Abstract
Purpose: The objectives were to investigate the effect of transscleral iontophoresis of macromolecules in vitro and in vivo, to study the importance of electroosmosis on macromolecules of low charge to mass ratio, and to evaluate transscleral iontophoresis efficacy in a choroidal neovascularization (CNV) animal model.
Methods: Through in vitro transport experiments, the permeability coefficients of macromolecules [eg, immunoglobulin G (IgG), dextran 70 kDa] were determined under different conditions. The effect of ionic strength formulations and iontophoretic conditions was studied on the distribution of IgG and bevacizumab into the eye in vivo. Magnetic resonance imaging (MRI) was utilized to evaluate in vivo real time distribution of gadolinium-labeled albumin (Galbumin) following iontophoresis. The efficacy between no treatment, intravitreal injection (IVT), and iontophoresis of bevacizumab on a CNV model of subretinal injection of adeno-associated virus encoding human VEGF-165 was investigated.
Results: The permeability data suggested a significant effect of ionic strength on the iontophoretic transport of macromolecules. Transscleral iontophoresis of IgG at 4 mA with a low ionic strength formulation was about 600 times greater than passive diffusion and 14-fold over a conventional formulation in vitro. Approximately 0.6 mg of bevacizumab can be delivered into the rabbit eye in vivo with a 20-min treatment of iontophoresis. MRI showed that Galbumin was in the posterior tissues after iontophoresis. In the CNV model, the iontophoresis and IVT methods of bevacizumab delayed retinal neovascularization by 4 and 8 weeks, respectively.
Conclusions: Transscleral iontophoresis is capable of delivering macromolecule drugs through the conjunctiva and sclera, eventually exposing the retina/choroid to the drugs.
Keywords: noninvasive ocular drug delivery, iontophoresis, electroosmosis, macromolecule, bevacizumab, choroidal neovascularization
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the United States. While wet AMD only accounts for 10%–15% of AMD cases, it accounts for ∼90% of all AMD-related blindness.1 More than 1.7 million Americans have the advanced form of the disease (ie, wet AMD).2 According to the Retina Foundation, AMD affects about 18 million people in the United States3 and up to 25 million people, worldwide.4 There are about 200,000 new cases of wet AMD diagnosed annually in North America. The prevalence of AMD is similar to that of all invasive cancers combined and more than double the prevalence of Alzheimer's disease.5,6 Due to an aging population, the National Eye Institute has estimated that the prevalence of advanced AMD will grow to 5.44 million cases by 2050.1
The eye presents several unique anatomic and physiologic barriers to drug delivery for the treatment of eye disease. Although significant progress has been made in optimizing ocular drug delivery in recent years,7,8 delivery of therapeutic doses of drugs to the posterior segment of the eye remains a significant challenge. Consequently, most of the new drug candidates are administered through intravitreal injections (IVTs). However, periodic IVTs impose a significant financial burden on patients and practices, as well as potential risks of bleeding, infection, traumatic injury, retinal detachment, and endophthalmitis. Thus, the development of an effective noninvasive ocular delivery system is needed.
Ocular iontophoresis particularly transscleral iontophoresis is a noninvasive drug administration method that potentially may circumvent adverse effects associated with IVTs.9–11 It could improve patient compliance with those who have limited access to retinal specialists since iontophoresis treatments potentially can be administered by nurses, technicians, optometrists, or patients themselves. Transscleral iontophoresis may take advantage of the suprachoroidal space of the eye, a postulated low barrier, rapid diffusion pathway beneath the initial intrascleral barrier, delivering the drug, in part, to the posterior pole of the eye.12,13 Ocular iontophoresis has been applied to various drug molecules for the treatment of various eye diseases.14 Moreover, it has been suggested that transscleral iontophoresis may be able to deliver macromolecule drugs to the back of the eye.15
Conventional thinking in ocular iontophoresis believed that electrophoresis, which depends on the charge of the molecule/drug, is the key driving force in delivering drugs to the eye.9,16 More recently, it has been learned that the effective net transport of a water-soluble active ion is based substantially on the interplay of both electrophoresis and electroosmosis.17–20 For neutral or low charge to mass ratio drug molecules particularly macromolecules, electroosmosis dominates such interplay whereby the effect due to electrophoresis has a negligible impact on the transport.17,20,21 Few researchers have recognized this potential of electroosmosis for noninvasive delivery of macromolecule drugs such as bevacizumab (150 kD) into the eye.17–20,22 There is no comparative information on drug delivery efficiency such as drug penetration and distribution for macromolecule drugs with respect to the parameters of iontophoresis (ie, surface area, current density, total current, and duration of application) under electroosmosis dominant delivery conditions and for drug delivery to the posterior segment of the eye in vivo. The research on iontophoretic delivery of macromolecules has mostly been done in vitro only. Importantly, to our knowledge, there is no study that has shown that iontophoresis can deliver macromolecules to treat a disease model in vivo.
Magnetic resonance imaging (MRI) permits real time monitoring of specific molecular targets and biological processes in living organisms. To evaluate the pharmacokinetics of macromolecules after transscleral iontophoresis, MRI and/or traditional pharmacokinetic studies are performed. Surrogate permeants for a macromolecule drug such as gadolinium-labeled albumin (Galbumin, 80 kDa) have been studied with MRI to monitor drug distribution both locally and systemically in real time following ocular iontophoresis.23–25
At the time of this study, there was no commercially available iontophoresis eye applicator. Since loss of proper contact between the drug electrode and eye application site can lead to intermittent variable current density or short circuiting that can deleteriously disrupt the iontophoresis treatment, the iontophoretic eye applicator like Visulex-I that fits well and has reliable contact with the surface of the eye was selected to test in vivo transscleral iontophoresis in this study. Visulex-I is a prototype of transscleral iontophoretic applicator developed by Aciont, Inc. (Salt Lake City, UT) specifically for the rabbit experiments in this study (Fig. 1).
FIG. 1.
Visulex-I, an iontophoresis applicator used for the studies in rabbit. The diameter was ∼2 cm, and the contact area on the eye surface was ∼1.1 cm2.
The hypothesis of this study was that transscleral iontophoresis (ie, anodal iontophoresis) can deliver macromolecules to the eye tissues at the therapeutic levels of choroidal neovascularization (CNV) suppression in a rabbit animal model. This hypothesis was tested by a series of experiments, and the objectives of each experiment are the following: (1) to examine the effect of ionic strength on small and large molecules in vitro using side by side diffusion cells for tissue transport studies, (2) to study the effect of anodal iontophoresis (ie, current density and iontophoretic duration) on the pharmacokinetic aspects with Visulex-I in vivo using the macromolecules, including immunoglobulin G (IgG), bevacizumab (Avastin®), and Galbumin, and (3) to evaluate the efficacy of bevacizumab administered by Visulex-I on a CNV animal model. The sequence of the experiments is represented in Fig. 2.
FIG. 2.
Experimental design.
Methods
Iontophoresis applicator
All animal experiments used in this investigation complied with the ARVO Statement for the use of Animals in Ophthalmic and Vision Research and was approved by The University of Utah Institutional Animal Care and Use Committee (Salt Lake City, UT). Iontophoretic eye applicator (Visulex-I; Aciont, Inc.) was utilized in all in vivo iontophoresis studies (Fig. 1). The applicator was made from a flexible biocompatible silicone that can conform to the shape of the eye. The applicator incorporated a corneal dome, which minimizes corneal drying and corneal seal which minimizes drug fluid leakage during the iontophoretic procedure. A silver-based electrode was incorporated into the applicator between the drug carrier sponge and the shell of the applicator. The vacuum bulb on the applicator created a slight negative pressure for self-adhering during the iontophoretic procedure and made it easy to handle during the placement of applicator onto the eye like a scleral lens.
In vitro transport experiments
The effect of the ionic strength of the formulation on transscleral drug transport in vitro was determined in this experiment. A set of in vitro transport experiments were performed with 14C-mannitol (>98% purity; Perkin-Elmer Life and Analytical Sciences, Boston, MA), fluorescence isothiocyanate (FITC)-labeled dextran 70 kDa (Sigma-Aldrich, St. Louis, MO), and human IgG (GAMMAGARD S/D, Baxter, Inc., Deerfield, IL, and Privigen, LEE Biosolutions, Maryland Heights, MO) as permeants of interest. A 2-chamber side-by-side diffusion cell system (Glass Shop at University of Utah, Salt Lake City, UT) with freshly excised rabbit sclera (with or without conjunctiva) was used in the experiments. Each compartment of the diffusion cell had a 2.0 mL volume and an effective diffusional area of 0.2 cm2. The sclera was sandwiched between the 2 half-cells with the choroid side facing the receiver chamber. IgG was used as a bevacizumab surrogate. IgG concentration used in vitro was ∼2.5–25 mg/mL.
The diffusion cell was placed in a circulating water bath at 37°C±1°C. The receiver solution was 2.0 mL of phosphate-buffered saline (PBS) for all the in vitro experiments. The IgG in PBS donor solution 2.0 mL was prepared in a range of ionic strength from 0.0015 to 1.5 M (∼3.28–3,280 mOsm). The 14C-mannitol and FITC labeled dextran 70 kDa donor solution 2.0 mL were prepared in 0.15 M PBS. The silver (Ag) foil and silver/silver chloride (Ag/AgCl) coated silver foil were used as the conductive elements in anodal iontophoresis (anode in the donor). The current used in the in vitro experiment was 2.0 mA, current density 10 mA/cm2. A Phoresor II Auto constant current iontophoretic device (Model No PM 850; Iomed, Inc., Salt Lake City, UT) was the iontophoretic dose controller for providing the electric current. Samples were withdrawn from the donor and receiver chambers. Typically, 20–100 μL-mL aliquots were taken from the donor chamber at the beginning and end of experiment, and 1.0 mL aliquots were withdrawn from the receiver chamber at predetermined time intervals. The same volume of fresh saline was added back to the receiver chamber after each aliquot removal to maintain a constant volume. The Ag and Ag/AgCl electrodes were also replaced at predetermined times during the experiments to prevent the change in pH due to electrolysis of the media.
The flux (J) and permeability coefficient (P) were calculated at steady state under sink conditions (receiver concentrations ≤10% of the donor concentrations):
| (1) |
where AD is the diffusional surface area; ΔQ/Δt is the slope of the cumulative amount of the permeant transported across the membrane into the receiver chamber versus time plot; and CD is the concentration of the permeant in the donor chamber. The donor and receiver samples were analyzed by a colorimetric method for 70 kDa dextran, enzyme-linked immunosorbent assay (ELISA) for IgG, and a liquid scintillation counter (Packard TriCarb Model 1900TR Liquid Scintillation Analyzer) for 14C-mannitol.
In vivo local pharmacokinetics and drug distribution experiments
Under various test conditions, the amounts of drug delivered to the eye tissues were quantified immediately after iontophoresis (t = 0 h). This experiment was to investigate the effects of key variables on the amount of drug delivered into the eye, including formulation ionic strength and iontophoretic dose. The test formulations were prepared using IgG at different ionic strength. The formulation ionic strength effect on bevacizumab delivery was then tested by comparing the results of 25 mg/mL commercial bevacizumab formulation (Avastin; Genentech, South San Francisco, CA) versus the low ionic strength formulation bevacizumab (12.5–15.0 mg/mL, prepared in our laboratory) at 4.0 mA, anodal iontophoresis in 3 iontophoresis durations (ie, 5, 10, and 20 min), and current density ∼1.8 mA/cm2. The low ionic strength formulation for bevacizumab was prepared by dialysis and reconstituting with low ionic strength vehicle. The concentration and stability of the low ionic strength formulation were confirmed with ELISA before the experiment and 1 week after preparation for stability.
New Zealand white rabbits of 3 to 4 kg from Western Oregon Rabbit Co. (Philomath, OR) were used as the animal model. The sample size of each individual experiment was indicated in the Results section. After the rabbits were anesthetized with ketamine and xylazine, the Visulex-I loaded with drug solution (500 μL) was placed on the conjunctiva/sclera. The return electrode (∼4 cm2 surface area) was placed on a gauze pad (∼40 cm2 surface area) wetted in saline and clamped to the ear on the side opposite to that of the treated eye. Anodal iontophoresis experiments were performed at predetermined iontophoretic setting. After 20 min of iontophoresis delivery, the rabbits were sacrificed and the eyes enucleated. The eyes were dissected into 7 tissue sections: cornea, aqueous humor, vitreous, conjunctiva, sclera, choroid, and retina. After dissection, the drug was extracted from each tissue overnight with 5 mL of the extraction solvent (ie, 1% bovine serum albumin in phosphate buffer solution). The tissue section was then separated from the extraction solution by centrifuge at 3,400 rpm for 10 min. A second extraction was also done overnight with 5 mL of the extraction solvent on all tissues to confirm full extraction. The extraction solutions were assayed for drug concentrations with ELISA. The contralateral eye and control no treatment eye samples were also performed and assayed with ELISA.
The ELISA protocol was taken from Bakri et al.,26 with minor changes tailored for bevacizumab. Briefly, the 165-amino acid variant of human recombinant VEGF was immobilized on a Microlite 2 (Thermo Labsystems, Franklin, MA). The samples were diluted within the linear range of the assay. The bound bevacizumab was detected with a goat antihuman IgG/Fc antibody labeled with horseradish peroxidase (HRP; Jackson Immuno Research Lab., Inc., PA). The detection of HRP was performed with HRP substrate solution and 1 M H2SO4 for stopping the reaction. The wells were then transferred to the transparent 96-well plate for a color absorbance measurement at 450 nm with a correction at 562 nm by a UV-VIS spectrophotometer plate reader machine (SpectraMax M2; Molecular Devices LLC, San Jose, CA).
In vivo MRI experiment
New Zealand white rabbits (n = 3) were used in this experiment. Gadolinium-labeled albumin (Galbumin™; BioPAL, Inc., Worcester, MA) at a concentration of 25 mg/mL (the same concentration as commercial bevacizumab) was used as a surrogate molecule to bevacizumab. The molecular weight of Galbumin is of ∼80 kDa. Galbumin is a bovine albumin conjugated with gadolinium in PBS. Visulex-I device was loaded with Galbumin, and anodal iontophoresis was applied to the eye at 4 mA for 20 min, current density ∼1.8 mA/cm2.
MRI experiments were conducted with a clinical 3-T MRI system Trio with Sonata gradient (Siemens Medical Solution, Erlangen, Germany) and a human wrist coil (a transmit/receive volume coil). Dynamic MR imaging was performed, with T1 (spin–lattice relaxation time) weighted spin-echo imaging technique.23,24 MRI spatial resolution is a function of the MRI protocol and equipment used. The spatial resolution was 0.28 × 0.28 × 1.0 mm3. Each scan provided at least 20 transaxial image slices to cover the whole eye. Imaging time for single time data was ∼26 min. Using a 3 T clinical scanner to accommodate the rabbits as the animal model does not provide high resolution where posterior tissues (sclera, choroid, and retina) could be differentiated.
The animal was in prone position (abdomen facing down), and transverse images of the head were taken. Each scan provided at least 20 transaxial image slices to cover the whole eye. MRIs obtained were analyzed with MRIcro and ImageJ (obtained from MRIcro and NIH websites). All MRI experiments were carried out at least in duplicate.
Qualitative assessment of adverse effects
New Zealand white rabbits (n = 3) were subjected to 20 min of anodal iontophoresis at 4 mA current (1.8 mA/cm2 current density), using the commercial bevacizumab formulation. The eyes were examined with an indirect ophthalmoscope before and immediately after iontophoresis at day 0, then again on days 2, 4, and 6. The eyes were graded according to a modified McDonald-Shaddock scale for conjunctival injection, chemosis, corneal haze, iritis, and other ocular signs.27 At day 6, the animals were sacrificed, the eyes enucleated and stored in Davidson's solution for 18 h, and then transferred to 70% ethanol. All eyes were examined by an independent pathologist for histopathological abnormality.
CNV in the new rabbit animal model
Nine Dutch belted rabbits were given subretinal injections of adeno-associated virus encoding human VEGF-165. Each eye had 2 subretinal injections. Approximately 20 μL/injection of 5 × 1011 vp/mL was injected using a 30-gauge blunt tip needle. From previous studies (unpublished data) this method required ∼4 ± 1 week to develop CNV in the rabbit. Three rabbits were control with no bevacizumab treatment (n = 6, eyes); 3 rabbits were treated bilaterally 1 time with IVT of 1.25 mg bevacizumab (n = 6, eyes); and 3 rabbits were treated 1 time unilaterally with anodal iontophoresis of bevacizumab (n = 3, eyes). The IVT and iontophoresis groups were treated at week 3 after the subretinal injections. Anodal iontophoresis was performed at 3 mA for 20 min (1.4 mA/cm2, current density) with the low ionic strength formulation of bevacizumab (12.5 mg/mL). Fluorescence angiography was performed at weeks 1, 2, 4, 5, 7, 8, 12, and 16.
Results
In vitro transport experiments
The effect of conjunctiva on iontophoretic transport can be observed based on the permeability coefficient results shown in Tables 1 and 2. In Table 1, the passive permeability of 2 model neutral molecules (ie, 14C-mannitol and dextran 70 kDa) was 2–3 times greater in sclera alone than in the conjunctiva+sclera composite. With anodal iontophoresis, the 14C-mannitol permeability values for sclera alone and for conjunctiva+sclera were similar, indicating little or no effect due to the presence of conjunctiva, while with dextran 70 kDa, the permeability value for sclera alone was around 2 times greater compared with conjunctiva+sclera. Table 2 shows that the presence of conjunctiva reduced the IgG transport by about a factor of 1.5 compared to sclera alone (permeability coefficient value of sclera alone was 4.8 × 10−5 cm/s compared to permeability coefficient value of conjunctiva+sclera which was 3.1 × 10−5 cm/s).
Table 1.
Passive and 2 mA Anodal Iontophoresis Transport Across Sclera+Conjunctiva and Sclera Alone (n = 3, mean ± SD)
| Permeability (P) × 105 cm/s | Sclera |
Conjunctiva+sclera |
||
|---|---|---|---|---|
| Passive | AI | Passive | AI | |
| 14C-Mannitol | 2.2 ± 0.9 | 6.3 ± 0.8 | 0.6 ± 0.2 | 6.5 ± 0.6 |
| Dextran 70 kDa | 0.4 ± 0.1 | 4.1 ± 0.8 | 0.2 ± 0.01 | 1.9 ± 1.0 |
AI, anodal iontophoresis.
Table 2.
IgG Transport Across Sclera+Conjunctiva and Sclera Alone Using 20 Min Anodal Iontophoresis with IgG in Phosphate-Buffered Saline Versus New Electroosmosis Formulation (Low Ionic Strength, IgG Concentration = 25 mg/mL), n = 3
| Sclera |
Conjunctiva+sclera |
||||||
|---|---|---|---|---|---|---|---|
| 2 mA PBS | 2 mA new formulation | Passive PBS | 2 mA PBS | 4 mA PBS | 2 mA new formulation | 4 mA new formulation | |
| Permeability (P) × 105 cm/s | 4.8 ± 1.8 | 16.9 ± 5.5 | 0.013 ± 0.007 | 3.1 ± 1.4 | 8.7 ± 4.1 | 8.3 ± 0.6 | 13.3 ± 3.2 |
| Amount delivered (μg) | 288 ± 108 | 1,020 ± 300 | 0.78 ± 0.42 | 234 ± 114 | 522 ± 246 | 498 ± 36 | 780 ± 180 |
IgG, immunoglobulin G; PBS, phosphate-buffered saline.
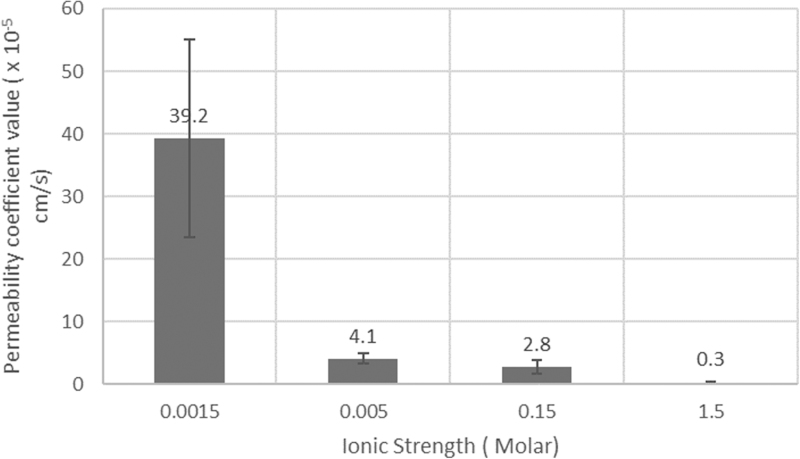
The effect of ionic strength formulation on iontophoretic transport can be observed based on the permeability coefficient results shown in Fig. 3 and Table 2. The permeability coefficient value of IgG, which corresponds to the amount being transported across tissue, was increased ∼14 times with the low ionic strength (0.0015 M) formulation compared to the standard ionic strength buffer (0.15 M). Table 2 shows the effects of current intensity (2 mA vs. 4 mA, 10 − 20 mA/cm2) on IgG transport values. Iontophoretic delivery of IgG at 4 mA is about 600 times greater than passive delivery (8.7 × 10−5 compared to 1.3 × 10−7 cm/s). Figure 3 presents results showing the influence of ionic strength upon IgG transport across conjunctiva+sclera. The lower the ionic strength, the higher in permeability value particularly when the ionic strength is lower than 0.005 M.
FIG. 3.
Effect of ionic strength on IgG transport across sclera with conjunctiva during anodal iontophoresis in vitro. IgG, immunoglobulin G.
In vivo ocular pharmacokinetics and drug distribution
The effect of ionic strength on bevacizumab delivery into the eye in vivo can be observed in Tables 3 and 4. In Table 3, a larger amount of bevacizumab is delivered into the eye with the low ionic strength formulation at a lower concentration (14 mg/mL) than with commercial bevacizumab (25 mg/mL). Table 4 shows the effect of iontophoretic duration, and Table 5 shows the effect of current density on IgG delivery into the eye in vivo at 4 mA (1.8 mA/cm2) using anodal iontophoresis and the Visulex-I applicator at low ionic strength conditions. These data confirm the effect of low ionic strength on enhancing the delivery effect across the eye tissue. The lower the ionic strength, the more IgG is being delivered. Under this optimized condition, ∼0.73 mg total bevacizumab was delivered into the eye. The total bevacizumab amount in the eye after iontophoresis between the commercial formulation (ie, standard ionic strength) and the low ionic strength formulation at the 5-, 10-, and 20-min application times of iontophoresis is shown in Table 4. In the case of commercial bevacizumab, the drug was delivered in a proportional manner up to 20 min, whereas there was no substantial increase in drug being delivered into the eye after 5 min for the low ionic strength formulation. This suggests that the enhanced electroosmosis effect with the low ionic strength formulation may be limited to the first 5 min of anodal iontophoresis in vivo. There was no observed conjunctival injection or inflammation after the 5- and 10-min application. Mild conjunctival injection was observed at the 20 min application of the low ionic strength formulation only and not standard ionic strength.
Table 3.
Amount of Bevacizumab Delivered into the Eye In Vivo Using Visulex-I: Comparing Standard Ionic Strength (Commercial Bevacizumab Formulation 25 mg/mL) Versus Low Ionic Strength [New Iontophoresis Bevacizumab Formulation (12.5–15.0) mg/mL]; 4 mA, 20 Min (Solution Volume = 500 μL, Contact Area = 2.2 cm2, n = 6)
| Formulation | Total amount delivered into the eye (μg) | Amount delivered to retina/choroid (μg) |
|---|---|---|
| Standard ionic strength | 353 ± 42 | 5 ± 3 |
| Low ionic strength | 616 ± 317 | 14 ± 9 |
Table 4.
Amount of Bevacizumab Delivered into the Eye In Vivo Using Visulex-I: Comparing Standard Ionic Strength (Commercial Bevacizumab Formulation 25 mg/mL) Versus Low Ionic Strength (New Iontophoresis Bevacizumab Formulation 12.5 mg/mL) at Different Iontophoretic Durations; 4 mA for 5, 10, and 20 Min (Solution Volume = 500 μL, Contact Area = 2.2 cm2, n = 6)
| Formulation | Total amount delivered to eye (μg) |
||
|---|---|---|---|
| 5 min | 10 min | 20 min | |
| Standard ionic strength | 79 ± 19 | n/a | 353 ± 42 |
| Low ionic strength | 496 ± 197 | 500 ± 115 | 616 ± 317 |
Table 5.
Eye Tissue Distributions of IgG After 20 Min Anodal Iontophoresis at Two Different Current Densities: 1.86 and 3.6 mA/cm2
| Tissue (n = 6) | 3.6 mA/cm2 |
1.86 mA/cm2 |
||
|---|---|---|---|---|
| Amount of IgG (μg) | Conc. [IgG (μg)/g tissue] | Amount of IgG (μg) | Conc. [IgG (μg)/g tissue] | |
| Cornea | 112.5 ± 36.6 | 1470.2 ± 478.4 | 46.1 ± 12.1 | 602.1 ± 158.4 |
| Aqueous humor | 1.8 ± 0.2 | 6.3 ± 0.8 | 1.9 ± 1.1 | 6.9 ± 3.9 |
| Lens | 1.9 ± 1.4 | 4.6 ± 3.5 | 0.8 ± 0.6 | 2.1 ± 1.6 |
| Vitreous | 7.2 ± 2.9 | 4.9 ± 2.0 | 2.6 ± 1.3 | 1.8 ± 0.9 |
| Retina/choroid | 42.1 ± 38.8 | 268.6 ± 247.5 | 7.5 ± 7.4 | 48.1 ± 47.0 |
| Sclera | 186.9 ± 75.4 | 539.1 ± 217.5 | 131.4 ± 37.3 | 378.9 ± 107.5 |
| Conjunctiva | 374.6 ± 113.9 | 545.3 ± 165.8 | 247.6 ± 42.5 | 360.4 ± 61.9 |
| Total | 727.1 ± 37.7 | 438.0 ± 63.3 | ||
The higher current density (3.6 mA/cm2), the applicator was replaced at 10 min to maintain neutral pH in the applicator.
Conc., concentration.
MRI in vivo
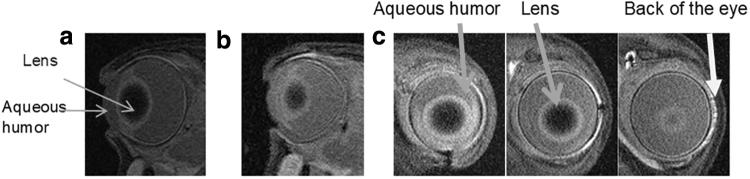
The MRIs indicated that Galbumin penetrated through the pars plana into the anterior chamber after anodal iontophoresis (Fig. 4). The MRIs also show that Galbumin penetrated into the posterior tissues. In Fig. 4c, the sagittal cuts show the penetration of Galbumin into the aqueous humor and surrounding tissues of the conjunctiva. The last image in Fig. 4c shows the presence of Galbumin in surrounding tissues, including sclera and retina/choroid; this indicates that the delivery was detectable behind the lens and toward the back of the eye within hours after treatment.
FIG. 4.
MRI of rabbit eye (a) control blank eye (axial cut), (b) 1 h after anodal iontophoresis of Galbumin (axial cut), and (c) the sagittal cuts at 1 h after the iontophoresis of Galbumin show the signal of Galbumin in the posterior chamber behind the lens. MRI, magnetic resonance imaging.
Treatment of CNV in the rabbit model
The CNV model used in this study provided pathology similar to human wet AMD for a long-term efficacy study (>6 months). A representative eye from each group is presented in Fig. 5. A comparison of retina neovascularization progression of the eyes in the 3 groups is shown: control (no treatment), 1.25 mg IVT of bevacizumab (25 mg/mL), and anodal iontophoresis of bevacizumab (12.5 mg/mL, 3 mA for 20 min, 1.4 mA/cm2). Retina neovascularization can be seen as the proliferation of new, tiny, abnormal, leaky blood vessels as shown by the arrows in the images. The eyes in the control group developed retina neovascularization within 4 ± 1 week. The bevacizumab treatments for both the iontophoresis and the IVT groups were given at week 3. The IVT group eyes showed suppression of retina neovascularization in all 6 eyes up to week 12. Of these, 4 of the 6 eyes were followed until week 16, and all 4 showed development of retina neovascularization between weeks 12 and 16. The iontophoresis group eyes showed suppression of retina neovascularization in all 3 eyes and had a delayed onset of retina neovascularization through week 8. Comparing iontophoresis treatment to control (no bevacizumab treatment), the iontophoresis treatment was capable of suppressing the retina neovascularization for an average of 4 weeks. These findings suggest that both treatment modalities of IVT and iontophoresis were effective. While the CNV suppression of IVT treatment lasted about 4 weeks longer compared with the anodal iontophoresis treatment, this outcome is not surprising because the bevacizumab amount delivered in the IVT treatment was significantly larger than that used in iontophoresis (ie, 1.25 mg vs. 0.6 mg).
FIG. 5.
Representative fluorescein angiogram images of the optic nerve and subretinal injection lesion sites. (a) Control (no bevacizumab treatment) n = 6; (b) iontophoresis delivery of bevacizumab n = 6; (c) intravitreal injection of bevacizumab n = 6.
Qualitative assessment of adverse effects after transscleral iontophoresis
The only adverse effect noted immediately after the iontophoresis at 4 mA for 20 min was slight conjunctival injection found in all 6 eyes. On day 2, the conjunctival injection in 2 eyes had resolved and 4 eyes showed improvement. By day 4, only 2 eyes, both on the same rabbit, showed minimal conjunctival injection, and this had resolved by day 6. Histopathology reports show no appearance damage due to the treatment. One rabbit showed mild inflammation in the limbus of both eyes. Minimal inflammation of the limbus of this type is occasionally observed in normal rabbit eyes; however, the inflammation in these eyes was graded mild by the pathologist. These results indicate that the intended treatment of iontophoresis has minimal safety concerns.
Discussion
Previous studies (eg, DSP-Visulex studies by Aciont, Inc. and EGP-437 studies by EyeGate Pharma) have shown that the delivery of small molecules through the sclera and conjunctiva at therapeutically relevant levels can be achieved passively and iontophoretically.27–30 However, the challenge remains in delivering macromolecules to the posterior segment of the eye. The results in this study indicate that anodal iontophoresis is capable of delivering macromolecules (ie, IgG, high molecular weight dextran, and bevacizumab) into and across the conjunctiva and sclera, eventually exposing the retina/choroid to the drug. The advantage of iontophoresis using the low ionic strength formulation is at least 600-fold increase over passive diffusion-based delivery and 14-fold over a conventional formulation in vitro. The results demonstrated that the amount of a macromolecule delivered into the eye and its relative penetration underneath the intrascleral barriers through anodal iontophoresis can be controlled by current density, formulations, and treatment duration.
This study suggests that anodal iontophoresis of the low ionic strength formulation can enhance the delivery of macromolecules into the eye significantly and deliver ∼0.7 mg of IgG (150 kDa molecule) and 0.6 mg of bevacizumab to the rabbit eye. Within the known well tolerated current range of iontophoresis treatments,31,32 ∼14 ± 9 μg of bevacizumab was delivered to the retina/choroid (ie, 89 ± 56 μg/g concentration). However, it appears that enhanced electroosmosis takes place with the low ionic strength formulation mainly in the first 5 min of anodal iontophoresis. This may be due to the limited volume (500 μL) in the applicator compared to 2 mL in the donor chamber in vitro or due to the presence of tears that may have raised the ionic strength of the formulation rapidly in the applicator in contact with the eye tissue.
It is surprising that Galbumin, which is a large molecule with significant negative charge (−27),23 can be delivered into the eye through anodal iontophoresis. This observation is not expected through the traditional thinking of electrophoresis. However, in our previous in vivo iontophoresis study,24,25 MRIs showed that cathodal iontophoresis primarily delivered Galbumin to the conjunctiva and the sclera under the site of application of the iontophoresis electrode. No Galbumin was detected in the anterior chamber or the vitreous after cathodal iontophoresis. In this study, the MRIs suggest that anodal iontophoresis is capable of delivering Galbumin across the sclera. Although it is difficult to determine from MRI with a clinical MRI scanner where Galbumin actually is localized in the posterior tissues between sclera, retina, and choroid, the drug distribution data (Table 5) support that the penetration with similar molecular weight molecules does reach the retina/choroid layers. The MRI result shows that the main pathway was through the pars plana and into the anterior chamber. Although Galbumin has a relatively large negative charge of −27, electroosmosis (through anodal iontophoresis) and not electrophoresis (through cathodal iontophoresis) appears to determine the direction of the Galbumin transport, and this illustrates the vital role of electroosmosis in macromolecule delivery. In addition, the movement of Galbumin is consistent with how contrast agents passively diffuse within a short time period through the suprachoroidal space upon the intrascleral infusion of such agents as was shown in a previous study using a similar MRI technique.33
While further research is needed to support a suitability of the CNV model for wet AMD and the safety of Visulex-I, the above results demonstrate the potential importance of the collective investigative animal models as they demonstrate the delivery, efficacy, and safety of the Visulex-I (anodal iontophoresis of bevacizumab) delivery system. Specifically, the efficacy of the bevacizumab treatments is measured in terms of the delays in the time of appearance of the retina neovascularization in rabbit and these delays occur within the time frame of clinical significance, acceptability of clinical practice, and patient compliance. While the number of rabbits in this initial efficacy study was limited, the results indicate that reproducibility with regard to the induction times for retina neovascularization in the rabbit should be adequate for assessing the performance of the bevacizumab iontophoretic treatment relative to the control (no treatment) and the IVT treatment. Thus, the present findings of the 4-week suppression of retina neovascularization in rabbit with the bevacizumab iontophoretic treatment are considered to be highly encouraging especially with the comparative inclusion of IVT bevacizumab as the positive control. According to a recent publication,34 the ionic strength could affect the size and charge of IgG and bevacizumab. This will be investigated for future studies by capillary electrophoresis and dynamic light scattering. The effect of altering ion competition in iontophoretic transport by the low ionic strength formulation could contribute to the observed enhanced delivery, and this should also be investigated. It will provide a more mechanistic understanding on the enhanced electroosmosis effect seen in this study.
In conclusion, transscleral anodal iontophoresis can deliver macromolecules to the eye tissues at the therapeutic levels of CNV suppression in a rabbit animal model. This investigation has demonstrated the importance of formulation ionic strength in delivering macromolecules using transscleral iontophoresis. While it is acknowledged that translating any rabbit model results to the human disease situation is uncertain, the noninvasive treatment of transscleral iontophoresis presents a promising opportunity that may provide better cost-effective access to care and compliance for patients with retinal diseases.
Acknowledgments
The authors thank Dr. David Miller at Keystone Pharmacy for his comments on iontophoresis, Dr. Nick Mamalis and Dr. Paul Bernstein at the Moran Eye Center for their medical insights and helpful discussion on the animal experiments, and Aciont research team for their contributions to this study.
Author Disclosure Statement
Visulex-I is a proprietary technology of Aciont, Inc.; S.M., S.K.L., and B.B are consultants at Aciont, Inc.; K.P. and J.W.H are employees at Aciont, Inc.; C.B. is a former employee at Aciont, Inc.; W.I.H. is a founder and CTO of Aciont, Inc.; and B.A. has no commercial relationship with Aciont, Inc.
Funding Information
This investigation was financially supported by NEI SBIR Grant 1R43EY020791 and Aciont, Inc.
References
- 1. National Eye Institute NIoH. Age-Related Macular Degeneration (AMD). 2019. Available at: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics Accessed February14, 2020
- 2. Prall F.R.C., Thomas A., Criswell M.H., and Harris A.. Exudative (wet) age-related macular degeneration (AMD). In: Dahl, A.A. ed. Medscape Reference. 2018. Available at: https://www.medscape.com/answers/1226030-3388/what-is-the-role-of-fluorescein-angiography-fa-in-the-diagnosis-of-exudative-wet-age-related-macular-degeneration-amd Accessed February14, 2020
- 3. Retina Foundation. Age-Related Macular Degeneration. 2019. Available at: https://retinafoundation.org/resources/#additional_info Accessed February14, 2020.
- 4. Taylor P. Novartis; longer-acting AMD drug matches archrival Eylea in phase 3 trials. FierceBiotech. Framingham, MA: Questex, 2017
- 5. Bright Focus. Sources for Macular Degeneration: Facts & Figures. 2015. Available at: https://www.brightfocus.org/sources-macular-degeneration-facts-figures Accessed February14, 2020
- 6. National Institute on Aging. About Alzheimer's Disease: Alzheimer's Basics. 2015. Available at: https://www.nia.nih.gov/health/alzheimers/basics Accessed February14, 2020
- 7. Patel A., Cholkar K., Agrahari V., and Mitra A.K.. Ocular drug delivery systems: an overview. World J. Pharmacol. 2:47–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sikandar M.K., Sharma P.K., and Visht S.. Ocular drud delivery system: an overview. IJPSR. 2:1168–1175, 2011 [Google Scholar]
- 9. Eljarrat-Binstock E., and Domb A.J.. Iontophoresis: a non-invasive ocular drug delivery. J. Control. Release. 110:479–489, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Leboulanger B., Fathi M., Guy R., and Delgado-Charro M.. Reverse iontophoresis as a noninvasive tool for lithium monitoring and pharmacokinetic profiling. Pharm. Res. 21:1214–1222, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Parkinson T.M., Ferguson E., Febbraro S., Bakhtyari A., King M., and Mundasad M.. Tolerance of ocular iontophoresis in healthy volunteers. J. Ocul. Pharmacol. Ther. 19:145–151, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Moisseiev E., Loewenstein A., and Yiu G.. The suprachoroidal space: from potential space to a space with potential. Clin. Ophthalmol. 10:173–178, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel S.R., Berezovsky D.E., McCarey B.E., Zarnitsyn V., Edelhauser H.F., and Prausnitz M.R.. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the EyeMicroneedle use for targeted drug delivery. Invest. Ophthalmol. Visual Sci. 53:4433–4441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang D., Chen Y.S., and Rupenthal I.D.. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 126:96–112, 2018 [DOI] [PubMed] [Google Scholar]
- 15. Gratieri T., Santer V., and Kalia Y.N.. Basic principles and current status of transcorneal and transscleral iontophoresis. Expert Opin. Drug Deliv. 14:1091–1102, 2017 [DOI] [PubMed] [Google Scholar]
- 16. Myles M.E., Neumann D.M., and Hill J.M.. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv. Drug Deliv. Rev. 57:2063–2079, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Chopra P., Hao J., and Li S.K.. Iontophoretic transport of charged macromolecules across human sclera. Int. J. Pharm. 388:107–113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li S.K., and Hao J.. Transscleral passive and iontophoretic transport: theory and analysis. Expert. Opin. Drug Deliv. 15:283–299, 2018 [DOI] [PubMed] [Google Scholar]
- 19. Li S.K., Liddell M.R., and Wen H.. Effective electrophoretic mobilities and charges of anti-VEGF proteins determined by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 55:603–607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pescina S., Santi P., Ferrari G., N, Sara. Trans-scleral delivery of macromolecules. Ther. Deliv. 2:1331–1349, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Andreev V.P. Electrophoresis and electroosmosis in the intracellular transport of macromolecules. In COMSOL Group proceedings of the 2011 COMSOL Conference. Boston; 2011. Available at: https://www.comsol.com/paper/download/100773/andreev_paper.pdf Accessed February14, 2020
- 22. Nicoli S., Ferrari G., Quarta M., Macaluso C., and Santi P.. In vitro transscleral iontophoresis of high molecular weight neutral compounds. Eur. J. Pharm. Sci. 36:486–492, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Li S.K., Lizak M.J., and Jeong E.-K.. MRI in ocular drug delivery. NMR Biomed. 21:941–956, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molokhia S.A., Jeong E.K., Higuchi W.I., and Li S.K.. Transscleral iontophoretic and intravitreal delivery of a macromolecule: study of ocular distribution in vivo and postmortem with MRI. Exp. Eye Res. 88:418–425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molokhia S., Liu X., Jeong E., Higuchi W., and Li K.. Transscleral iontophoretic delivery of a macromolecule in vivo. Invest. Ophthalmol. Visual Sci. ARVO Annual Meeting Abstract 49, 2008 [Google Scholar]
- 26. Bakri S.J., Snyder M.R., Reid J.M., Pulido J.S., Ezzat M.K., and Singh R.J.. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 114:2179–2182, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Papangkorn K., Prendergast E., Higuchi J.W., Brar B., and Higuchi W.I.. Noninvasive ocular drug delivery system of dexamethasone sodium phosphate in the treatment of experimental uveitis rabbit. J. Ocul. Pharmacol. Ther. 33:753–762, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papangkorn K., Higuchi J.W., Brar B., and Higuchi W.I.. Ocular drug distribution and safety of a noninvasive ocular drug delivery system of dexamethasone sodium phosphate in rabbit. J. Ocul. Pharmacol. Ther. 34:325–334, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papangkorn K., Truett K.R., Vitale A.T., et al. Novel dexamethasone sodium phosphate treatment (DSP-Visulex) for noninfectious anterior uveitis: a randomized phase I/II clinical trial. Curr. Eye Res. 44:185–193, 2019 [DOI] [PubMed] [Google Scholar]
- 30. Patane M.A., Cohen A., From S., Torkildsen G., Welch D., and Ousler G.W., 3rd. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin. Ophthalmol. 5:633–643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parkinson T.M., Ferguson E., Febbraro S., Bakhtyari A., and Mundasad M.. Tolerance of ocular iontophoresis in healthy volunteers. Invest. Ophthalmol. Visual Sci. 43, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Patane M.A., Cohen A.E., Sheppard J.D., and Nguyen Q.D.. Ocular iontophoresis for drug delivery. Retina Today. 64–66, 2011 [Google Scholar]
- 33. Kim S.H., Galban C.J., Lutz R.J., et al. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Invest. Ophthalmol. Visual Sci. 48:808–814, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Pindrus M.A., Shire S.J., Yadav S., and Kalonia D.S.. The effect of low ionic strength on diffusion and viscosity of monoclonal antibodies. Mol. Pharm. 15:3133–3142, 2018 [DOI] [PubMed] [Google Scholar]