Abstract
Salmonella enterica is a common contaminant of macadamia nut kernels in the subtropical state of Queensland (QLD), Australia. We hypothesized that nonhuman sources in the plantation environment contaminate macadamia nuts. We applied a modified Hald source attribution model to attribute Salmonella serovars and phage types detected on macadamia nuts from 1998 to 2017 to specific animal and environmental sources. Potential sources were represented by Salmonella types isolated from avian, companion animal, biosolids-soil-compost, equine, porcine, poultry, reptile, ruminant, and wildlife samples by the QLD Health reference laboratory. Two attribution models were applied: model 1 merged data across 1998–2017, whereas model 2 pooled data into 5-year time intervals. Model 1 attributed 47% (credible interval, CrI: 33.6–60.8) of all Salmonella detections on macadamia nuts to biosolids-soil-compost. Wildlife and companion animals were found to be the second and third most important contamination sources, respectively. Results from model 2 showed that the importance of the different sources varied between the different time periods; for example, Salmonella contamination from biosolids-soil-compost varied from 4.4% (CrI: 0.2–11.7) in 1998–2002 to 19.3% (CrI: 4.6–39.4) in 2003–2007, and the proportion attributed to poultry varied from 4.8% (CrI: 1–11) in 2008–2012 to 24% (CrI: 11.3–40.7) in 2013–2017. Findings suggest that macadamia nuts were contaminated by direct transmission from animals with access to the plantations (e.g., wildlife and companion animals) or from indirect transmission from animal reservoirs through biosolids-soil-compost. The findings from this study can be used to guide environmental and wildlife sampling and analysis to further investigate routes of Salmonella contamination of macadamia nuts and propose control options to reduce potential risk of human salmonellosis.
Keywords: source attribution, Salmonella, macadamia nuts, Australia, modeling, environmental transmission
Introduction
Australia is among the world's largest producers of macadamia nuts (Nock et al., 2019) with ∼40,000 tons of nuts harvested from 6 million trees annually. Macadamia is a genus within the Proteaceae family consisting of four species of which two, Macadamia integrifolia and Macadamia tetraphylla, produce edible nuts of high value due to a rich oil content (Wallace and Walton, 2011; Nock et al., 2019). The warm and humid climate of northeast New South Wales (NSW) and southeast Queensland (QLD) provides optimal growing conditions, and large amounts are harvested from March to October in these areas. Commercial macadamia orchards average ∼18 (NSW) to >100 hectares (QLD), the largest containing several 100,000 trees. These are planted in rows, and mulch; fertilizer and compost (more common in QLD) are applied. Wild animals, including kangaroos, dingoes, rabbits, foxes, and rodents, have access to trees, although livestock are generally excluded from orchards. Nuts are de-husked at harvesting, on farm or at processor, and dried before cracking, roasting, and processing.
Macadamias are a low-water activity food (Zhang et al., 2017) and not supportive of bacterial growth. Salmonella cannot grow in low-water activity foods, but can survive in and on nuts for over a year (Little et al., 2010).
Contamination of nuts is a public health concern due to Salmonella's ability to cause illness in humans at low concentrations and its potential to survive stomach acid in high fat content foods, such as macadamia nuts. A recent study of Salmonella sources of sporadic human salmonellosis cases in QLD, Australia, suggested that nuts, mainly macadamia nuts, may serve as a proxy indicator of environmental transmission of Salmonella to humans (Fearnley et al., 2018). Nuts are mostly sold as ready-to-eat foods, and consumers rarely cook nuts before consumption, which adds to the risk of salmonellosis. Salmonellosis outbreaks and recalls of nut products due to Salmonella contamination have become increasingly common, raising awareness of nuts as a potential vehicle for foodborne illness. Information about prevalence of human pathogens in nuts is limited (Brar and Danyluk, 2018). Zhang et al. (2017) found 4.2% (95% confidence interval 2.40–6.90) Salmonella prevalence in macadamia nuts in the United States. The NSW Food Authority (2012) estimated a 0–2.8% prevalence on ready-to-eat nuts from different countries and 0.1% for Australia-grown nuts specifically. In QLD, several serovars of Salmonella enterica contaminate macadamia nut kernels. The potential risk of outbreaks combined with consumer focus on exotic, high protein, and raw food (Newell et al., 2010) exemplified by the increased recognition of nuts as a healthy snack (CBI, 2008) emphasizes the importance of identifying possible sources of Salmonella contamination of nuts.
We investigated Salmonella contamination of Australian macadamia nuts and assessed routes and sources of contamination with the aim of contributing to understanding environmental transmission and reducing potential of human salmonellosis from contaminated macadamia nuts. Using historical Salmonella typing data from QLD isolates from various sources collected between 1998 and 2017, we applied a source attribution model to attribute Salmonella-positive macadamia nut samples to animal and environmental source(s). As Salmonella contamination of nuts may be introduced at the plantation site (on the tree or during harvest) or along the postharvest processing path (Brar and Danyluk, 2018), we hypothesized that nonhuman sources in the plantation environment contaminated macadamia nuts.
Materials and Methods
Data
Queensland Health Public Health Microbiology laboratory provided nonhuman Salmonella isolates referred from private and public laboratories in primarily Northern NSW and QLD from 1998 to 2017. Data were from research projects, animal health diagnostics and surveillance, food manufacturing/processing quality assurance, and foodborne outbreak investigations and included information about the isolate origin, isolation date, geographic information (city and/or postcode), Salmonella serovar, and for some isolates, phage type. The geographic area included postcodes 4000–4700 covering south-eastern QLD and postcodes 2000–2400 covering north-eastern NSW.
Potential sources of Salmonella contamination of macadamia nuts
Site visits to macadamia plantations and processing plants provided information regarding potential Salmonella contamination routes and sources. This information was used to group data into animal and environmental sources with potential to contaminate nuts. Samples unrelated to these sources, such as zoo animals, were excluded.
Salmonella isolates from macadamia nut kernels were included in the study, together with isolates from likely sources within the geographic areas of interest. Nine different source categories were identified as potential sources of contamination: avian, biosolids-soil-compost, companion animals, equine, poultry, porcine, reptile, ruminant, and wildlife. Equine, porcine, poultry, and ruminant were considered farm animals. Wildlife mainly consisted of isolates from kangaroos, while avian consisted of isolates from wild birds. The animal sources porcine, poultry, ruminant, and wildlife included isolates from animals and food products, whereas equine, companion animal, avian, and reptile included isolates from animals only. Poultry also included isolates from eggs. Biosolid, soil, and compost isolates were treated as a combined environmental source and contained mainly isolates from biosolids, followed by soil and a single compost isolate. Biosolids are typically the dried settled solids from municipal sewage treatment. See Supplementary Table S1 for a complete list of isolates included in each source.
The start of the study period was defined as the first year in which Salmonella positive samples from macadamia nuts and all other sources were reported (1998). Isolates with missing year were excluded. See online Supplementary Figure S1 for a flowchart of data management.
Salmonella types
“Salmonella types” refers to a single serovar or combined with a phage type and will be used throughout the article. All isolates were characterized by serovar, with the majority of isolates of Salmonella Bovismorbificans, Salmonella Enteritidis, Salmonella Heidelberg, Salmonella Typhimurium, and Salmonella Virchow also phage typed. Fourteen different phage types were registered for Salmonella Bovismorbificans, Salmonella Enteritidis, Salmonella Typhimurium, and Salmonella Virchow collectively. Isolates with unknown phage type from these specific serovars were excluded (n = 210). The criterion for Salmonella type inclusion in the final dataset was isolation from both macadamia nuts and any of the nine sources of interest. This comprised 2299 isolates from 1998 to 2017, representing 52 different Salmonella types.
Source attribution model
We modified the Hald et al. (2004) model, which relies on a Bayesian framework using Markov Chain Monte Carlo simulations, originally developed to attribute human salmonellosis cases to animal reservoirs, to estimate the relative contribution of animal and environmental sources to Salmonella contamination of macadamia nuts. A n ≥ 5 Salmonella isolates per source and time period criterion were applied. Two models were applied; model 1 (“Salmonella type”) estimated the number of macadamia nut isolates attributed to each source (j) per Salmonella type (i.e., sero- and phage-type) (i) for all 20 years combined. In model 2 (“Salmonella type and time”), five-year time intervals (t) were included as a third dimension: λijt = pijt × qi × ajt, where λ was the number of nut isolates, p was the relative prevalence, and q and a were Salmonella type and source-specific factors estimated by the model, respectively. The relative prevalence of each specific Salmonella type by source was calculated as the fraction of total number of Salmonella isolates from that source.
The a-factor described different sources' ability to act as a vehicle for Salmonella contamination and was assumed to vary over time in model 2. The q-factor described the ability of the different Salmonella types to contaminate macadamia nuts, including potential differences in their ability to survive in the environment. The q-factor value was calculated for Salmonella types occurring only in a single source (n = 5) and was assumed to remain constant over time (de Knegt et al., 2016). All other q-factors and all a-factors were included as noninformative prior distributions. Results were reported as mean percent cases of macadamia nut contamination attributed to the different sources with credible intervals (95% CrI). The unknown source category reflected any model over-, and under-estimation.
Microsoft Excel was used for data management. Figures were generated in R version 3.5.2 (December 20, 2018). Source attribution models were run with WinBUGS version 3.2.3. Five independent Markov Chain Monte Carlo simulations were run for 50,000 iterations with random starting values between 0 and 100. Burn-in period was 10,000 iterations with a thinning value of 1. Convergence was monitored using Gelman–Rubin diagnostics (Toft et al., 2007).
Results
The distribution of Salmonella types in macadamia nuts and the nine sources of interest are shown in Supplementary Figure S2. The six most prevalent Salmonella types in macadamia nuts were Salmonella Aberdeen (17%), Salmonella Birkenhead (7%), Salmonella Potsdam (7%), Salmonella Typhimurium PT135 (6%), Salmonella Waycross (6%), and Salmonella Zanzibar (6%). Distribution of Salmonella types in macadamia nuts appears similar to that of biosolids-soil-compost, companion animal, ruminant, equine, and wildlife contrasting that of reptile and avian. The attribution pattern of Salmonella types changed between each five-year interval, especially for biosolids-soil-compost for the time periods 2008–2012 and 2013–2017 (Supplementary Fig. S3).
The Salmonella type model (model 1) attributed 47% (CrI: 33.6–60.8) of all Salmonella detections in macadamia nuts to biosolids-soil-compost (Fig. 1 and Table 1), followed by wildlife (12.6% CrI: 3.5–23.3) and companion animals (11.5%, CrI: 0.8–27.2). The models estimated source specific a-factors and Salmonella type specific q-factors (Supplementary Table S2). The model 1 a-value for biosolids-soil-compost was ∼10 times that of any other source.
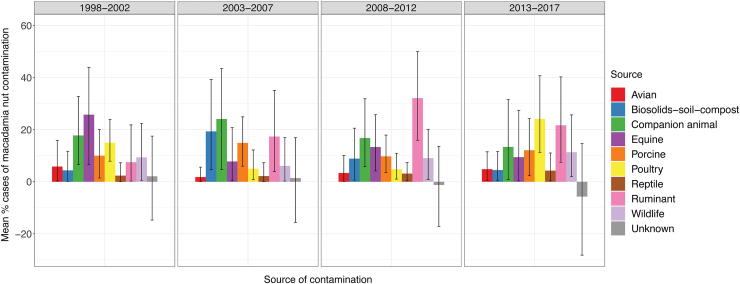
FIG. 1.
Source attribution results of the Salmonella type model (model 1). Results are reported as mean percent cases of macadamia nut contamination attributed to the different sources with 95% CrI reflected by the black vertical lines. The unknown source category reflects any model over- and underestimation. CrI, credible interval. Color images are available online.
Table 1.
Mean Percentages of Salmonella Macadamia Nut Isolates Attributed to the Potential Sources by Model 1 (“Salmonella Type Model”) and Model 2 (“Salmonella Type and Time Model”)
| Model 1, mean % (CrI) |
Model 2, mean % (CrI) |
||||
|---|---|---|---|---|---|
| 1998–2017 | 1998–2002 | 2003–2007 | 2008–2012 | 2013–2017 | |
| Avian | 4.9 (0.6–12.8) | 5.8 (0.2–15.9) | 1.8 (0.1–5.6) | 3.4 (0.1–10.0) | 4.8 (0.6–11.5) |
| Biosolids-soil-compost | 47.4 (33.6–60.8) | 4.4 (0.2–11.7) | 19.3 (4.6–39.4) | 8.9 (0.6–20.6) | 4.5 (0.6–11.6) |
| Companion animal | 11.5 (0.8–27.2) | 17.8 (6.6–32.8) | 24.1 (4.7–43.5) | 16.8 (5.8–31.9) | 13.4 (0.9–31.6) |
| Equine | 6.2 (0.4–15.9) | 25.8 (6.5–43.9) | 7.8 (0.4–20.8) | 13.4 (4.2–25.8) | 9.4 (0.3–27.5) |
| Porcine | 3.6 (0.2–9.3) | 10.0 (1.4–20.1) | 14.9 (6.0–24.9) | 9.7 (3.6–17.9) | 12.1 (2.3–24.3) |
| Poultry | 10.0 (3.7–17.5) | 15.0 (7.9–24.0) | 5.0 (0.8–12.2) | 4.8 (1.0–11.0) | 24.2 (11.3–40.7) |
| Reptile | 2.8 (0.1–8.3) | 2.3 (0.1–7.3) | 2.2 (0.1–7.3) | 3.1 (0.4–7.3) | 4.3 (0.3–11.1) |
| Ruminant | 2.6 (0.1–8.3) | 7.5 (0.3–21.9) | 17.4 (3.9–35.1) | 32.1 (16.0–50.1) | 21.7 (7.5–40.3) |
| Wildlife | 12.6 (3.5–23.3) | 9.4 (0.6–22.4) | 6.1 (0.3–17.0) | 9.1 (0.9–20.2) | 11.3 (2.0–25.7) |
| Unknown | −1.7 (−10.4 to 6.6) | 2.1 (−14.7 to 17.5) | 1.4 (−15.6 to 16.9) | −1.2 (−17.2 to 13.6) | −5.7 (−28.2 to 14.7) |
| Fit | 0.99 (0.91–1.07) | 1.03 (0.9–1.2) | 1.02 (0.87–1.20) | 0.99 (0.85–1.16) | 0.96 (0.78–1.17) |
| RSS | 745.6 (446.1–1259) | 283 (203.9–423.2) | 195.6 (120.9–332.8) | 331 (205.3–510) | 184.1 (116.4–300.3) |
Fit estimate obtained by dividing the number of observed number of sporadic positive Salmonella macadamia nuts with the sporadic number of positive Salmonella macadamia nuts estimated by the model.
RSS, residual sums of squares; CrI, credible interval.
Source attribution estimates from the model based on Salmonella type and time (model 2) varied between time periods; for example, Salmonella attributed to biosolids-soil-compost varied from 4.3% (CrI: 0.6–11.5) in 1998–2002 to 19.3% (CrI: 4.6–39.4) in 2003–2007 and poultry varied from 4.8% (CrI: 1–11) in 2008–2012 to 24% (CrI: 11.3–40.7) in 2013–2017 (Fig. 2 and Table 1).
FIG. 2.
Source attribution results of the Salmonella type and time model (model 2). Results are reported as mean percent cases of macadamia nut contamination attributed to the different sources with 95% CrI reflected by the black vertical lines. The unknown source category reflects any model over-, and under-estimation. CrI, credible interval. Color images are available online.
The unknown source category reflected any overestimation (negative mean value) and underestimation (positive mean value) by the model. The Salmonella type model (model 1, Fig. 1) overestimated around 2% (mean of −2%, CrI: −10.4 to 6.6) of the samples, whereas the Salmonella type and time model (model 2, Fig. 2) over- and underestimation varied from approximately −6% (CrI: −28.2 to 14.7) to +2% (CrI: −14.7 to 17.5). The large associated CrIs reflected low sample sizes when stratified for the different sources, Salmonella type, and time period.
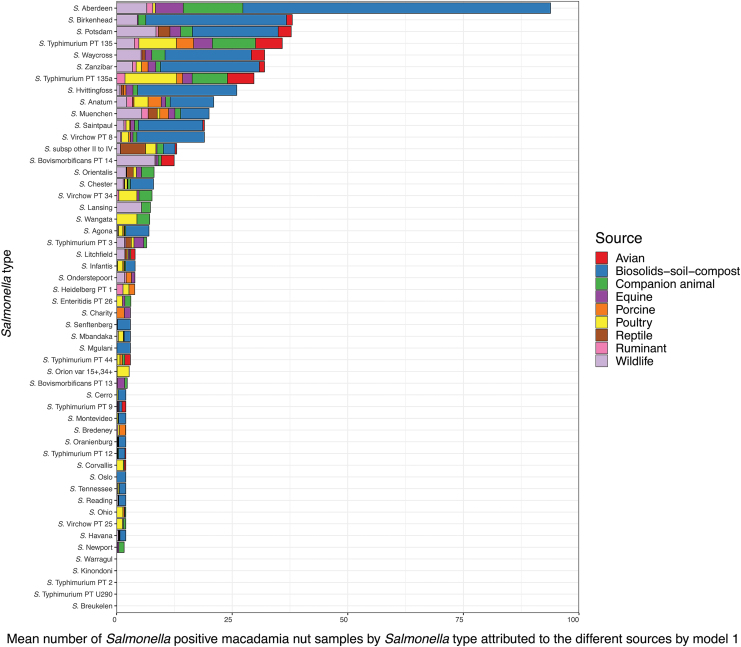
Attributed Salmonella nut isolates by Salmonella type and source assigned by model 1 and model 2 are shown in Figure 3 and Supplementary Figure S4a–d, respectively. In general, Salmonella Aberdeen was the main attributed Salmonella type and was found in most sources except for porcine and reptiles.
FIG. 3.
Salmonella types isolated from macadamia nuts and attributed to sources by the Salmonella type model (model 1). Salmonella types sorted after decreasing mean number with the largest value at the top (Salmonella Aberdeen). Color images are available online.
Salmonella Aberdeen was isolated from nearly one-fifth (17%) of 539 macadamia nut samples and almost one-tenth (9.6%) of companion animal and equine (8.2%) samples (Supplementary Fig. S2). Salmonella Aberdeen also represented 5.4% of biosolids-soil-compost sample isolates. The vast majority (72%) of the Salmonella Aberdeen isolates from macadamia nuts were attributed by model 1 to biosolids-soil-compost, followed by companion animal (14%), wildlife (8%), and equine (7%) (Fig. 3).
Discussion
Results suggest that biosolids-soil-compost was an important source of Salmonella contamination of macadamia nuts together with animals (e.g., wildlife and companion animals) with access to plantation grounds. These are likely routes of contamination as macadamia nuts may lie on the ground for several weeks before harvest (Little et al., 2010; Wall, 2013). Nuts that fall and/or are not picked up during harvesting rounds (∼4.5/yr) may remain on the ground for considerably longer periods. Nuts are thus susceptible to contamination likely introduced by biosolids-soil-compost used as fertilizer or manure from rodents and grazing animals such as kangaroos. Unless present as a component of commercial compost subject to controlled, cyclic thermophilic processing, use of biosolids in Australian horticulture is discouraged (Freshcare, n.d.). Therefore, while biosolid samples were included as part of the biosolids-soil-compost source, it is unlikely that biosolids as an individual source were applied on macadamia plantations.
The Salmonella type model (model 1) attributed 47% to biosolids-soil-compost, whereas the type and time model (model 2) attributed 4–19%. This difference is driven by the very high source-specific parameter (a) to biosolids-soil-compost found for model 1 compared to model 2. The importance and likelihood of biosolids as a source of contamination needs to be considered more carefully via further analysis. Findings from both models are presented, allowing the reader to assess the uncertainty of the attribution depending on the underlying model.
The time period(s) used in the source attribution model was crucial for evaluating the dynamics and routes of transmission. The Salmonella type model permitted Salmonella isolated from macadamia nut samples isolated at the beginning of the 20-year time period to be attributed to a source from which the organism was isolated much later or vice versa. The underlying assumption of this model was that the distribution of Salmonella types within each source varied little across the 20-year time period—an assumption that is not valid for all sources. The Salmonella type and time model assumed similar distribution across 5-year time intervals. This model provided higher resolution, enabling improved differentiation between animal reservoirs leading us to propose that the Salmonella found in the biosolids-soil-compost source likely originated from an animal reservoir (Fig. 2).
Salmonella Aberdeen was the main type attributed to macadamia nuts and found in most of the potential sources represented.
This is also the most commonly notified serovar to the food safety authority in QLD when Salmonella is detected by macadamia nut processors in kernel products (whole, halves, chips, meal) during quality control testing. Parisi et al. (2019) found that Salmonella Aberdeen ranked third in QLD in terms of overall numbers of notified invasive nontyphoidal Salmonella (iNTS), 6th in non-iNTS human clinical isolates and 11th of 37 Salmonella types in terms of age- and gender-adjusted odds ratio of invasiveness from 2007 to 2016. This and the findings of the present study support the results of Fearnley et al., which suggest macadamia nuts as a potential proxy indicator of environmental transmission of Salmonella Aberdeen to humans.
We found source attribution modeling useful for quantifying contamination sources of foods that are potential vehicles of human salmonellosis. Source attribution models have been applied in several countries to attribute human salmonellosis cases to animal reservoirs (Hald et al., 2004; Mullner et al., 2009; Glass et al., 2015). However, we are not aware of studies attributing contamination from animal and environmental reservoirs to a food source.
The significance of Salmonella contamination of macadamia nuts has been reflected in nut product recalls and nut-associated outbreaks more generally. Chocolate macadamia nut confections were recalled in Australia in 2011 (Wall, 2013) and ∼70 nut and nut products (coconut, pine nut, almond, cashew, macadamia, hazelnut, pistachios, walnut, pecan, peanut, and sesame) were recalled in the United States (U.S.) between 2001 and 2018, of which 21 were macadamia nut related. In 2001, internationally distributed peanuts caused a mixed serovar S. enterica outbreak affecting 109 people in Australia, Canada, England and Wales, and Scotland (Kirk et al., 2004). In 2003–2004, a North American outbreak of Salmonella Enteritidis in raw almonds affected 29 people and led to routine heat treatment of almonds (CDC, 2004). Most cases of salmonellosis in Australia are sporadic, and to date no outbreaks associated with macadamia nuts have been identified. They likely do not occur because of irregularly distributed contamination, levels representing subinfectious doses, or outbreaks which are undetected or misclassified as “mixed food”.
Processing practices (typically thermal, including controlled-condensation Pasteurization) are carried out to treat microbial contamination on ready-to-eat nuts, including macadamias. Such processes (other than roasting, which is not considered treatment principally for pathogen reduction) were introduced in ∼2012 in two of six Australian processors relevant to our study samples. However, even a post-Pasteurization prevalence as low as 0.1% on ready-to-eat nuts reported by Little et al. (2010) is of public health concern. Macadamia nut pieces and meal are commonly sold and used as ingredients in food products, which might be subject to temperature abuse and/or conditions conducive to Salmonella growth, increasing the risk of infection in the case of contaminated nut ingredients. However, although Zhang et al. (2017) detected Salmonella in 4.2% of macadamia nuts in a U.S. survey, 61% of these were at levels < 1 most probable number (MPN)/100 g, with the highest level in 0.1% of all samples at 9 MPN/100 g. This suggests that while Salmonella may represent a risk of growth to infectious doses in foods, it most frequently occurs at subinfectious doses through direct ready-to-eat nut consumption.
Limitations
The models lack data from other potential sources such as rodents (in particular rats) and irrigation water as data were not obtained by a formalized source-sampling scheme or systematic surveillance program. Owing to limited data, we chose to combine biosolids, soil, and compost into a single category. However, this restricts our ability to interpret this category since—while compost and soil are likely to be applied to plantations, biosolids are not. With a larger dataset, attribution of compost and soil to animal reservoirs would be informative.
The relative prevalence (p) of the specific Salmonella type by source was calculated directly from the dataset without information on how representative the samples were of their respective sources. A representative dataset is an underlying assumption of source attribution models (Hald et al., 2004). Prevalence of the different Salmonella subtypes in the different sources is an informative parameter in the source attribution model and can be confidently calculated directly from a representative dataset. However, source attribution based on nonrepresentative data has been performed in New Zealand (Mullner et al., 2009) and South Australia (Glass et al., 2015) applying Mullners' modified Hald model (Mullner et al., 2009) that estimates subtype-specific prevalences by combining counts of each subtype from each source with an overall estimate of the Salmonella prevalence in the given source (Glass et al., 2015). As overall prevalence estimates were not available for most of the animal and environmental sources, relative prevalences were applied in the model, which limited our ability to interpret the changing source patterns over time, as these changes may have been due to differences in sample sizes, survey design etc. This also prevented us from modeling changes in prevalence over time. Despite this limitation, the overall results allowed us to infer likely sources of contamination to guide future studies.
Salmonella-positive macadamia nut samples were not specifically collected from ready-to-eat products. Samples were either collected from the plantation site or along the production chain. Detailed information about the specific point of collection and whether the Salmonella was isolated from the husk, shell, or kernel itself would have added great value to this study and its interpretation. Salmonella samples from the husk or shell might not represent foodborne public health risk as these materials are components rejected during processing to obtain kernels, with the latter subject to processing practices to treat microbial contamination on ready-to-eat nuts. However, this study can be used to guide risk assessments and future attribution studies to understand and potentially mitigate routes of Salmonella contamination of macadamia nuts. Focused source sampling and genomic analysis may improve source attribution, and currently bioinformatic analysis of environmental, clinical, and macadamia-associated whole genome sequenced Salmonella Aberdeen isolates is investigating these areas.
Conclusions
Our findings suggest that macadamia nuts were most likely contaminated by Salmonella via direct transmission from animals with access to plantations or indirectly from animal reservoirs through biosolids-soil-compost. These findings can be used to guide focused environmental and wildlife sampling and analysis of Salmonella isolates to further investigate source attribution and inform control strategies to reduce potential human salmonellosis risk.
Supplementary Material
Acknowledgments
The authors thank the following for their assistance: Louise C. Brown, Dwayne Ingall, and Ty Jackson of the Wide Bay Hospital and Health Service Public Health Unit (sampling and on-site assessments); the staff of Public Health Microbiology, Forensic and Scientific Services, Health Support Queensland (sample analysis, Salmonella isolate database maintenance and provision); Wide Bay, Australia area macadamia growers; and processors, and the Australian Macadamia Society for valuable information regarding industry practices and operations.
Funding Information
Funding was provided from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 643476. The Australian National Health and Medical Research Council (NHMRC) supported this project through a European Union Grant (GNT1129770) and salary support for MDK (GNT1145997).
Supplementary Material
References
- Brar PK, Danyluk MD. Nuts and grains: Microbiology and preharvest contamination risks. Microbiol Spectr 2018;6 DOI: 10.1128/microbiolspec.PFS-0023-2018. [DOI] [PubMed] [Google Scholar]
- CBI. CBI Centre for the Promotion of Imports from developing countries. Market Survey: The Preserved Fruit and Vegetables Market in the EU, June 2008, the Netherlands Ministry of Foreign Affairs, 2008
- CDC. Outbreak of Salmonella Serotype Enteritidis Infections Associated with Raw Almonds—United States and Canada, 2003—2004. MMWR 2004. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5322a8.htm, accessed July29, 2019 [PubMed]
- de Knegt LV, Pires SM, Löfström C, Sørensen G, Pedersen K, Torpdahl M, et al. . Application of Molecular Typing Results in Source Attribution Models: The Case of Multiple Locus Variable Number Tandem Repeat Analysis (MLVA) of Salmonella Isolates obtained from Integrated Surveillance in Denmark. Risk Anal 2016;36:571–588 [DOI] [PubMed] [Google Scholar]
- Fearnley EJ, Lal A, Bates J, Stafford R, Kirk MD, Glass K. Salmonella source attribution in a subtropical state of Australia: Capturing environmental reservoirs of infection. Epidemiol Infect 2018;146:1903–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshcare. Using compost safely. A guide for the use of recycled organics in horticulture n.d. Available at: www.freshcare.com.au/wp-content/uploads/Compost-Factsheet-Growers.pdf, accessed May21, 2019
- Glass K, Fearnley E, Hocking H, Raupach J, Veitch M, Ford L, et al. . Bayesian source attribution of salmonellosis in South Australia. Risk Anal 2015;36:561–570 [DOI] [PubMed] [Google Scholar]
- Hald T, Vose D, Wegener HC, Koupeev T. A Bayesian approach to quantify the contribution of animal-food sources to human salmonellosis. Risk Anal 2004;24:255–269 [DOI] [PubMed] [Google Scholar]
- Kirk MD, Little CL, Lem M, Fyfe M, Genobile D, Tan A, et al. . An outbreak due to peanuts in their shell caused by Salmonella enterica serotypes Stanley and Newport—Sharing molecular information to solve international outbreaks. Epidemiol Infect 2004;132:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CL, Rawal N, de Pinna E, McLauchlin J. Survey of Salmonella contamination of edible nut kernels on retail sale in the UK. Food Microbiol 2010;27:171–174 [DOI] [PubMed] [Google Scholar]
- Mullner P, Jones G, Noble A, Spencer SEF, Hathaway S, French NP. Source attribution of food-borne zoonoses in New Zealand: A modified hald model. Risk Anal 2009;29:970–984 [DOI] [PubMed] [Google Scholar]
- Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, et al. . Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol 2010;139:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock CJ, Hardner CM, Montenegro JD, Termizi AAA, Hayashi S, Playford J, et al. . Wild origins of macadamia domestication identified through intraspecific chloroplast genome sequencing. Front Plant Sci 2019;10:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSW Food Authority. Report on the prevalence of Salmonella and E. coli in ready to eat nuts and nut products sold in Australia 2012
- Parisi A, Crump JA, Stafford R, Glass K, Howden BP, Kirk MD. Increasing incidence of invasive nontyphoidal Salmonella infections in Queensland, Australia, 2007–2016. PLoS Negl Trop Dis 2019;13:e0007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft N, Innocent GT, Gettinby G, Reid SWJ. Assessing the convergence of Markov Chain Monte Carlo methods: An example from evaluation of diagnostic tests in absence of a gold standard. Prev Vet Med 2007;79:244–256 [DOI] [PubMed] [Google Scholar]
- Wall MM. Improving the quality and safety of macadamia nuts. In: Improving the Safety and Quality of Nuts. Volume 250. Linda JH, ed. USA: Elsevier, 2013, pp. 274–296. [Google Scholar]
- Wallace HM, Walton DA. Macadamia (Macadamia integrifolia, Macadamia tetraphylla and hybrids). In: Postharvest Biology and Technology of Tropical and Subtropical Fruits. Elhadi MY, ed. Mexico: Woodhead Publishing, 2011, pp. 450–474e. [Google Scholar]
- Zhang G, Hu L, Melka D, Wang H, Laasri A, Brown EW, et al. . Prevalence of Salmonella in cashews, hazelnuts, macadamia nuts, pecans, pine nuts, and walnuts in the United States. J Food Prot 2017;80:459–466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.