Abstract
Aims/hypothesis
Physical inactivity, low mitochondrial function, increased intramyocellular lipid (IMCL) deposition and reduced insulin sensitivity are common denominators of chronic metabolic disorders, like obesity and type 2 diabetes. Yet, whether low mitochondrial function predisposes to insulin resistance in humans is still unknown.
Methods
Here we investigated, in an intervention study, whether muscle with low mitochondrial oxidative capacity, induced by one-legged physical inactivity, would feature stronger signs of lipid-induced insulin resistance. To this end, ten male participants (age 22.4 ± 4.2 years, BMI 21.3 ± 2.0 kg/m2) underwent a 12 day unilateral lower-limb suspension with the contralateral leg serving as an active internal control.
Results
In vivo, mitochondrial oxidative capacity, assessed by phosphocreatine (PCr)-recovery half-time, was lower in the inactive vs active leg. Ex vivo, palmitate oxidation to 14CO2 was lower in the suspended leg vs the active leg; however, this did not result in significantly higher [14C]palmitate incorporation into triacylglycerol. The reduced mitochondrial function in the suspended leg was, however, paralleled by augmented IMCL content in both musculus tibialis anterior and musculus vastus lateralis, and by increased membrane bound protein kinase C (PKC) θ. Finally, upon lipid infusion, insulin signalling was lower in the suspended vs active leg.
Conclusions/interpretation
Together, these results demonstrate, in a unique human in vivo model, that a low mitochondrial oxidative capacity due to physical inactivity directly impacts IMCL accumulation and PKCθ translocation, resulting in impaired insulin signalling upon lipid infusion. This demonstrates the importance of mitochondrial oxidative capacity and muscle fat accumulation in the development of insulin resistance in humans.
Trial registration
Funding
PS was supported by a ‘VICI’ Research Grant for innovative research from the Netherlands Organization for Scientific Research (Grant 918.96.618).
Electronic supplementary material
The online version of this article (10.1007/s00125-020-05128-1) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Fat oxidation, Insulin resistance, Intramyocellular lipid content, Mitochondrial function, Mitochondrial oxidative capacity, Physical inactivity, Unilateral lower-limb suspension
Introduction
Physical inactivity is a major determinant of the current epidemic of chronic metabolic disorders, like obesity and type 2 diabetes mellitus [1, 2]. Obese individuals may display accumulation of excessive fat in non-adipose tissues, such as liver, heart and skeletal muscle, termed as ectopic fat accumulation [3–5]. Ectopic fat accumulation in skeletal muscle is strongly associated with insulin resistance [6, 7]. Likewise, individuals with type 2 diabetes [8] (as well as first-degree relatives of diabetic individuals), who feature insulin resistance years before the onset of the disease and, therefore, are prone to develop type 2 diabetes [9–11], are characterised by excessive accumulation of fat in skeletal muscle. Increased accumulation of the intramyocellular lipid (IMCL) intermediate diacylglycerol (DAG) and the concomitant activation of protein kinase C (PKC) θ have been reported in the muscle of individuals with type 2 diabetes as an explanation for this lipid-induced insulin resistance [12–15]. However, IMCL content is also increased in highly insulin-sensitive endurance-trained humans (known as the athlete’s paradox) [8], suggesting that IMCL accumulation, per se, is not causal for skeletal-muscle insulin resistance. Studies in lipid-infused rodents have dissociated intramuscular triacylglycerol (TAG) from lipid-induced muscle insulin resistance [16]. In contrast to the well-known high mitochondrial capacity of endurance-trained athletes, several (but not all [17, 18]) studies have found that insulin-resistant individuals with type 2 diabetes are characterised by impaired mitochondrial oxidative capacity [19–22]. These findings suggest that the imbalance between skeletal muscle fat accumulation and a low mitochondrial oxidative capacity determines the development of insulin resistance.
However, studying the direct relationship between physical inactivity, mitochondrial function and insulin resistance in humans is difficult and is mainly derived from cross-sectional studies. A few human experimental studies have applied the model of bed rest to investigate the impact of whole-body altered physical activity on the development of whole-body insulin resistance. To investigate, however, the muscle-specific mechanisms underlying lipid-induced insulin resistance, an unilateral immobilisation study, as presented here, may be helpful. The unilateral lower-limb suspension (ULLS), as originally described by Berg et al. [23], provides a unique model that can be applied in humans to examine the effect of a local reduction in muscle mitochondrial oxidative capacity (as shown previously [24]) on fat accumulation and insulin sensitivity, and allows comparison with an internal unaffected muscle. Here, we aimed to investigate the direct effects of physical inactivity on skeletal muscle mitochondrial oxidative capacity, IMCL accumulation, PKCθ translocation and insulin signalling upon lipid infusion. To this end, we employed the model of ULLS for 12 days in young men, with the contralateral leg serving as an active internal control muscle.
Methods
Participants
Ten healthy, lean young men participated in this study. Participant characteristics, as determined before ULLS, are shown in Table 1. The participants included in the study performed exercise maximally two times per week (or less) and none of the volunteers participated in competitive sports. Individuals with unstable body weight (>3 kg change in the 6 months preceding recruitment) were excluded. The institutional medical ethics committee approved the study and all participants gave their informed written consent. The study has been registered with ClinicalTrial.gov (registration no. NCT01576250).
Table 1.
Participant characteristics (n = 10), as measured before ULLS
| Characteristic | Mean ± SEM |
|---|---|
| Age (years) | 22.4 ± 4.2 |
| BMI (kg/m2) | 21.3 ± 2.0 |
| Fat (%) | 14.6 ± 3.9 |
| Systolic blood pressure (mmHg) | 115 ± 12 |
| Diastolic blood pressure (mmHg) | 74 ± 11 |
| Plasma glucose (mmol/l) | 4.9 ± 0.3 |
| Plasma TAGs (mmol/l) | 1.0 ± 0.4 |
| Plasma NEFA (mmol/l) | 0.4 ± 0.2 |
| /kg (ml min−1 [kg body weight]−1) | 48 ± 10 |
Study design
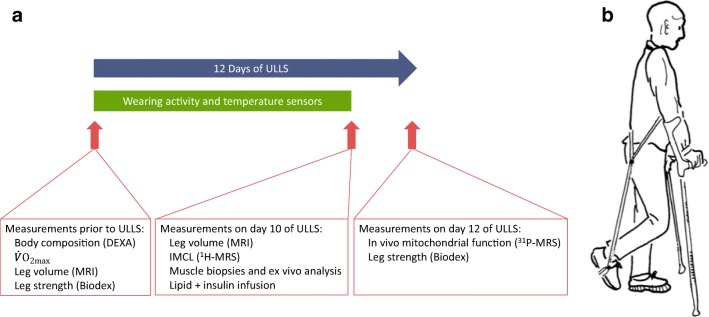
We examined the effects of one-legged restricted physical activity on skeletal muscle mitochondrial oxidative capacity in vivo, and ex vivo glucose and lipid metabolism, lipid content and insulin signalling. Participants were enrolled in the study between April 2012 until August 2013. Volunteers were subjected to 12 days of ULLS randomised to either their dominant or non-dominant leg. All outcome measurements were performed in the active and the inactivated leg and, where appropriate, in a randomised manner. Outcome parameters were compared within one individual in a paired design. See study design in Fig. 1a.
Fig. 1.
(a) Study design and (b) ULLS set up. Image in (b) from Berg et al. [23], reprinted with permission from the American Physiological Society. DEXA, dual energy x-ray absorptiometry
Before the start of the ULLS intervention, a basal blood sample was obtained after an overnight fast. Furthermore, resting blood pressure, body composition and maximal aerobic capacity was determined in all participants before ULLS.
Lower-limb suspension was performed as previously described [25]. Suspension was achieved by attachment of a sling to a non-rigid ankle brace and to a harness on the upper body. Thus, the suspended leg was unloaded from weight bearing (see Fig. 1b for illustration). The knee was slightly flexed at an angle of 130°, while the hip and the ankle maintained full mobility. The sling was worn during all locomotor activity. Participants were provided with crutches to support daily life mobility. During the night, the sling was taken off. Also, during periods of prolonged sitting and showering the sling was temporarily removed and participants were instructed to minimise activity of the suspended leg during sitting and showering. To monitor compliance and physical activity level, participants: (1) kept a diary of their daily activities; (2) wore temperature sensors on their lower front legs (iButtons; type DS1921H; Maxim Integrated, Dallas, TX, USA), as it is known that a lower physical activity leads to a lower temperature of the leg [26]; and (3) wore leg-specific accelerometers (CIRO Activity Monitors [CAMs]; Maastricht Instruments BV, Maastricht, NL) on both legs at all day times, except for when showering/bathing.
After 9 days of ULLS, participants reported to the laboratory after an overnight fast. Upper leg muscle volume and IMCL content in the muscle tibialis were determined by MRI and proton magnetic resonance spectroscopy (1H-MRS), respectively. Thereafter, a basal muscle biopsy was taken from musculus vastus lateralis of both legs, in randomised order, to examine the effect of ULLS on ex vivo lipid metabolism, IMCL content, and incorporation of labelled palmitate into DAG and TAG. Subsequently, a 5 h lipid infusion was initiated to elevate plasma NEFA concentrations to supraphysiological levels previously shown to compromise insulin sensitivity [16]. After 4.5 h of lipid infusion, a short-term hyperinsulinaemic-euglycaemic clamp was performed with simultaneous infusions of insulin and glucose. Insulin was infused at a constant rate and the co-infusion of 20% glucose was adjusted to keep participants in euglycaemia (~5 mmol/l). After 30 min of insulin infusion (and 4.5 h of lipid infusion), a second biopsy was taken from both legs, in a randomised order, to examine the effect of ULLS on markers of insulin sensitivity. On day 12 of ULLS, in vivo mitochondrial oxidative capacity (phosphocreatine [PCr] recovery) and isometric leg strength were measured in both legs using phosphorous magnetic resonance spectroscopy (31P-MRS) and Biodex (system 3; Biodex Medical Systems, New York, NY, USA), respectively. During the 2 days separating the clamp procedure and these functional assays, the individuals continued using the sling for ULLS to prevent any carry-over effects of the clamp procedure on subsequent functional assays.
Maximal performance and body composition
A routine incremental cycling test on a stationary bike to exhaustion was used to assess maximal exercise capacity, as described previously [27] and a dual energy x-ray absorptiometry (DEXA) scan was used to determine body composition. These measurements were done before ULLS.
Compliance
The activity monitors were attached to the upper part of the suspended and unsuspended limbs, just above the knee, using customised pouches [28]. The CAM contained a triaxial piezoresistive accelerometer and had a sample rate of 25 Hz. MATLAB R2014b (Mathworks, Natick, MA, USA) and algorithms were used to calculate movement intensity [29, 30]. Also skin temperature of the quadriceps and gastrocnemius muscle was monitored using iButtons [31] throughout the whole ULLS period, as a marker of physical activity.
Muscle volume and strength
As muscle mass is a determinant of insulin sensitivity [32], we aimed to design the intervention such that muscle mass loss would be minimised as a consequence of the suspension by using a relative short duration and a mild level of inactivity. However, as muscle mass can still be changed upon short-term interventions, we examined the muscle volume of the upper leg by serial T2-weighted MRI images, along with measurements of knee flexion and extension strength. Images to assess muscle volume were acquired using a turbo spin echo (TSE) sequence (TSE factor = 21), (Achieva 3Tx; Philips Healthcare, Best, the Netherlands). Twenty contiguous slices of 10 mm thickness were acquired (field-of-view [FOV] = 200× 400× 200 mm; echo time [TE] = 100 ms; repetition time [TR] = 5351 ms; flip angle = 90°, acquired by the body coil; scan duration = 4 min and 30 s). The most distal slice was positioned on the patella and all slices up to the hip were used for analysis. The magnetic resonance images (in-plane resolution 0.78× 0.78 mm) were segmented into muscle tissue and non-muscle tissue upon automated greyscale-based binning using a home-written script in MATLAB. The muscle area upon segmentation was computed for every single slice and multiplied by slice thickness to compute muscle volume.
Isometric knee extending and flexing strength at five different knee angles (70°, 80°, 90°, 100° and 110°) were measured using a Biodex III dynamometer [33]. Participants were seated upright in a chair and tightly fixed using straps. Subsequently, the knee was brought into position and participants were asked to conduct maximal knee extension (3 s) followed by maximal knee flexion (3 s), while the force traces were recorded on a computer. This procedure was repeated once for each evaluated joint angle. Participants were allowed 2 min of rest between contractions.
Lipid infusion plus clamp
In the morning after 9 days of limb suspension, participants reported to the laboratory after an overnight fast. After taking basal muscle biopsies (musculus vastus lateralis) from both legs in randomised order, a Teflon cannula was inserted into an antecubital vein for the heparinised (0.2 U kg−1 min−1) infusion of long-chain TAGs (1.35 ml/min intralipid; Intralipid, Fresenius-Kabi, the Netherlands) for 5 h. Blood was sampled from a second cannula, inserted retrogradely into a superficial dorsal hand vein, for later analysis of plasma glucose and NEFA. This venous blood was arterialised by placing the hand into a hot box that emits warm air (~65°C). After 4.5 h, a simultaneous infusion of insulin (40 mU m−2 min−1; Novorapid; Novo Nordisk, Copenhagen, Denmark) and glucose was started. During a 30 min hyperinsulinaemic-euglycaemic clamp, plasma glucose levels were clamped at ~5 mmol/l by variable co-infusion of 20% glucose. Thirty min after the start of the insulin infusion, a second muscle biopsy was taken from the vastus lateralis of both legs, in a randomised order.
Magnetic resonance spectroscopy measurements
In vivo mitochondrial oxidative capacity was determined by 31P-MRS in the afternoon after 12 days of ULLS, as previously described [22]. Briefly, a knee-extension protocol was performed on a custom-built magnetic resonance-compatible ergometer with a pulley system in a 3 T whole-body MRI scanner (Achieva 3Tx; Philips Healthcare) for 5 min, with weight corresponding to 50–60% of the participant’s pre-determined maximal knee-extension capacity. A transmit/receive surface coil (5 cm diameter) was positioned on the vastus lateralis muscle and a time series of 31P-MRS spectra (free induction decays) were acquired with a repetition time of 4 s. Post-exercise PCr kinetics was computed as previously described [22].
In vivo IMCL content in musculus tibialis was assessed by 1H-MRS on a 3.0 T whole-body magnetic resonance system (Achieva 3Tx; Philips Healthcare) as described previously [34]. T2-weighted images were acquired for accurate positioning of the voxel 12× 12× 12 mm3 in the tibialis anterior muscle. Point resolved spectroscopy (PRESS) was used with the following acquisition parameters: TR = 2000 ms; TE = 38 ms; number of signal averages (NSA) = 128; sample points = 2048. Water suppression was performed using a selective excitation pulse followed by dephasing gradients. A second non-water suppressed spectrum was acquired subsequently from the same volume (NSA = 8), which enabled us to use the water signal as an internal reference.
The 1H-MRS spectra obtained were analysed and fitted in the time domain by using the non-linear least-squares Advanced Method for Accurate, Robust, and Efficient Spectral (AMARES) algorithm [35, 36], in the java-based magnetic resonance user interface (jMRUI) software package [37] as described earlier [34]. IMCL is given as percentage of the CH2 peak compared with the water resonance and corrected for T1 and T2 relaxation time. IMCL could not be measured in one participant due to technical failure.
Muscle biopsies and analysis
Muscle biopsies were taken from the musculus vastus lateralis of both legs after 9 days of ULLS, under local anaesthesia (2% lidocaine; Fresenius-Kabi, Bad Homburg vor der Höhe, Germany), according to the Bergstrom technique [38], and instantaneously processed for ex vivo 14C oxidation assays. The remainder of the muscle tissue was stored at −80°C for future analysis of IMCL content by histology, and for markers of insulin action.
[14C]palmitate and glucose oxidation assays were performed as previously described [39, 40]. Briefly, palmitate oxidation was determined by measuring production of 14CO2 and acid-soluble metabolites (ASMs) in skeletal-muscle homogenates containing 250 mmol/l sucrose, 10 mmol/l Tris-HCl, 1 mmol/l EDTA and 2 mmol/l ATP. Reactions were initiated with 0.2 mmol/l palmitate and 0.0175 mmol/l [1-14C]palmitate, and terminated with 70% perchloric acid. CO2 was trapped in 1 mol/l NaOH. Lipid incorporation was measured by thin-layer chromatography. Radioactivity in bands corresponding to TAG and DAG were quantified by liquid scintillation, as described previously [41]. Glucose oxidation to CO2 was determined by replacing [1-14C]palmitate with [U-14C]glucose.
IMCL
IMCL was assessed in muscle cross-sections using a modified Oil Red O staining for fluorescence microscopy [42]. Images were analysed with ImageJ (type 1.51 J8 for windows; NIH, Bethesda, Maryland, USA). Freezing artefacts in sections of four participants prevented valid histological quantification of the IMCL content and these participants were, therefore, excluded from the analysis.
Insulin signalling
Muscle biopsies were prepared for analysis of the expression and phosphorylation of components of the insulin signalling pathway by western blotting, as described previously [43]. The blots were incubated overnight at 4°C with antibodies recognising the insulin receptor β-subunit (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and pyruvate dehydrogenase kinase 4 (PDK4) (Abcam, Cambridge, UK), and phosphorylated IRS1Ser1101, AktThr308, AktSer473, glycogen synthase kinase 3β (GSK3βSer9), glycogen synthase (GSSer641), Forkhead box O (FOXO1Thr24/FOXO3aThr32), AMP-activated protein kinase (AMPKThr172) and acetyl-CoA carboxylase (ACCSer79) (all from Cell Signaling Technology, Beverly, MA, USA). For an overview of all antibodies, please see electronic supplementary material (ESM) Table 1. Antibodies were diluted in 5% (wt/vol.) BSA dissolved in Tris-buffered saline and 0.1% Tween-20. Following incubation, the blots were washed with Tris-buffered saline and 0.1% Tween-20. Bound antibodies were detected by incubation with the appropriate secondary horseradish peroxidase (HRP)-conjugated antibodies (Promega, Mannheim, Germany), and visualised by enhanced chemiluminescence on a VersaDoc 4000 MP (Bio-Rad Laboratories, Irvine, CA, USA) workstation. Signals were quantified using ImageLab software (version 5.2.1; Bio-Rad), and normalised for abundance of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (blots shown in ESM Fig. 1).
PKCθ translocation
Membrane translocation of PKCθ was assessed as described [16]. Due to lack of muscle material, PKCθ translocation could only be measured in n = 8 participants. PKCθ translocation was expressed as the ratio of membrane to cytosol bands on the same film.
Blood sample analysis
Blood samples were collected in EDTA-containing tubes and immediately spun at high speed, frozen in liquid nitrogen and stored at −80°C. Plasma NEFA, TAGs, and glucose were measured with enzymatic assays automated on a Cobas Fara/Mira (NEFA: Wako NEFA C test kit, Wako Chemicals, Neuss, Germany; glucose: hexokinase method, Roche, Basel, Switzerland; TAGs: ABX Pentra CP reagents, Horiba ABX Diagnostics, Montpellier, France).
Statistics
All data were analysed in a blinded fashion. Data are presented as mean ± SEM. The samples size was determined based on insulin-stimulated skeletal muscle glucose disposal, with the expected effect size and SD based on previous research within our research group [44]. All statistics were performed using SPSS 16.0 for Mac (IBM, New York, NY, USA). For most parameters, differences between the suspended and the active leg were analysed with a two-tailed, paired Student’s t test, unless otherwise specified. The non-normally distributed insulin action data were tested for differences using a non-parametric Wilcoxon signed-rank test for related samples. Statistical significance was set at p ≤ 0.05.
Results
To check for compliance to the suspension protocol, we used both accelerometer data and data on skin temperature; activity counts in the suspended leg were lower in all participants than in the active leg (48,826 ± 7354 counts/day vs 65,969 ± 10,101 counts/day, respectively; p < 0.050; Fig. 2a). In line with lower activity, skin temperature was lower in the suspended leg compared with the active leg (lower leg: 31.5 ± 0.03°C vs 33.0 ± 0.02°C; upper leg: 32.2 ± 0.02°C vs 33.0 ± 0.04°C, respectively; p < 0.050; Fig. 2b). Jointly, these data indicate that all individuals were very compliant and adhered well to the ULLS protocol.
Fig. 2.
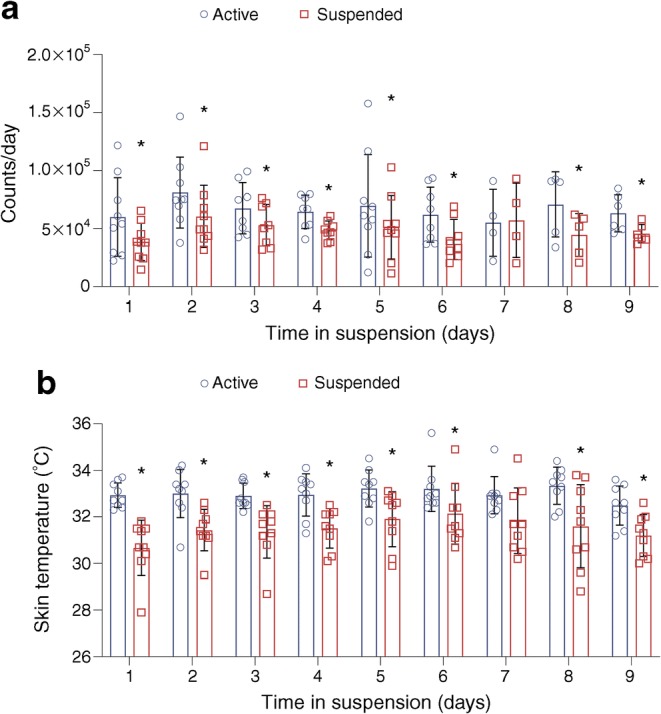
Participant compliance to the intervention as measured with (a) activity monitors (accelerometers) (n = 4–9) and (b) temperature sensors (n = 9). *p < 0.05. Data expressed as mean ± SEM
We were anticipating that upon 10 days of ULLS, mitochondrial oxidative capacity would decrease without large effects on muscle volume and strength. As anticipated, upper leg muscle volume upon ULLS was similar in the active vs the suspended leg (2766 ± 173 cm3 in the active leg vs 2794 ± 182 cm3 in the suspended leg; p = 0.33; data not shown). Furthermore, maximal isometric strength of the knee extensors and flexors was unaffected by ULSS (170 ± 21 Nm for the suspended leg extensor vs 178 ± 20 Nm for the active leg extensor, p = 0.601; 95 ± 9 Nm for the suspended leg flexor vs 95 ± 9 Nm for the active leg flexor, p = 0.948; data not shown). Data for the knee extension was obtained at an angle of 90° and data for the knee flexion was obtained at an angle of 70°.
Next, we investigated the effect of ULLS on mitochondrial oxidative capacity in vivo by 31P-MRS and ex vivo by [14C]palmitate oxidation assays. PCr-recovery half-time (T1/2) was significantly longer in the suspended leg compared with the active leg, (21.4 ± 2.3 s vs 16.7 ± 1.8 s, respectively; p = 0.019; Fig. 3a), demonstrating that mitochondrial oxidative capacity was indeed reduced in skeletal muscle of the inactive leg. Ex vivo palmitate oxidation to 14CO2 (‘complete oxidation’) was significantly lower in the suspended leg compared with the active leg after 9 days of suspension (0.14 ± 0.03 nmol 2 h−1 mg−1 vs 0.18 ± 0.03 nmol 2 h−1 mg−1, respectively; p = 0.013; Fig. 3b [n = 9]). The level of ASMs (reflecting incomplete oxidation) was non-significantly lower in the suspended leg compared with the active leg (1.15 ± 0.07 nmol [2 h]−1 mg−1 vs 1.29 ± 0.10 nmol [2 h]−1 mg−1, respectively; p = 0.065; Fig. 3c [n = 9]). In addition, incorporation of [14C]palmitate into TAGs was non-significantly higher in the suspended leg compared with the active leg (0.019 ± 0.005 nmol [2 h]−1 mg−1 vs 0.010 ± 0.002 nmol [2 h]−1 mg−1, respectively; p = 0.075; Fig. 3d [n = 9]), whereas [14C]palmitate incorporation into DAGs was not different between the two legs (0.048 ± 0.008 nmol [2 h]−1 mg−1 vs 0.046 ± 0.006 nmol [2 h]−1 mg−1 in the suspended vs the active leg; p = 0.822; Fig. 3e). No effects were observed on ex vivo glucose oxidation rates (0.429 ± 0.125 nmol [2 h]−1 mg−1 in the suspended leg vs 0.567 ± 0.167 nmol [2 h]−1 mg−1 in the active leg; p = 0.461; n = 9; data not shown).
Fig. 3.
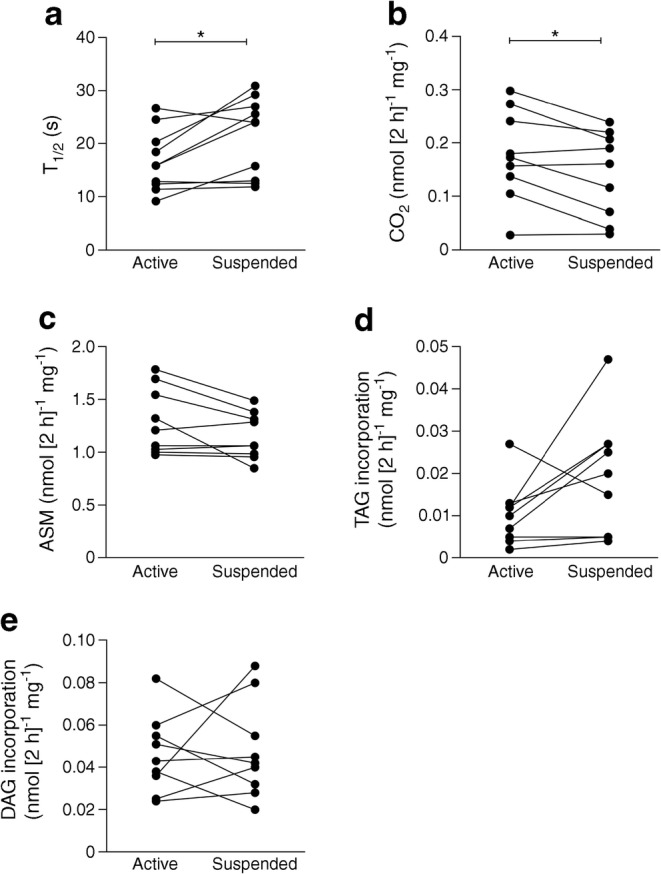
Mitochondrial oxidative capacity and incorporation of labelled palmitate into DAG and TAG in the active vs the suspended leg, post-suspension, in the overnight fasted state. (a–c) Mitochondrial oxidative capacity expressed as (a) PCr-recovery T1/2 in vivo (n = 10), and (b,c) ex vivo [14C]palmitate oxidation to CO2 (b; n = 9) and ASMs (c; n = 9). (d, e (both n = 9) [14C]palmitate incorporation into TAGs (d) and DAGs (e). *p < 0.05
We subsequently investigated if the reduction in mitochondrial oxidative capacity in the suspended leg was also paralleled by accumulation of IMCL. These examinations were performed in two different ways: in muscle biopsies taken prior to lipid infusion and with 1H-MRS-based measurements of IMCL, both in the fasted state. In vivo IMCL in the musculus tibialis anterior, as measured with 1H-MRS, was 23% higher in the suspended leg compared with the active leg (0.31% ± 0.05% vs 0.24% ± 0.04%; p = 0.003; Fig. 4a [n = 9]). This increase in IMCL upon leg suspension was confirmed in muscle biopsies taken from the musculus vastus lateralis, showing a 53% higher IMCL content in the suspended leg compared with the active leg (lipid area fraction: 1.79% ± 0.48% vs 0.85% ± 0.24%; p = 0.021; Fig. 4b [n = 6]).
Fig. 4.
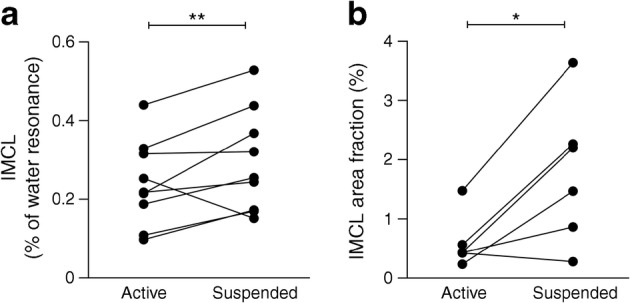
(a) In vivo IMCL in the musculus tibialis anterior, measured by 1H-MRS, in the active vs the suspended leg, post-suspension, in the overnight fasted state (n = 9). (b) Ex vivo IMCL in musculus vastus lateralis, measured with Oil Red O staining, in the overnight fasted state (n = 6). *p < 0.05, **p < 0.01
It has been shown that activation of PKCθ by lipid intermediates, leading to translocation of PKCθ from the cytosol to the muscle membrane, is pivotal in lipid-induced insulin resistance (reviewed in [45]). Although human studies are limited in the amount of muscle material that can be obtained, which prevented us from performing lipidomics, we did determine PKCθ activation. We found that PKCθ translocation, reflected as the ratio of membrane/cytosol PKCθ content, was significantly higher in the suspended leg compared with the active leg (0.91 ± 0.16 vs 0.29 ± 0.06; p = 0.01; Fig. 5 [n = 8]).
Fig. 5.
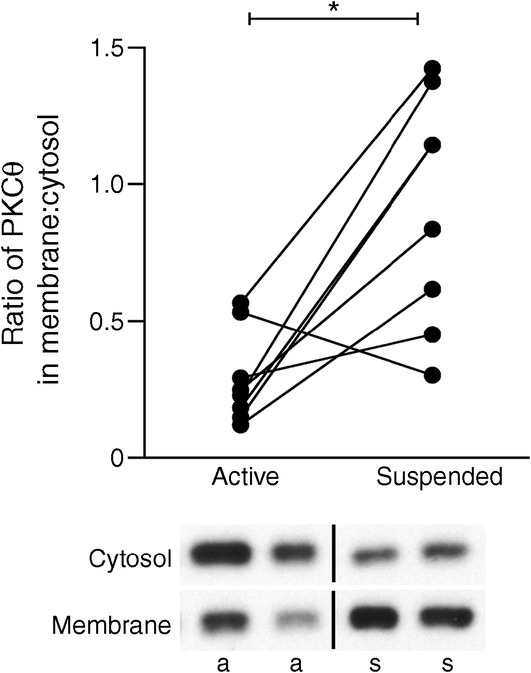
Ratio of PKCθ in the muscle membrane:cytosol (a measure of PKCθ activation) in the overnight fasted state. Representative blots are also shown (a, active; s, suspended). n = 8; *p < 0.05
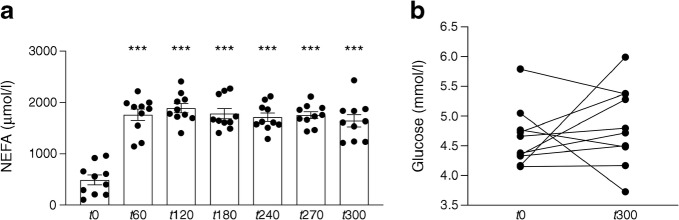
To investigate if compromised mitochondrial oxidative capacity contributed to lipid-induced insulin resistance, we applied a model of lipid infusion. As expected, systemic plasma NEFA concentrations increased from 492 ± 95 μmol/l at baseline to 1759 ± 111 μmol/l 1 h after the start of lipid infusion and remained elevated over the 5 h lipid-infusion period (p < 0.0001; Fig. 6a). As aimed, plasma glucose concentrations were stable and did not change significantly after lipid and insulin infusion (4.6 ± 0.2 mmol/l at baseline and 4.8 ± 0.2 mmol/l after 5 h of lipid infusion; p = 0.462; Fig. 6b).
Fig. 6.
Plasma concentrations of (a) NEFA (n = 10; data expressed as mean ± SEM) and (b) glucose during the insulin+lipid infusion (n = 10). ***p < 0.001, compared with baseline (time [t]0)
In contrast to rodent models, where 14C-labelled glucose can be used to determine leg-specific muscle glucose uptake, radioactive 14C cannot be applied in humans. Therefore, we measured insulin-induced activation of insulin signalling as a measure of insulin sensitivity in both the suspended and active leg upon a 5 h lipid infusion. As anticipated the expression level of insulin receptor between the suspended and the active leg after insulin+lipid infusion was unaffected (p = 0.161; Fig. 7a). Consistent with increased PKCθ activation in the suspended leg, we observed increased phosphorylation of IRS1 at Ser1101 which is a known target of PKCθ [46], in the suspended leg compared with the active leg (p = 0.018; Fig. 7b). Increased Ser1101 phosphorylation of IRS1, as well as attenuated phosphorylation of Akt at Thr308 and Ser473 residues, are expected under insulin-resistant conditions [12, 47]. Indeed, phosphorylation of Akt at Thr308 (but not Ser473) was significantly reduced in the suspended leg compared with the inactive leg upon insulin+lipid infusion (p-AktThr308: p = 0.036, Fig. 7c; p-AktSer473: p = 0.263; Fig. 7d). The abundance of PDK4 was borderline higher in the suspended leg upon insulin+lipid infusion compared with the active leg (p = 0.058; Fig. 7e). Furthermore, a higher phosphorylation of GS in the suspended leg compared with the active leg was observed (p = 0.050; Fig. 7f) and the phosphorylation of GSK3β at Ser9, inhibiting GSK3β activity, was significantly higher in the suspended leg compared with the active leg upon lipid infusion (p = 0.036; Fig. 7g). Finally, the phosphorylation of FOXO (p = 0.889; Fig. 7h), AMPK at Thr172 (p = 0.208; Fig. 7i) and ACC (p = 0.183; Fig. 7j) were not significantly different between legs. Collectively, these data indicate that the suspended leg featured more vigorous lipid-induced insulin resistance compared with the contralateral active leg.
Fig. 7.
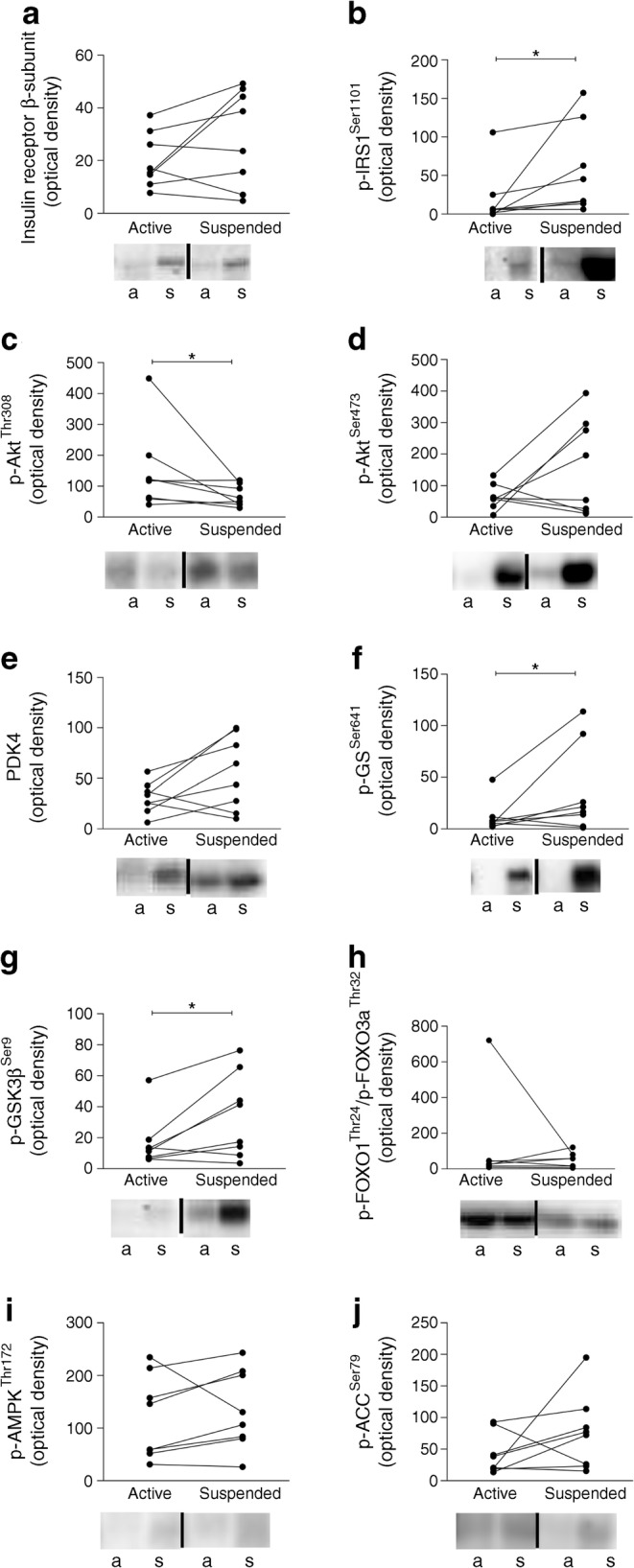
The expression level of (a) insulin receptor, (b) p-IRS1Ser1101, (c) p-AktThr308, (d) p-AktSer473, (e) PDK4, (f) p-GSSer641, (g) p-GSK3βSer9, (h) p-FOXO (FOXO1Thr24/FOXO3aThr32), (i) p-AMPKThr172 and (j) p-ACCSer79 in the active vs the suspended leg, in the insulin-stimulated state. Representative blots also shown (a, active; s, suspended). n = 8; *p < 0.05
Discussion
Physical inactivity-associated low skeletal muscle mitochondrial oxidative capacity is suggested to contribute to skeletal muscle fat accumulation and insulin resistance [19, 20] and thereby is thought to be involved in the development of type 2 diabetes. However, proof for a direct relationship between these variables is difficult to examine in humans, since physical inactivity has many systemic effects that may also contribute to insulin resistance. Here we employed a unique human model of unilateral leg suspension that allowed us to investigate the direct effects of inactivity-mediated lowering of mitochondrial oxidative capacity on IMCL content and lipid-induced insulin resistance, using the contralateral leg as an active control. We observed that local physical inactivity indeed leads to lower in vivo mitochondrial oxidative capacity, consistent with previous findings [24], as well as a reduced fat oxidative capacity ex vivo, paralleled by elevated IMCL content, PKCθ activation and a blunted insulin action upon lipid infusion. Together these data demonstrate that the direct effects of physical inactivity on key metabolic parameters, including lowered mitochondrial oxidative capacity in muscle, may underlie lipid-induced insulin resistance, via the activation of PKCθ.
To investigate the local effects of physical inactivity independently from the systemic effects, we used the ULLS model in humans. During the ULLS period, leg skin temperature and overall activity level was lower in the suspended leg compared with the active leg, showing good compliance to the intervention. Importantly, however, the immobilised leg was not completely inactivated, which could underlie differences seen with studies including bed rest or a full leg cast, with more pronounced physical inactivity [48, 49]. Importantly, in our hands, this model of inactivity did not induce muscle mass loss, as substantiated by the lack of changes in leg muscle volume or muscle strength, likely due to the relatively short duration and relative mild induction of physical inactivity. This lack of loss of muscle mass is helpful in the present study as muscle mass is a determinant of insulin sensitivity. Together, the ULLS model provides the characteristics of a good model to mimic the modest reductions in physical activity in society that are associated with the development of chronic metabolic diseases over time.
Interestingly, both mitochondrial oxidative capacity and skeletal muscle fat oxidative capacity (palmitate oxidation to 14CO2) appear to be ~22% lower in the suspended leg compared with the active leg. Previously, we have shown that young, lean insulin-resistant offspring of parents with type 2 diabetes [11] and individuals with diabetes have a 25–48% lower mitochondrial oxidative capacity [21, 22, 50] and fat oxidative capacity [51] compared with healthy control participants, which was associated with increased IMCL. In addition, ageing has been shown to be associated with a 35–40% reduction in mitochondrial skeletal muscle oxidative/phosphorylation activity, increased IMCL content and muscle insulin resistance [52]. This indicates that with the relatively mild inactivity induced by ULLS, we created a reduction in mitochondrial oxidative capacity and fat oxidative capacity comparable with individuals prone to type 2 diabetes or diagnosed with type 2 diabetes.
The aim of the current study was to test the hypothesis that an inactivity-induced reduction in mitochondrial function would result in the accumulation of IMCL in humans and impaired insulin signalling upon lipid infusion. Indeed, we observed that IMCL content was higher in the suspended inactive leg than in the active leg upon the intervention period, as measured by two independent measures. This result is consistent with previous bedrest studies, showing a reduction in fat oxidation [53, 54], favouring incorporation of dietary saturated fatty acids (palmitate) into IMCLs [55]. In addition, our results are consistent with a recent study showing that 2 weeks of immobilisation also resulted in IMCL accumulation in young and elderly volunteers [56]. Although less likely, our model cannot rule out that physical inactivity resulted in a primary change in IMCL and secondary impairment in mitochondrial oxidative capacity. To investigate this, we used an ex vivo approach and determined the incorporation of [14C]palmitate into TAGs in muscle homogenates of the active and inactive leg. Consistent with our hypothesis, the reduced mitochondrial and fat oxidative capacity in muscle of the inactive leg was accompanied by higher [14C]palmitate incorporation into TAGs, suggesting that a low oxidative capacity accelerates IMCL accumulation, although this measure did not reach statistical significance probably due to the low number of participants in our study.
It has been shown that the association between IMCL accumulation and insulin resistance can be mechanistically explained by the interference of lipid intermediates with insulin signalling. In this respect, activation of novel PKCs has been shown to link fat accumulation with impaired insulin signalling in both animal models and individuals with type 2 diabetes [7]. Thus, studies using rats fed a high-fat diet showed that the translocation of PKC was associated with skeletal muscle insulin resistance [57]. In human studies, PKCθ translocation in the basal state was found to be higher in skeletal muscle of individuals with type 2 diabetes compared with lean control participants [12]. Here, we observed a pronounced increase in membrane-associated PKCθ content in the skeletal muscle of the inactivated compared with active leg, further providing evidence for the cascade by which a reduction in mitochondrial function leads to the accumulation of IMCLs and activation of PKCθ. We hypothesised that muscle of the inactive leg would be more insulin resistant upon lipid infusion compared with the active leg. Indeed, upon lipid infusion we found significantly higher p-GSSer641, p-IRS1Ser1101 and lower p-AktThr308 in the suspended leg, all indicative of a more pronounced compromised insulin action in the suspended leg than in the active leg. Phosphorylation of IRS1 at Ser1101 inhibits Akt [12, 47], leading to reduced cellular glucose uptake. Phosphorylation of GS inactivates GS and results in lower glycogen production, which is suggestive of lower insulin sensitivity in the suspended leg. Also, an increase in PDK4 (which was non-significantly elevated in the suspended leg in our study) would be consistent with reduced insulin sensitivity in the inactive leg. Overall these data support the notion that the suspended, inactive leg, with compromised mitochondrial function, is more insulin resistant than the active leg upon lipid challenge. These data are consistent with our previous observation that highly trained athletes are protected against lipid-induced insulin resistance [43] and collectively suggest that a low mitochondrial function induced by physical inactivity predisposes humans to the development of lipid-induced insulin resistance. However, our results do not imply that mitochondrial function is the only determinant of insulin resistance; studies have shown that improvements in insulin sensitivity do not always coincide with improvements in mitochondrial function, for example, as was recently observed after gastric bypass-induced weight loss [58].
The intensive design of our study, including one-leg immobilisation for 12 consecutive days and two muscle biopsies, did prevent us from adding a control arm with the infusion of saline to compare difference in insulin sensitivity between legs without lipid infusion. Although this is a limitation of the study, the present design did allow us to carefully evaluate the differences between the active vs inactive leg under lipid-infused conditions, an intervention well-known to acutely induce insulin resistance of the periphery.
In conclusion, inactivity compromises in vivo mitochondrial oxidative capacity, along with reduced fat oxidative capacity and higher IMCL content, PKCθ translocation and an augmented decline in insulin action upon lipid infusion. Together, these data demonstrate the impact of physical inactivity on skeletal muscle insulin signalling, fat oxidation and IMCL storage and suggest that a reduced mitochondrial oxidative capacity may underlie these effects in healthy young men.
Electronic supplementary material
(PDF 1763 kb)
Acknowledgements
Some of the data were presented as an abstract at the 53rd EASD Annual Meeting in 2017.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- ASM
Acid-soluble metabolite
- CAM
CIRO Activity Monitor
- DAG
Diacylglycerol
- FOXO
Forkhead box O
- GS
Glycogen synthase
- GSK3β
Glycogen synthase kinase 3β
- 1H-MRS
Proton magnetic resonance spectroscopy
- IMCL
Intramyocellular lipid
- NSA
Number of signal averages
- PCr
Phosphocreatine
- PDK4
Pyruvate dehydrogenase kinase 4
- PKC
Protein kinase C
- 31P-MRS
Phosphorous magnetic resonance spectroscopy
- T1/2
Half-time
- TAG
Triacylglycerol
- TE
Echo time
- TR
Repetition time
- TSE
Turbo spin echo
- ULLS
Unilateral lower-limb suspension
Contribution statement
LB designed and performed the experiments, analysed data and wrote the manuscript. EP, TvdW, AG, MB, JAJ, GS, DZ, GIS and EM-K assisted during the acquisition, analysis and interpretation of data and reviewed the manuscript. KM contributed to the design of the study, interpretation of data and reviewed the manuscript. MH contributed to the design of the study and reviewed the manuscript. DMO contributed to the analysis of data, interpretation of data and reviewed the manuscript. MKCH, VBS-H and PS contributed to the design of the study, analysed and interpreted the data, and reviewed and edited the manuscript. All authors reviewed and approved the final version of the manuscript. PS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
PS was supported by a ‘VICI’ Research Grant for innovative research from the Netherlands Organization for Scientific Research (Grant 918.96.618).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Esther Phielix and Tineke van de Weijer contributed equally to this work.
References
- 1.Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18(6):737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- 2.Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni M, Rossi AP, Fantin F, et al. Predictors of ectopic fat in humans. Curr Obes Rep. 2014;3(4):404–413. doi: 10.1007/s13679-014-0126-7. [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 6.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 7.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 9.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48(8):1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 10.Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48(5):1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szendroedi J, Yoshimura T, Phielix E, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111(26):9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perreault L, Newsom SA, Strauss A, et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight. 2018;3(3):e96805. doi: 10.1172/jci.insight.96805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-θ knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114(6):823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 17.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50(4):790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol. 2008;158(5):643–653. doi: 10.1530/EJE-07-0756. [DOI] [PubMed] [Google Scholar]
- 19.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 20.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 21.Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50(1):113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 23.Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991;70(4):1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- 24.Gram M, Vigelso A, Yokota T, et al. Two weeks of one-leg immobilization decreases skeletal muscle respiratory capacity equally in young and elderly men. Exp Gerontol. 2014;58:269–278. doi: 10.1016/j.exger.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol. 2005;288(4):H1747–H1755. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- 26.Noddeland H, Winkel J. Effects of leg activity and ambient barometric pressure on foot swelling and lower-limb skin temperature during 8 h of sitting. Eur J Appl Physiol Occup Physiol. 1988;57(4):409–414. doi: 10.1007/bf00417985. [DOI] [PubMed] [Google Scholar]
- 27.Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6(4):197–201. doi: 10.1055/s-2008-1025839. [DOI] [PubMed] [Google Scholar]
- 28.Dudley GA, Duvoisin MR, Adams GR, Meyer RA, Belew AH, Buchanan P. Adaptations to unilateral lower limb suspension in humans. Aviat Space Environ Med. 1992;63(8):678–683. [PubMed] [Google Scholar]
- 29.Annegarn J, Spruit MA, Uszko-Lencer NH, et al. Objective physical activity assessment in patients with chronic organ failure: a validation study of a new single-unit activity monitor. Arch Phys Med Rehabil. 2011;92(11):1852–1857. doi: 10.1016/j.apmr.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Preece SJ, Goulermas JY, Kenney LP, Howard D, Meijer K, Crompton R. Activity identification using body-mounted sensors--a review of classification techniques. Physiol Meas. 2009;30(4):R1–R33. doi: 10.1088/0967-3334/30/4/R01. [DOI] [PubMed] [Google Scholar]
- 31.van Marken Lichtenbelt WD, Daanen HA, Wouters L, Fronczek R, Raymann RJ, Severens NM, van Someren E. Evaluation of wireless determination of skin temperature using iButtons. Physiol Behav. 2006;88(4–5):489–497. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and nutrition examination survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 33.Savelberg HH, Meijer K. The effect of age and joint angle on the proportionality of extensor and flexor strength at the knee joint. J Gerontol. 2004;59(11):1120–1128. doi: 10.1093/gerona/59.11.1120. [DOI] [PubMed] [Google Scholar]
- 34.Lindeboom L, Nabuurs CI, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am J Clin Nutr. 2015;101(1):65–71. doi: 10.3945/ajcn.114.094730. [DOI] [PubMed] [Google Scholar]
- 35.Krssak M, Mlynarik V, Meyerspeer M, Moser E, Roden M. 1H NMR relaxation times of skeletal muscle metabolites at 3 T. Magma. 2004;16(4):155–159. doi: 10.1007/s10334-003-0029-1. [DOI] [PubMed] [Google Scholar]
- 36.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 37.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12(2–3):141–152. doi: 10.1007/bf02668096. [DOI] [PubMed] [Google Scholar]
- 38.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- 39.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Phys Endocrinol Metab. 2000;279(5):E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 40.Sparks LM, Johannsen NM, Church TS, et al. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2013;98(4):1694–1702. doi: 10.1210/jc.2012-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sparks LM, Moro C, Ukropcova B, et al. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One. 2011;6(7):e21068. doi: 10.1371/journal.pone.0021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116(1):63–68. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 43.Phielix E, Meex R, Ouwens DM, et al. High oxidative capacity due to chronic exercise training attenuates lipid-induced insulin resistance. Diabetes. 2012;61(10):2472–2478. doi: 10.2337/db11-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Ligt M, Bruls YMH, Hansen J, et al. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol Metab. 2018;12:39–47. doi: 10.1016/j.molmet.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Soos TJ, Li X, et al. Protein kinase C θ inhibits insulin signaling by phosphorylating IRS1 at Ser1101. J Biol Chem. 2004;279(44):45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 47.Hojlund K, Glintborg D, Andersen NR, et al. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008;57(2):357–366. doi: 10.2337/db07-0706. [DOI] [PubMed] [Google Scholar]
- 48.Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 49.Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014;210(3):600–611. doi: 10.1111/apha.12190. [DOI] [PubMed] [Google Scholar]
- 50.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572–579. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sparks LM, Bosma M, Brouwers B, et al. Reduced incorporation of fatty acids into triacylglycerol in myotubes from obese individuals with type 2 diabetes. Diabetes. 2014;63(5):1583–1593. doi: 10.2337/db13-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergouignan A, Schoeller DA, Normand S, et al. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: results of a randomized trial. PLoS Clin Trials. 2006;1(5):e27. doi: 10.1371/journal.pctr.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanc S, Normand S, Pachiaudi C, Fortrat JO, Laville M, Gharib C. Fuel homeostasis during physical inactivity induced by bed rest. J Clin Endocrinol Metab. 2000;85(6):2223–2233. doi: 10.1210/jcem.85.6.6617. [DOI] [PubMed] [Google Scholar]
- 55.Bergouignan A, Trudel G, Simon C, et al. Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes. 2009;58(2):367–376. doi: 10.2337/db08-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vigelso A, Gram M, Dybboe R, et al. The effect of age and unilateral leg immobilization for 2 weeks on substrate utilization during moderate-intensity exercise in human skeletal muscle. J Physiol. 2016;594(8):2339–2358. doi: 10.1113/JP271712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz-Peiffer C, Browne CL, Oakes ND, et al. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46(2):169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 58.Lund MT, Larsen S, Hansen M, et al. Mitochondrial respiratory capacity remains stable despite a comprehensive and sustained increase in insulin sensitivity in obese patients undergoing gastric bypass surgery. Acta Physiol. 2018;223(1):e13032. doi: 10.1111/apha.13032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1763 kb)
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.