Abstract
Introduction: Bariatric surgery (BS) has gained popularity in order to treat morbid obesity. However, post-operative (PO) neurologic complications have become increasingly recognized. Our aim was to examine incidence, clinical presentation, and outcomes of neurologic disorders secondary to BS. Methods: Patients who underwent BS between the years 2012 and 2015 at Parma University were included in this survey, and assessed before (T0) and 1 year after surgery (T1). Baseline characteristics and medical comorbidities, type of surgery, and PO complications were retrieved. Patients with a previous history of peripheral neuropathic disease were excluded from the analysis. If a patient presented with a new onset neurologic symptom including extremity numbness, paresthesia, muscle weakness, the status was considered “positive” for PO-neuropathy. Results: Overall, we retrieved data from 61 patients (n=30 Roux-en-Y Gastric bypasses, n=31 Gastric banding; 81.0% females). Of them, 7 (11.4%) developed some signs of PO-neuropathy, that eventually disappeared at T+24 months. The most common manifestations were paresthesia (n=6) and muscle weakness (n=4), similarly distributed in Gastric Bypass (n=4) and Gastric Banding (n=3) groups. Although patients affected by PO-neuropathy exhibited higher SF-36 score at T0 (p=0.018), no significant differences were found regarding BMI (T0, T1), percentual weight loss, serological data (i.e. vitamin B1, B2, B6, B12: in all cases p>0.05). Conclusion: PO-BS neuropathy is usually associated with lower levels of vitamin B1, B2, B12. However, no differences in PO-BMI, excess weight loss, and metabolic data levels were found. Larger data and more extended follow-up are required to validate our results. (www.actabiomedica.it)
Keywords: peripheral nervous system diseases, bariatric surgery, malnutrition, obesity, morbid, obesity
Introduction
During the past five decades, prevalence of overweight (body mass index, BMI ≥25 kg/m2) and obesity (BMI ≥30 kg/m2) has globally risen, ultimately becoming of international public health concern (1-3). Morbid obesity (MOb, i.e. BMI >40 kg/m2) has been recognized by the World Health Organization (WHO) as a systemic disease, being associated with an increased risk of hypertension, diabetes mellitus, obstructive sleep apnea, osteoarthritis, and infertility, as well as with increased risk for certain types of cancer and musculoskeletal disorders (MSD), collectively causing a considerable increase in direct and indirect costs (1-10).
Even though lifestyle changes are essential long-lasting results (4), bariatric surgery (BaS) had been recognized as the most efficient long-term treatment for both MO and complicated obesity (COb, i.e. BMI > 35 kg/m2, with obesity-related complications such as hypertension, obstructive sleep apnea, diabetes mellitus, dyslipidemia, and disabling MSD) who have failed conservative treatment (5-7).
Even though BaS significantly improves the overall quality of life (QoL) of patients (8-11), acute and chronic complications have been diffusely reported, including a broad spectrum of neurologic disorders (7, 12, 13). For instance, available reports suggest that 1.3% up to 16% of all patients who have undergone BaS for MOb eventually complain some kinds of neurological complications months or years following the procedure, and no part of the neuraxis seems exempt (5-7, 14, 15).
Peripheral neuropathies (PN; i.e. acute, subacute, and chronic polyneuropathies, as well as focal entrapment neuropathies; radiculoplexopathy; and burning feet) following BaS seemly represent a significant issue. Not only half of the neurological complications of BaS usually affects peripheral nervous system, but complications of the peripheral nervous system have been described in around a tenth to a third of all BaS patients (5-7, 14), with an average time of 3.7 years following surgery to develop neuropathy (12, 13, 15).
Multiple etiologies have been proposed for the development of PN after BaS (15). Mechanisms of neural injury may include mechanical compression and entrapment in mononeuropathies. Still, as such complications are more frequently seen in patients who have rapid and significant early weight loss, with and without diarrhea, dumping syndrome or nausea and vomiting, the most important factors in their pathogenesis are usually identified in nutritional deficiencies due to malabsorpion or prolonged emesis (12, 13, 15).
The purpose of this study was therefore to determine the incidence rate of PN in a cohort of severe obese individuals treated at our institution and beneficing from a standardized follow up including pre- and post-operative nutritional assessment, in order to evaluate its potential risk factors.
Materials and Methods
1. Study Sample. This was a retrospective review of patients who underwent Roux-en-Y gastric bypass (RYB) or gastric banding (GB) at a specialized bariatric center between March 2010 and March 2015 at the Department of Medicine and Surgery, Section of General Surgery and Surgical Therapy, University of Parma. All the patients met the international criteria for bariatric surgery (16): age at surgery 18 to 60, BMI ≥40 kg/m2 or BMI 35-40 kg/m2 with comorbidities in which surgically-induced weight loss is expected to improve the disorder (i.e. metabolic disorders, cardio-respiratory disease, severe joint disease, obesity-related severe psychological problems, etc.). All patients underwent an interdisciplinary assessment prior to BaS (T1) and during the follow ups (T+24 months, T2): this specific protocol has been described in previous studies (8-11, 17-19), and included the collection of following data: general patient demographic characteristics (sex, and age), medical comorbidities (i.e. diabetes, hypertension, hyperlipidemia, psychiatric disorders, and neurologic diseases), preoperative BMI, postoperative course, BMI at follow-up (T2), re-hospitalization, neurologic signs/symptoms identified at follow-up (i.e. paresthesia, dysesthesia, abolition of deep tendon reflexes, ataxia, and Wernicke’s encephalopathy), treatment received, and progression of the neuropathy. Weight loss results were expressed as the change in BMI, percentage of excess weight loss (%EWL) and percentage of excess BMI loss (%EBMIL). Patients’ quality of life in physical and mental domains was measured using The Medical Outcomes Study Short-Form 36 Health Status Survey (SF-36) (20), which was submitted to the patients at T1 and T2. The SF-36 has been previously used in BaS and has good construct validity, high internal consistency and high test-retest reliability (10, 21-23). Moreover, an official Italian translation was previously validated (10, 22, 24).
2. Clinical assessment. PN was defined by clinical criteria encompassing all new onset neurologic symptom including extremity numbness, paresthesia, muscle weakness, neuropathy, imbalance, dizziness, or memory deficit that were either complained or clinically identified at follow-up. PN cases were included in the analyses if the patient had received one or more gastrointestinal operations for morbid obesity (i.e. BaS) and afterwards he developed symptomatic, clinically defined PN. On the other hand, PN cases excluded from the analyses if:
PN developed after operation but from another known cause such as alcohol abuse, heavy metal intoxication, monoclonal gammopathy, associated necrotizing vasculitis, or toxic exposure, or the patient had pre-existing PN preoperatively;
essential data were missing from medical records;
conditions that affect weight, such as metastatic cancer, untreated hyperthyroidism, pregnancy etc. had been previously identified;
the patient had received either before or after BaS the diagnosis of neurologic conditions that would interfere with assessment of PN symptoms, such as multiple sclerosis, severe cervical or lumbosacral radiculopathy, stroke etc.
3. Laboratory assessment. Routine lab work were assessed at T1, during follow up and included: blood count, metabolic profile, iron studies, lipid panel, folate level, vitamin B1, vitamin B2, vitamin B6, vitamin B12 level, and 25-hydroxy vitamin D levels.
4. Statistical analysis. Descriptive analyses were performed for all variables being examined. Patients who had neurological complications after BaS were compared to those who did not present any complications. Univariate analysis of continuous variables was performed through Student’s t test for paired or unpaired data, when appropriate, whereas association between discrete variables was analysed using Chi-squared tests or Fisher’s exact test. All tests were two-tailed and statistical significance was set at p<0.05. All statistical analyses were performed using IBM SPSS Statistics 25.0 for Macintosh (IBM Corp. Armonk, NY).
5. Ethical considerations. This paper details a part of a larger study that was carried out in accordance with the principles of the Declaration of Helsinki, and was specifically reviewed by an institutional board. Details are provided elsewhere (8,9-11). All patients received both written and oral information regarding the procedure, and all provided informed consent before undergoing the surgical procedure. Subjects refusing their consent were excluded from the study population.
Results
Among 76 patients scheduled for BaS at our institution during the study period, 74 (97.4%) signed their consent to the study and underwent the preoperative assessment. Six patients were excluded from the analyses (7.9%): for complications unrelated to BaS during the follow-up (n=1, 1.3%), because of a prior diagnosis of neurologic disease (n =1, 1.3%), or were lost to follow-up (n=4, 5.3%). A total of 61 patients attended post-surgery appointments and were included in the final analyses (83.3% of the original sample), including n=30 RYB (49.2%), and n=31 GB (50.8%).
The characteristics of the study population are shown in Table 1. Briefly, the sample consisted mostly of females (n=50, 82.0%), with a mean age at T0 of 41.8±11.4 years, and a preoperative BMI of 44.4±7.2 kg/m2. No cases of nutritional deficiencies regarding micronutrients (Vitamin B1, B2, B6, B12) were reported. Mean total weight loss was 59.8%±22.3 at T2.
Table 1.
Characteristics of the 61 Bariatric Surgery (BS) cases included in the analysis. Confrontations were performed through Student’s t test for unpaired data for continuous variables and by means of Chi squared test or Fisher’s test (= *) for dichotomous variables
| All cases (n=61, 100%) | NP (n=7, 11.5%) | Non-NP (n=54, 88.5%) | p value | |
| Age (years; mean±SD) | 41.8±11.4 | 42.7±13.9 | 41.7±11.2 | 0.831 |
| Female Sex (n, %) | 50, 82.0% | 6, 85.7% | 44, 81.5% | 1.000 |
| Smoking history (n, %) | 8, 26.7% | 0, - | 8, 30.8% | 0.550* |
| Diabetes (n, %) | 10, 16.4% | 2, 28.6% | 8, 14.8% | 0.702 |
| Surgical procedure | 0.963 | |||
| Gastric banding | 31, 50.8% | 3, 42.9% | 51.9% | |
| Gastric bypass | 30, 49.2% | 4, 57.1% | 48.1% | |
| BMI TO (Kg/m2; mean±SD) | 44.4±7.2 | 44.3±3.8 | 44.4±7.6 | 0.969 |
| BMI T1(Kg/m2; mean±SD) | 32.7±4.9 | 32.0±5.1 | 32.8±4.9 | 0.719 |
| T2 data | ||||
| EWL (%) | 59.8±22.3 | 61.9±30.8 | 59.5±21.4 | 0.843 |
| Vitamin B1 (nmol/L) | 113.3±36.3 | 111.5±37.1 | 126.8±28.3 | 0.297 |
| Vitamin B2 (^g/L) | 258.1±76.7 | 213.7±72.2 | 263.9.±76.0 | 0.103 |
| Vitamin B6 (^g/L) | 19.6±5.1 | 17.2±3.3 | 19.9±5.2 | 0.185 |
| Vitamin B12 (pg/mL) | 249.1±101.3 | 251.3±99.3 | 260.3±106.4 | 0.829 |
| SF-36 (TO) | 478.3±160.2 | 344.5±146.5 | 495.6±154.8 | 0.018 |
| SF-36 (T+24) | 686.8±152.4 | 589.6±167.5 | 699.4±147.3 | 0.141 |
Notes: NP=neuropathic pain; Non-NP=non neuropathic pain; BMI=Body Mass Index (Kg/m2); T0=Before Surgery; T1=at follow up (12 months after surgery); SF-36=Short Form (36) Health Survey; EWL=Percent excess body weight lost
Overall, 7 patients (11.4%) developed some signs of PO-neuropathy during follow up. In six cases, symptoms eventually disappeared within 6 months after T2 (Table 2). In the only case without spontaneous resolution, paresthesia was associated with significant forearm muscle weakness, and a subsequent medical assessment identified a case of previously not reported Carpal Tunnel Syndrome (CTS).
Table 2.
Characteristics of patients affected by peripheral neuropathies during the follow up
| Sex | Age | Operation | Upper limb | Lower Limb |
| F | 28 | RYGB | Paresthesia, Tibial region (left) | |
| F | 54 | RYGB | Muscle weakness, shoulder (right) Muscle weakness, arm (left) | Paresthesia, Feet (bilateral) Dysesthesia (thermal perception; tibial region) |
| F | 47 | RYGB | Paresthesia, hand and wrist (right) (CTS) | |
| M | 53 | RYGB | Muscle weakness, shoulder (right) | |
| M | 43 | GB | Paresthesia, Foot (right) | |
| F | 44 | GB | Dysesthesia (thermal perception; left foot) | |
| F | 55 | GB | Muscle weakness, ventral forearm (right) Parestesia, hand and wrist (right) |
Notes: RYGB=Roux-en-Y Gastric Bypass; GB=Gastric Banding; CTS=Carpal Tunnel Syndrome
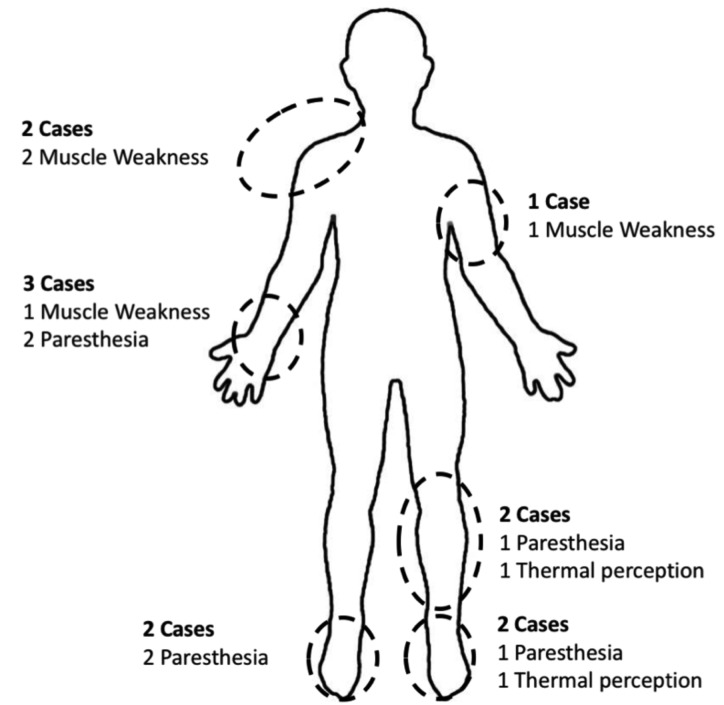
The most common manifestations were paresthesia/dysesthesia (n=5, 8.2%) and localized muscle weakness (n=3, 4.9%). As shown in Figure 1, all cases of muscle involvement were diagnosed at the upper limbs, and involved shoulder region (n=2, both cases affecting trapezius muscle), arm (n=1, muscular venter of the biceps) and ventral forearm (n=1, brachioradialis and flexor carpi ulnaris muscles). Regarding the laterality, right upper limb was involved in 3 out 4 cases, and all cases were right dominant. Conversely, paresthesia and dysesthesia predominantly affected lower limbs, as paresthesia of the feet was identified in 2 cases (one, of them bilaterally), while involvement of the tibial region was identified in 2 other cases. Simultaneous involvement of upper and lower limb was identified in one case.
Figure 1.
Graphical representation of neuropathic disorders reported by patients. Overall, 6 out of 7 patients reported paresthesia, 4 muscle weakness (all in the upper limbs), and 2 further cases had thermal perception disorders. On the contrary, no cases of neuropathic pain were referred
Cases of PN were similarly distributed among RYB (n=4) and GB (n=3, p=0.963) groups. Although patients affected by PN exhibited lower cumulative SF-36 score, both at T1 (344.5±146.5 vs. 495.6±154.8), and at T2 (589.6±167.5 vs. 699.4±147.3), the difference was significant only at T1 (p=0.018 and p=0.141, respectively).
Also regarding assessed serological data for micronutrients (i.e. vitamin B1, B2, B6, B12), lower values were reported for PN patients at T2, but the difference was not significant (all cases p>0.05).
Discussion
Peripheral neuropathies following BaS are a relatively common complication (12, 13, 15, 25, 26). BaS-associacted peripheral polyneuropathies typically develop as predominantly sensory disorders, characterized by painful paresthesias with stocking-glove distribution, i.e. affecting lower and upper extremities. An increased risk for mononeuropathies has been similarly described: median mononeuropathy at wrist, or CTS, develops in up to 7% of all gastric bypass patients, followed by a lesser risk reported for peroneal, ulnar and radial isolated mononeuropathies (15).
Even though multiple etiologies have been proposed for the development of peripheral neuropathies following BaS (15), the association with rapid and significant early weight loss, diarrhea, dumping syndrome or nausea and vomiting has suggested that the main effector may be found in nutritional deficiencies generally due to malabsorption or prolonged emesis (12, 13, 15). Despite their obesity, 20 to 30% patients usually have micronutrient deficiencies prior to BaS (i.e. low thiamine, vitamin C, iron, zinc, vitamin B12, and 25-hydroxyvitamin D3 deficiency), and such deficiencies may get worse after surgery because of prolonged vomiting, loss of absorptive surface, altered dietary patterns, loss of gastric acid, loss of intrinsic factor, encompassing calcium, zinc, selenium, vitamin A, 25-hydroxyvitamin D, and thiamine (5, 6, 12, 13). In a meta-analysis of nearly 1,000 patients, 25% of them were B12-deficient, 20% folate-deficient, and 1% thiamine deficient (5, 6, 12-14).
Although previous reports have suggested that the risk for PN after BaS may eventually depend on the extent of weight loss, and surgical procedure used, growing from gastric banding, vertical banded gastroplasty, sleeve gastrectomy, Roux-en-Y gastric bypass, partial biliopancreatic bypass (5, 6), in our sample patients with and without PN had significant differences regarding EWL, nutritional indices (i.e. vitamin B1, B2, B6, B12), surgical procedures. Moreover, nearly all cases of PN we identified had a spontaneous resolution during the follow up. Only one case requested subsequent treatment because of a diagnosis of CTS: even though CTS is the most commonly reported among patients receiving BaS, and rapid weight loss may make the nerves more susceptible to compression through loss of subcutaneous tissue, loss of protective far pads, or structural changes, accurate evaluation of the case suggested that CTS pre-existed BaS (5, 6, 27-29). It is therefore reasonable that BaS per se had no role in the pathogenesis of the mononeuropathy.
In our sample, no cases of nutritional deficiencies were identified prior to BaS. Moreover, although PN cases exhibited relatively lower concentrations of vitamin B1, B2, B6 at T2, not only none of them had significant deficiencies, but the difference between PN cases and non-PN cases was not significant. As a consequence, we can speculate that a surgical treatment performed before the patient eventually develops micronutrient deficiency as well as an accurate follow-up course, maintaining a good nutritional status, may be associated with a low risk for long-lasting PN and severe complications such as Wernicke’s encephalopathy.
Several other factors suggest that a good preoperative assessment of the patient may be useful in averting “classical” PN associated with nutritional deficiencies. First at all, PN are usually classified among “late” complications, i.e. developing between 6 weeks and 2 years after surgery BaS, whereas all cases we identified eventually resolved within less than three years from surgery. The transient nature of PN we identified in our sample may therefore be found in the transient inflammatory status that usually characterizes the early post-operative period (25, 26).
Moreover, the typical pattern of post-BaS peripheral neuropathy is represented by symmetric disorders (13), whereas in nearly all PN cases we reported a monolateral pattern was identified (5, 6, 13, 25, 26).
Our study is affected by several limits. First at all, it should be stressed that our study, as well as the majority of available reports, had a retrospective design, without pre-operative electrophysiology studies that may lead to an early, preoperative identification of borderline neurological disorders (5, 6). In other words, we are unable to assess whether PN cases had a previous, border-line disorder, similarly to the CTS we identified, that post-operative status may have enlighten rather than elicited.
Second, our study lacks an anatomopathological assessment. As a consequence, we can only speculate that the disorders we identified had an inflammatory nature. In this regard, nerve biopsies from BaS patients who had neurological complications (polineuropathy or radiculoplexus neuropathy with acute or subacute onset) suggest that a cachexia-like state with nutritional deficiencies may induce inflammatory changes and immune mechanisms of neuropathy (25, 26). Unfortunately, without specific biopsies we are unable to assess whether PN cases had a specific anatomopathological pattern of inflammation or not.
Third, we focused only on Vitamin B1, B2, B6, B12. As potential involvement of several other nutritional factors in post-operative PN has been previously suggested, our study is unable tu rule out that the specific pattern we identified as associated with low vitamin C, iron, zinc, and 25-hydroxyvitamin D3 deficiency (5, 6, 12, 13).
In conclusion, PN is a relatively common complication of BaS. In cases with a good pre-operative nutritional status and without significant deficiencies of micronutrients, it may be characterized by a transient nature, with no long-lasting consequences.
Conflict of interest:
The facts, conclusions, and opinions stated in the article represent the authors’ research, conclusions, and opinions and are believed to be substantiated, accurate, valid, and reliable. However, as this article includes the results of personal researches of the Authors, presenting correspondent, personal conclusions and opinions, parent employers are not forced in any way to endorse or share its content and its potential implications. Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N. Global, regional and national prevalence of overweight and obesity in children and adults 1980-2013: A systematic analysis. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. doi:10.1016/S0140-6736(14)60460-8. Global. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet. 2016;387(10031):1947–1956. doi: 10.1016/S0140-6736(16)00271-3. doi:10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 3.Caban AJ, Lee DJ, Fleming LE, Gómez-Márin O, LeBlanc W, Pitman T. Obesity in US workers: The National Health Interview Survey, 1986 to 2002. Am J Public Health. 2005;95(9):1614–1622. doi: 10.2105/AJPH.2004.050112. doi:10.2105/AJPH.2004.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabbara M, Carandina S, Bossi M, Polliand C, Genser L, Barrat C. Rare Neurological Complications After Sleeve Gastrectomy. Obes Surg. 2016;26(12):2843–2848. doi: 10.1007/s11695-016-2227-8. doi:10.1007/s11695-016-2227-8. [DOI] [PubMed] [Google Scholar]
- 5.Landais AF. Rare neurologic complication of bariatric surgery: Acute motor axonal neuropathy (AMAN), a severe motor axonal form of the Guillain Barré syndrome. Surg Obes Relat Dis. 2014;10(6):e85–e87. doi: 10.1016/j.soard.2014.02.019. doi:10.1016/j.soard.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Landais A. Neurological Complications of Bariatric Surgery. Obes Surg. 2014;24(10):1800–1807. doi: 10.1007/s11695-014-1376-x. doi:10.1007/s11695-014-1376-x. [DOI] [PubMed] [Google Scholar]
- 7.Berger JR, Singhal D. 1st ed. Vol. 120. Elsevier B.V.: 2014. The Neurologic Complications of Bariatric Surgery. doi:10.1016/B978-0-7020-4087-0.00039-5. [DOI] [PubMed] [Google Scholar]
- 8.Marchesi F, Tartamella F, De Sario G, et al. The Sleeping Remnant. Effect of Roux-En-Y Gastric Bypass on Plasma Levels of Gastric Biomarkers in Morbidly Obese Women: A Prospective Longitudinal Study. Obes Surg. 2017 doi: 10.1007/s11695-017-2724-4. doi:10.1007/s11695-017-2724-4. [DOI] [PubMed] [Google Scholar]
- 9.Marchesi F, De Sario G, Reggiani V, et al. Road Running After Gastric Bypass for Morbid Obesity: Rationale and Results of a New Protocol. Obes Surg. 2015;25(7):1162–70. doi: 10.1007/s11695-014-1517-2. doi:10.1007/s11695-014-1517-2. [DOI] [PubMed] [Google Scholar]
- 10.Riccò M, Marchesi F, Tartamella F, et al. The impact of bariatric surgery on health outcomes, wellbeing and employment rates: Analysis from a prospective cohort study. Ann di Ig. 2017;29(5):440–452. doi: 10.7416/ai.2017.2176. doi:10.7416/ai.2017.2176. [DOI] [PubMed] [Google Scholar]
- 11.Marchesi F, Giacosa R, Reggiani V, et al. Morphological Changes in the Carotid Artery Intima after Gastric Bypass for Morbid Obesity. Obes Surg. 2017;27(2):357–36. doi: 10.1007/s11695-016-2279-9. doi:10.1007/s11695-016-2279-9. [DOI] [PubMed] [Google Scholar]
- 12.Rudnicki SA. Prevention and treatment of peripheral neuropathy after bariatric surgery. Curr Treat Options Neurol. 2010;12(1):29–36. doi: 10.1007/s11940-009-0052-2. doi:10.1007/s11940-009-0052-2. [DOI] [PubMed] [Google Scholar]
- 13.Juhasz-Pocsine K, Rudnicki SA, Archer RL, Harik SI. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology. 2007 doi: 10.1212/01.wnl.0000262768.40174.33. doi: 10.1212/01.wnl.0000262768.40174.33. [DOI] [PubMed] [Google Scholar]
- 14.Koffman BM, Greenfield LJ, Ali II, Pirzada NA. Neurologic complications after surgery for obesity. Muscle and Nerve. 2006;33(2):166–176. doi: 10.1002/mus.20394. doi:10.1002/mus.20394. [DOI] [PubMed] [Google Scholar]
- 15.Clark N. Neuropathy following bariatric surgery. Semin Neurol. 2010;30(4):433–435. doi: 10.1055/s-0030-1267287. doi:10.1055/s-0030-1267287 [doi] [DOI] [PubMed] [Google Scholar]
- 16.Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European Guidelines on Metabolic and Bariatric Surgery. Obes Surg. 2014;24(1):42–55. doi: 10.1007/s11695-013-1079-8. doi:10.1007/s11695-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 17.Grieco MP, Bertozzi N, Simonacci F, et al. Quality of life in post-bariatric surgery patients undergoing aesthetic abdomi-noplasty: Our experience. Surg Chronicles. 2016;21(1):5–8. [Google Scholar]
- 18.Tartamella F, Petracca G, Romboli A, Marchesi F. Laparoscopic gastric bypass with remnant gastrectomy in a super-super obese patient with gastric metaplasia : a surgical hazard. Acta Biom. 2017;88(4):496–500. doi: 10.23750/abm.v88i4.6671. doi:10.23750/abm.v88i4.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Panfilis C, Generali I, Dall’Aglio E, Marchesi F, Ossola P, Marchesi C. Temperament and one-year outcome of gastric bypass for severe obesity. Surg Obes Relat Dis. 2014;10(1):144–148. doi: 10.1016/j.soard.2013.09.018. doi:10.1016/j.soard.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey ( SF-36 ): I. Conceptual Framework and Item Selection. Med Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-00002. [PubMed] [Google Scholar]
- 21.Adams TD, Pendleton RC, Strong MB, et al. Health Outcomes of Gastric Bypass Patients Compared to Nonsurgical, Nonintervened Severely Obese. Obesity. 2010;18(1):121–130. doi: 10.1038/oby.2009.178. doi:10.1038/oby.2009.178. Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apolone G, Mosconi P. The Italian SF-36 Health Survey. J Clin Epidemiol. 1998;51(11):1025–1036. doi: 10.1016/s0895-4356(98)00094-8. doi:10.1016/S0895-4356(98)00094-8. [DOI] [PubMed] [Google Scholar]
- 23.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. doi:10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donini LM, Brunani A, Sirtori A, et al. Assessing disability in morbidly obese individuals: The Italian Society of Obesity test for obesity-related disabilities. Disabil Rehabil. 2011;33(25-26):2509–2518. doi: 10.3109/09638288.2011.575529. doi:10.3109/09638288.2011.575529. [DOI] [PubMed] [Google Scholar]
- 25.Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63(8):1462–70. doi: 10.1212/01.wnl.0000142038.43946.06. doi: 10.1212/01.WNL.0000142038.43946.06. [DOI] [PubMed] [Google Scholar]
- 26.Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJB. Good nutritional control may prevent polyneuropathy after bariatric surgery. Muscle and Nerve. 2010;42(5):709–714. doi: 10.1002/mus.21802. doi:10.1002/mus.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riccò M, Signorelli C. Personal and occupational risk factors for carpal tunnel syndrome in meat processing industry workers in Northern Italy. Med Pr. 2017;68(2):199–209. doi: 10.13075/mp.5893.00605. doi: 10.13075/mp.5893.00605. [DOI] [PubMed] [Google Scholar]
- 28.Riccò M, Cattani S, Signorelli C. Personal risk factors for carpal tunnel syndrome in female visual display unit workers. Int J Occup Med Environ Health. 2016;29(6):927–936. doi: 10.13075/ijomeh.1896.00781. doi: 10.13075/ijomeh.1896.00781. [DOI] [PubMed] [Google Scholar]
- 29.Riccò M, Cattani S, Gualerzi G, Signorelli C. Work with visual display units and musculoskeletal disorders: A cross-sectional study. Med Pr. 2016;67(6):707–719. doi: 10.13075/mp.5893.00471. doi: 10.13075/mp.5893.00471. [DOI] [PubMed] [Google Scholar]