Abstract
Background:
Olive oil intake has been associated with lower risk of cardiovascular disease (CVD) in Mediterranean populations, but little is known about these associations in the U.S population.
Objectives:
To examine whether olive oil intake is associated with total CVD, coronary heart disease (CHD) and stroke risk.
Methods:
We included 61,181 women from the Nurses’ Health Study (1990-2014) and 31,797 men from the Health Professionals Follow-up Study (1990-2014) who were free of cancer, heart disease, and stroke at baseline. Diet was assessed using food frequency questionnaires at baseline and then every 4 years. Cox proportional hazards regressions were used to estimate hazard ratios (HR) and 95% confidence intervals (CI).
Results:
During 24 years of follow-up, we documented 9,797 incident cases of CVD, including 6,034 CHD cases and 3,802 stroke cases. After adjusting for major diet and lifestyle factors, compared with non-consumers, those with higher olive oil intake (>1/2 tablespoon/d or >7g/d) had 14% lower risk of CVD [pooled HR (95% CI): 0.86 (0.79, 0.94)] and 18% lower risk of CHD [pooled HR (95% CI): 0.82 (0.73, 0.91)]. No significant associations were observed for total or ischemic stroke. Replacing 5g/d of margarine, butter, mayonnaise, or dairy fat with the equivalent amount of olive oil was associated with 5-7% lower risk of total CVD and CHD. No significant associations were observed when olive oil was compared with other plant oils combined. In a subset of participants, higher olive oil intake was associated with lower levels of circulating inflammatory biomarkers and a better lipid profile.
Conclusions:
Higher olive oil intake was associated with lower risk of CHD and total CVD in two large prospective cohorts of U.S. men and women. The substitution of margarine, butter, mayonnaise, and dairy fat with olive oil could lead to lower risk of CHD and CVD.
Keywords: Olive Oil, Plant Oils, Cardiovascular Disease, Coronary Heart Disease, Stroke
CONDENSED ABSTRACT:
Findings from two large cohort studies, including 61,181 women from the Nurses’ Health Study (1990-2014) and 31,797 men from the Health Professional’s Follow-up Study (1990-2014), and 9,797 incident cases of cardiovascular disease (CVD), showed that higher olive oil intake was prospectively associated with a lower risk of coronary heart disease (CHD) and total CVD. The substitution of margarine, butter, mayonnaise, and dairy fat with olive oil could lead to a lower risk of CHD and CVD. These findings support current recommendations to replace saturated fat and animal fat with unsaturated plant oils, such as olive oil, for the prevention of CVD.
Introduction
Cardiovascular disease (CVD), a leading cause of global death, can be largely prevented with a healthy lifestyle (1). Current recommendations highlight the importance of dietary patterns including healthy sources of dietary fats, such as those high in unsaturated fat and low in saturated fat (SFA), for primary prevention of CVD (2). Olive oil is high in monounsaturated fat (MUFA), especially oleic acid, and other minor components including vitamin E, polyphenols, and lipid molecules that may contribute to its anti-inflammatory and antioxidant properties (3). Olive oil has been traditionally used as the main culinary and dressing fat in Mediterranean regions, and recently, it has become more popular worldwide.
Early ecological studies observed inverse associations between average country-level consumption of olive oil and the risk of CVD (4). Clinical trials have shown that the consumption of olive oil improves cardiovascular risk factors, including inflammatory and lipid biomarkers (5). In addition, observational studies found that olive oil intake is inversely associated with CVD (6–8) and all-cause death (7). Results from the PREDIMED trial also revealed that a Mediterranean diet supplemented with extra-virgin olive oil reduced the risk of a composite of CVD events by 31% compared to the control diet (9). A recent meta-analysis found an inverse association between olive oil consumption and risk of stroke, but there were inconsistencies between the studies which assessed CHD as the end-point (10). Of note, all of the included studies were conducted in Mediterranean countries.
The associations between olive oil intake and risk of CVD have not yet been evaluated in the U.S. population, whose olive oil consumption has increased in the recent years. Therefore, we examined the association between olive oil consumption with CVD in two large U.S. prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professional’s Follow-up Study (HPFS). We used statistical models to estimate risk of CVD when margarine, butter, mayonnaise, dairy fat, and plant oils were replaced by olive oil. In addition, we examined the associations between olive oil intake, plasma inflammatory biomarkers and lipids in a subpopulation of the cohorts.
Methods
Study population
The NHS is an ongoing prospective cohort study of 121,700 U.S. female registered nurses aged 30-55 at study baseline in 1976. The NHSII started in 1989 with 116,429 female nurses aged 25-42 years. The HPFS is a prospective cohort study of 51,529 male health professionals aged 40-75 at study baseline in 1986. Detailed information has been described previously elsewhere (11, 12).
Baseline for both cohots was 1990, when olive oil consumption was first included as part of the food frequency questionnaires (FFQ). Those participants who reported cancer, heart disease, or stroke at baseline, participants with missing information on olive oil questions, or those who had daily energy intakes <600 or >3,500 kcal for women and <800 or >4,200 kcal for men, were excluded. After exclusions, a total of 61,181 women and 31,797 men remained for analysis. The protocol was approved by the institutional review board of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
Ascertainment of CVD
The primary outcome measure was major CVD defined as a combined endpoint of non-fatal myocardial infarction, non-fatal stroke, or fatal CVD (fatal stroke, fatal myocardial infarction, and other cardiovascular death). The following secondary outcome measures were assessed: total CHD, which was defined as fatal CHD and nonfatal myocardial infarction; total stroke, which included all fatal and nonfatal stroke cases (ischemic, hemorrhagic and undetermined subtypes); and fatal CVD, which included all fatal CHD, fatal stroke and other cardiovascular death. When a participant (or family members of deceased participants) reported an incident event, permission was requested to examine their medical records by physicians who were blinded to the participant risk factor status. For each endpoint, the month and year of diagnosis were recorded as the diagnosis date. Nonfatal events were confirmed through review of medical records. Myocardial infarction was confirmed if the WHO criteria were met on the basis of symptoms plus diagnostic electrocardiogram changes or elevated cardiac enzymes. Strokes were confirmed if data in the medical records fulfilled the National Survey of Stroke criteria requiring evidence of a neurological deficit with sudden or rapid onset that persisted for >24h of until death (13). Strokes were classified as ischemic stroke (thrombotic or embolic occlusion of a cerebral artery), hemorrhagic stroke (subarachnoid and intraparenchymal hemorrhage), or stroke of probable/unknown subtype (a stroke was documented, but the subtype could not be ascertained).
Deaths were identified by reports of families, the U.S. postal system, or using death certificates obtained from state vital statistics departments and the National Death Index, and confirmed through review of medical records or autopsy reports. Follow-up for deaths was >98% complete.
Dietary assessment
Dietary intake was measured using a validated semiquantitative FFQ with over 130 items administered every 4 years. The reproducibility and validity of these FFQs have been described in previous reports (14). Participants were asked how often, on average, they had consumed specific foods, as well as types of fats, oils, and brand or type of oils used for cooking and added at the table in the preceding year. Total olive oil intake was calculated from the sum of three questionnaire questions related to olive oil intake: olive oil salad dressing, olive oil added to food or bread, and olive oil used for baking and frying at home. Olive oil intake was categorized into four categories: (1) never or less than once per month, (2) >0 to ≤1 teaspoon(>0 to ≤4.5g/d), (3) >1 teaspoon to ≤1/2 tablespoon(>4.5-≤7g/d), and (4) >1/2 tablespoon(>7g/d)]. We also analyzed olive oil intake as a continuous variable. One tablespoon was considered to be equivalent to 13.5g of olive oil. The amount of other plant oils (e.g., corn, safflower, soybean, canola) was calculated based on the participant’s reported oil brand and type of fat used for cooking at home, including frying, sautéing, baking, and salad dressing. Data about homemade baking items and frying fats at home was also incorporated. Total margarine was calculated based on the reported frequency of stick, tub, or soft margarine and the amount of margarine added from baking and frying at home. Butter intake was also calculated based on the frequency that butter was added to foods and used for frying, sauteing, and baking. Intakes of dairy and other fats and nutrients were calculated based on the USDA and Harvard University Food Composition Database (15) and our biochemical analyses.
Assessment of plasma inflammatory biomarkers and lipids
Plasma samples were collected in substudies of the NHS(n=32,862) during 1989-1990, NHS2(n=29,611) during 1996-1999, and HPFS(n=18,019) during 1993-1995 (16). Plasma concentrations of several inflammatory and lipid biomarkers were measured (16). Data from these sub-studies and corrected for batch effects were combined. After excluding outliers (identified by a generalized extreme studentized deviate many-outlier procedure (17)) in each sub-study, and duplicates across sub-studies, a total of 32,624 individuals were included in the biomarker analyses.
Assessment of covariates
Every 2 years, participants returned a mailed validated questionnaire that obtained updated information on age, body weight, smoking status, physical activity, aspirin and other medications use, multivitamin use, menopausal status and postmenopausal hormone use in women, and physician diagnosis of chronic diseases. Baseline history of hypertension, hypercholesterolemia, and type 2 diabetes mellitus were determined through self-reporting. Body mass index was calculated as weight in kilograms divided by the square of the height in meters.
Statistical analysis
Each individual person-time was calculated from the date of the return of the baseline questionnaire to the date of CVD diagnosis, death, or the end of follow-up (June 30, 2014 for the NHS, and January 31, 2014 for HPFS), whichever came first. Dietary variables were stopped updated on a report of cancer, coronary artery bypass, or angina because changes in diet after the development of these conditions may confound the associations. The cumulative average of food intake from all available FFQs was calculated to better represent long-term diet and to minimize within-person variation.
Multivariable Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of developing total CVD, CHD, and stroke according to olive oil intake categories. Separate analyses were conducted for ischemic stroke, fatal and nonfatal CVD. Hemorrhagic stroke was not analyzed separately due to the low number of cases. Multivariable models were adjusted for updated covariates: age, ethnicity, Southern European/Mediterranean ancestry, smoking status, alcohol intake, physical activity, family history of diabetes, family history of myocardial infarction, cancer, baseline diabetes mellitus, hypertension or antihypertensive medication use, hypercholesterolemia or cholesterol-lowering medication use, multivitamin use, aspirin use, in women postmenopausal status and menopausal hormone use, total energy intake, and body mass index. Model 3 was additionally adjusted for red meat, fruits and vegetables, nuts, soda, whole grain intake (in quintiles), and trans fat. To quantify a linear trend, we conducted a Wald test for linear trend by assigning the median intake within each quintile and modeling this as a continuous variable.
Stratified analysis and potential interactions with several pre-specified subgroups were evaluated using the Wald test on cross-product terms based on olive oil intake (continuous variable) and the stratification variables.
The risk of total CVD, CHD, and stroke was estimated when substituting 5g/d of olive oil for the equivalent amount of other types of fats [margarine, butter, mayonnaise, other plant oils (corn, safflower, soybean, and canola), dairy fat, and all other fats combined]. Both continuous variables were included in the multivariable model described above and mutually adjusted for other types of fat. The difference between regression β coefficients and variance and the covariance were used to derive the HRs and 95% CIs for the substitution associations.
Sensitivity analyses were conducted to test the robustness of the results. First, to test whether the results were affected by selectively stopping updating diet, diet was continuously updated until the end of follow-up. Second, instead of using the cumulative average of diet, the most recent measure of diet was used. Third, the models were mutually adjusted for other types of fats. Fourth, sensitivity analysis excluding BMI from the models were conducted. Fifth, the models were adjusted for modified AHEI (without PUFA:SFA ratio). Finally, the models were adjusted for updated history of diabetes, hypertension or medication and hypercholesterolemia or medication. Bonferroni corrections to account for multiple testing were conducted at alpha=0.016 (alpha corrected for three outcomes in the main Table 2) and alpha =0.008 (alpha corrected for six tests in the Central Figure, substitution analyses).
Table 2.
Relative risk (95% CI) of cardiovascular events according to categories of total olive oil intake
| Categories of olive oil intake | |||||||
|---|---|---|---|---|---|---|---|
| Never or <1/month | >0–≤4.5 g/d (>0 to ≤1 teaspoon) | >4.5–≤ 7 g/d >1 teaspoon to ≤1/2 | >7 g/d (>1/2 TBS) | P for trend | HR (95% CI) for 5 g increase in | ||
|
TOTAL CVD: fatal and nonfatal myocardial infarction + fatal and nonfatal stroke | |||||||
| NHS | Mean total olive oil | 0 | 1.5 (± 1.2) | 5.6 (± 0.7) | 11.7 (± 5.7) | ||
| N° cases/ Person-years | 1971/399686 | 2658/638583 | 367/106313 | 491/150743 | |||
| Incidence rate | 0.49% | 0.41% | 0.34% | 0.32% | |||
| Age-adjusted model 1 | 1 (Ref.) | 0.81 (0.77, 0.86) | 0.72 (0.64, 0.81) | 0.69 (0.62, 0.76) | <0.001 | 0.87 (0.84, 0.90) | |
| Multivariable model 2 | 1 (Ref.) | 0.90 (0.85, 0.96) | 0.86 (0.76, 0.96) | 0.86 (0.77, 0.95) | 0.01 | 0.94 (0.90, 0.97) | |
| Multivariable model 3 | 1 (Ref.) | 0.92 (0.86, 0.97) | 0.88 (0.78, 0.98) | 0.88 (0.79, 0.98) | 0.05 | 0.95 (0.91, 0.99) | |
| HPFS | Mean total olive oil | 0 | 1.5 (± 1.2) | 5.6 (± 0.7) | 11.2 (± 5.4) | ||
| N° cases/ Person-years | 1696/191480 | 2041/308406 | 258/45995 | 315/55468 | |||
| Incidence rate | 0.88% | 0.66% | 0.56% | 0.56% | |||
| Age-adjusted model 1 | 1 (Ref.) | 0.79 (0.74, 0.85) | 0.69 (0.60, 0.78) | 0.72 (0.64, 0.82) | <0.001 | 0.88 (0.84, 0.93) | |
| Multivariable model 2 | 1 (Ref.) | 0.85 (0.80, 0.91) | 0.77 (0.67, 0.88) | 0.82 (0.72, 0.93) | 0.001 | 0.93 (0.88, 0.97) | |
| Multivariable model 3 | 1 (Ref.) | 0.86 (0.80, 0.92) | 0.77 (0.68, 0.89) | 0.83 (0.73, 0.94) | 0.004 | 0.93 (0.89, 0.98) | |
| Pooled Model 3 | 1 (Ref.) | 0.89 (0.85, 0.93) | 0.83 (0.76, 0.91) | 0.86 (0.79, 0.94) | <0.001 | 0.94 (0.92, 0.97) | |
|
CORONARY HEART DISEASE: fatal and nonfatal myocardial infarction | |||||||
| NHS | N° cases/ Person-years | 1078/400215 | 1373/639428 | 181/106445 | 250/150908 | ||
| Incidence rate | 0.27% | 0.21% | 0.17% | 0.16% | |||
| Age-adjusted model 1 | 1 (Ref.) | 0.79 (0.72, 0.85) | 0.66 (0.56, 0.77) | 0.65 (0.57, 0.75) | <0.001 | 0.84 (0.79, 0.89) | |
| Multivariable model 2 | 1 (Ref.) | 0.89 (0.82, 0.96) | 0.81 (0.69, 0.95) | 0.84 (0.73, 0.98) | 0.03 | 0.92 (0.87, 0.97) | |
| Multivariable model 3 | 1 (Ref.) | 0.91 (0.84, 0.99) | 0.84 (0.72, 0.99) | 0.89 (0.76, 1.03) | 0.13 | 0.94 (0.89, 0.99) | |
| HPFS | N° cases/ Person-years | 1310/191843 | 1440/308891 | 193/46046 | 209/55564 | ||
| Incidence rate | 0.68% | 0.46% | 0.42% | 0.37% | |||
| Age-adjusted model 1 | 1 (Ref.) | 0.73 (0.68, 0.79) | 0.68 (0.58, 0.79) | 0.63 (0.54, 0.73) | <0.001 | 0.86 (0.81, 0.91) | |
| Multivariable model 2 | 1 (Ref.) | 0.80 (0.74, 0.86) | 0.78 (0.66, 0.91) | 0.74 (0.63, 0.86) | <0.001 | 0.91 (0.86, 0.97) | |
| Multivariable model 3 | 1 (Ref.) | 0.81 (0.75, 0.87) | 0.79 (0.67, 0.92) | 0.75 (0.64, 0.87) | 0.001 | 0.92 (0.87, 0.98) | |
| Pooled model 3 | 1 (Ref.) | 0.85 (0.81, 0.89) | 0.81 (0.73, 0.91) | 0.82 (0.73, 0.91) | 0.001 | 0.93 (0.89, 0.97) | |
|
STROKE: fatal and nonfatal stroke | |||||||
| NHS | N° cases/ Person-years | 906/400164 | 1308/639362 | 185/106427 | 245/150884 | ||
| Incidence rate | 0.22% | 0.20% | 0.17% | 0.16% | |||
| Age-adjusted model 1 | 1 (Ref.) | 0.88 (0.81, 0.96) | 0.81 (0.69, 0.95) | 0.77 (0.67, 0.89) | 0.001 | 0.91 (0.86, 0.96) | |
| Multivariable model 2 | 1 (Ref.) | 0.95 (0.87, 1.04) | 0.91 (0.78, 1.07) | 0.90 (0.77, 1.04) | 0.17 | 0.96 (0.91, 1.01) | |
| Multivariable model 3 | 1 (Ref.) | 0.95 (0.87, 1.04) | 0.92 (0.78, 1.09) | 0.92 (0.79, 1.07) | 0.31 | 0.97 (0.91, 1.02) | |
| HPFS | N° cases/ Person-years | 386/192081 | 601/309197 | 65/46093 | 106/55573 | ||
| Incidence rate | 0.20% | 0.19% | 0.14% | 0.19% | |||
| Age-adjusted model 1 | 1 (Ref.) | 1.00 (0.87, 1.14) | 0.73 (0.56, 0.95) | 1.02 (0.82, 1.27) | 0.43 | 0.95 (0.88, 1.04) | |
| Multivariable model 2 | 1 (Ref.) | 1.02 (0.89, 1.17) | 0.75 (0.58, 0.99) | 1.07 (0.86, 1.34) | 0.69 | 0.99 (0.97, 1.02) | |
| Multivariable model 3 | 1 (Ref.) | 1.03 (0.90, 1.18) | 0.75 (0.57, 0.99) | 1.07 (0.85, 1.35) | 0.68 | 0.99 (0.97, 1.02) | |
| Pooled model 3 | 1 (Ref.) | 0.99 (0.97, 1.02) | 0.90 (0.80, 1.01) | 0.95 (0.85, 1.12) | 0.29 | 0.96 (0.92, 1.01) | |
Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (95% CI). Abbreviations: NHS, Nurses’ Health Study, HPFS, Health Professionals Follow-up Study. Model 2 was adjusted for age (years), ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), smoking status (never, former, current smoker 1-14 cigarettes per day, 15-24 cigarettes per day, or ≥ 25 cigarettes per day), alcohol intake (0, 0.1-4.9,5.0-9.9,10.0-14.9, and ≥ 15.0 g/d), physical activity (<3.0,3.0-8.9,9.0-17.9,18.0-26.9,≥27.00 metabolic equivalent task-h/week), family history of diabetes (yes/no), family history of myocardial infarction (yes/no), family history of cancer (yes/no), baseline diabetes mellitus (yes/no), baseline hypertension or antihypertensive medication use (yes/no), baseline hypercholesterolemia or cholesterol-lowering medication use (yes/no), multivitamin use (yes/no), aspirin use (yes/no), in women postmenopausal status and menopausal hormone use [premenopausal, postmenopausal (no, past, or current hormone use), total energy intake (kilocalories per day) and body mass index (calculated as weight in kilograms divides by height in meters squared). Model 3 was additionally adjusted for red meat, fruits and vegetables, nuts, soda, whole grains intake (in quintiles), and trans fat. Results were pooled with the use of fixed-effect models. Adjusting for multiple testing using the Bonferroni corrections did not change the main results, as the P values for the pooled analyses were <0.001.
Central Illustration. Hazard Ratios for Cardiovascular Disease, Coronary Heart Disease and Stroke associated with substitution of 5 g/d of olive oil for equivalent amounts of other fats.
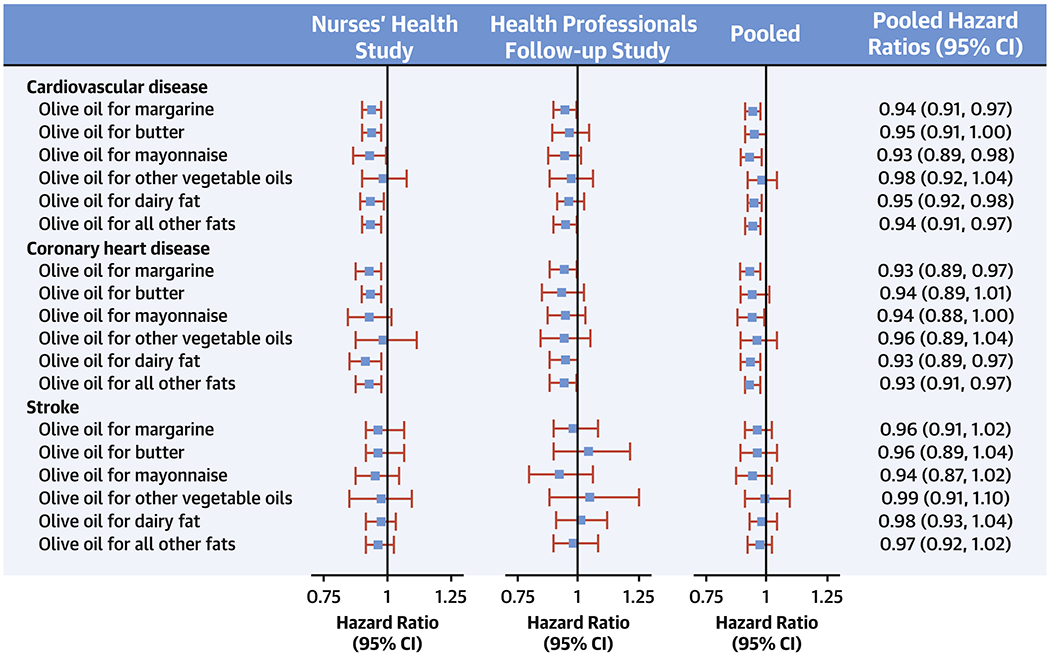
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professional’s Follow-up Study. Multivariate-adjusted models were adjusted for age (years), ethnicity (white, non-white), Southern European/Mediterranean ancestry, smoking status (never, former, current smoker 1-14 cigarettes per day, 15-24 cigarettes per day, or ≥ 25 cigarettes per day), alcohol intake (0, 0.1-4.9,5.0-9.9,10.0-14.9, and ≥ 15.0 g/d), physical activity (<3.0,3.0-8.9,9.0-17.9,18.0-26.9,≥27.00 metabolic equivalent task-h/week), family history of diabetes, family history of myocardial infarction, family history of cancer, baseline diabetes mellitus, baseline hypertension or antihypertensive medication use, baseline hypercholesterolemia or cholesterol-lowering medication use, multivitamin use, aspirin use, in women postmenopausal status and menopausal hormone use [premenopausal, postmenopausal (no, past, or current hormone use), total energy intake (kilocalories per day), body mass index (kg/m2), for red meat, fruits and vegetables, nuts, soda, whole grains intake (in quintiles), trans fat, and mutually adjusted for other types of fat. Results were pooled with the use of the fixed-effects model. Horizontal lines represent 95% confidence intervals.
Linear regressions were used to evaluate the associations between categories of olive oil intake, plasma levels of inflammatory biomarkers, and lipids. The average intake of olive oil was calucalted from the two FFQs administered closer to the data of blood collection (in NHS and HPFS, 1990 and 1994; and in NHSII, 1991 and 1995). Multivariable models were adjusted for the same covariates described above, with additional adjustment for study cohort, fasting and case-control status, steroid use, SFA and PUFA intake. Participants taking lipid-lowering medication or with hypercholesterolemia at baseline were excluded in the analyses of blood lipids.
The HRs from multivariable models in each cohort were pooled with the use of an inverse variance–weighted meta-analysis using a fixed-effects model. Analyses were performed with the SAS statistical package (version 9.4, SAS Institute). Statistical tests were 2 sided, and p values of <0.05 were considered to indicate statistical significance.
Results
During an average of 24 years of follow-up, a total of 9,797 CVD cases, 5,487 in the NHS and 4,310 in the HPFS were documented. Mean consumption of olive oil increased from 1.30g/d in 1990 to 4.2 g/d in 2010, whereas intake of margarine decreased over the course of follow-up (Supplemental Figure 1). The Spearman correlations between olive oil and other types of fat are presented in Supplemental Table 1. Characteristics of participants according to frequency of olive oil intake using updated variables over time are shown in Table 1. Men and women with a higher intake of also tended to have higher energy intake, and higher intakes of nuts, fruits and vegetables, and other plant oils. The mean intake of total olive oil in the highest category (>1/2 tablespoon/d) was about 12g/d (Table 1).
Table 1.
Characteristics of Participants according to categories of total olive oil intake
| Olive oil intake | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Never or <1/month | >0–≤4.5 g/d (>0 to ≤1 teaspoon) | >4.5–≤7 g/d >1 teaspoon to ≤1/2 TBS) | >7 g/d (>1/2 TBS) | Never or <1/month | >0–≤4.5 g/d (>0 to ≤1 teaspoon) | >4.5–≤7 g/d >1 teaspoon to ≤1/2 TBS) | >7 g/d (>1/2 TBS) |
| Nurses’ Health Study | Health Professional’s Follow-up Study | |||||||
| Number of participants | 32,673 | 22,918 | 2,412 | 3,178 | 16,073 | 12,853 | 1,246 | 1,625 |
| Total olive oil (g/d) | 0.0 (0.0) | 1.5 (1.2) | 5.6 (0.7) | 11.7 (5.7) | 0.0 (0.0) | 1.5 (1.2) | 5.6 (0.7) | 11.2 (5.4) |
| Age (y) | 65 (10) | 67 (10) | 67 (8) | 67 (8) | 65 (10) | 65 (10) | 65 (10) | 65 (10) |
| BMI (kg/m2) | 26.1 (5.9) | 26.1 (5.2) | 26.1 (5.0) | 25.8 (4.9) | 25.9 (3.6) | 26.0 (3.5) | 25.9 (3.5) | 25.8 (3.6) |
| Physical activity (MET-h/wk) | 15.5 (21.4) | 18.3 (23.2) | 21.4 (23.9) | 23.7 (25.6) | 35.1 (41.8) | 38.6 (42.2) | 41.5 (42.9) | 45.1 (46.2) |
| Family history of diabetes (%) | 31.1 | 29.4 | 28.4 | 27.7 | 20.8 | 21.6 | 20.9 | 21.6 |
| Family history of myocardial infarction (%) | 18.8 | 19.1 | 19.2 | 19.1 | 31.3 | 30.3 | 32.5 | 32.0 |
| Ethnicity, white (%) | 97.4 | 98.1 | 98.3 | 98.5 | 95.2 | 95.6 | 95.8 | 96.6 |
| Southern European/Mediterranean ancestry (%) | 13.9 | 16.8 | 21.8 | 28.2 | 20.2 | 22.3 | 27.9 | 36.0 |
| Current smoker (%) | 10.7 | 9.0 | 7.9 | 7.1 | 6.2 | 4.4 | 4.2 | 3.8 |
| Current menopausal hormone use (%) | 32.0 | 31.8 | 32.5 | 31.1 | - | - | - | - |
| Hypertension (%) | 43.8 | 47.2 | 47.3 | 45.2 | 33.7 | 37.4 | 39.0 | 38.2 |
| Hypercholesterolemia (%) | 53.7 | 59.2 | 60.6 | 58.7 | 38.2 | 45.8 | 48.7 | 47.9 |
| Total energy intake (kcal/day) | 1637 (515) | 1677 (517) | 1805 (523) | 1915 (547) | 1934 (605) | 1988 (615) | 2093 (618) | 2223 (632) |
| Alcohol (g/d) | 3.6 (8.1) | 5.5 (9.7) | 7.6 (11.2) | 9.1 (12.4) | 8.9 (13.7) | 11.9 (14.9) | 15.5 (16.4) | 17.7 (17.9) |
| Red meat (servings/d) | 0.9 (0.6) | 0.8 (0.5) | 0.8 (0.5) | 0.7 (0.5) | 1.1 (0.8) | 1.0 (0.7) | 0.9 (0.7) | 0.9 (0.6) |
| Nuts (servings/d) | 0.2 (0.7) | 0.4 (1.1) | 0.5 (1.5) | 0.7 (1.9) | 0.2 (0.3) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.4) |
| Whole grains (servings/d) | 1.7 (1.3) | 1.8 (1.2) | 1.9 (1.3) | 2.0 (1.3) | 1.2 (1.0) | 1.2 (0.9) | 1.3 (0.9) | 1.3 (1.0) |
| Fruits and vegetables (servings/d) | 4.8 (1.9) | 5.1 (1.9) | 5.7 (2.0) | 6.1 (2.1) | 5.2 (2.3) | 5.6 (2.3) | 6.4 (2.5) | 7.0 (2.7) |
| Coffee (servings/d) | 1.7 (1.5) | 1.7 (1.4) | 1.7 (1.3) | 1.7 (1.3) | 1.9 (1.7) | 1.9 (1.6) | 1.9 (1.5) | 2.0 (1.5) |
| Soda (servings/d) | 0.7 (0.8) | 0.6 (0.8) | 0.6 (0.8) | 0.6 (0.8) | 0.8 (0.9) | 0.7 (0.8) | 0.7 (0.8) | 0.6 (0.8) |
| Dairy fat (g/d) | 10.9 (6.5) | 11.0 (6.1) | 11.3 (6.2) | 11.7 (6.5) | 11.4 (7.7) | 11.3 (7.1) | 11.3 (6.9) | 11.6 (7.3) |
| Other plant oils (g/d) | 4.0 (3.6) | 3.7 (2.9) | 3.8 (3.1) | 4.1 (3.8) | 4.2 (3.8) | 3.8 (3.2) | 3.9 (3.2) | 3.9 (3.5) |
| Margarine (g/d) | 13.7 (15.2) | 11.3 (12.9) | 10.5 (12.5) | 9.8 (12.3) | 11.8 (14.9) | 9.1 (11.9) | 8.1 (11.2) | 7.5 (11.1) |
| Butter (g/d) | 1.1 (2.7) | 1.5 (2.7) | 1.9 (2.9) | 2.3 (3.2) | 1.2 (3.0) | 1.5 (2.9) | 1.8 (3.0) | 2.1 (3.2) |
| Mayonnaise (g/d) | 4.1 (5.5) | 3.3 (4.2) | 3.5 (4.5) | 3.9 (5.5) | 4.0 (5.6) | 3.3 (4.4) | 3.4 (4.6) | 3.4 (4.9) |
| All other types of fat (g/d) | 32.2 (20.6) | 28.8 (17.5) | 28.7 (17.4) | 28.9 (18.2) | 31.1 (21.2) | 27.0 (17.5) | 26.1 (17.1) | 25.8 (17.3) |
| Multivitamin supplement use (%) | 50.8 | 59.5 | 62.2 | 61.8 | 47.1 | 55.0 | 56.9 | 56.5 |
| Aspirin use (%) | 47.0 | 49.8 | 50.3 | 48.7 | 57.0 | 65.4 | 68.1 | 66.9 |
Characteristics of participants are presented using updated variables. Abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index; MET, metabolic equivalent task; TBS, tablespoon. Values are presented as mean (SD) or percentage, standardized to the age distribution of the study population. All other fat is the sum of dairy fat, other plant oils, margarine and mayonnaise.
After adjusting for demographic and lifestyle factors, compared with those who consumed olive oil less than once per month, those who consumed >1/2 tablespoon/d of olive oil had a 14% lower risk of CVD (HR: 0.86 (0.79, 0.94,Ptrend<0.001) (Table 2). When BMI was excluded from the models the results were consistent [pooled HR:0.83, 95%CI: 0.76, 0.91; Ptrend<0.001]. The pooled HR(95%CI) for CHD comparing extreme categories of olive oil intake after adjusting for potential confounders was 0.82 (0.73, 0.91;.Ptrend<0.001). Per each 5g/d increase in olive oil intake the HR for stroke was 0.96 (95%CI: 0.92, 1.01,P=0.14) (Table 2). Pooling estimates of the fully adjusted model from both cohorts resulted in an overall HR of ischemic stroke of 0.99 (95%CI: 0.93, 1.05,P=0.66) per each 5g/d increase in olive oil consumption (Supplemental Table 2).
When the models for total olive oil were mutually adjusted for other types of fat, the estimates were consistent with those in the primary analysis (Supplemental Table 3). Total olive oil intake was also associated with lower risk of fatal CVD, which was more pronounced than the risk of non-fatal CVD (Supplemental Table 4). In the pooled fully adjusted analysis, each 5g/d increase in olive oil consumption was associated with a 19% lower risk of fatal CVD (95%CI: 0.71, 0.93,Ptrend=0.01) and a 9% lower risk of non-fatal CVD (95%CI: 0.82, 1.01,Ptrend=0.02).
We found significant inverse associations in most of the pre-specified subgroup analyses (Table 3). No significant interactions were observed for any of the variables analyzed. Participants reporting Southern European/Mediterranean ancestry and higher olive oil intake had a 6% (HR: 0.94; 95%CI, 0.90,0.98) lower risk of CVD, which was similar to the association observed in the non-Mediterranean ancestry subgroups.
Table 3.
Subgroup analyses for risk of total CVD according to olive oil intake
| Adjusted HR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Subgroup | NHS | P for interaction | HPFS | P for interaction | Pooled | P for interaction |
| Age | ||||||
| Younger (< 65 years) | 0.83 (0.74, 0.92) | 0.01 | 0.94 (0.85, 1.04) | 0.82 | 0.90 (0.85, 0.97) | 0.54 |
| Older (≥ 65 years) | 0.97 (0.93, 1.01) | 0.93 (0.88, 0.98) | 0.95 (0.92, 0.99) | |||
| Body mass index, kg/m2 | ||||||
| <25 | 0.95 (0.91, 0.98) | 0.46 | 0.94 (0.88, 1.00) | 0.80 | 0.94 (0.90, 1.00) | 0.80 |
| ≥25 | 0.94 (0.89, 0.99) | 0.93 (0.86, 1.00) | 0.94 (0.88, 0.98) | |||
| Family history of myocardial infarction | ||||||
| No | 0.97 (0.92, 1.01) | 0.04 | 0.95 (0.89, 1.01) | 0.11 | 0.96 (0.91, 1.01) | 0.08 |
| Yes | 0.88 (0.81, 0.96) | 0.89 (0.82, 0.97) | 0.89 (0.82, 0.96) | |||
| Ancestry | ||||||
| Southern European/Mediterranean | 0.95 (0.90, 0.99) | 0.95 | 0.93 (0.87, 0.98) | 0.71 | 0.94 (0.90, 0.98) | 0.82 |
| Other ancestry | 0.94 (0.87, 1.02) | 0.93 (0.85, 1.01) | 0.94 (0.86, 1.01) | |||
| AHEI | ||||||
| Below median | 0.96 (0.90, 1.02) | 0.88 | 0.91 (0.83, 0.99) | 0.72 | 0.94 (0.90, 0.99) | 0.59 |
| Above median | 0.94 (0.89, 0.99) | 0.95 (0.89, 1.01) | 0.95 (0.89, 0.99) | |||
| AMED | ||||||
| Below median | 0.93 (0.87, 1.00) | 0.48 | 0.92 (0.85, 1.00) | 0.92 | 0.92 (0.87, 1.00) | 0.29 |
| Above median | 0.95 (0.90, 1.00) | 0.94 (0.88, 1.00) | 0.95 (0.90, 1.00) | |||
| Total vegetable intake | ||||||
| Below median | 0.94 (0.88, 0.99) | 0.42 | 0.88 (0.79, 0.96) | 0.21 | 0.92 (0.85, 0.97) | 0.12 |
| Above median | 0.95 (0.91, 1.00) | 0.96 (0.90, 1.02) | 0.96 (0.91, 1.00) | |||
| Green vegetable intake | ||||||
| Below median | 0.96 (0.90, 1.02) | 0.64 | 0.90 (0.82, 0.99) | 0.30 | 0.94 (0.90, 1.00) | 0.48 |
| Above median | 0.93 (0.88, 0.98) | 0.96 (0.90, 1.02) | 0.93 (0.89, 0.98) | |||
| Lettuce intake | ||||||
| Below median | 0.97 (0.91, 1.03) | 0.48 | 0.91 (0.83, 1.00) | 0.27 | 0.95 (0.90, 1.00) | 0.80 |
| Above median | 0.93 (0.88, 0.98) | 0.96 (0.91, 1.02) | 0.95 (0.91, 0.98) | |||
Results are expressed as hazard ratios (HR) and 95% confidence intervals (95% CI). HRs for 5g increase in olive oil intake in each subgroup category. Abbreviations: AHEI, Alternative Healthy Eating Index Score; AMED, Alternate Mediterranean Diet; HPFS, Health Professionals- Follow-up Study; NHS, Nurses’ Health Study, HPFS, Health Professionals Follow-up Study. Multivariable model was adjusted for age, ethnicity (white, non-white), Southern European/Mediterranean ancestry, smoking status (never, former, current smoker 1-14 cigarettes per day, 15-24 cigarettes per day, or ≥ 25 cigarettes per day), alcohol intake (0, 0.1-4.9,5.0-9.9,10.0-14.9, and ≥ 15.0 g/d), physical activity (<3.0,3.0-8.9,9.0-17.9,18.0-26.9,≥27.00 metabolic equivalent task-h/week), family history of diabetes, family history of myocardial infarction, family history of cancer, baseline diabetes mellitus, baseline hypertension or antihypertensive medication use, baseline hypercholesterolemia or cholesterol-lowering medication use, multivitamin use, aspirin use, in women postmenopausal status and menopausal hormone use, total energy intake, BMI, red meat, fruits and vegetables, nuts, soda, whole grains intake (in quintiles), and trans fat. No adjustment for multiple testing were made for this results.
Replacing 5g/d of margarine with 5g/d of olive oil was estimated to be associated with 6% lower risk of CVD (95%CI, 0.91, 0.97,P<0.001) (Central Illustration). The respective estimate for butter was 0.95(95%CI: 0.91, 1.00,P=0.06). For mayonnaise, the HR was 0.93(95% CI: 0.89, 0.98,P<0.001). Replacing 5g/d of dairy fat for the same amount of olive oil was associated with 5% lower risk of CVD (95%CI, 0.92, 0.98,P<0.001). Substituting olive oil for other plant oils was not significantly associated with CVD. Similar results were observed for CHD and no significant associations were observed for stroke (Central Illustration).
When we adjusted for multiple testing using the Bonferroni corrections, the main results and conclusions did not change, as the P values for the pooled analyses were <0.001.
In the sensitivity analysis without stop updating diet, associations for 5g/d increase in olive oil intake were consistent. The pooled HR(95% CI) were: 0.95(95%CI, 0.89, 0.99) for CVD, 0.93(95% CI, 0.86, 0.98) for CHD and 0.97(95%CI, 0.89, 1.06) for stroke. When using the most recent diet measurement, the respective estimates were: 0.93(0.89, 0.97) for CVD, 0.91(0.86, 0.96) for CHD, and 0.96(0.90, 1.01) for stroke. The results for the main analysis remained unchanged when the models were adjusted for the AHEI score (Supplemental Table 5). When the models were adjusted for updated history of diabetes, hypertension, and hypercholesterolemia, the pooled multivariable HR(95%CI) for CVD was 0.94 (95%CI, 0.91, 0.97,P<0.001) in the pooled models.
In secondary analyses in a subpopulation of the three cohorts with available biomarker data, higher olive oil intake was associated with lower levels of several inflammatory biomarkers including interleukin-6 (P=0.006), soluble intercellular adhesion molecule-1 (sICAM-1) (P=0.05), and tumor necrosis factor-α receptor 2 (TNFα-R2) (P=0.007) (Figure 1). For blood lipids, higher olive oil intake was associated with higher levels of HDL-C (P=0.004). No significant associations were observed for LDL-C (Supplementary Figure 2).
Figure 1. Associations between olive oil intake at baseline and inflammatory biomarkers in the NHS, NHS2, and HPFS.
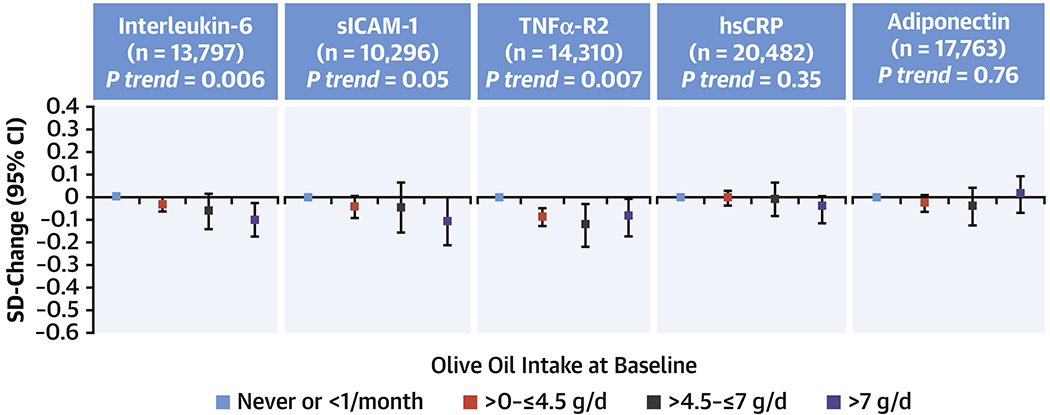
Linear regressions were used to analyze the association between olive oil intake categories (cumulative average scores of 1990 and 1994 in NHS and HPFS, and 1991 and 1995 in NHSII) and blood lipid levels assessed using blood samples (1989-1990 in NHS, 1993-1995 in HPFS, and 1996-1999 in NHSII). Multivariable models were adjusted for study cohort, age, fasting status, body mass index, ethnicity (white, non-white), Southern European/Mediterranean ancestry, smoking status, alcohol intake, physical activity, family history of diabetes, family history of myocardial infarction, baseline diabetes mellitus, baseline hypertension and antihypertensive medication, baseline hypercholesterolemia and lipid-lowering medication, steroid use, multivitamin use, aspirin use, in women postmenopausal status and menopausal hormone use [premenopausal, postmenopausal (no, past, or current hormone use), total energy intake, trans, saturated and polyunsaturated fatty acids and case-control status in original sub-studies. CI, confidence interval; SD, standard deviation; sICAM-1, soluble intercellular adhesion molecule-1; TNFα-R2, tumor necrosis factor-α receptor 1 and 2; and hsCRP, high-sensitive C-reactive protein.
Discussion
In two large prospective cohorts followed for 24 years, we found inverse associations between olive oil consumption and the incidence of cardiovascular events after adjusting for cardiovascular risk factors (Central Illustration). As compared with non-consumers, those with higher consumption of olive oil had 14% lower risk of CVD and 18% lower risk of CHD. Results were consistent across all subgroups, including participants with and without Southern European ancestries. In addition, it was estimated that compared with margarine, butter, mayonnaise, and dairy fat, olive oil was associated with lower risk of CVD and CHD, whereas when compared with other plant oils combined, olive oil was not associated with CVD. The present work generates new evidence suggesting that replacement of more saturated fats, such as butter and margarine, with healthy plant-based fats, like olive oil, is beneficial for the primary prevention of CVD. Of note, during the earlier part of the follow-up, many margarines contained substantial amounts of trans fatty acids and the results may not apply to current margarines. Furthermore, higher olive oil intake was associated with lower levels of inflammatory biomarkers and a better lipid profile, suggesting that moderate olive oil intake could have some benefits on surrogate markers of CVD.
Existing literature supports the association between olive oil intake and lower incidence of cardiovascular risk factors and chronic diseases (5). However, most of the previous studies have been conducted in Mediterranean and European populations (6, 8, 9, 18–21), where the average intake of olive oil and its between-person variability is higher than in the U.S. population. In the current study, the mean intake of olive oil was 12g/d while in Mediterranean populations, such as the Spanish participants of the PREDIMED Study, the mean intake of olive oil at baseline was as high as 40g/d (8). Moreover, some of the studies have been conducted in participants who had already suffered from CVD or who were at high cardiovascular risk (8, 19). Our findings provide further evidence that olive oil is associated with a lower risk of CVD in a healthy U.S. adults. Notably, and as shown in our supplementary graph (Supplementary Figure 1), the intake of olive oil has become more popular in the U.S. in recent years.
Our findings are in line with previous observational studies showing that olive oil consumption is inversely associated with CVD in Mediterranean populations (6–8, 21). In the EPIC-spain cohort, each 10g/d increase in olive oil intake was associated with a 7% lower CHD risk after 10 years of follow-up (7). Findings from the PREDIMED trial, demonstrated that a Mediterranean diet supplemented with extra virgin olive oil reduced the risk of a composite of cardiovascular events by 31% (95%CI: 0.53, 0.91) in a population at high cardiovascular risk (9). In a secondary analysis of the PREDIMED Study, for each 10g/d increase in total olive oil intake, CVD and CVD mortality risk was 13% and 16% lower, respectively (8). Regular consumption of olive oil was also associated with a 44% lower risk of CHD after 7.8 years of follow-up in Italian women who were survivors of myocardial infarction (6).
A recent meta-analysis of case-control, cohort, and intervention studies, concluded that epidemiological studies consistently demonstrate associations between olive oil intake and a reduced risk of stroke (as well as stroke and CHD combined), but no significant association for risk of CHD (10). These findings are somewhat different from our results showing stronger associations for CHD than stroke when those outcomes were analyzed separately. Olive oil consumption was lower in our cohorts compared to the included cohorts where estimates for continuous variable were reported for 25g increase. There is a possibility that the effect of polyphenolic components of olive oil, which are present in higher amounts in the virgin olive oil variety of olive oil, may contribute to lower risk of stroke (10). Given our findings, it would be of interest for future studies investigating the associations with stroke to test higher intakes of olive oil, including specific olive oil varieties.
To our knowledge, this study is the first to estimate the impact of replacing specific types of fat with olive oil in relation to the incidence of CVD. We projected that replacing other types of more saturated fat with olive oil was associated with a lower risk of total CVD and CHD. These findings are consistent with evidence that substitution of fats high in SFA or trans isomers, which increase LDL-C, with fats higher in unsaturated fatty acids can be beneficial for CVD prevention (22). A recent randomized controlled trial of replacing SFA with walnuts or vegetable oils showed reduced central diastolic blood pressure and improved blood lipid profile (23). Our secondary analysis, confirmed that olive oil intake was associated with increased levels of HDL-C. Moreover, in a randomized controlled trial including 92 participants with abdominal obesity and relatively low HDL-C concentrations, replacing SFA from butter or cheese with either MUFA or PUFA rich plant oils had major benefits on blood lipids (24). Replacement of SFA with unsaturated fatty acids from olive oil is a strategy that aligns with current dietary guidelines and recommendations to reduce the risk of cardiovascular outcomes (2). Recent studies have suggested that when MUFA from plant sources replaced MUFA from animal sources and SFA, lower risk of CHD and CVD mortality were observed (25, 26). Controlled feeding studies that examined vegetable oils rich in MUFAs, including olive oil, high-oleic-acid sunflower oil, high-oleic acid canola oil, and nuts, have consistently demonstrated beneficial effects of higher intake of these oils on reducing cardiovascular risk factors (23). Therefore, consumption of other plant oils could also be a healthy alternative when compared to animal fat, especially because they tend to be more affordable in the U.S. compared to olive oil. However, further research is needed to confirm the effects of plant oils on health outcomes.
Olive oil is high in oleic acid and is less susceptible to oxidation than more unsaturated fatty acids (27). It has also been observed that olive oil can have favorable effects on endothelial dysfunction, hypertension, inflammation, insulin sensitivity, and diabetes (3, 5, 28). Experimental studies and clinical trials have shown that olive oil, especially the virgin olive oil variety that is richer in polyphenolic compounds and other bioactive molecules, is associated with lower risk of CVD and its risk factors due to its antioxidant capacity (5). Our results showed that higher olive oil intake was associated with lower levels of inflammatory biomarkers and a better lipid profile. It is likely that higher intake of olive oil, and especially the virgin olive oil varieties, might have stronger inverse associations with inflammatory and lipid biomarkers. Despite olive oil being a high-fat, high-energy food, it has not been associated with weight gain (29).
Strengths and limitations
The strengths of the present study include the large sample size, long-term and high rates of follow-up, use of repeated measurements of diet and lifestyle variables, the use of validated FFQ, and analyses of several CVD outcomes including fatal and nonfatal CVD, CHD, and stroke. Our analyses were extended by including secondary analysis on biomarkers which are surrogate markers of CVD. The limitations of the present study also deserve consideration. First, because of the observational design, a causal association was not demonstrated and residual confounding remains a possibility even though the analyses were extensively adjusted for potential confounders. Second, these analyses were conducted in cohorts of predominantly non-Hispanic white nurses and health professionals, which minimizes potential confounding by socioeconomic status but may limit the generalizability. Still, there is no reason to expect that the underlying biological mechanisms may be different in other ethnic groups. Third, although validated, the FFQ and self-reported diet can produce measurement errors in intake of olive oil and other plant oils. However, the use of repeated measurements reduced random measurement errors caused by within-person variation. Fourth, because this information was not recorded, we could not distinguish between the different olive oil varieties. Finally, because we have conducted a large number of statistical tests it is possible that some of them were incorrectly discovered. Although not necessary due to the study desing, when Bonferroni corrections with a more conservative P value for multiple testing were applied, the main results and conclusions remained unchanged.
Conclusions
In summary, in this large study of U.S. men and women, higher intake of olive oil was associated with significantly lower risk of CVD and CHD. Replacing margarine, butter, mayonnaise, and dairy fat with olive oil was associated with lower incidence of cardiovascular events. Our study provides further evidence that the intake of plant-based healthy fats can improve diet quality and play a role in CVD prevention in the general population.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care:
Dietary intake of olive oil was inversely associated with cardiovascular disease in general and coronary artery disease in particular in two large patient cohorts in the U.S., and replacing other types of fat like margarine, butter, mayonnaise, and dairy fat (but not plant oils) with olive oil is associated with lower cardiovascular risk.
Translational Outlook:
Further research is needed to disclose the mechanisms underlying the associations between olive oil consumption and cardiovascular risk and identify population groups most likely to benefit from replacement of saturated and animal fats with unsaturated plant oils, such as olive oil.
Acknowledgements:
We thank the participants and staff of the Nurses’ Health Study and Health Professionals Follow-up Study for their valuable contributions.
Funding: This work was supported by research grants UM1 CA186107, UM1 CA176726, U01 CA167552, P01 CA87969, P01 CA055075, R01 HL034594, HL088521, HL35464, and HL60712 from the National Institutes of Health. MG-F is supported by the American Diabetes Association grant #1-18-PMF-029.
Abbreviations:
- AHEI
alternative healthy eating index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- FFQ
food frequency questionnaire
- HDL-C
high-density lipoprotein cholesterol
- HPFS
Health Professionals Follow-up study
- LDL-C
low-density lipoprotein cholesterol
- MUFA
monounsaturated fatty acids
- NHS
Nurses’ Health Study
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
- sICAM-1
soluble intercellular adhesion molecule-1
- TNFα-R2
tumor necrosis factor-α receptor 2
- U.S.
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JSS and MAM-G received olive oil used in the PREDIMED and PREDIMED-PLUS trials from The Fundacion Patrimonio Comunal Olivarero and Hojiblanca SA (Malaga, Spain). FBH received research support from California Walnut Commission, outside the submitted work. No conflicts of interest were reported for other authors.
Suggested Tweet (@MartaGuasch1): Findings from two large US cohort studies showed that higher olive oil intake was associated with lower CVD risk.
References
- 1.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–69. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaforio JJ, Visioli F, Alarcón-de-la-Lastra C, et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019;11(9), E2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keys A Olive oil and Coronary Heart Disease. Lancet 1987;1(8539):983–4. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Canela M, Martínez-González MA. Olive oil in the primary prevention of cardiovascular disease. Maturitas 2011;68:245–250. [DOI] [PubMed] [Google Scholar]
- 6.Bendinelli B, Masala G, Saieva C, et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: the EPICOR Study. Am J Clin Nutr. 2011;93(2):275–283 [DOI] [PubMed] [Google Scholar]
- 7.Buckland G, Travier N, Barricarte A, et al. Olive oil intake and CHD in the European Prospective Investigation into Cancer and Nutrition Spanish cohort. Br J Nutr. 2012;108(11):2075–82. [DOI] [PubMed] [Google Scholar]
- 8.Guasch-Ferré M, Hu FB, Martínez-González MA, et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estruch R, Ros E, Salas-Salvadó J, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018; 379(14):1388–1389. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-González MA, Dominguez LJ, Delgado-Rodríguez M. Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case–control, cohort and intervention studies. Br Jr Nutr. 2014; 112(2):248–59. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114-26-36. [DOI] [PubMed] [Google Scholar]
- 13.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12:113–44. [PubMed] [Google Scholar]
- 14.Yuan C, Spiegelman D, Rimm EB, et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol. 2018;187(5):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvard TH Chan School of Public Health Nutrition Department. Food composition table. https://regepi.bwh.harvard.edu/health/nutrition/. [Google Scholar]
- 16.Pai JK, Pischon T, Ma J, et al. Inflammatory Markers and the Risk of Coronary Heart Disease in Men and Women. N Engl J Med. 2004;351(25):2599–2610. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Rice MS, Huang T, et al. Circulating prolactin concentrations and risk of type 2 diabetes in US women. Diabetologia 2018;61(12):2549–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckland G, Mayen AL, Agudo A, et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am J Clin Nutr. 2012;96(1):142–149. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Jarne E, Martínez-Losa E, Prado-Santamaría M, et al. Risk of first non-fatal myocardial infarction negatively associated with olive oil consumption: a case-control study in Spain. Int J Epidemiol. 2002;31(2):474–80. [DOI] [PubMed] [Google Scholar]
- 20.Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A. Mediterranean diet and CHD: the Greek European Prospective Investigation into Cancer and Nutrition cohort. Br J Nutr. 2012;108(4):699–709. [DOI] [PubMed] [Google Scholar]
- 21.Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol. 2012;176(12):1185–92. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tindall AM, Petersen KS, Skulas-Ray AC, Richter CK, Proctor DN, Kris-Etherton PM. Replacing Saturated Fat With Walnuts or Vegetable Oils Improves Central Blood Pressure and Serum Lipids in Adults at Risk for Cardiovascular Disease: A Randomized Controlled-Feeding Trial. J Am Heart Assoc. 2019;8(9):e011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brassard D, Tessier-Grenier M, Allaire J, et al. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: a randomized controlled trial. Am J Clin Nutr. 2017;105(4):800–809. [DOI] [PubMed] [Google Scholar]
- 25.Zong G, Li Y, Sampson L, et al. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr. 2018;107(3):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guasch-Ferré M, Zong G, Willett WC, et al. Associations of Monounsaturated Fatty Acids From Plant and Animal Sources With Total and Cause-Specific Mortality in Two US Prospective Cohort Studies. Circ Res. 2019;124(8):1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covas MI, Konstantinidou V, Fito M. Olive oil and cardiovascular health. J Cardiovasc Pharmacol 2009;54(6):477–482. [DOI] [PubMed] [Google Scholar]
- 28.Tierney AC, Roche HM. The potential role of olive oil-derived MUFA in insulin sensitivity. Mol. Nutr. Food Res 2007;51:1235–48. [DOI] [PubMed] [Google Scholar]
- 29.Estruch R, Marténez-González MA, Corella D, et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):e6–e17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


