Abstract
Background:
Impairment of odor discrimination (D), identification (I), and threshold (T) are characteristic features of multiple sclerosis (MS).
Objective:
To identify patterns of gray matter concentration (GMC) associated with different qualities of olfactory function.
Methods:
Olfactory function (T and combined DI score) was measured by Sniffin’ Sticks-Test over 2 years longitudinally, and T1-weighted 3-T magnetic resonance imaging (MRI) was performed in 37 MS patients and 18 matched healthy controls (HCs). Statistical parametric mapping (SPM) was applied to objectively identify changes of voxel-wise-GMC throughout the entire brain volume and to correlate image parameters with odor function.
Results:
SPM localized significant GMC decreases in the anterior cingulum as well as temporomesial and frontobasal brain areas of the MS group compared with HCs, and revealed significant correlations between lower DI scores and GMC decreases in the olfactory gyrus, anterior cingulum, temporal regions including the parahippocampus, and putamen. Contrarily, no correlations were found between T and GMC. Patients with disability progression had significantly lower mean temporomesial/putamen GMC (0.782 vs 0.804, p = 0.004) compared to patients without Expanded Disability Status Scale (EDSS) progression.
Conclusion:
Impairment of DI, but not T is associated with GM atrophy in brain regions related to olfactory function. Further studies are warranted to investigate DI scores and temporomesial/putamen GMC as biomarkers for disability progression.
Keywords: Multiple sclerosis, identification, discrimination, threshold, MRI, atrophy, disability progression
Introduction
Dysfunction of various qualities of the olfactory sense has been increasingly recognized in multiple sclerosis (MS) with prevalences ranging from 30% to 75%, mostly depending on different MS populations and testing methods.1,2 The current paradigm of MS pathology comprises a disease process involving both inflammation and neurodegeneration with the former being the prominent feature of early and relapsing disease phases while the latter is deemed the key contributor to accumulation of disability and development of progressive disease stages.3
The capacity to correctly identify odors (identification) and to discriminate them (discrimination) is affected in MS patients suffering from disability progression, whereas olfactory threshold is transiently altered during acute relapses in early and active MS resolving in the absence of a relapse.4,5 In this regard, the sum score of discrimination and identification (DI score) was shown to be correlated with disease duration, physical disability, reduced cognitive function, and reduced retinal thickness of MS patients.6 Hence, odor identification and discrimination were suggested to serve as parameters reflecting neurodegeneration in MS, while decreased odor thresholds indicate short-term inflammatory disease activity.4,5
Structural assessment of olfactory dysfunction using magnetic resonance imaging (MRI) was shown to be associated with the number and activity of MS-related plaques in frontal and temporal brain regions, atrophy of the olfactory bulb and tract, a decrease in white matter integrity, and recently atrophy of olfactory-related gray matter volume.2,7–12
However, these studies have predominantly considered olfactory dysfunction as one domain and hence did not investigate associations of different qualities of olfactory function such as odor identification, discrimination, and threshold, and the corresponding brain structure. Also, there is a lack of longitudinal studies investigating gray matter volume and olfactory function.
Thus, in this study we aimed to identify patterns of gray matter alterations associated with different qualities of olfactory function applying voxel-based morphometry of gray matter concentration (GMC), an early marker of structural brain alteration, to a cohort of early-staged MS patients in a longitudinal design.
Methods
Between May 2015 and February 2016, we recruited 37 MS patients and 18 healthy controls (HCs) from the MS clinic of the Department of Neurology at the Medical University Innsbruck. HCs did not report any health-associated complaints. The study was approved by the ethics committee of the Medical University Innsbruck (ethical approval number: AM3743-281/4.3) and all participants gave written informed consent before inclusion. MS patients were diagnosed according to the 2010 McDonald’s criteria.7 Patients and HCs had to be aged between 18 and 55 years. Exclusion criteria were cognitive impairment defined as a score of 25 or lower in the Mini-Mental State Examination, or a history of chronic oto-rhino-laryngeal disease (such as chronic rhinitis, nasal polyposis, or sinus disease), head trauma, toxic exposures, upper respiratory infections at the time of assessment, previous radiation, nasal and/or oral surgery, and symptoms of nasal obstruction or other neurological diseases known to be associated with olfactory disturbances.
Study visits were conducted at baseline and at 2 years of follow-up. At every visit, demographic data, smoking habits, neurological and pharmacological history including occurrence and date of relapses and disease-modifying therapy, Expanded Disability Status Scale (EDSS), subjective olfactory function, and use of drugs and hormonal contraceptives were obtained from each participant.8 EDSS progression was defined as a confirmed EDSS increase of ⩾1.0 point in patients with a baseline score of ⩽5.5, or an increase of ⩾0.5 points in patients with a baseline score of >5.5 sustained for at least 12 months as compared to baseline.9 If a relapse, verified by the treating neurologist, had occurred within 6 months, EDSS was only considered when confirmed after 6 months following the visit.
The Beck Depression Inventory (BDI) was performed at each visit to screen for depression. Depression was defined as a score of 18 or higher on the BDI.10
Olfactory function was assessed at each visit using the extended version of the Sniffin’ Sticks test (Burghart Medizintechnik, Wedel, Germany) according to the manufacturer’s instruction including change of testing sticks every 6 months.11 The Sniffin’ Sticks is a three-stage test, based on pen-like odor-dispensing devices. In stage 1, odor threshold is assessed using n-butanol as a single odorant. Three sticks are presented to each subject in a randomized order, two containing solvent and the third containing the odorant at a certain dilution. The subject is asked to identify the stick with the odorant. In stage 2, the subject is presented with three pens and asked to discriminate between two pens containing the same odorant and one containing a different one. In stage 3, the ability of the subject to identify an odorant is assessed by testing 16 different odors from a single pen by a forced choice from four options. The threshold, discrimination, and identification scores were rated for each patient. The maximum score at each stage is 16 points and reflects optimal olfactory function. Lower scores are associated with impaired ability to identify and discriminate odors and an increased threshold for odor perception. The overall TDI score is not ideal for quantification of olfactory dysfunction in MS as it displays less robust correlations to clinical variables compared to its subscores.4 Alterations in threshold are masked by better performance in discrimination/identification or vice versa. Therefore, we regarded threshold as a separate domain to discrimination and identification summed in a composite score (DI score) providing better correlation to clinical variables than each subscore alone.4
The normative values are based on data from 3000 healthy subjects.12 Olfactory testing was postponed for 4 weeks, if the patient had a relapse or received corticosteroids within 4 weeks or if upper respiratory tract infections were present at the time of assessment.
MRI data acquisition
All MRI measurements were performed on a 3.0 T whole-body MR scanner (Magnetom Skyra, Siemens, Erlangen, Germany) equipped with a 20-channel head coil. The MRI protocol included 3D-T1-weighted and T2-SPACE sequences. T1-MPRAGE sequence parameters were 160 slices TR = 1850 milliseconds, TE = 3.01 milliseconds, inversion time = 900 milliseconds, slice thickness = 1 mm, matrix = 256 × 204 pixels, number of excitations = 1, flip angle = 8, and FOV = 220 mm × 178.75 mm. T2-SPACE parameters were set to the following: 160 slices, slice thickness = 1 mm, TR = 4800 milliseconds, TE = 388 milliseconds, flip angle = 120, and FOV = 230 × 222.81 mm.
Image postprocessing
T2-SPACE and T1-MPRAGE images were brain extracted. Registration of T2-SPACE and T1-MPRAGE was conducted by the FMRIB’s linear image registration tool FLIRT implemented into FSL software (FMRIB library 5.0, Oxford, UK).13 An automated lesion prediction algorithm as implemented in the lesion segmentation toolbox for statistical parametric mapping (SPM) was used to segment T2 hyperintense and gray matter hypointense lesions in SPACE and T1-weighted images. The tool gives total lesion volume and lesion number. The segmented lesions were reviewed by an expert (C.S.).14–16
To avoid a priori assumptions through region of interest (ROI) analysis on brain areas of potential interests, gray matter density measures were subjected to SPM (SPM12, Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB 9.2 (MathsWorks Inc., Sherborn, MA, USA), a technique that objectively localizes focal changes of voxel values throughout the entire brain volume.14 Voxel-based morphometry of the gray matter compartment was performed by applying the standard version of the diffeomorphic anatomical registration using exponentiated lie algebra toolbox implemented in SPM12 to have a high-dimensional normalization protocol.16
Segmented and unmodulated images were transformed from the study-specific diffeomorphic anatomical registration space into Montreal Neurological Institute (MNI) space and smoothed by a Gaussian kernel of 8 mm × 8 mm × 8 mm in order to accommodate interindividual anatomic variability and to improve signal-to-noise ratios for the statistical analysis. Brodmann areas (BAs) were obtained by the MNI atlas implemented into the software package MRICRON.17 A masking threshold of 10% of the lower image signal was applied to reduce signal noise.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 (SPSS Inc, Chicago, IL, USA). Categorical variables were expressed in frequencies and percentages. Continuous variables were tested for normal distribution by Kolmogorov–Smirnov test. In case of normal distribution, variables were displayed as mean and standard deviation or 95% confidence interval. If variables were not normally distributed, they were displayed as median and range. Univariate comparisons were done by chi-square-test, Fisher’s exact test, Mann–Whitney U test, or independent t-test as appropriate. Effect sizes have been calculated using Cohen’s d values where appropriate.
The obtained datasets allowed for comparisons of GMC in analogous voxel regions between healthy volunteers and MS patients. A general linear model was set up to compare GMC values between groups using a two-tailed t-contrast. MRI parameters and scores of olfactory testing (threshold and DI score) were entered into the design matrix of the general linear model and examined by t-contrast. All results were corrected for multiple comparisons at family-wise-error I (FWE) p < 0.05 with the statistical threshold set to p < 0.001 for group comparison and correlation analysis. Age and total intracranial volume were entered as covariates.
A post hoc t-test analysis of the mean GMC of the voxel-cluster displaying significant positive correlations of GMC decreases and DI score reductions was performed. Since the olfactory cortex refers to a constellation of processing modules distributed predominantly throughout the mediotemporal and inferofrontal cortices, the respective voxel-cluster was separated into a frontal and a temporomesial/putamen region with the regional boundaries defined by the MNI atlas.18–20
Subsequently, GMC values of these two ROIs were compared between patients with and without EDSS progression within 2 years from baseline using t-test analysis.
In an explorative approach, we performed receiver-operating characteristic (ROC) curve analysis to identify the best possible cutoff values discriminating between patients with and without EDSS progression in the 2-year span after the baseline visit, respectively.
Results
Characteristics of MS patients are displayed in Table 1. The patient and HC groups were matched for age and sex. Five MS patients were excluded from the SPM analyses for insufficient MRI quality. The cumulative white matter lesion volume of the entire MS cohort was 164.16 ccm. The filled-in gray matter lesion volume ranged from 0.01 to 3.74 ccm (mean 1.16 ± 1.09 ccm).
Table 1.
Characteristics of MS patients and HC.
| MS (n = 37) | HC (n = 18) | p-Value | |
|---|---|---|---|
| Femalea | 29 (78.4%) | 14 (77.7%) | 0.999d |
| Age at baseline (years)b | 33.8 (6.9) | 30.6 (6.6) | 0.107e |
| Disease duration (years)b | 5.5 (4.4) | na | |
| RRMSa | 33 (89.2%) | na | |
| SPMSa | 3 (8.1%) | na | |
| PPMSa | 1 (2.7%) | ||
| Number of relapses in last yearb | 0.73 (0.93) | na | |
| EDSS at baselinec | 2.0 (0–4.5) | na | |
| EDSS at year 2c | 2.5 (0–5.5) | na | |
| EDSS progression baseline—year 2a | 15 (40.5%) | na | |
| Treated with DMTa | 32 (86.5%) | na | |
| Interferon betaa | 13 (35.1%) | na | |
| Glatiramer acetatea | 10 (27.0%) | na | |
| Fingolimoda | 1 (2.7%) | na | |
| Natalizumaba | 8 (21.6%) | na | |
| Beck Depression Indexb | 5.1 (5.6) | na | |
| Depression (BDI ⩾18)a | 2 (5.4%) | na | |
| MMSEc | 30 (28–30) | na | |
| Smokersa | 11 (29.7%) | na | |
| Baseline thresholdc | 7.0 (4.0–10.0) | na | |
| Threshold age-adjusted hyposmiaa | 15 (40.5%) | na | |
| Baseline discriminationc | 13 (11–16) | na | |
| Discrimination age-adjusted hyposmiaa | 4 (10.8%) | na | |
| Baseline identificationc | 13 (9–16) | na | |
| Identification age-adjusted hyposmiaa | 5 (13.5%) | na | |
| 2-year thresholdc | 7.25 (4.25–10.0) | na | |
| Threshold age-adjusted hyposmiaa | 15 (40.5%) | na | |
| 2-year discriminationc | 12 (11–16) | na | |
| Discrimination age-adjusted hyposmiaa | 7 (18.9%) | na | |
| 2-year identificationc | 11 (8–16) | na | |
| Identification age-adjusted hyposmiaa | 9 (24.3%) | na |
BDI: Beck Depression Index; DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; HC: healthy controls; MMSE: Mini-Mental Status Examination; MS: multiple sclerosis; Na: not applicable; PMS: progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis.
Number (percentage).
Mean and standard deviation.
Median and range.
Calculated by Fisher’s exact test.
Calculated by t-test.
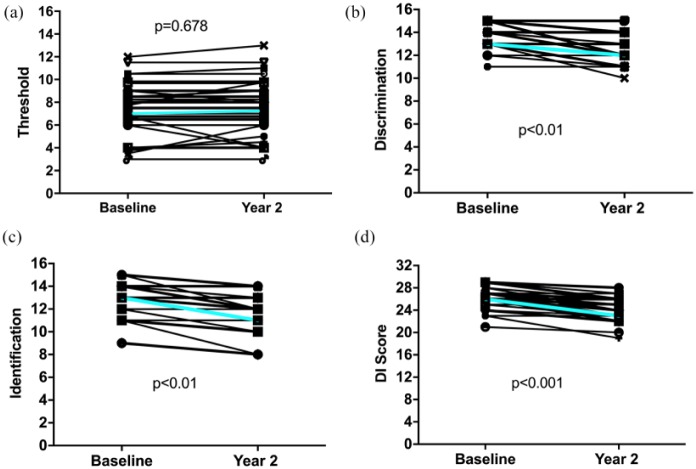
While DI score significantly decreased during the observation period, olfactory threshold did not change significantly (Table 1, Figure 1).
Figure 1.
Development of olfactory threshold (panel a), odor discrimination (b), identification (c), and combined DI score (d).
p-Values calculated by Mann–Whitney U test. Cyan lines indicate median scores.
SPM localized significant decreases in GMC between MS patients and HCs, most prominent bilaterally in the cingulum (BA 23 and 24), parahippocampal gyri (BA 28), and in the right straight gyrus, frontal superior and medial orbital gyrus, and olfactory gyrus (BA 11), at FWE corrected p < 0.001 (Supplemental Table 1, Supplemental Figure 1).
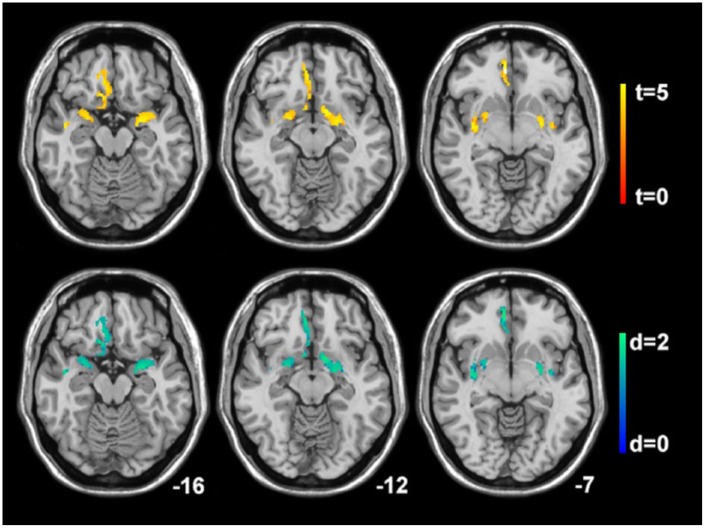
Decreases of the DI score correlated significantly with decreased GMC in the right olfactory gyrus and anterior cingulate (BA 11), the middle temporal gyrus and temporal pole (BA 20, 21), the left parahippocampus (BA 35), and bilaterally in the amygdala (BA 34) and the putamen (p < 0.001; FWE corrected; Table 2, Figure 2). No significant associations were found between olfactory threshold scores and GMC.
Table 2.
Brain regions of significant positive correlations of gray matter concentration decreases and combined olfactory function discrimination and identification score in patients with multiple sclerosis.
| Cerebral region | Cluster size (mm3) | MNI coordinates | t-value | Cohen’s d | Height threshold | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Right middle temporal gyrus and temporal pole (BA 20, 21) | 7780 | −50 | −8 | −18 | 4.6 | 1.91 | 0.001 |
| Right putamen | −34 | −8 | −5 | 4.45 | 1.85 | ||
| Right amygdala | 22 | −2 | −15 | 4.31 | 1.79 | ||
| Right frontal superior and medial orbital gyrus | −15 | 35 | −20 | 4.57 | 1.9 | ||
| Straight gyrus (BA 11) | −9 | 33 | −18 | 4.2 | 1.74 | ||
| Right olfactory gyrus (BA 25) | −10 | 12 | −15 | 3.77 | 1.57 | ||
| Right anterior cingulate (BA 11) | −8 | 39 | −4 | 4.2 | 1.76 | ||
| Left amygdala (BA 34) | 3490 | 29 | 2 | −18 | 4.41 | 1.84 | |
| Left putamen | 29 | −2 | −4 | 4.08 | 1.70 | ||
| Left parahippocampus (BA 35) | 19 | −4 | −27 | 3.75 | 1.56 | ||
BA: Brodmann area; MNI: Montreal Neurological Institute coordinates.
Cerebral regions are family-wise error corrected at the threshold of 0.001 of the cluster level.
Figure 2.
Correlations of gray matter concentration decreases and the combined olfactory function discrimination and identification score. Statistical parametric mapping (t-value) axial intensity projection maps showing areas of significant positive correlations of gray matter concentration decreases and the combined olfactory function discrimination and identification score (upper panel) and its effect size representing Cohen’s d values (lower panel) in a cohort of patients with multiple sclerosis rendered on to a stereotactically normalized magnetic resonance imaging scan. The number at the bottom right corner of each MRI scan corresponds to the z coordinate in Montreal Neurological Institute space.
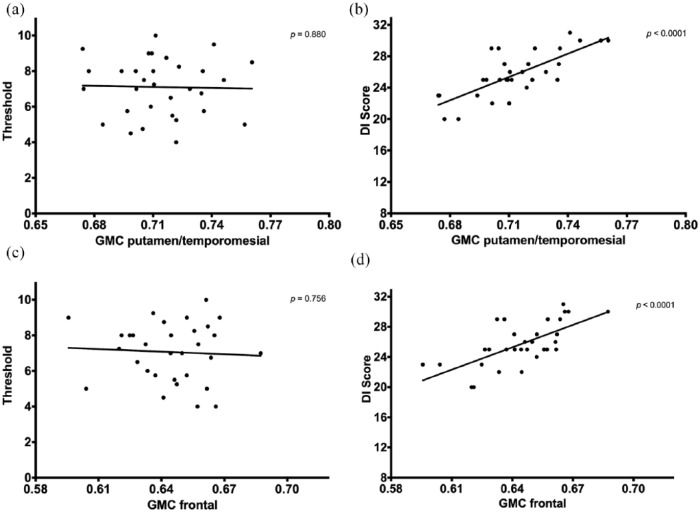
In both the frontal region and the temporomesial/putamen ROIs, GMC correlated significantly with DI score but not with threshold (Figure 3). Patients with EDSS progression had significantly lower mean GMC in the temporomesial/putamen ROI (0.782 vs 0.804, p = 0.004, Cohen’s d = 2.103) compared to patients without EDSS progression, while the frontal ROI was not statistically significant (0.688 vs 0.692, p = 0.074, Cohen’s d = 1.478). Mean DI score was significantly lower in patients suffering an EDSS progression (23.2 vs 27.0, p = 0.004, Cohen’s d = 2.354), while threshold scores did not differ (6.8 vs 7.4, p = 0.345, Cohen’s d = 0.921).
Figure 3.
Gray matter concentration in relation to olfactory threshold (a) and (c) and DI score (b) and (d).
DI score: combined score of discrimination and identification; GMC: gray matter concentration.
p-Values calculated by general linear models.
The DI score displayed the best discrimination ability at a cutoff lower than 24 points (specificity 94%, sensitivity 63%, area under the ROC curve (AUC) 0.86) followed by temporomesial/putamen GMC (specificity 82%, sensitivity 58%, AUC 0.81) with the cutoff at <0.79 (Table 3).
Table 3.
ROC analysis for prediction of EDSS progression within 2 years.
| Cutoff value | Specificity (%) | Sensitivity (%) | AUC (SD) | |
|---|---|---|---|---|
| Frontal ROI GMC | <0.69 | 74 | 34 | 0.68 (0.53–0.78) |
| Temporomesial/putamen ROI GMC | <0.79 | 82 | 58 | 0.81 (0.65–0.93) |
| DI-score | <24 | 94 | 63 | 0.86 (0.72–0.96) |
AUC: area under the ROC curve; DI: discrimination and identification sum score; EDSS: expanded Disability Status Scale; GMC: gray matter concentration; ROC: receiver-operating characteristic; ROI: region of interest; SD: standard deviation.
Discussion
In this study, we identified a robust correlation between impairment of DI score and decreased GMC in the putamen and temporomesial brain regions in MS patients. On the contrary, we did not find any significant associations between olfactory threshold and GMC alterations.
In recent years, detailed assessments of odor function in MS revealed the DI score as strongly correlated with clinical measures of neurodegeneration (i.e. EDSS, cognitive function) mostly affected in patients suffering from disability progression, but independent of inflammatory disease activity (i.e. occurrence and number of relapses).1,4,6,21 On the contrary, impairment of olfactory threshold is correlated with short-term inflammatory relapse activity, transiently resolving in the absence of relapse activity but independent of parameters of neurodegeneration.4–6,22
However, the underlying pathophysiology is unclear. It has been hypothesized that olfactory threshold is a function of more peripheral parts of the olfactory system (“cable function”) affected by a bystander inflammation of the olfactory tract during phases of clinical disease activity, while discrimination and identification are complex cortical functions (“processing functions”) likely to be mainly affected by neurodegenerative processes.4,22 The plausibility of this concept is strengthened by observations regarding the transient nature of threshold impairment compared to the irreversible affection of discrimination and identification.4,5 Also, retinal nerve fiber layer thickness, which is a reliable and robust surrogate biomarker of neuroaxonal degeneration, is correlated with DI score, but not with threshold.6,23
Gray matter atrophy quantified by means of MRI is a well-established and widely accessible imaging marker of neurodegeneration in MS.24 It was shown to occur in all MS phenotypes and to be associated with disability accumulation.25 In line with our findings, brain areas displaying early atrophy include the cingulate cortex, insular and temporal cortical GM, and the deep gray matter (i.e. putamen and caudate).25,26 Interestingly, histopathological examinations revealed the neostriatum (putamen and caudate) and the deep gray matter as brain regions exhibiting the greatest extent of demyelination in early MS.27 Since the striatum receives significant inputs from the motor cortex and the association cortices, it was speculated that enhanced vulnerability of these structures may arise from retrograde neurodegeneration secondary to higher lesion load.25
While former studies applied gray matter volume measures, a recent study investigated GMC and successfully revealed subtle changes in subcortical and frontoparietal regions of MS patients displaying little signs of physical disability (EDSS score <3.0) but cognitive impairment. Hence, reduction of GMC seems to precede overt brain atrophy measured by volumetric methods.28
In accordance with previously reported morphometric investigations in MS, categorical voxel-based analysis identified significant GMC decrease in the right olfactory gyrus, the anterior cingulate (BA 11), the middle temporal gyrus and temporal pole (BA 20, 21), the left parahippocampus (BA 35), and bilaterally in the amygdala (BA 34) and the putamen in MS patients compared to HCs.25,26
Linking GM atrophy and olfactory dysfunction in MS, the volume of “olfactory-related GM” (predominantly in the parahippocampal gyrus, PCG) was previously reported to be decreased in patients with olfactory dysfunction.22 However, this study did not differentiate between odor identification, discrimination, and threshold. In our study, we identified a highly significant association between PCG density and DI scores, but not olfactory threshold scores. The PCG along with the amygdala and parts of the anterior and posterior cingulate is involved in olfactory memory, semantic memory and recollection, and odor familiarity, features that are assessed by DI but not threshold.29,30
Current evidence suggests that olfactory dysfunction in MS has to be considered as a result of a dual pathology: (1) alteration of discrimination and identification reflects GM atrophy in regions directly related to olfactory function and disease progression, whereas (2) impairment of olfactory threshold does not (and might instead reflect inflammatory disease activity). Our study is supporting this concept by providing for the first time a direct link between impairment of DI score, disability progression, and GM atrophy in a longitudinal design.
In this context, dysfunction of distinct olfactory qualities is emerging as a biomarker of both inflammation and subsequent neurodegeneration. The spectrum of disease-modifying treatment options has significantly broadened over the last decade and, as a consequence, treatment decisions have become much more complex. To enable evidence-based decision making, it is critical to have biomarkers for the assessment of individual risk for short- to mid-term disability progression. Clinical disability assessment by the EDSS reflects neurodegenerative damage incompletely, with substantial delay and low sensitivity and does not provide a linear marker, as greater rates of change are observed for lower EDSS scores.31
Based on our results, both DI score and temporomesial/putamen GMC are suggested candidates for biomarkers for the prediction of short- to mid-term disability progression. However, as this is an explorative study with small sample size, our results certainly have to be interpreted cautiously and require validation in an independent larger cohort.
In conclusion, impairment of odor discrimination and identification, but not threshold, is associated with gray matter atrophy in regions related to olfactory function. This finding suggests to validate the assessment of DI score and temporomesial/putamen GMC as biomarkers to predict disability progression and monitor neurodegeneration in MS.
Supplemental Material
Supplemental material, MSJ838205_supplementary_figures for Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in MS by Gabriel Bsteh, Ruth Steiger, Noora Tuovinen, Harald Hegen, Klaus Berek, Sebastian Wurth, Michael Auer, Franziska Di Pauli, Elke R Gizewski, Florian Deisenhammer, Thomas Berger and Christoph Scherfler in Multiple Sclerosis Journal
Supplemental material, MSJ838205_supplementary_table_1 for Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in MS by Gabriel Bsteh, Ruth Steiger, Noora Tuovinen, Harald Hegen, Klaus Berek, Sebastian Wurth, Michael Auer, Franziska Di Pauli, Elke R Gizewski, Florian Deisenhammer, Thomas Berger and Christoph Scherfler in Multiple Sclerosis Journal
Footnotes
Author contribution: G.B. contributed to study concept and design, patient recruitment, acquisition of data, statistical analysis, and interpretation of data. R.S. contributed to patient recruitment, acquisition of MRI data, and critical revision of manuscript for intellectual content. N.T. contributed to patient recruitment, acquisition of MRI data, statistical analysis of MRI data, and critical revision of manuscript for intellectual content. H.H. contributed to patient recruitment, acquisition of data, and critical revision of manuscript for intellectual content. K.B. contributed to patient recruitment, acquisition of data, and critical revision of manuscript for intellectual content. S.W. contributed to patient recruitment, acquisition of data, and critical revision of manuscript for intellectual content. M.A. contributed to patient recruitment, acquisition of data, and critical revision of manuscript for intellectual content. F.D.P. contributed to patient recruitment, acquisition of data, and critical revision of manuscript for intellectual content. E.R.G. contributed to acquisition of MRI data and critical revision of manuscript for intellectual content. F.D. contributed to patient recruitment, acquisition of data, and critical revision of manuscript for intellectual content. T.B. contributed to patient recruitment, interpretation of data, critical revision of manuscript for intellectual content, and study supervision. C.S. contributed to study concept and design, patient recruitment, statistical analysis of MRI data, interpretation of data, critical revision of manuscript for intellectual content, and study supervision.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.B. has participated in meetings sponsored by, and received speaker honoraria or travel funding from Biogen, Merck, Novartis, Sanofi-Genzyme, and Teva, and received honoraria for consulting Teva. R.S. declares no conflicts of interest. N.T. declares no conflicts of interest. K.B. declares no conflicts of interest. H.H. has participated in meetings sponsored by, and received speaker honoraria or travel funding from Bayer, Biogen, Merck, Novartis, and Sanofi-Genzyme, and received honoraria for consulting Teva. S.W. has participated in meetings sponsored by, and received honoraria or travel funding from Biogen, Merck, Novartis, Sanofi-Genzyme, Teva, Allergan, Ipsen Pharma, and Roche. M.A. received speaker honoraria and/or travel grants from Merck, Novartis, and Biogen. F.D.P. has received speaking honoraria from Biogen and Sanofi-Genzyme. E.R.G. declares no conflicts of interest. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Bayer, Biogen, Sanofi-Genzyme, Merck, Novartis, and Roche. T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, and consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Celgene, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, and Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, Merck, Novartis, Sanofi Aventis, and Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, and Teva. C.S. has nothing to declare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Gabriel Bsteh  https://orcid.org/0000-0002-0825-0851
https://orcid.org/0000-0002-0825-0851
Noora Tuovinen  https://orcid.org/0000-0002-8835-5281
https://orcid.org/0000-0002-8835-5281
Sebastian Wurth  https://orcid.org/0000-0001-7122-0842
https://orcid.org/0000-0001-7122-0842
Florian Deisenhammer  https://orcid.org/0000-0003-4541-8841
https://orcid.org/0000-0003-4541-8841
Contributor Information
Gabriel Bsteh, Department of Neurology, Medical University of Innsbruck, Anichstrasse 35, 6020 Innsbruck, Austria; Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Ruth Steiger, Department of Neuroradiology, Medical University of Innsbruck, Innsbruck, Austria/Neuroimaging Research Core Facility, Medical University of Innsbruck, Innsbruck, Austria.
Noora Tuovinen, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria/Neuroimaging Research Core Facility, Medical University of Innsbruck, Innsbruck, Austria.
Harald Hegen, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Klaus Berek, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Sebastian Wurth, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Michael Auer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Franziska Di Pauli, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Elke R Gizewski, Department of Neuroradiology, Medical University of Innsbruck, Innsbruck, Austria/Neuroimaging Research Core Facility, Medical University of Innsbruck, Innsbruck, Austria.
Florian Deisenhammer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Thomas Berger, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Christoph Scherfler, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria/Neuroimaging Research Core Facility, Medical University of Innsbruck, Innsbruck, Austria.
References
- 1. Doty RL, Li C, Mannon LJ, et al. Olfactory dysfunction in multiple sclerosis. N Engl J Med 1997; 336(26): 1918–1919. [DOI] [PubMed] [Google Scholar]
- 2. Zorzon M, Ukmar M, Bragadin LM, et al. Olfactory dysfunction and extent of white matter abnormalities in multiple sclerosis: A clinical and MR study. Mult Scler 2000; 6(6): 386–390. [DOI] [PubMed] [Google Scholar]
- 3. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338(5): 278–285. [DOI] [PubMed] [Google Scholar]
- 4. Bsteh G, Hegen H, Ladstätter F, et al. Change of olfactory function as a marker of inflammatory activity and disability progression in MS. Mult Scler 2017; 25: 267–274. [DOI] [PubMed] [Google Scholar]
- 5. Bsteh G, Hegen H, Ladstätter F, et al. Transient impairment of olfactory threshold in acute multiple sclerosis relapse. Mult Scler Relat Disord 2018; 23: 74–77. [DOI] [PubMed] [Google Scholar]
- 6. Bsteh G, Berek K, Hegen H, et al. Smelling multiple sclerosis: Different qualities of olfactory function reflect either inflammatory activity or neurodegeneration. Mult Scler 2020; 26(1): 57–68. [DOI] [PubMed] [Google Scholar]
- 7. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 9. Hegen H, Bsteh G, Berger T. “No evidence of disease activity”—is it an appropriate surrogate in multiple sclerosis? Eur J Neurol 2018; 25(9): 1107–e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steer RA, Ball R, Ranieri WF, et al. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol 1999; 55(1): 117–128. [DOI] [PubMed] [Google Scholar]
- 11. Rumeau C, Nguyen DT, Jankowski R. How to assess olfactory performance with the Sniffin’ Sticks test®. Eur Ann Otorhinolaryngol Head Neck Dis 2016; 133(3): 203–206. [DOI] [PubMed] [Google Scholar]
- 12. Hummel T, Kobal G, Gudziol H, et al. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 2007; 264(3): 237–243. [DOI] [PubMed] [Google Scholar]
- 13. Duarte-Carvajalino JM, Sapiro G, Harel N, et al. A framework for linear and non-linear registration of diffusion-weighted MRIs using angular interpolation. Front Neurosci 2013; 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friston KJ, Ashburner J, Frith CD, et al. Spatial registration and normalization of images. Hum Brain Mapp 1995; 3: 165–189. [Google Scholar]
- 15. Schmidt P. Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging, 2017, urn:nbn:de:bvb:19-203731 [Google Scholar]
- 16. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38(1): 95–113. [DOI] [PubMed] [Google Scholar]
- 17. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000; 12(4): 191–200. [DOI] [PubMed] [Google Scholar]
- 18. Joseph A, DeLuca GC. Back on the scent: The olfactory system in CNS demyelinating diseases. J Neurol Neurosurg Psychiatry 2016; 87(10): 1146–1154. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Lopez L, Osher J, et al. Right orbitofrontal cortex mediates conscious olfactory perception. Psychol Sci 2010; 21(10): 1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doty RL. The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Ann Neurol 2008; 63(1): 7–15. [DOI] [PubMed] [Google Scholar]
- 21. Rolet A, Magnin E, Millot JL, et al. Olfactory dysfunction in multiple sclerosis: Evidence of a decrease in different aspects of olfactory function. Eur Neurol 2013; 69(3): 166–170. [DOI] [PubMed] [Google Scholar]
- 22. Li L-M, Yang L-N, Zhang L-J, et al. Olfactory dysfunction in patients with multiple sclerosis. J Neurol Sci 2016; 365: 34–39. [DOI] [PubMed] [Google Scholar]
- 23. Bsteh G, Hegen H, Teuchner B, et al. Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler 2019; 25: 196–203. [DOI] [PubMed] [Google Scholar]
- 24. Sastre-Garriga J, Pareto D, Rovira A. Brain atrophy in multiple sclerosis: Clinical relevance and technical aspects. Neuroimaging Clin N Am 2017; 27(2): 289–300. [DOI] [PubMed] [Google Scholar]
- 25. Eshaghi A, Prados F, Brownlee WJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol 2018; 83(2): 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steenwijk MD, Geurts JJG, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2016; 139(Pt 1): 115–126. [DOI] [PubMed] [Google Scholar]
- 27. Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry 2014; 85(12): 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mesaros S, Rovaris M, Pagani E, et al. A magnetic resonance imaging voxel-based morphometry study of regional gray matter atrophy in patients with benign multiple sclerosis. Arch Neurol 2008; 65(9): 1223–1230. [DOI] [PubMed] [Google Scholar]
- 29. Savic I, Berglund H. Passive perception of odors and semantic circuits. Hum Brain Mapp 2004; 21(4): 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iannilli E, Bitter T, Gudziol H, et al. Differences in anosmic and normosmic group in bimodal odorant perception: A functional-MRI study. Rhinology 2011; 49(4): 458–463. [DOI] [PubMed] [Google Scholar]
- 31. Damasceno A, Damasceno BP, Cendes F. No evidence of disease activity in multiple sclerosis: Implications on cognition and brain atrophy. Mult Scler 2016; 22(1): 64–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ838205_supplementary_figures for Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in MS by Gabriel Bsteh, Ruth Steiger, Noora Tuovinen, Harald Hegen, Klaus Berek, Sebastian Wurth, Michael Auer, Franziska Di Pauli, Elke R Gizewski, Florian Deisenhammer, Thomas Berger and Christoph Scherfler in Multiple Sclerosis Journal
Supplemental material, MSJ838205_supplementary_table_1 for Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in MS by Gabriel Bsteh, Ruth Steiger, Noora Tuovinen, Harald Hegen, Klaus Berek, Sebastian Wurth, Michael Auer, Franziska Di Pauli, Elke R Gizewski, Florian Deisenhammer, Thomas Berger and Christoph Scherfler in Multiple Sclerosis Journal