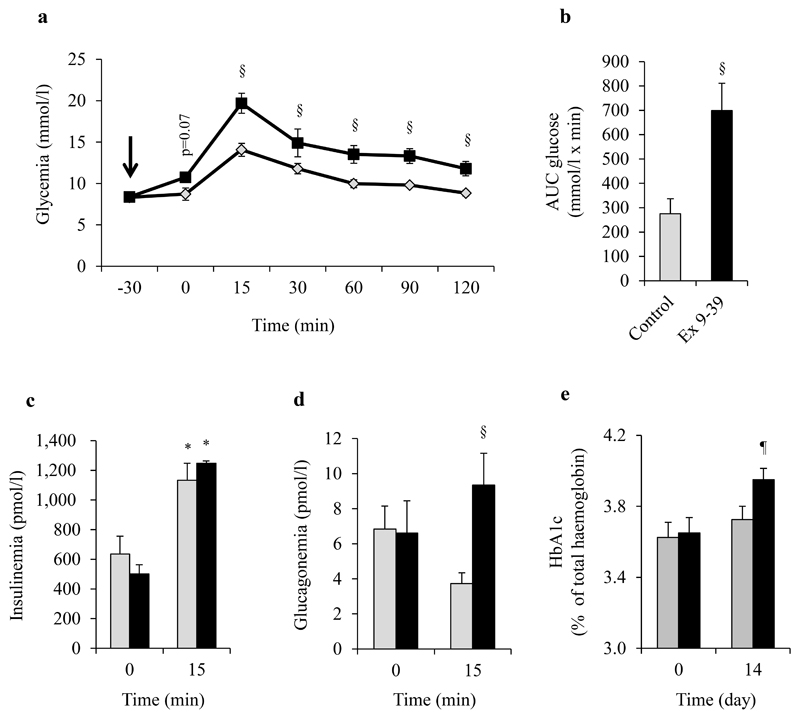
To evaluate the role of GLP-1 in the glycemic control of I-HFD mice; we administrated the GLP-1 antagonist Ex9-39 to I-HFD mice. First we injected Ex9-39 (I-HFD-Ex9-39) or NaCl (I-HFD-CTRL) to these mice 30min before glucose load during an OGTT. Ex9-39 administration led to a non-significant increase in glycemia before gavage but to a significant increase after glucose load, compared to I-HFD-CTRL mice as supported by AUC measurements (Fig.7a,b). While Ex9-39 injection did not impact insulin secretion during OGTT, glucagonemia, which was similar for both groups at T0, increased 15min after glucose gavage in I-HFD-Ex9-39 mice, indicating that blocking GLP-1 effects in I-HFD mice leads to dysregulated glucagon secretion in response to glucose, as observed in H-HFD mice (Fig.7d). We also evaluated chronic effects of 14 days treatment with Ex9-39 on I-HFD mice and showed that these mice exhibited a slight but significant increase of HbA1c, reflecting that partial blocking of GLP-1 action on compensated I-HFD mice impairs glycemic control (Fig.7e).
Figure 7. Exendin9-39 impairs glucose tolerance and glucagon secretion in I-HFD mice.
Glycemia (a), AUC-Glucose (b), plasma insulin (c) and glucagon (d) levels during OGTT (1g/kg) performed on I-HFD mice ip-injected with the GLP-1R antagonist Ex9-39 (150nmol/kg, black squares/bars) or with saline solution (light-grey losanges/bars) 30 minutes before gavage after 16 weeks of protocol. HbA1c before (Day0, 16 weeks of HFD) and 14 days after (Day14, 18 weeks of HFD) chronic Ex9-39 (150nmol/kg/day) or NaCl administration (e) (n=4 per group). *, § and ¶ mean significant compared to T0, controls (NaCl administration) and Day 0 respectively.
Our results suggest that the adaptive up-regulation of GLP-1 secretion in I-HFD mice is involved in the control of glycemia and alpha-cells function and may represent a compensatory phenomenon which contributes to maintain islet-cell function in obese mice.
Discussion
Although obese individuals do not always develop hyperglycemia, obesity is clearly linked to insulin resistance and an increased risk of diabetes (1). Functional alterations of pancreatic alpha-cells and enteroendocrine L-cells emerge as critical in the development of diabetes (24). Furthermore therapy aiming at blocking glucagon effects as well as mimicking GLP-1 action clearly improves glycemic control in type 2 diabetic patients as well as in rodent diabetic models (7,8,25,26). Thus understanding the functional and molecular changes in intestinal L- and pancreatic alpha-cells, in the transition from the lean to the obese and diabetic states could provide substantial new ways for therapeutic approaches. To better define these changes, we used the HFD-fed insulin resistant obese male mice characterized by variations in glucose homeostasis reflected by HbA1c values, ranging from relative normo (I-HFD) to hyperglycemia (H-HFD) despite similar weight-gain, plasma GIP levels, degree of insulin-resistance and basal hyperinsulinemia. I-HFD mice exhibit a relatively adequate compensation since these mice are barely glucose intolerant with similar HbA1c compared to LFD mice. The H-HFD group, by contrast, develops hyperglycemia with a decreased ability to compensate for insulin resistance. We show that the compensated glycemic state of I-HFD mice involves not only an adapted insulin secretion response to glucose, but also of L-cells of both the small intestine and colon with marked increases in GLP-1 biosynthesis and secretion in response to glucose. These adaptive changes occur in the absence of any increase in L-cell number. In I-HFD mice, alterations of alpha-cell function can be observed such as resistance to insulin and minor changes in gene expression but their response to glucose are preserved although slightly decreased. This is in marked contrast with the observed changes in H-HFD mice which mainly exhibit functional alterations of beta-cells, particularly in response to glucose, and of L-cells which fail to adapt adequately to obesity. This inability to functionally adapt is reflected by a lack of up-regulation of L-cell genes involved in GLP-1 biosynthesis and secretion and by an increase of beta- and L-cell numbers, which probably constitutes an attempt to compensate for the functional failure. Indeed, hyperplasia of beta-cells was previously described to be accompanied by alterations of the glucose response (27,28).
In H-HFD mice, alpha-cell dysfunction is clearly observed with resistance to insulin and an absence of response to glucose. Alpha-cell resistance to insulin is a probable contributor to the abnormal glucagon response to hyperglycemia; however, the absence of inhibition of glucagon secretion in response to glucose observed in H-HFD mice involves additional mechanisms such as insufficient GLP-1 adaptive responses as suggested by our results with Ex9-39 treatment of I-HFD mice.
Glucagon secretion is under tonic inhibition by insulin and glucose but also negatively regulated by GLP-1 independently of glucose concentration (29–32). Blocking GLP-1 action by Ex9-39 led to impairment of glycemia and a complete loss of the glucose inhibitory effects on glucagon secretion in I-HFD mice independently of insulin as suggested previously (33,34). These results indicate that GLP-1 acts as a key determinant in the regulation of glucagon secretion to maintain euglycemia but are in contradiction with those found in GLP-1 receptor knockout mice. Indeed, Ayala et al. showed that the deleterious impact of HFD on glucose metabolism was reduced in GLP-1 receptor knockout mice (35). However, GLP-1 receptor knock-out mice have developmental and chronic invalidation of the GLP-1 receptor which is not comparable to short-term pharmacological antagonism; our results clearly highlight that the increase of GLP-1 is correlated to better metabolic control confirming numerous works focusing on the effects of GLP-1 therapy on obese diabetic mice and patients (36,37).
We also show that pancreatic alpha-cells of H-HFD mice present specific molecular alterations including increased expression of Gcg as well as Foxa1 and cMaf genes shown to be also upregulated in insulinopenic streptozotocin-induced diabetic mice by our group (16). Furthermore, these cells express higher mRNA levels of Pcsk1/3 and produce more GLP-1, than alpha-cells from LFD mice as previously reported, an observation attributed to alpha-cell adaptation in response to hyperglycemia or to dedifferentiation (38,39). These observations illustrate a diabetic molecular footprint of alpha-cells independently of the presence or absence of insulin.
Human studies on GLP-1 secretion in control and obese diabetic patients indicate altered L-cell function (6,40) and no significant variation of L-cell number (41). Nevertheless, recent works have demonstrated that lipid-rich diets or short-chain fatty acids may increase L-cell number in obese patients and in human organoids through an increase of L-cell differentiation (42,43). Furthermore, a study including controls, pre-diabetic, drug-naive and medicated type 2 diabetic patients revealed higher plasma GLP-1 levels in diabetic patients compared to controls (44). These observations suggest that fat-enriched diet, obesity and diabetes may also control L-cell function and number in humans, similarly to our findings in DIO mice.
Previous studies on the adaptation of enteroendocrine L-cells to DIO generated controversial data depending on the experimental conditions. Indeed, HFD mice were previously described with elevated plasma GLP-1 levels compared to controls despite down- or up-regulation of L-cell number (42,45). Furthermore, HFD mice exhibited alterations of L-cell function including higher basal GLP-1 release and lower glucose-induced GLP-1 secretion (23,45). In the present study, the separation of obese mice based on their glycemic status allows a better characterization of L-cell function and adaptation. Indeed L-cells from I-HFD mice display a major adaptation with increases in the transcription of genes coding for proteins involved in GLP-1 biosynthesis and secretion leading to increases of cellular GLP-1 content and release without variation of L-cell number. By contrast, H-HFD mice presented much less gene expression adaptation to obesity and a molecular footprint of L-cells similar to the one observed in LFD mice however with increased L-cell number. Interestingly, these cells were still able to slightly increase basal and stimulated GLP-1 secretion despite decreases of critical gene expression in small intestine (46–50) such as Pcsk1/3 and InsR, without increase in Gcg gene expression as previously observed (23). Overall our observations suggest that L-cells from H-HFD mice exhibit specific molecular alterations but are still able to generate a GLP-1 response although insufficiently compared to I-HFD mice, leading to hyperglycemia.
We also observed that L-cells from small intestine of the I-HFD group had decreased Slc5a1 mRNA levels compared to the LFD group without impairment of glucose-stimulated GLP-1 secretion. Slc5a1 is sensitive to diet composition and stimulated by glucose as previously reported (51). Since LFD contains more sugar compared to HFD, our observation may suggest that relative variations of Slc5a1 may represent an adaptive response to diet composition or alternatively that chronic overfeeding with fat may lead to down-regulation of these genes as previously demonstrated (52).
Diabetes development under high-fat feeding is characterized by marked heterogeneity as reported by several groups (53,54); thus understanding the cause and the mechanisms is critical. We demonstrated that the variable impact of high-fat diet on glucose homeostasis is secondary to, at least partly, pancreatic and intestinal endocrine cell function and that L-cell adaptation emerges as a very important factor. Among the components which may regulate L-cell function, insulin, leptin, fatty acids and bile acids represent potential activators (48,55–58). Although circulating fatty-acids (NEFA) were higher in H-HFD mice compared to the LFD and I-HFD groups, I-HFD and H-HFD mice exhibited the same weight-gain. Furthermore, we observed that excessive levels of plasma insulin and leptin were similar between both HFD groups. These observations thus cannot explain L-cell adaptation of I-HFD mice.
Interestingly the metabolic phenotypes observed in HFD-fed mice were previously attributed to specific gut microbial profiles leading to diabetes sensitive or resistant mice (54). Indeed, the gut microbiome can influence the metabolic state through at least bacterial fermentation which generates short-chain fatty-acids which in turn modulate GLP-1 secretion (59). We can thus postulate that L-cell adaptation in I-HFD mice may be due to specific gut microbiota profiles. Further experiments such as analysis of microbiota composition as well as measurements of luminal fatty acid and bile acid composition in HFD mice could bring potential explanations concerning the L-cell adaptation in DIO mice.
Taken together our observations suggest that maintenance of normoglycemia in obesity requires hypersecretion of GLP-1 and insulin with controlled glucagon secretion; hyperglycemia occurs when beta- and L-cells are unable to functionally compensate, leading to increased numbers of dysfunctional beta- and L-cells. We propose that GLP-1 is critical for the regulation of glucagon secretion in response to glucose in obesity and central in the prevention of hyperglycemia in male obese mice.
Supplementary Material
Acknowledgments
We thank Pr David D’Alessio (Duke Molecular Physiology Institute, University of Wisconsin, Madison, WI, USA) for the generous gift of mouse anti-GLP-1 antibody. We also thank Dr Jean-Pierre Aubry and Mrs Cécile Gameiro of Flow cytometry core facility of Medical school of Geneva University for FACS as well as Mrs Marie-claude Brulhard Mennet (EDN, department of internal medicine, University of Geneva School of Medicine) for NEFA measurements.
Footnotes
1. Disclosure Statement: YG, RD, SH, SS, FV, CV, MHM, FG, FR and JP have nothing to declare. JP received grant support from Swiss National Fund (Main recipient, N°31003A-149749).
2. Disclosure Statement: The authors have nothing to disclose.
Authors Contribution
R.D and S.H are co-first authors since they are involved equally in the project.
All authors took part in the conception and design of the study, acquisition, analysis and interpretation of data as well as revision and approval. R.D, S.H, S.S, M.HM, F.V and C.V: researched data (design, acquisition and analysis of data) and revision; F.G, F.R and JP: contributed to researched data design, revision of manuscript. Y.G: project management, researched data (design, acquisition and analysis of data) and wrote manuscript.
Y.G and J.P are the guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The abbreviations used are HbA1c: glycated hemoglobin (%), InsR: Insulin Receptor, Gipr: GIP receptor, RPS9: ribosomic protein 9S, SEM: Standard Error of the Mean (=Standard Deviation/√(sample size)), NEFA: non-esterified fatty-acids.
Gene symbols used are Gcg: proglucagon, Gck: Glucokinase, Slc5a1: Sglt1, Slc5a5: Glut5, Slc2a1: Glut1, Slc2a2: Glut2, Ffar1: Gpr40, Ffar2: Gpr43, Ffar3: Gpr41, Ffar4: Gpr120, Pou3f4: Brain4, Cacna1C: Cav1.2, Cacna1A: Cav2.1, Scna3: Nav1.3, Kcnj11: Kir6.2, Abcc8: Sur1, Gpbar1: Tgr5.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Dunning BE, Foley JE, Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–1713. doi: 10.1007/s00125-005-1878-0. [DOI] [PubMed] [Google Scholar]
- 3.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine reviews. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 4.Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. The Journal of endocrinology. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142:521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 6.Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- 7.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48:1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 8.Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regulatory peptides. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 9.Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, Vilsboll T. Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2009;94:4679–4687. doi: 10.1210/jc.2009-0921. [DOI] [PubMed] [Google Scholar]
- 10.King AJ. The use of animal models in diabetes research. Br J Pharmacol. 166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 12.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958–1966. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 13.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutrition research reviews. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 14.He M, Su H, Gao W, Johansson SM, Liu Q, Wu X, Liao J, Young AA, Bartfai T, Wang MW. Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PloS one. 2010;5:e14205. doi: 10.1371/journal.pone.0014205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell metabolism. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dusaulcy R, Handgraaf S, Heddad-Masson M, Visentin F, Vesin C, Reimann F, Gribble F, Philippe J, Gosmain Y. Alpha-cell dysfunctions and molecular alterations in male insulinopenic diabetic mice are not completely corrected by insulin. Endocrinology. 2015 doi: 10.1210/en.2015-1725. en20151725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacini G, Omar B, Ahren B. Methods and models for metabolic assessment in mice. Journal of diabetes research. 2013;2013 doi: 10.1155/2013/986906. 986906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moolenbeek C, Ruitenberg EJ. The "Swiss roll": a simple technique for histological studies of the rodent intestine. Laboratory animals. 1981;15:57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 19.Whittem CG, Williams AD, Williams CS. Murine Colitis modeling using Dextran Sulfate Sodium (DSS) Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montanya E, Tellez N. Pancreatic remodeling: beta-cell apoptosis, proliferation and neogenesis, and the measurement of beta-cell mass and of individual beta-cell size. Methods in molecular biology. 2009;560:137–158. doi: 10.1007/978-1-59745-448-3_11. [DOI] [PubMed] [Google Scholar]
- 22.Andersson-Rolf A, Fink J, Mustata RC, Koo BK. A video protocol of retroviral infection in primary intestinal organoid culture. Journal of visualized experiments : JoVE. 2014:e51765. doi: 10.3791/51765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards P, Pais R, Habib AM, Brighton CA, Yeo GS, Reimann F, Gribble FM. High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides. 2015 doi: 10.1016/j.peptides.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 25.Winzell MS, Brand CL, Wierup N, Sidelmann UG, Sundler F, Nishimura E, Ahren B. Glucagon receptor antagonism improves islet function in mice with insulin resistance induced by a high-fat diet. Diabetologia. 2007;50:1453–1462. doi: 10.1007/s00125-007-0675-3. [DOI] [PubMed] [Google Scholar]
- 26.Kelly RP, Garhyan P, Raddad E, Fu H, Lim CN, Prince MJ, Pinaire JA, Loh MT, Deeg MA. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes, obesity & metabolism. 2015;17:414–422. doi: 10.1111/dom.12446. [DOI] [PubMed] [Google Scholar]
- 27.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. The Journal of clinical investigation. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. The Journal of biological chemistry. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 29.Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50:370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. The Journal of clinical investigation. 1984;74:2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010;59:2936–2940. doi: 10.2337/db10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 33.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut. 2006;55:243–251. doi: 10.1136/gut.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baggio L, Kieffer TJ, Drucker DJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology. 2000;141:3703–3709. doi: 10.1210/endo.141.10.7720. [DOI] [PubMed] [Google Scholar]
- 35.Ayala JE, Bracy DP, James FD, Burmeister MA, Wasserman DH, Drucker DJ. Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology. 2010;151:4678–4687. doi: 10.1210/en.2010-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, Yoon JW, Jun HS. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671–1679. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 37.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 38.Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, Grieco FA, Del Guerra S, D'Aleo V, Piro S, Marselli L, Boggi U, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55:3262–3272. doi: 10.1007/s00125-012-2716-9. [DOI] [PubMed] [Google Scholar]
- 39.Hansen AM, Bodvarsdottir TB, Nordestgaard DN, Heller RS, Gotfredsen CF, Maedler K, Fels JJ, Holst JJ, Karlsen AE. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus--an adaptive response to hyperglycaemia? Diabetologia. 2011;54:1379–1387. doi: 10.1007/s00125-011-2080-1. [DOI] [PubMed] [Google Scholar]
- 40.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. The Journal of clinical endocrinology and metabolism. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 41.Kampmann K, Ueberberg S, Menge BA, Breuer TG, Uhl W, Tannapfel A, Meier JJ. Abundance and turnover of GLP-1 producing L-cells in ileal mucosa are not different in patients with and without type 2 diabetes. Metabolism: clinical and experimental. 2016;65:84–91. doi: 10.1016/j.metabol.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Aranias T, Grosfeld A, Poitou C, Omar AA, Le Gall M, Miquel S, Garbin K, Ribeiro A, Bouillot JL, Bado A, Brot-Laroche E, et al. Lipid-rich diet enhances L-cell density in obese subjects and in mice through improved L-cell differentiation. Journal of nutritional science. 2015;4:e22. doi: 10.1017/jns.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen N, Reimann F, Bartfeld S, Farin HF, Ringnalda FC, Vries RG, van den Brink S, Clevers H, Gribble FM, de Koning EJ. Generation of L cells in mouse and human small intestine organoids. Diabetes. 2014;63:410–420. doi: 10.2337/db13-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chia CW, Odetunde JO, Kim W, Carlson OD, Ferrucci L, Egan JM. GIP contributes to islet tri-hormonal abnormalities in type 2 diabetes. The Journal of clinical endocrinology and metabolism. 2014 doi: 10.1210/jc.2013-3994. jc20133994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kappe C, Zhang Q, Nystrom T, Sjoholm A. Effects of high-fat diet and the anti-diabetic drug metformin on circulating GLP-1 and the relative number of intestinal L-cells. Diabetology & metabolic syndrome. 2014;6:70. doi: 10.1186/1758-5996-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustavsson N, Wang Y, Kang Y, Seah T, Chua S, Radda GK, Han W. Synaptotagmin-7 as a positive regulator of glucose-induced glucagon-like peptide-1 secretion in mice. Diabetologia. 2011;54:1824–1830. doi: 10.1007/s00125-011-2119-3. [DOI] [PubMed] [Google Scholar]
- 47.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, Brubaker PL. Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology. 2009;150:580–591. doi: 10.1210/en.2008-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du A, McCracken KW, Walp ER, Terry NA, Klein TJ, Han A, Wells JM, May CL. Arx is required for normal enteroendocrine cell development in mice and humans. Developmental biology. 2012;365:175–188. doi: 10.1016/j.ydbio.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill ME, Asa SL, Drucker DJ. Essential requirement for Pax6 in control of enteroendocrine proglucagon gene transcription. Molecular endocrinology. 1999;13:1474–1486. doi: 10.1210/mend.13.9.0340. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto K, Hase K, Takagi T, Fujii T, Taketani Y, Minami H, Oka T, Nakabou Y. Differential responses of intestinal glucose transporter mRNA transcripts to levels of dietary sugars. The Biochemical journal. 1993;295(Pt 1):211–215. doi: 10.1042/bj2950211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wisniewski JR, Friedrich A, Keller T, Mann M, Koepsell H. The impact of high-fat diet on metabolism and immune defense in small intestine mucosa. Journal of proteome research. 2015;14:353–365. doi: 10.1021/pr500833v. [DOI] [PubMed] [Google Scholar]
- 53.Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly E, Prentki M. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59:2178–2187. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serino M, Luche E, Gres S, Baylac A, Berge M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, Mariette J, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beysen C, Karpe F, Fielding BA, Clark A, Levy JC, Frayn KN. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia. 2002;45:1533–1541. doi: 10.1007/s00125-002-0964-9. [DOI] [PubMed] [Google Scholar]
- 56.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature medicine. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 57.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 58.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell metabolism. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.