Abstract
Neurodegenerative diseases, characterized by progressive loss of neurons, share common mechanisms such as apoptotic cell death, mitochondrial dysfunction, inflammation, and oxidative stress. Genus Boswellia is a genus in the Burseraceae family. It comprises several species traditionally used for treatment of chronic inflammatory diseases, cerebral edema, chronic pain syndrome, gastrointestinal diseases, tumors, as well as enhancing intelligence. Many studies have been carried out to discover therapeutic approaches for neurodegenerative diseases such as Alzheimer’s diseases, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis, stroke, and concomitant cognitive deficits. However, no curative treatment has been developed. This paper provides an overview of evidence about the potential of the Boswellia species and their main constituents, boswellic acids, as modulators of several mechanisms involved in the pathology of the neurodegenerative diseases. In vitro, animal, and clinical studies have confirmed that Boswellia species contain bioactive components that may enhance cognitive activity and protect against neurodegeneration. They exert the beneficial effects via targeting multiple pathological causes by antioxidative, anti-inflammatory, antiamyloidogenic, and anti-apoptotic properties. The Boswellia species, having neuroprotective potential, makes them a promising candidate to cure or prevent the neurodegenerative disorders.
Key Words: Alzheimer’s diseases, Boswellia, Cognitive, Neurodegenerative, diseases Neuroprotection
Introduction
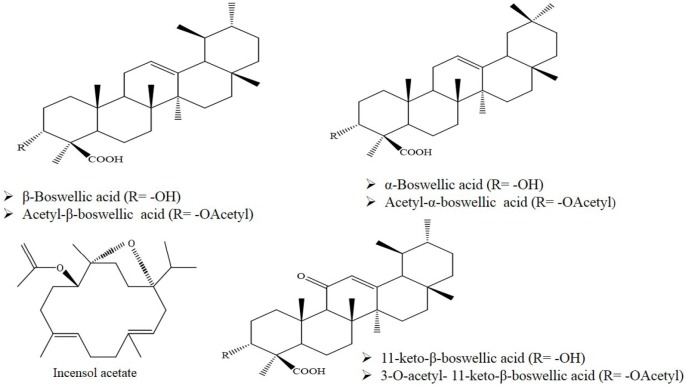
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis (MS), amyotrophic lateral sclerosis, and stroke are age-related disorders (1, 2). A number of common pathophysiological features have been proposed for these diseases, including elevated oxidative/nitrosative stress, mitochondrial dysfunction, protein misfolding/aggregation, synapse loss, and decreased neuronal survival (3, 4). Considering limitation of effective treatments for these diseases, there is an urgent need for new strategies using natural products that act through novel biological targets (5). The genus Boswellia belonging to the Burseraceae family comprises about 20 species. Species include B. serrata, B. sacra, B. frereana, B. neglecta, B. microphylla, B. papyrifera, B. ogadensis, B. pirottae, B. rivae, B. madagascariensis, B. socotrana, B. popoviana, B. nana, B. ameero, B. bullata, B. dioscoridis, B. elongata, and B. ovalifoliolata. B. neglectae, and B. dalzielii (6, 7). The genus is widespread in dry areas such as Arabia, northeastern coast of Africa, and India (8). The species have been useful in traditional medicine for treatment of inflammatory diseases, including asthma, arthritis, cerebral edema, chronic pain syndrome, gastrointestinal disease, tumors, and for enhancing memory and learning function (9-11). Frankincense, oleo-gum resins obtained from the genera Boswellia, is composed of essential oil (5-9%), mucopolysaccharides (20-23%), and resin (60%) (12, 13). The resinous part contains tetracyclic and pentacyclic triterpene acids. Boswellic acids (BAs) are considered the main biologically active components among the triterpene acids (Figure 1) (8, 14). Frankincense is responsible for anti-inflammatory and anti-cancer effects of BAs (15, 16). The anti-inflammatory mechanisms are applied through inhibition of 5-lipoxygenase, cathepsin G, and microsomal prostaglandin-E synthase (mPGES)-1 (17). Other mechanisms include suppression of nuclear transcription factor κB (NF-κB) and pro-inflammatory cytokines such as tumor necrosis factor (TNFα), interleukin (IL)-1β, IL-2, and IL-6 (15, 17). Also, BAs lead to induction of apoptosis in cancer cells via activation of caspase-8 and inhibition of topoisomerases-I and II-alpha (16, 18). In this review, the therapeutic effects of Boswellia and their major constituents on various neurodegenerative disease models have been summarized (Figure 2). Herein, pharmacological effects of the genus Boswellia in neurodegenerative diseases were classified as follows:
Figure 1.
Chemical structure of main constituents of genus Boswellia
1. Alzheimer’s disease
2. Parkinson’s disease
3. Cognitive dysfunction
4. Multiple sclerosis
5. Central nervous system trauma and brain ischemia
Methods
To prepare this review, an online search was performed by using some databases, including PubMed, Google Scholar, Science Direct, and Scopus. This review mainly focuses on the therapeutic/or pharmaceutical effects of genus Boswellia and its main active constituents, AKBA, on neurodegenerative diseases (such as AD, PD, MS, and cerebral ischemia). The search terms included “Neuropharmacology”, “Learning”, “Memory”, “Neurocognitive”, “Neurodegenerative”, “Neurological disorders”, “Alzheimer’s disease”, “Parkinson’s disease”, “Multiple sclerosis”, “Cerebral ischemia”, “Boswellia”, and “AKBA (3-acetyl-11-keto-β-boswellic acid)”
Alzheimer’s disease
Alzheimer’s disease is the most common type of neurodegenerative dementia in older people (19). It is characterized by amyloid-beta (Aβ) accumulation in plaques and hyper-phosphorylation of tau protein forming neurofibrillary tangles (20). Aβ aggregation and neurofibrillary tangles induce neuron and synapse loss and gross degeneration in the temporal lobe, parietal lobe, as well as parts of the frontal cortex and cingulate gyrus (21). The pathological alterations cause progressive memory loss, cognitive impairment and the inability to perform daily activities (21, 22). Aβ toxicity, cholinergic dysfunction, oxidative damage, apoptosis, synaptic dysfunction, and senile plaque-induced inflammation have been postulated to be involved in pathogenesis AD (21, 23). The possible prophylactic and therapeutic effects of B. serrata using an animal model AD induced by AlCl3 (17 mg/kg for 4 weeks, orally) were assessed. In this study, rivastigmine (0.3 mg/kg/day), as standardized medicine, and B. serrata (45 and 90 mg/kg/day) were given for 2 weeks before AlCl3 administration to rats. The results revealed that activity of rats increased, while the duration taken by rats to reach food in the T-maze test decreased. According to biochemical analysis, treatment with B. serrate led to elevation of acetylcholine (ACh) levels while acetylcholine esterase (AChE) activity was suppressed in brain homogenates. The histopathology findings indicated that amyloid plaques reduced in the hippocampus (24). In a preclinical investigation, therapeutic potential of B. serrata against neurodegeneration using an AlCl3-induced rat model of AD was claimed. Following treatment of AD animals with B. serrata as resin methanolic extract (137.5 mg/kg, 3 months, orally), Aβ plaques in histopathological samples disappeared. Biochemical analysis showed brain and serum levels of AChE, C-reactive protein (CRP), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), monocyte chemoattractant protein-1 (MCP-1), and leukotriene B4 (LTB4) were suppressed while brain ACh and Bcl-2 were elevated. The data represented preventing efficacy of B. serrata against neuro-inflammatory and apoptosis insults (25). Also, co-administration of ginger (Zingiber officinale, 108 and 216 mg/kg) and B. serrata (45 and 90 mg/kg) in rats treated with AlCl3. The B. serrata and ginger improved histopathologic changes and also behavior stress tests, including activity cage, rotarod, T- maze, as well as restoring ACh and AChE levels in brain homogenate (26). Recent evidence revealed that insulin resistance and metabolic dysfunction play an important role in the pathology of sporadic Alzheimer’s disease (sAD) (27). Intracerebral-ventricular injection of streptozotocin (STZ, 2- deoxy-2-(3-(methyl-3-nitrosoureido)-D-glucopyranose) is applied to mimic sAD (28). STZ -induced insulin resistance causes several features characterizing AD including oxidative stress, neuroinflammation, and dysfunctions in adult neurogenesis that are followed by progressive deficits in learning and memory (29-31). A study explored whether aqueous extract of frankincense from B. carteri could have therapeutic effects on STZ- induced memory impairment. The evaluation of learning using passive avoidance task (PAT) indicated that chronic administration of aqueous extract of frankincense (50 mg/kg, 42 days) improved memory in rats receiving STZ (1.5 mg/kg/2 μl/side, i.c.v.) in a time-dependent manner (32). SuHeXiang Wan (SHXW) is a traditional Chinese medicine comprising Liquidambar orientalis, Saussurea lappa, Aquilaria agallocha, Santalum album, B. carteri, Eugenia caryophyllata, Cyperus rotundus, Styrax benzoin, and Dryobalanops aromatica that has been used orally for the treatment of seizures, infantile convulsions, and stroke (33). The potential beneficial effects of SHXW essential oil were investigated on SH-SY5Y neuroblastoma cells and animal AD model induced by Aβ1-42 in mice. SHXW essential oil attenuated Aβ-induced cytotoxicity in SH-SY5Y cells through inhibition of apoptosis and ROS generation. Up-regulation of heme oxygenase-1 (HO-1), nuclear factor erythroid 2-related factor (Nrf2) expression, and increased Bcl-2/Bax protein ratio have been shown to be involved in the protective effects (34). SHXW essential oil ameliorated cognitive dysfunction in Aß1-42 treated mice, and it was associated with reduced p38, c-Jun N-terminal kinases, and tau phosphorylation (34). The findings suggested SHXW essential oil as a therapeutic agent for the prevention and treatment of AD and other tau protein pathology-related neurodegenerative diseases (34). Collectively, the experimental studies confirmed the inhibitory potential of Boswellia against formation of amyloid plaques and degeneration of cholinergic neurons induced by Aß. The medicinal herbs were found to induce anti-apoptotic activity through modulation of Bcl-2/Bax protein ratio. In addition, they counteracted oxidative damages through enhancement of HO-1/Nrf2 protein expression and restoring oxidative stress markers (Table 1).
Table 1.
A summary of pre-clinical and clinical studies on protective effects of genus Boswellia in the neurodegenerative diseases
| Protective agent/dose/reference | Study design or experimental model | Main results |
|---|---|---|
| B. serrata (45 and 90 mg/kg/day, 2 weeks) (24). | Animal model AD induced by AlCl3 in rat | Elevated ACh, suppressed AChE activity, improved histopathology changes, and reduced Aβ plaques in the hippocampus |
| B. serrata resin methanolic extract (137.5 mg/kg, 3 months) (25). | Animal model AD induced by AlCl3 in rat | Induced anti-neuro-inflammatory and anti-apoptotic properties indicated by suppression of serum level of AChE, CRP, NF-kB, MCP-1, LTB4, and elevation of brain ACh and Bcl-2. Aβ plaques disappeared |
| Co-administration of ginger (Zingiber officinale, 216 mg/kg) and B. serrata (45 and 90 mg/kg) (26). | Animal model AD induced by AlCl3 in rat | Improved histopathologic changes and behavior stress tests including activity cage, rotarod, and T- maze as well as restored ACh and AChE level in brain homogenate |
| Frankincense aqueous extract (50 mg/kg, 42 days) (32). | STZ (1.5 mg/kg/2 μl/side, i.c.v) - induced memory impairment | Evaluation of learning using passive avoidance task and improvement of memory |
| SHXW essential oil (1, 10, 100 µg/ml) (34). | SH-SY5Y neuroblastoma under Aβ1-42 (25 µM) toxicity | Attenuated Aβ-induced cytotoxicity through inhibition of apoptosis and ROS generation Up-regulated HO-1 and Nrf2 expression and Bcl-2/Bax protein ratio |
| Mouse AD models induced by Aβ1-42 | Ameliorated cognitive dysfunction in mice associated with reduced p38, c-Jun N-terminal kinases, and tau phosphorylation | |
| Boswellia resin extract (10 µg/ml) (38). | An in vitro PD model induced by MPTP in human dopaminergic SK-N-SH cell-line | Attenuated MPTP-induced neurotoxicity including inhibition of apoptosis |
| 1) B. serrata aqueous extract (0.1 g/kg/day) (47) 2) B. serrata (100 mg/kg/day) (48). |
Assessment of cognitive dysfunction in young Wistar rats whose mothers received Boswellia during gestation (3 weeks) | Induced more dendritic segments and branching density in the neurites of CA3 hippocampal cells |
| Frankinsense aqueous extract (50 and 100 mg/kg, 4 weeks) (49). | Assessment of learning and spatial memory in rats using Morris water maze test method | Facilitated the learning and spatial memory formation as reduction in escape latency and traveled distance |
| Frankincense aqueous extract (50 and 100 mg/kg) during gestation and lactation periods (50). | Assessment of the frankincense efficacy on memory formation during development of the rat brain | Enhanced memory performance and up-regulated CaMKII and CaMKIV mRNA levels in the hippocampus offspring rats |
| Frankincense aqueous extract (50 and 100 mg/kg/day, 4 weeks) (54). | Evaluation of the spatial memory parameters by MWM test | Improved spatial learning and memory and up-regulated expression of BDNF but not CREB |
| Boswellia papyrifera total extracts (300 mg/kg, three times a day) and boswellic acids fraction (100, 200, and 300 mg/kg) (55). | Assessment of spatial memory using MWM task | Enhanced the retention phase of spatial memory proposing the improvement of memory function |
| Olibanum (100 and 500 mg/kg, 180 days) (59). | Assessment of memory function using methimazole-induced hypothyroidism animal model | Counteracted memory deficit in the Morris water maze test |
| Ethyl acetate (0.1 mg/kg) and N-butanol (0.1 mg/kg) fractions of B. carterii gum resin (61). | Memory impairments induced by hyoscine-induced | Ethyl acetate fraction was much more significant than other fraction in enhancing the memory ability indicated by the MWM task |
| Combined administration of M. officinalis and B. serrata (200 and 400 mg/kg) (62). | Spatial memory against cognitive impairment related to scopolamine | Improved memory performance indicated by MWM method |
| Frankincense hydro-alcoholic extract (50 mg/kg) (67). | Memory loss following LPS administration (1 mg/kg) | Enhanced step-through latency in a passive avoidance task accompanied by reduced TNF-α level in the hippocampus |
| Aqueous extracts of B. serrata (0.1, 0.5, and 1 g/kg, IP) (71, 72). | Pentylenetetrazol-induced kindled rats were used to study epilepsy and its consequences on memory using shuttle box apparatus and step-through latency method | Improved passive-avoidance learning ability associated with an increase in the number of pyramidal neurons and dendritic spines in CA1 |
| B. serrate aqueous extract (100 mg/kg/d, for 8 weeks) (74). | Age-related morphological changes of hippocampal granule cells and concomitant cognitive deficits in escape latency and swimming distance | Enhanced dendritic complexity in the dentate granule cells and spine density associated with improvement of spatial learning capability |
| A tablet containing B. serrata and Melisa officinalis extract (290 and 27 mg) (75). | A randomized, parallel, double-blind, placebo-controlled clinical trial performed among 70 older adults | Improved memory function |
| Ethanolic extract of B. serrata oleo-gum resin (10, 20, 40, and 80 µg/ml) (79). | Oligodendroglia (OLN-93) cell injury induced by glutamate and quinolinic acid | Attenuated oxidative stress |
| The extract mixture of Portulaca olerace, Urtica dioica, and B. serrata (200 and 400 mg/kg) (81). | MS model induced by intra-hippocampal injection of ethidium bromide (stereotaxic surgery) in rats | Induced neurogenesis and memory improvement in the shuttle box test |
| Capsule containing B. papyrifera (300 mg, twice a day, 2 months) (82). | A randomized, double-blind, clinical trial in MS patients | Indicated therapeutic efficacy for cognitive dysfunction as improved visual-spatial memory |
| Capsule containing B. serrate extract (450 mg twice a day, two months) (83). | A double-blind clinical trial in MS patients with cognitive deficits | Improved cognitive deficits indicated by the improvement of auditory/verbal and visual/spatial memory in brief visuospatial memory test and California verbal learning test |
| B. serrata hydroalcoholic extract (1.5-6 µg/ml) and AKBA (0.5-2.5 µg/ml) (90). | Ischemia-induced cytotoxicity in PC12 cells following exposure to oxygen/glucose/serum deprivation condition | Increased cell survival and counteracted oxidative stress (ROS, lipid peroxidation, and oxidative DNA damage) |
| BSE (25, 50, 100 μg/ml) and AKBA (5 μm) (91). | Cell culture model of neurodegeneration induced by glutamate toxicity in PC12 and Neuro-2a cell | Inhibited oxidative damage and apoptotic cell death |
| B. serrata methanolic extract (50, 100, 250, 500, 1000, and 2000 µg/ml) (93). | In vitro assessment of antioxidant and anti-inflammatory activity | Exhibited DPPH free radical scavenging activity (IC50 = 54.06 µg/ml), ferric reducing power (IC50 = 62.12 µg/ml) stabilization towards human red blood cell membrane stabilization |
| Boswellia aqueous and ethanolic extracts (125, 250, and 500 mg/kg, IP) and AKBA (50 mg/kg, IP) (94). | An animal model of ischemia, MCAO | Improved neurological deficits and reduced brain infarction volume, neuronal apoptotic cell death accompanied by up-regulation of Bcl-2 and down-regulation of Bax and caspase-3. Reduced oxidative stress (counteracted lipid peroxidation and restored glutathione content and superoxide dismutase activity) in the cerebral cortex |
| Frankincense aqueous extract (100 and 150 mg/kg, 30 days) (95). 95 | MCAO surgery was performed to induce ischemia-reperfusion status | The level of blood-brain barrier (BBB) permeability and stroke-induced brain edema and reduction of infarction volume and neurological impairments |
| BS (4200 mg/day) during radiotherapy (96). | A prospective, randomized, placebo-controlled, double-blind, pilot trial in cerebral edema following brain radiotherapy | Suppressed the edema volume evaluated by T2-weighted magnetic resonance imaging (MRI) |
| B. serrata capsules (containing 215 mg gum resin) for 6 weeks (97). | Assessment of effect on neuro-recovery following diffuse axonal injury in a double-blind, randomized, cross-over study | Enhanced the cognitive outcome of patients with diffuse axonal injury |
| B. serrata (250 and 500 mg/ kg) (98). | Cerebral inflammation after induction of diffuse traumatic brain injury | Attenuated brain edema and disruption of blood-brain-barrier accompanied by improvement of vestibulomotor dysfunction and modulation of IL-1β and IL-10 in the brain tissue |
Ach: Acetylcholine; AChE: acetylcholine esterase; Aβ: amyloid Beta; Bcl-2: B-cell lymphoma 2; BDNF: Brain-derived neurotrophic factor; CREB: cAMP response element-binding protein; CRP: C-reactive protein; DPPH: 2,2-diphenyl-1-picrylhydrazyl; IL-10: Interleukin 10; LTB4: Leukotriene B4; MCAO: middle cerebral artery occlusion; MCP-1: Monocyte Chemoattractant Protein-1; MPTP: 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyryridine; MWM: Morris water maze; MS: Multiple sclerosis; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; ROS: reactive oxygen species content; SHXW: SuHeXiang Wan; CaMKII: calcium/calmodulin kinase II
Parkinson’s disease
Parkinson’s disease (PD), a common chronic, progressive neurodegenerative disorder of the elderly, is characterized by motor (including bradykinesia, tremor, and rigidity) and non-motor symptoms (35). The symptoms of PD result from the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and dopamine (DA) deficiency in the striatum (36). Moreover, the presence of α-synuclein containing Lewy bodies in the surviving neurons is also proposed in the neuropathology of PD (37). Oxidative stress, mitochondrial dysfunction, excitotoxicity, calcium cytotoxicity, trophic factor deficiency, inflammatory processes, genetic factors, and apoptosis are now considered to be key mechanisms that contribute to neurodegeneration in PD (36). Some evidences has demonstrated the neuroprotective potential of B. serrata on dopaminergic neurons that can be applicable in PD. Boswellia resin extract (10 µg/ml) attenuated MPP+ (1-methyl-4-phenylpyridinium, 1000 µM), an active metabolite of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyryridine (MPTP)- induced toxicity in human dopaminergic SK-N-SH cell-line. The protective effects were associated with increased cell viability and reduced apoptotic features (38) (Table 1).
Cognitive dysfunction
Learning means the process of acquiring knowledge from the outside environment while memory is retention and retrieval of learned information at a later date (39-41). Short-term plasticity (STP) and long-term potentiation (LTP), two types of synaptic plasticity, are mechanisms for memory storage (42-45). Learning and memory impairment are considered the most significant features of dementia (46). A number of experimental studies were conducted to evaluate the effect of maternal administration frankincense (an oleo-gum resin derived from trees of genus Boswellia) on the cognitive capabilities. Hosseini Sharifabad et al. assessed learning and memory in two-month-old male Wistar rats whose mothers orally received aqueous extract of B. serrata (0.1 g/kg/day) during gestation (3 weeks) using active avoidance learning test. Frankincense enhanced power of learning at post-learning stage, short-term memory, and long-term memory (47). The results were relevant to the alteration in the neurites of CA3 hippocampal cells reported in another study. Analysis of morphology of dendritic architecture of CA3 hippocampal neurons indicated more dendritic segments and branching density in young rats whose mothers were treated with B. serrata (100 mg/kg/day) during gestation compared with untreated rats (48). Administration of aqueous extract of frankincense (50 and 100 mg/kg, 4 weeks) facilitated the learning and spatial memory formation in rats. The results were demonstrated as reduction in escape latency and traveled distance by the Morris water maze test method (49). An in vivo study was performed to assess the efficacy of frankincense for memory formation during development of the rat brain. For this purpose, aqueous extract of frankincense (50 and 100 mg/kg) was orally administered into female rats during gestation and lactation periods. Memory performance and hippocampal calcium/calmodulin kinase II (CaMKII) and CaMKIV mRNA levels in the offspring rats were evaluated to identify potential molecular change during gestation and lactation periods (50). CaMKII and CaMKIV are involved in many signaling cascades and are thought to be crucial mediators of learning and memory (51). CaMKII, as an important component of the postsynaptic density of glutamatergic synapses (52), plays a role in regulation of synaptic transmission and induction of long-term potentiation (LTP) (53). According to the results, up-regulation of CaMKII-α mRNA expression of the hippocampus was concomitant with improvement of spatial memory retrieval in offspring rats (50). Evaluation of the spatial memory parameters by the Morris water maze (MWM) test revealed improvement of spatial learning and memory in rats treated with aqueous extract frankincense (50 and 100 mg/kg/day for 4 weeks). Frankincense up-regulated expression of brain-derived neurotrophic factor (BDNF) transcripts but not cAMP response element-binding (CREB). Therefore, the effects of the extract on memory formation may be attributed to another BDNF-related pathway other than BDNF–CREB–BDNF cycle (54). B. papyrifera total extracts (300 mg/kg, three times a day, orally) and boswellic acids fraction (100, 200, and 300 mg/kg) enhanced the retention phase of spatial memory of adult male rats in the MWM task. The results of the investigation proposed improving potential of Boswellia and boswellic acid fraction in memory function in normal subjects or neurodegenerative disorders (55). Impairment of cognitive function including memory, visuospatial organization, attention, and reaction time in overt hypothyroidism has been recognized for more than a century (56-58). Olibanum (resin of B. serrata) exhibited beneficial effects on memory deficit in methimazole-induced hypothyroidism model. Oral administration of olibanum (100 and 500 mg/kg, 180 days) improved memory and learning impairment in hypothyroid rats by the Morris water maze test (59). Animal models of amnesia induced by scopolamine are widely used to screen potential therapeutic value of compounds in treatment of dementia (60). Another study aimed to assess the effect of ethyl acetate and N-butanol fractions of B. carterii gum resin on intact memory and hyoscine-induced memory impairments using the MWM task. Ethyl acetate (0.1 mg/kg) and N-butanol (0.1 mg/kg) fractions remarkably enhanced intact memory. The ethyl acetate fraction was much more significant than other fractions in enhancing the memory (61). The combination of Melissa officinalis and B. serrata improved scopolamine-induced cognitive impairment. The MWM method revealed co-administration of M. officinalis and B. serrata (200 and 400 mg/Kg body weight) before scopolamine injection (0.1 mg/kg) led to improvement of memory function (62). Neuro-inflammation can cause cognitive deficits since it affects memory processing during consolidation and retrieval stages (63, 64). Considering anti-inflammatory activity of frankincense has been approved with an (15). Lipopolysaccharide (LPS) triggers the neuro-inflammatory process through activation of nuclear factor kappa B (NF-κB) pathway in microglia in the central nervous system (CNS) (65, 66). Administration of hydro-alcoholic extract of frankincense (50 mg/kg; orally) before LPS (1 mg/kg; IP) enhanced step-through latency (STL) in a passive avoidance task (PAT) via decreasing the TNF-α level in the hippocampus. Therefore, anti-inflammatory effects of frankincense may be involved in inhibition of memory loss (67). Clinical and pre-clinical studies have shown that prolonged frequent seizures cause cognitive, memory, and emotional impairments (68). These recurrent seizures affecting the hippocampus may lead to cell damage and death in the cornu ammonis (CA1) region (69). Function of CA1 neurons in the hippocampus plays a vital role in converting short-term memory to long-term memory (70). Pentylenetetrazol (PTZ)-induced kindled rats animal model was used for evaluation of epilepsy and its consequences on memory (71). The aqueous extracts of B. serrata (0.1, 0.5, and 1 g/kg, IP) improved passive-avoidance learning ability in kindled animals indicated by using shuttle box apparatus and step-through latency method. The findings were associated with increasing number of pyramidal neurons and dendritic spines in CA1 (71, 72). Therefore, consumption of B. serrata may be a therapeutic strategy for decreasing harmful effects of seizures on cognitive function (71). Age-related spatial learning deficits have been suggested to be due to changes that appear mostly in hippocampal connectivity and plasticity (73). The three main fields of the hippocampal region, CA1, CA3, and particularly dentate gyrus are vulnerable to aging (73). An experimental study conducted by Hosseini-Sharifabad et al. investigated the effects of chronic administration of B. serrata hydroalcoholic extract (BSE) on the learning performance and the morphology of hippocampal granule cells in aged rats (74). The rats (24 months old) received the aqueous extract of BSE (100 mg/kg/d, for 8 weeks, intragastrically), after this time, dendritic complexity in the dentate granule cells and spine density on the dendritic tree of the cells increased (74). These findings were observed along with improvement of spatial learning capability indicated as decrease in escape latency and swimming distance (74). Neuroprotective potential of Boswellia resin in age-related morphological changes and concomitant cognitive deficits may suggest it as a therapeutic agent in neurodegenerative diseases (74). In a randomized, parallel, double-blind, placebo-controlled clinical trial, administration of B. serrata and Melisa officinalis extracts (290 mg and 27 mg, for a month) improved memory in 70 older adults (75). Overall, this evidence provides preliminary support for the cognitive-enhancing efficacy of genus Boswellia. Potential beneficial actions may be attributed to BDNF up-regulation (Table 1).
Multiple sclerosis
Multiple sclerosis (MS) is a chronic autoimmune, inflammatory neurological disease of the CNS, which leads to the destruction of myelin, oligodendrocytes, and axons (76). Quinolinic acid (2, 3-pyridine dicarboxylic acid), a neuroactive metabolite of the kynurenine pathway, is an agonist of N-methyl-D-aspartate (NMDA). Inappropriate activation of the kynurenine pathway may increase quinolinic acid levels, which is often implicated in the pathogenesis of a number of neurological diseases such as MS (77, 78). In vitro study revealed that 24 hr pre-treatment of oligodendroglia (OLN-93) cells with ethanolic extract of B. serrata oleo-gum resin (10, 20, 40, and 80 µg/ml) prior to glutamate exposure reduced glutamate and quinolinic acid-induced oxidative injury (8 mM)(79). A mixture extract of Portulaca olerace, Urtica dioica, and B. serrata (200 and 400 mg/kg) has protective effects against ethidium bromide-induced MS model (80). The results revealed neurogenesis and memory improvement using the shuttle box test following treatment with the mixture (81). Cognitive deficits have been reported in up to 70% of MS patients (80). A clinical trial study was carried out in 80 patients with relapsing-remitting MS. In this randomized, double-blind, placebo-controlled study, effect of B. papyrifera on cognitive impairment in MS patients was investigated using brief international cognitive assessment for MS (BICAMS), symbol digit modality test (SDMT), and the California verbal learning test (CVLT). The patients received B. papyrifera (300 mg capsule, twice a day) and placebo with the same dose for 2 months. B. papyrifera remarkably improved visual-spatial memory while it was not effective in verbal memory and information processing speed, which may be due to the prescribed dose (82). Another double-blind clinical trial was carried out in 60 MS patients with cognitive deficits to assess the efficacy of B. serrata extract in treatment of cognitive dysfunction. The patients categorized randomly into two groups of treatment and placebo received 450 mg of B. serrate extract or placebo capsules, respectively, twice a day for two months. The extract remarkably enhanced auditory/verbal and visual/spatial memory using the brief visuospatial memory test (BVMT) and CVLT compared with the placebo group, which confirmed potential of B. serrata for MS patients suffering from cognitive impairments (83) (Table 1).
Central nervous systems trauma and brain ischemia
Stroke is the fourth cause of death and one of the main causes of disability worldwide (84). Ischemic stroke is the most common type of stroke, accounting for about 80 percent of all strokes, which results from transient or permanent cessation of cerebral blood flow (85, 86). Following brain ischemia, the level of glutamate increases, leading to over-activation of its receptors, including NMDA receptors and raised intracellular calcium (87). Brain ischemia also can trigger inflammatory responses and subsequently neuro-inflammation. Therefore, strategies targeting these pathways involve NMDA receptor antagonists, calcium channel blockers, and anti-inflammatory and antioxidant agents, which may be used as prophylactic or therapeutic for ischemia damage of brain tissue (87, 88). B. serrata and its constituent, AKBA (3-acetyl-11-keto-β-boswellic acid), exhibited potential neuroprotective and anti-oxidant activity (89). Neuroprotective potential of BSE and AKBA against ischemia-induced cytotoxicity was investigated. The survival of PC12 neural cells, pretreated with BSE (1.5-6 µg/ml) and AKBA (0.5–2.5 µg/ml) for 2 hr before exposure to oxygen/glucose/serum deprivation (OGSD) condition, increased. Moreover, BSE and AKBA counteracted oxidative stress indicated as restoring of intracellular reactive oxygen species content, lipid peroxidation, and oxidative DNA damage (90). Pre- and co-treatment with BSE and AKBA prevented glutamate-induced PC12 and Neuro-2a cell toxicity. The protective effect may be related to their inhibitory effects against oxidative damage and apoptotic cell death (91). An in vitro investigation aimed to explore anti-glycation and anti-oxidant potentials of B. sacra oleo-gum resin. Dichloromethane (CH2Cl2) fraction of the resin, 40% dichloromethane (CH2Cl2)/n-hexane sub-fraction, and frankincense oil exhibited l,l-Diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. In addition, moderate superoxide anion scavenging activity was exhibited by polar fraction, while the highest anti-glycation activity for polar fractions were reported (92). Another study was designed to investigate phytochemical screening, in vitro antioxidant activity of leaf extract of B. serrate. The methanolic extract of B. serrata contains alkaloids, terpenoids, saponins, and flavonoids (93). Methanolic extract exhibited significant DPPH free radical scavenging activity (IC50 = 54.06 µg/ml) and ferric reducing power (IC50 = 62.12 µg/ml), in a dose-dependent manner (93). Administration of the aqueous and ethanolic extracts of B. serrata (125, 250, and 500 mg/kg, IP) and AKBA (50 mg/kg, IP) just after middle cerebral artery occlusion (MCAO), for 30 min and reperfusion for 24 hr improved neurological deficits and reduced brain infarction volume. The extracts diminished neuronal apoptotic cell death through up-regulation of Bcl-2 and down-regulation of Bax and caspase-3. The modulated cerebral redox status was also indicated as inhibition of lipid peroxidation while increasing glutathione content and superoxide dismutase activity in the cerebral cortex (94). The neuroprotective effects of Boswellia against brain stroke were further confirmed by the reduction of infarction volume and neurological impairments. The aqueous extract of frankincense was administrated (50, 100, and 150 mg/kg, orally for 30 days). Two hours after the last treatment with frankincense extract, the rats were subjected to MCAO for 60 min followed by reperfusion for 24 hr. The level of blood-brain barrier (BBB) permeability and stroke-induced brain edema decreased in rats treated with aqueous extract of frankincense at doses of 100 and 150 mg/kg (95). The results of a prospective, randomized, placebo-controlled, double-blind, pilot trial confirmed the efficacy of BS on cerebral edema following brain radiotherapy (96). In this trial, forty-four patients with primary or secondary malignant cerebral tumors randomly received BS (4200 mg/day) or placebo during radiotherapy. Administration of BS suppressed the edema volume which was evaluated by T2-weighted magnetic resonance imaging (MRI) (96). To investigate the effect of B. serrata on neuro-recovery following diffuse axonal injury (DAI), a double-blind, randomized, cross-over study was designed. The outcome of diffuse axonal injury was assessed using the disability rating scale, a surrogate clinical marker for the pace of neuro-recovery. 38 patients randomly received either placebo or BS capsules (containing 215 mg BS gum resin) for 6 weeks. The BS resin enhanced the cognitive outcome of patients with DAI (97). The protective effects of the condor against cerebral inflammation after induction of diffuse traumatic brain injury were investigated. B. serrata (250 and 500 mg/ kg) attenuated brain edema and disruption of blood-brain-barrier induced by traumatic brain injury. The results were accompanied by improvement of vestibulomotor dysfunction as well as modulation of IL-1β and I-10 in brain tissue. Anti-inflammatory properties were suggested to be involved in the neuroprotective effects (98). Boswellia exhibited therapeutic potential for brain ischemia and injuries, which is most likely related at least in part to its anti-inflammatory, anti-apoptotic, as well as anti-oxidative and free radical scavenging activities (Table 1).
In this review, Tables 1 and 2 represent the brief description of pre-clinical and clinical studies on protective effects of genus Boswellia and AKBA in the neurodegenerative diseases, respectively.
Table 2.
A summary of in vitro and animal studies on neuroprotective potential of AKBA in the neurodegenerative diseases
| Agent | Type of study | Protocol | Results | Ref. |
|---|---|---|---|---|
| AKBA |
In vivo (MCAO) In vitro (OGD in primary cultured cortical neurons) |
20 mg/kg AKBA were given immediately after the onset of reperfusion Incubation with AKBA for 24 hr |
Treatment of AKBA : -reduced infarct volumes and apoptotic cells -increased neurologic scores by elevating the Nrf2 and HO-1 expression in brain -In primary cultured neurons: -increased the Nrf2 and HO-1 expression, -protection against OGD-induced oxidative stress |
(99). |
| AKBA |
In vivo (MCAO) In vitro (OGD in primary cultured astrocytes) |
KBA (25 mg/kg) applied 1 hr after reperfusion Incubation with KBA for 24 hr |
-reduced infarct volumes and apoptotic cells -increased neurologic scores -decreased MDA levels -restored the superoxide dismutase (SOD) activity -increased the protein Nrf2 and HO-1 expression in brain tissues -increased the Nrf2 and HO-1 expression -protection against OGD-induced oxidative stress |
(100). |
| AKBA | In vivo (cognitive impairment in mice induced by LPS) | dual therapy with AKBA (at a dose of 5 mg/kg, IP for 4 days) and celecoxib (at a dose of 30 mg/kg, IP for 7 days) | -reversed the behavioral and molecular changes -anti-inflammatory effect -antiglutamatergic effect -anti-amyloidogenic agent |
(101). |
| AKBA | In vivo (kainic acid-induced excitotoxicity and oxidative and nitrosative damage in mice) | the effects of COX inhibitors (indomethacin, nimesulide, and rofecoxib) and a 5-LOX inhibitor (AKBA) and the combination of these inhibitors in this model | -AKBA, indomethacin, and nimesulide did not produce any change in the behavioral parameters -rofecoxib increased the latency of clonic movement and decreased mortality rate -the effect of AKBA + rofecoxib was significantly more marked |
(102). |
| Nano formulation of AKBA |
In vivo (MCAO) In vitro (OGD) |
AKBA-NPs (containing AKBA 10 mg/kg), intravenously. OGD + AKBA-NP (10 mg/ml) treatment |
AKBA-NPs had better: -brain delivery efficacy -neuroprotection in OGD and MCAO models -modulation of antioxidant and anti-inflammatory pathways |
(103). |
| AKBA | In vivo (a single IP dose of LPS (0.8 mg/kg) was injected to induce cognitive dysfunction) | LPS-treated mice were administered for 7 days with AKBA(5 mg/kg, IP) or DEX (1 mg/kg, IP) | -AKBA and DEX reversed the behavioral dysfunction AKBA: -decreased P-IκB-α, miRNA-155 expression level, and carbonyl protein content -restored normal cytokine level -increased SOCS-1 expression level -showed anti-apoptotic and anti-amyloidogenic effects |
(104). |
| AKBA | In vivo (young and aged mice) | Chronic administration of AKBA (100 mg/kg, p.o.) and nimesulide (2.42 mg/kg, p.o.) for 15 days | -enhanced the cognitive performance -decreased oxidative damage -reversed the aging-induced motor dysfunction |
(105). |
| AKBA | In vivo (MCAO) | AKBA (50 mg/kg) was administered IP after MCAO induction | Improved neurological deficit -reduced brain infarction -decreased neuronal cell loss and apoptosis -attenuated lipid peroxidation -increased glutathione content and superoxide dismutase activity |
(94). |
| AKBA | In vitro (glutamate toxicity induced in PC12 and N2a cells) | Co- and pretreatment with AKBA (5 mM) was done on PC12 and N2a cells under glutamate toxicity (8 mM) | -↓ROS -↓lipid peroxidation -↓superoxide dismutase activity -↓oxidative DNA damage |
(91). |
MCAO: middle cerebral artery occlusion; OGD: oxygen-glucose deprivation; AKBA: acetyl-11-keto-β-boswellic acid; Nrf2: nuclear factor erythroid 2-related factor; MDA: malondialdehyde; LPS: lipopolysaccharide; COX: cyclooxygenase; DEX: dexamethasone; ROS: reactive oxygen species
Conclusion
Considering lack of effective therapy for clinical applications, pharmacologically active natural products, having neuroprotective activities are being focused, which makes them potential candidates for neurodegenerative disorders. The genus Boswellia has been suggested to target various molecular pathways involved in pathogenesis of neurodegenerative diseases. The genus regulates neurotrophic factors (including BDNF), apoptotic proteins (pro-apoptotic caspase-3 and anti-apoptotic bcl-2), and redox status. They were shown to be therapeutically effective at controlling inflammatory and cholinergic systems. Therefore, evidence suggests the importance of the genus in the prevention and treatment of neurodegenerative diseases even though further studies and clinical trials on these promising medicinal plants and their constituents should be strongly encouraged in the future.
References
- 1.Ayoobi F, Shamsizadeh A, Fatemi I, Vakilian A, Allahtavakoli M, Hassanshahi G, et al. Bio-effectiveness of the main flavonoids of Achillea millefolium in the pathophysiology of neurodegenerative disorders-a review. Iran J Basic Med Sci. 2017;20:604–612. doi: 10.22038/IJBMS.2017.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durães F, Pinto M, Sousa E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals. 2018;11:44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 5.Shirooie S, Nabavi SF, Dehpour AR, Belwal T, Habtemariam S, Argüelles S, et al. Targeting mTORs by omega-3 fatty acids: a possible novel therapeutic strategy for neurodegeneration? Pharmacol Res. 2018;135:37–48. doi: 10.1016/j.phrs.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Mertens M, Buettner A, Kirchhoff E. The volatile constituents of frankincense–a review. Flavour Fragr J. 2009;24:279–300. [Google Scholar]
- 7.Morikawa T, Matsuda H, Yoshikawa M. A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh. J Oleo Sci. 2017;66:805–814. doi: 10.5650/jos.ess16149. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui M. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. 2011;73:255–261. doi: 10.4103/0250-474X.93507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farshchi A, Ghiasi G, Farshchi S, Malek Khatabi P. Effects of Boswellia papyrifera gum extract on learning and memory in mice and rats. Iran J Basic Med Sci. 2010;13:9–15. [Google Scholar]
- 10.Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M. Frankincense (乳香 Rǔ Xiāng; Boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J Tradit Complement Med. 2013;3:221–226. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR, et al. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis. 2012;33:2441–2449. doi: 10.1093/carcin/bgs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E, Boon H, Heerema TD, Foppo I, Hashmi S, Hasskarl J, et al. Boswellia: An evidence-based systematic review by the natural standard research collaboration. J Herb Pharmacother. 2004;4:63–83. [PubMed] [Google Scholar]
- 13.Rijkers T, Ogbazghi W, Wessel M, Bongers F. The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. J Appl Ecol. 2006;43:1188–1195. [Google Scholar]
- 14.Goyal S. Novel anti-inflammatory topical herbal gels containing Withania somnifera and Boswellia serrata. Int J Pharm Biol Arch. 2011;2:1087–1094. [Google Scholar]
- 15.Ammon H. Boswellic acids and their role in chronic inflammatory diseases. In: Gupta SC, Prasad S, Aggarwal BB, editors , editors. Anti-inflammatory Nutraceuticals and Chronic Diseases. Springer; 2016. pp. 291–327. [Google Scholar]
- 16.Liu J-J, Nilsson A, Oredsson S, Badmaev V, Zhao W-Z, Duan R-D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23:2087–2093. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Boswellia serrata. Clin Pharmacokinet. 2011;50:349–369. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Syrovets T, Büchele B, Gedig E, Slupsky JR, Simmet T. Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIα. Mol Pharmacol. 2000;58:71–81. doi: 10.1124/mol.58.1.71. [DOI] [PubMed] [Google Scholar]
- 19.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Singh A. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy VS, Bukke S, Dutt N, Rana P, Pandey AK. A systematic review and meta-analysis of the circulatory, erythrocellular and CSF selenium levels in Alzheimer’s disease: A metal meta-analysis (AMMA study-I) J Trace Elem Med Biol. 2017;42:68–75. doi: 10.1016/j.jtemb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Wenk GL. Neuropathologic changes in Alzheimer’s disease: potential targets for treatment. J Clin Psychiatry. 2006;67:3–7. [PubMed] [Google Scholar]
- 24.Yassin N, El-Shenawy S, Mahdy KA, Gouda N, Marrie A, Farrag A, et al. Effect of Boswellia serrata on Alzheimer’s disease induced in rats. J Arab Soc Med Res. 2013;8:1–11. [Google Scholar]
- 25.Ahmed H, Mohamed E, El-Dsoki S. Evidences for the promising therapeutic potential of Boswellia serrata against Alzheimer’s disease: pre-clinical study. Int J Pharm Pharm Sci. 2014;6:384–392. [Google Scholar]
- 26.Ibrahim BMM. Experimental study of the effects of Boswellia serrata and ginger (Zingiber officinale) on Alzheimer’s Disease induced in rats. CU Theses; 2012. [Google Scholar]
- 27.Correia SC, Santos RX, Perry G, Zhu X, Moreira PI, Smith MA. Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res Rev. 2011;10:264–273. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal R, Tyagi E, Shukla R, Nath C. Insulin receptor signaling in rat hippocampus: a study in STZ (ICV) induced memory deficit model. Eur Neuropsychopharmacol. 2011;21:261–273. doi: 10.1016/j.euroneuro.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Qu Zq, Zhou Y, Zeng Ys, Lin Yk, Li Y, Zhong Zq, et al. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One. 2012;7:e29641. doi: 10.1371/journal.pone.0029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salkovic-Petrisic M, Knezovic A, Hoyer S, Riederer P. What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J Neural Transm. 2013;120:233–252. doi: 10.1007/s00702-012-0877-9. [DOI] [PubMed] [Google Scholar]
- 31.Sun P, Knezovic A, Parlak M, Cuber J, M Karabeg M, Deckert J, et al. Long-term effects of intracerebroventricular streptozotocin treatment on adult neurogenesis in the rat hippocampus. Curr Alzheimer Res. 2015;12:772–84. doi: 10.2174/1567205012666150710112147. [DOI] [PubMed] [Google Scholar]
- 32.Beheshti S, Aghaie R. Therapeutic effect of frankincense in a rat model of Alzheimer’s disease. Avicenna J Phytomed. 2016;6:468–475. [PMC free article] [PubMed] [Google Scholar]
- 33.Bensky D, Gamble A, Kaptchuk TJ. Chinese herbal medicine: materia medica. Eastland Press Seattle: 2004. [Google Scholar]
- 34.Jeon S, Hur J, Jeong HJ, Koo B-S, Pak SC. SuHeXiang Wan essential oil alleviates amyloid beta induced memory impairment through inhibition of tau protein phosphorylation in mice. Am J Chin Med. 2011;39:917–932. doi: 10.1142/S0192415X11009305. [DOI] [PubMed] [Google Scholar]
- 35.den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, Richard E. Apathy in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2015;30:759–769. doi: 10.1002/mds.26208. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Zhang ZW, Liang LW, Shen Q, Wang XD, Ren SM, et al. Treatment strategies for Parkinson’s disease. Neurosci Bull. 2010;26:66–76. doi: 10.1007/s12264-010-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2014;16:217–230. doi: 10.1007/s12017-014-8294-x. [DOI] [PubMed] [Google Scholar]
- 38.Kazmi S, Kafami L, Ebrahimi A, Jameie B, Joghataiee MT. The effects of Boswellia resin extract on dopaminergic cell line, SK-N-SH, against MPP+-induced neurotoxicity. Basic Clin Neurosci. 2011;3:16–21. [Google Scholar]
- 39.Baudry M, Bi X. Learning and memory: an emergent property of cell motility. Neurobiol Learn Mem. 2013;104:64–72. doi: 10.1016/j.nlm.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colciago A, Casati L, Negri-Cesi P, Celotti F. Learning and memory: steroids and epigenetics. J Steroid Biochem Mol Biol. 2015;150:64–85. doi: 10.1016/j.jsbmb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Gallistel CR, Balsam PD. Time to rethink the neural mechanisms of learning and memory. Neurobiol Learn Mem. 2014;108:136–144. doi: 10.1016/j.nlm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 43.Kandel ER, Schwartz JH, Jessell TM, Jessell MBT, Siegelbaum S, Hudspeth AJ. Principles of neural science. New York: McGraw-hill; 2000. [Google Scholar]
- 44.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 45.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 46.Arlt S. Non-Alzheimer’s disease-related memory impairment and dementia. Dialogues Clin Neurosci. 2013;15:465–473. doi: 10.31887/DCNS.2013.15.4/sarlt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseini SM, Esfandiari E, Alaei H. Effects of frankincense aqueous extract during gestational period on increasing power of learning and memory in adult offsprings. J Isfahan Med School. 2004;21:16–20. [Google Scholar]
- 48.Hosseini Sharifabad M, Esfandiary E. A morphometeric study on CA3 hippocampal field in young rats following maternal administration of Boswellia serrata resin during gestation. Iran J Basic Med Sci. 2007;10:176–182. [Google Scholar]
- 49.Sharifabad MH, Esfandiary E. The effects of maternal administration of boswellia gum resin (Frankincense) during lactation on stereological parameters of rat hippocampus. J Isfahan Med School. 2012;29:1–9. [Google Scholar]
- 50.Beheshti S, Shakakomi AG, Ghaedi K, Dehestani H. Frankincense upregulates the hippocampal calcium/calmodulin kinase II-α during development of the rat brain and improves memory performance. Int J Dev Neurosci. 2018;69:44–48. doi: 10.1016/j.ijdevneu.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- 52.Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 54.Khalaj-Kondori M, Sadeghi F, Hosseinpourfeizi MA, Shaikhzadeh-Hesari F, Nakhlband A, Rahmati-Yamchi M. Boswellia serrata gum resin aqueous extract upregulatesBDNF but not CREB expression in adult male rat hippocampus. Turk J Med Sci. 2016;46:1573–1578. doi: 10.3906/sag-1503-43. [DOI] [PubMed] [Google Scholar]
- 55.Mahmoudi A, Hosseini-Sharifabad A, Monsef-Esfahani HR, Yazdinejad AR, Khanavi M, Roghani A, et al. Evaluation of systemic administration of Boswellia papyrifera extracts on spatial memory retention in male rats. J Nat Med. 2011;65:519–525. doi: 10.1007/s11418-011-0533-y. [DOI] [PubMed] [Google Scholar]
- 56.Begin M, Langlois M, Lorrain D, Cunnane S. Thyroid function and cognition during aging. Curr Gerontol Geriatr Res. 2008;2008:474868. doi: 10.1155/2008/474868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller KJ, Parsons TD, Whybrow PC, Van Herle K, Rasgon N, Van Herle A, et al. Verbal memory retrieval deficits associated with untreated hypothyroidism. J Neuropsychiatry Clin Neurosc. 2007;19:132–136. doi: 10.1176/jnp.2007.19.2.132. [DOI] [PubMed] [Google Scholar]
- 58.Paz-Baruch N, Leikin M, Leikin R. Visual processing and attention abilities of general gifted and excelling in mathematics students. , Charles University in Prague, Faculty of Education; ERME, Feb 2015. Prague, Czech Republic: Charles University in Prague; pp. 1046–1051. [Google Scholar]
- 59.Hosseini M, Hadjzadeh MA-R, Derakhshan M, Havakhah S, Rassouli FB, Rakhshandeh H, et al. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010;33:463–468. doi: 10.1007/s12272-010-0317-z. [DOI] [PubMed] [Google Scholar]
- 60.Bejar C, Wang R-H, Weinstock M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur J Pharmacol. 1999;383:231–240. doi: 10.1016/s0014-2999(99)00643-3. [DOI] [PubMed] [Google Scholar]
- 61.Hosseinzadeh H, Ramezani M, Akhtar Y, Ziaei T. Effects Boswellia carterii gum resin fractions on intact memory and hyoscine-induced learning impairments in rats performing the Morris water maze task. J Medicinal Plants. 2010;2:95–101. [Google Scholar]
- 62.Mahboubi M, Taghizadeh M, Talaei SA, Firozeh SMT, Rashidi AA, Tamtaji OR. Combined administration of Melissa officinalis and Boswellia serrata extracts in an animal model of memory. Iran J Psychiatry Behav Sci. 2016;10:e681. doi: 10.17795/ijpbs-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF. Systemic lipopolysaccharide administration impairs retrieval of context–object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun. 2015;44:159–166. doi: 10.1016/j.bbi.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kranjac D, McLinden KA, Deodati LE, Papini MR, Chumley MJ, Boehm GW. Peripheral bacterial endotoxin administration triggers both memory consolidation and reconsolidation deficits in mice. Brain Behav Immun. 2012;26:109–121. doi: 10.1016/j.bbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao M, Zhou A, Xu L, Zhang X. The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroinflammation in hippocampal neurons. Neuroscience. 2014;269:93–101. doi: 10.1016/j.neuroscience.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 67.Beheshti S, Karimi B. Frankincense improves memory retrieval in rats treated with lipopolysaccharide. J Herbmed Pharmacol. 2016;5:12–16. [Google Scholar]
- 68.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5:21–24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Babb TL. Axonal growth and neosynaptogenesis in human and experimental hippocampal epilepsy. Adv Neurol. 1997;72:45–51. [PubMed] [Google Scholar]
- 70.Portavella M, Vargas J, Torres B, Salas C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res Bull. 2002;57:397–399. doi: 10.1016/s0361-9230(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 71.Jalili C, Salahshoor M, Pourmotabbed A, Moradi S, Roshankhah S, Darehdori AS, et al. The effects of aqueous extract of Boswellia serrata on hippocampal region CA1 and learning deficit in kindled rats. Res Pharm Sci. 2014;9:351–358. [PMC free article] [PubMed] [Google Scholar]
- 72.Jalili C, Salahshoor MR, Moradi S, Pourmotabbed A, Motaghi M. The therapeutic effect of the aqueous extract of Boswellia serrata on the learning deficit in kindled rats. Int J Prev Med. 2014;5:563–568. [PMC free article] [PubMed] [Google Scholar]
- 73.Burger C. Region-specific genetic alterations in the aging hippocampus: implications for cognitive aging. Front Aging Neurosci. 2010;2:140. doi: 10.3389/fnagi.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosseini-Sharifabad M, Kamali-Ardakani R, Hosseini-Sharifabad A. Beneficial effect of Boswellia serrata gum resin on spatial learning and the dendritic tree of dentate gyrus granule cells in aged rats. Avicenna J Phytomed. 2016;6:189–197. [PMC free article] [PubMed] [Google Scholar]
- 75.Taghizadeh M, Maghaminejad F, Aghajani M, Rahmani M. The effect of tablet containing Boswellia serrata and Melisa officinalis extract on older adults’ memory: A randomized controlled trial. Arch Gerontol Geriatr. 2018;75:146–150. doi: 10.1016/j.archger.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 76.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 77.Lopez YP, Kenis G, Rutten BP, Myint AM, Steinbusch HW, van den Hove DL. Quinolinic acid-immunoreactivity in the naïve mouse brain. J Chem Neuroanat. 2016;71:6–12. doi: 10.1016/j.jchemneu.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Sundaram G, Brew BJ, Jones SP, Adams S, Lim CK, Guillemin GJ. Quinolinic acid toxicity on oligodendroglial cells: relevance for multiple sclerosis and therapeutic strategies. J Neuroinflammation. 2014;11:204. doi: 10.1186/s12974-014-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahimi VB, Askari VR, Mehrdad A, Sadeghnia HR. Boswellia serrata has promising impact on glutamate and quinolinic acid-induced toxicity on oligodendroglia cells: in vitro study. Acta Pol Pharm. 2017;74:1803–1811. [Google Scholar]
- 80.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. The Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 81.Mirhosseini G, Tehranipour M, Shahri NM. The synergistic effects of mixture extract Portulaca olerace, Urtica Dioica, Boswellia serrate on multiple sclerosis in rats. J Gorgan Univ Med Sci. 2018;21:57–61. [Google Scholar]
- 82.Sedighi B, Pardakhty A, Kamali H, Shafiee K, Hasani BN. Effect of Boswellia papyrifera on cognitive impairment in multiple sclerosis. Iran J Neurol. 2014;13:149–153. [PMC free article] [PubMed] [Google Scholar]
- 83.Majdinasab N, Siahpush A, Mousavinejad SK, Malayeri A, Sajedi SA, Bizhanzadeh P. Effect of Boswellia serrata on cognitive impairment in multiple sclerosis patients. J Herb Med. 2016;6:119–127. [Google Scholar]
- 84.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 85.Della-Morte D, Guadagni F, Palmirotta R, Testa G, Caso V, Paciaroni M, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics. 2012;13:595–613. doi: 10.2217/pgs.12.14. [DOI] [PubMed] [Google Scholar]
- 86.Mahajan S, Kashyap R, Sood B, Jaret P, Mokta J, Kaushik N, et al. J Assoc Physicians India. 2004;52:699–702. [PubMed] [Google Scholar]
- 87.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 89.Assimopoulou A, Zlatanos S, Papageorgiou V. Antioxidant activity of natural resins and bioactive triterpenes in oil substrates. Food Chem. 2005;92:721–727. [Google Scholar]
- 90.Sadeghnia HR, Arjmand F, Ghorbani A. Neuroprotective effect of Boswellia serrata and its active constituent acetyl 11-keto-β-boswellic acid against oxygen-glucose-serum deprivation-induced cell injury. Acta Pol Pharm. 2017;74:911–920. [PubMed] [Google Scholar]
- 91.Rajabian A, Boroushaki MT, Hayatdavoudi P, Sadeghnia HR. Boswellia serrata Protects Against Glutamate-Induced Oxidative Stress and Apoptosis in PC12 and N2a Cells. DNA Cell Biol. 2016;35:666–679. doi: 10.1089/dna.2016.3332. [DOI] [PubMed] [Google Scholar]
- 92.Al-Harrasi A, Ali L, Ceniviva E, Al-Rawahi A, Hussain J, Hussain H, et al. Antiglycation and antioxidant activities and HPTLC analysis of Boswellia sacra Oleogum resin: the sacred frankincense. Trop J Pharm Res. 2013;12:597–602. [Google Scholar]
- 93.Afsar V, Reddy YM, Saritha K. In vitro antioxidant activity and anti-inflammatory activity of methanolic leaf extract of Boswellia serrata. Int J Life Sc Bt & Pharm Res. 2012;4:15–23. [Google Scholar]
- 94.Forouzanfar F, Hosseinzadeh H, Ebrahimzadeh Bideskan A, Sadeghnia HR. Aqueous and ethanolic extracts of Boswellia serrata protect against focal cerebral ischemia and reperfusion injury in rats. Phytother Res. 2016;30:1954–1967. doi: 10.1002/ptr.5701. [DOI] [PubMed] [Google Scholar]
- 95.Rahnema M. Effect of treatment with aqueous extracts of Boswellia serrata on blood-brain barrier permeability and brain edema in experimental model of stroke in rats. Qom Univ Med Sci J. 2017;11:56–65. [Google Scholar]
- 96.Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, et al. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer. 2011;117:3788–3795. doi: 10.1002/cncr.25945. [DOI] [PubMed] [Google Scholar]
- 97.Moein P, Abbasi Fard S, Asnaashari A, Baratian H, Barekatain M, Tavakoli N, et al. The effect of Boswellia Serrata on neurorecovery following diffuse axonal injury. Brain Inj. 2013;27:1454–1460. doi: 10.3109/02699052.2013.825009. [DOI] [PubMed] [Google Scholar]
- 98.Sheykhiyeh Golzardi Mahshid, Rezaenejad Rezvan, Kachouei Emadeddin Y, Siahposht-Khachaki Ali. Neuroscience J Shefaye Khatam. 2018;6 [Google Scholar]
- 99.Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J, et al. Neuroprotection by acetyl-11-keto-β-boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci Rep. 2014;4:7002–7010. doi: 10.1038/srep07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding Y, Chen M, Wang M, Li Y, Wen A. Post treatment with 11-keto-β-boswellic acid ameliorates cerebral ischemia-reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol. 2015;52:1430–1439. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 101.Sayed AS, El Sayed NSED. Co-administration of 3-acetyl-11-keto-beta-boswellic acid potentiates the protective effect of celecoxib in lipopolysaccharide-induced cognitive impairment in mice: possible implication of anti-inflammatory and antiglutamatergic pathways. J Mol Neurosci. 2016;59:58–67. doi: 10.1007/s12031-016-0734-7. [DOI] [PubMed] [Google Scholar]
- 102.Bishnoi M, Patil C, Kumar A, Kulkarni SK. Co-administration of acetyl-11-keto-β-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of COX-2 inhibitors in kainic acid-induced neurotoxicity in mice. Pharmacology. 2007;79:34–41. doi: 10.1159/000097627. [DOI] [PubMed] [Google Scholar]
- 103.Ding Y, Qiao Y, Wang M, Zhang H, Li L, Zhang Y, et al. Enhanced neuroprotection of acetyl-11-keto-β-boswellic acid (AKBA)-loaded O-carboxymethyl chitosan nanoparticles through antioxidant and anti-inflammatory pathways. Mol Neurobiol. 2016;53:3842–3853. doi: 10.1007/s12035-015-9333-9. [DOI] [PubMed] [Google Scholar]
- 104.Sayed AS, Gomaa IEO, Bader M, El Sayed NSED. Role of 3-acetyl-11-keto-beta-boswellic acid in counteracting LPS-induced neuroinflammation via modulation of miRNA-155. Mol Neurobiol. 2018;55:5798–5808. doi: 10.1007/s12035-017-0801-2. [DOI] [PubMed] [Google Scholar]
- 105.Bishnoi M, Patil C, Kumar A, Kulkarni S. Protective effects of nimesulide (COX Inhibitor), AKBA (5-LOX Inhibitor), and their combination in aging-associated abnormalities in mice. Methods Find Exp Clin Pharmacol. 2005;27:465–470. doi: 10.1358/mf.2005.27.7.920929. [DOI] [PubMed] [Google Scholar]