Abstract
Chronic non-cancer pain (CNCP) is a significant health burden among adults. Standard behavioral therapies typically focus on targeting negative affect (NA) and yield only modest treatment effects. The aims of this study were to systematically review and investigate the association between positive affect (PA) and pain severity among adults with CNCP. Databases search included MEDLINE (PubMed), PsycINFO, CINAHL, ProQuest Dissertations and Theses, OLASTER, Open Grey, and PsyArXiv (inception to July 23, 2019). We analyzed studies that: (1) employed observational, experimental, or intervention study designs; (2) enrolled individuals with CNCP (pain ≥ 12 weeks); and (3) reported full quantitative results on outcomes. Two researchers independently screened articles, extracted data, and assessed the risk of bias. The main meta-analysis was followed by subgroup analyses. All analyses were performed using random-effects models. Formal tests for heterogeneity (Q-statistic; I2) and publication bias (p-curve and p-uniform*) were performed. We meta-analyzed 29 studies with 3521 participants. Results demonstrated that PA inversely impacts pain severity in people with CNCP (r = −0.23). Subgroup analyses showed a significant effect for gender and marginally significant effects for age in studies that adjusted for NA. On average, effect sizes for observational studies were larger in studies with a higher proportion of female respondents and in studies that did not adjust for NA. Finally, larger effect sizes were found in intervention studies with older compared with younger samples.
Keywords: Positive affect, chronic pain, pain severity, systematic review, meta-analysis
1. Introduction
It is estimated that 70 million Americans—more than the number affected by diabetes, heart disease, and cancer combined—suffer from chronic non-cancer pain (hereafter referred to as CNCP) each year [11]. With an increased prevalence among persons ages 65 and over [84], chronic pain is a significant health burden—not just in terms of pain-related health care expenditures and disability, but also in terms of the inestimable costs to families and individual daily living and quality of life. Pain severity, a core clinical measure of chronic musculoskeletal pain [50], is associated with greater disability, sleep impairment, psychosocial difficulties [35; 83; 94] and increased prevalence of mental health disorders including depression, anxiety, and substance abuse among patients with CNCP [2; 74; 83]. Although there is increasing interest in the use of evidence-based non-pharmacological approaches to managing chronic pain severity [12; 62], standard behavioral therapies, such as cognitive-behavioral therapy (CBT) and mindfulness-based stress reduction (MBSR), typically focus on targeting negative affective states (e.g., anxiety and depression) [48; 49] and yield only modest treatment effects [25; 31]. Efforts are therefore needed to develop more effective psychological treatments for chronic pain by identifying new targets for intervention.
A growing body of theory and research suggest that positive affective states (e.g., gratitude and happiness) play a uniquely important role in promoting psychological adjustment in the face of chronic pain [40; 60; 64; 65]. Specifically, positive affect (PA) has been theorized to facilitate adaptive coping in the context of chronic pain by countering the negative effects of fear on attention [90]; buffering negative pain-related cognitions (i.e., rumination, helplessness, magnification) [63]; reducing inflammation [82]; promoting neutral reappraisal processes related to pain [46]; and enhancing engagement in valued activities in the face of pain [91].
Although narrative reviews have been conducted [28; 38; 57], to date, there has not been a comprehensive quantitative review relating PA to chronic pain severity. Howell et al. [45] meta-analyzed experimental studies and found that induced PA was associated with higher pain tolerance. More recently, Kushlev et al. [53] examined data from nearly 2.5 million U.S. respondents and found an inverse relationship between PA and previous day physical pain. Notably, both studies focused on acute pain responses. Thus, there is a need to establish whether these findings generalize to chronic pain. The primary aim of this systematic review and meta-analysis was to comprehensively review the literature examining the association of PA and pain severity in people with CNCP. We use systematic methods and standardized procedures [59; 72] for locating and evaluating the relevance and quality of observational, experimental, and intervention studies. Observational studies consisted of both ambulatory and longitudinal studies. Ambulatory studies used experience sampling methodology across several days or weeks to examine how changes in PA relate to pain. Longitudinal studies explored whether levels of PA predict future levels of pain across more extended periods. Experimental studies determined the effects of induced or manipulated PA on concurrent pain. Intervention studies examined the efficacy of PA-enhancing treatments on pain severity prospectively over time. The study’s secondary aims were to investigate moderators of the relation between PA and pain severity in people with CNCP, determine the quality of the studies, and examine potential publication bias.
2. Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [59]. All methods and planned analyses were preregistered on the Open Science Framework (OSF).
2.1. Data sources and searches
MEDLINE (PubMed), PsycINFO, and CINAHL databases were searched electronically from inception to March 21, 2017. An updated search was performed (from April 7, 2017 through September 24, 2018) to include research from gray literature sources (ProQuest Dissertations and Theses, OLASTER, Open Grey, and PsyArXiv) and to identify new publications. All databases and gray literature sources were searched again on July 23, 2019 to capture additional relevant literature published between 2017 and 2019. Search terms for PA used keywords drawn from prior reviews of health outcomes associated with PA [9; 15; 70], and included variations of happy, cheerful, joy, vigor, excited, elated, enthusiastic, energetic interest, content, amused, humor, calm, relaxed, grateful, satisfied, positive affect, positive emotions, and positive mood. Search terms for chronic pain included variations of widespread pain, recurrent pain, persistent pain, and long-term pain. The details of the full search strategy are presented in eAppendix 1.
2.2. Eligibility criteria and study selection
Studies were eligible for inclusion if they met the following criteria: (1) the study design was observational, experimental, or intervention (e.g., randomized controlled trial); (2) participants were adults (18 years or older) with CNCP (12 weeks or more in duration); and (3) results were reported in sufficient quantitative detail to discern a directional effect of PA on pain severity. For all intervention studies, data from the baseline to first follow-up were included, with the baseline vs. first follow-up contrast serving as the primary outcome. In the case of two or more groups receiving different PA interventions within one study, all were independently included.
Articles were excluded if they: (1) were not an empirical study; (2) did not involve human subjects; (3) did not include a measure of PA or a positive mood manipulation (e.g., humorous films, pleasant images); (4) used a reversed indicator of negative affect (NA) as a measure of PA (e.g., hopelessness vs. hopefulness; pessimism vs. optimism; fatigue vs. vigor/vitality); (5) did not include a subjective or objective measure of pain severity; (6) assessed affect only in regard to a specific life experience (e.g., “How happy are you about being pregnant?”); (7) examined only the directional effect of pain on PA or mean differences in PA between pain impaired and non-impaired samples; (8) did not enroll individuals identified as suffering from chronic pain; (9) assessed acute or experimentally induced pain; (10) did not employ an eligible study design; (11) did not examine or report a directional effect of PA on pain; (12) exclusively used a sample of patients with a primary cancer diagnosis; (13) were published in languages other than English; (14) did not include adults (18 years and above); (15) exclusively used a sample of patients presenting with a primary psychological disorder; (16) did not directly target PA; (17) did not include sufficient quantitative information on study outcomes; (18) full text of article could not be located; or (19) were a duplicate identified during full-text screening. When possible, we also contacted authors for further information.
After duplicates were removed, titles and abstracts were screened by two independent reviewers (K.R. and K.G.) to determine whether the citation met eligibility criteria. Subsequently, two independent authors (A.D.O. and F.T.) assessed the full text of potentially eligible studies for inclusion. Conflicts were resolved by consensus. Figure 1 presents the study selection process and indicates the number of articles excluded at each phase of screening.
Figure 1.
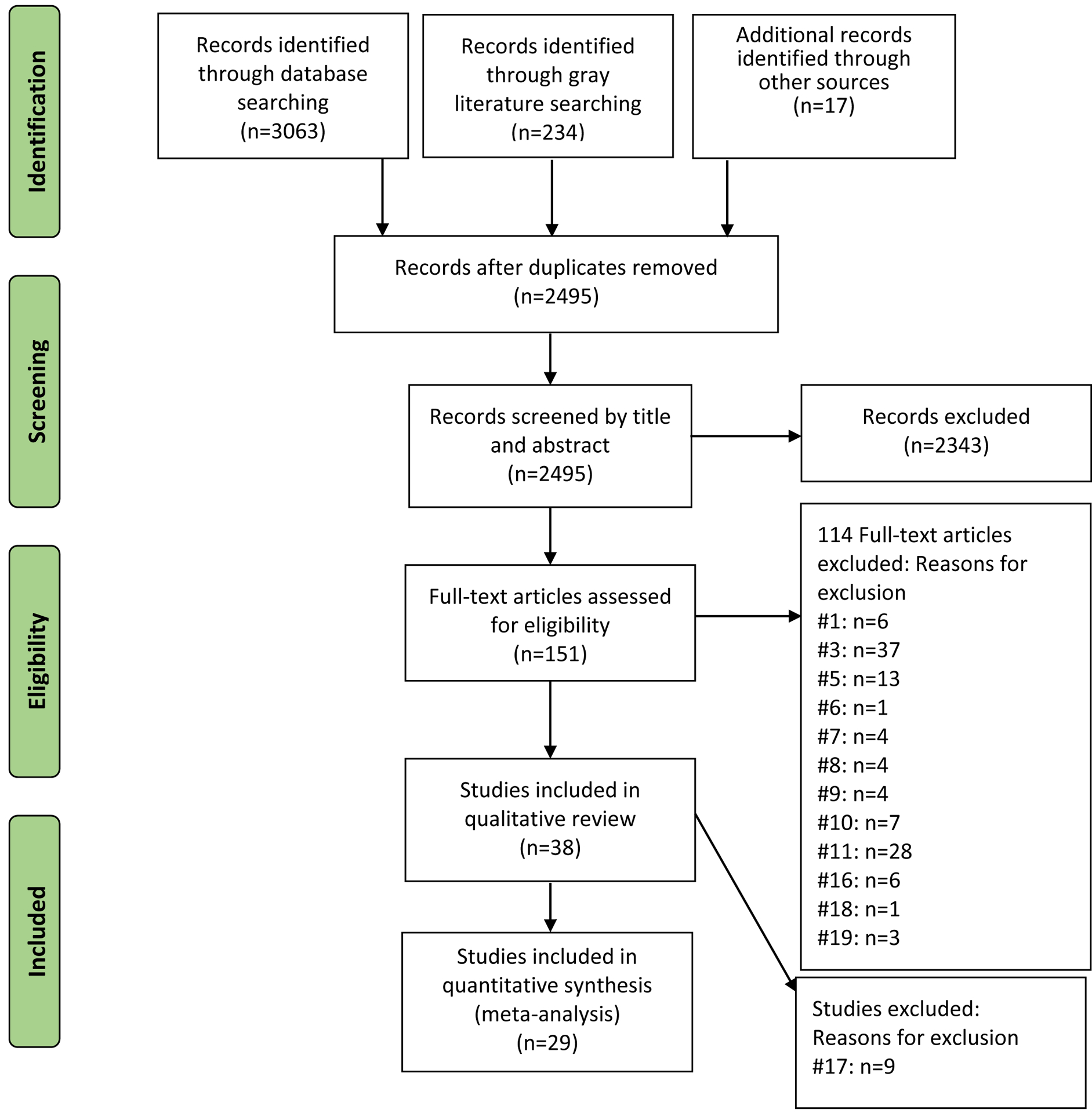
Study flow diagram. Articles were excluded if they met any one of the following criteria: 1) were not an empirical study; (2) did not involve human subjects; (3) did not include a measure of PA or a positive mood manipulation (e.g., humorous films, pleasant images); (4) used a reversed indicator of negative affect (NA) as a measure of PA (e.g., hopelessness versus hopefulness; pessimism versus optimism; fatigue versus vigor/vitality); (5) did not include a subjective or objective measure of pain severity; (6) assessed affect only in regard to a specific life experience (e.g., “How happy are you about being pregnant?”); (7) examined only the directional effect of pain on PA or mean differences in PA between pain impaired and non-impaired samples; (8) did not enroll individuals identified as suffering from chronic pain; (9) assessed acute or induced pain; (10) did not employ an eligible study design; (11) did not examine or report a directional effect of PA on pain; (12) exclusively used a sample of patients with a primary cancer diagnosis; (13) were published in languages other than English; (14) did not include adults (18 years and above); (15) exclusively used a sample of patients presenting with a primary psychological disorder; (16) did not directly target PA; (17) did not include full quantitative results on outcomes; (18) full text of article could not be located; or (19) were a duplicate identified during full-text screening.
2.3. Data extraction and quality assessment
Two reviewers (A.D.O. and F.T.) independently extracted study characteristics and outcome data from published articles. Risk of bias was independently assessed by two reviewers (A.O. and F.T.) using the Effective Public Health Practice Project (EPHPP) tool [72]. Specifically, the included studies were assessed for (1) selection bias, (2) study design, (3) confounders, (4) blinding, (5) data collection, and (6) withdrawals/dropouts. Each domain was rated as strong, moderate, or weak, and domain scores were averaged to provide a global rating for each study (inter-rater reliability 88%, Cohen’s kappa .79). Discrepancies were resolved by consensus.
2.4. Data synthesis and analysis
Meta-analyses were performed using the metafor and meta packages [76; 88] in R, version 3.4.3 (R Project for Statistical Computing). For each study, individual effect sizes were calculated within each independent sample. For observational studies (ambulatory and longitudinal), standardized regression coefficients were extracted and used as an effect size index [67]. For experimental and intervention studies, effects sizes (Hedges’ g) were extracted from descriptive statistics. Similar to Cohen’s d, Hedges’ g effect sizes of 0.00 to 0.32 can be considered as small, effect sizes of 0.33 to 0.55 as moderate, and effect sizes of 0.56 to 1.20 as large [54]. Although effect sizes were computed separately for each of the three study designs (observational, experimental, and intervention), we also computed an overall effect size (requivalent) [75] from exact t-values reported across studies. Specifically, we report requivalent effect sizes on the Fisher Z-transformed metric, and used a standard error, as suggested by Rosenthal and Rubin [75], defined as the square root of N −3. Meta-analyses yielded a point estimate, confidence interval, and p-value, along with statistics for heterogeneity (assessed using the Cochran QE-statistic and the Higgins-Thompson I2 values) [22]. Publication bias was evaluated using the Egger test (with p < .10 indicating asymmetry [26]), and visual inspection of funnel plots. For completeness, we conducted p-curves [79] (the distribution of statistically significant p values for a set of findings, with right-skewed p-curves suggesting findings that contain evidentiary value) and p-uniform* test (a publication bias test based on the effect size in a set of studies) [87].
2.5. Subgroup analyses
A priori subgroup analyses were performed to explore moderators of the PA-pain severity relation, including (1) risk of bias quality rating: weak, moderate, and strong; (2) demographics: percentage female, percentage racial minority, and mean age; (3) chronic pain status: fibromyalgia, rheumatoid arthritis, osteoarthritis, back pain, and multiple; (4) PA measurement: state and trait; and (5) covariate adjustment: unadjusted negative affect (NA) and adjusted NA. For categorical moderators that explained significant variance in the effect sizes (i.e., p < 0.05 for QM), post hoc contrasts were performed to determine which groups were statistically different. For continuous moderators, meta-regression analyses were used to determine whether variation in the effect sizes was explained by the moderator. A false discovery rate (FDR) Type I error control was used for all comparisons to correct for multiple testing [8].
3. Results
3.1. Study characteristics
From a total of 3,063 retrieved articles, 151 were identified based on title and abstract screening for full-text review. Of these, 38 studies fulfilled eligibility criteria and were included in the systematic review. Descriptive details of the studies are presented in Table 1. The included studies were published between 1981 [58] and 2018 [39]; came from ten countries, with 25 studies from the United States; had sample sizes ranging from 8 [58] to 360 [39], and included a total of 4,229 participants (mean [SD] age was 54 [9.57] years and 76% were women). It should be noted that not all studies reported the exact age or number of non-White participants.
Table 1:
Descriptive Characteristics of Included Studies
| Source [Country] | Year | Study type | Risk of bias | Sample size | Mean age | % white | % female | Pain condition | PA Manipulation/Measure | Pain Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Arnold et al [1][Germany] | 2008 | E | W | 120 | 49 | --- | 83 | M | Mood induction procedure using the IAPS; PANAS | Pain intensity via NRS |
| Baird et al [3][US] | 2006 | I | S | 28 | 73 | --- | 100 | O | GIR; mood measured as a subscale of HRQOL | Pain frequency and intensity via NRS |
| Basler et al [5][Germany] | 1997 | I | S | 76 | 49 | --- | 76 | B | CBT | Pain intensity and pain control via NRS |
| Baxter et al [6][New Zealand] | 2012 | I | M | 8 | 55 | --- | 50 | B | Character strengths & gratitude intervention; DESP; OHQSF | Pain intensity via VAS |
| Behrouz et al [7][Iran] | 2017 | I | M | 55 | 74 | --- | 71 | M | Humor therapy | Pain intensity via BPI (Modified German Version) |
| Carson et al[10] [US] | 2005 | I | M | 43 | 51 | 63 | 61 | B | Loving-kindness meditation | Pain intensity and pain rating index via MPQ; Usual and worst pain via BPI |
| Connelly et al [17][US] | 2007 | A | W | 94 | 56 | 91 | 72 | R | PANAS | Pain intensity via VAS |
| Davis & Zautra[20] [US] | 2013 | I | M | 79 | 46 | 83 | 98 | F | MB intervention targeting socioemotional regulation; PANAS | Daily pain intensity and coping via NRS |
| Davis et al [19][US] | 2014 | E | M | 110 | 57 | 91 | 100 | M | Positive and neutral mood induction; PANAS-X | Clinical pain via NRS |
| Dowd et al[23] [Ireland] | 2015 | I | W | 124 | 44 | --- | 90 | M | MBCT with emphasis on emotional regulation; SLS; MAAS | Pain intensity and interference via BPI |
| Finan et al [27][US] | 2009 | A | W | 260 | 57 | 88–93 | 100 | M | PANAS | Daily average pain via NRS |
| Finan et al[29] [US] | 2013 | A | W | 151 | 61 | 62 | 68 | O | PANAS-X; POMS - Bipolar | Pain severity via WOMAC |
| Fors, & Götestam[30] [Norway] | 2000 | I | M | 58 | 46 | --- | 100 | F | GI | Pain intensity via VAS |
| Garland et al[34] [US] | 2014 | I | S | 115 | 48 | 65 | 68 | M | MB, CBT | Pain intensity and interference via BPI |
| Garland et al [32][US] | 2017 | I | W | 55 | 49 | 75 | 62 | M | MB, CBT | Pain intensity via NRS |
| Gruszczynska et al[36] [Poland] | 2015 | A | W | 95 | 51 | --- | 100 | R | Folkman & Lazarus PA Scale | Daily pain via VAS |
| Guillory et al [37][US] | 2015 | I | M | 68 | 49 | 63 | 75 | M | Social support text messaging intervention; PAM | Pain intensity and interference via NRS |
| Hausmann et al [41] [US] | 2017 | I | S | 42 | 68 | 57 | 17 | O | PPI; PANAS; SLS | Pain severity and functional difficulty via WOMAC |
| Hausmann et al [39][US] | 2018 | I | S | 360 | 64 | 50 | 24 | O | PPI; International PANAS-SF; SLS | Pain severity and functional difficulty via WOMAC |
| Herrero et al [44][Spain] | 2014 | E | S | 40 | 49 | --- | 100 | F | Virtual reality; Mood state via VAS; Mood intensity via NRS | Pain intensity via NRS |
| Kamping et al [47][Germany] | 2013 | E | S | 32 | 52 | --- | 100 | F | Mood induction procedure using the IAPS | Pain intensity via VAS |
| Kruszewski [52][US] | 2010 | A | W | 143 | 58 | 89 | 100 | M | PANAS | Average daily pain via NRS |
| Litt et al [55][US] | 2004 | A | W | 30 | 36 | 80 | 87 | TMD | PANAS; Circumplex Model of Mood | Pain intensity via NRS |
| McKee [58] [US] | 1981 | E | M | 20 | 36 | 90 | 50 | M | GIR | Pain intensity via NRS |
| Mun et al [61][US] | 2017 | A | W | 220 | 51 | 78 | 87 | R | Shortened PANAS | Overall daily pain intensity via NRS |
| Müller et al[60] [US] | 2016 | I | M | 96 | 59 | 96 | 70 | O | Tailored PPI; PANAS; PWI-A | Pain severity via NRS |
| Peters et al[66] [the Netherlands] | 2017 | I | S | 284 | 49 | --- | 85 | F | Internet-based PPI; Present happiness via NRS; SCS-SF; BMIS | Pain intensity via NRS |
| Pintard [68][US] | 1986 | E | W | 60 | 52 | 77 | 65 | M | Humor induction; HA; POMS; CHS | Present pain intensity and pain rating index MPQ |
| Potter[69] [US] | 1999 | L | W | 285 | 63 | --- | 100 | M | PANAS | Aggregate of average level of pain over last week, |
| Raft et al[73] [US] | 1986 | E | M | 52 | --- | 85 | 60 | M | Pleasant imagery task | Pain intensity via VAS |
| Seebach et al[77] [US] | 2012 | L | W | 141 | 59 | 81 | 58 | B | PANAS | Pain intensity via BPI |
| Shaygan et al[78] [Germany] | 2017 | I | M | 88 | 53 | --- | 67 | M | Visual stimuli (e.g., pictures of loved ones, landscapes); Valence and arousal via SAM | Average pain intensity via NRS |
| Strand et al[81] [Norwary] | 2007 | L | W | 163 | 50 | --- | 79 | M | PANAS | Past week’s average pain via NRS |
| Tse et al[85] [Hong Kong] | 2010 | I | M | 70 | 79 | --- | 54 | M | Humor therapy; SHS; Revised LSI-A | Cantonese VRS |
| Wilkinson[92] [US] | 2003 | E | S | 60 | --- | --- | 75 | M | SHRQ | Current pain rating and location via NRS |
| Willmarth[93][US] | 1998 | E | M | 96 | 45 | --- | 41 | M | Hypnotically-induced positive mood | Sensory, affective and overall global pain ratings via VAS |
| Zautra et al[95] [US] | 2001 | A | W | 175 | 64 | 95 | 100 | M | PANAS | Average past week pain via NRS |
| W | 89 | 44 | --- | 100 | F | MAC | Current pain via NRS, averaged across body parts | |||
| Zautra et al[96] [US] | 2008 | I | S | 144 | 52 | 89 | 68 | R | CBT; MB intervention targeting emotion regulation and adaptation; PANAS | Average daily pain via NRS |
Notes. PA = Positive Affect; US = United States. Study type: A = ambulatory, L = longitudinal; E = experiment, I = intervention. Risk of bias: W = weak; M = moderate; S = strong. Pain conditions: F = fibromyalgia, O = osteoarthritis, R = rheumatoid arthritis, B = back pain, TMD = temporomandibular dysfunction, M = multiple. Measure and intervention abbreviations: BMIS = Brief Mood Introspection Scale; BPI = Brief Pain Inventory; Cantonese VRS = Cantonese Verbal Rating Scale; CBT = Cognitive Behavioral Therapy; CHS = Coping Humor Scale; DESP = Differential Emotions Scale Probe; GI or GIR = Guided Imagery or Guided Imagery Relaxation; HA = Humor Appreciation; HRQOL = Health-related Quality of Life; IAPS = International Affective Picture System; International PANAS-SF = International Positive and Negative Affect Schedule Short Form; MAAS = Mindful Attention Awareness Scale; MAC = Mood Adjective Checklist; MB = Mindfulness-based; MBCT = Mindfulness-based Cognitive Therapy; MPQ = McGill Pain Questionnaire; NRS = Numeric Rating Scale; OHQSF = Oxford Happiness Questionnaire Short Form; PAM = Photographic Affect Measure; PANAS = Positive and Negative Affect Schedule; PANAS-X = Positive and Negative Affect Schedule, Expanded Form; POMS = Profile of Mood States; POMS-Bipolar = Profile of Mood States – Bipolar; PPI = Positive Psychology Intervention; PWI-A = Personal Wellbeing Index (Adult version); Revised LSI-A = Revised Life Satisfaction Index – Form A; SAM = Self-assessment Manikin; SCS-SF = Self-compassion Scale Short Form; SF-12 = Short-Form Health Survey; SF-MPQ = Short-form McGill Pain Questionnaire; SF-MPQ-2 = Short-form McGill Pain Questionnaire 2; SHRQ = Situational Humor Response Questionnaire; SHS = Subjective Happiness Scale; SLS = Satisfaction with Life Scale; VAS = Visual Analog Scale; WOMAC = Western Ontario MacMaster Universities (Osteo-)Arthritis Index.
Among the 38 included studies, 11 were observational (eight ambulatory [17; 27; 29; 36; 52; 55; 61; 95], three were longitudinal studies [69; 77; 81]); 9 were experimental [1; 19; 44; 47; 58; 68; 73; 92; 93]; and 18 were interventions [3; 5–7; 10; 20; 23; 30; 32; 34; 37; 39; 41; 60; 66; 78; 85; 96]). Among observational studies, the majority used self-report adjective ratings of positive valence (e.g., active, energetic, happy, cheerful, joyful) to assess level of PA. State levels (momentary, daily) of PA were typically assessed in ambulatory studies, whereas trait levels (global ratings) of PA were typically measured in longitudinal studies. Among experimental studies, examples of PA-based inductions included viewing emotionally evocative images, humorous film clips, and guided imagery. Finally, in the intervention research reported here, a variety of methods were used to increase PA in people with CNCP, including expressing thanks, practicing acts of kindness, and savoring positive moments, among others [39].
3.2. Risk of bias
The assessment of the quality of the study methodology for the five domains (selection bias, study design, confounders, blinding, and data collection) is reported in eFigure 1 in the Supplement. Risk-of-bias assessments for individual studies included in the qualitative review are summarized in Table 1. Following the EPHPP tool, eight studies [3; 5; 34; 39; 41; 47; 66; 96] (21.05%) were classified as “strong” or having low risk of bias; 13 studies [6; 7; 10; 19; 20; 30; 37; 58; 60; 73; 78; 85; 93] (34.21%) were categorized as having “moderate” risk; and 17 studies [1; 17; 23; 27; 29; 32; 36; 44; 52; 55; 61; 68; 69; 77; 81; 92; 95] (44.74%) were categorized as “weak” or having high risk of bias. Weakness ratings derived from the inadequate control of confounders and insufficient information regarding study design, as well as lack of blinding.
3.3. Meta-analyses
A total of 29 studies (N = 3,521) fulfilled the eligibility criteria and were included in the final meta-analysis [5; 7; 10; 19; 20; 23; 29; 30; 32; 34; 36; 37; 39; 41; 44; 52; 58; 60; 61; 66; 69; 73; 77; 78; 81; 85; 92; 95; 96]. Pooling the results of the 29 studies, an average requivalent effect size of −0.23 (95% CI −0.36 to −0.13; P < .0001) was observed between PA and chronic pain severity.
3.3.1. Observational studies
Eight observational studies [29; 36; 52; 61; 69; 77; 81; 95] were included in the primary analysis, totaling 1,482 participants. Figure 2 displays the forest plots for the meta-analyses of the association between PA and pain severity in this group of studies. PA was associated with decreased chronic pain severity (β = −0.13, z = −4.60, P < 0.001), but heterogeneity across studies was substantial (I2 = 89.25%; 95% CI 73.6 to 96.3; QE (13) = 84.69, P < 0.001), indicating significant variation in the effect sizes. Egger’s test for funnel plot asymmetry yielded a result significant at the 0.10 level (z = −1.78, P = 0.08). The funnel plot however (eFigure 2 in the Supplement) suggested no readily detectable presence of bias, as effect sizes tended to cluster somewhat evenly within the funnel. We also conducted p-uniform* as an additional measure of publication bias. This test did not yield a significant result (Lpb = 0.83, P = 0.66), failing to indicate the presence of publication bias. Observed p-curve for observational studies are reported in eFigure 3 in the Supplement. The shape of the p-curve was signficantly right-skewed (z = −9.23 P < 0.001), indicating the set of studies contains evidentiary value.
Figure 2.
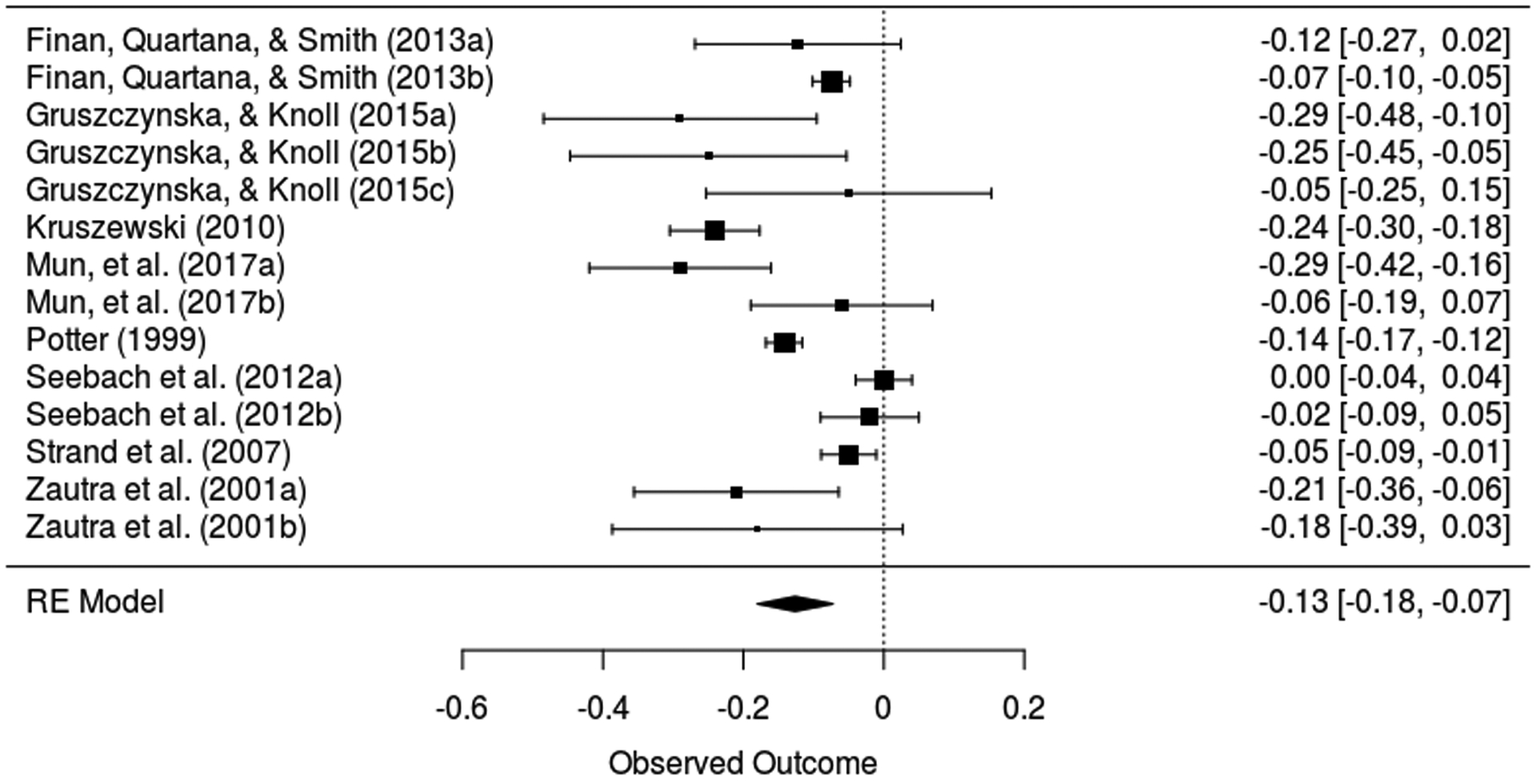
Forest plot for positive affect and pain in observational studies.
3.3.2. Experimental studies
Five experimental studies [19; 44; 58; 73; 92] provided data on pain severity for 282 participants. Contrary to expectations, PA was not associated with pain severity in experimental studies (Hedges’ g = −1.02; Z = −1.55; P = 0.12). Heterogeneity was significant and high (I2 96.1%; 95% CI 87.3 to 99.6; QE (4) = 33.87, P < .001), and funnel plots (eFigure 4 in the Supplement) and the Egger’s test (z = −5.18, p < 0.0001) suggested asymmetry. Likewise, the p-uniform* test yielded a significant result (Lpb = 5.83, P = 0.05), indicating the likely presence of publication bias. As shown in the forest plot in Figure 3, there were two studies with small sample sizes but relatively large effect sizes [58; 73], whereas the larger studies all had effect sizes that were much closer to zero. eFigure5 in Supplement reports the observed p-curve for expertimental studies, which was signficantly right-skewed (z = −10.1, P < 0.001), suggesting the set of significant findings contains evidentiary value.
Figure 3.
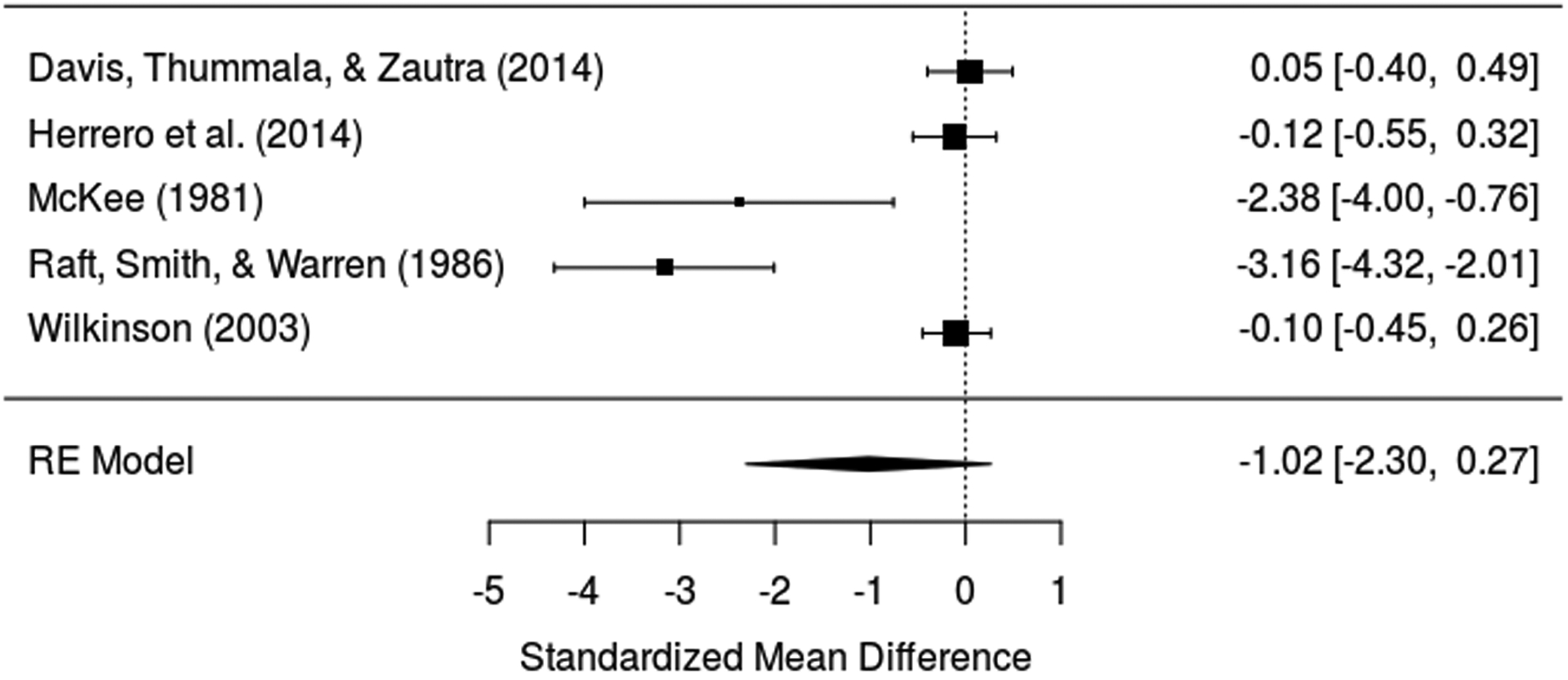
Forest plot for positive affect and pain in experimental studies.
3.3.3. Intervention studies
Sixteen intervention studies [5; 7; 10; 20; 23; 30; 32; 34; 37; 39; 41; 60; 66; 78; 85; 96] provided data on pain severity for 1,757 participants. As shown in Figure 4, PA was inversely associated with chronic pain severity (Hedges’ g = −0.36; Z = −3.54; P < 0.001). Heterogeneity was high (I2 = 73.8%; 95% CI 52.6 to 90.1; QE (17) = 59.53, P < 0.001), and funnel plots (eFigure 6 in the Supplement) and the Egger’s test (z = −2.34, P = 0.02) suggested potential asymmetry. However, the p-uniform* test yielded a non-significant result (Lpb = 0.31, P = 0.86), indicating lack of evidence for publication bias. eFigure 7 in Supplement reports the observed p-curve for expertimental studies, which was signficantly right-skewed (z = −9.69, P < 0.001), suggesting the set of significant findings contains evidentiary value.
Figure 4.
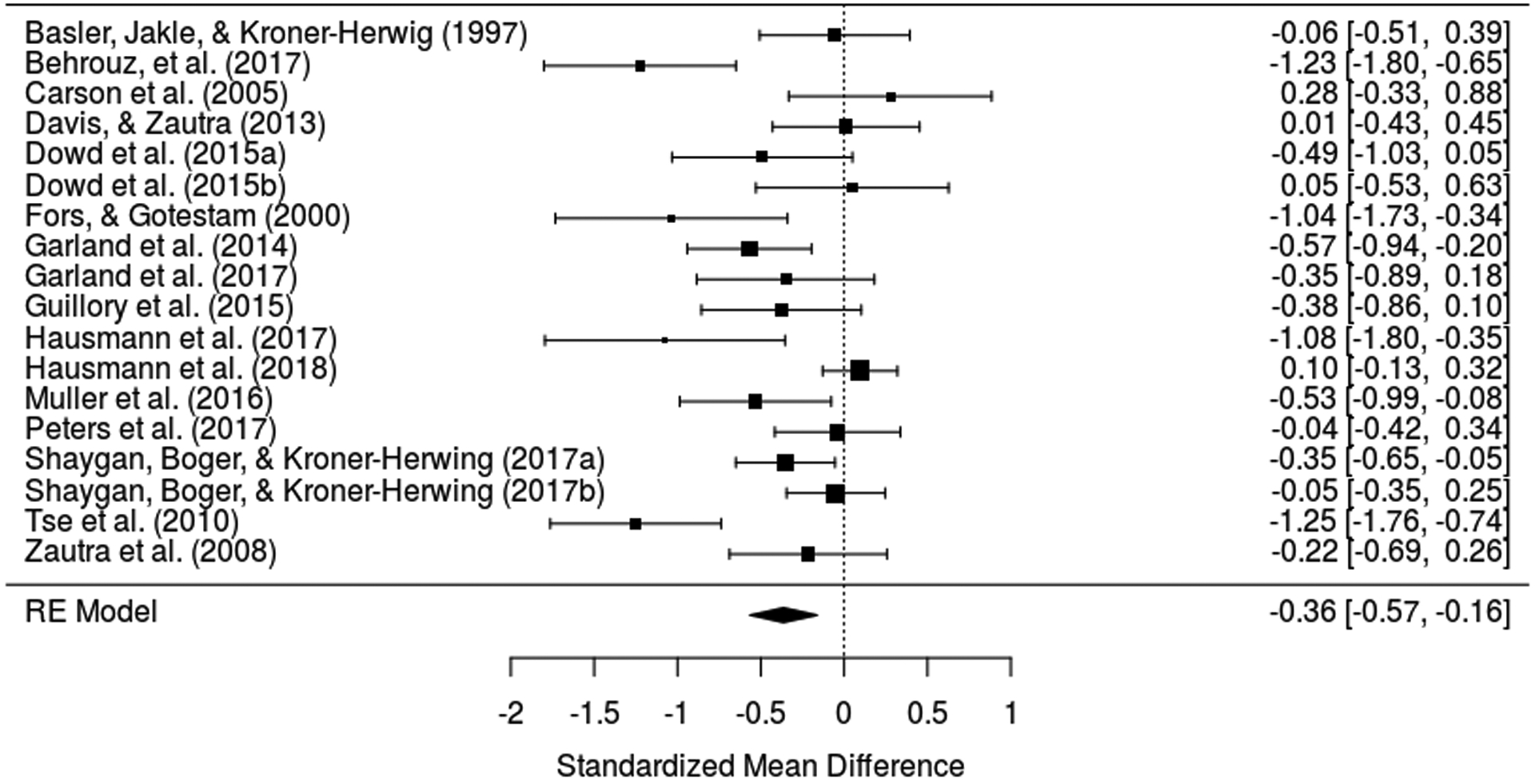
Forest plot for positive affect and pain in intervention studies.
3.4. Subgroup and exploratory analyses
A priori subgroup analyses within observational and intervention studies are shown in eTables 1–2 for categorical moderators and eTables 3–4 for continuous moderators (see Supplement). Subgroup analyses were not conducted on experimental studies due to the small number of studies in this cluster. With respect to the categorical moderators, lower effect sizes were found for observational studies that adjusted for NA (β = −0.09; Z = −2.91; P = 0.003) compared to those that did not (β = −0.18; Z = −4.39; P = < 0.0001); however, the difference between effect sizes did not reach conventional levels of statistical significance, QM = 3.52, p = 0.06. For continuous moderators, the gender composition of the sample moderated the relation between PA and chronic pain severity, such that observational study samples with a higher proportion of female participants reported larger effect sizes on average, QM= 22.68, p < 0.0001. Finally, larger effect sizes were found in intervention studies with older compared with younger samples, QM = 5.82, p = 0.015. This moderating effect, however, became non-significant following FDR correction, p = 0.125.
Post hoc exploratory analyses were conducted to examine intervention studies that reported data on the magnitude of change in PA (n = 6) and average rates of depression in the sample (n = 5). Among the included studies, effect sizes did not differ significantly as a function of reported PA change, QM= 0.05, p = 0.823. Studies reporting higher rates of depression had effect sizes that were not significantly different compared to those reporting lower rates of depression, QM= 0.17, p = 0.680.
4. Discussion
4.1. Main findings
This systematic review and meta-analysis provides quantitative evidence that PA is associated with reduced pain severity among adults with CNCP. Previous narrative reviews [28; 38; 57] have reported links between PA and pain. In the present review, we undertook a meta-analysis of observational, experimental, and intervention studies, enabling the quantification of these links and the exploration of key sources of heterogeneity across studies. Pooling the results of 29 observational, experimental, and interventions studies, we found an average r-effect size of −0.23 between PA and pain severity among people with CNCP. This effect size is similar to the effect size between PA and acute physical pain (r = −.18) found by Kushlev et al. [53], and smaller than the effect size reported by Howell et al. [45] in their meta-analyses examining the effects of laboratory-induced PA on pain tolerance (r = .32).
Effect sizes were relatively small in intervention studies (g = −.36). This finding is in line with two previous meta-analyses of RCT’s on the effects of PA-based interventions on other pain-relevant outcomes, including depression and anxiety. Hendriks et al. [43] reported relatively small effects on these outcomes (effect sizes ranged from −.35 to −.39), and a recent met-analysis by Chakssi et al. [13] conducted among clinical samples with psychiatric or somatic disorders also reported small effects for depression (g = −.23) and anxiety (g = −.36), respectively. Within observational studies, a small significant effect size of −.13 was found between PA and chronic pain severity. The magnitude of this effect is similar to that found in a prior meta-analysis examining the association between NA-based predictors (e.g., fear avoidance) and pain intensity [51].
In addition, we examined the impact of categorical (risk of bias, chronic pain status, state vs. trait PA measurement, NA adjustment) and continuous moderators (age, gender, race). Subgroup analyses revealed mainly non-significant associations between PA and chronic pain severity. These analyses showed only three significant moderating characteristics of the sample: gender and age. Notably, PA was associated with lower chronic pain severity in observational studies that adjusted for NA and had a higher percentage of female vs. male participants. Additionally, within intervention studies, age moderated the link between PA and pain severity, with larger effect sizes evident in studies with older compared with younger samples. Finally, in exploratory analyses, neither reported change in PA nor depression composition was found to significantly moderate the relation between PA and chronic pain severity.
4.2. Strengths and limitations
There are several strengths to this review, including its pre-registered design, comprehensive search strategy, systematic study inclusion, thorough assessment of study quality, use of a priori subgroup analyses, and formal tests for heterogeneity (Q-statistic; I2) and publication bias (p-curve and p-uniform*). There are also limitations to our review. First, although inspection of the funnel plot and Egger test did not identify strong evidence of publication bias in any of our analyses, we found high heterogeneity in terms of study population. Second, although moderating analyses revealed mainly non-significant associations, the small number of studies within each cluster prevented us from performing high-powered subgroup analyses [97]. Third, study quality did not prove to be a significant moderator of the PA-pain effect sizes. However, it is possible that our risk assessment instrument did not adequately capture the range of biases inherent in different types of study designs (e.g., observational, experimental, and intervention studies). That is, we assigned quality ratings based on an overall assessment of risk [72]; however, an alternative approach would be to use design-specific criteria to assess common sources of bias specific to certain types of study designs [89]. Fourth, only a small number of experimental (n = 2) and intervention studies (n = 6) reported data on the magnitude of change in PA, thus leaving unanswered the question of whether PA is the active psychological component in the causal chain. Thus, it is critical that researchers report data on the magnitude of change in the primary outcome (i.e., PA) as a means of assessing the efficacy of experimental and intervention procedures. Such data can then be used as sample-specific moderators in subsequent meta-analyses of PA-pain effect sizes. Likewise, few observational studies (n = 2 ambulatory; n = 3 longitudinal) examined relationships between PA and chronic pain severity while controlling for measures of NA. An important methodological issue in studies of PA and health is whether relationships are independent of negative affective states [71; 80]. In subgroup analyses, we found marginally lower effect sizes in observational studies that adjusted for NA compared to those that did not. However, with such a small number of studies, definitive conclusions cannot be drawn. Similarly, few of the included intervention studies (n = 5) assessed rates of depression. It is known that depression is highly comorbid with the occurrence of chronic pain [4; 18], and there is some evidence that depression may be an important moderator of interventions targeting PA regulation in chronic pain patients [20; 96]. Given the limited number of studies reporting on depression, firm conclusions on the effects of PA-enhancing interventions on chronic pain severity among clinical populations cannot yet be made. Fifth, the current meta-analysis focused on pain severity as the primary outcome, but the effects of PA on other salient outcomes (e.g., pain interference, pain catastrophizing) in people with CNCP need to be established in future research. Finally, despite the inclusion of gray literature, we used English search terms, which may have prevented us from identifying relevant studies published in other languages.
4.3. Implications for research and practice
Our findings support guideline recommendations [16; 24] that encourage clinicians to consider psychological treatments in the care of patients with CNCP, particularly interventions that have a PA component. Nevertheless, it is unclear whether existing nonpharmacological treatments for CNCP that incorporate elements of PA enhancement (e.g., MBSR [33]; acceptance and commitment therapy [ACT][42]; and emotional awareness and expression therapy [EAET][56]) are sufficient in reducing pain severity or whether the efficacy of these treatments to boost PA can be further strengthened [28]. An additional critical question is whether psychological treatments for CNCP that promote PA have greater benefits than those that are aimed at reducing NA. There is evidence that treatment modalities that incorporate mindfulness-based strategies (e.g., relaxation, present-focused awareness) may be an effective treatment alternative to standard CBT [14; 21; 86]. However, as Finan et al. [28] have noted, treatment approaches for CNCP like CBT, ACT, and MBSR typically emphasize minimizing negative thoughts and emotions associated with pain. As a consequence, it is currently unclear which therapeutic mechanisms (PA-enhancing or NA-reducing strategies) should be optimized in existing psychosocial treatments for CNCP. Finally, it also may be possible that interventions that integrate both PA-based and NA-based strategies could augment the therapeutic impact of current empirically-supported treatments for CNCP [39].
5. Conclusion
To our knowledge, this is the first pre-registered systematic review and meta-analysis to examine the association between PA and pain severity in adults with CNCP. The results indicated that PA is associated with a modest decrease in pain severity across observational, experimental, and intervention studies. The findings suggest that among adults with chronic non-cancer pain, PA may be a factor that promotes resilience in the face of chronic pain.
Supplementary Material
Footnotes
Conflicts of Interest
The authors have nothing disclose.
References
- [1].Arnold BS, Alpers GW, Sub H, Friedel E, Kosmutzky G, Geier A, Pauli P. Affective pain modulation in fibromyalgia, somatoform pain disorder, back pain, and healthy controls. European Journal of Pain 2008;12:329–338. [DOI] [PubMed] [Google Scholar]
- [2].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Archives of Internal Medicine 2003;163:2433–2445. [DOI] [PubMed] [Google Scholar]
- [3].Baird CL, Sands LP. Effect of guided imagery with relaxation on health-related quality of life in older women with Osteoarthritis. Research in Nursing & Health 2006;29:442–451. [DOI] [PubMed] [Google Scholar]
- [4].Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychological Bulletin 1996;119(1):95–110. [Google Scholar]
- [5].Basler H-D, Jäkle C, Kröner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: A controlled randomized study in German pain treatment centers. Patient Education and Counseling 1997;31(2):113–124. [DOI] [PubMed] [Google Scholar]
- [6].Baxter HJ, Johnson MH, Bean D. Efficacy of a character strength and gratitude intervention for people with chronic back pain. Australian Journal of Rehabilation Counseling 2012;18:135–147. [Google Scholar]
- [7].Behrouz S, Mazlom SR, Kooshiar H, Aghebati N, Asgharipour N, Vashani H. Investigating the effect of humor therapy on chronic pain in the elderly living in nursing homes in Mashhad, Iran. Evidence Based Care 2017;7:27–36. [Google Scholar]
- [8].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- [9].Boehm JK, Kubzansky LD. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychological Bulletin 2012;138:655–691. [DOI] [PubMed] [Google Scholar]
- [10].Carson JW, Keefe FJ, Lynch TR, Carson KM, Goli V, Fras AM, Thorp SR. Loving-kindness meditation for chronic low back pain: results from a pilot trial. Journal of holistic nursing : official journal of the American Holistic Nurses’ Association 2005;23(3):287–304. [DOI] [PubMed] [Google Scholar]
- [11].Centers for Disease Control and Prevention. Prevalence of chronic and high-impact pain among adults--United States, 2016, September 14, 2018. [Google Scholar]
- [12].Chang KL, Fillingim R, Hurley RW, Schmidt S. Chronic pain management: Nonpharmacological therapies for chronic pain. FP Essentials 2015;432:21–26. [PubMed] [Google Scholar]
- [13].Chaves C, Lopez-Gomez I, Hervas G, Vazquez C. A comparative study of the efficacy of a positive psychology intervention and a cognitive behavioral therapy for clinical depression. Cognitive Therapy and Research 2017;41:417–433. [Google Scholar]
- [14].Cherkin D, Sherman K, Balderson B, Cook A, Anderson M, Hawkes R, Hansen K, Turner JA. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: A randomized clinical trial. JAMA Internal Medicine 2016;315:1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine 2008;70(7):741–756. [DOI] [PubMed] [Google Scholar]
- [16].Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Wimer M, R F, Dana T, Kraegel P, Griffin J, Grusing S, Brodt ED. Nonpharmacologic therapies for low back pain: A systematic review for an American College of Physicians Clinical Practice Guideline. Annals of Internal Medicine 2017;166:493–505. [DOI] [PubMed] [Google Scholar]
- [17].Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain 2007;131(1–2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychosomatic Medicine 2005;35 1275–1282. [DOI] [PubMed] [Google Scholar]
- [19].Davis MC, Thummala K, Zautra AJ. Stress-related clinical pain and mood in women with chronic pain: moderating effects of depression and positive mood induction. Ann Behav Med 2014;48(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Davis MC, Zautra AJ. An online mindfulness intervention targeting socioemotional regulation in fibromyalgia: Results of a randomized controlled trial. Annals of Behavioral Medicine 2013;46(3):273–284. [DOI] [PubMed] [Google Scholar]
- [21].Day MA, Ward C, Ehde DM, thorn B, Burns J, Barnier A, Mattingley J, Jensen MP. A pilot randomized controlled trial comparing mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Pain Medicine 2019;20:2134–2148. [DOI] [PubMed] [Google Scholar]
- [22].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177– 188. [DOI] [PubMed] [Google Scholar]
- [23].Dowd H, Hogan MJ, McGuire BE, Davis MC, Sarma KM, Fish RA, Zautra AJ. Comparison of an online mindfulness-based cognitive therapy intervention with online pain management psychoeducation: A randomized controlled study. The Clinical Journal of Pain 2015;31(6):517–527. [DOI] [PubMed] [Google Scholar]
- [24].Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA Internal Medicine 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eccleston C, Morley SJ, Williams AC. Psychological approaches to chronic pain management: Evidence and challenges. British Journal of Anaesthesia 2013;111:59–63. [DOI] [PubMed] [Google Scholar]
- [26].Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Finan PA, Zautra AJ, Davis MC. Daily affect relations in Fibromyalgia patients reveal positive affective disturbance. Psychosomatic Medicine 2009;71:474–482. [DOI] [PubMed] [Google Scholar]
- [28].Finan PH, Garland EL. The role of positive affect in pain and its treatment. The Clinical Journal of Pain 2015;31(2):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Finan PH, Quartana PJ, Smith MT. Positive and negative affect dimensions in chronic knee osteoarthritis: Effects on clinical and laboratory pain. Psychosomatic Medicine 2013;75(5):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fors EA, Götestam KG. Patient education, guided imagery and pain related talk in fibromyalgia coping. European Journal of Psychiatry 2000;14(4):233–240. [Google Scholar]
- [31].Garcıa-Campayo J, Magdalena J, Magallon R, Fernandez-Garcia E, Salas M, Andreas E. A meta-analysis of the efficacy of fibromyalgia treatment according to level of care. Arthritis Care & Research 2008:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Garland EL, Bryan CJ, Finan PH, Thomas EA, Priddy SE, Riquino MR, Howard MO. Pain, hedonic regulation, and opioid misuse: Modulation of momentary experience by Mindfulness-Oriented Recovery Enhancement in opioid-treated chronic pain patients. Drug and Alcohol Dependence 2017;173:S65–S72. [DOI] [PubMed] [Google Scholar]
- [33].Garland EL, Howland EW. Mindfulness-oriented recovery enhancement reduces pain attentional bias in chronic pain patients. Psychotherapy and Psychosomatics 2013;82:311–318. [DOI] [PubMed] [Google Scholar]
- [34].Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology 2014;82(3):448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gaskin DJ, Richard P. The economic costs of pain in the United States. Paper commissioned by the Committee on Advancing Pain Research, Care, and Education, 2011. [Google Scholar]
- [36].Gruszczyńska E, Knoll N. Meaning-focused coping, pain, and affect: A diary study of hospitalized women with rheumatoid arthritis. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation 2015;24(12):2873–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guillory J, Chang P, Henderson CR Jr., Shengelia R, Lama S, Warmington M, Jowza M, Waldman S, Gay G, Reid MC. Piloting a text message-based social support intervention for patients with chronic pain: Establishing feasibility and preliminary efficacy. Clin J Pain 2015;31(6):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hanssen MH, Peters ML, Boselie JJ, Meulders A. Can positive affect attenuate (persistent) pain? State of the art and clinical implications. Current Rheumatology Reports 2017;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hausmann L, Youk A, Kwoh C, Gallagher R, Weiner D, Vina E, Obrosky S, Mauro G, McInnes S, Ibrahim S. Effect of a positive psychologicla intervention on pain and functional difficulty among adults with osteoarthritis: A randomized clinical trial. JAMA Open Network 2018;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hausmann LRM, Parks A, Youk AO, Kwoh CK. Reduction of bodily pain in response to an online positive activities intervention. Journal of Pain 2014;15:560–567. [DOI] [PubMed] [Google Scholar]
- [41].Hausmann LRM, Youk A, Kwoh CK, Ibrahim SA, Hannon MJ, Weiner DK, Gallagher RM, Parks A. Testing a positive psychological intervention for osteoarthritis. Pain Medicine 2017;18(10):1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behavioral Research Therapy 2006;44:1–25. [DOI] [PubMed] [Google Scholar]
- [43].Hendriks T, Schotanus-Dijkstra M, Hassankhan A, de Jong J, Bohlmeijer E. The efficacy of multi-component positive psychological interventions: A meta-analysis of randomized controlled trials. Journal of Happiness Studies in press. [Google Scholar]
- [44].Herrero R, Garcia-Palacios A, Castilla D, Molinari G, Botella C. Virtual reality for the induction of positive emotions in the treatment of fibromyalgia: a pilot study over acceptability, satisfaction, and the effect of virtual reality on mood. Cyberpsychol Behav Soc Netw 2014;17(6):379–384. [DOI] [PubMed] [Google Scholar]
- [45].Howell R, Kern M, Lyubomirsky S. Health benefits: Meta-analytically determining the impact of well-being on objective health outcomes. Health Psychology Review 2007;1:1–54. [Google Scholar]
- [46].Kalisch R, Müller MB, Tüscher O. A conceptual framework for the neurobiological study of resilience. Behavioral and Brain Sciences 2014:1–79. [DOI] [PubMed] [Google Scholar]
- [47].Kamping S, Bomba IC, Kanske P, Diesch E, Flor H. Deficient modulation of pain by a positive emotional context in fibromyalgia patients. PAIN 2013;154(9):1846–1855. [DOI] [PubMed] [Google Scholar]
- [48].Keefe FJ, Lumley M, Anderson T, Lynch T, Carson KL. Pain and emotion: New research directions. Journal of Clinical Psychology 2001;57:587–607. [DOI] [PubMed] [Google Scholar]
- [49].Koechlin H, Coakley R, Schechter N, Werner C, Kossowsky J. The role of emotion regulation in chronic pain: A systematic literature review. Journal of Psychosomatic Research 2018;107:38–45. [DOI] [PubMed] [Google Scholar]
- [50].Kroenke K, Krebs EE, Turk D, Von Korff M, Bair MJ, Allen KD, Sandbrink F, Cheville AL, DeBar L, Lorenz KA, Kerns RD. Core outcome measures for chronic musculoskeletal pain research: Recommendations from a Veterans Health Administration work group. Pain Medicine 2019;20:1500–1508. [DOI] [PubMed] [Google Scholar]
- [51].Kroska EB. A meta-analysis of fear-avoidance and pain intensity: The paradox of chronic pain. Scandanavian Journal of Pain 2016;13:43–58. [DOI] [PubMed] [Google Scholar]
- [52].Kruszewski DM. Positive affect and heart rate variability: Resources of resilience in the context of pain and stress. (Unpublished doctoral dissertation). Tempe, AZ, 2010. [Google Scholar]
- [53].Kushlev K, Drummond DM, Diener E. Subjective well-being and health behaviors in 2.5 million Americans. Applied Psychology: Healht and Well-Being in press. [DOI] [PubMed] [Google Scholar]
- [54].Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment: Confirmation from meta-analysis. American Psychologist 1993;48:1181–1209. [DOI] [PubMed] [Google Scholar]
- [55].Litt MD, Shafer D, Napolitano C. Momentary mood and coping processes in TMD pain . Health Psychology 2004;23(4):354–362. [DOI] [PubMed] [Google Scholar]
- [56].Lumley MA, Cohen JL, Stout RL, Neely LC, Sander LM, Burger AJ. An emotional exposure-based treatment of traumatic stress for people with chronic pain: preliminary results for fibromyalgia syndrome. Psychotherapy Theory, Research, Practice, Training 2008;45:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Markozannes G, Aretouli E, Rintou E, Dragioti E, Damigos D, Ntzani E, Evangelou E, tsilidis KK. An umbrella review of the literature on the effectiveness of psychological interventions for pain reduction. BMC Psychology 2017;31:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McKee PJ. The effects of a treatment program for chronic pain patients using enjoyable imagery with biofeedback induced relaxation, Vol. 42: ProQuest Information & Learning, 1981. pp. 2293–2293. [Google Scholar]
- [59].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- [60].Müller R, Gertz KJ, Molton IR, Terrill AL, Bombardier CH, Ehde DM, Jensen MP. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: A feasibility trial. The Clinical Journal of Pain 2016;32(1):32–44. [DOI] [PubMed] [Google Scholar]
- [61].Mun CJ, Thummala K, Davis MC, Karoly P, T H, Zautra AJ. Predictors and social consequences of daily pain expectancy among adults with chronic pain. Pain 2017;158:1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Niknejad B, Bolier R, Henderson CR, Delgado D, Kozlov E, Löckenhoff CE, Reid MC. Association between psychological interventions and chronic pain outcomes in older adults: A systematic review and meta-analysis. JAMA Internal Medicine 2018;178:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ong AD, Zautra AJ, Reid MC. Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychology and Aging 2010;25(3):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ong AD, Zautra AJ, Reid MC. Chronic pain and the adaptive significance of positive emotions. American Psychologist 2015;70:283–284. [DOI] [PubMed] [Google Scholar]
- [65].Peters ML, Smeets E, Feijge M, van Breukelen G, Andersson G, Buhrman M, Linton SJ. Happy despite pain: A randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patiens with chronic pain. Clinical Journal of Pain 2017;33:962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Peters ML, Smeets E, Feijge M, van Breukelen G, Andersson G, Buhrman M, Linton SJ. Happy despite pain: A randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patients with chronic pain. The Clinical Journal of Pain 2017;33(11):962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. Journal of Applied Psychology 2005;90:175–181. [DOI] [PubMed] [Google Scholar]
- [68].Pintard P. Stimulus-elicited laughter as a strategy for the modification of chronic pain responses. (Unpublished doctoral dissertation). Hattiesburg, MS: University of Southern Mississippi, 1986. [Google Scholar]
- [69].Potter PT. Stressful events and the internal structure of affect: Implications for health psychology, Vol. 9937416. Ann Arbor: Arizona State University, 1999. p. 133. [Google Scholar]
- [70].Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin 2005;131(6):925–971. [DOI] [PubMed] [Google Scholar]
- [71].Pressman SD, Jenkins BN, Moskowitz JT. Positive affect and health: What do we know and where next should we go? Annual Review of Psychology 2019;70:627–650. [DOI] [PubMed] [Google Scholar]
- [72].Project EPHP. Quality assessment tool for quantitative studies, 2009. [Google Scholar]
- [73].Raft D, Smith RH, Warren N. Selection of imagery in the relief of chronic and acute clinical pain. Journal of Psychosomatic Research 1986;30(4):481–488. [DOI] [PubMed] [Google Scholar]
- [74].Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C., Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 2003;289:(2370–2378). [DOI] [PubMed] [Google Scholar]
- [75].Rosenthal R, Rubin DB. r-sub(equivalent): A simple effect size indicator. Psychological Methods 2003;8:492–496. [DOI] [PubMed] [Google Scholar]
- [76].Schwarzer G. meta: An R package for meta-analysis. R News 2007;7(3):40–45. [Google Scholar]
- [77].Seebach CL, Kirkhart M, Lating JM, Wegener ST, Song Y, Riley LH, III, Archer KR. Examining the role of positive and negative affect in recovery from spine surgery. Pain 2012;153(3):518–525. [DOI] [PubMed] [Google Scholar]
- [78].Shaygan M, Böger A, Kröner-Herwig B. Valence and arousal value of visual stimuli and their role in the mitigation of chronic pain: What is the power of pictures? The Journal of Pain 2017;18(2):124–131. [DOI] [PubMed] [Google Scholar]
- [79].Simonsohn U, Nelson LD, Simmons JP. p-curve and effect size: Correcting for publication bias using only significant results. Perspectives on Psychological Science 2014;9:666–681. [DOI] [PubMed] [Google Scholar]
- [80].Steptoe A. Happiness and health. Annual Review of Public Health 2019;40:339–359. [DOI] [PubMed] [Google Scholar]
- [81].Strand EB, Kerns RD, Christie A, Haavik-Nilsen K, Klokkerud M, Finset A. Higher levels of pain readiness to change and more positive affect reduce pain reports - A weekly assessment study on arthritis patients. Pain 2007;127(3):204–213. [DOI] [PubMed] [Google Scholar]
- [82].Sturgeon J, Finan P, Zautra AJ. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nature Reviews Rheumatology 2016;12:532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Taylor DJ, Mallory LI, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep 2007;30:213–218. [DOI] [PubMed] [Google Scholar]
- [84].Thomas E. Pain in older people In: Croft P, Blyth FM, Windt Dvd, Chronic pain epidemiology: From aetiology to public health. Oxford: Oxford University Press, 2010. 186–199. [Google Scholar]
- [85].Tse MM, Lo AP, Cheng TL, Chan EK, Chan AH, Chung HS. Humor therapy: relieving chronic pain and enhancing happiness for older adults. Journal of aging research 2010;2010:343574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Turner JA, Anderson M, Balderson B, Cook A, Sherman K, Cherkin D. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain 2016;157:2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].van Assen MA, van Aert RC, Wicherts JM. Meta-analysis using effect size distributions of only statistically significant studies. Psychological Methods 2015;20:293–309. [DOI] [PubMed] [Google Scholar]
- [88].Viechtbauer W. Conducing meta-analysis in R with metafor package. Journal of Statistical Software 2010;36:1–48. [Google Scholar]
- [89].Viswanathan M, Berkman N. Development of the RTI item bank on risk of bias and precision of observational studies. Journal of Clinical Epidemiology 2011;65:163–178. [DOI] [PubMed] [Google Scholar]
- [90].Vlaeyen JW, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain 2016;157:1588–1589. [DOI] [PubMed] [Google Scholar]
- [91].White DK, Keysor JJ, Neogi T, Felson DT, LaValley M, Gross KD, Niu J, Nevitt M, Lewis CE, Tomer J, Fredman L. When it hurts, a positive attitude may help: association of positive affect with daily walking in knee osteoarthritis. Results from a multicenter longitudinal cohort study. Arthritis Care & Research 2012:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wilkinson LL. Love and laughter…a magical cure? The effects of humor and relationship statisfaction on chronic pain, Vol. 1416378. Ann Arbor: Lamar University - Beaumont, 2003. p. 51. [Google Scholar]
- [93].Willmarth EK. Modification of experienced pain with hypnotically induced positive mood. (Unpublished doctoral dissertation). Santa Barbara, CA: The Fielding Institute, 1999. [Google Scholar]
- [94].Wolf LD, Davis MC. Loneliness, daily pain, and perceptions of interpersonal events in adults with Fibromyalgia. Health Psychology 2014;33:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zautra A, Smith B, Affleck G, Tennen H. Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect. Journal of Consulting and Clinical Psychology 2001;69(5):786–795. [DOI] [PubMed] [Google Scholar]
- [96].Zautra AJ, Davis MC, Reich JW, Nicassario P, Tennen H, Finan P, Kratz A, Parrish B, Irwin MR. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. Journal of Consulting and Clinical Psychology 2008;76(3):408–421. [DOI] [PubMed] [Google Scholar]
- [97].Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care metaanalyses: A meta-epidemiological study. Critical Care 2013;17:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


