Highlights
-
•
We examined adolescent sleep quality and risky driving behaviors during fMRI.
-
•
Good sleep buffered against risky driving in most adolescents.
-
•
Sleep was not related to risk taking in high sensation-seeking adolescents.
-
•
Sleep was related to risk taking in teens with high ventral striatum activity.
-
•
Results identify two novel moderators of the sleep-risk association in adolescence.
Keywords: Adolescence, Risk taking, fMRI, Sleep, Ventral striatum
Abstract
The biological, environmental, and psychosocial changes that occur in adolescence engender an increase in risk taking often linked to the high rates of motor vehicle crashes amongst young drivers. Most U.S. adolescents suffer from poor sleep, which is known to exacerbate the risk of driving crashes; however, research has yet to uncover a neurobiological link between sleep and risky driving in adolescence. Here, we examined potential moderators of the sleep-risk relation in fifty-six adolescents (14–18y/o) as they completed a driving task during fMRI. While poor sleep was associated with increased risky driving (i.e., running more yellow lights), good sleep emerged as a novel buffer against risky driving in lower sensation-seeking adolescents. Neural activity in the ventral striatum (VS), a key node of the risk-taking circuit, also moderated the sleep-risk association: sleep was related to risk-taking in individuals demonstrating high, but not low, VS response during risky decision-making, suggesting that reward-related neural response may underly the connection between sleep and risk-taking in adolescence. This study sheds light on the risk of driving crashes in youth by highlighting sleep as both an exacerbator and a buffer of risky driving in adolescence. Taken together, these results underscore the importance of improving adolescent sleep.
1. Introduction
Adolescence is an essential period of cognitive and emotional development that is often characterized by an increase in exploration and risk taking. While frequently portrayed in a negative light, this increase in risk taking is a necessary and normative aspect of adolescence that can be observed across species (Brenhouse and Andersen, 2011; Steinberg, 2008) and cultures (Duell et al., 2018) and serves to promote independence, learning, and goal-directed behavior (Casey et al., 2008; Spear, 2000). In this way, risk taking is vital to the process of becoming an independent being.
Prior research has aimed to elucidate the neural underpinnings of this adolescent behavioral trend. During adolescence, limbic regions involved in processing reward and threat including the striatum and amygdala are hyperactive, increasing desire and sensitivity for positive feedback (Galván, 2013). Simultaneously, prefrontal regulatory systems are still maturing (Casey et al., 2008), which can result in heightened emotional reactivity and poor self-regulation. Along with the hormonal fluctuations of puberty, these brain dynamics may drive adolescents to make riskier choices than both children and adults (Cohen et al., 2010).
Despite the many normative outcomes of healthy risk-taking, one dangerous consequence is motor vehicle crashes, the leading cause of death for U.S. adolescents (CDC, 2017). Lack of driving experience combined with the tendency for risky decision making renders individuals ages 16–19 more likely to be involved in motor vehicle accidents than any other group (CDC, 2017). While the 16–19 age group makes up only ∼6.5 % of U.S. drivers, they are involved in ∼11 % of fatal crashes and account for an estimated $13.6 billion of the annual cost of all crashes (CDC, 2017; U.S. Census Bureau, 2017). The problem of reckless driving in adolescents has been examined from many disciplines—including law, policy, and public education—except neuroscience. Given the influential role neuroscience has had in policy and legal decisions relevant for adolescents (Steinberg, 2013), examination of risky driving through a neuroscientific lens is long overdue.
One factor that significantly impacts driving in individuals of all ages is sleep. Increasing importance has been placed on the role of sleep in healthy adolescent development; while research suggests that the need for sleep increases in adolescence (Carskadon, 1990; Carskadon et al., 1980), a majority of teens report getting insufficient or poor sleep (i.e., sleep that is not restful) which has been linked to negative outcomes such as mood disturbances, academic failure, health problems, substance abuse, and driving crashes (Carskadon, 2011; Colrain and Baker, 2011; Orzech et al., 2014). Evidence of the relation between poor sleep and risky driving in adolescence is abundant: sleep problems have been linked to enhanced risk-taking behaviors such as driving over the speed limit in adolescents (Pizza et al., 2010), while a 1 -h delay in school start times has been shown to reduce the risk of car crashes by improving adolescent sleep (Danner and Phillips, 2008). Even further, the AAA Foundation has estimated that 7 % of all crashes and 16.5 % percent of fatal crashes involve drowsy driving, with drivers ages 16–24 nearly twice as likely to be drowsy at the time of crash than are older drivers (Tefft, 2010). Despite the strong links between poor sleep and risk taking, no studies to date have examined the neurobiological link between poor sleep and risk of motor vehicle crashes in youth.
Research investigating the impact of sleep on neural functioning has highlighted a few plausible mechanistic explanations for the association between poor sleep and risk taking. In adults, frontal-reward circuits suffer after sleep deprivation (Krause et al., 2017), while mesolimbic regions such as the striatum and amygdala show a more vigorous response to emotionally pleasurable images and desirable food stimuli (Gujar et al., 2011). In rats, insufficient sleep interferes with dopaminergic function in the basal ganglia (Tufik, 1981; Volkow et al., 2012), results that are supported by studies of sleep-deprived humans demonstrating increased ventral striatum (VS) activity during anticipation and receipt of monetary rewards (Mullin et al., 2013; Venkatraman et al., 2007). In adolescents, poor sleep quality has been associated with increased risk taking paralleled by decreased recruitment of regulatory regions during cognitive control and decreased functional coupling of affective regions (VS, insula) with the dorsolateral prefrontal cortex (dlPFC) during reward processing (Telzer et al., 2013). Taken together, these studies suggest that poor sleep heightens reward system response while potentially dampening the influence of regulatory systems, often leading to unrestrained thrill-seeking following poor sleep. As adolescents already demonstrate a normative maturational shift in the connections among reward and regulatory systems, sleep may have the greatest impact on driving behaviors via these neural systems during this developmental period.
The goal of the present study was to determine the relation between poor sleep and behavioral and neural response to risky driving scenarios in adolescence. To account for heterogeneity in adolescent risk-taking perceptions, we examined how differences in risk-related traits (e.g., sensation seeking) moderated the relation between poor sleep and risk taking. We specifically examined neural response in the VS, a key reward region and node of the risk-taking brain network. The VS has been shown to exhibit altered functioning linked to increased risk taking after poor sleep (Telzer et al., 2013), while higher dopamine levels and greater dopaminergic response to reward in the VS have been associated with higher sensation seeking tendencies (Derringer et al., 2010; Riccardi et al., 2006; Zuckerman, 1985). Here, we predicted that poor sleep would relate to increased risky driving behaviors in adolescence, and that this relation would be moderated by individual differences in trait sensation seeking. We also hypothesized that VS activity during risky decision making would moderate the link between poor sleep and increased risk-taking behaviors. This study fills gaps in the literature by examining the neural correlates of the sleep-risk relation in an ecologically valid driving task, shedding light on the factors contributing to the abundance of motor vehicle crashes in adolescent drivers.
2. Methods
2.1. Participants
Fifty-nine typically developing adolescents ages 14–18 (MAge = 16.31 years; 29 F) were recruited to UCLA for this study. Of these, one participant was excluded from the fMRI scan due to a metal implant, one was excluded due to self-reported attention-deficit hyperactivity disorder (ADHD) diagnosis, and one was excluded for taking psychotropic medications. Data are presented for 56 participants (MAge = 16.90 years; 28 F), 91 % of whom reported post-pubertal status. Fifty-seven percent of our sample identified as Hispanic/Latino, 25 % Caucasian, 12.5 % African American, 3.5 % “other”, and 2 % Asian. All included participants were right-handed, free of metal, and reported no current medical or neurological disorders. Participants completed written consent and assent in accordance with UCLA’s Institutional Review Board and were compensated for their participation.
2.2. Eligibility criteria
Participants were screened for eligibility based on the following guidelines: (a) ages 14–18 years; (b) right-handed; (c) free of metal or other contraindications to imaging; (d) no medical or psychiatric conditions contraindicating study participation (e.g., suicidality, head trauma, pregnancy); (e) no current use of psychotropic medication; and (f) no history of claustrophobia.
2.3. Questionnaire measures
2.3.1. Sleep quality
Research has demonstrated that sleep quality, over sleep quantity, is crucial in adolescence; if an individual sleeps 10 h but is awakened during the night or deprived of one sleep stage, they will report symptoms similar to getting insufficient amounts of sleep (Bonnet and Arand, 2003; Ferrara et al., 2000). Here, subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989), a commonly-used measure of sleep quality in adolescent participants (Ji and Liu, 2016; Telzer et al., 2013). The PSQI consists of 19 questions describing participants’ subjective sleep quality and sleep disturbances over the past 30 days. These 19 questions are organized into 7 components, which are then summed together to obtain a global score of sleep quality ranging from 0 to 21. Higher scores indicate poorer sleep quality, and scores less than 6 indicate good sleep quality (Buysse et al., 1989). PSQI scores from this sample of participants ranged from 1 to 11 (M = 5.04, SD = 2.12).
2.3.2. Sensation seeking
The Sensation Seeking Scale (SSS; Zuckerman et al., 1978) was used to assess individual differences in trait sensation seeking, as characterized by a search for intense and novel experiences. The SSS is a 40-item scale that measures personality traits in four subscales: thrill and adventure seeking, disinhibition, experience seeking, and susceptibility to boredom. Each item is rated on an 11-point Likert scale ranging from 0 (strongly disagree) to 10 (strongly agree). For the purposes of this study, three items were removed before administration due to age-inappropriate and/or outdated topics (Gray and Wilson, 2007). Scores from each subscale were averaged to achieve an overall index of sensation-seeking tendencies, with higher scores indicating higher levels of sensation seeking (max score possible = 11). Scores from this sample of participants ranged from 1.97 to 7.68 (M = 4.31, SD = 1.35).
2.3.3. Impulsivity
As sensation seeking is often correlated with impulsivity, the UPPS-P Impulsive Behavior Scale (Cyders et al., 2007) was collected and used in control follow-up analyses to ensure results were driven by sensation seeking (over related constructs). The UPPS-P is a 59-item questionnaire that assesses five dimensions of impulsive behavior: negative urgency, premeditation, lack of perseverance, sensation seeking, and positive urgency. Each item was scored on a 4-point Likert scale ranging from 1 (strongly agree) to 4 (strongly disagree). For the purposes of this study, all subscales except the sensation seeking scale were averaged together to create a composite measure of impulsivity that was distinct from sensation seeking. Scores for this sample ranged from 0.67 to 2.30 (M = 1.48, SD = 0.40), with higher scores indicating higher impulsivity (max score possible = 4). Sensation seeking and impulsivity demonstrated a significant positive correlation (r(51) = 0.32, p = 0.02; Supplemental Fig. S1).
2.3.4. Real-world risky driving
To ensure ecological validity of the laboratory task, this study also collected information about real-world risky driving behaviors from 19 participants who reported having their license and driving regularly. Real-world risky driving behaviors were assessed using an adapted version of the Young Adult Driving Questionnaire which has been used to assess problematic driving behaviors in young adults (Donovan, 1993). Participants were asked how often they had engaged in 13 different risky driving behaviors (e.g., driving without a seatbelt, speeding up to beat a yellow light). Each item was scored on a 5-point Likert scale ranging from 0 (never) to 4 (almost always). Scores for this sample of participants ranged from 1 to 21.5 (M = 10.97, SD = 6.37), with higher scores indicating greater engagement in risky driving behaviors.
2.4. fMRI task
In the scanner, participants played two 8-minute runs of the Driving Game, an adapted version of the Stoplight Task originally designed by Chein and colleagues (Chein et al., 2011; Fig. 1). This game is a well-validated risky decision-making task for use in adolescents that involves driving a car on a simulated track and trying to reach the end as quickly as possible to maximize a monetary reward ($15 max). During the game, participants encountered a randomly presented series of green, yellow, and red traffic lights and were instructed to press a button (“1”) to go at green lights and (“2”) to stop at red lights. At yellow lights, the participants were given a choice whether to stop (cautious choice) or go (risky choice). Stopping at a yellow light resulted in the light turning red, adding a 3-second delay. Choosing to run the yellow light (risky choice) led to a 50/50 chance of a safe crossing, resulting in a monetary reward, or a crash, adding a longer delay (+6 s) to the route. Because the number of yellow trials for each participant was variable (stimuli were presented at random and were dependent on participant response), task risk taking was operationalized as the number of risky decisions divided by the number of yellow light trials with responses (“risk ratio”). The relation between sleep quality and task risk taking was marginally significant (r(56) = 0.24, p = 0.08), supporting the idea that poor sleep increases risky behavior.
Fig. 1.
The Driving Game. Participants encountered green, red, and yellow stoplights in the laboratory task and were instructed to press “1” to go for green lights, “2” to stop for red lights, and either “1” to go (risky choice) or “2” to stop (cautious choice) for yellow lights. A jittered inter-trial (ITI) stimulus followed each event. A risky choice at a yellow light was followed by either a reward (50 % chance), getting to the finish line faster and earning more money, or a crash (50 % chance), adding a 6 s delay. All trials began with 2–4 green lights and ended with either a red or yellow light. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.5. fMRI data acquisition
Scanning was performed using a 32-channel head coil on a 3-Tesla Siemens Trio MRI machine at the UCLA Center for Cognitive Neuroscience. Prior to scanning, participants completed a mock scanner session to ensure they were prepared, were not claustrophobic, and could easily remain still in the machine. All participants were screened for metal with a metal detector prior to entering the scanning suite. The scan task was presented on E-Prime, which collects behavioral responses and reaction times. Image acquisition parameters were voxel size = 3.0 × 3.0 × 4.0 mm, slices = 34, slice thickness = 4.0 mm, repetition time =2000 ms, echo time =30 ms, flip angle = 90°, interleaved slice geometry, field of view (FOV) = 192 mm, 118 vol. Preprocessing was conducted using FEAT (FMRI Expert Analysis Tool) version 6.00 within FSL (FMRIB Software Library; https://fsl.fmrib.ox.ac.uk/fsl/).
2.6. fMRI preprocessing
Preprocessing steps included non-brain removal using FSL BET, high-pass filtering (100-s cutoff), and spatial smoothing using a Gaussian kernel of FWHM 5 mm. Rigid body motion correction with six degrees of freedom was performed using MCFLIRT. A T2*weighted, matched bandwidth (MBW), high-resolution, anatomical scan and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan were acquired for registration purposes (TR: 1900 ms; TE 2.26 ms; FOV: 250 mm; slice thickness: 1 mm; 176 slices). Each participant’s functional data was registered to their MBW, then to the MPRAGE, and finally to Montreal Neurological Institute (MNI) stereotaxic space with 12° of freedom using FSL’s registration method via FLIRT. Participants did not exceed 1 mm mean relative motion and the majority did not exceed 3 mm max displacement (as determined using motion parameters generated by FSL). One participant exceeded 3 mm (3.34 mm) due to a single motion spike. Results remained the same with or without said participant, so to be as maximally inclusive of the data, this participant was included in all analyses. Independent components analysis (ICA) was performed using MELODIC (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC) to identify and remove artifacts in the data, after which FSLMotionOutliers (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers) was used to detect timepoints corrupted by a high degree of motion (> 0.90 mm framewise displacement (FD; Siegel et al., 2014). The resulting confound matrices were entered as regressors of no interest in the general linear model (GLM) for all analyses, removing the effects of these timepoints.
2.7. fMRI analysis
All analyses were performed using FEAT version 6 (www.fmrib.ox.ac.uk/fsl). For the Driving Game, a GLM was defined with eight regressors for the eight trial types: 1) Go (pressing “1” at a green light), 2) Inhibition (pressing “2” at a red light), 3) Risky (pressing “1” at a yellow light), 4) Cautious (pressing “2” at a yellow light), 5) Anticipation (the period between making a risky choice and receiving feedback), 6) Crash (crash following risky choice), 7) Reward (reward following risky choice), and 8) Junk (any trials of no interest or trials in which the participant did not respond to the stimulus). Each event was modeled with a canonical double-gamma hemodynamic response function (HRF) for a variable duration dependent on participant behavior. Rest periods and jittered inter-trial intervals (ITIs) were not explicitly modeled and were therefore used as the implicit baseline of interest. Temporal derivatives for all regressors, standard and extended motion parameters (6 standard motion parameters, their temporal derivatives, and squares of the above), and volumes exceeding 0.90 mm FD were included as covariates of no interest in all analyses. Individual-level models were defined with five main contrasts of interest: Risky vs. Baseline, Cautious vs. Baseline, Anticipation vs. Baseline, Risky vs. Cautious, and Yellow Lights vs. Other Lights. With these contrasts, we aimed to identify the neural correlates of risky and cautious driving behaviors, as well as the neural processes that distinguish risky decision-making from regular decision-making processes (Go and Inhibition) during a driving simulation. The two runs for each participant were combined using a fixed effect voxel-wise second-level model in FEAT.
Group-level analyses were performed using FMRIB Local Analysis of Mixed Effects module in FSL (Beckmann et al., 2003). Thresholded Z-statistic images were generated to visualize clusters determined by a corrected, cluster-forming threshold of Z > 3.1 and an extent threshold of p < 0.05 familywise error corrected using the Theory of Gaussian Random Fields (Poline et al., 1997). Statistical maps of all analyses were projected onto a standard MNI brain, and group activation maps were visualized using MRIcron software (http://www.sph.sc.edu/comd/rorden/mricron/).
2.8. Moderation
Simple moderation analyses were performed using Model 1 of Hayes’ PROCESS macro for SPSS (Hayes, 2012). Statistics were estimated using a bootstrapping method with 5000 samples, and significance was determined with 95 % bias-corrected confidence intervals. Predictor, moderator, and outcome variables were all continuous and demeaned in PROCESS to reduce concerns about multicollinearity. Significant interactions were depicted using 1 SD, mean, and +1 SD as plotted values of the moderator (Aiken and West, 1991). The Johnson-Neyman technique, a useful method of probing interactions with continuous moderators (Johnson and Fay, 1950), was used to determine where on the moderator continuum the effect of sleep on risk taking transitioned between statistically significant and nonsignificant (i.e., the “region of significance”). All analyses controlled for age and sex.
3. Results
3.1. Behavioral results
3.1.1. Sleep quality
Participants completed the PSQI to assess individual differences in quality of sleep over the previous month. Mean sleep quality for the group was 5.04 (SD = 2.12, range = 1–11), with 58.8 % of participants indicating good sleep quality (scores below 6; Buysse et al., 1989; Fig. 2a). Sleep quality was not significantly related to task risk taking (r(56) = 0.24, p = 0.08), sensation seeking (r(52) = 0.003, p = 0.98), impulsivity (r(51) = -0.05, p = 0.75), or age (r(56) = 0.04, p = 0.78). An independent samples t-test revealed no significant sex differences in sleep quality (t(54) = 1.27, p = 0.21).
Fig. 2.
Sleep quality scores and task risk-taking frequency. (A) Histogram of sleep quality scores on the PSQI. On average, participants achieved a sleep quality index of 5.04 (SD = 2.12, range = 1-11), with 58.8 % of participants reporting good sleep. (B) Histogram of risk-taking frequency in the Driving Game. Participants took risks on 37.47 % of the yellow light trials (SD = 26.46 %, range = 0-100 %).
3.1.2. Task risk taking
On average, participants took risks on 37.47 % of the yellow light trials (SD = 0.26, range = 0–100 %; Fig. 2b). Task risk taking was not significantly related to sensation seeking (r(56) = 0.05, p = 0.20) or age (r(56) = 0.07, p = 0.62). An independent samples t-test revealed no significant sex differences in risk-taking frequency (t(54) = 1.17, p = 0.25). Task risk taking was significantly related to average framewise displacement (FD) in the scanner (r(56) = 0.28, p = 0.03); therefore, we conducted additional follow-up analyses to ensure that all neural findings remained significant when controlling for average FD.
3.1.3. Real-world risky driving
In addition to completing a driving simulation in the laboratory task, 19 participants also reported on their previous real-world risky driving behaviors. When controlling for sleep quality, age, and sex, the number of real-world risky driving behaviors was associated with task risk-taking frequency (β = 0.02, p = 0.05; Fig. 3). For this analysis, we controlled for age (as we suspected that older adolescents may have had more time to engage in risky driving behaviors in the real world), self-reported sleep quality (to minimize the effects of recent sleep on task-based behavior), and sex (to remain consistent with the other analyses). There were no significant differences in task performance between regular and non-regular drivers (t(54) = -0.78, p = 0.44).
Fig. 3.
When controlling for sleep quality, age, and sex, self-reported real-world risky driving behaviors was associated with task risk-taking frequency (β = 0.02, p = 0.05).
3.1.4. Sensation seeking and the sleep-risk relation
A simple moderation analysis using sleep quality as the focal predictor, sensation seeking as the moderator, risk taking as the outcome variable, and age and sex as covariates revealed that trait sensation seeking significantly moderated the effect of sleep quality on risk-taking behaviors (β = 0.03, p = 0.02; Fig. 4). This suggests that as trait sensation seeking increases by one point, the effect (slope) of sleep on risk ratio decreases by 0.03. The negative slope of this interaction term suggests that as sensation seeking increases, the effect of sleep on risk taking will get closer to zero (i.e., high sensation seekers did not demonstrate a decrease in risk-taking frequency associated with better sleep). Simple slopes analysis demonstrated that better sleep was associated with decreased risk taking in individuals relatively low (1SD; β = 0.09, p = 0.004) and average (β = 0.05, p = 0.02) in sensation seeking, but not in individuals relatively high (+1SD; β = 0.00, p = 0.99) in sensation seeking. The Johnson-Neyman technique demonstrated that the relation between sleep and risk taking was significant when sensation seeking was more than 0.02 SDs below the mean, but not significant in individuals higher in sensation seeking.
Fig. 4.
Trait sensation seeking moderates the relation between poor sleep and risky driving behaviors. (A) Statistical diagram. A simple moderation analysis (Model 1; Hayes, 2012) using sleep quality as the focal predictor, sensation seeking as the moderator, risk taking as the outcome variable, and age and sex as covariates revealed that trait sensation seeking significantly moderated the effect of sleep quality on risk-taking behaviors (β = 0.03, p = 0.02). Neither age nor sex were significant predictors of task risk taking. (B) Analysis of conditional effects revealed that poor sleep was associated with increased risk taking in individuals low ( 1SD; β = 0.09, p = 0.004) and average (β = 0.05, p = 0.02) in sensation seeking, but not in individuals who were high (+1SD) in sensation seeking (β = 0.00, p = 0.99). * = p < .05, ** = p < .01, *** = p < .001.
As sensation seeking is often correlated with impulsivity, a variable that has also been related to poor sleep (Tashjian et al., 2017), we conducted follow-up analyses controlling for impulsivity in addition to age and sex to ensure effects were driven by sensation seeking and not by related constructs. All results remained significant. In a control moderation analysis, impulsivity did not significantly moderate the effect of sleep on risk taking behaviors (β = 0.004, p = 0.79; Supplemental Fig. S2).
3.2. Main effects of task
3.2.1. Yellow lights vs. other lights
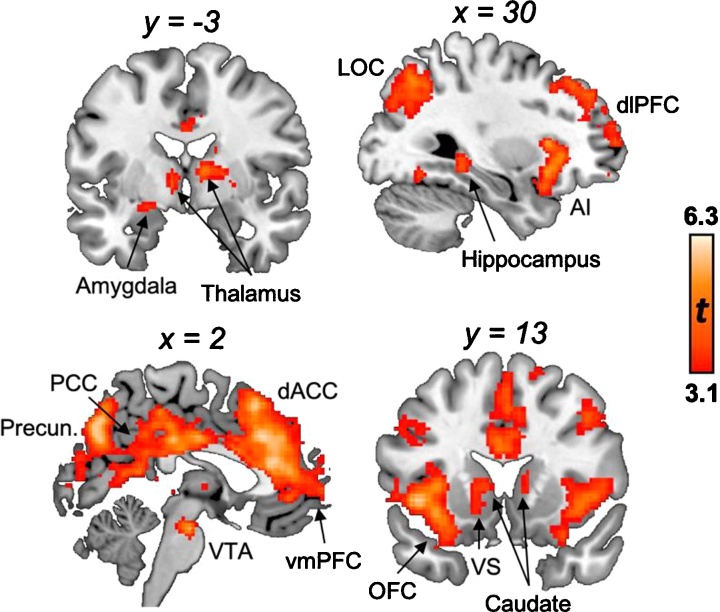
For our main contrast of interest, we aimed to isolate risky decision-making processes from typical decision-making processes by comparing risky and cautious decisions on yellow light trials to successful responses on green and red light trials. Whole-brain GLM analysis of Yellow Lights > Other Lights revealed activation of reward and limbic regions such as the ventral striatum (nucleus accumbens; NAcc) and bilateral thalamus, anterior insula (AI), and ventral tegmental area (VTA), bilateral DMN nodes such as the posterior cingulate cortex (PCC), precuneus, angular gyrus, dorsal anterior cingulate cortex (dACC), angular gyrus, and precuneus, and bilateral frontal regions such as the orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), and dorsolateral prefrontal cortex (dlPFC; Fig. 5). No voxels survived thresholding for the Other Lights > Yellow Lights contrast (Table 1).
Fig. 5.
Neural activation during risky decision making. Whole-brain activation for the Yellow Lights > Other Lights contrast revealed activation of mesolimbic regions such as the left ventral striatum (VS) and amygdala and bilateral thalamus, anterior insula (AI), and ventral tegmental area (VTA), bilateral default mode network (DMN) nodes such as the posterior cingulate cortex (PCC), precuneus (Precun), angular gyrus, dorsal anterior cingulate cortex (dACC), and bilateral frontal regions such as the orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), and dorsolateral prefrontal cortex (dlPFC). No voxels survived thresholding for the Other Lights > Yellow Lights contrast. All analyses cluster-corrected at Z > 3.1, p < .05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 1.
Neural activation for the Yellow Lights > Other Lights contrast.
| Region label | Peak MNI coordinates |
Z-max | Voxels (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L precuneus | −8 | −72 | −30 | 6.30 | 20,872 |
| R precuneus | 12 | −76 | 46 | 6.23 | |
| Dorsal anterior cingulate cortex | 3 | 32 | 17 | 5.74 | |
| Posterior cingulate cortex | −3 | −27 | 31 | 5.29 | |
| L dorsolateral prefrontal cortex | −34 | 54 | 12 | 5.58 | 1653 |
| L insula | −32 | 20 | 2 | 5.83 | 1357 |
| L orbitofrontal cortex | −28 | 24 | −6 | 5.80 | |
| L amygdala | −23 | −7 | −14 | 3.61 | |
| R thalamus | 10 | −4 | 8 | 4.57 | 1077 |
| L thalamus | −8 | −6 | 0 | 4.41 | |
| L caudate | −8 | 12 | 6 | 4.23 | |
| L nucleus accumbens | −11 | 14 | −5 | 3.61 | |
| R insula | 40 | 18 | −6 | 5.26 | 1015 |
| R orbitofrontal cortex | 30 | 16 | −18 | 4.35 | |
| L inferior temporal gyrus | −46 | −60 | −14 | 4.12 | 382 |
| R fusiform gyrus | 32 | −62 | −14 | 4.08 | 175 |
| Ventral tegmental area | 2 | −18 | −22 | 5.29 | 110 |
| L fusiform gyrus | −32 | −70 | −16 | 3.68 | 95 |
Note: x, y, and z refer to MNI coordinates; Z-max refers to the peak level of activation intensity; Voxels refers to the number of voxels in each significant cluster; L and R refer to left and right hemispheres.
3.2.2. Risky decisions
Whole-brain omnibus analysis of the Risky > Baseline condition using a GLM revealed bilateral activation in the dorsal (caudate and putamen) and ventral (NAcc) striatum, thalamus, AI, OFC, dACC, and dlPFC (Supplemental Fig. S3a). No voxels survived thresholding for the Baseline > Risky contrast.
3.2.3. Cautious decisions
Whole-brain omnibus GLM analysis of the Cautious > Baseline condition revealed bilateral activation of the dlPFC, AI, dACC, striatum, thalamus, and the PCC (Supplemental Fig. S3b). No voxels survived thresholding for the Baseline > Cautious contrast.
3.2.4. Anticipation
Anticipation in this context referred to the period of time between when the participant decided to take a risk (running a yellow light) and when feedback was provided (in the form of either a reward or a crash). The omnibus GLM analysis for the Anticipation > Baseline contrast identified activation in the dorsal (caudate and putamen) and ventral (NAcc) striatum, thalamus, AI, dACC, VTA, OFC, and the dlPFC (Supplemental Fig. S4). No voxels survived thresholding for the Baseline > Anticipation contrast.
3.2.5. Risky vs. Cautious
No voxels survived cluster correction at Z > 3.1, p < 0.05 for the Risky > Cautious or Cautious > Risky contrasts. In an exploratory analysis, we examined these contrasts using a corrected, cluster-forming threshold of Z > 2.3 and an extent threshold of p < 0.05. Whole-brain omnibus GLM analysis of the Risky > Cautious contrast revealed bilateral activation of the vmPFC, dACC, PCC, lateral occipital cortex (LOC), and precuneus cortex. No voxels survived thresholding for the Cautious > Risky contrast.
3.2.6. VS activity and the sleep-risk relation
A mask of the left NAcc (radius = 3 mm) was created by utilizing the reverse inference map from a meta-analysis of 194 studies using the term nucleus accumbens on Neurosynth (https://www.neurosynth.org/; Yarkoni et al., 2011). A central and peak (Z-score > 20) voxel (x = −12, y = 10, z = −12) was selected to be the center of the sphere, and analyses at the participant level were subsequently run through Featquery in FSL to determine mean VS activity for each participant during yellow light trials (Yellow Lights > Other Lights contrast). VS activation values, along with each participant’s risk ratio and PSQI score, were demeaned and entered into a simple moderation model (macro Model 1) in PROCESS v3.2.02 for SPSS (Hayes, 2012) with sleep quality as the focal predictor, VS activity as the moderator, and task risk taking as the outcome variable.
Sleep quality was related to increased risk taking, but VS activity significantly moderated this effect (β = 0.14, p = 0.01; Fig. 6). Worse sleep was associated with increased risky decisions and better sleep was associated with decreased risky decisions in adolescents demonstrating high (+1SD) VS activity during risk taking (β = 0.06, p = 0.006); however, this relationship was not observed in adolescents demonstrating average (β = 0.02, p = 0.22) or low (SD; β = 0.06, p = 0.51) VS activity during risk taking. The Johnson-Neyman technique (Johnson and Fay, 1950) indicated that the relation between sleep quality and risk taking was significant when VS activity was more than .016 SDs above the mean, but not significant in individuals demonstrating lower VS activity. VS activity was not significantly correlated with sensation seeking (r(52) = −0.15, p = 0.29), and results remained significant when controlling for sensation seeking. As mentioned previously, additional analyses were conducted controlling for average FD to ensure that results were not driven by motion in the scanner; the interaction between sleep quality and VS activity remained significant (β = 0.13, p = 0.03).
Fig. 6.
Ventral striatum activity moderates the association between poor sleep and risky driving behaviors. (A) An independent seed of the left ventral striatum (VS; radius = 3 mm) was generated using a meta-analysis of 194 studies on Neurosynth (https://www.neurosynth.org/). (B) Simple moderation analysis using sleep quality as the focal predictor, VS activity during risk taking as the moderator, and risk-taking frequency as the outcome variable revealed that VS activity significantly moderated the effect of sleep quality on risk-taking behaviors (β = 0 14, p = 0.01). (C) Analysis of conditional effects revealed that poor sleep was associated with increased risk taking in individuals demonstrating high ( 1SD) VS activity during yellow light trials (β = 0.06, p = 0.006), but not in individuals demonstrating average or low (-1SD) VS activity during yellow light trials (β = 0.02, p = 0.22; β = 0.06, p = 0.51). * = p < .05, ** = p < .01, *** = p < .001.
Separate moderation analyses identified two moderators of the sleep-risk association; however, these analyses did not address whether each interaction contributed uniquely to the sleep-risk association. To test this question, we utilized Model 2 of Hayes’ PROCESS macro for SPSS (Hayes, 2012) to specify two separate moderators (VS activity and sensation seeking). Both interactions remained significant in this model (Supplemental Fig. S5), suggesting that sensation seeking and VS activity function as independent moderators of the sleep-risk association.
4. Discussion
Poor sleep is common in adolescence (Beebe, 2011) and interferes with attention, impulse control, and behavioral regulation (Paavonen et al., 2009b, 2009a; Sadeh et al., 2002; Steenari et al., 2003), contributing to the abundance of motor vehicle crashes in adolescent drivers (Pizza et al., 2010). While the link between poor sleep and driving crashes has been shown (Carskadon, 2011; Danner and Phillips, 2008; Pizza et al., 2010), there is currently a dearth of research investigating how this sleep-risk relation interacts with other factors that influence the way adolescents react to risky driving scenarios. For example, trait sensation seeking has been associated with increased motor vehicle accidents in adolescence (Zuckerman, 2015), while ventral striatum (VS) activity has been linked to risk taking in both human (Casey et al., 2008; Chein et al., 2011; Galvan et al., 2006) and animal (Mitchell et al., 2014) adolescents. In the present study, we shed light on the complex interactions between these key variables by examining how individual differences in trait sensation seeking and VS response to risky driving scenarios moderate the relation between poor sleep and risk taking in adolescence.
In this sample of adolescents, individual differences in trait sensation seeking moderated the relation between sleep and risk taking such that better sleep was associated with a sharp decrease in risky behaviors (i.e., running less yellow lights) and worse sleep was associated with an increase in risky behaviors (i.e., running more yellow lights) in lower sensation-seeking youth. Higher sensation seeking youth, on the other hand, demonstrated no decrease in risk-taking frequency following good sleep, suggesting that sleep has the potential to diminish risky driving behaviors in those not naturally predisposed to taking frequent risks. Importantly, under poor sleep conditions, participants all performed similarly and individual differences in risk taking related to trait sensation seeking were washed out. In light of the widespread prevalence of sleep problems occurring adolescence, these results argue against a simplistic idea of adolescence as a time of uncontrolled risk taking, and instead frame adolescence as a period of burgeoning brain development and unideal circumstances that may accentuate the dynamic contributions of heightened reward response and ongoing development of the prefrontal cortex.
In this study, neural activity in the VS, a key node of the risk-taking neural circuit, during risky driving scenarios moderated the sleep-risk relation over and above sensation seeking; individuals demonstrating high VS activity during risky decision making showed a significant relation between sleep and risk-taking behaviors, with good sleep indicating lower risk taking and poor sleep indicating higher risk taking. Conversely, individuals demonstrating low and average VS activity during risky decision making showed no significant effect of sleep on risk-taking frequency. While poor sleep has been linked to increased VS activity in response to rewards (Mullin et al., 2013; Venkatraman et al., 2007), these results add nuance to this association by suggesting that VS activity may serve as a marker of the influence of sleep—whether good or bad in quality—on the adolescent brain. Furthermore, these results highlight the importance of considering both behavioral and neural influences when studying adolescent behavior; sensation seeking and VS activity both remained significant moderators of the sleep-risk association when included in the same model, suggesting that individual differences in risk-related traits and reward-related neural activation during risky decision-making each have a unique effect on the impact of sleep on adolescent risk taking.
It is important to acknowledge the limitations of the current study. Firstly, this research cannot make causal claims regarding the effects of sleep on risk taking, as sleep quality levels were observed rather than manipulated directly or examined longitudinally. Additionally, the distribution of task risk taking was slightly skewed, with most participants making cautious decisions on the majority of yellow light trials. The paucity of risky trials may have impacted the power of the Risky vs. Cautious contrast and contributed to the lack of results seen for this contrast under stringent thresholding. Furthermore, VS activity in response to yellow lights was only observed in the left hemisphere in this sample of participants, and the VS moderation analysis did not consider the role that fronto-limbic connectivity might play in the association between poor sleep and risk taking. Future research utilizing experimental and longitudinal designs, larger sample sizes, and network analyses will be crucial for achieving a deeper understanding of the causal mechanisms linking poor sleep to risky driving behaviors in adolescence.
While a large body of research has focused on the relation between poor sleep and impulsivity in adolescence, this study provides a new lens for examining sleep and risk taking by focusing on trait sensation seeking. Cross-sectional and longitudinal analyses demonstrate that sensation seeking, while often correlated with impulsivity, is its own distinct construct that plays an important role in adolescent risk taking by affecting differential expression of approach-avoidance reactions to same-intensity stimuli (Norbury and Husain, 2015). Sensation seeking is related to dopaminergic functioning and serves as a marker of intensity preference that can underlie thrill-seeking behaviors in adolescence. Furthermore, sensation seeking is linked to risk for psychopathology (Norbury and Husain, 2015; Perry et al., 2011; Zuckerman, 2015), rendering it a crucial trait to study in adolescence to prevent future onset of psychopathology. Of particular relevance to this study and adolescent health, sensation seeking has been linked with risky driving behaviors (e.g., speeding, driving recklessly) and increased risk of motor vehicle accidents (Jonah, 1997; Zuckerman, 2015), despite the fact that risky driving is not assessed on the Sensation Seeking Scale.
Interestingly, while sensation seeking has often been linked with negative outcomes such as substance abuse and gambling addiction (Norbury and Husain, 2015), studies investigating outcomes in response to environmental stressors have found that high sensation seekers adjusted better to environmental stressors such as wartime captivity and violence than did low sensation seekers (Neria et al., 2000; Solomon et al., 1995). These studies, combined with the adolescent results presented here, add nuance to our understanding of trait sensation seeking; while high sensation seeking may be a risk factor for a multitude of negative outcomes (Jonah, 1997; Zuckerman, 2015) related to higher levels of risk taking, it is possible that this intensity-craving trait also serves as a buffer for emotional and behavioral response to environmental stress (e.g., violence or poor sleep).
This study provides valuable insight into the high frequency of motor vehicle crashes involving adolescent drivers by demonstrating that not all adolescents make risky choices all the time – in fact, a majority of the participants in this study took a small fraction of risks in our ecologically valid driving task. While adolescence is often characterized by risk taking and suboptimal decisions and actions, it is crucial to also take into account the myriad of biological, environmental, and psychosocial changes occurring in adolescence that may steer adaptive risk taking to a maladaptive degree. On a promising note, we demonstrate here that good sleep can serve as an effective buffer from risky driving behaviors in adolescence. This study uncovers two moderating influences of the impact of sleep on risky driving behaviors in adolescence while highlighting good sleep as a protective mechanism to serve against risky driving; taken together, these results suggest promising mechanisms for intervention in adolescence.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Support for this study was provided by a Scholars Grant from the William T. Grant Foundation, the National Institutes of Health [1R01MH110476], and the Jeffrey/Wenzel Term Chair in Behavioral Neuroscience to AG, as well as by the National Science Foundation Graduate Research Fellowship and the National Institute of Child Health and Human Development T32 Doctoral Training Fellowship to AEB.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100790.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aiken L.S., West S.G. The effects of predictor scaling on coefficients of regression equations. Mult. Regres. Test. Interpret. Interact. 1991 [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003 doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beebe D.W. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr. Clin. North Am. 2011 doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet M.H., Arand D.L. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med. Rev. 2003 doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011 doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carskadon M.A. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990 [PubMed] [Google Scholar]
- Carskadon M.A. Sleep in adolescents: the perfect storm. Pediatr. Clin. North Am. 2011 doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon M.A., Harvey K., Duke P., Thomas F.A., Iris F.L., William C.D. Pubertal changes in daytime sleepiness. Sleep. 1980 doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008 doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, C. for D.C . 2017. Web-Based Injury Statistics Query and Reporting System (WISQARS) [WWW Document]. US Centers Dis. Control Prev. [Google Scholar]
- Chein J.M., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011 doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. A unique adolescent response to reward prediction errors. Nat. Neurosci. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain I.M., Baker F.C. Changes in sleep as a function of adolescent development. Neuropsychol. Rev. 2011;21:5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M.A., Smith G.T., Spillane N.S., Fischer S., Annus A.M., Peterson C. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol. Assess. 2007 doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Danner F., Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. J. Clin. Sleep Med. 2008 [PMC free article] [PubMed] [Google Scholar]
- Derringer J., Krueger R.F., Dick D.M., Saccone S., Grucza R.A., Agrawal A., Lin P., Almasy L., Edenberg H.J., Foroud T., Nurnberger J.I., Hesselbrock V.M., Kramer J.R., Kuperman S., Porjesz B., Schuckit M.A., Bierut L.J. Predicting sensation seeking from dopamine genes: a candidate-system approach. Psychol. Sci. 2010 doi: 10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J.E. Young adult drinking-driving: behavioral and psychosocial correlates. J. Stud. Alcohol. 1993 doi: 10.15288/jsa.1993.54.600. [DOI] [PubMed] [Google Scholar]
- Duell N., Steinberg L., Icenogle G., Chein J., Chaudhary N., Di Giunta L., Dodge K.A., Fanti K.A., Lansford J.E., Oburu P., Pastorelli C., Skinner A.T., Sorbring E., Tapanya S., Uribe Tirado L.M., Alampay L.P., Al-Hassan S.M., Takash H.M.S., Bacchini D., Chang L. Age patterns in risk taking across the world. J. Youth Adolesc. 2018 doi: 10.1007/s10964-017-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara M., De Gennaro L., Casagrande M., Bertini M. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology. 2000 doi: 10.1017/S0048577200981551. [DOI] [PubMed] [Google Scholar]
- Galván A. The teenage brain: sensitivity to rewards. Curr. Dir. Psychol. Sci. 2013 doi: 10.1177/0963721413480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006 doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.M., Wilson M.A. A detailed analysis of the reliability and validity of the sensation seeking scale in a UK sample. Pers. Individ. Dif. 2007 doi: 10.1016/j.paid.2006.08.019. [DOI] [Google Scholar]
- Gujar N., Yoo S.-S., Hu P., Walker M.P. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci. 2011 doi: 10.1523/jneurosci.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. PROCESS: a versatile computational tool for observed variable moderation, mediation, and conditional process modeling. Manuscr. Submitt. Publ. 2012 [Google Scholar]
- Ji X., Liu J. Subjective sleep measures for adolescents: a systematic review. Child Care Health Dev. 2016 doi: 10.1111/cch.12376. [DOI] [PubMed] [Google Scholar]
- Johnson P.O., Fay L.C. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950 doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Jonah B.A. Sensation seeking and risky driving: a review and synthesis of the literature. Accid. Anal. Prev. 1997 doi: 10.1016/S0001-4575(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Krause A.J., Simon E.Ben, Mander B.A., Greer S.M., Saletin J.M., Goldstein-Piekarski A.N., Walker M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017 doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.R., Weiss V.G., Beas B.S., Morgan D., Bizon J.L., Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin B.C., Phillips M.L., Siegle G.J., Buysse D.J., Forbes E.E., Franzen P.L. Sleep deprivation amplifies striatal activation to monetary reward. Psychol. Med. 2013 doi: 10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neria Y., Solomon Z., Ginzburg K., Dekel R. Sensation seeking, wartime performance, and long-term adjustment among Israeli war veterans. Pers. Individ. Dif. 2000 doi: 10.1016/S0191-8869(99)00243-3. [DOI] [Google Scholar]
- Norbury A., Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav. Brain Res. 2015 doi: 10.1016/j.bbr.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Orzech K.M., Acebo C., Seifer R., Barker D., Carskadon M.A. Sleep patterns are associated with common illness in adolescents. J. Sleep Res. 2014 doi: 10.1111/jsr.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen E.J., Porkka-Heiskanen T., Lahikainen A.R. Sleep quality, duration and behavioral symptoms among 5-6-year-old children. Eur. Child Adolesc. Psychiatry. 2009 doi: 10.1007/s00787-009-0033-8. [DOI] [PubMed] [Google Scholar]
- Paavonen E.J., Räikkönen K., Lahti J., Komsi N., Heinonen K., Pesonen A.K., Järvenpää A.L., Strandberg T., Kajantie E., Porkka-Heiskanen T. Short sleep duration and behavioral symptoms of attention-deficit/ hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009 doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- Perry J.L., Joseph J.E., Jiang Y., Zimmerman R.S., Kelly T.H., Darna M., Huettl P., Dwoskin L.P., Bardo M.T. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res. Rev. 2011 doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza F., Contardi S., Antognini A.B., Zagoraiou M., Borrotti M., Mostacci B., Mondini S., Cirignotta F. Sleep quality and motor vehicle crashes in adolescents. J. Clin. Sleep Med. 2010 [PMC free article] [PubMed] [Google Scholar]
- Poline J.B., Worsley K.J., Evans A.C., Friston K.J. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997 doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Riccardi P., Zald D., Li R., Park S., Ansari M.S., Dawant B., Anderson S., Woodward N., Schmidt D., Baldwin R., Kessler R. Sex differences in amphetamine-induced displacement of [18F]fallypride in striatal and extrastriatal regions: A PET study. Am. J. Psychiatry. 2006 doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Gruber R., Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002 doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Church J.A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014 doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon Z., Ginzburg K., Neria Y., Ohry A. Coping with war captivity: the role of sensation seeking. Eur. J. Pers. 1995 doi: 10.1002/per.2410090105. [DOI] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000 doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steenari M.R., Vuontela V., Paavonen E.J., Carlson S., Fjällberg M., Aronen E.T. Working memory and sleep in 6- to 13-year-old schoolchildren. J. Am. Acad. Child Adolesc. Psychiatry. 2003 doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. The influence of neuroscience on US Supreme Court decisions about adolescents’ criminal culpability. Nat. Rev. Neurosci. 2013 doi: 10.1038/nrn3509. [DOI] [PubMed] [Google Scholar]
- Tashjian S.M., Goldenberg D., Galván A. Neural connectivity moderates the association between sleep and impulsivity in adolescents. Dev. Cogn. Neurosci. 2017;27:35–44. doi: 10.1016/J.DCN.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefft B. Asleep at the Wheel : The Prevalence and Impact of Drowsy Driving. AAA Found. Traffic Saf. 2010 [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi: 10.1016/J.NEUROIMAGE.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology (Berl.) 1981 doi: 10.1007/BF00431826. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, P.D . 2017. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties and Puerto Rico Commonwealth and Municipios: April 1, 2010 to July 1, 2016. [Google Scholar]
- Venkatraman V., Chuah Y.M.L., Huettel S.A., Chee M.W.L. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007 doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Tomasi D., Wang G.-J., Telang F., Fowler J.S., Logan J., Benveniste H., Kim R., Thanos P.K., Ferre S. Evidence That sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J. Neurosci. 2012 doi: 10.1523/jneurosci.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011 doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking, mania, and monoamines. Neuropsychobiology. 1985 doi: 10.1159/000118174. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. International Encyclopedia of the Social & Behavioral Sciences. second edition. 2015. Sensation seeking: behavioral expressions and biosocial bases. [DOI] [Google Scholar]
- Zuckerman M., Eysenck S.B., Eysenck H.J. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J. Consult. Clin. Psychol. 1978 doi: 10.1037/0022-006X.46.1.139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.