Summary
Stroke can be a cause of death, while in non‐fatal cases it is a common cause of various disabilities resulting from associated brain damage. However, whether a specific periodontal pathogen is associated with increased risk of unfavorable outcome after stroke remains unknown. We examined risk factors for unfavorable outcome following stroke occurrence, including serum antibody titers to periodontal pathogens. The enrolled cohort included 534 patients who had experienced an acute stroke, who were divided into favorable (n = 337) and unfavorable (n = 197) outcome groups according to modified ranking scale (mRS) score determined at 3 months after onset (favorable = score 0 or 1; unfavorable = score 2–6). The associations of risk factors with unfavorable outcome, including serum titers of IgG antibodies to 16 periodontal pathogens, were examined. Logistic regression analysis showed that the initial National Institutes of Health stroke scale score [odds ratio (OR) = 1·24, 95% confidence interval (CI) = 1·18–1·31, P < 0·001] and C‐reactive protein (OR = 1·29, 95% CI = 1·10–1·51, P = 0·002) were independently associated with unfavorable outcome after stroke. Following adjustment with those, detection of the antibody for Fusobacterium nucleatum ATCC 10953 in serum remained an independent predictor of unfavorable outcome (OR = 3·12, 95% CI = 1·55–6·29, P = 0·002). Determination of the antibody titer to F. nucleatum ATCC 10953 in serum may be useful as a predictor of unfavorable outcome after stroke.
Keywords: Fusobacterium nucleatum, serum IgG antibody titer, unfavorable outcome after stroke
We examined risk factors for unfavorable outcome following stroke occurrence including serum antibody titers to periodontal pathogens. The enrolled cohort included 534 patients who had experienced an acute stroke, who were divided into favorable (n = 337) and unfavorable (n = 197) outcome groups according to modified ranking scale (mRS) score determined at 3 months after onset. Logistic regression analysis showed that detection of the antibody for F. nucleatum ATCC10953 in serum were independently associated with unfavorable outcome after stroke.
![]()
Introduction
A stroke causes damage to the brain due to sudden loss of blood circulation, and its occurrence is broadly categorized into ischemic and hemorrhagic [1]. Although considered to be one of the leading causes of death, a stroke in non‐fatal cases can lead to various types of disabilities, including physical, cognitive or emotional deficiency, resulting in requirement of partial or complete assistance with performing activities of daily living [2]. Furthermore, related costs, such as home‐ and hospital‐based rehabilitation and care, can place a heavy financial burden on individuals as well as society. Therefore, elucidation of clinical methods useful for prediction of an unfavorable functional outcome in the early stage following a stroke is important.
Periodontitis, a commonly encountered chronic oral inflammatory disease caused by interactions between host immune responses and periodontal bacteria, is characterized by loss of connective tissue and alveolar bone support, leading to tooth loss [3]. Some cohort studies have shown a relationship of periodontal disease with stroke occurrence. Recently, Lafson et al. evaluated the association between periodontal disease and incidence of ischemic and hemorrhagic stroke by performing a meta‐analysis of cohort studies, and found that the risk of stroke was significantly increased in individuals with periodontitis, while tooth loss was also shown to be a risk factor [4]. Furthermore, the Atherosclerosis Risk in Communities (ARIC) cohort study of 10 362 stroke‐free participants conducted during a 15‐year follow‐up period showed that periodontal disease was associated with incidence of cardioembolic and atherothrombotic stroke, and that regular dental care may lead to a lower adjusted risk [5].
Recent studies have also examined factors other than tooth loss as indicators of periodontal disease, such as performing measurements of concentrations of serum antibodies to periodontal pathogens, as those findings are considered to more accurately reflect the disease process and have become part of established criteria to identify causative organisms [6]. Recently, serum antibody titers related to a specific periodontal pathogen were revealed to be risk factors for systemic diseases, including ischemic stroke, coronary heart disease, non‐alcoholic fatty liver disease and Alzheimer’s disease [7, 8, 9, 10]. However, it remains unknown whether a serum antibody titer related to a specific periodontal pathogen is associated with increased risk of an unfavorable outcome after stroke. To determine the correlation of specific periodontal pathogens, we examined the relationships between unfavorable outcomes following stroke and associated risk factors, including serum titers of IgG antibodies to several different periodontal pathogens.
Materials and methods
Subjects
We enrolled acute stroke patients, diagnosed as ischemic or hemorrhagic, who had undergone treatment at Hiroshima University Hospital or Suiseikai Kajikawa Hospital from January 2013 to April 2016. The study design was approved by the Ethical Committee of Hiroshima University (Permission no. Epd‐614‐2) and Suiseikai Kajikawa Hospital (Permission no. 2015‐03), and each participant signed an informed consent agreement. All examinations were performed in accordance with relevant guidelines and regulations. Analysis of computed tomography or magnetic resonance imaging results of all patients was performed to determine a diagnosis of ischemic or hemorrhagic stroke. Patients who were disabled prior to stroke incidence corresponding to a premorbid modified Rankin scale (mRS) score ≥ 2 were excluded from analysis, while those with a premorbid mRS score of 0 or 1 were included as subjects. Favorable stroke outcome was defined as independence after 3 months, corresponding to an mRS score of 0 and 1, and an unfavorable outcome as an mRS score of 2–6 [11]. The study cohort included a total of 534 patients (mean age = 71·1 years, 57·1% male). The total sample size required for accurate unpaired t‐test, Mann–Whitney U‐test, χ2 test and Fisher’s exact test results, obtained using the G*Power software package (version 3.1.9.4; Heinrich‐Heine‐Universität, Düsseldorf, Germany), was calculated to be 278, 290, 88 and 100 subjects, respectively, with a statistical power of 80%, significance level 5% and effect size 0·3. Baseline clinical characteristic data, including gender, age, smoking status, alcohol drinking status and comorbidities (hypertension, diabetes, hyperlipidemia, atrial fibrillation, peripheral arterial disease, congestive heart failure), were obtained for all enrolled patients. Ischemic stroke subtypes were classified using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [12] by stroke specialists. Hemorrhagic infarction and trauma‐induced hemorrhage were excluded from intracerebral hemorrhage. Stroke severity upon admission was evaluated based on the National Institutes of Health Stroke Scale (NIHSS) score. Hypertension was defined as use of anti‐hypertensive medication before admission or confirmed blood pressure of ≥ 140/90 mmHg at rest when measured 2 weeks after onset. Diabetes mellitus was defined as a glycated hemoglobin level ≥ 6·5%, fasting blood glucose level ≥ 126 mg/dl or use of anti‐diabetes medication. Dyslipidemia was defined as total cholesterol level ≥ 220 mg/dl, low‐density lipoprotein cholesterol level ≥ 140 mg/dl, high‐density lipoprotein cholesterol level < 40 mg/dl, triglyceride level ≥ 150 mg/dl or use of anti‐hyperlipidemia medication. Atrial fibrillation was defined as history of sustained or paroxysmal atrial fibrillation, or atrial fibrillation detection upon arrival or during admission. Diagnosis of ischemic heart disease, peripheral arterial disease and congestive heart failure was determined by attending physicians. Blood samples were collected from all patients within 24 h of admission and C‐reactive protein (CRP) concentrations were measured using nephelometry.
Determination of serum immunoglobulin (Ig)G antibody titers to periodontal pathogens
Serum IgG antibody titers to periodontal pathogens were determined using an enzyme‐linked immunosorbent assay (ELISA), as previously described in detail [13]. Briefly, serum samples were collected from patients within 3 days after stroke occurrence and stored at −80°C. Sonicated preparations of the following periodontal pathogens were used as bacterial antigens in the present study: Porphyromonas gingivalis ATCC33277 (fimA type I), HW24D1 (fimA type II), 6/26 (fimA type III), W83 (fimA type IV) and HNA99 (fimA type V); Aggregatibacter actinomycetemcomitans ATCC29523 (serotype a), Y4 (serotype b) and AUNY67 (serotype c); Prevotella intermedia ATCC26511, P. nigrescens ATCC33563; Fusobacterium nucleatum ATCC25586 (subspecies nucleatum) and ATCC 10953 (subspecies polymorphum); Treponema denticola ATCC35405; Tannerella forsythensis ATCC43037; Campylobacter rectus ATCC33238; and Eikenella corrodens ATCC23834. Serum samples from five healthy subjects were also obtained and pooled, and used for calibration. Bacterial antigen‐coated wells were washed with phosphate‐buffered saline with Tween (PBST), then serum samples in PBST were added to the wells. After incubation at 4°C overnight, the wells were washed with PBST, then filled with alkaline phosphatase‐conjugated goat anti‐human IgG (gamma‐chain specific; Abcam, Cambridge, MA, USA) in PBST. After another incubation at 37°C for 2 h, the wells were again washed with PBST, then an aliquot of p‐nitrophenylphosphate at 1 mg/m; (Wako Pure Chemical Industries Ltd., Osaka, Japan) in 10% diethanolamine buffer was added to each well as a substrate and incubation was performed at 37°C for 30 min. Optical density at 405 nm was measured using a microplate reader (iMark; Bio‐Rad Laboratories Inc., Hercules, CA, USA). Values ≥ 1 were considered to represent more than 2 standard deviations of the mean of the controls and defined as antibody‐positive. Absolute serum antibody measurements were used to categorize the samples as positive or negative [8,9].
Statistical analysis
Statistical analysis was performed using the spss software package, version 24.0 (SPSS Inc., Chicago, IL, USA). Values are expressed as the mean ± standard deviation or median (minimum, maximum) for continuous variables, and as frequencies and percentages for discrete variables. Univariate analysis was performed to evaluate differences between the groups regarding baseline characteristics, risk factors, levels of serum CRP and serum IgG antibody titers to periodontal pathogens. Comparisons between the groups were made using an unpaired t‐test or Mann‐Whitney U‐test for continuous variables, and Fisher’s exact test or a χ2 test for discrete variables. Subsequently, to assess factors that may be associated with an unfavorable outcome, multivariate logistic regression analysis was performed using variables that showed a trend in univariate analysis (P < 0·2) with a forced‐entry method. All analyses were two‐tailed and a value of P < 0·05 was considered to indicate statistical significance.
Results
A total of 664 patients who had experienced an acute stroke were initially registered in this study, 130 of whom with a premorbid mRS score ≥ 2 were excluded from analysis. The stroke subtype of the 534 analyzed patients (mean age = 71·1 years, 57·1% male) was cerebral infarction in 447 (large vessel disease 88, cardioembolic disease 102, small vessel disease 92, other determined cause 20, undetermined cause 145) and cerebral hemorrhage in 87 patients. Subsequently, the 534 patients were categorized into favorable (n = 337) and unfavorable (n = 197) outcome groups according to the mRS score at 3 months after onset (Fig. 1). The initial clinical characteristics were compared between the groups (Table 1), which revealed that age, stroke subtype, initial NIHSS score and CRP level were significantly higher in the unfavorable group (P < 0·001) (Table 2). Additionally, patients with atrial fibrillation and congestive heart failure were more frequently found in the unfavorable group (P < 0·001, P = 0·01, respectively), whereas those with dyslipidemia were more frequently found in the favorable group (P = 0·048).
Fig. 1.
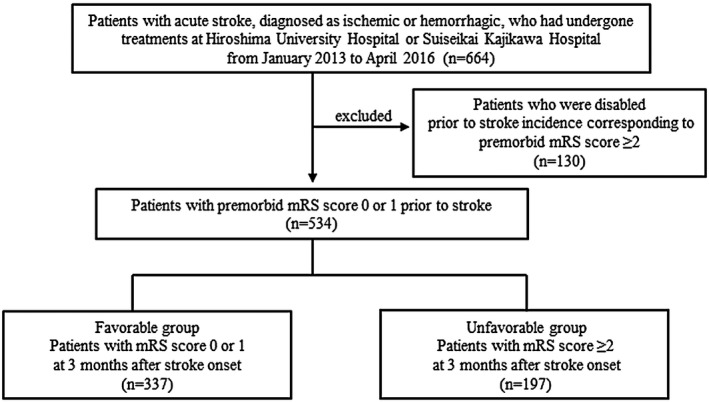
Flow‐chart showing patient selection.
Table 1.
Baseline clinical characteristics of patients in this study
| Clinical parameter n = 534 | n = 534 |
|---|---|
| Age, years | 71·1 ± 12·4 |
| Gender, male/female | 305/229 |
| BMI, kg/m2 | 23·1 ± 4·13 |
| Stroke subtype, ischemia/hemorrhage | 447/87 |
| NIHSS score, median (IQR) | 3 (1–7·5) |
| Smoking status, n (%) | |
| Never | 306 (57·3) |
| Past | 100 (18·7) |
| Current | 124 (23·2) |
| Alcohol status, n (%) | |
| No | 274 (51·3) |
| Occasionally | 109 (20·4) |
| Daily | 128 (24·0) |
| Hypertension, n (%) | 401 (75·1) |
| Diabetes mellitus, n (%) | 129 (24·2) |
| Dyslipidemia, n (%) | 227 (42·5) |
| Atrial fibrillation, n (%) | 93 (17·4) |
| Ischemic heart disease, n (%) | 43 (8·1) |
| Peripheral arterial disease, n (%) | 18 (3·4) |
| Congestive heart failure, n (%) | 21 (39) |
| CRP, mg/l, median (IQR) | 0·11 (0·04–0·36) |
NIHSS = National Institutes of Health Stroke Scale; IQR = interquartile range; CRP = C‐reactive protein; BMI = body mass index.
Table 2.
Comparison of clinical characteristics between favorable and unfavorable groups
| Clinical parameter | Favorable (n = 337) | Unfavorable (n = 197) | P‐value |
|---|---|---|---|
| Age, years | 69·4 ± 12·2 | 74·1 ± 12·5 | < 0·001* |
| Gender, male/female | 201/136 | 104/93 | 0·12 |
| BMI, kg/m2 | 23·3 ± 4·1 | 22·6 ± 9·2 | 0·03 |
| Stroke subtype, ischemia/hemorrhage | 302/35 | 145/52 | <0·001* |
| NIHSS score, median (IQR) | 2 (1–3) | 9·5 (4–18) | < 0·001* |
| Smoking status, n (%) | 0·39 | ||
| Never | 192 (57) | 114 (57·9) | |
| Past | 69 (20·5) | 31 (15·7) | |
| Current | 75 (22·3) | 49 (24·9) | |
| Alcohol status, n (%) | 0·12 | ||
| No | 175 (51·9) | 99 (50·3) | |
| Occasionally | 65 (19·3) | 44 (22·3) | |
| Daily | 92 (27·3) | 36 (18·3) | |
| Hypertension, n (%) | 246 (73·0) | 155 (78·7) | 0·16 |
| Diabetes mellitus, n (%) | 79 (23·4) | 50 (25·4) | 0·63 |
| Dyslipidemia, n (%) | 154 (45·7) | 73 (37·1) | 0·048* |
| Atrial fibrillation, n (%) | 40 (18·8) | 53 (26·9) | < 0·001* |
| Ischemic heart disease, n (%) | 24 (7·1) | 19 (9·6) | 0·31 |
| Peripheral arterial disease, n (%) | 9 (2·7) | 9 (4·7) | 0·25 |
| Congestive heart failure, n (%) | 6 (1·8) | 15 (7·6) | 0·01* |
| CRP, mg/l, median (IQR) | 0·09 (0·03–0·23) | 0·2 (0·06–0·81) | < 0·001* |
NIHSS = National Institutes of Health Stroke Scale; IQR = interquartile range; CRP = C‐reactive protein; BMI = body mass index.
P < 0·05 (statistically significant).
Next, the frequency of positive findings for serum IgG antibodies to periodontal pathogens were compared between the groups (Table 3). Antibodies to A. actinomycetemcomitans AUNY67 (serotype c), F. nucleatum ATCC25586 (subspecies nucleatum), F. nucleatum ATCC10953 (subspecies polymorphum) and C. rectus ATCC33238 were more frequently detected in the unfavorable compared with the favorable group, while detection of antibodies to the other examined periodontal pathogens was not significantly different between the groups.
Table 3.
Detection of serum IgG antibody titers to periodontal pathogens in favorable and unfavorable groups after stroke
| Periodontal pathogen | Positive for periodontal pathogen, n (%) | ||
|---|---|---|---|
| Favorable (n = 337) | Unfavorable (n = 197) | P‐value | |
| P. gingivalis ATCC33277 (fimA type I) | 163 (48·4) | 95 (48·2) | 0·96 |
| P. gingivalis HW24D1 (fimA type II) | 141 (41·8) | 90 (45·7) | 0·39 |
| P. gingivalis 6/26 (fimA type III) | 200 (59·3) | 117 (59·4) | 0·99 |
| P. gingivalis W83 (fimA type IV) | 148 (43·9) | 87 (44·1) | 0·96 |
| P. gingivalis HNA99 (fimA type V) | 191 (56·7) | 111 (56·3) | 0·94 |
| A. actinomycetemcomitans ATCC29523 (Serotype a) | 61 (18·1) | 45 (22·8) | 0·19 |
| A. actinomycetemcomitans Y4 (serotype b) | 100 (29·7) | 69 (35·0) | 0·20 |
| A. actinomycetemcomitans AUNY67 (Serotype c) | 87 (25·8) | 67 (34·0) | 0·044* |
| P. intermedia ATCC26511 | 92 (27·3) | 64 (32·5) | 0·25 |
| P. nigrescens ATCC33563 | 55 (16·3) | 40 (20·3) | 0·24 |
| F. nucleatum ATCC25586 | 50 (14·8) | 64 (32·5) | < 0·001* |
| F. nucleatum ATCC10953 | 26 (7·7) | 44 (22·3) | < 0·001* |
| T. denticola ATCC35405 | 20 (5·9) | 18 (9·1) | 0·17 |
| T. forsythensis ATCC43037 | 35 (10·4) | 21 (10·7) | 0·92 |
| C. rectus ATCC33238 | 76 (22·6) | 61 (31·0) | 0·032* |
| E. corrodens ATCC23834 | 46 (13·6) | 38 (19·3) | 0·08 |
P < 0·05 (statistically significant).
Variables with a P‐value < 0·20 in univariate analysis were entered into multivariate logistic regression analysis using a forced‐entry method (Table 4). Those results showed that initial NIHSS score [odds ratio (OR) = 1·24, 95% confidence interval (CI) = 1·18–1·31; P < 0·001] and CRP (OR = 1·29, 95% CI = 1·10–1·51; P = 0·002) were independently associated with unfavorable outcome after stroke. We also found that detection of the serum IgG antibody to F. nucleatum ATCC 10953 was an independent predictor of unfavorable outcome following stroke (OR = 3·12, 95% CI = 1·55–6·29; P = 0·002).
Table 4.
Multivariate logistic regression analysis to identify predictive factors for unfavorable outcome following stroke
| Risk factor | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Age | 1·02 | 1·00–1·05 | 0·07 |
| Gender | 0·93 | 0·54–1·58 | 0·08 |
| BMI | 0·97 | 0·91–1·04 | 0·42 |
| Stroke subtype, ischemia/hemorrhage | 1·90 | 0·97–3·75 | 0·06 |
| NIHSS score | 1·24 | 1·18–1·31 | < 0·001* |
| Alcohol status | 0·85 | 0·62–1·16 | 0·31 |
| Hypertension | 1·21 | 0·67–2·17 | 0·53 |
| Dyslipidemia | 0·89 | 0·53–1·48 | 0·65 |
| Atrial fibrillation | 1·05 | 0·53–2·09 | 0·89 |
| Congestive heart failure | 2·06 | 0·57–7·46 | 0·27 |
| CRP | 1·29 | 1·10–1·51 | 0·002* |
| P. gingivalis ATCC33277 (fimA type I) | 0·73 | 0·44–1·20 | 0·22 |
| A. actinomycetemcomitans ATCC29523 (serotype a) | 0·63 | 0·31–1·29 | 0·20 |
| A. actinomycetemcomitans Y4 (serotype b) | 1·04 | 0·55–1·99 | 0·90 |
| A. actinomycetemcomitans AUNY67 (serotype c) | 1·21 | 0·62–2·36 | 0·57 |
| F. nucleatum ATCC25586 | 1·63 | 0·87–3·03 | 0·13 |
| F. nucleatum ATCC10953 | 3·12 | 1·55–6·29 | 0·002* |
| T. denticola ATCC35405 | 1·16 | 0·46–2·93 | 0·75 |
| C. rectus ATCC33238 | 1·45 | 0·80–2·64 | 0·22 |
| E. corrodens ATCC23834 | 0·98 | 0·47–2·06 | 0·97 |
Based on 20 factors included for analysis with P‐values < 0·2 shown by univariate Fisher’s exact, χ2 or univariate logistic regression test results. *P < 0·05 (statistically significant in multivariate logistic regression via forced‐entry method). CI = confidence interval; BMI = body mass index.
Discussion
Although stroke is a leading cause of death, the rates of mortality of affected individuals have recently been decreasing due to development of advanced medical treatments, which, along with the ageing of society, has resulted in greater numbers who survive such an occurrence [14]. A study conducted in 2010 estimated that there were 50 million stroke survivors throughout the world at that time who showed various levels of functional disability, with 25–74% requiring some level of assistance or fully dependent on caregivers for activities of daily living [15]. NIHSS score has been shown to be associated with overall functional outcome in patients who have had a stroke [12], while a derivation cohort study conducted in Japan reported that specific NIHSS items, including leg weakness, intra‐ and extracranial vascular imaging abnormalities, advanced age and female gender, were associated with an unfavorable outcome following a minor ischemic stroke [16]. Additionally, C‐reactive protein (CRP), a sensitive inflammatory marker, has been reported to be associated with increased risk of stroke, with higher CRP level shown to be an independent predictor of survival after ischemic stroke and functional outcome after thrombolytic stroke [17, 18]. Similar to those previous studies, the present findings revealed that initial NIHSS score and CRP concentration had a dependent association with unfavorable outcome following a stroke in our patients.
Hypertension and smoking status are well‐established risk factors for stroke occurrence, with a strong association between those and stroke onset shown in many studies, and also reflected in presented guidelines [19, 20]. However, whether these factors are associated with unfavorable outcome after stroke remains unknown. Mahmoud et al. examined predictors of clinical outcome after 3 months in ischemic stroke patients and found that cardiovascular disease was associated with an unfavorable outcome, while hypertension status was not different between the unfavorable and favorable outcome groups [21]. Additionally, it has been reported that blood pressure management following an acute ischemic stroke is associated with clinical outcome [22]. Regarding smoking, its impact on unfavorable outcome is controversial. Smoking status in stroke patients was reported to be a predictor of good clinical outcome after intravenous thrombolysis (IVT) [23], although those beneficial effects of smoking after IVT and endovascular treatment were later shown to be mainly related to differences in baseline characteristics and the design of that observational study [24, 25]. In the present study, neither hypertension nor smoking status had a significant relationship with unfavorable or favorable outcome after stroke occurrence. It seems that consensus has not been reached regarding the relationship of unfavorable outcome following a stroke and these factors.
P. gingivalis has been identified as one of the main pathogens responsible for progression of periodontitis [26] and its fimbriae are considered to be an important virulence factor [27]. Furthermore, that bacterium has been classified into six genotypes (types I–V, Ib) based on the nucleotide sequence of the fimA gene encoding fimbriae [28]. Some investigators have reported that the risk of systemic disease is associated with an increased serum IgG antibody titer to P. gingivalis. Seror et al. noted that antibody titers for P. gingivalis were associated with occurrence of more severe rheumatoid arthritis in non‐smokers in a large cohort of patients with early rheumatoid arthritis [29]. Also, Yamazaki et al. found a high frequency of detection of the antibody for P. gingivalis Su63 (fim A type II), but not for FDC381 (fim A type I), in coronary heart disease patients [8]. In addition, Nakahara et al. reported a significant correlation between fibrosis progression in non‐alcoholic fatty liver disease patients and antibody titers for an fimA type IV strain of P. gingivalis [9]. In the present study, detection of antibody IgG titers for four different strains of P. gingivalis (fimA type I‐V) was not significantly different between the favorable and unfavorable outcome groups. Therefore, the presence of antibodies for P. gingivalis, a major periodontal pathogen associated with incidence of various severe systemic diseases, might not necessarily indicate increased risk of an unfavorable outcome following stroke.
F. nucleatum, a strictly anaerobic Gram‐negative rod bacterium normally found in the oral cavity [30], is considered to be a periodontal pathogen because it is frequently isolated from lesions, produces high numbers of tissue irritants and often aggregates with other periodontal pathogens as a bridge between early and late colonizers [30, 31]. In the present study, detection of serum IgG antibody titers for F. nucleatum ATCC 10953 was independently associated with unfavorable outcome following stroke. Sparks et al. examined serum IgG antibody levels of seven periodontal pathogens, including P. gingivalis and A. actinomycetemcomitans, in patients who eventually converted to Alzheimer’s disease (AD), and found that the titers of F. nucleatum and P. intermedia antibodies were significantly increased at baseline in patients who developed Alzheimer’s disease compared with the control group [10]. F. nucleatum is able to pass through the blood–brain barrier and has been found to be causative of brain abscesses in some case studies [32, 33], while it also has abilities to adhere to and invade host vascular endothelial cells via FadA adhesin molecules, as FadA binds to vascular endothelial–cadherin on the cell surface, which triggers breakdown of endothelial cell‐to‐cell junctions [34]. Additionally, F. nucleatum has been shown to be associated with portal vein thrombosis in some cases and may have inherent thrombogenic properties [35, 36]. Chang et al. reported unusual cases of cavernous sinus thrombosis associated with F. nucleatum and noted that those patients eventually developed ischemic stroke despite undergoing immediate antibiotic therapy [37]. Following passage through the blood–brain barrier, F. nucleatum organisms attack vascular endothelial cells in blood vessels in the brain, which can induce endothelial permeability via loosened cell junctions. Also, F. nucleatum is related to an unusual form of thrombosis, which may lead to rebleeding after hemorrhage and insufficient recanalization after infarction, thus promoting an unfavorable prognosis following onset in affected patients.
The virulence of F. nucleatum is mediated by endotoxin activity, hemagglutination, co‐aggregation and outer membrane proteins, such as FAP‐2 and RadD [38, 39], while it has also been reported to be induced by death of immune cells [40]. Conversely, this organism causes increased expression of genes associated with host immune responses, such as inflammatory chemokines and cytokines [41]. Some investigators have reported that the virulence and impact of the host immune response differs among F. nucleatum strains. Kugan et al. examined various strains, including ATCC 25586 and ATCC 10953, with regard to apoptosis, phagocytosis, superoxide generation and proinflammatory cytokine release by neutrophils, and found an F. nucleatum strain‐specific impact on neutrophil function [42]. Conversely, Bhattacharyya et al. noted that expression of mRNA of β‐defensin‐2 (hBD‐2), an anti‐microbial peptide, in oral epithelial cells was induced to a greater degree by live cells or cell walls of F. nucleatum strain ATCC 25586 compared to those of ATCC 10953, and that differences regarding the amino acid residue of FAD‐1 in those strains had effects on regulation of hBD‐2 expression [43]. In the present study, antibody titers for F. nucleatum ATCC 25586 and ATCC 10953 were significantly greater in the unfavorable group following stroke in univariate analysis, while multivariate analysis revealed that detection of the serum IgG antibody to F. nucleatum ATCC 10953 was an independent predictor of an unfavorable outcome. Differences regarding virulence and host immune responses among F. nucleatum strains may have varying impacts on unfavorable outcome after stroke.
This study has some limitations. First, we could not accurately evaluate the presence of oral periodontal bacteria in blood, as those organisms are often not detected because an inadequate number of certain bacterial types, such as anaerobic periodontal bacteria, remain alive during blood culture examinations [44]. Secondly, we were not able to evaluate the oral periodontal examination results in a comprehensive manner using indicators such as probing pocket depth. A third limitation is that IgG antibody titer may reflect either current or previous exposure of the host to periodontal pathogens, although previous reports have noted relationships of serum IgG antibody titers of periodontal pathogens with the severity of periodontitis and deep pockets [45]. Furthermore, periodontal treatments decrease serum IgG antibody titers of periodontal pathogens [45, 46]; thus, titer levels seem to reflect the current disease status of individuals with chronic periodontitis. Finally, the present results are limited by use of multiple comparisons. For this study, 20 factors regarding unfavorable outcome after stroke shown to have a P‐value < 0·20 in univariate analysis were subjected to multivariate logistic regression analysis. Although it is possible that such analysis can be affected by chance when several variable factors are examined together, we found that an unfavorable outcome after stroke was associated with detection of a serum IgG antibody titer to F. nucleatum ATCC 10953, as well as NIHSS score and CRP.
In summary, this study is the first to demonstrate that detection of a serum IgG antibody titer to F. nucleatum ATCC 10953 is independently associated with unfavorable outcome after stroke. We consider that such detection following stroke occurrence may be useful for predicting patient outcome.
Disclosures
The authors have no potential conflicts of interest to declare with respect to authorship and/or publication of this article.
Author contributions
H. N., N. H., K. O. and H. K. conceived the study and designed the protocol. H. N., K. O., T. S., T. O. and M. S. performed the experiments. H. N., N. H., H. S. and S. A. analyzed the data obtained. K. I., M. N., T. N., N. K. and Y. S. collected samples. H. O., H. M., H. K. and H. K. supervised the project. H. N., N. H. and K. O. wrote the manuscript. All authors have reviewed the final version of the manuscript and approved its submission for publishing.
Acknowledgements
We appreciate the expert advice from Tsuyoshi Miyagawa (Center for Integrated Medical Research, Hiroshima University Hospital, Japan). This work was supported by a Grant‐in‐aid for young scientists (B) (No. 17K17350, 17K17907) and a Grant‐in‐aid for Scientific Research (C) (No. 15K08615, 17K11839, 18K10746) from the Japanese Ministry of Education, Culture, Sports, and Technology.
References
- 1. Fisher M. The challenge of mixed cerebrovascular disease. Ann NY Acad Sci 2010; 207:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Langhorne P, Stott DJ, Robertson L et al Medical complications after stroke: a multicenter study. Stroke 2000; 31:1223–9. [DOI] [PubMed] [Google Scholar]
- 3. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366:1809–20. [DOI] [PubMed] [Google Scholar]
- 4. Lafon A, Pereira B, Dufour T et al Periodontal disease and stroke: a meta‐analysis of cohort studies. Eur J Neurol 2014; 21:1155–61. [DOI] [PubMed] [Google Scholar]
- 5. Sen S, Giamberardino LD, Moss K et al Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke 2018; 49:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dye BA, Herrera‐Abreu M, Lerche‐Sehm J et al Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol 2009; 80:634–47. [DOI] [PubMed] [Google Scholar]
- 7. Hosomi N, Aoki S, Matsuo K et al Association of serum anti‐periodontal pathogen antibody with ischemic stroke. Cerebrovasc Dis 2012; 34:385–92. [DOI] [PubMed] [Google Scholar]
- 8. Yamazaki K, Honda T, Domon H et al Relationship of periodontal infection to serum antibody levels to periodontopathic bacteria and inflammatory markers in periodontitis patients with coronary heart disease. Clin Exp Immunol 2007; 149:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakahara T, Hyogo H, Ono A et al Involvement of Porphyromonas gingivalis in the progression of non‐alcoholic fatty liver disease. J Gastroenterol 2018; 53:269–80. [DOI] [PubMed] [Google Scholar]
- 10. Sparks Stein P, Steffen MJ, Smith C et al Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement 2012; 8:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tu WJ, Qiu HC, Zhang Y et al Lower serum retinoic acid level for prediction of higher risk of mortality in ischemic stroke. Neurology 2019; 92:e1678–87. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP Jr, Bendixen BH, Kappelle LJ et al Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993; 24:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Murayama Y, Nagai A, Okamura K et al Serum immunoglobulin G antibody to periodontal bacteria. Adv Dent Res 1988; 2:339–45. [DOI] [PubMed] [Google Scholar]
- 14. Go AS, Mozaffarian D, Roger VL et al Executive summary: heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 2014; 129:399–410. [DOI] [PubMed] [Google Scholar]
- 15. Miller EL, Murray L, Richards L et al Comprehensive overview of nursing andinterdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke 2010; 41:2402–48. [DOI] [PubMed] [Google Scholar]
- 16. Sato S, Uehara T, Ohara T, Suzuki R, Toyoda K, Minematsu K. Factors associated with unfavorable outcome in minor ischemic stroke. Neurology 2014; 83:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muir KW, Weir CJ, Alwan W, Squire IB, Lees KR. C‐reactive protein and outcome after ischemic stroke. Stroke 1999; 30:981–5. [DOI] [PubMed] [Google Scholar]
- 18. Rocco A, Ringleb PA, Grittner U, Nolte CH, Schneider A, Nagel S. Follow‐up C‐reactive protein level is more strongly associated with outcome in stroke patients than admission levels. Neurol Sci 2015; 36:2235–41. [DOI] [PubMed] [Google Scholar]
- 19. Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiol 2019;14:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther 2010; 8:917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fouad MM, Farag SM, Hegazy MI, Aziz MA. Prediction of functional outcome in ischemic stroke patients: an observational study on Egyptian population. Neurology 2012; 79:1440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ntaios G, Lambrou D, Michel P. Blood pressure changes in acute ischemic stroke and outcome with respect to stroke etiology. Neurology 2012; 79:1440–8. [DOI] [PubMed] [Google Scholar]
- 23. Kvistad CE, Oeygarden H, Logallo N, Thomassen L, Waje‐Andreassen U, Naess H. Is smoking associated with favourable outcome in tPA‐treated stroke patients? Acta Neurol Scand 2014; 130:299–304. [DOI] [PubMed] [Google Scholar]
- 24. Kurmann R, Engelter ST, Michel P et al Impact of smoking on clinical outcome and recanalization after intravenous thrombolysis for stroke: multicenter cohort study. Stroke 2018; 49:1170–5. [DOI] [PubMed] [Google Scholar]
- 25. Gralla J, Mordasini P, El Koussy M et al Impact of smoking on stroke outcome after endovascular treatment. PLoS One 2018; 13:e0194652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang HW, Huang YF, Chou MY. Occurrence of Porphyromonas gingivalis and Tannerella forsythensis in periodontally diseased and healthy subjects. J Periodontol 2004;75:1077–83. [DOI] [PubMed] [Google Scholar]
- 27. Hamada S, Amano A, Kimura S, Nakagawa I, Kawabata S, Morisaki I. The importance of fimbriae in the virulence and ecology of some oral bacteria. Oral Microbiol Immunol 1998; 13:129–38. [DOI] [PubMed] [Google Scholar]
- 28. Dickinson DP, Kubiniec MA, Yoshimura F, Genco RJ. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis . J Bacteriol 1988; 170:1658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seror R, Le Gall‐David S, Bonnaure‐Mallet M et al Association of anti‐Porphyromonas gingivalis antibody titers with nonsmoking status in early rheumatoid arthritis: results from the prospective French cohort of patients with early rheumatoid arthritis. Arthritis Rheumatol 2015; 67:1729–37. [DOI] [PubMed] [Google Scholar]
- 30. Han YW. Fusobacterium nucleatum: a commensal‐turned pathogen. Curr Opin Microbiol 2015; 23:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feuille F, Ebersole JL, Kesavalu L, Stepfen MJ, Holt SC. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergistic effects on virulence. Infect Immun 1996; 64:2094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heckmann JG, Lang CJ, Hartl H, Tomandl B. Multiple brain abscesses caused by Fusobacterium nucleatum treated conservatively. Can J Neurol Sci 2003; 30:266–8. [DOI] [PubMed] [Google Scholar]
- 33. Kai A, Cooke F, Antoun N, Siddharthan C, Sule O. A rare presentation of ventriculitis and brain abscess caused by Fusobacterium nucleatum . J Med Microbiol 2008; 57:668–71. [DOI] [PubMed] [Google Scholar]
- 34. Fardini Y, Wang X, Témoin S et al Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol Microbiol 2011; 82:1468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bultink IE, Dorigo‐Zetsma JW, Koopman MG, Kuijper EJ. Fusobacterium nucleatum septicemia and portal vein thrombosis. Clin Infect Dis 1999; 28:1325–6. [DOI] [PubMed] [Google Scholar]
- 36. El Braks R, Harnois F, Boutros N et al Mesenteric adenitis and portal vein thrombosis due to Fusobacterium nucleatum . Eur J Gastroenterol Hepatol 2004; 16:1063–6. [DOI] [PubMed] [Google Scholar]
- 37. Chang CS, Liou CW, Huang CC, Lui CC, Chang KC. Cavernous sinus thrombosis and cerebral infarction caused by Fusobacterium nucleatum infection. Chang Gung Med J 2004; 27:459–63. [PubMed] [Google Scholar]
- 38. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum . Clin Microbiol Rev 1996; 9:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaplan CW, Ma X, Paranjpe A et al Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun 2010; 78:4773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huynh T, Kapur RV, Kaplan CW et al The role of aggregation in Fusobacterium nucleatum‐induced immune cell death. J Endod 2011; 37:1531–5. [DOI] [PubMed] [Google Scholar]
- 41. Signat B, Roques C, Poulet P, Duffaut D. Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol 2011; 13:25–36. [PubMed] [Google Scholar]
- 42. Kurgan Ş, Kansal S, Nguyen D et al Strain‐specific impact of Fusobacterium nucleatum on neutrophil function. J Periodontol 2017; 88:380–9. [DOI] [PubMed] [Google Scholar]
- 43. Bhattacharyya S, Ghosh SK, Shokeen B et al FAD‐I, a Fusobacterium nucleatum cell wall‐associated diacylated lipoprotein that mediates human beta defensin 2 induction through Toll‐like receptor‐1/2 (TLR‐1/2) and TLR‐2/6. Infect Immun 2016; 84:1446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoen B, Selton‐Suty C, Lacassin F et al Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one‐year nationwide survey in France. Clin Infect Dis 1995; 20:501–6. [DOI] [PubMed] [Google Scholar]
- 45. Kudo C, Naruishi K, Maeda H et al Assessment of the plasma/serum IgG test to screen for periodontitis. J Dent Res 2012; 91:1190–5. [DOI] [PubMed] [Google Scholar]
- 46. Sugi N, Naruishi K, Kudo C et al Prognosis of periodontitis recurrence after intensive periodontal treatment using examination of serum IgG antibody titer against periodontal bacteria. J Clin Lab Anal 2011; 25:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


