Abstract
Episodic memory reflects the ability to recollect the temporal and spatial context of past experiences. Episodic memories depend on the hippocampus, but have been proposed to undergo forgetting unless consolidated during off-line periods like sleep to neocortical areas for long-term storage. Here, we propose an alternative to systems consolidation theory — a contextual binding account — in which the hippocampus binds item- and context-related information. We compare this account with behavioral, lesion, neuroimaging and sleep studies of episodic memory, and contend that forgetting is largely due to contextual interference. Accordingly, episodic memory remains dependent on the hippocampus across time, contextual drift produces post-encoding activity, and sleep benefits memory by reducing contextual interference.
One of the central goals of memory research is to understand why we remember some events and forget others. Over 100 years ago, memory consolidation was proposed as a way of partially answering this question1,2. The main idea is that new memories will be rapidly forgotten unless they undergo an active post-encoding consolidation process that fixes those memories into long term storage. Consolidation is thought to occur at both the cellular and the systems levels3,4. Cellular consolidation is essential for memory retention and it refers to the cascade of molecular processes that occur immediately after learning that stabilize the cellular and synaptic changes produced by learning3;5. In contrast, systems consolidation (SC) refers to the idea that memories for events or episodes are only temporarily dependent on the hippocampus and so they will be forgotten unless they go through a consolidation process that effectively transfers the content of those memories to the neocortex such that they are no longer dependent on the hippocampus4,6–10. Systems consolidation is assumed to occur during off-line periods such as sleep, during which the hippocampus replays previously encoded events to the neocortex, leading to the gradual strengthening of cortical associations without strengthening hippocampal associations.
SC theory has been widely accepted in the cognitive neuroscience literature, and it has garnered support from various areas of research, including behavioral studies of forgetting, lesion and neuroimaging studies, and studies of sleep6–9,11 (but also see12–14). In the current paper, we describe an alternative approach that we refer to as ‘contextual binding’ (CB) theory which assumes that episodic memory is not consolidated to the cortex and that instead gradually changing context leads to forgetting and temporally extended encoding activity. This approach is then assessed in light of the research that has been used as evidence for SC. Although some findings are found to be equally well explained by both accounts, a growing body of research directly challenges the assumptions of SC and provides support for CB (Box 1). We argue that a CB account of episodic memory and the medial temporal lobe provides a useful way of understanding when forgetting will occur, and how memory is gradually altered over time.
Box 1 |. Current results and future studies.
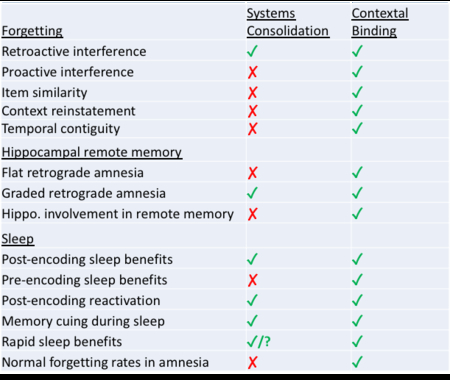
a | Well established empirical results relevant in assessing the systems consolidation and contextual binding theories. A check mark indicates results that are accurately predicted by the theory, whereas an x indicates that the results either cannot be explained or they require additional post hoc assumptions.
b | Further tests of contextual binding theory:
1. What is the relationship between memory encoding activity and pre-encoding activity?
Whereas the consolidation construct leads one to examine brain activity that occurs after the study event has been encoded, CB suggests that neural activity that occurs prior to encoding will also play a critical role. To the extent that pre-encoding activity reflects processing of ongoing context that is shared with the subsequent encoding event, one should observe ‘memory pre-activation’ that is predictive of subsequent memory. Because time moves in a forward direction, post-encoding activity should be more similar to the encoding activity than pre-encoding activity, but it should be possible to observe pre-activation effects that parallel the reactivation effects.
2. What is the effect of changing context on pre- and post-encoding activity?
The CB approach suggests that studies examining the effects of changing context prior to and after encoding will be useful in understanding phenomenon once thought to involve consolidation. For example, to the extent that memory reactivation results reflect lingering context-related activity, those effects should be reduced under conditions in which the physical or mental context changes between the encoding and the post-encoding period. Evidence in support of this possibility comes from studies of post-encoding stress, which have indicated that stress-related increases in cortisol immediately after learning can slow forgetting, presumably by facilitating consolidation. Importantly, recent work has indicated that these consolidation effects only occur when the stressor occurs in the same spatial context as the initial learning161. See also:
3. How does pre-encoding sleep impact encoding?
Although most prior memory studies of sleep have focused on post-encoding sleep, more work examining the effects of sleep immediately prior to encoding will be important. For example, given the importance of SWS in reducing retroactive interference, one would predict that pre-encoding SWS should also reduce proactive interference. Moreover, CB predicts that sleep effects should have larger effects on episodic tasks that have a heavy contextual component, as is seen in studies of post-encoding sleep, but whether this holds for pre-encoding sleep is not yet known.
4. Under what conditions does post-encoding memory-cuing improve memory?
CB suggest these effects should be most pronounced for contextual driven episodic memory tests. Moreover, these effects should not be limited to sleep, as some studies have suggested, rather cueing an earlier memory while the subject is awake should also lead to benefits in memory. Moreover, if post-encoding cueing enhances memory by leading subjects to re-encode the item, one would expect it to lead to an increase in hippocampal activation during the time of final retrieval, whereas a consolidation account presumably would not predict an increase in hippocampal involvement.
5. Are there conditions that consistently lead to graded retrograde amnesia?
In the limited number of cases in which hippocampal damage does lead to temporally graded retrograde amnesia, if those results are due to CB rather than SC, then context manipulations should be found to modulate those effects. For example, evidence for temporal gradients should be decreased if the stimuli are less likely to lead to re-encoding (e.g., less aversive or less appetitive learning in rodent studies) or under conditions in which the delay context is less likely to cue the initial encoding event and thus reduce the likelihood that the initial memory is incidentally retrieved.
6. Under what conditions does the neocortex exhibit rapid learning without the hippocampus?
Systems consolidation is sometimes thought to be necessary because the cortex can only learn very slowly, and it needs the hippocampus to gradually train it8. However, there are cases in which the cortex learns very quickly, such as in amnesic patients when random associations are treated as single units162, or in rodents when learning is related to well learned schemas163. Now that we know that the cortex can support instantaneous learning, this opens up opportunities to try to reduce hippocampal based memory deficits, such as those seen in aging ().
The contextual binding model
The CB approach (see Figure 1) assumes that episodic memory is dependent on the hippocampus and it reflects the ability to retrieve the context in which items or objects were previously encountered. Context refers to any aspect of the study episode that links the test item to the specific study event, such as remembering the spatial, temporal or other details of that event. So, for example, subjects might encounter several objects that are presented in an experimental context, and then at some later time they are either required to retrieve the objects that occurred in that study context (i.e., a recall task), or indicate if an object was or was not encoded in that context (i.e., a recognition task). Similarly, a rodent might be required to navigate to an earlier learned location (i.e., a water maze task) or discriminate between recently studied and relatively novel objects (i.e., object recognition). The hippocampus is assumed to bind together item and context information that it receives from other regions including the neocortex, and so is critical for the recollection of previous episodes. In contrast, the neocortex plays less of a role in learning detailed contextual information, and instead supports familiarity (i.e., discriminating between recently presented and novel items) and semantic memory (i.e., the acquisition of knowledge about the world)15–18. In addition, we assume that context gradually changes as the physical and mental state of the subject changes17,23,24,152, and as such, the episodic memory event that is being remembered cannot be treated as being limited to the time period in which the study item or object is presented, but rather it extends in time to include the time prior to and after the nominal study item is presented.
Fig. 1 |. Contextual binding theory.
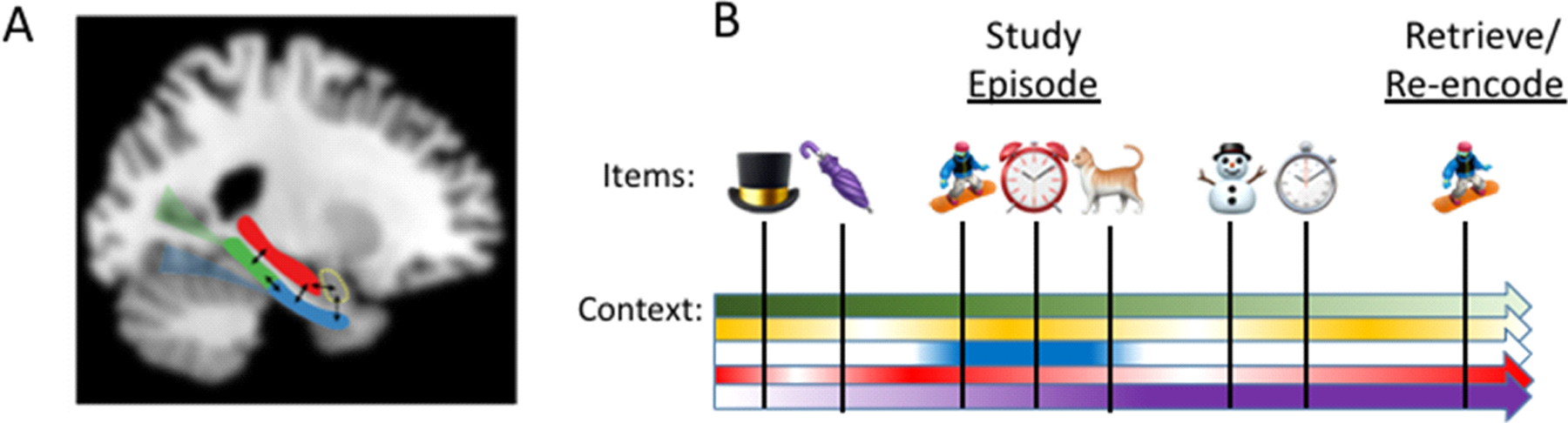
a | Contextual binding theory assumes that the hippocampus (red) is necessary for episodic memory because it binds together the item and context information that makes up the study event. The hippocampus receives information from various regions including the perirhinal cortex and the ventral ‘what’ stream, and is thought to provide information about the items in an event (e.g., objects and people), the amygdala (dotted yellow) which provides information about the emotional aspects of the event, and the parahippocampal cortex (green) which receives spatial information from the dorsal ‘where’ stream. The regions outside the hippocampus are assumed to support the learning of simple associations, and so can learn about regularities and occurrences in the environment, whereas the hippocampus is unique in supporting memory for individual episodes, and so is said to support complex or high-resolution bindings179, in the sense that it links together the multiple objects and detailed contextual information that makes up an event. This approach is consistent with neurocomputational models that propose that the hippocampus supports memory via a process of pattern separation and completion8,17,180. b | According to the CB model, context can reflect any aspect of the study episode that links the test item to the specific study event, such as the spatial, temporal or cognitive details of that event. Some aspects of context can change quickly like moving to a new room or initiating a new cognitive task (e.g., the blue arrow), whereas other aspects of context can change gradually like changes in the subject’s mood or changes in lighting throughout the day. Because context gradually drifts, the study event will extend in time beyond the occurrence of the study items themselves. In this way, forgetting is assumed to be due to interference from other memories that share similar content or context. That is, because episodic memory requires subjects to recollect which items (i.e., snowboarder, clock, cat) occurred in a specific experimental context (i.e., the related portion of the context arrows), other episodic memories that share a similar context to the studied items (i.e., umbrella and snowman) or that have similar content (i.e., stopwatch) will interfere with memory retrieval because they are confusable and effectively compete with each other. Importantly, because forgetting is the result of contextual interference, forgetting will be produced not only by events that occur after the study event, but also by events that occur prior to the study event (i.e., top-hat and umbrella). In addition, manipulations that reduce the encoding of interfering materials, such as allowing subjects to rest or sleep, are expected to benefit memory by reducing contextual interference. Moreover, if an item is repeated (e.g., the item may be re-studied or the initial event may be remembered) it will be re-encoded along with new context information. Finally, neural activity that is related to the encoding of the study event will be temporally extended because of the gradually changing context, such that encoding related activity will linger after the nominal study event is over (i.e., re-activation), and in fact, may even be observed prior to the onset of the study event (i.e., pre-activation).
Because context drifts, this will impact what will be forgotten, and it will produce temporally extended encoding related neural activity. For example, items that occur immediately before or after the study event will share a similar context and thus will interfere with recollection for the study event. Conversely, conditions such as sleep that reduce the encoding of new interfering memories will benefit subsequent recollection, essentially acting like a switch to a non-interfering, novel context. Moreover, context should be beneficial for memory in the sense that it should enhance the likelihood of remembering temporally contiguous items in an event, and should improve memory if it can be reinstated at the time of retrieval. In addition, because context gradually drifts, encoding related brain activity should be observed for some time after the nominal encoding event is over. Moreover, encoding related activity related to the study context, should also be observable even before the study event is initiated. We note that such temporal context effects should be largest in the forward direction because the context of the post encoding materials will presumably include information from the prior study event19.
CB differs from SC in that the CB assumes that the hippocampus plays a necessary - and not just temporary - role in episodic memory. Moreover, according to the CB account, the hippocampus and neocortex are assumed to largely support different types of memory (i.e., recollection and familiarity/semantic memory, respectively) rather than the same types of memory at different times. In addition, forgetting is assumed to reflect interference from events that can occur before or after the study event, rather than as reflecting the failure of systems consolidation that occurs after an event is encoded. Finally, context-related activity should be observed shortly before and after the study event.
Forgetting
Both CB and SC theories provide explanations for how memories can be forgotten, but CB provides a more general account of forgetting and it accounts for a number of forgetting effects that SC cannot explain without making additional assumptions.
Graded retroactive interference.
One of the initial motivations for consolidation theory was the finding by Müller and Pilzecker2, illustrated in Figure 2A, that forgetting is greatest when interfering information occurs shortly after encoding rather than later in the retention interval2,20–22. This graded retroactive interference was taken as evidence that memories are actively consolidated after encoding, and that this process is disrupted if interfering information is encountered before consolidation is complete. Moreover, it was argued that the results strongly compel a consolidation account because an interference account would predict that interfering materials should have a similar impact on memory whether they occur early or late in the retention interval.
Fig. 2 |. Results that have historically been taken as evidence in support of systems consolidation theory.
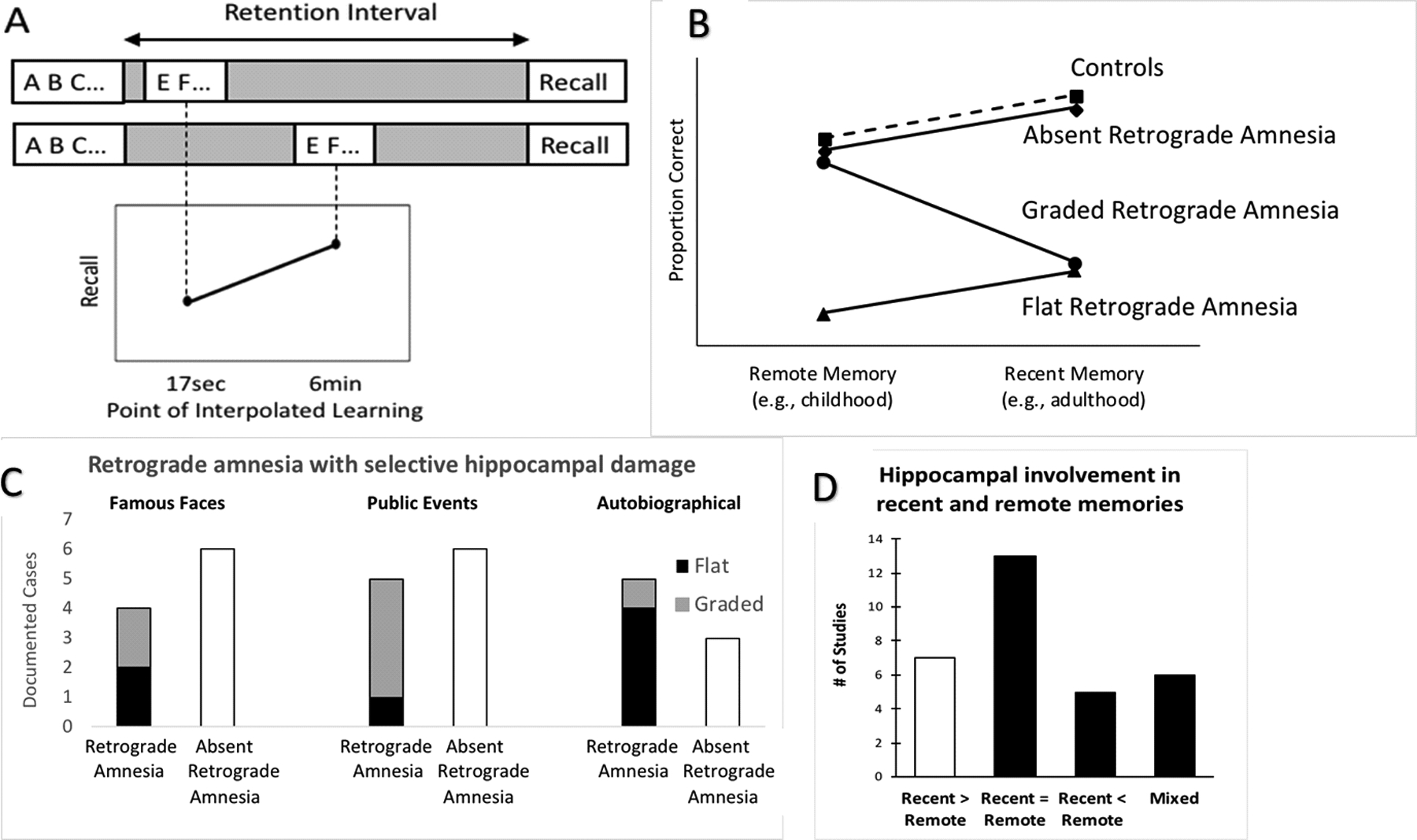
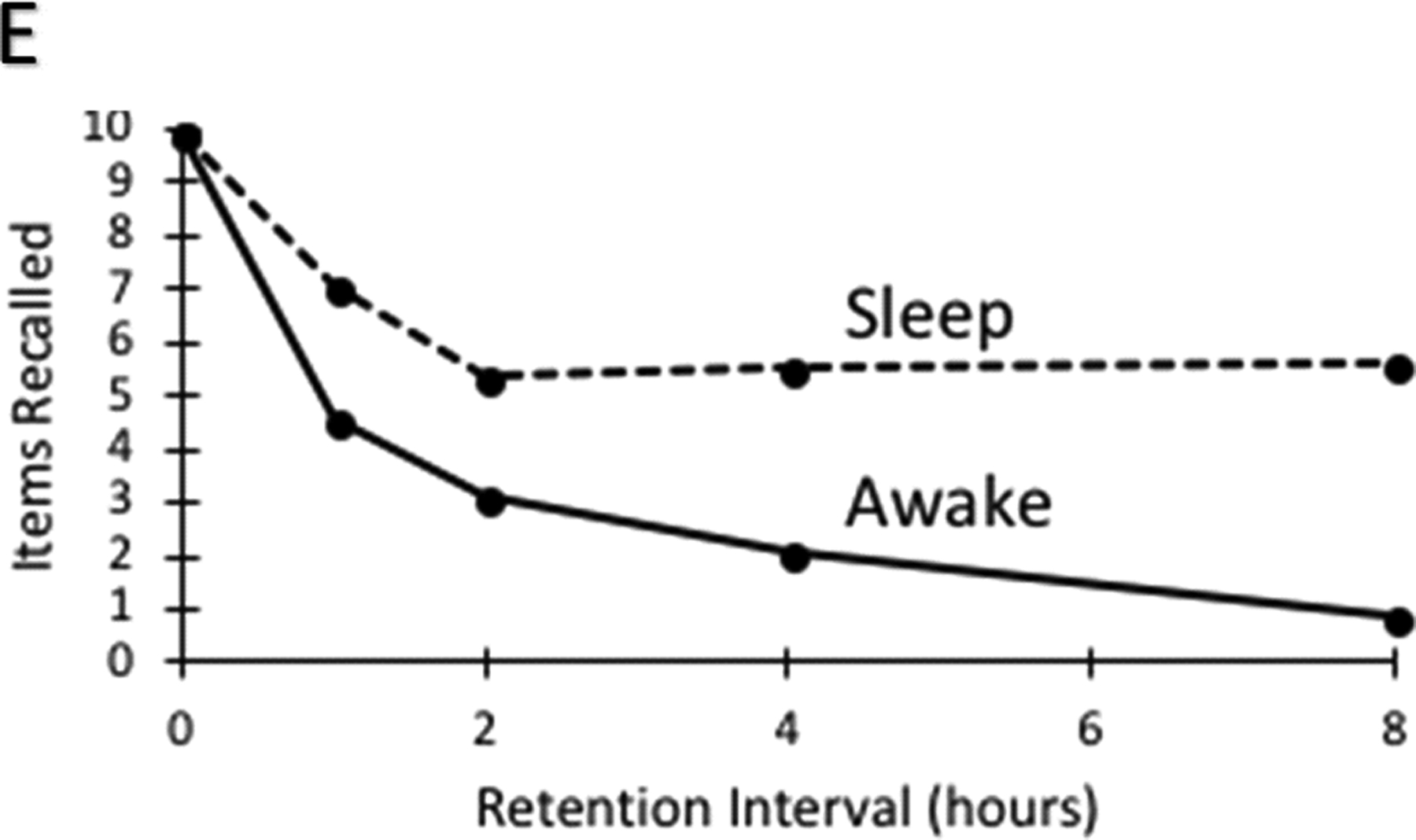
a | Graded retroactive interference. Forgetting of an event is greater when subsequent interfering information is encountered shortly after encoding. Müller and Pilzecker2 presented subjects with nonsense syllables (represented here by letters) and tested cued recall after a 1.5 hour retention interval. Importantly, they found that if subjects were presented with additional nonsense syllables to learn shortly after the initial encoding phase (top row; left on graph), subjects recalled fewer of the initial items than if the additional nonsense syllables were presented later in the retention interval (bottom row; right on graph). The finding can be explained if the interpolated task disrupts consolidation, or if it produces more contextual interference. b | Retrograde amnesia. Amnesic patients may be, impaired at retrieving memory from recent but not remote periods (i.e., graded retrograde amnesia) - a pattern consistent with SC - but they may also be impaired at retrieving memories for both remote and recent events (i.e., flat retrograde amnesia) or they may be unimpaired for both time periods (i.e., absent retrograde amnesia). c | Reports of graded retrograde amnesia in patients with selective hippocampal damage are rare. A number of patients with selective hippocampal damage have been assessed on standard tests of retrograde amnesia52,164,182–187. In the famous faces test, only 2 patients (i.e., patients LM and WH52) out of 10 revealed evidence of graded retrograde amnesia. Similarly, in tests of memory for public events, only 4 patients (i.e. GD, LM & WH52, and patient YK164) out of 11 showed evidence of a temporal gradient. On the autobiographical memory interview (AMI), only one patient out of 8 (i.e., YK11,164) exhibited a greater impairment for recent than remote periods. Interestingly, the authors of the lattermost study indicated that YK’s memory reports were entirely lacking in episodic details, suggesting impairments in both remote and recent autobiographical memory. Four additional patients with selective hippocampal damage were assessed on the AMI182, and were unimpaired at the remote period, but the recent period results were not reported, so they are not included in this figure. Nevertheless, in a subsequent reanalysis, those patients were included with additional patients that could not be scanned and as a group they exhibited only a mild memory impairment for recent items that was limited to autobiographical memory questions and not the personal semantic memory items of the AMI53. d | Retrieval related hippocampal activity for remote and recent memories. Human neuroimaging studies suggest that the hippocampus is involved in retrieving both recent and remote memories. That is, the most common finding is that the hippocampus is similarly involved during the retrieval of both remote and recent memories54–57,188–196. A few studies reported either greater197–203 or less204–208 hippocampal activity for recent than for remote memories, whereas others reported mixed results209–215 such as214 the left hippocampus being equally involved in remote and recent memories whereas the right hippocampus was more active for recent than remote memory (the figure was based on an informal review by searching Pubmed using the search string “(remote) AND (memory) AND (hippocampus)”, as well as well as examining citations and references. These studies are characterized in more detail in supplementary Table 1). e | The effects of sleep on forgetting in episodic memory (based on Table 3 from Jenkins and Dallenbach, 1924101). Subjects learned a sequence of nonsense syllables and were then tested for recall after delays varying in length from 1 to 8 hours that were filled either with sleep or wake. Compared to the awake condition, subjects who slept exhibited significantly slower forgetting rates. The finding can be explained if sleep facilitates consolidation or if it reduces the encoding of interfering information.
However, these results are predicted quite naturally by CB without making assumptions about consolidation (also see12,23–25). That is, when interfering items are presented immediately after the initial encoding event, they interfere to a greater extent than items presented later in the retention period because the initial encoded items and the immediately presented interfering items involve greater contextual overlap. Moreover, contextual interference should occur even if the intervening items are not highly similar to the study materials, as was reported by Müller and Pilzecker2. We suspect that the reason these graded retroactive interference results are often thought to challenge interference explanations is that earlier theories of interference focused exclusively on interference from similar items, and they attributed little or no role to the importance of context in episodic memory.
Related forgetting effects.
CB, and interference theories in general, also naturally explain a number of other well-established forgetting effects that SC cannot account for without making additional assumptions. For example, forgetting is produced not only when interfering information is presented shortly after the study event, but also when it is presented just before the study event - a well-established experimental phenomenon referred to as proactive interference26,27,28. CB predicts both retroactive and proactive interference effects, while SC only predicts retroactive effects because consolidation occurs after the study event. Moreover, although Müller and Pilzecker found that the forgetting effects could occur even when the interfering materials were quite dissimilar from the studied materials, forgetting generally increases as the interfering materials become more similar to the study materials29,30. This increased forgetting due to similarity is predicted by interference theories — as forgetting is expected to arise due to interference from similar events — but this is not predicted by consolidation theory.
Context models also provide a way of explaining why context can sometimes have beneficial effects on memory. For example, retroactive interference effects, such as those seen in the study of Müller and Pilzecker2, are critically dependent on the context in which the interfering information occurs. Thus, forgetting effects are greatly reduced if the interfering materials are learned in a spatial context than the study materials31–33. In addition, in ‘context reinstatement’ studies, memory performance is generally increased if the test context matches that of the study context34,35, and items that have been forgotten can often be rescued by reinstating the physical or mental context of the original study phase36,37. These types of results are predicted by models that assume that episodic memory involves the binding of item and context information, but are not predicted by SC. In addition, studies of ‘temporal contiguity’ have shown that subjects tend to successively recall and are more likely to recognize items that were presented in nearby positions on a studied list19,38, with such contiguity effects spanning potentially many other intervening memories39. In addition, studies of contiguity effects have indicated that context-related signals in the medial temporal lobe change gradually during the presentation of a study list, and then are reinstated when an item is remembered during test40–44. These results provide direct support for contextual binding models.
One might argue that SC should not be expected to account for these forgetting effects because these studies examine delays of only minutes or days and so may not be relevant if SC takes years or decades to occur, as some have suggested8,45,46. However, others have assumed that SC occurs very rapidly7,11,47, such that even a 60-minute nap immediately after learning can benefit consolidation and, accordingly, neural reactivation immediately after encoding is assumed to reflect SC (see below). Ambiguity about the duration of the proposed consolidation process has led to some degree of theoretical drift and vagueness that has been criticized by some13 but because SC theory is used to account for results from studies of both short and long delay periods, all of these results are relevant.
Hippocampal remote memory
If the hippocampus is only temporarily involved in episodic memory then it should be involved in retrieving recently encoded events, but should not be involved in remotely encoded events. However, contrary to the predictions of SC, it is now clear that the hippocampus is critically involved in remote episodic memory.
Graded retrograde amnesia.
Another cornerstone of SC is the observation that patients with hippocampal damage, such as the famous patient HM, exhibit graded retrograde amnesia (Fig2a): they cannot remember events that occurred during the years just before the lesion, but can retrieve memories from more remote time periods, such as childhood48–50. This pattern is consistent with SC, but an examination of the existing literature indicates that graded retrograde amnesia is rare in patients with selective hippocampal lesions, and that, even when it is observed, it is quite variable. For example, more recent work with patient HM revealed that he was severely impaired at retrieving episodic details for both remote and recent time periods (i.e., flat retrograde amnesia), and that he showed relative sparing of semantic memory across those same periods51 (i.e., absent retrograde amnesia).
In addition, studies examining retrograde amnesia in patients with more selective hippocampal lesions also fail to find compelling evidence for the graded retrograde amnesia predicted by SC. For example, Figure 2c illustrates the published reports of retrograde amnesia as assessed on formal tests in patients with reported selective hippocampal lesions. The results indicate that on tests of semantic memory (i.e., tests for famous faces and public events), in most cases, retrograde memory is unaffected by hippocampal damage. Even in the few patients that appear to exhibit graded retrograde amnesia, the duration over which the gradient is observed is quite variable. For example, some patients exhibit retrograde impairments that extend to periods well over 15 years prior to the lesion52, whereas others exhibit deficits extending only 1 to 5 years53. In tests of autobiographical memory, on the other hand, retrograde amnesia is more common, and it tends to lead to similar deficits in both recent and remote time periods, indicating that episodic memory is always dependent on the hippocampus.
Neuroimaging of remote and recent episodic memory.
Several neuroimaging studies have aimed to determine if the hippocampus is more involved in the retrieval of recent than remote memories, as predicted by SC. However, as illustrated in Figure 2d, the hippocampus is most often found to be equally involved in the retrieval of both recent and remote memories. One potential concern is that recent memories may be more vivid or stronger than remote memories and so might lead to greater activation, or conversely, remote memories may be weaker and thus they may lead to additional encoding into memory during the retrieval test, thus leading to greater retrieval-related activation. However, studies controlling for the vividness or strength of memory54–57 have reported hippocampal involvement during remote and recent memory retrieval.
Although there is little support for the assumption that the hippocampus becomes less involved as episodic memories become more remote, do cortical regions such as the medial prefrontal cortex (mPFC) become more involved as memories age? The evidence is mixed. For example, some human neuroimaging studies have shown greater frontal activity for older memories, whereas others have reported less or similar activity for remote memories (for reviews58,59). This is consistent with studies showing that damage to the mPFC does not generally lead to remote memory impairments59,60, but rather is associated with confabulation61, and decreases in self-related processing62. We note however, that rodent studies have indicated that disruption of mPFC within a 1–2 hour period after learning can lead to memory impairments whereas disruption outside this window does not63. The latter results suggest that the mPFC may play a role in cellular rather than systems consolidation.
Animal studies.
Studies of hippocampal lesions in rodents are consistent with those in the human literature in showing that tasks requiring the retrieval of detailed contextual memories, such as remembering the location of a hidden escape platform in a water maze, or finding a food-well location containing food, hippocampal damage leads to flat retrograde amnesia64–73. Moreover, consistent with the lesion studies, activation studies in rodents that have used the water maze report similar or higher levels of hippocampal activation for remote compared to recent memories74–78.
In contrast, in memory tasks requiring less precise contextual information the results are quite mixed, with some studies showing flat retrograde amnesia and others showing graded effects. For example, several studies of context fear conditioning – in which rodents exhibit freezing behaviors when placed in an enclosure that was previously paired with foot shocks – report that hippocampal lesions equally reduce freezing behavior for remote and for recently learned associations (i.e., flat retrograde amnesia)71,79–86, whereas others report that recent memories are more disrupted than remote memories87–96. Activation studies examining contextual fear conditioning are also quite mixed, with some reporting evidence for hippocampal involvement in remote memory and others failing to80,97–99. However, similar to the effect of lesions, hippocampal activity appears to be correlated with memory precision rather than the age of the memory94,100.
In sum, the results from lesion and activation studies of both humans and rodents indicate that the hippocampus plays a critical role in supporting both remote and recent episodic memory, which is consistent with the CB model and contradicts the predictions of the SC model. There are a few reported cases of graded retrograde impairments in humans rodents that require less contextually rich memory such as fear conditioning. These later results are consistent with SC but they can also be explained by CB. That is, because remote memories will have had more opportunity than recent memories to be remembered and re-encoded before the lesion, they can be supported by the neocortex.
Sleep
In one of the first systematic studies of sleep on memory, Jenkins and Dallenbach101 found that forgetting was slower if subjects were allowed to sleep immediately after learning than if they were required to remain awake (Figure 2e). It is possible that sleep slowed forgetting because memories were consolidated to the cortex during sleep. Another potential explanation, however, is that forgetting was slowed because subjects encoded less interfering information when they were asleep than when they remained awake. As described below, there are other aspects of sleep that are equally well explained by these two different accounts, but there are several findings that appear to preferentially support the CB.
Post-encoding slow wave sleep benefits episodic memory.
After an event has been encoded, it appears to be slow-wave sleep (SWS; i.e., periods of low-frequency oscillations containing brief periods of high-frequency activity that dominates early night sleep), rather than rapid eye movement sleep (REM; i.e., periods of wake-like neural activity that is associated with vivid dreaming that dominates late night sleep), which is the most beneficial for episodic memory102. Why SWS is more important for episodic memory than REM sleep is currently debated102–104, but this finding is broadly consistent with both the SC and CB accounts. From the perspective of SC, SWS may be particularly important if slow wave activity in the cortex were to drive repeated reactivation of hippocampal representations (via fast-wave ripples) that might then entrain cortical regions102,105. In contrast, from the perspective of CB theory, SWS may be particularly effective at reducing contextual interference because it is deeper than REM sleep in the sense that electrophysiological activity, heart rate and blood pressure observed during SWS are least like that observed during wake. In addition, SWS tends to occur earlier in sleep when interference effects are greatest (i.e., the period shortly after encoding).
The beneficial effects of post-encoding sleep are most pronounced on memory tasks that require subjects to remember associative compared to item information106–110but see111,112, and for recollection-based recognition responses more than familiarity-based responses107,108,110,113–116. These results are consistent with CB in the sense that if sleep reduces contextual interference it should be memory for contextual information that is particularly sensitive to sleep, but how the SC approach would account for these results is not clear.
Reactivation.
There is a growing body of research showing that encoding-related activity can be observed during periods of post-encoding sleep102,117–119, with such reactivation results consistent with both SC and CB. For example, hippocampal activity during SWS is increased after a navigation task, and correlates with subsequent memory119. These results are similar to rodent studies of ‘replay’ that have indicated that during offline states such as sleep, hippocampal place cells fire in a temporal sequence that correlates with the order of place fields recorded during earlier exploration120,121. These reactivation effects were thought to be limited to sleep, thus reflecting off-line hippocampal replay of recent memories. Recent results, however, have indicated that sleep per se does not have a priveledged roles in this process in the sense that replay in rodents also occur in awake animals, reactivation effects in humans are observed during periods of quiet wakefulness, and when subjects are completing demanding math tasks102,122–125. In addition, so far such reactivation effects have only been well established for a short period after learning (e.g., less than an hour after learning in rodents [Kudrimotri et al], and within a hour after learning in humans [refs]). Thus, reactivation is not limited to sleep or rest, but instead seems to occur regardless of sleep status for a short period of time after information has been encoded.
Such reactivation results could reflect the hippocampus replaying memories in order to transfer those memories to the cortex, or they could reflect neural activity arising from contextual binding. According to the CB model, during encoding, one would expect to see the activity of the hippocampus and cortical regions supporting the information being bound to the study context. Importantly however, to the extent that learning increases the activation sensitivity of related synapses, encoding-related regions will show enhanced levels of spontaneous activity and co-activity for a short period after learning. Thus, one would expect to see a reflection of this “reverberative” activity (i.e., reactivation) after the study event. This should be particularly evident if the context remains unchanged. Moreover, because effective encoding should lead to greater residual activity and better subsequent retrieval, the residual post-encoding activation would be correlated with memory performance, as has been reported. Given that context is drifting, the variance captured by activity during the study event and the post-encoding event would not be expected to be identical. This idea is consistent with work indicating that learning temporarily increases the excitability of neurons and causes them to be activated during the formation of memories for other events that occur close in time, resulting in these events becoming linked because they involve some of the same neurons126–128. These results are also consistent with the finding that neural activity related to a specific encoding context (i.e., words presented in the context of complex visual scenes) can linger for several minutes during the encoding of subsequent words, even when the context (i.e., visual scenes) is no longer present129.
Interestingly, there have been studies that have suggested that reactivation is stronger for weakly encoded materials130, a finding that is not predicted by the CB account. However, other studies have shown that reactivation effects are stronger for well encoded materials131,132, so further empirical work in this area is warranted. Moreover, given the reactivation results observed so far have been limited to a short period after encoding, another interpretation of these results is that they reflect cellular consolidation rather than SC (see Box 2). Other studies have indicated that disrupting sharp-wave ripples in rodents impairs subsequent memory133, indicating that ripples may play a critical role in post-encoding sleep; however, whether these results reflect a disruption of systems consolidation, a disruption of context-related residual activity, or a disruption of cellular consolidation is unknown. As far as we are aware, there are no studies indicating that naturally occurring post-encoding reactivation causes hippocampal episodic memories to be forgotten while simultaneously strengthening cortical traces.
Box 2 |. The role of the hippocampus in non-episodic forms of memory.
As presented in the main text, we contend that episodic memories are not consolidated from the hippocampus to the cortex, as is suggested by systems consolidation theory. However, does the hippocampus play a time-dependent role in shaping non-episodic memory forms of memory, such as semantic memory, implicit memory and skill learning? We believe that future studies will be necessary in order to adequately answer this question.
One possibility that is consistent with the CB theory is that subjects might remember, or be reminded of, an earlier event, and this could lead to a new encoding event that would be expected to impact both the hippocampus and the neocortex. In this way, neocortical representations that support semantic memory could be influenced by hippocampal representations over multiple remindings. Such remindings certainly occur and can explain why amnestic patients are sometimes able to recall salient childhood memories11,164, and why rodents sometimes show preserved context fear conditioning for remote learning87–96. However, the extent to which this occurs is unclear, as many patients show severe deficits for remote memory and many studies of animal context fear conditioning do not show preserved remote memory. Thus, the factors that determine when semantic memory might benefit from hippocampal training is not yet clear.
Additional studies examining the role of the hippocampus in implicit memory and skill learning will also be important. For example, implicit memory and skill learning are well preserved in patients with medial temporal lobe damage165, and there little indication that this damage leads to faster forgetting in these tasks, even across delays that include many nights of sleep, as one would expect if the hippocampus gradually trained these non-episodic forms of memory. For example, amnestic patients exhibit normal rates of forgetting on perceptual implicit memory tasks including picture naming priming, across 7 day retention intervals166; conceptual implicit memory, such as sentence puzzle tasks, across 7 days167; rotor pursuit learning, across 2 years168; and mirror reading, across 3 months169. Thus, these forms of memory do not seem to benefit from a hippocampally dependent retention or transformation process, at least over the delay periods examined so far. Nevertheless, there are a number of reports of ‘incubation effects’ where performance on non-episodic memory tasks can significantly improve over time, such as sleep-related benefits in transitive inference170,171, probabilistic learning172,173, remote associates174,175, and mathematical insight problems171. In addition, there is evidence that sleep can lead to enhancements in motor learning102,176, although whether these enhancements reflect some form of consolidation is controversial177. In either case, studies designed to determine if the hippocampus plays a causal role in producing these memory benefits will be important.
Memory-cueing during sleep.
Another finding that can be explained by both SC and CB is the observation that cueing recently encoded memories during SWS enhances subsequent episodic memory134,135. For example, if subjects learn a set of location-object associations in the presence of an odor, and then are allowed to sleep, subsequent memory is improved if the odor cue is re-presented during SWS136–139. Although the conditions under which these cueing effects are observed are not yet well understood, they have been observed using olfactory and auditory retrieval cues, and they appear to be reduced if the cue is presented later in the night such as during REM sleep or if presented to awake subjects who are engaged in a demanding primary task140–142. Whether retrieval cueing results in consolidation of episodic information from the hippocampus to the cortex, or whether it simply reflects the retrieval and re-encoding of the initial study episode is not yet known, and further work will be needed to further assess these two accounts (Box 1).
Beneficial effects of sleep on memory are rapid.
Although much of the sleep literature seems equally consistent with both the SC and CB approaches, other findings seem to favor the CB account. For example, an examination of Figure 2e indicates that the beneficial effects of sleep occur remarkably quickly. That is, even after only one hour, sleep effectively increased recall from 46% to 71%. If this sleep benefit reflects SC, this means that the hippocampus transferred a large proportion of memories from the hippocampus to the cortex that normally would have been forgotten during this time. This rapid induction of sleep benefits in episodic memory has now been well documented. For example, even 60 minute naps are found to produce sizable sleep benefits in episodic memory that are often similar to those observed after an entire night of sleep143,144. Moreover, sleep has been found to slow forgetting if sleep occurs immediately after learning and has reduced or no impact on materials learned earlier in the day109,145,146.
The fact that sleep benefits memory primarily when it occurs immediately after learning is explained by CB theory, which predicts that interference effects will be largest when the study materials and the interfering materials occur close in time. Although the results are often interpreted as being consistent with SC, they do present some puzzles for the approach. If the observed sleep benefits reflect consolidation it means that a large proportion of memories that would have been forgotten are transferred to the cortex within an hour, which is difficult to reconcile with results and theoretical proposals suggesting that SC is a slow process that takes years or decades8,9. In addition, the results suggest that SC does not preserve memory for the events from across the day, but rather is limited primarily to storing events that happen immediately before falling asleep. Another possibility is that the sleep effects that have been reported in the literature reflect something other than SC, such as cellular consolidation, which would be expected to occur at this very short time scale.
Proactive effects of sleep.
If the beneficial effects of sleep are due to a reduction of contextual interference, then in the same way that interfering materials encoded before learning can interfere with memory (i.e., proactive interference), sleep should also benefit memory if it is inserted just prior to learning. Consistent with these expectations, memory for pictures is improved if subjects nap immediately before or after learning147. In addition, the capacity to encode new episodic memories is reduced after sleep deprivation148,149, and even mild sleep disruption, which decreases slow wave activity without reducing total sleep time, can reduce subsequent episodic encoding150. Moreover, the ability to encode new episodic memories decreases gradually across the day, yet can be restored by a brief nap151. Although these proactive effects of sleep on memory are consistent with the CB model, they are not predicted by SC theory, which instead proposes that consolidation facilitates recently encoded events, rather than augmenting the encoding of future events.
Normal forgetting rates in amnesia.
If the hippocampus is critical for consolidating memories to the cortex during sleep, then patients with hippocampal damage should show accelerated forgetting across delays that include sleep152–154. There is little evidence to support this prediction, however. For example, an early study in patient H.M. examined recognition memory at 10 minute, 1 day, and 7 day retention intervals under conditions in which healthy controls were matched by increasing study duration at the 10 minute delay155. Although the patient performed normally at the 10 minute delay, he seemed to perform worse than the controls at the later intervals, suggesting that he might exhibit accelerated forgetting. However, subsequent studies failed to replicate this effect, and instead showed that his forgetting rate was normal across a retention interval of 7 days in one study156, and 6 months in another157. Thus, even extensive MTL damage, as seen in HM, does not appear to lead to accelerated forgetting across delays that include periods of sleep.
Several subsequent studies with various groups of individuals with memory disorders have verified this pattern. For example, the amnesic patient NA, who suffered a diencephalic lesion, exhibited normal forgetting across a retention interval of 32 hours158, similar to amnestics with Korsakoff syndrome155,158,159. In addition, normal forgetting rates have been reported in a group of patients with extensive MTL lesions, as well as a group with diencephalic lesions160. Thus, lesion results indicate that the hippocampus does not play a causal role in slowing episodic forgetting, at least across retention intervals varying from days to months. Thus, the pronounced beneficial effects of sleep that have been observed in healthy subjects after brief periods of sleep must then be explained in some other way, such as arising because of a reduction in contextual interference.
Conclusions
SC has been a useful scientific construct in accounting for results from across various different research domains. As we show above, however, SC fails to account for a growing body of findings from the same research paradigms that motivated its original development; that is, from studies of forgetting, lesion, and activation studies of remote memory, as well as studies of the effects of sleep on memory. Overall, such results provide support for a CB account of episodic memory consistent with memory theories that do no depend on systems consolidation.
Moving forward, one important aspect of episodic memory that current work discussed above highlights is that an episodic memory should not be treated limited to the period in which the study item or object is presented, but rather extending in time before and after the nominal study event (for additional predictions of the CB theory see box 1). Thus, manipulations that occur during the retention period need not impact a hypothetical consolidation process, but rather may affect the temporally extended encoding of the study event itself. Such considerations provide an account of the existing literature once thought to support the notion of consolidation, leading to a number of novel predictions that we hope will be useful in guiding future studies.
In sum, we propose that the construct of systems consolidation may have outlived its usefulness and should be replaced by theories that acknowledge the critical role of context in episodic memory and forgetting such as CB theory. Even if CB is found to be wanting, and alternative accounts are proposed, we hope that the ideas discussed here will lead researchers to consider a wider variety of theoretical explanations when they find that memory performance or brain activity changes during a retention interval.
Supplementary Material
Box 3 |. How is contextual binding related to cellular consolidation and synaptic downregulation?
According to the CB account of episodic memory, contextual interference continues to act on memory after the nominal learning event is over. How does contextual interference relate to other post-encoding processes that affect memory? For example, cellular consolidation, which involves a cascade of molecular processes that occur in the hours shortly after learning3 could be involved in transforming memory during retention. How these processes are related to contextual binding is not yet known, and so the current proposal gives rise to new questions that will need to be empirically addressed. For example, does cellular consolidation differentially impact forms of hippocampally based contextual memory such as recollection, compared to other forms of memory such as familiarity and semantic memory178? Conversely, to what extent does contextual binding impact cellular consolidation? Given the importance of context in producing interference effects in episodic memory, cellular consolidation process might be disrupted by changes in spatial and mental context.
In addition, synaptic activity is known to be downregulated during periods of rest and sleep, to counteract the increased levels of activity induced by prolonged periods of active wake104. How CB might be related to synaptic downregulation not yet known. Although the processes underlying synaptic downregulation are thought to be observed across the cortex, do the regions that support episodic memory play any special role in governing which synapses — and thus, which memories — benefit from downregulation? For example, downregulation is thought to suppress weakly represented information while preferentially preserving information that is strongly represented in memory either because it is consistent with preexisting representations or is strongly encoded. One possibility that has yet to be explored is whether items that are well integrated with the ongoing experimental context may be preferentially protected from the effects of downregulation.
CB borrows from several earlier theoretical approaches12–15,18,25,181, but shares a number of core assumptions with ‘multiple-trace/transformation’ theory13,16 in the sense that the hippocampus is assumed to be necessary for the storage and retrieval of detail-rich episodic memories, whereas the neocortex supports the acquisition of less contextually-detailed information such as semantic knowledge. Moreover, according to both of these approaches, the retrieval of a memory is expected to lead to the re-encoding that would impact both the hippocampus and the neocortex. If this occurs frequently enough, it would lead to a strong neocortical semantic representations that could support decontextualized memory for remote events. Thus, the two approaches predict that the hippocampus will be necessary for retrieving recent and remote contextually rich memories, whereas the neocortex can support decontextualized memories, and may be particularly effective for repeatedly remembered remote memories. However, CB goes further by specifying the critical role of context in accounting for episodic memory and forgetting, and so it gives rise to additional predictions about manipulations such as interference and sleep, areas that multiple-trace/transformation theory does not make any specific predictions about. Given that the interference and sleep literatures have been interpreted as providing support for SC, this is a significant shortcoming of the multiple trace approach that we believe the CB approach overcomes. By focusing on the critical role of context in episodic memory, the CB approach builds on other theoretical work that highlight the role that context plays in both facilitating memory and producing forgetting17,23,24,152, and as described in the main text, it converges with recent empirical work showing how the medial temporal lobe supports memory for spatiotemporal context.
Acknowledgements
The paper was based on discussions with students and faculty participating in a graduate seminar at UC Davis, including Alyssa Borders, Grant Shields, Robin Goodrich, Michelle Ramey, Trevor Baer, Ilse Pastor, Cameron Riddell, Matt Sazma, Andrew McCollough, Carlos Carrasco, Maureen Ritchey, Dan Ragland, Michael Starrett, Mingli Liang,…..lots of folks from charans and brians and arne’s lab………. ☺
This work was supported by the National Eye Institute of the National Institutes of Health under Award Number R01EY025999 (APY), NIH/NINDS grants NS076856 (ADE) and NS093052 (ADE/APY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Ribot T Diseases of memory: An essay in the positive psychology. Diseases of memory: An essay in the positive psychology. Vol. 43. D. Appleton, 1882. Vol. 43 D. Appleton (1882). [Google Scholar]
- 2.Müller GE & Pilzecker A Experimentelle beiträge zur lehre vom gedächtniss Vol 1 JA Barth, (1900). [Google Scholar]
- 3.Kandel ER, Dudai Y & Mayford MR The molecular and systems biology of memory. Cell 157, 163–186, doi: 10.1016/j.cell.2014.03.001 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55, 51–86, doi: 10.1146/annurev.psych.55.090902.142050 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Bekinschtein P et al. Persistence of long-term memory storage: new insights into its molecular signatures in the hippocampus and related structures. Neurotox Res 18, 377–385, doi: 10.1007/s12640-010-9155-5 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Frankland PW & Bontempi B The organization of recent and remote memories. Nat Rev Neurosci 6, 119–130, doi: 10.1038/nrn1607 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Marr D Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci 262, 23–81 (1971). [DOI] [PubMed] [Google Scholar]
- 8.McClelland JL, McNaughton BL & O’Reilly RC Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Squire LR & Alvarez P Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 5, 169–177 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Squire LR, Genzel L, Wixted JT & Morris RG Memory consolidation. Cold Spring Harb Perspect Biol 7, a021766, doi: 10.1101/cshperspect.a021766 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diekelmann S & Born J The memory function of sleep. Nat Rev Neurosci 11, 114–126, doi: 10.1038/nrn2762 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Lewandowsky S, Ecker Ullrich KH, Farrell S & Brown GD Models of cognition and constraints from neuroscience: A case study involving consolidation. Australian Journal of Psychology 64, 37–45. (2012). [Google Scholar]
- 13.Nadel L & Moscovitch M Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7, 217–227 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Sutherland RJ & Lehmann H Alternative conceptions of memory consolidation and the role of the hippocampus at the systems level in rodents. Curr Opin Neurobiol 21, 446–451, doi: 10.1016/j.conb.2011.04.007 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Eichenbaum H, Yonelinas AP & Ranganath C The medial temporal lobe and recognition memory. Annu Rev Neurosci 30, 123–152, doi: 10.1146/annurev.neuro.30.051606.094328 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscovitch M, Nadel L, Winocur G, Gilboa A & Rosenbaum RS The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology 16, 179–190, doi: 10.1016/j.conb.2006.03.013 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Norman KA & O’Reilly RC Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev 110, 611–646, doi: 10.1037/0033-295X.110.4.611 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Yonelinas AP Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci 356, 1363–1374, doi: 10.1098/rstb.2001.0939 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahana MJ Associative retrieval processes in free recall. Mem Cognit 24, 103–109 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Dewar M, Alber J, Cowan N & Della Sala S Boosting long-term memory via wakeful rest: intentional rehearsal is not necessary, consolidation is sufficient. PLoS One 9, e109542, doi: 10.1371/journal.pone.0109542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewar MT, Cowan N & Sala SD Forgetting due to retroactive interference: a fusion of Muller and Pilzecker’s (1900) early insights into everyday forgetting and recent research on anterograde amnesia. Cortex 43, 616–634 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner HA, Squire LR & Byrne JH 100 years of consolidation--remembering Muller and Pilzecker. Learn Mem 6, 77–87 (1999). [PubMed] [Google Scholar]
- 23.Estes WK Statistical theory of spontaneous recovery and regression. Psychol Rev 62, 145–154 (1955). [DOI] [PubMed] [Google Scholar]
- 24.Mensink GM & R. JGW A model for contextual fluctuation. Journal of Mathematical Psychology 33, 172–186. (1989). [Google Scholar]
- 25.Polyn SM, Norman KA & Kahana MJ A context maintenance and retrieval model of organizational processes in free recall. Psychol Rev 116, 129–156, doi: 10.1037/a0014420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postman L & Keppel G Conditions of Cumulative Proactive-Inhibition. Journal of Experimental Psychology-General 106, 376–403 (1977). [Google Scholar]
- 27.Watkins OC, and Watkins Michael J.. Buildup of proactive inhibition as a cue-overload effect. Journal of Experimental Psychology: Human Learning and Memory 1, no. 4 (1975): 442, 442. (1975). [PubMed] [Google Scholar]
- 28.Dallett K & Wilcox GS Contextuall stimui and proactive inhibition. Journal of experimental psychology 78, 475–480 (1968). [Google Scholar]
- 29.McGeocH JA, & McDonald WT Meaningful relation and retroactive inhibition. Amer. J. Psychol, 43 579–588. (1931). [Google Scholar]
- 30.Melton AW & von Lackum WJ Retroactive and proactive inhibition in retention: Evidence for a two-factor theory of retroactive inhibition. American Journal of Psychology 54, 157–173, doi:Doi 10.2307/1416789 (1941). [DOI] [Google Scholar]
- 31.Bilodeau IM & Schlosberg H Similarity in stimulating conditions as a variable in retroactive inhibition. J Exp Psychol 41, 199–204 (1951). [DOI] [PubMed] [Google Scholar]
- 32.Greenspoon J & Ranyard R Stimulus conditiopns and retroactive inhibition. Journal of experimental psychology 53, 55–59 (1957). [DOI] [PubMed] [Google Scholar]
- 33.Strand BZ Change of Context and Retroactive Inhibition. Journal of Verbal Learning and Verbal Behavior 9, 202-&, doi:Doi 10.1016/S0022-5371(70)80051-2 (1970). [DOI] [Google Scholar]
- 34.Godden DR & Baddeley AD Context-Dependent Memory in 2 Natural Environments - Land and Underwater. Brit J Psychol 66, 325–331, doi:DOI 10.1111/j.2044-8295.1975.tb01468.x (1975). [DOI] [Google Scholar]
- 35.Tulving E & Thomson DM Word-Blindness in Episodic Memory. Psychon Sci 29, 262-& (1972). [Google Scholar]
- 36.Gardiner JM, Craik Fergus IM, and Birtwistle John. Retrieval cues and release from proactive inhibition. Journal of Verbal Learning and Verbal Behavior 11, no 6 778–783. (1972). [Google Scholar]
- 37.Kroll NEA, Ogawa KH & Nieters JE Eyewitness Memory and the Importance of Sequential Information. B Psychonomic Soc 26, 395–398 (1988). [Google Scholar]
- 38.Schwartz G, Howard MW, Jing B, & Kahana MJ. Shadows of the past: Temporal retrieval effects in recognition memory. Psychological Science 16, 898–904 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard MW, Youker TE & Venkatadass VS The persistence of memory: Contiguity effects across hundreds of seconds. Psychon B Rev 15, 58–63, doi: 10.3758/Pbr.15.1.58 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkerts S, Rutishauser U, & Howard MW. Human episodic memory retrieval is accompanied by a neural contiguity effect. Journal of Neuroscience 3, 2312–2317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard MW, Viskontas IV, Shankar KH & Fried I Ensembles of human MTL neurons “jump back in time” in response to a repeated stimulus. Hippocampus 22, 1833–1847, doi: 10.1002/hipo.22018 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning JR, Polyn SM, Baltuch GH, Litt B & Kahana MJ Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. P Natl Acad Sci USA 108, 12893–12897, doi: 10.1073/pnas.1015174108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palombo DJ D. L. JM;Howard MW; Verfaellie M Medial Temporal lobe amnesia is associated with a deficit in recoveing temporal context. J Cognitive Neurosci X, 1–13 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Yaffe RB et al. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. P Natl Acad Sci USA 111, 18727–18732, doi: 10.1073/pnas.1417017112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Squire LR Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99, 195–231 (1992). [DOI] [PubMed] [Google Scholar]
- 46.Squire LR, Slater PC & Chace PM Retrograde amnesia: temporal gradient in very long term memory following electroconvulsive therapy. Science 187, 77–79 (1975). [DOI] [PubMed] [Google Scholar]
- 47.Tse D et al. Schemas and memory consolidation. Science 316, 76–82, doi: 10.1126/science.1135935 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Corkin S Lasting Consequences of Bilateral Medial Temporal Lobectomy - Clinical Course and Experimental Findings in Hm. Semin Neurol 4, 249–259, doi:DOI 10.1055/s-2008-1041556 (1984). [DOI] [Google Scholar]
- 49.Penfield W & Milner B Memory Deficit Produced by Bilateral Lesions in the Hippocampal Zone. Arch Neuro Psychiatr 79, 475–497, doi:DOI 10.1001/archneurpsyc.1958.02340050003001 (1958). [DOI] [PubMed] [Google Scholar]
- 50.Scoville WB & Milner B Loss of Recent Memory after Bilateral Hippocampal Lesions. J Neurol Neurosur Ps 20, 11–21, doi:DOI 10.1136/jnnp.20.1.11 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinvorth S, Levine B & Corkin S Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from HM and WR. Neuropsychologia 43, 479–496, doi: 10.1016/j.neuropsychologia.2005.01.001 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Rempel-Clower NL, Zola SM, Squire LR & Amaral DG Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci 16, 5233–5255 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bayley PJ, Hopkins RO & Squire LR The fate of old memories after medial temporal lobe damage. J Neurosci 26, 13311–13317, doi: 10.1523/JNEUROSCI.4262-06.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Addis DR, Moscovitch M, Crawley AP & McAndrews MP Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus 14, 752–762, doi: 10.1002/hipo.10215 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Gilboa A, Winocur G, Grady CL, Hevenor SJ & Moscovitch M Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex 14, 1214–1225, doi: 10.1093/cercor/bhh082 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Sheldon S & Levine B Same as it ever was: vividness modulates the similarities and differences between the neural networks that support retrieving remote and recent autobiographical memories. Neuroimage 83, 880–891, doi: 10.1016/j.neuroimage.2013.06.082 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Viard A et al. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cereb Cortex 17, 2453–2467, doi: 10.1093/cercor/bhl153 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cabeza R & St Jacques P Functional neuroimaging of autobiographical memory. Trends Cogn Sci 11, 219–227, doi: 10.1016/j.tics.2007.02.005 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Dede AJ, and Smith Christine N.. The functional and structural neuroanatomy of systems consolidation for autobiographical and semantic memory.”. 1–32 (2016). [DOI] [PubMed]
- 60.Nieuwenhuis IL, & Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behavioural brain research 218, 325–334. (2011). [DOI] [PubMed] [Google Scholar]
- 61.(2008), S. A The confabulating mind. (Oxford University Press, USA, 2008). [Google Scholar]
- 62.Philippi CL, D. M, Denburg NL, Tranel D, Rudrauf D Medial PFC damage abolishes the self-reference effect. J Cogn Neurosci 24, 475–481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Euston DR, Gruber AJ, & McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolhuis JJ, Stewart CA & Forrest EM Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculum. Q J Exp Psychol B 47, 129–150 (1994). [PubMed] [Google Scholar]
- 65.Clark RE, Broadbent NJ & Squire LR Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus 15, 340–346, doi: 10.1002/hipo.20076 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clark RE, Broadbent NJ & Squire LR Hippocampus and remote spatial memory in rats. Hippocampus 15, 260–272, doi: 10.1002/hipo.20056 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark RE, Broadbent NJ & Squire LR The hippocampus and spatial memory: findings with a novel modification of the water maze. J Neurosci 27, 6647–6654, doi: 10.1523/JNEUROSCI.0913-07.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hollup SA, Kjelstrup KG, Hoff J, Moser MB & Moser EI Impaired recognition of the goal location during spatial navigation in rats with hippocampal lesions. J Neurosci 21, 4505–4513 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin SJ, de Hoz L & Morris RG Retrograde amnesia: neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding. Neuropsychologia 43, 609–624, doi: 10.1016/j.neuropsychologia.2004.07.007 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Mumby DG, Astur RS, Weisend MP & Sutherland RJ Retrograde amnesia and selective damage to the hippocampal formation: memory for places and object discriminations. Behav Brain Res 106, 97–107 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Ocampo AC, Squire LR & Clark RE Hippocampal area CA1 and remote memory in rats. Learn Mem 24, 563–568, doi: 10.1101/lm.045781.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutherland RJ et al. Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus 11, 27–42, doi: (2001). [DOI] [PubMed] [Google Scholar]
- 73.Winocur G, Moscovitch M, Caruana DA & Binns MA Retrograde amnesia in rats with lesions to the hippocampus on a test of spatial memory. Neuropsychologia 43, 1580–1590, doi: 10.1016/j.neuropsychologia.2005.01.013 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Barry DN, Coogan AN & Commins S The time course of systems consolidation of spatial memory from recent to remote retention: A comparison of the Immediate Early Genes Zif268, c-Fos and Arc. Neurobiol Learn Mem 128, 46–55, doi: 10.1016/j.nlm.2015.12.010 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Bonaccorsi J et al. System consolidation of spatial memories in mice: effects of enriched environment. Neural Plast 2013, 956312, doi: 10.1155/2013/956312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kee N, Teixeira CM, Wang AH & Frankland PW Imaging activation of adult-generated granule cells in spatial memory. Nat Protoc 2, 3033–3044, doi: 10.1038/nprot.2007.415 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Lopez J et al. Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus 22, 827–841, doi: 10.1002/hipo.20943 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Teixeira CM, Pomedli SR, Maei HR, Kee N & Frankland PW Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci 26, 7555–7564, doi: 10.1523/JNEUROSCI.1068-06.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Broadbent NJ & Clark RE Remote context fear conditioning remains hippocampus-dependent irrespective of training protocol, training-surgery interval, lesion size, and lesion method. Neurobiol Learn Mem 106, 300–308, doi: 10.1016/j.nlm.2013.08.008 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Goshen I et al. Dynamics of retrieval strategies for remote memories. Cell 147, 678–689, doi: 10.1016/j.cell.2011.09.033 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Lehmann H, Lacanilao S & Sutherland RJ Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur J Neurosci 25, 1278–1286, doi: 10.1111/j.1460-9568.2007.05374.x (2007). [DOI] [PubMed] [Google Scholar]
- 82.Lehmann H, Rourke BK, Booker A & Glenn MJ Single session contextual fear conditioning remains dependent on the hippocampus despite an increase in the number of context-shock pairings during learning. Neurobiol Learn Mem 106, 294–299, doi: 10.1016/j.nlm.2012.10.011 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Quinn JJ, Ma QD, Tinsley MR, Koch C & Fanselow MS Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem 15, 368–372, doi: 10.1101/lm.813608 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sparks FT, Spanswick SC, Lehmann H & Sutherland RJ Neither time nor number of context-shock pairings affect long-term dependence of memory on hippocampus. Neurobiol Learn Mem 106, 309–315, doi: 10.1016/j.nlm.2013.05.008 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Sutherland RJ, O’Brien J & Lehmann H Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus 18, 710–718, doi: 10.1002/hipo.20431 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Lehmann H et al. Making context memories independent of the hippocampus. Learn Mem 16, 417–420, doi: 10.1101/lm.1385409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anagnostaras SG, Maren S & Fanselow MS Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci 19, 1106–1114 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corcoran KA et al. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci 31, 11655–11659, doi: 10.1523/JNEUROSCI.2107-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frankland PW et al. Stability of recent and remote contextual fear memory. Learn Mem 13, 451–457, doi: 10.1101/lm.183406 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim JJ & Fanselow MS Modality-specific retrograde amnesia of fear. Science 256, 675–677 (1992). [DOI] [PubMed] [Google Scholar]
- 91.Kitamura T et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139, 814–827, doi: 10.1016/j.cell.2009.10.020 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Maren S, Aharonov G & Fanselow MS Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res 88, 261–274 (1997). [DOI] [PubMed] [Google Scholar]
- 93.Wang SH, Teixeira CM, Wheeler AL & Frankland PW The precision of remote context memories does not require the hippocampus. Nat Neurosci 12, 253–255, doi: 10.1038/nn.2263 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Wiltgen BJ et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol 20, 1336–1344, doi: 10.1016/j.cub.2010.06.068 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winocur G, Frankland PW, Sekeres M, Fogel S & Moscovitch M Changes in context-specificity during memory reconsolidation: selective effects of hippocampal lesions. Learn Mem 16, 722–729, doi: 10.1101/lm.1447209 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Winocur G, Sekeres MJ, Binns MA & Moscovitch M Hippocampal lesions produce both nongraded and temporally graded retrograde amnesia in the same rat. Hippocampus 23, 330–341, doi: 10.1002/hipo.22093 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Frankland PW, Bontempi B, Talton LE, Kaczmarek L & Silva AJ The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883, doi: 10.1126/science.1094804 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Lux V, Atucha E, Kitsukawa T & Sauvage MM Imaging a memory trace over half a life-time in the medial temporal lobe reveals a time-limited role of CA3 neurons in retrieval. Elife 5, e11862, doi: 10.7554/eLife.11862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tayler KK, Tanaka KZ, Reijmers LG & Wiltgen BJ Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol 23, 99–106, doi: 10.1016/j.cub.2012.11.019 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Guo N et al. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat Med 24, 438–449, doi: 10.1038/nm.4491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jenkins JG & D’allenbach KM Obivscence during sleep and waking. The americal journal of psychology 35, 605–612 (1924). [Google Scholar]
- 102.Rasch B & Born J About sleep’s role in memory. Physiol Rev 93, 681–766, doi: 10.1152/physrev.00032.2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luthi A Sleep Spindles: Where They Come From, What They Do. Neuroscientist 20, 243–256, doi: 10.1177/1073858413500854 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Tononi G & Cirelli C Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34, doi: 10.1016/j.neuron.2013.12.025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buzsaki G Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570 (1989). [DOI] [PubMed] [Google Scholar]
- 106.Lewis PA, Cairney S, Manning L, & Critchley HD. The impact of overnight consolidation upon memory for emotional and neutral encoding contexts. Neuropsychologia 49, 2619–2629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plihal W & Born J Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9, 534–547, doi: 10.1162/jocn.1997.9.4.534 (1997). [DOI] [PubMed] [Google Scholar]
- 108.Studte S, Bridger E & Mecklinger A Nap sleep preserves associative but not item memory performance. Neurobiology of Learning and Memory 120, 84–93, doi: 10.1016/j.nlm.2015.02.012 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Talamini LM, Nieuwenhuis IL, Takashima A & Jensen O Sleep directly following learning benefits consolidation of spatial associative memory. Learn Mem 15, 233–237, doi: 10.1101/lm.771608 (2008). [DOI] [PubMed] [Google Scholar]
- 110.van der Helm E, Gujar N, Nishida M & Walker MP Sleep-dependent facilitation of episodic memory details. PLoS One 6, e27421, doi: 10.1371/journal.pone.0027421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mawdsley M, Grasby K & Talk A The effect of sleep on item recognition and source memory recollection among shift-workers and permanent day-workers. J Sleep Res 23, 538–544, doi: 10.1111/jsr.12149 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Schonauer M, Pawlizki A, Kock C & Gais S Exploring the effect of sleep and reduced interference on different forms of declarative memory. Sleep 37, 1995–2007, doi: 10.5665/sleep.4258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Atienza M & Cantero JL Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res 17, 285–294, doi: 10.1111/j.1365-2869.2008.00661.x (2008). [DOI] [PubMed] [Google Scholar]
- 114.Daurat A, Terrier P, Foret J & Tiberge M Slow wave sleep and recollection in recognition memory. Conscious Cogn 16, 445–455, doi: 10.1016/j.concog.2006.06.011 (2007). [DOI] [PubMed] [Google Scholar]
- 115.Drosopoulos S, Wagner U & Born J Sleep enhances explicit recollection in recognition memory. Learn Mem 12, 44–51, doi: 10.1101/lm.83805 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sterpenich V et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol 5, e282, doi: 10.1371/journal.pbio.0050282 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bergmann TO, Molle M, Diedrichs J, Born J & Siebner HR Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59, 2733–2742, doi: 10.1016/j.neuroimage.2011.10.036 (2012). [DOI] [PubMed] [Google Scholar]
- 118.Deuker L et al. Memory consolidation by replay of stimulus-specific neural activity. J Neurosci 33, 19373–19383, doi: 10.1523/JNEUROSCI.0414-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peigneux P et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44, 535–545, doi: 10.1016/j.neuron.2004.10.007 (2004). [DOI] [PubMed] [Google Scholar]
- 120.Foster DJ & Wilson MA Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683, doi: 10.1038/nature04587 (2006). [DOI] [PubMed] [Google Scholar]
- 121.Lee AK & Wilson MA Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002). [DOI] [PubMed] [Google Scholar]
- 122.Staresina BP, Alink A, Kriegeskorte N & Henson RN Awake reactivation predicts memory in humans. Proc Natl Acad Sci U S A 110, 21159–21164, doi: 10.1073/pnas.1311989110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tambini A & Davachi L Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc Natl Acad Sci U S A 110, 19591–19596, doi: 10.1073/pnas.1308499110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tambini A, Ketz N & Davachi L Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65, 280–290, doi: 10.1016/j.neuron.2010.01.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tompary A, Duncan K & Davachi L Consolidation of Associative and Item Memory Is Related to Post-Encoding Functional Connectivity between the Ventral Tegmental Area and Different Medial Temporal Lobe Subregions during an Unrelated Task. J Neurosci 35, 7326–7331, doi: 10.1523/JNEUROSCI.4816-14.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai DJ et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118, doi: 10.1038/nature17955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dragoi G & Tonegawa S Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397–401, doi: 10.1038/nature09633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rogerson T et al. Synaptic tagging during memory allocation. Nat Rev Neurosci 15, 157–169, doi: 10.1038/nrn3667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manning JR et al. A neural signature of contextually mediated intentional forgetting. Psychon B Rev 23, 1534–1542, doi: 10.3758/s13423-016-1024-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schapiro AC M. EA; Rogers TT; Mednick SC; Norman KA Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nature Communications, 3920–3931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gruber MJ, Ritchey M, Wang SF, Doss MK & Ranganath C Post-learning Hippocampal Dynamics Promote Preferential Retention of Rewarding Events. Neuron 89, 1110–1120, doi: 10.1016/j.neuron.2016.01.017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murty VP, Tompary A, Adcock RA & Davachi L Selectivity in Postencoding Connectivity with High-Level Visual Cortex Is Associated with Reward-Motivated Memory. J Neurosci 37, 537–545, doi: 10.1523/JNEUROSCI.4032-15.2016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Girardeau G, Benchenane K, Wiener SI, Buzsaki G & Zugaro MB Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12, 1222–1223, doi: 10.1038/nn.2384 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Paller KA Sleeping in a Brave New World: Opportunities for Improving Learning and Clinical Outcomes through Targeted Memory Reactivation. Curr Dir Psychol Sci 26, 532–537, doi: 10.1177/0963721417716928 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schouten DI, Pereira SI, Tops M & Louzada FM State of the art on targeted memory reactivation: Sleep your way to enhanced cognition. Sleep Med Rev 32, 123–131, doi: 10.1016/j.smrv.2016.04.002 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Diekelmann S, Buchel C, Born J & Rasch B Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci 14, 381–386, doi: 10.1038/nn.2744 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Rasch B, Buchel C, Gais S & Born J Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315, 1426–1429, doi: 10.1126/science.1138581 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Rihm JS, Diekelmann S, Born J & Rasch B Reactivating memories during sleep by odors: odor specificity and associated changes in sleep oscillations. J Cogn Neurosci 26, 1806–1818, doi: 10.1162/jocn_a_00579 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Schreiner T & Rasch B Boosting Vocabulary Learning by Verbal Cueing During Sleep. Cereb Cortex 25, 4169–4179, doi: 10.1093/cercor/bhu139 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Cairney SA, Lindsay S, Sobczak JM, Paller KA & Gaskell MG The Benefits of Targeted Memory Reactivation for Consolidation in Sleep are Contingent on Memory Accuracy and Direct Cue-Memory Associations. Sleep 39, 1139–1150, doi: 10.5665/sleep.5772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Donohue KC & Spencer RM Continuous Re-Exposure to Environmental Sound Cues During Sleep Does Not Improve Memory for Semantically Unrelated Word Pairs. J Cogn Educ Psychol 10, 167–177, doi: 10.1891/1945-8959.10.2.167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rudoy JD, Voss JL, Westerberg CE & Paller KA Strengthening individual memories by reactivating them during sleep. Science 326, 1079, doi: 10.1126/science.1179013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tucker MA & Fishbein W Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep 31, 197–203 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tucker MA et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem 86, 241–247, doi: 10.1016/j.nlm.2006.03.005 (2006). [DOI] [PubMed] [Google Scholar]
- 145.Gais S, Lucas B & Born J Sleep after learning aids memory recall. Learn Mem 13, 259–262, doi: 10.1101/lm.132106 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Payne JD, Chambers AM & Kensinger EA Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci 6, 108, doi: 10.3389/fnint.2012.00108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cellini N, Torre J, Stegagno L & Sarlo M Sleep before and after learning promotes the consolidation of both neutral and emotional information regardless of REM presence. Neurobiol Learn Mem 133, 136–144, doi: 10.1016/j.nlm.2016.06.015 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Drummond SP & Brown GG The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology 25, S68–73, doi: 10.1016/S0893-133X(01)00325-6 (2001). [DOI] [PubMed] [Google Scholar]
- 149.Drummond SP, Gillin JC & Brown GG Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res 10, 85–92 (2001). [DOI] [PubMed] [Google Scholar]
- 150.Van Der Werf YD et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci 12, 122–123, doi: 10.1038/nn.2253 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Mander BA, Santhanam S, Saletin JM & Walker MP Wake deterioration and sleep restoration of human learning. Curr Biol 21, R183–184, doi: 10.1016/j.cub.2011.01.019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Elliott G, Isaac CL & Muhlert N Measuring forgetting: a critical review of accelerated long-term forgetting studies. Cortex 54, 16–32, doi: 10.1016/j.cortex.2014.02.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Isaac CL & Mayes AR Rate of forgetting in amnesia: I. Recall and recognition of prose. J Exp Psychol Learn Mem Cogn 25, 942–962 (1999). [DOI] [PubMed] [Google Scholar]
- 154.Kopelman MD Organic retrograde amnesia. Cortex 38, 655–659 (2002). [DOI] [PubMed] [Google Scholar]
- 155.Huppert FA & Piercy M Normal and abnormal forgetting in organic amnesia: effect of locus of lesion. Cortex 15, 385–390 (1979). [DOI] [PubMed] [Google Scholar]
- 156.Freed DM, Corkin S & Cohen NJ Forgetting in H.M.: a second look. Neuropsychologia 25, 461–471 (1987). [DOI] [PubMed] [Google Scholar]
- 157.Freed DM & Corkin S Rate of forgetting in H.M.: 6-month recognition. Behav Neurosci 102, 823–827 (1988). [DOI] [PubMed] [Google Scholar]
- 158.Squire LR Two forms of human amnesia: an analysis of forgetting. J Neurosci 1, 635–640 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huppert FA & Piercy M Recognition memory in amnesic patients: effect of temporal context and familiarity of material. Cortex 12, 3–20 (1976). [DOI] [PubMed] [Google Scholar]
- 160.McKee RD & Squire LR Equivalent forgetting rates in long-term memory for diencephalic and medial temporal lobe amnesia. J Neurosci 12, 3765–3772 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shields GS, Sazma MA, McCullough AM & Yonelinas AP The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol Bull 143, 636–675, doi: 10.1037/bul0000100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tse D et al. Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895, doi: 10.1126/science.1205274 (2011). [DOI] [PubMed] [Google Scholar]
- 163.Quamme JR, Yonelinas AP & Norman KA Effect of unitization on associative recognition in amnesia. Hippocampus 17, 192–200, doi: 10.1002/hipo.20257 (2007). [DOI] [PubMed] [Google Scholar]
- 164.Hirano M & Noguchi K Dissociation between specific personal episodes and other aspects of remote memory in a patient with hippocampal amnesia. Percept Mot Skills 87, 99–107, doi: 10.2466/pms.1998.87.1.99 (1998). [DOI] [PubMed] [Google Scholar]
- 165.Schacter DL, Chiu CY & Ochsner KN Implicit memory: a selective review. Annu Rev Neurosci 16, 159–182, doi: 10.1146/annurev.ne.16.030193.001111 (1993). [DOI] [PubMed] [Google Scholar]
- 166.Cave CB & Squire LR Intact and long-lasting repetition priming in amnesia. J Exp Psychol Learn Mem Cogn 18, 509–520 (1992). [DOI] [PubMed] [Google Scholar]
- 167.McAndrews MP, Glisky EL & Schacter DL When priming persists: long-lasting implicit memory for a single episode in amnesic patients. Neuropsychologia 25, 497–506 (1987). [DOI] [PubMed] [Google Scholar]
- 168.Tranel D, Damasio AR, Damasio H & Brandt JP Sensorimotor skill learning in amnesia: additional evidence for the neural basis of nondeclarative memory. Learn Mem 1, 165–179 (1994). [PubMed] [Google Scholar]
- 169.Cohen NJ & Squire LR Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science 210, 207–210 (1980). [DOI] [PubMed] [Google Scholar]
- 170.Ellenbogen JM, Hu PT, Payne JD, Titone D & Walker MP Human relational memory requires time and sleep. Proc Natl Acad Sci U S A 104, 7723–7728, doi: 10.1073/pnas.0700094104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wagner U, Gais S, Haider H, Verleger R & Born J Sleep inspires insight. Nature 427, 352–355, doi: 10.1038/nature02223 (2004). [DOI] [PubMed] [Google Scholar]
- 172.Djonlagic I et al. Sleep enhances category learning. Learn Mem 16, 751–755, doi: 10.1101/lm.1634509 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Durrant SJ, Taylor C, Cairney S & Lewis PA Sleep-dependent consolidation of statistical learning. Neuropsychologia 49, 1322–1331, doi: 10.1016/j.neuropsychologia.2011.02.015 (2011). [DOI] [PubMed] [Google Scholar]
- 174.Cai DJ, Mednick SA, Harrison EM, Kanady JC & Mednick SC REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A 106, 10130–10134, doi: 10.1073/pnas.0900271106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sio UN, Monaghan P & Ormerod T Sleep on it, but only if it is difficult: effects of sleep on problem solving. Mem Cognit 41, 159–166, doi: 10.3758/s13421-012-0256-7 (2013). [DOI] [PubMed] [Google Scholar]
- 176.Laureys S, Peigneux P, Perrin F & Maquet P Sleep and motor skill learning. Neuron 35, 5–7 (2002). [DOI] [PubMed] [Google Scholar]
- 177.Pan SC & Rickard TC Sleep and motor learning: Is there room for consolidation? Psychol Bull 141, 812–834, doi: 10.1037/bul0000009 (2015). [DOI] [PubMed] [Google Scholar]
- 178.Miranda M & Bekinschtein P Plasticity Mechanisms of Memory Consolidation and Reconsolidation in the Perirhinal Cortex. Neuroscience 370, 46–61, doi: 10.1016/j.neuroscience.2017.06.002 (2018). [DOI] [PubMed] [Google Scholar]
- 179.Yonelinas AP The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res 254, 34–44, doi: 10.1016/j.bbr.2013.05.030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rolls ET A theory of hippocampal function in memory. Hippocampus 6, 601–620, doi:Doi (1996). [DOI] [PubMed] [Google Scholar]
- 181.Kahana MJ, Howard MW, Zaromb F & Wingfield A Age dissociates recency and lag recency effects in free recall. J Exp Psychol Learn Mem Cogn 28, 530–540 (2002). [DOI] [PubMed] [Google Scholar]
- 182.Bayley PJ, Hopkins RO & Squire LR Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron 38, 135–144 (2003). [DOI] [PubMed] [Google Scholar]
- 183.Bright P et al. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learn Mem 13, 545–557, doi: 10.1101/lm.265906 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cipolotti L et al. Long-term retrograde amnesia…the crucial role of the hippocampus. Neuropsychologia 39, 151–172 (2001). [DOI] [PubMed] [Google Scholar]
- 185.Kapur N & Brooks DJ Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus 9, 247–254, doi: (1999). [DOI] [PubMed] [Google Scholar]
- 186.Reed JM & Squire LR Retrograde amnesia for facts and events: findings from four new cases. J Neurosci 18, 3943–3954 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zola-Morgan S, Squire LR & Amaral DG Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 6, 2950–2967 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bernard FA et al. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage 22, 1704–1714, doi: 10.1016/j.neuroimage.2004.03.036 (2004). [DOI] [PubMed] [Google Scholar]
- 189.Bonnici HM, Chadwick MJ & Maguire EA Representations of recent and remote autobiographical memories in hippocampal subfields. Hippocampus 23, 849–854, doi: 10.1002/hipo.22155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maguire EA, Henson RN, Mummery CJ & Frith CD Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport 12, 441–444 (2001). [DOI] [PubMed] [Google Scholar]
- 191.Rissman J, Chow TE, Reggente N & Wagner AD Decoding fMRI Signatures of Real-world Autobiographical Memory Retrieval. J Cogn Neurosci 28, 604–620, doi: 10.1162/jocn_a_00920 (2016). [DOI] [PubMed] [Google Scholar]
- 192.Ryan L et al. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus 11, 707–714, doi: 10.1002/hipo.1086 (2001). [DOI] [PubMed] [Google Scholar]
- 193.Soderlund H, Moscovitch M, Kumar N, Mandic M & Levine B As time goes by: hippocampal connectivity changes with remoteness of autobiographical memory retrieval. Hippocampus 22, 670–679, doi: 10.1002/hipo.20927 (2012). [DOI] [PubMed] [Google Scholar]
- 194.Stark CE & Squire LR fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus 10, 329–337, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 195.Steinvorth S, Corkin S & Halgren E Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage 30, 285–298, doi: 10.1016/j.neuroimage.2005.09.025 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tsukiura T et al. Medial temporal lobe activation during context-dependent relational processes in episodic retrieval: an fMRI study. Functional magnetic resonance imaging. Hum Brain Mapp 17, 203–213, doi: 10.1002/hbm.10068 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Haist F, Bowden Gore J & Mao H Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci 4, 1139–1145, doi: 10.1038/nn739 (2001). [DOI] [PubMed] [Google Scholar]
- 198.Milton F et al. An fMRI study of long-term everyday memory using SenseCam. Memory 19, 733–744, doi: 10.1080/09658211.2011.552185 (2011). [DOI] [PubMed] [Google Scholar]
- 199.Piefke M, Weiss PH, Zilles K, Markowitsch HJ & Fink GR Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 126, 650–668 (2003). [DOI] [PubMed] [Google Scholar]
- 200.Smith CN & Squire LR Medial temporal lobe activity during retrieval of semantic memory is related to the age of the memory. J Neurosci 29, 930–938, doi: 10.1523/JNEUROSCI.4545-08.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Takashima A et al. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci 29, 10087–10093, doi: 10.1523/JNEUROSCI.0799-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Takashima A et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A 103, 756–761, doi: 10.1073/pnas.0507774103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yamashita K et al. Formation of long-term memory representation in human temporal cortex related to pictorial paired associates. J Neurosci 29, 10335–10340, doi: 10.1523/JNEUROSCI.1328-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bosshardt S et al. One month of human memory consolidation enhances retrieval-related hippocampal activity. Hippocampus 15, 1026–1040, doi: 10.1002/hipo.20105 (2005). [DOI] [PubMed] [Google Scholar]
- 205.Bosshardt S et al. Effects of memory consolidation on human hippocampal activity during retrieval. Cortex 41, 486–498 (2005). [DOI] [PubMed] [Google Scholar]
- 206.Gais S et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A 104, 18778–18783, doi: 10.1073/pnas.0705454104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Piolino P et al. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage 22, 1371–1383, doi: 10.1016/j.neuroimage.2004.02.025 (2004). [DOI] [PubMed] [Google Scholar]
- 208.Rekkas PV & Constable RT Evidence that autobiographic memory retrieval does not become independent of the hippocampus: an fMRI study contrasting very recent with remote events. J Cogn Neurosci 17, 1950–1961 (2005). [DOI] [PubMed] [Google Scholar]
- 209.Donix M et al. Age-dependent differences in the neural mechanisms supporting long-term declarative memories. Arch Clin Neuropsychol 25, 383–395, doi: 10.1093/arclin/acq037 (2010). [DOI] [PubMed] [Google Scholar]
- 210.Douville K et al. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia 43, 693–703, doi: 10.1016/j.neuropsychologia.2004.09.005 (2005). [DOI] [PubMed] [Google Scholar]
- 211.Furman O, Mendelsohn A & Dudai Y The episodic engram transformed: Time reduces retrieval-related brain activity but correlates it with memory accuracy. Learn Mem 19, 575–587, doi: 10.1101/lm.025965.112 (2012). [DOI] [PubMed] [Google Scholar]
- 212.Harand C et al. The hippocampus remains activated over the long term for the retrieval of truly episodic memories. PLoS One 7, e43495, doi: 10.1371/journal.pone.0043495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Janzen G, Jansen C & van Turennout M Memory consolidation of landmarks in good navigators. Hippocampus 18, 40–47, doi: 10.1002/hipo.20364 (2008). [DOI] [PubMed] [Google Scholar]
- 214.Maguire EA & Frith CD Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci 23, 5302–5307 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ritchey M, Montchal ME, Yonelinas AP & Ranganath C Delay-dependent contributions of medial temporal lobe regions to episodic memory retrieval. Elife 4, doi: 10.7554/eLife.05025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


