Abstract
The culinary herb Satureja montana, known as winter savory, is an ingredient of traditional dishes known in different parts of the world. As an ingredient of foods it has the potential to improve their safety. In this study, the herb’s activity was investigated against Campylobacter jejuni, the leading cause of the most prevalent bacterial gastroenteritis worldwide. The ethanolic extract and essential oil of the herb were chemically characterized and six pure compounds—carvacrol, thymol, thymoquinone, p-cymene, γ-terpinene, and rosmarinic acid—were chosen for further analysis. The antimicrobial activity of the ethanolic extract (MIC 250 mg/L) was 4-fold higher compared to the essential oil. Carvacrol, thymol and thymoquinone had the strongest antimicrobial effect (MIC 31.25 mg/L) and a strong synergistic activity between carvacrol and thymol was determined (FICi 0.2). Strong inhibitory effect on C. jejuni efflux pumps (2-fold inhibition) and disruption of membrane integrity (> 80% disruption) of the herb were determined as modes of action. For resistance against the herb, C. jejuni need efflux pumps, although increased resistance against this herb does not co-occur with increased efflux pump activity, as for antibiotics. This study shows the potential of a common culinary herb for the reduction of the food pathogen C. jejuni without increasing resistance.
Keywords: Satureja montana herb, Campylobacter jejuni, essential oil, ethanolic extract, antimicrobial, synergistic activity, efflux pump inhibition, membrane disruption, carvacrol, thymol
1. Introduction
Campylobacter spp. are the most commonly reported bacterial cause of gastroenteritis in the European Union, which is mainly due to infection by Campylobacter jejuni [1,2]. Campylobacteriosis is a disease that presents itself as watery diarrhea, fever and cramps, and can lead to the development of Guillain-Barre syndrome, a severe neurological condition [2,3]. With the number of confirmed campylobacteriosis cases reaching more than 250,000 per year in the EU, this is a burden for human health as well as national economies [1,4].
The present-day antibiotic resistance of bacteria is a considerable challenge, and C. jejuni is no exception. Due to the overuse of antibiotics in veterinary and human medicine, C. jejuni resistance levels to ciprofloxacin, nalidixic acid, and tetracycline are high. With more than 50% of isolates from poultry now resistant to at least one antibiotic, the risk of resistant C. jejuni spreading through the food chain is also high [5]. C. jejuni resistance nodulation division (RND) efflux pumps CmeABC and CmeDEF, and the major facilitator superfamily (MFS) efflux pump CmeGH are great contributors to these resistances. These efflux pumps and their overexpression cause resistance against antibiotics, such as erythromycin and ciprofloxacin, heavy metals, and bile acids, although, even without overexpression they can confer other resistance determinants for greater antibiotic and stress resistance in C. jejuni [6,7,8,9]. The inhibition of the major efflux pumps can thus lead to increased C. jejuni susceptibility to antimicrobials [6]. The worrying resistance levels in bacteria make it necessary to find alternative solutions for treatment and protection against pathogenic bacteria and efflux pumps are a promising target. More than 200 species can be found among the herbs collectively known as savory (Satureja), although summer (S. hortensis) and winter savory (S. montana) are the best known. Although use of the summer savory species is more widespread, winter savory has been gaining in popularity. The reason is the easier cultivation of S. montana, even in colder climates, and its more intense taste. S. montana is used in traditional dishes in the Karst region of Slovenia [10], but its use is also documented in different parts of the world [11,12,13]. S. montana extracts have shown good anti-oxidative potential and antimicrobial activities against Staphylococcus aureus, Escherichia coli, Bacillus cereus, Salmonella enterica, and Listeria monocytogenes [14,15,16], and when added to mortadella cured pork, it was shown to reduce the levels of Clostridium perfringens [17]. Similar effects were shown for L. monocytogenes in wine-marinated beef [16]. These characteristics make S. montana an interesting and promising product to study in the fight against foodborne pathogens. It can easily be added to food and thus serve as a supporting therapeutic for bacterial gastroenteritis.
The aim of this study was to determine the antimicrobial activity of the herb S. montana as a crude ethanolic extract and essential oil against the major foodborne pathogen C. jejuni. Furthermore, six compounds identified in the herb were investigated in their pure form to determine their potential synergistic effects against C. jejuni: carvacrol, thymol, thymoquinone, γ-terpinene, p-cymene, and rosmarinic acid. The modes of action of the ethanolic extract, essential oil, and the pure compounds were determined in terms of efflux pump inhibition and membrane disruption. Additionally, the involvement of the C. jejuni efflux pumps CmeABC, CmeDEF, and CmeGH in C. jejuni resistance against S. montana was examined
2. Materials and Methods
2.1. Ethanolic Extract and Essential Oil Preparation and Chemical Analysis
S. montana was supplied as dried herb by Kottas Pharma (Vienna, Austria). Ethanol was selected as solvent due to its ability to extract medium polarity as well as non-polar compounds and its compatibility with food processing. For extraction, 2 L of 96% denatured ethanol (Roth, Karlsruhe, Germany) was added to 100 g of herbal material, then mixed with a magnetic stirrer at room temperature for 48 h and filtered (Rotilabo pleated paper filters; Roth). The filtrate was evaporated to dryness using a rotary evaporator (Rotavapor, Büchi, Flawil, Switzerland) under vacuum at 40 °C and 175 mbar pressure. The final drying was carried out under a nitrogen flow, affording a yield of 3 g of dried extract from 100 g plant material. This ethanolic extract was analyzed by ultra-high-performance liquid chromatography–photodiode array–electrospray ionization mass spectrometry (UHPLC–PDA–ESI-MS). The UHPLC system (Dionex Ultimate 3000 RS; Thermo Scientific, Waltham, MA, USA) included pump, autosampler, column compartment, and PDA detector. Separation was carried out on an analytical column (Zorbax SB-C18 Rapid Resolution HD; 100 × 2.1 mm, 1.8 μm; Agilent, Santa Clara, CA, USA). The mobile phases were water plus 0.1% formic acid (A) and non-acidified acetonitrile (B), with gradient elution of: 0.0 → 9.0 min, 8% → 45% B; 9.0 → 15.0 min, 45% → 100% B; 15.0 → 15.5 min, 100% → 8% B; 15.5 → 21 min, 8% B. The column temperature was 35 °C and the flow rate was 0.390 mL/min. A solution of 5 mg/mL ethanolic extract was prepared in methanol. Control samples of 0.5 mg/mL rosmarinic acid and 0.5 µL/mL carvacrol were used. The injection volume was 2.0 µL. The PDA detection was in the wavelength range of 190 nm to 500 nm.
MS detection was achieved using a linear ion-trap mass spectrometer (LTQ XL; Thermo Scientific) equipped with an ESI source (Thermo Scientific). Mass spectra were recorded in negative and positive ion modes for the m/z range from 50 amu to 2000 amu, with data-dependent fragmentation (normalized collision energy, 35%). The further settings included: source voltage, 3.5 kV (ESI negative), 5.0 kV (ESI positive); capillary temperature, 350 °C; source temperature, 300 °C; sheath gas flow, 40 units (machine setting); auxiliary gas flow, 10 units (machine setting).
The essential oil was collected after hydro-distillation (Clevenger apparatus) of 30 g of plant material for 2 h. Gas chromatography (GC)-MS analysis was performed using a GC system (7890A; Agilent) interfaced to a mass selective detector (5975C; Agilent), with: electron ionization, 70 eV; ion source temperature, 230 °C; and interface temperature, 280 °C. The split injection (injection volume, 0.2 µL; split ratio, 50:1) at 240 °C injected the sample onto a fused silica capillary column (5% phenyl, 95% methyl polysiloxane; HP-5MS; 30 m × 250 µm × 0.25 µm; Agilent). Quantification of the compounds of interest was performed by comparison of the relative peak areas after normalization obtained by GC–flame ionization detector (FID) analysis, using the area % method without considering calibration factors. A gas chromatograph (GC 2014; Shimadzu, Kyoto, Japan) equipped with a FID and a fused silica column (5% polysilarylene, 95% polymethylsiloxane; Zebron ZB-5MSi; 30 m × 250 µm × 0.25 µm; Phenomenex, Torrance, CA, USA) was used, with experimental conditions identical to those for the GC-MS analysis. Reference compounds were obtained from Sigma Aldrich (Munich, Germany) with the following purities: carvacrol 98.0%, thymol ≥ 98.5%, thymoquinone ≥ 98.0%, γ-terpinene 98.0%, p-cymene 98.0%, and rosmarinic acid ≥ 98.0%.
2.2. Bacterial Strains and Growth Conditions
The C. jejuni strains shown in Supplementary Table S1 were stored at −80 °C in 20% glycerol and 80% Mueller Hinton broth (MHB; Oxoid, Basingstoke, UK) and were grown on Mueller-Hinton agar (MHA; Oxoid) at 42 °C under microaerobic conditions (5% O2, 10% CO2, in N2) for 24 h. The second passage of each culture was used in the experiments. When necessary, MHA was supplemented with 30 mg/L kanamycin (Sigma Aldrich) or 4 mg/L chloramphenicol (Merck, Darmstadt, Germany).
2.3. Preparation of the C. jejuni 11168ΔcmeG Mutant Strain
Natural transformation of C. jejuni [18] was used to generate the cmeG insertional mutant strain. The donor DNA was C. jejuni genomic DNA prepared from a previously published mutant strain [6], and the recipient strain was C. jejuni 11168. The cmeG transformants were selected on MHA with 30 mg/L kanamycin, and their DNA was isolated following the protocol described by Klančnik et al. [19]. Successful transformation was confirmed by PCR using the specific primers Cj1375F (CATCTACACAAATGCCACTATCATCACTTAA) and Cj1375R (GCCACAAGCCAAATTAGAGC TAAAATTACTAA), as described previously [6].
2.4. Antimicrobial Activity Assay
The minimal inhibitory concentrations (MICs) of S. montana ethanolic extract and essential oil, and of the pure compounds carvacrol, thymol, thymoquinone, γ-terpinene, p-cymene and rosmarinic acid were determined using the broth microdilution method as described previously [6] for C. jejuni 11,168 and its efflux pump mutants ∆cmeB, ∆cmeR, ∆cmeF, and ∆cmeG. All tested compounds were freshly dissolved in 100% dimethylsulfoxide (DMSO, Sigma Aldrich) before the test. For broth microdilution the tested compounds prepared in DMSO were diluted in MHB (to a final concentration of 2.5% DMSO). The bacterial respiration was determined with 10 μL of resazurin solution (0.25 mg/mL resazurin, 0.14 mg/mL menadion, in MHB; Sigma Aldrich). As resazurin detects C. jejuni numbers over 5 × 105 CFU/mL, the MICs were assessed as the lowest concentration of a compound at which bacterial growth/respiration was inhibited. All tests were carried out in triplicate.
2.5. Checkerboard Assay
The checkerboard method was used to determine the potential synergistic activity of the pure compounds carvacrol, thymol, thymoquinone, γ-terpinene, p-cymene, and rosmarinic acid on C. jejuni 11168, as described previously [20]. Briefly, the tested compounds were prepared in MHB with 2.5% DMSO, and serially diluted in a microtiter plate, where the first compound was two-fold serially diluted in columns, and the second in rows, to a final volume of 100 µL. Then 100 µL C. jejuni 11,168 culture in MHB at 5 × 105 CFU/mL was added to each well. The plates were incubated at 42 °C under microaerobic conditions for 24 h. The MICs of the combinations of compounds were determined with the resazurin solution as described for the antimicrobial activity assay. The tests were carried out in triplicate. The fractional inhibitory concentration index (FICI) was used as the measure of the synergistic activity of these compounds. The FICI is defined as the sum of FICA and FICB as it is shown in Equation (3), where FICA is the MIC of compound A supplemented with compound B (Amix), divided by the MIC of compound A alone (Apure), as shown in Equation (1), and FICB the MIC of compound B supplemented with compound A (Bmix), divided by the MIC of compound B alone (Bpure), as shown in Equation (2):
| FICA = MICAmix/MICApure | (1) |
| FICB = MICBmix/MICBpure | (2) |
| FICI = FICA + FICB | (3) |
These values were interpreted as follows: for FICI ≤ 0.5, a synergistic effect; for FICI > 0.5 and ≤ 4, an additive effect; and for FICI ˃ 4, an antagonistic effect [21].
2.6. Ethidium Bromide Accumulation Assay
To determine whether S. montana ethanolic extract and essential oil, and the pure compounds were efflux pump inhibitors against C. jejuni 11168, an ethidium bromide accumulation assay was carried out, as previously described by Kovač et al. [22], with some modifications. Briefly, cultures of C. jejuni 11,168 previously adjusted to OD600 0.2 in MHB and incubated at 42 °C under microaerobic conditions for 4 h were washed and resuspended in phosphate-buffered saline (Oxoid), and adjusted to OD600 0.4. The prepared ethanolic extract, essential oil, and pure compounds were added to the cultures to a final volume of 100 µL in black microtiter plates (Nunc, Thermo Scientific). Then 0.5 mg/L ethidium bromide (final concentration; Sigma Aldrich) was added. Ethidium bromide is an intercalating agent that binds to the DNA strand within the bacterial cell and emits fluorescence. The accumulation of intracellular ethidium bromide was measured as increasing fluorescence at ʎex 500 nm and ʎem 608 nm, using a microplate reader (Varioskan Lux; Thermo Fisher Scientific) at 1 min intervals over 1 h. As the positive control, the reference efflux pump inhibitor reserpine (Sigma Aldrich) at 10 mg/L was used. The fold-changes in the ethidium bromide accumulation determined after 1 h were used as the measure of the efflux pump inhibition. These measurements were carried out in triplicate.
2.7. Membrane Integrity Assay
The disruptive effects of S. montana ethanolic extract and essential oil, and the pure compounds (i.e., carvacrol, thymol, thymoquinone, γ-terpinene, p-cymene, rosmarinic acid) on the membranes of C. jejuni 11,168 were evaluated using Live/Dead bacterial viability kits (L-7012; Molecular Probes, Eugene, OR, USA), as previously described [22]. Briefly, mid-exponential phase cells (i.e., after an 8-h incubation) were harvested and washed twice with warm phosphate-buffered saline (42 °C), with OD600 adjusted to 0.4. The compounds were added to the prepared cultures at sub-inhibitory concentrations, with no effects on C. jejuni growth. The Live/Dead solution was prepared according to the manufacturer instructions and added to the cultures (1:1, v/v). The SYTO9 fluorescence was measured at λex 481 nm and λem 510 nm at 60 s intervals over 1 h, in black microtiter plates (Nunc). A heat-treated culture (80 °C, 15 min) was used as the negative control, and an untreated culture as the positive control. The relative rates of disruption were calculated considering 100% disruption in the heat-treated culture and 0% disruption in the untreated culture. These experiments were carried out in triplicate.
2.8. Statistics
Statistical analysis was carried out using the SPSS software version 22 (IBM, Armonk, NY, USA). Ethidium bromide accumulation and membrane disruption were compared between untreated and treated cultures using Student’s t-tests or Mann Whitney U tests. In the checkerboard assays, significant synergism was set at FICI ≤ 0.5. Differences in the MICs of the tested compounds between C. jejuni 11,168 wild type and the mutant strains at ≥2 were set as biologically significant.
3. Results
3.1. Chemical Analysis
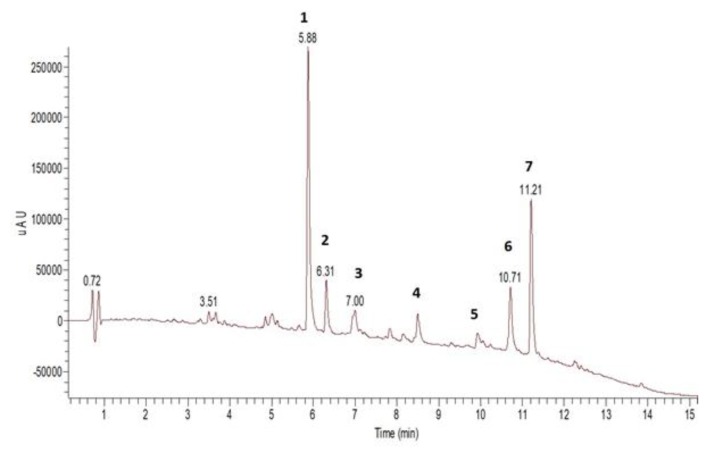
The S. montana ethanolic extract was analysed by UHPLC with PDA and ESI-MS detection. Supplementary Table S2 shows the peak assignments with UV and MS data, and Figure 1 shows a total scan UV chromatogram. Rosmarinic acid (Figure 1, peak 1) was identified as a major constituent of the ethanolic extract. Carvacrol (Figure 1, peak 7) and thymoquinone (Figure 1, peak 6) followed next in terms of quantities. In addition, a caffeoyl rosmarinic acid isomer was identified. A number of methoxylated flavones were identified according to their UV data and their fragmentation patterns with ESI-MS (positive and negative modes); however, to determine the definite substitution patterns, isolation of the pure compounds and structural elucidation by nuclear magnetic resonance would be necessary. Although no easily volatile compounds were detected in the ethanolic extract it became apparent that less volatile compounds of the essential oil like carvacrol and thymoquinone were still present. Hence, in addition the composition of the essential oil was analyzed by GC-MS and GC-FID (Supplementary Table S3). According to these analyses, carvacrol, thymol, thymoquinone, p-cymene, γ-terpinene, and rosmarinic acid were selected as the individual and combined pure test compounds.
Figure 1.
UHPLC separation of Satureja montana ethanolic extract. Total scan UV chromatogram (190-500 nm). Peak assignments: 1 = rosmarinic acid, 2 = caffeoyl rosmarinic acid isomer, 3 = apigeninmethyl ether hexosyldeoxyhexoside, 4 = trihydroxy-trimethoxyflavone, 5 = dihydroxy-dimethoxyflavone, 6 = thymoquinone, 7 = carvacrol.
3.2. Anti-Campylobacter Activity of S. montana Ethanolic Extract and Essential Oil
The antimicrobial activity of the S. montana ethanolic extract and essential oil and the six chosen pure compounds were tested against C. jejuni 11,168 (Table 1). The ethanolic extract and essential oil had medium anti-Campylobacter activity, with MICs of 250 mg/L and 1000 mg/L, respectively.
Table 1.
Antimicrobial activity of Satureja montana ethanolic extract and essential oil, and of the six pure compounds against Campylobacter jejuni 11168, in terms of their minimal inhibitory concentrations (MICs) in mg/L.
| Treatment | MIC (mg/L) |
|---|---|
| Ethanolic extract | 250 |
| Essential oil | 1000 |
| Carvacrol | 31.25 |
| Thymol | 31.25 |
| Thymoquinone | 31.25 |
| p-cymene | 1000 |
| γ-terpinene | 1000 |
| Rosmarinic acid | 250 |
Further, the pure carvacrol, thymol, and thymoquinone had greater antimicrobial activities (MICs of 31.25 mg/L for all three) compared to the ethanolic extract and essential oil. The opposite was seen for the pure p-cymene and γ-terpinene (MICs of 1000 mg/L for both), which showed 4-fold greater MICs compared to the ethanolic extract, but no difference compared to the essential oil. Rosmarinic acid (MIC 250 mg/L) was as active as the ethanolic extract, with a lower MIC compared to the essential oil.
3.3. Synergistic Activity of the Six Pure Compounds
The potential synergistic activity of carvacrol, thymol, thymoquinone, p-cymene, γ-terpinene, and rosmarinic acid were examined using the checkerboard assay and determined through the calculation of the FICI values as: FICI ≤ 0.5, synergistic; FICI > 0.5 and ≤ 4, additive; and FICI ˃ 4, antagonistic [21].
The most effective combinations of these pure compounds that showed strong synergistic activity according to the FICI were (see Table 2 for details): carvacrol plus thymoquinone (FICI, 0.5), carvacrol plus thymol (FICI, 0.2), thymol plus thymoquinone (FICI, 0.3), and thymoquinone plus rosmarinic acid (FICI, 0.5). A second combination of carvacrol (3.9 µg/mL) plus thymol (7.81 µg/mL) was also synergistic (FICI, 0.3). All the other tested combinations showed additive effects, with no antagonistic effects seen. The antimicrobial activity of carvacrol, thymoquinone, and rosmarinic acid in these synergistic combinations was significantly increased compared to the pure compounds alone.
Table 2.
Analysis of the synergistic activity of the six pure compounds tested as binary combinations (compound A + compound B) against Campylobacter jejuni 11168, in terms of the fractional inhibitory concentration index (FICI) calculated from the minimal inhibitory concentrations (MICs) of the pure compounds alone (Apure, Bpure) and when tested together (Amix, Bmix). See Equations (1)–(3) for further details.
| Combination | MIC (mg/L) | FICi | ||||
|---|---|---|---|---|---|---|
| Pure Compounds | Mixed Compounds | |||||
| Compound A | Compound B | Apurea | Bpureb | Amixc | Bmixd | |
| Carvacrol | Thymol | 62.5 | 31.25 | 7.81 | 1.95 | 0.2 |
| Thymoquinone | 62.5 | 31.25 | 15.62 | 7.81 | 0.5 | |
| p-cymene | 62.5 | 500 | 62.5 | 500 | 2.0 | |
| γ-terpinene | 31.25 | 2000 | 31.25 | 2000 | 2.0 | |
| Rosmarinic acid | 31.25 | 250 | 31.25 | 250 | 2.0 | |
| Thymol | Thymoquinone | 31.25 | 31.25 | 3.9 | 3.9 | 0.3 |
| p-cymene | 31.25 | 31.25 | 31.25 | 31.25 | 2 | |
| γ-terpinene | 125 | 1000 | 62.5 | 1000 | 1.5 | |
| Rosmarinic acid | 31.25 | 250 | 31.25 | 250 | 2 | |
| Thymoquinone | p-cymene | 15.62 | 1000 | 7.81 | 1000 | 1.5 |
| γ-terpinene | 31.25 | 1000 | 7.81 | 500 | 0.8 | |
| Rosmarinic acid | 31.25 | 250 | 7.81 | 62.50 | 0.5 | |
| p-cymene | γ-terpinene | 250 | 1000 | 125 | 1000 | 1.5 |
| Rosmarinic acid | 1000 | 250 | 500 | 125 | 1.0 | |
| γ-terpinene | Rosmarinic acid | 1000 | 250 | 1000 | 125 | 1.5 |
a—MIC of compound A when tested alone (e.g., carvacrol alone); b—MIC of compound B when tested alone (e.g., thymol); c—the concentration of compound A in AB mixture (e.g., carvacrol in carvacrol + thymol mixture) at MIC of AB; d—the concentration of compound B in AB mixture (e.g., thymol in carvacrol + thymol mixture) at MIC of AB.
3.4. Efflux Pump Inhibition in C. jejuni
The efflux pump inhibitory effects of S. montana ethanolic extract and essential oil, and their six major constituents, were determined in C. jejuni 11,168, using the ethidium bromide accumulation assay. Ethidium bromide accumulation is used as a measure of efflux pump activity of a culture. When ethidium bromide accumulates in the cells it is toxic, and therefore the cells use their efflux pumps to eliminate it. Within the cell, the intercalation of ethidium bromide in the DNA strand results in a fluorescent signal. Thus, for this assay, higher fluorescence indicates higher accumulation of ethidium bromide, which in turn translates into lower efflux pump activity, and hence their inhibition.
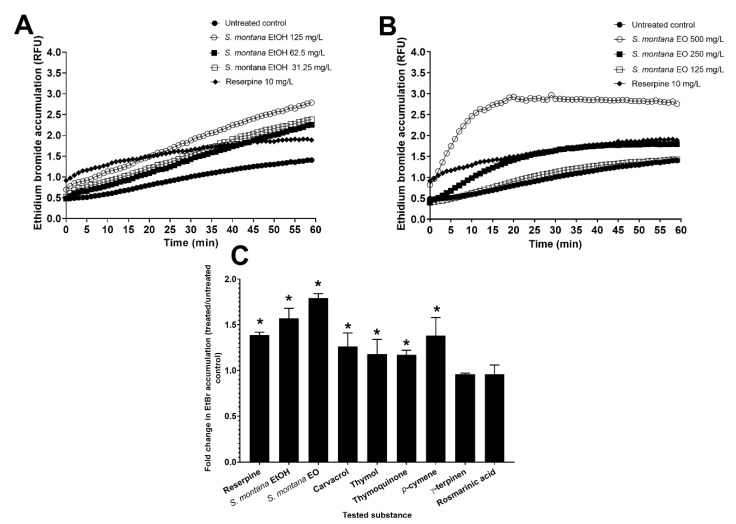
The highest tested concentration of S. montana ethanolic extract (125 mg/L) resulted in a 1.9-fold increase in C. jejuni ethidium bromide accumulation after 1 h of treatment (Figure 2A). The greatest accumulation of ethidium bromide, 2.07-fold as compared to the untreated control (Figure 2B), was observed for the cultures treated with S. montana essential oil at 0.5× MIC (500 mg/L). Both the ethanolic extract and the essential oil showed a stronger effect than reserpine (1.39-fold), the known efflux pump inhibitor, and in both cases the effects were concentration dependent, as at lower concentrations they had lesser effects on ethidium bromide accumulation.
Figure 2.
Effect of S. montana (A) ethanolic extract (EtOH) and (B) essential oil on membrane integrity disruption in C. jejuni NCTC 11,168 in sub-inhibitory concentrations of 0.5× (125 and 500 mg/L), 0.25× (62.5 and 250 mg/L), and 0.125× (31.25 and 125 mg/L) of the minimal inhibitory concentration (MIC), presented in relative fluorescent units (RFU) measured for 1 h in 1 min intervals. Fold increase in EtBr accumulation (C) in the treated culture compared to untreated culture is shown for S. montana ethanolic extract and essential oil, and 6 pure compounds, at the subinhibitory concentration of 0.25× MIC. Reserpine at 10 mg/L was used as a positive control. A higher value presents higher accumulation of ethidium bromide and thus a lower activity and inhibition of C. jejuni efflux pumps. *, p < 0.05 (Student’s t-test).
The treatment of C. jejuni with the ethanolic extract and essential oil at 0.25× MICs increased the ethidium bromide accumulation by 1.57-fold and 1.79-fold, respectively (Figure 2C). Across all six pure compounds, only p-cymene showed any appreciable efflux pump inhibitory activity, with a 1.38-fold increase in ethidium bromide accumulation. Carvacrol had a weak effect, with a 1.26-fold increase in ethidium bromide accumulation. Thymol, thymoquinone, γ-terpinene, and rosmarinic acid only increased the ethidium bromide accumulation by up to 1.2-fold.
3.5. Disruption of Membrane Integrity in C. jejuni
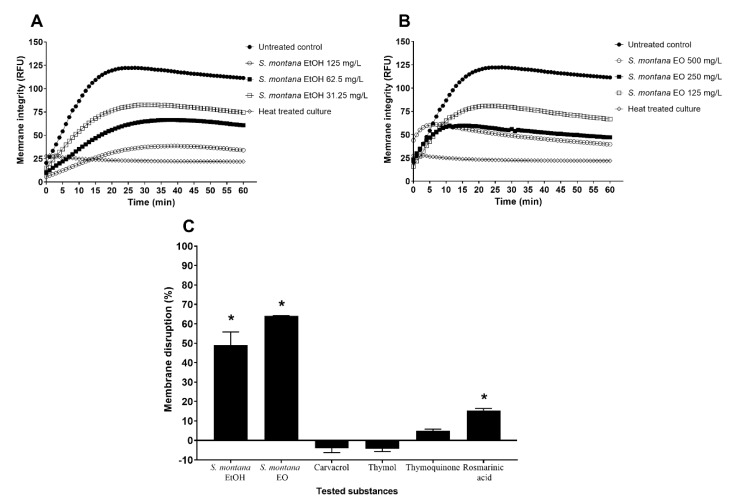
All the tested concentrations of S. montana ethanolic extract and essential oil showed disruptive effects on the membranes of C. jejuni 11,168, and these effects were concentration dependent. At 0.5× MICs of S. montana ethanolic extract and essential oil, treatment for 1 h decreased C. jejuni membrane integrity by 88% and 83%, respectively (Figure 3A,B). The concentrations of 0.25× MICs for all these samples were chosen for further testing, as they had no effects on the growth of C. jejuni. The ethanolic extract and essential oil at 0.25× MICs damaged the C. jejuni membranes by 49% and 64%, respectively. Of the tested pure compounds, again at 0.25× MICs, only rosmarinic acid showed any disruption of the membranes (15%).
Figure 3.
Effect of S. montana (A) ethanolic extract (EtOH) and (B) essential oil (EO) on membrane integrity disruption of C. jejuni 11,168 in sub-inhibitory concentrations of 0.5 × (125 and 500 mg/L), 0.25 × (62.5 and 250 mg/L), and 0.125 × (31.25 and 125 mg/L) of the minimal inhibitory concentration (MIC), presented in relative fluorescent units (RFU) measured for 1 h in 1 min intervals. A higher value presents higher accumulation of ethidium bromide and thus a lower activity and inhibition of C. jejuni efflux pumps. The disruption of C. jejuni membrane (C) in the treated culture compared to the untreated culture, is shown for S. montana ethanolic extract and essential oil, and 6 pure compounds, at the subinhibitory concentration of 0.25× MIC as the percentage (%) of disruption. Membrane disruption of p-cymene and γ-terpinene could not be measured because of autofluorescence of compounds. *, p < 0.05 (Student’s t-test).
3.6. Involvement of Efflux Systems in Resistance of C. jejuni to S. montana Ethanolic Extract and Essential Oil and Their Six Major Constituents
To examine the involvement of the efflux systems of C. jejuni in its resistance to the antimicrobial activity of S. montana ethanolic extract and essential oil, and their six major constituents, their MICs were determined for wild-type C. jejuni 11,168 and for the efflux pump mutants: ΔcmeF, which lacks a functional CmeDEF efflux pump; ΔcmeG, which lacks a functional CmeGH efflux pump; ΔcmeB, which lacks a functional CmeABC efflux pump; and ΔcmeR, which lacks a functional CmeR repressor of the CmeABC efflux pump (Table 3).
Table 3.
Antimicrobial activity of Satureja montana ethanolic extract and essential oil, and of the six pure compounds against the Campylobacter jejuni 11,168 efflux pump mutants, in terms of their minimal inhibitory concentrations (MICs). ΔcmeB, lacking functional efflux pump CmeABC; ΔcmeR, lacking repressor of efflux pump CmeABC; ΔcmeF, lacking functional efflux pump CmeDEF; and ΔcmeG, lacking functional efflux pump CmeGH.
| Treatment | ΔcmeB | ΔcmeR | ΔcmeF | ΔcmeG | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) | FCa | MIC (mg/L) | FCa | MIC (mg/L) | FCa | MIC (mg/L) | FCa | |
| Ethanolic extract | 31.25 | 4 | 125 | 1 | 62.5 | 2 | 31.25 | 4 |
| Essential oil | 1000 | 1 | 1000 | 1 | 1000 | 1 | 500 | 2 |
| Carvacrol | 15.25 | 2 | 7.81 | 4 | 15.62 | 2 | 0.97 | 32 |
| Thymol | 7.81 | 4 | 7.81 | 4 | 31.25 | 1 | 1.95 | 16 |
| Thymoquinone | 15.25 | 2 | 7.81 | 4 | 15.62 | 2 | 0.97 | 32 |
| p-cymene | 1000 | 1 | 500 | 2 | 500 | 2 | 500 | 2 |
| γ-terpinene | 500 | 2 | 250 | 4 | 250 | 4 | 500 | 2 |
| Rosmarinic acid | 62.5 | 4 | 125 | 4 | 125 | 2 | 125 | 2 |
aFC, Fold change increase in antimicrobial activity of the tested compound in the mutant strain, compared to wild type.
Here, the CmeGH efflux pump had a major role in C. jejuni resistance to both S. montana ethanolic extract and essential oil, whereas the CmeABC efflux pump was involved only in resistance to the ethanolic extract. The antimicrobial activity of S. montana ethanolic extract against C. jejuni ΔcmeB and ΔcmeG was 4-fold greater than for C. jejuni wild-type. The lack of the CmeDEF efflux pump resulted in only a halving of the MIC of the ethanolic extract, with no changes seen for the activity of the essential oil.
Inactivation of the CmeABC efflux pump resulted in 4-fold the antimicrobial activity of both thymol and rosmarinic acid, and in 2-fold the antimicrobial activity of both carvacrol and γ-terpinene. Inactivation of the CmeDEF efflux pump increased the γ-terpinene antimicrobial activity by 4-fold, and to a lesser degree (2-fold) for carvacrol, thymoquinone, p-cymene, and rosmarinic acid. The highest impact on the C. jejuni resistance to the pure compounds was for the inactivation of the CmeGH efflux pump. Here, carvacrol and thymoquinone showed 32-fold increased antimicrobial activities in the mutant strain compared to the wild-type, with 16-fold increase seen for thymol. This indicates the strong involvement of the CmeGH efflux pump in the C. jejuni resistance to these compounds. However, the effects of inactivation of the CmeGH efflux pump on the antimicrobial activity of p-cymene, γ-terpinene, and rosmarinic acid were minor (2-fold increase). Surprisingly, the CmeR deficient mutant was more sensitive to carvacrol, thymol, thymoquinone, γ-terpinene, and rosmarinic acid, with 4-fold antimicrobial activities seen.
4. Discussion
In this study, we analyzed the anti-Campylobacter activity of S. montana with a comprehensive approach in which we chemically characterized the ethanolic extract and essential oil and their antimicrobial action against C. jejuni. The modes of action of S. montana and its major constituents were determined, but also the importance of efflux pump involvement in Campylobacter resistance against S. montana was shown.
The chemical composition and the anti-Campylobacter activity of an ethanolic extract and an essential oil from the common culinary herb winter savory (S. montana) were examined. Rosmarinic acid was the major compound in the ethanolic extract, already reported as one of the most common caffeic acid esters in Lamiaceae [23]. In addition, a caffeoyl rosmarinic acid isomer was detected. It’s UV and MS data are similar to those for melitric acid A, which was recently described for Satureja biflora [24]. Carvacrol was seen to make up about 63% of the essential oil, as already observed by other authors [25], and was also present as a major compound in the ethanolic extract, together with thymoquinone. Conversely, thymoquinone was found at a low level in the essential oil (1.8%); however, it was extracted as a higher proportion of the ethanolic extract and might be of relevance for anti-Campylobacter effects.
S. montana ethanolic extract and essential oil proved to have good anti-microbial activity against C. jejuni, higher than what was reported on other foodborne pathogens such as E. coli, S. aureus, and B. cereus [15]. These differences might be due to the different origin of the same plant, the different strain investigated, and/or the reaction of these foodborne pathogens to the extracts. The difference between antimicrobial activity of the ethanolic extract and essential oil (4-fold) against C. jejuni may be due to their different compositions and thus differences in their stability in an aqueous solution with an organic solvent, as they were prepared in this study.
S. montana ethanolic extract and essential oil were seen to contain many components, and based on the chemical analyses, the pure forms of the six major components were investigated individually, as: carvacrol, thymol, thymoquinone, p-cymene, γ-terpinene, and rosmarinic acid. The antimicrobial and synergistic activity of these compounds ranged from very strong to weak. In detail, for carvacrol and thymol, whose anti-Campylobacter activity was also studied by Alphen et al. [26], Kelly et al. [27], Upadhyay [28] and others, our data confirmed the published data, in terms of good antimicrobial activity against C. jejuni. On the other side, when thymoquinone was studied for its activity against S. aureus and P. aeruginosa, no effects were observed, although it was reported to be effective against Vibrio parahaemolyticus [29] and E. coli [30]. In the present study, thymoquinone showed antimicrobial activity against C. jejuni that was comparable to carvacrol and thymol, which also confirms the better antimicrobial activity of thymoquinone against Gram-negative bacteria. p-cymene has been shown to be effective against the enteric pathogens L. monocytogenes, E. coli, and Salmonella spp. [31], but its anti-Campylobacter activity in the present study was weak. Weak antimicrobial activity of γ-terpinene against E. coli and S. aureus was reported by Burt et al. [32], which is confirmed in the present study for C. jejuni. Finally, rosmarinic acid, a well-known polyphenol, has been shown to have antibacterial activity against several foodborne pathogens as S. aureus, E. coli, and Shigella spp. [33]. The results of this study confirmed the observations of Klančnik et al. [19] of a medium anti-Campylobacter activity of rosmarinic acid.
The major constituents of S. montana ethanolic extract and essential oil had both strong and weak antimicrobial activity, whereby the overall activity of S. montana ethanolic extract and essential oil reflects the antimicrobial activities of the pure compounds.
Natural extract and essential oils are mixtures of many different compounds, and thus synergistic or antagonistic activities of their constituents are likely. Previous studies have shown increased activity for p-cymene when combined with carvacrol [34], and for carvacrol in combination with thymol [32,35], which were confirmed for C. jejuni in the present study. Here, synergism was also seen for the combinations of thymoquinone with carvacrol, thymol, and rosmarinic acid. These tested major constituents of S. montana have been shown to affect the cell membrane of bacteria [36]. Here we see that, even when we combine two compounds with apparently similar action, e.g., carvacrol and thymol, their antimicrobial activity will be potentiated. This points to different targets of these constituents in the C. jejuni cell.
This study shows that the antimicrobial activity of the S. montana ethanolic extract and essential oil cannot be attributed to the action of any one single compound but will be the result of the combined, be it strong or weak, effects of their constituents. It should be noted that only binary combinations of only six pure compounds were tested here, therefore we cannot conclude what the exact effect of multiple combinations in one extract/essential oil would be. In C. jejuni, efflux pump inhibitors can increase its sensitivity to various antimicrobials, including antibiotics [22,37]. The inhibitory effects on the C. jejuni efflux pumps of S. montana ethanolic extract were weak compared to those of the resistance modulator α-pinene [22], which is also a constituent of S. montana herb, but were still stronger than those of the known efflux pump inhibitor reserpine. This makes S. montana ethanolic extract an important subject for future consideration in studies on resistance modulation of C. jejuni.
The inhibitory effects on C. jejuni efflux pumps of S. montana ethanolic extract and essential oil were not reflected in the activity of the six pure compounds tested in this study (Figure 2). This is an important issue to consider when searching for new efflux pump inhibitors derived from natural extracts, as the pure compounds might not reflect the activity of the whole phytocomplex.
The Lamiaceae family has been known to harbor plants with vast biological properties, including antimicrobial activity. Modes of action of these essential oils and their constituents are targeting the cell membrane with protein degradation and membrane disruption [36]. The Gram-negative cell membrane is a formidable barrier for antimicrobials to bypass, and thus the search for membrane disruptors is an important challenge. Both S. montana ethanolic extract and essential oil had membrane disruptive effects on C. jejuni, although again, these were not confirmed by data of the six pure compounds (Figure 3). Indeed, although La Storia et al. [38] reported that carvacrol acts as a membrane disruptor, the present study does not confirm. In this respect, it should be taken into account that we tested the modes of action at sub-inhibitory concentrations, in contrast to the higher inhibitory concentrations that are usually evaluated.
In particular, the efflux pump inhibitory effects and membrane disruptive effects of S. montana ethanolic extract and essential oil, and the lack of these effects in the pure compounds tested, indicate the presence of further compounds in the ethanolic extract that are present at lower concentrations, but that act as potentially more potent efflux pump inhibitors and membrane disruptors.
When a new antimicrobial compound is tested, the resistance mechanisms of bacteria against the compound in question need to be considered. It has been shown that C. jejuni major efflux pumps that are involved in resistance against antibiotics and bile salts [6,8] also contribute to the resistance of C. jejuni to natural extracts and phenolic compounds [19,22]. In the present study, both the CmeABC and CmeDEF efflux pump systems were seen to be involved in the resistance against S. montana ethanolic extract and essential oil, and their major constituents, which is not surprising, as their substrates often overlap [39]. Interestingly, the C. jejuni 11168∆cmeR mutant showed increased sensitivity to the tested compounds. CmeR acts as a repressor of the promotor of the CmeABC multidrug efflux pump, and mutation of the cmeR gene leads to enhanced production of this pump, and higher resistance of C. jejuni to antibiotics [8,40]. This shows the involvement of the CmeABC efflux pump in the excretion of these compounds, and its importance as part of the C. jejuni resistance to S. montana ethanolic extract and essential oil, although there is no threat for the development of a higher resistance against S. montana with increased efflux.
The involvement of the CmeGH efflux pumps in the resistance of C. jejuni to S. montana ethanolic extract and essential oil is similar to that of the CmeABC and CmeDEF efflux pumps. Interestingly, the involvement of the CmeGH efflux pump was most prominent in the resistance against thymol, carvacrol, and thymoquinone. This increase in sensitivity of the mutant strain that lacked a functional CmeGH efflux pump was much higher than previously reported for antibiotic susceptibility of C.jejuni 11,168∆cmeG mutant strain. Jeon et al. [6] showed increased susceptibility of the C. jejuni 11168∆cmeG mutant strain to erythromycin (8-fold), ciprofloxacin (4-fold), gentamycin (16-fold), tetracycline (4-fold), and ethidium bromide (8-fold). In the present study, there was a 32-fold increase in the susceptibility of C. jejuni 11168∆cmeG mutant strain for carvacrol and thymoquinone. Our data show, for the first time, a major role for the CmeGH efflux pump in the resistance of C. jejuni against carvacrol, thymol, and thymoquinone, and therefore against natural extracts and essential oils that contain these compounds.
5. Conclusions
Although the antimicrobial activity of the pure compounds surpassed that of either S. montana ethanolic extract and essential oil, this was not reflected in the efflux pump inhibition or the membrane disruption. This shows how such a complex mixture of different compounds cannot be described only by its major constituents, and that the action of multiple compounds is necessary to achieve the desired effects. With their good efflux pump inhibitory activity, S. montana ethanolic extract and essential oil show promise as resistance modulators. Although to confirm this, further testing is necessary, including the possible synergistic activity of the extracts with antibiotics of clinical importance (e.g., ciprofloxacin and erythromycin) in vitro and in vivo. The C. jejuni major efflux pump also has a vital role in C. jejuni resistance to S. montana ethanolic extract and essential oil, although the present study does not allow us to conclude that the occurrence of resistance against S. montana ethanolic extract and essential oil would be due to increased CmeABC efflux pump activity. However, this study demonstrates the use of natural compounds to increase Campylobacter susceptibility, and thus the possibility of alternative control strategies.
Acknowledgments
The authors thank Kaja Erzar for her contribution and technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/4/537/s1, Table S1: Strain designation, description and reference of strains used in the study, Table S2: UHPLC-PDA-ESI-MS analysis of the ethanolic extract of Satureja montana herbal parts, Table S3: Chemical analysis of S. montana essential oil.
Author Contributions
Conceptualization, K.Š., F.B., A.K., and S.S.M.; data curation, K.Š., and F.B.; formal analysis, K.Š., F.B., and F.P.; funding acquisition, F.B., A.P., and S.S.M.; investigation, K.Š., F.B., F.P. and S.S.M.; methodology, K.Š., F.B., and A.K.; project administration, F.B., A.K., A.P., and S.S.M.; resources, F.B., and S.S.M.; supervision, K.Š., F.B., A.P., and S.S.M.; validation, K.Š., F.B., and A.K.; visualization, K.Š., and F.B.; writing—original draft preparation, K.Š., F.B., F.P., and A.K.; writing—review and editing, A.K., F.B., A.P., and S.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (ARRS) grant P4-0116, and bilateral grant of ARRS and OEAD AT-SLO-2016/2017. K.Š. was supported with the CEEPUS and GoStyria exchange scholarships. F.P. was supported with the ERASMUS exchange scholarship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.European Food Safety Authority. European Centre for Disease Prevention and Control The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaakoush N.O., Castaño-Rodríguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachamkin I., Allos B.M., Ho T. Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 1998;11:555–567. doi: 10.1128/CMR.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel on Biological Hazards European Food Safety Authority Scientific opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9 doi: 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 5.EFSA. ECDC The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019;17:e05598. doi: 10.2903/j.efsa.2019.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon B., Wang Y., Hao H., Barton Y.-W., Zhang Q. Contribution of CmeG to antibiotic and oxidative stress resistance in Campylobacter jejuni. J. Antimicrob. Chemother. 2011;66:79–85. doi: 10.1093/jac/dkq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J., Michel L.O., Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J., Akiba M., Sahin O., Zhang Q. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 2005;49:1067–1075. doi: 10.1128/AAC.49.3.1067-1075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieira A., Ramesh A., Seddon A.M., Karlyshev A.V. CmeABC multidrug efflux pump contributes to antibiotic resistance and promotes Campylobacter jejuni survival and multiplication in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 2017;15:83. doi: 10.1128/AEM.01600-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumpert M., Kreft S. Folk use of medicinal plants in Karst and Gorjanci, Slovenia. J. Ethnobiol. Ethnomed. 2017;13:16. doi: 10.1186/s13002-017-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrier J., Saciragic L., Trakić S., Chen E.C.H., Gendron R.L., Cuerrier A., Balick M.J., Redžić S., Alikadić E., Arnason J.T. An ethnobotany of the Lukomir Highlanders of Bosnia & Herzegovina. J. Ethnobiol. Ethnomed. 2015;11:81. doi: 10.1186/s13002-015-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasser S., Schunko C., Vogl C.R. Gathering “tea”—From necessity to connectedness with nature. Local knowledge about wild plant gathering in the Biosphere Reserve Grosses Walsertal (Austria) J. Ethnobiol. Ethnomed. 2012;1:31. doi: 10.1186/1746-4269-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieroni A., Rexhepi B., Nedelcheva A., Hajdari A., Mustafa B., Kolosova V., Cianfaglione K., Quave C.L. One century later: The folk botanical knowledge of the last remaining Albanians of the upper Reka Valley, Mount Korab, Western Macedonia. J. Ethnobiol. Ethnomed. 2013;9:22. doi: 10.1186/1746-4269-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano C., Matos O., Teixeira B., Ramos C., Neng N., Nogueira J., Nunes M.L., Marques A. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 2011;91:1554–1560. doi: 10.1002/jsfa.4347. [DOI] [PubMed] [Google Scholar]
- 15.Ćetković G.S., Čanadanović-Brunet J.M., Djilas S.M., Tumbas V.T., Markov S.L., Cvetković D.D. Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L. subsp. kitaibelii Extracts. Int. J. Mol. Sci. 2007;8:1013–1027. [Google Scholar]
- 16.Vasilijević B., Mitić-Ćulafić D., Djekic I., Marković T., Knežević-Vukčević J., Tomasevic I., Velebit B., Nikolić B. Antibacterial effect of Juniperus communis and Satureja montana essential oils against Listeria monocytogenes in vitro and in wine marinated beef. Food Control. 2019;100:247–256. doi: 10.1016/j.foodcont.2019.01.025. [DOI] [Google Scholar]
- 17.De Oliveira T.L.C., de Araújo Soares R., Ramos E.M., das Graças Cardoso M., Alves E., Piccoli R.H. Antimicrobial activity of Satureja montana L. essential oil against Clostridium perfringens type A inoculated in mortadella-type sausages formulated with different levels of sodium nitrite. Int. J. Food Microbiol. 2011;144:546–555. doi: 10.1016/j.ijfoodmicro.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Taylor D.E. Natural transformation in Campylobacter species. J. Bacteriol. 1990;172:949–955. doi: 10.1128/JB.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klančnik A., Smole Možina S., Zhang Q. Anti-Campylobacter activities and resistance mechanisms of natural phenolic compounds in Campylobacter. PLoS ONE. 2012;7:e51800. doi: 10.1371/journal.pone.0051800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh M.H., Yu C.M., Yu V.L., Chow J.W. Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect. Dis. 1993;16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 21.EUCAST Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000;6:503–508. doi: 10.1046/j.1469-0691.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 22.Kovač J., Šimunović K., Wu Z., Klančnik A., Bucar F., Zhang Q., Smole Možina S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE. 2015;10:e0122871. doi: 10.1371/journal.pone.0122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zgórka G., Głowniak K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001;26:79–87. doi: 10.1016/S0731-7085(01)00354-5. [DOI] [PubMed] [Google Scholar]
- 24.Moghadam S.E., Ebrahimi S.N., Gafner F., Ochola J.B., Marubu R.M., Lwande W., Frei Haller B., Salehi P., Hamburger M. Metabolite profiling for caffeic acid oligomers in Satureja biflora. Ind. Crops Prod. 2015;76:892–899. doi: 10.1016/j.indcrop.2015.07.059. [DOI] [Google Scholar]
- 25.Pellegrini M., Ricci A., Serio A., Chaves-Lopez C., Mazzarrino G., D’Amato S., Sterzo C.L., Paparella A. Characterization of essential oils obtained from abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods. 2018;7:19. doi: 10.3390/foods7020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Alphen L.B., Burt S.A., Veenendaal A.K.J., Bleumink-Pluym N.M.C., van Putten J.P.M. The natural antimicrobial carvacrol inhibits Campylobacter jejuni motility and infection of epithelial cells. PLoS ONE. 2012;7:e45343. doi: 10.1371/journal.pone.0045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly C., Gundogdu O., Pircalabioru G., Cean A., Scates P., Linton M., Pinkerton L., Magowan E., Stef L., Simiz E., et al. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog. Dis. 2017;14:341–349. doi: 10.1089/fpd.2016.2265. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyay A., Arsi K., Wagle B.R., Upadhyaya I., Shrestha S., Donoghue A.M., Donoghue D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017 doi: 10.3389/fmicb.2017.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaieb K., Kouidhi B., Jrah H., Mahdouani K., Bakhrouf A. Antibacterial activity of thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011;11:29. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad Z., Laughlin T.F., Kady I.O. Thymoquinone inhibits Escherichia coli ATP synthase and cell growth. PLoS ONE. 2015;10:e0127802. doi: 10.1371/journal.pone.0127802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchese A., Arciola C.R., Barbieri R., Silva A.S., Nabavi S.F., Tsetegho Sokeng A.J., Izadi M., Jafari N.J., Suntar I., Daglia M., et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials. 2017;10:947. doi: 10.3390/ma10080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burt S.A., Vlielander R., Haagsman H.P., Veldhuizen E.J.A. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 2005;68:919–926. doi: 10.4315/0362-028X-68.5.919. [DOI] [PubMed] [Google Scholar]
- 33.Ekambaram S.P., Perumal S.S., Balakrishnan A., Marappan N., Gajendran S.S., Viswanathan V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016;5:358–363. doi: 10.5455/jice.20160906035020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rattanachaikunsopon P., Phumkhachorn P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010;110:614–619. doi: 10.1016/j.jbiosc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Gavaric N., Smole Možina S., Kladar N., Bozin B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants. 2015;18:1013–1021. doi: 10.1080/0972060X.2014.971069. [DOI] [Google Scholar]
- 36.Nieto G. Biological activities of three essential oils of the Lamiaceae family. Medicines. 2017;4:63. doi: 10.3390/medicines4030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovač J., Gavaric N., Bucar F., Smole Možina S. Antimicrobial and resistance modulatory activity of Alpinia katsumadai seed phenolic extract, essential oil and post-distillation extract. Food Technol. Biotechnol. 2014;52:248–254. [Google Scholar]
- 38.La Storia A., Ercolini D., Marinello F., Di Pasqua R., Villani F., Mauriello G. Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Res. Microbiol. 2011;162:164–172. doi: 10.1016/j.resmic.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Pumbwe L., Randall L.P., Woodward M.J., Piddock L.J.V. Evidence for multiple-antibiotic resistance in Campylobacter jejuni not mediated by CmeB or CmeF. Antimicrob. Agents Chemother. 2005;49:1289–1293. doi: 10.1128/AAC.49.4.1289-1293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T., Cheng Y., Luo Q., Lu Q., Dong J., Zhang R., Wen G., Wang H., Luo L., Wang H., et al. Correlation between gyrA and CmeR box polymorphism and fluoroquinolone resistance in Campylobacter jejuni isolates in China. Antimicrob. Agents Chemother. 2017;61:e00422-17. doi: 10.1128/AAC.00422-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.