Abstract
The current study investigates anti-neoplastic and immunomodulatory activities of co-treatment based on bovine lactoferrin (bLF) and/or muramyl dipeptide (MDP) with or without cisplatin (Cis) in tumor-bearing mice. In the present study, bLF (100 mg/kg; orally) and MDP (0.5 mg/kg; subcutaneously) was administered alone or together. MDP or bLF was co-treated with Cis (1 mg/kg; intraperitoneally) in mice-bearing Ehrlich solid carcinoma. Tumor size, tumor mass proliferation, apoptosis using immunohistochemistry, the alteration in spleen cell proliferation, phenotype using flow cytometry and white blood cells total and differential counts were detected. Treatment with Cis or (bLF and MDP) significantly reduced tumor size, upregulated the pro-apoptotic p53 expression and downregulated the anti-apoptotic Bcl-2 and proliferative marker PCNA expression compared to non-treated tumor-bearing animals. Moreover, co-treatment of MDP and Cis significantly potentiated the reduction of the tumor size, downregulated the Bcl-2 and PCNA expression and upregulated the p53 expression compared to Cis-treated animals. While bLF and Cis co-treatment positively controlled PCNA and p53 expression compared to tumor-bearing animals, it significantly potentiated the reduction of the tumor size and downregulated the Bcl-2 expression compared to Cis-treated animals. Co-treatment of (bLF and MDP), (bLF and Cis) or (MDP and Cis) increased the spleen cell proliferation and altered the immunological profile of the CD3+CD4+, CD3+CD8+, CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells to achieve better immune response against tumor. In conclusion, co-treatments based on bLF and/or MDP are promising therapies against cancer, through their potency to control proliferation, enhance apoptosis and improve the immune status against tumor cells.
Keywords: lactoferrin, muramyl dipeptide, cisplatin, immunity, tumor, apoptosis
Graphical Abstract
Graphical Abstract.

Introduction
In different communities, cancers have been reported to be one of the main causes of death [1]. Radiotherapy, chemotherapy and surgery are the conventional strategies to treat cancers for several decades [2]. However, chemotherapy, including cisplatin (Cis), is one of the critical therapeutic approaches for cancer; the success of this therapeutic approach is limited due to several reasons such as the demonstration of drug-resistant cancer cells, lack of selectivity for the target cells and toxic adverse effects [3]. For these reasons, the development of new alternative or complementary treatment approaches to conquer the cancer cells are warranted [4–6].
Over the past years, tumor immunotherapy, which uses the immune system of the body to enhance response against the tumor, is becoming an attractive approach against cancer [7, 8]. Tumor immunotherapy could be classified into passive or active immune-based cancer therapies [9, 10]. Passive immune-based cancer therapies include the inoculation of several agents such as monoclonal antibodies, lymphocytes or dendritic cells that augment the existing anti-cancer response [11]. Active immune-based cancer therapies attempt to stimulate self-immune system to eliminate the cancer cells through specific vaccines or non-specific immunomodulatory agents [11].
Although some immunomodulatory agents are targeting the innate immunity and others stimulating the adaptive immunity, the most effective scenario is dealing with agents utilized in combination in order to stimulate both innate and adaptive immune responses against tumors. Several studies proved important roles of both innate and adaptive immune systems against tumors [12, 13]. Muramyl dipeptide (MDP) is a small component of cell walls from Mycobacterium species that can stimulate both humoral and cellular immune responses [14]. MDP is mainly bound to nucleotide-binding oligomerization domain 2 (NOD2), which is determined on different kinds of innate immune cells including monocytes and macrophages, and induce the production of many cytokines [e.g. interleukin (IL)-1, IL-6, IL-8, IL-12 and TNF-α] [15]. Therefore, it enables the activated immune cells to attack cancer cells, but not other normal cells [16]. Many reports detected several therapeutic uses for MDP and its derivatives such as anti-viral, immunostimulant and tumoricidal activities [17–21].
On the other side, lactoferrin (LF), an approximately 80-kDa iron-binding glycoprotein, is present in exocrine secretions of mammals such as breast milk, seminal fluid, tears, bronchial mucus and the neutrophil secondary granules [22–24]. Through T cells-dependent manner and activation of T helper, T cytotoxic and natural killer cells, LF inhibited the tumor progression in different cancer models [25–29]. Moreover, many studies demonstrated strong anti-tumor activities of bovine LF (bLF) on gastric cancer cells, melanoma cells and colorectal cancer cells in vitro [30–32], without any harm effects on the normal cells [33].
Based on the biological activities and mechanisms of action of MDP and bLF, we thought in this study that the combination of MDP and bLF could stimulate both innate and adaptive immune responses in order to obtain the optimal therapeutic immune response in an Ehrlich carcinoma murine model.
Materials and Methods
Experimental model
Female Swiss CD1 mice (weighted 26–30 g) were purchased from the National Research Center, Giza, Egypt. All mice were kept under designed laboratory conditions with 12-hour dark/light cycle. Standard rodent food and clean water were supplied ad libitum. The mice were acclimatized to laboratory condition for 12 days before the start of the current experiments. This study was approved by the Institutional Animal Ethical Committee, Menoufia University (approval ID: MUFS/F/IM/1/16).
Reagents and antibodies
Concanavalin A (ConA) from Canavalia ensiformis, Cis, bLF and MDP were obtained from Sigma (Sigma, St. Louis, CA, USA). Fluorescein isothiocyanate (FITC)-labeled anti-mouse CD3 mAb (clone: 17A2), FITC-labeled anti-mouse Ly6G mAb (clone: RB6-8C5), Phycoerythrin (PE)-PE.Cy5-labeled anti-mouse CD8 mAb (clone: S3–6.7), PE.Cy7-labeled anti-mouse CD69 mAb (clone: H1.2F3), Allophycocyanin (APC)-labeled anti-mouse CD4 mAb (clone: GK1.5) and APC-labeled anti-mouse CD11b mAb (clone: M1/70) (Fc Block™ were obtained from BD Bioscience company (BD Bioscience, CA, USA). Utilized reagents and chemicals were of the highest purity available.
Ehrlich ascites tumor model and experimental design
Ehrlich ascites carcinoma (EAC) cells were obtained from the National Cancer Institute, Cairo University, Egypt, and maintained by means of serial intraperitoneal (i.p.) transplantation of 0.5 × l06 viable tumor cells in 0.2 ml of saline into female Swiss CD1 mice. Tumor cell viability was detected by trypan blue assay and viable cells were counted by hemocytometer. EAC cells (2.5 × l06) were inoculated by intramuscular (i.m.) injection in the right thigh of the hind limb of each mouse in order to induce Ehrlich solid tumor (EST) on day 0 [34]. Eight groups contained nine animals per each were categorized as follows: Group I—normal, healthy control (naive) inoculated with 0.2 ml of PBS, i.m.; Group II—EST-bearing mice without treatment inoculated with EAC cells 2.5 × 106 cells in 0.2 ml of PBS/mouse on day 0 as previously mentioned; Group III—positive control Cis (1 mg/kg; i.p.) [35] injected three times each other day from day 11 to day 15; Group IV—bLF (100 mg/kg; orally) administrated 10 successive days from day 11 [25]; Group V—bLF (100 mg/kg; orally) and Cis (1 mg/kg; i.p.) injected as the same as Groups III and IV; Group VI—MDP (0.5 mg/kg; subcutaneously) injected three times each other day from day 11 [17]; Group VII—MDP (0.5 mg/kg; subcutaneously) and Cis (1 mg/kg; i.p.) injected as the same as Groups III and VI and Group VIII—bLF (100 mg/kg; orally) and MDP (0.5 mg/kg; subcutaneously) injected as the same as Groups IV and VI. On day 21, blood was collected using orbital bleeding, mixed with EDTA, and then the mice were sacrificed and spleen and right thigh muscles to be prepared for further analysis.
Tumor size determination
Starting from the day 7 of tumor induction, dimensions of the right thigh of the lower limb were measured using two-end electronic digital caliber (Switzerland), each other day till day 21 and the tumor volume was calculated [36] as follows: tumor volume (mm3) = 1/2 × (Tumor’s higher diameter × Tumor’s lower diameter2).
White blood cells total and relative differential counts
Blood samples mixed with EDTA were utilized in order to determine the white blood cell (WBC) counts as described previously [37].
Splenocyte phenotypes
The preparation and count of the splenocyte suspensions were performed [38]. The splenocyte viability was detected by trypan blue dye exclusion test. T-helper CD3+CD4+, CD3+CD4+CD69+, T-cytotoxic CD3+CD8+, CD3+CD8+CD69+ and myeloid-derived suppressor CD11b+Ly6G+ cells were analyzed with BD FACS Canto II flow cytometer using BD FACS DIVA™ software (BD Biosciences). After incubation with the indicated conjugated mAbs for 30 minutes on ice, cells were post-fixed with 0.3 ml of Cell Fix 1× (BD, Biosciences), kept at 4°C in the dark, and then the indicated surface marker expression was subjected to the flow cytometer.
Splenocyte proliferation assay
Spleen cells were washed twice in PBS and 5 × 107 cells were resuspended in PBS and labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (BioLegend, USA) for 8 minutes at 37°C. Then, 10% of FBS was used to stop the reaction. Cells were washed three times in PBS. The CFSE-labeled cells were seeded onto six-well plates with or without the Con-A and cultured for 3 days. Data were analyzed using a BD FACS Canto II flow cytometer and Flow Jo software (BD Biosciences). In order to estimate the Con-A-induced spleen cell proliferation, the percentage of unstimulated spleen cells in the absence of the ConA was subtracted from the percentage of ConA-stimulated cells [39].
Immunohistochemical examination
Right thigh muscle samples from examined mice were fixed with 10% neutral formalin and reacted specifically with proliferating cell nuclear antigen (PCNA) p53 (Thermo Fisher Scientific, USA) and Bcl-2 (BioGenex, USA) monoclonal antibodies according to the manufacturer’s instructions. Immunohistochemistry (IHC) was performed [40]. In order to quantify the IHC, 1 × 103 cells from at least five separate tissue sections were analyzed using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. For statistical analysis, the SPSS (IBM SPSS statistics for Windows Version 22, Armonk, NY) computer program was used. Data were evaluated by analysis of variance (ANOVA) test followed by post hoc analysis of group differences that was accomplished by the least significant difference (LSD) test. Correlation coefficients were calculated using the Pearson rank test; P < 0.05 was considered to be statistically significant.
Results
Co-treatment with bLF and MDP reduced tumor size
Co-treatment of EST-bearing mice with bLF and MDP significantly (P < 0.05) decreased the tumor size from day (d13) to the end of the experiment (d21) as compared to EST-non-treated mice (Fig. 1).
Figure 1.

Effect of co-treatment of bLF, MDP or each agent in combination with chemotherapeutic agent cisplatin (Cis) on tumor growth in EST-bearing mice. Data are expressed as the mean ± SD, n = 7. Statistical difference was calculated with an ANOVA and follow-up test (LSD). *P < 0.05 indicates significant difference compared to EST group.
Moreover, bLF or MDP mono-treatment and co-treatment (bLF and Cis) or (MDP and Cis) significantly (P < 0.05) lowered the tumor size compared to EST-non-treated animals. On the other hand, no significant changes were observed in the tumor size when compared to Cis-treated mice from day (d15) to the end of the experiment (d21). Co-treatment of (bLF and Cis) or (MDP and Cis) showed lower tumor size compared to co-treatment of bLF and MDP or Cis-mono-treatment. The current data indicate that bLF and MDP co-treatment have a significant anti-neoplastic potency.
Co-treatment with bLF and MDP decrease the tumor cells proliferation and augment their apoptosis
In order to examine tumor proliferation activities, immunohistochemistry was utilized to evaluate the related cell cycle antigen expression, PCNA (Table 1; Fig. 2). Cis or bLF mono-treatment significantly reduced (P < 0.05) the PCNA expression compared to EST-non-treated mice (Table 1).
Table 1.
Effect of co-treatment of bLF, MDP or each combined with Cis on the percentage of positively stained cells of proliferation and apoptosis-related proteins detected by immunohistochemistry
| PCNA % | Bcl-2% | P53% | |
|---|---|---|---|
| EST | 24.77 ± 0.25 | 23.1 ± 0.35 | 0.6 ± 0.1 |
| Cis | 15.2 ± 0.15* | 17.2 ± 0.37* | 17.73 ± 0.2* |
| Cis + bLF | 13.5 ± 2.5* | 0.67 ± 0.21*,# | 11.18 ± 2.78*,# |
| Cis + MDP | 12.06 ± 0.05*,# | 1.06 ± 0.05*,# | 25.26 ± 0.55*,# |
| bLF | 15.9 ± 2.52* | 0.67 ± 0.15*,# | 16.34 ± 1.7*,# |
| MDP | 9.1 ± 1.39*,# | 1.91 ± 0.1*,# | 24.2 ± 0.26*,# |
| MDP + bLF | 11.8 ± 2.3*,# | 0.43 ± 0.07*,# | 16.1 ± 0.76* |
On day 21, thin sections in the right thigh muscles were prepared for immunohistochemical examination. Data are presented as mean ± SD, n = 5.
* P < 0.05 indicates significant difference compared to EST group.
# P < 0.05 indicates significant difference compared to Cis-treated mice.
Figure 2.
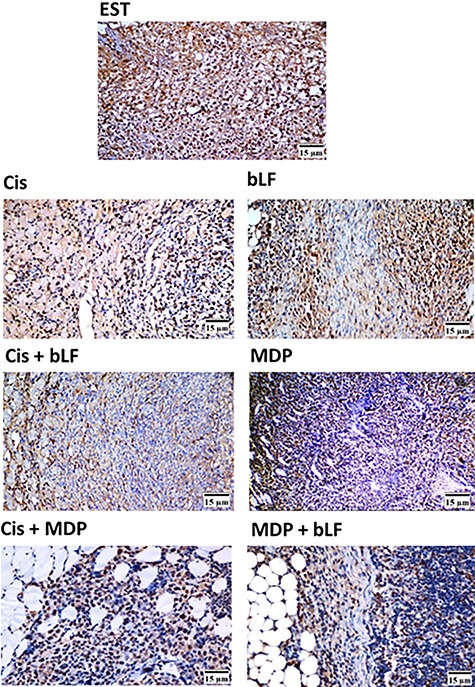
Representative light micrographs of the proliferation marker PCNA protein expression in tumor mass after co-treatment with bLF and MDP. On day 21, thin sections in the right thigh muscles were prepared for immunohistochemical examination. The brown stain referred to positive immunohistochemical reaction; blue stain is hematoxylin counter satin (×400), n = 1.
Interestingly, co-treatment of EST-bearing mice with bLF and MDP produced a marked significant inhibition (P < 0.05) in the PCNA expression compared to EST-non-treated mice or Cis-treated mice (Table 1; Fig. 2). MDP mono-treatment showed the lowest PCNA expression compared to the different treated groups (Table 1; Fig. 2). Co-treatment (bLF and Cis) or (MDP and Cis) showed remarkable reduction in the PCNA expression compared to Cis-treated animals (Table 1).
Because it is well known that different pro- and anti-apoptotic proteins control apoptosis, the current study examined the capacity of the co-treatment based on bLF and/or MDP to modulate the expression of growth arresting and pro-apoptotic protein, p53, and anti-apoptotic protein, Bcl-2, in EST-bearing mice (Table 1; Figs 3 and 4). Mono-treatment of Cis, MDP or bLF demonstrated marked significant increase (P < 0.05) in the expression of p53 associated with an obvious significant decrease (P < 0.05) in the Bcl-2 expression compared to EST-non-treated animals. Co-treatment (bLF and MDP), (bLF and Cis) or (MDP and Cis) significantly (P < 0.05) potentiated the reduction in the expression of Bcl-2 when compared to Cis-treated mice (Table 1, Fig. 4). However, bLF and MDP co-treatment illustrated almost similar elevation in the p53 expression when compared to Cis-treated animals (Table 1, Fig. 3); only MDP and Cis co-treatment significantly (P < 0.05) potentiated the elevation in the p53 expression when compared to Cis-treated mice (Table 1; Fig. 3). From the above results, co-treatment (bLF and MDP), (bLF and Cis) or (MDP and Cis) of anti-neoplastic potency might be attributed to their abilities to inhibit the tumor cells proliferation and induce their apoptosis.
Figure 3.

Representative light micrographs of the pro-apoptotic p53 protein expression in tumor mass after co-treatment with bLF and MDP. On day 21, thin sections in the right thigh muscles were prepared for immunohistochemical examination. The brown stain referred to positive immunohistochemical reaction; blue stain is hematoxylin counter satin (×400), n = 1.
Figure 4.

Representative light micrographs of the anti-apoptotic Bcl-2 protein expression in tumor mass after co-treatment with bLF and MDP. On day 21, thin sections in the right thigh muscles were prepared for immunohistochemical examination. The brown stain referred to positive immunohistochemical reaction; blue stain is hematoxylin counter satin (×400), n = 1.
Co-treatment with bLF and MDP controlled the total and relative differential WBC counts
An elevation was detected in the total WBC count from EST-bearing mice compared to the normal healthy naïve mice (Table 2). Moreover, significant increase (P < 0.05) in the relative granulocyte count accompanied by a significant reduction (P < 0.05) in the relative lymphocyte count was observed in EST-bearing mice or Cis-treated mice compared to normal healthy mice. Treatment with MDP alone or bLF and MDP co-treatment caused significant (P < 0.05) increase in the total WBC count and the relative granulocyte count associated with a decrease in the relative lymphocyte count compared to the EST group.
Table 2.
Effect of co-treatment of bLF, MDP or each combined with Cis on the total and the differential leukocyte counts in EST-bearing mice
| WBCs (103/mm3) | Granulocytes (%) | Lymphocytes %) | Monocytes (%) | |
|---|---|---|---|---|
| Naïve | 7.56 ± 2.3 | 34.6 ± 3.7 | 60.4 ± 2.9 | 5.0 ± 1.0 |
| EST | 8.64 ± 2.3 | 53.6 ± 8.32 | 41.2 ± 7.8 | 5.2 ± 0.83 |
| Cis | 6.9 ± 0.15 | 59.8 ± 1.7* | 34.6 ± 1.14* | 5.6 ± 1.14 |
| Cis + bLF | 6.94 ± 0.11 | 51.6 ± 2.3 | 43.8 ± 1.3# | 5.6 ± 1.14 |
| Cis + MDP | 9.28 ± 0.94 | 59.2 ± 4.43* | 36.2 ± 4.38* | 4.6 ± 1.67 |
| bLF | 10.7 ± 3.63*,# | 41.6 ± 2.4*,# | 53.2 ± 2.16*,# | 5.2 ± 0.83 |
| MDP | 14.04 ± 2.95*,# | 56 ± 5.01* | 39.6 ± 5.02# | 4.4 ± 0.5 |
| MDP + bLF | 10.08 ± 0.4*,# | 58.8 ± 5.5* | 35.6 ± 4.3* | 4.8 ± 2.2 |
On day 21, blood was collected using orbital bleeding. Data are presented as mean ± SD, n = 7.
* P < 0.05 indicates significant difference compared to EST group.
# P < 0.05 indicates significant difference compared to Cis-treated mice.
On the contrary, treatment with bLF alone caused significant (P < 0.05) elevations in the total WBC count and the relative lymphocyte count accompanied by significant (P < 0.05) decrease in the relative granulocyte count compared to the EST group or Cis-treated mice. No significant changes were recorded in the relative monocyte counts. Co-treatment of MDP and Cis minimally increase the total WBC count and the relative lymphocyte count compared to the Cis-treated mice. Co-treatment (bLF and Cis) tend to partially normalize the total and differential WBC counts.
Co-treatment with bLF and MDP immunomodulatory potency on spleen cells
A significant sharp reduction (P < 0.05) in the percentage of splenocyte proliferation was observed after Cis mono-treatment compared to the normal healthy mice or EST-bearing mice. Co-treatment (bLF and Cis) or (MDP and Cis) significantly (P < 0.05) improved spleen cell proliferation percentage when compared to Cis-treated mice. Interestingly, treatment with bLF or MDP alone or bLF and MDP co-treatment resulted in significant (P < 0.05) elevations in the spleen cell proliferation percentage compared to Cis-treated mice, EST group or even normal healthy control mice (Fig. 5).
Figure 5.

Effect of co-treatment of bLF, MDP or each agent in combination with cisplatin on splenocyte proliferation rate in EST-bearing mice. On day 21, spleen was collected and 5 × 107 spleen cells were labeled with 1 μM CFSE. The data obtained from four individual mice per group. Asterisk denotes P < 0.05 statistically compared with EST control group. Hash denotes P < 0.05 statistically compared with Cis-treated group.
In order to examine the effect of co-treatment of bLF and/or MDP in changing spleen cell immunological profile, the expression of CD3+CD4+, CD3+CD8+, CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells (Table 3, Fig. 6) after treatment with (bLF and MDP), (bLF and Cis) or (MDP and Cis) were determined using flow cytometry. A significant reduction (P < 0.05) was detected in the percentages of CD3+CD4+ and CD3+CD8+ cells from EST-bearing mice compared to the normal healthy mice. On the other hand, the percentages of CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells were found to be increased significantly (P < 0.05) in EST group compared to the healthy naive mice. Cis-treated mice showed minimal significant (P < 0.05) elevation the percentage of CD3+CD8+ accompanied by a significant reduction (P < 0.05) in the percentage of CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells compared to the EST-bearing mice.
Table 3.
Effect of co-treatment of bLF, MDP or each agent in combination with Cis on splenocytes phenotyping in EST-bearing mice
| CD3+CD4+ | CD3+CD8+ | CD3+CD8+ CD69+ | CD3+CD4+ CD69+ | CD11b+Ly6G+ | |
|---|---|---|---|---|---|
| Naïve | 36.9 ± 0.08 | 13.9 ± 2.05 | 3.86 ± 1.5 | 0.075 ± 0.05a | 0.23 ± 0.04 |
| EST | 13.6 ± 1.5 | 8.06 ± 1.6 | 32.6 ± 3.5 | 15.75 ± 2.1b | 15.4 ± 2.9 |
| Cis | 13.7 ± 0.5 | 11.2 ± 1.1* | 25.9 ± 2.7* | 6.7 ± 1.6* | 7.1 ± 2.5* |
| Cis + bLF | 29 ± 2.8*,# | 30.02 ± 2.8*,# | 3.4 ± 0.7*,# | 1.8 ± 0.4*,# | 0.12 ± 0.05*,# |
| Cis + MDP | 15.6 ± 3.3* | 13.5 ± 2.7* | 2.3 ± 0.2*,# | 0.2 ± 0.08*,# | 0.11 ± 0.02*,# |
| bLF | 18.2 ± 3.8*,# | 10.5 ± 1.6* | 1.9 ± 0.47*,# | 1.25 ± 0.05*,# | 0.3 ± 0.1*,# |
| MDP | 18.9 ± 4.4*,# | 14 ± 3.04* | 6.5 ± 1.7*,# | 0.2 ± 0.12*,# | 0.12 ± 0.05*,# |
| MDP + bLF | 18.8 ± 1.*,# | 28.2 ± 5.85*,# | 5.5 ± 0.48*,# | 0.1 ± 0.0*,# | 0.0 ± 0.0*,# |
On day 21, spleens were collected and 1 × 106 spleen cells were stained with the indicated surface marker and subjected to the flow cytometer. Data were presented as mean ± SD, n = 4.
* P < 0.05 indicates significant difference compared to EST group.
# P < 0.05 indicates significant difference compared to Cis-treated mice.
Figure 6.

Flow cytometric histogram plots of splenocytes phenotyping in EST-bearing mice showing the effect of co-treatment of bLF, MDP or each agent in combination with cisplatin. On day 21, spleens were collected and 1 × 106 spleen cells were stained with the indicated surface marker and subjected to the flow cytometer. Numbers correspond to the percentage of double-positive fluorescence-labeled cells, n = 1.
Moreover, treatment with bLF or MDP alone or bLF and MDP co-treatment resulted in a significant (P < 0.05) increases in CD3+CD4+cell percentage, associated with a significant (P < 0.05) decreases in CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cell percentages compared to the EST-bearing mice and Cis-treated mice. Furthermore, bLF and MDP co-treatment caused a significant elevation (P < 0.05) in the percentages of CD3+CD8+ cells compared to the EST-bearing mice and Cis-treated mice. Co-treatment (bLF and Cis) or (MDP and Cis) significantly (P < 0.05) improved CD3+CD4+, CD3+CD8+, CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells compared to the Cis-treated mice. Altogether, bLF and MDP co-treatment exhibit immunomodulatory effects in EST-bearing mice.
In order to link the bLF/MDP mono-treatment or combinational treatments with immune cell-mediated anti-tumor effect, we detect the correlation between immune cell changes and bLF/MDP anti-tumor effects (Table 4). The tumor size was significantly correlated with the percentage of CD3+CD4+ cells after (bLF and MDP) or (bLF and Cis) co-treatment, while they were highly correlated after (bLF) mono-treatment without a significant difference. In mice co-treated with (bLF and MDP) or mono-treated with (bLF), the expression of Bcl2 was significantly correlated with the percentage of CD3+CD8+ cells. Moreover, the expression of Bcl2 was significantly correlated with the percentage of CD3+CD4+ cells after (bLF) mono-treatment. The expression of Bcl2 was also significantly correlated with the percentage of CD3+CD8+CD69+ cells in mice co-treated with after (bLF and Cis), while they were highly correlated after (MDP and Cis) co-treatment without any significant difference. Finally, the expression of p53 was significantly correlated with the percentage of CD3+CD8+ cells in mice co-treated with (bLF and Cis) or (MDP and Cis), while they were highly correlated after (MDP) mono-treatment without any significant difference. On the level of PCNA expression, correlation was detected in mice mono-treated with (bLF) without any significant difference (data not shown).
Table 4.
Correlation between immune cell changes and anti-tumor effect of bLF and/or MDP
| bLF | MDP | bLF + MDP | Cis + bLF | Cis + MDP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | P value | R | P value | R | P value | R | P value | R | P value | |
| Tumor size vs. CD3+CD4+ | 0.917 | 0.08 | 0.719 | 0.28 | 0.951** | 0.045 | 0.987** | 0.01 | 0.414 | 0.58 |
| Bcl-2 vs. CD3+CD8+ | 0.991** | 0.009 | 0.431 | 0.56 | 0.979** | 0.02 | 0.861 | 0.13 | 0.302 | 0.69 |
| Bcl-2 vs. CD3+CD4+ | 0.989** | 0.01 | 0.298 | 0.7 | 0.298 | 0.7 | 0.126 | 0.87 | 0.161 | 0.83 |
| Bcl-2 vs. CD3+CD8+CD69+ | 0.381 | 0.61 | 0.601 | 0.39 | 0.655 | 034 | 0.957** | 0.04 | 0.902 | 0.09 |
| p53 vs. CD3+CD8+ | 0.489 | 0.51 | 0.901 | 0.09 | 0.245 | 0.75 | 0.951** | 0.045 | 0.957** | 0.045 |
R = Pearson correlation coefficient.
** P < 0.05.
Discussion
In this study, bLF and MDP co-treatment potentiated anti-tumor effects in EST-bearing mice through decreasing the tumor size without any significant difference when compared to Cis-treated mice from day 15 to the end of the current experiment. A similar pattern was demonstrated after treatment with bLF or MDP alone in EST-bearing mice. Moreover, bLF or MDP in combination with Cis enhanced the chemotherapy anti-tumor efficacy in EST-bearing mice through reduction of the tumor size without any significant differences when compared to Cis-treated mice from day 17 to day 21. The anti-neoplastic properties of bLF or MDP and its derivatives were recorded previously [14, 17, 32, 41–43].
Uncontrolled and undesirable multiplication and escaping from the apoptotic mechanisms are the main characteristics of tumor cells [44]. The current study investigated the tumor cell proliferation using PCNA expression and apoptosis using p53 and Bcl-2 to distinguish the reasons behind the anti-neoplastic properties of bLF- and/or MDP-based therapies in EST-bearing animals. In the bLF and MDP co-treated mice, significant upregulation in the pro-apoptotic p53 expression accompanied by a significant downregulation in the anti-apoptotic Bcl-2 and PCNA expression was illustrated compared to the EST-bearing mice. The significant downregulation in the anti-apoptotic protein Bcl-2 and PCNA expression after co-treatment with bLF and MDP was obviously significant compared to the Cis-treated mice. A significant upregulation in the pro-apoptotic p53 expression accompanied by a significant downregulation in the anti-apoptotic Bcl-2 and PCNA expression was illustrated after bLF mono-treatment or bLF and Cis co-treatment compared to the EST-bearing mice. Marked significant downregulation in the anti-apoptotic Bcl-2 expression after bLF mono-treatment or bLF and Cis co-treatment was demonstrated compared to the Cis-treated mice. Interestingly, the proliferative and anti-apoptotic expression of the tumor cells were significantly reduced in MDP mono-treatment or (MDP and Cis) co-treated mice, while the pro-apoptotic expression was significantly augmented as compared to EST-bearing mice or the Cis-treated mice. Consistent with the present data, MDP as NOD2 agonist, inhibited oral squamous cell carcinoma (OSCC) proliferation and induced their apoptosis [45].
NOD2, as well as NOD1, undergoes conformational alterations upon binding with its ligands, resulting in self-oligomerization through the central NOD domain and binding to receptor-interacting protein-like interacting caspase-like apoptosis regulatory protein kinase, a serine threonine kinase that leads to nuclear factor-kappa B (NF-κB) activation, which leads to autophagy, apoptosis and inflammation [46, 47]. In the same way, bLF exerted antitumor activities through induced apoptosis and reduced the viability of colon cancer cells (HT-29) [32]. Luzi et al. [48] reported that iron-free bLF (apo-bLF) triggered apoptosis of human epithelial cancer (HeLa) cells through the modulation of various apoptotic regulators including Bax, Bcl-2, Sirt1, Mcl-1, PARP-1 and caspase activation. bLF suppressed the growth of different breast cancer cells, such as MCF-7, Hs578T, MDA-MB-231 and T-47D, via the induction of cell cycle arrest and the inhibition of mammalian target of rapamycin (mTOR) signaling [49]. Furthermore, bLF selectively inhibited OSCC proliferation via mTOR/S6K and JAK/STAT3 pathways and enhanced their apoptosis [43]. bLF selectively enhanced apoptosis of highly metastatic breast cancer cells (MDA-MB-231 and Hs 578T) via its ability to inhibit the plasmalemmal vacuolar H+-ATPase [50]. Potentially, the detected effects of bLF and/or MDP on the tumor cell proliferation (PCNA expression) and apoptosis (p53 and Bcl-2 expression) supporting their anti-neoplastic activities.
Immune response plays critical role in the identification and elimination of the tumor cells [51]. Several reports emphasized the involvement of T helper (CD3+CD4+) and T-cytotoxic (CD3+CD8+) cells in the vanishing of the antigen-specific tumor [52–54]. On the other side, tumor cells have different mechanisms to escape or evade from the immune response. These mechanisms include the suppression of the protective anti-tumor immunity through the expansion of different immune subsets such as CD69+, CD4+CD25+ and CD11b+Ly6G+ cells in the tumor microenvironment [55–59]. CD69 activation plays critical role in enhancing the tumor-infiltrating T-cell exhaustion, resulting in the production of T-reg cells (CD4+CD25+), and inducing the tumor progression [60–62]. In tumor-bearing animals, anti-CD69 mAb administration obviously reduced the exhaustion of T-cells and inhibited the tumor progression [62].
In the present study, the decrease in the percentage of CD3+CD4+ and CD3+CD8+ cells in the spleen of EST-bearing mice was associated with an expansion and elevation in the percentage of CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells compared to the healthy naive mice. Furthermore, Cis treatment resulted in a significant suppression in the relative lymphocyte count, spleen cell proliferation, CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cell percentages associated with a significant increase in the relative granulocyte count and CD3+CD8+ cells when compared to the EST-bearing animals. Miller et al. [63] recorded that treatment with Cis had a lymphopenic effect as it increased the number of neutrophils in blood and decreased the number of the cells of the bone marrow. The immunosuppressive effect of Cis in albino mice was recently reported [64].
In the present study, bLF and MDP co-treatment improved CD3+CD4+, CD3+CD8+, CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cell percentages compared to Cis-treated animals. Where the increase in the percentages of CD3+CD4+ and CD3+CD8+ cells was associated with a decrease in the percentages of CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells. bLF or MDP mono-treatment had a partial tendency to normalize CD3+CD4+, CD3+CD8+, CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cell percentages. In the same way, co-treatment (bLF and Cis) or (MDP and Cis) significantly decreased CD3+CD4+CD69+, CD3+CD8+CD69+ and CD11b+Ly6G+ cells compared to the Cis-treated mice. While bLF and Cis co-treatment significantly elevated the percentage of CD3+CD4+ and CD3+CD8+ cell population, compared to the Cis-treated mice, MDP and Cis co-treatment increased the percentage of CD3+CD4+ and CD3+CD8+ cells without any significant difference compared to the Cis-treated mice. On the level of spleen cell proliferation in all treatments (bLF and MDP co-treatment, bLF and Cis co-treatment, MDP and Cis co-treatment, bLF or MDP mono-treatment) significantly increased the spleen cell proliferation compared to the Cis-treated mice. Regarding WBCs total and differential counts while bLF and MDP co-treatment or MDP and Cis co-treatment exhibited similar Cis effects on the differential counts, these co-treatments elevated total WBC count. Co-treatment of bLF and Cis showed a tendency to normalize the differential counts. Altogether, the immunomodulatory capabilities of bLF or MDP might support their anti-neoplastic activities. Consistent with the current observations, as immunomodulator agent, LF can modulate and support the proliferation, differentiation, maturation, activation and function of immune cells through the activation of NF-κB and MAP kinase [65–67]. Oral treatment with recombinant human LF inhibited head and neck squamous cell carcinoma in mice via T cell-dependent manner [29]. Moreover, LF treatment is associated with strong Th1-response, upregulation and/or enhanced activation of CD4+, CD8+ T-cells and natural killer cells in different cancer models [25–28].
Muramyl peptides have the ability to modulate the immune response through stimulation of both humoral and cell-mediated immunity mainly through their abilities to restore the hematopoiesis and control cytokine production [68–70]. Furthermore, MDP together with other muropeptides enhances the immunity by elevating the interferon (IFN)-γ and other cytokine release, potentiating the proliferation and differentiation of lymphocytes, which mainly defend the host bodies against foreign invaders [14, 71–73]. Moreover, conjugation of MDP to maleylated bovine serum albumin activated anti-tumor efficacy of macrophages and stimulated the secretion of IL-12 [74], which in turn could enhance IFN-𝛾 production, and generate the cytotoxic effects of CD4+ and CD8+ lymphocytes [75]. In acute leukemia children, co-administration of MDP and TNF-α modulated differentiation, maturity of dendritic cells (DC) and anti-tumor effect of DC through inducing T-cell proliferation, producing higher levels of IFN-γ and presenting the antigens more effective to cytotoxic T-cells in a dose-dependent manner [76]. Tada et al. [77] demonstrated IL-12 release followed by synergistic production of IFN-γ from T-cells after stimulation of DC by MDP plus lipid A and suggested that DC-derived IL-12 enhanced Th1 activation and/or differentiation. MDP adjuvant, N-acetyl-D glucosaminyl-(beta 1–4)-N-acetylmuramyl-L-alanyl-D-isoglutamine, ability to induce class I-restricted, CD8+ cytotoxic T-cells response was established [78]. At the level of immune cell proliferation, MDP oral administration into mice enhanced proliferation of lymphocytes derived from Peyer’s patches to various mitogens, produced more immunoglobulins than non-treated cells [79].
In conclusion, bLF and/or MDP (combinatorial treatment or mono-treatment) anti-neoplastic properties may be rendered to their abilities to control the tumor cell proliferation, augment their apoptosis and modulate the immune response in the tumor-bearing host. In a successful way, bLF and MDP sensitized Cis-anti-neoplastic potency and minimized its detrimental effects. Further studies using different doses of bLF and MDP are required to emphasize their anti-tumor synergistic effects.
Authors’ contributions
H.M.I. and M.L.S. designed the study. D.S.M., H.M.I., A.H.M. and M.L.S. performed most of the experiments, analyzed and interpreted the data. H.M.I. wrote the first version of the manuscript. H.M.I., A.H.M., M.L.S., G.Y.O. and D.S.M. assisted during the acquisition, analysis and interpretation of data and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgements
The authors thank Dr Amany E. Nofal, Zoology Department, Faculty of Science, Menoufia University, for her technical assistance during IHC section quantification. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest
The authors declare no competing financial interests.
References
- 1. Lacombe J, Armstrong MEG, Wright FL et al. The impact of physical activity and an additional behavioural risk factor on cardiovascular disease, cancer and all-cause mortality: a systematic review. BMC Public Health 2019;19:900. doi: 10.1186/s12889-019-7030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang H, Chen J. Current status and future directions of cancer immunotherapy. J Cancer 2018;9:1773–81. doi: 10.7150/jca.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Padma VV. An overview of targeted cancer therapy. Biomedicine 2015;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salem ML, Shoukry NM, Teleb WK et al. In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. Springerplus 2016;5:570. doi: 10.1186/s40064-016-2269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adusumilli PS, Cha E, Cornfeld M et al. New cancer immunotherapy agents in development: a report from an associated program of the 31st Annual Meeting of the Society for Immunotherapy of Cancer, 2016. J Immunother Cancer 2017;5:50. doi: 10.1186/s40425-017-0253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim HM, Abdul Ghaffar FR, El-Elaimy IA et al. Antitumor and immune-modulatory efficacy of dual-treatment based on levamisole and/or taurine in Ehrlich ascites carcinoma-bearing mice. Biomed Pharmacother 2018;106:43–9. [DOI] [PubMed] [Google Scholar]
- 7. Subramaniam DS, Liu SV, Giaccone G. Novel approaches in cancer immunotherapy. Discov Med 2016;21:267–74. [PubMed] [Google Scholar]
- 8. Choi BK, Kim SH, Kim YH et al. Cancer immunotherapy using tumor antigen-reactive T cells. Immunotherapy 2018;10:235–45. doi: 10.2217/imt-2017-0130. [DOI] [PubMed] [Google Scholar]
- 9. Dustin ML. Cancer immunotherapy: killers on sterols. Nature 2016;531:583–4. doi: 10.1038/nature17310. [DOI] [PubMed] [Google Scholar]
- 10. Munhoz RR, Postow MA. Recent advances in understanding antitumor immunity. F1000 Res 2016;5:2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kakimi K, Karasaki T, Matsushita H et al. Advances in personalized cancer immunotherapy. Breast Cancer 2017;24:16–24. doi: 10.1007/s12282-016-0688-1. [DOI] [PubMed] [Google Scholar]
- 12. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 2004;4:11–22. [DOI] [PubMed] [Google Scholar]
- 13. Lakshmi-Narendra B, Eshvendar-Reddy K, Shantikumar S et al. Immune system: a double-edged sword in cancer. Inflamm Res 2013;62:823–34. doi: 10.1007/s00011-013-0645-9. [DOI] [PubMed] [Google Scholar]
- 14. Ogawa C, Liu Y-J, Kobayashi KS. Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr Bioact Compd 2011;7:180–97. doi: 10.2174/157340711796817913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inohara N, Ogura Y, Fontalba A et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s disease. J Biol Chem 2003;278:5509–12. [DOI] [PubMed] [Google Scholar]
- 16. Thundimadathil J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids 2012;2012:967347. doi: 10.1155/2012/967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bloksma N, Hothuis F, Willers J. Muramyl dipeptide is a powerful potentiator of the antitumor action of various tumor necrotizing agents. Cancer Immunol Immunother 1984;17:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Killion JJ, Fidler IJ. Therapy of cancer metastasis by tumoricidal activation of tissue macrophages using liposome-encapsulated immunomodulators. Pharmacol Ther 1998;78:141–54. [DOI] [PubMed] [Google Scholar]
- 19. Darcissac EC, Truong MJ, Dewulf J et al. The synthetic immunomodulator murabutide controls human immunodeficiency virus type 1 replication at multiple levels in macrophages and dendritic cells. J Virol 2000;74:7794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Srividya S, Roy RP, Basu SK et al. Selective activation of antitumor activity of macrophages by the delivery of muramyl dipeptide using a novel polynucleotide-based carrier recognized by scavenger receptors. Biochem Biophys Res Commun 2000;268:772–7. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Yu J, Xu S et al. Chemical conjugation of muramyl dipeptide and paclitaxel to explore the combination of immunotherapy and chemotherapy for cancer. Glycoconj J 2008;25:415–25. [DOI] [PubMed] [Google Scholar]
- 22. Masson PL, Heremans JF, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med 1969;130:643–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farnaud S, Evans RW. Lactoferrin – a multifunctional protein with antimicrobial properties Mol. Immunology 2003;40:395–405. [DOI] [PubMed] [Google Scholar]
- 24. Lonnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care 2009;12:293–7. doi: 10.1097/MCO.0b013e328328d13e. [DOI] [PubMed] [Google Scholar]
- 25. Iigo M, Kuhara T, Ushida Y et al. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin Exp Metastasis 1999;17:35–40. [DOI] [PubMed] [Google Scholar]
- 26. Kuhara T, Iigo M, Itoh T et al. Orally administered lactoferrin exerts an anti-metastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr Cancer 2000;38:192–9. [DOI] [PubMed] [Google Scholar]
- 27. Wang WP, Iigo M, Sato J et al. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn J Cancer Res 2000;91:1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer R, Debbabi H, Dubarry M et al. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol 2006;84:303–11. [DOI] [PubMed] [Google Scholar]
- 29. Wolf JS, Li G, Varadhachary A et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin Cancer Res 2007;13:1601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan WR, Chen PW, Chen YL et al. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J Dairy Sci 2013;96:7511–20. doi: 10.3168/jds.2013-7285. [DOI] [PubMed] [Google Scholar]
- 31. Riedl S, Rinner B, Schaider H et al. Killing of melanoma cells and their metastases by human lactoferricin derivatives requires interaction with the cancer marker phosphatidylserine. Biometals 2014;27:981–97. doi: 10.1007/s10534-014-9749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang R, Lonnerdal B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem Cell Biol 2017;95:99–109. doi: 10.1139/bcb-2016-0094. [DOI] [PubMed] [Google Scholar]
- 33. Mader JS, Salsman J, Conrad DM et al. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol Cancer Ther 2005;4:612–24. doi: 10.1158/1535-7163.MCT-04-0077. [DOI] [PubMed] [Google Scholar]
- 34. Noaman E, Badr El-Din K, Bibars M et al. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett 2008;268:348–59. [DOI] [PubMed] [Google Scholar]
- 35. Chen PC, Chen CC, Ker YB et al. Anti-metastatic effects of antrodan with and without cisplatin on Lewis lung carcinomas in a mouse xenograft model. Int J Mol Sci 2018;19:1565. doi: 10.3390/ijms19061565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goto T, Nishi T, Tamura T et al. Highly efficient electro-gene therapy of solid tumor by using an expression plasmid for the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A 2000;97:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dacie SJV, Lewis SM. Practical Hematology. UK: Churchill Livingstone, 1984, 24–45. [Google Scholar]
- 38. Ibrahim HM, Xuan X, Nishikawa Y. Toxoplasma gondii cyclophilin 18 regulates the proliferation and migration of murine macrophages and spleen cells. Clin Vaccine Immunol 2010;17:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ibrahim HM, El-Elaimy IA, Saad Eldien HM et al. Blocking type I interferon signaling rescues lymphocytes from oxidative stress, exhaustion and apoptosis in a streptozotocin-induced mouse model of type I diabetes. Oxid Med Cell Longev 2013;2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arriazu R, Pozuelo JM, Henriques-Gil N et al. Immunohistochemical study of cell proliferation, Bcl-2, p53, and caspase-3 expression on preneoplastic changes induced by cadmium and zinc chloride in the ventral rat prostate. J Histochem Cytochem 2006;54:981–90. [DOI] [PubMed] [Google Scholar]
- 41. Worth LL, Jia SF, An T et al. ImmTher, a lipophilic disaccharide derivative of muramyl dipeptide, up-regulates specific monocyte cytokine genes and activates monocyte-mediated tumoricidal activity. Cancer Immunol Immunother 1999;48:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arias M, Hilchie AL, Haney EF et al. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem Cell Biol 2017;95:91–8. doi: 10.1139/bcb-2016-0175. [DOI] [PubMed] [Google Scholar]
- 43. Chea C, Miyauchi M, Inubushi T et al. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem Biophys Res Commun 2018;507:142–7. doi: 10.1016/j.bbrc.2018.10.193. [DOI] [PubMed] [Google Scholar]
- 44. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 45. Yoon HE, Ahn MY, Kwon SM et al. Nucleotide-binding oligomerization domain 2 (NOD2) activation induces apoptosis of human oral squamous cell carcinoma cells. J Oral Pathol Med 2016;45:262–7. doi: 10.1111/jop.12354. [DOI] [PubMed] [Google Scholar]
- 46. Inohara N, Koseki T, Lin J et al. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem 2000;275:27823–31. [DOI] [PubMed] [Google Scholar]
- 47. Yamaguchi N, Suzuki Y, Mahbub MH et al. The different roles of innate immune receptors in inflammation and carcinogenesis between races. Environ Health Prev Med 2017;22:70. doi: 10.1186/s12199-017-0678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luzi C, Brisdelli F, Iorio R et al. Apoptotic effects of bovine apo-lactoferrin on HeLa tumor cells. Cell Biochem Funct 2017;5:33–41. doi: 10.1002/cbf.3242. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Nicolau A, Lima CF et al. Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells. Nutr Cancer 2014;66:1371–85. doi: 10.1080/01635581.2014.956260. [DOI] [PubMed] [Google Scholar]
- 50. Pereira CS, Guedes JP, Gonçalves M et al. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget 2016;7:62144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vesely MD, Kershaw MH, Schreiber RD et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–71. [DOI] [PubMed] [Google Scholar]
- 52. Segura JA, Barbero LG, Márquez J. Ehrlich ascites tumour unbalances splenic cell populations and reduces responsiveness of T cells to Staphylococcus aureus enterotoxin B stimulation. Immunol Lett 2000;74:111–5. [DOI] [PubMed] [Google Scholar]
- 53. Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol 2001;167:6497–502. [DOI] [PubMed] [Google Scholar]
- 54. Shankaran V, Ikeda H, Bruce AT et al. IFN gamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107–11. [DOI] [PubMed] [Google Scholar]
- 55. Hiura T, Kagamu H, Miura S et al. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J Immunol 2005;175:5058–66. [DOI] [PubMed] [Google Scholar]
- 56. Liu Z, Kim JH, Falo LD Jr et al. Tumor regulatory T cells potently abrogate antitumor immunity. J Immunol 2009;182:6160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naga-Anusha P, Siddiqui A, Hima-Bindu A. Immuno defense mechanism against tumors. J Cancer Sci Ther 2011;S17:1–5. doi: 10.4172/1948-5956.S17-005. [DOI] [Google Scholar]
- 58. Källberg E, Stenström M, Liberg D et al. CD11b+Ly6C++Ly6G- cells show distinct function in mice with chronic inflammation or tumor burden. BMC Immunol 2012;13:69. doi: 10.1186/1471-2172-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moran AE, Polesso F, Weinberg AD. Immunotherapy expands and maintains the function of high-affinity tumor-infiltrating CD8 T cells in situ. J Immunol 2016;197:2509–21. doi: 10.4049/jimmunol.1502659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu J, Feng A, Sun J et al. Increased CD4+CD69+CD25- T cells in patients with hepatocellular carcinoma are associated with tumor progression. J Gastroenterol Hepatol 2011;26:1519–26. doi: 10.1111/j.1440-1746.2011.06765.x. [DOI] [PubMed] [Google Scholar]
- 61. Dong B, Dai G, Xu L et al. Tumor cell lysate induces the immunosuppression and apoptosis of mouse immunocytes. Mol Med Rep 2014;10:2827–34. doi: 10.3892/mmr.2014.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mita Y, Kimura MY, Hayashizaki K et al. Crucial role of CD69 in anti-tumor immunity through regulating the exhaustion of tumor-infiltrating T cells. Int Immunol 2018;30:559–67. doi: 10.1093/intimm/dxy050. [DOI] [PubMed] [Google Scholar]
- 63. Miller RP, Tadagavadi RK, Ramesh G et al. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shruthi S, Vijayalaxmi KK, Shenoy KB. Immunomodulatory effects of Gallic acid against cyclophosphamide- and cisplatin-induced immunosuppression in Swiss albino mice. Indian J Pharm Sci 2018;80:150–60. [Google Scholar]
- 65. Gahr M, Speer C, Damerau B et al. Influence of lactoferrin on the function of human polymorphonuclear leukocytes and monocytes. J Leukoc Biol 1991;49:427–33. [DOI] [PubMed] [Google Scholar]
- 66. Adlerova L, Bartoskova A, Faldyna M. Lactoferrin: a review. Vet Med 2008;53:457–68. [Google Scholar]
- 67. Ibrahim HM, Mohammed-Geba K, Tawfic AA et al. Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immunotoxicity in rats. Food Funct 2019;10:7523–32. doi: 10.1039/C9FO01914F. [DOI] [PubMed] [Google Scholar]
- 68. Galelli A, Chedid L. Modulation of myelopoiesis in vivo by synthetic adjuvant-active muramyl peptides: induction of colonystimulating activity and stimulation of stem cell proliferation. Infect Immun 1983;42:1081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bahr GM, Chedid L. Immunological activities of muramyl peptides. Fed Proc 1986;45:2541–4. [PubMed] [Google Scholar]
- 70. Bahr GM, Darcissac E, Bevec D. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on murabutide. Int J Immunopharmacol 1995;17:117–31. [DOI] [PubMed] [Google Scholar]
- 71. Saiki I, Fidler IJ. Synergistic activation by recombinant mouse interferon-gamma and muramyl dipeptide of tumoricidal properties in mouse macrophages. J Immunol 1985;135:684–8. [PubMed] [Google Scholar]
- 72. Souvannavong V, Brown S, Adam A. Muramyl dipeptide (MDP) synergizes with interleukin 2 and interleukin 4 to stimulate, respectively, the differentiation and proliferation of B cells. Cell Immunol 1990;126:106–16. [DOI] [PubMed] [Google Scholar]
- 73. Traub S, Aulock S, Hartung T et al. MDP and other muropeptides--direct and synergistic effects on the immune system. J Endotoxin Res 2006;12:69–85. [DOI] [PubMed] [Google Scholar]
- 74. Srividya S, Roy RP, Basu SK et al. Scavenger receptor-mediated delivery of muramyl dipeptide activates antitumor efficacy of macrophages by enhanced secretion of tumor-suppressive cytokines. J Leukoc Biol 2000;67:683–90. [DOI] [PubMed] [Google Scholar]
- 75. Okamoto I, Kohno K, Tanimoto T et al. Development of CD8+ effector T cells is differentially regulated by IL-18 and IL-12. J Immunol 1999;162:3202–11. [PubMed] [Google Scholar]
- 76. Wang LZ, Li XL, Pang XY et al. Muramyl dipeptide modulates differentiation, maturity of dendritic cells and anti-tumor effect of DC-mediated T cell in acute leukemia children. Hum Vaccin 2011;7:618–24. [DOI] [PubMed] [Google Scholar]
- 77. Tada H, Aiba S, Shibata K et al. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun 2005;73:7967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hornung RL, Longo DL, Gowda VL et al. Induction of a CD8+ cytotoxic T lymphocyte response to soluble antigen given together with a novel muramyl dipeptide adjuvant, N-acetyl-D-glucosaminyl-(beta 1-4)-N-acetylmuramyl-L-alanyl-D-isoglutamine (GMDP). Ther Immunol 1995;2:7–14. [PubMed] [Google Scholar]
- 79. Zunić M, Kricek F, Dukor P et al. Oral administration of muramyl dipeptide into mice modulates cell proliferation, immunoglobulin synthesis and cytokine mRNA levels in gut associated lymphoid tissues. Int J Immunopharmacol 1996;18:155–62. [DOI] [PubMed] [Google Scholar]


