Abstract
Purpose
This study investigated the effects of an intensive cognitive-communication rehabilitation (ICCR) program for young individuals with chronic acquired brain injury.
Method
ICCR included classroom lectures; metacognitive instruction, modeling, and application; technology skills training; and individual cognitive–linguistic therapy. Four individuals participated in the intensive program (6 hr with 1-hr lunch break × 4 days × 12 weeks of treatment): 3 participants completed 3 consecutive semesters, and 1 participant completed 1 semester. Two controls did not receive treatment and completed assessments before and after the 12-week treatment interval only.
Results
All 4 experimental participants demonstrated significant improvements on at least 1 standardized cognitive–linguistic measure, whereas controls did not. Furthermore, time point significantly predicted participants' scores on 2 of the 4 standardized outcome measures, indicating that as duration in ICCR increased, scores also increased. Participants who completed multiple semesters of ICCR also improved in their therapy and personal goals, classroom behavior, life participation, and quality of life.
Conclusion
After ICCR, participants showed gains in their cognitive–linguistic functioning, classroom participation, and individual therapy. They also demonstrated improvements outside the classroom and in their overall well-being. There is a gap between the large population of young adults with acquired brain injury who wish to return to higher education and a lack of rehabilitation programs supporting reentry into academic environments; ICCR is a first step in reducing that gap.
Individuals with acquired brain injury (ABI) as a result of traumatic brain injury (TBI) or stroke (cerebrovascular accident [CVA]) typically experience cognitive and/or language deficits that persist for years postonset (Chapey, 2008; Cicerone et al., 2011; Kennedy, Coelho, et al., 2008; Sohlberg & Mateer, 1989). 1 Young adults are a frequently affected and growing population (i.e., ages 18–36) within ABI (“TBI: Get the Facts,” 2017; “Young Stroke Survivors,” 2016).
Unfortunately, when a young adult has a brain injury during high school or college, essential aspects of the college experience (e.g., following a schedule, taking notes, studying, writing papers, giving presentations) become challenging due to deficits in executive function, attention, memory, and language skills. Post–secondary education may be challenging for individuals with ABI but has the potential to be quite valuable for them as it would tax their problem-solving skills, provide opportunities to interact with peers (Cicerone, 2004; Lyon, 1992), and build independence (Kennedy, O'Brien, & Krause, 2012). Regrettably, individuals with ABI are typically offered transition services that prepare them for employment or independent living rather than a college/university setting (Todis & Glang, 2008). When individuals with ABI do pursue post–secondary education, they often do not seek out available support services (e.g., notetakers, counselors; Kennedy, Krause, & Turkstra, 2008). Not surprisingly then, degree completion is rare for this population and requires extensive support and extraordinary personal motivation (Todis & Glang, 2008).
Currently, cognitive rehabilitation (CR; Cicerone et al., 2000) is the gold standard treatment for individuals with ABI. Most CR can be organized into two broad categories: impairment-based therapy (e.g., attention process training; Sohlberg, McLaughlin, Pavese, Heidrich, & Posner, 2000) and functional therapy (e.g., training on the use of external memory aids, Promoting Aphasics' Communicative Effectiveness; Cicerone et al., 2011; Davis, 2005). Ideally, a comprehensive CR program would employ a holistic approach: targeting the body structure/function or impairment level, and the activity/participation or functional level (World Health Organization, 2002). Furthermore, it would consider psychosocial, environmental, and personal factors (Bayley et al., 2014; Cicerone et al., 2011; Corrigan & Hammond, 2013; Kennedy & Coelho, 2005; Kennedy, Coelho, et al., 2008; Scottish Intercollegiate Guidelines Network, 2013). One could further argue that CR service delivery models for young individuals with ABI should include academic instruction, vocational counseling/rehabilitation, opportunities for community reentry with typical peers, and/or programming for age-appropriate social and leisure activities. Yet, few programs currently exist for young individuals with ABI who wish to enroll in college that include all of these components.
There is a wealth of literature demonstrating that individuals with ABI respond variably to rehabilitation (e.g., Brady, Kelly, Godwin, Enderby, & Campbell, 2016; Cicerone et al., 2011). One individual may respond well to a particular treatment, but then, that same treatment approach may be less effective for another individual, despite similar clinical profiles (Coelho, DeRuyter, & Stein, 1996; Holland, Fromm, DeRuyter, & Stein, 1996). Many behavioral, neurological, and psychosocial factors influence treatment recovery in ABI (Bonilha, Gleichgerrcht, Nesland, Rorden, & Fridriksson, 2016; Leininger, Strong, & Donders, 2014). Thus, one underlying driver of rehabilitation is how the brain reorganizes as a response to specific training (i.e., experience-dependent neural plasticity; Kerr, Cheng, & Jones, 2011; Kleim, 2011; Kleim & Jones, 2008; Power & Schlaggar, 2017; Warraich & Kleim, 2010). Based on this research, brain reorganization occurs according to behavioral, sensory, and cognitive experiences that encourage specific skill use and repetitive, intensive practice. These tenets have since been implemented in effective rehabilitation techniques for neurogenic populations (e.g., constraint-induced language therapy; Pulvermüller et al., 2001).
The following principles of neural plasticity are particularly relevant to CR for young individuals with ABI interested in pursuing higher education: age (i.e., younger brains may change more and faster than aging brains, although both are responsive to experience), intensity (i.e., increased length and frequency of treatment), salience (i.e., stimuli must be sufficiently interesting and engaging), and repetition (i.e., skill is elicited a sufficient number of times for learning). However, to our knowledge, no CR programs to date incorporate all of these principles into one design to optimize the potential for neural plasticity. The following sections detail programs that incorporate some, but not all, of these principles.
Currently, most CR is provided in a hospital or clinic setting (e.g., TBI Model System Centers). Although this intervention may include academic support and training, it may not be formal and is unlikely to involve real-time clinician support in the classroom setting.
In recent years, intensive comprehensive aphasia programs (ICAPs) have also become a popular option for individuals with aphasia as a result of ABI. ICAPs are efficacious treatments that are generally hosted at an aphasia center or in a university clinic (Babbitt, Cherney, & Worrall, 2016; Hoover, Caplan, Waters, & Carney, 2017; Persad, Wozniak, & Kostopoulos, 2013; Rodriguez et al., 2013; Rose, Cherney, & Worrall, 2013; Winans-Mitrik et al., 2014). Although these treatment programs were built using principles of neural plasticity (e.g., intensity, salience) and target both the impairment and activity/participation levels, they do not appear to be the most appropriate CR choice for young individuals with ABI interested in enrolling in higher education for a number of reasons. First, although younger individuals have participated in ICAPs, they were primarily surrounded by much older individuals (M = 53, range 16–86; Persad et al., 2013). Second, most ICAP participants have already graduated from college and/or worked in professional careers (Persad et al., 2013). Third, caregivers are heavily involved in ICAPs, which is at odds with typical goals for college-age individuals (i.e., to increase their independence from their guardians). Most important, although ICAPs provide intensive treatment (≥ 3 hr of daily therapy, 2–4.5 weeks; Rose et al., 2013), cognitive–linguistic skills are not specifically targeted in an academic context.
In addition to the efficacy of ICAPs, recent work in CR for TBI has investigated its effect on military service members. Although differences in TBI etiology and their subsequent sequelae exist between civilians and service members, 2 a brief discussion of the approaches used in a few of these studies is relevant to this study. As veterans often pursue academic goals upon deployment, MacLennan and MacLennan (2008) investigated the readiness of three veterans with TBI to enter the post–secondary setting via a simulated college experience. The intervention involved sixteen 60-min sessions and consisted of 12 lectures, seven of which focused on the effects of brain injury (e.g., pathophysiology of brain injury) and five on study skills (e.g., study skills: reading college textbooks). The benefits of compensatory strategies (i.e., notetakers, extra time for tests, video-recorded lectures, and audio-recorded textbooks) were assessed for each student. They were quizzed using short answer, multiple-choice, and true/false questions to capture both their recall and recognition memory function. Following the intervention, two students decided not to enroll in school due to the severity of their impairments, and the third student was similarly encouraged to pursue vocation rather than school. Although referred to as a simulated college experience, this program was used primarily as an assessment. It did not provide students with academic content, nor did it provide support for those who wanted to return to school but did not possess the necessary skills.
In addition, the Study of Cognitive Rehabilitation Effectiveness (SCORE; Cooper et al., 2017), consisting of four treatment arms, (a) psychoeducation, (b) computer-based CR, (c) therapist-directed CR, and (d) integrated therapist-directed CR, combined with cognitive–behavioral psychotherapy, was recently completed. The fourth arm was the most comprehensive, holistic, and intensive, providing 10 hr total of individual CR, metacognitive group therapy, psychoeducational counseling, and computerized therapy, and thus would be hypothesized to be the most effective. Yet, all four arms resulted in significant cognitive, psychological, and behavioral improvements, suggesting that no particular treatment arm was significantly more effective than any other arm. Furthermore, the SCORE program was tested with individuals with mild TBI only and did not focus on transition to an academic environment.
Some treatments have in fact been developed to support individuals with TBI in the academic environment, but these are offered to students who are already actively enrolled. The College Program for Students with Brain Injury (Kennedy & Krause, 2011) at the University of Minnesota is one such opportunity (eligibility criteria: completed rehabilitation, accepted to a 2- or 4-year college, and need to have returning to college as a “realistic” goal). While providing a valuable service to students with ABI in the academic setting, such programs are not available to individuals who have not yet been accepted to a university due to the severity of their cognitive–linguistic impairments. Furthermore, this program teaches strategies to cope in the classroom, not the academic material. Some community colleges also provide opportunities for individuals with ABI (e.g., Coastline Community College's ABI program), although the efficacy of these programs has not been established through experimental means.
Although some of the aforementioned programs incorporated intensity (e.g., SCORE, ICAPs) and others implemented specificity of training (e.g., MacLennan & MacLennan, 2008), age (e.g., College Program for Students with Brain Injury), and/or repetition (e.g., TBI Model Systems of Care), none of them included all the key principles of neural plasticity in one program (i.e., repetition, salience, specificity of training, intensity, age). Given this literature, it appears rare for individuals with ABI to receive postacute CR that focuses directly on the necessary skills for a successful transition to higher education (Masel & DeWitt, 2010). Therefore, in this study, we developed a comprehensive CR program, entitled Intensive Cognitive-Communication Rehabilitation (ICCR), to address the rehabilitation needs of young individuals with ABI interested in enrolling in higher education with full inclusion. To determine the initial efficacy of this novel treatment approach, we investigated the following research questions:
1. Do participants demonstrate changes in cognitive–linguistic skills as a result of this novel intervention program?
Hypothesis: ICCR incorporated key aspects of evidence-based CR (i.e., targeting impairment, function, and psychosocial aspects of ABI within individual and group settings) and principles of experience-dependent neural plasticity (i.e., age, repetition, salience, and intensity). Therefore, we hypothesized that participants with chronic ABI would improve in their cognitive–linguistic skills as measured by standardized outcome measures after treatment.
2. Do participants improve in their classroom participation over time?
Hypothesis: In this program, participants were provided instructional material at a reduced pace with repetition and instructed to use metacognitive strategies in the academic context. Given this design, we hypothesized that they would answer questions, make comments, and ask questions at an increased frequency and with greater accuracy and appropriateness over the course of treatment.
3. Do participants progress toward therapy and personal goals over the course of treatment?
Hypothesis: Participants received intensive speech-language and cognitive therapy (one to four times per week), targeting both therapy (e.g., improve auditory comprehension of complex questions) and personal goals (e.g., self-transportation) throughout the program. Therefore, we hypothesized that they would show improvements in these areas.
4. Do participants demonstrate changes at the activity and participation levels, as well as changes to their quality of life (QOL), as a result of this program?
Hypothesis: Individuals in ICCR participated in a semester-long academic program within a real university setting. They engaged in a college experience with a cohort of age-matched peers and used academic facilities with other university students. They became part of the college milieu, an opportunity otherwise unavailable to them because of the severity of their brain injuries. We hypothesized that treatment in the group setting, in a college environment, would increase not only their life participation but also their QOL, as assessed via standardized measures and subjective reports.
Method
Participants
Six individuals with ABI (four male individuals, two female individuals) as the result of a TBI (n = 4) or CVA were recruited from the New England region of the United States through referral from physicians, speech-language pathologists, neuropsychologists, and word of mouth. Recruitment materials were e-mailed to professionals (e.g., physicians, speech-language pathologists) working with this population in the greater Boston area and nationally (e.g., hospitals, rehabilitation clinics, community colleges). Fliers were also posted on various academic and clinical listservs. Interested individuals who were not within commuting distance of Boston University temporarily relocated to participate in the program, as dormitory housing was not available.
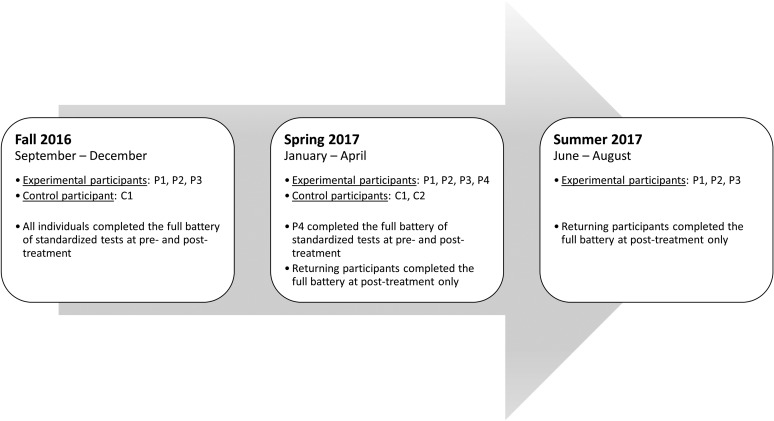
Participants ranged in age from 21 to 35 years (M = 27.49, SD = 5.41), months postonset from 38 to 97 months (M = 68.44, SD = 24.32), and education from 12 to 15 years (M = 13, SD = 1.41). The study utilized a quasiexperimental design, with treatment administered to four experimental participants (P1, P2, P3, and P4) and deferred for two control participants (C1 and C2). It should be noted that P1, P2, and P3 participated in three consecutive semesters, C1 participated in the first two semesters, and P4 and C2 participated in the spring semester only (displayed in Figure 1).
Figure 1.
Schedule of standardized assessment testing for experimental participants and controls.
Participants met several inclusion criteria: (a) between the ages of 18 and 36 years, (b) ABI after the onset of adolescence (age 13 years or older), 3 (c) interest in post–secondary education, (d) cognitive and/or linguistic deficits that precluded enrollment in post–secondary education, and (e) adequate vision and hearing for functional reading and conversation. Participants with cognitive and/or linguistic deficits solely as the result of a congenital or developmental disorder and/or concomitant neurological disease were excluded. Participants consented in writing before any assessments were administered in accordance with the Boston University Institutional Review Board protocol.
The diagnosis of cognitive–linguistic impairment was made using the battery of standardized assessments outlined below. Medical records were also reviewed to determine the nature and etiology of their ABI (see Table 1 for relevant demographic information and treatment assignment). Notably, P4 had severe language deficits and mild-to-moderate cognitive deficits secondary to TBI. As we hypothesized that young individuals with any severity of ABI would improve in their cognitive–linguistic skills as a function of ICCR and he met the selection criteria for the study, he was not excluded based on his initial test scores but rather was enrolled with support in the classroom to augment his auditory comprehension (i.e., supported communication techniques).
Table 1.
Demographic information.
| Characteristic | P1 | P2 | P3 | P4 | C1 | C2 |
|---|---|---|---|---|---|---|
| Etiology | TBI | CVA | TBI | TBI | CVA | TBI |
| Age | 21 | 29 | 25 | 34 | 31 | 23 |
| Sex | M | M | M | M | F | F |
| Education (years) | 12 | 15 | 12 | 16 | 14 | 12 |
| Months postonset | 49 | 70 | 96 | 97 | 59 | 38 |
| Cognitive–linguistic profile | Moderate–severe cognitive deficits, moderate aphasia, mild–moderate AOS | Mild–moderate aphasia, mild cognitive deficits | Severe cognitive deficits, severe spastic dysarthria | Severe Broca's aphasia, mild-to-moderate cognitive deficits, moderate AOS | Mild aphasia, mild AOS | Moderate cognitive deficits, moderate-to-severe hypokinetic dysarthria |
Note. TBI = traumatic brain injury; CVA = cerebrovascular accident; M = male; F = female; AOS = apraxia of speech.
Standardized Assessments
The following assessments were administered: (a) Western Aphasia Battery–Revised (WAB-R; Kertesz, 2006) to assess broad language function; (b) Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, 2012) to measure cognitive function; (c) Scales of Cognitive and Communicative Ability for Neurorehabilitation (SCCAN; Holland & Milman, 2012) to evaluate cognitive and linguistic skills; (d) Discourse Comprehension Test (DCT; Brookshire & Nicholas, 1993) to examine listening and reading comprehension at the narrative level; (e) Child and Adolescent Scale of Participation (CASP; McDougall, Bedell, & Wright, 2013) to assess participation at home, school, and in the community; and (f) subtests of the TBI Quality of Life (TBI-QOL; Tulsky et al., 2016) or Neurologic Quality of Life (Neuro-QOL; Gershon et al., 2012, i.e., communication, anxiety, depression, positive affect and well-being, and cognitive function), depending on etiology, to evaluate health-related QOL. 4 Goal Attainment Scaling (GAS; King, McDougall, Palisano, Gritzan, & Tucker, 2000) was also incorporated to allow participants to develop personal goals for the semester. All participants were assessed with this battery both before and after each semester of the intervention. For all individuals who participated in multiple semesters of ICCR, the postintervention scores from the first semester were used as the preintervention scores for the next semester. Figure 1 depicts the schedule of assessments for each participant.
Treatment
Similar to a typical undergraduate student, ICCR students took four classes per semester, were administered weekly quizzes and final exams, prepared presentations and wrote papers, asked and answered questions requiring both memorization and critical thinking, discussed course content with the lecture facilitator and their peers, accessed course content online (i.e., video lectures to review later and class notes), asked the lecture facilitator to meet with them to review course content outside the class as needed, traveled from one classroom to another, took certain courses on one day and others on a different day, and stayed in an apartment near campus and/or commuted from home. One possible difference from a traditional liberal arts education was that students in ICCR reviewed video-recorded lectures, which were facilitated by a speech-language pathologist. However, online courses have increased in prevalence in the last 20 years (Miller et al., 2013), and therefore, watching lectures on a screen may actually be reflective of some typical students' experiences. ICCR further diverged from a typical college environment in that students were not regularly expected to complete assignments outside the class and were reminded of assignments/course topics by the clinician as opposed to having to follow a syllabus only.
According to the principle of intensity, each treatment phase consisted of a 12-week semester, during which participants received treatment 4 days per week, 6 hr per day with a 1-hr lunch break. The only restriction for control subjects was that they do not engage in other intensive rehabilitation during the deferred treatment phase. In each day of ICCR, students participated in academic classes and metacognitive therapy, individual speech-language-cognitive therapy, and technology skill training. Lecture content was repeated throughout the day, and the schedule of activities did not vary, which allowed for specificity and repetition of training. Each of these elements is described below.
Academic coursework consisted of open source academic material (e.g., “Khan Academy,” 2017; Open Yale Courses, Bloom, 2012) spanning subjects from psychology to personal finance. Each semester was designed to simulate a liberal arts curriculum, thus comprising the functional component of ICCR, wherein students applied discretely trained skills in the relevant, salient classroom environment. Academic content was presented through video-recorded lectures moderated by a trained research assistant or clinical fellow in speech-language pathology (CF-SLP; e.g., stopping the lecture to ask discussion questions, review information). Classes included lecture material, academic projects, and training and application of strategies. Course subjects were selected based on the complexity of the material and with consideration of participants' interests to align with the principle of salience. The reader is referred to Appendix A for more details on the academic component of ICCR.
Speech-language-cognitive therapy served as the impairment-based complement to the functional classroom. Short- and long-term goals were developed according to the participants' individual profiles, as determined from case histories, client and caregiver report, and formal testing, which also supported salience. Therapy could address the following goal areas: attention, memory, executive functioning, auditory comprehension, verbal expression, reading comprehension, written expression, motor speech, social pragmatics, and/or augmentative–alternative communication. Metacognitive strategy training and supported practice were provided at the individual and group levels.
Participants targeted their cognitive–linguistic functioning during a technology skills session, which included various programs (e.g., ICCR website, Microsoft Office, Google Suite, Constant Therapy). During this time, they could access previously watched lectures to review content about which they were confused, enter information into Google sheets related to a class activity, write a paragraph/essay/paper for a class assignment, work on a presentation for finance or public speaking, and/or target impairment-based cognitive–linguistic skills using application-based therapy.
In terms of the clinician's role, during class, the clinician targeted ICCR students' attention (e.g., redirected students during lecture preview), memory (e.g., asked students to recall lecture content immediately and after a delay), problem solving (e.g., during moments of confusion, irritability, hypersensitivity, and within academic tasks), executive function skills (e.g., promoted students to self-monitor, self-correct, self-advocate), auditory comprehension (e.g., repeated information on request), verbal expression (e.g., facilitated lexical retrieval with semantic, phonologic, orthographic cueing and/or encouraged self-cueing), reading (e.g., supported students' oral reading), writing (e.g., assisted students' note writing), metacognitive skills (e.g., provided strategy instruction and modeling, facilitated application in a natural context), and pragmatic skills (e.g., targeted appropriateness such as turn-taking, topic, and timing; extinguished negative behaviors; increased social communication). The clinician was also responsible for creating the weekly quizzes and lecture notes and keeping the website current for students. During the technology skills training, the clinician supported students with application-based therapy as indicated, encouraged students to maintain attention to the tasks, and provided assistance to students reviewing classroom material, as needed.
Treatment Data
Classroom Performance
Students' classroom behavioral performance was measured by online tracking of the frequency of “positive” behaviors (i.e., answering questions accurately [i.e., cued and uncued], asking appropriate questions, and making appropriate comments) and “negative” behaviors (i.e., answering questions inaccurately, asking inappropriate questions, and making inappropriate comments) exhibited during coursework on a daily basis. The instructor would tally each time the participants performed one of the previously described behaviors on a paper datasheet, which was then entered into research electronic data capture (REDCap; Harris et al., 2009) for later analysis. The reader is referred to Appendix B for more information regarding the classroom performance data collection process. Progress in SLP sessions was measured across different cognitive–linguistic domains (e.g., attention, auditory comprehension), with specific metrics for the task at hand (e.g., accuracy, duration, frequency).
Weekly Quizzes
Participants were administered quizzes in two courses each week. Quizzes consisted of five questions (i.e., four multiple-choice and one true/false) pertaining to academic content that had been repeated multiple times during the lecture and was provided in supplemental lecture notes. Quizzes were administered to (a) hold the students accountable for the material they were learning each day, (b) provide a context for them to apply metacognitive strategies, and (c) facilitate retention of information in line with the testing effect (i.e., more likely to recall information later when you have been tested on it; Batsell, Perry, Hanley, & Hostetter, 2017).
Data Analysis
First, a group-level analysis was performed using logistic mixed-effect regression models to determine if time point significantly predicted item score on the standardized measures. Fixed effects included time point as a numerical predictor (Pre = “0,” Post1 = “1,” Post2 = “2,” Post3 = “3”). Random effects included random intercepts for subjects and items with by-subject random slopes for time point.
Second, to supplement the group-level analysis, data from four experimental patients and two controls were analyzed on an individual basis. McNemar's tests were performed comparing item-level accuracy between different time periods: (a) before treatment/no treatment to the final treatment/no treatment time point (P1–P3: Pre to Post3, P4 and C2: Pre to Post1, C1: Pre to Post2) and (b) between each treatment/no treatment time point (i.e., P1–P3 and C2: Post1 to Post2, P1–P3: Post2 to Post3) to assess for statistical improvements on standardized measures (i.e., WAB-R, RBANS, SCCAN, and DCT). For items with gradient scoring (e.g., WAB-R object naming scores range 0–3), responses that received full credit were assigned a 1, and responses below this threshold were assigned a 0. This type of analysis summed all of the incorrect responses (0) and correct responses (1) and then compared the proportion to see if there were significantly more positive or negative responses between two time points.
Linear mixed-effects regression models were used to analyze classroom participation (i.e., summed frequency of classroom behaviors) as the dependent variable and time (i.e., weeks), behavior type (i.e., positive/negative), and their interaction as independent variables. These data were collected for each semester. However, only the data for Semester 3 could be analyzed because the same coding system was employed throughout the entire semester by the same clinician, which was not the case for the other two semesters.
Although gains in individual speech-language-cognitive therapy were measured during each therapy session by the treating clinician, qualitative improvements were noted through inspection for changes in the complexity of short-term goals across the duration of the program, which are available in Table 2.
Table 2.
Speech-language pathology treatment goal areas.
| Participant | Initial goal areas | Final goal areas |
|---|---|---|
| P1 (August 2016 to August 2017) |
• Selective attention in a nondistracting environment with minimal cues | • Alternating/divided attention in a mildly distracting environment with minimal cues |
| • Concrete problem solving with moderate cues and extra time | • Mixed concrete–abstract problem solving with minimal–moderate cues and extra time | |
| • 1-paragraph auditory comprehension | • Word-to-phrase–level reading and writing | |
| P2 (August 2016 to August 2017) |
• 1- to 2-paragraph auditory comprehension with moderate cues and extra time | • Multistep functional problem solving with moderate cues |
| • Concrete problem solving | • Organization and cognitive flexibility in functional situations with moderate–maximal cues | |
| • Organization and cognitive flexibility in concrete, discrete scenarios with maximal cues | ||
| P3 (August 2016 to August 2017) |
• 1–5 min sustained attention in a minimally distracting environment with moderate–maximal cues | • 10 min sustained and selective attention in a classroom environment with minimal cues |
| • Basic concrete problem solving with maximal cues and extra time | • Minimally moderately complex concrete problem solving with moderate–maximal cues and extra time | |
| • < 15 automatic utterances per session with maximum cues | • < 10 automatic, inappropriate utterances per session with minimal cues | |
| P4 (January to May 2016) |
• Use total communication on 3 occasions to repair breakdowns given maximal cues | • Use total communication on 4–5 occasions to repair breakdowns given moderate cues |
| • Identify basic familiar pictures by name from a field of 3 | • Identify basic familiar pictures by name from a field of 4 |
Note. Date ranges refer to the time periods during which the participant was enrolled in intensive cognitive-communication rehabilitation.
Improvements in participation and QOL were determined by visual inspection for increases in t scores on the TBI-QOL, the Neuro-QOL, and the CASP summary and domain scores, which are available in Table 3.
Table 3.
Standardized tests scores at each time point for each participant.
| Test | P1 |
P2 |
P3 |
P4 |
C1 |
C2 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post1 | Post2 | Post3 | Pre | Post1 | Post2 | Post3 | Pre | Post1 | Post2 | Post3 | Pre | Post | Pre | Post1 | Post2 | Pre | Post | ||
| WAB-R | Language Quotient | 56.8 | 60.4 | 65.9 | 64.5 | 72.5 | 80.3 | 82.4 | 85.3 | 69.9 | 78.9 | 81.0 | 82.8 | 24.1 | 25.1 | 85.0 | 84.5 | 87.0 | 90.6 | 91.3 |
| Cortical Quotient | 65.2 | 66.5 | 73.9 | 73.2 | 76.4 | 81.8 | 86.3 | 89.1 | 71.6 | 81.0 | 83.6 | 84.8 | 34.0 | 32.9 | 88.0 | 89.4 | 91.0 | 90.3 | 89.8 | |
| Aphasia Quotient | 61.9 | 66.6 | 78.3 | 74.9 | 78.8 | 85.8 | 88.1 | 93.3 | 62.5 | 76.3 | 83.0 | 83.0 | 18.8 | 17.5 | 84.3 | 91.0 | 91.8 | 91.3 | 92.8 | |
| RBANS | Imm. Mem. | 44.0 | 44.0 | 44.0 | 61.0 | 69.0 | 76.0 | 83.0 | 73.0 | 44.0 | 40.0 | 40.0 | 44.0 | 40.0 | 40.0 | 73.0 | 76.0 | 73.0 | 69.0 | 87.0 |
| V/C | 69.0 | 72.0 | 72.0 | 69.0 | 72.0 | 87.0 | 87.0 | 100.0 | 66.0 | 75.0 | 69.0 | 72.0 | 96.0 | 84.0 | 84.0 | 92.0 | 84.0 | 60.0 | 53.0 | |
| Language | 40.0 | 44.0 | 40.0 | 47.0 | 82.0 | 87.0 | 78.0 | 74.0 | 47.0 | 74.0 | 47.0 | 74.0 | 40.0 | 40.0 | 87.0 | 78.0 | 54.0 | 74.0 | 85.0 | |
| Attention | 43.0 | 49.0 | 40.0 | 43.0 | 40.0 | 40.0 | 43.0 | 46.0 | 53.0 | 64.0 | 53.0 | 43.0 | 43.0 | 46.0 | 49.0 | 40.0 | 56.0 | 56.0 | 43.0 | |
| Del. Mem. | 44.0 | 44.0 | 48.0 | 52.0 | 94.0 | 88.0 | 97.0 | 94.0 | 40.0 | 44.0 | 44.0 | 40.0 | 40.0 | 40.0 | 94.0 | 83.0 | 97.0 | 44.0 | 44.0 | |
| Total | 45.0 | 46.0 | 45.0 | 49.0 | 64.0 | 69.0 | 72.0 | 71.0 | 46.0 | 52.0 | 46.0 | 49.0 | 47.0 | 46.0 | 71.0 | 67.0 | 66.0 | 52.0 | 54.0 | |
| SCCAN | Oral Expr. | 42.1 | 47.4 | 57.9 | 57.9 | 78.9 | 78.9 | 73.7 | 89.5 | 47.4 | 47.4 | 57.9 | 52.6 | 15.8 | 5.3 | 89.5 | 84.2 | 94.7 | 100.0 | 100.0 |
| Orient. | 58.3 | 83.3 | 91.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 58.3 | 91.7 | 66.7 | 66.7 | 50.0 | 91.7 | 100.0 | 100.0 | 100.0 | 100.0 | 83.3 | |
| Memory | 42.1 | 36.8 | 47.4 | 57.9 | 42.1 | 52.6 | 84.2 | 84.2 | 21.1 | 26.3 | 31.6 | 42.1 | 21.1 | 21.1 | 57.9 | 68.4 | 89.5 | 31.6 | 26.3 | |
| Speech | 61.5 | 69.2 | 69.2 | 69.2 | 69.2 | 100.0 | 92.3 | 100.0 | 84.6 | 100.0 | 92.3 | 100.0 | 30.8 | 46.2 | 76.9 | 100.0 | 92.3 | 100.0 | 92.3 | |
| Reading Comp. | 33.3 | 83.3 | 66.7 | 83.3 | 83.3 | 83.3 | 83.3 | 91.7 | 58.3 | 66.7 | 58.3 | 41.7 | 66.7 | 33.3 | 83.3 | 91.7 | 83.3 | 75.0 | 66.7 | |
| Writing | 57.1 | 57.1 | 57.1 | 57.1 | 57.1 | 57.1 | 57.1 | 57.1 | 85.7 | 71.4 | 85.7 | 85.7 | 42.9 | 42.9 | 57.1 | 71.4 | 57.1 | 71.4 | 71.4 | |
| Attention | 43.8 | 56.3 | 62.5 | 81.3 | 75.0 | 75.0 | 81.3 | 93.8 | 43.8 | 50.0 | 56.3 | 50.0 | 50.0 | 56.3 | 87.5 | 100.0 | 93.8 | 68.8 | 75.0 | |
| Prob. Solv. | 47.8 | 69.6 | 82.6 | 87.0 | 87.0 | 82.6 | 78.3 | 100.0 | 56.5 | 52.2 | 56.5 | 56.5 | 47.8 | 60.9 | 95.7 | 95.7 | 91.3 | 69.6 | 82.6 | |
| Total | 46.0 | 60.0 | 63.0 | 71.0 | 70.0 | 75.0 | 80.0 | 86.0 | 54.0 | 59.0 | 58.0 | 58.0 | 37.0 | 41.0 | 64.0 | 68.0 | 72.0 | 63.0 | 55.0 | |
| DCT | List. + Read. Total | 45.0 | 51.0 | 54.0 | 48.0 | 60.0 | 53.0 | 60.0 | 57.0 | 38.0 | 41.0 | 43.0 | 46.0 | 0.0 | 40.0 | 64.0 | 68.0 | 72.0 | 63.0 | 55.0 |
| CASP | Home | 79.2 | 100.0 | 79.2 | 100.0 | 91.7 | 83.3 | 87.5 | 83.3 | 87.5 | 83.3 | 79.2 | 79.2 | 79.2 | 70.8 | 91.7 | 100.0 | 100.0 | 87.5 | |
| Neigh. and Comm. | 50.0 | 68.8 | 50.0 | 81.3 | 75.0 | 62.5 | 75.0 | 93.8 | 81.3 | 75.0 | 68.8 | 75.0 | 68.8 | 68.8 | 93.8 | 93.8 | 100.0 | 100.0 | ||
| School | 0.0 | 75.0 | 80.0 | 90.0 | 0.0 | 85.0 | 85.0 | 85.0 | 0.0 | 75.0 | 75.0 | 85.0 | 0.0 | 65.0 | 0.0 | 0.0 | 0.0 | 100.0 | ||
| Home and Comm. | 100.0 | 50.0 | 58.3 | 95.0 | 80.0 | 68.8 | 68.8 | 85.0 | 81.3 | 62.5 | 62.5 | 45.0 | 37.5 | 75.0 | 100.0 | 100.0 | 100.0 | 87.5 | ||
| Total | 75.0 | 76.3 | 69.4 | 92.5 | 83.3 | 76.3 | 80.3 | 86.3 | 83.9 | 75.0 | 72.4 | 71.3 | 64.3 | 70.0 | 95.0 | 98.3 | 100.0 | 93.8 | ||
| Neuro-TBI and TBI-QOL | Anxiety | 41.2 | 43.9 | 54.2 | 48.4 | 41.2 | 36 | 49.4 | 42.1 | 52.0 | ||||||||||
| Cog. Fxn. | 43.0 | 43.8 | 43.9 | 42.9 | 32.8 | 24.1 | 47.5 | 44.9 | 38.2 | |||||||||||
| Communication | 45.4 | 45.4 | 20.0 | 19.0 | 57.5 | 47.6 | 40.3 | 19 | 49.9 | |||||||||||
| Depression | 68.3 | 61.0 | 45.3 | 46.8 | 38.3 | 38.3 | 70.5 | 36.9 | 63.0 | |||||||||||
| Pos. Aff. | 33.7 | 38.4 | 57.8 | 56.8 | 67.8 | 67.8 | 43.1 | 68.0 | 41.5 | |||||||||||
| GAS | Change | 20.0 | 0.0 | 3.1 | 0.0 | 0.0 | 30.0 | 10.0 | 12.4 | 0.0 | ||||||||||
Note. WAB-R = Western Aphasia Battery–Revised (Kertesz, 2006); RBANS Update = Repeatable Battery for the Assessment of Neuropsychological Status Update (Randolph, 2012); SCCAN = Scales of Cognitive and Communicative Ability for Neurorehabilitation (Holland & Milman, 2012); DCT = Discourse Comprehension Test (Brookshire & Nicholas, 1993); CASP = Child and Adolescent Scale of Participation (McDougall et al., 2013); TBI-QOL = TBI Quality of Life (Tulsky et al., 2016); Neuro-QOL = Neurologic Quality Of Life (Gershon et al., 2012); GAS = Goal Attainment Scaling (King et al., 2000); Imm. Mem. = Immediate Memory; V/C = Visuospatial Constructional; Del. Mem. = Delayed Memory; Oral Expr. = Oral Expression; Orient. = Orientation; Reading Comp. = Reading Comprehension; Prob. Solv. = Problem Solving; List. + Read. Total = Listening and Reading; Neigh. and Comm. = Neighborhood and Community; Home and Comm. = Home and Community Living; Cog. Fxn. = Cognitive Function; Pos. Aff. = Positive Affect and Well-Being.
Results
Standardized Assessments
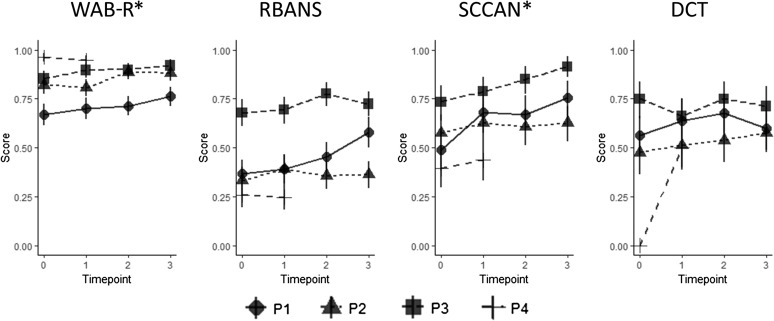
Logistic mixed-effects regression analyses accounting for differences in baseline performance across participants/items and different rates of improvement showed a positive effect of treatment on cognitive–linguistic functioning. Time point significantly predicted participants' scores on the WAB-R (β = 0.45, SE = 0.12, t = 3.88, p < .001) and the SCCAN (β = 0.44, SE = 0.16, t = 2.74, p = .01), indicating that participants' scores increased as the number of semesters they spent in ICCR increased, as depicted in Figure 2.
Figure 2.
Proportion of correct responses on each standardized test for experimental participants at each time point. Time point significantly predicted participants' scores on the Western Aphasia Battery–Revised (WAB-R) and Scales of Cognitive and Communicative Ability for Neurorehabilitation (SCCAN), indicating that, as the number of semesters in intensive cognitive-communication rehabilitation increased, participants' scores on those tests increased. * indicates that time point was a significant predictor of score. DCT = Discourse Comprehension Test; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
Follow-up analyses conducted at the individual participant level support the results reported above. All four experimental participants made statistically significant gains on at least one standardized assessment by the third semester of intervention. The reader is referred to Table 3 for subtest and total scores on these measures and Table 4 for statistical results of the McNemar's tests. On the WAB-R, P1 made statistically significant gains from the second to third time points (i.e., semester) and from pretreatment to the final semester, P2 showed statistically significant gains from the first to second semesters and from pretreatment to the final semester, and P3 demonstrated statistically significant gains from pretreatment to the first semester and from pretreatment to the final semester. On the RBANS, P1 made statistically significant improvements from the second to third semesters and from pretreatment to the final semester. On the SCCAN, P1 exhibited statistically significant gains after the first semester and from pretreatment to the final semester, and P2 showed statistically significant increases from the second to third semesters and from pretreatment to the final semester. On the DCT, only P4 demonstrated statistically significant improvements from pretreatment to the final semester. Importantly, none of the control participants exhibited statistically significant gains on any of the standardized assessments after a period without intervention, suggesting that the gains seen in the experimental group were not due to practice effects. Furthermore, the experimental participants did not demonstrate steady gains on each subtest each semester, as would have been if their improvements were due to repeated exposure to the tests.
Table 4.
Statistical results of McNemar's tests used to test for item-level gains in standardized measures after each assessment time point.
| Time point | P1 |
P2 |
P3 |
P4 |
C1 |
C2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre–Post1 | Post1–Post2 | Post2–Post3 | Pre–Post3 | Pre–Post1 | Post1–Post2 | Post2–Post3 | Pre–Post3 | Pre–Post1 | Post1–Post2 | Post2–Post3 | Pre–Post3 | Pre–Post1 | Pre–Post1 | Post1–Post2 | Pre–Post2 | Pre–Post1 | |
| WAB-R | χ2 = 5.0, p = .025 | χ2 = 10.1, p = .001 | χ2 = 15.6, p < .001 | χ2 = 8.0, p < .01 | p = .007 | χ2 = 9.6, p < .01 | |||||||||||
| RBANS | χ2 = 8.5, p = 0.004 | χ2 = 18.3, p < .001 | |||||||||||||||
| SCCAN | p < .001 | χ2 = 19.9, p < .001 | p = .031 | p < .01 | |||||||||||||
| DCT | χ2 = 38.0, p < .01 | ||||||||||||||||
Note. Blank cell = change was nonsignificant; Time point: Pre = Baseline testing, Post1 = Semester 1, Post2 = Semester 2, Post3 = Semester 3/final time point; WAB-R = Western Aphasia Battery–Revised (Kertesz, 2006); RBANS Update = Repeatable Battery for the Assessment of Neuropsychological Status Update (Randolph, 2012); SCCAN = Scales of Cognitive and Communicative Ability for Neurorehabilitation (Holland & Milman, 2012); DCT = Discourse Comprehension Test.
Classroom Performance
Attendance
Overall, participants who committed to the program (i.e., P1, P2, P3, P4) attended consistently (see Table 5). In the first semester, P1 and P3 attended ICCR very regularly, as evidenced by attendance records of 98% and 95%, respectively. P2 committed at the start of the first semester to a less intensive schedule (i.e., 3 days/week); thus, he attended 68% of the 4-day week. Of note, he attended 86% of his 3-day week schedule. In the spring semester, attendance ranged from 93% to 98% for all four participants. In the summer semester, attendance ranged from 95% to 100%.
Table 5.
Intensive cognitive-communication rehabilitation attendance (% of days attended).
| Participant | Semester |
||
|---|---|---|---|
| Fall | Spring | Summer | |
| P1 | 98.0 | 95.0 | 100.0 |
| P2 | 68.0 a | 98.0 | 100.0 |
| P3 | 95.0 | 98.0 | 95 |
| P4 | n/a | 93.0 | n/a |
Note. Attendance was based on the number of days the participant attended per semester (e.g., 4 days/week for 12 weeks). n/a = not applicable.
P3 attended 86% of his agreed upon 3-day week schedule in the fall semester. He attended 4 days per week in the spring and summer semesters.
In-Class Participation
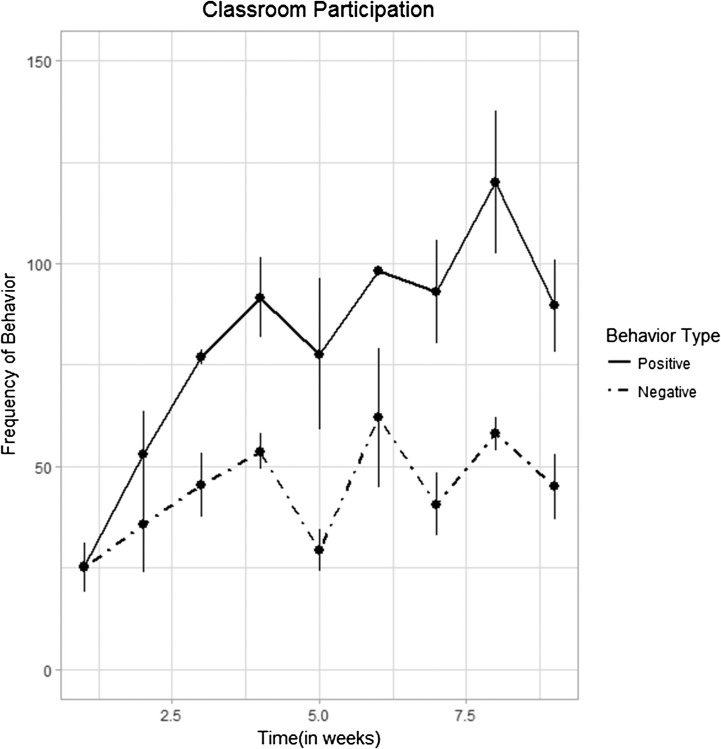
To capture changes in the classroom over time, participation (i.e., summed frequency of tracked behaviors) for each week of the third semester served as the dependent variable in a linear mixed-effects model. Fixed effects in this model included time (i.e., weeks), behavior type (i.e., positive/negative), and their interaction. To account for individual variability, participant was used as a random effect in the model. The first finding was a main effect of time, F(1, 51) = 37.75, p < .001, suggesting that classroom behavior increased significantly as the third semester progressed. Second and more important, as depicted in Figure 3, there was a significant time-by-behavior type interaction effect, F(1, 51) = 11.249, p = .002, such that the effect of time was significantly less for negative behaviors than positive behaviors (β = −5.850, SE = 1.744), t(1, 51) = −3.34, p = .002. In other words, the frequency of positive behaviors (e.g., asking appropriate questions) increased at a greater rate over time than negative behaviors (e.g., asking inappropriate questions), suggesting that participants were more positively engaged in the classroom with the duration of the third semester. It is important to note that, although these results reflect the data that were collected in the third semester, they may have been influenced by the classroom experience of previous semesters. Furthermore, reliability checks could not be completed for the classroom data. Thus, these findings should be interpreted with caution.
Figure 3.
Results of linear mixed-effects regression revealed that time (i.e., weeks) had a significantly greater effect on the positive classroom behaviors (solid line) than the negative classroom behaviors (dotted line), supporting that intensive cognitive-communication rehabilitation students demonstrated more accurate and appropriate classroom participation with the duration of the third semester.
Quiz Performance
Participants demonstrated variable accuracy on the weekly quizzes, as reflected in Table 6. Anecdotally, P1 consistently studied for his quizzes across all semesters, P2 started consistently studying for quizzes in the spring semester, and P3 did not regularly study for quizzes outside the ICCR, as he needed support to do so and that was not available through his group home. These observations were relatively consistent with their performance. Notably, one may not expect to see a linear upward trend in accuracy on the quizzes across the semester(s) as the complexity of the test varied each week.
Table 6.
Participant's quiz data (i.e., proportion of correct items/total items represented as a percent correct).
| Quiz No. | P1 |
P2 |
P3 |
P4 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fall |
Spring |
Summer |
Fall |
Spring |
Summer |
Fall |
Spring |
Summer |
Fall |
Spring |
Summer |
|||||||||||||
| C1 | C2 | C3 | C4 | C5 | C6 | C1 | C2 | C3 | C4 | C5 | C6 | C1 | C2 | C3 | C4 | C5 | C6 | C1 | C2 | C3 | C4 | C5 | C6 | |
| 1 | 80 | 60 | 80 | 60 | 60 | 60 | n/a | 80 | 100 | 100 | 80 | 80 | 20 | 40 | 60 | 80 | 40 | 40 | n/a | n/a | 60 | 60 | n/a | n/a |
| 2 | 40 | 40 | 80 | 100 | 100 | 100 | 60 | 20 | 100 | 100 | 40 | 60 | 60 | 60 | 80 | 80 | 60 | 60 | n/a | n/a | 80 | n/a | n/a | n/a |
| 3 | 50 | 100 | 60 | 100 | 40 | 40 | 100 | 100 | 100 | 100 | 40 | 80 | 83 | 40 | 100 | 100 | 40 | 40 | n/a | n/a | 60 | 60 | n/a | n/a |
| 4 | 40 | 40 | 100 | 100 | 80 | 80 | 60 | 60 | 80 | 80 | 40 | 100 | 40 | 60 | 80 | 100 | 40 | 40 | n/a | n/a | 20 | 20 | n/a | n/a |
| 5 | 80 | 40 | 60 | 40 | 60 | 60 | 80 | 80 | 100 | 100 | 20 | 100 | 40 | 60 | 80 | 100 | 60 | 60 | n/a | n/a | 60 | 40 | n/a | n/a |
| 6 | 80 | 69 | 80 | 40 | 60 | 60 | 100 | 31 | 80 | 40 | 80 | 80 | 40 | 38 | 80 | 60 | 20 | 20 | n/a | n/a | 80 | 60 | n/a | n/a |
| 7 | 100 | 60 | 60 | 80 | 80 | 80 | 80 | 80 | 100 | 60 | 60 | 60 | 80 | 60 | 100 | 80 | 60 | 60 | n/a | n/a | 60 | 80 | n/a | n/a |
| 8 | 80 | 80 | 80 | 80 | 40 | 40 | 60 | 80 | 100 | 80 | 40 | 60 | 40 | 80 | 100 | 60 | 20 | 20 | n/a | n/a | 80 | 20 | n/a | n/a |
| 9 | 100 | 100 | 0 | 0 | 100 | 100 | 80 | 40 | 80 | 80 | 20 | 20 | ||||||||||||
| 10 | 80 | 60 | 60 | 80 | 60 | 40 | 100 | 60 | 60 | |||||||||||||||
Note. All quizzes were five questions (i.e., four multiple-choice and one true/false), with the exception of Quiz 6 in C2, which was out of 16 points. C1 = Psychology I; C2 = Economics I; C3 = Psychology II; C4 = Biology I; C5 = Psychology III; C6 = Biology II; n/a = not applicable.
SLP Performance
Three to five impairment-level cognitive–linguistic goals per semester were generated for each participant and targeted during individual SLP sessions via drills and structured exercises. Participants' performance in these goal areas was regularly monitored, and once the established criterion was achieved, the goal was revised. All participants who committed to at least one full semester of ICCR targeted more complex goals in their SLP sessions at the end of the treatment than at baseline.
At the time of enrollment, P1 exhibited moderate–severe cognitive deficits, moderate anomic aphasia, and mild–moderate apraxia of speech. After three semesters, he demonstrated improvements across goal areas and now exhibits moderate impairments. P2 initially exhibited mild–moderate anomic aphasia and mild cognitive deficits, particularly in executive function. P2 also demonstrated broad gains across goal areas, ultimately presenting with only mild cognitive–linguistic deficits. Furthermore, after completing his third semester, P2 discharged from ICCR and reenrolled at a local community college to finish his associate's degree, a goal he had abandoned prior to ICCR. P3 initially was classified as having moderate-to-severe cognitive–linguistic deficits, particularly in attention and memory, as well as a severe spastic dysarthria. At the end of Semester 3, he continued to demonstrate moderate-to-severe cognitive-communication deficits, though he demonstrated incremental gains across goal areas. Finally, P4 demonstrated severe Broca's aphasia, mild-to-moderate cognitive deficits of attention and executive function, and moderate apraxia of speech. Following his one semester of treatment, his profile also remained generally stable, though incremental progress was noted in his goals as well.
GAS was used to assist participants in generating specific goals that they wanted to prioritize in therapy. P1 improved in his immediate/delayed memory in the first semester (+20) and ability to navigate a city in the third semester (+3.10). P2 was interested in securing alternative transportation (i.e., wanted to begin driving lessons, take public transportation, etc.) and made gains on that goal in the third semester (+30). P3 made gains in reducing his use of a maladaptive speech intelligibility strategy (i.e., reduce finger occlusion of nose) and ability to make a daily schedule in the fall (+10) and spring (+12.40) semesters. P4 was interested in obtaining employment and did not make gains toward that goal, which may not be surprising given that ICCR is focused on furthering academic versus vocational goals specifically. These results are also available in Table 3.
Participation and QOL
In order to measure participation, CASP responses from pretreatment and the final available time point were compared. All experimental participants transitioned from a score of 0 (“unable to participate”) in the school domain to a score of 65 or greater. P1, P2, and P4 all exhibited increases in their total CASP scores (+17.5, +2.9, +5.7), as did C1 (+5.0), though P3 exhibited a decrease (−12.7).
Responses from the TBI-QOL and the Neuro-QOL were compared for all patients who completed the measure more than once (i.e., P1, P2, and P3). All values refer to t scores, with the exception of Communication on the Neuro-QOL, which does not provide a t score so raw scores were used. P1 demonstrated an improvement in Cognitive Function (+0.8), Depression (−7.3; lower scores indicate fewer symptoms of depression), and Positive Affect and Well-Being (+4.7). He also showed an increase in Anxiety (+2.7; higher scores indicate more symptoms of anxiety). Positively, P2 demonstrated a reduction in his anxiety levels (i.e., Anxiety: −5.8), but slight decreases in the remaining QOL domains (Cognitive Function: −1, Communication: −1, Depression: +1.5, Positive Affect and Well-Being: −1). Finally, P3 demonstrated improved Anxiety levels (−5.2), decreased Cognitive Function (−8.7) and Communication (−9.9), and stable report in the remaining domains.
Treatment Fidelity
A speech-language pathologist on the project who was not directly involved in the day-to-day treatment administration documented observation of approximately 10% of the classroom instruction across the fall, spring, and summer semesters. No gross deviations from the treatment protocol (see Appendix A) were noted during those times. Treatment protocols detailing procedures for different aspects of the project were written before the start of the semester for the speech-language pathologist and/or classroom facilitators to follow.
Discussion
The results of this study provide initial evidence that an ICCR program resulted in improved cognitive–linguistic skills for young adults with chronic ABI. Following ICCR, all four experimental participants demonstrated statistically significant gains on at least one standardized measure of cognitive–linguistic functioning, whereas control participants did not. Furthermore, there was a significant linear effect of time point for the WAB-R and SCCAN, such that as the number of semesters students were in the program increased, their assessment scores increased. Extending those results, all three participants who completed multiple semesters of ICCR demonstrated significantly more positive classroom behaviors than negative behaviors over time and more complex individual SLP goals and made gains on their personal (GAS) goals across at least one semester. Finally, those same three participants improved in their school participation and on at least one aspect of their health-related QOL.
This study's findings support growing evidence that principles of experience-dependent neural plasticity are indeed advantageous for rehabilitation (Persad et al., 2013). ICCR deliberately incorporated many of these principles into its design (i.e., age, intensity, repetition, specificity of training, and salience). The benefits of CR for individuals with ABI are well known, and during ICCR, the classroom setting provided a much-needed context for learning and generalization 4 days a week (Peach, Nathan, & Beck, 2017; i.e., intensity, repetition, age). Behaviors and metacognitive strategies targeted during individual therapy, group therapy, and technology skills training could be immediately applied within the functional setting of the classroom, as also seen by Kennedy and Krause (2011) with their coaching intervention. These results indicate that a systematic, structured, and intensive rehabilitation program that includes both impairment and activity-level components in its treatment approach has the potential to improve functional skills in young adults with chronic TBI. Although the key component of ICCR cannot be determined without future carefully designed research, it is likely that the intensive, repetitive training of discrete skills, while providing a natural context for their application, is driving the improvements seen in ICCR students.
By participating in ICCR, students also became aware of strategies and accommodations that were specifically beneficial to them. Of note, P2 has returned to community college to complete an associate's degree and is an excellent example of a student utilizing the skills and services promoted in ICCR. He and his family met with disability services prior to reenrolling, they utilized SLP progress notes to ensure appropriate accommodations, and they petitioned for one ICCR class to fill an educational maintenance requirement during his absence from school.
Outside the cognitive gains, students also expressed changes in their participation, social engagement, and well-being after the program. It would seem that ICCR supported the students' ability to increase their involvement in activities in their home (e.g., caregivers reported anecdotally that one participant initiated more conversation over the weekends with family), school (e.g., all students asked/answered more questions and made more comments in class), and community (e.g., one student invited another to go to an amusement park together to celebrate their birthday). Of note, social communication was encouraged in the program (i.e., clinicians suggested that students eat lunch together) but not targeted directly to allow conversation and friendships to develop organically. In the future, this aspect of the program will be investigated objectively to determine the advantages/disadvantages of this approach.
The above-described reports are supported objectively in that three of the four experimental participants made gains on the CASP total participation scale by their final semester. These improvements may be partly attributable to the cognitive-communication gains they made in the program, which may have allowed them to engage more frequently and successfully in their homes and communities. In addition, as ICCR courses took place in Sargent College of Rehabilitation Sciences, ICCR students attended classes on the same semester schedule as Boston University students and thus had the opportunity to observe and interact with them on a regular basis in the building. The positive effects of this environment on ICCR students' psychosocial gains cannot be understated, particularly given the propensity for individuals with ABI to experience social isolation postinjury (McLean, Jarus, Hubley, & Jongbloed, 2012; Northcott & Hilari, 2011).
Gains in QOL were expected as a result of ICCR for both individuals with cognitive–linguistic impairments secondary to TBI and/or stroke. According to the literature, social integration, a key component of ICCR, has been shown to be related to better QOL in both of these populations (Hilari, Cruice, Sorin-Peters, & Worrall, 2016; Johnston & Miklos, 2002). Furthermore, in persons with aphasia, participation in ICAPs and group therapy have also been associated with gains in QOL, again suggesting that QOL should improve with ICCR. Interestingly, post-ICCR, participants who had completed multiple semesters of ICCR reported improvements in at least one domain, as well as decreases in at least one domain. The decreases are likely attributable to two factors: (a) increased insight into deficits and (b) response shift, a change in self-evaluation due to improved understanding of QOL on follow-up test administration (Megari, 2013).
Although ICCR resulted in consistent positive gains on standardized assessment measures for participants who enrolled in consecutive semesters, not all participants responded similarly to the treatment. This responsiveness primarily appeared to be due to severity, insight, and motivation, which will be described in some detail below. P4 had high levels of family/caregiver support and initial motivation to attend, and so he was enrolled with accommodations for his language impairments. Despite these adaptations, he struggled to attend, comprehend, and participate. Although he demonstrated the smallest objective gains in ICCR, he did initiate use of total communication in the classroom, which was a therapy goal for him. Nonetheless, he elected to seek vocational employment upon completion of one semester, which in certain cases may be more appropriate for some young individuals with ABI (MacLennan & MacLennan, 2008).
With regard to the control subjects, both C1 and C2 were invited to enroll as experimental participants after deferring for one semester. However, neither decided to do so after reevaluating their current levels of functioning and/or educational goals. C2 agreed to continue as a control subject for one additional semester. Notably, neither C1 nor C2 demonstrated statistically significant gains during their involvement in the study.
There are many ongoing and future avenues available for ICCR's improvement. First, although this study demonstrated preliminary evidence of efficacy, it needs to be studied with a larger, more diverse patient sample. Thus, these results, as well as any limitations, should be interpreted with caution, especially given the small sample size. Second, time postonset (i.e., all participants were in the chronic phase of recovery; Moss & Nicholas, 2006) and etiology (i.e., participants with TBI and stroke significantly improved) did not appear to influence treatment outcome. Yet, severity did seem to play a role (i.e., P1 and P2: less severe, robust treatment responses; P3 and P4: more severe, less favorable treatment responses). Thus, ICCR does look to be a better fit for individuals with chronic ABI with deficits in the mild and moderate range. Third, it would be ideal if ICCR could be scaled to other colleges and rehabilitation clinics, and partnerships with the local community and state may be useful in meeting that goal. Fourth, it may also be beneficial to explore the effects of neuromodulation (e.g., transcranial direct current stimulation [TDCS]) concurrent with behavioral intervention to enhance ongoing treatment. Finally, although no participants thus far have been safe to undergo magnetic resonance imaging, we hope to explore the effects of ICCR on brain reorganization (i.e., functional near-infrared spectroscopy, electroencephalography, functional magnetic resonance imaging, diffusion tensor imaging) if possible.
Conclusion
Currently, there exists a gap in rehabilitation services for young individuals with chronic ABI who want to continue their education but are not yet able to do so. ICCR is a first step in filling that gap. This program was designed using principles of experience-dependent neural plasticity and CR to support young adults with ABI to build the skills necessary to enroll in post–secondary education. Following ICCR, participants demonstrated improved cognitive–linguistic skills, more appropriate classroom behavior, and increased complexity of targeted SLP goals. Beyond these improvements, participants reported increased life participation and QOL. They enjoyed ICCR and found it beneficial; they learned from one another, supported each other, and even became friends. Overall, these findings demonstrate initial effectiveness of the ICCR program and support its use with this population.
Acknowledgments
This project was supported by an internal grant through Boston University (PI: Swathi Kiran) and T32DC0130170 (PI: Christopher Moore). The authors thank the participants, families, and caregivers for their support and belief in the program. In addition, they extend their gratitude to Natalie Albrittain-Ross for developing course content and serving as a course instructor for two semesters. The authors thank Rachel Ryskin for her assistance with data analysis and plotting using R and R Studio. Finally, they appreciate Carrie Des Roches, Deirdre McLaughlin, Heather Wolfe, Shreya Chaturvedi, and Lindsey Foo for their assistance with data collection, classroom support, and program development.
Appendix A
ICCR Treatment Protocol
Semester 1 classes: Psychology, Economics, US History, Personal Finance, Public Speaking
Semester 2 classes: Biology, Psychology, US History, Finance, Communications
Semester 3 classes: Biology, Psychology, Finance, US History
Pre-semester preparation
Determine semester schedule
Decide on courses from content available on Khan Academy, Open Yale Courses, other on-line sources and students’ interests
Choose units/sub-topic videos with 30-40 minutes lecture content/hour
Administer pre-treatment test battery unless student is returning for a consecutive semester, in which case use previous post-treatment scores
Create the following documents for each class:
Semester Tracker - plan and track completion of video lectures
Syllabus – instructor information, class description, dates/schedules, lecture topics, and grading rubric
Weekly PowerPoints – presentations with linked videos for core and elective classes
Generate materials for individual treatment sessions to target:
Impairment-based cognitive-linguistic goals based on pre-testing for 1:1 sessions and classroom
Functional GAS goal(s) developed with clients in pre-testing
During the semester
Classes
Core classes: “Preview, Review/Discuss, Quiz Review” Model
First hour (preview): watch lecture with no distractions, pause only for student questions.
Second hour (review/discussion): re-watch content presented in the first hour; introduce metacognitive strategies (e.g., RITA: Rehearse, Imagine, Take Time, Activate; STEP BACK: Self-care, Take Breaks, Exercise, Pace yourself, Be open to help, Avoid interruptions, Cut distractions, Keep it simple) and mnemonics while providing rationale for their use; provide visual aids, written support, and repetition to support memory and auditory comprehension; facilitate conversation between the students to recap what they have learned and generate connections between new and old content.
Third hour (quiz review): answer sample quiz questions that have been prepared by the clinician and apply metacognitive strategies they learned in the previous hour to encode salient information from the lecture that they may be quizzed on later in the week
Elective classes:
-
Option 1
60 minutes (discussion): watch the lecture and clinician stops it and asks students to recall salient information that was just presented taxing their immediate recall. Then, they discuss the meaning/significance/relevance and relate it to current events and their environment
-
Option 2
Find an article relevant to the subject
Read as a group with participants taking turns
Discuss main ideas following each paragraph/section
Generate a slide presentation as a group to be presented by students on final day of article discussion
ICCR Website
Student “Lecture notes” – Clinician posts notes in basic outline format on academic content in CORE subject
Research Assistants (RAs) create these outside of class
Videos – post lecture content for all classes at the end of each day
Individual sessions
60 minute cognitive-linguistic therapy
Technology Time
60 minutes of Constant Therapy, individual or supported lecture review, or classroom assignment completion (i.e., may involve use of internet, Google Docs, Microsoft Word)
RA provides ICCR students support during this hour
Testing
Quizzes – 5 question multiple choice (1 True/False) questions regarding content from that week’s lecture material
Administered Mondays/Tuesdays prior to lecture in CORE classes ONLY
Hand back to students for guided self-correction during “Quiz Review” hour
Final exam – compilation of quizzes; CORE classes ONLY
Appendix B
Additional Details Regarding Classroom Data
Operational definitions used for coding classroom participation behaviors:
Accurately answered question: Student gave an accurate response reflecting comprehension of the material
Accurately answered question cued: Clinician provided support for the student to answer the question accurately (i.e., choices, phonemic (first sound of the word), semantic (related word), orthographic (wrote part of the word)) or, if the clinician asked the student to elaborate.
Inaccurately answered question: Clinician explicitly stated that the student was incorrect and asked them to try again, or asked another student to answer. Also, relevant if the student replied “I don’t know” when asked a question
Appropriate question: Student generated a question that was on topic, relevant, and added value to the conversation (e.g. While discussing addictive food product, a student asked if cigarettes have similar addictive properties? Although class is currently discussing food, student connected addictive properties to another known source).
Inappropriate question: When the student produced questions that were directed at discontinuing a productive academic activity such as, “Why do we have to do this?” or questions that were irrelevant to the current topic?, “Did you know last summer I scraped my knee and got a bruise?”
Appropriate comment: When the student’s response was relevant to the current topic, and/or added value to the subject, or when students made comments that related information in the current class to information they were learning in another class.
Inappropriate comment: Student produced a comment that was not irrelevant to the current topic, was distracting to other students, or included information that should be discussed individually (if at all) and not with the group during class
| Sample datasheet used to collect classroom participation data | |||
| Classes_________________ | Date_________ | ||
| Clinician________________ | ICCR Day_____ | ||
| P1 | P2 | P3 | |
| Answered Question | |||
| Asked Question | |||
| Made Comment | |||
| Notes | |||
Key: accurate/appropriate = +, accurate/appropriate cued = (+), inaccurate/inappropriate = -
Funding Statement
This project was supported by an internal grant through Boston University (PI: Swathi Kiran) and T32DC0130170 (PI: Christopher Moore). The authors thank the participants, families, and caregivers for their support and belief in the program. In addition, they extend their gratitude to Natalie Albrittain-Ross for developing course content and serving as a course instructor for two semesters.
Footnotes
Although individuals with brain injury due to TBI and CVA do not present with exactly the same deficit profiles and needs, there is considerable overlap. For example, it is common for both groups to experience deficits in attention, memory, language, and executive function (Bonini & Radanovic, 2015; Rao & Lyketsos, 2000); mood and anxiety disorders (Mukherjee, Levin, & Heller, 2006); and fatigue (Colle, Bonan, Gellez Leman, Bradai, & Yelnik, 2006).
The primary causes of TBI in the military population are blasts, blasts plus motor vehicle accidents (MVA), MVAs, and gunshot wounds. TBI in civilians is often caused by falls, MVAs, being hit with something, and/or assault. TBIs in veterans may result in different symptoms that require additional intervention than those needed by civilians with ABI (i.e., postconcussive symptoms for longer periods of time, posttraumatic stress disorder, chronic pain, substance abuse, other medical injuries; Summerrall, 2017).
Individuals with language and cognitive deficits as a result of stroke and/or TBI were included in this study, as the primary inclusion criteria was that participants were young and interested in enrolling in college but could not due to the severity of their cognitive–linguistic profile.
Both patient-reported outcome measures were developed according to the Patient-Reported Outcomes Measurement Information System (PROMIS) standards; the Neuro-QOL has good internal consistency, test–retest reliability, and responsiveness to change, and the TBI-QOL has good construct validity and internal consistency (Tulsky et al. 2016).
References
- Babbitt E. M., Cherney L. R., & Worrall L. (2016). Who benefits from an intensive comprehensive aphasia program? Topics in Language Disorders, 36(2), 168–184. [Google Scholar]
- Batsell W. R., Perry J. L., Hanley E., & Hostetter A. B. (2017). Ecological validity of the testing effect: The use of daily quizzes in introductory psychology. Teaching of Psychology, 44(1), 18–23. https://doi.org/10.1177/0098628316677492 [Google Scholar]
- Bayley M. T., Tate R., Douglas J. M., Turkstra L. S., Ponsford J., Stergiou-Kita M., … Bragge P. (2014). INCOG guidelines for cognitive rehabilitation following traumatic brain injury: Methods and overview. Journal of Head Trauma Rehabilitation, 29(4), 290–306. https://doi.org/10.1097/HTR.0000000000000070 [DOI] [PubMed] [Google Scholar]
- Bloom P. (2012, April 5). Psych 110: Introduction to psychology. Retrieved from https://oyc.yale.edu/introduction-psychology/psyc-110
- Bonilha L., Gleichgerrcht E., Nesland T., Rorden C., & Fridriksson J. (2016). Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabilitation and Neural Repair, 30(3), 266–279. https://doi.org/10.1177/1545968315593808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini M. V., & Radanovic M. (2015). Cognitive deficits in post-stroke aphasia. Arquivos de Neuro-Psiquiatria, 73(10), 840–847. https://doi.org/10.1590/0004-282X20150133 [DOI] [PubMed] [Google Scholar]
- Brady M. C., Kelly H., Godwin J., Enderby P., & Campbell P. (2016). Speech and language therapy for aphasia following stroke. In The Cochrane Collaboration (Ed.), Cochrane database of systematic reviews. Chichester, United Kingdom: Wiley; Retrieved from http://doi.wiley.com/10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookshire R. H., & Nicholas L. E. (1993). Discourse Comprehension Test. Minneapolis, MN: BRK Publishers. [Google Scholar]
- Chapey R. (2008). Language intervention strategies in aphasia and related neurogenic communication disorders (5th ed.). Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- Cicerone K. D. (2004). Participation as an outcome of traumatic brain injury rehabilitation. Journal of Head Trauma Rehabilitation, 19(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Cicerone K. D., Dahlberg C., Kalmar K., Langenbahn D. M., Malec J. F., Bergquist T. F., … Morse P. A. (2000). Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Archives of Physical Medicine and Rehabilitation, 81(12), 1596–1615. https://doi.org/10.1053/apmr.2000.19240 [DOI] [PubMed] [Google Scholar]
- Cicerone K. D., Langenbahn D. M., Braden C., Malec J. F., Kalmar K., Fraas M., … Ashman T. (2011). Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation, 92(4), 519–530. https://doi.org/10.1016/j.apmr.2010.11.015 [DOI] [PubMed] [Google Scholar]
- Coelho C. A., DeRuyter F., & Stein M. (1996). Treatment efficacy: Cognitive-communicative disorders resulting from traumatic brain injury in adults. Journal of Speech and Hearing Research, 39(5), S5–S17. https://doi.org/10.1044/jshr.3905.s5 [PubMed] [Google Scholar]
- Colle F., Bonan I., Gellez Leman M. C., Bradai N., & Yelnik A. (2006). Fatigue after stroke. Annales de Réadaptation et de Médecine Physique, 49(6), 361–364. https://doi.org/10.1016/j.annrmp.2006.04.010 [DOI] [PubMed] [Google Scholar]
- Cooper D. B., Bowles A. O., Kennedy J. E., Curtiss G., French L. M., Tate D. F., & Vanderploeg R. D. (2017). Cognitive rehabilitation for military service members with mild traumatic brain injury: A randomized clinical trial. Journal of Head Trauma Rehabilitation, 32(3), E1–E15. https://doi.org/10.1097/HTR.0000000000000254 [DOI] [PubMed] [Google Scholar]
- Corrigan J. D., & Hammond F. M. (2013). Traumatic brain injury as a chronic health condition. Archives of Physical Medicine and Rehabilitation, 94, 1199–1201. [DOI] [PubMed] [Google Scholar]
- Davis G. A. (2005). PACE revisited. Aphasiology, 19(1), 21–38. https://doi.org/10.1080/02687030444000598 [Google Scholar]
- Gershon R. C., Lai J. S., Bode R., Choi S., Moy C., Bleck T., … Cella D. (2012). Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Quality of Life Research, 21(3), 475–486. https://doi.org/10.1007/s11136-011-9958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., & Conde J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilari K., Cruice M., Sorin-Peters R., & Worrall L. (2016). Quality of life in aphasia: State of the art. Folia Phoniatrica et Logopaedica, 67(3), 114–118. https://doi.org/10.1159/000440997 [DOI] [PubMed] [Google Scholar]
- Holland A. L., Fromm D. S., DeRuyter F., & Stein M. (1996). Treatment efficacy: Aphasia. Journal of Speech and Hearing Research, 39, S27–S36. [DOI] [PubMed] [Google Scholar]
- Holland A. L., & Milman L. (2012). Scales of Cognitive and Communicative Ability for Neurorehabilitation (SCCAN). Austin, TX: Pro-Ed. [Google Scholar]
- Hoover E. L., Caplan D. N., Waters G. S., & Carney A. (2017). Communication and quality of life outcomes from an interprofessional intensive, comprehensive, aphasia program (ICAP). Topics in Stroke Rehabilitation, 24(2), 82–90. [DOI] [PubMed] [Google Scholar]
- Johnston M. V., & Miklos C. S. (2002). Activity-related quality of life in rehabilitation and traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 83, S26–S38. https://doi.org/10.1053/apmr.2002.37100 [DOI] [PubMed] [Google Scholar]
- Kennedy M. R., & Coelho C. (2005). Self-regulation after traumatic brain injury: A framework for intervention of memory and problem solving. Seminars in Speech and Language, 26(4), 242–255. [DOI] [PubMed] [Google Scholar]
- Kennedy M. R., Coelho C., Turkstra L., Ylvisaker M., Sohlberg M. M., Yorkston K., … Kan P.-F. (2008). Intervention for executive functions after traumatic brain injury: A systematic review, meta-analysis and clinical recommendations. Neuropsychological Rehabilitation, 1, 1–43. [DOI] [PubMed] [Google Scholar]
- Kennedy M. R., & Krause M. O. (2011). Self-regulated learning in a dynamic coaching model for supporting college students with traumatic brain injury: Two case reports. Journal of Head Trauma Rehabilitation, 26(3), 212–223. https://doi.org/10.1097/HTR.0b013e318218dd0e [DOI] [PubMed] [Google Scholar]
- Kennedy M. R., Krause M. O., & Turkstra L. S. (2008). An electronic survey about college experiences after traumatic brain injury. NeuroRehabilitation, 23(6), 511–520. [PubMed] [Google Scholar]
- Kennedy M. R., O'Brien K. H., & Krause M. O. (2012). Bridging person-centered outcomes and therapeutic processes for college students with traumatic brain injury. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 22(4), 143–151. [Google Scholar]
- Kerr A. L., Cheng S.-Y., & Jones T. A. (2011). Experience-dependent neural plasticity in the adult damaged brain. Journal of Communication Disorders. 44(5), 538–48. https://doi.org/10.1016/j.jcomdis.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. (2006). Western Aphasia Battery–Revised. San Antonio, TX: PsychCorp; Retrieved from http://www.pearsonclinical.com/language/products/100000194/western-aphasia-batteryrevised.html [Google Scholar]
- Khan Academy. (2017). Retrieved from https://www.khanacademy.org/
- King G. A., McDougall J., Palisano R. J., Gritzan J., & Tucker M. A. (2000). Goal Attainment Scaling: Its use in evaluating pediatric therapy programs. Physical & Occupational Therapy in Pediatrics, 19(2), 31–52. https://doi.org/10.1080/J006v19n02_03 [Google Scholar]
- Kleim J. A. (2011). Neural plasticity and neurorehabilitation: Teaching the new brain old tricks. Journal of Communication Disorders, 44(5), 521–528. https://doi.org/10.1016/j.jcomdis.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Kleim J. A., & Jones T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research, 51(1), S225–S239. [DOI] [PubMed] [Google Scholar]
- Leininger S., Strong C.-A. H., & Donders J. (2014). Predictors of outcome after treatment of mild traumatic brain injury: A pilot study. Journal of Head Trauma Rehabilitation, 29(2), 109–116. https://doi.org/10.1097/HTR.0b013e3182860506 [DOI] [PubMed] [Google Scholar]
- Lyon J. G. (1992). Communication use and participation in life for adults with aphasia in natural settings: The scope of the problem. American Journal of Speech-Language Pathology, 1(3), 7–14. [Google Scholar]
- MacLennan D. L., & MacLennan D. C. (2008). Assessing readiness for post–secondary education after traumatic brain injury using a simulated college experience. NeuroRehabilitation, 23(6), 521–528. [PubMed] [Google Scholar]
- Masel B. E., & DeWitt D. S. (2010). Traumatic brain injury: A disease process, not an event. Journal of Neurotrauma, 27(8), 1529–1540. https://doi.org/10.1089/neu.2010.1358 [DOI] [PubMed] [Google Scholar]
- McDougall J., Bedell G., & Wright V. (2013). The youth report version of the Child and Adolescent Scale of Participation (CASP): Assessment of psychometric properties and comparison with parent report. Child: Care, Health and Development, 39(4), 512–522. https://doi.org/10.1111/cch.12050 [DOI] [PubMed] [Google Scholar]
- McLean A. M., Jarus T., Hubley A. M., & Jongbloed L. (2012). Differences in social participation between individuals who do and do not attend brain injury drop-in centres: A preliminary study. Brain Injury, 26(1), 83–94. https://doi.org/10.3109/02699052.2011.635353 [DOI] [PubMed] [Google Scholar]
- Megari K. (2013). Quality of life in chronic disease patients. Health Psychology Research, 1(3), e27 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4768563/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Benke M., Chaloux B., Ragan L. C., Schroeder R., Smutz W., & Swan K. (2013). Leading the e-learning transformation of higher education: Meeting the challenges of technology and distance education. Sterling, VA: Stylus Publishing. [Google Scholar]
- Moss A., & Nicholas M. (2006). Language rehabilitation in chronic aphasia and time postonset—A review of single-subject data. Stroke, 37(12), 3043–3051. https://doi.org/10.1161/01.STR.0000249427.74970.15 [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Levin R. L., & Heller W. (2006). The cognitive, emotional, and social sequelae of stroke: Psychological and ethical concerns in post-stroke adaptation. Topics in Stroke Rehabilitation, 13(4), 26–35. [DOI] [PubMed] [Google Scholar]
- Northcott S., & Hilari K. (2011). Why do people lose their friends after a stroke? International Journal of Language & Communication Disorders, 46(5), 524–534. https://doi.org/10.1111/j.1460-6984.2011.00079.x [DOI] [PubMed] [Google Scholar]
- Peach R., Nathan M., & Beck K. (2017). Language-specific attention treatment for aphasia: Description and preliminary findings. Seminars in Speech and Language, 38(1), 5–16. https://doi.org/10.1055/s-0036-1597260 [DOI] [PubMed] [Google Scholar]
- Persad C., Wozniak L., & Kostopoulos E. (2013). Retrospective analysis of outcomes from two intensive comprehensive aphasia programs. Topics in Stroke Rehabilitation, 20(5), 388–397. https://doi.org/10.1310/tsr2005-388 [DOI] [PubMed] [Google Scholar]
- Power J. D., & Schlaggar B. L. (2017). Neural plasticity across the lifespan: Neural plasticity across the lifespan. Wiley Interdisciplinary Reviews: Developmental Biology, 6(1), e216 https://doi.org/10.1002/wdev.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F., Neininger B., Elbert T., Mohr B., Rockstroh B., Koebbel P., & Taub E. (2001). Constraint-induced therapy for chronic aphasia after stroke. Stroke, 32(7), 1621–1626. [DOI] [PubMed] [Google Scholar]
- Randolph C. (2012). Repeatable Battery for the Assessment of Neuropsychological Status Update (RBANS). Bloomington, MN: Pearson Clinical; Retrieved from http://www.pearsonclinical.com/psychology/products/100000726/repeatable-battery-for-the-assessment-of-neuropsychological-status-update-rbans-update.html [Google Scholar]
- Rao V., & Lyketsos C. (2000). Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics, 41(2), 95–103. [DOI] [PubMed] [Google Scholar]
- Rodriguez A. D., Worrall L., Brown K., Grohn B., McKinnon E., Pearson C., … Copland D. A. (2013). Aphasia LIFT: Exploratory investigation of an intensive comprehensive aphasia programme. Aphasiology, 27(11), 1339–1361. https://doi.org/10.1080/02687038.2013.825759 [Google Scholar]
- Rose M. L., Cherney L. R., & Worrall L. E. (2013). Intensive comprehensive aphasia programs: An international survey of practice. Topics in Stroke Rehabilitation, 20(5), 379–387. [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network. (2013). Brain injury rehabilitation in adults: A national clinical guideline (A. McMillan, Ed.). Edinburgh, United Kingdom: Author; Retrieved from https://www.sign.ac.uk/sign-130-brain-injury-rehabilitation-in-adults.html [Google Scholar]
- Sohlberg M. M., & Mateer C. A. (1989). Introduction to cognitive rehabilitation: Theory and practice. New York, NY: Guilford Press. [Google Scholar]
- Sohlberg M. M., McLaughlin K. A., Pavese A., Heidrich A., & Posner M. I. (2000). Evaluation of attention process training and brain injury education in persons with acquired brain injury. Journal of Clinical and Experimental Neuropsychology, 22(5), 656–676. [DOI] [PubMed] [Google Scholar]
- Summerrall E. L. (2017, November 6). PTSD: National Center for PTSD; Retrieved from https://www.ptsd.va.gov/professional/co-occurring/traumatic-brain-injury-ptsd.asp [Google Scholar]
- TBI: Get the Facts. (2017, April 6). Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/traumaticbraininjury/get_the_facts.html
- Todis B., & Glang A. (2008). Redefining success: Results of a qualitative study of postsecondary transition outcomes for youth with traumatic brain injury. Journal of Head Trauma Rehabilitation, 23(4), 252–263. [DOI] [PubMed] [Google Scholar]
- Tulsky D. S., Kisala P. A., Victorson D., Carlozzi N., Bushnik T., Sherer M., … Cella D. (2016). TBI-QOL: Development and calibration of item banks to measure patient reported outcomes following traumatic brain injury. Journal of Head Trauma Rehabilitation, 31(1), 40–51. https://doi.org/10.1097/HTR.0000000000000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warraich Z., & Kleim J. A. (2010). Neural plasticity: The biological substrate for neurorehabilitation. PM&R, 2(12), S208–S219. [DOI] [PubMed] [Google Scholar]
- Winans-Mitrik R. L., Hula W. D., Dickey M. W., Schumacher J. G., Swoyer B., & Doyle P. J. (2014). Description of an intensive residential aphasia treatment program: Rationale, clinical processes, and outcomes. American Journal of Speech-Language Pathology, 23(2), S330–S342. https://doi.org/10.1044/2014_AJSLP-13-0102 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2002). Towards a common language for functioning, disability and health: ICF. Retrieved from http://www.who.int/classifications/icf/icfbeginnersguide.pdf?ua=1
- Young Stroke Survivors. (2016, December 19). Retrieved from http://www.stroke.org/understand-stroke/impact-stroke/young-stroke-survivors