Abstract
Rapid shifts in biotic communities due to environmental variability challenge the detection of anthropogenic impacts by current biomonitoring programs. Metacommunity ecology has the potential to inform such programs, because it combines dispersal processes with niche-based approaches and recognizes variability in community composition. Using intermittent rivers—prevalent and highly dynamic ecosystems that sometimes dry—we develop a conceptual model to illustrate how dispersal limitation and flow intermittence influence the performance of biological indices. We produce a methodological framework integrating physical- and organismal-based dispersal measurements into predictive modeling, to inform development of dynamic ecological quality assessments. Such metacommunity-based approaches could be extended to other ecosystems and are required to underpin our capacity to monitor and protect ecosystems threatened under future environmental changes.
Keywords: bioassessment, connectivity, dispersal, intermittent rivers and streams, climate change
Ecosystems are increasingly affected by anthropogenic impacts such as land-use change and climate change, altering the structure and function of biological communities (Haddad et al. 2015, Tonkin et al. 2019). However, our knowledge of how different biodiversity metrics are affected by human activities is still limited, which hampers their effective use in the detection and biomonitoring of anthropogenic impacts (Heino et al. 2015, Donohue et al. 2016). This challenge is especially pertinent for highly dynamic ecosystems (HDEs), which experience wide temporal and spatial environmental variability because of recurrent natural disturbances such as droughts, fires, and floods, that act at relatively short timescales (Datry et al. 2016a, Ryo et al. 2019). HDEs include ecosystems such as estuaries, coastal lagoons, floodplains, temporary ponds, intermittent rivers, fire-prone shrublands, and coastal dune systems. Their inherent dynamism poses a challenge for assessing and predicting the ecological effects of anthropogenic activities. This is because reference conditions (i.e., natural undisturbed conditions) are also variable and therefore difficult to define (Ghazoul et al. 2015) and concurrent ecological responses to both natural and human-induced disturbances can be complex and difficult to disentangle (box 1; Donohue et al. 2016).
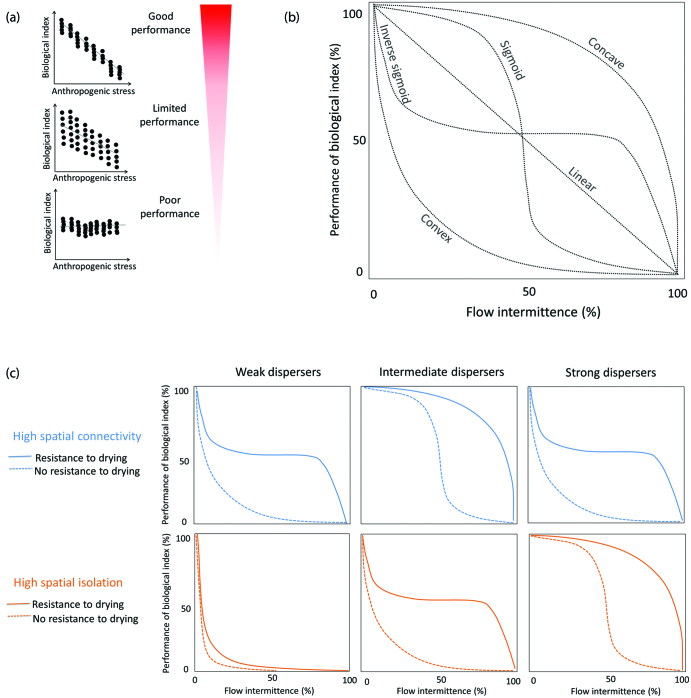
Box 1. High variability limits the performance of biological indices.
In general, biomonitoring requires that biological variables, including indices designed to summarize community structure and composition, respond predictably to anthropogenic stressors (Bonada et al. 2006). Therefore, a biological index performs effectively if it changes in response to increasing anthropogenic stress levels (figure 3a). Effective indices therefore enable sites to be classified according to their level of biological impairment. One way to assess if a site is affected is to compare its observed (O) index value with the expected (E) value obtained from unimpaired reference sites (Clarke et al. 2003) or, in the absence of unimpaired sites (e.g., sites in catchments dominated by human land uses), E can be estimated by modeling biotic communities on the basis of environmental filters (Chessman and Royal 2004). In either case, when the index responds to anthropogenic stress but values are highly variable, its performance is limited and unimpaired sites may be misclassified as impaired and vice versa. Finally, index performance is poor when it cannot detect anthropogenic stress, even if variability in index values is low. Both situations may occur in HDEs, principally because of two major challenges: setting reference conditions and distinguishing between the effects of natural environmental dynamism and anthropogenic stress.
In HDEs such as intermittent rivers—that is, those that recurrently cease to flow or dry (figure 1)—current biomonitoring methods rely on those developed for perennial watercourses. If monitored, biological communities are sampled only during the flowing phase, and biological quality is assessed using reference values obtained from perennial rivers (Stubbington et al. 2018). This usually leads to poor performance of biological indices (Chessman et al. 2010, Soria et al. 2020) as a result of lower taxa richness (Soria et al. 2017) and a higher number of tolerant species (Bogan et al. 2017). In regions in which nonperennial rivers prevail, many efforts have been made to adapt traditional biomonitoring methods (Chiu et al. 2017). In Mediterranean-climate regions, characterization of the flow regime represents an important step toward the adaptation of reference conditions (Gallart et al. 2017). Still, spatiotemporal variability has not been adequately addressed. In highly isolated sites, dispersal limitation can influence the composition of local communities (Cañedo-Argüelles et al. 2015) and having information only on the flow regime may not be sufficient. For example, in sites with comparable river typology and flow intermittence regimes but different distances to the nearest perennial water body, the biomonitoring index IBMWP (Alba-Tercedor et al. 2002) can show very different bioassessment results (figure 2). HDEs also show high temporal species turnover (Tonkin et al. 2017), and only considering the flowing phase could lead to underestimation of overall diversity at reference sites. Therefore, a metacommunity perspective that also incorporates temporal dynamics (Sarremejane et al. 2017a) has a considerable potential to inform the biomonitoring of HDEs.
Current biomonitoring methods assume that biological communities are relatively stable and governed by local abiotic conditions through environmental filtering (i.e., a niche-based approach). However, communities typically occur as part of metacommunities that are connected by dispersal across the landscape, and are therefore influenced by regional processes (Leibold et al. 2004). In HDEs, the importance of the metacommunity context may be stronger than in other ecosystems because of recurrent community reassembly following disturbances, which creates spatiotemporally variable metacommunities (Datry et al. 2016a). Despite considerable efforts to adapt traditional biomonitoring approaches for HDEs (e.g., Elliott and Quintino 2007, Stubbington et al. 2018, Pitacco et al. 2019), no approach has adopted a metacommunity perspective that considers dispersal. Moving beyond a niche-based approach and recognizing regional dispersal processes could improve the accuracy of biomonitoring methods and inform effective ecosystem management strategies.
Intermittent rivers are archetypal HDEs (box 1, figure 1). They are globally dominant and support considerable biodiversity and provide many ecosystem functions and services (Datry et al. 2014), The in-stream conditions of intermittent rivers change substantially in time and space among and within lotic (flowing), lentic (nonflowing), and terrestrial (dry) phases (Datry et al. 2014, 2016a, Gallart et al. 2017). Reflecting the relative duration and predictability of these phases, such systems include streams characterized by long dry periods interrupted by short, unpredictable flow events after rain, and systems with longer, seasonal flowing phases during which disconnected pools may persist. Their biotic communities also change continuously, reflecting local colonization and extinction events associated with shifts between phases (Sarremejane et al. 2017a). Regional processes (i.e., dispersal) are key influences on aquatic community reassembly after habitats become reconnected by flow resumption, and contribute substantially to the maintenance of local and regional aquatic biodiversity (Sarremejane et al. 2017a).
Figure 1.

Intermittent rivers in contrasting climatic regions during flowing and dry phases: (a) Cérvol, in Mediterranean-climate Spain. (b) Manifold, oceanic England, United Kingdom. Photographs: (a) Núria Cid. (b) Nick Mott.
The dynamic nature of intermittent rivers hampers their adequate biomonitoring and management (Fritz et al. 2017). In a biomonitoring context, their highly variable reference conditions (Cid et al. 2017) and the different responses of biological indices across gradients of anthropogenic stress (Soria et al. 2020) can reduce the accuracy of biological quality assessments (box 1). As a result, the implementation of sustainable water resource management strategies is less well developed in intermittent compared with perennial rivers, which hinders compliance with international and national legislation (Fritz et al. 2017, Marshall et al. 2018). As intermittent rivers increase in extent because of climate change and water demands (Döll and Schmied 2012), adapting tools and methods to enable their assessment and conservation is increasingly urgent.
In the present article, using intermittent rivers as typical HDEs, we demonstrate how a metacommunity approach could integrate niche- and dispersal-based processes to guide biomonitoring. We present a conceptual model illustrating how flow intermittence and dispersal proxies may limit the performance of biomonitoring methods. We then convert these concepts into recommendations for river basin management by establishing a methodological framework that incorporates regional variables on the basis of dispersal (i.e., physical- and organismal-based dispersal proxies) into biomonitoring (Heino et al. 2017).
The application of metacommunity ecology to biomonitoring
Metacommunity organization has recently received considerable interest both from fundamental and applied perspectives (Holyoak et al. 2006, Leibold and Chase 2018). From an applied perspective, metacommunity studies have highlighted the relevance of regional processes for the biomonitoring (Heino 2013, Siqueira et al. 2014), restoration (Kitto et al. 2015, Swan and Brown 2017), and conservation of ecosystems (Guichard et al. 2006, Ruhí et al. 2017). Despite rapid progress, applied metacommunity ecology is an emerging research field that remains in its infancy (Marini et al. 2019).
The niche-based principles that underpin current biomonitoring (Heino 2013) state that local communities comprise taxa that differ in their responses to environmental variation and therefore inhabit different niches. Local abiotic environmental conditions therefore determine the occurrence of taxa at individual sites and, consequently, changes in environmental conditions alter local communities. This idea assumes that dispersal enables all species to reach all sites and that the match between community composition and the environment is optimal. Although it is often valid, this view needs expansion to recognize regional metacommunity influences on and, specifically, dispersal processes for local community composition (Leibold et al. 2004). In particular, mass effects mean that organisms temporarily inhabit in suboptimal habitats because of high dispersal rates, changing community composition irrespective of local environmental conditions. This can decouple biological indices from habitat conditions, causing overestimation or underestimation of biological quality (Brown et al. 2011). In contrast, dispersal limitation can prevent species from colonizing sites with suitable abiotic conditions (Heino et al. 2017), typically causing underestimation of biological quality such that fewer species inhabit unaffected isolated sites compared with equivalent sites supplied by more colonists and dispersal pathways.
In river networks, regional influences are especially important because dispersal can be strongly affected by network connectivity (Tonkin et al. 2018). For example, dispersal may be more prevalent in central areas such as at confluences than in peripheral areas such as headwaters (Brown and Swan 2010). Also, flow variability may affect dispersal rates, which are typically related to both landscape configuration and species’ dispersal abilities (Liu et al. 2013). High discharge increases dispersal rates of passively dispersing lotic organisms, although these rates may be contingent on dispersal-related species traits. Equally, low discharge can lead to network fragmentation, thereby decreasing the dispersal rates of aquatic organisms (Cañedo-Argüelles et al. 2015, de Campos et al. 2019).
Despite previous efforts to minimize the effects of regional processes on index performance (e.g., Hawkins et al. 2000, Aroviita et al. 2009, Frimpong and Angermeier 2010), dispersal processes have yet to be effectively incorporated into biomonitoring. For example, regional stratification generates smaller and more ecologically meaningful spatial units (e.g., ecoregion, drainage basin) that potentially decrease the effects of dispersal limitation on local communities and may promote more effective biomonitoring (Heino 2013). However, biomonitoring programs done to assess compliance with national and international legislation are conducted across larger spatial scales, including multiple bioregions, ecoregions, and drainage basins. Moreover, regional stratification does not directly recognize important aspects from a metacommunity perspective such as physical dispersal barriers (i.e., spatial connectivity) or organisms’ dispersal abilities (e.g., low, high) or modes (e.g., aerial, aquatic), despite their effects on local community structure (Cañedo-Argüelles et al. 2015).
Studies on the application of metacommunity approaches to biomonitoring are very limited and focused on perennial rivers. For example, by generating different scenarios of spatial connectivity between sites, organisms’ dispersal abilities, and anthropogenic impact levels in a hypothetical river network, Siqueira and colleagues (2014) found that biological quality was underestimated at isolated sites. This evidences that both the spatial configuration of sites and species’ dispersal abilities should be considered in biomonitoring (Heino et al. 2017). However, no studies focusing on biomonitoring at the metacommunity level have considered the effects of the wide spatiotemporal variability that characterizes HDEs such as intermittent rivers (box 1). In these systems, there is a particular need to account for recurrent spatial isolation of aquatic habitats and for among-site variability generated by natural flow intermittence (Datry et al. 2016a). For example, the position of intermittent river reaches in relation to perennial waters is particularly relevant to the dispersal of aquatic communities, because perennial habitats can act as stepping stones during overland dispersal (Cañedo-Argüelles et al. 2015) and can be a source of colonists after flow resumption (Sarremejane et al. 2017a). As well as spatial factors, there is a need to include the temporal dimension of dispersal (Buoro and Carlson 2014). In intermittent rivers, resilience strategies of aquatic organisms usually involve spatial dispersal, either using strictly aquatic (e.g., fish) or combining aerial and aquatic (e.g., aquatic insects) modes, whereas resistance strategies allow local survival in wet refuges or as desiccation-tolerant life stages in drying sediments and can be viewed as temporal dispersal (Bonada et al. 2017). Spatial and temporal dispersal covary (Wisnoski et al. 2019), and, in a context of limited spatial dispersal, temporal dispersal is key to maintaining local communities under harsh conditions (Bogan et al. 2015). As climate change increases the frequency and magnitude of extreme events (Trenberth et al. 2015) such as floods and drying in rivers, integrating dispersal into biomonitoring could enable managers to conserve and monitor ecosystems more effectively.
A conceptual model for metacommunity biomonitoring in highly dynamic ecosystems
In the present article, we use intermittent rivers as an example HDE to develop a conceptual model that illustrates how the performance of biological indices or metrics can be negatively affected by environmental harshness resulting from flow intermittence (Chessman et al. 2010, Soria et al. 2020). We predict that when incorporating a metacommunity perspective, the index performance in conditions of flow intermittence depends on the degree of spatial connectivity, and organisms’ spatial dispersal abilities and resistance to drying. The model assumes that both dispersal limitation and mass effects limit the performance of biological indices (Heino et al. 2017). In intermittent rivers, resistance to drying acts as temporal dispersal (Bonada et al. 2017) and will therefore influence index performance. For example, index performance may decrease because of dispersal limitation, but decreases may be compensated by temporal dispersal in regions that often support more resistant taxa (e.g., Mediterranean-climate regions; Bonada and Resh 2013). The model also assumes that dispersal rates reflect the balance between dispersal ability and spatial connectivity (Sarremejane et al. 2017b, Tonkin et al. 2018). This means that, for example, dispersal limitation is higher at more isolated sites and in communities dominated by weak dispersers, but strong dispersal can offset the effects of spatial isolation. According to these assumptions, the model predicts five basic relationships between the performance of a hypothetical biological index and flow intermittence: concave, convex, sigmoid, inverse sigmoid, and linear (box 2, figure 3b).
Box 2. A conceptual model of the performance of biological indices in intermittent rivers.
Our conceptual model illustrates how the performance of an index is negatively affected by flow intermittence, and predicts different relationships depending on the degree of spatial connectivity, organisms’ dispersal ability (i.e., weak, intermediate and strong dispersers), and resistance to drying (figure 3b):
Concave. An increase in flow intermittence is accompanied by a decrease in index performance only at high levels of intermittence (i.e., at sites experiencing long dry phases). This pattern occurs when sufficient among-site dispersal rates (i.e., communities dominated by intermediate dispersers at high spatial connectivity, or by strong dispersers at high spatial isolation) and the resistance of local communities compensate for the effects of flow intermittence (figure 3c). In this case, both spatial and temporal dispersal contribute to community assembly.
Convex. An increase in flow intermittence causes a major decrease in index performance, limiting performance at low levels of intermittence (i.e., near-perennial sites) or even perennial sites. This occurs when insufficient dispersal rates (i.e., communities dominated by weak dispersers at high spatial connectivity, or by intermediate dispersers at high spatial isolation) and limited resistance lead to taxon absences from unaffected sites (figure 3c). This convexity is reinforced when weak dispersers inhabit highly isolated sites. A convex relationship could also occur when high dispersal rates (i.e., strong dispersers at high spatial connectivity) lead to mass effects and reduce index performance because of community homogenization (i.e., species disperse everywhere).
Sigmoid. An increase in flow intermittence results in a minor decrease in index performance at low intermittence levels until a threshold is reached (in the present article, 50% intermittence) at which performance abruptly declines. This relationship reflects sufficient among-site dispersal rates to compensate for the effects of flow intermittence, but only up to a certain threshold (figure 3c), because taxa lacking resistance strategies dominate communities and these taxa rely primarily on spatial dispersal.
Inverse sigmoid. An increase in flow intermittence is accompanied by a major decrease in index performance at even low intermittence levels (i.e., near-perennial sites), reaching a transitory steady state (in the present article, 50% of index performance) until a threshold is reached (in the present article, 80% intermittence) at which performance declines abruptly. This occurs when insufficient dispersal rates prevent resilient taxa from recolonizing unimpaired sites or when high dispersal rates reduce index performance, but resistance (i.e., temporal dispersal) compensates for the effects of flow intermittence on local communities (figure 3c).
Linear. Index performance declines with flow intermittence at a constant rate. This relationship represents the traditional niche-based approach in assuming that all species can reach all sites, and that the match between community composition and flow intermittence is proportional.
Figure 3.
The performance of traditional biological indices used in river biomonitoring (a) is reduced by increasing flow intermittence (percentage) following different relationships (b) depending on spatial connectivity and species’ dispersal and resistance traits (c). Typically, an effective index classifies 80%–100% of sites correctly. See box 2 for a detailed description. The percentage of flow intermittence refers to the annual amount of time that the river has no flow.
Each of the five relationships (figure 3b) may vary in its threshold of change, as a result of the different contributions of spatial and temporal dispersal in a metacommunity. For example, the shape of an inverse sigmoid curve may differ among communities with contrasting resistance strategies, reflecting the different thresholds at which index performance abruptly declines because of the effect of flow intermittence. These relationships may also vary in relation to additional spatiotemporal features of intermittent rivers, such as spatial drying configurations, the temporal predictability of drying, and the number of drying events (box 3; Datry et al. 2016a).
Box 3. Key questions on metacommunity ecology applied to the biomonitoring of intermittent rivers.
What is the relative contribution of flow permanence and spatial connectivity to the applicability of biological indices? Does this contribution vary among taxonomic groups?
What is the covariation between spatial and temporal dispersal in a metacommunity context? How do the predictability, duration and frequency of different hydrological phases, in particular dry phases, and spatial drying configurations influence community composition? How will this affect our ability to predict site-specific community composition and improve biomonitoring?
Can biomonitoring be improved by using only a subset of taxa—that is, those with high resilience and resistance to drying? Can these taxa recolonize after flow resumes, track environmental conditions that match their habitat preferences, and therefore act as biological indicators?
Which set of dispersal traits and spatial connectivity measurements best help predicting the probability of finding a certain taxon at a site?
Which community metrics will better distinguish responses to anthropogenic impacts and flow intermittence?
Identifying these relationships can enable characterization of reference conditions, reliable metrics, and realistic environmental targets in intermittent rivers. Our conceptual model may also be applicable to other HDEs experiencing other recurrent natural short-timescale disturbances (e.g., fire-prone ecosystems, coastal lagoons). Regardless of HDE type, incorporating ecologically relevant variables such as spatial connectivity represents a step toward better integrating the natural range of variation of ecosystems into biomonitoring.
Proposed methodological framework
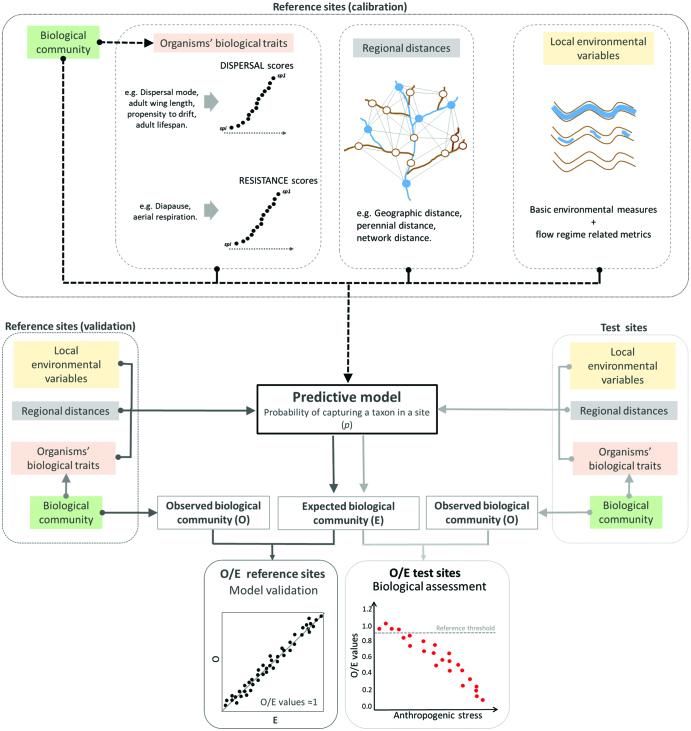
Ecological modeling is a powerful tool to support ecosystem management that has been used in the assessment of anthropogenic stressors in many ecosystems (Lynam et al. 2016, Tonkin et al. 2019). One of the most extensive methods in river biomonitoring is predictive modeling. It has been tested using different biotic groups (Feio and Poquet 2011) and also in other freshwater ecosystems (Reynoldson et al. 1995, Dziock et al. 2006). Predictive modeling is based on empirical statistical models (e.g., general linear models) and predicts site-specific communities on the basis of selected environmental variables. When used in intermittent river biomonitoring, RIVPACS (River Invertebrate Prediction and Classification System)-type predictive models may not detect anthropogenic impacts (Chessman et al. 2010, but see Poquet et al. 2009), corroborating the limitation of current bioassessment methods in such systems. Here, we address these limitations by proposing a methodological framework to incorporate regional, as well as local variables into current biomonitoring by using predictive modeling. Specifically, we describe how to effectively account for the components influencing a hypothetical biomonitoring index in our conceptual model: flow intermittence, the degree of spatial connectivity, organisms’ spatial dispersal ability, and resistance to drying. This methodological framework has five main steps.
Step 1: Obtaining the reference biological data set
As in traditional biomonitoring methods, the first step is to obtain a data set of reference sites selected according to objective criteria that capture enough natural environmental variability to represent a study area (Feio and Poquet 2011). In our framework, this variability encompasses both local environmental and regional variables, and reference sites should include biological data representing different levels of flow intermittence and spatial connectivity. Reflecting the spatial scale at which regional processes operate, the catchment-scale application of metacommunity-based biomonitoring is typically appropriate (e.g., Heino 2013). However, the spatial scale at which metacommunity processes operate is not well defined yet and might be specific to each region (Viana and Chase 2019). For example, in very large catchments (e.g., the Rhône River; 98,000 square kilometers), communities that are not connected by dispersal do not constitute a metacommunity, and smaller (e.g., subcatchment) spatial scales may therefore enable more effective management actions. The spatial scale could also vary according to the dispersal abilities of the targeted biotic group. For instance, diatoms have been ranked as having high dispersal ability, followed by macroinvertebrates, fish, and macrophytes (Padial et al. 2014). Larger spatial scales could therefore be considered when the biotic group has high dispersal abilities (e.g., diatoms). Overall, we suggest obtaining data from reference sites over relatively small spatial scales (Heino 2013) with the possibility of testing the aggregation of small spatial extents into larger ones when building the predictive model (step 4). Standard, well-established methods can be used to obtain the biological data set once sites are selected; for example, many quantitative and semiquantitative methods are used to sample aquatic invertebrates (Birk et al. 2012). However, because local aquatic community composition is characterized by temporal variability in intermittent rivers, we need to consider whether one or multiple sampling periods are included in the predictive model (Chessman et al. 2010). Predictive modeling used in traditional biomonitoring allows model development on the basis of one or multiple sampling occasions (Linke et al. 1999). In our case, this enables generation of models specific for each phase supporting aquatic communities in intermittent rivers (e.g., flowing waters and disconnected pools), models integrating all phases, or models adapted to the prevailing aquatic phase. Where resources permit, we recommend modeling reference data to test the performance of options 1–3 (e.g., Linke et al. 1999) and to obtain spatiotemporally variable and distinct reference conditions (Tonkin et al. 2017).
Step 2: Obtaining local environmental variables
Following the standard methodology for predictive modeling, we propose obtaining information on local environmental variables (Clarke et al. 2003), including detailed site-specific hydrological information. The aquatic phase (i.e., flowing, disconnected pools) at the time of sampling and the long-term flow regime (i.e., the percentage annual flow permanence) require characterization. The inclusion of such hydrological variables is crucial for the bioassessment of intermittent rivers (Gallart et al. 2017, Stubbington et al. 2018, Soria et al. 2020). Although hydrological information in intermittent rivers remains difficult to obtain, new tools and methods include wet–dry mapping, remote sensing techniques, field loggers, and modeling (Costigan et al. 2017). For example, the open-access software TREHS (Temporary Rivers Ecological and Hydrological Status) provides basic hydrological metrics to characterize the flow regime and detect potential hydrological alterations (Gallart et al. 2017). TREHS
uses information including hydrological models, gauging station data, interviews, aerial photographs, or site visits to identify hydrological phases, and provides metrics such as the percentage annual flow permanence and the associated predictability. Other methods based on citizen science and wet–dry mapping could be used (Datry et al. 2016b, Allen et al. 2019).
Step 3: Obtaining regional variables
Organisms’ dispersal rates in a landscape can be measured using direct and indirect methods. Direct methods include tagging (Beirinckx et al. 2006), stable isotopes (Lancaster and Downes 2013), and genetics (Heino et al. 2017). Indirect methods involve dispersal proxies such as biological traits or spatial modeling using different connectivity levels (Sarremejane et al. 2017a, Heino et al. 2017). Direct methods give more detailed information on species dispersal rates but are typically applied at small spatial scales (i.e., stream; Downes et al. 2017) and can be misleading because of low recapture rates (Keller et al. 2010). In the present article, we suggest using indirect methods combining measures of spatial connectivity and dispersal-related species traits (e.g., Sarremejane et al. 2017b), which practitioners can incorporate into existing biomonitoring protocols in a standardized way.
To account for spatial connectivity, different spatial measures can be easily obtained using GIS. For example, assuming that lower stream orders are less connected, site-specific connectivity measures could be obtained by dividing sites by order, then measuring the length of the upstream channel and of tributaries in a 2-kilometers buffer and the distance to the nearest perennial reach (Sarremejane et al. 2017b). Another more complex option is to calculate the connectivity between sites within a catchment using physical distance measures that characterize landscape connectivity in intermittent river networks (Cañedo-Argüelles et al. 2015): overland distance, the shortest linear distance between sites; topographic distance, the shortest distance between sites considering landscape configuration; perennial distance, the shortest distance between sites considering perennial water bodies; and network distance, the shortest distance between sites following the river network. In addition, flow distance could be incorporated to indicate upstream versus downstream aquatic dispersal when intermittent rivers are flowing (Heino et al. 2017), and the influence of wind directions on aerial dispersal could also be assessed. There are many other methods to obtain spatial variables—for example, resistance surfaces (Zeller et al. 2012), Moran's eigenvector maps (Dray et al. 2006), and the dendritic connectivity index (Cote et al. 2009). We therefore suggest that spatial variables derived from different approaches could be tailored to be used in a specific context.
To account for species’ dispersal ability, we propose using a database of biological traits of species within the study area, on the basis of their dispersal abilities and resistance to drying, to incorporate both temporal and spatial dispersal. Data on organisms’ biological traits is increasingly freely available online (e.g., www.freshwaterecology.info, https://ecoevorxiv.org/kac45), and compiled information on dispersal can be complemented by the literature and expert knowledge. In this regard, a new open-access dispersal database has been developed including aquatic invertebrate traits linked to both aquatic and aerial dispersal (Sarremejane et al. 2020). These traits correspond to morphological (e.g., body size, wing length, wing pair type), behavioral (e.g., dispersal mode, drift propensity), and life history attributes (e.g., fecundity, voltinism), which, combined, can be used to assign spatial dispersal scores. For example, active aerial adult insects with two pairs of long wings (e.g., Odonata) would have higher aerial dispersal compared with those with smaller wings (e.g., Ephemeroptera). However, lower aerial dispersal could be compensated by higher aquatic dispersal resulting from high drift propensity and fecundity (e.g., Baetis mayflies). Using these data, together with information on resistance to drying, each taxon can be scored according to its spatial and temporal dispersal ability, then incorporated into predictive modeling, to calculate the probability of a certain taxon occurring at a site.
Step 4: Building the predictive model
To build the predictive model (e.g., RIVPACS-type) and ultimately predict the community at a site, the probability of capturing each taxon (p) is calculated (figure 4). Such methods use cluster analysis to allocate reference biological data to groups, and select those variables that best discriminate groups (usually using discriminant analysis). In the present article, we suggest incorporating expanded local environmental variables (e.g., site-specific flow regime metrics, aquatic phase during sampling) and regional distances (e.g., perennial distance) into the variable selection, in addition to those already considered by current predictive modeling (figure 4). We also recommend building models from reference data obtained during different aquatic phases, to capture temporal variability (see step 1). The p for each taxon is then calculated by summing each site's probability of being part of each group, weighted by the probability of finding that taxon within each group (Clarke et al. 2003). By calculating p for each taxon, the expected community (E) at a site is obtained. In our approach, when calculating p, the weighting will also account for taxon-specific dispersal and resistance scores. Model validation can be performed using an independent data set of reference sites, for which E is predicted using the model and then is compared with the observed community (O) using a regression analysis (O/E reference sites, figure 4). The closer the slope of the O/E regression (the O/E value) is to 1, the more accurate the model (Linke et al. 2005).
Figure 4.
Steps for the incorporation of metacommunity-based measurements into bioassessment. Black lines represent the process of model building, dark grey lines (left) the model validation, and light lines (right) the biological assessment of test sites.
Step 5: Biological quality assessment
The expected (E) community in a new site (i.e., a test site, figure 4) is also calculated by the predictive model and an O/E value obtained. This O/E value can be calculated for any index or community metric from the taxa present in E and O, making our framework applicable to both existing and new indices. O/E values closer to 1 indicate that O and E are similar and that the site is closer to reference conditions; the closer the O/E values are to 0, the higher the level of biological impairment. Finally, quality classes can be banded and created from O/E values across a gradient of anthropogenic stress, as is required by biomonitoring programs.
Conclusions
An understanding of how temporal and spatial variability affect the performance of biomonitoring methods in HDEs is needed to underpin a robust methodological framework that recognizes current ecological theory. Our methodological framework integrates metacommunity influences on local communities, and its alignment with existing biomonitoring methods will facilitate uptake by regulatory agencies. This approach accounts for the high spatiotemporal variability of HDEs, because predictive models could be built on the basis of single or multiple sampling occasions, depending on the context (e.g., taxonomic group, climate, ecosystem type), and local and regional variables. As a result, any test site can be located within an O/E gradient and be assessed accordingly (figure 4). This will, for example, support accurate estimation of biological quality at intermittent river sites with different levels of spatial isolation despite the effects of dispersal limitation (figure 2). We used intermittent rivers as model systems, but our approach could be extended to other HDEs. Predictive models could be applied to different ecosystem types, and selected regional and local variables could be adapted for different contexts. For example, the regional variables we propose could also be used in freshwater rock pools, but instead of flow intermittence, frequency of filling should be considered (Jocque et al. 2010). Similarly, in routinely monitored coastal lagoons, frequency of marine water inputs could be included (Mouillot 2007).
Figure 2.
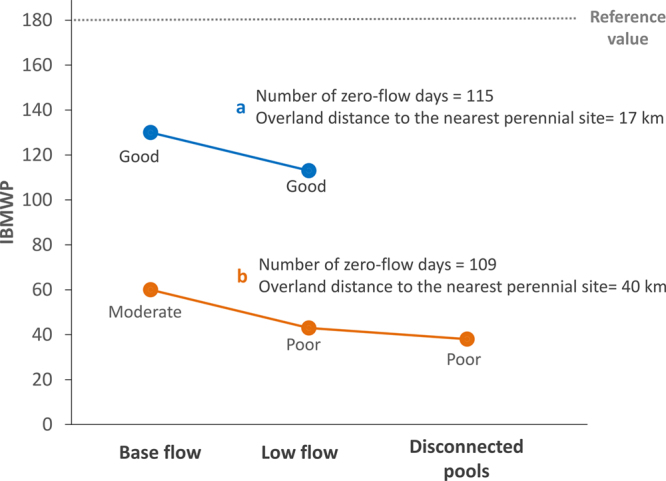
Values for the biomonitoring index IBMWP and associated biological quality (moderate, poor, bad) in two headwater intermittent rivers: (a) Cérvol and (b) Monlleó (Castelló, Spain). Although they may have disconnected pools for a short period during transitions between flowing and dry phases (i.e., Monlleó), both sites completely dry out during the dry season. The number of zero-flow days was assessed using temperature data loggers (Soria et al. 2020). Both sites are within the same official river typology (i.e., limestone rivers of Mediterranean lowland mountains) and, therefore, their quality is assessed using the same reference values. Neither site is exposed to significant anthropogenic pressures. Even when sampled during the flowing phase, as was indicated in standard protocols developed for perennial rivers, biological quality is underestimated for the site with the greatest distance to the nearest perennial site. Quality assessments are not performed when the river is dry. Data obtained from the TRivers Project.
Our framework could also inform conservation management practices. First, by identifying reference conditions when building a predictive model, the connectivity between sites will be documented, providing information to inform strategies that seek to maintain and enhance both local and regional biodiversity. Second, incorporating both local and regional dispersal-related variables could improve the accuracy of niche-based models predicting the effects of global change on biodiversity (Heino et al. 2017, Sofaer et al. 2019). For example, our approach could enable prediction of expected communities (E) in intermittent river networks under future habitat conditions at the local (e.g., increased dry phase durations) and regional (e.g., increased isolation from perennial habitats) scale and could identify key refuges in which species could persist during dry phases.
Our approach represents a starting point and requires empirical testing. Several aspects of the model building and practical implementation require consideration. An important issue is that anthropogenic activities may also create dispersal barriers. For example, urbanization effects on terrestrial and aquatic environments can fragment river metacommunities (Urban et al. 2006), and dams can prevent fish movements and migrations (Fuller et al. 2015). Polluted river sections may also increase dispersal distances between near-pristine sites because sensitive species excluded from polluted sites may lack stepping stones that enable their dispersal (Heino et al. 2017). Anthropogenic land uses and occurrence of artificial dispersal barriers require identification and where isolation results from human activity, the sites should be assessed against unaffected conditions and restoration initiatives designed to reestablish natural dispersal pathways. The local-scale influence of biotic interactions also requires recognition (Heino 2013, Siqueira and Wunderlich 2018) and may be particularly important in intermittent rivers, where biotic interactions may intensify and become more important than local abiotic conditions in determining community composition as aquatic habitats contract (Datry et al. 2016a). Our approach indirectly considers their potential effects by integrating multiple sampling periods.
Intermittent rivers dominate networks in some global regions—in particular, drylands—and are estimated to account for half the global river length (Datry et al. 2014). With increasing aridity and extreme climatic events such as drought, as is expected in many global regions, fragmented river networks including intermittent rivers will become increasingly dominant, and aquatic populations and communities will therefore become less well connected (Datry et al. 2016a, Ogden 2017). In this context, there is an urgent need to reconceptualize and adapt biomonitoring methods to accurately assess ecological quality. Our framework provides an opportunity for academic and manager collaborators to work toward the implementation of adapted biomonitoring practices, optimizing efforts to protect HDEs in a context of ongoing global change. Our framework can also be developed to support future research that uses both empirical and modeling approaches to address the questions outlined in box 3. We encourage the use of data obtained from theoretical metacommunity studies and from regulatory agencies as well as the collection of new biomonitoring data in pilot river networks to extend our metacommunity methodological framework.
Acknowledgments
The study was supported by the French research program Make Our Planet Great Again. We acknowledge the work from COST (the European Cooperation in Science and Technology) Action CA15113 (Science and Management of Intermittent Rivers and Ephemeral Streams, www.smires.eu), and from the MECODISPER project (grant no. CTM2017- 89295-P), funded by the Spanish Ministerio de Economía, Industria y Competitividad, Agencia Estatal de Investigación and cofunded by the European Regional Development Fund.
Author Biographical
Núria Cid (nuria.cid-puey@inrae.fr) is a postdoctoral researcher, Julie Crabot is a PhD student, and Thibault Datry is a researcher and head of the DYNAM Lab at INRAE, in Lyon, France. Núria Bonada is a Serra Húnter associate professor and Miguel Cañedo-Argüelles is a postdoctoral researcher at the Freshwater Ecology, Hydrology, and Management research group at the University of Barcelona, in Barcelona, Catalonia, Spain. Rachel Stubbington is an associate professor and Romain Sarremejane is a postdoctoral researcher at Nottingham Trent University, in Trent, United Kingdom. Jani Heino is a senior research fellow at the Finnish Environment Institute, Freshwater Centre, in Oulu, Finland. Janne Soininen is a professor in the Department of Geosciences and Geography at the University of Helsinki, in Helsinki, Finland.
References cited
- Alba-Tercedor J et al.. 2002. Caracterización del estado ecológico de ríos mediterráneos ibéricos mediante el índice IBMWP (antes BMWP’). Limnética 21: 175–185. [Google Scholar]
- Allen DC, Kopp DA, Costigan KH, Datry T, Hugueny B, Turner DS, Bodner GS, Flood TJ. 2019. Citizen scientists document long-term streamflow declines in intermittent rivers of the desert southwest, USA. Freshwater Science 38: 244–256. [Google Scholar]
- Aroviita J, Mykrä H, Muotka T, Hämäläinen HE. 2009. Influence of geographical extent on typology- and model-based assessments of taxonomic completeness of river macroinvertebrates. Freshwater Biology 54: 1774–1787. [Google Scholar]
- Beirinckx K, Van Gossum HJ, Lajeunesse M R, Forbes M. 2006. Sex biases in dispersal and philopatry: Insights from a meta-analysis based on capture–mark–recapture studies of damselflies. Oikos 113: 539–547. [Google Scholar]
- Birk S, Bonne W, Borja A, Brucet S, Courrat A, Poikane S, Solimini A, van de Bund W, Zampoukas N, Hering D. 2012. Three hundred ways to assess Europe's surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators 18: 31–41. [Google Scholar]
- Bogan MT, Boersma KS, Lytle DA. 2015. Resistance and resilience of invertebrate communities to seasonal and supraseasonal drought in arid-land headwater streams. Freshwater Biology 60: 2547–2558. [Google Scholar]
- Bogan MT, Chester ET, Datry T, Murphy AL, Robson BJ, Ruhi A, Stubbington R, Whitney JE. 2017. Resistance, Resilience, and community recovery in intermittent rivers and ephemeral sreams. Pages349–376 in Datry T, Bonada N, Boulton A, eds. Intermittent Rivers and Ephemeral Streams. Academic Press. [Google Scholar]
- Bonada N, Carlson SM, Datry T, Finn DS, Leigh C, Lytle DA, Monaghan MT, Tedesco PA. 2017. Genetic, evolutionary, and biogeographical processes in intermittent rivers and ephemeral streams. Pages405–431 in Datry T, Bonada N, Boulton A, eds. Intermittent Rivers and Ephemeral Streams. Academic Press. [Google Scholar]
- Bonada N, Prat N, Resh VH, Statzner B. 2006. Developments in aquatic insects biomonitoring: A comparative analysis of recent approaches. Annual Review of Entomology 51: 495–523. [DOI] [PubMed] [Google Scholar]
- Bonada N, Resh VH.. 2013. Mediterranean-climate streams and rivers: Geographically separated but ecologically comparable freshwater systems. Hydrobiologia 719: 1–29. [Google Scholar]
- Brown BL, Swan CM.. 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580. [DOI] [PubMed] [Google Scholar]
- Brown BL, Swan CM, Auerbach DA, Campbell Grant EH, Hitt NP, Maloney KO, Patrick C. 2011. Metacommunity theory as a multispecies, multiscale framework for studying the influence of river network structure on riverine communities and ecosystems. Journal of the North American Benthological Society 30: 310–327. [Google Scholar]
- Buoro M, Carlson SM.. 2014. Life-history syndromes: Integrating dispersal through space and time. Ecology Letters 17: 756–767. [DOI] [PubMed] [Google Scholar]
- de Campos R, Oliverira da Conceição E, Martens K, Higuti J. 2019. Extreme drought periods can change spatial effects on periphytic ostracod metacommunities in river-floodplain ecosystems. Hydrobiologia 828: 369–381. [Google Scholar]
- Cañedo-Argüelles M, Boersma KS, Bogan MT, Olden JD, Phillipsen I, Schriever TA, Lytle DA. 2015. Dispersal strength determines meta-community structure in a dendritic riverine network. Journal of Biogeography 42: 778–790. [Google Scholar]
- Chessman BC, Jones HA, Searle NK, Growns IO, Pearson MR. 2010. Assessing effects of flow alteration on macroinvertebrate assemblages in Australian dryland rivers. Freshwater Biology 55: 1780–1800. [Google Scholar]
- Chessman BC, Royal MJ. 2004. Bioassessment without reference sites: Use of environmental filters to predict natural assemblages of river macroinvertebrates. Journal of the North American Benthological Society 23: 599–615. [Google Scholar]
- Chiu MC, Leigh C, Mazor R, Cid N, Resh V. 2017. Anthropogenic Threats to intermittent rivers and ephemeral Streams. Pages433–454 in Datry T, Bonada N, Boulton A, eds. Intermittent Rivers and Ephemeral Streams. Academic Press. [Google Scholar]
- Cid N, Bonada N, Carlson S, Grantham T, Gasith A, Resh V. 2017. High variability is a defining component of Mediterranean-climate rivers and their biota. Water 9: 52. [Google Scholar]
- Clarke RT, Wright JF, Furse MT. 2003. RIVPACS models for predicting the expected macroinvertebrate fauna and assessing the ecological quality of rivers. Ecological Modelling 160: 219–233. [Google Scholar]
- Costigan KH, Kennard MJ, Leigh C, Sauquet E, Boulton AJ. 2017. Flow Regimes in intermittent rivers and ephemeral streams. Pages51–78 in Datry T, Bonada N, Boulton A, eds. Intermittent Rivers and Ephemeral Streams. Academic Press. [Google Scholar]
- Cote D, Kehler DG, Bourne C, Wiersma YF. 2009. A new measure of longitudinal connectivity for stream networks. Landscape Ecology 24: 101–113. [Google Scholar]
- Datry T, Bonada N, Heino J. 2016a. Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos 125: 149–159. [Google Scholar]
- Datry T, Larned ST, Tockner K. 2014. Intermittent rivers: A challenge for freshwater ecology. BioScience 64: 229–235. [Google Scholar]
- Datry T, Pella H, Leigh C, Bonada N, Hugueny B. 2016b. A landscape approach to advance intermittent river ecology. Freshwater Biology 61: 1200–1213. [Google Scholar]
- Döll P, Schmied HM.. 2012. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environmental Research Letters 7: 014037. [Google Scholar]
- Donohue I et al.. 2016. Navigating the complexity of ecological stability. Ecology Letters 19: 1172–1185. [DOI] [PubMed] [Google Scholar]
- Downes BJ, Lancaster J, Glaister A, Bovill WD. 2017. A fresh approach reveals how dispersal shapes metacommunity structure in a human-altered landscape. Journal of Applied Ecology 54: 588–598. [Google Scholar]
- Dray S, Legendre P, Peres-Neto PR. 2006. Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecological Modelling 196: 483–493. [Google Scholar]
- Dziock F, Henle K, Foeckler F, Follner K, Sholz M. 2006. Biological indicator systems in floodplains – A review. International Review of Hydrobiology 91: 271–291. [Google Scholar]
- Elliott M, Quintino V.. 2007. The Estuarine Quality Paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Marine Pollution Bulletin 54: 640–645. [DOI] [PubMed] [Google Scholar]
- Feio MJ, Poquet JM.. 2011. Predictive models for freshwater biological assessment: Statistical approaches, biological elements and the Iberian Peninsula experience: A review. International Review of Hydrobiology 96: 321–346. [Google Scholar]
- Frimpong EA, Angermeier PL.. 2010. Comparative utility of selected frameworks for regionalizing fish-based bioassessments across the United States. Transactions of the American Fisheries Society 139: 1872–1895. [Google Scholar]
- Fritz K, Cid N, Autrey B. 2017. Governance, legislation, and protection of intermittent rivers and rphemeral streams. Pages447–507 in Datry T, Bonada N, Boulton A, eds. Intermittent Rivers and Ephemeral Streams. Academic Press. [Google Scholar]
- Fuller MR, Doyle MW, Strayer DL. 2015. Causes and consequences of habitat fragmentation in river networks. Annals of the New York: Academy of Sciences 1355: 31–51. [DOI] [PubMed] [Google Scholar]
- Gallart F et al.. 2017. TREHS: An open-access software tool for investigating and evaluating temporary river regimes as a first step for their ecological status assessment. Science of the Total Environment 607–608: 519–540. [DOI] [PubMed] [Google Scholar]
- Ghazoul J, Burivalova Z, Garcia-Ulloa J, King LA. 2015. Conceptualizing Forest Degradation. Trends in Ecology and Evolution 30: 622–632. [DOI] [PubMed] [Google Scholar]
- Guichard F, Levin SA, Hastings A, Siegel D. 2006. Toward a dynamic metacommunity approach to marine reserve theory. BioScience 54: 1003–1011. [Google Scholar]
- Haddad NM et al.. 2015. Habitat fragmentation and its lasting impact on Earth's ecosystems. Science Advances 1: e1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CP, Norris RH, Gerritsen J, Hughes RM, Jackson SK, Johnson RK, Stevenson RJ. 2000. Evaluation of the Use of landscape classifications for the prediction of freshwater biota: Synthesis and recommendations. Journal of the North American Benthological Society 19: 541–556. [Google Scholar]
- Heino J. 2013. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biological Reviews 88: 166–178. [DOI] [PubMed] [Google Scholar]
- Heino J et al.. 2015. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution 5: 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J et al.. 2017. Integrating dispersal proxies in ecological and environmental research in the freshwater realm. Environmental Reviews 25: 334–349. [Google Scholar]
- Holyoak M, Leibold MA, Holt RD. 2006. Metacommunities: Spatial Dynamics and Ecological Communities. University of Chicago Press. [Google Scholar]
- Jocque M, Vanschoenwinkel B, Brendock L. 2010. Freshwater rock pools: A review of habitat characteristics, faunal diversity and conservation value. Freshwater Biology 55: 1587–1602. [Google Scholar]
- Keller D, Brodbeck S, Flöss I, Vonwil G, Holderegger R. 2010. Ecological and genetic measurements of dispersal in a threatened dragonfly. Biological Conservation 143: 2658–2663. [Google Scholar]
- Kitto JAJ, Gray DP, Greig HS, Niyogi DK, Harding JS. 2015. Meta-community theory and stream restoration: Evidence that spatial position constrains stream invertebrate communities in a mine impacted landscape. Restoration Ecology 23: 284–291. [Google Scholar]
- Lancaster J, Downes BJ.. 2013. Aquatic Entomology. Oxford University Press. [Google Scholar]
- Leibold MA et al.. 2004. The metacommunity concept: A framework for multi-scale community ecology. Ecology Letters 7: 601–613. [Google Scholar]
- Leibold M, Chase J.. 2018. Metacommunity Ecology. Princeton University Press. [Google Scholar]
- Linke S, Bailey RC, Schwindt J. 1999. Temporal variability of stream bioassessments using benthic macroinvertebrates. Freshwater Biology 42: 575–584. [Google Scholar]
- Linke S, Norris RH, Faith DP, Stockwell D. 2005. ANNA: A new prediction method for bioassessment programs. Freshwater Biology 50: 147–158. [Google Scholar]
- Liu J, Soininen J, Han BP, Declerck SAJ. 2013. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. Journal of Biogeography 40: 2238–2248. [Google Scholar]
- Lynam CP et al.. 2016. Uses of innovative modeling tools within the implementation of the Marine Strategy Framework Directive. Frontiers in Marine Science 3: 1–18. [Google Scholar]
- Marini L, Bartomeus I, Rader R, Lami F. 2019. Species–habitat networks: A tool to improve landscape management for conservation. Journal of Applied Ecology 56: 923–928. [Google Scholar]
- Marshall JC et al.. 2018. Protecting U.S. temporary waterways. Science 361: 856–857. [DOI] [PubMed] [Google Scholar]
- Mouillot D. 2007. Niche-assembly versus dispersal-assembly rules in coastal fish metacommunities: Implications for management of biodiversity in brackish lagoons. Journal of Applied Ecology 44: 760–767. [Google Scholar]
- Ogden LE. 2017. Dried out: Aquatic biodiversity faces challenges in a drying climate. BioScience 67: 949–956. [Google Scholar]
- Padial AA et al.. 2014. Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLOS ONE 9: e111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitacco V, Reizopoulou S, Sfriso A, Sfriso A, Mistri M, Munari C. 2019. The difficulty of disentangling natural from anthropogenic forcing factors makes the evaluation of ecological quality problematic: A case study from Adriatic lagoons. Marine Environmental Research 150: 104756. [DOI] [PubMed] [Google Scholar]
- Poquet JM et al.. 2009. The MEDiterranean Prediction and Classification System (MEDPACS): An implementation of the RIVPACS/AUSRIVAS predictive approach for assessing Mediterranean aquatic macroinvertebrate communities. Hydrobiologia 623: 153–171. [Google Scholar]
- Reynoldson TB, Bailey RC, Day KE, Norris RH. 1995. Biological guidelines for freshwater sediment based on BEnthic Assessment of SedimenT (the BEAST) using a multivariate approach for predicting biological state. Australian Journal of Ecology 20: 198–219. [Google Scholar]
- Ruhí A, Datry T, Sabo JL. 2017. Interpreting beta-diversity components over time to conserve metacommunities in highly dynamic ecosystems. Conservation Biology 31: 1459–1468. [DOI] [PubMed] [Google Scholar]
- Ryo M, Aguilar-Trigueros CA, Pinek L, Muller LAH, Rillig MC. 2019. Basic principles of temporal dynamics. Trends in Ecology and Evolution 34: 723–733. [DOI] [PubMed] [Google Scholar]
- Sarremejane R, Cañedo-Argüelles M, Prat N, Mykrä H, Muotka T, Bonada N. 2017a. Do metacommunities vary through time? Intermittent rivers as model systems. Journal of Biogeography 44: 2752–2763. [Google Scholar]
- Sarremejane R, Mykrä H, Bonada N, Aroviita J, Muotka T. 2017b. Habitat connectivity and dispersal ability drive the assembly mechanisms of macroinvertebrate communities in river networks. Freshwater Biology 62: 1073–1082. [Google Scholar]
- Sarremejane R et al.. 2020. DISPERSE: A trait database to assess the dispersal potential of aquatic macroinvertebrates. BioRxiv (24 February 2020, art. 953737). 10.1101/2020.02.21.953737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira T, Durães LD, de Oliveira Roque F. 2014. Predictive modelling of insect metacommunities in biomonitoring of aquatic networks. Pages109–126 in Ferreira CP, Godoy WAC, eds. Ecological Modelling Applied to Entomology. Springer International Publishing. [Google Scholar]
- Siqueira T, Wunderlich A.. 2018. Dispersal dilemmas. Nature Ecology and Evolution 2: 1836–1837. [DOI] [PubMed] [Google Scholar]
- Sofaer HR et al.. 2019. Development and delivery of species distribution models to inform decision-making. BioScience 69: 544–557. [Google Scholar]
- Soria M, Leigh C, Datry T, Bini LM, Bonada N. 2017. Biodiversity in perennial and intermittent rivers: A meta-analysis. Oikos 126: 1078–1089. [Google Scholar]
- Soria M et al.. 2020. Natural disturbances may produce misleading bioassessment results: Identifying metrics to detect anthropogenic impacts in intermittent rivers. Journal of Applied Ecology 57: 283–295. [Google Scholar]
- Stubbington R et al.. 2018. Biomonitoring of intermittent rivers and ephemeral streams in Europe: Current practice and priorities to enhance ecological status assessments. Science of the Total Environment 618: 1096–1113. [DOI] [PubMed] [Google Scholar]
- Swan CM, Brown BL.. 2017. Metacommunity theory meets restoration: Isolation may mediate how ecological communities respond to stream restoration. Ecological Applications 27: 2209–2219. [DOI] [PubMed] [Google Scholar]
- Tonkin JD, Altermatt F, Finn DS, Heino J, Olden JD, Pauls SU, Lytle DA. 2018. The role of dispersal in river network metacommunities: Patterns, processes, and pathways. Freshwater Biology 63: 141–163. [Google Scholar]
- Tonkin JD, Bogan MT, Bonada N, Rios-Touma B, Lytle DA. 2017. Seasonality and predictability shape temporal species diversity. Ecology 98: 1201–1216. [DOI] [PubMed] [Google Scholar]
- Tonkin JD, Poff NL, Bond NR, Horne A, Merritt DM, Reynolds LV, Olden JD, Ruhi A, Lytle DA. 2019. Prepare river ecosystems for an uncertain future. Nature 570: 301–303. [DOI] [PubMed] [Google Scholar]
- Trenberth KE, Fasullo JT, Shepherd TG. 2015. Attribution of climate extreme events. Nature Climate Change 5: 725–730. [Google Scholar]
- Urban MC, Skelly DK, Burchsted D, Price W, Lowry S. 2006. Stream communities across a rural–urban landscape gradient. Diversity and Distributions 12: 337–350. [Google Scholar]
- Viana DS, Chase JM. 2019. Spatial scale modulates the inference of metacommunity assembly processes. Ecology 100: 1–9. [DOI] [PubMed] [Google Scholar]
- Wisnoski NI, Leibold MA, Lennon JT. 2019. Dormancy in Metacommunities. The American Naturalist 194: 135–151. [DOI] [PubMed] [Google Scholar]
- Zeller KA, McGarigal K, Whiteley AR. 2012. Estimating landscape resistance to movement: A review. Landscape Ecology 27: 777–797. [Google Scholar]