Abstract
INTRODUCTION
Smartphone-based smoking cessation interventions are increasingly used around the world. However, the effects of smartphone applications on applicability and efficacy on cessation rate and prevention of relapses are not often evaluated. Therefore, this review aims to assess the evidence on effectiveness of smartphone applications as an intervention tool for smoking cessation support.
METHODS
We conducted the search using Ovid Medline/PubMed, CENTRAL and Scopus databases dated (January 2007-June 2016). Inclusion criteria include randomized control trials or intervention studies with mobile applications that offer smoking cessation support. Two assessors independently extracted and evaluated the data from each included study.
RESULTS
The review of eight selected studies illustrate the use of smartphone applications in increasing quit rates among smokers, however adherence to app features influences quit rates. Audiovisual features followed by a quit plan, tracking progress and sharing features are most accepted and utilised app features. However, inconsistency was observed in their association with abstinence or quit rate. App engagement features increase the statistical significance in the quit rate. Development of smartphone applications was supported by behavior change theories in all studies nevertheless; heterogeneous forms of intervention were adopted within studies. Similarly, reduction in relapse attributed to enhanced discussion among quitters using social media applications was observed.
CONCLUSIONS
Quality evidence is warranted with large sample size to measure effect size of the intervention. Future research on effectiveness and efficacy of smartphone alone and comparisons with other mHealth interventions, such as text messaging would be useful.
Keywords: Smoking cessation, mHealth, randomized control trials, intervention study, smartphone mobile application, relapse prevention
INTRODUCTION
The health consequences of active and passive smoking are causally linked to nicotine addiction, cancer, respiratory disease, cardiovascular disease, and diabetes1. Smoking is also attributed to one in every six non-communicable disease (NCD) deaths in the world2. There are about one billion smokers in the world and six million people die each year from tobacco use3, 4. Evidence from the tobacco control program suggests that pharmacological, psychological and behavioural assistance are key to achieving smoking cessation, as only three percent of the smokers manage to quit without the help of intervention5. The search for personalised behavioural intervention along with the pharmacological treatment of addiction is inevitable as this combination produces large effect size for abstinence rate of more than 6 months. The benefits of smoking cessation are not questionable as cessation by 40 years and 60 years reduces the risk of premature death by 90% and 40%, respectively6. However, reduced number of cigarettes smoked per day is much less effective than complete cessation to avoid the excess risk of premature death from smoking1.
A previous review suggests mobile phone technology has enormous potential for behaviour change7. Smartphone applications (apps) are well accepted among mobile phone users. More than 3 billion mobile health (mHealth) apps are estimated to be downloaded worldwide in 20158. Mobile applications can easily be downloaded and a large number of users can receive tailored text messages and information at low cost. There is a sky-rocketing growth in mobile phone technology and users around the world. A World Health Organization (WHO) report suggests the global penetration of mobile phones has potential to enhance availability, accessibility, innovation, cost effectiveness, real-time access to information, and portability to health service and promotion interventions9. The use of mHealth intervention for smoking cessation represents one of the best buys to curb the global public health threat of the tobacco epidemic9, 10.
A 2015 report shows that 43% of the global population owns a smartphone whilst an estimated 12 % does not have access to cellular technology11. The 2015 estimate shows mobile broadband penetration has reached 47% and the 3G (third generation of wireless mobile telecommunications technology) coverage of about 70% of the world population12. Recently, mobile phone applications have delivered health promotion interventions and services successfully such as regulation of physical activity13–16, mental health monitoring15, nutrition and diet improvement17, 18. One comparative study suggested a large number of smokers use the smartphone to send and receive texts, download apps, use Facebook, and browse health-related internet sites19.
Evaluation of evidence of the impact and cost-effectiveness of mHealth services is imperative, as the modifiable gap in communication persists between healthcare professionals and smokers20. Review studies have shown the efficacy of clinicians and doctor’s brief advice on smoking cessation; however, unique individual behavioural issues during the cessation course are often conflicting to be addressed by health care professionals alone. Multiple channels and personalised behavioural intervention are required to reach unmotivated smokers. The study on the behavioural functionality of mobile apps shows mobile apps are well accepted among users but research still lacks scientific rigour needed to determine the efficacy of and establish quality evidence on mobile apps for best practices21. A review of the mobile health technology-based health behaviour change or disease management interventions found that only six of the forty-nine interventions used apps22. Among more than 100,000 health apps available23, a number of downloads, information retrieval and application features are measured daily but very little is explored on its implication for behaviour change.
The current literature on mHealth application involving smoking cessation intervention lacks evidence on various issues such as low generalizability power, reporting bias, short follow-up duration, and inconsistency in the measurement of dose-effect relationship24. Consolidation of evidence from this review on smoking cessation mobile apps will be significant; to shape future research as there is a growing body of literature on the use of apps to support behaviour change communication. Evaluations of the impact of mHealth, including mobile applications in behaviour modification are becoming an urgent need as technology changes quickly. One recent review found a growing body of positive evidence demonstrating the use of mobile phone-based technologies to support smoking cessation7. However, most of this evidence consists of studies evaluating the efficacy of mobile phone SMS text messaging interventions7, 25. With the continuous growth in mobile phone health applications alone, its impact remains difficult to measure. One study in 2015 reported about 400 smoking cessation applications available in the US, UK and Australian market. While a limited number of extensive randomized control trials (RCTs) are conducted solely using mobile apps in various settings, the findings from these trials have not been evaluated systematically.
The aim of this review is to assess the effectiveness of using smartphone mobile applications for smoking cessation among adult smokers resulting in smoking outcomes, engagement and utilisation of the application.
METHODS
Search strategy
We searched electronic databases (Ovid MEDLINE/PubMed, CENTRAL and Scopus) from January 2007 to June 2016 to identify relevant studies. Publicly available trials register; ClinicalTrials.Gov was searched for all trials. The combination of free text words, medical subject heading (MeSH) and index terms relating to the use of the mobile applications and smoking cessation were used during the search in the specific database. Restriction to study participants, intervention, comparison, outcome, setting and timing (PICOST/PICO) was applied. Indexed words such as smoking cessation, tobacco use, randomised control trials, intervention study, mHealth, or mobile health were used for the literature search. Medical Subject Heading (MeSH) also included: “Smartphone” (MeSH): A cellular phone with advanced computing and connectivity capability built on an operating system. “Mobile Applications” or “Apps” (MeSH): Computer programs or software installed on mobile electronic devices which support a wide range of functions and uses that include visual graphics, audio, video, music, text processing and internet service.
Inclusion and Exclusion criteria
The papers were selected based on the following eligibility criteria:
Subjects: Adolescent or adult current smokers using a mobile application in the smartphone, tablets or portable device that are capable of computing.
Study design: Studies using interventional design, quasi-experimental studies or randomized control trials or control trials.
Intervention: Mobile application designed with the smoking cessation support materials (quit plan, motivational audio-visual materials, and smoking calculators).
Comparison: usual health education delivered through mobile phones application technology (e.g. text messaging) or traditional methods (leaflets, talks, counselling)
Primary outcomes measured: smoking outcomes (cessation rate, relapse rate), engagement to application and utilisation rate.
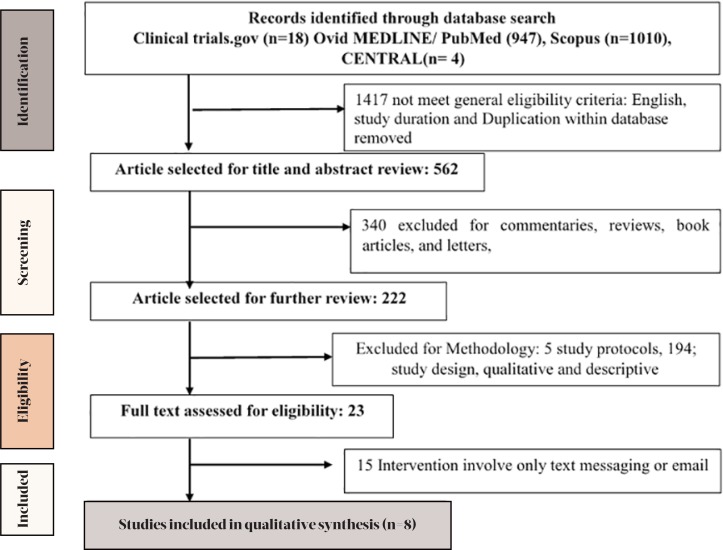
Reviews, conference papers, commentaries and letters along with studies that presented methodological issues or include app development analysis were excluded. Titles, abstracts, and methodology section of all potential articles meeting the inclusion criteria were studied by two authors independently. Figure 1 shows the detailed flow of study selection. Titles and abstracts of articles obtained from the search were screened following the inclusion and exclusion criteria. We obtained the full-text article for each eligible study for further assessment.
Figure 1.
Search details
Data extraction process
The extraction of information from the individual articles was performed on the predefined framework based on the PICOs framework. The framework includes; study title, author, date and place published, design features of the study, research question assessed, description of the intervention (mobile application, participants, intervention design, comparison, outcome measures, duration of the intervention, and key findings). The assessment of risk and bias was conducted using the framework suggested by the Cochrane tool for assessing risk and bias in intervention studies26. A risk and bias assessment consists of elements of the study design; sample size, allocation concealment, blinding, free of selective reporting, free of other individual and design bias and outcome reporting. The risk and bias table gives a high, low or unclear level of risk. The kappa inter-rated reliability of 85% was achieved with the use of SPSS v1627. The preferred reporting items for systematic reviews and meta-analysis (PRISMA) guideline28 was followed to give the whole structure of the report.
RESULTS
Search details
A total of 1979 studies were identified in all databases. Two hundred and twenty-four unique articles met the eligibility criteria and therefore were screened. Only eight articles meeting the inclusion and exclusion criteria were included in the review. See Figure 1 for details of the search result and reasons for exclusion. All included studies were conducted in the past two years.
Study characteristics Participants
The participants included in the study were adult daily smokers aged 18 or older with minimal computing literacy in using smartphones. The majority of the studies were conducted in the United States29–33. One unique intervention of mobile apps on the tablet was conducted among randomly selected hospital patients. Most studies recruited the participants online. Five randomised control trials and quasi-experimental design studies examined a total of 628 participants ranging from 96 to 196 (Table 1).
Table 1.
Summary of included Studies.
| Author/Country | Finkelstein& Cha, 2016 USA | Buller DB, et al. 2014, Australia | Bricker JB, et al. 2014, USA | Heffner LJ, et al. 2014, USA | Ubhi KH, et al. 2015, England | Zeng et al. 2015, USA | Zeng et al. 2016, USA | Cheung et al 2015 Hong Kong |
|---|---|---|---|---|---|---|---|---|
| Title | Using Mobile app to promote smoking cessation in Hospitalized patient. | Randomized trial of smartphone mobile application compared to text messaging to support smoking cessation | Randomized controlled pilot trial of smartphone app for smoking cessation using acceptance and commitment therapy | Feature level analysis of novel smartphone application for smoking cessation | A Mobile App to Aid Smoking Cessation: Preliminary Evaluation of SmokeFree28 | Predictors of utilization of a Novel Smoking cessation smartphone App. | Get with the program: Adherence to a smartphone app for smoking cessation | Using WhatsApp and Facebook Online Social Groups for Smoking Relapse Prevention for Recent Quitters: A Pilot Pragmatic Cluster Randomized Controlled Trial |
| Design features | Prospective Intervention design N=55 | RCT (Pre-test, post-test Two group design) N=102 | Pilot RCT, stratified randomization N=196 | Single arm Post hoc analysis of RCT N=96 | Interventional Study N=1135 | Pilot RCT, Two arm N=98 | Single arm pilot study (Quasi experiment) N=84 | Pilot single-blinded, parallel, 3-arm pilot, cluster randomized controlled trial N=136 |
| Description of the Mobile application | Name: Computer assisted Education system (CO-ED) Theory: Adult learning and instructional technology, Information processing theory, Constructive theory, Cognitive flexibility theory, Submission theory, Drive Reduction theory, Cognitive load theory. Features: Knowledge repository containing educational content and user interface supporting content delivered via multiple platform; tablet, smartphone, gaming, touch screens Available platform: NA |
Name: Real E Quit Mobile application (REQ-Mobile) Theory: Unclear Features: Receive test message, Support document (benefit of quitting, strategies for stopping NRT, coping and withdrawal), quit plan supported by automated messages. |
Name: Smart Quit Theory: Action and Commitment Theory (ACT) Features: 1) Staying motivated focus on ACT values via testimonials of formers smokers describing how quitting smoking has help them do things that deeply matter them e.g. time spent with family, Pictorial message with reasons of quit2) Personalized Quit plan 3) audio and text base skill presented for coping with cravings to smoke, 4) Audio and text-smoking lapsed and self-judgement tool 5) Tracking of actions. Name: Quit Guide app: from National Cancer Institute Theory : Not clear Based on Clinical practice guideline Features: Features and content drawn from smokefree.gov website.1)Reason based motivation to quit 2) Personalized quit plan 3) Social support 4) Information on FDA approved medication 5)Teach skills to cope carving, give technique, share success |
Name: Smart Quit Theory : ACT and Cognitive Behavioural Therapy (CBT) Features: ACT specific exercise are grouped for motivation text and video, skills to accept urges to smoke, and coping skills. Non ACT/CBT features: self-monitoring with feedback, (tracking and viewing progress), positive reinforcement, creating a quit plan and sharing progress via email, text and social media. Name: Quit Guide app: from National Cancer Institute Theory : NA Features: NA |
Name: SmokeFree28 Theory: Behaviour change theories PRIME theory (Plans, Responses, Impulses, Motives, and Evaluations). Features: Quit plan and preparatory behavioural modification to quit before Specific daily message and planning activities for 28 days. |
Name: Smart Quit Theory: Action and Commitment Theory Features: Exercise designed to increase willingness to experience trigger situation without smoking, Increase recovery skills for smoking lapses and develop self-compassion |
Name: ACT-based cessation App Theory: ACT Evidence based: YES65 Features: 1) creating a quit plan 2) completing eight daily ACT modules 3) tracking letting ten urges pass visiting t 4) Anytime Coaching section at least once |
Name: Whats app and Online Facebook Theory: Unclear Feature: Social group function of the application Reminders from the moderators texts, pictures, and videos, were based on the “Treatments for the Recent Quitter” of the US Clinical Practice Guidelines on Treating Tobacco Use and Dependence [2], including (1) encourage to maintain abstinence, (2) remind about the importance of remaining abstinence, (3) prevent smoking triggers, (4) remind about the withdrawal symptoms and lapse, (5) advise about stress and mood management, and (6) advise about weight control |
| Participants | Two US Hospital smokers Age: Sampling: consecutive selection, sample size: 55 Recruitment: Hospital admission | US Adult smokers (18-30) Sampling : Online recruitment, Probability sampling sample 102 | US adults 18+or older smokers smoking at least 5 cigarettes daily for at least 12 month Sampling : probability 196 Recruitment: Online (Facebook, website, search engine ) Offline: TV advertisement | Exploratory study of randomized app users | Automated data collected on each time potential user open the app. Participant set the quit date and each day of abstinence was rewarded by the app |
Two arm randomized | Single arm Pilot randomization Intervention study | WhatsApp group chat Facebook chat as intervention platform 3 reminders per week Cluster Randomization using random number Masking of Clients And recruiters were weekly notified |
| Comparison | Post Intervention comparison | Mobile application Vs Text messaging | Smart Quit app based on Acceptance and commitment therapy VS Quit guide app | Smart Quit Vs Quit Guide App | None | None | None | WhatsApp Vs Facebook group online discussion and booklet Control : No-group discussion |
| Outcome measures | Difference in knowledge test score pre and post App use, Process of smoking cessation (Stage of TTM) Smoking self-Qualitative verbatim transcription | Questionnaires: Baseline,6 weeks post-test, smokers reported smoking status Readiness to quit Point prevalence abstinence of smoking |
Thirty day point prevalence abstinence | App utilization Smoking cessation point prevalence rates Questionnaire | 28 days abstinence | User characteristics (by Education, Heavier smoking, No of close friend who smoke, Anxiety, Depression) and utilization of app | Smoking cessation (two-month post-randomization 7-day point prevalence abstinence via self-report,) Adherence rate | Relapse prevention rate in Facebook, WhatsApp and Control group |
| Duration of intervention | 45 minutes for a session, Duration of intervention not reported. | 12 weeks | 8 weeks | 60 day post randomization | 28 days | 8 weeks | 2 month | 6 month |
| Key findings | Knowledge gain was the main predictor of more favourable attitudes towards mobile app (OR 4.8, CI 1.1, 20.0) | 30 day point prevalence abstinence r=0.32, p=0.14 and continuous abstinence r=0.31, p=0.09. | Smart app quit rate with ACT was 13% Smart app with quick guide was 8% (OR 2.7;95% C.I, 0.8-20.7) | Viewing and staying motivated video (OR 4.1 95% CI (0.9-17.6), Urge exercise “Leaves on stream” video (OR 4.1 95% CI (0.9-17.6 ), predicted smoking abstinence n=15 users) |
The self-reported smoking cessation rate for 28 days or longer was 18.9% (95% CI 16.7-21.1). Recorded abstinence was significantly associated with older age, non-manual occupational group, and use of a stop-smoking medicine but not with daily cigarette consumption | Heavier smoking, depression and lower education were predictive of app utilization Heavier smoking (RR 0.95; p=0.003) Lower Education (RR:0.492; p=0.021 ) Depression (RR: 0.958; p=0.017) |
Fully adherent users (24%) were over four times more likely to quit smoking (OR = 4.45; 95% CI = 1.13, 17.45; p = 0.032). | Fewer participants in the WhatsApp group reported relapse than the control group at 2-month (OR 0.27, 95% CI 0.10-0.71) and 6-month; OR 0.43, 95% CI 0.19-0.99) follow-ups. The Facebook group had an insignificantly lower relapse rate than the control group at 2-month (OR 0.58, 95% CI 0.24-1.37) and 6-month OR 0.70, 95% CI 0.31-1.61) follow-ups. |
RCT- Randomized control trials, ACT- Action and Commitment Theory, OR- odds ratio, RR- Relative Risk, CI- Confidence Interval NA- Not Available/Unclear
Design features of the studies
Studies were pilot randomized control trials, control trials or quasi-interventional by design. Two studies included were a post-hoc analysis of the single arm pilot randomised control trials31, 32. Three studies did not have a control group29, 33, 34.
Mode of Recruitment
Most studies used online recruitment methods predominately using social media sites such as Facebook or Google advertisement. The traditional method of recruitment was also included along with online advertisement in a few studies. Two studies included hospital patient and smoking cessation clinic clients29, 35. One study used an online screening survey for recruitment32. The format of delivery of intervention varied with each study.
Mobile application
Studies reported unique and catchy names of the mobile application that relates to quitting or smoking cessation. Mobile applications available on different platforms were reported. One was a tablet-based education app for hospital inpatient, while others were predominately smartphone based, delivered over online application platforms. One study was confined to the I-phone based platform. Another study used a social media application such as WhatsApp and Facebook to deliver the smoking cessation service35. Six studies adopted automated data extraction from the application. Four studies30, 31, 33, 34, 36 included automated motivational messages while another included a specific daily educational module on smoking cessation29. One study employed exercise as the medium to adhere to for post cessation relapse and withdrawal symptoms32. Most studies have adopted a quit plan, pictorial and audio-visual, cost saving, coping skills, and social support features in the application for smoking cessation.
Theory adapted for intervention
Mobile applications applied behavior change theories to bring changes in the behavior of the smokers. Two studies reported adoption of multiple behavioral theories used to design intervention features29, 34. Multiple behavior theories include; adult learning, PRIME, action and commitment theories, and features of cognitive behaviour change theories (CBT); such as drive reduction, cognitive flexibility, submission theory, positive reinforcement. Three studies reported the use of action and commitment theory only. Only one study reported the evidence-based design of the intervention30. However, all studies reported used features derived from behavior change theories. (Table 1)
Nature of Intervention and working modality
The included study involved a mobile application inbuilt with features designed to help smokers in two ways. First, the application content motivates smokers for smoking cessation through knowledge repositories, on benefits of cessation, and planning for the cessation attempt. This process includes planning, tracking, visualising and learning behaviour change techniques, including audio and visual messages. Studies using only this technique reported a significant change in knowledge, attitude and self-efficacy to avoid cigarettes. One study reported a significant change in both knowledge and attitude but there was no significant result over self-efficacy to avoid smoking temptations29. The statistics on the usage of mobile applications reveal a significant proportion (on average 60%) of the users in the experimental group used all features of the mobile application36. Secondly, some mobile applications attempt to engage smokers within the application content. Techniques included setting a quit date, push-notification, maintaining quit diaries, sharing features, email reminders, and prescription of theory based exercises designed to mitigate cravings, and creating a quit plan30, 31, 36. These app engagement features reported a statistical significant increase in the quit rate.
Study quality and potential sources of bias
The risk and bias assessment of the studies are presented in Table 3. The quality assessment criteria were adapted from the Cochrane handbook of quality assessment of intervention studies. The quality assessment shows the apparent heterogeneity in the selection of study participants, and measurement of outcome that’s prevented us from employing meta-analysis to draw a quantitative conclusion. The selection bias was the most prominent source of bias in the included studies. One-third of the studies had a high level of risk on selection bias. The source of selection bias was randomization and allocation to treatment and control group. Only two studies have appropriately masked the potential source of outcome bias33, 34. A few studies suffered selective outcome analysis as they were derived from the post hoc analysis of long-term randomized control trials. (Table 2). As variability in study objective and design was observed in the selected studies we extracted information according to their outcome measured to summarise the effect on three different outcomes: smoking cessation, app utilization/engagement and relapse prevention.
Table 2.
Risk and bias analysis of included studies
| Studies | Finkelstein& Cha, 2016 USA | Buller DB, et al. 2014, Australia | Heffner LJ, et al. 2014, USA | Ubhi KH, et al. 2015, England | Zeng et al. 2015, USA | Zeng et al. 2016, USA | Cheung et al 2015 |
|---|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | High | Low | Low | High | Low | High | Low |
| Allocation concealment (selection bias) | High | Low | Low | High | Unclear | High | Low |
| Blinding (performance bias and detection bias) all outcomes | High | Unclear | High | Low | Unclear | Low | High |
| Incomplete outcome data (attrition bias) | Low | Low | Low | Low | Low | Low | Low |
| Other bias (analysis bias, publication, ) | Low | Low | Low | High | Low | High | Unclear |
High – reviewers observed the high level risk and bias
Low- reviewers observed low level of risk and bias
Unclear: Not enough information to judge the criteria
Effect of intervention on the outcome
Smoking cessation: Three studies reported higher smoking cessation/quit rate among app users30, 33, 34. One interventional study showed the self-reported smoking cessation rate for 28 days or longer was about 19%, (95% CI 16.7-21.1)34. Other randomised controlled trials comparing two mobile applications using action and commitment theory reported 13% (95% CI,6-22%) quit rate in the intervention (ATC based smart application) versus 8% (95% CI, 3-16%) in control groups (Quit Guide app)36. The odds of quitting were 2.9%, (95% CI 0.8-10.3).Two studies measuring 8-weeks cessation rate compared to conventional treatment only and full app adherence post-intervention were two times and four times more likely to achieve cessation, respectively30, 32. When comparing the smartphone mobile applications with text message smoking cessation support, significantly higher number of quitters were found among the text message support group. Inconsistency in abstinence by quit duration was observed among the smokers in the text message support group. Among those in the smartphone application group, the frequency of use was positively associated with smoking cessation at 12 weeks36.
App Utilization and Adherence: The average number of application openings in the experimental group of the three studies was twenty-four. The least number of app openings was eleven times followed by twenty-six times and the highest was thirty-seven times. However, in interventional studies, the average number of the application openings was only 8.5 (SD = 9). Young age, knowledge level, heavier smoking, depression were predictive factors for app utilization studied in three studies29, 32, 34. Audio-visual features were most used aspects of the application followed by quit plan, tracking process and sharing features. One study shows quit plan was positively associated while tracking practice was opposing the quitting practice among smartphone app users31. The effect of the use of applications positively and significantly increases knowledge on smoking hazards and cessation rate from baseline29, 34. One interventional study34 showed a strong positive association between the number of application openings and 4 weeks abstinence rate (OR 1.17, 95% CI (1.15-1.19). A study specifically measuring adherence found that out of twenty-four percent (n=99) of app users who fully adhered (completed all program components) to seven-day point prevalence was 4.5 times higher (95% CI 1.13-17.45) when compared to users who were not fully adherent33.
Relapse Prevention: One study taking into account relapse prevention intervention, delivered via social media applications WhatsApp and Facebook, reported a lower relapse rate than non-users at 2 months and 6 months35. The difference was significant in the WhatsApp group. Fewer participants in the WhatsApp group (17%, 7/42) reported relapse compared to the control group (42.6%, 23/54) at 2-month (OR 0.27, 95% CI 0.10-0.71) and 6-month (40.5%, 17/42 vs 61.1%, 33/54; OR 0.43, 95% CI 0.19-0.99) follow-up. However, the power analysis in this study showed that the Facebook group and control group had large type II error. Overall studies conclude enhance discussion decreases the relapse rate.
DISCUSSION
The study illustrates that evidence-based smartphone apps have been recently introduced and are continuously developing. All the evidence is representative of the high-income countries published in last two years. The use of smartphone applications fosters quit rates among smokers, however full adherence to application features is identified as a most important aspect. Audio-visual features followed by quit plan, tracking progress and sharing features are the most accepted and utilized app features. However, inconsistency is observed in their association with abstinence or quit rate. Studies using smartphone mobile applications were only included in our study, and we evaluated the evidence on smoking cessation, and relapse prevention interventions among smokers.
Mobile health research continues to expand rapidly with the innovation in mobile technology. Undeniably, smoking cessation intervention delivered via mobile text messages has shown beneficial impact1. This clearly suggests viability and applicability in using the mobile applications to deliver health services and interventions. The use of the smartphone-based mobile applications to deliver health interventions is relatively new to public health practice but the mHealth initiative is believed to be continuously revolutionizing the health sector since 194937. Initial research focused on the potential use of apps by health care professionals and students. The access to the apps was further enhanced in 2008 with the introduction of apple store for iPhone, iPad and play store for android devices38. Global data from the International Telecommunications Union (ITU) and subsequent global research has confirmed the use of the internet in smart mobile devices to be rapidly replacing traditional devices8, 12. The review studies also suggest the possible benefit of application as they can provide audio, video and text-based intervention under a single platform. They are possibly cost effective, easy to deliver and implement39–41.
All studies have reported the positive influence of mobile applications on quit rate. The quit rate ranges from 13 to 24 percent. The review of high-quality mobile phone based intervention, predominately text messaging intervention, has shown quit rates of about 10 percent1. The population-level efficacy of text messaging also favoured quit rates42. Smartphone application in addition to text message engage smokers with audio-visuals, tutorials, cessation planning and tracking progress that might have favoured high quit rate43–45. The behavioural change programs that involve diet and physical activity have demonstrated the feasibility of using applications16–18, 46. However, our review could only find small scale trials with high risk of bias. Most studies are pilot randomized trials with adequacy in measuring large effects only. However, small-scale studies have good strength and precision on testing new technology47.
Studies have shown inconsistent correlation on app utilization and acceptance. Online and mHealth studies face challenges regarding ideal participant yield and fluctuating costs of online recruitment19, 48. The theory based app features are employed in our studies with limited information on the quality of this evidence. Individual app features and their possible implication for cessation, acceptance and utilization have not yet performed or are limited43, 44, 49. Content analysis of smartphone applications have identified calculators, trackers, and motivators that are tailored with two-way communication, are the most downloaded50. Our studies are consistent with the deployment of the common application features but lack consistency in reporting application features. Recently guidelines on reporting mHealth evidence were published, future research is warranted to report technological, fidelity, access and feedback features including context and replicability51.
Limited and moderate quality evidence support the precision of mobile applications for relapse prevention. One of our studies has evaluated social media smartphone applications WhatsApp and Facebook to prevent relapses. The differences between those two applications are justified by the role of moderators. Online discussion and expert-moderated engagement facilitate relapse prevention. However, behavioural approaches to relapse prevention studies with virtual methods, such as mobile phone and applications, are still in infancy52. Review of relapse prevention intervention suggested no long-term benefit of behavioural intervention but recommends extended pharmacological treatment intervention53. The dose-response relationship is demonstrated in randomized control trial of web-based computer tailored intervention53, 54. We assume low relapse rate in our study due to the influence of frequent feedback and interaction sharing features.
Overall, the proliferation and penetration of smartphone and smartphone applications have provided a platform to support smoking cessation. The evidence on smartphone apps are yet to reach the maturity to address gaps of generalizability, but the evidence on content43, 45, 50, 55, design consideration56, 57, quality reporting guidelines51 support future evidence. This difference also adds to the variability in duration of measurements of smoking cessation outcome. Despite a limited number of studies met the selection criteria few study protocols were found to be published39, 58. This review is expected to guide future research with improved methods, sample size, and evidence-based design consideration to measure effect size.
The strength of the present study is that it is one of the first reviews attempting to consolidate efficacy and effectiveness of mobile applications for smoking cessation. Attempts were made to make a comprehensive search strategy. We followed best practice to avoid bias in the sample of studies we have retrieved. We checked the studies included for relevance and methodological rigour using PICOST framework. One limitation of this review was we extensively relied on published literature. The exclusion of grey literature might have left some potential work in this field. Our study was limited to few accessible databases. Other precisely used databases such as PsycINFO and EMBASE could extend our search coverage. Rigorous reviews were made to differentiate studies with the use of the mobile applications and other online, web-based and text messaging interventions.
CONCLUSIONS
Smartphone mobile applications have demonstrated a positive influence in fostering an increase in cessation rate. However, the quality evidence is warranted with large sample size to measure effect size of the intervention. Efficacy on relapse prevention, addressing craving post cessation and sequencing of effective application features need further research. In addition, effectiveness and efficacy of smartphone alone and its comparisons with other mHealth interventions such as text messaging and emails are urgently needed. We also recommend uniformity in design consideration and adoption of either theory-based content development or evidence-based content development and testing of smoking cessation mobile applications.
CONFLICT OF INTERESTS
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
FUNDING
There was no source of funding for this research.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed
REFERENCES
- 1.Whittaker R., et al. Mobile phone-based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016. 2016 Aug;16 doi: 10.1002/14651858.CD006611.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaglehole R., et al. Priority actions for the non-communicable disease crisis. The Lancet. 377(9775):1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 3.WHO Tobacoo Fact sheet. [Internet] 2016. 2016. Jun, [cited 2016 June 12]; Available from: http://www.who.int/mediacentre/factsheets/fs339/en/ (accessed 3 April 2017)
- 4.WHO, W.H.O W.H. Organization, editor. Global status report on noncommunicable diseases 2010. 2011. p. 176.
- 5.Stead L.F., et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2016(3) doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R., et al. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker R., et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4:Cd006611. doi: 10.1002/14651858.CD006611.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Research2Guidance mHealth App Developer Economics 2015:The current status and trends of the mHealth app market. [online research report] 2015. [cited 2016 13, July]; Available from: http://research2guidance.com/r2g/r2g-mHealth-App-Developer-Economics-2015.pdf (accessed 3 April 2017)
- 9.WHO Mobile health (mHealth) for tobacco control. Tobacco Free Initiative (TFI) [Internet] 2015. [cited 2016 June 28]; Available from: http://www.who.int/tobacco/mhealth/en/ (accessed 3 April 2017)
- 10.Pujari S. Tobacco control and mobile health (mHealth) - a new initiative, in The intersection of mobile health technology and tobacco control. WHO; 2011. http://www.who.int/tobacco/mhealth/en/ (accessed 3 April 2017) [Google Scholar]
- 11.Smartphone Ownership and Internet Usage Continues to Climb in Emerging Economies. 2016. Febuary
- 12.Union I.T. ICT Facts & Figures: The World in 2015. [Internet] 2016. [cited 2016 June 14]; Available at: http://www.itu.int/en/ITU-D/Statistics/Pages/facts/default.aspx (accessed 3 April 2017)
- 13.Fanning J., Mullen S.P., McAuley E. Increasing Physical Activity With Mobile Devices: A Meta-Analysis. Journal of Medical Internet Research. 2012;14(6):e161. doi: 10.2196/jmir.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey M., et al. Patients' experiences of using a smartphone application to increase physical activity: the SMART MOVE qualitative study in primary care. Br J Gen Pract. 2014;64(625):e500–8. doi: 10.3399/bjgp14X680989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macias C., et al. Using Smartphone Apps to Promote Psychiatric and Physical Well-Being. Psychiatr Q. 2015;86(4):505–19. doi: 10.1007/s11126-015-9337-7. [DOI] [PubMed] [Google Scholar]
- 16.Smith J.J., et al. Smart-phone obesity prevention trial for adolescent boys in low-income communities: the ATLAS RCT. Pediatrics. 2014;134(3):e723–31. doi: 10.1542/peds.2014-1012. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland J., et al. Using a Smartphone Application to Promote Healthy Dietary Behaviours and Local Food Consumption. Biomed Res Int. 2015;2015:841368. doi: 10.1155/2015/841368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson E., et al. Development and feasibility testing of a smart phone based attentive eating intervention, in BMC Public Health. 2013. p. 639. [DOI] [PMC free article] [PubMed]
- 19.Borrelli B., et al. Prevalence and Frequency of mHealth and eHealth Use Among US and UK Smokers and Differences by Motivation to Quit. J Med Internet Res. 2015;17(7):e164. doi: 10.2196/jmir.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twyman L., et al. Perceived barriers to smoking cessation in selected vulnerable groups: a systematic review of the qualitative and quantitative literature. BMJ Open. 2014;4(12) doi: 10.1136/bmjopen-2014-006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne H.E., et al. Behavioral Functionality of Mobile Apps in Health Interventions: A Systematic Review of the Literature. JMIR mHealth uHealth. 2015;3(1):e20. doi: 10.2196/mhealth.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Free C., et al. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Res Notes. 2010;3:250. doi: 10.1186/1756-0500-3-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W., Liu Y. mHealthApps: A Repository and Database of Mobile Health Apps. JMIR mHealth uHealth. 2015;3(1):e28. doi: 10.2196/mhealth.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eapen Z.J., Peterson E.D. Can mobile health applications facilitate meaningful behavior change?: Time for answers. JAMA. 2015;314(12):1236–1237. doi: 10.1001/jama.2015.11067. [DOI] [PubMed] [Google Scholar]
- 25.Ghorai K., et al. mHealth for Smoking Cessation Programs: A Systematic Review. Journal of Personalized Medicine. 2014;4(3):412–423. doi: 10.3390/jpm4030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J.P., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leech N.L., Barrett K.C., Morgan G.A. IBM SPSS for intermediate statistics: Use and interpretation. Routledge; 2014. [Google Scholar]
- 28.Moher D., et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelstein J., Cha M.E. Using a Mobile App to Promote Smoking Cessation in Hospitalized Patients. JMIR mHealth uHealth. 2016;4(2):e59. doi: 10.2196/mhealth.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bricker J.B., et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014;143:87–94. doi: 10.1016/j.drugalcdep.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffner J.L., et al. Feature-level analysis of a novel smartphone application for smoking cessation. The American Journal of Drug and Alcohol Abuse. 2015;41(1):68–73. doi: 10.3109/00952990.2014.977486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng E.Y., et al. Predictors of Utilization of a Novel Smoking Cessation Smartphone App. Telemedicine and e-Health. 2015;21(12):998–1004. doi: 10.1089/tmj.2014.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng E.Y., et al. Get with the program: Adherence to a smartphone app for smoking cessation. Addictive Behaviors. 2016;63:120–124. doi: 10.1016/j.addbeh.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ubhi H.K., et al. A Mobile App to Aid Smoking Cessation: Preliminary Evaluation of SmokeFree28. Journal of Medical Internet Research. 2015;17(1):e17. doi: 10.2196/jmir.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung Y.T.D., et al. Using WhatsApp and Facebook Online Social Groups for Smoking Relapse Prevention for Recent Quitters: A Pilot Pragmatic Cluster Randomized Controlled Trial. J Med Internet Res. 2015;17(10):e238. doi: 10.2196/jmir.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buller D.B., et al. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed J E Health. 2014;20(3):206–14. doi: 10.1089/tmj.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruessner V. The History of Mobile Health: From Cell Phones to Wearables. [Website] 2015. [cited 2016 11 August]; News]. Available at: http://mhealthintelligence.com/news/the-history-of-mobile-health-from-cell-phones-to-wearables (accessed 3 April 2017)
- 38.Ventola C.L. Mobile Devices and Apps for Health Care Professionals: Uses and Benefits. Pharmacy and Therapeutics. 2014;39(5):356–364. [PMC free article] [PubMed] [Google Scholar]
- 39.BinDhim N.F., McGeechan K., Trevena L. Assessing the effect of an interactive decision-aid smartphone smoking cessation application (app) on quit rates: a double-blind automated randomised control trial protocol. BMJ Open. 2014;4(7):e005371. doi: 10.1136/bmjopen-2014-005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.“Tobacco Quit and Save” app tracks daily savings. Home Healthc Nurse. 2014;32(7):391. doi: 10.1097/NHH.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 41.Witkiewitz K., et al. Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychol Addict Behav. 2014;28(3):639–50. doi: 10.1037/a0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Head K.J., et al. Efficacy of text messaging-based interventions for health promotion: A meta-analysis. Social Science & Medicine. 2013;97:41–48. doi: 10.1016/j.socscimed.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Abroms L.C., et al. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med. 2013;45(6):732–6. doi: 10.1016/j.amepre.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi J., Noh G.Y., Park D.J. Smoking cessation apps for smartphones: content analysis with the self-determination theory. J Med Internet Res. 2014;16(2):e44. doi: 10.2196/jmir.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs M.A., et al. Facebook apps for smoking cessation: a review of content and adherence to evidence-based guidelines. J Med Internet Res. 2014;16(9):e205. doi: 10.2196/jmir.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q., et al. Diet and Physical Activity Apps: Perceived Effectiveness by App Users. JMIR mHealth and uHealth. 2016;4(2):e33. doi: 10.2196/mhealth.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hackshaw A. Small studies: strengths and limitations. European Respiratory Journal. 2008;32(5):1141–1143. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- 48.Lane T.S., Armin J., Gordon J.S. Online Recruitment Methods for Web-Based and Mobile Health Studies: A Review of the Literature. Journal of Medical Internet Research. 2015;17(7):e183. doi: 10.2196/jmir.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson A.M., et al. Content Analysis of Anti-Tobacco Videogames: Characteristics, Content, and Qualities. Games for Health Journal. 2016;5(3):216–223. doi: 10.1089/g4h.2015.0096. [DOI] [PubMed] [Google Scholar]
- 50.Hoeppner B.B., et al. How Smart are Smartphone Apps for Smoking Cessation? A Content Analysis. Nicotine & Tobacco Research. 2016;18(5):1025–1031. doi: 10.1093/ntr/ntv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal S., et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352 doi: 10.1136/bmj.i1174. [DOI] [PubMed] [Google Scholar]
- 52.Maher C.A., et al. Are Health Behavior Change Interventions That Use Online Social Networks Effective? A Systematic Review. J Med Internet Res. 2014;16(2):e40. doi: 10.2196/jmir.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajek P., et al. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2013(8) doi: 10.1002/14651858.CD003999.pub4. [DOI] [PubMed] [Google Scholar]
- 54.Elfeddali I., et al. Preventing Smoking Relapse via Web-Based Computer-Tailored Feedback: A Randomized Controlled Trial. J Med Internet Res. 2012;14(4):e109. doi: 10.2196/jmir.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClure J.B., et al. Evaluating an Adaptive and Interactive mHealth Smoking Cessation and Medication Adherence Program: A Randomized Pilot Feasibility Study. JMIR Mhealth Uhealth. 2016;4(3):e94. doi: 10.2196/mhealth.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pulverman R., Yellowlees P.M. Smart Devices and a Future of Hybrid Tobacco Cessation Programs. Telemedicine and e-Health. 2014;20(3):241–245. doi: 10.1089/tmj.2013.0096. [DOI] [PubMed] [Google Scholar]
- 57.Roth W.R., et al. Practical considerations in the design and development of smartphone apps for behavior change. Journal of Contextual Behavioral Science. 2014;3(4):269–272. doi: 10.1016/j.jcbs.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valdivieso-Lopez E., et al. Efficacy of a mobile application for smoking cessation in young people: study protocol for a clustered, randomized trial. BMC Public Health. 13:704. doi: 10.1186/1471-2458-13-704. [DOI] [PMC free article] [PubMed] [Google Scholar]