Abstract
Significance: From studies of diabetic animal models, the downregulation of peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α)–heme oxygenase 1 (HO-1) axis appears to be a crucial event in the development of obesity and diabetic cardiomyopathy (DCM). In this review, we discuss the role of metabolic and biochemical stressors in the rodent and human pathophysiology of DCM. A crucial contributor for many cardiac pathologies is excessive production of reactive oxygen species (ROS) pathologies, which lead to extensive cellular damage by impairing mitochondrial function and directly oxidizing DNA, proteins, and lipid membranes. We discuss the role of ROS production and inflammatory pathways with multiple contributing and confounding factors leading to DCM.
Recent Advances: The relevant biochemical pathways that are critical to a therapeutic approach to treat DCM, specifically caloric restriction and its relation to the PGC-1α–HO-1 axis in the attenuation of DCM, are elucidated.
Critical Issues: The increased prevalence of diabetes mellitus type 2, a major contributor to unique cardiomyopathy characterized by cardiomyocyte hypertrophy with no effective clinical treatment. This review highlights the role of mitochondrial dysfunction in the development of DCM and potential oxidative targets to attenuate oxidative stress and attenuate DCM.
Future Directions: Targeting the PGC-1α–HO-1 axis is a promising approach to ameliorate DCM through improvement in mitochondrial function and antioxidant defenses. A pharmacological inducer to activate PGC-1α and HO-1 described in this review may be a promising therapeutic approach in the clinical setting.
Keywords: diabetes, diabetic cardiomyopathy, antioxidant, heme oxygenase, PGC-1α
Introduction
Obesity is a major risk factor for vascular dysfunction, diabetes, hyperlipidemia, and hypertension (62). The prevalence of obesity and diabetes effects are on the rise in the United States of America (119), have doubled in many developed countries, and continue to increase in most developing countries (30). Clinical and experimental evidence reveals the existence of specific cardiomyopathy in diabetic patient and animal models, termed “diabetic cardiomyopathy” (DCM) (38). DCM was first described as a distinct pathology, in a small cohort of diabetic patients with adverse myocardial structural changes at postmortem (156). Considerable effort was invested in identifying and characterizing this cardiomyopathy, bringing forth the awareness of diabetes as a risk factor for heart failure (HF). The Framingham Heart Study found that the risk of developing HF increases by two-fold in diabetic males, and by five-fold in diabetic females, independent of age, obesity, dyslipidemia, and coronary heart disease (83). Besides being a risk factor for HF (45, 128), diabetes is also a predictor of poor prognosis in patients with HF (76, 139).
DCM Phenotype
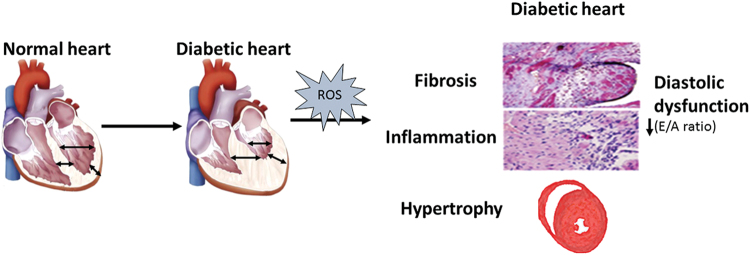
DCM is a complex pathology that develops due to a combination of several metabolic disorders: hyperglycemia, insulin resistance in type 2 diabetes mellitus (DM2), hypertension, and obesity (78, 112, 208). DCM phenotype caused by metabolic co-morbidities (obesity/diabetes and hypertension) is summarized in Figure 1.
FIG. 1.
The phenotype and characteristic of diabetic cardiomyopathy. Diabetic cardiomyopathy phenotype caused by metabolic co-morbidities (obesity/diabetes and hypertension) is characterized by left ventricle hypertrophy (wall thickening and smaller cavity), fibrosis, and diastolic dysfunction; this is defined as increased resistance to filling and is diagnosed by doppler echo when the E/A waves ratio is reduced (<1). Color images are available online.
Diastolic dysfunction
Diastolic HF is defined as increased resistance to the filling of one or both cardiac ventricles. Diastolic HF may be the result of structural abnormalities that increase resistance to ventricular inflow or physiologic abnormalities, resulting in impaired myocardial relaxation. In advanced myocardial hypertrophy, the increased resistance to ventricular diastolic inflow is due to both structural alterations (increased wall thickness and myocardial fibrosis) and impaired diastolic relaxation of the hypertrophied myocardium (60). Diastolic dysfunction is the main functional change occurring in DCM, and it precedes left ventricle (LV) remodeling and systolic dysfunction (40). In the clinical setting, defining and diagnosing diastolic dysfunction poses a challenge. As per the European Society of Cardiology guidelines for the diagnosis of diastolic dysfunction, the E wave velocity is reduced, resulting in E/A reversal (ratio <1.0) as measured by using Doppler echocardiography (136). Besides the impaired cardiac filling, diastolic HF symptoms must comprise: (i) congestive HF; (ii) normal or mild systolic dysfunction; and (iii) elevated levels of brain natriuretic peptide (BNP). The development of diastolic dysfunction with preserved/normal systolic was shown to be associated with: reduced insulin sensitivity (10), increased cardiomyocyte stiffness (122, 204), impaired glucose metabolism and increased fatty acid utilization (14), oxidative stress (67, 68, 152), and Ca2+ overload (63).
Cardiac hypertrophy
LV hypertrophy is a major phenotype in patients with diabetes and is a risk factor for myocardial infarction (MI), stroke, and HF (120). In diabetic patients, LV hypertrophy initially develops as an adaptive response to elevated hemodynamic stress but, in the case of pathological stimuli, it becomes maladaptive (116). This hypertrophy is characterized by increased LV posterior wall thickness and cardiac mass, in addition to a higher ratio of wall thickness to chamber diameter together with molecular and histopathological evidence of cardiomyocyte hypertrophy, fibrosis (40, 100), reduced cardiomyocyte contractility (203), and neurohormonal activation (107). Although LV hypertrophy is frequently associated with increased afterload in diabetic patients with hypertension (15), it can also occur independently of pressure overload (53, 198). The clinical phenotype diabetes-induced hypertrophy was replicated in animal models that displayed increases in a heart weight-to-body weight ratio, cardiomyocyte size, and elevated hypertrophic gene expression (e.g., β-myosin heavy chain, atrial natriuretic peptide [ANP], BNP) (19, 25, 69, 152, 153).
Cardiac fibrosis
Hyperglycemia is sufficient to activate pro-growth (230), promoting cardiac fibroblast and vascular smooth muscle cell proliferation (13, 175). As a result, a major characteristic feature of the diabetic myocardium is extensive fibrosis (176). Tissue fibrosis is a result of abnormally elevated extracellular matrix (ECM) deposition, composed of collagen, elastin, laminin, and fibronectin. The role of the ECM is to provide a scaffold for cardiomyocytes to maintain myocardial structure, shape, and LV wall thickness (37). Elevated myocardial levels and gene expression of ECM proteins (particularly collagen) were observed in animal models of DM2 (122), and they were shown to be related to the impairments in LV diastolic filling, increased myocardial stiffness, and impaired LV relaxation [reviewed in ref. (158)]. Elevated myocardial content and gene expression of ECM proteins (particularly collagen) were observed in experimental models of DM2 (122), including an increase in the insoluble form (176). Importantly, an increase in Collagen was observed in the human diabetic heart (9). The accumulation of ECM is controlled by the degrading enzymes, matrix metalloproteinases (MMP) and their inhibitors (tissue inhibitors of metalloproteinases). They have been suggested as a mechanism underlying ECM accumulation in the diabetic heart (205, 218). MMP-9 knock out (KO) mice are protected from diabetes-induced contractile dysfunction (142). In addition to an increase in ECM, upregulation of transforming growth factor β1 (TGF-β1), its receptor TGF-β receptor II, and its downstream target, connective tissue growth factor promotes fibroblast proliferation in the diabetic heart (12, 158, 212, 231).
Apoptosis, Autophagy, and Mitophagy in the Diabetic Heart
Apoptosis, a form of programmed cell death, is essential for maintaining homeostasis under normal physiological conditions (52, 102). In the diabetic heart, increased apoptosis is involved in the transition from compensated to decompensated hypertrophic state (52) and plays an important role in the development of DCM (18, 49, 52, 94). Apoptosis in the diabetic heart is also associated with pathological structural changes, including increased fibrosis and cardiomyocyte hypertrophy (52, 68, 152). In the diabetic heart, apoptosis is triggered by hyperglycemia-induced caspase-3 activation (18).
Autophagy is another form of programmed cell death, whose role is to remove defective cells and organelles, as well as to recycle damaged proteins (46, 58, 151). The autophagosome is a double-membrane vacuole containing cell components or proteins marked for degradation, before vacuole–lysosome fusion. Well-established markers to detect autophagy include the microtubule-associated protein light chain 3 (LC3) puncta, the ratio of active LC3-II isoform to the inactive LC3-I isoform levels, visualizing double-membrane vesicles on electron microscopy, and increased levels of the autophagy-related genes Atg5, Atg7, Beclin-1, and p62 (43, 46, 151, 171, 228). Autophagy is mainly a cell survival mechanism, regulating the turnover proteins and protecting cells during starvation and other cell stressors (43, 46, 58, 151, 229). However, dysregulated autophagy can result in excessive cell death, and it has been implicated in various diseases, including cancer, neurodegenerative disorders, and diabetes (43, 46, 118).
In the animal model of fructose-fed, insulin-resistant mice exhibited, there is an increase in autophagy but not apoptosis, accompanied by elevated oxidative stress and fibrosis (117). In contrast, in diabetic OVE26 mice, reduced cardiomyocyte autophagy is observed, with reduced adenosine monophosphate-activated protein kinase (AMPK) activity and cardiac dysfunction (222). In view of this conflicting evidence of excessive or insufficient autophagy in the pathophysiology of cardiomyocyte death and cardiac dysfunction (58, 151, 170), the contribution of the autophagic response to the pathogenesis of DCM requires further elucidation.
Mitochondrial function is essential to maintain cellular homeostasis and metabolism. When energy is abundant, excess mitochondria generate excessive reactive oxygen species (ROS) and can start consuming ATP (215). Damaged or unstable mitochondria release ROS and apoptosis-promoting factors that cause damage to neighboring mitochondria and the entire cell (35). Therefore, autophagy-mediated regulation of mitochondria numbers termed “mitophagy,” in response to metabolic demands, is an important mechanism to prevent mitochondria-related cellular damage. The fact that mitophagy occurs after apoptosis (225), and in the absence of apoptosis (226), reinforces the idea that mitophagy's primary role is to preserve the cell when there is mitochondrial dysfunction (57, 199). Mitophagy is activated in cardiomyocytes at baseline and in response to stress to degrade both damaged and excess mitochondria (70, 179). Together with the process of mitochondrial fission, fusion, and mitochondrial biogenesis, mitophagy is one of the most important steps that are necessary to regulate mitochondrial function (171). Previous studies have shown that autophagy, which nonselectively degrades mitochondria, and mitophagy are either downregulated (74, 79, 172, 222, 224) or upregulated (117, 159) in the heart with metabolic syndrome. The occurrence of mitophagy in the heart during the development of DCM and its underlying mechanism is not unequivocally documented. General autophagy, evaluated with LC3II in the whole-cell fraction, is activated by high-fat diet (HFD) feeding, peaking at 6 weeks, but declining thereafter. Interestingly, both LC3II in the mitochondrial fraction and mitophagy were increased. LC3II found predominantly in the mitochondrial fraction and the majority (>75%) of GFP-LC3 ring-like structures colocalized with mitochondria. Thus, activation of autophagy may take place primarily in mitochondria during HFD consumption. These data suggest that the activities of autophagy in the whole-cell fraction and in mitochondria are differentially regulated. Alternatively, additional mechanisms of mitophagy, such as LC3-independent mitophagy, may be activated after conventional mechanisms of autophagy are inactivated. Mitophagy after HFD is attenuated in the absence of Atg7- or Parkin, suggesting that Atg7- or Parkin-dependent mitophagy is activated in the heart during HFD consumption. Cardiac hypertrophy, diastolic dysfunction, and fibrosis are exacerbated in the atg7-cKO mice. These results suggest that Atg7-dependent activation of autophagy/mitophagy acts as an adaptive mechanism to protect the heart against DCM (200).
Mitochondrial Function and Oxidative Stress
Despite the clinical challenges in diagnosing and treating DCM, the specific cellular and molecular mechanisms driving the adverse cardiac remodeling and diastolic dysfunction have not been fully elucidated. However, it is frequently attributed and associated with increased oxidative stress.
Molecular oxygen is inert (174). But the enzyme-driven addition of electrons to oxygen molecules increases their reactivity, producing different types of ROS molecules. The oxidizing potential of ROS is used by aerobic cells to regulate the function and activity of cell signaling molecules. But this process must be tightly regulated by antioxidant molecules to prevent oxidative damage (114, 174, 196).
The endogenous superoxide dismutase (SOD) enzymes degrade •O2− to the more stable forms, H2O2 and O2. Then, H2O2 is further broken down to H2O by glutathione peroxidase and catalase. Under pathological states, H2O2 also generates •OH, which is a highly reactive molecule. In addition, the interaction between •O2− and NO• produces Peroxynitrite (ONOO−) (173).
Excessive ROS production is a significant factor in the development of various pathologies, due to the oxidizing capacity of DNA, proteins, and lipid membranes (219) (Fig. 2), and to activate stress pathways that regulate cellular damage such as endoplasmic reticulum stress (132, 157). Figure 2 describes the role of oxidative stress formation and its consequences in the diabetic heart. Studies linked diabetes with an increase in ROS and a reduction in antioxidant molecules (26, 41, 147). In obese and diabetic patients, elevated levels of the oxidative DNA damage marker 8-hydroxy 2′-deoxyguanosine were detected and they positively correlated with the body mass index (5). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and uncoupled nitrite oxide synthases (NOS) are a major cellular source of •O2− generated from the mitochondrial activity that contribute to cardiac ROS (193).
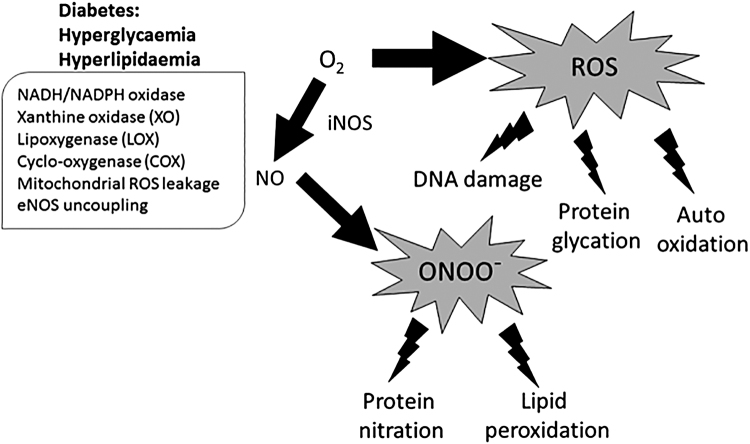
FIG. 2.
Oxidative stress formation and consequences in the diabetic heart. Increase in NADH oxidation, mitochondrial leakage leading to increased O2 and XO production, and eNOS uncoupling promote the formation of ROS and via iNOS, RNS. ROS and RNS are responsible for cellular damage through direct DNA damage, protein glycation, nitration, and peroxidation. eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; RNS, reactive nitrogen species; ROS, reactive oxygen species; XO, xanthine oxidase.
Under normal conditions, the endogenous antioxidant system is sufficient to break down the small amount of ROS produced during mitochondrial respiration to water. But the impaired antioxidant capacity observed in HF increases oxidative stress (133, 194). Another source of oxidative stress in the cardiovascular system is the uncoupling of endothelial nitric oxide synthase (eNOS) (85). Under physiological conditions, eNOS functions as a homodimer enzyme to produce NO• to transfer electrons from NADPH (via the) to the heme molecule in the oxygenase domain, using flavin adenine dinucleotide and flavinmononucleotide as cofactors. Finally, the substrate l-arginine is oxidized to l-citrulline and NO•; therefore, uncoupling of the eNOS complex is critical for the formation of NO, ROS, and reactive nitrogen species (51). When eNOS is in its uncoupled state, electrons transferred from the reductase to the oxygenase domain are donated to molecular oxygen instead of l-arginine, producing •O2−. Therefore, the regulation of eNOS activity is crucial in preventing the uncoupling of O2 reduction from NO• generation (47, 98, 197). HMG-CoA reductase was demonstrated as a regulator of eNOS uncoupling and its inhibition normalized endothelial function and reduced oxidative stress in diabetic rats by inhibiting the activation of NADPH oxidase and preventing eNOS uncoupling (217). Alterations in the function of NOS, predominantly the endothelial isoform eNOS, are generally accepted as responsible for the diabetes-induced dysfunction of NO signaling, production, and bioavailability (28, 108). Increased ROS promote LV hypertrophy via several mechanisms, including the elevated release of vasoconstrictor peptides such as Angiotensin II (Ang II) and ET-1, activation of protein kinases such as c-jun N-terminal protein kinase and p38 mitogen-activated protein kinase (MAPK), and increased mechanical pressure (59, 160, 213, 233, 234). Treating with antioxidant compounds such as butylated hydroxyanisole vitamin E, catalase, probucol, tempol, and N-acetylcysteine was beneficial in preventing tumor necrosis factor (TNF)-α and Ang II-induced cardiomyocyte hypertrophy, de novo protein synthesis, and the elevation in ANP expression (125, 126). Treating mouse papillary muscles with hydroxyl radical for 2 min was sufficient to impair both developed and diastolic force, indicative of systolic and diastolic dysfunction (65). Exposure of cardiomyocytes to H2O2, which is a less reactive species, is sufficient to impair cell contractility (96). These direct effects of ROS on cardiac function in isolated cardiomyocytes also occur in vivo, as evidenced by the favorable effects of antioxidant compounds and overexpression of protein with antioxidative activity in models of HF and DCM (75, 106, 127). Increased systematic activation of xanthine oxidase (XO) has been reported in rats and mice (66), including the heart (146). The inhibition of XO attenuates most pathological alteration of DCM and improves both systolic and diastolic dysfunctions in type I diabetic mouse (146) and rat (54) hearts. In patients with ischemic heart disease, treatment with the XO inhibitor allopurinol reduced left ventricular mass and improved clinical symptoms (195). These studies suggest that increased ROS production plays an important role in the pathophysiology and progression of cardiomyocyte hypertrophy. Thus, reduction of ROS generation and restoration of redox balance, using antioxidative treatment, is a promising approach to prevent and treat myocardial fibrosis and hypertrophy in the failing heart.
The development of DCM requires the occurrence of one or more comorbidities that lead to the upregulation of inflammation ROS, impair NO signaling responses and passive tension at both the systemic and the endothelial level. These impact the adjacent cardiomyocytes to induce hypertrophy (6, 135).
In a novel “2 hits” mouse model of DCM, combining HFD and inhibition of constitutive nitric oxide synthase using Nω-nitro-l-arginine methyl ester increased the activity of inducible nitric oxide synthase (iNOS), and S-nitrosylation was observed. Importantly, in patients with similar diastolic dysfunction and HF, NOS2 was elevated as well as increased protein nitrosylation was observed. Pharmacological and genetic suppression of iNOS ameliorated the phenotype (167).
In summary, maintaining the balance between the generation of ROS and their removal by the antioxidant system is critical for normal cardiac function. In the diabetic heart, the excess ROS that are not eliminated by the endogenous antioxidant system cause irreparable damage to cellular organelles and proteins, affecting cardiomyocyte contractility and promoting hypertrophy and fibrosis.
Activation of the Antioxidant Defense Mechanism by Caloric Restriction in the Diabetic Heart
There are several modes of therapy for DCM and lifestyle modification comprising caloric restriction (CR), and physical activity is considered the main therapy. Ultimately, glycemic control over time ameliorates the hazardous effects of obesity and glucose on atherosclerosis and cardiac muscle (17). Physiological studies demonstrated a similar energy intake in HF patients and higher energy expenditure in HF patients, which leads to a negative energy balance associated with increased catecholamines and proinflammatory cytokines levels that are manifested in the development of cachexia and increased mortality (7, 36, 104, 166). On the other side, obesity increases the risk of HF and contributes to its pathophysiology (87).
CR is a regimen that reduces the caloric intake in about 30%–50% below the energy demand that is required to maintain normal body weight and adiposity but still maintains essential nutrients. CR is well known for its ability to increase longevity and to delay or slow the progression of multiple age-related diseases in many laboratory animal models (31, 115, 190). Studies following individuals who practice long-term CR demonstrate that it favorably affects chronic disease risk factors and alleviates oxidative stress. Beyond the direct metabolic response, CR also influences the activity of transcription factors and gene expression (214).
In the aging cardiovascular system, CR has profound beneficial effects, likely related to a reduction in inflammation and oxidative stress (214). CR also protects against endothelial dysfunction, arterial stiffness and attenuates atherogenesis by reducing metabolic risk factors (214).
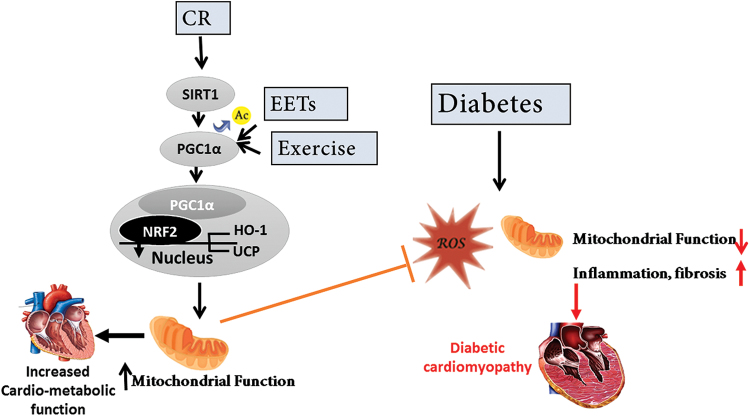
Adiponectin levels rise under CR, concomitant with a significant decline in circulating insulin and glucose levels. In the heart, CR promotes a shift from carbohydrate to fat metabolism and reduces oxidative stress. Several metabolic regulators/pathways are believed to mediate the CR effect with a focus on the insulin-like growth factor insulin signaling, AMPK, sirtuin (SIRT)1, and peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α). After CR, adiponectin and phospho-AMPK increase in the heart and are involved in cardioprotection (44, 92, 154, 177, 178). The beneficial effects on the aging cardiovascular system of CR include inhibition of nuclear nuclear factor Kappa-light-chain-enhancer of activated B cells (NFκβ) activation and the subsequent inflammatory response, protection against fibrosis, cardiomyocyte apoptosis, and preservation of left ventricular diastolic function (214). The generalized cellular mechanism of CR is illustrated in Figure 3. Figure 3 illustrates the cardiometabolic protective pathway activated by CR. A unique metabolic case of spontaneously reduced food intake and cardioprotection is depicted by transgenic alpha MUPA mice carrying the urokinase-type plasminogen activator (uPA) (105, 121) and is caused by increased levels of leptin, an anorectic satiety hormone. These mice show a set of metabolic changes compared with their control wild-type mice, including reduced food intake when fed at libitum, resistance to obesity, cardioprotection, increased lifespan, and several other benefits seen in CR mice. In contrast, leptin is strongly reduced after experimentally imposed CR, leading to sustained hunger. Leptin/JAK2/PI3K/AKT and leptin/JAK2/STAT3 cascades appear to mediate the leptin-induced cardioprotection in aMUPA mice, possibly by activating myocardial survival pathways (105, 121). In diabetic mice, CR attenuates the development of cardiomyopathy and improves metabolic markers. CR in angiotensin II-stressed-diabetic mice reduces body weight, improves the metabolic profile, and attenuates the cardiomyopathy phenotype. CR ameliorates both fibrosis and inflammation. CR was associated with increased levels of cardiac peroxisome proliferator-activated receptor (PPAR)α followed by improved fatty acids (FAs) utilization, and a reduction in the inflammatory markers TNF-α, toll-like receptor (TLR)2, and TLR4 (29).
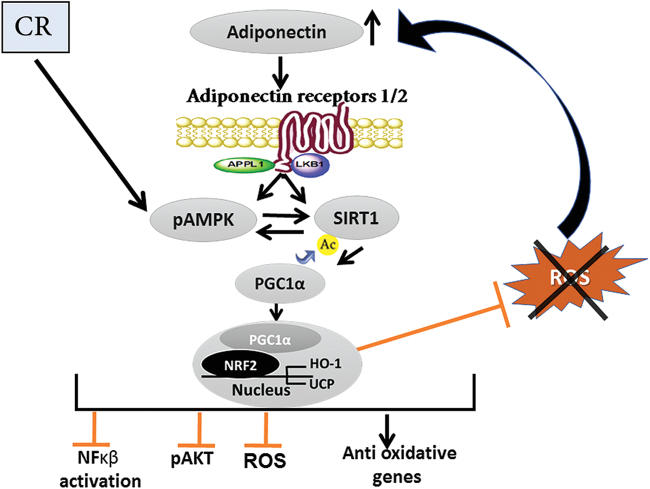
FIG. 3.
The cardiometabolic protective pathway activated by CR. After CR, SIRT1 and AMPK are activated. SIRT1, through deacetylation, increases PGC-1α translocation to the nucleus and through binding to NRF2 activates the expression of HO-1 and UCP. Ultimately, CR attenuates the inflammatory response (by preventing NFκB activation), inhibits AKT overactivation, and reduces oxidative stress. Further, the reduction in ROS prevents the degradation of circulating adiponectin levels and through the adiponectin receptors the activation of this protective pathway is enhanced. AMPK, adenosine monophosphate-activated protein kinase; CR, caloric restriction; HO-1, heme oxygenase 1; NFκB, nuclear factor Kappa-light-chain-enhancer of activated B cells; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; SIRT, sirtuin; UCP, uncoupling protein. Color images are available online.
Sirtuins
SIRTs are class III histone deacetylases that are nicotinamide adenine dinucleotide dependent for their deacetylation reaction (86, 123). In mammalians, there are seven SIRT homologs (SIRT1–SIRT7). These proteins are energy sensors that regulate a major of biological processes, including: glucose metabolism (55), gene expression (206), aging and longevity (16, 61), and cellular stress response (134). It was first demonstrated in yeast in which increased expression of SIRT2 extended the lifespan by 50%, whereas its deletion reduced lifespan. In mice, the deletion of SIRT6 showed the worst phenotype of premature aging and severe metabolic impairment (48). On the other hand, overexpression of SIRT6 in mice on HFD prevented the metabolic dysfunction and the development of comorbidities associated with cardiomyopathy (71, 81, 82, 235) and extended lifespan similarly to CR (81). Similarly, SIRT1 activation also promotes longevity and mimics CR (16, 220). The cellular protection afforded by overexpressing SIRTs includes activation of the AMPKα pathway, inhibition of apoptosis, induction of autophagy, attenuation of inflammation through the inhibition of NFκβ, a decrease in ROS production, and a reduction in the activation of protein kinase B (AKT). All these pathways protect cardiomyocytes from metabolic stress and increase cell survival and longevity (113).
Heme oxygenase 1
Heme oxygenase 1 (HO-1) is the HO-inducible isoform that degrades heme to biliverdin to release CO and iron (1, 2). Biliverdin is then reduced to form bilirubin (4, 84). HO-1 and HO-2 are similar in terms of mechanism, cofactor, and substrate requirements. HO-1 is competitively inhibited by synthetic metalloporphyrins in which the central iron atom is replaced by tin, zinc, cobalt, or chromium (3). HO-1 expression is regulated by various transcription factors, including nuclear factor erythroid-2-related factor 2, and several intracellular signaling molecules, such as MAPK and AKT. HO-1 is induced by an extraordinarily wide variety of drugs, including statins, aspirin, niacin, prostaglandins, eicosanoids, and metallic compounds (1, 161, 162). The degradation products of HO-1 iron, bilirubin, and CO have important cellular functions of their own. Iron is vital for the synthesis of hemoglobin and ferritin, and HO-1 deficiency leads to anemia (140). HO-1 deficiency leads to reduced stress defense (141) and accelerates the formation of arterial thrombosis (201). Importantly, activating the HO-1 signaling was demonstrated to reduce inflammation (99, 129) and fibrosis (33, 88) and to improve both adiposity and endothelial cell dysfunction. (137). HO-1 induction in diabetic mice reduces oxidative stress and inflammation after MI and improves heart function. In cardiomyocytes, an increase in HO-1 levels improved mitochondria membrane potential after hypoxia (73) and reduced ROS production in cardiomyocytes exposed to high glucose levels whereas inhibition of heme degradation promoted ROS production (211). In diabetic rats, increased levels of HO-1 activated AMPK and improved insulin sensitivity (129). The protective role of HO-1 against stress and metabolic dysfunction makes it a very interesting therapeutic target in the battle against obesity and its related disorders.
Peroxisome proliferator-activated receptor-gamma coactivator-1α
PGC-1α is a transcriptional coactivator of nuclear receptors and transcription factors that regulates and enhances their activity (50, 91). PGC-1α regulates oxidative metabolism, adipogenesis, mitochondrial function, and mitochondrial biogenesis (97, 143). PGC-1α was first discovered as a coactivator of the PPARs in a yeast two-hybrid system designed to identify the proteins that regulate the differentiation of brown adipose tissue (97, 144). Another metabolically important target includes nuclear respiratory factor-1 and -2 (207, 221). The induction of PGC-1α decreases lipid accumulation and mitochondrial ROS and increases adiponectin (97, 216). However, PGC-1α KO impairs mitochondrial biogenesis, reduces FA oxidation, as well as increases glucose and insulin resistance (91). The main physiological inducers are CR, fasting (103), exercise (77), and thyroid hormone (56). The activation of PGC-1α is either through deacetylation by SIRT1 or through phosphorylation by AMPK, leading to its translocation to the nucleus (97). Reduction in the expression of PGC-1α has been found to be associated with cardiac dysfunction (180). The regulation of mitochondrial biogenesis by PGC-1α is integral to normal cardiac function, and models of PGC-1α KO or deficiency have significant alterations in cardiac function and structure (8). Data from both pharmacological and genetic intervention, targeting PGC-1α and its downstream targets, support its role in maintaining mitochondrial function and the critical role of mitochondrial dysfunction in the pathogenesis of cardiomyopathy (180).
Cumulatively, these pathways lead to metabolic adaptation to glucose and energy deficiency by promoting gluconeogenesis; increasing mitochondrial function and FA oxidation; and attenuating oxidative stress (24, 101), thus playing a crucial role in maintaining cardiac function.
Ang II-Induced Hypertension, Oxidative Stress, and PGC-1α–HO-1 Signaling
Bartter's syndromes/Gitelman's syndromes (BS/GS) are rare diseases caused by genetic mutations in kidney transporters and ion channels. The patients suffer from hypokalemia, sodium depletion, and activation of the renin–angiotensin–aldosterone system. But surprisingly, these patients have normal blood pressure, reduced vascular resistance and are not responsive to pressor agents (124). In BS/GS patients, SIRT1 and HO-1 are elevated compared with patients with hypertension and healthy subjects, pointing toward a role for reduced SIRT1 and HO-1 in the endothelial dysfunction caused by hypertension (39). A link between oxidative stress mediated by Ang II and PGC-1α was shown in PGC-1α KO mice that exhibited vascular dysfunction and inflammation during chronic Ang II infusion by increasing mitochondrial ROS production (93). However, endothelial-specific PGC-1α gain of function suppressed Ang II-induced hypertension (34). Mechanistically, Ang II stimulates the phosphorylation of PGC-1 by Akt at Ser570. Sequentially, this phosphorylation is required for the binding of general control nonderepressible 5 and the lysine acetylation of PGC-1α. This chain of events disrupts the PGC-1α forkhead transcription factor 1 (FoxO1) complex bound to the FoxO1 response element in the catalase promoter, downregulating the expression of catalase, increasing ROS levels, and promoting hypertrophy (202, 223). In conclusion, hypertension and increased oxidative stress caused by Ang II induction can be attenuated by PGC-1α and HO-1 signaling.
Activation of PGC-1α and HO-1 in Response to Exercise As Antifatigue and Antioxidant Mechanism
The production of ROS and nitric oxide plays a critical role in the controlling contractile force in skeletal muscle (148), and it is evident that physical exercise increases ROS production [reviewed in ref. (130)]. When muscle cells are not stressed, ROS and nitric oxide favor a more pro-oxidant state that is necessary for optimal contractility. Conversely, the treatment of an unfatigued muscle with antioxidants can lead to a suppression of force production (32, 149). However, the production of excessive ROS decreases maximal force production and increases muscle fatigue (187). Therefore, a strenuous exercise that produces high levels of ROS negatively affects contractility and contributes to the development of fatigue (150). To counteract this, exercise training elicits several adaptive mechanisms in skeletal muscle that improve cellular metabolism: It increases the transcription of uncoupling protein 3, pyruvate dehydrogenase kinase 4, HO-1, and PGC-1α during the recovery period (138, 186). The antioxidant mechanism involving the activation of PGC-1α and HO-1 after exercise might be of similar importance for cardiomyocyte contractility.
The Role of PGC-1α–HO-1 Axis in the Attenuation of DCM
In the hearts of diabetic animals, HO-1 levels are reduced (95, 211), contributing to an increase in O2− production (95). In mice fed an HFD, HO-1 overexpression, specifically in adipose tissue, leads to weight loss; reduced blood glucose, blood pressure, and inflammatory cytokines. It also improved insulin sensitivity, adiponectin levels and increased vascular relaxation in response to acetylcholine. (20). In diabetic mice (induced by streptozotocin) treated with cobalt protoporphyrin (CoPP) to induce HO-1 expression, the increase in HO-1 was linked to an increase in adiponectin, pLKB1, pAKT, pAMPK, pGSK-3, and peNOS levels; a decrease in myocardial superoxide and 3-nitrotyrosine levels; and reduced coronary resistance (95). HO-1 ablation in adipose tissue reduced PGC-1α expression, promoted mitochondrial dysfunction, and contributed to an increase of pro-inflammatory visceral fat (182). In 3T3–L1 preadipocytes subjected to adipogenesis, induction of the PGC-1α signaling cascade prevented the differentiation of the pre-adipocyte cells into large and inflamed adipocytes, maintaining mitochondrial function and increasing HO-1 expression (209). Human studies aimed at investigating the levels of signaling molecules and inflammatory adipokines that regulate cardiovascular risk in epicardial fat from patients with HF who underwent coronary artery bypass surgery. The study revealed a decrease in mitochondrial signaling of HO-1–PGC-1α and an increase of the inflammatory adipokine cellular communication network factor family member 3 levels. Induction of HO-1 in epicardial fat attenuated cardiometabolic dysfunction and improved LV fractional shortening in obese mice, supporting the role of HO-1 signaling in cardiac metabolism and function (184). Individuals with shorter (GT)n repeats in the HO-1 gene, when compared with individuals with longer ones, have a higher transcriptional activity and thus higher HO-1 levels. The shorter (GT)n repeats polymorphism was associated with higher oxidative stress and increased risk of developing coronary artery disease and atherosclerosis (27, 227). Individuals with shorter (<27) (GT)n repeats, despite having significant risk factors (hyperlipidemia, diabetes, and smoking) for coronary artery disease, had better prognosis and reduced risk (80). These data show that polymorphisms correlated with a higher expression of HO-1 are protective against cardiovascular disease in the clinical setting.
In rats on HFD, the induction of HO-1 using CoPP not only improved cardiac function but also prevented myocardial and perivascular fibrosis. These beneficial effects were accompanied by increased levels of cardiac adiponectin levels, and the activation of AMPK, eNOS, and iNOS. Inhibition of HO activity by tin mesoporphrin abolished these beneficial effects, confirming the role of the HO-1 in the cardioprotection (22).
In summary, by examining induction/overexpression or inhibition/knocking down approaches to target HO-1, it is clear that HO-1 and HO activity is central for preventing and even reversing cardiac complications caused by the metabolic syndrome and vascular diseases [reviewed in ref. (42)].
In db/db mice, the induction of PGC-1α reduced the levels of fasting blood glucose; improved cardiac function; increased O2; elevated pro-inflammatory adipokines; activated the nephroblastoma overexpressed (NOV) signaling, HO-1 levels, and insulin receptor phosphorylation; and improved mitochondrial function. Importantly, these beneficial effects were reversed by the deletion of PGC-1α (21, 183). Significantly, the deletion of PGC-1α resulted in inhibition of HO-1, suggesting that PGC-1α regulates the expression of HO-1 (185). In a model of DCM of db/db mice treated with Ang II, reduced levels of cardiac PGC-1α were observed and were associated with increased oxidative stress. CR attenuated the cardiomyopathy and increased SIRT1 activity and PGC-1α levels. The increased activity of the PGC-1α in CR elevated the expression of cardiac HO-1 and reduced the oxidative stress (210). Inhibition of HO activity promoted the hypertrophic response in diabetic mice, abolished the protective effects of CR on the diabetic heart, increased ROS production, and dramatically reduced PGC-1α levels (211), indicating a crucial role of HO-1 and HO activity in maintaining metabolically “healthy” cardiomyocytes.
These findings suggest the critical role of PGC-1α HO-1 and their interaction, in regulating obesity, diabetes, and heart function, and that targeting this axis constitutes a novel and promising therapy for DCM. The role of HO-1 and PGC-1α in maintaining mitochondrial function is illustrated in Figure 4.
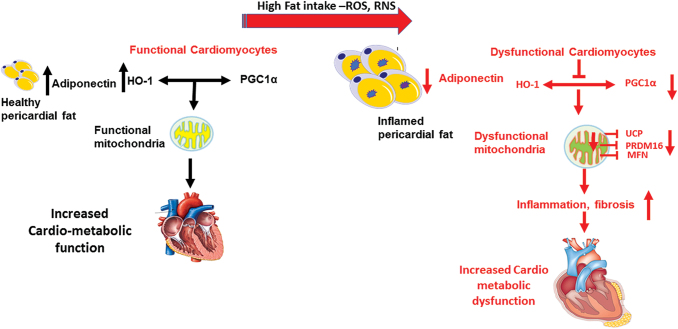
FIG. 4.
The role of HO-1 and PGC-1α in maintaining mitochondrial function. Under normal diet, high levels of adiponectin secreted for “healthy” adipocytes increase the activity of HO-1 and PGC-1α that are important to mitochondrial and, subsequently, cardiac function. Under high-fat diet condition, ROS and RNS levels are increased, and inflamed dysfunctional adipocytes secrete less adiponectin, resulting in reduced levels and activity of HO-1 and PGC-1α, reduced level of mitochondrial fusion proteins, and impaired mitochondrial function, culminating in cardiac metabolic dysfunction, inflammation, and fibrosis and the development of diabetic cardiomyopathy. Color images are available online.
Epoxyeicosatrienoic acids As Inducers of PGC-1α and HO-1
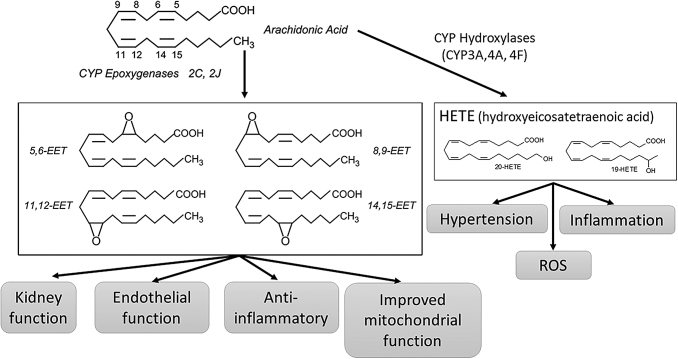
Arachidonic acids, a group of polyunsaturated fatty acids that are part of the membranal phospholipids membranes, are degraded by the enzyme cytochrome P450 to form a group of monooxygenase metabolites called “epoxyeicosatrienoic acids” (EETs) and two isoforms of hydroxyeicosatrienoic acids (HETEs). The metabolism of arachidonic acid to EETs and its physiological function is shown in Figure 5 (169). HETEs and EETs have a contrasting effect on the vascular tone, whereas HETEs function primarily as vasoconstrictors in the microcirculation. EET functions as a vasodilator (64). Soluble epoxide hydrolase (sEH) metabolizes EET to dihydroxyeicosatrienoic acid, a metabolite that has only 20% the activity of EETs (191, 232). sEH inhibition or deletion increases cellular and circulating EETs (mainly 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET) (131, 192); it results in a significant decrease in adipocyte size, inflammatory adipokines NOV and TNF-α, while increasing adiponectin. The differentiation into beige-like fat, and the expression of mitochondrial and thermogenic genes are promoted. Overexpression of HO-1 further increased the levels of EETs, suggesting that the antioxidant HO-1 system protects EETs from degradation by ROS. This also suggests that EETs and HO-1 act synergistically and their effects on adipocytes and vascular function are greater than the effects of sEH deletion alone. (110). Treatment with EET agonists prevented both vascular dysfunction (72) and adiposity in vitro (90) and in vivo in mice fed an HFD (189) and increased antioxidant levels, including HO-1 (3, 11, 188). EET-mediated increase in HO-1 upregulates Akt signaling cascade and the activity of eNOS (23, 89), resulting in vasodilation (163, 164). In addition, EET induction reduced the expression of adipogenic markers, affecting adipocyte differentiation (89). This is highlighted by the evidence in obese and diabetic mice where treatment with EET analog (EET-A [(S)-2-(11-(nonyloxy) undec-8(Z)-enamido) succinic acid]) diminished fatty acid accumulation, fibrosis, improved renal function, and attenuated non-alcoholic fatty liver disease through upregulation of HO-1 and PGC-1α (145, 168). This was mirrored by a similar metabolic improvement by HO-1 induction (165). EET agonist as well as overexpression of CYP2J2 attenuated cardiomyopathy in diabetic mice (111, 188). These results suggest that EET has the potential to have a profound effect on hypertension, metabolic syndrome, and cardiac failure. In the 3T3 cells model of adipocyte differentiation, an EET-A induced the PGC-1α expression and protein levels, whereas silencing of PGC-1α abolished EET-mediated elevation of HO-1 levels (181, 209). In mice fed an HFD treated with EET-A, levels of PGC-1α and HO-1 were elevated in adipose tissue, an increase that was abolished in the absence of PGC-1α. The EET-PGC1α-mediated reduction in adiposity in mice fed an HFD involved upregulated mitochondrial function, demonstrated by an increase in MnSOD and SIRT3, as well as in three fusion mediators, Mitofusin 1 and 2, and OPA1, regulating adipocyte cell differentiation, blood pressure and improving metabolism (185). In db/db mice, EET-A ameliorated cardiomyopathy and improved mitochondrial function. Similar to the finding in adipocytes, EET-A's beneficial effects against DCM were mediated by PGC-1α (21, 183). These studies demonstrate that both in vitro and in vivo EET is upstream of the PGC-1α signaling. Thus, an EETs agonist can serve as an inducer of PGC-1α and its downstream signaling. Further, the increase in the activity of HO-1 by EET is dependent on PGC-1α.
FIG. 5.
The metabolism of arachidonic acid to EETs and its physiological function. Arachidonic acid through CYP epoxygenases is metabolite to EETs or through CYP hydroxylases to HETEs. EETs that can have local effects or endocrine effects improve kidney and endothelial function, attenuate inflammation and oxidative stress. On the other hand, HETEs promote hypertension inflammation and ROS production. EET, epoxyeicosatrienoic acid; HETE, hydroxyeicosatrienoic acid.
To summarize, EETs agonists or sEH inhibitors emerge as a promising strategy for pharmacological treatment in metabolic disorders and some of them are already in clinical trials. EETs, through PGC-1α (acting as an inducer of PGC-1α), upregulate HO-1, to reduce inflammation, improve mitochondrial function, and increase vasodilation (109, 155). As there is no known direct pharmacological inducer for PGC-1α, its induction by EETs agonists is a novel and promising therapeutic approach.
Summary and Future Perspectives
Diabetes mellitus is posing a significant clinical challenge and burden around the globe, mainly due to the increased risk of cardiovascular diseases. Diabetic patients have a higher risk of developing diastolic dysfunction and atherosclerosis and some develop DCM. Despite extensive research, there are no specific and effective therapies for DCM. Therefore, unraveling the complex molecular and pathophysiological mechanisms that define the disease process, mainly the precise mechanisms linking chronic metabolic dysfunction observed in diabetes with the unique structural and functional changes, is important to the development of effective treatments. Clinical and preclinical models indicate that the combination of metabolic and biochemical stressors promotes heart dysfunction present in diabetic patients. It has become apparent that increased oxidative/nitrosative stress and inflammation are central triggers to the pathological processes associated with DCM. Despite the high prevalence and mortality of patients with DCM, the precise factors that trigger DCM remain unclear; therefore, research focusing on metabolic pathways that are disturbed in the diabetic heart to develop personalized and precise medicine is needed (78).
CR has profound beneficial effects on the aging or injured cardiovascular system, by reducing oxidative stress and inflammation in the vascular system and the heart as well as preventing adiposity and insulin resistance. From studies of diabetic models, the downregulation of the PGC-1α—HO-1 axis seems to be a crucial event in the development of obesity and DCM. Thus, targeting the PGC-1α–HO-1 axis is a promising approach to ameliorate DCM through improvement in mitochondrial function and antioxidant defenses. Since CR is hard to employ and maintain, a pharmacological inducer to activate PGC-1α and HO-1 and gain the beneficial effects of CR and/or exercise, as this review suggests, may be a promising alternative in the clinical setting as shown in Figure 6.
FIG. 6.
Inducers of the PGC-1α pathway improve mitochondrial function. CR, EETs, or exercise promotes the deacetylation and activation of PGC-1α. Deacetylated PGC-1α translocates to the nucleus and together with the transcription factor NRF2 induces the expression of mitochondrial and antioxidant genes such as HO-1 and UCP. Targeting this pathway has a therapeutic potential to improve mitochondrial function and prevent or attenuate the development of diabetic cardiomyopathy. NRF2, nuclear factor erythroid-2-related factor 2. Color images are available online.
Abbreviations Used
- AMPK
adenosine monophosphate-activated protein kinase
- Ang II
angiotensin II
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- BS
Bartter's syndromes
- CoPP
cobalt protoporphyrin
- CR
caloric restriction
- DM2
type 2 diabetes mellitus
- ECM
extracellular matrix
- EET
epoxyeicosatrienoic acid
- EET-A
EET analog
- eNOS
endothelial nitric oxide synthase
- FoxO1
forkhead transcription factor 1
- GCN5
general control nonderepressible 5
- GS
Gitelman's syndromes
- HETE
hydroxyeicosatrienoic acid
- HF
heart failure
- HFD
high-fat diet
- HO-1
heme oxygenase 1
- iNOS
inducible nitric oxide synthase
- KO
knock out
- LC3
microtubule-associated protein light chain 3
- LV
left ventricle
- MAPK
mitogen-activated protein kinase
- MFN
mitofusin
- MMP
matrix metalloproteinases
- MI
myocardial infarction
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor Kappa-light-chain-enhancer of activated B cells
- NOS
nitrite oxide synthases
- NRF2
nuclear factor erythroid-2-related factor 2
- NOV
nephroblastoma overexpressed
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator-1α
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- sEH
soluble epoxide hydrolase
- SIRT
sirtuin
- SOD
superoxide dismutase
- TGF-β1
transforming growth factor β1
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- UCP
uncoupling protein
- XO
xanthine oxidase
Funding Information
This work was supported by the National Institutes of Health (grant 1R56HL139561; N.G.A.).
References
- 1. Abraham N, Drummond G, Lutton J, and Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem 6: 129–168, 1996 [Google Scholar]
- 2. Abraham NG, Junge JM, and Drummond GS. Translational significance of heme oxygenase in obesity and metabolic syndrome. Trends Pharmacol Sci 37: 17–36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham NG and Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Ahmad Z, Salim M, and Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J Biol Chem 277: 9226–9232, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Al-Aubaidy HA and Jelinek HF. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol 164: 899–904, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Alagiakrishnan K, Banach M, Jones LG, Datta S, Ahmed A, and Aronow WS. Update on diastolic heart failure or heart failure with preserved ejection fraction in the older adults. Ann Med 45: 37–50, 2013 [DOI] [PubMed] [Google Scholar]
- 7. Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E, and Pastoris O. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol 42: 1218–1223, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, and Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Asbun J and Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 47: 693–700, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Bajraktari G, Koltai MS, Ademaj F, Rexhepaj N, Qirko S, Ndrepepa G, and Elezi S. Relationship between insulin resistance and left ventricular diastolic dysfunction in patients with impaired glucose tolerance and type 2 diabetes. Int J Cardiol 110: 206–211, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Balla J, Jacob HS, Balla G, Nath K, Eaton JW, and Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A 90: 9285–9289, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ban CR and Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag 4: 575–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Begum N and Ragolia L. High glucose and insulin inhibit VSMC MKP-1 expression by blocking iNOS via p38 MAPK activation. Am J Physiol Cell Physiol 278: C81–C91, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Belke DD, Larsen TS, Gibbs EM, and Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 279: E1104–E1113, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Bell DS. Hypertension and diabetes: a toxic combination. Endocr Pract 14: 1031–1039, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, and Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6: 759–767, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Brahma MK, Pepin ME, and Wende AR. My sweetheart is broken: role of glucose in diabetic cardiomyopathy. Diabetes Metab J 41: 1–9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai L, Li W, Wang G, Guo L, Jiang Y, and Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 51: 1938–1948, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, and Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92: 785–792, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG, and Kappas A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension 60: 467–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao J, Singh SP, McClung JA, Joseph G, Vanella L, Barbagallo I, Jiang H, Falck JR, Arad M, Shapiro JI, and Abraham NG. EET intervention on Wnt1, NOV, and HO-1 signaling prevents obesity-induced cardiomyopathy in obese mice. Am J Physiol Heart Circ Physiol 313: H368–H380, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao J, Sodhi K, Puri N, Monu SR, Rezzani R, and Abraham NG. High fat diet enhances cardiac abnormalities in SHR rats: protective role of heme oxygenase-adiponectin axis. Diabetol Metab Syndr 3: 37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao J, Tsenovoy PL, Thompson EA, Falck JR, Touchon R, Sodhi K, Rezzani R, Shapiro JI, and Abraham NG. Agonists of epoxyeicosatrienoic acids reduce infarct size and ameliorate cardiac dysfunction via activation of HO-1 and Wnt1 canonical pathway. Prostaglandins Other Lipid Mediat 116–117: 76–86, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chalkiadaki A and Guarente L. Metabolic signals regulate SIRT1 expression. EMBO Rep 12: 985–986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang KC, Tseng CD, Chou TF, Cho YL, Chi TC, Su MJ, and Tseng YZ. Arterial stiffening and cardiac hypertrophy in a new rat model of type 2 diabetes. Eur J Clin Invest 36: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Chattopadhyay M, Khemka VK, Chatterjee G, Ganguly A, Mukhopadhyay S, and Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol Cell Biochem 399: 95–103, 2015 [DOI] [PubMed] [Google Scholar]
- 27. Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, and Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Cheng CP, Cheng H-J, Shao Q, Zhang L, Callahan MF, Kitzman D, Zhao D, and Wagner JD. Upregulation of cardiac beta3-adrenergic receptor-activated inducible nitric oxide synthase (iNOS)-coupled pathway promotes diabetic cardiomyopathy in spontaneous diabetic cynomolgus monkeys: role of oxidant stress. Circulation 138: A11401, 2018 [Google Scholar]
- 29. Cohen K, Waldman M, Abraham NG, Laniado-Schwartzman M, Gurfield D, Aravot D, Arad M, and Hochhauser E. Caloric restriction ameliorates cardiomyopathy in animal model of diabetes. Exp Cell Res 350: 147–153, 2017 [DOI] [PubMed] [Google Scholar]
- 30. Collaborators GO. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377: 13–27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, and Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5: 3557, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coombes JS, Powers SK, Rowell B, Hamilton KL, Dodd SL, Shanely RA, Sen CK, and Packer L. Effects of vitamin E and alpha-lipoic acid on skeletal muscle contractile properties. J Appl Physiol (1985) 90: 1424–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Correa-Costa M, Semedo P, Monteiro AP, Silva RC, Pereira RL, Goncalves GM, Marques GD, Cenedeze MA, Faleiros AC, Keller AC, Shimizu MH, Seguro AC, Reis MA, Pacheco-Silva A, and Camara NO. Induction of heme oxygenase-1 can halt and even reverse renal tubule-interstitial fibrosis. PLoS One 5: e14298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Craige SM, Kroller-Schon S, Li C, Kant S, Cai S, Chen K, Contractor MM, Pei Y, Schulz E, and Keaney JF Jr. PGC-1alpha dictates endothelial function through regulation of eNOS expression. Sci Rep 6: 38210, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341 (Pt 2): 233–249, 1999 [PMC free article] [PubMed] [Google Scholar]
- 36. Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, and Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 165: 55–61, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, and van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 195: 321–338, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Davidoff AJ, Davidson MB, Carmody MW, Davis ME, and Ren J. Diabetic cardiomyocyte dysfunction and myocyte insulin resistance: role of glucose-induced PKC activity. Mol Cell Biochem 262: 155–163, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Davis PA, Pagnin E, Dal Maso L, Caielli P, Maiolino G, Fusaro M, Paolo Rossi G, and Calo LA. SIRT1, heme oxygenase-1 and NO-mediated vasodilation in a human model of endogenous angiotensin II type 1 receptor antagonism: implications for hypertension. Hypertens Res 36: 873–878, 2013 [DOI] [PubMed] [Google Scholar]
- 40. Devereux RB, Roman MJ, Paranicas M, O'grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, and Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 101: 2271–2276, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Dong K, Ni H, Wu M, Tang Z, Halim M, and Shi D. ROS-mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes. Biochem Biophys Res Commun 476: 204–211, 2016 [DOI] [PubMed] [Google Scholar]
- 42. Drummond GS, Baum J, Greenberg M, Lewis D, and Abraham NG. HO-1 overexpression and underexpression: clinical implications. Arch Biochem Biophys 673: 108073, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, and Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 8: 325–332, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, and Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev 131: 739–742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Einarson TR, Acs A, Ludwig C, and Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol 17: 83, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eskelinen EL and Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793: 664–673, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Faria AM, Papadimitriou A, Silva KC, Lopes de Faria JM, and Lopes de Faria JB. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 61: 1838–1847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Finkel T, Deng CX, and Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, and Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-dependent. Lab Invest 80: 513–527, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, and Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem 387: 1521–1533, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, and Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Galderisi M, Anderson KM, Wilson PW, and Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 68: 85–89, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Gao X, Xu Y, Xu B, Liu Y, Cai J, Liu HM, Lei S, Zhong YQ, Irwin MG, and Xia Z. Allopurinol attenuates left ventricular dysfunction in rats with early stages of streptozotocin-induced diabetes. Diabetes Metab Res Rev 28: 409–417, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, and Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goldenthal MJ, Weiss HR, and Marin-Garcia J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem 265: 97–106, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Gottlieb RA and Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol 299: C203–C210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gottlieb RA and Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol 72: 45–59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gray MO, Long CS, Kalinyak JE, Li HT, and Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res 40: 352–363, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Grossman W. Diastolic dysfunction in congestive heart failure. N Engl J Med 325: 1557–1564, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Haigis MC and Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Hall JE. The kidney, hypertension, and obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Hamilton S and Terentyev D. Proarrhythmic remodeling of calcium homeostasis in cardiac disease; implications for diabetes and obesity. Front Physiol 9: 1517, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harder D, Campbell W, and Roman R. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32: 79–92, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Hiranandani N, Bupha-Intr T, and Janssen PM. SERCA overexpression reduces hydroxyl radical injury in murine myocardium. Am J Physiol Heart Circ Physiol 291: H3130–H3135, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Huynh FK, Hershberger KA, and Hirschey MD. Targeting sirtuins for the treatment of diabetes. Diabetes Manag (Lond) 3: 245–257, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huynh K, Kiriazis H, Du XJ, Love JE, Jandeleit-Dahm KA, Forbes JM, McMullen JR, and Ritchie RH. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia 55: 1544–1553, 2012 [DOI] [PubMed] [Google Scholar]
- 69. Huynh K, McMullen JR, Julius TL, Tan JW, Love JE, Cemerlang N, Kiriazis H, Du XJ, and Ritchie RH. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 59: 1512–1520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, and Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116: 264–278, 2015 [DOI] [PubMed] [Google Scholar]
- 71. Imai S and Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci 31: 212–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opin Drug Metab Toxicol 4: 165–174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Issan Y, Kornowski R, Aravot D, Shainberg A, Laniado-Schwartzman M, Sodhi K, Abraham NG, and Hochhauser E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS One 9: e92246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jaishy B, Zhang Q, Chung HS, Riehle C, Soto J, Jenkins S, Abel P, Cowart LA, Van Eyk JE, and Abel ED. Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. J Lipid Res 56: 546–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ji L, Liu F, Jing Z, Huang Q, Zhao Y, Cao H, Li J, Yin C, Xing J, and Li F. MICU1 Alleviates diabetic cardiomyopathy through mitochondrial Ca2+-dependent antioxidant response. Diabetes 66: 1586–1600, 2017 [DOI] [PubMed] [Google Scholar]
- 76. Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, and Norhammar A. Risk factors, treatment and prognosis in men and women with heart failure with and without diabetes. Heart 101: 1139–1148, 2015 [DOI] [PubMed] [Google Scholar]
- 77. Joseph AM, Pilegaard H, Litvintsev A, Leick L, and Hood DA. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem 42: 13–29, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Kain V and Halade GV. Metabolic and biochemical stressors in diabetic cardiomyopathy. Front Cardiovasc Med 4: 31, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M, Seishima M, and Minatoguchi S. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 11: 1146–1160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, Aizawa T, Ishizaka N, and Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol 22: 1680–1685, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, and Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483: 218–221, 2012 [DOI] [PubMed] [Google Scholar]
- 82. Kanfi Y, Peshti V, Gil R, Naiman S, Nahum L, Levin E, Kronfeld-Schor N, and Cohen HY. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 9: 162–173, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Kannel WB, Hjortland M, and Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34: 29–34, 1974 [DOI] [PubMed] [Google Scholar]
- 84. Kapitulnik J and Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci 30: 129–137, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Karbach S, Wenzel P, Waisman A, Munzel T, and Daiber A. eNOS uncoupling in cardiovascular diseases-the role of oxidative stress and inflammation. Curr Pharm Des 20: 3579–3594, 2014 [DOI] [PubMed] [Google Scholar]
- 86. Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, and Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136: 62–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, and Vasan RS. Obesity and the risk of heart failure. N Engl J Med 347: 305–313, 2002 [DOI] [PubMed] [Google Scholar]
- 88. Kie JH, Kapturczak MH, Traylor A, Agarwal A, and Hill-Kapturczak N. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, and Schwartzman ML. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARγ. Stem Cells Dev 19: 1863–1873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, and Abraham NG. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev 19: 1863–1873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, and Spiegelman BM. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci U S A 109: 9635–9640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, and Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem 284: 1718–1724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kroller-Schon S, Jansen T, Schuler A, Oelze M, Wenzel P, Hausding M, Kerahrodi JG, Beisele M, Lackner KJ, Daiber A, Munzel T, and Schulz E. Peroxisome proliferator-activated receptor gamma, coactivator 1alpha deletion induces angiotensin II-associated vascular dysfunction by increasing mitochondrial oxidative stress and vascular inflammation. Arterioscler Thromb Vasc Biol 33: 1928–1935, 2013 [DOI] [PubMed] [Google Scholar]
- 94. Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, and Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Horm Metab Res 39: 672–676, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Kusmic C, L'Abbate A, Sambuceti G, Drummond G, Barsanti C, Matteucci M, Cao J, Piccolomini F, Cheng J, and Abraham NG. Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling. J Cell Biochem 109: 1033–1044, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao R, Siwik DA, and Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med 48: 1182–1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, and Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 98. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, and Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Laniado-Schwartzman M, Abraham NG, Conners M, Dunn MW, Levere RD, and Kappas A. Heme oxygenase induction with attenuation of experimentally induced corneal inflammation. Biochem Pharmacol 53: 1069–1075, 1997 [DOI] [PubMed] [Google Scholar]
- 100. Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, Szklo M, and Ward BJ. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: the Cardiovascular Health Study. Am Heart J 133: 36–43, 1997 [DOI] [PubMed] [Google Scholar]
- 101. Lee S-J, Zhang J, Choi AM, and Kim HP. Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid Med Cell Longev 2013: 327167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lee SC and Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol 39: 497–504, 2007 [DOI] [PubMed] [Google Scholar]
- 103. Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, and Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lennie TA. Nutrition self-care in heart failure: state of the science. J Cardiovasc Nurs 23: 197–204, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Levy E, Kornowski R, Gavrieli R, Fratty I, Greenberg G, Waldman M, Birk E, Shainberg A, Akirov A, Miskin R, and Hochhauser E. Long-lived alphaMUPA mice show attenuation of cardiac aging and leptin-dependent cardioprotection. PLoS One 10: e0144593, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li L, Luo W, Qian Y, Zhu W, Qian J, Li J, Jin Y, Xu X, and Liang G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 59: 152774, 2019 [DOI] [PubMed] [Google Scholar]
- 107. Lijnen P and Petrov V. Renin-angiotensin system, hypertrophy and gene expression in cardiac myocytes. J Mol Cell Cardiol 31: 949–970, 1999 [DOI] [PubMed] [Google Scholar]
- 108. Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, and Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front Physiol 6: 20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu L, Huang X, Gao J, Guo Y, and Di Y. EET intervention on HO-1 prevent obesity derived cardiovascular diseases. J Biomol Res Ther 7: 2, 2018 [Google Scholar]
- 110. Liu L, Puri N, Raffaele M, Schragenheim J, Singh SP, Bradbury JA, Bellner L, Vanella L, Zeldin DC, Cao J, and Abraham NG. Ablation of soluble epoxide hydrolase reprogram white fat to beige-like fat through an increase in mitochondrial integrity, HO-1-adiponectin in vitro and in vivo. Prostaglandins Other Lipid Mediat 138: 1–8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ma B, Xiong X, Chen C, Li H, Xu X, Li X, Li R, Chen G, Dackor RT, Zeldin DC, and Wang DW. Cardiac-specific overexpression of CYP2J2 attenuates diabetic cardiomyopathy in male streptozotocin-induced diabetic mice. Endocrinology 154: 2843–2856, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Maisch B, Alter P, and Pankuweit S. Diabetic cardiomyopathy—fact or fiction? Herz 36: 102–115, 2011 [DOI] [PubMed] [Google Scholar]
- 113. Maksin-Matveev A, Kanfi Y, Hochhauser E, Isak A, Cohen HY, and Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp Cell Res 330: 81–90, 2015 [DOI] [PubMed] [Google Scholar]
- 114. Maritim AC, Sanders RA, and Watkins JB 3rd.. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17: 24–38, 2003 [DOI] [PubMed] [Google Scholar]
- 115. Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, and de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. McMullen JR and Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34: 255–262, 2007 [DOI] [PubMed] [Google Scholar]
- 117. Mellor KM, Bell JR, Young MJ, Ritchie RH, and Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 50: 1035–1043, 2011 [DOI] [PubMed] [Google Scholar]
- 118. Mellor KM, Reichelt ME, and Delbridge LM. Autophagy anomalies in the diabetic myocardium. Autophagy 7: 1263–1267, 2011 [DOI] [PubMed] [Google Scholar]
- 119. Menke A, Casagrande S, Geiss L, and Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 314: 1021–1029, 2015 [DOI] [PubMed] [Google Scholar]
- 120. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, and Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. Jama 317: 912–924, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miskin R and Masos T. Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J Gerontol A Biol Sci Med Sci 52: B118–B124, 1997 [DOI] [PubMed] [Google Scholar]
- 122. Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, Ohmori K, and Matsuo H. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation 101: 899–907, 2000 [DOI] [PubMed] [Google Scholar]
- 123. Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, and Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124: 315–329, 2006 [DOI] [PubMed] [Google Scholar]
- 124. Naesens M, Steels P, Verberckmoes R, Vanrenterghem Y, and Kuypers D. Bartter's and Gitelman's syndromes: from gene to clinic. Nephron Physiol 96: 65–78, 2004 [DOI] [PubMed] [Google Scholar]
- 125. Nakagami H, Takemoto M, and Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol 35: 851–859, 2003 [DOI] [PubMed] [Google Scholar]
- 126. Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, and Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 98: 794–799, 1998 [DOI] [PubMed] [Google Scholar]
- 127. Ni R, Cao T, Xiong S, Ma J, Fan G-C, Lacefield JC, Lu Y, Le Tissier S, and Peng T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med 90: 12–23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nichols GA, Gullion CM, Koro CE, Ephross SA, and Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes care 27: 1879–1884, 2004 [DOI] [PubMed] [Google Scholar]
- 129. Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, and Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53: 508–515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Niess AM and Simon P. Response and adaptation of skeletal muscle to exercise—the role of reactive oxygen species. Front Biosci 12: 4826–4838, 2007 [DOI] [PubMed] [Google Scholar]
- 131. Node K, Huo YQ, Ruan XL, Yang BC, Spiecker M, Ley K, Zeldin DC, and Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ochoa CD, Wu RF, and Terada LS. ROS signaling and ER stress in cardiovascular disease. Mol Aspects Med 63: 18–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Okonko DO and Shah AM. Heart failure: mitochondrial dysfunction and oxidative stress in CHF. Nat Rev Cardiol 12: 6, 2015 [DOI] [PubMed] [Google Scholar]
- 134. Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, and Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science 317: 516–519, 2007 [DOI] [PubMed] [Google Scholar]
- 135. Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013 [DOI] [PubMed] [Google Scholar]
- 136. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, and Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539–2550, 2007 [DOI] [PubMed] [Google Scholar]
- 137. Peterson SJ, Rubinstein R, Faroqui M, Raza A, Boumaza I, Zhang Y, Stec D, and Abraham NG. Positive effects of heme oxygenase upregulation on adiposity and vascular dysfunction: gene targeting vs. pharmacologic therapy. Int J Mol Sci 20: pii:, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pilegaard H, Ordway GA, Saltin B, and Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279: E806–E814, 2000 [DOI] [PubMed] [Google Scholar]
- 139. Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJV, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, and Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 27: 65–75, 2005 [DOI] [PubMed] [Google Scholar]
- 140. Poss KD and Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Poss KD and Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A 94: 10925–10930, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Prathipati P, Metreveli N, Nandi SS, Tyagi SC, and Mishra PK. Ablation of matrix metalloproteinase-9 prevents cardiomyocytes contractile dysfunction in diabetics. Front Physiol 7: 93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Puigserver P and Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Puigserver P, Wu Z, Park CW, Graves R, Wright M, and Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 145. Raffaele M, Bellner L, Singh SP, Favero G, Rezzani R, Rodella LF, Falck JR, Abraham NG, and Vanella L. Epoxyeicosatrienoic intervention improves NAFLD in leptin receptor deficient mice by an increase in PGC1alpha-HO-1-PGC1alpha-mitochondrial signaling. Exp Cell Res 380: 180–187, 2019 [DOI] [PubMed] [Google Scholar]
- 146. Rajesh M, Mukhopadhyay P, Batkai S, Mukhopadhyay B, Patel V, Hasko G, Szabo C, Mabley JG, Liaudet L, and Pacher P. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med 13: 2330–2341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Raza H, John A, and Howarth FC. Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell Physiol Biochem 35: 1241–1251, 2015 [DOI] [PubMed] [Google Scholar]
- 148. Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc 33: 371–376, 2001 [DOI] [PubMed] [Google Scholar]
- 149. Reid MB, Khawli F, and Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol 75: 1081–1087, 1993 [DOI] [PubMed] [Google Scholar]
- 150. Reid MB, Stokic DS, Koch SM, Khawli FA, and Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94: 2468–2474, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rifki OF and Hill JA. Cardiac autophagy: good with the bad. J Cardiovasc Pharmacol 60: 248–252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ritchie RH, Love JE, Huynh K, Bernardo BC, Henstridge DC, Kiriazis H, Tham YK, Sapra G, Qin C, Cemerlang N, Boey EJ, Jandeleit-Dahm K, Du XJ, and McMullen JR. Enhanced phosphoinositide 3-kinase(p110alpha) activity prevents diabetes-induced cardiomyopathy and superoxide generation in a mouse model of diabetes. Diabetologia 55: 3369–3381, 2012 [DOI] [PubMed] [Google Scholar]
- 153. Ritchie RH, Quinn JM, Cao AH, Drummond GR, Kaye DM, Favaloro JM, Proietto J, and Delbridge LM. The antioxidant tempol inhibits cardiac hypertrophy in the insulin-resistant GLUT4-deficient mouse in vivo. J Mol Cell Cardiol 42: 1119–1128, 2007 [DOI] [PubMed] [Google Scholar]
- 154. Rohrbach S, Aurich AC, Li L, and Niemann B. Age-associated loss in adiponectin-activation by caloric restriction: lack of compensation by enhanced inducibility of adiponectin paralogs CTRP2 and CTRP7. Mol Cell Endocrinol 277: 26–34, 2007 [DOI] [PubMed] [Google Scholar]
- 155. Romashko M, Schragenheim J, Abraham NG, and McClung JA. Epoxyeicosatrienoic acid as therapy for diabetic and ischemic cardiomyopathy. Trends Pharmacol Sci 37: 945–962, 2016 [DOI] [PubMed] [Google Scholar]
- 156. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, and Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602, 1972 [DOI] [PubMed] [Google Scholar]
- 157. Ruiz-Ramírez A, López-Acosta O, Barrios-Maya MA, and El-Hafidi M. Cell death and heart failure in obesity: role of uncoupling proteins. Oxid Med Cell Longev 2016: 9340654, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Russo I and Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 90: 84–93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, and Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 122: 3919–3930, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ruwhof C and van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47: 23–37, 2000 [DOI] [PubMed] [Google Scholar]
- 161. Ryter SW and Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal 4: 625–632, 2002 [DOI] [PubMed] [Google Scholar]
- 162. Ryter SW, Otterbein LE, Morse D, and Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234–235: 249–263, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, and Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol 291: H1999–H2002, 2006 [DOI] [PubMed] [Google Scholar]
- 164. Sacerdoti D, Colombrita C, Di Pascoli M, Schwartzman ML, Bolognesi M, Falck JR, Gatta A, and Abraham NG. 11,12-epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat 82: 155–161, 2007 [DOI] [PubMed] [Google Scholar]
- 165. Sacerdoti D, Singh SP, Schragenheim J, Bellner L, Vanella L, Raffaele M, Meissner A, Grant I, Favero G, Rezzani R, Rodella LF, Bamshad D, Lebovics E, and Abraham NG. Development of NASH in obese mice is confounded by adipose tissue increase in inflammatory NOV and oxidative stress. Int J Hepatol 2018: 3484107, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sandek A, Doehner W, Anker SD, and von Haehling S. Nutrition in heart failure: an update. Curr Opin Clin Nutr Metab Care 12: 384–391, 2009 [DOI] [PubMed] [Google Scholar]
- 167. Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, and Wang ZV. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568: 351, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Schragenheim J, Bellner L, Cao J, Singh SP, Bamshad D, McClung JA, Maayan O, Meissner A, Grant I, Stier CT Jr., and Abraham NG. EET enhances renal function in obese mice resulting in restoration of HO-1-Mfn1/2 signaling, and decrease in hypertension through inhibition of sodium chloride co-transporter. Prostaglandins Other Lipid Mediat 137: 30–39, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Schwartzman ML, Martasek P, Rios AR, Levere RD, Solangi K, Goodman AI, and Abraham NG. Cytochrome P450-dependent arachidonic acid metabolism in human kidney. Kidney Int 37: 94–99, 1990 [DOI] [PubMed] [Google Scholar]
- 170. Sciarretta S, Boppana VS, Umapathi M, Frati G, and Sadoshima J. Boosting autophagy in the diabetic heart: a translational perspective. Cardiovasc Diagn Ther 5: 394, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sciarretta S, Maejima Y, Zablocki D, and Sadoshima J. The role of autophagy in the heart. Annu Rev Physiol 80: 1–26, 2018 [DOI] [PubMed] [Google Scholar]
- 172. Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, and Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 125: 1134–1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Seddon M, Looi YH, and Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93: 903–907, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Selemidis S, Sobey CG, Wingler K, Schmidt HH, and Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120: 254–291, 2008 [DOI] [PubMed] [Google Scholar]
- 175. Shamhart PE, Luther DJ, Adapala RK, Bryant JE, Petersen KA, Meszaros JG, and Thodeti CK. Hyperglycemia enhances function and differentiation of adult rat cardiac fibroblasts. Can J Physiol Pharmacol 92: 598–604, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, Okada Y, and Nakanishi I. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol 46: 32–36, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Shinmura K, Tamaki K, and Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol 39: 285–296, 2005 [DOI] [PubMed] [Google Scholar]
- 178. Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, and Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 116: 2809–2817, 2007 [DOI] [PubMed] [Google Scholar]
- 179. Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, and Sadoshima J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 133: 1249–1263, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sihag S, Cresci S, Li AY, Sucharov CC, and Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol 46: 201–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Singh SP, Bellner L, Vanella L, Cao J, Falck JR, Kappas A, and Abraham NG. Downregulation of PGC-1alpha prevents the beneficial effect of EET-heme oxygenase-1 on mitochondrial integrity and associated metabolic function in obese mice. J Nutr Metab 2016: 9039754, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Singh SP, Grant I, Meissner A, Kappas A, and Abraham NG. Ablation of adipose-HO-1 expression increases white fat over beige fat through inhibition of mitochondrial fusion and of PGC1α in female mice. Horm Mol Biol Clin Investig 31: [Epub ahead of print]; DOI: 10.1515/hmbci-2017-0027, 2017 [DOI] [PubMed] [Google Scholar]
- 183. Singh SP, McClung JA, Bellner L, Cao J, Waldman M, Schragenheim J, Arad M, Hochhauser E, Falck JR, Weingarten JA, Peterson SJ, and Abraham NG. CYP-450 epoxygenase derived epoxyeicosatrienoic acid contribute to reversal of heart failure in obesity-induced diabetic cardiomyopathy via PGC-1 alpha activation. Cardiovasc Pharm Open Access 7: pii:, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Singh SP, McClung JA, Thompson E, Glick Y, Greenberg M, Acosta-Baez G, Edris B, Shapiro JI, and Abraham NG. Cardioprotective heme oxygenase-1-PGC1α signaling in epicardial fat attenuates cardiovascular Risk in humans as in obese mice. Obesity (Silver Spring) 27: 1634–1643, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, and Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat 125: 8–18, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Skovgaard C, Brandt N, Pilegaard H, and Bangsbo J. Combined speed endurance and endurance exercise amplify the exercise-induced PGC-1α and PDK4 mRNA response in trained human muscle. Physiol Rep 4: e12864, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Smith MA and Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol 151: 229–241, 2006 [DOI] [PubMed] [Google Scholar]
- 188. Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, and Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther 331: 906–916, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, and Abraham NG. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prostaglandins Other Lipid Mediat 98: 133–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Speakman JR and Mitchell SE. Caloric restriction. Mol Aspects Med 32: 159–221, 2011 [DOI] [PubMed] [Google Scholar]
- 191. Spector AA, Fang X, Snyder GD, and Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43: 55–90, 2004 [DOI] [PubMed] [Google Scholar]
- 192. Spiecker M and Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys 433: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- 193. Sugamura K, Keaney JF Jr.. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51: 978–992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sverdlov AL, Elezaby A, Qin F, Behring JB, Luptak I, Calamaras TD, Siwik DA, Miller EJ, Liesa M, and Shirihai OS. Mitochondrial reactive oxygen species mediate cardiac structural, functional, and mitochondrial consequences of diet-induced metabolic heart disease. J Am Heart Assoc 5: e002555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Szwejkowski BR, Gandy SJ, Rekhraj S, Houston JG, Lang CC, Morris AD, George J, and Struthers AD. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol 62: 2284–2293, 2013 [DOI] [PubMed] [Google Scholar]
- 196. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6: 456, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, and Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 56: 666–674, 2007 [DOI] [PubMed] [Google Scholar]
- 198. Tokuda K, Kai H, Kuwahara F, Yasukawa H, Tahara N, Kudo H, Takemiya K, Koga M, Yamamoto T, and Imaizumi T. Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension 43: 499–503, 2004 [DOI] [PubMed] [Google Scholar]
- 199. Tong M and Sadoshima J. Mitochondrial autophagy in cardiomyopathy. Curr Opin Genet Dev 38: 8–15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Tong M, Saito T, Zhai P, Oka S-I, Mizushima W, Nakamura M, Ikeda S, Shirakabe A, and Sadoshima J. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res 124: 1360–1371, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, and Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res 101: 893–901, 2007 [DOI] [PubMed] [Google Scholar]
- 202. Ushio-Fukai M, Alexander RW, Akers M, and Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273: 15022–15029, 1998 [DOI] [PubMed] [Google Scholar]
- 203. van Empel VP and De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res 63: 487–499, 2004 [DOI] [PubMed] [Google Scholar]
- 204. van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, and Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117: 43–51, 2008 [DOI] [PubMed] [Google Scholar]
- 205. Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, Richter U, Fischer JW, Bohm M, Pauschinger M, Schultheiss HP, and Tschope C. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 103: 319–327, 2008 [DOI] [PubMed] [Google Scholar]
- 206. Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, and Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16: 93–105, 2004 [DOI] [PubMed] [Google Scholar]
- 207. Vercauteren K, Gleyzer N, and Scarpulla RC. PGC-1-related coactivator complexes with HCF-1 and NRF-2β in mediating NRF-2 (GABP)-dependent respiratory gene expression. J Biol Chem 283: 12102–12111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Voulgari C, Papadogiannis D, and Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag 6: 883–903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Waldman M, Bellner L, Vanella L, Schragenheim J, Sodhi K, Singh SP, Lin D, Lakhkar A, Li J, Hochhauser E, Arad M, Darzynkiewicz Z, Kappas A, and Abraham NG. Epoxyeicosatrienoic acids regulate adipocyte differentiation of mouse 3T3 cells, via PGC-1alpha activation, which is required for HO-1 expression and increased mitochondrial function. Stem Cells Dev 25: 1084–1094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, and Hochhauser E. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc Diabetol 17: 111, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Waldman M, Nudelman V, Shainberg A, Zemel R, Kornwoski R, Aravot D, Peterson SJ, Arad M, and Hochhauser E. The role of heme oxygenase 1 in the protective effect of caloric restriction against diabetic cardiomyopathy. Int J Mol Sci 20: pii:, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, Sandusky GE, Pechous PA, Vlahos CJ, Wakasaki H, and King GL. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes 51: 2709–2718, 2002 [DOI] [PubMed] [Google Scholar]
- 213. Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, and Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol 288: H37–H42, 2005 [DOI] [PubMed] [Google Scholar]
- 214. Weiss EP and Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol 301: H1205–H1219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Weiss JN, Korge P, Honda HM, and Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93: 292–301, 2003 [DOI] [PubMed] [Google Scholar]
- 216. Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, and Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 106: 20405–20410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 217. Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Forstermann U, and Munzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis 198: 65–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Westermann D, Rutschow S, Jäger S, Linderer A, Anker S, Riad A, Unger T, Schultheiss H-P, Pauschinger M, and Tschöpe C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56: 641–646, 2007 [DOI] [PubMed] [Google Scholar]
- 219. Wold LE, Ceylan-Isik AF, and Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin 26: 908–917, 2005 [DOI] [PubMed] [Google Scholar]
- 220. Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, and Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689, 2004 [DOI] [PubMed] [Google Scholar]
- 221. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, and Scarpulla RC. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 222. Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, and Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60: 1770–1778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Xiong S, Salazar G, San Martin A, Ahmad M, Patrushev N, Hilenski L, Nazarewicz RR, Ma M, Ushio-Fukai M, and Alexander RW. PGC-1 alpha serine 570 phosphorylation and GCN5-mediated acetylation by angiotensin II drive catalase down-regulation and vascular hypertrophy. J Biol Chem 285: 2474–2487, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, Gulick J, Yue Z, Robbins J, Epstein PN, and Liang Q. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem 288: 18077–18092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Xue L, Fletcher GC, and Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci 14: 180–198, 1999 [DOI] [PubMed] [Google Scholar]
- 226. Xue L, Fletcher GC, and Tolkovsky AM. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol 11: 361–365, 2001 [DOI] [PubMed] [Google Scholar]
- 227. Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, and Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 66: 187–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Yamaguchi M, Matoba K, Sawada R, Fujioka Y, Nakatogawa H, Yamamoto H, Kobashigawa Y, Hoshida H, Akada R, Ohsumi Y, Noda NN, and Inagaki F. Noncanonical recognition and UBL loading of distinct E2s by autophagy-essential Atg7. Nat Struct Mol Biol 19: 1250–1256, 2012 [DOI] [PubMed] [Google Scholar]
- 229. Yamaguchi O and Otsu K. Role of autophagy in aging. J Cardiovasc Pharmacol 60: 242–247, 2012 [DOI] [PubMed] [Google Scholar]
- 230. Yeshao W, Gu J, Peng X, Nairn AC, and Nadler JL. Elevated glucose activates protein synthesis in cultured cardiac myocytes. Metabolism 54: 1453–1460, 2005 [DOI] [PubMed] [Google Scholar]
- 231. Yue Y, Meng K, Pu Y, and Zhang X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract 133: 124–130, 2017 [DOI] [PubMed] [Google Scholar]
- 232. Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276: 36059–36062, 2001 [DOI] [PubMed] [Google Scholar]
- 233. Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, and Muslin AJ. The role of the Grb2–p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest 111: 833–841, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Zhang W, Elimban V, Nijjar MS, Gupta SK, and Dhalla NS. Role of mitogen-activated protein kinase in cardiac hypertrophy and heart failure. Exp Clin Cardiol 8: 173–183, 2003 [PMC free article] [PubMed] [Google Scholar]
- 235. Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, and Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]