Abstract
Given that multiple neurobiological systems, as well as components within these systems are impacted by stress, and may interact in additive, compensatory and synergistic ways to promote or mitigate PTSD risk, severity, and recovery, we thought that it would be important to consider the collective, as well as separate effects of these neurobiological systems on PTSD risk. With this goal in mind, we conducted a proof-of-concept study utilizing cerebrospinal fluid (CSF) collected from unmedicated, tobacco- and illicit substance-free men with PTSD (n = 13) and trauma-exposed healthy controls (TC) (n = 17). Thirteen neurobiological factors thought to contribute to PTSD risk or severity based on previous studies were assayed. As the small but typical sample size of this lumbar puncture study limited the number of factors that could be considered in a hierarchical regression model, we included only those five factors with at least a moderate correlation (Spearman rho > 0.30) with total Clinician-Administered PTSD Scale (CAPS-IV) scores, and that did not violate multicollinearity criteria. Three of the five factors meeting these criteria—CSF allopregnanolone and pregnanolone (Allo + PA: equipotent GABAergic metabolites of progesterone), neuropeptide Y (NPY), and interleukin-6 (IL-6)—were found to account for over 75% of the variance in the CAPS-IV scores (R2 = 0.766, F = 8.75, p = 0.007). CSF Allo + PA levels were negatively associated with PTSD severity (β = −0.523, p = 0.02) and accounted for 47% of the variance in CAPS-IV scores. CSF NPY was positively associated with PTSD severity (β = 0.410, p = 0.04) and accounted for 14.7% of the CAPS-IV variance. There was a trend for a positive association between PTSD severity and CSF IL-6 levels, which accounted for 15.3% of the variance in PTSD severity (β = 0.423, p = 0.05). Z-scores were then computed for each of the three predictive factors and used to depict the varying relative degrees to which each contributed to PTSD severity at the individual PTSD patient level. This first of its kind, proof-of-concept study bears replication in larger samples. However, it highlights the collective effects of dysregulated neurobiological systems on PTSD symptom severity and the heterogeneity of potential biological treatment targets across individual PTSD patients—thus supporting the need for precision medicine approaches to treatment development and prescribing in PTSD.
Abbreviations: Allo + PA, sum of allopregnanolone and pregnanolone; EIA, enzyme immunoassay; GC-MS, gas chromatography-mass spectrometry; HPLC, high pressure liquid chromatography; LP, lumbar puncture; PE, prolonged exposure therapy; PFC, prefrontal cortex; RIA, radioimmunoassay; 3α-HSD, 3α-hydroxysteroid dehydrogenase; TC, trauma-exposed control
1. Introduction
Currently available treatments for posttraumatic stress disorder (PTSD) have limited efficacy in many patients, likely due to the multiple, heterogenous and individually variable pathophysiological processes underlying the PTSD symptom phenotype (Friedman and Bernardy, 2017; Raber et al., 2019; Rasmusson and Abdallah, 2015; Rasmusson and Pineles, 2018). Given that stress itself has wide-ranging and individually variable effects on central and peripheral neurobiological systems, we can assume that these systems, as well as components within them, interact in compensatory, additive, redundant, and synergistic ways to promote or mitigate PTSD risk, severity, and recovery (Rasmusson and Pineles, 2018). It is therefore important to consider the collective, as well as separate effects of these neurobiological systems on PTSD risk.
PTSD symptom severity within 60 days of trauma is highly predictive of risk for PTSD up to 15 months later (Shalev et al., 2019). This suggests that identification of neurobiological systems that contribute to PTSD severity generally, and to PTSD symptom severity at the individual patient level may, respectively, inform development and individual prescribing of interventions for PTSD. With these goals in mind, we conducted a proof-of-concept study utilizing cerebrospinal fluid (CSF) collected from trauma-exposed men with and without PTSD to analyze a range of neurobiological factors thought to contribute to PTSD risk or severity based on previous studies, as detailed in the Supplementary Information section. We then used a data-driven approach to assess the relationship of these neurobiological factors to PTSD severity in the current sample. Given the limited but typical sample size of this lumbar puncture (LP) study, we developed a parsimonious statistical model by first computing correlations between each neurobiological factor and PTSD severity. We then entered only those factors yielding at least a moderate correlation into a hierarchical regression to evaluate their individual and composite contributions to PTSD severity. Finally, we plotted the relative degrees to which the final three neurobiological factors found to substantially contribute to PTSD severity in the sample as a whole were dysregulated in individual PTSD patients.
2. Materials and methods
This study was an extension of a previous study conducted by Rasmusson et al. (2019) at the VA National Center for PTSD, Women's Health Science Division, VA Boston Healthcare System, and was approved by the Institutional Review Boards of VA Boston Healthcare System and Boston University School of Medicine. The previous study (Rasmusson et al., 2019) focused on CSF GABAergic neurosteroids, while the current study analyzed the individually variable and composite contributions of multiple neurobiological factors assayed in CSF to PTSD severity in trauma-exposed men with PTSD.
2.1. Screening and lumbar puncture
Thirteen unmedicated, tobacco-free, fasting male veterans with chronic PTSD and 17 trauma-exposed healthy male controls without current or past PTSD participated in the study. As previously described (Rasmusson et al., 2019), participants were screened for DSM-IV PTSD Criterion A1/A2 trauma using the Trauma Life Event Questionnaire (TLEQ) and Childhood Trauma Questionnaire (CTQ) (Fink et al., 1995; Kubany et al., 2000). PTSD diagnosis and severity were determined using the Clinician-Administered PTSD Scale (CAPS-IV one-month version) (Blake et al., 1995; Weathers et al., 2001). Other psychiatric diagnoses were established using the DSM-IV SCID (First et al., 2005). A medical history was taken, and a physical examination, electrocardiogram, routine clinical laboratory tests and urine toxicology tests were performed. Tested substances included amphetamine, benzodiazepines, cannabinoids, cocaine, opiates, oxycodone, and cotinine. Participants were required to be free of chronic or acute medical problems, and to have normal clinical laboratory results, as well as negative urine toxicology and cotinine tests at screening and the lumbar puncture (LP). Current and past psychiatric diagnoses except a past history of single episode major depression (MDD) were exclusionary for the healthy trauma-exposed controls. A lifetime diagnosis of a schizophreniform or bipolar disorder, or substance abuse/dependence within 6 months of the LP were exclusionary for participants with PTSD. All participants were asked to abstain from alcohol, nicotine, illicit substances, and all medication prior to the LP for 4 weeks, or 6 weeks for selective serotonin reuptake inhibitors with long half-lives. Over-the-counter medications approved by the study PI, such as ibuprofen, acetaminophen, or loratadine could be taken on an intermittent, as needed basis up to one-week before the LP.
Participants fasted except for water intake after midnight the night before the LP. They were asked to arrive for the LP by 7:30 am for urine drug and cotinine testing, vital signs, and an ophthalmologic exam. Blood was drawn ~60 min after the participants were seated (TC: 66.6 ± 25.8 min; PTSD: 72.4 ± 21.6 min; p = 0.77). The LP was performed by an anesthesiologist ~30 min after the blood draw (TC: 34.2 ± 21.9 min; PTSD: 31.8 ± 12.0 min; p = 0.67). Participants remained seated during the LP and lay prone for 30 min after the LP, during which the CAPS-IV one-week version was administered. Blood samples were placed on wet ice and immediately processed in a refrigerated centrifuge to obtain plasma before storage at −80 °C. CSF samples (20 1cc-aliquots) were placed on dry ice and immediately stored at −80 °C.
2.2. CSF assays
Thirteen neurobiological factors were assayed in CSF: norepinephrine (NE) (1), neuropeptide Y (NPY) (2), gamma-amino-butyric-acid (GABA) (3), GABAergic neurosteroids synthesized from progesterone [allopregnanolone (Allo) + pregnanolone (PA),which were summed because these stereoisomers are equipotent)] (4), a less potent GABAergic neurosteroid synthesized from testosterone (3α-androstanediol) (5), the steroid precursors for these GABAergic steroids: progesterone (6), dihydroprogesterone (5α-DHP) (7), testosterone (8) and 5α-dihydrotestosterone (5α-DHT) (9), adrenal steroids that negatively modulate GABAA receptor function and positively modulate N-methyl-D-aspartate (NMDA) receptor function: dehydroepiandrosterone (DHEA) (10) and DHEA-sulfate (DHEAS) (11), 17β-estradiol (12), and the inflammatory cytokine, interleukin-6 (IL-6) (13).
CSF NE was measured after alumina extraction of 0.5 mL of CSF by HPLC using a Thermo Fisher Scientific ESA coulometric detector (E1/E2 = +450/-350). The intra- and inter-assay coefficients of variation (CVs) were less than 5% and 8%, respectively (Anderson et al., 1988).
A direct, highly sensitive radioimmunoassay (RIA) (Euro Diagnostica- ALPCO Diagnostics, Salem, NH) was used to assay NPY-like immunoreactivity. The antibody has <0.1% cross-reactivity with NPY22-36, peptide YY, pancreatic polypeptide, and other neuropeptides. Assay sensitivity was ~12.81 pg/mL. The intra- and inter-assay CVs were 4.7 ± 0.3% and 8.4 ± 0.8%, respectively.
CSF GABA was determined using a slight modification of the method of Schur et al. (2016). D6-GABA (40 ng) (Sigma-Aldrich) and 500 μL of acetonitrile were added to 100 μL of plasma; the mixture was vortexed, placed on ice for 10 min and then centrifuged. The supernatant was poured into a 1.5 mL tube and evaporated under vacuum with centrifugation (Savant Speed Vac), after which 100 μL of a 4:1 mixture of butanol: acetyl chloride (99.8% and 98%, respectively, both from Sigma-Aldrich) was added and heat applied at 60 °C for 15 min. After evaporation under vacuum centrifugation, the residue was dissolved in 100 μL of acetonitrile and stored at −70 °C until analysis. HPLC-mass spectrometric (LC-MS/MS) analysis was performed with separation on a Waters Acquity 1.7 μm BEH Amide column (2.1 × 100 mm, with a 2.1 mm × 5 mm guard column) with mobile phases of 50 mM ammonium formate (A) and acetonitrile with 0.1% formic acid (B) delivered as follows: 0–1.0 min, 250 μL/min, 70% A; 1.0–2.5 min 70%–5% A gradient; 2.5 min–7.0 min, 400 μL/min, 5% A; 7–10 min, 250 μL/min, 70% A. An API 4000 Qtrap mass spectrometer was used with declustering, entrance, and collision cell exit potentials of 41, 10, and 10 V, respectively, and a collision energy of 25 V. The ion spray voltage was 4200 eV and source temperature 400 °C. The curtain gas, ion source gas 1, and ion source gas 2 pressures were 15, 1, and 50 psi respectively. The transitions monitored for GABA and D6-GABA, respectively, were 160.1/87.0 and 166.1/93. GABA measures in a pooled quality assessment plasma sample (24.7 ng/mL) had intra-assay and inter-assay CVs of 0.9–5.1% and 6.7%, respectively.
Gas chromatography, mass spectrometry (GC-MS) was used after HPLC separation of the CSF steroids of interest to measure progesterone, 5α-DHP, Allo, PA, testosterone, 5α-DHT, 3α-androstanediol, DHEA, DHEAS, and 17β-estradiol (Pinna et al., 2000; Rasmusson et al., 2019). Tritiated neuroactive steroids (American Radiolabeled Chemicals, St. Louis, MO, USA) were added to monitor the HPLC retention profile. Deuterated internal standards consisting of 1 pmol of deuterium-labeled neuroactive steroid (CDN Isotopes, Pointe-Claire, QC, and Steraloids, Newport, RI, USA) were used to allow quantification of the compounds of interest and correct for procedural losses. The sensitivity was ~1.6 pg/ml. The intra- and inter-assay CVs were <5% and <10%, respectively.
IL-6 levels were assayed with a highly specific enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 0.7 pg/mL, range: 3.1–300 pg/mL, and cross-reactivity of <0.5% with other cytokines (R&D Systems, Minneapolis, MN).
2.3. Statistical analyses
Descriptive statistics for demographic characteristics, DSM-IV diagnoses, and screening Clinician-Administered PTSD Scale (CAPS)-IV scores for PTSD and trauma-exposed healthy participants were previously reported (Rasmusson et al., 2019) and are reported again in Table 1; group mean CSF biomarker levels also were computed. Independent t-tests with Welch's correction for unequal variance were used when appropriate to compare group means. Subjects with missing CSF biomarker data (due to technical problems) were excluded from analyses to avoid making assumptions about variable distributions. Given that emotional stress triggered by the screening evaluation or LP might have variably influenced participant ratings of PTSD symptoms, we used the average of the CAPS-IV scores at screening (rates for the past month) and the LP (rated for the past week), for all study analyses as a more integrated assessment of chronic PTSD severity.
Table 1.
Demographic characteristics of PTSD and trauma control groups.
| Total Sample Mean ± SD (n = 30) |
PTSD Mean ± SD (n = 13) |
TC Mean ± SD (n = 17) |
Welch's t-test/Chi square t | |
|---|---|---|---|---|
| Age (years) | 37.2 ± 10.6 | 40.1 ± 9.3 | 35.0 ± 11.2 | - 1.35 |
| BMI (kg/m2) | 27.0 ± 4.7 | 28.5 ± 4.0 | 26.0 ± 5.0 | −1.49 |
| TLEQ | 13.9 ± 14.8 | 21.9 ± 15.7 | 7.8 ± 11.0 | −2.77* |
| CTQ | 48.2 ± 23.9 | 56.2 ± 22.8 | 42.1 ± 23.6 | −1.67 |
| CAPS Average | 33.5 ± 34.6 | 70.38 ± 18.70 | 6.78 ± 8.43 | -11.45* |
| BDI | 8.7 ± 13.1 | 18.5 ± 15.1 | 1.1 ± 1.7 | −4.15* |
| Current Axis I Dx | n (%) | n (%) | n (%) | Χ2 |
| Anxiety Disorder | 3 (10.00) | 3 (23.08) | 0 (0) | 4.36 |
| Depression | 7 (23.33) | 7 (53.85) | 0 (0) | 11.94* |
| Alcohol Abuse | 1 (3.33) | 1 (7.69) | 0 (0) | 1.35 |
| Lifetime Axis I Dx | n (%) | n (%) | n (%) | Χ2 |
| Anxiety Disorder | 1 (3.33) | 1 (7.69) | 0 (0) | 1.35 |
| Depression | 1 (3.33) | 1 (7.69) | 0 (0) | 1.35 |
| Alcohol Abuse | 9 (30.00) | 7 (53.84) | 2 (11.76) | 6.21* |
| Substance Abuse | 6 (20.00) | 4 (30.78) | 2 (11.76) | 1.66 |
*p < 0.05; TC: trauma control; BMI: body mass index; TLEQ: Traumatic Life Events Questionnaire; CTQ: Childhood Traumatic Questionnaire; BDI: Beck Depression Inventory; CAPS: Clinician-Administered PTSD Scale. Anxiety Disorder: panic disorder, specific phobia, obsessive compulsive disorder, social phobia.
As the first step in data analysis addressing the main aim of the study, we computed Spearman's rank-order correlations between each of the 13 CSF neurobiological factors and average CAPS-IV scores. The small PTSD sample size (typical for LP studies) limited the total number of factors that could be included in a hierarchical regression model. Therefore, the factors considered for the regression model had to be at least moderately correlated with CAPS-IV average scores (rho > 0.30) (Cohen, 2013). The hierarchical regression then evaluated incremental increases in explanation of the variance in average CAPS-IV scores (i.e. R2 change) by these predictive factors. The predictor accounting for the most variance at each step in the regression was retained. Predictors that resulted in minimal R2 changes (i.e., R2 change < 0.005 or < 0.5%) were eliminated from the final model.
Several standard statistical tests were used to ensure the validity of these analyses. With the addition of each predictor to the model, we assessed potential relationships among the predictors, by a) calculating severity of multicollinearity using the Variance Inflation Factor (VIF), and b) examining the correlation matrix. The VIF cutoff was set at a conservative level of 2.00. Any predictor that violated the multicollinearity diagnostics and/or strongly correlated with another predictor in the model (rho > 0.8) was eliminated. In addition, regression diagnostics were performed to confirm the assumptions of homogeneity of variance, linearity and the normality of residuals. We also examined the initial correlations between average CAPS-IV scores and each neurobiological factor for influential points by computing Cook's Distance (>0.33), standardized residuals (>2.00) and leverage (>0.66); no variables met the criteria for an influential point. In addition, we examined participant age and BMI as potential confounders of the relationship between the CSF neurobiological factors and CAPS average scores. Potential confounders are defined as variables that show significant correlations with both dependent (CAPS average) and independent variables (CSF neurobiological factors) and are not on the causal pathway between the independent and dependent variables (Greenland et al., 1999). Age and BMI were not associated with CAPS average in the univariate correlation (r = −0.152, p = 0.675; r = 0.30, p = 0.370, respectively). Thus, age and BMI could not be considered confounders and were not included as covariates in the linear regression models.
Z-scores of the three predictive factors in the final multiple linear regression model were then computed to illustrate variations in the relative degrees to which each factor contributed to PTSD severity at the individual subject level. First, Z-scores for each neurobiological factor were calculated across the diagnostic groups to see whether patterns of variability tended to vary between individuals with and without PTSD. Z-scores for each factor also were calculated within the PTSD group, as it is the clinically relevant group for which such biomarkers potentially could be characterized to help target individually relevant treatments.
Finally, as part of a post hoc analysis discussed in section 4.3.2, plasma NPY levels were examined for diagnostic group mean differences and correlations with CAPS-IV scores in the PTSD group.
Analyses were conducted using SPSS Statistics (Version 25). All statistical tests were two-sided. A p-value < 0.05 was used as the cut-off for reporting statistically significant results for the final multiple regression model.
3. Results
3.1. Participant characteristics
As previously reported (Rasmusson et al., 2019), the PTSD and trauma-exposed healthy groups did not differ significantly in age, weight, body mass index, ethnicity or education. The PTSD group had higher rates of past alcohol abuse, and higher TLEQ and CAPS scores. Approximately half of the PTSD participants were diagnosed with current comorbid MDD. Table 1 is an abbreviated version of a similar table reported previously by Rasmusson et al. (2019).
3.2. Levels of CSF neurobiological factors in PTSD and trauma-exposed healthy participants
Table 2 shows diagnostic group means and the results of t-tests for the CSF neurobiological factors assayed. CSF NPY levels were significantly higher in the PTSD participants than controls. There also was a trend for higher IL-6 levels in the PTSD vs. control groups. There were no other significant differences between groups in the levels of neurobiological factors assayed.
Table 2.
Unadjusted t-Tests Comparing CSF Neurobiological Factors in PTSD Subjects & Trauma Controls.
| Cerebrospinal Fluid Neurobiological Factor Levels (pg/ml) | TC Mean ± SD (n) |
PTSD Mean ± SD (n) |
ta-Test | dfa | p-Valuea | Cohen's d [95% CI]a,b |
|---|---|---|---|---|---|---|
| Norepinephrine (NE) | 140.2 ± 55.6 (17) | 150.7 ± 32.7 (13) | 0.65 | 26.5 | 0.524 | 0.23 [-22.9; 43.9] |
| Neuropeptide Y (NPY) | 846.0 ± 68.5 (17) | 905.8 ± 74.3 (13) | −2.28 | 28.0 | 0.03a | 0.84 [6.18; 113.4] |
| Gamma-amino-butyric-acid (GABA) | 19.8 ± 7.5 (17) | 17.9 ± 4.2 (13) | −0.86 | 25.9 | 0.397 | 0.31 [-6.30; 2.59] |
| Progesterone (PROG) | 13.0 ± 12.2 (14) | 17.1 ± 11.6 (12) | 0.87 | 23.7 | 0.39 | 0.34 [-0.47; 1.16] |
| 5α-Dihydroprogesterone (5α-DHP) | 573.7 ± 669.7 (15) | 309.4 ± 293.7 (12) | −1.37 | 20.1 | 0.19 | 0.5 [-1.34; 0.28] |
| Allopregnanolone (Allo) + Pregnanolone (PA) |
23.3 ± 10.6 (15) | 32.1 ± 23.7 (12) | 1.20 | 14.5 | 0.25 | 0.48 [-0.34; 1.27] |
| Testosterone | 39.4 ± 12.3 (16) | 37.3 ± 14.0 (11) | −0.39 | 19.7 | 0.70 | 0.16 [-13.0; 8.85] |
| 5α-Dihydrotestosterone (5α-DHT) | 20.8 ± 24.7 (15) | 16.1 ± 7.9 (12) | −0.70 | 17.4 | 0.49 | 0.26 [-19.0; 9.50] |
| 3α-Androstanediol | 1.4 ± 2.6 (15) | 2.9 ± 3.2 (11) | 0.51 | 18.8 | 0.62 | 0.21 [-1.87; 3.08] |
| Dehydroepiandrosterone (DHEA) | 839.4 ± 889.0 (17) | 618.9 ± 479.3 (13) | −0.87 | 25.6 | 0.39 | 0.31 [-1.08; 0.44] |
| DHEA-Sulfate (DHEAS) | 2121 ± 1572 (16) | 2059 ± 2087 (12) | −0.09 | 19.7 | 0.93 | 0.03 [-0.82; 0.75] |
| 17β-Estradiol | 4.9 ± 1.7 (16) | 4.3 ± 2.4 (11) | 0.36 | 16.6 | 0.72 | 0.15 [-1.50; 2.11] |
| Interleukin 6 (IL-6) | 2.0 ± 0.8 (17) | 3.0 ± 1.8 (13) | 1.86 | 15.9 | 0.08 | 0.72 [-.137; 2.13] |
ap < 0.05; PTSD: Posttraumatic stress disorder; TC: Trauma-exposed healthy control; df: degrees of freedom; CI: confidence interval.
t-test using Welch's correction for unequal variances.
CI for the mean difference comparing the PTSD to TC groups.
3.3. Correlations between CSF biomarkers and CAPS-IV scores in PTSD subjects
The results of Spearman's rank-order correlations between the CSF neurobiological factors of interest and average CAPS-IV scores are presented in Table 3 for the PTSD group. There was a significant, large negative correlation between the CSF Allo + PA levels and average total CAPS-IV scores (r = −0.69, p = 0.01). There was a trend for a large positive correlation between CSF IL-6 levels and average CAPS scores (r = 0.52, p = 0.07), while there were non-significant, moderate positive correlation of CSF NPY, 3α-androstanediol and DHEAS levels with average CAPS score (rho's > 0.3, p's = 0.22 to 0.31).
Table 3.
Spearman correlations between CSF biomarkers and total CAPS-IV scores in PTSD.
| Neurobiological Factor Assayed in CSF (Ordered by Strength of Correlation) | Correlation Coefficient (rho) | p-Value |
|---|---|---|
| Allopregnanolone + Pregnanolone (Allo + PA) | −0.69 | 0.01* |
| Interleukin 6 (IL-6) | 0.52 | 0.07 |
| 3α-Androstanediol | −0.39 | 0.24 |
| Neuropeptide Y (NPY) | 0.37 | 0.22 |
| Dehydroepiandrosterone-Sulfate (DHEAS) | 0.32 | 0.31 |
| Dehydroepiandrosterone (DHEA) | 0.26 | 0.34 |
| Testosterone | −0.26 | 0.45 |
| 5α-Dihydroprogesterone (5α-DHP) | −0.25 | 0.43 |
| Gamma-amino-butyric acid (GABA) | 0.20 | 0.52 |
| 5α-Dihydrotestosterone (5α-DHT) | −0.13 | 0.68 |
| 17β-Estradiol | −0.06 | 0.85 |
| Progesterone | 0.06 | 0.85 |
| Norepinephrine (NE) | −0.04 | 0.91 |
3.4. Hierarchical regression analysis
As planned, neurobiological factors correlating with CAPS-IV average scores at rho ≤ 0.30 (Table 3: DHEA, testosterone, 5α-DHP, GABA, 5α-DHT, 17β-estradiol, progesterone and NE) were not included in the hierarchical regression analysis, leaving 5 factors for consideration as predictors of CAPS-IV average scores. In the hierarchical regression analysis (Table 4), we entered Allo + PA into Step 1 because it had the strongest association with CAPS-IV average scores, explaining 47% of the variance. In Step 2, each of the remaining 4 factors (IL-6, 3α-androstanediol, NPY, and DHEAS) was entered alone, in addition to Allo + PA, to assess incremental change in explained variance; NPY was retained in the model, as its entry caused the biggest incremental change in explained variance (i.e., 14.7%). In Step 3, each of the remaining 3 factors (IL-6, 3α-androstanediol, and DHEAS) was entered alone, in addition to Allo + PA and NPY. IL-6 was retained in the model, as it effected the largest change in explained variance (15.3%). The remaining two predictors, DHEAS and 3α-androstanediol, did not meet the threshold of R2 change >0.005 (R2 change = 0.002 and R2 change = < 0.001, respectively), meaning they each contributed only 0.2% and <0.1% to the explained variance. Hence, DHEAS and 3α-Androstanediol were excluded from the final model.
Table 4.
Hierarchical regression predicting PTSD symptom severity.
| Predictors entered | R2/R2 Change | Adjusted R2 | p valuea |
|---|---|---|---|
| Step 1 | |||
| Allo + PA | 0.466 | 0.413 | 0.014 |
| Step 2 | |||
| Allo + PA, NPY | 0.613/0.147 | 0.527 | 0.014 |
| Step 3 | |||
| Allo + PA, NPY, IL-6 | 0.766/0.153 | 0.679 | 0.007 |
ap values for each step of the linear regression model after strongest predictor retained.
As can be seen (Table 5), the final model including Allo + PA, NPY, and IL-6 explained more than 75% of the variance in average total CAPS scores (R2 = 0.766, F = 8.75, p = 0.007). The sum of the CSF Allo + PA levels was significantly and negatively associated with PTSD severity (β = −0.523, p = 0.02) after controlling for IL-6 and NPY levels. NPY had a significant positive association with PTSD severity (β = 0.410, p = 0.04) while controlling for CSF GABAergic neurosteroid and IL-6 levels. There was a borderline significant positive association between CSF IL-6 levels and PTSD severity (β = 0.423, p = 0.05), while controlling for NPY and the progesterone-derived GABAergic neurosteroid levels.
Table 5.
Final linear regression model predicting PTSD CAPS-IV average scores by CSF biomarkers.
| Variables | B | SE | β | t | p-Value |
|---|---|---|---|---|---|
| CSF Allo + PA | −0.423 | 0.149 | −0.523 | −2.84 | 0.02 |
| CSF NPY | 0.101 | 0.042 | 0.410 | 2.40 | 0.04 |
| CSF IL-6 | 4.35 | 1.90 | 0.423 | 2.29 | 0.05 |
| N = 12. R2 = .766. Adjusted R2 = .697. | |||||
B: unstandardized coefficient; SE: standard error; β: standardized coefficient; t: t statistic.
3.5. Results plotted at the individual patient level
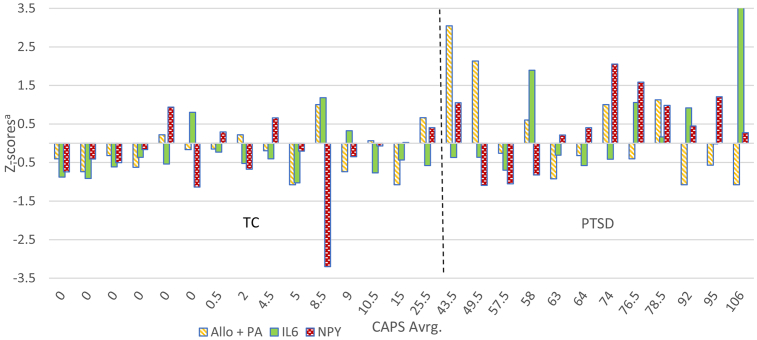
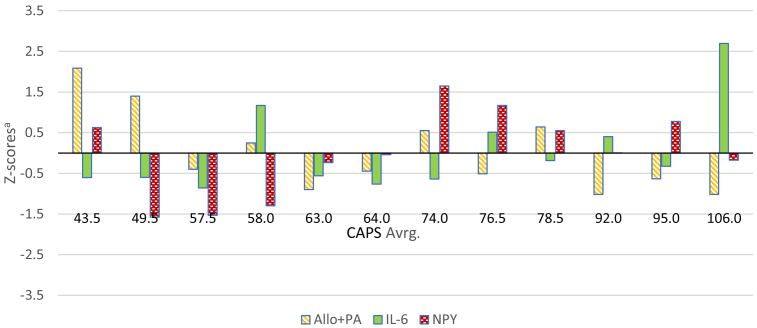
Illustrating the findings at the individual patient level, Fig. 1 depicts Z-scores for CSF Allo + PA, NPY and IL-6 across the PTSD and TC groups considered together, and Fig. 2 depicts Z-scores calculated for these three CSF factors within the PTSD group alone.
Fig. 1.
Z-scores of the CSF neurobiological factors retained in the multiple regression model predicting PTSD severity across the diagnostic groups. CAPS Avrg. = average Clinician-Administered PTSD Scale score; Allo + PA = allopregnanolone + pregnanolone; IL-6 = Interleukin-6; NPY = neuropeptide Y; TC = Trauma Control group; PTSD = PTSD group; aCalculated across both PTSD and TC groups.
Fig. 2.
Z-scores for the CSF neurobiological factors retained in the multiple regression model predicting PTSD severity in the PTSD group. CAPS Avrg. = average Clinician-Administered PTSD Scale score; Allo + PA = allopregnanolone + pregnanolone; IL-6 = interleukin-6; NPY = neuropeptide Y; aCalculated within the PTSD group.
4. Discussion
4.1. Collective effects of multiple CSF neurobiological factors on PTSD Severity and clinical relevance
This proof-of-concept study is unique in analyzing the composite contributions of multiple neurobiological factors to PTSD severity in men. Observed differences in a range of neurobiological factors between patients with and without PTSD (Pitman et al., 2012), and increases in medical illness burden experienced by PTSD patients with dysregulation of multiple neurobiological systems (Frayne et al., 2011) have led researchers to conceptualize PTSD as a manifestation of multi-system dysregulation (Chakraborty et al., 2017; Mellon et al., 2019; Thakur et al., 2015). Nevertheless, most studies of PTSD risk, severity and response to treatment have focused on single biomarkers, even when multiple biomarkers were available for analysis (e.g., Yehuda et al., 2014).
In the current study, a multiple linear regression model including three CSF neurobiological factors (Allo + PA, NPY, and IL-6), which correlated with PTSD severity at a moderate or higher level when considered separately, was significant and accounted for over 75% of the variance in PTSD severity. This study thus demonstrates the importance of considering the collective impact of multiple dysregulated neurobiological systems on PTSD severity—and joins recent studies from fields outside and inside of psychiatry examining multiple biological factors from the same patient to better ascertain diagnosis or account for illness severity or morbidity (Lindstrom and Robinson, 2010). This proof-of-concept study even more importantly illustrates the biological heterogeneity of PTSD at the individual patient level and suggests that it may be possible in the future to query the function of multiple PTSD-relevant systems to help guide the prescription of therapeutics—just as current routine panels of laboratory tests querying the function of several somatic systems help medical providers sort out disorders with overlapping symptom presentations in order to select best treatments. Assessing multiple neurobiological factors potentially involved in the pathophysiology of a heterogeneous symptom-based disorder such as ‘PTSD’ is thus in line with the movement towards personalized precision medicine whereby treaters target underlying pathophysiological mechanisms specific to an individual patient.
4.2. Biological profiling of individual PTSD patients
As depicted in Fig. 1, one can see that there is substantially more variability in the levels of these three CSF biomarkers among the participants with PTSD than in the TCs. In addition, the direction and amplitude of apparent dysregulation (or perhaps stress adaptation) of each of these factors varied across the subjects with PTSD in relation to PTSD severity (Fig. 1, Fig. 2). A second key observation (Fig. 2) is that PTSD subjects with markedly different biological profiles are categorized under one symptom-based diagnosis. For example, the relationships among Allo + PA, NPY and IL-6 differ, as do the amplitudes and relative direction of dysregulation in these factors between the PTSD group subjects with the lowest (43.5) and highest (106) CAPS-IV scores.
4.3. The significant neurobiological contributors to PTSD severity
4.3.1. Allopregnanolone and pregnanolone
The current study found that the combined levels of Allo and PA contributed the most (as indicated by the standard coefficient beta in Table 5) to PTSD severity in the multiple linear regression model. This finding aligns with our previously reported LP study in men with PTSD (Rasmusson et al., 2019), as well as CSF and plasma studies in women with PTSD (Rasmusson et al., 2006; Pineles et al., 2018) reporting strong associations between deficits in the capacity for synthesis of these GABAergic neurosteroids and PTSD symptoms. Interventions promoting the biosynthesis of these neurosteroids thus may be relevant to treating PTSD in par individuals with Allo + PA deficits. In a mouse model of PTSD, levels of Allo in the prefrontal cortex (PFC) and hippocampus were reduced after exposure to experimental stress and renormalized by the administration of sertraline and ginsenoside Rg2 along with normalization of behavior (Gao et al., 2018). In a male mouse model of PTSD, in which prolonged social isolation markedly reduced brain Allo levels, administration of the Allo analogue ganaxolone (Pinna and Rasmusson, 2014) or the endocannabinoid congener N-palmitoylethanolamine (PEA), which activates peroxisome proliferator activated receptor-alpha (PPAR-α) and induces neurosteroidogenesis (Locci and Pinna, 2019), improved PTSD-like symptoms. Another novel treatment that raises levels of Allo is brexanolone, an IV Allo formulation recently approved by the FDA for treatment of post-partum depression (Meltzer-Brody et al., 2018). It should be noted, however, that ganaxolone treatment was beneficial only in Allo-deficient mice (Pinna, 2019; Pinna and Rasmusson, 2014), highlighting the possible importance of our observation that only some PTSD patients have ‘deficient’ Allo + PA levels (Fig. 1, Fig. 2; Rasmusson et al., 2019).
4.3.2. Neuropeptide Y (NPY)
In contrast to the LP study by Sah et al. (2014) in male combat veterans with PTSD compared to trauma-exposed combat veterans without PTSD, our study found higher rather than lower levels of CSF NPY in the men with PTSD. In addition, CSF NPY levels correlated positively, although not significantly, with PTSD severity in the univariate analyses (Table 3). The absolute CSF NYP levels in the current study also were considerably higher than in the studies by Sah et al. (2009, 2014). There could be several explanations for these discrepancies, including differences in the study populations and/or experimental conditions.
For example, seven of 11 PTSD patients and just five of 14 trauma controls in the study by Sah et al. (2014) smoked, whereas smokers were excluded from the current study. Hussain et al. (2012) found lower resting plasma NPY levels in smokers than non-smokers, which normalized three months after smoking cessation. Further, NPY is released from sympathetic neurons and the adrenal medulla in response to intense stress and crosses the blood brain barrier via non-saturable transport (Kastin and Akerstrom, 1999). Familoni et al. (2016) showed dampening effects on sympathetic system reactivity in trauma-exposed smokers with and without PTSD compared to the non-smokers in these groups.
The PTSD subjects in the current study also had higher mean CAPS-IV scores (70.4 ± 5.2) than those in the study by Sah et al. (2014) (57.5 ± 4.8) (Table 6), suggesting that they may have been more susceptible to the “stress” of the LP. Peripheral NPY is colocalized with NE in sympathetic neurons. Under resting or mildly stressful conditions, NPY acts at NPY-Y2 receptors on these neurons to negatively modulate the release of NE. During intense stress, however, NPY is released in addition to NE from sympathetic neurons and acts at post-synaptic NPY-Y1 receptors to potentiate the post-synaptic effects of NE. Consistent with these phenomena, Rasmusson et al. (2000) demonstrated a negative correlation between resting plasma NPY levels and plasma 3-methyl-4-hydroxyphenylglycol (MHPG, the major metabolite of NE) responses to intense SNS activation in combat veterans with PTSD (r = −0.49, p = 0.04), but a positive correlation between the increases in plasma NPY and MHPG after SNS activation (r = 0.52, p = 0.03). As NPY freely crosses the blood brain barrier, it is plausible that more severely affected PTSD patients with greater amygdala reactivity and downstream SNS activation may have higher CSF NPY levels. However, the release of NPY from brain neurons also may contribute to CSF NPY levels. In the brain, NPY expression is widely distributed, and relevant to PTSD, is present in cortex, hippocampus, hypothalamus, and amygdala. In rodents, greater stress-induced reductions in amygdala NPY expression are associated with more extreme PTSD-like behaviors (Cohen et al., 2012), and in humans, low NPY expression haplotypes are associated with increased amygdala reactivity to negatively-valenced stimuli (e.g., Gutman et al., 2008). In contrast, McGuire et al. (2011) demonstrated delayed increases in PFC NPY immunoreactivity after stress. In the PFC, NPY is colocalized and released with GABA from stress-activated infralimbic non-parvalbumin containing projection neurons that inhibit prelimbic pyramidal projection neurons that potentiate amygdala stress responses (Vollmer et al., 2016). However, NPY also acts to inhibit infralimbic GABAergic neurons that project to prelimbic cortex—thus disinhibiting stress responses. It is therefore notable that an allelic variation of the NPY gene at rs16147 has been linked both to increased NPY gene expression in post-mortem PFC, and to anxiety and depression in young adults with early childhood adversity (Sommer et al., 2010).
Table 6.
Comparison of Study Design and Assay Methodologies: Kim et al. vs. Sah et al. (2014).
| Study | Subjects/Trauma Type | CSF NPY Assays | CSF NPY (pg/ml) Mean ± SE |
Age of Subjects Mean ± SE |
CAPS-IV Score* Mean ± SE |
Medication Use | Smoking Status |
|---|---|---|---|---|---|---|---|
| Kim et al. (current study) | Male: 13 PTSD 17 TC Trauma type: Multiple |
Radioimmunoassay (RIA) (Euro Diagnostica-ALPCO Diagnostics, Salem, NH). | PTSD: 905.8 ± 20.6 TC: 846.0 ± 16.6 |
PTSD: 40.1 ± 2.57 TC: 35.0 ± 2.78 |
70.4 ± 5.2 (PTSD); 6.8 ± 2.0 (TC) *Average of CAPS-IV (1 month version) at screen and CAPS-IV (1 week version) immediately after LP |
Medication free for > 4 weeks; > 6 weeks for SSRIs with long half-lives. | All non-smokers |
| Sah et al. (2014) | Male: 11 PTSD 14 TC Trauma: Combat; likely other |
Enzyme immunoassay (EIA) kit (Peninsula Laboratories Inc., Bachem, San Carlos, CA | PTSD: 180 ± 12.6 TC: 258.6 ± 21.6 |
PTSD: 30.7 ± 2.7 TC: 32.1 ± 1.4 |
57.5 ± 4.8 (PTSD) 7.11 ± 2.2 (TC) Assessed the day before the LP |
Medication free for at least 10 disappearance half-lives | PTSD: 7 of 11; TC: 5 of 14. Overnight abstinence before LP. |
PTSD: posttraumatic stress disorder; TC: trauma-exposed control; NPY: neuropeptide Y; CSF: cerebrospinal fluid; LP: lumbar puncture; SSRI: selective serotonin reuptake inhibitor.
Finally, the use of different immunoassay types may account for the striking differences in absolute CSF NPY levels between our study and that by Sah et al. (2014). Therefore, to ensure the consistency of our own methodology and face validity of our results, we compared our resting plasma NPY results in our PTSD group (see Methods) with resting plasma NPY results from previous studies using the same ALPCO RIA, a similar RIA (Allen et al., 1991), or one of several commercially available enzyme immunoassays (EIAs) (Table 7). As expected, given that trauma exposure rather than PTSD is associated with reductions in resting plasma NPY (Morgan et al., 2003), the resting plasma NPY levels in the current study did not differ between the PTSD subjects (303.9 ± 73.6 pg/ml) and TCs (293.5 ± 48.0 pg/ml). In addition, plasma NPY levels correlated negatively with PTSD symptoms (r = −0.75, p = 0.007), as reported for male veterans with PTSD in a study using a similar RIA (Rasmusson et al., 2000), and did not correlate with CSF NPY (r = −0.08, p = 0.70), as in a study of healthy male subjects by (Baker et al., 2013) using the same RIA. The absolute CSF NPY levels, and the difference between CSF and plasma NPY levels, also were similar to those observed by Baker et al. (2013).
Table 7.
Comparison of Immunoassay Kits Measuring NPY levels.
| Assay | Source | Sensitivity | Specificity |
|---|---|---|---|
| RIA | Euro Diagnostica-ALPCO Diagnostics, Salem, NH | ~12.81 pg/ml | <0.1% cross-reactivity with NPY22-36, peptide YY, pancreatic polypeptide, and other neuropeptides. |
| RIA | Allen et al. (1991) | 20 pg/mL with intra- and inter-assay coefficients of variation of 8% and 10%, respectively. | Percent binding of NPY and NPY 2–36 to [NPY]-α-globulin 3-5 was 100% and 100%, respectively. |
| EIA | RayBiotech, Norcross, GA | 3 ng/ml or 3000 pg/ml (Reijnen et al., 2018) | Detects human, mouse, and rat active NPY (1–36)a |
| EIA | EMD Millipore Corporation, Billerica, MA | 2 pg/ml using a 50 μL sample sizeb | Human, Rat NPY: 100%, NPY 2–36: 67%, NPY 3–36: 68%, NPY (free acid): 6%, NPY 13–36: 8%, (Leu31, Pro34) Human, Rat NPY: 41%, Porcine NPY: 44%, Porcine NPY 3–36: 41%. Pancreatic polypeptide and other human peptides: 0%b |
NPY: Neuropeptide Y; RIA: Radioimmunoassay; EIA: Enzyme Immunoassay.
http://www.emdmillipore.com/US/en/product/Human-Neuropeptide-Y-NPY-ELISA,MM_NF-EZHNPY-25K#anchor_PR
The plasma NPY levels in the current sample of male civilians and veterans with PTSD (303.9 ± 73.6 pg/ml) were similar to those reported by Scioli-Salter et al. (2016), who used the same RIA in male and female civilians and veterans with chronic PTSD (~150–300 pg/ml). As may be expected, they were lower than those measured by the same RIA in a large Marine Resilience Study cohort with high cardiorespiratory fitness and very low rates of PTSD (~400–900 pg/ml) (Reijnen et al., 2018). As expectable, the upper, but not lower, end of the range of resting plasma NPY levels in the current sample of PTSD subjects was higher than that measured by the similar RIA in male combat veterans with more uniformly severe PTSD (~90–240 pg/ml) (Rasmusson et al., 2000). In contrast, plasma NPY measured by an EIA (Ray Biotech) in the PRISMO Dutch military cohort with low rates of PTSD (~41–100 ng/ml) (Reijnen et al., 2018) were more than 300-fold higher than ours, and 100-fold higher than NPY levels in the Marine Resiliency cohort, consistent with the low sensitivity (3 ng/ml) and specificity reported for that EIA (Table 7). Other EIAs also have been problematic. Hauger et al. (personal communication) compared resting and acute exercise-induced increases in NPY by EIA (EMD Millipore; Table 7) and the ALPCO RIA. Resting NPY levels measured by EIA were at or below the limit of detection, while the RIA yielded results comparable to those in other studies using RIAs. Post-exercise NPY levels measured by EIA were also much lower than those measured by RIA. Unfortunately, plasma NPY levels have not been reported in the LP studies by Sah et al. (2009, 2014) and so can't be compared.
4.3.3. Interleukin 6 (IL-6)
CSF IL-6 levels correlated positively with CAPS-IV scores and made a significant independent contribution to PTSD severity in the multiple linear regression model. While not statistically significant, there also was a strong trend for higher CSF IL-6 levels in this relatively small sample of men with PTSD compared to trauma-exposed men without PTSD. While peripheral inflammatory markers have been examined extensively in relation to PTSD, there are few studies on inflammatory factors in CSF (Baker et al., 2001; Bonne et al., 2011). Baker et al. (2001) reported CSF IL-6 levels to be over 50% higher and correlated with NE among male combat veterans compared to healthy combat-unexposed controls. Bonne et al. (2011) found no difference in CSF IL-6 between civilians with PTSD and healthy trauma-unexposed controls. Our finding in unmedicated non-smokers with PTSD compared to trauma-exposed controls thus constitutes a meaningful addition to the studies in this area. As the association between inflammation and depression has been well-documented (Dowlati et al., 2010; Raison and Miller, 2013), some suggest that comorbid MDD may be a confounder in the relationship of inflammation to PTSD (Gill et al., 2010). However, in our study, 7 of 13 PTSD subjects had comorbid MDD, yet there was no significant difference in IL-6 levels between the PTSD subjects with and without MDD (t = 1.32, p = 0.216). In addition, Lindqvist et al. (2014) found higher levels of pro-inflammatory cytokines in combat-related PTSD even after accounting for depression (Lindqvist et al., 2014) and a meta-analysis by Passos et al. (2015) found increased inflammatory biomarkers, including IL-6, in PTSD subjects in the absence of comorbid MDD. Future studies thus should be aimed at elucidating the causes and role of inflammation in PTSD, as well as the potential therapeutic benefits of suppressing inflammation in PTSD.
4.4. Study limitations
Considering that the participants were free from medication, tobacco and illicit psychoactive drugs, and considering the well-known challenges of conducting LP studies, we believe that our data make a meaningful addition to the growing CSF database in PTSD. However, the current study has important limitations. The number of subjects in the study was small, although comparable to other CSF studies in PTSD (Baker et al., 2001; Sah et al., 2014). Finding such strong relationships between theoretically and empirically supported systems and PTSD severity suggests the potential clinical utility of this approach to querying PTSD pathophysiology at the individual patient level. Replication thus will be important, hopefully in larger cohorts that may allow: a) replication of the contributions of GABAergic neurosteroids, NPY and IL-6 to PTSD severity, b) stratification and covariation for participant characteristics such as sex and smoking status, c) elucidation of other CSF neurobiological factors with limited previous empirical links to PTSD, and d) analysis of the relationships of molecular factors to symptom clusters defined by factor analytic models of PTSD symptoms. With their limited sample sizes, this and previous CSF studies also have been restricted to consideration of only linear relationships between neurobiological factors of possible relevance to PTSD and PTSD risk or severity. NE, for example, is likely to have a more complex non-linear relationship to PTSD symptom expression (Pitman et al., 2012). Linking molecular findings to phenomena from other translational levels of inquiry, including neuroimaging, psychophysiology, genomics, and epigenetics, also may be useful in characterizing endophenotypes that inform PTSD treatment (Chakraborty et al., 2017). For the current cross-sectional study, data regarding rates of PTSD improvement over time or response to previous PTSD interventions were not available. Thus, this study could not gauge (either retrospectively or prospectively) whether the neurobiological factors profiled might indicate capacity for recovery. A longitudinal study following subjects from pre-trauma to post-trauma also may be helpful in clarifying whether changes in measurable CSF neurobiological factors in reaction to trauma confer risk or predict capacity to recover from traumatic stress. Finally, it would be important to obtain both resting and stress activated plasma markers while doing an LP study to see if they could as effectively query these systems, as plasma sampling is much less burdensome to patients.
5. Conclusion
Most studies of the neurobiology of PTSD have focused on the link between single neurobiological factors and PTSD risk or severity. Variations in study methodology and the heterogeneity of PTSD biophenotypes are likely to have contributed to inconsistencies across previous single biomarker studies. Our multiple regression model that identified substantial composite contributions of CSF GABAergic neurosteroids, NPY, and IL-6 levels to PTSD severity demonstrates the importance of considering the collective effects of multiple neurobiological systems on PTSD severity. This study also demonstrated the heterogeneity of PTSD biophenotypes across individual PTSD subjects, suggesting the need for integrated assessments to inform individualized treatment targeting.
As recently reviewed (Mellon et al., 2018), our understanding of PTSD as a ‘systemic’ disorder beyond a purely psychological condition continues to expand. A broader and more precise understanding of the multiple neurobiological mechanisms contributing to PTSD risk, severity and recovery will require further research as the field pushes towards precision medicine for the treatment of PTSD. In addition, the field must aspire to development of clinically feasible and relevant neuromolecular tests, the use of uniform, reliable, and accurate assay methodologies, and establishment of subpopulation specific normative ranges for the molecular diagnostics and prognostics of interest yet to be defined.
Funding
This work was supported by NIMH R21 MH31113 (Rasmusson, PI) and the National Center for PTSD, Women's Health Science Division, Department of Veterans Affairs at VA Boston Healthcare System in Boston, MA. R. Hauger was supported by the VISN22 Center for Excellence in Stress and Mental Health (CESMAH) and NIA RO1 grants AG022381 and AG050595. G. Pinna was supported by the United States Department of Defense Grant W81XWH-15-10521.
CRediT authorship contribution statement
Byung Kil Kim: Conceptualization, Writing - original draft, Writing - review & editing, Methodology, Formal analysis, Visualization. Jennifer R. Fonda: Formal analysis, Writing - review & editing, Validation. Richard L. Hauger: Investigation, Writing - review & editing, Validation. Graziano Pinna: Investigation, Writing - review & editing, Validation. George M. Anderson: Investigation, Writing - review & editing, Validation. Ivan T. Valovski: Investigation. Ann M. Rasmusson: Methodology, Investigation, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
Regarding potential conflicts of interest: Dr. Rasmusson has been an unpaid consultant to Cohen Veterans Bioscience during the past three years. Dr. Pinna has been compensated for measurement of GC/MS neurosteroid levels for Marinus Pharmaceuticals. Dr. Anderson has consulted to Pepper Hamilton representing the Eli Lilly Co. Dr. Hauger, Dr. Fonda and Dr. Kim have no conflicts of interest to disclose.
Acknowledgements
We would like to thank the study participants who generously contributed to this study. We would also like to thank Kristin Gregor, Mohamed Hamouda, Erica Scioli-Salter, Suzanne Pineles, Marianela Nelson and Sandra Braun who contributed much time and expertise to this study, and Benjamin Johnides, Maggie Bauer, Leah Brogan, Rachel Maskin and Joyce Ambrosino, our dedicated research assistants and nurse who recruited and guided study participants through the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100220.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Allen R., Boublik J., Hauger R.L., Scott N., Rivier J., Brown M.R. Neuropeptide Y radio-immunoassay: characterization and application. Clin. Exp. Pharmacol. Physiol. 1991;18:825–833. doi: 10.1111/j.1440-1681.1991.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Anderson G.M., Durkin T.A., Morton J.B., Cohen D.J. Liquid chromatographic determination of urinary catecholamines after one-step alumina extraction. J. Chromatogr. 1988;424:373–377. doi: 10.1016/s0378-4347(00)81115-9. [DOI] [PubMed] [Google Scholar]
- Baker D.G., Bertram T.M., Patel P.M., Barkauskas D.A., Clopton P., Patel S., Geracioti T.D., Jr., Haji U., O'Connor D.T., Nievergelt C.M., Hauger R.L. Characterization of cerebrospinal fluid (CSF) and plasma NPY levels in normal volunteers over a 24-h timeframe. Psychoneuroendocrinology. 2013;38:2378–2382. doi: 10.1016/j.psyneuen.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Baker D.G., Ekhator N.N., Kasckow J.W., Hill K.K., Zoumakis E., Dashevsky B.A., Chrousos G.P., Geracioti T.D., Jr. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bonne O., Gill J.M., Luckenbaugh D.A., Collins C., Owens M.J., Alesci S., Neumeister A., Yuan P., Kinkead B., Manji H.K., Charney D.S., Vythilingam M. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine. J. Clin. Psychiatr. 2011;72:1124–1128. doi: 10.4088/JCP.09m05106blu. [DOI] [PubMed] [Google Scholar]
- Chakraborty N., Meyerhoff J., Jett M., Hammamieh R. Genome to phenome: a systems biology approach to PTSD using an animal model. Methods Mol. Biol. 2017;1598:117–154. doi: 10.1007/978-1-4939-6952-4_6. [DOI] [PubMed] [Google Scholar]
- Cohen H., Liu T., Kozlovsky N., Kaplan Z., Zohar J., Mathe A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Routledge; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Familoni B.O., Gregor K.L., Dodson T.S., Krzywicki A.T., Lowery B.N., Jr., Orr S.P., Suvak M.K., Rasmusson A.M. Sweat pore reactivity as a surrogate measure of sympathetic nervous system activity in trauma-exposed individuals with and without posttraumatic stress disorder. Psychophysiology. 2016;53:1417–1428. doi: 10.1111/psyp.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink L.A., Bernstein D., Handelsman L., Foote J., Lovejoy M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am. J. Psychiatr. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. patient edition. Biometrics Research Department, Columbia University; New York, NY: 2005. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. [Google Scholar]
- Frayne S.M., Chiu V.Y., Iqbal S., Berg E.A., Laungani K.J., Cronkite R.C., Pavao J., Kimerling R. Medical care needs of returning veterans with PTSD: their other burden. J. Gen. Intern. Med. 2011;26:33–39. doi: 10.1007/s11606-010-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M.J., Bernardy N.C. Considering future pharmacotherapy for PTSD. Neurosci. Lett. 2017;649:181–185. doi: 10.1016/j.neulet.2016.11.048. [DOI] [PubMed] [Google Scholar]
- Gao Z.W., Ju R.L., Luo M., Wu S.L., Zhang W.T. The anxiolytic-like effects of ginsenoside Rg2 on an animal model of PTSD. Psychiatr. Res. 2018;279:130–137. doi: 10.1016/j.psychres.2018.12.034. [DOI] [PubMed] [Google Scholar]
- Gill J., Luckenbaugh D., Charney D., Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol. Psychiatry. 2010;68:999–1006. doi: 10.1016/j.biopsych.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Greenland S., Pearl J., Robins J.M. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Gutman A.R., Yang Y., Ressler K.J., Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J. Neurosci. : Off. J. Soc. Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T., Al-Daghri N.M., Al-Attas O.S., Draz H.M., Abd Al-Rahman S.H., Yakout S.M. Plasma neuropeptide Y levels relate cigarette smoking and smoking cessation to body weight regulation. Regul. Pept. 2012;176:22–27. doi: 10.1016/j.regpep.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Kastin A.J., Akerstrom V. Nonsaturable entry of neuropeptide Y into brain. Am. J. Physiol. 1999;276:E479–E482. doi: 10.1152/ajpendo.1999.276.3.E479. [DOI] [PubMed] [Google Scholar]
- Kubany E.S., Leisen M.B., Kaplan A.S., Watson S.B., Haynes S.N., Owens J.A., Burns K.J.P.a. vol. 12. 2000. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure; p. 210. (The Traumatic Life Events Questionnaire). [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Wolkowitz O.M., Mellon S., Yehuda R., Flory J.D., Henn-Haase C., Bierer L.M., Abu-Amara D., Coy M., Neylan T.C., Makotkine I., Reus V.I., Yan X., Taylor N.M., Marmar C.R., Dhabhar F.S. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Lindstrom T.M., Robinson W.H. Biomarkers for rheumatoid arthritis: making it personal. Scand. J. Clin. Lab. Invest. Suppl. 2010;242:79–84. doi: 10.3109/00365513.2010.493406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A., Pinna G. Stimulation of Peroxisome Proliferator-Activated Receptor-alpha by N-Palmitoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biol. Psychiatry. 2019;85:1036–1045. doi: 10.1016/j.biopsych.2019.02.006. [DOI] [PubMed] [Google Scholar]
- McGuire J.L., Larke L.E., Sallee F.R., Herman J.P., Sah R. Differential regulation of neuropeptide Y in the amygdala and prefrontal cortex during recovery from chronic variable stress. Front. Behav. Neurosci. 2011;5:54. doi: 10.3389/fnbeh.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S.H., Bersani F.S., Lindqvist D., Hammamieh R., Donohue D., Dean K., Jett M., Yehuda R., Flory J., Reus V.I., Bierer L.M., Makotkine I., Abu Amara D., Henn Haase C., Coy M., Doyle F.J., 3rd, Marmar C., Wolkowitz O.M. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PloS One. 2019;14 doi: 10.1371/journal.pone.0213839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S.H., Gautam A., Hammamieh R., Jett M., Wolkowitz O.M. Metabolism, metabolomics, and inflammation in posttraumatic stress disorder. Biol. Psychiatry. 2018;83:866–875. doi: 10.1016/j.biopsych.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S., Colquhoun H., Riesenberg R., Epperson C.N., Deligiannidis K.M., Rubinow D.R., Li H., Sankoh A.J., Clemson C., Schacterle A., Jonas J., Kanes S. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058–1070. doi: 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- Morgan C.A., 3rd, Rasmusson A.M., Winters B., Hauger R.L., Morgan J., Hazlett G., Southwick S. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol. Psychiatry. 2003;54:1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhaes P.V., Kapczinski F., Kauer-Sant'Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet. Psy. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Pineles S.L., Nillni Y.I., Pinna G., Irvine J., Webb A., Arditte Hall K.A., Hauger R., Miller M.W., Resick P.A., Orr S.P., Rasmusson A.M. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. doi: 10.1016/j.psyneuen.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Pinna G. Animal models of PTSD: the socially isolated mouse and the biomarker role of allopregnanolone. Front. Behav. Neurosci. 2019;13:114. doi: 10.3389/fnbeh.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Rasmusson A.M. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front. Cell. Neurosci. 2014;8:256. doi: 10.3389/fncel.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Uzunova V., Matsumoto K., Puia G., Mienville J.M., Costa E., Guidotti A. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J., Arzy S., Bertolus J.B., Depue B., Haas H.E., Kangas M., Kensinger E., Lowry C.A., Marusak H.A., Minnier J., Mouly A.M., Muehlberger A., Norrholm S.D., Peltonen K., Pinna G., Rabinak C., Shiban Y., Soreq H., van der Kooij M.A., Lowe L., Weingast L.T., Yamashita P., Boutros S.W. Current understanding of fear learning and memory in humans and animal models and the value of a linguistic approach for analyzing fear learning and memory in humans. Neurosci. Biobehav. Rev. 2019;105:136–177. doi: 10.1016/j.neubiorev.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Raison C.L., Miller A.H. Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. Mod. Trends Pharmacopsychiatr. 2013;28:33–48. doi: 10.1159/000343966. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M., Abdallah C. Biomarkers for treatment and diagnosis. PTSD Research Quarterly. 2015;26:1–14. [Google Scholar]
- Rasmusson A.M., Hauger R.L., Morgan C.A., Bremner J.D., Charney D.S., Southwick S.M. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol. Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M., King M.W., Valovski I., Gregor K., Scioli-Salter E., Pineles S.L., Hamouda M., Nillni Y.I., Anderson G.M., Pinna G. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104. doi: 10.1016/j.psyneuen.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson A.M., Pineles S.L. Neurotransmitter, peptide, and steroid hormone abnormalities in PTSD: biological endophenotypes relevant to treatment. Curr. Psychiatr. Rep. 2018;20:52. doi: 10.1007/s11920-018-0908-9. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M., Pinna G., Paliwal P., Weisman D., Gottschalk C., Charney D., Krystal J., Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol. Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Reijnen A., Geuze E., Eekhout I., Maihofer A.X., Nievergelt C.M., Baker D.G., Vermetten E. Biological profiling of plasma neuropeptide Y in relation to posttraumatic stress symptoms in two combat cohorts. Biol. Psychol. 2018;134:72–79. doi: 10.1016/j.biopsycho.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Sah R., Ekhator N.N., Jefferson-Wilson L., Horn P.S., Geracioti T.D., Jr. Cerebrospinal fluid neuropeptide Y in combat veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:277–283. doi: 10.1016/j.psyneuen.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R., Ekhator N.N., Strawn J.R., Sallee F.R., Baker D.G., Horn P.S., Geracioti T.D., Jr. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol. Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur R.R., Boks M.P., Geuze E., Prinsen H.C., Verhoeven-Duif N.M., Joels M., Kahn R.S., Vermetten E., Vinkers C.H. Development of psychopathology in deployed armed forces in relation to plasma GABA levels. Psychoneuroendocrinology. 2016;73:263–270. doi: 10.1016/j.psyneuen.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Scioli-Salter E., Forman D.E., Otis J.D., Tun C., Allsup K., Marx C.E., Hauger R.L., Shipherd J.C., Higgins D., Tyzik A., Rasmusson A.M. Potential neurobiological benefits of exercise in chronic pain and posttraumatic stress disorder: pilot study. J. Rehabil. Res. Dev. 2016;53:95–106. doi: 10.1682/JRRD.2014.10.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A.Y., Gevonden M., Ratanatharathorn A., Laska E., van der Mei W.F., Qi W., Lowe S., Lai B.S., Bryant R.A., Delahanty D.J.W.P. Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict. PTSD (ICPP) 2019;18:77–87. doi: 10.1002/wps.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer W.H., Lidstrom J., Sun H., Passer D., Eskay R., Parker S.C., Witt S.H., Zimmermann U.S., Nieratschker V., Rietschel M., Margulies E.H., Palkovits M., Laucht M., Heilig M. Human NPY promoter variation rs16147:T>C as a moderator of prefrontal NPY gene expression and negative affect. Hum. Mutat. 2010;31:E1594–E1608. doi: 10.1002/humu.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur G.S., Daigle B.J., Jr., Dean K.R., Zhang Y., Rodriguez-Fernandez M., Hammamieh R., Yang R., Jett M., Palma J., Petzold L.R., Doyle F.J., 3rd Systems biology approach to understanding post-traumatic stress disorder. Mol. Biosyst. 2015;11:980–993. doi: 10.1039/c4mb00404c. [DOI] [PubMed] [Google Scholar]
- Vollmer L.L., Schmeltzer S., Schurdak J., Ahlbrand R., Rush J., Dolgas C.M., Baccei M.L., Sah R. Neuropeptide Y impairs retrieval of extinguished fear and modulates excitability of neurons in the infralimbic prefrontal cortex. J. Neurosci. 2016;36:1306–1315. doi: 10.1523/JNEUROSCI.4955-13.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Keane T.M., Davidson J.R. Clinician-administered PTSD scale: a review of the first ten years of research. Depress. Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Pratchett L.C., Elmes M.W., Lehrner A., Daskalakis N.P., Koch E., Makotkine I., Flory J.D., Bierer L.M. Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus. 2014;4:20140048. doi: 10.1098/rsfs.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.