Abstract
The metabolic syndrome, by definition, is not a disease but is a clustering of individual metabolic risk factors including abdominal obesity, hyperglycemia, hypertriglyceridemia, hypertension, and low high-density lipoprotein cholesterol levels. These risk factors could dramatically increase the prevalence of type 2 diabetes and cardiovascular disease. The reported prevalence of the metabolic syndrome varies, greatly depending on the definition used, gender, age, socioeconomic status, and the ethnic background of study cohorts. Clinical and epidemiological studies have clearly demonstrated that the metabolic syndrome starts with central obesity. Because the prevalence of obesity has doubly increased worldwide over the past 30 years, the prevalence of the metabolic syndrome has markedly boosted in parallel. Therefore, obesity has been recognized as the leading cause for the metabolic syndrome since it is strongly associated with all metabolic risk factors. High prevalence of the metabolic syndrome is not unique to the USA and Europe and it is also increasing in most Asian countries. Insulin resistance has elucidated most, if not all, of the pathophysiology of the metabolic syndrome because it contributes to hyperglycemia. Furthermore, a major contributor to the development of insulin resistance is an overabundance of circulating fatty acids. Plasma fatty acids are derived mainly from the triglycerides stored in adipose tissues, which are released through the action of the cyclic AMP-dependent enzyme, hormone sensitive lipase. This review summarizes the latest concepts in the definition, pathogenesis, pathophysiology, and diagnosis of the metabolic syndrome, as well as its preventive measures and therapeutic strategies in children and adolescents.
Keywords: Obesity, Diabetes, Insulin resistance, Dyslipidemia, Hyperglycemia
INTRODUCTION
The metabolic syndrome is defined as a clustering of metabolic abnormalities that include central obesity, insulin resistance, hypertriglyceridemia, hypercholesterolemia, hypertension, and reduced high-density lipoprotein (HDL)-cholesterol concentrations [1,2,3,4]. It is also associated with other comorbidities including the proinflammatory state, prothrombotic state, nonalcoholic fatty liver disease (NAFLD), cholesterol gallstone disease, and reproductive disorders [5,6,7,8,9,10,11,12,13,14,15,16]. The metabolic syndrome has been considered to be one of the most risk factors for the epidemic of type 2 diabetes and cardiovascular disease in the 21 century [17,18,19,20,21,22]. Furthermore, it could be mainly caused by inactive lifestyle, overconsumption of food, and the resulting abdominal obesity. However, it has been recognized that the metabolic syndrome is not a disease but is a common complex entity that emerges as a worldwide epidemic and major public health concern with a prevalence rate of approximately 25% in American adults [23,24,25,26]. Moreover, the prevalence of the metabolic syndrome is increasing not only in the USA and Europe, but also in Asian countries such as China, India, and South Korea [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Most studies have found that the metabolic syndrome is associated with an approximate doubling of cardiovascular disease risk and a 5-fold increased risk for type 2 diabetes. Although it is unclear whether there is a unifying pathogenic mechanism that could decipher the pathophysiology of the metabolic syndrome, it is highly likely that abdominal obesity and insulin resistance could play a central role in promoting the development of the metabolic syndrome [42,43,44]. Therefore, lifestyle modification and weight loss should be considered to be the first step for preventing or treating the metabolic syndrome [45,46,47]. In addition, other cardiac risk factors should be actively managed in individuals with the metabolic syndrome [48].
Although there are many different definitions of the metabolic syndrome, almost all the metabolic abnormalities include central obesity, hypercholesterolemia, reduced HDL-cholesterol levels, hypertension, and elevated plasma triglycerides, with insulin resistance as the potential uniting pathogenic factor [49]. The importance of the metabolic syndrome is not just related to its high prevalence rate worldwide but also because it could help predict the development of type 2 diabetes and cardiovascular disease [50,51,52]. Both NAFLD and cholesterol gallstone disease are now recognized to be the two major hepatic components of the metabolic syndrome, a kind of “fellow travelers” [6,53,54,55]. Moreover, high triglyceride and cholesterol concentrations in the liver together with other risk components, particularly insulin resistance, elevated plasma fatty acid concentrations, and diabetes, are the major risk factors for the development of nonalcoholic steatohepatitis (NASH), the most severe form of NAFLD [56,57,58,59]. However, the importance of the metabolic syndrome lies not in a specific definition but rather in its causative role and association with the worldwide epidemics of diabetes, cardiovascular disease, NAFLD, and NASH. According to the Guidelines of the National Cholesterol Education Program (NCEP) expert panel on the detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III) (ATPIII), the main goal of identifying patients with the metabolic syndrome is to find individuals at high risk of cardiovascular disease that extends beyond low-density lipoprotein (LDL)-cholesterol levels and is highly obesity-related morbidity and mortality [60].
Similar to adults, overweight and obesity in children and adolescents have become major public health issues as their prevalent rates have substantially increased not only in the USA and European countries, but also in Asian countries during the past three decades [61,62,63,64,65,66,67,68]. Based on the definition of the metabolic syndrome for adults, some modified definitions of this syndrome for children and adolescents have been proposed over the past 13 years; however, no unified definition has been proposed to assess risk or outcomes of the metabolic syndrome specially in pubertal subjects [69]. Obviously, a simple easy-to-use clinical definition is strongly needed to identify the metabolic syndrome in young people globally. Because obesity is associated with increased risk of cardiovascular disease and type 2 diabetes, which may persist from childhood and adolescence into young adulthood [70,71,72], it is important to make early identification of children and adolescents who are at high risk of developing the metabolic syndrome. Therefore, it would be possible to give early preventive measures, including lifestyle modification, to these young people to halt the development of the metabolic abnormalities. In this review, we summarize recent advances in the definition, pathogenesis, pathophysiology, and diagnosis of the metabolic syndrome, as well as its prevention and treatment interventions in children and adolescents.
DEFINITION AND CLINICAL DIAGNOSIS
Although the term, the metabolic syndrome, has become widely used since the 1990s, it is recently that the concept of “clustering” metabolic disorders and cardiovascular disease risk factors is intensively discussed in the literature [73,74,75,76,77]. In 1923, Kylin [78] first reported a relationship between hypertension, hyperglycemia, and gout in adult patients. In 1978, Phillips [79] found the coexistence of impaired glucose metabolism with hyperinsulinemia, hyperlipidemia, and hypertension, thus increasing the risk of developing cardiovascular disease. In 1980, Albrink [80] reported a relationship between obesity, hypertriglyceridemia, and hypertension. In 1988, Reaven [81] described a clustering of the metabolic abnormalities with insulin resistance as the central pathophysiological feature and used the term, the syndrome X, to depict the potential relationship between insulin resistance, hypertension, type 2 diabetes, and cardiovascular disease. In addition, the syndrome X and other terms, such as the deadly quartet and the insulin resistance syndrome, have been largely used in the literature to describe the clustering of cardiovascular and metabolic risk factors for several decades [82,83,84,85]. However, the metabolic syndrome is most widely used for describing the metabolically associated disorders including obesity, insulin resistance, type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, NAFLD, NASH, and cholesterol gallstone disease [1,5,6,11,86,87,88,89].
Notably, many different definitions have been proposed to describe the metabolic syndrome in adults. In general, the metabolic syndrome is defined as a cluster of risk factors for cardiovascular disease and type 2 diabetes, including visceral obesity, dyslipidemia, impaired glucose tolerance, and hypertension. In 1998, the World Health Organization (WHO) was the first to propose a definition and diagnostic criteria [90], in which insulin resistance is considered to be the major pathogenic factor underlying the metabolic syndrome. However, the WHO definition has been criticized because of these weaknesses: (i) it is difficult to use the clamp technique to analyze insulin resistance and apply it to large-scale epidemiological surveys; (ii) it is not a precise method to use the ratio of waist to hip to measure abdominal obesity; and (iii) it is unclear about the relationship between insulin resistance and microalbuminuria [90].
Subsequently, the European Group for the Study of Insulin Resistance (EGIR) used fasting plasma glucose and insulin levels instead of the insulin clamp technique to define insulin sensitivity and proposed a new criterion for non-diabetic patients based on these parameters [91,92]. In addition, the EGIR definition used the waist circumference, but not the ratio of waist to hip, to measure abdominal obesity. This significantly promoted wide-ranging epidemiological surveys.
In 2001, the NCEP:ATPIII proposed a new criterion to define the metabolic syndrome in adults, which worked as part of the educational program for the prevention of cardiovascular disease [93]. The NCEP:ATPIII definition did not list insulin resistance as a component. In contrast, it made all five components equally important, which greatly facilitates the diagnosis of the metabolic syndrome in clinical practice. Using the NCEP:ATPIII guidelines, the diagnosis of the metabolic syndrome requires the presence of at least three of the five components [93]. In general, the NCEP:ATPIII and the WHO criteria identify the same prevalence of the metabolic syndrome in adult patients, but the NCEP:ATPIII definition may be superior in identifying patients at increased risk of cardiovascular disease and for predicting type 2 diabetes. Nevertheless, these existing criteria for diagnosing the metabolic syndrome are less accurate in predicting clinical endpoints compared to other established models such as the Framingham risk score of cardiovascular disease. In addition, the American Association of Clinical Endocrinologists proposed a new criterion with a focus on insulin resistance and an exclusion of patients with type 2 diabetes.
Because of the use of different criteria for defining the metabolic syndrome in adults, the prevalence rates collected from epidemiological surveys vary greatly from country to country, as well as from region to region even in the same country. Thus, it is quite difficult to compare the reported prevalence rates of the metabolic syndrome around the world, as well as the data between studies due to these different diagnostic criteria. In 2005, the International Diabetes Federation (IDF) proposed a unifying definition that emphasizes the importance of central obesity and has it as a necessary condition to diagnose the metabolic syndrome [94,95]. In other words, the diagnosis of the metabolic syndrome requires central obesity plus two additional components. It should point out that the IDF criteria do not emphasize insulin resistance, but instead focus on fasting plasma glucose levels. In addition, a new set of criteria with ethnic/racial specific cutoffs were defined. For example, the guideline for measuring waist circumference was proposed and the ranges of waist circumference were specified for different ethnic/racial groups. In 2007, harmonizing the definition of the metabolic syndrome was proposed by comparison of the criteria of the NCEP:ATPIII and the IDF criteria in American and European populations [96]. In 2009, this harmonized definition for the metabolic syndrome was revised, which reached an agreement: (i) there should not be an obligatory component, but waist measurement would continue to be a useful preliminary screening tool; (ii) three abnormal findings out of 5 would qualify a person for the metabolic syndrome; (iii) a single set of cut points would be used for all components except waist circumference, for which further work is required; and (iv) in the interim, national or regional cut points for waist circumference can be used [97]. Nevertheless, clinical practice and epidemiological investigations strongly demand a simple easy-to-use unifying definition of the metabolic syndrome.
For children and adolescents, the IDF Task Force on the Epidemiology and Prevention of Diabetes set a practical clinical criterion for the diagnosis of the metabolic syndrome in 2007 [98,99]. Based on a modification of previous adult standards, the IDF has promoted a new criterion for the diagnosis of the metabolic syndrome mainly for children and adolescents between the ages of 10 and 16 years [98,99], as shown in Table 1. The definition of the metabolic syndrome in this age group is central obesity (≥90th percentile) plus the presence of two or more other components (Fig. 1), including hypertriglyceridemia (≥1.7 mmoL/L; ≥150 mg/dL), high blood glucose (≥5.6 mmoL/L; ≥100 mg/dL), high blood pressure (≥130 mmHg systolic or ≥85 mmHg diastolic), or low HDL-cholesterol levels (≤1.03 mmoL/L; ≤40 mg/dL). To date, the available data, however, were not sufficient to make a recommendation for children aged <6 years. For children aged between 6 and 10 years, as the metabolic syndrome cannot be diagnosed, they should be strongly recommended weight loss, especially those with abdominal obesity. For adolescents aged 16 years or older, the adult criteria could be used.
Table 1. The IDF definition of the at-risk group and the metabolic syndrome in children and adolescents (2007).
| Age group (yr) | Obesity (WC) | Triglycerides | HDL-C | Blood pressure | Plasma glucose |
|---|---|---|---|---|---|
| 6–<10* | ≥90th percentile | ||||
| 10–<16 | ≥90th percentile or adult cut-off if lower | ≥1.7 mmoL/L (≥150 mg/dL) | <1.03 mmoL/L (<40 mg/dL) | Systolic BP ≥130 or Diastolic BP ≥85 mmHg | FPG ≥5.6 mmoL/L (100 mg/dL)‡ or known T2DM |
| 16+ (adult criteria) | WC ≥94 cm for Europid males and ≥80 cm for Europid females, with ethnic-specific values for other groups†) | ≥1.7 mmoL/L (≥150 mg/dL) or specific treatment for high triglycerides | <1.03 mmoL/L (<40 mg/dL) in males and <1.29 mmoL/L (<50 mg/dL) in females, or specific treatment for low HDL | Systolic BP ≥130 or diastolic BP ≥85 mmHg or treatment of previously diagnosed hypertension | FPG ≥5.6 mmoL/L (100 mg/dL)‡ or known T2DM |
IDF: International Diabetes Federation, WC: waist circumference, HDL-C: high-density lipoprotein cholesterol, BP: blood pressure, FPG: fasting plasma glucose; T2DM: type 2 diabetes mellitus.
*The metabolic syndrome cannot be diagnosed, but further measurements should be made if there is a family history of the metabolic syndrome, type 2 diabetes, dyslipidemia, cardiovascular disease, hypertension, and/or obesity.
†For those of South and South-East Asian, Japanese, and ethnic South and Central American origin, the cutoffs should be ≥90 cm for men, and ≥80 cm for women. The IDF Consensus Group recognize that there are ethnic, gender and age differences, but research is still needed on outcomes to establish risk.
‡For clinical purposes, but not for diagnosing the metabolic syndrome, if fasting plasma glucose is 5.6–6.9 mmoL/L (100–125 mg/dL) and it is not known to have diabetes, an oral glucose tolerance test should be performed.
Diagnosing the metabolic syndrome requires the presence of central obesity plus any two of the other four factors. Modified and reproduced with permission from reference [98].
Fig. 1. Criteria for clinical diagnosis of the metabolic syndrome in childhood and adolescence. The definition of the metabolic syndrome in this age group is central obesity plus the presence of two or more than two components.
HDL-C: high-density lipoprotein cholesterol.
In 2014, a new criterion for defining the metabolic syndrome in prepubertal children was proposed by the identification and prevention of dietary- and lifestyle-induced health effects in children and infants (IDEFICS) study [100], which addressed the limitations of previous definitions in children and the need for early diagnosis in young people. Using reference values from the study of 18,745 children in eight European countries, the IDEFICS study set up the age-specific and sex-specific (and height-specific in the case of blood pressure) percentiles to identify cutoffs for the components of the metabolic syndrome in children at the age of 2-11 years [100]. However, the proposed cutoffs were based on a statistical definition and these did not allow to quantify the risk of subsequent diseases such as type 2 diabetes and cardiovascular disease.
Obviously, the diagnostic standards for adults cannot be simply used in children and adolescents, particularly in toddlers and even younger children, because of significant changes in body size and continuous growth and development with age. Furthermore, puberty has a drastic effect on fat redistribution in the body, leading to an enhanced insulin sensitivity in the liver, adipose tissues, and muscle, as well as an increased insulin secretion by the pancreatic β cells. In other words, compared to that in adults, insulin sensitivity is lower by 25 to 50% during childhood and returns to normal after pubertal development. Growth and developmental changes with age are also associated with physiological adjustments in blood pressure, plasma lipid levels, and energy metabolism, as well as glucose and lipid metabolism in the liver and adipose tissues. All these factors make it difficult to develop a precise definition for the diagnosis of the metabolic syndrome in young people with different ages, different ethnic/racial groups, and genders. In particular, because of the lack of reference values for some of the components of metabolic syndrome in children and adolescents, a consensus definition is not proposed easily. Notably, over the past 30 years, there is a significant increase in the prevalent rate of obesity-related complications in children and adolescents [101,102,103,104,105,106,107,108]. This high prevalence underscores the urgent need to develop a new definition for the diagnosis of the metabolic syndrome in young people. In addition, further research is imperative to identify biomarkers of the metabolic syndrome in early childhood. Long-term studies are also needed in children and adolescents with obesity, with a particular focus on intervention strategies. This will allow early diagnosis and timely action, including lifestyle modification and pharmaceutical intervention, to halt the development of metabolic abnormalities, thereby preventing long-term metabolic and cardiovascular consequences in children and adolescents [109,110,111].Obviously, the diagnostic standards for adults cannot be simply used in children and adolescents, particularly in toddlers and even younger children, because of significant changes in body size and continuous growth and development with age. Furthermore, puberty has a drastic effect on fat redistribution in the body, leading to an enhanced insulin sensitivity in the liver, adipose tissues, and muscle, as well as an increased insulin secretion by the pancreatic β cells. In other words, compared to that in adults, insulin sensitivity is lower by 25 to 50% during childhood and returns to normal after pubertal development. Growth and developmental changes with age are also associated with physiological adjustments in blood pressure, plasma lipid levels, and energy metabolism, as well as glucose and lipid metabolism in the liver and adipose tissues. All these factors make it difficult to develop a precise definition for the diagnosis of the metabolic syndrome in young people with different ages, different ethnic/racial groups, and genders. In particular, because of the lack of reference values for some of the components of metabolic syndrome in children and adolescents, a consensus definition is not proposed easily. Notably, over the past 30 years, there is a significant increase in the prevalent rate of obesity-related complications in children and adolescents [101,102,103,104,105,106,107,108]. This high prevalence underscores the urgent need to develop a new definition for the diagnosis of the metabolic syndrome in young people. In addition, further research is imperative to identify biomarkers of the metabolic syndrome in early childhood. Long-term studies are also needed in children and adolescents with obesity, with a particular focus on intervention strategies. This will allow early diagnosis and timely action, including lifestyle modification and pharmaceutical intervention, to halt the development of metabolic abnormalities, thereby preventing long-term metabolic and cardiovascular consequences in children and adolescents [109,110,111].
EPIDEMIOLOGY AND PREVALENCE
The prevalence rates of the metabolic syndrome have markedly increased not only in adults, but also in children and adolescents throughout the world over the past 30 years [112,113,114,115]. However, there is a striking difference in the prevalence rates of the metabolic syndrome in the USA and other countries, greatly depending on which definition is used to categorize individuals and determine inclusion and exclusion (Table 2) [62,63,65,98,438,439,440,441], as well as to analyze the composition of the population, e.g., gender, age, race, and ethnicity [116,117,118]. Additionally, lifestyle habits and socioeconomic status have a marked impact on the prevalence rates of the metabolic syndrome across gender, age, and race/ethnicity cohorts, as found by some epidemiological studies [112,119,120].
Table 2. Different definitions of the metabolic syndrome in children and adolescents [62,63,65,98,438,439,440,441].
| Cook (2003) | Cruz (2004) | Weiss (2004) | de Ferranti (2004) | Ford (2005) | Viner (2005) | IDF (2007) | IDEFICS (2012) |
|---|---|---|---|---|---|---|---|
| Fasting glucose ≥110 mg/dL | Impaired glucose tolerance (ADA criterion) | Impaired glucose tolerance (ADA criterion) | Fasting glucose ≥6.1 mmoL/L (≥110 mg/dL) | Fasting glucose ≥110 mg/dL (additional analysis with ≥100 mg/dL) | Hyperinsulinemia ≥104.2 pmoL/L (15 mU/L) or impaired fasting glucose ≥6.11 mmoL/L (110 mg/dL) | Impaired fasting glucose ≥5.55 mmoL/L (100 mg/dL) | HOMA-insulin resistance ≥90th percentile or fasting glucose ≥90th percentile |
| WC ≥90th percentile (age and sex specific, NHANES III) | WC ≥90th percentile (age, sex and race specific, NHANES III) | BMI–Z score ≥2.0 (age and sex specific) | WC >75th percentile | WC ≥90th percentile (sex specific, NHANES III) | BMI ≥95th percentile | WC ≥90th percentile | WC ≥90th percentile |
| Triglycerides ≥110 mg/dL (age specific, NCEP) | Triglycerides ≥90th percentile (age and sex specific, NHANES III) | Triglycerides >95th percentile (age, sex and race specific, NGHS) | Triglycerides ≥1.1 mmoL/L (≥100 mg/dL) | Triglycerides ≥110 mg/dL (age specific, NCEP) | Triglycerides ≥1.69 mmoL/L (150 mg/dL) | Triglycerides ≥1.69 mmoL/L (150 mg/dL) | Triglycerides ≥90th percentile |
| HDL-C ≤40 mg/dL (all ages/sexes, NCEP) | HDL-C ≤10th percentile (age and sex specific, NHANES III) | HDL-C <5th percentile (age, sex and race specific, NGHS) | HDL-C <1.3 mmoL/L (<50 mg/dL) | HDL-C ≤40 mg/dL (all ages/sexes, NCEP) | HDL-C <0.91 mmoL/L (35 mg/dL) or high total cholesterol ≥95th percentile | HDL-C <1.03 mmoL/L (40 mg/dL) | HDL-C ≤10th percentile |
| Blood pressure ≥90th percentile (age, sex and height specific, NHBPEP) | Blood pressure >90th percentile (age, sex and height specific, NHBPEP) | Blood pressure >95th percentile (age, sex and height specific, NHBPEP) | Blood pressure >90th percentile | Blood pressure ≥90th percentile (age, sex and height specific, NHBPEP) | SBP ≥95th percentile | SBP ≥17.3 kPa (130 mmHg) or DBP ≥11.3 kPa (85 mm Hg) | SBP ≥90th percentile or DBP ≥90th percentile |
IDF: International Diabetes Federation, IDEFICS: identification and prevention of dietary- and lifestyle-induced health effects in children and infants, WC: waist circumference, NHANES: National Health and Nutrition Examination Survey, NCEP: National Cholesterol Education Program, HDL-C: high-density lipoprotein cholesterol, NHBPEP: National High Blood Pressure Education Program, ADA: American Diabetes Association, BMI: body mass index, NGHS: National Growth and Health Study, SBP: systolic blood pressure, DBP: diastolic blood pressure, HOMA: homeostatic model assessment.
The WHO and the NCEP:ATPIII definitions for adults are basically similar in the diagnostic criteria on obesity, hypertension, and dyslipidemia [90,93]. However, type 2 diabetes, insulin resistance, and/or impaired glucose tolerance are a prerequisite for the WHO definition, making the criterion relatively more restrictive [90]. The exclusion of patients with type 2 diabetes from the EGIR definition also makes its definition less inclusive [91,92]. In contrast, the IDF definition has central obesity as its prerequisite and this may make it relatively less restrictive comparted to the NCEP:ATPIII definition [94,95]. Prevalence became more inclusive after the original NCEP:ATPIII criteria were revised to include the cutoff for impaired fasting glucose, i.e., ≤100 mg/dL vs. 110 mg/dL, as recommended by the 2003 criteria of the American Diabetes Association [121]. Because the vast majority of diabetic patients meet the minimum criteria for the diagnosis of the metabolic syndrome, inclusion or exclusion of patients with type 2 diabetes has a marked impact on the prevalence rates while using the WHO or the NCEP:ATPIII definitions for epidemiological studies. In general, higher prevalence may be estimated using the IDF definition compared to the NCEP:ATPIII definition, as well as using the revised NCEP:ATPIII definition compared to the original one. Moreover, there is a difference in the age-adjusted prevalence rates among the various definitions of metabolic syndrome [122,123,124]. It is worth noting that definition-related differences in prevalence are not consistent among countries, whereas these differences may be attributed, in part, to the race-specific waist circumference guidelines.
Although one definition with cutoffs specific for gender and ethnic origin could be appropriate for at-risk adults, using such definition for children and adolescents is problematic because lipid concentrations in the liver, adipose tissues, and plasma, as well as anthropometric variables, blood pressure, and insulin sensitivity change dramatically with age and pubertal development [124]. Furthermore, for children aged 10 years or older, the diagnosis of the metabolic syndrome is often made by abdominal obesity plus the presence of two or more other clinical features, i.e., hyperglycemia, hypertriglyceridemia, hypertension, or low plasma HDL-cholesterol levels (Table 3) [100]. Despite this, a major problem with the diagnosis of the metabolic syndrome in children and adolescents is that no established criteria for this young population are available to date. The uniqueness of pubertal growth patterns, effects of hormonal changes of puberty on insulin sensitivity and lipid profile, redistribution of adipose tissues in the body, and the impact of ethnic background on components of the metabolic syndrome make such criteria difficult to establish for children and adolescents [34,64,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152].
Table 3. The IDEFICS definition of the metabolic syndrome in children aged 2–11 years (2014).
| · Obesity: ≥90th percentile as assessed by waist circumference |
| · Triglycerides: ≥90th percentile |
| · HDL cholesterol: ≤10th percentile |
| · Blood pressure: systolic ≥90th percentile or diastolic ≥90th percentile |
| · Glucose: insulin ≥90th percentile or fasting glucose ≥90th percentile, according to homeostasis model assessment |
Each category counts as one risk criterion.
IDEFICS: identification and prevention of dietary- and lifestyle-induced health effects in children and infants, HDL: high-density lipoprotein.
Modified and reproduced with permission from reference [100].
As shown by epidemiological investigations, the presence of the metabolic syndrome in children and adolescents could be an important predictor of future risk for type 2 diabetes and cardiovascular disease in adulthood [63,64,153,154]. The results from the Bogalusa Heart Study suggested that cardiovascular risk factors present in childhood could be predictive of coronary artery disease in adulthood [155,156,157,158,159,160,161,162]. This study pointed out that plasma LDL-cholesterol concentrations and BMI determined in childhood could be used to predict carotid intima-media thickness in young adults. In addition, visceral obesity is the major determinant of insulin resistance in children and adolescents [163,164,165,166,167,168,169,170], which increases the risk not only for the metabolic syndrome in young people, but also for the development of cardiovascular disease and type 2 diabetes in adulthood [124].
Table 4 [15] shows the recommended waist circumference thresholds for abdominal obesity in adults by different organizations. Although numerous variables have been used to define obese children, waist circumference is an important predictor that is independent of insulin resistance, plasma lipid levels, and blood pressure in young people [171,172,173,174,175,176,177], which is consistent with the situation in adults [178,179,180,181,182]. Even if children and adolescents are obese and have similar BMI, insulin sensitivity is lower in those with large amounts of visceral adipose tissues compared to those with small amounts [183,184,185,186,187]. For the new IDF criteria, it is critical to use waist circumference to define central obesity in children and adolescents [94,95]. Moreover, percentiles, but not absolute values, of waist circumference are used by the new IDF definition to compensate for variation in child development and ethnic origin [94,95]. Children and adolescents with a waist circumference higher than the 90th percentile have multiple risk factors for cardiovascular disease compared to those with lower one. Many studies have used the 90th percentile as a cutoff for waist circumference in young people. As a result, the concept of using percentiles of waist circumference, which is specific to ethnic origin, is becoming increasingly acceptable for clinical studies [188,189,190,191,192,193]. In addition, the 90th percentile as a cutoff for waist circumference has been used as the essential standard for the diagnosis of the metabolic syndrome in children and adolescents [94,95,194].
Table 4. Recommended waist circumference thresholds for abdominal obesity in adults by different organizations (2009).
| Population | Organization | Recommended waist circumference threshold for abdominal obesity | |
|---|---|---|---|
| Men | Women | ||
| Europid | IDF | ≥94 cm | ≥80 cm |
| Caucasian | WHO | ≥94 cm (increased risk) | ≥80 cm (increased risk) |
| ≥102 cm (still higher risk) | ≥88 cm (still higher risk) | ||
| United States | AHA/NHLBI (ATP III)* | ≥102 cm | ≥88 cm |
| Canada | Health Canada | ≥102 cm | ≥88 cm |
| European | European Cardiovascular Societies | ≥102 cm | ≥88 cm |
| Asian (including Japanese) | IDF | ≥90 cm | ≥80 cm |
| Asian | WHO | ≥90 cm | ≥80 cm |
| Japanese | Japanese Obesity Society | ≥85 cm | ≥90 cm |
| China | Cooperative Task Force | ≥85 cm | ≥80 cm |
| Middle East, Mediterranean | IDF | ≥94 cm | ≥80 cm |
| Sub-Saharan African | IDF | ≥94 cm | ≥80 cm |
| Ethnic Central and South American | IDF | ≥90 cm | ≥80 cm |
IDF: International Diabetes Federation, WHO: World Health Organization, NHLBI: National Heart, Lung, and Blood Institute, ATPIII: the adult treatment panel III.
*The guidelines of the American Heart Association and the National Heart, Lung, and Blood Institute for the metabolic syndrome recognize an increased risk for cardiovascular disease and diabetes at waist-circumference thresholds of ≥94 cm in men and ≥80 cm in women and identify these as optional cut points for individuals or populations with increased insulin resistance.
Modified and reproduced with permission from reference [15].
The reported prevalence rates of the metabolic syndrome in young people vary greatly depending on the age and population studied and the definition used. Because it is more difficult to assess the prevalence rates of the metabolic syndrome in children and adolescents compared to adults, this has greatly prompted the development of new simple-to-use definitions. Many new definitions of the metabolic syndrome in young people builds on previous studies that used the modified adult criteria to investigate prevalence in children and adolescents [195,196,197,198,199,200]. A study using the modified NCEP:ATPIII criteria found that the prevalence of metabolic syndrome in adolescents is 12% [98]. The National Health and Nutrition Examination Survey III (NHANES) reported approximately 10% of children and adolescents aged 12–19 years suffer from the metabolic syndrome [62]. As estimated by population-weighted studies, it is highly likely that more than 2 million American youngsters suffer from the phenotypes of the metabolic syndrome [61,62,63]. The metabolic syndrome is most frequent in obese adolescents, with a prevalence of 32.1%, compared with only 7.1% in overweight adolescents [65,201]. Because of the increasing prevalence of overweight and obesity in children and adolescents, as well as the compelling relationship between obesity and the metabolic syndrome, it is well understood that the prevalence rates of the metabolic syndrome have augmented in American youngsters over the past 30 years.
In addition, there are racial/ethnic differences in the prevalence rates of the metabolic syndrome, as well as of individual components of the metabolic syndrome in children and adolescents. Using the 1986–1994 NHANES data as analyzed by the modified NCEP:ATPIII criteria, the overall prevalence rates of the metabolic syndrome in 2,430 American adolescents are 4.2%, with 6.1% being boys and 2.1% being girls, respectively [62,71,202,203]. Among obese and overweight adolescents, the prevalence rates of the metabolic syndrome are 28.7% and 6.8%, respectively [62,71,202,203]. Similar to adults, the prevalence of individual components of the metabolic syndrome differed by race/ethnicity, e.g., the prevalence of hypertension is higher in African American adolescents compared to that in young non-Hispanic Caucasians or Mexican Americans. In contrast, the prevalence of hypertriglyceridemia and low plasma HDL-cholesterol concentrations is lower in the former than in the latter [204,205,206,207,208,209,210,211,212,213].
PATHOPHYSIOLOGY
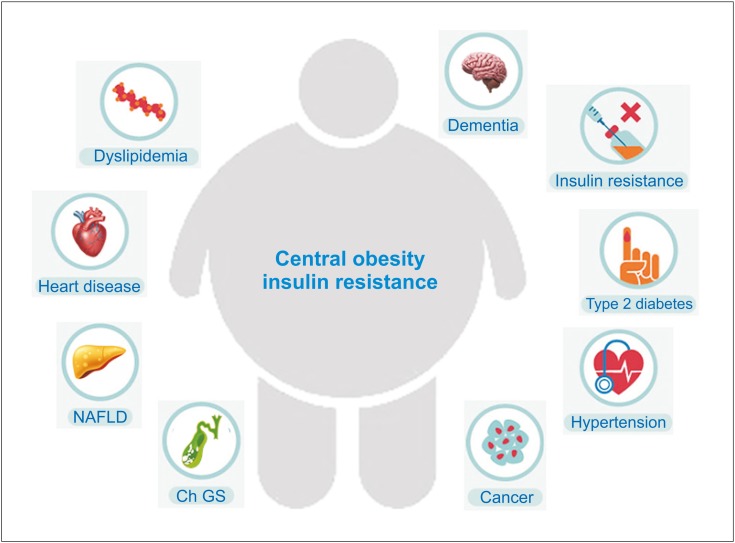
Clinical and epidemiological studies have clearly demonstrated that obesity is often associated with many metabolic abnormalities including insulin resistance, impaired glucose tolerance, hypertension, hypercholesterolemia, and hypertriglyceridemia [214], as shown in Fig. 2. The cluster of these metabolic abnormalities is defined as the metabolic syndrome, a state associated with increased prevalence of several metabolic diseases such as type 2 diabetes and cardiovascular disease, and all-cause mortality not only during adulthood, but also in children and adolescents [215]. The prevalence rates of obesity in adolescents (≥95th percentile of BMI for age) have dramatically increased from 15 to 37% over the last 30 years [107,216,217,218,219,220]. There is a risk of 50 to 77% for obese adolescents to become obese adults [221,222,223]. If parents are obese, this risk can increase to 80% for obese adolescents [224]. In addition, obese children and adolescents are at high risk of developing obesity-related complications and have shown an increased risk of adult morbidity and mortality [225,226,227].
Fig. 2.
Obesity and insulin resistance play a key role in the pathogenesis of the metabolic syndrome in childhood and adolescence.
Ch GS: cholesterol gallstones, NAFLD: nonalcoholic fatty liver disease.
Although the exact etiology of the metabolic syndrome is not fully understood, insulin resistance is considered as a key factor for the development of the metabolic syndrome and is largely involved in the pathogenesis of individual metabolic components of the syndrome [49,50,51]. As found by the insulin-modified, frequently-sampled intravenous glucose tolerance assay, insulin sensitivity is significantly lower in patients with two or more than two components of the metabolic syndrome compared to those with none of these components [228,229,230]. It is well known that insulin plays a critical role in the regulation of glucose, lipid, and energy metabolism in many organs and tissues such as the liver, adipose tissue, muscle, heart, and gastrointestinal track [231]. Therefore, early identification of children and adolescents who are at risk of developing the metabolic syndrome, type 2 diabetes, and cardiovascular disease in later life is extremely important [232,233,234,235]. Conditions in utero and in early childhood could predispose a child to metabolic disorders such as obesity, insulin resistance, hypertension, hypercholesterolemia, hypertriglyceridemia, type 2 diabetes, NAFLD, NASH, cardiovascular disease, and the metabolic syndrome [236,237,238,239,240]. Furthermore, urbanization, overconsumption of nutrients, unhealthy diet, and sedentary lifestyle have been found to be major contributors to such disorders [241,242,243]. Epidemiological investigations have clearly shown that obesity is associated with increased risk of type 2 diabetes and cardiovascular disease [244,245,246], which may persist from childhood and adolescence into young adulthood [247,248,249,250].
PREVENTION AND TREATMENT
Because obesity is associated with increased risk of type 2 diabetes and cardiovascular disease, this may continue into childhood and adolescence until adulthood. Therefore, lifestyle changes are the main options for the prevention of the metabolic syndrome in childhood and adolescence with a special focus on keeping weight within normal range [251,252]. Lifestyle modification includes eating healthy diet and appropriate amounts of total calories, increasing physical activity, and maintaining the right weight. In general, the therapeutic interventions are divided into (i) lifestyle modification, (ii) pharmaceutical therapy, and (iii) bariatric surgery [253,254,255].
Lifestyle modification
1. Diet
It is well known that Western diet contains high total calories, cholesterol, saturated fatty acids, refined carbohydrates, proteins, and salt, as well as low fibers, and it is highly associated with the metabolic abnormalities [256,257,258]. Moreover, overconsumption of fast foods in combination with inactive physical activity is strongly associated with the high prevalence of overweight, obesity, dyslipidemia, hypertension, type 2 diabetes, and cardiovascular disease in children and adolescents over the past 30 years [259]. Clearly, eating healthy diet has a significant impact on all the components of the metabolic syndrome [260]. Although each case should be treated individually, it is important to recommend a healthy diet with low total calories, cholesterol, saturated fat, and sodium, as well as high unsaturated fat, complex carbohydrates, and fiber [261]. This should be the first step in halting the development of metabolic abnormalities in children and adolescents [262,263,264].
It is well established that weight loss has a great benefit for the treatment of all the components of the metabolic syndrome, including excessive adiposity, dyslipidemia, hypertension, insulin resistance, and hyperglycemia [265,266,267,268]. The intensive lifestyle intervention with a special focus on a significant decrease in daily caloric intake could lead to weight loss [269]. It is worthwhile noting that even if the magnitude of weight loss is not drastic, some metabolic abnormalities could be improved. As shown by the Finnish Diabetes Prevention Study [270], lifestyle intervention with modest weight loss could significantly reduce the prevalence of the metabolic syndrome compared with the control group. A modest weight loss often improves blood pressure regulation and decreases the risk of developing hypertension [271,272,273]. In addition, weight loss may increase plasma HDL-cholesterol concentrations, as well as reduce plasma triglyceride and fasting blood glucose levels, and hemoglobin A1c values [274,275,276]. A 7-day negative energy balance without measurable weight loss has found that reducing daily caloric intake may improve insulin sensitivity [277,278,279].
It has been recognized that high dietary cholesterol is a risk factor for cardiovascular disease, dyslipidemia, NAFLD, NASH, and cholesterol gallstone disease, with all of these being the major components of the metabolic syndrome [280,281,282]. Therefore, it is important to recommend a low cholesterol diet to children and adolescents. The NCEP:ATPIII guidelines and the recommendations from the American Heart Association and the American College of Cardiology have proposed a lower (<100 mg/dL) target for plasma LDL-cholesterol levels for individuals at high risk for adverse cardiovascular events [283,284,285,286]. Therefore, the low cholesterol diet could reduce not only total cholesterol concentrations in the plasma, liver, and bile, but also plasma LDL-cholesterol concentrations [287].
Dietary carbohydrates are often divided into two types: simple and complex. It is recommended that complex carbohydrates should make up most of daily carb intake [288,289,290]. In contrast, simple carbohydrates, especially refined carbohydrates such as added sugars, should be limited [291,292,293]. Common sources of added sugars include soft and fruit drinks, as well as candies, cakes, cookies, dairy desserts, pies, and purified sugars [294,295,296]. Although chemical structures are identical between added sugars and naturally occurring simple sugars, e.g., sugars found in fruit, added sugars contain less or no vitamins and minerals. Thus, large amounts of added sugar intake could lead to a lack of nutrients found in foods. In addition, added sugars quickly raise blood glucose levels and increase the risk of insulin resistance. Based on these observations, a concept was proposed that carbohydrates are classified as “good” or “bad” for disease risk, as indicated by the glycemic index [297,298,299]. For example, low glycemic index foods may improve components, i.e., hyperlipidemia and hyperglycemia, of the metabolic syndrome, whereas high glycemic index foods may increase the risk of insulin resistance and the metabolic syndrome [299,300,301,302]. Moreover, the Nurses' Health Study showed that a lower glycemic load is associated with a decreased risk of developing cardiovascular disease [303].
For most people, a carbohydrate intake of 45 to 65% of total daily calories is appropriate, as recommended by the US Department of Agriculture. In general, a diet high in complex, unrefined carbohydrates with an emphasis on fiber (25 g per day) and low in added sugars (<25% of caloric intake) is recommended for individuals with or at risk for the metabolic syndrome [304,305,306,307]. Clinical studies found that high daily carbohydrate intake is associated with increased plasma total cholesterol, LDL-cholesterol, and triglyceride concentrations, and reduced HDL-cholesterol levels [292,293,308,309,310]. In contrast, low carbohydrate diets may improve glucose metabolism in subjects with insulin resistance and/or type 2 diabetes [170,311,312]. It is unclear whether low daily carbohydrate intake may influence lipid metabolism and reduce the risk of hypercholesterolemia and hypertriglyceridemia. Another explanation is that low daily carbohydrate intake may enhance insulin sensitivity, thus improving cholesterol and triglyceride metabolism in the liver and plasma [30,313,314,315]. Although lower carbohydrate diets may be helpful in weight loss in the short term, the effects on long-term weight loss have been mixed and further studies are needed.
Most studies suggested that high fat intake, i.e., 20 to 40% of caloric intake, may increase the risk of overweight and obesity, thereby leading to insulin resistance. In addition, high fat intake may increase the prevalence of NAFLD, NASH, hypertension, type 2 diabetes, and cardiovascular disease [57,316,317,318]. However, because some conflicting results have been reported, it is unclear whether increased fat intake per se may have an impact on insulin sensitivity or may impair glucose metabolism [319]. Although the average fat intake in the USA has been reduced from 36.9 to 32.8% in men and from 36.1 to 32.8% in women over the past 50 years, there has been a marked increase in overweight, obesity, and the metabolic syndrome during the same time period [106,107,320]. This suggested that it may be the type of fats consumed, rather than the total amount of intake, producing a greater effect on the components of the metabolic syndrome [321,322].
The fatty acids in fat are often divided into two types: saturated and unsaturated fatty acids, with the latter being subclassified to monounsaturated and polyunsaturated fatty acids [57]. In general, saturated, but not unsaturated, fatty acids are associated with impaired glucose tolerance and obesity, as well as increased risk of developing NAFLD, NASH, hypertension, type 2 diabetes, and cardiovascular disease [323,324,325]. It is highly likely that a diet with high unsaturated fatty acids and low saturated fatty acids may improve insulin sensitivity and plasma lipid and lipoprotein metabolism [326]. The Nurses' Health Study has found that a 5% increase in saturated fat intake is associated with a 17% increment in risk of coronary heart disease [327]. In contrast, increased monounsaturated and polyunsaturated fat intake may be associated with a reduced risk of coronary heart disease [328].
Low sodium intake has a good benefit for blood pressure regulation because clinical and epidemiological studies have revealed a clear positive relationship between sodium intake and blood pressure [329]. It is well known that excessive sodium intake can cause hypertension not only in adults, but also in children and adolescents [260]. As shown by the Dietary Approaches to Stop Hypertension Study [330,331,332], lower sodium intake reduces blood pressure in people with mild or moderate hypertension, as well as sodium restriction may be associated with decreased risk of cardiovascular disease and congestive heart failure. Therefore, it is strongly recommended that sodium restriction or low sodium diet should be given to children and adolescents, especially to obese young people. This is a key step for the prevention and the treatment of hypertension, a major component of the metabolic syndrome [333].
Because of a lack of sufficient clinical and epidemiological data, it is unclear whether protein intake has an association with the development of the metabolic syndrome [334]. A daily protein intake of 10 to 35% of total calories has been recommended for the general population [335]. Nevertheless, appropriate daily protein intake is good for people, regardless of whether they have normal weight or are obese, except for patients with nephropathy [336].
2. Physical activity
Epidemiological surveys have found that a sedentary lifestyle in combination with unhealthy eating habits likely increases the risk of insulin resistance, type 2 diabetes, cardiovascular disease, NAFLD, and NASH [337]. Thus, increasing excise to reduce and/or maintain weight is another important approach for preventing or treating the metabolic syndrome [338,339,340]. Many epidemiological reports have shown that low physical activity is associated with increased prevalence of the metabolic syndrome, whereas high physical activity is likely to protect against the development of the metabolic syndrome [341]. Indeed, higher cardiorespiratory fitness and extensive physical activity have been shown to improve glucose metabolism and insulin sensitivity and reduce cardiovascular disease mortality, as well as the risk of type 2 diabetes, NAFLD, and NASH [342,343,344,345]. It is likely that increasing physical activity could reduce the risk of cardiovascular disease and the prevalence of type 2 diabetes, NAFLD, and NASH through weight loss [346,347,348]. Furthermore, cardiorespiratory fitness and intensive physical activity prevent the development of the metabolic syndrome likely through their effects on each of the individual components [343,349,350]. Clinical studies have revealed that combining with healthy dietary intake, high-intensity exercise, i.e., aerobic exercise, is very effective at enhancing insulin sensitivity and reducing weight, particularly abdominal adiposity, as well as potentially improving hypertension, hyperglycemia, and dyslipidemia [351,352,353,354].
As shown by a systematic review of the literature, aerobic exercise may reduce visceral adiposity in a dose-dependent manner [355,356,357]. However, it is unclear whether exercise could reduce visceral adipose tissue in the absence of weight loss [358]. To achieve continued benefit of exercise on insulin action, the American Heart Association and the American College of Sports Medicine have recommended exercise at least 30 minutes/day most days of the week [359]. Aerobic exercise may produce a persistent effect on glucose tolerance and insulin action beyond the immediate post-exercise effects and possibly through weight loss [360,361,362]. More importantly, while maintaining weight, regular aerobic exercise is still critical to reducing abdominal fat tissue and preventing weight regain in individuals who have successfully lost weight [362,363,364].
Pharmaceutical therapy
Because there are no published papers reporting double-blind, randomized controlled trials on the management of the metabolic syndrome, no guidelines or specific recommendations are currently available for treating the metabolic syndrome [365,366,367,368,369,370,371]. In addition, because the cellular and molecular mechanisms underlying the pathogenesis of the metabolic syndrome are not completely understood, the therapeutic options have not yet been developed [10]. The currently available therapeutic strategies focus mainly on treating the individual components of the metabolic syndrome, with the overall goals of reducing the risk of cardiovascular disease and type 2 diabetes or preventing them [86,372,373,374,375,376] Moreover, some therapeutic options may have a marked impact on two or more than two components of the metabolic syndrome [377,378,379]. Nevertheless, many therapeutic efforts on the treatment of the visceral obesity and insulin resistance associated with the metabolic syndrome may provide the most overall success in achieving these goals [380,381,382,383,384,385,386,387].
Bariatric surgery
For adults, if a body mass index (BMI) is ≥40 or a BMI is 35 in patients with significant obesity-related comorbidities, bariatric surgery can be considered as a weight loss procedure [388,389,390,391,392]. Because of a lack of clear evidence concerning surgical treatment for children and adolescents, bariatric surgery is not recommended by the NIH Consensus Panel as an acceptable alternative that can achieve sustained weight loss in young patients [393,394,395,396]. However, when lifestyle modification and standard pharmaceutical therapy are not effective in reducing body weight and BMI, bariatric surgery may be considered for children and adolescents [397,398,399,400]. In general, the surgical interventions include Roux-en-Y gastric bypass (RYGBP), adjustable gastric band, sleeve gastrectomy, and biliopancreatic diversions for obese children and adolescents [401,402,403,404,405,406,407,408]. The limited experience with bariatric surgery in young patients suggests that RYGBP surgery and adjustable gastric banding can effectively treat the comorbidities of adolescent obesity. The surgery can be open and/or laparoscopic procedures [409]. However, because children and adolescents are still developing, both physically and mentally, they and/or their parents may show less willingness to give consent to surgery [410]. Moreover, bariatric surgery may dramatically change their lives after operation. Therefore, careful consideration must be given to whether or not surgery is performed on obese children and adolescents [411,412,413,414]. Table 5 [404] lists indications and contraindications for bariatric surgery in children and adolescents.
Table 5. Indications and contraindications for bariatric surgery in children and adolescents.
| Indications: | |
| · Failure of at least 6 months of organized, medically supervised weight loss attempts | |
| · Ages 13 to 18 for girls, and 14 to 18 for boys | |
| · BMI ≥40 with presence of severe obesity-related comorbidity | |
| · BMI ≥50 with less severe obesity-related comorbidities | |
| Contraindications: | |
| · Substance abuse problem within the preceding year | |
| · Psychiatric diagnosis that would impair ability to adhere to postoperative dietary or medication regimen (e.g., psychosis) | |
| · Medically correctable cause of obesity | |
| · Inability or unwillingness of patient or parent to fully comprehend the surgical procedure and its medical consequences | |
| · Inability or refusal to participate in lifelong medical surveillance | |
BMI: body mass index.
Modified and reproduced with permission from reference [404].
As shown in the data from the US National Inpatient Sample, 2,744 adolescents have received bariatric surgeries in the USA from 1996 to 2003 [415]. Because many children and adolescents with clinically severe obesity are interested in a bariatric surgical option to attain a healthier weight, it is estimated that more and more bariatric operations have been done since this time [415]. Similar to adults, bariatric surgery leads to very good short-term weight outcomes in children and adolescents [415]. Among different surgical methods, RYGB produces the greatest weight loss, as shown by the most reliable clinical evidence [416,417,418,419]. Because no other evidence-based medical interventions lead to a similar magnitude of weight loss, bariatric surgery could be considered a treatment option especially for children and adolescents who have obesity-related complications and comorbidities [420,421,422,423].
Although there are compelling reasons to offer bariatric procedures to some children and adolescents in whom prior weight management attempts have not been successful [424,425,426,427,428,429], there are also disagreements and dissenting voices because of the limited experience with bariatric surgery in young patients. Furthermore, children and adolescents can lose weight more effectively with lifestyle modification compared to adults, but they have not always made their best attempts at nonsurgical weight loss [430,431,432]. More aggressive steps should be taken to improve young people's lifestyles to achieve sustained weight loss. In addition, children and adolescents are still growing with age, which could be adversely affected by nutritional consequences of a bariatric operation [433,434,435]. Although many seriously obese adolescents are interested in bariatric procedures to achieve weight loss, they are not psychologically prepared for bariatric surgery [436]. Therefore, it is necessary to propose more strict bariatric surgery standards for young people compared to adults [404]. In addition, to more carefully evaluate the harms and benefits of bariatric surgery for children and adolescents, it is imperative to perform long-term, prospectively designed clinical studies, with clear inclusion and exclusion criteria and reporting of beneficial effects, side effects, complications, and comorbidity resolution, as well as measures of health-related quality of life after operation [437].
CONCLUSIONS AND FUTURE DIRECTIONS
Clearly, the metabolic syndrome, by definition, is not a disease but is a clustering of individual metabolic risk factors that could dramatically increase the prevalence of type 2 diabetes and cardiovascular disease. Although many definitions of the metabolic syndrome in children and adolescents have been proposed, no unified definition exists to assess risk or outcomes in pubertal subjects to date. Because dysfunctional glucose, lipid, and energy metabolism across several organs and tissues occurs under insulin resistant conditions, together creating the observed interplay of several concurrent metabolic abnormalities, early identification of children and adolescents at risk of developing the metabolic syndrome is extremely important. A clinically accessible diagnostic tool is needed to identify the metabolic syndrome in young people globally. This will greatly prompt the development of a new simple definition that is easy to apply for clinical practice and epidemiological surveys.
For future research, it is imperative to decipher the relationship between body fat and its distribution in the body in children and adolescents and investigate whether early growth patterns predict future adiposity and other features of the metabolic syndrome. More importantly, it is urgent to perform long-term cohort studies on children and adolescents of different ethnic origin into adulthood by investigating the natural history and effectiveness of interventions, especially those related to lifestyle. As indicated in Fig. 3, early detection followed by treatment, particularly lifestyle intervention, is vital to halt the progression of the metabolic syndrome in children and adolescents. Such an action should reduce morbidity and mortality in adulthood and help minimize the global burden of cardiovascular disease and type 2 diabetes.
Fig. 3. Because obesity is associated with increased risk of type 2 diabetes and cardiovascular disease, this may continue into childhood and adolescence until adulthood. Therefore, lifestyle modifications, including eating healthy diet and appropriate amounts of total calories, increasing physical activity, and maintaining the right weight, are the main options for the prevention of the metabolic syndrome by halting the development of metabolic abnormalities in childhood and adolescence.
Lifestyle interventions and other non-pharmacological treatments are often considered to be the first option but, such interventions have variable outcomes. Eating healthy diet and appropriate amounts of total calories, increasing physical activity, and maintaining the right weight could substantially improve insulin resistance, blood pressure, and plasma lipid and lipoprotein metabolism. Pharmacological treatment appears to have modest effectiveness. However, when combined with lifestyle interventions, it is associated with more adverse effects than lifestyle interventions alone. Several surgical procedures are available for children and adolescents; however, long-term effects of bariatric surgery from high quality studies are strongly needed.
ACKNOWLEDGEMENTS
This work was supported in part by research grants DK106249 and DK114516 (to DQ-HW), as well as P30 DK041296 (to Marion Bessin Liver Research Center), all from the National Institutes of Health (US Public Health Service).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Dommermuth R, Ewing K. Metabolic syndrome: systems thinking in heart disease. Prim Care. 2018;45:109–129. doi: 10.1016/j.pop.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Després JP, Ndumele CE, et al. The cardiometabolic health alliance: working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66:1050–1067. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 5.D'Adamo E, Marcovecchio ML, Giannini C, Capanna R, Impicciatore M, Chiarelli F, et al. The possible role of liver steatosis in defining metabolic syndrome in prepubertal children. Metabolism. 2010;59:671–676. doi: 10.1016/j.metabol.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr. 2004;80:1–2. doi: 10.1093/ajcn/80.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 10.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM. Overnutrition, ectopic lipid and the metabolic syndrome. J Investig Med. 2016;64:1082–1086. doi: 10.1136/jim-2016-000155. [DOI] [PubMed] [Google Scholar]
- 12.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24:283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- 14.Stenholm S, Koster A, Alley DE, Visser M, Maggio M, Harris TB, et al. Adipocytokines and the metabolic syndrome among older persons with and without obesity: the InCHIANTI study. Clin Endocrinol (Oxf) 2010;73:55–65. doi: 10.1111/j.1365-2265.2009.03742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim LJ, Nalls MA, Eiriksdottir G, Sigurdsson S, Launer LJ, Koster A, et al. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES-Reykjavik study. Obesity (Silver Spring) 2011;19:1265–1271. doi: 10.1038/oby.2010.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 18.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 20.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes. 2005;54:3252–3257. doi: 10.2337/diabetes.54.11.3252. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 22.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 24.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 26.McCullough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12:333–340. doi: 10.1111/j.1751-2980.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007-2014. Int J Cardiol. 2018;259:216–219. doi: 10.1016/j.ijcard.2018.01.139. [DOI] [PubMed] [Google Scholar]
- 29.Cheung BM, Ong KL, Man YB, Wong LY, Lau CP, Lam KS. Prevalence of the metabolic syndrome in the United States National Health and Nutrition Examination Survey 1999-2002 according to different defining criteria. J Clin Hypertens (Greenwich) 2006;8:562–570. doi: 10.1111/j.1524-6175.2006.05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastorini CM, Panagiotakos DB, Georgousopoulou EN, Laskaris A, Skourlis N, Zana A, et al. Metabolic syndrome and 10-year cardiovascular disease incidence: the ATTICA study. Nutr Metab Cardiovasc Dis. 2016;26:223–231. doi: 10.1016/j.numecd.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. doi: 10.1016/j.phrs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T, et al. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans. The MORGAM prospective cohort project. PLoS One. 2014;9:e107294. doi: 10.1371/journal.pone.0107294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. 2013;57:867–871. doi: 10.1016/j.ypmed.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 35.Cheng TO. Prevalence of metabolic syndrome in Chinese adults has been underestimated by using US-Based National Cholesterol Education Programs Adult Treatment Panel III and World Health Organization criteria. Am J Cardiol. 2006;98:422–423. doi: 10.1016/j.amjcard.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 36.He Y, Li Y, Bai G, Zhang J, Fang Y, Zhao L, et al. Prevalence of metabolic syndrome and individual metabolic abnormalities in China, 2002-2012. Asia Pac J Clin Nutr. 2019;28:621–633. doi: 10.6133/apjcn.201909_28(3).0023. [DOI] [PubMed] [Google Scholar]
- 37.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: a community study from urban Eastern India. J Cardiovasc Dis Res. 2012;3:204–211. doi: 10.4103/0975-3583.98895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katulanda P, Ranasinghe P, Jayawardana R, Sheriff R, Matthews DR. Metabolic syndrome among Sri Lankan adults: prevalence, patterns and correlates. Diabetol Metab Syndr. 2012;4:24. doi: 10.1186/1758-5996-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SR, Cha MJ, Kang DY, Oh KC, Shin DH, Lee HY. Increased prevalence of metabolic syndrome among hypertensive population: ten years' trend of the Korean National Health and Nutrition Examination Survey. Int J Cardiol. 2013;166:633–639. doi: 10.1016/j.ijcard.2011.11.095. [DOI] [PubMed] [Google Scholar]
- 40.Lee SE, Han K, Kang YM, Kim SO, Cho YK, Ko KS, et al. Trends in the prevalence of metabolic syndrome and its components in South Korea: findings from the Korean National Health Insurance Service Database (2009-2013) PLoS One. 2018;13:e0194490. doi: 10.1371/journal.pone.0194490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh JH, Kang DR, Jang JY, Shin JH, Kim JY, Choi S, et al. Metabolic syndrome epidemic among Korean adults: Korean survey of Cardiometabolic Syndrome (2018) Atherosclerosis. 2018;277:47–52. doi: 10.1016/j.atherosclerosis.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Genser L, Casella Mariolo JR, Castagneto-Gissey L, Panagiotopoulos S, Rubino F. Obesity, type 2 diabetes, and the metabolic syndrome: pathophysiologic relationships and guidelines for surgical intervention. Surg Clin North Am. 2016;96:681–701. doi: 10.1016/j.suc.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome. Lancet. 2005;366:1921–1922. doi: 10.1016/S0140-6736(05)67778-1. author reply 1923-4. [DOI] [PubMed] [Google Scholar]
- 44.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48:1684–1699. doi: 10.1007/s00125-005-1876-2. [DOI] [PubMed] [Google Scholar]
- 45.Ekelund U, Ward HA, Norat T, Luan J, May AM, Weiderpass E, et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC) Am J Clin Nutr. 2015;101:613–621. doi: 10.3945/ajcn.114.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emili A, Abushomar H, Nair K. Treating metabolic syndrome: lifestyle change or medication? Can Fam Physician. 2007;53:1203–1205. [PMC free article] [PubMed] [Google Scholar]
- 47.Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134:e262–79. doi: 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 48.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535–78. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 49.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 Suppl 1):S12–8. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–2190. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 51.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120(9 Suppl 1):S10–6. doi: 10.1016/j.amjmed.2007.06.006. discussion S16-7. [DOI] [PubMed] [Google Scholar]
- 52.Bodhini D, Mohan V. Mediators of insulin resistance & cardiometabolic risk: newer insights. Indian J Med Res. 2018;148:127–129. doi: 10.4103/ijmr.IJMR_969_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 54.Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–198. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 55.Neuschwander-Tetri BA. Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci. 2005;330:326–335. doi: 10.1097/00000441-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 57.Wang DQ, Portincasa P, Neuschwander-Tetri BA. Steatosis in the liver. Compr Physiol. 2013;3:1493–1532. doi: 10.1002/cphy.c130001. [DOI] [PubMed] [Google Scholar]
- 58.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 59.Banini BA, Sanyal AJ. Current and future pharmacologic treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:134–141. doi: 10.1097/MOG.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American Heart Association. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 61.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 62.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 63.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 64.Bokor S, Frelut ML, Vania A, Hadjiathanasiou CG, Anastasakou M, Malecka-Tendera E, et al. Prevalence of metabolic syndrome in European obese children. Int J Pediatr Obes. 2008;3(Suppl 2):3–8. doi: 10.1080/17477160802404509. [DOI] [PubMed] [Google Scholar]
- 65.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 66.Park J, Hilmers DC, Mendoza JA, Stuff JE, Liu Y, Nicklas TA. Prevalence of metabolic syndrome and obesity in adolescents aged 12 to 19 years: comparison between the United States and Korea. J Korean Med Sci. 2010;25:75–82. doi: 10.3346/jkms.2010.25.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park MJ, Boston BA, Oh M, Jee SH. Prevalence and trends of metabolic syndrome among Korean adolescents: from the Korean NHANES survey, 1998-2005. J Pediatr. 2009;155:529–534. doi: 10.1016/j.jpeds.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 68.Friend A, Craig L, Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. 2013;11:71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 69.Higgins V, Adeli K. Pediatric metabolic syndrome: pathophysiology and laboratory assessment. EJIFCC. 2017;28:25–42. [PMC free article] [PubMed] [Google Scholar]
- 70.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–451. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 71.Cook S. The metabolic syndrome: antecedent of adult cardiovascular disease in pediatrics. J Pediatr. 2004;145:427–430. doi: 10.1016/j.jpeds.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Agirbasli M, Tanrikulu AM, Berenson GS. Metabolic syndrome: bridging the gap from childhood to adulthood. Cardiovasc Ther. 2016;34:30–36. doi: 10.1111/1755-5922.12165. [DOI] [PubMed] [Google Scholar]
- 73.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 74.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 75.Grundy SM. A constellation of complications: the metabolic syndrome. Clin Cornerstone. 2005;7:36–45. doi: 10.1016/s1098-3597(05)80066-3. [DOI] [PubMed] [Google Scholar]
- 76.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 77.Grundy SM. Point: the metabolic syndrome still lives. Clin Chem. 2005;51:1352–1354. doi: 10.1373/clinchem.2005.050989. [DOI] [PubMed] [Google Scholar]
- 78.Kylin E. Studien ueber das Hypertonie-Hyperglyka “mie-Hyperurika” miesyndrom. Zentralblatt fuer Innere Medizin. 1923;44:105–127. [Google Scholar]
- 79.Phillips GB. Sex hormones, risk factors and cardiovascular disease. Am J Med. 1978;65:7–11. doi: 10.1016/0002-9343(78)90685-x. [DOI] [PubMed] [Google Scholar]
- 80.Albrink MJ, Krauss RM, Lindgrem FT, von der Groeben J, Pan S, Wood PD. Intercorrelations among plasma high density lipoprotein, obesity and triglycerides in a normal population. Lipids. 1980;15:668–676. doi: 10.1007/BF02534017. [DOI] [PubMed] [Google Scholar]
- 81.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 82.Cheal KL, Abbasi F, Lamendola C, McLaughlin T, Reaven GM, Ford ES. Relationship to insulin resistance of the adult treatment panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes. 2004;53:1195–1200. doi: 10.2337/diabetes.53.5.1195. [DOI] [PubMed] [Google Scholar]
- 83.Kim SH, Reaven GM. The metabolic syndrome: one step forward, two steps back. Diab Vasc Dis Res. 2004;1:68–75. doi: 10.3132/dvdr.2004.010. [DOI] [PubMed] [Google Scholar]
- 84.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33:283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Reaven GM. Insulin resistance, cardiovascular disease, and the metabolic syndrome: how well do the emperor's clothes fit? Diabetes Care. 2004;27:1011–1012. doi: 10.2337/diacare.27.4.1011. [DOI] [PubMed] [Google Scholar]
- 86.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26:364–373. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Salazar MR, Carbajal HA, Espeche WG, Dulbecco CA, Aizpurúa M, Marillet AG, et al. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diab Vasc Dis Res. 2011;8:109–116. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 88.Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, et al. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53:600–605. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- 89.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 91.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 92.Balkau B, Eschwège E. Insulin resistance: an independent risk factor for cardiovascular disease? Diabetes Obes Metab. 1999;1(Suppl 1):S23–31. doi: 10.1046/j.1463-1326.1999.0010s1023.x. [DOI] [PubMed] [Google Scholar]
- 93.Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 94.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 95.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 96.Assmann G, Guerra R, Fox G, Cullen P, Schulte H, Willett D, et al. Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European populations. Am J Cardiol. 2007;99:541–548. doi: 10.1016/j.amjcard.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 97.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 98.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 99.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 100.Ahrens W, Moreno LA, Mårild S, Molnár D, Siani A, De Henauw S, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes (Lond) 2014;38(Suppl 2):S4–14. doi: 10.1038/ijo.2014.130. [DOI] [PubMed] [Google Scholar]
- 101.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157:26–31.e2. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 103.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 104.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319:1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 107.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 108.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M. Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care. 2004;27:1798–1811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 110.Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 111.Dietz WH, Robinson TN. Clinical practice. Overweight children and adolescents. N Engl J Med. 2005;352:2100–2109. doi: 10.1056/NEJMcp043052. [DOI] [PubMed] [Google Scholar]
- 112.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 113.Arai H, Yamamoto A, Matsuzawa Y, Saito Y, Yamada N, Oikawa S, et al. Prevalence of metabolic syndrome in the general Japanese population in 2000. J Atheroscler Thromb. 2006;13:202–208. doi: 10.5551/jat.13.202. [DOI] [PubMed] [Google Scholar]
- 114.Saito I, Mori M, Shibata H, Hirose H, Tsujioka M, Kawabe H. Prevalence of metabolic syndrome in young men in Japan. J Atheroscler Thromb. 2007;14:27–30. doi: 10.5551/jat.14.27. [DOI] [PubMed] [Google Scholar]
- 115.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adams RJ, Appleton S, Wilson DH, Taylor AW, Dal Grande E, Chittleborough C, et al. Population comparison of two clinical approaches to the metabolic syndrome: implications of the new International Diabetes Federation consensus definition. Diabetes Care. 2005;28:2777–2779. doi: 10.2337/diacare.28.11.2777. [DOI] [PubMed] [Google Scholar]
- 117.Athyros VG, Ganotakis ES, Elisaf M, Mikhailidis DP. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin. 2005;21:1157–1159. doi: 10.1185/030079905x53333. [DOI] [PubMed] [Google Scholar]
- 118.Guerrero-Romero F, Rodríguez-Morán M. Concordance between the 2005 International Diabetes Federation definition for diagnosing metabolic syndrome with the National Cholesterol Education Program Adult Treatment Panel III and the World Health Organization definitions. Diabetes Care. 2005;28:2588–2589. doi: 10.2337/diacare.28.10.2588a. [DOI] [PubMed] [Google Scholar]
- 119.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lovre D, Mauvais-Jarvis F. Trends in prevalence of the metabolic syndrome. JAMA. 2015;314:950. doi: 10.1001/jama.2015.8625. [DOI] [PubMed] [Google Scholar]
- 121.Marchesini G, Forlani G, Cerrelli F, Manini R, Natale S, Baraldi L, et al. WHO and ATPIII proposals for the definition of the metabolic syndrome in patients with Type 2 diabetes. Diabet Med. 2004;21:383–387. doi: 10.1111/j.1464-5491.2004.01115.x. [DOI] [PubMed] [Google Scholar]
- 122.Hu G, Lindström J, Jousilahti P, Peltonen M, Sjöberg L, Kaaja R, et al. The increasing prevalence of metabolic syndrome among Finnish men and women over a decade. J Clin Endocrinol Metab. 2008;93:832–836. doi: 10.1210/jc.2007-1883. [DOI] [PubMed] [Google Scholar]
- 123.Ilanne-Parikka P, Eriksson JG, Lindström J, Hämäläinen H, Keinänen-Kiukaanniemi S, Laakso M, et al. Prevalence of the metabolic syndrome and its components: findings from a Finnish general population sample and the Diabetes Prevention Study cohort. Diabetes Care. 2004;27:2135–2140. doi: 10.2337/diacare.27.9.2135. [DOI] [PubMed] [Google Scholar]
- 124.Reinehr T, de Sousa G, Toschke AM, Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: a critical approach. Arch Dis Child. 2007;92:1067–1072. doi: 10.1136/adc.2006.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jørgensen ME, Bjerregaard P, Gyntelberg F, Borch-Johnsen K Greenland Population Study. Prevalence of the metabolic syndrome among the Inuit in Greenland. A comparison between two proposed definitions. Diabet Med. 2004;21:1237–1242. doi: 10.1111/j.1464-5491.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- 126.Császár A, Kékes E, Abel T, Papp R, Kiss I, Balogh S. Prevalence of metabolic syndrome estimated by International Diabetes Federation criteria in a Hungarian population. Blood Press. 2006;15:101–106. doi: 10.1080/08037050600772284. [DOI] [PubMed] [Google Scholar]
- 127.Liu J, Hanley AJ, Young TK, Harris SB, Zinman B. Characteristics and prevalence of the metabolic syndrome among three ethnic groups in Canada. Int J Obes (Lond) 2006;30:669–676. doi: 10.1038/sj.ijo.0803179. [DOI] [PubMed] [Google Scholar]
- 128.Al-Qahtani DA, Imtiaz ML, Saad OS, Hussein NM. AComparison of the prevalence of metabolic syndrome in Saudi adult females using two definitions. Metab Syndr Relat Disord. 2006;4:204–214. doi: 10.1089/met.2006.4.204. [DOI] [PubMed] [Google Scholar]
- 129.Balasubramanyam A, Rao S, Misra R, Sekhar RV, Ballantyne CM. Prevalence of metabolic syndrome and associated risk factors in Asian Indians. J Immigr Minor Health. 2008;10:313–323. doi: 10.1007/s10903-007-9092-4. [DOI] [PubMed] [Google Scholar]
- 130.Baracco R, Mohanna S, Seclén S. A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in peru. Metab Syndr Relat Disord. 2007;5:55–62. doi: 10.1089/met.2006.0019. [DOI] [PubMed] [Google Scholar]
- 131.Alexander CM, Landsman PB, Grundy SM. The influence of age and body mass index on the metabolic syndrome and its components. Diabetes Obes Metab. 2008;10:246–250. doi: 10.1111/j.1463-1326.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 132.Basit A, Shera AS. Prevalence of metabolic syndrome in Pakistan. Metab Syndr Relat Disord. 2008;6:171–175. doi: 10.1089/met.2008.0005. [DOI] [PubMed] [Google Scholar]
- 133.Bee YT, Jr, Haresh KK, Rajibans S. Prevalence of metabolic syndrome among Malaysians using the International Diabetes Federation, National Cholesterol Education Program and modified World Health Organization definitions. Malays J Nutr. 2008;14:65–77. [PubMed] [Google Scholar]
- 134.Caceres M, Teran CG, Rodriguez S, Medina M. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008;8:31. doi: 10.1186/1471-2431-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Calcaterra V, Klersy C, Muratori T, Telli S, Caramagna C, Scaglia F, et al. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clin Endocrinol (Oxf) 2008;68:868–872. doi: 10.1111/j.1365-2265.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- 136.Bener A, Zirie M, Musallam M, Khader YS, Al-Hamaq AO. Prevalence of metabolic syndrome according to Adult Treatment Panel III and International Diabetes Federation criteria: a population-based study. Metab Syndr Relat Disord. 2009;7:221–229. doi: 10.1089/met.2008.0077. [DOI] [PubMed] [Google Scholar]
- 137.Bindraban NR, van Valkengoed IG, Mairuhu G, Koster RW, Holleman F, Hoekstra JB, et al. A new tool, a better tool? Prevalence and performance of the International Diabetes Federation and the National Cholesterol Education Program criteria for metabolic syndrome in different ethnic groups. Eur J Epidemiol. 2008;23:37–44. doi: 10.1007/s10654-007-9200-8. [DOI] [PubMed] [Google Scholar]
- 138.Caranti DA, Lazzer S, Dâmaso AR, Agosti F, Zennaro R, de Mello MT, et al. Prevalence and risk factors of metabolic syndrome in Brazilian and Italian obese adolescents: a comparison study. Int J Clin Pract. 2008;62:1526–1532. doi: 10.1111/j.1742-1241.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 139.Chien KL, Lee BC, Hsu HC, Lin HJ, Chen MF, Lee YT. Prevalence, agreement and classification of various metabolic syndrome criteria among ethnic Chinese: a report on the hospital-based health diagnosis of the adult population. Atherosclerosis. 2008;196:764–771. doi: 10.1016/j.atherosclerosis.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 140.Churilla JR, Fitzhugh EC, Thompson DL. The metabolic syndrome: how definition impacts the prevalence and risk in U.S. adults: 1999-2004 NHANES. Metab Syndr Relat Disord. 2007;5:331–342. doi: 10.1089/met.2007.0010. [DOI] [PubMed] [Google Scholar]
- 141.Dhanaraj E, Bhansali A, Jaggi S, Dutra P, Jain S, Tiwari P, et al. Prevalence and predictors of metabolic syndrome in non-obese Asian Indians with newly detected type 2 diabetes mellitus. J Indian Med Assoc. 2008;106:366–368. 370–362. [PubMed] [Google Scholar]
- 142.Ekelund U, Anderssen S, Andersen LB, Riddoch CJ, Sardinha LB, Luan J, et al. Prevalence and correlates of the metabolic syndrome in a population-based sample of European youth. Am J Clin Nutr. 2009;89:90–96. doi: 10.3945/ajcn.2008.26649. [DOI] [PubMed] [Google Scholar]
- 143.Erem C, Hacihasanoglu A, Deger O, Topbaş M, Hosver I, Ersoz HO, et al. Prevalence of metabolic syndrome and associated risk factors among Turkish adults: Trabzon MetS study. Endocrine. 2008;33:9–20. doi: 10.1007/s12020-008-9044-3. [DOI] [PubMed] [Google Scholar]
- 144.Fiuza M, Cortez-Dias N, Martins S, Belo A VALSIM study investigators. Metabolic syndrome in Portugal: prevalence and implications for cardiovascular risk--results from the VALSIM Study. Rev Port Cardiol. 2008;27:1495–1529. English, Portuguese. [PubMed] [Google Scholar]
- 145.Malik M, Razig SA. The prevalence of the metabolic syndrome among the multiethnic population of the United Arab Emirates: a report of a national survey. Metab Syndr Relat Disord. 2008;6:177–186. doi: 10.1089/met.2008.0006. [DOI] [PubMed] [Google Scholar]
- 146.Martínez MA, Puig JG, Mora M, Aragón R, O'Dogherty P, Antón JL, et al. Metabolic syndrome: prevalence, associated factors, and C-reactive protein: the MADRIC (MADrid RIesgo Cardiovascular) Study. Metabolism. 2008;57:1232–1240. doi: 10.1016/j.metabol.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 147.Matei C, Pop I, Jurcut R, Suceveanu M, Predescu D, Nechita E, et al. ROmanian multicentric study of the prevalence of metabolic syndrome--ROMES. Hellenic J Cardiol. 2008;49:303–309. [PubMed] [Google Scholar]
- 148.Sen Y, Kandemir N, Alikasifoglu A, Gonc N, Ozon A. Prevalence and risk factors of metabolic syndrome in obese children and adolescents: the role of the severity of obesity. Eur J Pediatr. 2008;167:1183–1189. doi: 10.1007/s00431-007-0658-x. [DOI] [PubMed] [Google Scholar]
- 149.Seo SJ, Lee HY, Lee SW. The prevalence of the metabolic syndrome in Korean children and adolescents: comparisons of the criteria of Cook et al., Cruz and Goran, and ferranti et al. Yonsei Med J. 2008;49:563–572. doi: 10.3349/ymj.2008.49.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shimajiri Y, Tsunoda K, Furuta M, Kadoya Y, Yamada S, Nanjo K, et al. Prevalence of metabolic syndrome in Japanese type 2 diabetic patients and its significance for chronic vascular complications. Diabetes Res Clin Pract. 2008;79:310–317. doi: 10.1016/j.diabres.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 151.Yoneda M, Yamane K, Jitsuiki K, Nakanishi S, Kamei N, Watanabe H, et al. Prevalence of metabolic syndrome compared between native Japanese and Japanese-Americans. Diabetes Res Clin Pract. 2008;79:518–522. doi: 10.1016/j.diabres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 152.Halley Castillo E, Borges G, Talavera JO, Orozco R, Vargas-Alemán C, Huitrón-Bravo G, et al. Body mass index and the prevalence of metabolic syndrome among children and adolescents in two Mexican populations. J Adolesc Health. 2007;40:521–526. doi: 10.1016/j.jadohealth.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 153.Xu H, Li Y, Liu A, Zhang Q, Hu X, Fang H, et al. Prevalence of the metabolic syndrome among children from six cities of China. BMC Public Health. 2012;12:13. doi: 10.1186/1471-2458-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lucove JC, Kaufman JS, James SA. Association between adult and childhood socioeconomic status and prevalence of the metabolic syndrome in African Americans: the Pitt County Study. Am J Public Health. 2007;97:234–236. doi: 10.2105/AJPH.2006.087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen W, Srinivasan SR, Berenson GS. Path analysis of metabolic syndrome components in black versus white children, adolescents, and adults: the Bogalusa Heart Study. Ann Epidemiol. 2008;18:85–91. doi: 10.1016/j.annepidem.2007.07.090. [DOI] [PubMed] [Google Scholar]
- 156.Harville EW, Srinivasan S, Chen W, Berenson GS. Is the metabolic syndrome a “small baby” syndrome?: the bogalusa heart study. Metab Syndr Relat Disord. 2012;10:413–421. doi: 10.1089/met.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27:1398–1404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H, Zhang T, Li S, Li Y, Hussain A, Fernandez C, et al. Long-term impact of childhood adiposity on adult metabolic syndrome is modified by insulin resistance: the Bogalusa Heart study. Sci Rep. 2015;5:17885. doi: 10.1038/srep17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Metabolic syndrome variables at low levels in childhood are beneficially associated with adulthood cardiovascular risk: the Bogalusa Heart Study. Diabetes Care. 2005;28:126–131. doi: 10.2337/diacare.28.1.126. [DOI] [PubMed] [Google Scholar]
- 160.Magnussen CG, Koskinen J, Chen W, Thomson R, Schmidt MD, Srinivasan SR, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122:1604–1611. doi: 10.1161/CIRCULATIONAHA.110.940809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care. 2008;31:2044–2049. doi: 10.2337/dc08-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Srinivasan SR, Myers L, Berenson GS. Changes in metabolic syndrome variables since childhood in prehypertensive and hypertensive subjects: the Bogalusa Heart Study. Hypertension. 2006;48:33–39. doi: 10.1161/01.HYP.0000226410.11198.f4. [DOI] [PubMed] [Google Scholar]
- 163.Wang J, Zhu Y, Cai L, Jing J, Chen Y, Mai J, et al. Metabolic syndrome and its associated early-life factors in children and adolescents: a cross-sectional study in Guangzhou, China. Public Health Nutr. 2016;19:1147–1154. doi: 10.1017/S1368980015002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.DuBose KD, Stewart EE, Charbonneau SR, Mayo MS, Donnelly JE. Prevalence of the metabolic syndrome in elementary school children. Acta Paediatr. 2006;95:1005–1011. doi: 10.1080/08035250600570553. [DOI] [PubMed] [Google Scholar]
- 166.Invitti C, Maffeis C, Gilardini L, Pontiggia B, Mazzilli G, Girola A, et al. Metabolic syndrome in obese Caucasian children: prevalence using WHO-derived criteria and association with nontraditional cardiovascular risk factors. Int J Obes (Lond) 2006;30:627–633. doi: 10.1038/sj.ijo.0803151. [DOI] [PubMed] [Google Scholar]
- 167.Kaler SN, Ralph-Campbell K, Pohar S, King M, Laboucan CR, Toth EL. High rates of the metabolic syndrome in a First Nations Community in western Canada: prevalence and determinants in adults and children. Int J Circumpolar Health. 2006;65:389–402. doi: 10.3402/ijch.v65i5.18139. [DOI] [PubMed] [Google Scholar]
- 168.Kim S, So WY. Prevalence of metabolic syndrome among Korean adolescents according to the National Cholesterol Education Program, Adult Treatment Panel III and International Diabetes Federation. Nutrients. 2016;8:E588. doi: 10.3390/nu8100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Molnár D. The prevalence of the metabolic syndrome and type 2 diabetes mellitus in children and adolescents. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S70–4. doi: 10.1038/sj.ijo.0802811. [DOI] [PubMed] [Google Scholar]
- 170.Papoutsakis C, Yannakoulia M, Ntalla I, Dedoussis GV. Metabolic syndrome in a Mediterranean pediatric cohort: prevalence using International Diabetes Federation-derived criteria and associations with adiponectin and leptin. Metabolism. 2012;61:140–145. doi: 10.1016/j.metabol.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 171.Agredo-Zúñiga RA, Aguilar-de Plata C, Suárez-Ortegón MF. Waist:height ratio, waist circumference and metabolic syndrome abnormalities in Colombian schooled adolescents: a multivariate analysis considering located adiposity. Br J Nutr. 2015;114:700–705. doi: 10.1017/S0007114515002275. [DOI] [PubMed] [Google Scholar]
- 172.Bitsori M, Linardakis M, Tabakaki M, Kafatos A. Waist circumference as a screening tool for the identification of adolescents with the metabolic syndrome phenotype. Int J Pediatr Obes. 2009;4:325–331. doi: 10.3109/17477160902914597. [DOI] [PubMed] [Google Scholar]
- 173.Choi DH, Hur YI, Kang JH, Kim K, Cho YG, Hong SM, et al. Usefulness of the waist circumference-to-height ratio in screening for obesity and metabolic syndrome among Korean children and adolescents: Korea National Health and Nutrition Examination Survey, 2010-2014. Nutrients. 2017;9:E256. doi: 10.3390/nu9030256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hirschler V, Aranda C, Calcagno ML, Maccalini G, Jadzinsky M. Can waist circumference identify children with the metabolic syndrome? Arch Pediatr Adolesc Med. 2005;159:740–744. doi: 10.1001/archpedi.159.8.740. [DOI] [PubMed] [Google Scholar]
- 175.McCarthy HD. Body fat measurements in children as predictors for the metabolic syndrome: focus on waist circumference. Proc Nutr Soc. 2006;65:385–392. doi: 10.1017/s0029665106005143. [DOI] [PubMed] [Google Scholar]
- 176.Wu XY, Hu CL, Wan YH, Su PY, Xing C, Qi XY, et al. Higher waist-to-height ratio and waist circumference are predictive of metabolic syndrome and elevated serum alanine aminotransferase in adolescents and young adults in mainland China. Public Health. 2012;126:135–142. doi: 10.1016/j.puhe.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 177.Spolidoro JV, Pitrez Filho ML, Vargas LT, Santana JC, Pitrez E, Hauschild JA, et al. Waist circumference in children and adolescents correlate with metabolic syndrome and fat deposits in young adults. Clin Nutr. 2013;32:93–97. doi: 10.1016/j.clnu.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 178.Arimura ST, Moura BM, Pimentel GD, Silva ME, Sousa MV. Waist circumference is better associated with high density lipoprotein (HDL-c) than with body mass index (BMI) in adults with metabolic syndrome. Nutr Hosp. 2011;26:1328–1332. doi: 10.1590/S0212-16112011000600020. [DOI] [PubMed] [Google Scholar]
- 179.Zanchetti A, Hennig M, Baurecht H, Tang R, Cuspidi C, Carugo S, et al. Prevalence and incidence of the metabolic syndrome in the European Lacidipine Study on Atherosclerosis (ELSA) and its relation with carotid intima-media thickness. J Hypertens. 2007;25:2463–2470. doi: 10.1097/HJH.0b013e3282f063d5. [DOI] [PubMed] [Google Scholar]
- 180.Cardinal TR, Vigo A, Duncan BB, Matos SM, da Fonseca MJ, Barreto SM, et al. Optimal cut-off points for waist circumference in the definition of metabolic syndrome in Brazilian adults: baseline analyses of the Longitudinal Study of Adult Health (ELSA-Brasil) Diabetol Metab Syndr. 2018;10:49. doi: 10.1186/s13098-018-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee WY, Park JS, Noh SY, Rhee EJ, Kim SW, Zimmet PZ. Prevalence of the metabolic syndrome among 40,698 Korean metropolitan subjects. Diabetes Res Clin Pract. 2004;65:143–149. doi: 10.1016/j.diabres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 182.Miccoli R, Bianchi C, Odoguardi L, Penno G, Caricato F, Giovannitti MG, et al. Prevalence of the metabolic syndrome among Italian adults according to ATP III definition. Nutr Metab Cardiovasc Dis. 2005;15:250–254. doi: 10.1016/j.numecd.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 183.Ali O, Cerjak D, Kent JW, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes. 2014;9:e58–62. doi: 10.1111/j.2047-6310.2014.218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xi B, Zong X, Kelishadi R, Litwin M, Hong YM, Poh BK, et al. International waist circumference percentile cutoffs for central obesity in children and adolescents aged 6 to 18 years. J Clin Endocrinol Metab. 2020;105:dgz195. doi: 10.1210/clinem/dgz195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mladenova S, Andreenko E. Prevalence of underweight, overweight, general and central obesity among 8-15-years old Bulgarian children and adolescents (Smolyan region, 2012-2014) Nutr Hosp. 2015;31:2419–2427. doi: 10.3305/nh.2015.31.6.8805. [DOI] [PubMed] [Google Scholar]
- 186.Hosseini M, Kelishadi R, Yousefifard M, Qorbani M, Bazargani B, Heshmat R, et al. Height-adjusted percentiles evaluated central obesity in children and adolescents more effectively than just waist circumference. Acta Paediatr. 2017;106:112–119. doi: 10.1111/apa.13622. [DOI] [PubMed] [Google Scholar]
- 187.Ejtahed HS, Kelishadi R, Qorbani M, Motlagh ME, Hasani-Ranjbar S, Angoorani P, et al. Utility of waist circumference-to-height ratio as a screening tool for generalized and central obesity among Iranian children and adolescents: the CASPIAN-V study. Pediatr Diabetes. 2019;20:530–537. doi: 10.1111/pedi.12855. [DOI] [PubMed] [Google Scholar]
- 188.Fernández JR, Bohan Brown M, López-Alarcón M, Dawson JA, Guo F, Redden DT, et al. Changes in pediatric waist circumference percentiles despite reported pediatric weight stabilization in the United States. Pediatr Obes. 2017;12:347–355. doi: 10.1111/ijpo.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 190.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148:188–194. doi: 10.1016/j.jpeds.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 191.Maffeis C, Corciulo N, Livieri C, Rabbone I, Trifirò G, Falorni A, et al. Waist circumference as a predictor of cardiovascular and metabolic risk factors in obese girls. Eur J Clin Nutr. 2003;57:566–572. doi: 10.1038/sj.ejcn.1601573. [DOI] [PubMed] [Google Scholar]
- 192.Maffeis C, Grezzani A, Pietrobelli A, Provera S, Tatò L. Does waist circumference predict fat gain in children? Int J Obes Relat Metab Disord. 2001;25:978–983. doi: 10.1038/sj.ijo.0801641. [DOI] [PubMed] [Google Scholar]
- 193.Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tatò L. Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res. 2001;9:179–187. doi: 10.1038/oby.2001.19. [DOI] [PubMed] [Google Scholar]
- 194.Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr. 2006;149:809–816. doi: 10.1016/j.jpeds.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 195.Cameron AJ, Magliano DJ, Zimmet PZ, Welborn T, Shaw JE. The metabolic syndrome in Australia: prevalence using four definitions. Diabetes Res Clin Pract. 2007;77:471–478. doi: 10.1016/j.diabres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 196.de Simone G, Devereux RB, Chinali M, Best LG, Lee ET, Galloway JM, et al. Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the Strong Heart Study. Diabetes Care. 2007;30:1851–1856. doi: 10.2337/dc06-2152. [DOI] [PubMed] [Google Scholar]
- 197.Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7:220. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. 2007;77:251–257. doi: 10.1016/j.diabres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 199.Pirkola J, Tammelin T, Bloigu A, Pouta A, Laitinen J, Ruokonen A, et al. Prevalence of metabolic syndrome at age 16 using the International Diabetes Federation paediatric definition. Arch Dis Child. 2008;93:945–951. doi: 10.1136/adc.2007.132951. [DOI] [PubMed] [Google Scholar]
- 200.Brown TM, Vaidya D, Rogers WJ, Waters DD, Howard BV, Tardif JC, et al. Does prevalence of the metabolic syndrome in women with coronary artery disease differ by the ATP III and IDF criteria? J Womens Health (Larchmt) 2008;17:841–847. doi: 10.1089/jwh.2007.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Caprio S, Weiss R. The metabolic phenotype of pre-diabetes in obese youth. Nutr Metab Cardiovasc Dis. 2004;14:270–275. doi: 10.1016/s0939-4753(04)80054-1. [DOI] [PubMed] [Google Scholar]
- 202.Cook S, Auinger P, Huang TT. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr. 2009;155:S6.e15–26. doi: 10.1016/j.jpeds.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 204.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 205.Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI. Fat distribution and insulin response in prepubertal African American and white children. Am J Clin Nutr. 1998;67:821–827. doi: 10.1093/ajcn/67.5.821. [DOI] [PubMed] [Google Scholar]
- 206.Cruz ML, Huang TT, Johnson MS, Gower BA, Goran MI. Insulin sensitivity and blood pressure in black and white children. Hypertension. 2002;40:18–22. doi: 10.1161/01.hyp.0000019972.37690.ef. [DOI] [PubMed] [Google Scholar]
- 207.Ball GD, Huang TT, Gower BA, Cruz ML, Shaibi GQ, Weigensberg MJ, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr. 2006;148:16–22. doi: 10.1016/j.jpeds.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 208.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res. 2001;9:423–431. doi: 10.1038/oby.2001.56. [DOI] [PubMed] [Google Scholar]
- 209.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 210.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29:1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 211.Linder B, Imperatore G. Research updates on type 2 diabetes children. NASN Sch Nurse. 2013;28:138–140. doi: 10.1177/1942602X13479402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bell RA, Mayer-Davis EJ, Beyer JW, D'Agostino RB, Jr, Lawrence JM, Linder B, et al. Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S102–11. doi: 10.2337/dc09-S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bobo N, Evert A, Gallivan J, Imperatore G, Kelly J, Linder B, et al. An update on type 2 diabetes in youth from the National Diabetes Education Program. Pediatrics. 2004;114:259–263. doi: 10.1542/peds.114.1.259. [DOI] [PubMed] [Google Scholar]
- 214.Bosevski M, Borozanov V, Gucev F, Bosevska G, Tosev S, Georgievska-Ismail L. Prevalence of metabolic syndrome components in the type 2 diabetic population who presented coronary artery disease. Prilozi. 2007;28:161–169. [PubMed] [Google Scholar]
- 215.Machado-Rodrigues AM, Leite N, Coelho-e-Silva MJ, Martins RA, Valente-dos-Santos J, Mascarenhas LP, et al. Independent association of clustered metabolic risk factors with cardiorespiratory fitness in youth aged 11-17 years. Ann Hum Biol. 2014;41:271–276. doi: 10.3109/03014460.2013.856471. [DOI] [PubMed] [Google Scholar]
- 216.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 217.Ogden CL, Troiano RP, Briefel RR, Kuczmarski RJ, Flegal KM, Johnson CL. Prevalence of overweight among preschool children in the United States, 1971 through 1994. Pediatrics. 1997;99:E1. doi: 10.1542/peds.99.4.e1. [DOI] [PubMed] [Google Scholar]
- 218.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 219.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101(3 Pt 2):497–504. [PubMed] [Google Scholar]
- 220.Flegal KM. The effects of age categorization on estimates of overweight prevalence for children. Int J Obes Relat Metab Disord. 2000;24:1636–1641. doi: 10.1038/sj.ijo.0801441. [DOI] [PubMed] [Google Scholar]
- 221.Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:E5. doi: 10.1542/peds.101.3.e5. [DOI] [PubMed] [Google Scholar]
- 222.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 223.Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 225.Steinberger J, Daniels SR American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young); American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 226.Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol. 2011;8:513–525. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smith SC, Jr, Clark LT, Cooper RS, Daniels SR, Kumanyika SK, Ofili E, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111:e134–9. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 228.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–591. doi: 10.1016/j.annepidem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 229.St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004;27:2222–2228. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- 230.Sumner AE, Luercio MF, Frempong BA, Ricks M, Sen S, Kushner H, et al. Validity of the reduced-sample insulin modified frequently-sampled intravenous glucose tolerance test using the nonlinear regression approach. Metabolism. 2009;58:220–225. doi: 10.1016/j.metabol.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mayer-Davis EJ, D'Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, et al. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–674. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 232.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45:235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 233.Maffeis C, Moghetti P, Grezzani A, Clementi M, Gaudino R, Tatò L. Insulin resistance and the persistence of obesity from childhood into adulthood. J Clin Endocrinol Metab. 2002;87:71–76. doi: 10.1210/jcem.87.1.8130. [DOI] [PubMed] [Google Scholar]
- 234.Thivel D, Malina RM, Isacco L, Aucouturier J, Meyer M, Duché P. Metabolic syndrome in obese children and adolescents: dichotomous or continuous? Metab Syndr Relat Disord. 2009;7:549–555. doi: 10.1089/met.2008.0085. [DOI] [PubMed] [Google Scholar]
- 235.Katzmarzyk PT, Pérusse L, Malina RM, Bergeron J, Després JP, Bouchard C. Stability of indicators of the metabolic syndrome from childhood and adolescence to young adulthood: the Québec Family Study. J Clin Epidemiol. 2001;54:190–195. doi: 10.1016/s0895-4356(00)00315-2. [DOI] [PubMed] [Google Scholar]
- 236.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–107. [PubMed] [Google Scholar]
- 237.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 238.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101(3 Pt 2):518–525. [PubMed] [Google Scholar]
- 239.Faienza MF, Chiarito M, Molina-Molina E, Shanmugam H, Lammert F, Krawczyk M, et al. Childhood obesity, cardiovascular and liver health: a growing epidemic with age. World J Pediatr. 2020 doi: 10.1007/s12519-020-00341-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Faienza MF, Wang DQ, Frühbeck G, Garruti G, Portincasa P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern Emerg Med. 2016;11:175–182. doi: 10.1007/s11739-015-1382-6. [DOI] [PubMed] [Google Scholar]
- 241.Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Prev Med Rep. 2017;7:211–215. doi: 10.1016/j.pmedr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Parsons TJ, Manor O, Power C. Television viewing and obesity: a prospective study in the 1958 British birth cohort. Eur J Clin Nutr. 2008;62:1355–1363. doi: 10.1038/sj.ejcn.1602884. [DOI] [PubMed] [Google Scholar]
- 243.Freedman DS, Ogden CL, Flegal KM, Khan LK, Serdula MK, Dietz WH. Childhood overweight and family income. MedGenMed. 2007;9:26. [PMC free article] [PubMed] [Google Scholar]
- 244.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 245.Michaud A, Laforest S, Pelletier M, Nadeau M, Simard S, Daris M, et al. Abdominal adipocyte populations in women with visceral obesity. Eur J Endocrinol. 2016;174:227–239. doi: 10.1530/EJE-15-0822. [DOI] [PubMed] [Google Scholar]
- 246.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–225. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 247.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 248.Lorenzo C, Serrano-Ríos M, Martínez-Larrad MT, Gabriel R, Williams K, Gómez-Gerique JA, et al. Central adiposity determines prevalence differences of the metabolic syndrome. Obes Res. 2003;11:1480–1487. doi: 10.1038/oby.2003.198. [DOI] [PubMed] [Google Scholar]
- 249.Haffner SM, Mykkänen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101:975–980. doi: 10.1161/01.cir.101.9.975. [DOI] [PubMed] [Google Scholar]
- 250.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 251.Wagh A, Stone NJ. Treatment of metabolic syndrome. Expert Rev Cardiovasc Ther. 2004;2:213–228. doi: 10.1586/14779072.2.2.213. [DOI] [PubMed] [Google Scholar]
- 252.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects) Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bassi N, Karagodin I, Wang S, Vassallo P, Priyanath A, Massaro E, et al. Lifestyle modification for metabolic syndrome: a systematic review. Am J Med. 2014;127:1242.e1–1242.10. doi: 10.1016/j.amjmed.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 254.Grundy SM. Drug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacy. Nat Rev Drug Discov. 2006;5:295–309. doi: 10.1038/nrd2005. [DOI] [PubMed] [Google Scholar]
- 255.DePaula AL, Macedo AL, Rassi N, Vencio S, Machado CA, Mota BR, et al. Laparoscopic treatment of metabolic syndrome in patients with type 2 diabetes mellitus. Surg Endosc. 2008;22:2670–2678. doi: 10.1007/s00464-008-9808-0. [DOI] [PubMed] [Google Scholar]
- 256.Danaei G, Singh GM, Paciorek CJ, Lin JK, Cowan MJ, Finucane MM, et al. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 2013;127:1493–1502. 1502.e1–1498. doi: 10.1161/CIRCULATIONAHA.113.001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 260.Geleijnse JM, Kok FJ, Grobbee DE. Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. Eur J Public Health. 2004;14:235–239. doi: 10.1093/eurpub/14.3.235. [DOI] [PubMed] [Google Scholar]
- 261.Pacifico L, Anania C, Martino F, Poggiogalle E, Chiarelli F, Arca M, et al. Management of metabolic syndrome in children and adolescents. Nutr Metab Cardiovasc Dis. 2011;21:455–466. doi: 10.1016/j.numecd.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 262.Reinehr T, Kleber M, Toschke AM. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis. 2009;207:174–180. doi: 10.1016/j.atherosclerosis.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 263.Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism. 2006;55:871–878. doi: 10.1016/j.metabol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 264.Roberts CK, Chen AK, Barnard RJ. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis. 2007;191:98–106. doi: 10.1016/j.atherosclerosis.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 265.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401–406. [PubMed] [Google Scholar]
- 266.Van Gaal LF, Wauters MA, De Leeuw IH. The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(Suppl 1):S5–9. [PubMed] [Google Scholar]
- 267.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 268.Case CC, Jones PH, Nelson K, O'Brian Smith E, Ballantyne CM. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab. 2002;4:407–414. doi: 10.1046/j.1463-1326.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 269.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ilanne-Parikka P, Eriksson JG, Lindström J, Peltonen M, Aunola S, Hämäläinen H, et al. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31:805–807. doi: 10.2337/dc07-1117. [DOI] [PubMed] [Google Scholar]
- 271.Forman D, Bulwer BE. Cardiovascular disease: optimal approaches to risk factor modification of diet and lifestyle. Curr Treat Options Cardiovasc Med. 2006;8:47–57. doi: 10.1007/s11936-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 272.Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153:849–858. [PubMed] [Google Scholar]
- 273.Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–1041. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 274.Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29:1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 275.Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Serdula M, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med. 2004;117:762–774. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 276.Hofsø D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163:735–745. doi: 10.1530/EJE-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.McAuley KA, Williams SM, Mann JI, Goulding A, Chisholm A, Wilson N, et al. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care. 2002;25:445–452. doi: 10.2337/diacare.25.3.445. [DOI] [PubMed] [Google Scholar]
- 279.Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115:1447–1463. doi: 10.1016/j.jand.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 280.Grundy SM. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin Cornerstone. 2006;8(Suppl 1):S21–7. doi: 10.1016/s1098-3597(06)80005-0. [DOI] [PubMed] [Google Scholar]
- 281.Wyszynski DF, Waterworth DM, Barter PJ, Cohen J, Kesäniemi YA, Mahley RW, et al. Relation between atherogenic dyslipidemia and the Adult Treatment Program-III definition of metabolic syndrome (Genetic Epidemiology of Metabolic Syndrome Project) Am J Cardiol. 2005;95:194–198. doi: 10.1016/j.amjcard.2004.08.091. [DOI] [PubMed] [Google Scholar]
- 282.Grundy SM. Cardiovascular and metabolic risk factors: how can we improve outcomes in the high-risk patient? Am J Med. 2007;120(9 Suppl 1):S3–8. doi: 10.1016/j.amjmed.2007.06.005. discussion S9. [DOI] [PubMed] [Google Scholar]
- 283.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 284.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. A summary of implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24:1329–1330. doi: 10.1161/01.ATV.0000139012.45265.e0. [DOI] [PubMed] [Google Scholar]
- 285.See R, Lindsey JB, Patel MJ, Ayers CR, Khera A, McGuire DK, et al. Application of the screening for Heart Attack Prevention and Education Task Force recommendations to an urban population: observations from the Dallas Heart Study. Arch Intern Med. 2008;168:1055–1062. doi: 10.1001/archinte.168.10.1055. [DOI] [PubMed] [Google Scholar]
- 286.Grundy SM. United States Cholesterol Guidelines 2001: expanded scope of intensive low-density lipoprotein-lowering therapy. Am J Cardiol. 2001;88(7B):23J–7J. doi: 10.1016/s0002-9149(01)01931-2. [DOI] [PubMed] [Google Scholar]
- 287.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Astrup A, Hjorth MF. Classification of obesity targeted personalized dietary weight loss management based on carbohydrate tolerance. Eur J Clin Nutr. 2018;72:1300–1304. doi: 10.1038/s41430-018-0227-6. [DOI] [PubMed] [Google Scholar]
- 289.Chu L, Morrison KM, Riddell MC, Raha S, Timmons BW. Effect of 7 days of exercise on exogenous carbohydrate oxidation and insulin resistance in children with obesity. Appl Physiol Nutr Metab. 2018;43:677–683. doi: 10.1139/apnm-2017-0358. [DOI] [PubMed] [Google Scholar]
- 290.Churuangsuk C, Kherouf M, Combet E, Lean M. Low-carbohydrate diets for overweight and obesity: a systematic review of the systematic reviews. Obes Rev. 2018;19:1700–1718. doi: 10.1111/obr.12744. [DOI] [PubMed] [Google Scholar]
- 291.Bailes JR, Strow MT, Werthammer J, McGinnis RA, Elitsur Y. Effect of low-carbohydrate, unlimited calorie diet on the treatment of childhood obesity: a prospective controlled study. Metab Syndr Relat Disord. 2003;1:221–225. doi: 10.1089/154041903322716697. [DOI] [PubMed] [Google Scholar]
- 292.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 293.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 294.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100:43–51. doi: 10.1016/S0002-8223(00)00018-3. quiz 49-50. [DOI] [PubMed] [Google Scholar]
- 295.Dietz WH. Sugar-sweetened beverages, milk intake, and obesity in children and adolescents. J Pediatr. 2006;148:152–154. doi: 10.1016/j.jpeds.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 296.Dietz WH. Television, obesity, and eating disorders. Adolesc Med. 1993;4:543–550. [PubMed] [Google Scholar]
- 297.Dowla S, Pendergrass M, Bolding M, Gower B, Fontaine K, Ashraf A, et al. Effectiveness of a carbohydrate restricted diet to treat non-alcoholic fatty liver disease in adolescents with obesity: trial design and methodology. Contemp Clin Trials. 2018;68:95–101. doi: 10.1016/j.cct.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hall KD, Chung ST. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2018;21:308–312. doi: 10.1097/MCO.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 299.Sartorius K, Sartorius B, Madiba TE, Stefan C. Does high-carbohydrate intake lead to increased risk of obesity? A systematic review and meta-analysis. BMJ Open. 2018;8:e018449. doi: 10.1136/bmjopen-2017-018449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kaartinen NE, Knekt P, Kanerva N, Valsta LM, Eriksson JG, Rissanen H, et al. Dietary carbohydrate quantity and quality in relation to obesity: a pooled analysis of three Finnish population-based studies. Scand J Public Health. 2016;44:385–393. doi: 10.1177/1403494815622860. [DOI] [PubMed] [Google Scholar]
- 301.Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Nagasawa SY, et al. Dietary carbohydrate intake, presence of obesity and the incident risk of type 2 diabetes in Japanese men. J Diabetes Investig. 2016;7:343–351. doi: 10.1111/jdi.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sartorius B, Sartorius K, Aldous C, Madiba TE, Stefan C, Noakes T. Carbohydrate intake, obesity, metabolic syndrome and cancer risk? A two-part systematic review and meta-analysis protocol to estimate attributability. BMJ Open. 2016;6:e009301. doi: 10.1136/bmjopen-2015-009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 304.Frugé AD, Byrd SH, Fountain BJ, Cossman JS, Schilling MW, Gerard P. Increased physical activity may be more protective for metabolic syndrome than reduced caloric intake. An analysis of estimated energy balance in U.S. adults: 2007-2010 NHANES. Nutr Metab Cardiovasc Dis. 2015;25:535–540. doi: 10.1016/j.numecd.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 305.Cano-Ibáñez N, Bueno-Cavanillas A, Martínez-González MA, Corella D, Salas-Salvadó J, Zomeño MD, et al. Dietary intake in population with metabolic syndrome: is the prevalence of inadequate intake influenced by geographical area? Cross-sectional analysis from PREDIMED-Plus study. Nutrients. 2018;10:E1661. doi: 10.3390/nu10111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Navarro JCA, Antoniazzi L, Oki AM, Bonfim MC, Hong V, Bortolotto LA, et al. Prevalence of metabolic syndrome and framingham risk score in apparently healthy vegetarian and omnivorous men. Arq Bras Cardiol. 2018;110:430–437. doi: 10.5935/abc.20180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.de Mello Fontanelli M, Sales CH, Carioca AAF, Marchioni DM, Fisberg RM. The relationship between carbohydrate quality and the prevalence of metabolic syndrome: challenges of glycemic index and glycemic load. Eur J Nutr. 2018;57:1197–1205. doi: 10.1007/s00394-017-1402-6. [DOI] [PubMed] [Google Scholar]
- 308.Ma Y, Li Y, Chiriboga DE, Olendzki BC, Hebert JR, Li W, et al. Association between carbohydrate intake and serum lipids. J Am Coll Nutr. 2006;25:155–163. doi: 10.1080/07315724.2006.10719527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Liese AD, Gilliard T, Schulz M, D'Agostino RB, Jr, Wolever TM. Carbohydrate nutrition, glycaemic load, and plasma lipids: the Insulin Resistance Atherosclerosis Study. Eur Heart J. 2007;28:80–87. doi: 10.1093/eurheartj/ehl389. [DOI] [PubMed] [Google Scholar]
- 310.Lewis CJ, Park YK, Dexter PB, Yetley EA. Nutrient intakes and body weights of persons consuming high and moderate levels of added sugars. J Am Diet Assoc. 1992;92:708–713. [PubMed] [Google Scholar]
- 311.Slyper AH. The influence of carbohydrate quality on cardiovascular disease, the metabolic syndrome, type 2 diabetes, and obesity - an overview. J Pediatr Endocrinol Metab. 2013;26:617–629. doi: 10.1515/jpem-2012-0419. [DOI] [PubMed] [Google Scholar]
- 312.Tran BT, Jeong BY, Oh JK. The prevalence trend of metabolic syndrome and its components and risk factors in Korean adults: results from the Korean National Health and Nutrition Examination Survey 2008-2013. BMC Public Health. 2017;17:71. doi: 10.1186/s12889-016-3936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kastorini CM, Panagiotakos DB, Chrysohoou C, Georgousopoulou E, Pitaraki E, Puddu PE, et al. Metabolic syndrome, adherence to the Mediterranean diet and 10-year cardiovascular disease incidence: The ATTICA study. Atherosclerosis. 2016;246:87–93. doi: 10.1016/j.atherosclerosis.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 314.He DH, Yang M, Zhang RH, Ma XG, Huang LC, Huang ES, et al. Dietary patterns associated metabolic syndrome in Chinese adults. Biomed Environ Sci. 2015;28:370–373. doi: 10.3967/bes2015.051. [DOI] [PubMed] [Google Scholar]
- 315.Lockard B, Earnest CP, Oliver J, Goodenough C, Rasmussen C, Greenwood M, et al. Retrospective analysis of protein- and carbohydrate-focused diets combined with exercise on metabolic syndrome prevalence in overweight and obese women. Metab Syndr Relat Disord. 2016;14:228–237. doi: 10.1089/met.2015.0096. [DOI] [PubMed] [Google Scholar]
- 316.Um YJ, Oh SW, Lee CM, Kwon HT, Joh HK, Kim YJ, et al. Dietary fat intake and the risk of metabolic syndrome in Korean adults. Korean J Fam Med. 2015;36:245–252. doi: 10.4082/kjfm.2015.36.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yubero-Serrano EM, Delgado-Lista J, Tierney AC, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, et al. Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: the LIPGENE study. Am J Clin Nutr. 2015;102:1509–1517. doi: 10.3945/ajcn.115.111286. [DOI] [PubMed] [Google Scholar]
- 318.Melanson EL, Astrup A, Donahoo WT. The relationship between dietary fat and fatty acid intake and body weight, diabetes, and the metabolic syndrome. Ann Nutr Metab. 2009;55:229–243. doi: 10.1159/000229004. [DOI] [PubMed] [Google Scholar]
- 319.Vessby B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol. 2003;14:15–19. doi: 10.1097/00041433-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 320.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 321.Uusitupa M, Schwab U, Mäkimattila S, Karhapää P, Sarkkinen E, Maliranta H, et al. Effects of two high-fat diets with different fatty acid compositions on glucose and lipid metabolism in healthy young women. Am J Clin Nutr. 1994;59:1310–1316. doi: 10.1093/ajcn/59.6.1310. [DOI] [PubMed] [Google Scholar]
- 322.Julibert A, Bibiloni MDM, Bouzas C, Martínez-González MÁ, Salas-Salvadó J, Corella D, et al. Total and subtypes of dietary fat intake and its association with components of the metabolic syndrome in a mediterranean population at high cardiovascular risk. Nutrients. 2019;11:E1493. doi: 10.3390/nu11071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Vollmer WM, Sacks FM, Svetkey LP. New insights into the effects on blood pressure of diets low in salt and high in fruits and vegetables and low-fat dairy products. Curr Control Trials Cardiovasc Med. 2001;2:71–74. doi: 10.1186/cvm-2-2-071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 325.Kris-Etherton P, Daniels SR, Eckel RH, Engler M, Howard BV, Krauss RM, et al. Summary of the scientific conference on dietary fatty acids and cardiovascular health: conference summary from the nutrition committee of the American Heart Association. Circulation. 2001;103:1034–1039. doi: 10.1161/01.cir.103.7.1034. [DOI] [PubMed] [Google Scholar]
- 326.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–456. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 327.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 328.Tierney AC, McMonagle J, Shaw DI, Gulseth HL, Helal O, Saris WH, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome--LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond) 2011;35:800–809. doi: 10.1038/ijo.2010.209. [DOI] [PubMed] [Google Scholar]
- 329.Zoccali C, Mallamaci F. Background dietary patterns and the time course of the blood pressure response to low sodium intake. Hypertension. 2017;70:890–892. doi: 10.1161/HYPERTENSIONAHA.117.10129. [DOI] [PubMed] [Google Scholar]
- 330.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 331.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, 3rd, Lin PH, et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74:80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 332.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 333.Campanozzi A, Avallone S, Barbato A, Iacone R, Russo O, De Filippo G, et al. High sodium and low potassium intake among Italian children: relationship with age, body mass and blood pressure. PLoS One. 2015;10:e0121183. doi: 10.1371/journal.pone.0121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Campbell WW, Kim JE, Amankwaah AF, Gordon SL, Weinheimer-Haus EM. Higher total protein intake and change in total protein intake affect body composition but not metabolic syndrome indexes in middle-aged overweight and obese adults who perform resistance and aerobic exercise for 36 weeks. J Nutr. 2015;145:2076–2083. doi: 10.3945/jn.115.213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 336.Van Elswyk ME, Weatherford CA, McNeill SH. A systematic review of renal health in healthy individuals associated with protein intake above the US recommended daily allowance in randomized controlled trials and observational studies. Adv Nutr. 2018;9:404–418. doi: 10.1093/advances/nmy026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Goran MI. Metabolic precursors and effects of obesity in children: a decade of progress, 1990-1999. Am J Clin Nutr. 2001;73:158–171. doi: 10.1093/ajcn/73.2.158. [DOI] [PubMed] [Google Scholar]
- 338.Bakker EA, Lee DC, Sui X, Artero EG, Ruiz JR, Eijsvogels TMH, et al. Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clin Proc. 2017;92:1214–1222. doi: 10.1016/j.mayocp.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bateman LA, Slentz CA, Willis LH, Shields AT, Piner LW, Bales CW, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) Am J Cardiol. 2011;108:838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Christ M, Iannello C, Iannello PG, Grimm W. Effects of a weight reduction program with and without aerobic exercise in the metabolic syndrome. Int J Cardiol. 2004;97:115–122. doi: 10.1016/j.ijcard.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 341.Fatone C, Guescini M, Balducci S, Battistoni S, Settequattrini A, Pippi R, et al. Two weekly sessions of combined aerobic and resistance exercise are sufficient to provide beneficial effects in subjects with Type 2 diabetes mellitus and metabolic syndrome. J Endocrinol Invest. 2010;33:489–495. doi: 10.1007/BF03346630. [DOI] [PubMed] [Google Scholar]
- 342.Kim JW, Kim DY. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord. 2012;10:452–457. doi: 10.1089/met.2012.0036. [DOI] [PubMed] [Google Scholar]
- 343.Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64:90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wewege MA, Thom JM, Rye KA, Parmenter BJ. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274:162–171. doi: 10.1016/j.atherosclerosis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 346.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 347.Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 348.Christiansen T, Paulsen SK, Bruun JM, Overgaard K, Ringgaard S, Pedersen SB, et al. Comparable reduction of the visceral adipose tissue depot after a diet-induced weight loss with or without aerobic exercise in obese subjects: a 12-week randomized intervention study. Eur J Endocrinol. 2009;160:759–767. doi: 10.1530/EJE-08-1009. [DOI] [PubMed] [Google Scholar]
- 349.Xiao T, Fu YF. Resistance training vs. aerobic training and role of other factors on the exercise effects on visceral fat. Eur Rev Med Pharmacol Sci. 2015;19:1779–1784. [PubMed] [Google Scholar]
- 350.Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. 2017;7:43029. doi: 10.1038/srep43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yoshimura E, Kumahara H, Tobina T, Matsuda T, Ayabe M, Kiyonaga A, et al. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes. 2014;2014:197216. doi: 10.1155/2014/197216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lee S, Deldin AR, White D, Kim Y, Libman I, Rivera-Vega M, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305:E1222–9. doi: 10.1152/ajpendo.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Thomas EL, Brynes AE, McCarthy J, Goldstone AP, Hajnal JV, Saeed N, et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35:769–776. doi: 10.1007/s11745-000-0584-0. [DOI] [PubMed] [Google Scholar]
- 354.van der Heijden GJ, Wang ZJ, Chu ZD, Sauer PJ, Haymond MW, Rodriguez LM, et al. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity (Silver Spring) 2010;18:384–390. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 355.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 2007;31:1786–1797. doi: 10.1038/sj.ijo.0803683. [DOI] [PubMed] [Google Scholar]
- 356.Okura T, Nakata Y, Ohkawara K, Numao S, Katayama Y, Matsuo T, et al. Effects of aerobic exercise on metabolic syndrome improvement in response to weight reduction. Obesity (Silver Spring) 2007;15:2478–2484. doi: 10.1038/oby.2007.294. [DOI] [PubMed] [Google Scholar]
- 357.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68–91. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 358.Davis JN, Ventura EE, Shaibi GQ, Byrd-Williams CE, Alexander KE, Vanni AK, et al. Interventions for improving metabolic risk in overweight Latino youth. Int J Pediatr Obes. 2010;5:451–455. doi: 10.3109/17477161003770123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 360.Molina-Molina E, Lunardi Baccetto R, Wang DQ, de Bari O, Krawczyk M, Portincasa P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur J Clin Invest. 2018;48:e12958. doi: 10.1111/eci.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Morales-Palomo F, Ramirez-Jimenez M, Ortega JF, Mora-Rodriguez R. Effectiveness of aerobic exercise programs for health promotion in metabolic syndrome. Med Sci Sports Exerc. 2019;51:1876–1883. doi: 10.1249/MSS.0000000000001983. [DOI] [PubMed] [Google Scholar]
- 362.Crist LA, Champagne CM, Corsino L, Lien LF, Zhang G, Young DR. Influence of change in aerobic fitness and weight on prevalence of metabolic syndrome. Prev Chronic Dis. 2012;9:E68. doi: 10.5888/pcd9.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wang X, Hsu FC, Isom S, Walkup MP, Kritchevsky SB, Goodpaster BH, et al. Effects of a 12-month physical activity intervention on prevalence of metabolic syndrome in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67:417–424. doi: 10.1093/gerona/glr187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–152. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 365.Robins SJ. Cardiovascular disease with diabetes or the metabolic syndrome: should statins or fibrates be first line lipid therapy? Curr Opin Lipidol. 2003;14:575–583. doi: 10.1097/00041433-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 366.Scott R, Donoghoe M, Watts GF, O'Brien R, Pardy C, Taskinen MR, et al. Impact of metabolic syndrome and its components on cardiovascular disease event rates in 4900 patients with type 2 diabetes assigned to placebo in the FIELD randomised trial. Cardiovasc Diabetol. 2011;10:102. doi: 10.1186/1475-2840-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Scott R, O'Brien R, Fulcher G, Pardy C, D'Emden M, Tse D, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Burguera B, Schauer P, Kahan S. What to offer the 99% of patients with severe obesity who do not undergo bariatric surgery? Mayo Clin Proc. 2019;94:957–960. doi: 10.1016/j.mayocp.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 369.Younis A, Younis A, Tzur B, Peled Y, Shlomo N, Goldenberg I, et al. Metabolic syndrome is independently associated with increased 20-year mortality in patients with stable coronary artery disease. Cardiovasc Diabetol. 2016;15:149. doi: 10.1186/s12933-016-0466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Tenenbaum A, Fisman EZ. Which is the best lipid-modifying strategy in metabolic syndrome and diabetes: fibrates, statins or both? Cardiovasc Diabetol. 2004;3:10. doi: 10.1186/1475-2840-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tenenbaum A, Fisman EZ, Motro M, Adler Y. Atherogenic dyslipidemia in metabolic syndrome and type 2 diabetes: therapeutic options beyond statins. Cardiovasc Diabetol. 2006;5:20. doi: 10.1186/1475-2840-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002–3009. doi: 10.1161/CIRCULATIONAHA.107.713438. [DOI] [PubMed] [Google Scholar]
- 373.Keaney JF, Jr, Curfman GD, Jarcho JA. A pragmatic view of the new cholesterol treatment guidelines. N Engl J Med. 2014;370:275–278. doi: 10.1056/NEJMms1314569. [DOI] [PubMed] [Google Scholar]
- 374.Bays HE, Jones PH, Brown WV, Jacobson TA National Lipid Association. National Lipid Association Annual Summary of Clinical Lipidology 2015. J Clin Lipidol. 2014;8(6 Suppl):S1–36. doi: 10.1016/j.jacl.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 375.Jacobson TA. Combination lipid-altering therapy: an emerging treatment paradigm for the 21st century. Curr Atheroscler Rep. 2001;3:373–382. doi: 10.1007/s11883-001-0075-y. [DOI] [PubMed] [Google Scholar]
- 376.Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care. 2003;26:1513–1517. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 377.Dujovne CA, Williams CD, Ito MK. What combination therapy with a statin, if any, would you recommend? Curr Atheroscler Rep. 2011;13:12–22. doi: 10.1007/s11883-010-0150-3. [DOI] [PubMed] [Google Scholar]
- 378.Boden WE, Pearson TA. Raising low levels of high-density lipoprotein cholesterol is an important target of therapy. Am J Cardiol. 2000;85:645–650. A10. doi: 10.1016/s0002-9149(99)00826-7. [DOI] [PubMed] [Google Scholar]
- 379.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 380.Jacobson TA. Combination lipid-lowering therapy with statins: safety issues in the postcerivastatin era. Expert Opin Drug Saf. 2003;2:269–286. doi: 10.1517/14740338.2.3.269. [DOI] [PubMed] [Google Scholar]
- 381.Blaha MJ, Blumenthal RS, Brinton EA, Jacobson TA National Lipid Association Taskforce on Non-HDL Cholesterol. The importance of non-HDL cholesterol reporting in lipid management. J Clin Lipidol. 2008;2:267–273. doi: 10.1016/j.jacl.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 382.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. J Clin Lipidol. 2015;9:129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 383.Windler E, Zyriax BC, Bamberger C, Rinninger F, Beil FU. Current strategies and recent advances in the therapy of hypercholesterolemia. Atheroscler Suppl. 2009;10:1–4. doi: 10.1016/S1567-5688(09)00065-8. [DOI] [PubMed] [Google Scholar]
- 384.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Robins SJ. Lipid therapy for cardiovascular disease with insulin resistance, diabetes, or the metabolic syndrome. Curr Cardiol Rep. 2005;7:457–464. doi: 10.1007/s11886-005-0064-9. [DOI] [PubMed] [Google Scholar]
- 386.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 388.Alsumali A, Al-Hawag A, Samnaliev M, Eguale T. Systematic assessment of decision analytic models for the cost-effectiveness of bariatric surgery for morbid obesity. Surg Obes Relat Dis. 2018;14:1041–1059. doi: 10.1016/j.soard.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 389.Busetto L. Timing of bariatric surgery in people with obesity and diabetes. Ann Transl Med. 2015;3:94. doi: 10.3978/j.issn.2305-5839.2015.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Obesity Management Task Force of the European Association for the Study of Obesity Released “Practical Recommendations for the Post-Bariatric Surgery Medical Management”. Obes Surg. 2018;28:2117–2121. doi: 10.1007/s11695-018-3283-z. [DOI] [PubMed] [Google Scholar]
- 391.Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the obesity management task force of the European association for the study of obesity for the post-bariatric surgery medical management. Obes Facts. 2017;10:597–632. doi: 10.1159/000481825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Busetto L, Dixon J, De Luca M, Shikora S, Pories W, Angrisani L. Bariatric surgery in class I obesity : a Position Statement from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Obes Surg. 2014;24:487–519. doi: 10.1007/s11695-014-1214-1. [DOI] [PubMed] [Google Scholar]
- 393.Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16:175–247. doi: 10.1016/j.soard.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 394.Rega-Kaun G, Kaun C, Jaegersberger G, Prager M, Hackl M, Demyanets S, et al. Roux-en-Y-bariatric surgery reduces markers of metabolic syndrome in morbidly obese patients. Obes Surg. 2020;30:391–400. doi: 10.1007/s11695-019-04190-y. [DOI] [PubMed] [Google Scholar]
- 395.Reiter-Purtill J, Ley S, Kidwell KM, Mikhail C, Austin H, Chaves E, et al. Change, predictors and correlates of weight- and health-related quality of life in adolescents 2-years following bariatric surgery. Int J Obes (Lond) 2019 doi: 10.1038/s41366-019-0394-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mital S, Nguyen HV. Incremental cost-effectiveness of aspiration therapy vs bariatric surgery and no treatment for morbid obesity. Am J Gastroenterol. 2019;114:1470–1477. doi: 10.14309/ajg.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 397.Wee CC, Huskey KW, Bolcic-Jankovic D, Colten ME, Davis RB, Hamel M. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med. 2014;29:68–75. doi: 10.1007/s11606-013-2603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Dixon JB, Zimmet P, Alberti KG, Rubino F International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011;28:628–642. doi: 10.1111/j.1464-5491.2011.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Allen SR, Lawson L, Garcia V, Inge TH. Attitudes of bariatric surgeons concerning adolescent bariatric surgery (ABS) Obes Surg. 2005;15:1192–1195. doi: 10.1381/0960892055002176. [DOI] [PubMed] [Google Scholar]
- 400.Desai NK, Wulkan ML, Inge TH. Update on adolescent bariatric surgery. Endocrinol Metab Clin North Am. 2016;45:667–676. doi: 10.1016/j.ecl.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 401.Al-Qahtani AR. Laparoscopic adjustable gastric banding in adolescent: safety and efficacy. J Pediatr Surg. 2007;42:894–897. doi: 10.1016/j.jpedsurg.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 402.O'Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25:1358–1365. doi: 10.1111/j.1440-1746.2010.06391.x. [DOI] [PubMed] [Google Scholar]
- 403.Horgan S, Holterman MJ, Jacobsen GR, Browne AF, Berger RA, Moser F, et al. Laparoscopic adjustable gastric banding for the treatment of adolescent morbid obesity in the United States: a safe alternative to gastric bypass. J Pediatr Surg. 2005;40:86–90. doi: 10.1016/j.jpedsurg.2004.09.034. discussion 90-1. [DOI] [PubMed] [Google Scholar]
- 404.Inge TH, Garcia V, Daniels S, Langford L, Kirk S, Roehrig H, et al. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004;39:442–447. doi: 10.1016/j.jpedsurg.2003.11.025. discussion 446-7. [DOI] [PubMed] [Google Scholar]
- 405.Breaux CW. Obesity surgery in children. Obes Surg. 1995;5:279–284. doi: 10.1381/096089295765557647. [DOI] [PubMed] [Google Scholar]
- 406.Strauss RS, Bradley LJ, Brolin RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001;138:499–504. doi: 10.1067/mpd.2001.113043. [DOI] [PubMed] [Google Scholar]
- 407.Stanford A, Glascock JM, Eid GM, Kane T, Ford HR, Ikramuddin S, et al. Laparoscopic Roux-en-Y gastric bypass in morbidly obese adolescents. J Pediatr Surg. 2003;38:430–433. doi: 10.1053/jpsu.2003.50074. [DOI] [PubMed] [Google Scholar]
- 408.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–108. doi: 10.1016/S1091-255X(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 409.Fernandez AZ, Jr, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, et al. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc. 2004;18:193–197. doi: 10.1007/s00464-003-8926-y. [DOI] [PubMed] [Google Scholar]
- 410.Helmrath MA, Brandt ML, Inge TH. Adolescent obesity and bariatric surgery. Surg Clin North Am. 2006;86:441–454. doi: 10.1016/j.suc.2005.12.015. x. [DOI] [PubMed] [Google Scholar]
- 411.Goldschmidt AB, Khoury J, Jenkins TM, Bond DS, Thomas JG, Utzinger LM, et al. Adolescent loss-of-control eating and weight loss maintenance after bariatric surgery. Pediatrics. 2018;141:e20171659. doi: 10.1542/peds.2017-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Inge TH, Coley RY, Bazzano LA, Xanthakos SA, McTigue K, Arterburn D, et al. Comparative effectiveness of bariatric procedures among adolescents: the PCORnet bariatric study. Surg Obes Relat Dis. 2018;14:1374–1386. doi: 10.1016/j.soard.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Bianciardi E, Di Lorenzo G, Niolu C, Betrò S, Zerbin F, Gentileschi P, et al. Body image dissatisfaction in individuals with obesity seeking bariatric surgery: exploring the burden of new mediating factors. Riv Psichiatr. 2019;54:8–17. doi: 10.1708/3104.30935. [DOI] [PubMed] [Google Scholar]
- 414.Price PH, Kaizer AM, Daniels SR, Jenkins TM, Inge TH, Eckel RH. Physical activity improves lipid and weight-loss outcomes after metabolic bariatric surgery in adolescents with severe obesity. Obesity (Silver Spring) 2019;27:989–996. doi: 10.1002/oby.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161:217–221. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 416.Inge TH, Donnelly LF, Vierra M, Cohen AP, Daniels SR, Garcia VF. Managing bariatric patients in a children's hospital: radiologic considerations and limitations. J Pediatr Surg. 2005;40:609–617. doi: 10.1016/j.jpedsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 417.Stefater MA, Inge TH. Bariatric surgery for adolescents with Type 2 diabetes: an emerging therapeutic strategy. Curr Diab Rep. 2017;17:62. doi: 10.1007/s11892-017-0887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Coutant R, Bouhours-Nouet N, Donzeau A, Fauchard M, Decrequy A, Malka J, et al. Bariatric surgery in adolescents with severe obesity: Review and state of the art in France. Ann Endocrinol (Paris) 2017;78:462–468. doi: 10.1016/j.ando.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 419.Canoy D, Yang TO. Obesity in children: bariatric surgery. BMJ Clin Evid. 2015;2015:0325. [PMC free article] [PubMed] [Google Scholar]
- 420.Borgès Da Silva V, Borgès Da Silva R, Prud'homme A, Campan P, Azorin JM, Belzeaux R. Association between binge eating disorder and psychiatric comorbidity profiles in patients with obesity seeking bariatric surgery. Compr Psychiatry. 2018;87:79–83. doi: 10.1016/j.comppsych.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 421.Field AE, Inge TH, Belle SH, Johnson GS, Wahed AS, Pories WJ, et al. Association of obesity subtypes in the longitudinal assessment of bariatric surgery study and 3-year postoperative weight change. Obesity (Silver Spring) 2018;26:1931–1937. doi: 10.1002/oby.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ryder JR, Gross AC, Fox CK, Kaizer AM, Rudser KD, Jenkins TM, et al. Factors associated with long-term weight-loss maintenance following bariatric surgery in adolescents with severe obesity. Int J Obes (Lond) 2018;42:102–107. doi: 10.1038/ijo.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Michalsky MP, Inge TH, Jenkins TM, Xie C, Courcoulas A, Helmrath M, et al. Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics. 2018;141:e20172485. doi: 10.1542/peds.2017-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Inge TH, Krebs NF, Garcia VF, Skelton JA, Guice KS, Strauss RS, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 425.O'Brien PE. Controversies in bariatric surgery. Br J Surg. 2015;102:611–618. doi: 10.1002/bjs.9760. [DOI] [PubMed] [Google Scholar]
- 426.O'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29:3–14. doi: 10.1007/s11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.O'Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257:87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 428.O'Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–1040. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 429.van Geelen SM, Bolt IL, van der Baan-Slootweg OH, van Summeren MJ. The controversy over pediatric bariatric surgery: an explorative study on attitudes and normative beliefs of specialists, parents, and adolescents with obesity. J Bioeth Inq. 2013;10:227–237. doi: 10.1007/s11673-013-9440-0. [DOI] [PubMed] [Google Scholar]
- 430.Epstein LH, McCurley J, Valoski A, Wing RR. Growth in obese children treated for obesity. Am J Dis Child. 1990;144:1360–1364. doi: 10.1001/archpedi.1990.02150360086029. [DOI] [PubMed] [Google Scholar]
- 431.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264:2519–2523. [PubMed] [Google Scholar]
- 432.Valoski A, Epstein LH. Nutrient intake of obese children in a family-based behavioral weight control program. Int J Obes. 1990;14:667–677. [PubMed] [Google Scholar]
- 433.Xanthakos SA, Khoury JC, Inge TH, Jenkins TM, Modi AC, Michalsky MP, et al. Nutritional risks in adolescents after bariatric surgery. Clin Gastroenterol Hepatol. 2020;18:1070–81.e5. doi: 10.1016/j.cgh.2019.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Anker SD, Anker MS, von Haehling S. Weight loss and health status after bariatric surgery in adolescents. N Engl J Med. 2016;374:1988. doi: 10.1056/NEJMc1602007. [DOI] [PubMed] [Google Scholar]
- 435.Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017;5:165–173. doi: 10.1016/S2213-8587(16)30315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Hofmann B. Bariatric surgery for obese children and adolescents: a review of the moral challenges. BMC Med Ethics. 2013;14:18. doi: 10.1186/1472-6939-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Black JA, White B, Viner RM, Simmons RK. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev. 2013;14:634–644. doi: 10.1111/obr.12037. [DOI] [PubMed] [Google Scholar]
- 438.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 439.Ford ES, Ajani UA, Mokdad AH National Health and Nutrition Examination. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005;28:878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 440.Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–14. doi: 10.1136/adc.2003.036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Mellerio H, Alberti C, Druet C, Capelier F, Mercat I, Josserand E, et al. Novel modeling of reference values of cardiovascular risk factors in children aged 7 to 20 years. Pediatrics. 2012;129:e1020–9. doi: 10.1542/peds.2011-0449. [DOI] [PubMed] [Google Scholar]