Abstract
The emerging coronavirus SARS-CoV-2 has caused a COVID-19 pandemic. SARS-CoV-2 causes a generally mild, but sometimes severe and even life-threatening infection, known as COVID-19. Currently, there exist no effective vaccines or drugs and, as such, global public authorities have so far relied upon non pharmaceutical interventions (NPIs). Since COVID-19 symptoms are aspecific and may resemble a common cold, if it should come back with a seasonal pattern and coincide with the influenza season, this would be particularly challenging, overwhelming and straining the healthcare systems, particularly in resource-limited contexts, and would increase the likelihood of nosocomial transmission. In the present study, we devised a mathematical model focusing on the treatment of people complaining of influenza-like-illness (ILI) symptoms, potentially at risk of contracting COVID-19 or other emerging/re-emerging respiratory infectious agents during their admission at the health-care setting, who will occupy the detection kits causing a severe shortage of testing resources. The model is used to assess the effect of mass influenza vaccination on the spread of COVID-19 and other respiratory pathogens in the case of a coincidence of the outbreak with the influenza season. Here, we show that increasing influenza vaccine uptake or enhancing the public health interventions would facilitate the management of respiratory outbreaks coinciding with the peak flu season, especially, compensate the shortage of the detection resources. However, how to increase influenza vaccination coverage rate remains challenging. Public health decision- and policy-makers should adopt evidence-informed strategies to improve influenza vaccine uptake.
Keywords: Coronavirus, Pandemic outbreak, Limited resources, Influenza season, Influenza vaccination
Highlights
-
•
A novel COVID-19 mathematical model incorporating cross-infection and limited detection capability is proposed.
-
•
Mass influenza vaccination is verified to be effective in mitigating the COVID-19 epidemics.
-
•
Improving the detection capability is a key in controlling the COVID-19 outbreak in the early stage.
-
•
Public health interventions can compensate the shortage of detection resource.
1. Introduction
An emerging coronavirus, currently known as “Severe Acute Respiratory Syndrome coronavirus type 2” (SARS-CoV-2) and previously termed as “2019 novel coronavirus” (2019-nCoV), has spread out from its first reported epicenter and quickly become a pandemic [1], [2].
SARS-CoV-2 causes a generally mild, but sometimes severe and even life-threatening infection, known as “coronavirus disease” (COVID-19). Currently, there exist no effective vaccines or drugs that can effectively prevent or treat COVID-19 patients. As such, global public authorities have so far relied upon behavioral, non pharmaceutical interventions (NPIs), such as use of masks, social distancing, self-isolation, quarantine and even lock-down of entire territories and communities, to contain or, at least, mitigate the burden of the ongoing pandemic [3], [4], [5]. Despite the unprecedented nature of some of these measures, western countries have found it difficult to fully suppress/eradicate the outbreak and have preferred to mitigate it, deciding to opt for a short-period of NPIs, which will be followed by gradual reopening the economy and return to a new normal. Resumption of daily working and social activities is expected to cause further outbreak waves, due to the re-increasing of contact rates.
In [6], Sajadi et al. provided the evidence that COVID-19 could perhaps prevail at low levels and begin to rise again in late fall and winter in temperate regions in the upcoming year. Since COVID-19 symptoms are aspecific and may resemble a common cold, if it should come back with a seasonal pattern and coincide with the influenza season, this would be particularly challenging. From a clinical standpoint, it would be difficult to distinguish between the two infectious agents, with the definition of COVID-19 cases rather problematic. Moreover, diagnostic tests as well as human resources are limited. Furthermore, currently commercially available rapid diagnostic tests are characterized by a good specificity but have a limited sensitivity, as such not enabling a quick and reliable diagnosis of COVID-19. Altogether, this would significantly impact downstream public health efforts to properly identify COVID-19 cases and contain the outbreak, overwhelming and straining the healthcare systems, particularly in resource-limited contexts, and would increase the likelihood of nosocomial transmission.
When the “Severe Acute Respiratory Syndrome” (SARS) outbreak caused by the coronavirus SARS-CoV-1 occurred in mainland China in 2002, the World Health Organization (WHO) has recommended to increase influenza vaccination, considering that the actual coverage rate is still sub-optimal and below the threshold. In particular, the WHO recommended a campaign targeting high-risk groups, such as healthcare workers, the elderly and disabled people, to be able to differentiate more quickly between the two infections and to be more effective in counteracting the outbreak [7], [8], [9].
A similar strategy would be valuable also for COVID-19. To test such a hypothesis, we devised a mathematical model incorporating the treatment of people complaining of ILI symptoms, potentially at risk of contracting COVID-19 or other emerging/re-emerging respiratory infectious agents during their admission at the health-care setting, where the competition of the detection resources between the COVID-19 infected population and individuals with ILI symptoms is considered. The main purpose of this study is using the model to assess the effect of mass influenza vaccination and public health interventions on the spread of COVID-19 in the case of a coincidence of the outbreak with the influenza season.
2. Methodology
2.1. Data
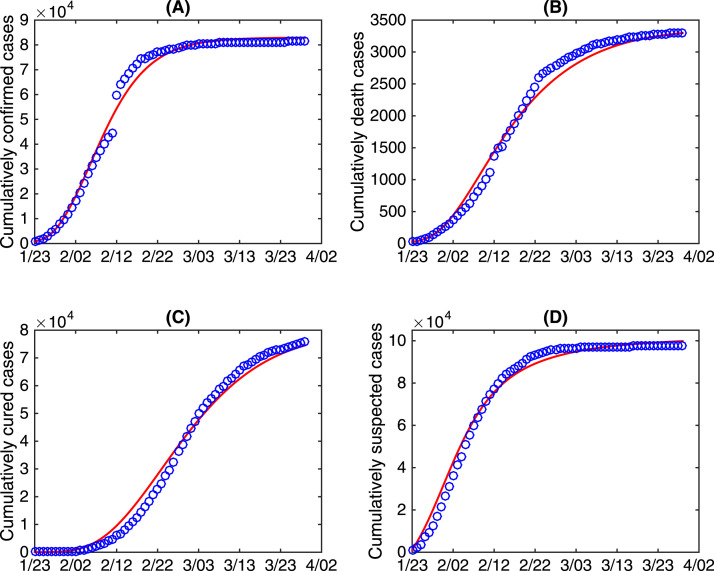
We obtained the data of COVID-19 cases in China from January 23rd to March 29th 2020 from the National Health Commission of the People’s Republic of China [10]. The data information includes the cumulative number of confirmed cases, the cumulative number of death cases, the cumulative number of cured cases, and the cumulative number of suspected cases, shown in Fig. 1. It should be mentioned that the number of suspected cases includes the number of quarantined COVID-19 exposed cases, the number of quarantined COVID-19 infected cases but not confirmed yet, and the number of quarantined individuals with clinical fever symptoms who are susceptible to COVID-19.
Fig. 1.
The data of COVID-19 in China from January to March 2020. (A) The cumulative number of confirmed cases; (B) The cumulative number of death cases; (C) The cumulative number of cured cases; (D) The cumulative number of suspected cases.
2.2. Model
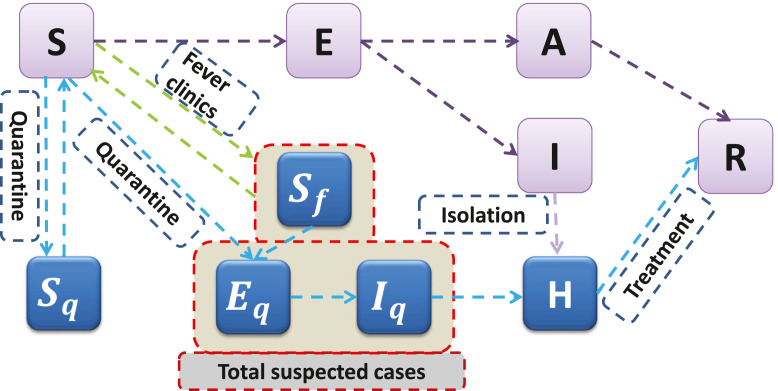
Based on the epidemical progression of COVID-19 and the intervention measures, we extended the classical SEIR model by including social distancing measures and including “cross-infected” individuals, those who are having clinical fever symptoms and are considered as COVID-19 suspected (and thus quarantined) due to their exposure to COVID-19 infected individuals. The transmission diagram is shown in Fig. 2. In the model, we divide the total population into ten compartments: susceptible (), exposed (), symptomatic infected (), asymptomatic infected (), quarantined susceptible (), quarantined susceptible with fever symptoms (), quarantined exposed (), quarantined infected (), confirmed and hospitalized (), and recovered ().
Fig. 2.
Diagram of the model adopted in the study for illustrating the COVID-19 infection dynamics. Interventions including intensive contact tracing followed by quarantine and isolation are indicated. The total suspected cases consisting of quarantined susceptible with clinical fever symptoms (), quarantined exposed (), and quarantined infected ().
With the implementation of contact tracing, a proportion of of individuals exposed to the virus is quarantined. Let the transmission probability be and the contact rate be , then the quarantined individuals can move to compartment (or ) at a rate of (or ) if they are effectively infected (or not effectively infected). While the other proportion, , missed from the contact tracing, will move to the exposed compartment at a rate of once effectively infected or stay in the susceptible compartment otherwise.
Note that, due to clinical fever or illness-like symptoms, susceptible individuals may also be quarantined and move to the compartment at a transition rate of , and they can be infected by the quarantined infected individuals at a rate of . Based on the above assumptions and previous studies [5], [11], [12], the transmission dynamics is governed by the following model:
| (1) |
The detailed definitions of all the parameters and variables are listed in Table 1.
Table 1.
Parameter estimates for the COVID-19 epidemics in China.
| Parameter | Definition | Value | Source | |
|---|---|---|---|---|
| Contact rate at the initial time | 14.781 | [11] | ||
| Minimum contact rate with control strategies | 2.0 | Estimated | ||
| Exponential decreasing rate of contact rate | 0.1 | Estimated | ||
| Transmission probability from to per contact | 0.18 | Estimated | ||
| Quarantined rate at the initial time | [12] | |||
| Maximum quarantined rate with control strategies | 0.9 | Estimated | ||
| Exponential increasing rate of quarantined rate | 0.1 | Estimated | ||
| Transmission probability from to per contact | 0.01 | Estimated | ||
| Quarantined rate of susceptible population with fever symptoms at the initial time | Estimated | |||
| Minimal quarantined rate of susceptibles with fever symptoms | Estimated | |||
| Exponential decreasing rate of quarantined rate | 0.0567 | Estimated | ||
| Releasing rate of quarantined susceptibles | [11] | |||
| Releasing rate of quarantined susceptibles with fever symptoms | 0.1 | Estimated | ||
| Ratio of symptomatic infection | 0.5 | Estimated | ||
| Transition rate of exposed individuals to the infected class | [13] | |||
| Fast diagnose rate of infected individuals | 0.5 | Estimated | ||
| Initial number of detection kits per day | 2000 | Estimated | ||
| Maximal number of detection kits per day | Estimated | |||
| Exponential increasing rate of the number of detection kits | 0.885 | Estimated | ||
| Recovery rate of asymptotic infected individuals | 0.13978 | [11] | ||
| Disease-induced death rate at the initial time | 0.012 | Estimated | ||
| Minimal disease-induced death rate with treatment | 0.0012 | Estimated | ||
| Exponential increasing rate of disease-induced death rate | 0.1129 | Estimated | ||
| Transmission rate from to | Estimated | |||
| Contact rate of suspected cases | 2.0 | Estimated | ||
| Transition rate of quarantined exposed individuals to the quarantined infected class | 0.2 | Estimated | ||
| Fast diagnose rate of quarantined individuals | 1.0 | Estimated | ||
| Recovery rate of confirmed individuals at the initial time | 0.001 | Estimated | ||
| Maximal recovery rate of confirmed individuals with treatment | 0.15 | Estimated | ||
| Exponential increasing rate of recovery rate | 0.0123 | Estimated | ||
| Variable | Definition | Initial value | Source | |
| Susceptible population | Estimated | |||
| Exposed population | 8216 | Estimated | ||
| Infected symptomatic population | 1000 | Estimated | ||
| Infected asymptomatic population | 1000 | Estimated | ||
| Quarantined susceptible population | 7347 | Data | ||
| Quarantined susceptible population with fever symptoms | 499.9975 | Estimated | ||
| Quarantined exposed population | 100.0003 | Estimated | ||
| Quarantined infected population | 250.0005 | Estimated | ||
| Confirmed and hospitalized population | 771 | Data | ||
| Recovered population | 34 | Data | ||
A significant difference between the COVID-19 transmission dynamics model in our previous studies [11], [12], [14] and the current study is the introduction of two saturated functions
and
to describe the impact of “cross-infection” on the diagnose rate of the infected class () and the detection rate of the quarantined infected class () with limited testing capacity, respectively. and are the fastest diagnose rate of infected individuals and quarantined infected individuals, respectively, that the medical resources permit, is the maximum number of individuals who can be tested per unit time (day) (limited by the maximum testing kits and staff to administrate the test) with and . In other word, measures the testing capacity per day. Here, we set the daily capacity as an increasing function of time because of the increasing production of detection kits and the improvement of detection techniques. The function of is of the following form:
where is the total number of available detection kits at the initial time (i.e. January ) with , is the maximum number of available tests permitted with , and is the exponential increasing rate.
The saturation functions and are decreasing functions with respect to . This is because the quarantined COVID-19 susceptible individuals with fever symptoms belong to the COVID-19 suspected population, and will be tested to confirm if they are COVID-19 positive or not. These individuals will consume the detection kits and require staff time, resulting in a slower detection rate of the COVID-19 infected cases.
Similarly to the previous studies [12], [14], as a result of the improvement of medical treatment and the implementation of a series of strict control interventions adopted by the Chinese government since January 23rd, we assume that the contact rate , the quarantine rate , the quarantine rate of susceptible population with clinical fever symptoms , disease-induced death rate , and recovery rate of confirmed individuals are time-dependent functions. In more details, the contact rate is a decreasing function with respect to time , which is given by
where is the contact rate at the initial time with , is the minimum contact rate under control measures and self-isolation with , and is the exponential decreasing rate.
The quarantined rate is an increasing function with respect to time due to the strengthened contact tracing, which takes the following form
where is the quarantined rate at the initial time with , is the maximum quarantined rate under control measures with , and is the exponential increasing rate.
The quarantined rate of susceptible population with clinical fever symptoms is a decreasing function with respect to time , given by
where denotes the quarantined rate of susceptible population with fever symptoms at the initial time with , is the minimum quarantined rate of susceptible population with fever symptoms with , and is the exponential decreasing rate.
Due to the improvements of medical treatments and the strengthening of the production and supply of prevention and control products [15], disease-induced death rate decreases with respect to time , and the recovery rate of confirmed individuals increases with respect to time . Thus and take the following forms,
where is the disease-induced death rate at the initial time with , is the minimum disease-induced death rate with , and is the exponential decreasing rate. denotes the recovery rate of confirmed individuals at the initial time with , denotes the maximum recovery rate of confirmed individuals with , and denotes the exponential increasing rate.
Using the next generation matrix, we can define and calculate the effective reproduction number as follows:
where
and
Note that represents the effective reproduction number of cross-infected individuals.
3. Main results
3.1. Parameter estimation process
In order to fit the model to the data, we firstly fix some parameters of our model from previous literature to reduce the complexity. In particular, the contact rate and the quarantined rate at the initial time are fixed as [11] and [12], respectively. The incubation period is fixed as days [13], i.e. , the releasing rate of quarantined susceptible individuals is fixed as [11], while the recovery rate of the asymptomatic infected individuals is fixed as [11]. In addition, we fix the initial quarantined susceptible population, confirmed and hospitalized population, and recovered population as , and respectively according to the data information.
By simultaneously fitting the proposed model to the cumulative number of confirmed cases, cumulative number of death cases, cumulative number of cured cases and cumulative number of suspected cases, we first estimate the rest parameters and initial conditions using the least square method. The best fitting curves are marked as red in Fig. 3 with the blue circles representing the data from January 23rd to March 29th 2020. The detailed estimated values of the parameters and initial conditions are listed in Table 1.
Fig. 3.
Best model fitting result. The red curves are the best fitting curves, and the blue circles denote the cumulatively confirmed cases, cumulatively death cases, cumulatively cured cases and cumulatively suspected cases.
It is worth mentioning that we use four time series of data to fit the model, simultaneously, which can cross-validate the estimation results. Furthermore, based on the available information, the least squares method with a priori distribution for each parameter is used in this study. In another word, we implicitly utilize a penalized least square method to select reasonable parameter values falling in the ranges which were estimated in other published studies. Particularly, our estimated minimum contact rate with control strategies is 2.0 which is consistent with contact surveys in study [16], and the transition rate of quarantined exposed individuals to the quarantined infected class is estimated as being in line with the incubation period [13]. It was illustrated in [17] that it takes about 2 days from the start of sampling to the return of results, indicating that our estimation value of fast diagnose rate of infected individuals is reasonable. Furthermore, the estimated initial number of detection kits per day is highly consistent with the news reported in the Beijing News [17].
Furthermore, we use the coefficient of determination () to estimate the goodness of fit for our model fitting results. Given a data set with observed value . The corresponding estimated values from the model are defined as and is the average observed value, then the coefficient of determination value () can be defined as with denoting the sum of squares of residuals and denoting the total sum of squares. Therefore, we obtain the coefficients of determination for the model fitting results in Fig. 3(A-D) being 0.9905, 0.988, 0.9884 and 0.9868, respectively. This indicates that the model fits the data very well.
3.2. Impacts of limited detection kits
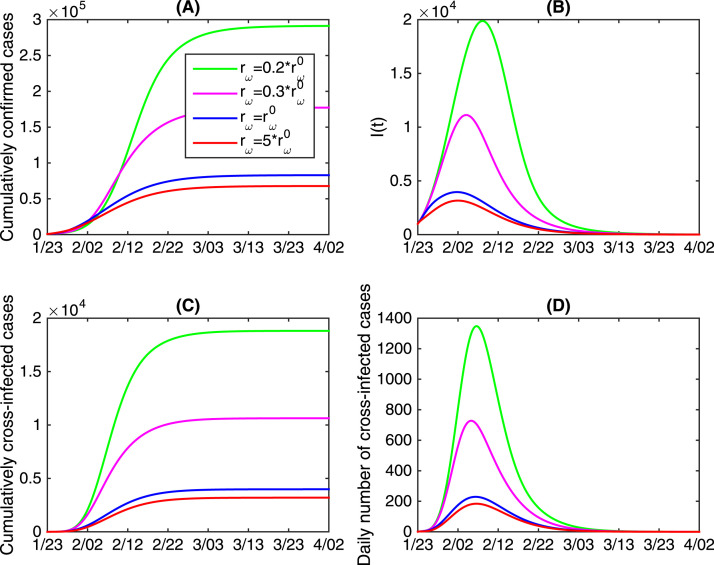
Detection kits for COVID-19 were firstly introduced and used on January 16th 2020, but with very limited number. Since January 23rd 2020, detection kits were delivered to Hubei province from other provinces in China. Since it took time to increase the production of kits and improve the level of production technology, there was a serious shortage of detection kits during the initial stage of the COVID-19 epidemics. To examine the impacts of the limited detection kits on the COVID-19 epidemics in terms of the cumulative number of confirmed cases, the cumulative number of cross-infected cases and the infected population (), we vary the increasing rate of the available detection kits in Fig. 4. It follows from Fig. 4 that reducing will significantly increase both the cumulative number of confirmed cases and cross-infected cases, and the infected population of and daily cross-infected population at the peak time. Particularly, the cumulatively confirmed cases and cumulatively cross-infected cases will increase 3.5 times (about cases) and 4.7 times (about cases), respectively, if the exponential increasing rate decreases by percent. This means that deficiency and delay of detection cause more serious outbreaks. In another word, speeding up the production of detection kits and improve the detection capability play an important role in reducing the final size of infections.
Fig. 4.
The effects of varying the increasing rate of available detection kits on the COVID-19 epidemic in mainland China. denotes the estimated value of .
When faced with limited detection kits supply, we show the impacts of public health interventions on the outbreak of COVID-19, as seen in Table 2. In particular, increasing or remarkably reduces the cumulative number of confirmed cases. Specifically, when is very small (), i.e. the testing kits production increases very slowly, then (1). If increases by times, the cumulative number of confirmed cases will decrease by 93.6%; (2). If increases by times, the cumulative number of confirmed cases will decrease by 78.7%. In addition, comparing the results in Table 2, we find that the impact of increasing or on mitigating the epidemics weakens as increases. This implies that rapid implementation of public health interventions, such as reducing contact rate and enhancing quarantined rate, is a good way to compensate the shortage of detection kits, and it is more indispensable for countries with severe limited resource of detection kits.
Table 2.
The impacts of public health interventions on the cumulative number of confirmed cases when faced with limited detection kits supply.
| Value of | Cumulative number of confirmed cases with varying |
Cumulative number of confirmed cases with varying |
||||
|---|---|---|---|---|---|---|
| (−80.6%) | (−93.6%) | (−58.4%) | (−78.7%) | |||
| (−56.4%) | (−80.5%) | (−23.0%) | (−42.8%) | |||
| (−52.3%) | (−76.6%) | (−19.2%) | (−36.9%) | |||
| (−51.0%) | (−75.1%) | (−18.1%) | (−35.0%) | |||
Note that , and are the estimated value of , and , respectively.
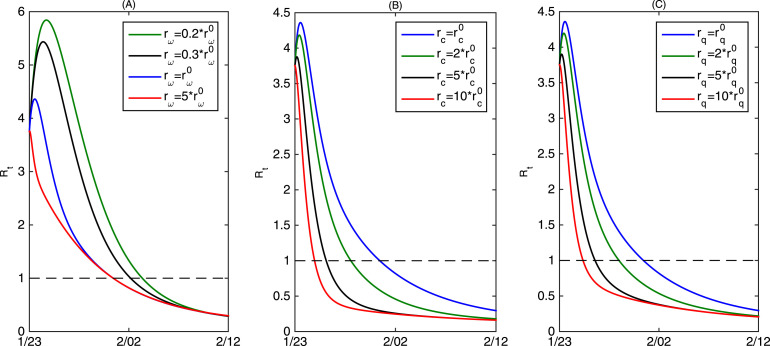
It is also interesting to observe from Fig. 5(A) that the estimated effective reproduction number (the blue curve) will first experience a short-period increasing before it decreases below the threshold . However, we further observe that if we increase the rate of by times (the red curve in Fig. 5(A)), the effective reproduction number will decrease below the threshold directly. This indicates that due to the limited resource of the testing kits, the fast-increased infected population will result in more infections as they are not confirmed and hospitalized. And the situation will become worse if is smaller, shown in Fig. 5(A) as well. Furthermore, it follows from Fig. 5(B) and (C) that increasing or can avoid the magnification of the effective reproduction number and make the threshold value reduce to ahead of time, which implies that reducing the contact rate quickly and increasing the quarantined rate can effectively avert the short-term intensification of the outbreak caused by the limited detection kits.
Fig. 5.
(A) Estimated effective reproduction number (blue curve) and the variation of the effective reproduction number by varying ; (B) The variation of the effective reproduction number by varying ; (C) The variation of the effective reproduction number by varying . Here denotes the estimated value of the increasing rate of available detection kits , denotes the estimated value of the decreasing rate of contact rate , and denotes the estimated value of the increasing rate of quarantined rate .
3.3. Benefits of getting vaccinated against influenza
The COVID-19 outbreak in China coincided partially with flu season, and it was difficult to distinguish COVID-19 accurately and rapidly from influenza-like-illnesses. As a result, individuals with clinical fever symptoms required medical treatment in high risk settings of COVID-19, and the risk of cross-infection increased. Here we seek to use our transmission dynamics model to explore the impact of mass influenza vaccination prior to the onset of flu season on controlling the transmission of COVID-19.
Note that, in our model, we assumed that due to the influenza-like-illness (ILI), the susceptible individuals () can be quarantined at a rate of (move to ), which is proportion to the susceptible population. Further, we assume that the susceptible population is vaccinated against influenza with an effective vaccination coverage rate . Thus, the vaccinated population will not be quarantined because of the clinical fever symptoms, consequently, the rate at which the susceptible population is quarantined due to the ILI becomes . Based on the above assumptions, we evaluate the impact of the mass influenza vaccination on the transmission dynamics of COVID-19.
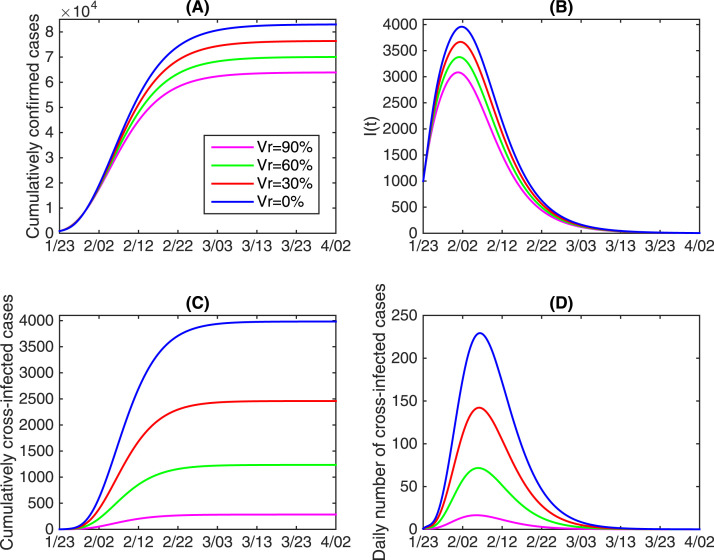
In Fig. 6, by changing the vaccination coverage rate of and fixing all the other parameters as the estimated baseline values, we examine the impact of vaccination against influenza on the COVID-19 epidemics in China in terms of the final size and the peak values. It follows from Fig. 6 that increasing the vaccination coverage rate against influenza can remarkably reduce the cumulative number of COVID-19 confirmed cases and cross-infected cases, and also reduce the peak number of and daily cross-infected population. In more detail, we find that by a vaccination coverage rate of 90%, the cumulative number of confirmed cases can reduce by 23.0% (about 19062 total cases), moreover, the cumulative number of cross-infected cases can reduce by 92.9% (about 3700 cases). This implies that mass influenza vaccination could contribute significantly to the control of the outbreak of COVID-19 and significantly reduce the risk of cross-infection.
Fig. 6.
The impacts of getting vaccinated against influenza on the COVID-19 epidemic in mainland China. Here “Vr” represents the vaccination coverage rate against influenza.
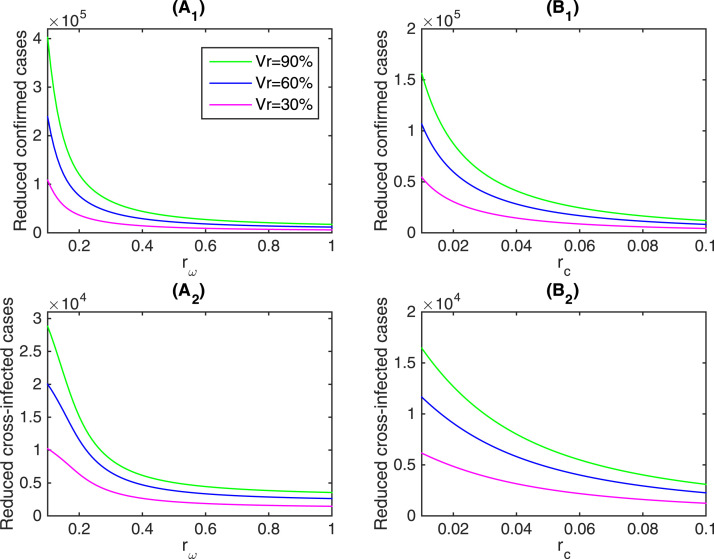
In addition, in Fig. 7 we illustrate the impact of getting vaccine against influenza on the reduction of the cumulative number of confirmed cases and cross-infected cases incorporating the effect of limited testing kits and public health interventions, that is, with different increasing rate of available detection kits or different decreasing rate of contact rate . As shown in Fig. 6, vaccination against influenza could reduce the cumulative numbers of confirmed cases and cumulative cross-infected cases, hereafter referred as the reduced confirmed cases and reduced cross-infected cases, respectively. It follows from Fig. 7() and () that getting vaccinated against influenza could significantly reduce the cumulative number of confirmed cases and cross-infected cases with respect to . We give the specific reduction rate with respect to different and vaccination coverage rate in Table 3. Specifically, when the vaccination coverage rate was 90%, the cumulative number of confirmed cases and cross-infected cases could reduce by 51.5% and 91.4% with , respectively. We can further observe that the effects of getting vaccinated against influenza weakens as increases. It means that getting vaccinated against influenza could effectively control the outbreak of COVID-19. Moreover, mass influenza vaccination is more necessary when detection kits are severely limited and under seriously shortage of supply. In another words, mass influenza vaccination can be a much more effective control measure in mitigating the COVID-19 epidemics in the early stage with a rapid growth and the countries or areas with limited testing kits.
Fig. 7.
The total reduction number of cumulatively confirmed cases and the reduction of cumulatively cross-infected cases by different vaccination coverage rate against influenza with respect to (A1 and A2) and (B1 and B2). Here “Vr” represents the vaccination coverage rate against influenza.
Table 3.
The impacts of getting vaccinated against influenza on the cumulative number of confirmed cases and the cumulative number of cross-infected cases with respect to .
| Vaccination coverage rate | Cumulative number of confirmed cases |
Cumulative number of cross-infected cases |
||||
|---|---|---|---|---|---|---|
| 0 | ||||||
| (−13.5%) | (−12.4%) | (−9.4%) | (−31.9%) | (−41.0%) | (−39.2%) | |
| (−30.1%) | (−25.1%) | (−18.7%) | (−62.8%) | (−72.6%) | (−70.2%) | |
| 410.8 | 309.2 | |||||
| (−51.5%) | (−38.2%) | (−27.9%) | (−91.4%) | (−94.5%) | (−93.4%) | |
Here is the estimated value of .
Similarly, Fig. 7() and () illustrate the impact of getting vaccinated on reducing the cumulative number of confirmed cases and cross-infected cases with respect to , and the detailed reduction rate with respect to different and vaccination coverage rate is given in Table 4. Specifically, for a 90% vaccination coverage rate, the cumulative number of confirmed cases could reduce by 30.8%, and the cumulative number of cross-infected cases could reduce by 92.0% with , showing that if the contact rate was not fast controlled and reduced, mass vaccination could effectively aid the containment of COVID-19 outbreak.
Table 4.
The impacts of getting vaccinated against influenza on the cumulative number of confirmed cases and the cumulative number of cross-infected cases with respect to .
| Vaccination coverage rate | Cumulative number of confirmed cases |
Cumulative number of cross-infected cases |
||||
|---|---|---|---|---|---|---|
| 0 | ||||||
| (−10.7%) | (−9.2%) | (−7.9%) | (−34.7%) | (−37.6%) | (−38.2%) | |
| (−21.0%) | (−18.1%) | (−15.6%) | (−65.6%) | (−68.4%) | (−69.0%) | |
| 601.2 | 283.0 | |||||
| (−30.8%) | (−26.8%) | (−23.0%) | (−92.0%) | (−92.8%) | (−92.9%) | |
Here is the estimated value of .
4. Discussions and conclusions
In the present mathematical modeling study, we have tested the hypothesis that a mass influenza vaccination campaign would have a positive effect on the management of people with non-specific symptoms and complaining of ILIs, potentially at risk of developing the COVID-19 (or other emerging respiratory infections) during their admission at the health-care setting. Our findings show that increasing influenza vaccination coverage rate to an optimal threshold would facilitate the efforts of containing the COVID-19 outbreak.
According to some researchers, the SARS-CoV-2 had already been circulating much earlier than late December 2019 but its correct identification was hindered by a considerable amount of people complaining of influenza-like-illness (ILI) symptoms. In the case of the implementation of a mass influenza vaccination campaign well before the onset of the influenza season, the exposure of emerging/re-emerging respiratory pathogens would be unmasked, facilitating their identification and the design of ad hoc public health interventions. This would significantly alleviate the pressure on health-care facilities, reducing the total number of people complaining of ILI symptoms, and decreasing the transmission probability of COVID-19 or other emerging infectious agents both among healthcare workers and, subsequently, among people under investigation for their disease.
Influenza generates a relevant burden worldwide, both in terms of healthcare resources consumption and socio-economic impact. Despite the existence of effective vaccines and their importance as cost-effective preventative tool, vaccination coverage rate still remains suboptimal. With respect to the general population, healthcare workers are at a higher risk of exposure to circulating respiratory pathogens, including influenza, potentially threatening their own health and compromising patients’ safety. Influenza vaccine uptake is suboptimal also among other targeted categories, including the elderly and disabled people. An inadequate disease risk perception, a low health literacy, perception of the societal effects of vaccination and alleged side-effects are among the drivers of vaccine hesitancy.
Influenza vaccination would enable to better control and contain the spread of COVID-19 or other emerging/re-emerging pathogens, in case of coincidence of the outbreak with the influenza season. Mass influenza vaccination prior to the onset of the peak influenza season would significantly decrease the number of ILIs among the general population and specifically the elderly, with fewer persons with ILIs seeking for medical advice, particularly those in high-risk settings, with frailty, underlying co-morbidities or disabled. As such, this would minimize the probability of not quickly and accurately identifying circulating respiratory pathogens as well as the possibility of ongoing nosocomial transmission.
However, how to increase influenza vaccination coverage rate remains challenging. Mandatory policies for targeted categories are under debate in several countries. Also, in this study, we did not consider explicitly the efficacy of the influenza vaccine, and the vaccination coverage should be an effective vaccination rate. However, we believe that this study provided a fundamental framework for quantitatively evaluating the impact of vaccination against influenza on mitigating the COVID-19 epidemics. Public health decision- and policy-makers should adopt evidence-informed strategies to improve influenza vaccine uptake, given its impact on respiratory pandemic outbreaks coinciding with the peak influenza season and the shortage of medical personnel and equipment, including diagnostic tests.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
YX was supported by the National Natural Science Foundation of China (NSFC, 11631012), JW was supported by the Canadian Institute of Health Research (CIHR) 2019 Novel Coronavirus (COVID-19) rapid research program .
References
- 1.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;14 doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395(10233):1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Q.Y., Zhao S., Gao D.Z., Wang W.M., Yang L., He D.H. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngonghala C.N., Iboi E., Eikenberry S., Scotch M., MacIntyre C.R., Bonds M.H., Gumel A.B. 2020. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel coronavirus. arXiv preprint arXiv:2004.07391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. 2020. Temperature, humidity and latitude analysis to predict potential spread and seasonality for COVID-19. Available at SSRN: https://ssrn.com/abstract=3550308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlagenhauf P. Influenza vaccine enlisted to prevent SARS confusion. Lancet. 2003;362(9386):809. doi: 10.1016/S0140-6736(03)14301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dini G., Toletone A., Sticchi L., Orsi A., Bragazzi N.L., Durando P. Influenza vaccination in healthcare workers: A comprehensive critical appraisal of the literature. Hum. Vaccin Immunother. 2018;14(3):772–789. doi: 10.1080/21645515.2017.1348442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas R.E., Lorenzetti D.L. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst. Rev. 2018;5 doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2020. National Health Commission of the People’s Republic of China. http://www.nhc.gov.cn/xcs/yqfkdt/gzbd_index.shtml. (Accessed 5 April 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang B., Wang X., Li Q., Bragazzi N.L., Tang S.Y., Xiao Y.N., Wu J.H. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J. Clin. Med. 2020;9(2):462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang B., Xia F., Tang S.Y., Bragazzi N.L., Li Q., Sun X.D., Liang J.H., Xiao Y.N., Wu J.H. The effectiveness of quarantine and isolation determine the trend of the COVID-19 epidemics in the final phase of the current outbreak in China. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine Association, The Chinese Preventive Medicine Association An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19) Chin. J. Epidemiol. 2020;41:139–144. doi: 10.3760/cma.j.issn.0254-6450.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Tang B., Bragazzi N.L., Li Q., Tang S.Y., Xiao Y.N., Wu J.H. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infect. Dis. Model. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubei Provincial People’s Government. http://hubei.gov.cn/zwgk/hbyw/hbywqb/202001/t20200123_2014361.shtml.
- 16.Zhang J.J., Litvinova M., Liang Y.X., Wang Y., Wang W., Zhao S.L., Wu Q.H., Merler S., Viboud C., Vespignani A., Ajelli M., Yu H.J. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020 doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beijing News. http://www.bjnews.com.cn/news/2020/01/24/679052.html.