Abstract
A novel endophytic actinomycete strain GKU 173T isolated from the roots of Acacia mangium Willd. showed potential plant growth promoting (PGP) activity. Phylogenetic analysis, based on 16S rRNA gene, indicated that strain GKU 173T was the most closely related to Fodinicola feengrottensis HKI 0501T—the only species in the genus Fodinicola. Morphology and chemotaxonomy of strain GKU 173T indicated that it belongs to the genus Fodinicola by having meso-diaminopimelic acid in the cell wall and xylose as the characteristic cell-wall sugars. The cellular fatty acid profile mainly comprised iso-C16:0, anteiso-C17:0, iso-C18:0, and iso-C17:0. The major menaquinones were MK-9(H4), MK-9(H6), and MK-9(H8). The main polar phospholipids contained diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), and phosphatidylinositol (PI). Genome analysis signified DNA G+C content of 67.81 mol%. The level of digital DNA-DNA relatedness between strain GKU 173T and the type strain was 21.30%. On the basis of polyphasic characteristics, strain GKU 173T clearly represents a novel species of the genus Fodinicola, for which the name Fodinicola acaciae sp. nov. (= TBRC 10620T = NBRC 114213T) is proposed. Furthermore, genome analysis of both strains suggested that members of the genus Fodinicola are promising sources of beneficial PGP-actinomycetes and novel secondary metabolites.
Keywords: endophytic actinomycete, new species, Fodinicola, genome analysis
1. Introduction
Endophytic actinomycetes colonize the internal plant tissues and usually have a beneficial effect to the host plant by promoting growth and protecting the plant from biotic and abiotic stresses without any damage or morphological changes to the plant [1,2]. Isolation and identification of novel genus and species of endophytic actinomycetes are so far attractive since these organisms are potential sources for plant growth-promoting (PGP) bacteria. Various PGP activity, including production of auxin to enhance plant growth, siderophores to chelate iron and other elements, 1-aminocyclopropane-1-carboxylate (ACC) deaminase to reduce plant stress ethylene, and solubilization of inorganic phosphate, were identified from several endophytic actinomycetes [3,4,5]. Reports evidencing that those endophytic actinomycetes directly improve and promote plant growth and protect plants against pathogens and abiotic stresses have been gradually increasing [5,6,7]. Furthermore, endophytic actinomycetes are recognized for their capability to produce new antimicrobial metabolites [8]. Recent genome mining of actinomycetes has revealed a remarkably large number of secondary metabolite biosynthetic gene clusters (BGC) [9], including substantial numbers of silent/cryptic BGCs that are potential sources for novel bioactive compounds which have not been detected under conventional method [10].
The genus Fodinicola was first proposed by Carlsohn et al. [11] and is a member in the family Cryptosporangiaceae [12] within the order Cryptosporangiales [13]. Member of the genus only comprises a single species, Fodinicola feengrottensis HKI 0501T, which was isolated from acidic metal-containing rocks of a medieval alum slate mine in Germany [11]. Generally, the species of this genus produce branched substrate mycelium and irregular rod-like fragmented aerial hyphae. The genus is characterized chemotaxonomically by the presence of meso-diaminopimelic acid in the cell wall, and xylose as the diagnostic cell-wall sugars. The predominant menaquinones are MK-9(H4), MK-9(H6) and MK-9(H8). The polar lipids comprise diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol. Phylogenetically, the genus is closely affiliated to the genus Cryptosporangium [14]. In this work, a new species of endophytic actinomycete belonging to the genus Fodinicola was described by polyphasic taxonomy. Furthermore, the whole genome sequences of the novel species and the single member of the genus Fodinicola were investigated for PGP related genes and specialized BGCs.
2. Material and Methods
2.1. Isolation and Culture Conditions
The roots of a black wattle tree (Acacia mangium Willd.), collected at Kasetsart University, Bangkok, Thailand (13°50′34″ N, 100°34′30″ E), were surface-sterilized following the previously described method [15]. Colonies were appeared on starch-casein agar [16] supplemented with ketocanozol (50 µg/mL), nalidixic acid (25 µg/mL), and nystatin (50 µg/mL) after incubation at 28 °C for two weeks. A single colony was purified and cultured on mannitol-soybean (MS) agar [17] and maintained in 20% (v/v) glycerol at ‒80 °C.
2.2. Characterization of Plant Growth-Promoting Traits
Indole-3-acetic acid (IAA) was determined by colorimetric method [18]. Strain GKU 173T was grown in glucose-beef extract broth supplemented with 10 mM L-tryptophan at 28 °C for 7 days in dark. The culture was then centrifuged and 2 mL of supernatant was mixed with 1 mL of Salkowski’s reagent (0.5 M FeCl3, 70% perchloric acid). The positive reaction was indicated by development of a pink-red in color and was quantified by measuring absorbance at 530 nm compared with a standard curve of pure IAA [18]. Phosphate solubilization was determined by the development of clear zone around the bacterial plug on Pikovskaya’s agar [19] supplemented with 2% (w/v) tricalcium phosphate after incubation at 28 °C for three weeks. Siderophore production was detected by the development of orange-yellow halo around the bacterial plug on chrome azurol S (CAS) agar [20] after incubation at 28 °C for three weeks. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase activity was verified by positive growth on minimal medium containing 3mM ACC as a sole nitrogen source after incubation at 28 °C for 21 days in comparison with (NH4)2SO4 (2 g/L) [21].
2.3. 16S rRNA Gene Sequencing and Phylogenetic Analysis
Genomic DNA of strain GKU 173T was extracted from the culture grown on MS agar at 28 °C for seven days according to standard procedure [22]. 16S rRNA gene was amplified using specific primers and PCR condition, as previously described [23]. The PCR product was purified by GenepHlowTM Gel/PCR kit (Geneaid, New Taipei City, Taiwan) and was sent for sequencing at Macrogen (Seoul, Republic of Korea). The almost full-length 16S rRNA gene sequences of strain GKU 173T was compared with 16S rRNA gene sequences of reference type strains using the EzBioCloud server database [24]. Multiple sequence alignments were performed using ClustalW tool in MEGA version 7.0 [25] and trimmed manually where necessary. Phylogenetic evolutionary trees were constructed with the neighbor-joining [26], maximum-likelihood [27] and maximum-parsimony [28] algorithms using the MEGA software. Evolutionary distance was calculated using Kimura’s two-parameter model [29]. The topologies of the phylogenetic trees were evaluated using the bootstrap method [30] base on 1000 resampled datasets.
2.4. Morphological and Physiological Characteristics
Morphological and physiological characteristics of strain GKU 173T and Fodinicola feengrottensis HKI 0501T (JCM 14718T) were determined under the same condition. Gram staining was carried out using standard Gram staining method. Both strains were cultivated on Bennett’s agar [31], MS agar, organic medium 79 [32], and International Streptomyces Project (ISP) media [33] namely, yeast extract-malt extract agar (ISP 2), oatmeal agar (ISP 3), inorganic salt-starch-agar (ISP 4), glycerol-asparagine agar (ISP 5), peptone-yeast extract-iron agar (ISP 6), and tyrosine agar (ISP 7) for up to 21 to 28 days at 28 °C. The color of mycelia and soluble pigment were determined by comparison with color chips from Methuen Handbook of Color [34]. Strain GKU 173T was grown on oatmeal-nitrate agar (ON: 0.02% KNO3, 0.02% MgSO4.7H2O, 0.05% K2HPO4, 0.3% oatmeal, and 1.5% agar) at 28 °C for 21 days for observation of hyphae and spore morphology under scanning electron microscopy (JSM-5600, JEOL, Tokyo, Japan). Spore motility was observed under light microscope followed the method of Tamura et al. [35].
Growth of strain GKU 173T at different temperatures (5‒50 °C) was tested on MS agar slants for 15 days using a temperature gradient incubator (model TN-3, Toyo Kagaku Sangyo, Tokyo, Japan). The pH range for growth (pH values 3‒11, at an interval of 1.0 pH unit) was performed on MS agar adjusting pH values with 1 M NaOH and 1 M HCl. Growth at different NaCl concentration [0‒7% (w/v), at an interval of 1%] was determined on ISP 3 agar for three weeks. Catalase and oxidase activities were observed with 3% (v/v) hydrogen peroxide solution and 1% (v/v) tetramethyl-p-phenylenediamine solution [36], respectively. Acid production from carbohydrates was determined based on the method of Gordon et al. [37]. The utilization of carbon and nitrogen sources were determined on ISP 9 and basal medium supplemented with a final concentration of 1% carbon and 0.1% nitrogen sources, respectively [38]. Degradation of Tweens 20 and 80 [1% (w/v)] were tested on Sierra medium [39] after incubation at 28 °C for 21 days. Decomposition of adenine and tyrosine [0.5% (w/v) each], xanthine, hypoxanthine, xylan [0.4% (w/v) each], casein [1% (w/v) skimmed milk] and urea were determined according to the previous methods [37,38]. Hydrolysis of starch was tested by flooding the culture growing on ISP 4 agar with Lugol’s solution [40]. Milk coagulation and peptonization were performed in 10% (w/v) skimmed milk broth (Difco, BD, Franklin Lakes, NJ, USA) and observed after incubation at 37 °C for 21 days. Gelatin liquefaction was examined on gelatin medium consisted of 0.5% peptone (Difco, BD, Franklin Lakes, NJ, USA), 2% glucose, 20% gelatin, pH 7. Reduction of nitrate was performed using nitrate broth (Difco, BD, Franklin Lakes, NJ, USA). Enzyme activities were tested by the API ZYM test kit (bioMérieux, Durham, NC, USA), according to the manufacturer’s instruction.
2.5. Chemotaxonomic Characterization
For chemotaxonomic analysis, biomass was prepared by growing strain GKU 173T in Bennett’s broth on a rotary sharker at 28 °C for 14 days. Cells were harvested by centrifugation, washed with distilled water, and freeze-dried. Cell wall peptidoglycan was prepared using the modified method described by Kawamoto et al. [41] to analyze the cell-wall amino acids and sugars. The amino acid composition of the peptidoglycan hydrolysate (6 M HCl, 100 °C, 16 h) was determined by one-dimensional TLC on a cellulose plate (Merck, Burlington, MA, USA), using solvent system n-buthanol:acetic acid:water (4:1:2, v/v) and then analysed by LC/MS following the method of Také et al. [42] after Nα-(5-fluoro-2,4-dinitrophenyl)-D-leucinamide (FDLA) derivatization according to the method of Fujii et al. [43]. The LC/MS analysis was performed by AB Sciex TripleTOF 5600+ System with a CAPCELL CORE C18 (3.0 mm x 100 mm) column (Shiseido, Tokyo, Japan) using eluent A (water with 0.1% formic acid) and eluent B (methanol with 0.1% formic acid) by gradient elution: 0–2 min, 50% B; 2–18 min, 50–100% B; 18–20 min, 100% B; flow rate, and 0.5 mL/min. Cell wall sugar was determined by one dimensional TLC on cellulose plate (Merk, Burlington, MA, USA) using solvent system described by Becker et al. [44] and by LC/MS (AB Sciex TripleTOF 5600+ System) with a CAPCELL CORE C18 (3.0 mm x 100 mm) column using 40% methanol with 0.1% formic acid as the mobile phase after 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization [42]. The isomer of diaminopimelic acid (DAP) in the cell wall was analyzed using thin-layer chromatography (TLC) as previously described [45]. N-acyl group of muramic acid in the peptidoglycan was examined using the previously described method [46]. Mycolic acids were determined using TLC according to the procedure developed by Tomiyasu [47]. Phospholipids in the cell were extracted and determined by two-dimensional TLC following the method of Minikin et al. [48]. Cellular fatty acid methyl esters were extracted and analyzed using the Sherlock microbial identification system (version 6.1; MIDI database: TSBA6, Newark, DE, USA) according to procedure of Sasser [49] by the Scientific Instrument Center, King Mongkut’s Institute of Technology Ladkrabang (KMITL), Thailand. Menaquinones were extracted and purified, using a previously described method [50], and analysed by LC-MS (JSM-T100LP; JEOL, Tokyo, Japan) with a Capcell pak C18 UG 120 column (Shiseido) using methanol:iso-propanol (7:3, v/v) as the mobile phase.
2.6. Genome Sequencing and Analysis
Genomic DNA of strain GKU 173T and F. feengrottensis HKI 0501T were extracted from the culture grown in Bennett’s broth at 28 °C for seven days according to standard procedure [22]. Whole genomes of strain GKU 173T and F. feengrottensis HKI 0501T were sequenced using Illumina Hiseq PE 150 serviced by Novogene (Beijing, China) and PacBio Sequel systems at Chulabhorn Royal Academy, Thailand. Prior to assembly, the reads were trimmed for adapter sequences and filtered for sequence quality and checked by Fast QC tool [51]. Sequencing data were assembled with Unicycler [52] and determined by QUAST [53]. G+C content was calculated from the whole genome sequences.
Average Nucleotide Identity (ANI) and Ortho ANI [54] were compared using ChunLab’s online ANI calculator [55] and the Orthologous Average Nucleotide Identity tool version 0.93.1 (OAT) [50]. Digital DNA-DNA hybridization (dDDH) was performed using Genome-to-Genome Distance Calculator (GGDC) [56]. Contamination Estimator by 16S software (ContEst16S) was used to determine contamination of 16S rRNA gene from the query genome [57]. Functional annotation was carried out using the Rapid Annotation using Subsystem Technology (RAST) server with the seed database [58] and the potential biosynthetic gene clusters were predicted by antiSMASH (version.5.0.0) [59].
3. Results and Discussion
3.1. Isolation and Plant Growth Promoting (PGP) Traits
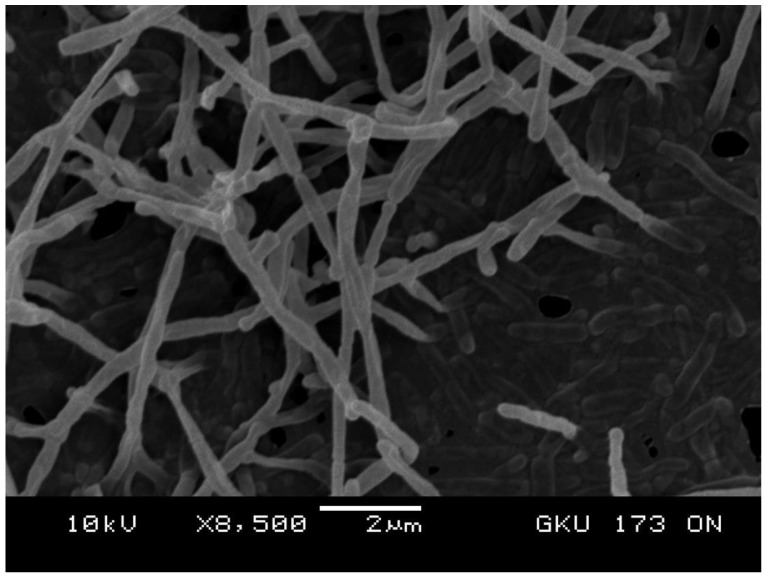
Strain GKU 173T was isolated from surface-sterilized roots of the black wattle tree (Acacia mangium Willd.) on starch-casein agar. It is an aerobic Gram-stain-positive non-sporulating actinobacterium. Morphological observation on ON agar indicated that strain GKU 173T formed a branched substrate mycelium and abundant white aerial hyphae that fragmented into irregular rod-like elements (Figure 1). Strain GKU 173T showed good to abundant growth on all tested media. The substrate mycelium was brownish red, greyish red, high red, and deep red to violet brown associated with red soluble pigment on ISP 2, ISP 3, organic medium 79, Bennett’s agar, and MS agar (Table S1).
Figure 1.
Scanning electron micrograph of fragmentation of aerial hyphae of strain GKU 173T on ON agar at 28 °C after 21 days. Bar, 2 μm.
Strain GKU 173T produced IAA at low concentration at an average of 3 ± 0.3 μg mL − 1. It produced a clear and visible dissolution halo on Pikovskaya’s medium supplemented with 2% (w/v) tricalcium phosphate (Figure S1a) indicating its phosphate solubilization activity. It produced siderophores by showing a yellow halo surrounding the bacterial plug on blue agar CAS assay (Figure S1b). The strain was unable to grow on minimum medium supplemented with 3 mM ACC as a nitrogen source, implying no ACC deaminase activity (data not shown). By carrying these PGP-traits, strain GKU 173T might be able to promote plant growth and play a beneficial role to the plants similar to other endophytic actinomycetes [3,4,5].
3.2. Phylogenetic Analysis of 16S rRNA Gene
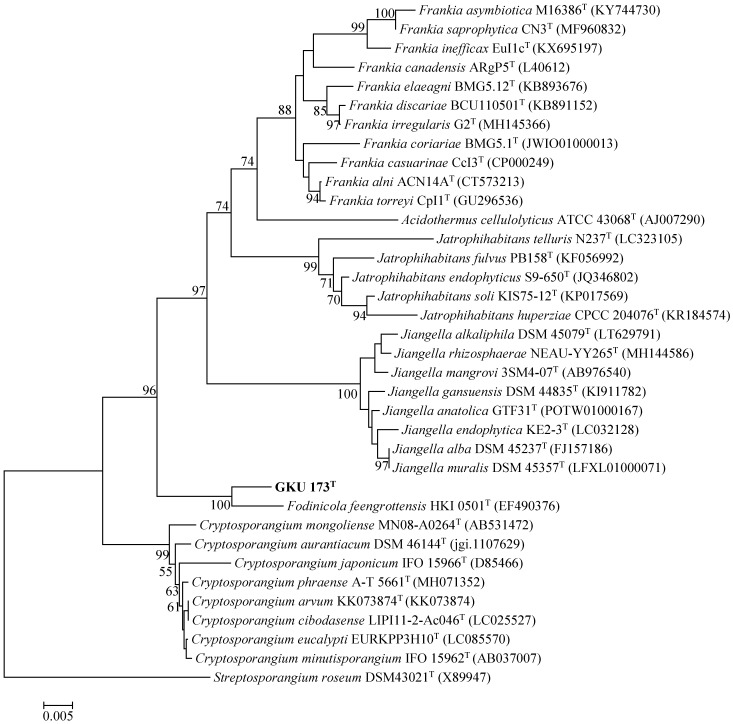
Results of 16S rRNA gene sequence analysis (1498 bp, GenBank accession number MK323078) indicated that strain GKU 173T was the most closely related to the genus Fodinicola. The highest similarity values were detected with the only species in the genus, Fodinicola feengrottensis HKI 0501T (97.13%). The strain also showed a remote relationship (<95% similarity) with other species including Cryptosporangium eucalypti EURKPP3H10T (94.64%), Cryptosporangium phraense A-T 5661T (94.57%), Cryptosporangium aurantiacium DSM 46144T (94.50%), Jiangella anatolica GTF31T (94.36%), Cryptosporangium minutisporangium IFO 15962T (94.36%), and Frankia coriariae BMG5.1T (94.34%). Neighbor-joining phylogenetic analysis indicated that strain GKU 173T grouped tightly with the only member of the genus Fodinicola (Figure 2). The positions of strain GKU 173T in all reconstruction trees were in agreement and supported by the high bootstrap values (Figures S2 and S3). Genus Fodinicola proposed by Carlsohn et al. [11] is monophyletic and forms a distinct cluster with the genus Cryptosporangium [14] as a member of the family Cryptosporangiaceae [12] within the order Cryptosporangiales [13]. At the time of writing, this genus comprises only a single species, F. feengrottensis HKI 0501T, which was isolated from acidic rocks of a medieval alum slate mine in Germany in 2008 [11]. Based on phylogenetic analysis, strain GKU 173T is likely to belong to the genus Fodinicola.
Figure 2.
Neighbor-joining phylogenetic tree on the basis of 16S rRNA gene sequences of strain GKU 173T and related species. Streptosporangium roseum DSM43021T was used as an outgroup. Numbers at nodes refer to bootstrap values (based on 1000 replicates; only values >50% are shown). Bar, 0.005 nucleotide substitutions per site.
3.3. Phenotypic Characterization
Strain GKU 173T and its closely related species, F. feengrottensis HKI 0501T, were phenotypically characterized. Strain GKU 173T showed better growth than F. feengrottensis HKI 0501T in most tested media (Table S1). Its substrate mycelium was in range of red associated with red soluble pigment, while mycelium of strain HKI 0501T was pale orange with no soluble pigment (Table S1). Strain GKU 173T was able to grow between 14 to 42 °C, with optimal growth at 24 to 30 °C. The pH range for growth was 4–11, with optimal growth at pH 7 and 8. The strain grew in NaCl concentrations of 0%–6% (w/v) with good growth at concentration up to 3% (w/v). Strain GKU 173T showed oxidase-negative and catalase-positive. The difference of acidic production, and carbon/nitrogen utilization of strain GKU 173T and F. feengrottensis HKI 0501T are summarized in Table 1. Based on morphological and phenotypical data, strain GKU 173T can be distinguished from F. feengrottensis HKI 0501T.
Table 1.
Differential characteristics of strain GKU 173T and closely related Fodinicola species. All data obtained from the present study unless otherwise indicated. +, Positive; w, weakly positive; -, negative.
| Characteristics | GKU 173T | Fodinicola feengrottensis HKI 0501T |
|---|---|---|
| Isolation source | Roots of plants | Acidic rocks * |
| Cell morphology | Fragmentation of aerial hyphae | Fragmentation of aerial hyphae * |
| Growth Substrate mycelium Soluble pigment |
Good Red Red |
Moderate Pale orange None |
| Temperature range for growth (°C) | 14–42 | 14–40 |
| pH range for growth | 4–11 | 4–9 |
| NaCl range for growth (%, w/v) | 0–6 | 0–3 |
| Urea | - | + |
| Acid production: | ||
| - Adonitol | + | - |
| - Myo-inositol | + | - |
| - Rhamnose | - | + |
| - D-sorbitol | + | - |
| Carbon utilization: | ||
| - Adonitol | + | - |
| - Lactose | w | + |
| - Myo-inositol | + | - |
| - Rhamnose | + | w |
| - D-sorbitol | + | - |
| - D-xylose | + | w |
| Nitrogen utilization: | ||
| - L-histidine | + | w |
| - L-isoleucine | + | w |
| - L-tryptophan | + | - |
| - L-valine | + | w |
| Enzyme activities: | ||
| - Βeta-glucosidase | + | - |
| - Cystine arylamidase | w | + |
| - Esterase | + | w |
| - Lipase | + | w |
* Data obtained from [11].
3.4. Chemotaxonomic Analysis
The analysis of the cell wall peptidoglycan by TLC (Figure S4) and LC/MS showed that strain GKU 173T contained meso-diaminopimelic acid, D-alanin, D-glutamic acid, and glycine. Cell wall sugars were xylose and mannose. The N-acyl type of muramic acid in the peptidoglycan was N-acetyl. Mycolic acids were absent. The polar phospholipids comprised of diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), phosphatidylinositol (PI), several unknown phospholipids, and unknown ninhydrin-positive compounds (Figures S5 and S6). The fatty acid profiles predominantly contained iso-C16:0 (58.22%), anteiso-C17:0 (9.94%), iso-C18:0 (9.35%), and iso-C17:0 (6.58%); and a small proportion of 10-methyl C17:0 (3.87%), 10-methyl C18:0 (1.9%), iso-C16:1 (1.63%), sum in feature 8 (C18:1 ω6c, 1.3%), and summed feature 8 (C18:1 ω7c or C18:1 ω6c, 1.3%). The menaquinones were MK-9(H4) (25%), MK-9(H6) (36%) and MK-9(H8) (39%). All chemotaxonomic results revealed that strain GKU 173T shared apparent characteristics with the only member of the genus Fodinicola particularly, the presence of xylose as the diagnostic cell-wall sugar [11]. Altogether, chemotaxonomic and phenotypic data evidently constitute strain GKU 173T as a novel species within the genus Fodinicola.
3.5. Whole Genome Sequencing and Digital DNA-DNA Hybridization (dDDH)
Genomes of strain GKU 173T and strain HKI 0501T were sequenced and compared. The assembled draft genomes of strain GKU 173T and strain HKI 0501T contained 4 and 107 contigs with a total length of 8.86 Mbp (GenBank accession number WOTN00000000) and 8.71 Mbp (GenBank accession number WOTO00000000), respectively. The N50 value of genomes of strains GKU 173T and HKI 0501T was 2.35 Mbps (L50 = 2) and 1.15 Mbps (L50 = 26), respectively. The ANI value between strain GKU 173T and strain HKI 0501T was 77.92% which was much lower than the ANI threshold range (95%–96%) for species delineation [60]. The dDDH value between both strains was 21.30%, which was much lower than the recommended cut-off value of 70% for species recognition [61]. The calculated genomic G+C contents of strain GKU 173T and F. feengrottensis HKI 0501T were 67.81% and 66.61%, respectively. The difference in G+C value was higher than 1% indicating distinct species according to Meier-Kolthoff et al. [62]. On the basis of polyphasic characteristics, strain GKU 173T was distinguished from the closely related type strain and represents a novel species of the genus Fodinicola, for which the name Fodinicola acaciae sp. nov. (= TBRC 10620T = NBRC 114213T) is proposed.
3.6. Description of Fodinicola acaciae sp. nov.
Fodinicola acaciae (a.ca.ci’ae. L. n. acacia, the acacia-tree and also the name of a botanical genus; L. gen. n. acaciae, of Acacia, isolated from roots of a black wattle tree (Acacia mangium Willd.)
Gram-stain-positive, aerobic, non-motile, catalase-positive, oxidase-negative actinomycete that produces branched substrate mycelium and abundant aerial mycelium. Aerial hyphae break up into irregular rod-like elements. Colonies are wrinkled, beige to orange in color. Red-series diffusible pigment is produced on Bennett, ISP 2, ISP 3, organic medium 79, and MS media. Growth occurs between 14 and 42 °C, good growth at 28 °C, and no growth below 14 °C or above 42 °C. Good growth occurs at pH 7.0 and pH 8.0, but no growth occurs at pH 3 nor pH 12. NaCl tolerance is up to 6% (w/v). Nitrate is not reduced. Gelatin liquefaction, milk coagulation and peptonization are positive. Acid production from adonitol, L-arabinose, D-fructose, glycerol, D-glucose, myo-inositol, lactose, mannitol, D-raffinose, D-sorbitol, sucrose, D-tretalose, and D-xylose, but not from rhamnose. L-tyrosine, Tween 20, and Tween 80 are degraded, but adenine, hypoxanthine, starch, urea, xanthine, and xylan are not. Adonitol, L-arabinose, D-fructose, D-glucose, glycerol, myo-inositol, lactose (weakly), mannitol, D-raffinose, rhamnose, D-sorbitol, sucrose, D-trehalose, and D-xylose are used as carbon sources. L-arginine, L-cysteine, L-histidine, L-isoleucine, L-phenylalanine, potassium nitrate, L-tryptophan, and L-valine are used as nitrogen sources. By API ZYM system, production of acid phosphatase, alkaline phosphatase, α-chymotrypsin (weakly), cysteine arylamidase (weakly), esterase (C4), α-galactosidase, β-galactosidase, N-acetyl-β-glucosaminidase, α-glucosidase, β-glucosidase, leucine arylamidase, lipase (C8), lipase (C14), α-mannosidase, naphthol-AS-BI-phosphohydrolase, and trypsine are positive, but not α-fucosidase and β-glucuronidase. The cell wall peptidoglycan contains meso-A2pm, D-alanine, D-glutamic acid, and glycine. The muramic acid in peptidoglycan is N-acetylated. The cell-wall sugars are xylose and mannose. The predominant menaquinones are MK-9(H4), MK-9(H6), and MK-9(H8). The polar phospholipids comprise diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), phosphatidylinositol (PI), several unknown phospholipids, and unknown ninhydrine-positive compounds. Mycolic acids are absent. The predominant cellular fatty acid profiles contain iso-C16:0, anteiso-C17:0, iso-C18:0, and iso-C17:0. The G+C content is 67.81% (determined from the genome sequence).
Type strain GKU 173T (= TBRC 10620T = NBRC 114213T) was isolated from the roots of black wattle tree (Acacia mangium Willd.) collected at Kasetsart University, Bangkok, Thailand.
3.7. PGP-Trait and Biosynthetic Gene Cluster (BGC) Prediction
RAST annotation of genome sequences of strain GKU 173T showed 8705 coding sequences and 55 RNAs, while F. feengrottensis HKI 0501T showed 11,978 coding sequences and 57 RNAs. The genome annotation revealed genes related to the PGP traits according to those in vitro activities of strain GKU 173T and HKI 0501T (Table 2). Indole-3-acetamide (IAM) hydrolase representing the key enzyme converting IAM to IAA in IAA biosynthetic pathway [63] was located in the genome of strain GKU 173T, while absent in that of strain HKI 0501T. Both genomes consisted of two to three copies of acid phosphatase genes that are likely involved with inorganic phosphate solubility [64]. The genome of strain GKU 173T exhibited siderophore biosynthetic gene cluster (BGC) of lucA/lucC family protein [65].
Table 2.
Genome analysis of PGP-trait and biosynthetic gene cluster (BGC) prediction of strain GKU173T and Fodinicola feengrottensis HKI 0501T.
| Gene Feature | GKU 173T | HKI 0501T |
|---|---|---|
| PGP-trait Prediction | ||
| - IAA production | Indole-3-acetamide (IAM) hydrolase | - |
| - Phosphate solubilization | Acid phosphatases | Acid phosphatases |
| - Siderophore production | - IucA/IucC family siderophore biosynthesis proteins - N-acetyltransferase - Aminotransferase |
- IucA/IucC family siderophore biosynthesis proteins - N-acetyltransferase - Aminotransferase |
| BGC Prediction (number) | ||
| Known BGCs | ||
| - Bacteriocin | 1 | 1 |
| - Ectoine | 1 | 1 |
| - Geosmin | 1 | 0 |
| - Hopene | 1 | 0 |
| - 2-methylisoborneol | 1 | 0 |
| Silent/cryptic BGCs | ||
| - Lanthipeptide | 5 | 2 |
| - Lassopeptide | 1 | 0 |
| - Non-ribosomal peptide (NRP) | 0 | 3 |
| - Siderophore | 1 | 1 |
| - Type I polyketide (PK) | 0 | 2 |
| - Type I PK-NRP hybrid | 1 | 1 |
| - Type II PK | 1 | 2 |
| - Terpene | 2 | 3 |
The BGCs from genomes of strain GKU 173T and F. feengrottensis HKI 0501T were investigated by antiSMASH. The results showed that strain GKU 173T carried 16 BGCs including 5 known BGCs and 11 uncharacterized BGCs (Table 2). The silent/cryptic BGCs encoded 6 ribosomally synthesized and post-translationally modified peptides (RiPPs), 1 siderophore, 1 Type I polyketide (PK)-non-ribosomal peptide (NRP) hybrid, 1 Type II PK, and 2 terpenes. The genome of strain HKI 0501T consisted of 16 BGCs including 2 known BGCs and 14 uncharacterized BGCs (Table 2). The silent/cryptic BGCs encoded 3 NRPs, 2 RiPPs, 1 siderophore, 2 Type I PKs, 2 Type II PKs, 1 Type I PK-NRP hybrid, and 3 terpenes. Interestingly, the Type I PK-NRP BGCs of both strains were nearly identical (98%) and organized in the same pattern, thus, they might be involved in the same biosynthesis of new compounds. The in-silico genome analysis above supported that rare actinomycetes carried significant number of BGCs, particularly the silent/cryptic clusters [10,66]. Therefore, members of genus Fodinicola are potential species for production of novel competent compounds.
4. Conclusions
Based on the polyphasic taxonomic study demonstrated that strain GKU 173T isolated from roots of a black wattle tree (Acacia mangium Willd.) constitutes a novel species within the genus Fodinicola of which Fodinicola acaciae sp. nov. was proposed under the type strain GKU 173T (= TBRC 10620T = NBRC 114213T). Genome analysis of the novel strain and the closely related strain, F. feengrottensis HKI 0501T, revealed potential PGP-traits including genes involved in phosphate solubilization, IAA and siderophore production; and a variety of uncharacterized BGCs comprising NRPs, RiPPs, Type I PKs, Type II PKs, Type I PK-NRP hybrid, and terpenes. The results suggested that members in the genus Fodinicola have potential activity as beneficial PGP-bacteria and specialized secondary metabolite producers.
Acknowledgments
H.P. was awarded a MS scholarship from the Kasetsart University Scholarships for ASEAN for Commemoration of the 60th Birthday Anniversary of Her Royal Highness Princess Chulabhorn Mahidol, the Capacity Building of KU Students on Internationalization (KUCSI), and Faculty of Science, Kasetsart University. We thank Chakrit Bunyoo and Sasithorn Chotewutmontri, Chulabhorn Royal Academy, Thailand for bacterial whole genome sequencing by PacBio Sequel systems. This work was financially supported by OmiKU, International SciKU Branding (ISB), and Kasetsart University Research and Development Institute (KURDI).
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/4/467/s1.
Author Contributions
Investigation, H.T.T.P.; Formal Analysis, H.T.T.P., W.K.; Resources, W.S.; Conceptualization, Supervision, Project Administration, and Funding Acquisition, A.T. (Arinthip Thamchaipenet); Methodology, A.T. (Arinthip Thamchaipenet), Y.I., A.T. (Akira Také), and A.M.; Writing—Original Draft Preparation, H.T.T.P., W.K., and A.T. (Arinthip Thamchaipenet); Writing—Review & Editing, A.T. (Arinthip Thamchaipenet), Y.I., A.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hallmann J., Quadt-Hallmann A., Mahaffee W., Kloepper J. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997;43:895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- 2.Hasegawa S., Meguro A., Shimizu M., Nishimura T., Kunoh H. Endophytic actinomycetes and their interactions with host plants. Actinomycetologica. 2006;20:72–81. doi: 10.3209/saj.20.72. [DOI] [Google Scholar]
- 3.Rungin S., Indananda C., Suttiviriya P., Kruasuwan W., Jaemsaeng R., Thamchaipenet A. Plant growth enhancing effects by a siderophore-producing endophytic streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105) Antonie Van Leeuwenhoek. 2012;102:463–472. doi: 10.1007/s10482-012-9778-z. [DOI] [PubMed] [Google Scholar]
- 4.Jog R., Pandya M., Nareshkumar G., Rajkumar S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology. 2014;160:778–788. doi: 10.1099/mic.0.074146-0. [DOI] [PubMed] [Google Scholar]
- 5.Kruasuwan W., Thamchaipenet A. Diversity of culturable plant growth-promoting bacterial endophytes associated with sugarcane roots and their effect of growth by co-inoculation of diazotrophs and actinomycetes. J. Plant Growth Regul. 2016;35:1074–1087. doi: 10.1007/s00344-016-9604-3. [DOI] [Google Scholar]
- 6.Franco C., Michelsen P., Percy N., Conn V., Listiana E., Moll S., Loria R., Coombs J. Actinobacterial endophytes for improved crop performance. Austral. Plant Pathol. 2007;36:524–531. doi: 10.1071/AP07067. [DOI] [Google Scholar]
- 7.Jaemsaeng R., Jantasuriyarat C., Thamchaipenet A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep. 2018;8:1950. doi: 10.1038/s41598-018-19799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto A., Takahashi Y. Endophytic actinomycetes: Promising source of novel bioactive compounds. J. Antibiot. 2017;70:514–519. doi: 10.1038/ja.2017.20. [DOI] [PubMed] [Google Scholar]
- 9.Baltz R.H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 2017;44:573–588. doi: 10.1007/s10295-016-1815-x. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.-S., Kim H.-J., Lee H.-S., Kim P., Kim E.-S. Genome mining of rare actinomycetes and cryptic pathway awakening. Process Biochem. 2015;50:1184–1193. doi: 10.1016/j.procbio.2015.04.008. [DOI] [Google Scholar]
- 11.Carlsohn M.R., Groth I., Saluz H.P., Schumann P., Stackebrandt E. Fodinicola feengrottensis gen. nov., sp. nov., an actinomycete isolated from a medieval mine. Int. J. Syst. Evol. Microbiol. 2008;58:1529–1536. doi: 10.1099/ijs.0.65512-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhi X.Y., Li W.J., Stackebrandt E. An update of the structure and 16S rRNA gene sequence-based definition of higher ranks of the class Actinobacteria, with the proposal of two new suborders and four new families and emended descriptions of the existing higher taxa. Int. J. Syst. Evol. Microbiol. 2009;59:589–608. doi: 10.1099/ijs.0.65780-0. [DOI] [PubMed] [Google Scholar]
- 13.Nouioui I., Carro L., García-López M., Meier-Kolthoff J.P., Woyke T., Kyrpides N.C., Pukall R., Klenk H.P., Goodfellow M., Göker M. Genome-based taxonomic classification of the phylum Actinobacteria. Front. Microbiol. 2018;9:2007. doi: 10.3389/fmicb.2018.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura T., Hayakawa M., Hatano K. A new genus of the order Actinomycetales, Cryptosporangium gen. nov., with descriptions of Cryptosporangium arvum sp. nov. and Cryptosporangium japonicum sp. nov. Int. J. Syst. Bacteriol. 1998;48:995–1005. doi: 10.1099/00207713-48-3-995. [DOI] [PubMed] [Google Scholar]
- 15.Indananda C., Matsumoto A., Inahashi Y., Takahashi Y., Duangmal K., Thamchaipenet A. Actinophytocola oryzae gen. nov., sp. nov., isolated from the roots of Thai glutinous rice plants, a new member of the family Pseudonocardiaceae. Int. J. Syst. Evol. Microbiol. 2010;60:1141–1146. doi: 10.1099/ijs.0.008417-0. [DOI] [PubMed] [Google Scholar]
- 16.Küster E., Williams S.T. Selection of media for isolation of streptomycetes. Nature. 1964;202:928–929. doi: 10.1038/202928a0. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs G., Frazer C.M., Gardner D.C.J., Cullum J.A., Oliver S.G. Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 1989;31:272–277. doi: 10.1007/BF00258408. [DOI] [Google Scholar]
- 18.Gordon S.A., Weber R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pikovskaya R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya. 1948;17:362–370. [Google Scholar]
- 20.Schwyn B., Neilands J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 21.El-Tarabily K.A. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil. 2008;308:161–174. doi: 10.1007/s11104-008-9616-2. [DOI] [Google Scholar]
- 22.Kieser T., Bibb M.J., Buttner M.J., Chater K.F., Hopwood D.A. Practical Streptomyces Genetics. The John Innes Foundation; Norwich, UK: 2000. [Google Scholar]
- 23.Rachniyom H., Matsumoto A., Indananda C., Duangmal K., Takahashi Y., Thamchaipenet A. Nonomuraea syzygii sp. nov., an endophytic actinomycete isolated from the roots of a jambolan plum tree (Syzygium cumini L. Skeels) Int. J. Syst. Evol. Microbiol. 2015;65:1234–1240. doi: 10.1099/ijs.0.000085. [DOI] [PubMed] [Google Scholar]
- 24.Yoon S.H., Ha S.M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Felsenstein J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 28.Fitch W.M. Toward defining the course of evolution: Minimum change for a specific tree topology. Syst. Zool. 1971;20:406–416. doi: 10.2307/2412116. [DOI] [Google Scholar]
- 29.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 30.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones K.L. Fresh isolates of actinomycetes in which the presence of sporogenous aerial mycelia is a fluctuating characteristic. J. Bacteriol. 1949;57:141–145. doi: 10.1128/JB.57.2.141-145.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prauser H., Falta R. Phagensensibilität, Zellwand-Zusammensetzung und Taxonomie von Actinomyceten. J. Basic Microbiol. 1968;8:39–46. doi: 10.1002/jobm.3630080106. [DOI] [PubMed] [Google Scholar]
- 33.Shirling E.B., Gottlieb D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 1966;16:313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- 34.Kornerup A., Wanscher J.H. Methuen Handbook of Colour. 3rd ed. Eyre Methuen; London, UK: 1978. [Google Scholar]
- 35.Tamura T., Hayakawa M., Hatano K. Sporichthya brevicatena sp. nov. Int. J. Syst. Bacteriol. 1999;49:1779–1784. doi: 10.1099/00207713-49-4-1779. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956;178:703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- 37.Gordon R.E., Barnett D.A., Handerhan J.E., Pang C.H.N. Nocardia coeliaca, Nocardia autotrophica, and the nocardin strain. Int. J. Syt. Evol. Microbiol. 1974;24:54–63. doi: 10.1099/00207713-24-1-54. [DOI] [Google Scholar]
- 38.Williams S.T., Goodfellow M., Alderson G., Wellington E.M., Sneath P.H., Sackin M.J. Numerical classification of Streptomyces and related genera. J. Gen. Microbiol. 1983;129:1743–1813. doi: 10.1099/00221287-129-6-1743. [DOI] [PubMed] [Google Scholar]
- 39.Sierra G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie van Leeuwenhoek. 1957;23:15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- 40.Barrow G.I., Feltham R.K.A. In Cowan and Steel’s Manual for the Identification of Medical Bacteria. 3rd ed. Cambridge University Press; Cambridge, UK: 1993. [Google Scholar]
- 41.Kawamoto I., Oka T., Nara T. Cell wall composition of Micromonospora olivoasterospora, Micromonospora sagamiensis, and related organisms. J. Bacteriol. 1981;146:527–534. doi: 10.1128/JB.146.2.527-534.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Také A., Nakashima T., Inahashi Y., Shiomi K., Takahashi Y., Ōmura S., Matsumoto A. Analyses of the cell-wall peptidoglycan structures in three genera Micromonospora, Catenuloplanes, and Couchioplanes belonging to the family Micromonosporaceae by derivatization with FDLA and PMP using LC/MS. J. Gen. Appl. Microbiol. 2016;62:199–205. doi: 10.2323/jgam.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Fujii K., Ikai Y., Oka H., Suzuki M., Harada K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997;69:5146–5151. doi: 10.1021/ac970289b. [DOI] [Google Scholar]
- 44.Becker B., Lechevalier M.P., Lechevalier H.A. Chemical composition of cell-wall preparations from strains of various form-genera of aerobic actinomycetes. Appl. Microbiol. 1965;13:236–243. doi: 10.1128/AEM.13.2.236-243.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasegawa T., Takizawa M., Tanida S. A rapid analysis for chemical grouping of aerobic actinomycetes. J. Gen. Appl. Microbiol. 1983;29:319–322. doi: 10.2323/jgam.29.319. [DOI] [Google Scholar]
- 46.Uchida K., Aida K. An improved method for the glycolate test for simple identification of the acyl type of bacterial cell walls. J. Gen. Appl. Microbiol. 1984;30:131–134. doi: 10.2323/jgam.30.131. [DOI] [Google Scholar]
- 47.Tomiyasu I. Mycolic acid composition and thermally adaptative changes in Nocardia asteroides. J. Bacteriol. 1982;151:828–837. doi: 10.1128/JB.151.2.828-837.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minnikin D., O’Donnell A. Actinomycete envelope lipid and peptidoglycan composition. In: Goodfellow M., Mordarski M., Williams S.T., editors. The Biology of the Actinomycetes. Academic Press; London, UK: 1884. pp. 337–388. [Google Scholar]
- 49.Sasser M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids. Microbial ID Inc.; Newark, DE, USA: 2001. Technical Note #101. [Google Scholar]
- 50.Collins M.D., Pirouz T., Goodfellow M., Minnikin D.E. Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 1977;100:221–230. doi: 10.1099/00221287-100-2-221. [DOI] [PubMed] [Google Scholar]
- 51.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. [(accessed on 24 March 2019)];2010 Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 52.Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee I., Ouk Kim Y., Park S.C., Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 55.Yoon S.H., Ha S.M., Lim J., Kwon S., Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 56.Auch A.F., von Jan M., Klenk H.P., Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genomic Sci. 2010;2:117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee I., Chalita M., Ha S.M., Na S.I., Yoon S.H., Chun J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017;67:2053–2057. doi: 10.1099/ijsem.0.001872. [DOI] [PubMed] [Google Scholar]
- 58.Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A., Formsma K., Gerdes S., Glass E.M., Kubal M., et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S.Y., Medema M.H., Weber T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richter M., Rosselló-Móra R., Oliver Glöckner F., Peplies J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32:929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wayne L.G., Brenner D.J., Colwell R.R., Grimont P.A.D., Kandler O., Krichevsky M.I., Moore L.H., Moore W.E.C., Murray R.G.E., Stackebrandt E., et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Evol. Microbiol. 1987;37:463–464. doi: 10.1099/00207713-37-4-463. [DOI] [Google Scholar]
- 62.Meier-Kolthoff J.P., Klenk H.P., Göker M. Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. Int. J. Syst. Evol. Microbiol. 2014;64:352–356. doi: 10.1099/ijs.0.056994-0. [DOI] [PubMed] [Google Scholar]
- 63.Manulis S., Shafrir H., Epstein E., Lichter A., Barash I. Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology. 1994;140:1045–1050. doi: 10.1099/13500872-140-5-1045. [DOI] [PubMed] [Google Scholar]
- 64.Rodríguez H., Fraga R., Gonzalez T., Bashan Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. In: Velázquez E., Rodríguez-Barrueco C., editors. First International Meeting on Microbial Phosphate Solubilization. Developments in Plant and Soil Sciences. Volume 102. Springer; Dordrecht, The Netherlands: 2007. pp. 15–21. [Google Scholar]
- 65.Bagg A., Neilands J.B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 1987;51:509–518. doi: 10.1128/MMBR.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruasuwan W., Hoskisson P.A., Thamchaipenet A. Draft genome sequence of root-associated sugarcane growth promoting Microbispora sp. GKU 823. Genome Announc. 2017;5:e00647-17. doi: 10.1128/genomeA.00647-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.