Abstract
Recent years have witnessed a revolution in our understanding of microglia biology, including their major role in the etiology and pathogenesis of neurodegenerative diseases. Technological advances have enabled the identification of microglial signatures in health and disease, including the development of new models to investigate and manipulate human microglia in vivo in the context of disease. In parallel, genetic association studies have identified several gene risk factors associated with Alzheimer’s disease that are specifically or highly expressed by microglia in the central nervous system (CNS). Here, we discuss evidence for the effect of stress, diet, sleep patterns, physical activity, and microbiota composition on microglia biology and consider how lifestyle might influence an individual’s predisposition to neurodegenerative diseases. We discuss how different lifestyles and environmental factors might regulate microglia, potentially leading to increased susceptibility to neurodegenerative disease, and we highlight the need to investigate the contribution of modern environmental factors on microglia modulation in neurodegeneration.
Microglia are the resident phagocytes of the innate immune system in the central nervous system (CNS). They are the first responders to neuroinflammation or damage and rapidly adapt their phenotype and functions in response to the brain milieu. They are important for several physiological functions, such as phagocytic activity and cytokine production, and they support other brain cells. New technological advances, including single-cell genomics, quantitative proteomics, and epigenetic studies, identified a role for the molecular and functional regulation of microglia in health and disease (Gosselin et al., 2014; Li et al., 2019; Masuda et al., 2019; Tay et al., 2018b). As an example, transforming growth factor β (TGF-β) and colony-stimulating factor 1 (CSF1) signaling, as well as other transcriptional factors including Sall1, Mafb, Irf8, and Pu.1, were identified as regulators of homeostatic microglia (Butovsky et al., 2014; Lund et al., 2018; Qin et al., 2018).
Neurodegeneration consists of age-related progressive loss and death of neuronal structures and functions in the CNS, leading to potential alterations of cognitive performance and dementia (Ramanan and Saykin, 2013). During neurodegeneration, microglia acquire neurodegenerative signatures, including those driven by Triggering receptor expressed on myeloid cells 2 (TREM2)-Apolipoprotein E (APOE) (Keren-Shaul et al., 2017; Krasemann et al., 2017). On the basis of these findings, human induced pluripotent stem cell (iPSC)-derived microglia were generated, allowing researchers to investigate the contribution of microglia-enriched risk genes to human disease. It also resulted in the development of novel humanized mouse models to study human microglia in vivo in the context of neurodegenerative disease.
Environmental factors such as chronic stress might influence microglia, which might in turn affect an individual’s susceptibility to neurodegeneration, because microglial dysfunction is a potential risk factor for Alzheimer’s disease (AD) development (Bisht et al., 2018; Katsumoto et al., 2018; Phan and Malkani, 2018). Stress during the perinatal period has also been linked to increases in cognitive deficits and psychiatric diseases later in life associated with neuroinflammation through potential alteration of microglial functions during development (Knuesel et al., 2014; Meyer and Hamel, 2014; Tay et al., 2018a). Dietary factors affect gut microbiota composition and modulate the immune system. Gut microbiota dysbiosis can regulate neuroinflammtion and microglia and has been associated with increases in the severity of neurodegeneration in animal models (Dodiya et al., 2019; Erny et al., 2015; Thion et al., 2018). Western diet has also been associated with an exacerbation of AD pathogenesis potentially through the alteration of microbiota (Drasar et al., 1973; Reddy et al., 1975; Wu et al., 2011). Therefore, diet intervention might be a promising approach for modulating disease predisposition and progression. Modern lifestyle is also associated with abnormal sleep patterns, which potentially promotes neurodegenerative functions of microglia (Bellesi et al., 2017; Wadhwa et al., 2017a; Wadhwa et al., 2017b). In this review, we discuss studies defining the mechanistic regulators and upstream molecular pathways that could be therapeutic targets to ameliorate the negative effects of lifestyle factors and their potential consequences on the brain. Altogether, recognition of the negative consequences resulting from certain lifestyles and the importance of microglia in the etiology and progression of AD might allow people to adopt different lifestyles to modulate their susceptibility to neurodegenerative disease, in addition to other treatments.
Chronic Stress and Microglial Dysregulation in Neurodegeneration: From Perinatal Stages to Aging
Suffering from chronic lifestyle stress during certain periods of life has become a common feature of modern societies. Chronic lifestyle stress and, in particular, psychosocial stress might confer a risk factor for late-onset AD (LOAD) and associated cognitive deficits. AD is characterized pathologically by the accumulation of phosphorylated tau and β-amyloid (Aβ) plaques. AD patients have been shown to present 83% increased cortisol levels in their cerebrospinal fluid (CSF) compared with healthy age-matched controls (Sapolsky et al., 1985; Swaab et al., 1994). Reactive gliosis, which consists of a range of molecular, morphological, and functional changes of glia in the CNS, and neuroinflammation are prominent hallmarks of AD (McGeer et al., 1987; Sarlus and Heneka, 2017). Microglia surround amyloid plaques in both human AD and animal models, although their role in disease progression is unclear (Sarlus and Heneka, 2017).
Several epidemiological and preclinical studies have shown that environmental factors might influence AD incidence (Qiu et al., 2009) (Figure 1). Individuals with a life-long history of stress are at higher risk for developing brain atrophy, cognitive deficits, and AD (Alkadhi, 2012; Gracia-García et al., 2015). Consistent with clinical studies, a retrospective study showed that aged primates stressed during early life presented significantly higher levels of Aβ plaque deposition and a reduction in synapse number compared with non-stressed primates (Merrill et al., 2011). Another study reported that restraint stress, or an unavoidable stress situation, elevates glucocorticoid hormones, which are key regulators of essential physiological functions in mammals, including the immune system (Smith and French, 1997). In rodent models of AD, chronic stress exacerbates neurodegeneration and cognitive impairments (Alkadhi and Tran, 2015; Carroll et al., 2011; Srivareerat et al., 2009) and increases Aβ accumulation and tau phosphorylation (Catania et al., 2009; Green et al., 2006; Joshi et al., 2012). Post-traumatic stress disorder (PTSD) is a neuropsychiatric disorder occurring in response to traumatic stress such as a dangerous or shocking event. A retrospective study of veterans showed that PTSD is associated with a higher risk of developing dementia (Yaffe et al., 2010), AD, and frontotemporal dementia (Bonanni et al., 2018). In addition, rodent models of PTSD present accelerated Aβ plaque formation and release (Rothman et al., 2012). Thus, chronic lifestyle stress is associated with increased vulnerability to neurodegenerative diseases along the lifespan. Frontal cortex and hippocampus are particularly vulnerable to the effects of stress. Interestingly, these regions are among the first regions to be affected in AD pathology. However, potentially common underlying mechanisms between stress and AD pathology have not yet been well characterized.
Figure 1. Lifestyle Factors Might Hijack Microglia Regulation and Predispose Individuals to Neurodegeneration.
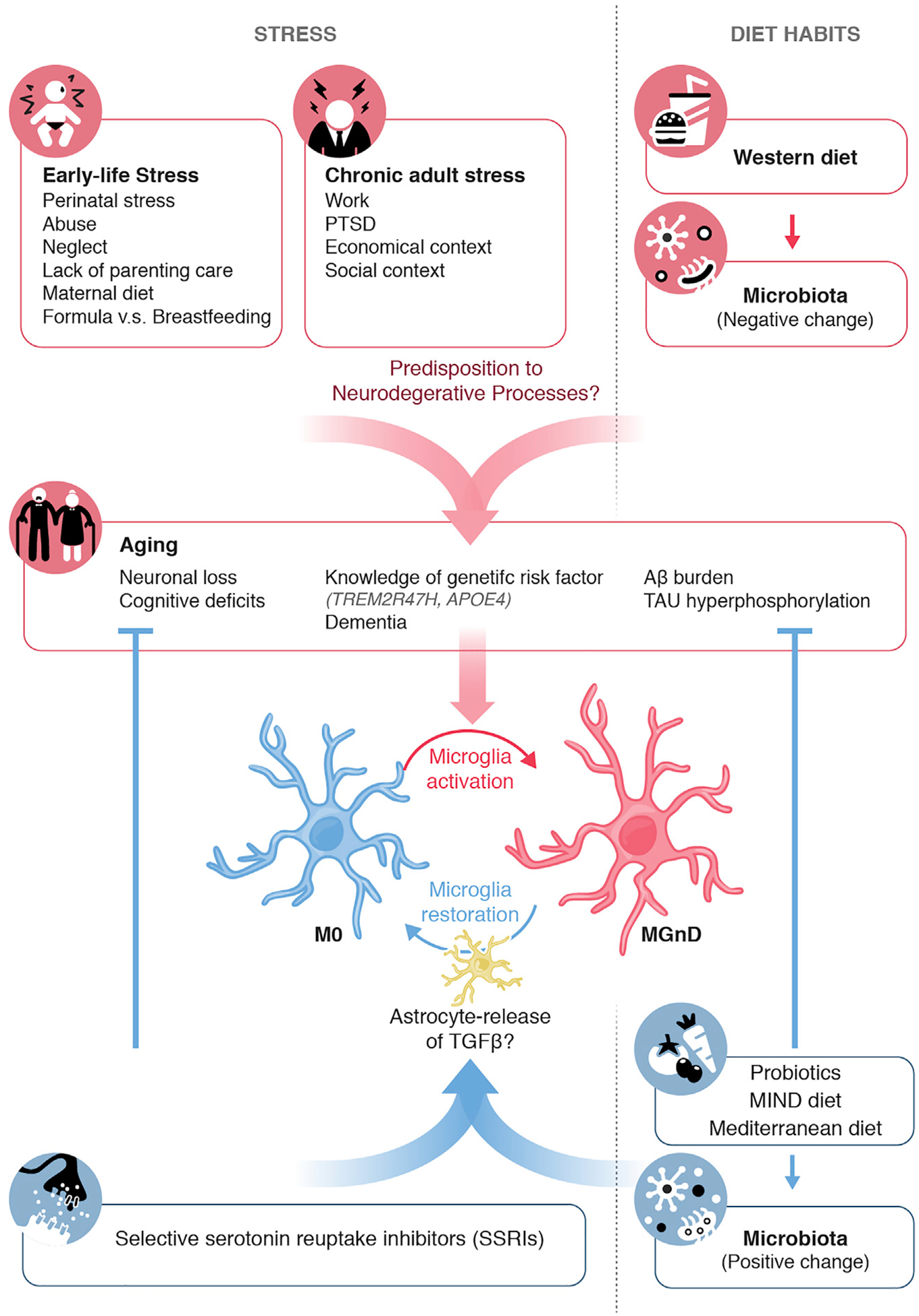
ELS, chronic adult stress, or changes in diet, microbiota, or social contexts might predispose individuals to neurodegenerative disease onset, as observed by associations with neuronal loss, cognitive deficits, and AD-related pathology. These phenotypes during aging could be influenced by a change in an individual’s dietary pattern, changes in an individual’s microbiota, and drug medications such as SSRIs. This preventive mechanism could be acting through astrocyte release of TGF-b that could modulate microglial functions and restore a phenotype that can stop neurodegeneration.
Neuroinflammation is a consequence of chronic stress and has been well characterized in AD. Epidemiological studies showed that PTSD is associated with immune system dysregulation (Hori and Kim, 2019). In particular, chronic psychosocial stress induces peripheral and CNS inflammation in the adult (Eidson et al., 2019). Restraint stress increased neuroinflammation, characterized by gliosis, and increased inflammatory gene transcription and lipid peroxidation independent of Aβ levels in an AD mouse model (Perez Nievas et al., 2011). Microglia are thought to play a pivotal role in AD pathology and plaque removal (Gandy and Heppner, 2013). Transcriptomic analyses have revealed a neurodegenerative signature termed MGnD (neurodegenerative microglia) or DAM (disease-associated microglia) in plaque-associated microglia from both human and mouse AD models (Kamphuis et al., 2016; Keren-Shaul et al., 2017; Krasemann et al., 2017; Yin et al., 2017) that was largely absent from microglia from non-plaque areas. This disease-associated signature includes upregulated expression of Apoe, Axl, Clec7a, Itgax, and Lgals3, forming a common core of neurodegenerative (Krasemann et al., 2017) and disease-associated transcriptomes demonstrated by several groups (Holtman et al., 2015; Keren-Shaul et al., 2017; Srinivasan et al., 2016). This core phenotype is characterized by the induction of genes related to APOE signaling and the suppression of TGF-b signaling in microglia associated with cellular damage and neurodegeneration. The MGnD and DAM phenotypes includes expression of chemokines that can facilitate recruitment of inflammatory monocytes and activation of astrocytes (Liddelow et al., 2017). Association of MGnD/DAM microglia with Aβ plaques is clear; however, major questions remain regarding their potential protective or disease-promoting functions. Innate immune cells can be protective in AD by reducing accumulation of Aβ (Frenkel et al., 2008; Simard et al., 2006), which happens early in the disease. Microglia might also lose their ability to phagocytose Aβ as they age (Hickman et al., 2013; Streit and Xue, 2009) and become neurotoxic with increasing Aβ deposition (Sastre et al., 2006; Streit et al., 2004). Chronic lifestyle stress sensitizes microglia toward a primed phenotype and induces neuroinflammation in the adult brain (Ramirez et al., 2016; Wohleb et al., 2014). Thus, stress might compromise the supportive roles of microglia for neurons and synapses, leading to deteriorating cognitive functions during aging. Once stressed, microglia change their gross morphology toward a more amoeboid, de-ramified phenotype with shorter and thicker processes (Kreisel et al., 2014; Wohleb et al., 2014). A marker for phagocytic activity, CD68, is increased on microglia in chronically stressed mice associated with augmented phagocytosis (Lehmann et al., 2016). Acute stress increases the number of microglia (Lehmann et al., 2016), whereas chronic stress decreases it, largely in the hippocampus (Tong et al., 2017). Thus, the intensity and duration of microglial activation by stress are important features that might lead to different pathological outcomes (Stein et al., 2017). However, persistent microglial pro-inflammatory activation after long exposure to stress could exacerbate neuronal damages and amyloidosis already present in AD pathology (Heppner et al., 2015). The potential contribution of somatic mutations toward chronic activation of microglia in neurodegeneration has to be taken into consideration. Indeed, somatic mutations in the line-age of erythro-myeloid progenitors (EMPs), to which microglia belong, might drive late-onset neurodegeneration by leading to constant microglial nuclear factor κB (NF-κB) activation and resulting in neuronal loss (Mass et al., 2017).
During postnatal development, many environmental factors, transmitted via the mother-child interaction, can affect proper brain development. For example, an infant depends on maternal care during the first weeks of life. However, in developed countries, child abuse, neglect, and lack of quality and time of parenting care have been linked to increased risk of negative long-term consequences on child health (Kaplan, 2001; Mueller et al., 2010; Nelson, 2007). In 2012, the U.S. Department of Health and Human Services referred 3.4 million cases to Child Protective Services, of which most related to child abuse or neglect. Early-life stress (ELS), in the form of childhood maltreatment, is the most common and potentially preventable cause of abnormal brain development (Kaffman and Meaney, 2007). ELS has been associated with social, emotional, cognitive impairment, and psychiatric disorders later in life (Delpech et al., 2016; Hanson et al., 2015; Pechtel and Pizzagalli, 2011). Studies in the early 1950s showed that glucocorticoid levels were higher in premature infants (Levine et al., 1951), potentially because of the neonatal intensive care period (Smith et al., 2011). Stress-related glucocorticoids—cortisol in primates and corticosterone in rodents—have been associated with a reduction in synapse number and altered neuronal assemblies and wiring (Popoli et al., 2011), and they are known to affect neurocognitive development (Carson et al., 2016). Thus, these neuroendocrine factors might affect neurodevelopment early in life. However, how ELS alters brain development associated with multiple clinical outcomes is not yet well understood in humans.
Chronic inflammatory conditions induce age-associated development of an AD-like neuropathology in mice, including increased amyloid precursor protein (APP) loads (Krstic et al., 2012). Thus, there is an emerging need to understand whether ELS affects the risk of developing AD by activating the brain’s innate immunity (Hoeijmakers et al., 2018). ELS aggravates neuropathology and alters disease progression in an AD mouse model (Hoeijmakers et al., 2017). However, the mechanism of microglial contribution to the effects of ELS is still unknown. It is possible that ELS drives neuroinflammation, which will alter microglial activity. ELS increases peripheral inflammation, which might play a role in many psychiatric disorders and alters microglial morphology and density, mainly in the rodent frontal cortex and hippocampus (Baldy et al., 2018; Gómez-González and Escobar, 2010; Roque et al., 2016). ELS is also associated with increased pro-inflammatory cytokines in the brain (Banqueri et al., 2019; Delpech et al., 2016; Gracia-Rubio et al., 2016). Thus, the link between early-life immune activation and deleterious consequences on the brain might affect microglial functions during development. Microglia mature during the second postnatal week (Butovsky et al., 2014; Matcovitch-Natan et al., 2016). The perinatal microglial phenotype resembles the MGnD and DAM phenotypes observed in neurodegenerative diseases (Hagemeyer et al., 2017; Krasemann et al., 2017; Wlodarczyk et al., 2017) and is represented by an APOE expression peak around postnatal day (P) 3, displaying gene characteristics of APOE-dependent MGnD signature (Butovsky et al., 2014; Hagemeyer et al., 2017; Matcovitch-Natan et al., 2016; Wlodarczyk et al., 2017) in a structure-dependent manner. This phenotype is associated with induction of MGnD genes such as Gpnmb, Spp1, and Apoe and an absence of expression of microglial homeostatic genes such as P2ry12, Tgfbr2, Mafb, Mef2a, and Sall1. However, this phenotype is considered non-toxic during development (Hagemeyer et al., 2017; Wlodarczyk et al., 2017).
Chronic stress might have a direct influence on microglial immune genes such as APOE and TREM2 associated with LOAD in genome-wide association study (GWAS) (Karch and Goate, 2015). These genes are associated with AD cognitive deficits and might be involved in stress-induced neuroinflammation. Human APOE has three alleles: ε2, ε3, and ε4. The APOE ε4 allele is found in about 15% of the population and in about 60% of patients with AD dementia (Mahley and Rall, 2000). Each additional copy of the ε4 allele is associated with a higher risk of developing AD and an earlier age of dementia onset (Corder et al., 1993; Strittmatter and Roses, 1995). APOE ε4 allele is the major genetic risk factor for LOAD (Corder et al., 1993; Strittmatter et al., 1993) acting in an age- and gene-dose-dependent manner (Corder et al., 1993; Farrer et al., 1997). APOE ε4 increases Aβ pathology and is associated with impaired memory performance (Liu et al., 2013). However, the pathophysiological mechanisms underlying the genetic association of APOE ε4 with AD remain elusive. One hypothesis is that long term exposure to chronic stress in older patients, which is associated with increased cortisol levels, might be associated with memory deficits in APOE ε4 variant carriers (Peavy et al., 2007). Chronic stress was shown to impair cognition in an APOE4-humanized mouse model at adult stages (Lin et al., 2016). Although cerebral Aβ deposition might begin 1 to 2 decades before the onset of cognitive impairment (Bateman et al., 2012; Price and Morris, 1999), recent studies suggest functional and structural brain alterations might precede the onset of Aβ deposition in APOE ε4 carriers (Bookheimer et al., 2000; Shaw et al., 2007). Young adult APOE ε4 carriers present impaired functional brain activity in regions preferentially affected by AD, decades before their average age at possible dementia onset (Shaw et al., 2007). Moreover, infant APOE ε4 carriers have decreased brain volumes in areas affected by AD (Dean et al., 2014). APOE ε4 adult carriers whom had childhood epilepsy exhibit increased brain Aβ load at late middle age (Joutsa et al., 2017). Thus, pathological conditions associated with activation of APOE signaling, i.e., induced apoptosis during CNS development or activation of glucocorticoids, might lead to an altered developmental APOE-MGnD phenotype, which might lead to later-life cognitive disorders and neurodegenerative diseases such as AD. Genetic variants of TREM2, most strongly R47H, also result in an increased risk of developing LOAD and other neurodegenerative diseases (Guerreiro et al., 2013; Ulrich and Holtzman, 2016). AD patients carrying the TREM2 mutation have increased brain atrophy and cognitive deficits (Jonsson et al., 2013). Moreover, naive Trem2−/− mice display impaired microglia-mediated synapse elimination (Filipello et al., 2018). However, how chronic stress affects TREM2 functions in microglia has yet to be investigated. A recent study reported that after chronic stress there is the presence of dark microglia that are TREM2+ and associated with AD pathology in APP/PS1 mice (Bisht et al., 2016). However, there is no study investigating cortisol levels or other measure of chronic stress in TREM2 variant carriers with AD or frontotemporal dementia. With the increase of personalized genetic testing in individuals, a patient’s knowledge of their genotype and potential risk of developing AD should be taken into consideration when cognition is measured in the elderly. Living with the knowledge of carrying the APOE ε4 variant and therefore being at increased risk for developing AD might adversely change the patient’s perception of their memory abilities and, thus, their performance on cognitive tests (Lineweaver et al., 2014). More importantly, it can be a source of chronic mental and emotional stress that could enhance the risk of developing neurodegeneration and AD pathology. An interesting observation is that women who breastfed had a lower AD incidence than women who did not (Fox et al., 2013). Ovarian hormone deprivation and/or insulin sensitivity present during breastfeeding might be responsible for this risk reduction. Promoting formula feeding and reducing maternity leave time in developed countries could be directly linked to an increase of AD in women.
Potential treatments for anxiety reduction such as selective serotonin reuptake inhibitors (SSRIs) might be used as preventive measures and treatments for AD (Figure 1). In clinical studies, long-term treatment with SSRIs is associated with reduced rates of dementia and delayed AD progression (Bartels et al., 2018; Kessing et al., 2009). Recently, a study showed that treating APP-PS1 mice with the SSRI fluoxetine improved memory performance, prevented loss of synapses and reduced Aβ plaques levels in the hippocampus (Zhou et al., 2019). Interestingly, these beneficial outcomes are potentially mediated through different cellular and molecular pathways including increased neurogenesis and gliogenesis (Banasr and Duman, 2007; Banasr et al., 2007), but also via activation of the TGF-β pathway in microglia mediated by astrocytic secretion (Caraci et al., 2018). However, genetic risk variants such as APOE4 and TREM2 R47H, which play a role in microglia modulation in AD, should be taken into consideration in future studies.
Diet Habits and Microglia Regulation: Influence of Microbiota
The gut microbiome has attracted significant attention from neuroscientists, because it modulates the CNS in both health and neurodegeneration. The adult microbiota depends on both genetics (Spor et al., 2011) and environmental factors including diet, maternal environment, and exposure to new microbiota (Sommer and Bäckhed, 2013).
Nutritional habits might influence neurodegenerative disease onset and progression by acting through microglia via peripheral inflammatory pathways such as microbiota. Nutrients and metabolites are known to modulate the immune system (Calder et al., 2017). However, the mechanisms of diet-induced effects are unclear. For example, peripheral inflammation and gut microbiota dysbiosis can tune neuroinflammation and increase the incidence of neurodegeneration (Heneka et al., 2015; Kowalski and Mulak, 2019; Pistollato et al., 2018) but the mechanisms are not well studied. Moreover, many studies have been conducted based on one nutrient at a time. However, the effects of food and nutrients might need to be studied in combination, because they are consumed together (Féart et al., 2009). A lot of attention has focused on the Mediterranean diet, especially in regard to cardiovascular diseases. However, the Mediterranean diet has also been positively associated with a slower rate of cognitive decline (van de Rest et al., 2015) and reduced risk of developing AD (Lourida et al., 2013) (Figure 1). The traditional dietary pattern in Mediterranean countries is characterized by high intake of fruits, vegetables, and wholegrains; moderate intake of fish, poultry, and alcohol (particularly red wine) and low intake of red and processed meats with olive oil used as the main fat source (Davis et al., 2015). On the opposite end of the spectrum, consumption of a Westernized diet, which is more prevalent in Western developed countries, has been associated with an increased risk of developing chronic inflammatory diseases (Uranga et al., 2016). The Western diet is characterized by a high content of proteins (derived from processed meats), saturated fats, refined grains, increased sugar and salt, alcohol, and mostly corn-derived fructose syrup and a decrease in fruit and vegetable consumption (Mozaffarian et al., 2011; Tilg and Moschen, 2015). This diet directly contributes to the development of obesity, metabolic syndrome, and cardiovascular diseases (Shoelson et al., 2007; Tilg and Moschen, 2015). In addition, dietary composition of Western-like diets increases the release of cortisol, influencing the response to stress (Maurer et al., 2003). Western diet has been shown to have an effect on intestinal microbiota, leading to a decrease in total bacteria, as well as specific species such as Bifidobacterium and Eubacterium (Drasar et al., 1973; Reddy et al., 1975; Wu et al., 2011). On the opposing end, the Mediterranean diet leads to increased levels of microbiota metabolites (short-chain fatty acids [SCFAs]), Prevotella bacteria, and other Firmicutes (De Filippis et al., 2016). Beneficial aspects of the Mediterranean diet are potentially because of increases in Lactobacillus, Bifidobacterium, and Prevotella and decreases in Clostridium (Bialonska et al., 2010; Clemente-Postigo et al., 2012; Fava et al., 2013; Furet et al., 2010; Koloverou et al., 2016; Queipo-Ortuño et al., 2012), although the specific effects of these alterations on microglia are unknown. Several studies show that obesity, which is associated with the Western diet, and chronic low-grade peripheral inflammation are a risk factor for AD at midlife (Anstey et al., 2011; Chuang et al., 2016; Kivipelto et al., 2005) (Figure 1). Obesity might induce inflammation through alterations of metabolic hormones levels such as insulin (Procaccini et al., 2016). Changes in insulin signaling might affect cognitive deficits and dementia onset as it impairs the control of neuronal excitability for example. Lower serum levels of insulin-like growth factor 1 (IGF-1) have been observed in obese patients (Galli et al., 2012) and might contribute to neuronal loss, as observed in Igf-1-deficient mice (Beck et al., 1995). Consumption of a Western diet also induces astrocytosis and TREM2+ microglial activation during aging and in AD mouse model (Cope et al., 2018; Graham et al., 2016). In addition, obesity accelerates AD pathology in APOE4-humanized 53FAD mice associated with an increase in Aβ pathology and microglial activation (Moser and Pike, 2017). Thus, obesity associated with a Western diet induces chronic neuroinflammation that might exacerbate AD pathogenesis.
Microglia from adult germ-free (GF) mice, which are not exposed to bacteria throughout life, display altered cell proportions and an immature phenotype, characterized by longer processes and greater numbers of branching and terminal points. This phenotype was associated with impaired CNS immune responses toward immune challenges (Figure 2) (Brown et al., 2019; Erny et al., 2015). Recolonization with complex microbiota or administration of bacterial metabolites, a mix of SCFAs, was able to restore defects in microglia density, proportions, and surface marker expression (Erny et al., 2015). A model of limited microbial diversity was used to examine the impact of a non-diverse gut microbiome (Erny et al., 2015). These mice displayed similarly altered microglia relative to GF mice, suggesting that a non-diverse microbiome is insufficient for normal microglial development. However, recolonization of these mice with a more diverse microbiome allowed microglial maturation to a phenotype similar to adult specific pathogen-free animals. Thus, it might be that the presence of particular microbiota species or a sufficiently diverse microbiome is required for proper microglial function. In humans, an individual’s microbiota is relatively stable with day-to-day variation (Costello et al., 2009). Large disruptions occur after antibiotic administration, infection, and large diet changes (David et al., 2014). Depending on the disruption frequency and length, microbiota recovery might be impaired (Dethlefsen et al., 2008; Levy et al., 2017; Ubeda and Pamer, 2012). The use of antibiotics might affect microglial function, which must be taken into account when considering the large-scale use of antibiotics. Interventions to enrich or entire reconstruction of an individual’s microbiome by probiotics or fecal matter transplant (FMT) might become viable options.
Figure 2. Microbiome Depletion Modulates Microglial Phenotype and Neurodegenerative Disease Pathology.
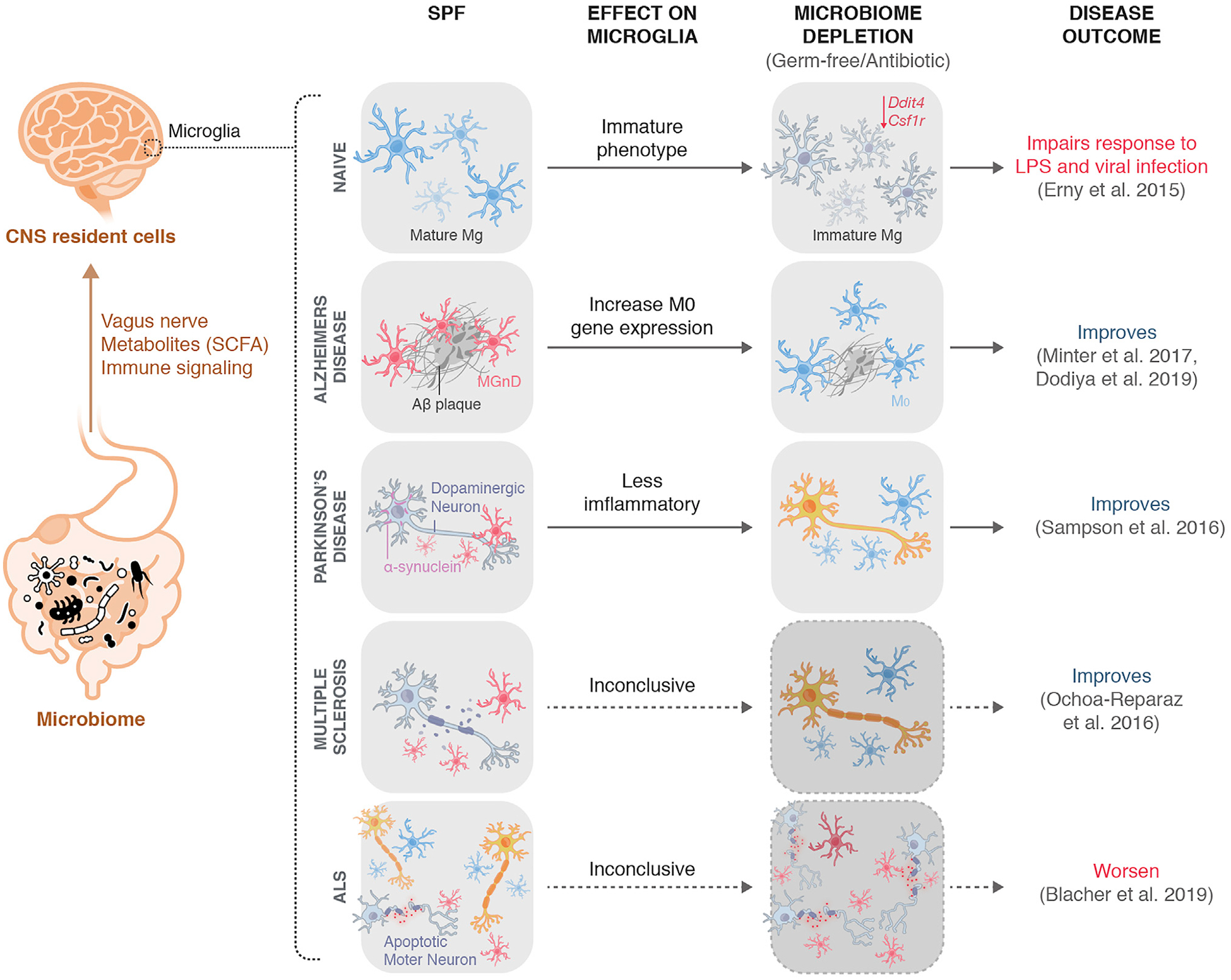
The microbiome can communicate with CNS resident cells, including microglia, through various pathways, including the vagus nerve, microbial metabolites (SCFAs), and direct and in-direct immune signaling pathways. Upon microbiome depletion either in a GF context or after antibiotic administration, microglia adopt an immature phenotype associated with impaired responses to LPS and viral infections. In the context of neurodegeneration, microbiome depletion in AD and PD is associated with amelioration of disease progression and pathology, with increased M0-homeostatic gene expression in microbiome-deplete AD mice and evidence of less inflammatory microglia in microbiome-deplete PD mice. In ALS, disease pathology is markedly worsened in a microbiome-depleted context. Microglial phenotype remains to be explored in microbiome-depleted ALS mice.
The maternal gut microbiome is also important for microglial development and maturation. The maternal gut microbiome helps shape microglial development close to birth. At embryonic day (E) 14.5, embryonic microglia from offspring of GF dams display only minor differences compared with controls; however, microglia isolated closer to birth, at E18.5, displayed considerable differences in transcriptomes, morphology, and distribution. Importantly, a strong gender effect was observed; major differences were specifically observed in embryonic males, whereas females displayed the largest differences later in adulthood (Thion et al., 2018). Metabolites such as SCFAs have been shown to influence microglial maturation and phenotype in health and disease (Hara et al., 1999; Raybould, 2010). Mice lacking free fatty acid receptor 2 (FFAR2), a G protein-coupled receptor required for SCFA signaling in the gut, exhibited a similar microglial phenotype to that observed in GF mice (Erny et al., 2015), although the mechanism of action is still unclear (Chen et al., 2007; Erny et al., 2015). These important observations open up interesting avenues to explore the role of microbiome modulation of microglia in development and its potential consequences on neurodegeneration in adulthood (Figure 2). In humans, maternal anxiety during pregnancy (Hechler et al., 2019) and ELS (Jacquot et al., 2011; Rougé et al., 2010) can affect microbial composition in infants, linking psychological symptoms in pregnancy to microbiota alterations in offspring. The use of probiotics may ameliorate these effects (Cowan et al., 2019).
Several bidirectional signaling pathways have been identified by which the gut microbiota affects microglia, including immune and neural pathways (Figure 2) (Bravo et al., 2011; Erny et al., 2015; Tan et al., 2014b). Efferent signals can modulate gastroin-testinal (GI) motility, secretions, and permeability, modifying the microbiome environment and its composition (Collins et al., 2012). The vagus nerve connects the gut and the brainstem, serving as the afferent conduit for communicating satiety, stress, and mood, and recognizes gut microbial products (Forsythe et al., 2014; Goehler et al., 2005). The inflammatory state of the gut can directly activate chemoreceptors located at vagal nerve endings to influence microglia and the level of inflammation in the CNS (Forsythe et al., 2014). A peripheral immune challenge can cause upregulation of anti-inflammatory pathways in the brain via the vagus nerve, including decreased microglial pro-inflammatory cytokines (Frasch et al., 2016; Kaczmarczyk et al., 2017; Meneses et al., 2016). Microbiota can influence the CNS through direct modulation of the peripheral immune system or by regulating intestinal permeability to control the entry of pathogenic, immune-stimulating, and neuroactive substances (Karczewski et al., 2010; Kelly et al., 2015). Astrocytes might also control microglia regulation during aging and in disease. Indeed, it has been shown that in combination with microbiota changes, dietary tryptophan is metabolized by the gut into aryl hydrocarbon receptor (AHR) agonists that have an effect on astrocytes to limit neuroinflammation via interferon type I (IFN-I) signaling (Rothhammer et al., 2016). In addition, microbial metabolites might limit pathogenic activities of microglia and astrocytes and suppress CNS inflammation via transforming growth factor α (TGF-α) and vascular endothelial growth factor B (VEGF-B) produced by microglia (Rothhammer et al., 2018). Mice fed a high-fat diet show elevated levels of Firmicutes and Proteobacteria compared with Bacteroidetes, a finding replicated in obese patients. Akkermansia muciniphila is one identified Bacteroidetes that is less abundant in obese individuals and has been implicated in intestinal integrity (Dao et al., 2016). A pro-inflammatory response could compromise the integrity of the blood-brain barrier (BBB) (Rochfort et al., 2014), and increased circulation of BBB-permeable cytokines and neurotoxic compounds could directly lead to microglial activation (Qin et al., 2008; Riazi et al., 2008). Microbiome-microglia communication might rely on specific microbiota and is likely mediated via multiple mechanisms. Identification of individual metabolites and their effects on microglia is a critical step in developing any therapeutic strategy. The effects of the microbiome on microglial phenotype and functions, such as phagocytosis of various substrates, milieu sensing, tolerance, and migration, remain to be investigated. Interesting questions remaining to be explored are whether and how microglia might modulate the composition or function of the microbiome.
Microbiome-Microglia Modulation in Neurodegeneration
Preclinical and human cross-sectional studies have associated microbiome alterations with several neurodegenerative diseases, including Parkinson’s disease (PD) (Fasano et al., 2013; Keshavarzian et al., 2015; Tan et al., 2014a), AD (Harach et al., 2017; Ho et al., 2018; Minter et al., 2016), amyotrophic lateral sclerosis (ALS) (Rowin et al., 2017; Zhang et al., 2017b), and multiple sclerosis (MS) (Cekanaviciute et al., 2017; Kosmidou et al., 2017; Miyake et al., 2015).
PD is the second most common neurodegenerative disorder after AD and is characterized by dopaminergic neuron death in the substantia nigra and neuronal accumulation of α-synuclein (Goedert et al., 2013). Alterations of the microglial microenvironment might trigger a pathogenic phenotype associated with release of pro-inflammatory cytokines and other neurotoxic compounds (Zhang et al., 2005). Large-scale microglial molecular profiling in animal models of PD has not yet been conducted, but activated microglia are known to be prominent in both mouse models and human PD (Joers et al., 2017), including expression of AXL associated with MGnD/DAM microglia (Keren-Shaul et al., 2017; Krasemann et al., 2017). One hypothesis contends that sporadic PD begins in the gut by α-synuclein aggregation, spreading via the vagus nerve (Braak et al., 2004; Rietdijk et al., 2017). Indeed, vagotomy might be associated with reduced risk of PD development in humans (Svensson et al., 2015a). In support of this hypothesis, a recent study found that gut injection of α-synuclein fibrils converts endogenous α-synuclein into a pathological species that spreads to the brain, leading to PD features. Consistently, vagotomy or α-synuclein deficiency prevented neuropathological and neurobehavioral deficits (Kim et al., 2019). Gut-associated symptoms, including chronic constipation and GI distress, precede motor symptoms in up to 80% of PD patients (O’Sullivan et al., 2008). Thus, it would be important to investigate whether patients with GI distress exhibit increased levels of α-synuclein production in the gut, thereby linking the GI issues with the potential mechanism of spreading to the brain. Moreover, PD patients exhibit significantly altered gut microbiome (Hill-Burns et al., 2017) and reduced SCFA concentrations (Unger et al., 2016). GF mice that overexpress human α-synuclein display reduced motor deficits, GI dysfunction, and microglial activation (Figure 2) (Sampson et al., 2016). Furthermore, administering SCFAs to these GF transgenic mice increased microglial activation and induced motor deficits (Sampson et al., 2016). Interestingly, in a toxin-induced model of PD, FMT attenuated microglial activation, along with motor deficits and decreased SCFAs (Sun et al., 2018). This supports the idea that microbiome components might have a beneficial effect and, if identified, could be therapeutic targets. Microglial phenotype in PD still needs to be defined to put their role into context with other neurodegenerative diseases.
The gut microbiome might be an important factor in the etiology of AD. Microbial metabolites were measured in the CSF of AD patients and associated with AD biomarkers, including phosphorylated tau and Aβ (Vogt et al., 2018). In addition, aged individuals exhibit a different microbiome (Claesson et al., 2011), which supports the possibility that an altered microbiome mediated by aging is associated with the AD etiology and pathogenesis. When comparing fecal microbiomes and SCFAs between AD and wild-type (WT) mice at different ages, the proportions of certain microbiota were different, including elevations in Verrucomicrobia and Proteobacteria, whereas the levels of SCFAs were reduced (Zhang et al., 2017a). An interesting approach would be to investigate whether FMT from old to young AD mice would exacerbate disease progression, as well as whether young-to-old microbiome transfers may be a treatment for AD. The oral microbiome has also become of interest after the pathogen Porphyromonas gingivalis, involved in chronic periodontitis, was identified in the brain of AD patients, and oral infection in mice resulted in brain colonization and increased Aβ production (Dominy et al., 2019). GF AD mice displayed a reduction in Aβ pathology, and colonization of these mice increased pathology (Harach et al., 2017). Furthermore, long-term antibiotic treatment in an AD mouse model decreased Aβ plaque deposition associated with altered cytokine and chemokine signatures and microglial morphology (Figure 2; Minter et al., 2016). Strikingly, short-term postnatal antibiotic treatment (P14–P21) resulted in lasting gut microbiome alterations associated with a reduction in Aβ deposition later in life, along with alterations in the CNS inflammatory milieu and reduced plaque-associated microglia (Minter et al., 2017). Aβ pathology amelioration by antibiotics occurs only in brains of male mice associated with restoration of M0-homeostatic microglia. In addition, FMT of microbiota from age-matched AD male mice into antibiotic-treated AD males restored the gut microbiome and partially restored Aβ pathology and microglial morphology (Dodiya et al., 2019). In contrast to depleting the microbiome, administration of Lactobacillus plantarum was able to ameliorate cognitive deficits in AD mice (Nimgampalle and Kuna, 2017).
ALS is a fatal neurodegenerative disease characterized by the progressive loss of motor neurons. Most ALS patients die within three to five years of diagnosis because of respiratory paralysis (Alonso et al., 2009). Microglia isolated from the SOD1 mouse model of ALS upregulate MGnD/DAM pathways similar to microglia from AD mice, including APOE expression (Butovsky et al., 2015; Chiu et al., 2013; Krasemann et al., 2017), a finding confirmed in human ALS (Butovsky et al., 2015). The M0-homeostatic microglial signature is lost as early as 2 months before disease onset in SOD1 mice (Butovsky et al., 2015). Similar to AD, ALS is an age-dependent disease, potentially implicating age-related changes in the microbiome to disease vulnerability. SOD1 mice had an altered microbiome profile with reduced levels of Butyrivibrio fibrisolvens, Escherichia coli, and Fermicus, which was detected before disease onset (Wu et al., 2015), potentially linking changes in the gut microbiome to microglial changes. Importantly, Butyrivibrio fibrisolvens is a butyrate-producing bacterium, and feeding SOD1 mice with the SCFA restored microbial homeostasis, improved gut integrity, and prolonged lifespan (Zhang et al., 2017b). In a small pilot study, the gut microbiome of 5 human ALS patients was examined, and all displayed altered gut microbiome characterized by low diversity with relatively intact abundance (Rowin et al., 2017). A recent study found that GF or antibiotic-treated SOD1 mice had significantly exacerbated disease (Figure 2; Blacher et al., 2019). This work identified 11 distinct microbiota that correlated with disease severity and showed through individual supplementation that Akkermensia muciniphila ameliorated disease and Ruminococcus torques and Parabacteroides distasonis exacerbated disease. Interestingly, the authors identified that Akkermensia muciniphila-treated SOD1 mice displayed an accumulation of nicotinamide (NAM) in the CNS. They went on to systemically administer NAM, which significantly improved the performance of SOD1 mice in both behavioral and neurological motor tests and produced a trend toward increased survival. The authors compared the microbiome composition of ALS patients with healthy control household members and found significant differences overall, but only five specific bacterial species reached near significance. Functionally, the ALS microbiomes showed differences in bacterial gene content, with decreases in several key genes involved in NAM metabolism (Blacher et al., 2019). These findings that microbiome depletion worsens disease in SOD1 mice stand in contrast to those in other neurodegenerative models. However, they are consistent with the opposing effects of minocycline in MS and ALS (Gordon et al., 2007; Metz and Eliasziw, 2017). Although the diseases share a common symptom, neurodegeneration, these discordant effects of microbiome depletion emphasize the consideration that opposing, disease-specific treatment approaches might be necessary. It is also possible that microglia might not be involved in disease pathogenesis in all neurodegenerative diseases. Nonetheless, defining microglia in the context of microbiome-depleted ALS models will likely be an area of intense research to investigate how their function in disease might differ in these models and to discover mechanisms that could serve as therapeutic targets.
MS is a chronic, inflammatory disease characterized by immune-mediated CNS demyelination resulting in neurological disorders. Microglia play an important role in MS, including during neurodegeneration in later stages. In the experimental autoimmune encephalomyelitis (EAE) mouse model of MS, microglia have different transcriptional phenotypes associated with disease stages. At disease peak, their phenotype is similar to that observed in other neurodegenerative disease models, such as AD and ALS (Chiu et al., 2013; Krasemann et al., 2017). In human MS, microglia also have multiple phenotypes dependent on disease stage (relapsing/remitting or progressive) and lesion type (active or chronic) (Zrzavy et al., 2017). Microglia lose the expression of homeostatic markers, including P2RY12, and express pro-inflammatory markers, including phagocytic, antigen presentation, and reactive oxygen species markers (Zrzavy et al., 2017). Minocycline, which has been shown to specifically affect microglia (Kobayashi et al., 2013; Sriram et al., 2006), showed positive trends in a trial in MS (Metz and Eliasziw, 2017), although it worsened disease in ALS patients (Gordon et al., 2007). However, it is possible that minocycline affected the microbiota, which then affected disease progression (Vaughn et al., 2017). EAE studies showed that oral administration of antibiotics significantly reduced disease severity (Ochoa-Repáraz et al., 2009) and GF mice display attenuated EAE development (Lee et al., 2011; Figure 2). In a clinical study, elevated levels of specific microbiota (Akkermansia muciniphila and Acinetobacter calcoaceticus) were observed in MS patients (Jangi et al., 2016). Transplantation of these bacteria from patients with MS into GF mice leads to EAE exacerbation (Cekanaviciute et al., 2017). Further studies have shown that microbiota from pediatric MS patients exhibit relatively greater pro-inflammatory trends and that depletion of certain flora might be linked to increased relapse risk (Tremlett et al., 2016; Tremlett and Waubant, 2018). Treatment of MS with the probiotic VSL3, a cocktail of eight bacteria with a good safety profile, enriched specific microbiota in the intestines and inhibited monocyte-mediated peripheral inflammation, an effect that disappeared after discontinuation (Tankou et al., 2018). Although the microglial phenotype in EAE and MS has been defined and significant investigation into the role of microbiota is ongoing, defining work into how microglia and the microbiome interact in MS and EAE remains to be conducted. One important question to be addressed is how microbiome alterations at different stages of the disease course might modulate disease progression and microglial phenotype.
A significantly altered gut microbiome is a common feature in neurodegenerative disease. Altered microbiomes might have many effects on brain physiology, including the consistent observation of an immature and functionally impaired phenotype in microglia. It is also possible that in microbiome-depletion conditions, microglia might be reset to a homeostatic state. This hypothesis is supported by the appearance of M0-homeostatic microglia in the antibiotic-treated AD model (Dodiya et al., 2019). The identification of specific microbiota associated with worsening or ameliorated pathology is a critical step to investigate precise underlying mechanisms. Putative molecules are being identified that can potentially move to clinical trials to investigate their function in humans. Further work is needed to explore microbiome-mediated maintenance and polarization of M0 and MGnD and DAM microglia and to further define specific microbiome-dependent microglial phenotypes and functions in neurodegeneration. It is clear that lifestyle, including dietary choices and treatments like antibiotics, can significantly affect the human microbiome and microglia, possibly affecting predisposition to neurodegeneration.
Circadian Rhythm and Sleep Patterns Influence on Microglia Regulation in Neurodegeneration
We spend one-third of our lives sleeping. Sleep is required to maintain normal neurocognitive and immune functions (Dumaine and Ashley, 2015). During sleep, the brain processes information, consolidates newly formed memories (Abel et al., 2013), promotes spatial learning (Nguyen et al., 2013b), and clears the brain (Xie et al., 2013). Sleep loss might result from sleep deprivation (SD), chronic sleep restriction, and sleep fragmentation. Sleep fragmentation is commonly seen in sleep disorders such as obstructive sleep apnea or restless legs syndrome (Reynolds and Banks, 2010). SD and chronic sleep restriction are mainly caused by work, lifestyle, drugs, and aging.
Sleep plays an important role in Aβ clearance (Xie et al., 2013). The sleep-wake cycle can regulate interstitial fluid (ISF) and CSF levels of Aβ (Figure 3). It has been shown that Aβ clearance pre-dominantly occurs during sleep (Kang et al., 2009), which was ascribed to the glymphatic pathway operating most efficiently during sleep (Iliff et al., 2012; Lee et al., 2015; Louveau et al., 2015; Xie et al., 2013). Using real-time in vivo two-photon microscopy, it has also been shown that patrolling monocytes might crawl onto the luminal walls of blood vessels to clear and carry Aβ out of the brain (Michaud et al., 2013). Interestingly, neuroimaging studies showed that short sleeping duration and poor sleep quality in cognitively healthy middle-aged and aged people might be associated with higher Aβ accumulation (Brown et al., 2016; Spira et al., 2013; Sprecher et al., 2015). A human positron emission tomography (PET) study showed that just one night of SD significantly induced Aβ levels in the brain (Shokri-Kojori et al., 2018). Acute SD is enough to elevate Aβ levels in mouse ISF (Kang et al., 2009) and human CSF (Ooms et al., 2014). Chronic sleep restriction significantly increases the ISF level of Aβ in Drosophila (Tabuchi et al., 2015) and mouse (Kang et al., 2009) models of AD. Sleep disturbance and circadian rest-activity pattern alterations observed in preclinical AD studies might be risk factors for developing AD (Jagust, 2016; Mucke and Selkoe, 2012; Musiek et al., 2018). Furthermore, in human brains, the rhythmic DNA methylation of brain and muscular Arnt-like 1 (BMAL1) is altered in AD, which might be one reason for the circadian alterations in AD brains (Cronin et al., 2017). In addition to Ab, CSF tau and α-synuclein might increase in sleep-deprived humans. In a transgenic P301S tau mouse model and in human CSF, tau levels were increased after chronic SD that could facilitate the spread of tau pathology (Holth et al., 2019) (Figure 3).
Figure 3. Sleep Loss Might Promote Neurodegenerative Functions of Microglia and Can Be Prevented by Physical Exercise.
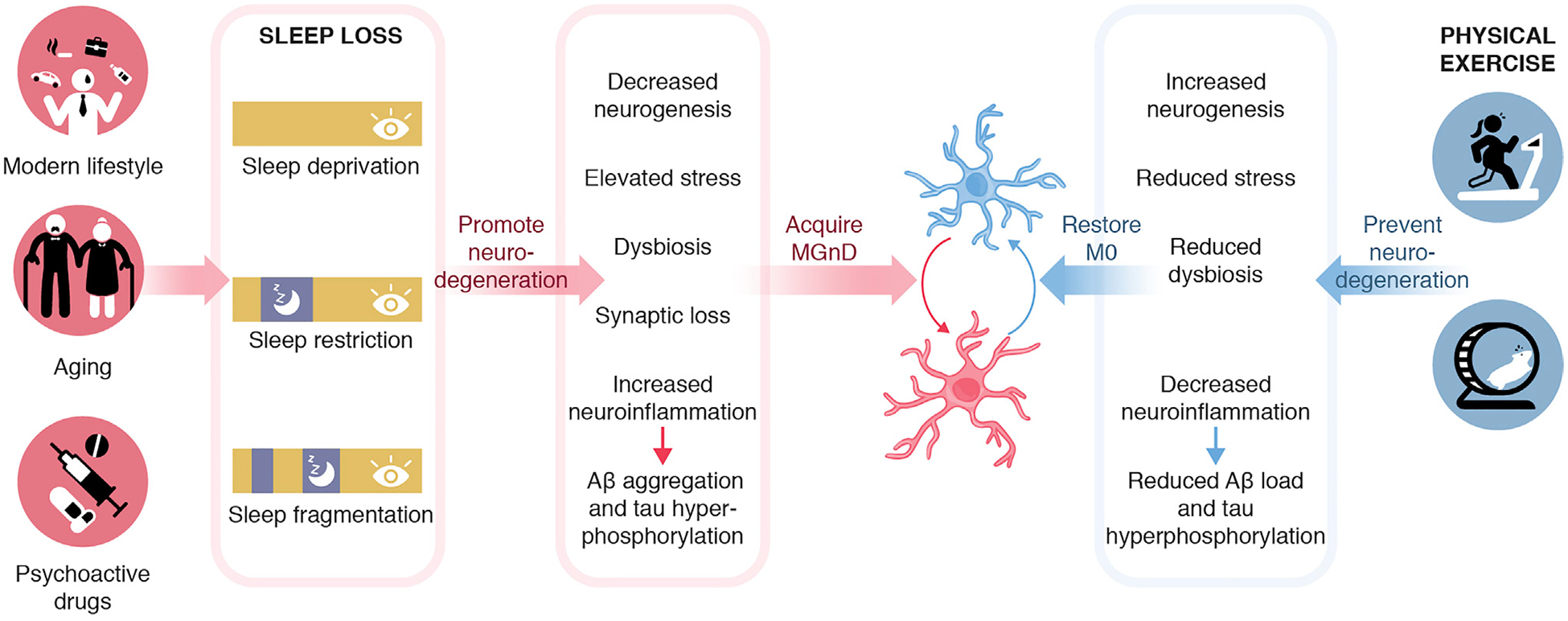
Forms of sleep loss, including deprivation, restriction, and fragmentation, could promote neurodegeneration by decreased neurogenesis, elevated stress, altered microbiome composition, synaptic loss, increased neuroinflammation, and protein aggregation. These conditions could lead to the acquisition of MGnD, which could exacerbate disease. Conversely, physical exercises might support increased neurogenesis, reduced stress, altered microbiome composition, reduced neuroinflammation, and protein aggregation, which might restore microglial phenotype and prevent neurodegeneration.
SD and sleep restriction alter brain functions at molecular, cellular, and network levels, which might lead to severe cognitive and emotional problems (Musiek and Holtzman, 2016; Pires et al., 2016). SD-induced cognitive impairment is associated with elevated inflammatory cytokine levels in the hippocampus, gliosis, and morphological changes of microglia and astrocytes (Wadhwa et al., 2017a). Several studies have shown that SD modulates astrocytes and microglial phenotypes and functions, which might contribute to neurodegeneration (Figure 3). Interestingly, inhibiting microglial activation with minocycline during SD decreases the hippocampal immunoreactivity and improves cognition and adult hippocampal neurogenesis (Wadhwa et al., 2017b). Tyrosine kinase receptor Mertk and its ligand GAS6, which mediate microglial process extension and induce phagocytosis (Fernandes et al., 2016; van der Meer et al., 2014), are upregulated in both microglia and astrocytes during SD (Grommes et al., 2008). Both acute SD and chronic SD impair the cognition and immune functions, in which microglia play an active role. Further studies on microglial molecular signature alteration during SD are needed. The sleep-wake cycle affects synaptic remodeling via microglia. Mice lacking cathepsin S, which is a microglial-specific lysosomal cysteine protease, present impaired diurnal variations in spine density and activity of cortical neurons (Hayashi et al., 2013). Phagocytic microglia mediate synapse elimination via C1q and C3 complement factors (Schafer et al., 2012; Stevens et al., 2007). In addition, early synaptic loss in AD mouse models is mediated by microglia through C1q and C3 (Hong et al., 2016) (Figure 3). Interestingly, C3 levels are upregulated in the brain after acute and chronic SD (Bellesi et al., 2017). Thus, complement-mediated synaptic pruning might be exacerbated by SD, which might contribute to early synaptic loss and AD onset. Potential molecular pathways and upstream regulators could be therapeutic targets for preventing brain impairments caused by sleep loss.
Microglial inflammatory responses can be tightly controlled by the circadian clock and, if altered, might predispose individuals to neurodegeneration. When immune challenged, microglia display higher expression of pro-inflammatory cytokines, and their process extension is significantly increased during the light phase (Fonken et al., 2015; Takayama et al., 2016). In addition, chronic stress during the light phase could amplify microglial responses (Fonken et al., 2016b). Glucocorticoids play a pivotal role in stress-induced priming of neuroinflammatory responses (Frank et al., 2012, 2014). Thus, the circadian dependency of microglia responsiveness to glucocorticoids might be one mechanism for the diurnal rhythm of microglia toward inflammatory stimuli (Fonken et al., 2016b). Another possibility could be that circadian genes are involved in regulating immunological activities of microglia. For example, circadian protein circadian locomotor output cycles kaput (CLOCK) regulates NF-κB-mediated transcription (Spengler et al., 2012). Circadian gene BMAL1 has been found to regulate diurnal oscillation of Ly6CHi classical inflammatory monocytes in trafficking to sites of inflammation (Nguyen et al., 2013a). BMAL1−/− mice have been shown to present massive gliosis and degeneration of synaptic terminals (Musiek et al., 2013). miR-155 plays an important role in induction of inflammatory microglial phenotype in neurodegeneration (Butovsky et al., 2012, 2015; Koval et al., 2013). BMAL1 inhibits NF-kB activation and suppresses miR-155 in peripheral myeloid cells (Curtis et al., 2015). In addition, depletion of miR-155 diminishes the circadian rhythm of cytokine responses to lipopolysaccharide (LPS) (Curtis et al., 2015). Thus, circadian genes regulate the immune response of microglia and might contribute to neurodegeneration directly by enhancing neuroinflammation or via alterations of the microbiome. A jetlag circadian paradigm can change gut microbiota (Phan and Malkani, 2018) and thus potentially microglial responses. Future studies are needed to investigate the association between circadian rhythm disorder and microglial functional alterations, as well as their contribution to neurodegeneration.
Aging is associated with altered sleep patterns and disrupted circadian rhythm of microglia. Sleep in aged people is characterized by an increased number of arousals from sleep, decreases in total sleep time and sleep efficiency, and a reduction of nonrapid eye movement (non-REM) sleep (Miner and Kryger, 2017). In addition, the common feature of age-related change in circadian rhythmicity is the shift of sleep to earlier hours, i.e., early morning awakening hours, that are often earlier than desired (Duffy et al., 2015). Microglia isolated from aged animals displayed suppressed expression of diurnal clock genes and inflammatory cytokines compared with young microglia (Fonken et al., 2016a). Glucocorticoid rhythm is dysregulated in aged hippocampus (Herbert et al., 2006), despite aged microglia maintaining the responsiveness to corticosterone (Fonken et al., 2016a). The dysregulated glucocorticoid rhythm in aging subjects is associated with chronic stress, which contributes to neurodegeneration (Vyas et al., 2016). Aging-related microglia-specific circadian genes expression profiling is still lacking. In the future, targeted therapies that enhance molecular rhythmicity might be a potential approach to prevent age-related sleep-wake change and improve cognition. Deeper understanding of how the circadian clock change during aging influences microglial function might create insight to regulate circadian clock and improve health and longevity.
Exercise Modulates Microglia in Neurodegeneration
The neuroprotective roles of physical exercise and environmental enrichment have been recognized in several studies (Chen et al., 2016; Ziv et al., 2006). Beneficial effects of physical exercise include anti-inflammatory effects, improvement of hippocampal neurogenesis, and stimulation of brain-derived neurotrophic factor (BDNF) release (Gleeson et al., 2011). In addition, physical exercises reduce anxiety and modulate the gut microbiota, independent of diet, and have positive health effects in the brain (Mailing et al., 2019) (Figure 3). An hour of wheel running by mice can increase the relative abundance of Lachnospiraceae, which is positively associated with reduced anxiety-like behavior in mice (Kang et al., 2014). Accumulating evidence also suggest that aerobic exercise could protect the brain from systemic inflammation by directly regulating inflammatory cytokines (Beavers et al., 2010), by mediating the secretion of anti-inflammatory adipokines and myokines through the muscle-adipose crosstalk (Kelly, 2018; Leal et al., 2018; Mazur-Bialy et al., 2017), or via the hypothalamic-pituitary-adrenal axis (Ortega, 2016). The effects of short- or long-term physical exercises on brain functions have been shown in various AD mouse models and include improvement of cognitive performance, reduction of pro-inflammatory cytokine levels, and most importantly, amelioration of Aβ deposition and tau pathology (Kelly, 2018). Physical exercises reduce the production of cerebral pro-inflammatory cytokines by promoting clearance of Aβ (Prado Lima et al., 2018) and reduce GFAP+ astrocyte density in the hippocampus of APP-PS1 (Tapia-Rojas et al., 2016). Environmental enrichment has been shown to reduce Aβ levels in an AD mouse model (Lazarov et al., 2005) and to prevent microglia-mediated neuroinflammation in injected with human Aβ oligomers (Xu et al., 2016). Physical exercise and environmental enrichment ameliorate Aβ and tau pathology not only when they are provided before the pathology starts to show but also at the late stage of the disease (Leem et al., 2011; Tapia-Rojas et al., 2016). In a 16-month-old transgenic tau mouse model, treadmill running for 3 months could significantly decrease tau phosphorylation, as well as microglia-induced neuroinflammation (Leem et al., 2011). The anti-inflammatory effects of exercises were also shown in a PD mouse model (Svensson et al., 2015b). Beneficial effects include amelioration of motor deficits and reduction of pro-inflammatory cytokine secretion (Svensson et al., 2015b). Treadmill running for 30 min/day for 2 weeks in rats leads to reduced expression of downstream targets of Toll-like receptor (TLR) pathways, such as myeloid differentiation primary response gene 88 (MyD88) and NF-κB (Altmeppen et al., 2013). The reduction in TLR signaling in microglia could be one mechanism for the anti-inflammatory effects of physical exercise. Exercise could reverse aging and infection-induced memory deficits (Barrientos et al., 2011) by directly increasing the levels of interleukin-10 (IL-10), an anti-inflammatory cytokine in the hippocampus of aged rat (Gomes da Silva et al., 2013). CD86+ and major histocompatibility complex class II-positive (MHC class II+) microglia are increased with aging (Kohman et al., 2013). Wheel running by mice has been shown to decrease the proportion of both CD86+ and MHC class II+ microglia in the hippocampus. However, these effects are gender dependent, and aged male mice showed a decrease in CD86+ microglia and an increase in MHC class II+ microglia (Kohman et al., 2013). Microglial functions might also be restored in an enriched environment promoting neuronal support. In addition, the beneficial effect of exercise on cognition in a transgenic AD mouse model has been suggested to be mediated by both improvement of neurogenesis and elevation of BDNF secretion genetically and pharmacologically (Choi et al., 2018). Interestingly, elevated neurogenesis alone could not restore cognitive impairment in 53FAD mice. The effect has to be combined with increased BDNF. This will be a potential way to improve cognition in neurodegeneration. However, it remains to be determined whether these beneficial effects are microglia mediated. In summary, exercise and enriched environment could play a strong protective role in aging and neurodegeneration by reducing neuroinflammation and modulating microglial phenotypes and functions.
Concluding Remarks
Here we reviewed current knowledge of how modern societal lifestyle factors such as diet, sleep patterns, physical activity, and microbiota affect microglia regulation and neurodegeneration in both animal models and humans. Determining the true role and importance of microglia in the onset and progression of neurodegenerative diseases remains an active area of research, as does how different lifestyle factors regulate microglia to potentially lead to increased susceptibility to neurodegeneration. It is important to consider that neurodegenerative diseases are complex and multi-factorial, and an individual’s predisposition to neurodegenerative disease is likely driven by numerous other factors as well. Recent findings have made clear the importance and impact of the gut microbiome on microglial phenotype and pathology in models of neurodegenerative diseases. However, further work remains to be done on how microglia and the gut microbiome might interact and whether findings will be validated in human studies. Furthermore, precisely how the composition of the human gut microbiome over the lifespan, potentially changing with dietary choices, living environments, and age, contributes to predisposition to neurodegenerative disease remains a necessary question to answer. Such information would allow individuals to make informed choices, as well as allowing potential governmental regulatory oversight over food options. Microglial immune response is controlled by the circadian clock. Circadian rhythm sleep disorders and sleep loss contribute to neurodegeneration via microglia-induced neuroinflammation and elevated protein aggregation. Circadian rhythm also strongly affects stress level and the composition of the gut microbiome, which have been shown to closely relate to neurodegeneration. Physical exercises and environmental enrichment play a neuroprotective role by mediating anti-inflammatory effects, promoting neurogenesis, and regulating the gut microbiota. Mediterranean diet patterns have been observed in different countries but are particularly effective in so-called blue zones. In these areas, including Sardinia, Italy; Okinawa, Japan; Loma Linda, California; Nicoya Peninsula (Costa Rica); and Icaria, Greece, populations share similarities in their diet pattern but also common features regarding lifestyle stress factors such as stress-free environment, regular physical exercise, and familial and social life (Buettner and Skemp, 2016), and a higher rate of longevity (Pes et al., 2013). In Sardinia, they also present lower cognitive deficits, better working memory performance, and lower levels of depressive symptoms associated to their lifestyle pattern (Fastame, 2014; Fastame et al., 2015; Fastame and Penna, 2014). Microbiome composition and sleep patterns should be investigated in these populations to put findings from animal models in a human context. Altogether, better use and control of our diet, environment, and our way of living might lead to decreased susceptibility to neurodegenerative disorders.
ACKNOWLEDGMENTS
O.B. is supported by the NIH National Institute of Neurological Disorders and Stroke (R01 NS088137, R21 NS104609, and R21 NS101673), National Institute on Aging (NIA; R01 AG051812 and R01 AG054672), and National Eye Institute (R01 EY027921); the National Multiple Sclerosis Society (5092A1); National Health and Medical Research Council Australia (RG180378); a Nancy Davis Foundation Faculty Award; a Cure Alzheimer’s Fund Award; the Amyotrophic Lateral Sclerosis Association; and Sanofi.
REFERENCES
- Abel T, Havekes R, Saletin JM, and Walker MP (2013). Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol 23, R774–R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkadhi KA (2012). Chronic psychosocial stress exposes Alzheimer’s disease phenotype in a novel at-risk model. Front. Biosci. (Elite Ed.) 4, 214–229. [DOI] [PubMed] [Google Scholar]
- Alkadhi KA, and Tran TT (2015). Chronic stress decreases basal levels of memory-related signaling molecules in area CA1 of at-risk (subclinical) model of Alzheimer’s disease. Mol. Neurobiol 52, 93–100. [DOI] [PubMed] [Google Scholar]
- Alonso A, Logroscino G, Jick SS, and Hernán MA (2009). Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study. Eur. J. Neurol 16, 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmeppen HC, Prox J, Puig B, Dohler F, Falker C, Krasemann S, and Glatzel M (2013). Roles of endoproteolytic a-cleavage and shedding of the prion protein in neurodegeneration. FEBS J 280, 4338–4347. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, and Young J (2011). Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes. Rev 12, e426–e437. [DOI] [PubMed] [Google Scholar]
- Baldy C, Fournier S, Boisjoly-Villeneuve S, Tremblay ME, and Kinkead R (2018). The influence of sex and neonatal stress on medullary microglia in rat pups. Exp. Physiol 103, 1192–1199. [DOI] [PubMed] [Google Scholar]
- Banasr M, and Duman RS (2007). Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol. Disord. Drug Targets 6, 311–320. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, and Duman RS (2007). Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol. Psychiatry 62, 496–504. [DOI] [PubMed] [Google Scholar]
- Banqueri M, Méndez M, Gómez-Lázaro E, and Arias JL (2019). Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 22, 563–570. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, and Maier SF (2011). Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J. Neurosci 31, 11578–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C, Wagner M, Wolfsgruber S, Ehrenreich H, and Schneider A; Alzheimer’s Disease Neuroimaging Initiative (2018). Impact of SSRI Therapy on Risk of Conversion From Mild Cognitive Impairment to Alzheimer’s Dementia in Individuals With Previous Depression. Am. J. Psychiatry 175, 232–241. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. ; Dominantly Inherited Alzheimer Network (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers KM, Brinkley TE, and Nicklas BJ (2010). Effect of exercise training on chronic inflammation. Clin. Chim. Acta 411, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, and Hefti F (1995). Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14, 717–730. [DOI] [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, and Cirelli C (2017). Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J. Neurosci 37, 5263–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, and Ferreira D (2010). The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol 140, 175–182. [DOI] [PubMed] [Google Scholar]
- Bisht K, Sharma K, and Tremblay ME (2018). Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 9, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht K, Sharma KP, Lecours C, Sánchez MG, El Hajj H, Milior G, Olmos-Alonso A, Gómez-Nicola D, Luheshi G, Vallières L, et al. (2016). Dark microglia: A new phenotype predominantly associated with pathological states. Glia 64, 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Franciotti R, Martinotti G, Vellante F, Flacco ME, Di Giannan-tonio M, Thomas A, and Onofrj M (2018). Post traumatic stress disorder heralding the onset of semantic frontotemporal dementia. J. Alzheimers Dis 63, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, and Small GW (2000). Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med 343, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, and Del Tredici K (2004). Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318, 121–134. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, and Cryan JF (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 108, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, Laws SM, Taddei K, Macaulay SL, Ames D, et al. ; AIBL Research Group (2016). The Relationship between sleep quality and brain amyloid burden. Sleep (Basel) 39, 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DG, Soto R, Yandamuri S, Stone C, Dickey L, Gomes-Neto JC, Pastuzyn ED, Bell R, Petersen C, Buhrke K, et al. (2019). The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. eLife 8, e47117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner D, and Skemp S (2016). Blue Zones: lessons from the world’s longest lived. Am. J. Lifestyle Med 10, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, Greco DJ, Wu PM, Doykan CE, Kiner O, et al. (2015). Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol 77, 75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. (2014). Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, et al. (2012). Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Invest 122, 3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, and Visioli F (2017). Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev 40, 95–119. [DOI] [PubMed] [Google Scholar]
- Caraci F, Spampinato SF, Morgese MG, Tascedda F, Salluzzo MG, Giambirtone MC, Caruso G, Munafò A, Torrisi SA, Leggio GM, et al. (2018). Neurobiological links between depression and AD: The role of TGF-β1 signaling as a new pharmacological target. Pharmacol. Res 130, 374–384. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, and Trojanowski JQ (2011). Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a cortico-tropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci 31, 14436–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R, Monaghan-Nichols AP, DeFranco DB, and Rudine AC (2016). Effects of antenatal glucocorticoids on the developing brain. Steroids 114, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, and Almeida OF (2009). The amyloidogenic potential and behavioral correlates of stress. Mol. Psychiatry 14, 95–105. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. (2017). Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 114, 10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB, Gean PW, Chuang DM, and Hong JS (2007). Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 149, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Zhang X, and Huang WJ (2016). Role of physical exercise in Alzheimer’s disease. Biomed. Rep 4, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, et al. (2013). A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4, 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, et al. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, Breitner JC, Ferruci L, Resnick SM, and Thambisetty M (2016). Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol. Psychiatry 21, 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 108 (Suppl 1), 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Postigo M, Queipo-Ortuño MI, Murri M, Boto-Ordoñez M, Perez-Martinez P, Andres-Lacueva C, Cardona F, and Tinahones FJ (2012). Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res 53, 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, and Bercik P (2012). The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol 10, 735–742. [DOI] [PubMed] [Google Scholar]
- Cope EC, LaMarca EA, Monari PK, Olson LB, Martinez S, Zych AD, Katchur NJ, and Gould E (2018). Microglia play an active role in obesity-associated cognitive decline. J. Neurosci 38, 8889–8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, and Pericak-Vance MA (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, and Knight R (2009). Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CSM, Stylianakis AA, and Richardson R (2019). Early-life stress, microbiota, and brain development: probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev. Cogn. Neurosci 37, 100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, De Jager PL, Bennett DA, and Desplats P (2017). Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement 13, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, et al. (2015). Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 112, 7231–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. ; MICRO-Obes Consortium (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Bryan J, Hodgson J, and Murphy K (2015). Definition of the Mediterranean diet; a literature review. Nutrients 7, 9139–9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, et al. (2016). High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821. [DOI] [PubMed] [Google Scholar]
- Dean DC 3rd, Jerskey BA, Chen K, Protas H, Thiyyagura P, Roontiva A, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, et al. (2014). Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol 71, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech JC, Wei L, Hao J, Yu X, Madore C, Butovsky O, and Kaffman A (2016). Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun 57, 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, and Relman DA (2008). The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 6, e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya HB, Kuntz T, Shaik SM, Baufeld C, Leibowitz J, Zhang X, Gottel N, Zhang X, Butovsky O, Gilbert JA, and Sisodia SS (2019). Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med 216, 1542–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv 5, eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar BS, Crowther JS, Goddard P, Hawksworth G, Hill MJ, Peach S, Williams RE, and Renwick A (1973). The relation between diet and the gut microflora in man. Proc. Nutr. Soc 32, 49–52. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zitting KM, and Chinoy ED (2015). Aging and circadian rhythms. Sleep Med. Clin 10, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaine JE, and Ashley NT (2015). Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am. J. Physiol. Regul. Integr. Comp. Physiol 308, R1062–R1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, deSousa Rodrigues ME, Johnson MA, Barnum CJ, Duke BJ, Yang Y, Chang J, Kelly SD, Wildner M, Tesi RJ, and Tansey MG (2019). Chronic psychological stress during adolescence induces sex-dependent adulthood inflammation, increased adiposity, and abnormal behaviors that are ameliorated by selective inhibition of soluble tumor necrosis factor with XPro1595. Brain Behav. Immun 81, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, and van Duijn CM; APOE and Alzheimer Disease Meta Analysis Consortium (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, Barbaro F, Piano C, Fortuna S, Tortora A, et al. (2013). The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord 28, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Fastame MC (2014). Exploring the effect of depressive symptoms and ageing on metamemory in an Italian adult sample. Psychol. Health Med 19, 127–135. [DOI] [PubMed] [Google Scholar]
- Fastame MC, Hitchcott PK, and Penna MP (2015). Do self-referent meta-cognition and residential context predict depressive symptoms across late-life span? A developmental study in an Italian sample. Aging Ment. Health 19, 698–704. [DOI] [PubMed] [Google Scholar]
- Fastame MC, and Penna MP (2014). Psychological well-being and meta-cognition in the fourth age: an explorative study in an Italian oldest old sample. Aging Ment. Health 18, 648–652. [DOI] [PubMed] [Google Scholar]
- Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, and Lovegrove JA (2013). The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes 37, 216–223. [DOI] [PubMed] [Google Scholar]
- Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, and Barberger-Gateau P (2009). Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 302, 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes NC, Sriram U, Gofman L, Cenna JM, Ramirez SH, and Potula R (2016). Methamphetamine alters microglial immune function through P2X7R signaling. J. Neuroinflammation 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, Erreni M, Markicevic M, Starvaggi-Cucuzza C, Otero K, et al. (2018). The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48, 979–991. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, and Maier SF (2015). Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav. Immun 45, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitt MM, Gaudet AD, Barrientos RM, Watkins LR, and Maier SF (2016a). Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol. Aging 47, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Weber MD, Daut RA, Kitt MM, Frank MG, Watkins LR, and Maier SF (2016b). Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology 66, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Bienenstock J, and Kunze WA (2014). Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol 817, 115–133. [DOI] [PubMed] [Google Scholar]
- Fox M, Berzuini C, and Knapp LA (2013). Maternal breastfeeding history and Alzheimer’s disease risk. J. Alzheimers Dis 37, 809–821. [DOI] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, and Maier SF (2014). Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology 40, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, and Maier SF (2012). Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun 26, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch MG, Szynkaruk M, Prout AP, Nygard K, Cao M, Veldhuizen R, Hammond R, and Richardson BS (2016). Decreased neuroinflammation correlates to higher vagus nerve activity fluctuations in near-term ovine fetuses: a case for the afferent cholinergic anti-inflammatory pathway? J. Neuroinflammation 13, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Puckett L, Petrovic S, Xia W, Chen G, Vega J, Dembinsky-Vaknin A, Shen J, Plante M, Burt DS, and Weiner HL (2008). A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann. Neurol 63, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, et al. (2010). Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Pinchera A, Piaggi P, Fierabracci P, Giannetti M, Querci G, Scartabelli G, Manetti L, Ceccarini G, Martinelli S, et al. (2012). Serum insulin-like growth factor-1 concentrations are reduced in severely obese women and raise after weight loss induced by laparoscopic adjustable gastric banding. Obes. Surg 22, 1276–1280. [DOI] [PubMed] [Google Scholar]
- Gandy S, and Heppner FL (2013). Microglia as dynamic and essential components of the amyloid hypothesis. Neuron 78, 575–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, and Nimmo MA (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol 11, 607–615. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Del Tredici K, and Braak H (2013). 100 years of Lewy pathology. Nat. Rev. Neurol 9, 13–24. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, and Lyte M (2005). Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun 19, 334–344. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Simôes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graça Naffah-Mazzacoratti M, and Arida RM (2013). Exercise-induced hippocampal anti-inflammatory response in aged rats. J. Neuroinflammation 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González B, and Escobar A (2010). Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol 119, 303–315. [DOI] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, et al. ; Western ALS Study Group (2007). Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 6, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, and Glass CK (2014). Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-García P, de-la-Cámara C, Santabárbara J, Lopez-Anton R, Quintanilla MA, Ventura T, Marcos G, Campayo A, Saz P, Lyketsos C, and Lobo A (2015). Depression and incident Alzheimer disease: the impact of disease severity. Am. J. Geriatr. Psychiatry 23, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Rubio I, Moscoso-Castro M, Pozo OJ, Marcos J, Nadal R, and Valverde O (2016). Maternal separation induces neuroinflammation and long-lasting emotional alterations in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 65, 104–117. [DOI] [PubMed] [Google Scholar]
- Graham LC, Harder JM, Soto I, de Vries WN, John SW, and Howell GR (2016). Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of Alzheimer’s disease. Sci. Rep 6, 21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, and LaFerla FM (2006). Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J. Neurosci 26, 9047–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, and Landreth GE (2008). Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J. Neuroimmune Pharmacol 3, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. ; Alzheimer Genetic Analysis Group (2013). TREM2 variants in Alzheimer’s disease. N. Engl. J. Med 368, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, and Prinz M (2017). Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol 134, 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, and Davidson RJ (2015). Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry 77, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Haga S, Aoyama Y, and Kiriyama S (1999). Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr 129, 942–948. [DOI] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T, and Bolmont T (2017). Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep 7, 41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Koyanagi Y, Kusunose N, Okada R, Wu Z, Tozaki-Saitoh H, Ukai K, Kohsaka S, Inoue K, Ohdo S, and Nakanishi H (2013). The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin. Sci Rep 3, 2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler C, Borewicz K, Beijers R, Saccenti E, Riksen-Walraven M, Smidt H, and de Weerth C (2019). Association between Psychosocial Stress and Fecal Microbiota in Pregnant Women. Sci. Rep 9, 4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Fein-stein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, and Becher B (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, and Seckl JR (2006). Do corticosteroids damage the brain? J. Neuroendocrinol 18, 393–411. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, and El Khoury J (2013). The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci 16, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, et al. (2017). Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord 32, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ono K, Tsuji M, Mazzola P, Singh R, and Pasinetti GM (2018). Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother 18, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Lesuis SL, Krugers H, Lucassen PJ, and Korosi A (2018). A preclinical perspective on the enhanced vulnerability to Alzheimer’s disease after early-life stress. Neurobiol. Stress 8, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Ruigrok SR, Amelianchik A, Ivan D, van Dam AM, Lucassen PJ, and Korosi A (2017). Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain Behav. Immun 63, 160–175. [DOI] [PubMed] [Google Scholar]
- Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, et al. (2019). The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman IR, Raj DD, Miller JA, Schaafsma W, Yin Z, Brouwer N, Wes PD, Möller T, Orre M, Kamphuis W, et al. (2015). Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol. Commun 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, and Kim Y (2019). Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci 73, 143–153. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. (2012). A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot A, Neveu D, Aujoulat F, Mercier G, Marchandin H, Jumas-Bilak E, and Picaud JC (2011). Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J. Pediatr 158, 390–396. [DOI] [PubMed] [Google Scholar]
- Jagust W (2016). Is amyloid-b harmful to the brain? Insights from human imaging studies. Brain 139, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. (2016). Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun 7, 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joers V, Tansey MG, Mulas G, and Carta AR (2017). Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol 155, 57–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med 368, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Chu J, and Praticò D (2012). Stress hormone leads to memory deficits and altered tau phosphorylation in a model of Alzheimer’s disease. J. Alzheimers Dis 31, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Rinne JO, Hermann B, Karrasch M, Anttinen A, Shinnar S, and Sillanpää M (2017). Association Between Childhood-Onset Epilepsy and Amyloid Burden 5 Decades Later. JAMA Neurol 74, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk R, Tejera D, Simon BJ, and Heneka MT (2017). Microglia modulation through external vagus nerve stimulation in a murine model of Alzheimer’s disease. J. Neurochem 10.1111/jnc.14284. [DOI] [PubMed] [Google Scholar]
- Kaffman A, and Meaney MJ (2007). Neurodevelopmental sequelae of post-natal maternal care in rodents: clinical and research implications of molecular insights. J. Child Psychol. Psychiatry 48, 224–244. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Kooijman L, Schetters S, Orre M, and Hol EM (2016). Transcriptional profiling of CD11c-positive microglia accumulating around amyloid plaques in a mouse model for Alzheimer’s disease. Biochim. Biophys. Acta 1862, 1847–1860. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, and Holtzman DM (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Jeraldo PR, Kurti A, Miller ME, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, and Fryer JD (2014). Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan C (2001). Immune thrombocytopenia in the foetus and the newborn: diagnosis and therapy. Transfus. Clin. Biol 8, 311–314. [DOI] [PubMed] [Google Scholar]
- Karch CM, and Goate AM (2015). Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 77, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, and Wells JM (2010). Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol 298, G851–G859. [DOI] [PubMed] [Google Scholar]
- Katsumoto A, Takeuchi H, Takahashi K, and Tanaka F (2018). Microglia in Alzheimer’s Disease: risk factors and inflammation. Front. Neurol 9, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM (2018). Exercise-induced modulation of neuroinflammation in models of Alzheimer’s disease. Brain Plast 4, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, and Hyland NP (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. (2017). A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, and Shannon KM (2015). Colonic bacterial composition in Parkinson’s disease. Mov. Disord 30, 1351–1360. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Søndergård L, Forman JL, and Andersen PK (2009). Antidepressants and dementia. J. Affect. Disord 117, 24–29. [DOI] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, et al. (2019). Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, and Nissinen A (2005). Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol 62, 1556–1560. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, and Prinssen EP (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol 10, 643–660. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Saka-moto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, and Kado-matsu K (2013). Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4, e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, and Rhodes JS (2013). Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation 10, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Grekas A, Christou A, Chatzigeorgiou M, Skoumas I, Tousoulis D, and Stefanadis C; ATTICA Study Group (2016). Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab. Res. Rev 32, 73–81. [DOI] [PubMed] [Google Scholar]
- Kosmidou M, Katsanos AH, Katsanos KH, Kyritsis AP, Tsivgoulis G, Christodoulou D, and Giannopoulos S (2017). Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J. Neurol 264, 254–259. [DOI] [PubMed] [Google Scholar]
- Koval ED, Shaner C, Zhang P, du Maine X, Fischer K, Tay J, Chau BN, Wu GF, and Miller TM (2013). Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet 22, 4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K, and Mulak A (2019). Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil 25, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, and Yirmiya R (2014). Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 19, 699–709. [DOI] [PubMed] [Google Scholar]
- Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, et al. (2012). Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflammation 9, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, and Sisodia SS (2005). Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120, 701–713. [DOI] [PubMed] [Google Scholar]
- Leal LG, Lopes MA, and Batista ML Jr. (2018). Physical exercise-induced myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front. Physiol 9, 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, and Benveniste H (2015). The effect of body posture on brain glymphatic transport. J. Neurosci 35, 11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, and Mazmanian SK (2011). Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108 (Suppl 1), 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem YH, Lee YI, Son HJ, and Lee SH (2011). Chronic exercise ameliorates the neuroinflammation in mice carrying NSE/htau23. Biochem. Biophys. Res. Commun 406, 359–365. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Cooper HA, Maric D, and Herkenham M (2016). Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J. Neuroinflammation 13, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SZ, Barnett HL, Bierman CW, and McNAMARA H (1951). Some clinical and metabolic responses of premature infants to corticotrophin (ACTH). AMA Am. J. Dis. Child 82, 236–237. [PubMed] [Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CA, and Elinav E (2017). Dysbiosis and the immune system. Nat. Rev. Immunol 17, 219–232. [DOI] [PubMed] [Google Scholar]
- Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, Gulati G, Bennett ML, Sun LO, Clarke LE, et al. (2019). Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell rna sequencing. Neuron 101, 207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LY, Zhang J, Dai XM, Xiao NA, Wu XL, Wei Z, Fang WT, Zhu YG, and Chen XC (2016). Early-life stress leads to impaired spatial learning and memory in middle-aged ApoE4-TR mice. Mol. Neurodegener 11, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineweaver TT, Bondi MW, Galasko D, and Salmon DP (2014). Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am. J. Psychiatry 171, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Liu CC, Kanekiyo T, Xu H, and Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, and Llewellyn DJ (2013). Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 24, 479–489. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H, Pieber M, Parsa R, Grommisch D, Ewing E, Kular L, Han J, Zhu K, Nijssen J, Hedlund E, et al. (2018). Fatal demyelinating disease is induced by monocyte-derived macrophages in the absence of TGF-b signaling. Nat. Immunol 19, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, and Rall SC Jr. (2000). Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet 1, 507–537. [DOI] [PubMed] [Google Scholar]
- Mailing LJ, Allen JM, Buford TW, Fields CJ, and Woods JA (2019). Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport Sci. Rev 47, 75–85. [DOI] [PubMed] [Google Scholar]
- Mass E, Jacome-Galarza CE, Blank T, Lazarov T, Durham BH, Ozkaya N, Pastore A, Schwabenland M, Chung YR, Rosenblum MK, et al. (2017). A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature 549, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, Scheiwe C, Nessler S, Kunz P, van Loo G, et al. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, et al. (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670. [DOI] [PubMed] [Google Scholar]
- Maurer M, Riesen W, Muser J, Hulter HN, and Krapf R (2003). Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol 284, F32–F40. [DOI] [PubMed] [Google Scholar]
- Mazur-Bialy AI, Bilski J, Pochec E, and Brzozowski T (2017). New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J. Physiol. Pharmacol 68, 243–251. [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, and McGeer EG (1987). Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett 79, 195–200. [DOI] [PubMed] [Google Scholar]
- Meneses G, Bautista M, Florentino A, Díaz G, Acero G, Besedovsky H, Meneses D, Fleury A, Del Rey A, Gevorkian G, et al. (2016). Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J. Inflamm. (Lond.) 13, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Masliah E, Roberts JA, McKay H, Kordower JH, Mufson EJ, and Tuszynski MH (2011). Association of early experience with neurodegeneration in aged primates. Neurobiol. Aging 32, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz LM, and Eliasziw M (2017). Trial of minocycline in clinically isolated syndrome of multiple sclerosis. N. Engl. J. Med 377, 789. [DOI] [PubMed] [Google Scholar]
- Meyer JS, and Hamel AF (2014). Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. ILAR J 55, 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud JP, Bellavance MA, Préfontaine P, and Rivest S (2013). Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep 5, 646–653. [DOI] [PubMed] [Google Scholar]
- Miner B, and Kryger MH (2017). Sleep in the aging population. Sleep Med. Clin 12, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, Oyler-Castrillo P, Zhang X, Musch MW, Shen X, et al. (2017). Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1ΔE9 murine model of Alzheimer’s disease. Sci. Rep 7, 10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, et al. (2016). Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep 6, 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, et al. (2015). Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS ONE 10, e0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VA, and Pike CJ (2017). Obesity accelerates Alzheimer-related pathology in APOE4 but not APOE3 mice. eNeuro 4, ENEURO.0077–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, and Hu FB (2011). Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med 364, 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, and Selkoe DJ (2012). Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb. Perspect. Med 2, a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, and Ernst M (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia 48, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, and Holtzman DM (2016). Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, and Ju YS (2018). Circadian Rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol 75, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, et al. (2013). Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest 123, 5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM (2007). Maternal-newborn nurses’ experiences of inconsistent professional breastfeeding support. J. Adv. Nurs 60, 29–38. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, and Chawla A (2013a). Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341, 1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ND, Tucker MA, Stickgold R, and Wamsley EJ (2013b). over-night sleep enhances hippocampus-dependent aspects of spatial memory. Sleep (Basel) 36, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgampalle M, and Kuna Y (2017). Anti-alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in alzheimer’s Disease induced albino rats. J. Clin. Diagn. Res 11, KC01–KC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SS, Williams DR, Gallagher DA, Massey LA, Silveira-Moriyama L, and Lees AJ (2008). Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov. Disord 23, 101–106. [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, and Kasper LH (2009). Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol 183, 6041–6050. [DOI] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, and Claassen JA (2014). Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 71, 971–977. [DOI] [PubMed] [Google Scholar]
- Ortega E (2016). The “bioregulatory effect of exercise” on the innate/inflammatory responses. J. Physiol. Biochem 72, 361–369. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, Mills PJ, Khandrika S, and Galasko D (2007). The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol. Psychiatry 62, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, and Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl.) 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Nievas BG, Hammerschmidt T, Kummer MP, Terwel D, Leza JC, and Heneka MT (2011). Restraint stress increases neuroinflammation independently of amyloid b levels in amyloid precursor protein/PS1 transgenic mice. J. Neurochem 116, 43–52. [DOI] [PubMed] [Google Scholar]
- Pes GM, Tolu F, Poulain M, Errigo A, Masala S, Pietrobelli A, Battistini NC, and Maioli M (2013). Lifestyle and nutrition related to male longevity in Sardinia: an ecological study. Nutr. Metab. Cardiovasc. Dis 23, 212–219. [DOI] [PubMed] [Google Scholar]
- Phan TX, and Malkani RG (2018). Sleep and circadian rhythm disruption and stress intersect in Alzheimer’s disease. Neurobiol. Stress 10, 100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires GN, Bezerra AG, Tufik S, and Andersen ML (2016). Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med 24, 109–118. [DOI] [PubMed] [Google Scholar]
- Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, Manna PP, Giampieri F, and Battino M (2018). Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: a focus on human studies. Pharmacol. Res 131, 32–43. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, and Sanacora G (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci 13, 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado Lima MG, Schimidt HL, Garcia A, Daré LR, Carpes FP, Izquierdo I, and Mello-Carpes PB (2018). Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc. Natl. Acad. Sci. USA 115, E2403–E2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, and Morris JC (1999). Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol 45, 358–368. [DOI] [PubMed] [Google Scholar]
- Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L, de Candia P, Galgani M, De Rosa V, and Matarese G (2016). Role of metabolism in neurodegenerative disorders. Metabolism 65, 1376–1390. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, and Crews FT (2008). Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Garrison BS, Ma W, Wang R, Jiang A, Li J, Mistry M, Bronson RT, Santoro D, Franco C, et al. (2018). A milieu molecule for TGF-b required for microglia function in the nervous system. Cell 174, 156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Kivipelto M, and von Strauss E (2009). Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci 11, 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, and Tinahones FJ (2012). Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr 95, 1323–1334. [DOI] [PubMed] [Google Scholar]
- Ramanan VK, and Saykin AJ (2013). Pathways to neurodegeneration: mechanistic insights from GWAS in Alzheimer’s disease, Parkinson’s disease, and related disorders. Am. J. Neurodegener. Dis 2, 145–175. [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Niraula A, and Sheridan JF (2016). GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav. Immun 51, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE (2010). Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton. Neurosci 153, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BS, Weisburger JH, and Wynder EL (1975). Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J. Nutr 105, 878–884. [DOI] [PubMed] [Google Scholar]
- Reynolds AC, and Banks S (2010). Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog. Brain Res 185, 91–103. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, and Pittman QJ (2008). Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA 105, 17151–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, and Kraneveld AD (2017). Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochfort KD, Collins LE, Murphy RP, and Cummins PM (2014). Down-regulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for inter-endothelial adherens and tight junctions. PLoS ONE 9, e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque A, Ochoa-Zarzosa A, and Torner L (2016). Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun 55, 39–48. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, et al. (2016). Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, and Mattson MP (2012). 3xTgAD mice exhibit altered behavior and elevated Aβ after chronic mild social stress. Neurobiol. Aging 33, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougé C, Goldenberg O, Ferraris L, Berger B, Rochat F, Legrand A, Göbel UB, Vodovar M, Voyer M, Rozé JC, et al. (2010). Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe 16, 362–370. [DOI] [PubMed] [Google Scholar]
- Rowin J, Xia Y, Jung B, and Sun J (2017). Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep 5, e13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s Disease. Cell 167, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, and McEwen BS (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci 5, 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlus H, and Heneka MT (2017). Microglia in Alzheimer’s disease. J. Clin. Invest 127, 3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Klockgether T, and Heneka MT (2006). Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int. J. Dev. Neurosci 24, 167–176. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, and Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, and Giedd JN (2007). Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol 6, 494–500. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, and Naaz A (2007). Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180. [DOI] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, et al. (2018). β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 115, 4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, and Rivest S (2006). Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502. [DOI] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur C, Wallendorf M, Neil J, and Inder T (2011). Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol 70, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, and French JA (1997). Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli). Physiol. Behav 62, 225–232. [DOI] [PubMed] [Google Scholar]
- Sommer F, and Bäckhed F (2013). The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol 11, 227–238. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, and Antoch MP (2012). Core circadian protein CLOCK is a positive regulator of NF-kB-mediated transcription. Proc. Natl. Acad. Sci. USA 109, E2457–E2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, and Resnick SM (2013). Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol 70, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spor A, Koren O, and Ley R (2011). Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol 9, 279–290. [DOI] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, and Benca RM (2015). Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol. Aging 36, 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Friedman BA, Larson JL, Lauffer BE, Goldstein LD, Appling LL, Borneo J, Poon C, Ho T, Cai F, et al. (2016). Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat. Commun 7, 11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Miller DB, and O’Callaghan JP (2006). Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J. Neurochem 96, 706–718. [DOI] [PubMed] [Google Scholar]
- Srivareerat M, Tran TT, Alzoubi KH, and Alkadhi KA (2009). Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer’s disease. Biol. Psychiatry 65, 918–926. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Vasconcelos MF, Albrechet-Souza L, Ceresér KMM, and de Almeida RMM (2017). Microglial Over-Activation by Social Defeat Stress Contributes to Anxietyand Depressive-Like Behaviors. Front. Behav. Neurosci 11, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, and Sparks DL (2004). Dystrophic microglia in the aging human brain. Glia 45, 208–212. [DOI] [PubMed] [Google Scholar]
- Streit WJ, and Xue QS (2009). Life and death of microglia. J. Neuroimmune Pharmacol 4, 371–379. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, and Roses AD (1995). Apolipoprotein E and Alzheimer disease. Proc. Natl. Acad. Sci. USA 92, 4725–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, and Roses AD (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, and Shen YQ (2018). Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-a signaling pathway. Brain Behav. Immun 70, 48–60. [DOI] [PubMed] [Google Scholar]
- Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, and Sørensen HT (2015a). Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol 78, 522–529. [DOI] [PubMed] [Google Scholar]
- Svensson M, Lexell J, and Deierborg T (2015b). Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabil. Neural Repair 29, 577–589. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Raadsheer FC, Endert E, Hofman MA, Kamphorst W, and Ravid R (1994). Increased cortisol levels in aging and Alzheimer’s disease in postmortem cerebrospinal fluid. J. Neuroendocrinol 6, 681–687. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, and Wu MN (2015). Sleep interacts with Aβ to modulate intrinsic neuronal excitability. Curr. Biol 25, 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama F, Hayashi Y, Wu Z, Liu Y, and Nakanishi H (2016). Diurnal dynamic behavior of microglia in response to infected bacteria through the UDP-P2Y6 receptor system. Sci. Rep 6, 30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM, Ng SW, Ang SP, Chow SK, Tan CT, et al. (2014a). Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord 20, 535–540. [DOI] [PubMed] [Google Scholar]
- Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, and Macia L (2014b). The role of short-chain fatty acids in health and disease. Adv. Immunol 121, 91–119. [DOI] [PubMed] [Google Scholar]
- Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, Vanande IP, Cook S, Gandhi R, Glanz B, et al. (2018). Investigation of probiotics in multiple sclerosis. Mult. Scler 24, 58–63. [DOI] [PubMed] [Google Scholar]
- Tapia-Rojas C, Aranguiz F, Varela-Nallar L, and Inestrosa NC (2016). Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol 26, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Béchade C, D’Andrea I, St-Pierre MK, Henry MS, Roumier A, and Tremblay ME (2018a). Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan. Front. Mol. Neurosci 10, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Sagar, Dautzenberg J, Grün D, and Prinz M (2018b). Unique microglia recovery population revealed by single-cell RNAseq following neurodegeneration. Acta Neuropathol. Commun 6, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, et al. (2018). Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, and Moschen AR (2015). Food, immunity, and the microbiome. Gastroenterology 148, 1107–1119. [DOI] [PubMed] [Google Scholar]
- Tong L, Gong Y, Wang P, Hu W, Wang J, Chen Z, Zhang W, and Huang C (2017). Microglia loss contributes to the development of major depression induced by different types of chronic stresses. Neurochem. Res 42, 2698–2711. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, Graves J, Lynch S, and Waubant E; US Network of Pediatric MS Centers (2016). Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol 23, 1308–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, and Waubant E (2018). Gut microbiome and pediatric multiple sclerosis. Mult. Scler 24, 64–68. [DOI] [PubMed] [Google Scholar]
- Ubeda C, and Pamer EG (2012). Antibiotics, microbiota, and immune defense. Trends Immunol 33, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JD, and Holtzman DM (2016). TREM2 function in Alzheimer’s disease and neurodegeneration. ACS Chem. Neurosci 7, 420–427. [DOI] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, and Schäfer KH (2016). Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord 32, 66–72. [DOI] [PubMed] [Google Scholar]
- Uranga JA, López-Miranda V, Lombó F, and Abalo R (2016). Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol. Rep 68, 816–826. [DOI] [PubMed] [Google Scholar]
- van de Rest O, Berendsen AA, Haveman-Nies A, and de Groot LC (2015). Dietary patterns, cognitive decline, and dementia: a systematic review. Adv. Nutr 6, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer JH, van der Poll T, and van ‘t Veer C (2014). TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood 123, 2460–2469. [DOI] [PubMed] [Google Scholar]
- Vaughn AC, Cooper EM, DiLorenzo PM, O’Loughlin LJ, Konkel ME, Peters JH, Hajnal A, Sen T, Lee SH, de La Serre CB, and Czaja K (2017). Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. (Warsz.) 77, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, Asthana S, Blennow K, Zetterberg H, Bendlin BB, and Rey FE (2018). The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther 10, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S, Rodrigues AJ, Silva JM, Tronche F, Almeida OF, Sousa N, and Sotiropoulos I (2016). Chronic stress and glucocorticoids: from neuronal plasticity to neurodegeneration. Neural Plast 2016, 6391686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa M, Kumari P, Chauhan G, Roy K, Alam S, Kishore K, Ray K, and Panjwani U (2017a). Sleep deprivation induces spatial memory impairment by altered hippocampus neuroinflammatory responses and glial cells activation in rats. J. Neuroimmunol 312, 38–48. [DOI] [PubMed] [Google Scholar]
- Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, Kishore K, Kumar S, and Panjwani U (2017b). Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J. Neuroinflammation 14, 222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, de Boer-Bergsma JJ, Martin NA, Karram K, et al. (2017). A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J 36, 3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, and Godbout JP (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Yi J, Zhang YG, Zhou J, and Sun J (2015). Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep 3, e12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen M, Liao Y, Thiyagarajan M, O’Donnell J, Christensen D, Nicholson C, Iliff J, et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Gelyana E, Rajsombath M, Yang T, Li S, and Selkoe D (2016). environmental enrichment potently prevents microglia-mediated neuroinflammation by human amyloid β-protein oligomers. J. Neurosci 36, 9041–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, Kluse M, and Marmar C (2010). Posttraumatic stress disorder and risk of dementia among US veterans. Arch. Gen. Psychiatry 67, 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Raj D, Saiepour N, Van Dam D, Brouwer N, Holtman IR, Eggen BJL, Möller T, Tamm JA, Abdourahman A, et al. (2017). Immune hyper-reactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiol. Aging 55, 115–122. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu YF, Huang L, et al. (2017a). Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis 60, 1241–1257. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, and Zhang J (2005). Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19, 533–542. [DOI] [PubMed] [Google Scholar]
- Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J, and Sun J (2017b). Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther 39, 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CN, Chao FL, Zhang Y, Jiang L, Zhang L, Fan JH, Wu YX, Dou XY, and Tang Y (2019). Fluoxetine delays the cognitive function decline and synaptic changes in a transgenic mouse model of early Alzheimer’s disease. J. Comp. Neurol 527, 1378–1387. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, and Schwartz M (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci 9, 268–275. [DOI] [PubMed] [Google Scholar]
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, and Lassmann H (2017). Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]


