Abstract
Chondrocyte hypertrophy represents a crucial turning point during endochondral bone development. This process is tightly regulated by various factors, constituting a regulatory network that maintains normal bone development. Histone deacetylase 4 (HDAC4) is the most well-characterized member of the HDAC class IIa family and participates in different signalling networks during development in various tissues by promoting chromatin condensation and transcriptional repression. Studies have reported that HDAC4-null mice display premature ossification of developing bones due to ectopic and early-onset chondrocyte hypertrophy. Overexpression of HDAC4 in proliferating chondrocytes inhibits hypertrophy and ossification of developing bones, which suggests that HDAC4, as a negative regulator, is involved in the network regulating chondrocyte hypertrophy. Overall, HDAC4 plays a key role during bone development and disease. Thus, understanding the role of HDAC4 during chondrocyte hypertrophy and endochondral bone formation and its features regarding the structure, function, and regulation of this process will not only provide new insight into the mechanisms by which HDAC4 is involved in chondrocyte hypertrophy and endochondral bone development, but will also create a platform for developing a therapeutic strategy for related diseases.
Cite this article: Bone Joint Res. 2020;9(2):82–89.
Keywords: Histone deacetylase 4, Chondrocyte hypertrophy, Endochondral bone development
Article focus
The role of histone deacetylase 4 (HDAC4) during chondrocyte hypertrophy and endochondral bone development.
Key messages
HDAC4 represses chondrocyte hypertrophy and endochondral bone formation by inhibiting the function of myocyte-specific enhancer factor 2C (MEF2C) and runt-related transcription factor 2 (RUNX2).
New breakthroughs might be achieved by controlling microRNA (miRNA), post-translational modifications (PTMs), or cleavage of HDAC4 to regulate chondrocyte hypertrophy and endochondral bone formation.
The breakthroughs of the review is that the article explains how HDAC4 functions by analyzing the features of HDAC4 in the structure, function, and regulation of chondrocyte hypertrophy during endochondral bone formation.
Strengths and limitations
This article not only reviews the role of HDAC4 during chondrocyte hypertrophy and endochondral bone development, but also systematically introduces the structural basis and regulatory mechanism of HDAC4.
Although the authors have tried their best to retrieve the relevant articles in recent years, there may still be some relevant content not included in this article.
Introduction
Endochondral bone formation is critical in vertebrate skeletal development, including in the long bones and vertebrae, via successive steps of mesenchymal condensation, chondrogenesis, chondrocyte maturation, hypertrophy, and finally vasculogenesis and osteoblast recruitment.1 Chondrocyte hypertrophy is essential for vascular invasion, osteoblast differentiation, and endochondral ossification and represents a crucial turning point from chondrocyte differentiation to bone formation. Anomalous regulation of chondrocyte hypertrophy disrupts the normal differentiation programme, resulting in a distorted growth plate architecture that leads to skeletal dysplasia with pronounced limb shortening.2,3 However, a systematic review regarding how HDAC regulates endochondral bone formation has been lacking. This review focuses on the mechanism by which HDAC is involved in chondrocyte hypertrophy based on its structure, function, and regulation.
Histone deacetylase 4 (HDAC4) is a member of the HDAC class IIa family (HDAC-4, -5, -6, -7, and -10) that promotes chromatin condensation and represses transcription by acetylating lysine to inhibit histone–DNA, histone–histone, and other protein–DNA interactions. These interactions have evolved into different signalling networks of development in different tissues, including adult tissues that adapt to environmental changes.4 Initial studies on HDAC4 addressed skeletal muscle development,5 and HDAC4 was found at high levels not only in skeletal muscle but also in the brain,6 heart,7 vasculature,8 cartilage,9 and liver.10 The study has shown that mice lacking HDAC4 display a remarkable phenotype characterized by inappropriate chondrocyte hypertrophy that leads to ectopic bone formation. Furthermore, overexpression of HDAC4 in proliferating chondrocytes in vivo inhibits hypertrophy and, thus, ossification of developing bone.11 These results have established that by controlling chondrocyte hypertrophy, HDAC4 acts as a central regulator of growth plate development.12
In addition, recent studies have discovered that during osteoarthritis (OA), chondrocytes lose their stable phenotype and undergo hypertrophic differentiation. Cartilage degeneration in OA is therefore similar to cartilage degradation in the growth plate.13,14 Decreases in HDAC4 contribute, at least in part, to the pathogenesis of OA cartilage degeneration.11,15 Overall, an understanding of the role of HDAC4 during chondrocyte hypertrophy and endochondral bone formation might provide a new therapeutic strategy for OA.
In this review, we focus on the role of HDAC4 in regulating chondrocyte hypertrophy and endochondral bone formation. The review not only provides new insight into the mechanisms of HDAC4 in chondrocyte hypertrophy and endochondral bone development but also creates a platform for developing a therapeutic strategy for related diseases, including skeletal dysplasias such as pronounced limb shortening and OA.
HDAC4 suppresses chondrocyte hypertrophy and endochondral bone formation by inhibiting the function of MEF2C and RUNX2
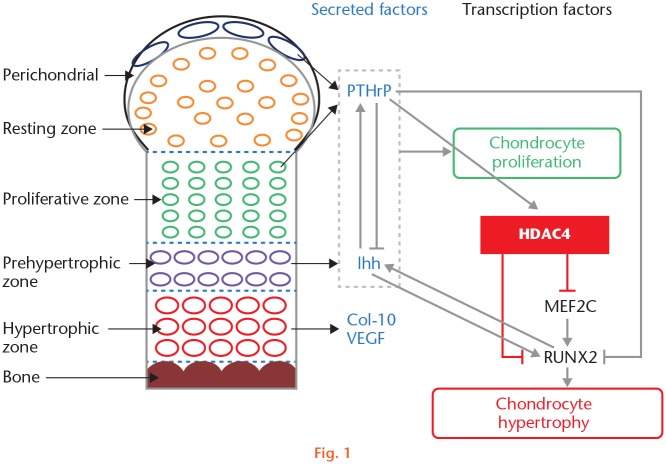
During endochondral bone development, chondrocyte hypertrophy is tightly regulated by various factors. The most well-known factors include secreted factors Indian hedgehog (Ihh), parathyroid hormone-related protein (PTHrP), type-X collagen (Col-X), matrix metalloproteinase-13 (MMP-13), vascular endothelial growth factor (VEGF), and transcription factors runt-related transcription factor 2 (RUNX2) and myocyte-specific enhancer factor 2C (MEF2C) (Figure 1).16
Fig. 1.
Schematic diagram of histone deacetylase 4 (HDAC4) regulation of chondrocyte hypertrophy and endochondral bone development. Col-10, type-X collagen; Ihh, Indian hedgehog; MEF2C, myocyte-specific enhancer factor 2C; PTHrP, parathyroid hormone-related protein; RUNX2, runt-related transcription factor 2; VEGF, vascular endothelial growth factor.
Ihh is a member of the Hedgehog family including sonic hedgehog (Shh), Desert hedgehog (Dhh), and Ihh, which are mainly produced and secreted by prehypertrophic chondrocytes, suggesting a critical role for the Ihh-PTHrP regulatory axis in chondrocyte proliferation and differentiation.17,18 Studies have found that Ihh stimulates proliferating chondrocytes to produce PTHrP, which in turn accelerates the proliferation of periarticular cells and prevents the onset of chondrocyte hypertrophy, maintaining chondrocytes in a proliferating state. This negative feedback loop regulates the balance between proliferation and maturation of chondrocytes, ensuring orderly bone formation.19 Furthermore, Kobayashi et al20 discovered that Ihh stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP.
PTHrP is a member of the parathyroid hormone (PTH) family. It was noted by Albright in 1941,21 and it has since been found in many tissues.22 PTHrP plays a key role during endochondral ossification;23 it is expressed by perichondrial cells and early proliferating chondrocytes and then diffuses to act on PTH/PTHrP receptor-bearing cells to inhibit expression of RUNX2 and suppress chondrocyte hypertrophy.24 In addition to regulating the proliferation and differentiation of chondrocytes by limiting Ihh expression, a recent study showed that PTHrP signalling promotes HDAC4 localization to the nucleus and increases HDAC4-induced inhibition of chondrocyte hypertrophy.25
Col-X is secreted by hypertrophic chondrocytes.26 MMP-13 is a matrix metalloproteinase involved in the degradation of the extracellular matrix molecule type-II collagen.27 VEGF, an important mediator of endochondral ossification expressed by hypertrophic chondrocytes, is required for chondrocyte survival, cartilage angiogenesis, and endochondral bone development.28 All these factors are considered to be major biological markers of chondrocyte hypertrophy in endochondral bone development.16
RUNX2, also named core binding factor alpha 1 (Cbfa1), polyoma enhancer binding protein 2 alpha 1 (PEBP2a1), and acute myeloid leukemia gene-3 (AML-3), is a member of the runt family of transcription factors that was originally isolated on the basis of its ability to activate transcription of the osteoblast-specific osteocalcin gene.29 Studies have shown that loss of RUNX2 function slows bone development and that gain of RUNX2 function accelerates bone formation, with an important role in maintaining the balance between proliferation and hypertrophy in chondrocytes.30 During endochondral bone formation, RUNX2 not only regulates expression of Col-X and MMP-13 in chondrocytes but also activates the Ihh promoter and stimulates Ihh expression, participating in the Ihh/PTHrP negative feedback loop.31 Most importantly, HDAC4 directly inhibits the function of RUNX2 to control chondrocyte hypertrophy during skeletogenesis.11
MEF2 is a MADS (MCM1, Agamous, Deficiens, Serum response factor) box factor implicated in muscle and cardiovascular development. There are four mammalian MEF2 genes: MEF2A, MEF2B, MEF2C, and MEF2D.32 MEF2C was shown to be necessary for chondrocyte hypertrophy, and knocking it down inhibits expression of RUNX2, Col-X, and VEGF. Thus, MEF2C acts upstream of RUNX2 during chondrocyte hypertrophy, and MEF2C promotes RUNX2 expression and chondrocyte hypertrophy. HDAC4 repression in chondrocyte hypertrophy is mediated by MEF2C transcription factors.33
All of the factors mentioned above constitute a complex and regulatory network that maintains normal bone development. HDAC4 is involved in the network by interacting directly with MEF2C and RUNX2 and inhibiting their expression and function, thereby suppressing chondrocyte hypertrophy and endochondral bone development. HDAC4 is believed to be a central regulator in the network.
Structure of the HDAC4 protein
HDAC4 has two distinct domains. The highly conserved carboxyl-terminal (C-terminal) domain of approximately 400 to 450 amino acids (residues 648 to 1057)34 with a hydrophobic nuclear export sequence (NES, 1051 to 1084)35 enables HDAC4 to shuttle between the nucleus and cytoplasm. The long amino-terminal (N-terminal) domain of approximately 450 to 60034 amino acids includes a nuclear localization signal (NLS, 247 to 285)35 that is related to its ability to shuttle between the cytoplasm and nucleus. The C-terminus mainly mediates HDAC function. The N-terminus is believed to be the key feature that distinguishes class I HDAC proteins; it is involved in interacting with different transcription factors to exert specific functions in different contexts.34 Many HDAC4 partners have been identified, such as MEF2C,36,37 RUNX2,11 calmodulin (CaM),38 14-3-3 protein,39 salt-inducible kinase 3 (SIK3),40 E1A C-terminal binding protein (CtBP),41 and serum response factor (SRF).42 The first discovered and main partner that interacts with the N-terminal domain of HDAC4 is MEF2C, and studies have shown that MEF2C binds to amino acids 118 to 180 of HDAC4.36,37 Vega et al11 found that the RUNX2 interaction domain of HDAC4 is localized within the first 220 amino acids of the protein, which also coincides with the MEF2-binding region. Further studies in vitro demonstrated that HDAC4 binds to CaM in a Ca2+-dependent manner via amino acids 150 to 220 of HDAC4 and that CaM competes for HDAC4 with MEF2.38 HDAC4 binding to 14-3-3 proteins depends on the phosphorylation of serine residues S246, S467, and S632,39 and SIK3 reportedly binds to 351aa to 620aa of HDAC4.40 CtBP interacts with HDAC4 via a P-X-D-L-R motif.41 Coimmunoprecipitation assays have shown that HDAC4 2aa to 289aa binds to SRF with greater affinity than does HDAC4 2aa to 201aa, suggesting that the region between 201aa and 289aa contains a critical part of the SRF-binding domain42 (Figure 2). In addition to SRF, interaction of HDAC4 with other proteins has crucial roles during chondrocyte hypertrophy and endochondral bone formation. The function of the above interactions will be discussed below.
Fig. 2.
The structure of histone deacetylase 4 (HDAC4). CaM, calmodulin; CtBP, C-terminal binding protein; MEF2, myocyte-specific enhancer factor 2; NES, nuclear export sequence; NLS, nuclear localization signal; RUNX2, runt-related transcription factor 2.
Function of the HDAC4 protein
Studies indicate that HDAC4 exhibits very little or no deacetylase activity, similar to the other members of the HDAC class IIa protein family. Indeed, only upon recruiting HDAC3, nuclear-hormone receptor corepressor (NcoR), and silencing-mediator-repressor-transcription (SMRT) to form the HDAC4-HDAC3/NCoR/SMRT complex does HDAC4 engage in enzymatic activity.43 In other words, HDAC4 is mainly responsible for recruitment in the deacetylase process. During chondrocyte hypertrophy and endochondral bone development, the HDAC4 N-terminus can also recruit target proteins through protein–protein interactions.
Inhibiting chondrocyte hypertrophy and endochondral bone development by binding and suppressing transactivation of MEF2C
MEF2 was initially identified as a transcription factor that activates expression of skeletal and cardiac muscle structural genes.32 Later, MEF2 was reported to be expressed in lymphocytes, the neural crest, smooth muscle, endothelium, and bone, and it plays central roles in diverse developmental programmes.44 MEF2C is one of the isoforms of MEF2; as mentioned earlier, it is a key regulator during chondrocyte hypertrophy and endochondral bone development through regulation of the function of Col-X, RUNX2, Ihh, and VEGF.33,45
Studies have demonstrated the affinity of HDAC4 for MEF2C in vitro and in vivo, showing that their interaction can inhibit chondrocyte hypertrophy by suppressing MEF2C transactivation.46 Binding of CaM to HDAC4 leads to HDAC4 dissociation from MEF2, relieving MEF2 from the transcriptional repression induced by HDAC4.38 Therefore, the authors of the study contended that the balance between the opposing actions of MEF2C and HDAC4 have a key function in chondrocyte hypertrophy and bone formation.38 However, research on the mechanism by which HDAC4 suppresses MEF2C in chondrocytes and bone formation is scarce. We review three different reported strategies by which HDAC class IIa proteins influence MEF2 transcription: 1) DNA-bound MEF2 recruits HDAC class IIa proteins, and local chromatin is deacetylated by HDAC class IIa proteins through interaction with the N-terminus of HDAC class IIa proteins, ultimately repressing MEF2 transcriptional activity;47 2) HDAC4 suppresses MEF2 transcription activity by promoting the sumoylation of MEF2 through association of the N-terminal domain with corepressors such as CtBP;48 3) the N-terminal HDAC4 cleavage product, HDAC4-NT, accumulates in the nucleus where it interacts with MEF2 to inhibit its activity in cardiomyocytes.49 These findings are expected to be useful in future research on the mechanism of HDAC4-mediated suppression of MEF2C in chondrocytes and bone formation.
Inhibiting chondrocyte hypertrophy and endochondral bone development by binding and suppressing transactivation of RUNX2
RUNX2 is necessary for mesenchymal condensation, osteoblast development, chondrocyte hypertrophy, vascular invasion of the developing skeleton, and activation of bone marrow endothelial cell migration and invasion.50 RUNX2 exerts its function both alone and in conjunction with numerous proteins to integrate a variety of signals and organize gene expression.51 HDAC4 directly inhibits the function of RUNX2 to control chondrocyte hypertrophy during skeletogenesis.11 However, the interactions of HDAC4 and RUNX2 during chondrocyte hypertrophy remain mechanistically and functionally poorly characterized. The first potential explanation is that HDAC4 specifically perturbs associations of RUNX2 with its target sequence in chromatin and suppresses acetylation of histone H3 on the RUNX2 promoter.11 In the second reported mechanism, HDAC4 directly deacetylates RUNX2 and RUNX3 to initiate their ubiquitin-mediated degradation.52 Interestingly, it was recently discovered that the RUNX2-induced osteoblast differentiation programme of C3H10T1/2 cells is unaffected by the nuclear/cytoplasmic location of HDAC4.53
The 14-3-3 protein interacts with HDAC4 to inhibit HDAC4 translocation into the nucleus to prevent HDAC4 inhibition of targeted proteins
Nucleocytoplasmic transport is considered to be an important process for controlling the transcription corepressor function of HDAC4 because, as researchers have argued, HDAC4 localization to the nucleus is necessary to repress transcription.34 The 14-3-3 protein interacts with HDAC4 via S246, S467, and S632 phosphoserines to escort phospho-HDAC4 from the nucleus to the cytoplasm, thus relieving HDAC4-induced inhibition of target proteins. It is well known that calcium/calmodulin-dependent protein kinase (CaMK)-I, -II, and -IV preferentially phosphorylate S246 or S467 and S632 of HDAC4 to promote its nuclear export and interaction with the 14-3-3 protein; the decreased level of nuclear HDAC4 inhibits its ability to repress the transcription of targets.35 Recently, a study showed that PTHrP signalling induces HDAC4 to separate from the 14-3-3 protein and migrate to the nucleus to inhibit chondrocyte hypertrophy through decreased phosphorylation of the 14-3-3-binding sites of HDAC4.25 Furthermore, SIK3 is reported to be essential for chondrocyte hypertrophy during skeletal development in mice, and the mechanism is similar to that of the 14-3-3 protein: SIK3 binds to HDAC4 and prevents it from leaving the cytoplasm.40
All of the above results indicate that HDAC4 recruits its target proteins via its N-terminus and that it forms the HDAC4-HDAC3/NCoR/SMRT complex to exert its deacetylase function on target proteins via its C-terminus. The most interesting finding is that HDAC3 is located mainly in the nucleus, which means HDAC4 is located in the nuclei and might be necessary for HDAC4 to work. This can explain why interaction between HDAC4 and the 14-3-3 protein prevents HDAC4 inhibition of targeted proteins. The hypothesis needs more work to be verified.
Regulation of the HDAC4 protein
The function of HDAC4 depends on its subcellular location, which is regulated by miRNA, post-translational modification (PTM), cleavage, the 14-3-3 protein, and mechanical loading during chondrocyte hypertrophy and endochondral bone formation.
miR-1 and miR-365 inhibit expression of HDAC4 during chondrocyte hypertrophy and endochondral bone formation
MicroRNAs (miRNAs) play a crucial role in the genetic regulation of HDAC4. To date, approximately 20 miRNAs have been discovered that regulate HDAC4 expression in different tissues, namely: miR-1,54,55 miR-133,54 miR-365,56 miR-206,57,58 miR-206-3p,59 miR-22,60 miR-140,61 miR-125a-5p,62 miR-155,63 miR-20a-5p,64 miR-548ah,65 miR-520b,66 miR-145-3p,67 miR-378a-3p,68 miR-381,69 miR-9,70 miR-483-5p,71 miR-29a,72 miR-200a,73 and others (Table I).Of these miRNAs, miR-1 and miR-381 are reported to be specifically expressed in growth plate cartilage and to regulate the chondrocyte phenotype by suppressing HDAC4 expression during growth plate development.74,69 Expression of miR-365 is elevated in the prehypertrophic zone of the growth plate, which stimulates chondrocyte differentiation by targeting HDAC4.75
Table I.
MicroRNAs targeting histone deacetylase 4.
| microRNA | Function | References |
|---|---|---|
| miRNA-1 | Promoting the differentiation of duck myoblasts; blunting cardiomyocyte hypertrophy elicited by thyroid hormone; regulating chondrocyte phenotype. | 54,55,74 |
| miRNA-133 | Affecting SRF and TGFBR1 expression to promote proliferation of duck myoblasts. | 54 |
| miRNA-365 | Ameliorating dexamethasone-induced suppression of osteogenesis, stimulating chondrocyte differentiation. | 56,75 |
| miRNA-206 | Attenuating denervation-induced skeletal muscle atrophy represses hypertrophy of myogenic cells. | 57,58 |
| miRNA-206-3p | Alleviating chronic constriction injury-induced neuropathic pain. | 59 |
| miRNA-22 | Promoting Th17 cell differentiation in inflammatory intestinal disease progression. | 60 |
| miRNA-140 | Inhibiting proliferation of osteosarcoma cells. | 61 |
| miRNA-125a-5p | Suppressing breast tumourigenesis. | 62 |
| miRNA-155 | Impairing transcriptional activity of B-cell lymphoma 6 (BCL6). | 63 |
| miRNA-20a-5p | Attenuating allergic inflammation in HMC-1 cells. | 64 |
| miRNA-548ah | Promoting the replication and expression of hepatitis B virus. | 65 |
| miRNA-520b | Restraining cell growth in lung cancer. | 66 |
| miRNA-145-3p | Suppressing proliferation and promotes apoptosis and autophagy of osteosarcoma cell. | 67 |
| miRNA-378a-3p | Promoting differentiation and inhibiting proliferation of myoblasts in skeletal muscle development. | 68 |
| miRNA-381 | Regulating chondrocyte hypertrophy. | 69 |
| miRNA-9 | miR-9 upregulation integrates post-ischemic neuronal survival and regeneration in vitro. | 70 |
| miRNA-483-5p | Regulating HDAC4 mRNA during embryogenesis. | 71 |
| miRNA-29a | Modulates the profibrogenic phenotype of the activated hepatic stellate cells. | 72 |
| miRNA-200a | Downregulation of miR-200a enhances the proliferation and migration of HCC cells. | 73 |
HCC, hepatocellular carcinoma; HDAC4, histone deacetylase 4; HMC, human mast cell; miR, microRNA; mRNA, messenger RNA; SRF, serum response factor; TGFBR1, transforming growth factor beta receptor 1; Th17, T-helper 17 cell.
Post-translational modifications regulate the subcellular localization of HDAC4
Regarding regulation at the protein level, PTMs are believed to mainly control spatial and temporal histone deacetylase functions by regulating subcellular localization. These PTMs include phosphorylations/dephosphorylations, sumoylation, disulfide bond formation, and ubiquitination and proteolytic cleavage.35
Phosphorylation and dephosphorylation of HDAC4 regulate its subcellular localization. Most kinases including CaMK, protein kinase D (PKD), adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK), salt-induced kinase (SIK), and dual specificity tyrosine-phosphorylation-regulated kinase 1B (DyrKB1) phosphorylate HDAC proteins and enhance their nuclear efflux76,77. The phosphorylation at HDAC4 S246, S467, and S632 can promote HDAC4 movement from the nucleus such that it can interact with the 14-3-3 protein and remain in the cytoplasm; dephosphorylation of these three sites leads to the opposite results. Nonetheless, protein kinase A (PKA) phosphorylates S265 and S266 of HDAC4 in cardiac and skeletal muscle, which results in HDAC accumulation in the nucleus.78 A recent study showed that PKA can mediate novel S584 phosphorylation of HDAC4 and thus enhance its function through a mechanism similar to that mediated by phosphorylation of S265 and S266.79 Furthermore, members of the protein phosphatase 2A (PP2A) family can remove HDAC4 phosphorylation and promote HDAC4 nuclear translocation to promote its transcriptional-repressive function.80
Sumoylation of HDAC4 regulates the subcellular distribution of HDAC4. HDAC4 is modified at K559 by the ubiquitin-related SUMO-1 modifier, and a sumoylation-deficient point mutant (HDAC4-K559R) slightly impairs its ability to repress transcription and reduces its histone deacetylase activity. During the process, the ability of HDAC4 to self-aggregate is a prerequisite for proper sumoylation in vivo. These findings suggest that sumoylation may be an important regulatory mechanism for controlling the transcriptional repression mediated by HDAC4.81 Furthermore, proteasome-dependent degradation of HDAC4 is associated with K559 sumoylation.82
Formation of disulfide bonds promotes HDAC4 translocation from the nucleus. A study showed that reactive oxygen species (ROS)-generating hypertrophic stimuli promote the oxidation of both Cys-274/Cys-276 in DnaJb5 and of C667/C669 in HDAC4 and the formation of intramolecular disulfide bonds, which signal HDAC4 nuclear export and thus inhibit its function.83
Ubiquitination of HDAC4 mediates the degradation of HDAC4. Some studies have shown that the stability of HDAC4 is influenced by its polyubiquitination and degradation, and serum and growth factor deprivation induces polyubiquitination and proteasome-mediated degradation of HDAC4 and thus achieves HDAC4 regulation.84,85 However, the specific mechanism remains unclear.
Caspase cleavage and PKA-dependent cleavage of HDAC4
There is evidence that caspase 2 and caspase 3 can induce the cleavage of HDAC4 at Asp289.86 The N-terminal fragment generated can lead to markedly increased apoptosis via the suppression of RUNX2 action in chondrocytes.87,88 Additionally, HDAC4 can be cleaved by PKA too. One study showed that PKA induces HDAC4 cleavage at T201,49 a site not found in other HDAC class IIa proteins, by a serine protease that has not yet been identified. This cleavage results in the production of an N-terminal fragment (HDAC4-NT) that accumulates in the nucleus to suppress MEF2 activity, enabling cardiomyocytes to exhibit differential hypertrophy49 and/or to protect against heart failure.89 Few studies to date have examined the role of the C-terminal fragment of HDAC4.
Mechanical loading promotes translocation of HDAC4 to the nucleus
Recent studies have shown that mechanical loading can regulate the function of HDAC4 in chondrocytes, and the results also showed that compressive loading induces HDAC4 relocation from the cytoplasm to the nucleus in chondrocytes via PP2A-dependent HDAC4 dephosphorylation at the sites of S246/S467/S632, which leads to suppression of RUNX2 expression and inhibition of chondrocyte hypertrophy.90,91 Moreover, in OA pathogenesis mechanical pressure is reported to downregulate HDAC4 expression by upregulating miR-365, which in turn causes cartilage degradation.92
Regulating the subcellular localization of HDAC4 is one of the important ways to regulate its function. Research into controlling miRNA, PTMs, cleavage of HDAC4, or mechanical loading to regulate the subcellular localization of HDAC4 and chondrocyte hypertrophy and endochondral bone formation may lead to new breakthroughs. Other approaches include mutating the caspase cleavage site to increase HDAC4.
In conclusion, HDAC4 plays a crucial role in chondrocyte hypertrophy and endochondral bone development. This review discusses the role of HDAC4 in the signalling networks regulating chondrocyte hypertrophy and endochondral bone development and the features of HDAC4 with regard to its structure, function, and regulation. In summary, HDAC4 located in the nucleus might be necessary for its function, and new breakthroughs might be achieved by controlling miRNA, PTMs, cleavage of HDAC4, or mechanical loading to regulate chondrocyte hypertrophy and endochondral bone formation.
Acknowledgments
The content is the sole responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors gratefully acknowledge all authors whose work contributed to this review, all funding that support the project (SXNSF 2014021039-1 and ISTCP 2015DFA33050), and American Journal Experts (AJE) for help with the manuscript preparation and editorial services.
Footnotes
Author Contributions: Z. Chen: Drafted and revised the manuscript.
Z. Zhang: Revised the manuscript.
L. Guo: Revised the manuscript.
X. Wei: Modified the manuscript.
Y. Zhang: Participated in the document retrieval and analysis.
X. Wang: Participated in the document retrieval and analysis.
L. Wei: Conceptualized, designed, and coordinated the study, Drafted the manuscript.
Z. Chen and Z. Zhang are co-first authors.
ICMJE COI statement: None declared
Ethical review statement: This study did not require ethical approval.
Follow us @BoneJointRes
Funding statement
The authors report institutional grants (paid to Second Hospital of Shanxi Medical University) from the Natural Science Foundation of Shanxi Province and China International Science and Technology Cooperation. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other non- profit organization with which one or more of the authors are associated.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2(4):389-406. [DOI] [PubMed] [Google Scholar]
- 2. Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun J, Wei X, Li S, et al. The Effects of Indian Hedgehog Deletion on Mesenchyme Cells: Inducing Intermediate Cartilage Scaffold Ossification to Cause Growth Plate and Phalange Joint Absence, Short Limb, and Dwarfish Phenotypes. Stem Cells Dev. 2018;27(20):1412-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parra M. Class IIa HDACs - new insights into their functions in physiology and pathology. FEBS J. 2015;282(9):1736-1744. [DOI] [PubMed] [Google Scholar]
- 5. Cohen TJ, Choi MC, Kapur M, Lira VA, Yan Z, Yao TP. HDAC4 regulates muscle fiber type-specific gene expression programs. Mol Cells. 2015;38(4):343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mielcarek M, Zielonka D, Carnemolla A, Marcinkowski JT, Guidez F. HDAC4 as a potential therapeutic target in neurodegenerative diseases: a summary of recent achievements. Front Cell Neurosci. 2015;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang LX, Du J, Zhao YT, et al. Transgenic overexpression of active HDAC4 in the heart attenuates cardiac function and exacerbates remodeling in infarcted myocardium. J Appl Physiol (1985). 2018;125(6):1968-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang D, Xiao C, Long F, et al. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc Res. 2018;114(7):1016-1028. [DOI] [PubMed] [Google Scholar]
- 9. Wang P, Mao Z, Pan Q, et al. Histone deacetylase-4 and histone deacetylase-8 regulate interleukin-1β-induced cartilage catabolic degradation through MAPK/JNK and ERK pathways. Int J Mol Med. 2018;41(4):2117-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbier-Torres L, Beraza N, Fernández-Tussy P, et al. Histone deacetylase 4 promotes cholestatic liver injury in the absence of prohibitin-1. Hepatology. 2015;62(4):1237-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vega RB, Matsuda K, Oh J, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555-566. [DOI] [PubMed] [Google Scholar]
- 12. Faulkner B, Astleford K, Mansky KC. Regulation of Osteoclast Differentiation and Skeletal Maintenance by Histone Deacetylases. Molecules. 2019;24(7):E1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8(4):294-302. [DOI] [PubMed] [Google Scholar]
- 14. Pfander D, Swoboda B, Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol. 2001;159(5):1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge NC. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem. 2010;285(13):9616-9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142(5):817-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13(16):2072-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273(5275):613-622. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T, Chung UI, Schipani E, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129(12):2977-2986. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Soegiarto DW, Yang Y, et al. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115(7):1734-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albright F. Case Records of the Massachusetts General Hospital–Case 39061. N Engl J Med. 1941;225:789-796. [Google Scholar]
- 22. Chen X, Macica CM, Dreyer BE, et al. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006;21(1):113-123. [DOI] [PubMed] [Google Scholar]
- 23. Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol. 1994;126(6):1611-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin TJ. Parathyroid Hormone-Related Protein, Its Regulation of Cartilage and Bone Development, and Role in Treating Bone Diseases. Physiol Rev. 2016;96(3):831-871. [DOI] [PubMed] [Google Scholar]
- 25. Nishimori S, Lai F, Shiraishi M, et al. PTHrP targets HDAC4 and HDAC5 to repress chondrocyte hypertrophy. JCI Insight. 2019;4(5):97903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong L, Huang X, Karperien M, Post JN. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int J Mol Sci. 2015;16(8):19225-19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rose BJ, Kooyman DL. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis Markers. 2016;2016:4895050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagao M, Hamilton JL, Kc R, et al. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci Rep. 2017;7(1):13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747-754. [DOI] [PubMed] [Google Scholar]
- 30. Inada M, Yasui T, Nomura S, et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999;214(4):279-290. [DOI] [PubMed] [Google Scholar]
- 31. Komori T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol. 2017;962:83-93. [DOI] [PubMed] [Google Scholar]
- 32. Shore P, Sharrocks AD. The MADS-box family of transcription factors. Eur J Biochem. 1995;229(1):1-13. [DOI] [PubMed] [Google Scholar]
- 33. Arnold MA, Kim Y, Czubryt MP, et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12(3):377-389. [DOI] [PubMed] [Google Scholar]
- 34. Fischle W, Kiermer V, Dequiedt F, Verdin E. The emerging role of class II histone deacetylases. Biochem Cell Biol. 2001;79(3):337-348. [PubMed] [Google Scholar]
- 35. Mathias RA, Guise AJ, Cristea IM. Post-translational modifications regulate class IIa histone deacetylase (HDAC) function in health and disease. Mol Cell Proteomics. 2015;14(3):456-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miska EA, Karlsson C, Langley E, Nielsen SJ, Pines J, Kouzarides T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 1999;18(18):5099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang AH, Bertos NR, Vezmar M, et al. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol Cell Biol. 1999;19(11):7816-7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Youn HD, Grozinger CM, Liu JO. Calcium regulates transcriptional repression of myocyte enhancer factor 2 by histone deacetylase 4. J Biol Chem. 2000;275(29):22563-22567. [DOI] [PubMed] [Google Scholar]
- 39. Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A. 2000;97(14):7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sasagawa S, Takemori H, Uebi T, et al. SIK3 is essential for chondrocyte hypertrophy during skeletal development in mice. Development. 2012;139(6):1153-1163. [DOI] [PubMed] [Google Scholar]
- 41. Zhang CL, McKinsey TA, Lu JR, Olson EN. Association of COOH-terminal-binding protein (CtBP) and MEF2-interacting transcription repressor (MITR) contributes to transcriptional repression of the MEF2 transcription factor. J Biol Chem. 2001;276(1):35-39. [DOI] [PubMed] [Google Scholar]
- 42. Davis FJ, Gupta M, Camoretti-Mercado B, Schwartz RJ, Gupta MP. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J Biol Chem. 2003;278(22):20047-20058. [DOI] [PubMed] [Google Scholar]
- 43. Fischle W, Dequiedt F, Hendzel MJ, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9(1):45-57. [DOI] [PubMed] [Google Scholar]
- 44. Clapham KR, Singh I, Capuano IS, Rajagopal S, Chun HJ. MEF2 and the right ventricle: from development to disease. Front Cardiovasc Med. 2019;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong C, Yang XZ, Zhang CY, et al. Myocyte enhancer factor 2C and its directly-interacting proteins: A review. Prog Biophys Mol Biol. 2017;126:22-30. [DOI] [PubMed] [Google Scholar]
- 46. Papaioannou G, Mirzamohammadi F, Lisse TS, Nishimori S, Wein MN, Kobayashi T. MicroRNA-140 Provides Robustness to the Regulation of Hypertrophic Chondrocyte Differentiation by the PTHrP-HDAC4 Pathway. J Bone Miner Res. 2015;30(6):1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martin M, Kettmann R, Dequiedt F. Class IIa histone deacetylases: regulating the regulators. Oncogene. 2007;26(37):5450-5467. [DOI] [PubMed] [Google Scholar]
- 48. Grégoire S, Yang XJ. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol Cell Biol. 2005;25(6):2273-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Backs J, Worst BC, Lehmann LH, et al. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J Cell Biol. 2011;195(3):403-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15(4):467-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jensen ED, Nair AK, Westendorf JJ. Histone deacetylase co-repressor complex control of Runx2 and bone formation. Crit Rev Eukaryot Gene Expr. 2007;17(3):187-196. [DOI] [PubMed] [Google Scholar]
- 52. Jin YH, Jeon EJ, Li QL, et al. Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J Biol Chem. 2004;279(28):29409-29417. [DOI] [PubMed] [Google Scholar]
- 53. Mu P, Deepak V, Kang L, et al. Ratjadone C-mediated nuclear accumulation of HDAC4: implications on Runx2-induced osteoblast differentiation of C3H10T1/2 mesenchymal stem cells. Z Naturforsch C J Biosci. 2014;69(11-12):471-478. [DOI] [PubMed] [Google Scholar]
- 54. Wu N, Gu T, Lu L, et al. Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle. J Cell Physiol. 2019;234(4):3490-3499. [DOI] [PubMed] [Google Scholar]
- 55. Diniz GP, Lino CA, Moreno CR, Senger N, Barreto-Chaves MLM. MicroRNA-1 overexpression blunts cardiomyocyte hypertrophy elicited by thyroid hormone. J Cell Physiol. 2017;232(12):3360-3368. [DOI] [PubMed] [Google Scholar]
- 56. Xu D, Gao Y, Hu N, Wu L, Chen Q. miR-365 Ameliorates Dexamethasone-Induced Suppression of Osteogenesis in MC3T3-E1 Cells by Targeting HDAC4. Int J Mol Sci. 2017;18(5):E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang QK, Qiao HY, Fu MH, et al. MiR-206 Attenuates Denervation-Induced Skeletal Muscle Atrophy in Rats Through Regulation of Satellite Cell Differentiation via TGF-β1, Smad3, and HDAC4 Signaling. Med Sci Monit. 2016;22:1161-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Winbanks CE, Beyer C, Hagg A, Qian H, Sepulveda PV, Gregorevic P. miR-206 represses hypertrophy of myogenic cells but not muscle fibers via inhibition of HDAC4. PLoS One. 2013;8(9):e73589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wen J, He T, Qi F, Chen H. MiR-206-3p alleviates chronic constriction injury-induced neuropathic pain through targeting HDAC4. Exp Anim. 2019;68(2):213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pei XF, Cao LL, Huang F, et al. Role of miR-22 in intestinal mucosa tissues and peripheral blood CD4+ T cells of inflammatory bowel disease. Pathol Res Pract. 2018;214(8):1095-1104. [DOI] [PubMed] [Google Scholar]
- 61. Xiao Q, Huang L, Zhang Z, et al. Overexpression of miR-140 Inhibits Proliferation of Osteosarcoma Cells via Suppression of Histone Deacetylase 4. Oncol Res. 2017;25(2):267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsieh TH, Hsu CY, Tsai CF, et al. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2015;6(1):494-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sandhu SK, Volinia S, Costinean S, et al. miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Eμ-miR-155 transgenic mouse model. Proc Natl Acad Sci U S A. 2012;109(49):20047-20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lu Y, Li Z, Xie B, Song Y, Ye X, Liu P. hsa-miR-20a-5p attenuates allergic inflammation in HMC-1 cells by targeting HDAC4. Mol Immunol. 2019;107:84-90. [DOI] [PubMed] [Google Scholar]
- 65. Xing T, Zhu J, Xian J, et al. miRNA-548ah promotes the replication and expression of hepatitis B virus by targeting histone deacetylase 4. Life Sci. 2019;219:199-208. [DOI] [PubMed] [Google Scholar]
- 66. Jin K, Zhao W, Xie X, Pan Y, Wang K, Zhang H. MiR-520b restrains cell growth by targeting HDAC4 in lung cancer. Thorac Cancer. 2018;9(10):1249-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu G, Yu W, Zhang M, Yin R, Wu Y, Liu Q. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells Nanomed Biotechnol. 2018;46(sup2):579-586. [DOI] [PubMed] [Google Scholar]
- 68. Wei X, Li H, Zhang B, et al. miR-378a-3p promotes differentiation and inhibits proliferation of myoblasts by targeting HDAC4 in skeletal muscle development. RNA Biol. 2016;13(12):1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen W, Sheng P, Huang Z, et al. MicroRNA-381 Regulates Chondrocyte Hypertrophy by Inhibiting Histone Deacetylase 4 Expression. Int J Mol Sci. 2016;17(9):E1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nampoothiri SS, Rajanikant GK. miR-9 Upregulation Integrates Post-ischemic Neuronal Survival and Regeneration In Vitro. Cell Mol Neurobiol. 2019;39(2):223-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Han K, Gennarino VA, Lee Y, et al. Human-specific regulation of MeCP2 levels in fetal brains by microRNA miR-483-5p. Genes Dev. 2013;27(5):485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang YH, Tiao MM, Huang LT, et al. Activation of Mir-29a in Activated Hepatic Stellate Cells Modulates Its Profibrogenic Phenotype through Inhibition of Histone Deacetylases 4. PLoS One. 2015;10(8):e0136453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yuan JH, Yang F, Chen BF, et al. The histone deacetylase 4/SP1/microrna-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology. 2011;54(6):2025-2035. [DOI] [PubMed] [Google Scholar]
- 74. Li P, Wei X, Guan Y, et al. MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development. FASEB J. 2014;28(9):3930-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guan YJ, Yang X, Wei L, Chen Q. MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011;25(12):4457-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parra M, Verdin E. Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol. 2010;10(4):454-460. [DOI] [PubMed] [Google Scholar]
- 77. McGee SL, Hargreaves M. Histone modifications and exercise adaptations. J Appl Physiol (1985). 2011;110(1):258-263. [DOI] [PubMed] [Google Scholar]
- 78. Liu Y, Schneider MF. Opposing HDAC4 nuclear fluxes due to phosphorylation by β-adrenergic activated protein kinase A or by activity or Epac activated CaMKII in skeletal muscle fibres. J Physiol. 2013;591(14):3605-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Doddi SK, Kummari G, M V J, Kalle AM. Protein kinase A-mediated novel Serine 584 phosphorylation of HDAC4. Biochem Cell Biol. 2019;97(5):526-535. [DOI] [PubMed] [Google Scholar]
- 80. Paroni G, Cernotta N, Dello Russo C, et al. PP2A regulates HDAC4 nuclear import. Mol Biol Cell. 2008;19(2):655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kirsh O, Seeler JS, Pichler A, et al. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J. 2002;21(11):2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Du J, Zhang L, Zhuang S, Qin GJ, Zhao TC. HDAC4 degradation mediates HDAC inhibition-induced protective effects against hypoxia/reoxygenation injury. J Cell Physiol. 2015;230(6):1321-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ago T, Liu T, Zhai P, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133(6):978-993. [DOI] [PubMed] [Google Scholar]
- 84. Cernotta N, Clocchiatti A, Florean C, Brancolini C. Ubiquitin-dependent degradation of HDAC4, a new regulator of random cell motility. Mol Biol Cell. 2011;22(2):278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Potthoff MJ, Wu H, Arnold MA, et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest. 2007;117(9):2459-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu F, Dowling M, Yang XJ, Kao GD. Caspase-mediated specific cleavage of human histone deacetylase 4. J Biol Chem. 2004;279(33):34537-34546. [DOI] [PubMed] [Google Scholar]
- 87. Cao K, Wei L, Zhang Z, et al. Decreased histone deacetylase 4 is associated with human osteoarthritis cartilage degeneration by releasing histone deacetylase 4 inhibition of runt-related transcription factor-2 and increasing osteoarthritis-related genes: a novel mechanism of human osteoarthritis cartilage degeneration. Arthritis Res Ther. 2014;16(6):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhou J, Li P, Chen Q, et al. Mitogen-activated protein kinase p38 induces HDAC4 degradation in hypertrophic chondrocytes. Biochim Biophys Acta. 2015;1853(2):370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lehmann LH, Jebessa ZH, Kreusser MM, et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med. 2018;24(1):62-72. [DOI] [PubMed] [Google Scholar]
- 90. Chen C, Wei X, Wang S, et al. Compression regulates gene expression of chondrocytes through HDAC4 nuclear relocation via PP2A-dependent HDAC4 dephosphorylation. Biochim Biophys Acta. 2016;1863(7 Pt A):1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen C, Wei X, Lv Z, et al. Cyclic Equibiaxial Tensile Strain Alters Gene Expression of Chondrocytes via Histone Deacetylase 4 Shuttling. PLoS One. 2016;11(5):e0154951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang X, Guan Y, Tian S, Wang Y, Sun K, Chen Q. Mechanical and IL-1β Responsive miR-365 Contributes to Osteoarthritis Development by Targeting Histone Deacetylase 4. Int J Mol Sci. 2016;17(4):436. [DOI] [PMC free article] [PubMed] [Google Scholar]