Abstract
The infraorder Cephalobomorpha is a diverse and ecologically important nematode group found in almost all terrestrial environments. In a recent nematode classification system based on SSU rDNA, Cephalobomorpha was classified within the suborder Tylenchina with Panagrolaimomorpha, Tylenchomorpha and Drilonematomorpha. However, phylogenetic relationships among species within Tylenchina are not always consistent, and the phylogenetic position of Cephalobomorpha is still uncertain. In this study, in order to examine phylogenetic relationships of Cephalobomorpha with other nematode groups, we determined the complete mitochondrial genome sequence of Acrobeloides varius, the first sequenced representative of Cephalobomorpha, and used this sequence for phylogenetic analyses along with 101 other nematode species. Phylogenetic analyses using amino acid and nucleotide sequence data of 12 protein-coding genes strongly support a sister relationship between the two cephalobomorpha species A. varius and Acrobeles complexus (represented by a partial mt genome sequence). In this mitochondrial genome phylogeny, Cephalobomorpha was sister to all chromadorean species (excluding Plectus acuminatus of Plectida) and separated from Panagrolaimomorpha and Tylenchomorpha, rendering Tylenchina non-monophyletic. Mitochondrial gene order among Tylenchina species is not conserved, and gene clusters shared between A. varius and A. complexus are very limited. Results from phylogenetic analysis and gene order comparison confirms Tylenchina is not monophyletic. To better understand phylogenetic relationships among Tylenchina members, additional mitochondrial genome information is needed from underrepresented taxa representing Panagrolaimomorpha and Cephalobomorpha.
Keywords: Acrobeloides varius, Cephalobomorpha, Tylenchina, Mitochondrial genome, Phylogeny
Introduction
The suborder Tylenchina sensu De Ley & Blaxter (2002) represents one of the most morphologically and ecologically diverse arrays of nematodes and contains many different trophic types: microbivores, fungivores, and plant- and animal-parasitic forms (Baldwin, Ragsdale & Bumbarger, 2004; Bert et al., 2008; Siddiqi, 1980). In the SSU rDNA-based classification system for the phylum Nematoda, the suborder Tylenchina was initially classified to include four infraorders (Cephalobomorpha, Drilonematomorpha, Panagrolaimomorpha and Tylenchomorpha) (De Ley & Blaxter, 2002; De Ley & Blaxter, 2004).
Cephalobomorphs, one of the infraordinal groups in Tylenchina, are the dominant fauna together with other nematodes in nutrient-poor soil such as deserts and dry Antarctic valleys and may play important roles in shaping ecological systems there (Andriuzzi et al., 2018; De Tomasel et al., 2013; Freckman & Mankau, 1986; Freckman & Virginia, 1997; Lee et al., 2019; Sylvain et al., 2014). Additionally, this group constitutes a microbivorous or saprophagous clade that is found in diverse terrestrial environments including tropical rainforests and hot deserts (Nadler et al., 2006; Timm, 1971; Waceke et al., 2005). Historically, relationships between cephalobomorphs and other chromadorean nematodes inferred using morphology have produced considerably different hypotheses. On the basis of similarities in stoma and oesophagus features, cephalobomorphs were considered closely related to rhabditomorphs in many previous nematode taxonomies (Chitwood & Chitwood, 1977; Maggenti, 1981; Poinar, 1983). On the other hand, other authors proposed that cephalobomorphs had a close relationship with parasitic, fungivore and plant parasitic tylenchs (Siddiqi, 1980; Siddiqi, 2000), the entomopathogenic steinernematids (Poinar, 1993) and/or annelid-parasitic drilonematids (Coomans & Goodey, 1965; De Ley & Coomans, 1990; Spiridonov, Ivanova & Wilson, 2005).
In the first phylum-wide molecular phylogeny of nematodes using nuclear small subunit ribosomal DNA (SSU rDNA) sequences, cephalobs were placed in Clade IV with panagrolaimids, strongyloids, steinernematids and tylenchs (Blaxter et al., 1998). In a further revised nematode classification system (De Ley & Blaxter, 2004) following the SSU phylogeny, cephalobs were treated as the infraorder Cephalobomorpha and placed in the suborder Tylenchina, along with Drilonematomorpha, Panagrolaimomorpha and Tylenchomorpha. These relationships were supported in subsequent phylogenetic analyses using SSU rDNA data with more extensive taxon sampling (Holterman et al., 2006; Van Megen et al., 2009). However, relationships among subordinate taxa within Tylenchina were not always consistent, depending on the size of the datasets analyzed (Holterman et al., 2006; Van Megen et al., 2009). Thus, alternative genetic markers are needed to elucidate the relationships that SSU rDNA data are not sufficient to resolve.
Complete mitochondrial genomes have been used for phylogenetic analyses of diverse animal groups and are a powerful molecular marker for resolving not only deep phylogeny but also relationships between groups with relatively recent common ancestry (Hegedusova et al., 2014; Hu & Gasser, 2006; Littlewood et al., 2006; Liu et al., 2013; Park et al., 2007; Waeschenbach et al., 2006). In nematode phylogenetic analyses based on complete mitochondrial genome sequences, inferred relationships have been mostly similar to SSU rRNA or morphology-based phylogenies. However, a mitochondrial genome approach has provided alternative hypotheses that differed from nuclear gene phylogeny in some cases, such as non-monophyly of clade III (sensu Blaxter et al., 1998), non-monophyly of Tylenchomorpha, and strong support for a clade consisting of the infraorders Rhabditomorpha, Diplogasteromorpha, Ascaridomorpha and Rhigonematomorpha (Kim et al., 2017; Kim et al., 2014).
Although available mt genome information for nematodes has been increasing in recent years, some nematode groups, including free-living microbivores and insect-associated taxa, are still underrepresented. In fact, among the 200 currently available nematode mt genomes (in GenBank as of December 2019), no representatives from Cephalobomorpha are yet available: only a partial fragment (contains cox1-cox3, nad2-nad6, nad4l and 15 tRNA genes) of the Acrobeles complexus mt genome (see Kim et al., 2015 for details). In this study, we determined the complete mitochondrial genome sequence of Acrobeloides varius, the first full representative of the infraorder Cephalobomorpha, compared its genome structure and organization to other nematodes, and used it to infer phylogenetic relationships among chromadorean nematodes.
Material and Methods
Sampling, culturing and identification of specimens
Specimens were obtained from soil collected from agricultural (rice) farmland in Hapcheon-gun, Gyeongsangnam Province, South Korea. Species identification was performed by a careful examination of morphological characters and morphometric analysis following the protocols of a recently published study (Kim, Kim & Park, 2017). For culturing, one individual nematode was transferred onto a soil agar plate (25 mg/ml autoclaved soil, 5 ug/ml cholesterol and 1% agar) at room temperature. Specimens were subsequently selected from the culture plate; roughly half of all selected specimens were used for species identification (see Kim, Kim & Park, 2017); the other half were preserved in absolute ethanol and stored at −80 °C until total genomic DNA was extracted. To reconfirm species identification, cox1 sequences from each individual were compared with cox1 sequences of the specimens published in Kim, Kim & Park (2017).
Molecular techniques
Total genomic DNA was extracted from about 10 individuals using a lysis buffer containing 0.2 M NaCl, 0.2 M Tris-HCL (pH 8.0), 1% (v/v) β-mercaptoethanol and 800 µg/ml proteinase-K (Holterman et al., 2006). Four partial fragments were initially amplified by polymerase chain reaction (PCR) for four different genes (cox1, cob, rrnL and nad5) using nematode-specific primer sets (Cepha_CO1_F/Cepha_CO1_R for cox1, Chroma_Cob_F/Chroma_Cob_R for cob, Nema_16S_F/Nema_16S_R for rrnL and Nema_ND5_F/Nema_ND5_R for nad5) designed from conserved regions of other nematode mitochondrial gene sequences available on GenBank (Table 1). PCR amplification was carried out in total 50 µL-volume reactions consisting of 0.2 mM dNTP mixture, 1X Ex Taq buffer containing MgCl2, 10 pmol primers (for both forward and reverse primers), 1.25 U Taq polymerase (TaKaRa Ex Taq), and 2 µL template with the following amplification conditions: one cycle of initial denaturing at 95 °C for 1 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 47 °C for 30 s, extension at 72 °C for 1 min, and one cycle of final extension at 72 °C for 10 min. The nucleotide sequences obtained from respective partial DNA fragments were then used to design species-specific primer sets (Table 1) for long PCR amplification. PCR reactions were carried out in 50 µL total volumes consisting of 0.4 mM dNTP mixture, 1X LA Taq buffer, 2.5 mM MgCl2, 10 pmol primers (both forward and reverse primers), 2.5 U Taq polymerase (TaKaRa LA Taq), and 2 µL template with the following amplification conditions: one cycle of initial denaturing at 95 °C for 1 min, followed by 35 cycles of denaturation at 95 ° C for 30 s, annealing and extension at 55 °C to 70 °C for 2 min to 10 min, and one cycle of final extension at 68 °C for 10 min. The amplified PCR products were isolated on 1% agarose gels using a QIAquick Gel Extraction Kit (QIAGEN Co.) following standard protocols. Two long-PCR products (Acva_16S_F/Acva_ND5_R and Acva_ND5_F/Acva_Cob_R) were used directly for primer walking sequencing. The other long-PCR products (Acva_CO1_F/Acva_16S_R and Acva_Cob_F/Acva_CO1_R) were ligated into a pCR-XL-TOPO vector using a TOPO XL PCR Cloning Kit (Invitrogen Co.) and then transformed into One Shot TOP10 Electrocompetent E. coli using electroporation (1.8 kV). Plasmid DNAs were extracted from competent cells using a QIAprep Spin Miniprep Kit (QIAGEN Co.) following standard protocols. Sequences of the PCR-amplified and/or cloned DNA fragments were determined using a Big Dye Terminator Cycle-Sequencing kit and ABI PRISM 3730XL Analyzer (Applied Biosystem Co.). A complete strand of the entire mitochondrial DNA sequence was then assembled by reconfirming the sequences of the overlapping regions of the long PCR fragments and partial fragments.
Table 1. Primers used to sequence the complete mitochondrial genome of Acrobeloides varius.
| Primers | Sequence (5′→3′) | Estimated size of PCR products |
|---|---|---|
| Cepha_CO1_F | ATGATTTTTTTTATGGTGATGCC | 700 bp |
| Cepha_CO1_R | ACTACAAAATATGTGTCATG | |
| Nema_16S_F | WWTAAATGGCAGYCTTAGCGTGA | 450 bp |
| Nema_16S_R | TCTYMCRAYGAAYTAAACTAATATC | |
| Nema_ND5_F | GTTCATAGAAGTACTTTGGTKACTGCTG | 500 bp |
| Nema_ND5_R | AAGACGMWAACWATAAMHAAAAGT | |
| Chroma_Cob_F | CARATRWSNTWTTGRGC | 370 bp |
| Chroma_Cob_R | TAYCAYTCNGGNACAAYATG | |
| Acva_CO1_F | GAGCGCATCATATGTATGTAACTGG | 6 kb |
| Acva_16S_R | CTACCCAAGGCGTCGTGTTCATCAC | |
| Acva _16S_F | CTTAGCGTGCGGACTTTAATGTAGC | 2 kb |
| Acva _ND5_R | GAACCCACAGAATTTCACTTGCGTC | |
| Acva _ND5_F | CATGTGGGTTCTAATCAACAAAATTGACG | 2.1 kb |
| Acva _Cob_R | ACACCTCTGTGAACGTAAAGCACAC | |
| Acva _Cob_F | GGTTATTAATGATAATCCTTATTATAGGTGC | 8.5 kb |
| Acva _CO1_R | GATCTACAGAAGCTCCATAATGAC |
Gene annotation and phylogenetic analyses
The 12 protein-coding genes (PCGs) and two ribosomal RNA genes were identified using the web-based automatic annotation programs DOGMA (http://dogma.ccbb.utexas.edu) (Wyman, Jansen & Boore, 2004) and MITOS (http://mitos.bioinf.uni-leipzig.de/index.py) (Bernt et al., 2013). The boundaries of each gene were confirmed by comparing nucleotide sequences with those from closely related nematodes. Putative secondary structures of 24 tRNAs were inferred using tRNAscan-SE Search Server v.1.21 (Lowe & Eddy, 1997), MITOS (Bernt et al., 2013) and DOGMA (Wyman, Jansen & Boore, 2004), and tRNA secondary structures and anticodon sequences were then checked manually.
For phylogenetic analysis, a representative nematode species was chosen from each genus for which a complete mitochondrial genome was available from GenBank (Table S1). Nucleotide and amino acid sequences of the 12 PCGs of 102 nematode species including A. varius and two arthropod species (outgroups) were concatenated for the analyses. In the nucleotide (NT) dataset, two datasets (3rd codon positions included and excluded were prepared for the analysis to examine whether tree topology is affected by nucleotide substitution saturation, which is most likely to occur in the third codon position. Multiple alignments of amino acid sequences of 12 PCGs were done individually using MAFFT with default options (Katoh & Standley, 2013). Nucleotide sequences of the 12 individual PCGs were also aligned with a scaffold of aligned amino acid sequences using RevTrans (Wernersson & Pedersen, 2003).
For phylogenetic analysis, individual amino acid and nucleotide alignments of 12 PCGs were both concatenated using Geneious Pro 10.2.3 (Biomatters Co.). The phylogenetic analyses utilizing each concatenated sequence dataset (amino acid (AA) sequences (4,532 AA residues) and nucleotide (NT) sequences with third codon position included (13,596 bp) or excluded (9,064 bp)) were performed using maximum likelihood (ML) with RAxML 8.2.10 (Stamatakis, 2014) and using Bayesian inference (BI) with MrBayes version 3.2.6 (Huelsenbeck & Ronquist, 2001); both programs were run through the CIPRES portal (Miller, Pfeiffer & Schwartz, 2010). The AA and NT sequence alignments were deposited in TreeBASE (https://www.treebase.org (ID number: 25588)). For phylogenetic analyses, the best-fit substitution model for each amino acid sequence dataset was estimated using the Akaike Information Criterion (AIC) as implemented in ProtTest 3.4.2 (Darriba et al., 2011) (Table S2). Substitution models for the two types of nucleotide sequence datasets (including versus excluding 3rd codon positions) were estimated separately for each gene using the AIC as implemented in jModelTest (Darriba et al., 2012). For the ML analysis, each of the 12 PCGs was treated as a separate partition and the best-fit substitution model (JTT, LG, MtArt or Vt) for the amino acid dataset was applied (Table S2). Similarly, for the ML analysis of the two nucleotide sequence datasets, each of the 12 PCGs was also treated as a separate, unlinked data partition; for nucleotide datasets RAxML uses the GTR GAMMA model for finding the optimal tree, with the shape of the gamma distribution estimated separately for each partition. Bootstrap maximum likelihood analysis was performed using the rapid bootstrapping option with 1000 iterations. For the BI analysis, the LG+I+G model of substitution (the closest fit model available for use in CIPRES) was used for the amino acid dataset, and the best-fit models for each of the 12 PCGs were used for the two nucleotide datasets (Table S2). Bayesian analysis was performed using four MCMC chains for 1 × 106 generations, sampled every 100 generations. Bayesian posterior probability (BPP) values were estimated after discarding the initial 25 × 104 generations as burn-in.
Results and Discussion
The mitochondrial genome of A. varius
The complete mitochondrial genome of A. varius (GenBank accession no. MK559448) is composed of 17,650 bp and contains 12 PCGs (atp6, cob, cox1-3, nad1-6 and nad4L), 22 tRNA genes and 2 rRNA genes (Fig. 1 and Table 2). The overall nucleotide composition of the A. varius mitochondrial DNA is 45.7% T, 8.8% C, 30.9% A, and 14.6% G, and the overall A+T content is 76.6% (Table S3). The overall A+T content is similar to that of other chromadorean nematodes, which ranges from 67.0% (Ascaridia sp. GHL-2013 (Liu et al., 2013) to 85.4% (Radopholus similis (Jacob et al., 2009)). In the 12 protein-coding mtDNA genes (PCGs), the most commonly used start codon is ATT, which is used for five genes (atp6, cob, nad1, nad4 and nad6) (Table 2). Two genes (cox2 and nad3) start with ATA, whereas nad2 and nad4l are inferred to initiate with ATG and cox1 with GTG, while cox3 starts with TTG. The gene nad5 uses TTT as the start codon. For termination codons, eight genes (cob, cox2, cox3, nad2, nad3, nad4l, nad5 and nad6) appear to use TAA and four genes (atp6, cox1, nad1 and nad4) use TAG. The codon usage of A. varius mtDNA is biased towards T-rich codons, similar to other nematode species. The four most frequently used codons are all T-rich (more than 2 Ts per triplet): TTT (10.6%), TTA (11.2%), ATT (7.5%) and TAT (5.4%), accounting for 34.7% of all PCGs (Table S4). In contrast, the frequency of C-rich codons (more than 2 Cs per triplet) is very low (2.9%).
Figure 1. Representation of circular mitochondrial genome of Acrobeloides varius.
All genes are encoded in the clockwise direction and 22 tRNA genes are marked by a single-letter amino acid code, based on the International Union of Pure and Applied Chemistry (IUPAC) code. The two leucine and two serine tRNA genes are labeled according to their individual anticodon sequence, as L1 (trnL1-uag), L2 (trnL2-uaa), S1 (trnS1-ucu), and S2 (trnS2-uga), respectively.
Table 2. Mitochondrial genome organization of Acrobeloides varius.
| Gene/ region | Position | Size | Codons | Intergenic sequence | |||
|---|---|---|---|---|---|---|---|
| Start | Finish | No. of nta | No. of aaa | Initiation | Termination | ||
| cox1 | 1 | 1,575 | 1,575 | 524 | GTG | TAG | 48 |
| trnG | 1,624 | 1,680 | 57 | 0 | |||
| trnL1 | 1,681 | 1,738 | 58 | 2 | |||
| nad1 | 1,741 | 2,613 | 873 | 290 | ATT | TAG | −1 |
| trnY | 2,613 | 2,668 | 56 | 9 | |||
| atp6 | 2,678 | 3,238 | 561 | 186 | ATT | TAG | 11 |
| trnV | 3,250 | 3,305 | 56 | 3 | |||
| trnL2 | 3,309 | 3,365 | 57 | 32 | |||
| trnT | 3,398 | 34,58 | 61 | 22 | |||
| nad4 | 3,481 | 4,671 | 1,191 | 396 | ATT | TAG | 49 |
| nad2 | 4,721 | 5,545 | 825 | 274 | ATG | TAA | 0 |
| trnI | 5,546 | 5,608 | 63 | 13 | |||
| trnH | 5,622 | 5,676 | 55 | 0 | |||
| rrnL | 5,677 | 6,859 | 1,183 | 0 | |||
| nad3 | 6,860 | 7,204 | 345 | 114 | ATA | TAA | 18 |
| trnC | 7,223 | 7,280 | 58 | 17 | |||
| trnA | 7,298 | 7,353 | 56 | 4 | |||
| nad5 | 7,358 | 8,950 | 1,593 | 530 | TTT | TAA | 12 |
| nad6 | 8,963 | 9,400 | 438 | 145 | ATT | TAA | −1 |
| nad4l | 9,400 | 9,633 | 234 | 77 | ATG | TAA | 7 |
| trnW | 9,641 | 9,699 | 59 | 47 | |||
| trnS1 | 9,747 | 9,804 | 58 | 1 | |||
| cob | 9,806 | 10,900 | 1,095 | 364 | ATT | TAA | 40 |
| trnN | 10,941 | 10,997 | 57 | 10 | |||
| trnF | 11,008 | 11,067 | 60 | 3 | |||
| cox3 | 11,071 | 11,844 | 774 | 257 | TTG | TAA | 0 |
| NCR1 | 11,845 | 12,408 | 564 | 0 | |||
| trnE | 12,409 | 12,466 | 58 | 61 | |||
| trnE | 12,528 | 12,589 | 62 | 12 | |||
| trnK | 12,602 | 12,658 | 57 | 0 | |||
| NCR2 | 12,659 | 13,906 | 1,248 | 0 | |||
| trnD | 13,907 | 13,963 | 57 | 124 | |||
| trnS2 | 14,088 | 14,144 | 57 | 28 | |||
| trnQ | 14,173 | 14,231 | 59 | 11 | |||
| trnP | 14,243 | 14,303 | 61 | 5 | |||
| trnM | 14,309 | 14,371 | 63 | 0 | |||
| NCR3 | 14,372 | 15,820 | 1,449 | 0 | |||
| rrnS | 15,821 | 16,727 | 907 | 0 | |||
| cox2 | 16,728 | 17,429 | 702 | 233 | ATA | TAA | 2 |
| trnR | 17,432 | 17,487 | 56 | 58 | |||
| trnK | 17,546 | 17,608 | 63 | 42 | |||
Notes.
Stop codons were not included.
- nt
- nucleotide
- aa
- amino acid
The tRNA predicted secondary structures, ranging from 55 bp (trnH) to 63 bp (trnI, trnK and trnM) in size, are similar to those found in other nematode species (Fig. S1 and Table 2; See Kim et al., 2017 for details): 22 tRNAs lack a TΨC arm which is instead replaced by a TV-replacement loop, whereas trnS1 and trnS2 lack a dihydrouridine arm (DHU) and instead have a TΨC structure. Note that tRNAs encoding trnE and trnK are inferred to be duplicated: two nucleotide sequence regions, with respective sizes of 58 bp (trnE-1) and 62 bp (trnE-2), are inferred for trnE (Fig. S1 and Table 2). In addition, two trnK gene copies (consisting of 57 bp (trnK-1) and 63 bp (trnK-2)) are inferred. These duplicated tRNA copies are assumed to be functional, since they are predicted to form typical nematode tRNA secondary structures (Fig. S1). Duplication of tRNA genes has been occasionally reported from other nematode mitochondrial genomes such as Camallanus cotti (Zou et al., 2017), Dracunculus medinensis (Genbank accession no. NC_016019, Clark et al., 2016) and Ruizia karukerae (Kim et al., 2018).
The small ribosomal RNA (rrnS) is located between NCR3 and cox2, with a length of 907 bp, and the large ribosomal RNA (rrnL) is located between trnH and nad3, with a length of 1,183 bp (Table 2). These two rRNA genes are among the largest in nematode mtDNA thus far reported. The predicted secondary structures of rrnS and rrnL of A. varius (Figs. S2 and S3) were inferred based on secondary structures of the rRNAs of other nematodes (Caenorhabditids elegans and Ascaris suum (Okimoto et al., 1992) and Steinernema carpocapsae (Montiel et al., 2006)), the 16S and 23S rRNAs of Escherichia coli (Dams et al., 1988; Gutell, 1994; Gutell, Gray & Schnare, 1993) and the LSU rRNA of Tetrahymena pyriformis (De Rijk et al., 1999). The secondary structures of the two rRNAs are generally conserved in nematode mitochondrial genomes, but some elements differ among nematode species. For example, helices 5 and 16 are absent from rrnS in Dirofilaria immitis and Strongyloides stercoralis (Hu, Chilton & Gasser, 2003; Hu et al., 2003), and helices C1, D6-10, D21, H3 and H4 are absent from rrnL in Xiphinema americanum (He et al., 2005). The predicted secondary structures of both rRNAs in the A. varius mt genome are similar to those reported from other nematodes such as C. elegans and A. suum. However, helices 35 and 39 and helices G9-G14 are predicted in rrnS and rrnL, respectively (Figs. S2 and S3). These elements are found in the 16S and 23S rRNAs of E. coli, but have not yet been reported from any other nematode species.
Three non-coding regions (NCRs) (ranging from 564 bp to 1,449 bp in size) are present in the A. varius mt genome, with high A+T content ranging from 80.5% (NCR1) to 83.7% (NCR3) (Fig. 1 and Table 2). Stem-and-loop structures formed by tandemly repeated sequences are found in all NCRs (Fig. S4).
Mitochondrial genome phylogeny
Phylogenetic analyses involved two tree-building methods (ML and BI) for three different datasets (amino acid (AA) sequences, and nucleotide (NT) sequences with third codon positions either included or excluded) for all 12 PCGs from 102 nematode species. In all phylogenetic analyses, the respective monophyly of chromadorean and enoplean nematodes was strongly supported (Fig. 2 and Figs. S5–S9). Although supporting values among some chromadorean infraordinal groups were low, and some relationships varied depending on tree building methods and datasets, many elements of previously published mitogenome phylogenies were robustly supported: the non-monophyly of the suborder Spirurina, non-monophyly of Tylenchomorpha, and the nested position of Diplogasteromorpha within Rhabditomorpha (see Kim et al., 2017 for more details).
Figure 2. Maximum likelihood (ML) tree based on the amino acid sequences of 12 protein-coding genes from the mitochondrial genomes of 102 nematodes and two outgroups.
Bootstrap percentages (BP) were calculated using the rapid bootstrapping method. BP values <70% are not shown.
In this study, mitochondrial genome trees depicted the monophyly of two cephalobs (A. varius and A. complexus) with high branch support value (1.00 Bayesian posterior probability (BPP) in the BI tree and 100% bootstrap percentage (BP) in the ML tree) in all phylogenetic analyses (Fig. 2 and Figs. S5–S9). Cephalobomorpha represented by the two cephalob species (one partial and one complete sequence) was found to be basal to all chromadorean species (excluding Plectus acuminatus of Plectida) and separated from Panagrolaimomorpha and Tylenchomorpha, rendering Tylenchina non-monophyletic. In addition, we recently recognized that the Panagrellus redivivus mitochondrial genome sequence (GenBank accession #: KM192364) published in an earlier article (Kim et al., 2015) was incorrect, possibly due to PCR contamination with a different nematode species. In order to check its misidentification, we compared the mtDNA cox1 and nuclear 28S rDNA sequences of the P. redivivus specimens used in Kim et al. (2015) with previously published and/or unpublished sequences of some Tylenchina species: the 28S rDNA sequence of the P. redivivus specimen of Kim et al. (2015) is very different (77.3% sequence identity) from the P. redivivus sequence (GenBank accession #: DQ145695; Nadler et al., 2006), but it is very similar to A. complexus (GenBank accession #: DQ145668; Nadler et al., 2006) (95.5% sequence identity) and A. varius sequence (99.5% sequence identity; GenBank accession #: MT233103 (this study)). The mtDNA cox1 sequences between and A. varius (this study) and the P. redivivus specimens of Kim et al. (2015) differed by 9.6%. Based on this sequence comparison, the P. redivivus specimen reported in Kim et al. (2015) is assumed to be some Cephalobidae species, likely to be close to Acrobeloides species. Therefore, the mitochondrial genome sequence labelled as P. redivivus (sensu Kim et al., 2015) was retracted from Genbank and we replaced it with other P. redivivus sequence that is newly available on GenBank (GenBank accession no. AP017464.1; Clark et al., 2016). Unlike the non-sister relationship between P. redivivus and Halicephalobus gingivalis representing one of the Panagrolaimomorpha families (i.e., Panagrolaimidae) depicted from Kim et al. (2015), phylogenetic trees grouped the new P. redivivus sequence with H. gingivalis as a sister species (Fig. 2 and Figs. S5–S9). These relationships received high support values (1.00 BPP in BI tree and 93–100% BP in the ML tree). In previous phylogenetic studies, relationships among major groups within Tylenchina, including the position of Cephalobomorpha within Tylenchina, varied depending on the data source (i.e., morphology vs. molecular data) and taxon sampling used for phylogenetic inference. Traditional morphology-based hypotheses depicted a close relationship between cephalobomorphs and rhabditomorphs based on similarities in stoma and oesophagus features (Chitwood & Chitwood, 1977; Maggenti, 1981; Poinar, 1983). On the other hand, cephalobomorphs were inferred to be closely allied to tylenchs based on some morphological characters (Siddiqi, 1980; Siddiqi, 2000): a thickened internal cuticle with irregular basal bulb lumen of some tylenchomorphs was considered as a degenerate form of the valve in the basal bulb of cephalobomorphs; a common origin of the inverted U-shaped excretory system of cephalobomorphs and the asymmetrical system of tylenchomorphs; morphological similarity between phasmid-like structure in Tylenchidae species and hypodermal pores of some Nothacrobeles species; similarity of cuticle and lateral field in their structure; prodelphic reproductive system; reduction of papillae on the male tail. Moreover, in previous analyses of SSU rDNA, relationships among cephalobomorphs and other Tylenchina nematodes varied depending on dataset size (Blaxter et al., 1998; Holterman et al., 2006; Van Megen et al., 2009). The first phylum-wide nematode molecular phylogenies (Blaxter et al., 1998; Holterman et al., 2006) depicted Cephalobomorpha as sister to a Tylenchoidea + Aphelenchidae clade. In contrast, phylogenetic analysis with extended taxon coverage (van Megen et al., 2009) depicted Cephalobomorpha was sister to all Tylenchina species except Steinernematidae (Strongyloidoidea). Inconsistency in relationships among the groups within Tylenchina was interpreted as an artifact of long-branch attraction due to the high A+T contents of some aphelenchoids (Aphelenchoididae, Parasitaphelenchidae and Seinuridae) and the Panagrolaimomorpha (ca 54% and 57%, respectively; Holterman et al., 2006) (Holterman et al., 2006; Van Megen et al., 2009). Meanwhile, a molecular phylogenetic study of the suborder Cephalobina sensu (Andrássy, 1974) using LSU rDNA data (Nadler et al., 2006) depicted different relationships depending on analysis methods: a parsimonious tree showed a sister relationship between cephalobs and tylenchs, whereas an ML tree depicted a sister relationship between cephalobs and Panagrolaimidae (excluding Brevibucca sp.). Minimum evolution analysis found chambersiellids (infraorder Panagrolaimomorpha) to be the sister group of cephalobs. Such inconsistent relationships from different methods were assumed due to nucleotide compositional bias and/or long-branch attraction in LSU phylogeny (Nadler et al., 2006); thus, these relationships have needed validation using independent molecular markers and broader taxon sampling.
In the present study, mitochondrial genome trees from different methods strongly supported a paraphyletic relationship between Cephalobomorpha and Tylenchoidea of Tylenchomorpha, separate from Panagrolaimomorpha and Aphelenchoidea (another branch of Tylenchomorpha), rendering Tylenchina non-monophyletic. This result is not consistent with earlier studies including molecular phylogenies (SSU rDNA; Blaxter et al., 1998; Holterman et al., 2006) and morphology-based phylogenies (Siddiqi, 1980; Siddiqi, 2000) that supported close relationship between cephalobomorphs and tylenchs. The non-monophyly of Tylenchina inferred from mt genome trees contradicts previous SSU rDNA phylogenies that supported monophyly of Tylenchina members (Blaxter et al., 1998; Holterman et al., 2006; Van Megen et al., 2009). Although the present study strongly indicated non-monophyly of Tylenchina, relationships within Tylenchina are not fully resolved due to the instability of some non-monophyletic groups including Tylenchomorpha and Panagrolaimomorpha. Phylogenetic algorithms are not able to easily handle compositional heterogeneity across lineages, which may exist in both rDNA and mitochondrial genome markers (Liu et al., 2018; Yang et al., 2018). This factor limits the potential implications of phylogenetic estimation using mitochondrial genomes. However, it has been shown that mitochondrial genome composition across chromadorean species is not very heterogeneous (Kim et al., 2014). For better resolution of the phylogenetic relationships among Cephalobomorpha, Tylenchomorpha, and Panagrolaimomorpha, additional mitochondrial genome sequences from underrepresented taxa and/or further phylogenetic analyses using additional genetic markers are needed.
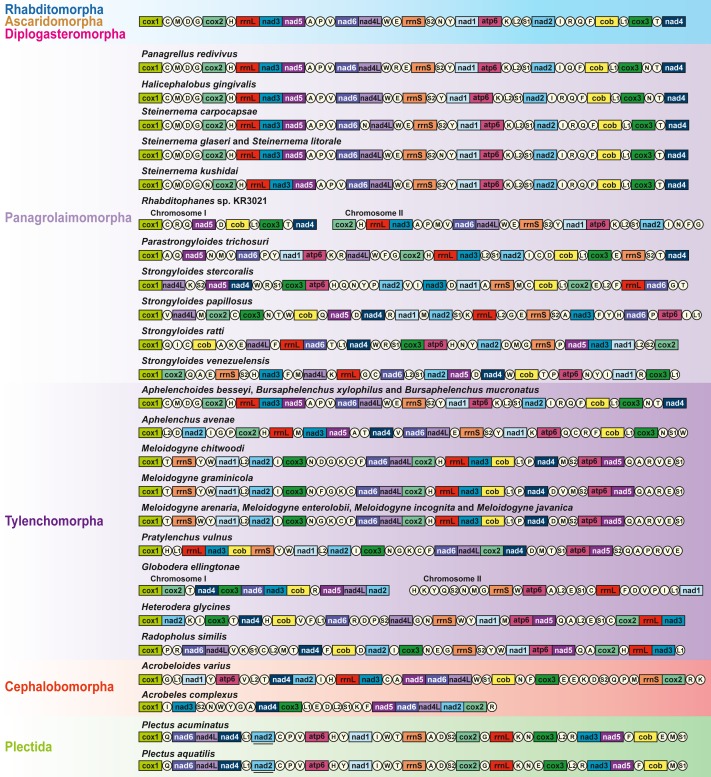
Comparison of mitochondrial gene arrangement
Compared to enoplean species, the mitochondrial gene order of chromadorean nematodes is relatively conserved, and gene order similarity among certain groups has been interpreted as additional supporting evidence for their phylogenetic affinity, although an idiosyncratic pattern has also been reported in some species (Kim et al., 2014; Kim et al., 2017). Among nematode mt genomes reported thus far, gene arrangement patterns in most members of Rhabditomorpha (70 of 72 species), Ascaridomorpha (24 of 28 species), Diplogasteromorpha (one of two species), Aphelenchoididae (all three species except for trnN) and Steinernema (2 out of 4 species) are identical except for some idiosyncratic gene arrangements and a few minor translocations of tRNAs in some species (Jex et al., 2010; Kim et al., 2018; Liu et al., 2013; Montiel et al., 2006; Wang et al., 2016).
A sister relationship between A. varius and A. complexus is recovered in phylogenetic analyses, but gene clusters shared between these two cephalobomorpha species (although the mtDNA of A. complexus is a partial sequence) are very limited: nad5+nad6+nad4l and cox2+trnR. The gene arrangement of A. varius is also very different from other Tylenchomorpha (Tylenchina) species, with very limited shared gene clusters (Fig. 3): nad2+trnI, trnH+rrnL+nad3 and nad6+nad4l are shared with Meloidogyne spp. (Besnard et al., 2014; Humphreys-Pereira & Elling, 2014; Humphreys-Pereira & Elling, 2015), nad2+trnI, rrnL+nad3, nad6+nad4l and trnS2+trnQ with Pratylenchus vulnus (Sultana et al., 2013), trnL1+nad1 and trnT+nad4 with Globodera ellingtonae (Phillips et al., 2016), trnT+nad4 and rrnL+nad3 with Heterodera glycines (Gibson et al., 2011) and nad6+nad4l, trnT+nad4, nad2+trnI and trnH+rrnL+nad3 with R. similis (Jacob et al., 2009). Shared gene clusters between A. varius and Panagrolaimomorpha species are also very limited: trnT+nad4, trnH+rrnL+nad3, trnP+trnM, nad6+nad4L+trnW, nad2+trnI and trnN+trnF are shared with Rhabditophanes sp. KR3021 (Hunt et al., 2016), nad4L+trnW, trnH+rrnL+nad3, nad2+trnI, cox3+trnE and trnT+nad4 are shared with Parastrongyloides trichosuri (Hunt et al., 2016), trnH+rrnL+nad3, nad4L+trnW, nad2+trnI and trnT+nad4 are shared with S. carpocapsae (Montiel et al., 2006) and trnH+rrnL+nad3, nad6+nad4L+trnW, nad2+trnI and trnT+nad4 are shared with S. glaseri, S. litorale, S. kushidai (Kikuchi, Afrin & Yoshida, 2016), H. gingivalis (Kim et al., 2015) and P. redivivus (GenBank accession no. AP017464.1; Clark et al., 2016). Although gene arrangement of Tylenchina species is not conserved, the most common gene arrangement pattern of Rhabditomorpha, Ascaridomorpha and Diplogasteromorpha is also found in some Tylenchina species (Fig. 3): Aphelenchoides besseyi, Bursaphelenchus xylophilus, B. muronatus (Tylenchomorpha), P. redivivus, H. gingivalis and four Steinernema species (Panagrolaimomorpha), where gene order is almost identical and/or very similar to the most common gene arrangement type. Further mitochondrial genome survey of gene order information is needed to understand whether the gene order data can be useful to estimate phylogenetic relationships among Tylenchina species.
Figure 3. Linearized comparison of the gene arrangement of chromadorean nematode species including Acrobeloides varius, the first sequenced representative of Cephalobomorpha.
All genes are encoded in the same direction and are not to scale. 22 tRNA genes are designated by a single letter amino acid code, based on the International Union of Pure and Applied Chemistry code. The two leucine and two serine tRNA genes are labeled according to their distinct anticodon sequences as L1 (trnL1-uag), L2 (trnL2-uaa), S1 (trnS1-ucu), and S2 (trnS2-uga). The non-coding regions are not included. Genes underlined in black (e.g., nad2 in Plectus species) indicate the genes that are transcribed in a reverse direction. Bipartite mitochondrial genomes of Rhabditophanes sp. KR3021 and Globodera ellingtonae are presented as two split gene clusters, respectively.
In conclusion, phylogenetic trees based on mitochondrial genome sequences indicate non-monophyly of Tylenchina (and a basal position of Cephalobomorpha to all chromadorean species excluding Plectus, separated from other Tylenchina members Panagrolaimomorpha and Tylenchomorpha). Nevertheless, phylogenetic relationships among Tylenchina nematodes are not clearly established due to inconsistent placement of some taxa including panagrolaimomorphs and aphelenchids. Along with employing independent molecular markers, further mitochondrial genome information from the underrepresented taxa of Panagrolaimomorpha and Cephalobomorpha needs to be obtained to clarify unresolved relationships within Tylenchina.
Supplemental Information
Watson-Crick base pairings are denoted by lines and G-U pairs are indicated by dots. The numbers identify the conserved secondary structure elements defined by Dams et al. (1988).
Watson-Crick base pairings are denoted by lines and G-U pairs are indicated by dots. The numbers identify the conserved secondary structure elements defined by De Rijk et al. (1999).
Bootstrap percentages (BP) were calculated using the rapid bootstrapping method. BP values <70% are not shown.
Bootstrap percentages (BP) were calculated using the rapid bootstrapping method. BP values <70% are not shown.
Bayesian posterior probabilities (BPP) were estimated after discarding the initial 250 trees (the first 25 × 104 generations) as burn-in. BPP values <0.7 are not shown.
Bayesian posterior probabilities (BPP) were estimated after discarding the initial 250 trees (the first 25 × 104 generations) as burn-in. BPP values <0.7 are not shown.
Bayesian posterior probabilities (BPP) were estimated after discarding the initial 250 trees (the first 25 × 104 generations) as burn-in. BPP values <0.7 are not shown.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2005898), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1I1A1A01041191), the Marine Biotechnology Program of the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) (No. 20170431), and the National Institute of Biological Resources (NIBR) funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202002109). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Taeho Kim conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yucheol Lee analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Hyun-Jong Kil analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Joong-Ki Park conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
References
- Andrássy (1974).Andrássy I. A nematodák evolúciója és rendszerezése. A Magyar Tudomanyos Akadémia Biológiai Tudományok Osztályának közleményei. 1974;17:13–58. [Google Scholar]
- Andriuzzi et al. (2018).Andriuzzi WS, Adams BJ, Barrett JE, Virginia RA, Wall DH. Observed trends of soil fauna in the Antarctic Dry Valleys: early signs of shifts predicted under climate change. Ecology. 2018;99:312–321. doi: 10.1002/ecy.2090. [DOI] [PubMed] [Google Scholar]
- Baldwin, Ragsdale & Bumbarger (2004).Baldwin JG, Ragsdale EJ, Bumbarger D. Revised hypotheses for phylogenetic homology of the stomatostylet in tylenchid nematodes. Nematology. 2004;6:623–632. doi: 10.1163/1568541042843559. [DOI] [Google Scholar]
- Bernt et al. (2013).Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution. 2013;69:313–319. doi: 10.1016/j.ympev.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Bert et al. (2008).Bert W, Leliaert F, Vierstraete AR, Vanfleteren JR, Borgonie G. Molecular phylogeny of the Tylenchina and evolution of the female gonoduct (Nematoda: Rhabditida) Molecular Phylogenetics and Evolution. 2008;48:728–744. doi: 10.1016/j.ympev.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Besnard et al. (2014).Besnard G, Jühling F, Chapuis É, Zedane L, Lhuillier É, Mateille T, Bellafiore S. Fast assembly of the mitochondrial genome of a plant parasitic nematode (Meloidogyne graminicola) using next generation sequencing. Comptes Rendus Biologies. 2014;337:295–301. doi: 10.1016/j.crvi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Blaxter et al. (1998).Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Chitwood & Chitwood (1977).Chitwood BG, Chitwood MB. Introduction to nematology. University Park Press; Baltimore: 1977. [Google Scholar]
- Clark et al. (2016).Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Research. 2016;44:D67–D72. doi: 10.1093/nar/gkv1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans & Goodey (1965).Coomans A, Goodey JB. Drilocephalobus congoensis n.g., n. sp. Nematologica. 1965;11:116–120. doi: 10.1163/187529265X00546. [DOI] [Google Scholar]
- Dams et al. (1988).Dams E, Hendriks L, Van de Peer Y, Neefs J-M, Smits G, Vandenbempt I, De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Research. 1988;16:87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba et al. (2011).Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba et al. (2012).Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley & Blaxter (2002).De Ley P, Blaxter ML. Systematic position and phylogeny. In: Lee DL, editor. The Biology of Nematodes. Taylor & Francis; New York: 2002. pp. 1–30. [Google Scholar]
- De Ley & Blaxter (2004).De Ley P, Blaxter ML. A new system for Nematoda: combining morphological characters with molecular trees, and translating clades into ranks and taxa. Nematology Monographs and Perspectives. 2004;2:633–653. [Google Scholar]
- De Ley & Coomans (1990).De Ley P, Coomans A. Drilocephalobus moldavicus Lisetskaya, 1968 from Senegal: an odd nematode adds to its reputation under the scanning electron microscope (Nematoda: Rhabditida) Revue de Nematologie. 1990;13:37–43. [Google Scholar]
- De Rijk et al. (1999).De Rijk P, Robbrecht E, De Hoog S, Caers A, Van de Peer Y, De Wachter R. Database on the structure of large subunit ribosomal RNA. Nucleic Acids Research. 1999;27:174–178. doi: 10.1093/nar/27.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tomasel et al. (2013).De Tomasel CM, Adams BJ, Tomasel FG, Wall DH. The life cycle of the Antarctic nematode Plectus murrayi under laboratory conditions. Journal of Nematology. 2013;45:39. [PMC free article] [PubMed] [Google Scholar]
- Freckman & Mankau (1986).Freckman DW, Mankau R. Abundance, distribution, biomass and energetics of soil nematodes in a northern Mojave Desert ecosystem. Pedobiologia. 1986;29:129–142. [Google Scholar]
- Freckman & Virginia (1997).Freckman DW, Virginia RA. Low-diversity Antarctic soil nematode communities: distribution and response to disturbance. Ecology. 1997;78:363–369. doi: 10.1890/0012-9658(1997)078[0363:LDASNC]2.0.CO;2. [DOI] [Google Scholar]
- Gibson et al. (2011).Gibson T, Farrugia D, Barrett J, Chitwood DJ, Rowe J, Subbotin S, Dowton M, Bonen L. The mitochondrial genome of the soybean cyst nematode, Heterodera glycines. Genome. 2011;54:565–574. doi: 10.1139/g11-024. [DOI] [PubMed] [Google Scholar]
- Gutell (1994).Gutell RR. Collection of small subunit (16S-and 16S-like) ribosomal RNA structures: 1994. Nucleic Acids Research. 1994;22:3502–3507. doi: 10.1093/nar/22.17.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell, Gray & Schnare (1993).Gutell RR, Gray MW, Schnare MN. A compilation of large subunit (23S and 23S-like) ribosomal RNA structures: 1993. Nucleic Acids Research. 1993;21:3055–3074. doi: 10.1093/nar/21.13.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2005).He Y, Jones J, Armstrong M, Lamberti F, Moens M. The mitochondrial genome of Xiphinema americanum sensu stricto (Nematoda: Enoplea): considerable economization in the length and structural features of encoded genes. Journal of Molecular Evolution. 2005;61:819–833. doi: 10.1007/s00239-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Hegedusova et al. (2014).Hegedusova E, Brejova B, Tomaska L, Sipiczki M, Nosek J. Mitochondrial genome of the basidiomycetous yeast Jaminaea angkorensis. Current Genetics. 2014;60:49–59. doi: 10.1007/s00294-013-0410-1. [DOI] [PubMed] [Google Scholar]
- Holterman et al. (2006).Holterman M, Van der Wurff A, Van Den Elsen S, Van Megen H, Bongers T, Holovachov O, Bakker J, Helder J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Molecular Biology and Evolution. 2006;23:1792–1800. doi: 10.1093/molbev/msl044. [DOI] [PubMed] [Google Scholar]
- Hu, Chilton & Gasser (2003).Hu M, Chilton NB, Gasser RB. The mitochondrial genome of Strongyloides stercoralis (Nematoda) –idiosyncratic gene order and evolutionary implications. International Journal for Parasitology. 2003;33:1393–1408. doi: 10.1016/S0020-7519(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Hu & Gasser (2006).Hu M, Gasser RB. Mitochondrial genomes of parasitic nematodes—progress and perspectives. Trends in Parasitology. 2006;22:78–84. doi: 10.1016/j.pt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2003).Hu M, Gasser RB, El-Osta YGA, Chilton NB. Structure and organization of the mitochondrial genome of the canine heartworm, Dirofilaria immitis. Parasitology. 2003;127:37–51. doi: 10.1017/S0031182003003275. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck & Ronquist (2001).Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Humphreys-Pereira & Elling (2014).Humphreys-Pereira DA, Elling AA. Mitochondrial genomes of Meloidogyne chitwoodi and M. incognita (Nematoda: Tylenchina): comparative analysis, gene order and phylogenetic relationships with other nematodes. Molecular and Biochemical Parasitology. 2014;194:20–32. doi: 10.1016/j.molbiopara.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Humphreys-Pereira & Elling (2015).Humphreys-Pereira DA, Elling AA. Mitochondrial genome plasticity among species of the nematode genus Meloidogyne (Nematoda: Tylenchina) Gene. 2015;560:173–183. doi: 10.1016/j.gene.2015.01.065. [DOI] [PubMed] [Google Scholar]
- Hunt et al. (2016).Hunt VL, Tsai IJ, Coghlan A, Reid AJ, Holroyd N, Foth BJ, Tracey A, Cotton JA, Stanley EJ, Beasley H, Bennett HM, Brooks K, Harsha B, Kajitani R, Kulkarni A, Harbecke D, Nagayasu E, Nichol S, Ogura Y, Quail MA, Randle N, Xia D, Brattig NW, Soblik H, Ribeiro DM, Sanchez-Flores A, Hayashi T, Itoh T, Denver DR, Grant W, Stoltzfus JD, Lok JB, Murayama H, Wastling J, Streit A, Kikuchi T, Viney M, Berriman M. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nature Genetics. 2016;48:299–309. doi: 10.1038/ng.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob et al. (2009).Jacob JE, Vanholme B, Van Leeuwen T, Gheysen G. A unique genetic code change in the mitochondrial genome of the parasitic nematode Radopholus similis. BMC Research Notes. 2009;2:192. doi: 10.1186/1756-0500-2-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jex et al. (2010).Jex AR, Hall RS, Littlewood DTJ, Gasser RB. An integrated pipeline for next-generation sequencing and annotation of mitochondrial genomes. Nucleic Acids Research. 2010;38:522–533. doi: 10.1093/nar/gkp883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, Afrin & Yoshida (2016).Kikuchi T, Afrin T, Yoshida M. Complete mitochondrial genomes of four entomopathogenic nematode species of the genus Steinernema. Parasites and Vectors. 2016;9:430. doi: 10.1186/s13071-016-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2017).Kim J, Kern E, Kim T, Sim M, Kim J, Kim Y, Park C, Nadler SA, Park J-K. Phylogenetic analysis of two Plectus mitochondrial genomes (Nematoda: Plectida) supports a sister group relationship between Plectida and Rhabditida within Chromadorea. Molecular Phylogenetics and Evolution. 2017;107:90–102. doi: 10.1016/j.ympev.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2018).Kim T, Kern E, Park C, Nadler SA, Bae YJ, Park J-K. The bipartite mitochondrial genome of Ruizia karukerae (Rhigonematomorpha. Nematoda). Scientific Reports. 2018;8:7482. doi: 10.1038/s41598-018-25759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2014).Kim T, Kim J, Cho S, Min GS, Park C, Carreno RA, Nadler SA, Park JK. Phylogeny of Rhigonematomorpha based on the complete mitochondrial genome of Rhigonema thysanophora (Nematoda: Chromadorea) Zoologica Scripta. 2014;43:289–303. doi: 10.1111/zsc.12047. [DOI] [Google Scholar]
- Kim, Kim & Park (2017).Kim T, Kim J, Park J-K. Acrobeloides varius sp. n. (Rhabditida: Cephalobidae) from South Korea. Nematology. 2017;19:489–496. doi: 10.1163/15685411-00003064. [DOI] [Google Scholar]
- Kim et al. (2015).Kim J, Lee SH, Gazi M, Kim T, Jung D, Chun JY, Kim S, Seo TK, Park C, Baldwin JG. Mitochondrial genomes advance phylogenetic hypotheses for Tylenchina (Nematoda: Chromadorea) Zoologica Scripta. 2015;44:446–462. doi: 10.1111/zsc.12112. [DOI] [Google Scholar]
- Lee et al. (2019).Lee CK, Laughlin DC, Bottos EM, Caruso T, Joy K, Barrett JE, Brabyn L, Nielsen UN, Adams BJ, Wall DH, Hopkins DW, Pointing SB, McDonald IR, Cowan DA, Banks JC, Stichbury GA, Jones I, Zawar-Reza P, Katurji M, Hogg ID, Sparrow AD, Storey BC, Green TGA, Cary SC. Biotic interactions are an unexpected yet critical control on the complexity of an abiotically driven polar ecosystem. Communications Biology. 2019;2:62. doi: 10.1038/s42003-018-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood et al. (2006).Littlewood DTJ, Lockyer AE, Webster BL, Johnston DA, Le TH. The complete mitochondrial genomes of Schistosoma haematobium and Schistosoma spindale and the evolutionary history of mitochondrial genome changes among parasitic flatworms. Molecular Phylogenetics and Evolution. 2006;39:452–467. doi: 10.1016/j.ympev.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2013).Liu G-H, Shao R, Li J-Y, Zhou D-H, Li H, Zhu X-Q. The complete mitochondrial genomes of three parasitic nematodes of birds: a unique gene order and insights into nematode phylogeny. BMC Genomics. 2013;14:414. doi: 10.1186/1471-2164-14-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu Y, Song F, Jiang P, Wilson J-J, Cai W, Li H. Compositional heterogeneity in true bug mitochondrial phylogenomics. Molecular Phylogenetics and Evolution. 2018;118:135–144. doi: 10.1016/j.ympev.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Lowe & Eddy (1997).Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Research. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggenti (1981).Maggenti A. General nematology. Springer-Verlag; New York: 1981. [Google Scholar]
- Miller, Pfeiffer & Schwartz (2010).Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE); 2010. pp. 1–8. [Google Scholar]
- Montiel et al. (2006).Montiel R, Lucena MA, Medeiros J, Simões N. The complete mitochondrial genome of the entomopathogenic nematode Steinernema carpocapsae: insights into nematode mitochondrial DNA evolution and phylogeny. Journal of Molecular Evolution. 2006;62:211–225. doi: 10.1007/s00239-005-0072-9. [DOI] [PubMed] [Google Scholar]
- Nadler et al. (2006).Nadler SA, De Ley P, Mundo-Ocampo M, Smythe AB, Stock SP, Bumbarger D, Adams BJ, De Ley IT, Holovachov O, Baldwin JG. Phylogeny of Cephalobina (Nematoda): molecular evidence for recurrent evolution of probolae and incongruence with traditional classifications. Molecular Phylogenetics and Evolution. 2006;40:696–711. doi: 10.1016/j.ympev.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Okimoto et al. (1992).Okimoto R, Macfarlane JL, Clary DO, Wolstenholme DR. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992;130:471–498. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2007).Park J-K, Kim K-H, Kang S, Kim W, Eom KS, Littlewood D. A common origin of complex life cycles in parasitic flatworms: evidence from the complete mitochondrial genome of Microcotyle sebastis (Monogenea: Platyhelminthes) BMC Evolutionary Biology. 2007;7:11. doi: 10.1186/1471-2148-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips et al. (2016).Phillips WS, Brown AMV, Howe DK, Peetz AB, Blok VC, Denver DR, Zasada IA. The mitochondrial genome of Globodera ellingtonae is composed of two circles with segregated gene content and differential copy numbers. BMC Genomics. 2016;17:706. doi: 10.1186/s12864-016-3047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar (1983).Poinar GO. The natural history of nematodes. Prentice-Hall; Englewood Cliffs: 1983. [Google Scholar]
- Poinar (1993).Poinar GO. Origins and phylogenetic relationships of the entomophilic rhabditids, Heterorhabditis and Steinernema. Fundamental and Applied Nematology. 1993;16:333–338. [Google Scholar]
- Siddiqi (1980).Siddiqi MR. The origin and phylogeny of the nematode orders Tylenchida Thorne, 1949 and Aphelenchida n. ord. Helminthological Abstracts, Series B. 1980;49:143–170. [Google Scholar]
- Siddiqi (2000).Siddiqi MR. Tylenchida: parasites of plants and insects. 2nd edition CABI Publishing; Wallingford: 2000. [Google Scholar]
- Spiridonov, Ivanova & Wilson (2005).Spiridonov SE, Ivanova ES, Wilson MJ. The nematodes of the genus Dicelis Dujardin, 1845 parasitic in earthworms: the interrelationships of four Eurasian populations. Russian Journal of Nematology. 2005;13:61–81. [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana et al. (2013).Sultana T, Kim J, Lee S-H, Han H, Kim S, Min G-S, Nadler SA, Park J-K. Comparative analysis of complete mitochondrial genome sequences confirms independent origins of plant-parasitic nematodes. BMC Evolutionary Biology. 2013;13:12. doi: 10.1186/1471-2148-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvain et al. (2014).Sylvain ZA, Wall DH, Cherwin KL, Peters DPC, Reichmann LG, Sala OE. Soil animal responses to moisture availability are largely scale, not ecosystem dependent: insight from a cross-site study. Global Change Biology. 2014;20:2631–2643. doi: 10.1111/gcb.12522. [DOI] [PubMed] [Google Scholar]
- Timm (1971).Timm RW. Antarctic soil and freshwater nematodes from the McMurdo Sound region. Proceedings of the Helminthological Society of Washington. 1971;38:42–52. [Google Scholar]
- Van Megen et al. (2009).Van Megen H, Van den Elsen S, Holterman M, Karssen G, Mooyman P, Bongers T, Holovachov O, Bakker J, Helder J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology. 2009;11:927–950. doi: 10.1163/156854109X456862. [DOI] [Google Scholar]
- Waceke et al. (2005).Waceke JW, Bumbarger DJ, Mundo-Ocampo M, Subbotin SA, Baldwin JG. Zeldia spannata sp. n. (Nematoda: Cephalobidae) from the Mojave Desert, California. Journal of Nematode Morphology and Systematics. 2005;8:57–67. [Google Scholar]
- Waeschenbach et al. (2006).Waeschenbach A, Telford MJ, Porter JS, Littlewood DTJ. The complete mitochondrial genome of Flustrellidra hispida and the phylogenetic position of Bryozoa among the Metazoa. Molecular Phylogenetics and Evolution. 2006;40:195–207. doi: 10.1016/j.ympev.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang B-J, Gu X-B, Yang G-Y, Wang T, Lai W-M, Zhong Z-J, Liu G-H. Mitochondrial genomes of Heterakis gallinae and Heterakis beramporia support that they belong to the infraorder Ascaridomorpha. Infection, Genetics and Evolution. 2016;40:228–235. doi: 10.1016/j.meegid.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Wernersson & Pedersen (2003).Wernersson R, Pedersen AG. RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Research. 2003;31:3537–3539. doi: 10.1093/nar/gkg609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman, Jansen & Boore (2004).Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2018).Yang H, Li T, Dang K, Bu W. Compositional and mutational rate heterogeneity in mitochondrial genomes and its effect on the phylogenetic inferences of Cimicomorpha (Hemiptera: Heteroptera) BMC Genomics. 2018;19:264. doi: 10.1186/s12864-018-4650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou et al. (2017).Zou H, Jakovlić I, Chen R, Zhang D, Zhang J, Li W-X, Wang G-T. The complete mitochondrial genome of parasitic nematode Camallanus cotti: extreme discontinuity in the rate of mitogenomic architecture evolution within the Chromadorea class. BMC Genomics. 2017;18:840. doi: 10.1186/s12864-017-4237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Watson-Crick base pairings are denoted by lines and G-U pairs are indicated by dots. The numbers identify the conserved secondary structure elements defined by Dams et al. (1988).
Watson-Crick base pairings are denoted by lines and G-U pairs are indicated by dots. The numbers identify the conserved secondary structure elements defined by De Rijk et al. (1999).
Bootstrap percentages (BP) were calculated using the rapid bootstrapping method. BP values <70% are not shown.
Bootstrap percentages (BP) were calculated using the rapid bootstrapping method. BP values <70% are not shown.
Bayesian posterior probabilities (BPP) were estimated after discarding the initial 250 trees (the first 25 × 104 generations) as burn-in. BPP values <0.7 are not shown.
Bayesian posterior probabilities (BPP) were estimated after discarding the initial 250 trees (the first 25 × 104 generations) as burn-in. BPP values <0.7 are not shown.
Bayesian posterior probabilities (BPP) were estimated after discarding the initial 250 trees (the first 25 × 104 generations) as burn-in. BPP values <0.7 are not shown.
Data Availability Statement
The following information was supplied regarding data availability:
The mt genome sequence and the 28S sequence of Acrobeloides varius are available at GenBank: MK559448 and MT233103.