Abstract
Enhancing LRP5 signaling and inhibiting TGFβ signaling have each been reported to increase bone mass and improve bone strength in wild-type mice. Monotherapy targeting LRP5 signaling, or TGFβ signaling, also improved bone properties in mouse models of Osteogenesis Imperfecta (OI). We investigated whether additive or synergistic increases in bone properties would be attained if enhanced LRP5 signaling was combined with TGFβ inhibition. We crossed an Lrp5 high bone mass (HBM) allele (Lrp5A214V) into the Col1a2G610C/+ mouse model of OI. At 6-weeks-of-age we began treating mice with an antibody that inhibits TGFβ1, β2, and β3 (mAb 1D11), or with an isotype-matched control antibody (mAb 13C4). At 12-weeks-old, we observed that combining enhanced LRP5 signaling with inhibited TGFβ signaling produced an additive effect on femoral and vertebral trabecular bone volumes, but not on cortical bone volumes. Although enhanced LRP5 signaling increased femur strength in a 3-point bending assay in Col1a2G610C/+ mice, femur strength did not improve further with TGFβ inhibition. Neither enhanced LRP5 signaling nor TGFβ inhibition, alone or in combination, improved femur 3-point-bending post-yield displacement in Col1a2G610C/+ mice. These pre-clinical studies indicate combination therapies that target LRP5 and TGFβ signaling should increase trabecular bone mass in patients with OI more than targeting either signaling pathway alone. Whether additive increases in trabecular bone mass will occur in, and clinically benefit, patients with OI needs to be determined.
Keywords: Osteogenesis Imperfecta, TGFβ, Sclerostin, LRP5, WNT
1. Introduction
1.1. Osteogenesis Imperfecta
Osteogenesis Imperfecta (OI) is a genetic disorder characterized by skeletal fragility [1]. The majority of patients with OI have dominant mutations in one of the 2 genes encoding type 1 collagen, COL1A1 and COL1A2 [2]. Recessive forms of OI are less common and generally associated with mutations affecting proteins involved in collagen assembly or secretion [1]. Patients with OI can experience multiple fractures and deformities, which produce pain, immobility, and reduced quality of life [3,4].
1.2. Pharmacologic therapy for OI
Several pharmacologic therapies that have been FDA approved to reduce skeletal fragility in patients with age-related and post-menopausal osteoporosis are currently being employed off-label or are in clinical trials for patients with OI. Bisphosphonates, which inhibit osteoclast-mediated bone resorption, were predicted to improve skeletal strength in patients with OI by allowing bone anabolism to outpace catabolism [5]. Numerous studies report significantly increased bone density in patients with OI who have been treated with bisphosphonates; however, the extent to which bisphosphonate-mediated increases in bone density reduce fracture risk remains unclear [6]. Fewer studies have examined another anti-resorptive therapy that targets osteoclasts, the anti-RANKL neutralizing antibody (Denosumab), in patients with OI but clinical trials are ongoing.(www.clinicaltrials.gov) [7–9].
Therapies that primarily promote bone anabolism, rather than inhibit bone catabolism, have also been studied in patients with OI. Teriparatide (bioactive recombinant PTH 1–34) exerts modest effects on BMD in individuals with OI, but the patient cohorts were too small to observe a significant change in fracture incidence [10–12]. Sclerostin, an endogenous inhibitor of the Wnt co-receptors LRP5 and LRP6, is another target for pro-anabolic therapies [13,14]. Sclerostin neutralizing antibody (e.g., Romosozumab) significantly increases bone mass and reduces fracture incidence in patients with osteoporosis and osteopenia, and this antibody has been submitted to the FDA for approval for these indications [15–19].
1.3. LRP5 signaling and OI
Mirroring the effect of neutralizing antibodies targeting Sclerostin are missense mutations in LRP5, which make the receptor resistant to Sclerostin-mediated inhibition [20–22]. Knockin mice with these missense mutations (e.g., Lrp5A214V) have increased bone mass and strength. [23] Importantly, these alleles can be crossed into mouse models of OI to determine the effect of enhancing LRP5 signaling on bone properties. When the Lrp5A214V allele was crossed into 4 different mouse OI models, bone mass and strength increased in each model [24,25] (Unpublished data). Consistent with these genetic experiments, administering sclerostin neutralizing antibodies to OI mouse models also improved the animals’ bone mass and strength [24]. These data provide pre-clinical support for a clinical trial of sclerostin inhibition in patients with OI that is underway (www.clinicaltrials.gov).
1.4. TGFβ and OI
Another potential anabolic strategy currently in clinical trials for patients with OI utilizes a TGFβ neutralizing antibody. The rationale for this approach is based on consistent therapeutic skeletal effects of TGFβ inhibition in several pre-clinical contexts, including genetic mouse models, small molecule inhibitors, and neutralizing antibody studies [26–30]. In two mouse models of OI, TGFβ inhibition significantly increased bone mass [31]. However, a more recent study in a severe mouse model of dominant OI did not show any improvement in bone properties from TGFβ inhibition [32].
Although current studies involving humans and pre-clinical mouse models have provided encouraging results, no single anti-resorptive or pro-anabolic therapy appears to completely solve the problem of skeletal fragility in OI. Consequently, better outcomes may be obtained by combining therapies. Here we report the effect of combining enhanced LRP5 signaling and TGFβ inhibition in the Col1a2G610C mouse model of OI.
2. Materials & methods
2.1. Mouse strains and genotyping
Col1a2G610C/+ (G610C OI) mice [33] were maintained on a fixed C57BL/6 background (Jackson Labs, Bar Harbor, ME, USA). Lrp5A214V/+ (A214V HBM) mice [23] were maintained on a fixed 129/SvJ background. Tail-snip DNA was recovered for PCR genotyping using the HotSHOT method [34]. Genotyping was performed as described previously [23,33].
2.2. Mouse care and handling
Male G610C OI mice were mated with female A214V HBM mice to generate offspring with the following four genotypes: wild-type (WT) (Col1a2+/+;Lrp5+/+), OI (Col1a2G610C/+;Lrp5+/+), OI and HBM (Col1a2G610C/+;Lrp5A214V/+), and HBM (Col1a2+/+;Lrp5A214V/+). Mice were tail-clipped for DNA and ear tagged for identification before 10-days-old, weaned by 28-days-old, and then group-housed as same-sex littermates. Only F1 offspring, male and female, were used in these studies. WT mice were not included since 2 laboratories have already independently described the effect of anti-TGFβ neutralizing antibody in WT mice [29,31].
2.3. Antibody treatment
Mice were randomly assigned by sex and genotype to receive thrice-weekly intraperitoneal (IP) injections of either a pan-TGFβ neutralizing antibody (1D11 at 10 mg/kg) or an isotype matched control antibody that does not recognize TGFβ (13C4 at 10 mg/kg). This dose has previously been shown to be effective at increasing bone properties in mice with OI [31]. Sanofi Genzyme provided both antibodies. Concentrated antibody stocks were stored at −20 °C. Working dilutions (1 mg/ml) were made as needed using sterile phosphate-buffered saline (PBS) (Gibco-Life Technologies, Grand Island, NY, USA) and stored at 4 °C for up to a month.
IP injections began when mice were 6-weeks-old and continued until the mice reached 12-weeks-old. Animals were weighed every other week and the antibody dose was adjusted accordingly. In total, each mouse received 19 injections of antibody 1D11 or 13C4. A minimum of 8 male and 8 female mice with each genotype were studied. The final number of mice available for endpoint analysis is variable because some bones were very fragile and broke during removal, preparation or transport.
2.4. Bone labeling
When 6-weeks-old, mice received a single IP injection of demeclocycline HCl Sigma-Aldrich, St. Louis, MO, USA; 75 mg/kg), or alizarin complexone (Sigma-Aldrich; 20 mg/kg). At 11-weeks-old, mice received a single IP injection of calcein green (Sigma-Aldrich; 10 mg/kg), followed 4 days later by a single IP injection of alizarin complexone (Sigma-Aldrich; 20 mg/kg).
2.5. Dual energy X-ray absorptiometry (DXA) analysis and tissue harvesting
Prior to euthanizing animals with CO2, DXA measures of whole-body (minus the cranium) bone mineral density (BMD) and bone mineral content (BMC) were obtained under isoflurane anesthesia using a Piximus II (GE Lunar, Madison, WI, USA). After sacrifice, both femurs and the L5 vertebra were removed. The left femur and L5 vertebrae were fixed in 10% formalin. The right femur was wrapped in sterile PBS-soaked gauze (Gibco-Life Technologies, Grand Island, NY, USA), and stored frozen at −20 °C.
2.6. Microcomputed tomography (μCT), quantitative histomorphometry, and 3-point-bending tests
Left femur midshaft cortical bone, distal femur trabecular bone, and L5 vertebral trabecular bone were measured by μCT as previously described [23,24]. Femurs from 3 to 4 mice per sex, genotype and treatment arm were subsequently embedded in methyl methacrylate, sectioned and imaged for quantitative histomorphometry as previously described (female only mice were used for the trabecular bone measures) [23,35]. Right femurs were subjected to 3-point bending tests as previously described [23,24].
2.7. Statistical analysis
We confirmed that each bone measure followed a distribution appropriate for parametric analysis. For each measure separately, we fitted a general linear model comprising 3 interacting binary factors (genotype, antibody, sex) and a single continuous covariate (weight change). The 3-factor interaction (genotype × antibody × sex) proved non-significant in every case, indicating that genotype and antibody acted consistently between males and females. Consequently, to estimate the mean measures for each combination of genotype and antibody, and to test the effect of TGFβ neutralizing antibody within each genotype, we constructed sex-adjusted means and contrasts from parameters of the fitted model (i.e., combining within-sex estimates to produce a common estimate). An additional contrast was constructed to assess the difference between OI and OI + HBM mice in the absence of TGFβ inhibition. The presence of weight change as a covariate further adjusted the estimated means to a common level of weight change. SAS software (version 9.4, Cary, NC) was used for statistical computations.
3. Results
3.1. TGFβ neutralizing antibody improves trabecular bone density
Consistent with our previous observations [24], mice with OI and HBM alleles had higher bone mass and bone strength compared with mice with OI (Table 1 and Figs. 1 and 2). For instance, DXA-measured bone density, μCT-measured midshaft cortical volume, μCT-measured distal femur and L5 trabecular BV/TV, and 3-point-bending tests for ultimate force were all significantly increased in OI and HBM mice compared to OI mice (Figs. 1 and 2) (Table 1). Also, animals that received TGFβ neutralizing antibody had significantly increased trabecular BV/TV compared to those that received control antibody (Fig. 1), an observation that is consistent with previous reports for WT [29] and G610C OI mice [31]. Although we did not include a WT control group in this study, as expected OI mice with either an Lrp5 HBM mutation or those that received TGFβ neutralizing antibody had trabecular BV/TV near or above reported WT values [24,31]. Mice with an OI allele and an Lrp5 HBM who also received TGFβ neutralizing antibody had trabecular BV/TV significantly above reported WT mouse values [36]. However, in contrast to the improved bone mechanical properties that were observed in WT mice treated with TGFβ neutralizing antibody [29], 3-point bending strength did not improve in OI mice. (Table 1 and Fig. 2).
Table 1.
Mean (±1 SE) values for trabecular thickness, trabecular number, trabecular space, stiffness and energy to ultimate force.
| Lrp5+/+;Colla2G610C/+ | Lrp5A214V/+;Colla2G610C/+ | Lrp5A214V/+;Colla2+/+ | ||||
|---|---|---|---|---|---|---|
| 13C4 Control Antibody | 1D11 TGFβ Antibody | 13C4 Control Antibody | 1D11 TGFβ Antibody | 13C4 Control Antibody | 1D11 TGFβ Antibody | |
| N = | 14 | 17 | 17 | 17 | 19 | 19 |
| Cortical Bone Volume (mm3) | 2.09 (0.07) | 2.18 (0.07) | 2.70† (0.07) | 2.86 (0.07) | 3.60 (0.06) | 3.46 (0.06) |
| (Femoral) Trabecular Thickness (mm) | 0.04 (0.001) | 0.05* (0.001) | 0.07† (0.002) | 0.09* (0.002) | 0.08 (0.002) | 0.10* (0.002) |
| (Femoral) Trabecular Number (1/mm) | 2.61 (0.10) | 4.32* (0.09) | 3.78† (0.09) | 5.54* (0.09) | 4.66 (0.09) | 5.85* (0.09) |
| (Femoral) Trabecular Separation (mm) | 0.39 (0.008) | 0.23* (0.007) | 0.25† (0.007) | 0.15* (0.007) | 0.19 (0.007) | 0.13* (0.007) |
| MS Mean Polar Moment of Inertia (mm4) | 0.33 (0.026) | 0.34 (0.024) | 0.44† (0.024) | 0.43 (0.024) | 0.65 (0.023) | 0.60 (0.022) |
| N = | 15 | 17 | 17 | 17 | 16 | 19 |
| (Vertebral) Trabecular Thickness (mm) | 0.04 (0.002) | 0.05* (0.002) | 0.07† (0.002) | 0.08* (0.002) | 0.08 (0.002) | 0.10* (0.002) |
| (Vertebral) Trabecular Number (1/mm) | 3.30 (0.20) | 4.79* (0.19) | 4.24† (0.19) | 5.65* (0.19) | 5.78 (0.20) | 6.20* (0.18) |
| (Vertebral) Trabecular Separation (mm) | 0.31 (0.01) | 0.20* (0.01) | 0.24† (0.01) | 0.17* (0.01) | 0.18 (0.01) | 0.14* (0.01) |
| N = | 16 | 17 | 17 | 17 | 19 | 19 |
| Stiffness (N/mm) | 77.01 (5.01) | 86.44 (4.90) | 111.75† (4.88) | 119.30 (4.95) | 147.83 (4.69) | 143.47(4.61) |
| Energy to Ultimate Force (J) | 3.10 (0.35) | 2.36 (0.35) | 3.15 (0.34) | 3.13 (0.35) | 8.30 (0.33) | 7.83 (0.33) |
p < 0.05 when compared to mice of the same genotype receiving control antibody. Note an Lrp5 HBM allele significantly increases (†p < 0.05) every measure, except for energy to ultimate force, in OI mice.
Fig. 1.
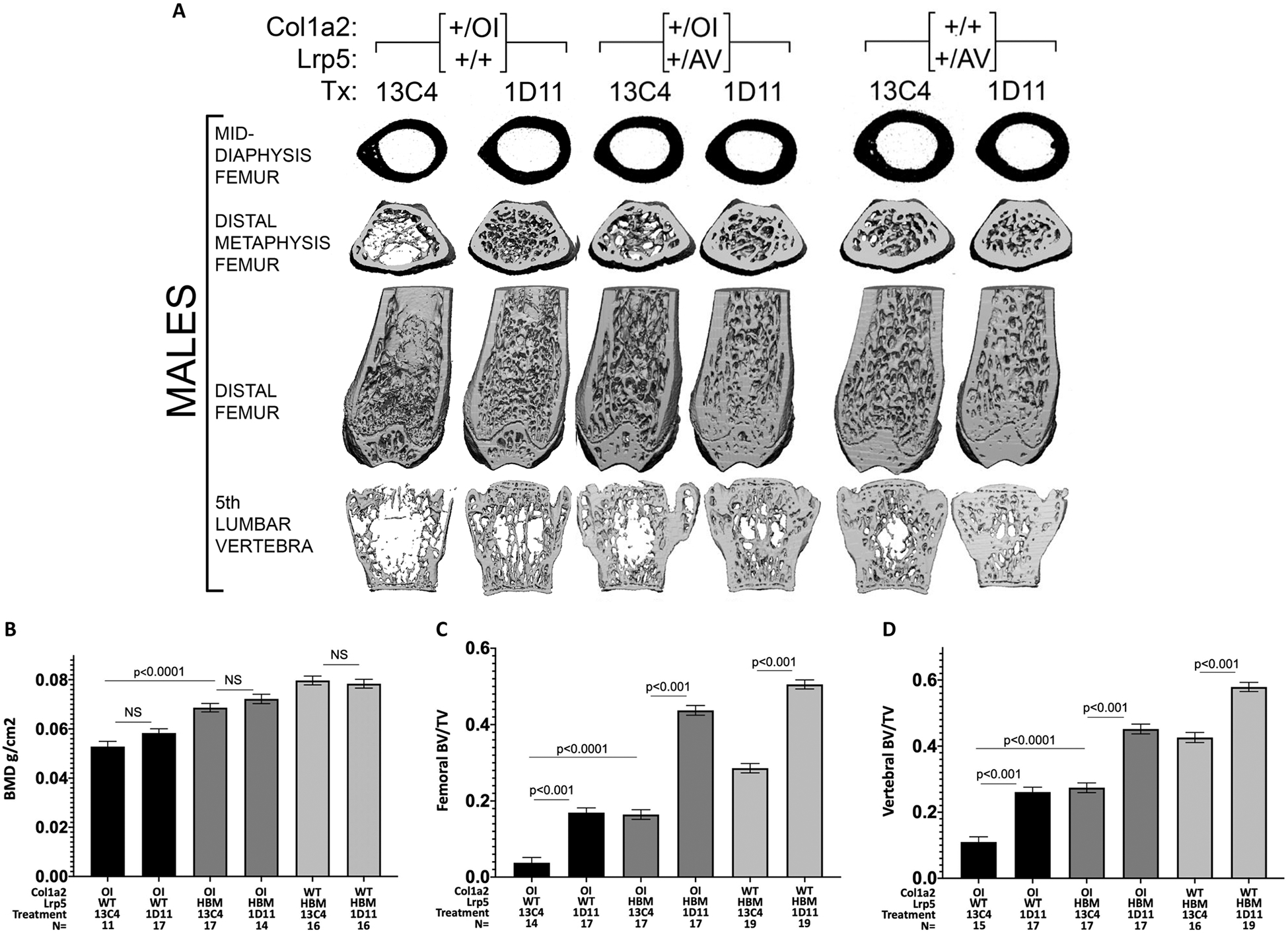
A. Reconstructed μCT skeletal images from male OI and WT mice with and without an Lrp5 HBM allele, and with TGFβ neutralizing antibody or control antibody. Midshaft femur cortical thickness only increases with the Lrp5 HBM allele, whereas femoral and vertebral bone mass increase with either the Lrp5 HBM allele or TGFβ neutralizing antibody. B–D. Bar graphs depicting mean (± 1 SE) total body BMD by DXA and μCT measured femoral and vertebral BV/TV in combined male and female OI and WT mice with and without an Lrp5 HBM allele, and TGFβ neutralizing antibody or control antibody. Note that combining the Lrp5 HBM allele with TGFβ neutralizing antibody further increases femoral and vertebral trabecular BV/TV.
Fig. 2.
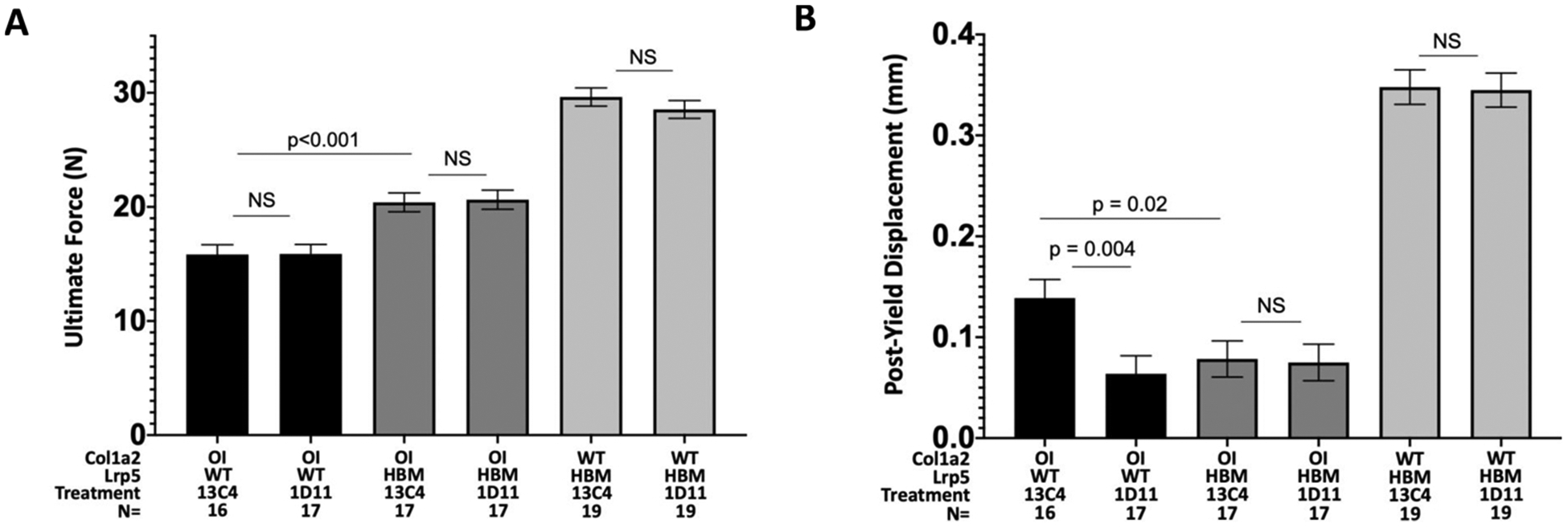
A. Bar graph depicting mean (± 1 SE) ultimate force measurements from 3-point bending tests. Note the Lrp5 HBM allele increased ultimate force and TGFβ neutralizing antibody did not. B. Bar graph depicting mean (± 1SE) post-yield displacement from 3-point bending tests. Neither the Lrp5 HBM allele nor TGFβ neutralizing antibody improved this measure of bone brittleness.
3.2. Combination therapy has an additive effect on trabecular bone density, but not on bone strength
OI and HBM mice that were given TGFβ neutralizing antibody had significantly higher trabecular BV/TV, trabecular thickness, and trabecular number than OI and HBM mice that were given control antibody (Table 1). Whereas an additive effect of combining the HBM allele and TGFβ neutralizing antibody was observed for trabecular bone, no additive effect was observed for femur cortical bone volume or for femur 3-point-bending tests (Fig. 2 and Table 1). In other words, the increase in cortical bone volume and femoral strength conferred by the HBM allele was not enhanced further by anti-TGFβ neutralizing antibody treatment (Table 1).
3.3. Combination therapy did not affect bone brittleness but caused a decrease in some bone formation rates
Although TGFβ neutralizing antibody treatment improves bone quality in WT mice [29], anti-TGFβ therapy alone or in combination with the HBM allele did not improve post-yield displacement during 3-point-bending (an indicator of bone brittleness) in OI mice (Fig. 2).
No significant difference was seen in most measures of periosteal or endosteal mineralizing surface/bone surface (MS/BS), mineral apposition rate (MAR) or bone formation rate (BFR) based on either genotype or treatment (Table 2). However, there was a significant increase in periosteal MS/BS in OI and HBM mice treated with TGFβ neutralizing antibody compared to mice receiving control antibody (Table 2). Interestingly, when we separated out male and female mice, this was due entirely to a large change in the male mice, with no difference in the female mice. We saw similar effects in male OI mice for periosteal MAR and BFR. In our previous study of the effects of the Lrp5 HBM mutation alone on G610C OI mice, we saw changes in some histomorphometric measures at 12 weeks of age [24]. We observed that the G610C OI mice retain fluorescein labeling on their endosteal cortical surface for at least 6 weeks, independent of whether they received TGFβ neutralizing antibody or the HBM allele.
Table 2.
Mean (± 1 SE) values for periosteal and endosteal MS/BS, MAR and BFR rates.
| Lrp5+/+;Colla2G610C/+ | Lrp5A214V/+;Col1a2G610C/+ | |||
|---|---|---|---|---|
| 13C4 Control Antibody | 1D11 TGFβ Antibody | 13C4 Control Antibody | 1D11 TGFβ Antibody | |
| N = | 8 | 7 | 8 | 8 |
| Periosteal MS/BS (%) | 51.28 (4.50) | 43.22 (4.94) | 40.26 (4.50) | 55.53 (4.54)* |
| Perisoteal MAR (μm/day) | 0.85 (0.11) | 0.75 (0.12) | 0.79 (0.11) | 0.88 (0.11) |
| Periosteal BFR (μm3/μm2/year) | 0.48 (0.07) | 0.33 (0.07) | 0.32 (0.07) | 0.49 (0.07) |
| Endosteal MS/BS (%) | 89.36 (3.46) | 96.85 (3.80) | 99.65 (3.46) | 103.05 (3.49) |
| Endosteal MAR (μm/day) | 1.29 (0.09) | 1.04 (0.10) | 0.96 (0.09) | 1.07 (0.09) |
| Endosteal BFR (μm3/μm2/year) | 1.15 (0.09) | 1.01 (0.10) | 0.98 (0.09) | 1.11 (0.09) |
p < 0.05 when compared to mice of the same genotype receiving control antibody. While most measures were not significant, there was one measure that showed a significant increase in bone formation in OI + HBM mice treated with TGFB neutralizing antibody at the periosteal surface.
We also saw a significant decrease in most measures of bone formation and mineralization at the trabecular bone when comparing OI mice with an Lrp5 HBM mutation treated with TGFβ neutralizing antibody (Fig. 3). This suggests that in order to produce the large increases in bone mass seen on μCT the antibody must be reducing bone turnover (anti-catabolic effect) by acting through osteoclasts. Unfortunately, we were unable to detect any osteoclasts on further TRAP staining of our existing bone sections.
Fig. 3.
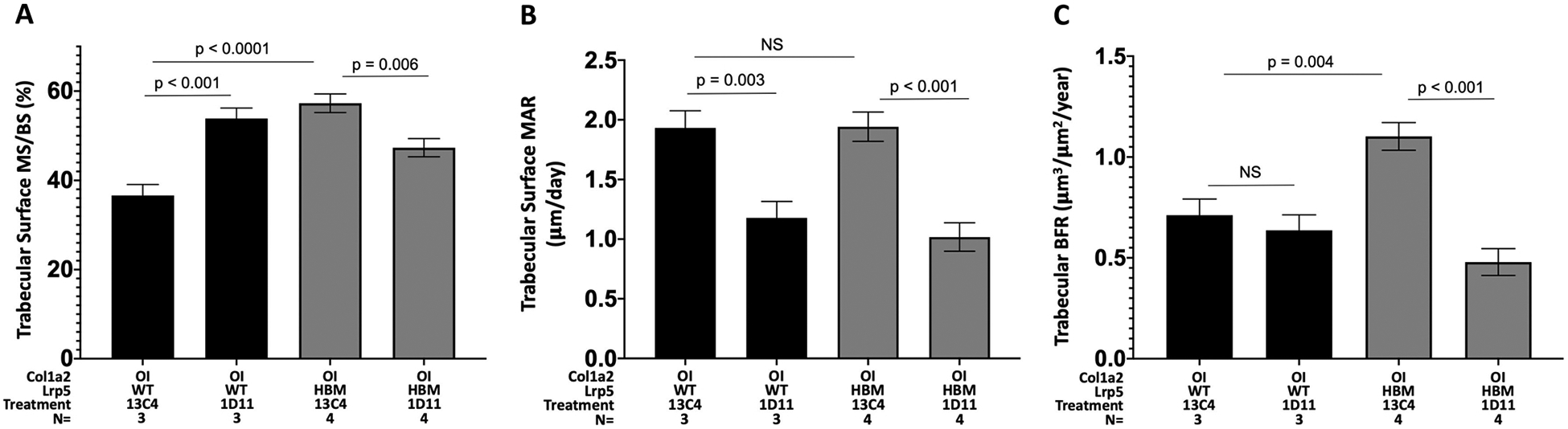
A–C. Bar graphs depicting mean ((± 1 SE) MS/BS, MAR and BFR at trabecular bone surfaces in female mice. Note the significant decrease (or trend towards a decrease) in bone formation and mineralization between mice treated with TGFβ neutralizing antibody compared to those with an OI allele with or without an Lrp5 HBM allele except at one measure in OI mice.
4. Discussion
4.1. Combination therapies have been effective in osteoporosis and OI
The effect of combination therapies on bone properties has been studied in patients with osteoporosis and osteopenia [37–46]. Aside from a single study of both growth hormone and bisphosphonates [47], combination therapy studies have been limited in patients with OI, but some have been tested in pre-clinical mouse models of OI [48,49]. In contrast to the study we performed, prior combination therapies in mice attempted to consolidate gains achieved with a pro-anabolic therapy by adding an anti-catabolic therapy [49]. In the present study, we attempted to concurrently modulate 2 different pathways in OI mice, that appear pro-anabolic in wild type mice [29].
4.2. Anabolic Lrp5 therapies and anti-catabolic TGFβ neutralization have variable effects in different mouse models of OI
While our work and others has shown modulation of the LRP5 pathway to be effective at improving bone properties in several mouse models of OI, including recessive models [24,25,50–54], it has not been effective at improving bone strength in at least one severe mouse model of dominant OI [55]. Further, combination therapy with sclerostin antibody and bisphosphonates together did not have an effect on bone properties greater than either alone when used in the G610C OI mouse model [48]. Together these data suggest that either the phenotypic severity of OI, the specific mutation or the timing of therapy may affect the response. More recently, a study was published reporting results of TGFβ neutralization in a severe mouse model of OI [32]. Although there was clear evidence of TGFβ neutralization, there was no effect on bone properties or any evidence of increased bone formation or turnover. The mouse model utilized in the study was the same that did not respond previously to sclerostin inhibition [55]. This again suggests that response to therapy in OI may be dependent on the degree of phenotypic severity or the specific collagen mutation. Further study is required to determine how phenotype-genotype interactions affect response to specific therapies as they enter widespread clinical use.
4.3. Anabolic Lrp5 HBM mutations and anti-catabolic TGFβ neutralization have an additive effect in OI
Consistent with prior work, we found that enhancing LRP5 signaling or inhibiting TGFβ signaling led to increased bone mass in G610C OI mice. With regard to trabecular bone parameters (BV/TV, Th, and Tb no.) the effects of the Lrp5 genotype or TGFβ neutralizing antibody were similar and there appeared to be an additive effect of combining therapies in OI and in WT mice. With regard to cortical bone, the HBM allele significantly increased bone volume and bone strength as assessed by 3-point-bending. In contrast to the HBM effect, antibody mediated TGFβ neutralization did not increase cortical bone volume or strength in G610C OI mice or in OI and HBM mice. Our data suggest that at least in this model of OI the increase in trabecular BV/TV caused by antibody mediated TGFβ neutralization is due to decreased osteoclast resorption, rather than increased formation. An anti-catabolic, rather than pro-anabolic effect of TGFβ neutralizing antibody therapy in OI is consistent with what other investigators have reported previously [31]. However, genetic, pharmacologic and antibody-mediated neutralization of TGFβ signaling were reported to improve cortical bone parameters in WT mice; additionally, antibody-mediated neutralization of TGFβ improved cortical bone parameters in a different mouse OI model (Crtap−/−) [54]. Cortical bone response, like overall response to TGFβ neutralization in OI, may be dependent on the degree of clinical severity or the specific mutation; alternatively, the length and/or timing of treatment may affect treatment outcomes. Therefore, we cannot preclude the possibility that cortical bone in G610C and other OI mice would improve if we treated younger animals or if we treated for longer periods of time.
4.4. Further study is needed to determine if combination therapy will have effects on trabecular bone strength
Although we did not observe additive effects of combination therapy on cortical bone volume or strength, the additive effects we observed on trabecular BV/TV has the potential to improve bone strength in regions where a considerable portion of the load is borne by cancellous bone, such as the vertebral bodies. Although we failed to collect vertebral specimens for compressive loading studies in this cohort, TGFβ neutralizing antibody has been shown to increase vertebral strength in WT mice [29]. Further study is required to determine whether combination therapy can increase vertebral strength in mouse models of OI, even when other skeletal properties (e.g., brittleness) are unaffected by combination therapy.
Funding sources
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, grants AR067388 (MLW) and AR063813 (CMJ).
References
- [1].Forlino A, Marini JC, Osteogenesis imperfecta, Lancet 387 (2016) 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Garnero P, The role of collagen organization on the properties of bone, Calcif. Tissue Int 97 (2015) 229–240. [DOI] [PubMed] [Google Scholar]
- [3].Dahan-Oliel N, Oliel S, Tsimicalis A, Montpetit K, Rauch F, Dogba MJ, Quality of life in osteogenesis imperfecta: a mixed-methods systematic review, Am. J. Med. Genet. A 170A (2016) 62–76. [DOI] [PubMed] [Google Scholar]
- [4].Tsimicalis A, Denis-Larocque G, Michalovic A, Lepage C, Williams K, Yao TR, Palomo T, Dahan-Oliel N, Le May S, Rauch F, The psychosocial experience of individuals living with osteogenesis imperfecta: a mixed-methods systematic review, Qual. Life Res 25 (2016) 1877–1896. [DOI] [PubMed] [Google Scholar]
- [5].Trejo P, Rauch F, Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment, Osteoporos. Int 27 (12) (2016) 3427–3437. [DOI] [PubMed] [Google Scholar]
- [6].Dwan K, Phillipi CA, Steiner RD, Basel D, Bisphosphonate therapy for osteogenesis imperfecta, Cochrane Database Syst. Rev 10 (2016) CD005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Uehara M, Nakamura Y, Takahashi J, Kamimura M, Ikegami S, Suzuki T, Uchiyama S, Yamaguchi T, Kosho T, Kato H, Efficacy of denosumab for osteoporosis in three female patients with osteogenesis imperfecta, Tohoku J. Exp. Med 242 (2) (2017) 115–120. [DOI] [PubMed] [Google Scholar]
- [8].Hoyer-Kuhn H, Netzer C, Koerber F, Schoenau E, Semler O, Two years’ experience with denosumab for children with osteogenesis imperfecta type VI, Orphanet J. Rare Dis 9 (2014) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, Hero B, Schoenau E, Semler O, Safety and efficacy of denosumab in children with osteogenesis imperfect—a first prospective trial, J. Musculoskelet. Neuronal Interact 16 (1) (2016) 24–32. [PMC free article] [PubMed] [Google Scholar]
- [10].Gatti D, Rossini M, Viapiana O, Povino MR, Liuzza S, Fracassi E, Idolazzi L, Adami S, Teriparatide treatment in adult patients with osteogenesis imperfecta type I, Calcif. Tissue Int 93 (5) (2013) 448–452. [DOI] [PubMed] [Google Scholar]
- [11].Orwoll ES, Shapiro J, Veith S, Wang Y, Lapidus J, Vanek C, Reeder JL, Keaveny TM, Lee DC, Mullins MA, Nagamani SC, Lee B, Evaluation of teriparatide treatment in adults with osteogenesis imperfecta, J. Clin. Invest 124 (2) (2014) 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Trejo P, Rauch F, Ward L, Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI, J. Musculoskelet. Neuronal Interact 18 (1) (2018) 76–80. [PMC free article] [PubMed] [Google Scholar]
- [13].Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D, Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling, J. Biol. Chem 280 (20) (2005) 19883–19887. [DOI] [PubMed] [Google Scholar]
- [14].Balemans W, Piters E, Cleiren E, Ai M, Van Wesenbeeck L, Warman ML, Van Hul W, The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations, Calcif. Tissue Int 82 (6) (2008) 445–453. [DOI] [PubMed] [Google Scholar]
- [15].McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG, Romosozumab in postmenopausal women with low bone mineral density, N. Engl. J. Med 370 (5) (2014) 412–420. [DOI] [PubMed] [Google Scholar]
- [16].Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A, Romosozumab treatment in postmenopausal women with osteoporosis, N. Engl. J. Med 375 (16) (2016) 1532–1543. [DOI] [PubMed] [Google Scholar]
- [17].Genant HK, Engelke K, Bolognese MA, Mautalen C, Brown JP, Recknor C, Goemaere S, Fuerst T, Yang YC, Grauer A, Libanati C, Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass, J. Bone Miner. Res 32 (1) (2017) 181–187. [DOI] [PubMed] [Google Scholar]
- [18].Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A, Romosozumab or alendronate for fracture prevention in women with osteoporosis, N. Engl. J. Med 377 (2017) 1417–1427. [DOI] [PubMed] [Google Scholar]
- [19].Genant HK, Engelke K, Bolognese MA, Mautalen C, Brown JP, Recknor C, Goemaere S, Fuerst T, Yang YC, Grauer A, Libanati C, Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass, J. Bone Miner. Res 32 (2017) 181–187. [DOI] [PubMed] [Google Scholar]
- [20].Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP, High bone density due to a mutation in LDL-receptor-related protein 5, N. Engl. J. Med 346 (2002) 1513–1521. [DOI] [PubMed] [Google Scholar]
- [21].Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML, A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait, Am. J. Hum. Genet 70 (2002) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R, Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity, J. Bone Miner. Res 21 (2006) 1738–1749. [DOI] [PubMed] [Google Scholar]
- [23].Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG, Lrp5 functions in bone to regulate bone mass, Nat. Med 17 (2011) 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, Weis M, Eyre D, Zurakowski D, Robling AG, Warman ML, Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta, J. Bone Miner. Res 29 (2014) 2297–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jacobsen CM, Schwartz MA, Roberts HJ, Lim KE, Spevak L, Boskey AL, Zurakowski D, Robling AG, Warman ML, Enhanced Wnt signaling improves bone mass and strength, but not brittleness, in the Col1a1(+/mov13) mouse model of type I Osteogenesis Imperfecta, Bone 90 (2016) 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dick IM, Devine A, Li S, Dhaliwal SS, Prince RL, The T869C TGF beta polymorphism is associated with fracture, bone mineral density, and calcaneal quantitative ultrasound in elderly women, Bone 33 (2003) 335–341. [DOI] [PubMed] [Google Scholar]
- [27].Hinke V, Seck T, Clanget C, Scheidt-Nave C, Ziegler R, Pfeilschifter J, Association of transforming growth factor-beta1 (TGFbeta1) T29 –> C gene polymorphism with bone mineral density (BMD), changes in BMD, and serum concentrations of TGF-beta1 in a population-based sample of postmenopausal German women, Calcif. Tissue Int 69 (2001) 315–320. [DOI] [PubMed] [Google Scholar]
- [28].Filvaroff E, Erlebacher A, Ye J, Gitelman SE, Lotz J, Heillman M, Derynck R, Inhibition of TGF-beta receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass, Development 126 (1999) 4267–4279. [DOI] [PubMed] [Google Scholar]
- [29].Edwards JR, Nyman JS, Lwin ST, Moore MM, Esparza J, O’Quinn EC, Hart AJ, Biswas S, Patil CA, Lonning S, Mahadevan-Jansen A, Mundy GR, Inhibition of TGF-beta signaling by 1D11 antibody treatment increases bone mass and quality in vivo, J. Bone Miner. Res 25 (2010) 2419–2426. [DOI] [PubMed] [Google Scholar]
- [30].Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S, Evans DS, Lang TF, Zhang B, Ritchie RO, Mohammad KS, Alliston T, Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling, Cell Rep. 21 (2017) 2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, Ishikawa Y, Weis MA, Sampath TK, Ambrose C, Eyre D, Bachinger HP, Lee B, Excessive transforming growth factor-beta signaling is a common mechanism in osteogenesis imperfecta, Nat. Med 20 (2014) 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tauer JT, Abdullah S, Rauch F, Effect of Anti-TGF-beta treatment in a mouse model of severe osteogenesis imperfecta, J. Bone Miner. Res 34 (2019) 207–214. [DOI] [PubMed] [Google Scholar]
- [33].Daley E, Streeten EA, Sorkin JD, Kuznetsova N, Shapses SA, Carleton SM, Shuldiner AR, Marini JC, Phillips CL, Goldstein SA, Leikin S, McBride DJ Jr, Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model, J. Bone Miner. Res 25 (2010) 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML, Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT), Biotechniques 29 (52) (2000) 54. [DOI] [PubMed] [Google Scholar]
- [35].Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH, The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment, J. Biol. Chem 281 (2006) 23698–23711. [DOI] [PubMed] [Google Scholar]
- [36].Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, Chen Y, Jiang MM, Bertin T, Dawson B, Asuncion F, Ke HZ, Ominsky MS, Lee B, Sclerostin antibody treatment improves the bone phenotype of Crtap mice, a model of recessive osteogenesis imperfecta, J. Bone Miner. Res 31 (5) (2016) 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li X, Ominsky MS, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Dwyer D, Grisanti M, Stolina M, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ, Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats, Endocrinology 152 (2011) 3312–3322. [DOI] [PubMed] [Google Scholar]
- [38].Nyman JS, Merkel AR, Uppuganti S, Nayak B, Rowland B, Makowski AJ, Oyajobi BO, Sterling JA, Combined treatment with a transforming growth factor beta inhibitor (1D11) and bortezomib improves bone architecture in a mouse model of myeloma-induced bone disease, Bone 91 (2016) 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aloia JF, Zanzi I, Vaswani A, Ellis K, Cohn SH, Combination therapy for osteoporosis with estrogen, fluoride, and calcium, J. Am. Geriatr. Soc 30 (1982) 13–17. [DOI] [PubMed] [Google Scholar]
- [40].Lou S, Lv H, Yin P, Li Z, Tang P, Wang Y, Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: a systematic review and meta-analysis of randomized controlled trials, Osteoporos. Int 30 (2019) 59–70. [DOI] [PubMed] [Google Scholar]
- [41].Lou S, Lv H, Li Z, Zhang L, Tang P, Combination therapy of anabolic agents and bisphosphonates on bone mineral density in patients with osteoporosis: a meta-analysis of randomised controlled trials, BMJ Open 8 (2018) e015187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lou S, Wang L, Wang Y, Jiang Y, Liu J, Wang Y, Combination therapy of anabolic and nonbisphosphonates antiresorptive agents for the treatment of osteoporosis: a meta-analysis, Medicine (Baltimore) 96 (2017) e9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shao Y, Hernandez-Buquer S, Childress P, Stayrook KR, Alvarez MB, Davis H, Plotkin LI, He Y, Condon KW, Burr DB, Warden SJ, Robling AG, Yang FC, Wek RC, Allen MR, Bidwell JP, Improving combination osteoporosis therapy in a preclinical model of heightened osteoanabolism, Endocrinology 158 (2017) 2722–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Walker MD, Cusano NE, Sliney J Jr, Romano M, Zhang C, McMahon DJ, Bilezikian JP, Combination therapy with risedronate and teriparatide in male osteoporosis, Endocrine 44 (2013) 237–246. [DOI] [PubMed] [Google Scholar]
- [45].Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, Glass EV, Myers SL, Krege JH, Combination teriparatide and raloxifene therapy for post-menopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial, J. Bone Miner. Res 20 (2005) 1905–1911. [DOI] [PubMed] [Google Scholar]
- [46].Evio S, Tiitinen A, Laitinen K, Ylikorkala O, Valimaki MJ, Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis, J. Clin. Endocrinol. Metab 89 (2004) 626–631. [DOI] [PubMed] [Google Scholar]
- [47].Antoniazzi F, Monti E, Venturi G, Franceschi R, Doro F, Gatti D, Zamboni G, Tato L, GH in combination with bisphosphonate treatment in osteogenesis imperfecta, Eur. J. Endocrinol 163 (2010) 479–487. [DOI] [PubMed] [Google Scholar]
- [48].Little DG, Peacock L, Mikulec K, Kneissel M, Kramer I, Cheng TL, Schindeler A, Munns C, Combination sclerostin antibody and zoledronic acid treatment outperforms either treatment alone in a mouse model of osteogenesis imperfecta, Bone 101 (2017) 96–103. [DOI] [PubMed] [Google Scholar]
- [49].Olvera D, Stolzenfeld R, Marini JC, Caird MS, Kozloff KM, Low dose of bisphosphonate enhances sclerostin antibody-induced trabecular bone mass gains in Brtl/+ osteogenesis imperfecta mouse model, J. Bone Miner. Res 33 (2018) 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM, Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta, J. Bone Miner. Res 28 (2013) 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sinder BP, White LE, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM, Adult Brtl/+ mouse model of osteogenesis imperfecta demonstrates anabolic response to sclerostin antibody treatment with increased bone mass and strength, Osteoporos. Int 25 (2014) 2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM, Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment, Bone 71 (2015) 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sinder BP, Lloyd WR, Salemi JD, Marini JC, Caird MS, Morris MD, Kozloff KM, Effect of anti-sclerostin therapy and osteogenesis imperfecta on tissue-level properties in growing and adult mice while controlling for tissue age, Bone 84 (2016) 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, Chen Y, Jiang MM, Bertin T, Dawson B, Asuncion F, Ke HZ, Ominsky MS, Lee B, Sclerostin antibody treatment improves the bone phenotype of Crtap(−/−) mice, a model of recessive osteogenesis imperfecta, J. Bone Miner. Res 31 (2016) 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, Rauch F, Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta, Bone 66 (2014) 182–188. [DOI] [PubMed] [Google Scholar]


